Short abstract
Background
Three-dimensional (3D) reconstruction has been used for various diseases, but few reports have described its application in pelvic reconstruction after removal of giant chondrosarcoma. Case reports describing the clinical application of personalized 3D-printed titanium implants are needed for future clinical reference.
Case presentation: We herein describe a 29-year-old woman with a giant chondrosarcoma treated with a personalized 3D titanium implant. The surgery was successful, and the patient recovered with significant pain relief and good functional recovery after the surgery. No implant-related complications occurred during the 12-month follow-up. The current case represents successful application of 3D printing technology to the treatment of a massive bone defect due to the removal of a giant osteoporotic tumor.
Conclusions
Personalized 3D titanium implants can be used in the reconstruction of massive bone defects after the removal of giant pelvic sarcomas. The methodology and results described in the current case report can be a used as reference in the treatment of similar cases in future.
Keywords: Pelvis, chondrosarcoma, three-dimensional printing, titanium, implant, case report
Background
The incidence of malignant tumors is consistently on the rise because of environmental changes and the aging of the population. In patients with massive pelvic sarcomas, the bone defect after resection of an osteogenic tumor requires a large bone transplantation and implant fixation.1–3 However, complex implant systems are likely to fall apart in many patients shortly after surgery. Three-dimensional (3D) printing technology uses a digitized model and specific materials to print out the structure.4,5 Since its creation in the 1980s, 3D printing has been widely applied in various fields including the military, architecture, and medical fields.6,7 With the application of neoadjuvant chemotherapy, limb-salvage surgery has become a common procedure in the treatment of osseous sarcomas of the extremities. In patients who undergo massive tissue removal, individualized implants can be extremely helpful because many large bone defects cannot be easily bridged by standard implants.8,9 However, although 3D printing technology has been widely used for preoperative surgical planning, customized 3D-printed implants have rarely been used in clinical practice. We herein describe a patient treated with 3D-printed implants for reconstruction of the pelvic bone after removal of a giant chondrosarcoma.
Case presentation
A 29-year-old woman presented to our clinic with a 10-year history of a growing lump in the left thigh. The patient had experienced no pain associated with the lump, no pain or analgesia of the lower extremities, and no difficulty in urination or defecation; however, she reported some difficulty in mobility. Physical examination revealed normal mobility of the spine and lower extremities. A hard bump with a diameter of 15 × 15 cm was palpated in the right pelvis. Examination revealed no redness, swelling, or pain around the lump; no muscular atrophy or weakness; no dislocation or malformation of the joints; and no varicose veins in the lower extremities. The length of both legs was 71 cm; no discrepancy was observed in the length of the two legs. The Harris hip score before surgery was 86 as evaluated based on the patient’s self-reported pain, functional capacity, malformation of the lower extremity, and range of motion.
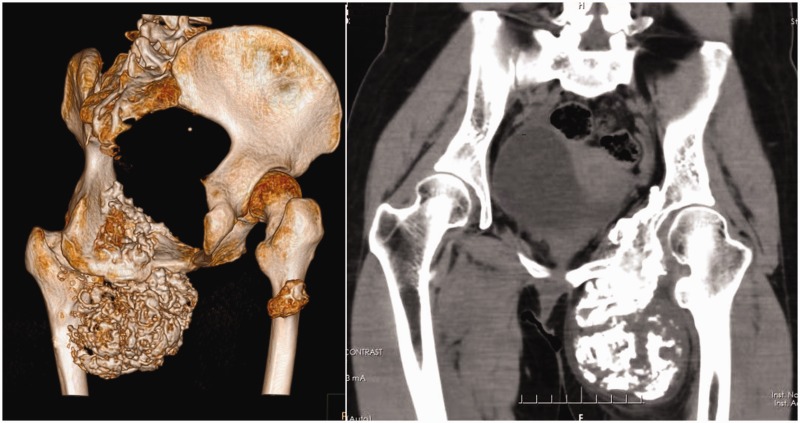
The outpatient X-ray showed a large bone and soft tissue tumor eroding the pubic bone, ischium, and acetabulum. The patient was admitted to the ward, and further radiological studies were carried out. Computed tomography (CT) and magnetic resonance imagery showed that the tumor had invaded the inner left thigh (Figure 1). CT angiography showed that the left femoral artery and deep femoral artery had been shifted by the tumor. A diagnosis of mucinous chondrosarcoma was suggested by fine needle biopsy.
Figure 1.
Three-dimensional reconstruction of the patient’s computed tomography scan.
After ruling out deficiencies of the circulatory, respiratory, urinary, and gastrointestinal systems, a multidisciplinary discussion was conducted among the oncologists, orthopedic surgeons, and researchers. Considering the large structural defect that would be present after removal of the tumor, intraoperative application of a 3D-printed implant was suggested. No chemotherapy was administered because of the nature of chondrosarcoma. A surgery was scheduled after the patient provided written informed consent. The 3D-printed implant was prepared by reconstruction of the patient’s CT scan (Figure 2). A chordoma was also found in the patient’s right femur. No surgical intervention was scheduled because the chordoma was not causing pain, analgesia, or loss of mobility.
Figure 2.
Design and mold of the three-dimensional implant used in the current case.
CT scans of the pelvic bone and upper one-third of the femoral bone were obtained by a CT scanner (Siemens Healthineers, Erlangen, Germany) with 1.0-mm slices. The data were stored and analyzed by Mimics 17.0 software (Materialise, Leuven, Belgium) in Digital Imaging and Communications in Medicine® format. After a 3D model of the pelvic bone had been constructed by Mimics 17.0 software, it was imported into Siemens NX software (Siemens PLM Software, Plano, TX, USA) to design the guiding template. When designing the template, the resection margin was extended 1 to 2 cm beyond the perimeter of the tumor. The design of the guiding template was stored in stereolithography format and printed by a UP BOX+ 3D printer (Beijing Tiertime Technology Co., Ltd., Beijing, China) using polylactic acid.
Although titanium alloys are widely used in orthopedic surgeries because of their outstanding biocompatibility and biomechanical characteristics, some reports have described implant loosening and bone resorption due to the stress shielding effect after the application of titanium implants.10 To avoid this effect, we used a porous structure 3D painted implant in which the diameter of each aperture was 200 µm, the extent of porosity was 38%, and the density was 2.8 g/m3. Suture anchors for fixation of the muscles and ligaments were designed on the implant according to the 3D-reconstructed CT data.
Surgical procedure
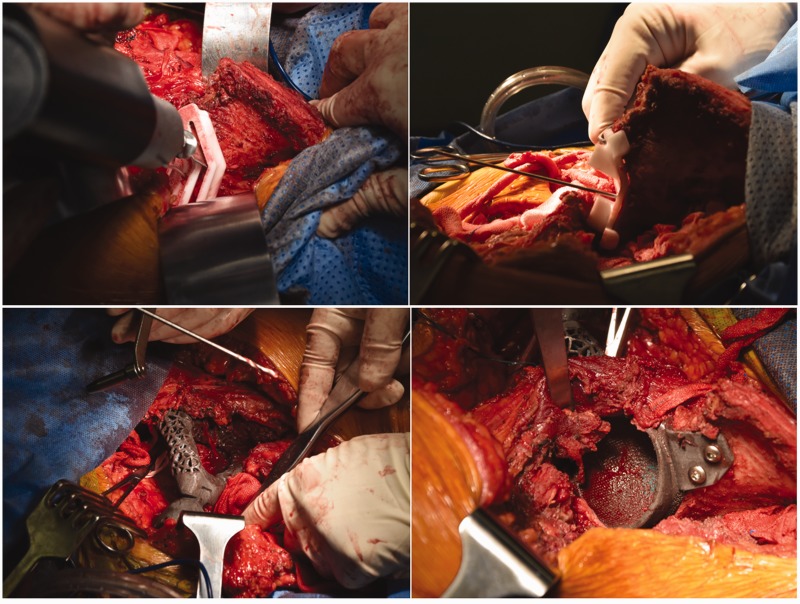
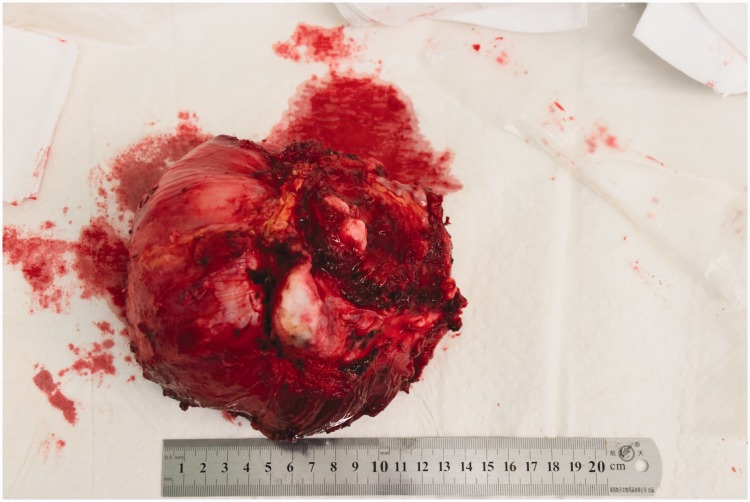
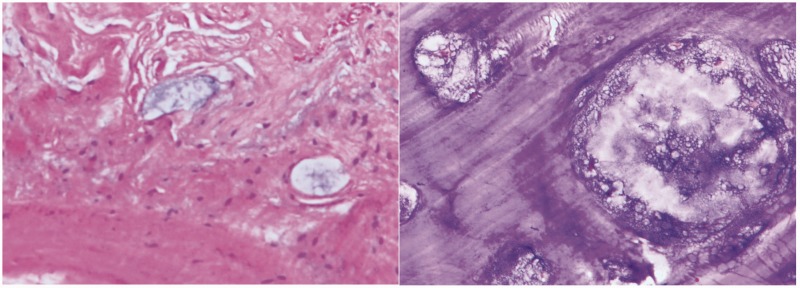
After all preoperative preparations were complete, en bloc tumor resection and 3D implant fixation were performed with the consent of the patient and her family (Figure 3). The surgery was carried out with the patient under general anesthesia. The patient was fixed in the right lateral position. After disinfection of the surgical field, the first incision was made horizontally on the crest of the ilium along the groin; a second incision was made longitudinally along the tumor and femoral vessels. The added length of the incision was 40 cm. After exposing the iliac periosteum, the tumor was isolated and resected using the 3D-printed guiding template. The left superior acetabulum, pubic symphysis, and femoral neck were resected and sent out for pathologic diagnosis. The cartilage on the right pubic symphysis was removed, and the implant was fixed on the bleeding bone bed with screws. The hip was abducted and externally rotated to expose the femoral neck, which was then sawed 1 cm superior to the lesser trochanter. After the reaming was deemed satisfactory, the hip joint was repositioned with a standard femoral neck and head. Intraoperative C-arm X-ray was used to confirm the position of the implants. The hip joint was dislocated, the bone cement was used to fill the 3D-printed acetabulum, and a polyethylene socket (#48; Johnson & Johnson, New Brunswick, NJ, USA) was fixed in the acetabulum in the 40° abduction and 20° anteversion position. After the bone cement was solid, a femoral stem prosthesis (#10; Johnson & Johnson) and femoral head prosthesis (M-SPEC metal Φ28 mm; Johnson & Johnson) were inserted, and the hip joint was restored. After assuring the mobility of the joint, the surgical field was washed with sterile physiological saline. The iliotibial tract was repaired; the quadriceps femoris muscle, iliacus muscle, and biceps femoris muscles were sutured onto the ischial tuberosity; and the incision was sutured in layers over a drainage tube. The whole surgery lasted 5 hours, and the intraoperative hemorrhage volume was 2000 mL. The removed tumor was a solid mass of 12 × 8 × 6 cm with clear margins (Figure 4). When the tumor was dissected after the surgery, white bone-like tissue with little tissue necrosis was observed. Pathological studies of the intraoperative specimen confirmed the preoperative diagnosis of mucinous chondrosarcoma (Figure 5).
Figure 3.
Surgical procedure of tumor resection and three-dimensional implant fixation.
Figure 4.
Tumor extraction during surgery.
Figure 5.
Pathological studies of the intraoperative specimen confirmed the preoperative diagnosis of mucinous chondrosarcoma.
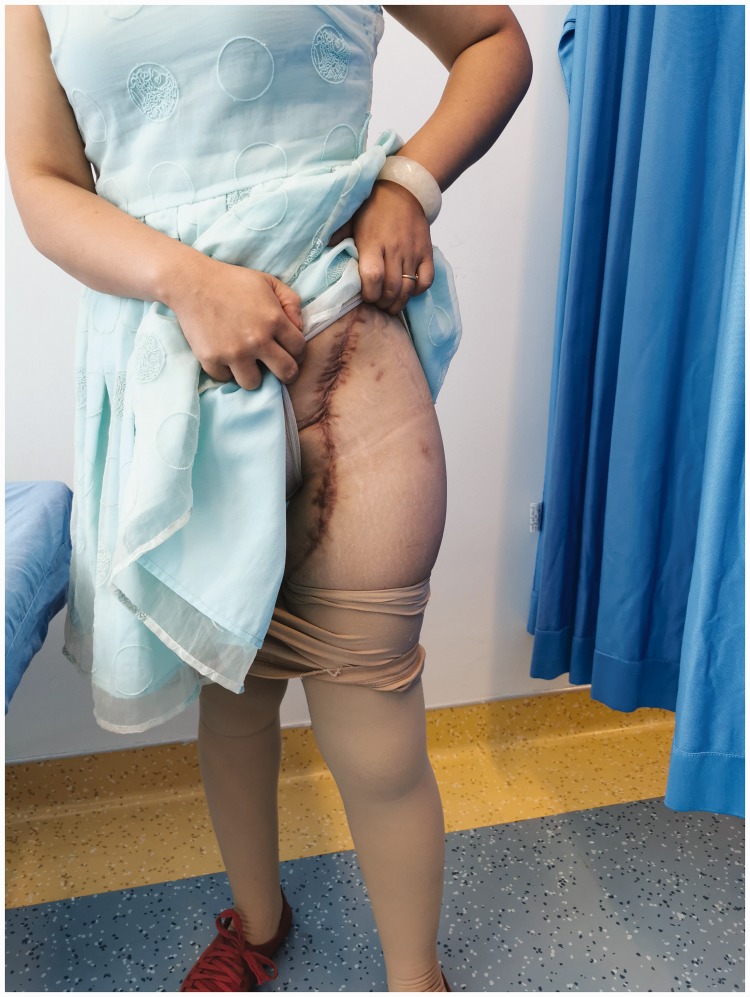
After the surgery, cefuroxime and vancomycin were used to prevent surgical site infection. The patient’s blood hemoglobin concentration was <80 g/L; therefore, 200 and 300 mL of suspended red blood cells were infused 48 and 72 hours postoperatively, respectively. The drainage tube was removed 7 days after surgery, and the surgical site healed without infection (Figure 6). The sutures were removed 14 days after surgery, and physiotherapy was started 1 month after surgery. The patient was scheduled for outpatient visits every 3 months.
Figure 6.
The surgical site healed without infection.
Results
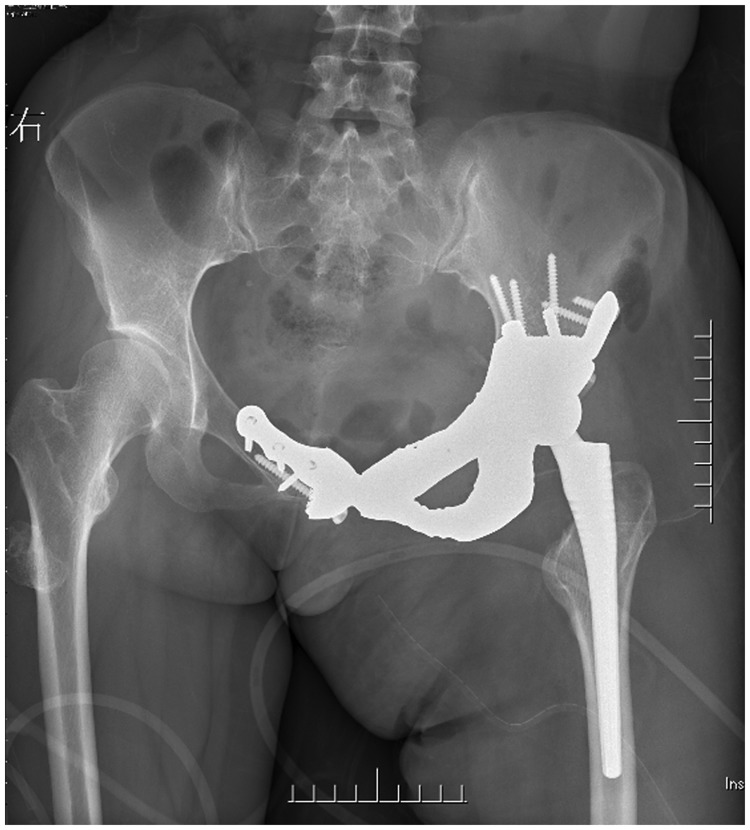
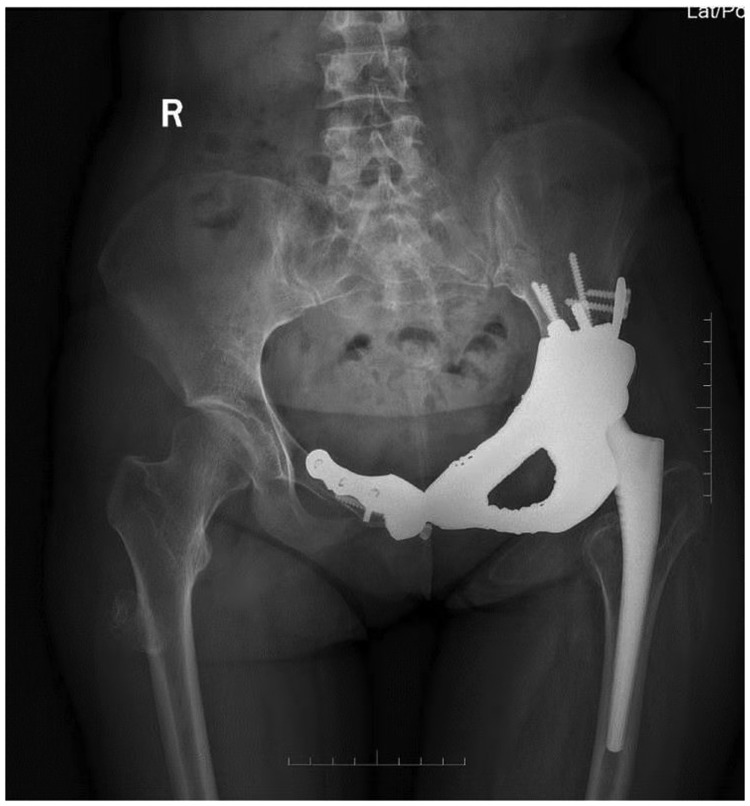
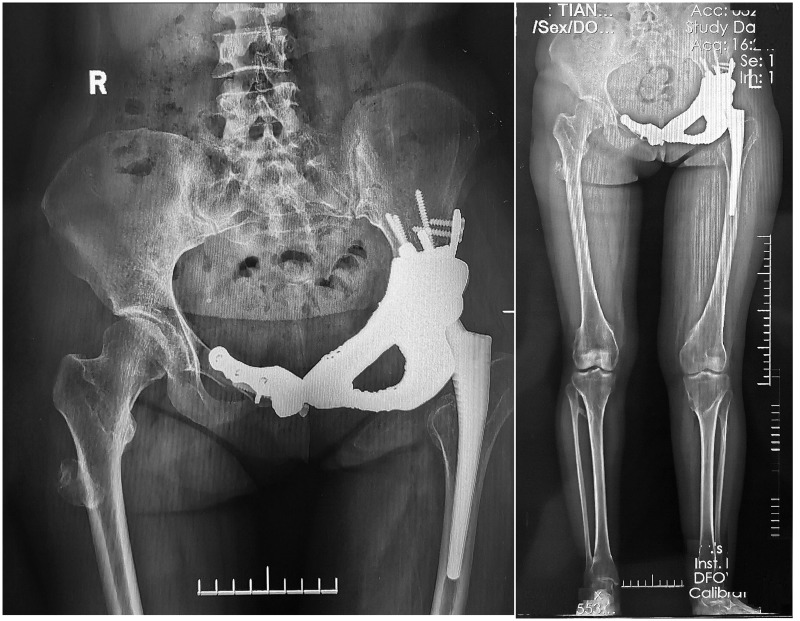
The patient achieved rapid recovery after the surgery, and no failure or loosening of the implant occurred after the patient began walking without an external aid (Figure 7). The length of the left leg and right leg was 72.5 and 71.0 cm, respectively. The Harris hip score was 73 at 1 month postoperatively, 79 at 6 months postoperatively, and 92 at 12 months postoperatively. No failure or loosening of the implant was evident at 6 months postoperatively (Figure 8, Video 1) or 12 months postoperatively (Figure 9, Video 2). The chordoma in the right femur did not progress during follow-up.
Figure 7.
No failure or loosening of the implant occurred when the patient started walking after the surgery.
Figure 8.
Video 1: No failure or loosening of the implant had occurred 6 months postoperatively.
Figure 9.
Video 2: No failure or loosening of the implant had occurred 12 months postoperatively.
Discussion
Chondrosarcoma may go unnoticed because of its typical clinical manifestations, such as lack of pain and minimal effect on patient mobility. Patients in rural areas may go without treatment of chondrosarcoma for many years. In the current case, the patient ignored the obvious lump in her left thigh for as long as 10 years. By the time she sought medical attention, the tumor had eroded the pelvic bone to an extent that the postoperative bone defect could not be easily bridged by conventional metallic implants and bone grafts. Biological and prosthetic reconstruction has been used as the standard for pelvic reconstruction. However, 3D printing technology has certain advantages over conventional techniques.11–13 It allows for printing of precise personalized implants using the patient’s X-ray, CT, and magnetic resonance imaging data, eliminating the need for a second surgery to harvest autologous bone grafts to fill the voids that might have been present with conventional implants. The accurate personalized design of the implants also reduces the need for allogeneic bone transplants, significantly reducing the duration of surgery.14,15 The total cost in our case was 96,380 Yuan ($13,768), which is similar to patients treated with conventional methods. However, because this technique is relatively new and not yet widely applied in clinical practice, the design and manufacturing of the customized implant could be costly.
Conventionally, the precision of en bloc resection of osseous sarcomas is dependent upon the surgeon’s experience. In our case, considering that the iliac tumor was an Enneking grade Ib chondrosarcoma, the consensus of the orthopedic, oncologic, and pathologic surgeons was removal of 1 to 2 cm of normal tissue, and the patient achieved good functional recovery with no recurrence.
With the application of 3D-printed intraoperative guidelines, high-precision tumor removal can be achieved with adequate preoperative planning by most surgeons.16,17 Because of the anatomic variations and differences in bone defects due to tumor removal among different patients, a more personalized implant with soft tissue docks can be beneficial for functional recovery. 3D printing can provide precise locations for the attachment of muscles and ligaments on the implants, increasing the stability of the implant and making it possible for the patients to achieve better functional recovery after surgery.18–20 We have herein reported a typical case of a complex pelvic tumor requiring a prosthetic replacement. 3D printing is currently a hot technology that conforms to the principle of precision therapy. It is an appropriate choice in complex orthopedic surgeries. In our case, despite the removal of a large portion of the pelvic bone and surrounding soft tissues, the patient achieved satisfactory mobility without implant loosening. Because 3D printing allows precise treatment, usually without the need for multiple intraoperative attempts to mold the acetabular cup, the duration of surgery was controlled within 5 hours, and most of this time was spent on removal of the giant tumor.
There are apparent disadvantages associated with this technique. The designing and printing process of 3D implants can be time-consuming. The patient in our case had to wait 1 month for completion of her 3D implant. Additional ethical and technical regulations may be needed for the application of this technique. However, considering that its advantages far outweigh its disadvantages and that most technical problems can be solved with further research, we are confident that 3D-printed implants will be widely applied in future to benefit millions of patients.
Abbreviations
3D: three-dimensional
CT: computed tomography
Authors’ contributions
GC and AM planned the research, analyzed the data, and wrote the manuscript. LY and HF carried out the surgical procedure. JL and CC stored the clinical materials and were in charge of patient follow-up. XW and PH analyzed the radiological data and prepared the 3D implant. FW and ZL planned the study, acquired the funds, and coordinated the whole study. All the authors read and approved the manuscript.
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Consent to publish
The patient provided written consent to publish her clinical materials.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics, consent, and permissions
The study was approved by the ethics committee of the Southwest Hospital of the Third Military Medical University of China.
Funding
This work was funded by the National Key Research and Development Plan (2016YFB11014040), Chongqing Municipal/Technology Platform and Base Construction (International Science and Technology Cooperation Base Construction) (cstc2014gjhz110003), Southwest Hospital Managed Project/Clinical Major New Technology Transformation and Breakthrough Plan (SWH2016ZDCX2010), and Southwest Hospital Managed Project/Platform Construction Special Project (SWH2016PTJS-04).
ORCID iD
Fuyou Wang https://orcid.org/0000-0001-9584-0851
Supplemental material
Supplemental material for this article is available online.
References
- 1.Wong KC. 3D-printed patient-specific applications in orthopedics. Orthop Res Rev 2016; 8: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017; 9: 024102. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PA. Clinical applications of 3D printing. Spine (Phila Pa 1976) 2017; 42: S30–S31. [DOI] [PubMed] [Google Scholar]
- 4.Xu N, Wei F, Liu X, et al. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with Ewing sarcoma. Spine (Phila Pa 1976) 2016; 41: E50–E54. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Xu L, Wang Y, et al. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Programs Biomed 2016; 125: 66–78. [DOI] [PubMed] [Google Scholar]
- 6.Liang H, Ji T, Zhang Y, et al. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Joint J 2017; 99: 267–275. [DOI] [PubMed] [Google Scholar]
- 7.Fan H, Fu J, Li X, et al. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: a case series and review of the literature. World J Surg Oncol 2015; 13: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan K, Sgro A, Maharaj MM, et al. Application of a 3D custom printed patient specific spinal implant for C1/2 arthrodesis. J Spine Surg 2016; 2: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamhamedi-Cherradi SE, Santoro M, Ramammoorthy V, et al. 3D tissue-engineered model of Ewing’s sarcoma. Adv Drug Deliv Rev 2014; 79: 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noyama Y, Miura T, Ishimoto T, et al. Bone loss and reduced bone quality of the human femur after total hip arthroplasty under stress-shielding effects by titanium-based implant. Mater Trans 2012; 53: 565–570. [Google Scholar]
- 11.Choy WJ, Mobbs RJ, Wilcox B, et al. Reconstruction of thoracic spine using a personalized 3D-printed vertebral body in adolescent with T9 primary bone tumor. World Neurosurg 2017; 105: 1032.e13–1032.e17. [DOI] [PubMed] [Google Scholar]
- 12.Gao S, Shen J, Hornicek F, et al. Three-dimensional (3D) culture in sarcoma research and the clinical significance. Biofabrication 2017; 9: 032003. [DOI] [PubMed] [Google Scholar]
- 13.Provaggi E, Leong JJH, Kalaskar DM. Applications of 3D printing in the management of severe spinal conditions. Proc Inst Mech Eng H 2017; 231: 471–486. [DOI] [PubMed] [Google Scholar]
- 14.Tappa K, Jammalamadaka U. Novel biomaterials used in medical 3D printing techniques. J Funct Biomater 2018; 9: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TM, Jin J, Lau TW, et al. The use of three-dimensional printing technology in orthopaedic surgery: a review. J Orthop Surg (Hong Kong) 2017; 25: 2309499016684077. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Zhu J, Peng X, et al. The application of 3D printed surgical guides in resection and reconstruction of malignant bone tumor. Oncol Lett 2017; 14: 4581–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartiaux O, Paul L, Francq BG, et al. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann Biomed Eng 2014; 42: 205–213. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Hao Y, Pu F, et al. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. Int Orthop 2018; 42: 687–694. [DOI] [PubMed] [Google Scholar]
- 19.Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery 2016; 159: 1485–1500. [DOI] [PubMed] [Google Scholar]
- 20.Mok SW, Nizak R, Fu SC, et al. From the printer: potential of three-dimensional printing for orthopaedic applications. J Orthop Translat 2016; 6: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.