Abstract
Bupropion and varenicline are widely prescribed pharmacological treatments for smoking cessation. These treatments are only marginally effective in clinical populations but most preclinical studies show that they are effective in decreasing self-administration in rats on a group level. The present study investigated individual differences in responding to bupropion or varenicline in a preclinical model of long-access to nicotine (0.03 mg/kg/inf; 12 h/day) in female rats. Rats were first assessed for their individual economic demand for nicotine and for their individual performance in open field and elevated plus maze prior to nicotine access and during withdrawal. Rats were then tested for the acute effects of bupropion, varenicline, and yohimbine. We found that neither bupropion nor varenicline decreased responding for nicotine on test days. On the contrary, a moderate dose of bupropion (30 mg/kg) significantly increased responding for nicotine. We also found that rats with higher demand for nicotine were more sensitive to pretreatment with yohimbine which resulted in increased responding for nicotine during the dose-effect tests. Finally, we show that rats that had a higher demand for nicotine also were more persistent in seeking nicotine during extinction and reinstatement tests with nicotine or yohimbine as triggers. Our findings suggest that the length of access to daily nicotine may be an important factor underlying the response to pharmacological treatments like bupropion or varenicline. Future studies modeling chronic treatment approaches that include both sexes will be needed to further extend our findings.
Keywords: Individualized treatment, Smoking cessation, Nicotine, Self-administration, Bupropion, Varenicline
1. Introduction
The use of tobacco products is responsible for more than 8 million deaths each year around the world [1]. The use of tobacco products is the leading cause of death, illness, and impoverishment worldwide [1]. In the United States alone, the cost of smoking-related illnesses is more than $300 billion each year including close to $170 billion for direct medical care and over $156 billion in lost productivity [2–4]. Although many tobacco users report a desire to quit, only a few are successful in doing so. A recent meta-analysis shows that nicotine replacement therapy increases cessation rates by about 7 %, bupropion by about 8.5 %, and varenicline by about 17 % over 10 % reported with placebo treatment [5]. In addition, there are currently no individualized treatment approaches that take into account consumption levels, length of tobacco use habit, or other individual factors. Thus, there is a significant gap in our understanding of how individual history with tobacco products contributes to nicotine dependence and whether or not individual history with tobacco products can predict treatment outcomes.
The current top three pharmacological interventions for smoking cessation include nicotine replacement therapies (NRT) and two medications that do not contain nicotine - bupropion and varenicline. Because bupropion and varenicline are more effective for smoking cessation than NRT they are widely prescribed around the world as a first treatment option. Bupropion, a medication that is also used to treat a major depressive disorder, is a selective inhibitor of noradrenaline and dopamine reuptake while also acting as a non-competitive antagonist at nicotinic acetylcholine receptors [nAChR’s; 6,7]. The exact mechanism by which bupropion enhances smoking cessation rates is not completely understood but the existing evidence implicates reduction of dopamine reuptake in the mesolimbic system and reduction of noradrenaline reuptake in the locus coeruleus [7–9]. Preclinical studies show mixed effects of bupropion pretreatment on nicotine self-administration in rodents that depend on the experimental design or dose. For example, some studies show no effect of acute or repeated bupropion treatment on nicotine self-administration [10–60 mg/kg; 10,11], while others show increased nicotine intake at lower doses [9 and 15 mg/kg; 12] or decreased responding at higher doses [30–75 mg/kg; 13,14–17]. Varenicline, on the other hand, is a partial agonist for the α4β2 and full agonist for α3β2 and α7 nAChR’s [6,7,18,19]. Varenicline is able to induce dopaminergic tone in the nucleus accumbens - a mechanism thought to be involved in reducing cravings and withdrawal effects associated with nicotine use. Acute and repeated treatment with varenicline reduces nicotine self-administration rates in rodents [19–21]. Both bupropion and varenicline can be discriminated from saline and share similar interoceptive properties with nicotine [for review see 22–24]. Importantly, there is evidence of individual variability in responding to these treatments in a preclinical model of nicotine use [17,25] and the underlying factors contributing to this variability are virtually unexplored [for review see 26].
There is a significant gap in understanding how individual history with nicotine contributes to nicotine use, relapse, and treatment outcomes. The majority of preclinical studies investigated the effectiveness of treatment strategies using grouped study designs. Although group study designs are important for the identification of potential treatments and treatment targets, they limit our understanding of individual factors that may predict or explain the favorable or unfavorable response to treatment. Likewise, most clinical studies also use group study designs and often use different inclusion criteria that further complicates generalizability of the outcomes to a wide range of users. For example, most clinical studies recruit smokers with high consumption levels and exclude light smokers that regularly use tobacco but do not meet predetermined levels of consumption for a study [27–29]. These recruitment strategies often result in studies that include a subset of tobacco users with a history of high consumption and with high motivation to quit (i.e., those responded to a recruitment call). One of the approaches that can increase the generalizability of study outcomes is to incorporate an individual level of assessment into experimental designs. By focusing on the individual, studies can use individual variability in nicotine consumption, length of use or other individual characteristics to predict response to treatment. Knowing the differences in treatment outcomes between individuals with higher or lower nicotine consumption can provide an initial step towards individualized prevention and treatment strategies.
We previously reported that rats vary in their responding for nicotine and that this variability relates to individual treatment outcomes [17]. In that study, male rats were self-administering nicotine using short-access (2 h) protocol and we used a behavioral economics approach to access individual variability in responding for nicotine [30–32]. Using this approach, responding for a substance is first stabilized on a variable schedule of reinforcement (VR3) where on average every third response is followed by an infusion. Rats then progress to earn each infusion on a fixed ratio (FR) schedule of reinforcement that is escalated daily (e.g., 1, 3, 5, 8, 12, etc.). Each rat progresses through these schedules until failing to earn at least one infusion over the course of a session. The individual economic demand for a drug is then derived from the amount of substance (mg/kg) consumed over each FR schedule of reinforcement [price; 32]. The essential value (EV) from the demand model is used to estimate the individual demand for a reinforcer and is calculated from the nonlinear least squares regression model fit to the individual consumption data from each schedule of reinforcement. The main advantage of using EV is that it is a unifying measure based on several critical parameters forming the exponential-demand equation. For example, EV takes into consideration consumption when the price of a reinforcer is low (e.g., FR1), consumption when the price of the reinforcer is high (e.g., higher or terminal FR schedules), and the slope of the demand curve - also referred to as elasticity. Although there are many other parameters that can be derived from the behavioral economics model, the main simplified question of “just how hard is an animal willing to work?” to produce the goods it consumes can be answered using a single value - EV - derived from the model [32]. Using this approach, we recently showed that pretreatment with bupropion or varenicline decreased overall responding for nicotine during a 2 h progressive ratio (PR) schedule of reinforcement tests [17]. These results are consistent with the findings from previous studies that also used short-access nicotine self-administration protocols [10–16,33]. In addition to grouped effects, we also showed in that study that rats with a higher demand for nicotine had a greater decrease in nicotine self-administration following pretreatment with bupropion or varenicline. Moreover, in the same study, rats with higher demand for nicotine had higher rates of nicotine seeking in extinction and during nicotine-triggered reinstatement tests. Although our findings from that study for the first time demonstrated how individual history with a primary reinforcer like nicotine can be used to predict treatment outcomes on an individual level, the translational relevance of these findings is limited because short-access self-administration protocols do not closely resemble nicotine use in clinical populations.
We recently developed a preclinical approach to study behavioral and biological mechanisms associated with substance use disorders that emphasize individual variability [17,30]. This approach is based on the established preclinical drug self-administration model, the use of behavioral economics, and a multivariate systems perspective that is especially suited for the study of individuals. This approach is especially suited to study prognostic and predictive markers associated with modeled substance use. Prognostic marker is a biological or behavioral characteristic that provides information about the likelihood of a clinical event while predictive markers are used to identify individuals who are more likely to experience favorable or unfavorable effects from the treatment. With this in mind, the primary goal of the present study was to a) identify prognostic markers associated with high economic demand for nicotine, resistance to extinction, and higher magnitude of simulated relapse; b) to identify predictive markers associated with response to acute bupropion or varenicline treatment; and c) assess the aforementioned effects using a long-access (12 h) self-administration protocol that better resembles human nicotine consumption than short-access models. Because the majority of preclinical studies investigating the effects of bupropion or varenicline previously used male rats, the secondary goal of the present study was to address this gap by investigating these effects in female rats. To achieve these goals we designed a study where we sampled behaviors along the natural continuum of the modeled addiction cycle that includes behaviors prior to access to nicotine, patterns of nicotine self-administration and response to treatment, behaviors in withdrawal, and responding in modeled abstinence or relapse. To this end, in addition to nicotine self-administration and dose response-tests, we selected open field and elevated plus maze tests to assess the exploration of novel environment or responding to mild anxiogenic contexts prior to access to nicotine and during withdrawal. We also assessed the effect of yohimbine, an alpha-2 adrenergic antagonist often characterized as a pharmacological stressor, on responding for nicotine or nicotine seeking in extinction as another modality of responding to anxiogenic stimuli.
2. Materials and methods
Major phases of experimental progression and additional pertinent information about this study are outlined in Fig. 1.
Fig. 1.
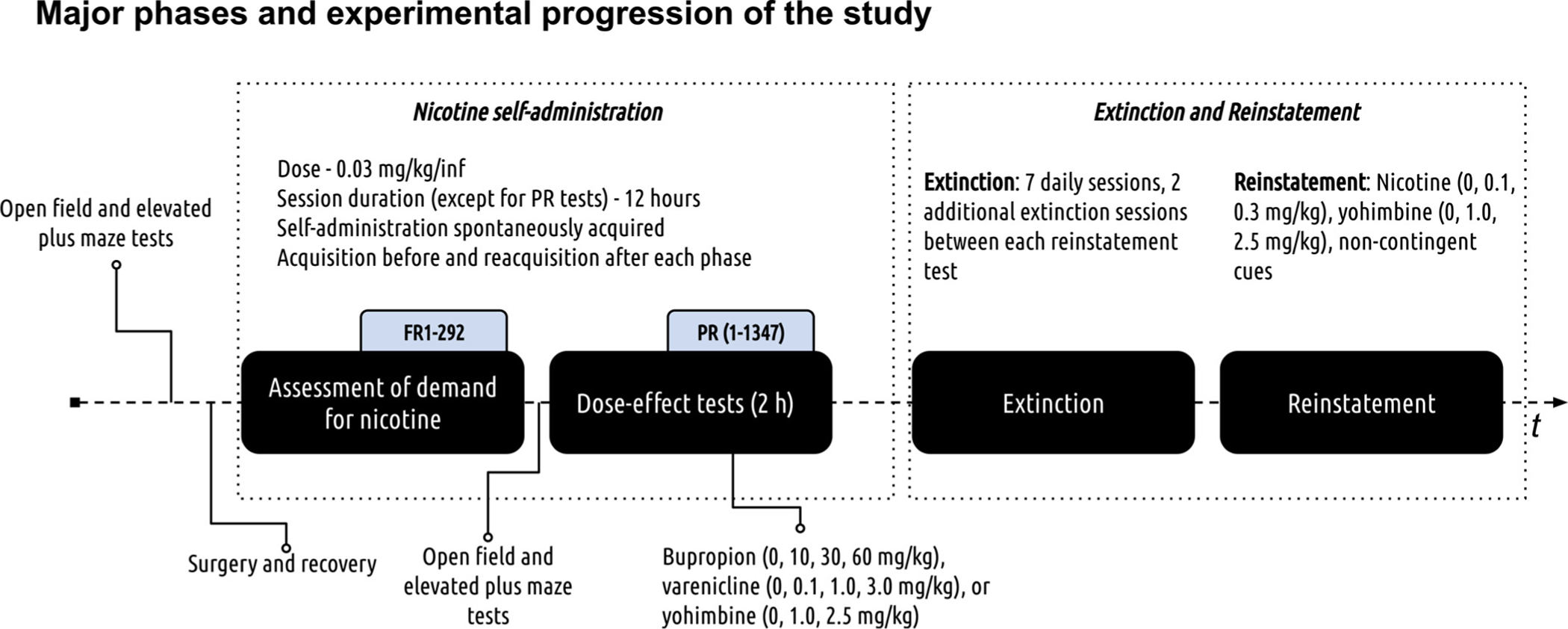
Experimental progression. The study used a within-subjects design with several distinct experimental phases outlined in the figure above (refer to black filled rounded rectangles and elbow brackets). The first nicotine self-administration phase modeled drug taking and assessed individual demand for nicotine. The second self-administration phase assessed the effects of acute bupropion, varenicline, or yohimbine pretreatment on responding for nicotine (doses for all treatments can be found in the figure). The third extinction phase modeled abstinence. The fourth phase modeled relapse and in this phase resurgence of active lever responding in extinction was triggered by non-contingent presentation of cues that were previously paired with nicotine infusions, nicotine, or yohimbine.
2.1. Animals
30 female Sprague Dawley rats (150–174 g) were purchased from Envigo (Indianapolis, IN, USA). Rats were postnatal days 60–70 at the start of experimental procedures. Upon arrival at the vivarium, rats were singly housed and acclimated to a colony for at least 1 week prior to experimentation. The vivarium was maintained on a 12 h light/dark cycle with lights on at 0700. For all rats, food and water were available ad libitum until day 7 of recovery from self-administration surgeries; thereafter, rats were food-deprived to 85 % of their free-feeding weight and this free-feeding weight was increased by 2 g every 30 days. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, DC, 2011) and were reviewed and approved by the University of New Hampshire Institutional Animal Care and Use Committee.
2.2. Apparatus
Conditioning chambers (ENV-018MD; Med Associates, Inc.; St. Albans, VT, USA; 30.5 × 24.1 × 21.0 cm), were enclosed in a sound-and light-attenuating cubicle equipped with an exhaust fan. Each chamber had aluminum sidewalls, metal rod floors with polycarbonate front, back, and ceiling. Two nosepokes2 were mounted on the right side of the chamber. A house light (two white 28 V, 100 mA lamps) was located 10 cm above the conditioning chamber. A speaker was mounted on the left side of the chamber. Two infrared beams that monitored gross chamber locomotion were located 4 cm above the floor and 5.4 cm from each aluminum sidewall. The infusion pump (PMH-100VS; Med Associates; St. Albans, VT, USA) for each chamber was located outside the sound-attenuating cubicle. A 5-mL syringe mounted on the infusion pump was connected to Tygon® tubing (AAQ04103; VWR; West Chester, PA, USA). The tubing was then attached to a swivel coupled with a spring leash (C313C; Plastics One; Roanoke, VA, USA) which were suspended over the ceiling of the chamber on a balanced metal arm. Med Associates interface and software (Med-PC for Windows, version IV) were used to record data and all programmed events. Open-field tests were conducted in an open-top square plywood box (120 cm × 120 cm × 25 cm; l × w×h) painted with flat black enamel. Test sessions were video recorded from a camera mounted above the apparatus and processed using ANY-maze software (version 6.10; Stoelting Co., IL, USA). Elevated plus maze tests were conducted using an open-top apparatus (Stoelting Co., IL, USA; 10 cm lane width, 50 cm arm width, 40 cm all height, 50 cm leg height) and video recorded from a camera mounted above.
2.3. Drugs
Nicotine bitartrate (MP Biomedicals, OH, USA), varenicline tartrate (Sigma-Aldrich, MO, USA), bupropion hydrochloride (Toronto Research Chemicals, ON, Canada), and yohimbine hydrochloride (Sigma-Aldrich, MO, USA) were dissolved in 0.9 % sterile saline. Intravenous nicotine dose (0.03 mg/kg/inf;) and nicotine subcutaneous injection dose (0.4 mg/kg) were chosen based on previous research [14,17,23]. All doses and administration protocols were adopted from previous research [11,14,20,33,34]. Nicotine doses are reported as base, whereas bupropion, varenicline, and yohimbine doses are reported as salt.
2.4. Open field and elevated plus maze tests
One of the goals of this study was to assess whether baseline behaviors, sampled before access to a drug, can be used to predict the economic demand for nicotine in the later phase of the study. Another goal of this study was to assess whether individual performance on common behavioral tests in withdrawal relates to individual demand for nicotine or can be predictive of performance on dose-effect tests. With these goals in mind, we selected open field and elevated plus maze tests to assay general locomotor activity, exploration of novel environments, and response to mild anxiogenic environments. For these tests, rats in their home cages were moved to the room adjacent to a testing room for at least 1 h before testing to minimize the effects of stress on behavior during testing. For the open field tests, rats were placed individually into the center of the open field apparatus for 10 min, after which they were returned to the vivarium. Locomotor activity, defined as the distance traveled (path length), and time spent in the center vs. perimeter (thigmotaxis) were measured using ANY-maze software (version 6.10; Stoelting Co., IL, USA). For the elevated plus maze tests, rats were placed in the center square region of the apparatus and allowed to move through apparatus for 10 min, after which they were returned to the vivarium. Activity (distance traveled, time and frequency in open or closed arms) during elevated plus maze tests was measured using ANY-maze software (version 6.10; Stoelting Co., IL, USA). All dependent measures were divided into the first 5 min (habituation; 0–5 min) and last 5 min (test; 5–10 min) of the test. Behaviors during the second 5 min bin were used for data analyses [30]. For the tests prior to access to nicotine rats were tested between 12 and 6 pm. For the tests in withdrawal, rats were tested between 4 and 7 pm.
2.5. Catheter implantation surgery
Rats were anesthetized using a 5% isoflurane and oxygen admixture for the induction phase and maintained at 1–3 % thereafter. Polyurethane catheter (RJVR-23; Strategic Applications Inc.; Lake Villa, IL, USA) with a rounded tip and double suture beads, one secured internally and other externally, was implanted into the right external jugular vein. The other end of the catheter was subcutaneously placed around the shoulder and exited below the scapula via subcutaneously implanted polycarbonate back-mount access port (313–000BM; Plastics One Inc.; Roanoke, VA, USA). Immediately following the surgery, catheters were flushed with 0.2 mL of cefazolin (50 mg/mL) diluted in sterile heparinized saline (30 U/mL). Thereafter, these catheter flushes occurred daily until the end of the self-administration phase of the experiment to ensure patency. For pain-management rats were pretreated with butorphanol (0.1 mg/kg; SC) and meloxicam (2 mg/kg; SC) 5 min before the surgery. Meloxicam (2 mg/kg; SC) was also administered daily for three days after the surgery. Catheter patency was assessed when patency loss was suspected or upon completion of the self-administration phase of the study using an infusion of 0.05 mL xylazine (20 mg/mL; IV). This xylazine concentration produces clear motor ataxia within 5–10 s [35,36]. Rats that did not exhibit noticeable motor ataxia within 5–10 s following xylazine infusion were considered non-patent. Resulting Ns are provided in figure captions.
2.6. Nicotine self-administration and assessment of individual demand for nicotine
Prior to nicotine self-administration phase of the study, rats were pretreated with nicotine (0.4 mg/kg; SC) for 3 consecutive days. These nicotine injections were used to alleviate the initial aversive effects of self-administering nicotine. All rats spontaneously acquired nicotine self-administration using nosepokes as manipulandum. Each session began with a termination of the house light, illumination of both nosepoke inlets, and a 0.9 s infusion to flush approximately 90 % of catheter volume. Completion of the required response resulted in a ~1 s infusion, termination of both nosepoke lights for 3 s accompanied by illumination of a house light and activation of white noise (70 dB). After 3 s of signaled timeout during which responses on the active nosepoke had no programmed consequences, white noise and house lights were turned-off and nosepoke lights were illuminated. All rats self-administered the exact dose of nicotine using a variation in infusion duration that was automatically controlled by the program based on their pre-session weight (approximate infusion time range: 0.91–1.12 s). Rats self-administered nicotine (0.03 mg/kg/inf; 12 h sessions) during their active night cycle from 7 p.m. to 7 a.m. Rats started nicotine self-administration on a VR1.5 for 3 days and on the VR3 schedule of reinforcement for additional 3 days. Rats then were allowed to earn nicotine on the daily escalated FR schedules of reinforcement (1, 3, 5, 8, 12, 18, 26, 38, 58, 86, 130, 195, and 292). Each rat progressed through the range of FR schedules until failing to earn at least 1 infusion; thereafter rats were allowed to self-administer nicotine for additional 2 days on an FR1 schedule. Rats then self-administered nicotine using a combination of PR and VR3 schedules for additional 5 days. Thus, these retraining sessions consisted of 2 h of responding on the PR schedule and additional 10 h of responding on the VR3 schedule of reinforcement. These hybrid sessions allowed us to stabilize responding on the PR schedule in anticipation for 2 h dose-effect tests that were conducted using identical PR schedules. The progression in the number of lever presses required to earn infusions on the PR schedule of reinforcement was a modified version of the following exponential equation: 5× e(Reinforcer number×0.2) −5 [37]. We modified the ratio by replacing first six values (1, 2, 3, 6, 9, and 12) with 1, 3, 6, 10 and then continued with the progression.
2.7. Dose-effect tests
Following the reacquisition of nicotine self-administration using hybrid PR-VR3 sessions, rats progressed to 2 h dose-effect tests using a PR schedule of reinforcement that was described above. Prior to each test session, rats were pretreated with bupropion (10, 30, or 60 mg/kg), varenicline (0.1, 1.0, or 3.0 mg/kg), yohimbine (1.0, or 2.5 mg/kg), or saline (0 mg/kg). Doses (3 bupropion, 3 varenicline, 3 yohimbine, and 0 mg/kg; 10 total test days) were assigned to each rat using Latin square design and were administered intraperitoneally 30 min prior to nicotine self-administration sessions. Between each treatment, rats were retrained to at least 80 % of their baseline responding for nicotine that was calculated based on the last two sessions prior to initiation of dose-effect tests. Following this dose-effect assessment, rats were allowed to self-administer nicotine on the VR3 schedule for additional 5 days.
2.8. Extinction and reinstatement
Extinction sessions were identical to self-administration sessions, except that nosepoke entries had no programmed consequences. There were 7 extinction sessions prior to the initiation of reinstatement tests. There were 2 additional extinction sessions between each reinstatement test. Non-contingent cue presentations, nicotine, or yohimbine were used as triggers for the 2 h reinstatement tests. The order of reinstatement tests with non-contingent cue presentations, nicotine or yohimbine doses were assigned using a Latin square design. For reinstatement tests with pharmacological triggers, rats were pretreated with either nicotine (0.1, 0.3 mg/kg; 5 min before the test) or yohimbine (1.0, 2.5 mg/kg; 30 min before the test) before 2 h extinction session. There was one test day with 0 mg/kg where half of the rats were pretreated 5 min before the test and other half 30 min before the test (pseudo-random assignment). During non-contingent cue presentation test rats were exposed to non-contingent cues every 5 min from the start of the session. These non-contingent cues were identical to those previously paired with nicotine infusions and consistent of nosepoke lights-off, house light-on, and white noise-on for 3 s. During all reinstatement tests, responding on the nosepokes had no programmed consequences.
2.9. Dependent measures and statistical analyses
Active nosepoke entries and gross chamber locomotion (total number of times two photocell beams were interrupted during a session) were used as primary dependent measures. Economic demand parameters were assessed using the demand model by Hursh and Silberberg [32,38]. The essential value, conceptualized as a strength of the reinforcer to maintain operant behavior, was derived from the economic demand model. Additional estimates for comparison of parameters from previous short-access and current long-access study were derived from the economic demand model and were assessed as differences in Q0, α, Pmax, and Omax. Analyses were performed in R 3.6.1 [39] using {stats} package for t-tests and {nlme} package for all analyses associated with linear mixed-effects modeling [40]. Linear regression analysis and least-squares nonlinear fit for the assessment of economic demand parameters were performed using GraphPad Prism version 8.2.1 (GraphPad Software, Inc., La Jolla, CA).
2.9.1. Analytical approach
To assess the effects of the bupropion, varenicline, or yohimbine on the responding for nicotine and to assess responding during reinstatement tests we used two levels of assessments - grouped and individual. These grouped and individual effects were analyzed using a linear mixed-effect modeling approach [17,35,41]. The linear mixed-effects modeling approach provides a number of advantages when compared to ANOVAs. For example, this analysis does not require the assumption that the relation between the covariate and the outcome is the same across the groups and thus does not require meeting the assumption of homogeneity. Furthermore, unlike ANOVA, linear mixed-effects modeling does not assume that the different cases of data were independent and hence can model relations between different outcomes which may be interrelated. Linear mixed-effects modeling is also more robust in dealing with missing data or unequal group sizes which is often the case in preclinical animal models. Finally, this approach is especially effective at analyzing individual effects - like those investigated in this study. For these reasons, most of the effects in this study were analyzed using linear mixed-effects modeling.
2.9.2. Essential value assessment
The essential value was derived from the economic demand model that was calculated from the nonlinear least squares regression model fit to the individual consumption data from each schedule of reinforcement using the following formula: . In this formula Q represents reinforcer consumption, Q0 is a consumption when price is zero or free, κ is a constant for the range of demand, e is the base of the natural logarithm, C is the varying cost of each reinforcer, and α is the rate of decline in relative log consumption with increases in price. The essential value was calculated from the demand model using the following formula EV = 1 ÷ (100 × α × κ1.5).
2.9.3. Grouped effects
Grouped effects were analyzed by building a model with a maximum likelihood fit from a baseline that does not include any predictors other than an intercept. The model was then built by first adding predictor 1 (Dose), then predictor 2 (Locomotion), and finally an interaction between predictor 1 and 2 (Dose × Locomotion). The predictor or interaction was declared significant when its addition improved the model by accounting for significantly more variance (the fit was examined using the Likelihood Ratio test of fixed effects; p < 0.05). Pairwise comparisons were performed using estimates from the model.
2.9.4. Individual effects
Individual effects for dose-effect tests were analyzed using linear mixed-effects modeling approach as described above with Dose as a predictor 1, Demand as predictor 2, and interaction between Dose × Demand as a final predictor. Additional analyses including parameters from the open field or elevated plus maze tests were performed as described above with the exception that the second predictor was a parameter from one of these tests. Significance was declared as described above. Pairwise comparisons and assessment of contrasts were performed using estimates from the model.
2.9.5. Reinstatement
The effect of non-contingent cue presentations on the responding during extinction tests was analyzed using a pairwise t-test by comparing responding during reinstatement test to the average of responding during the first 2 h of the last two extinction sessions. The effect of nicotine or yohimbine pretreatment on responding in extinction was analyzed using linear mixed-effects modeling described above with Dose or a combination of Dose and Demand as predictors.
3. Results
Analysis of responding over three VR3 schedule of reinforcement sessions preceding the transition to the demand assessment, showed a main effects of Nosepoke [active vs inactive; F (1, 36) = 61.52; p < 0.001], no effect of Session (p = 0.13), and no interaction (p = 0.14). During the last session of the acquisition phase (6 th overall self-administration session or 3 rd VR3 session), there were on average 128.5 active nosepoke entries, 14.4 inactive nosepoke entries, and 40.5 infusions indicating robust lever discrimination and relatively high nicotine consumption.
3.1. Exponential demand for nicotine
Because this long-access study extends our previous report demonstrating the individual effects of bupropion and varenicline using a short-access self-administration model [17], we are first comparing economic demand parameters between these two studies. Because data that were used for these analyses were gathered in two different studies, there are some limitations to these analyses. For example, Kazan and Charntikov (2019) study used male rats while here we are using female rats. In addition, the compared studies have different experimental manipulations prior to the acquisition of demand self-administration. For example, rats in the Kazan and Charntikov (2019) study were trained first to respond on levers for liquid sucrose, then were assessed for economic demand for liquid sucrose using levers as manipulandum, and then spontaneously acquired nicotine self-administration on nosepokes and were assessed for nicotine demand using nosepokes as well. Rats in this study were assessed for baseline activity in the open field and elevated plus maze before the spontaneous acquisition of nicotine self-administration and subsequent assessment of the economic demand. On the other hand, the equations that were used to compare economic demand parameters between the two studies are well suited for this task and have been previously shown to accurately model variations in consumption between different studies [32]. For example, the equations used here were previously shown to adequately normalize demand curves with a goal of isolating demand parameters for drugs with different potencies or different doses of the same drug [32,42–44]. With these limitations in mind, the comparison of the economic demand for nicotine between the two studies provides important information relevant to the interpretation of the principal effects of this study. The comparison of economic demands from the two studies presented below also provides an important contribution to the field because it demonstrates for the first time how the length of access to nicotine self-administration shifts overall nicotine demand and individual demand parameters.
Rats with long-access to nicotine had higher economic demand for nicotine than rats with short-access to nicotine (Fig. 2A; compare two curves). Specifically, rats with long-access consumed more nicotine at a simulated zero price (Q0; Fig. 2B), had lower elasticity of the economic demand (α; Fig. 2C), overall worked harder for nicotine infusions (EV; Fig. 2D), had higher perseverance of responding in the face of fluctuations in price (Pmax; Fig. 2E), and had higher maximal expenditure sustained by a reinforcer (Omax; Fig. 2F). Rats with long-access to nicotine also showed high degree of variability across most parameters derived from the economic demand equation. This increased variability is important for studies focusing on individual effects as it increases statistical power to detect individual effects using regression types of analyses.
Fig. 2.

Comparison of economic demand for nicotine between rats with short- (N = 21) or long-access (N = 19). Short-access data were acquired from a separate previously published Kazan and Charntikov (2019) study. (A) Grouped demand curves for short- and long-access nicotine. (B) Bar graph with individual values for Q0. (C) Bar graph with individual values for α. (D) Bar graph with individual values for EV. (E) Bar graph with individual values for Pmax. (F) Bar graph with individual values for Omax.
3.2. Individual performance in open field and elevated plus maze did not predict nicotine demand
To minimize type I error, analyses with behavioral responses from the open field and elevated plus maze tests prior to access to nicotine were restricted to a priori comparisons with individual economic demand (EV) for nicotine. Although individual performance on these baseline tests could have been used to predict responding on other tests throughout this study, the results of these additional analyses would be difficult to interpret because of the complexity of employed within-subjects experimental design. With these a priori stipulations in mind, individual performance in the open field or elevated plus maze tests across sampled dependent measures did not predict individual demand for nicotine (see Table 1 for means of parameters derived from these tests). Overall, rats spend significantly more time in the center of elevated plus maze, had less number of entries into the center of elevated plus maze, and spent less time in the closed arms of the elevated plus maze during withdrawal (test 2) than during tests prior to access to nicotine (test 1).
Table 1.
Parameters derived from OF and EPM tests expressed as group means and p-values derived from t-tests.
| Test 1 | Test 2 | P-value | |
|---|---|---|---|
| OF | |||
| Distance | 32.10 | 33.12 | 0.77 |
| Center entries | 7.53 | 6.26 | 0.23 |
| Center time | 26.52 | 25.62 | 0.83 |
| EPM | |||
| Distance | 13.80 | 12.86 | 0.37 |
| Open entries | 10.22 | 10.78 | 0.77 |
| Open time | 84.19 | 109.96 | 0.10 |
| Closed entries | 21.55 | 23.50 | 0.34 |
| Closed time | 134.13 | 69.94 | 0.00 |
| Center entries | 26.78 | 15.33 | 0.00 |
| Center time | 77.58 | 118.23 | 0.00 |
OF - Open Field; EPM - Elevated plus maze. Significant p-values are indicated in bold.
3.3. The effect of varenicline on nicotine self-administration
3.3.1. Grouped effects
There was no effect of varenicline on active nosepoke entries (p = 0.12). However, the active nosepoke entries differed depending on the general chamber Locomotion (χ2(4) = 8.68, p < 0.01; Fig. 3A; see point size for locomotion effects). There was no Dose × Locomotion interaction (p = 0.48). General chamber locomotion decreased from the mean of 637.84 after pretreatment with saline to the mean of 488.89 after pretreatment with 3 mg/kg.
Fig. 3.

Grouped (A, C, E) and individual (B, D, F) effects of bupropion, varenicline, or yohimbine on active nosepoke entries (N = 19). *Indicates significant difference in responding from 0 mg/kg. Chamber locomotion on panels A, C, and D is visualized using a point size.
3.3.2. Individual effects
To test whether responding after pretreatment with varenicline varied based on individual demand for nicotine, we performed additional tests with EV, derived from Hursh’s demand model for each subject, as additional predictor. These tests showed that entries into active nosepokes did not differ depending on varenicline Dose (p = 0.12) but did significantly differ depending on the Demand for nicotine (EV; χ2(4) = 12.04, p < 0.001; Fig. 3B). There was no significant Dose × Demand interaction (p = 0.87).
The significant effect of EV indicates that responding during 2 h PR schedule of reinforcement tests correlates to the individual demand for nicotine that was assessed using long-access sessions (12 h). In contrast to our previous short-access study [17], we here report that varenicline did not decrease nicotine self-administration rates in rats with a long-access history of nicotine self-administration (no effect of Dose).
3.4. The effect of bupropion on nicotine self-administration
3.4.1. Grouped effects
Entries into active nosepokes significantly differed depending on the bupropion Dose (χ2(3) = 19.03, p < 0.001; Fig. 3C). Entries into active nosepokes did not differ according to general chamber Locomotion (p = 0.09), and there was no Dose × Locomotion interaction (p = 0.99). Active nosepoke entries following pretreatment with 30 mg/kg were significantly higher than after pretreatment with 0 mg/kg (b = 221.47, t(54) = 3.39, p < 0.01; for locomotion compare point size).
3.4.2. Individual effects
To test whether responding after pretreatment with bupropion varied based on individual demand for nicotine, we performed additional tests with EV as additional predictor. These tests show that entries into active nosepokes significantly differed depending on the bupropion Dose (χ2(3) = 19.03, p < 0.001) and Demand for nicotine (χ2(4) = 5.61, p < 0.05; Fig. 3D). There was also significant Dose × Demand interaction (χ2(7) = 8.86, p < 0.05). Assessment of contrasts indicated that responding across nicotine demand spectrum was trending towards significance after pretreatment with 30 mg/kg of bupropion when comparing to responding after 0 mg/kg (b = 354.18, t (51) = 1.97, p = 0.053; see upward arrow in Fig. 3D).
In contrast to previous reports, we here show that bupropion increased nicotine self-administration rates in rats with a long-access history of nicotine self-administration. Rats with higher demand for nicotine seem to be more sensitive to pretreatment with bupropion (Fig. 3D; see arrow for trending effect).
3.5. The effect of yohimbine on nicotine self-administration
3.5.1. Grouped effects
Entries into active nosepokes significantly differed depending on the yohimbine Dose (χ2(2) = 7.16, p < 0.05; Fig. 3E), general chamber Locomotion (χ2(3) = 23.71, p < 0.001), and there was significant Dose × Locomotion interaction (χ2(5) = 12.44, p < 0.01). Responding following pretreatment with 1.0 mg/kg was significantly higher than responding after pretreatment with 0 mg/kg (b = 108.10, t (36) = 2.68, p < 0.05). Locomotor activity after pretreatment with yohimbine (1.0 or 2.5 mg/kg) did not differ from locomotor activity after pretreatment with 0 mg/kg.
3.5.2. Individual effects
The entries into active nosepokes significantly differed depending on the yohimbine Dose (χ2(2) = 7.16, p < 0.05) and Demand for nicotine (χ2(3) = 7.14, p < 0.05; Fig. 3F). There was no Dose × Demand interaction (p = 0.27). Assessment of contrasts did not show that responding after pretreatment with yohimbine differed across the nicotine demand spectrum.
3.6. Individual performance in open field and elevated plus maze in withdrawal compared to individual demand for nicotine and individual performance on dose-effect tests
As previously rationalized, analyses with individual performance in open field and elevated plus maze tests in withdrawal were restricted to a priori comparisons. Individual performance in these tests in withdrawal was assessed 8–10 h after the end of the daily access to nicotine. Recall that the open field and elevated plus maze tests after the chronic daily access to nicotine were conducted to assess the individual effects of withdrawal. However, because all rats were previously exposed to bupropion, varenicline and yohimbine the possible effect of exposure to these substances on performance in open field and elevated plus maze tests need to be taken into consideration when interpreting these effects (see Table 1 for means of parameters derived from these tests). Demand for nicotine predicted distance traveled in the open field in withdrawal (χ2(1) = 11.04, p < 0.0001; Fig. 4A). Rats that had a higher demand for nicotine had higher distance traveled during the open field test. None of the other measures from either the open field or elevated plus maze related to the individual demand for nicotine.
Fig. 4.

Individual performance in an open field and elevated plus maze tests in withdrawal compared to individual demand for nicotine (A; N = 19) and individual performance on dose effect tests (B-D; N = 19). OF - open field. EPM - elevated plus maze. *Indicates significant difference in responding from 0 mg/kg.
Active nosepoke entries following pretreatment with yohimbine significantly differed depending on the yohimbine Dose (χ2(2) = 7.16, p < 0.05), open field Distance traveled in withdrawal (χ2(3) = 10.48, p < 0.01), and there was significant Dose × Distance interaction (χ2(5) = 6.44, p < 0.05). Responding after pretreatment with 1.0 mg/kg across distance traveled in the withdrawal spectrum was significantly different than responding after pretreatment with 0 mg/kg (b = 7.45, t(34) = 2.49, p < 0.05; see arrow highlighting this effect in Fig. 4B). These results suggest that an increase in active nosepoke entries after pretreatment with yohimbine (1 mg/kg) was driven by rats that had higher open field distance traveled in withdrawal.
Adding time spent in the center of the open field in withdrawal to the linear regression model with Dose as another predictor revealed a significant effect of yohimbine Dose (χ2(2) = 7.16, p < 0.05) and a Dose × open field Center Time interaction (χ2(5) = 6.43, p < 0.05). There was no effect of open field Center Time in withdrawal (p = 0.08). Assessment of interaction effects revealed that responding after pretreatment with 1.0 mg/kg across open field Center Time spectrum was significantly different than responding after pretreatment with 0 mg/kg (b = 5.92, t(34) = 2.24, p < 0.05; see arrow highlighting this effect in Fig. 4C). This effect suggests that the difference in active nosepoke entries after pretreatment with these yohimbine (1.0 mg/kg) was driven by rats that spent more time in the center of open field in withdrawal.
Adding the number of entries into the open arms of the elevated plus maze in withdrawal to the linear regression model with Dose as another predictor revealed that active nosepoke entries significantly differed depending on the yohimbine Dose (χ2(2) = 7.16, p < 0.05) but not Open Arm Entries (p = 0.83). However, there was significant Dose × Open Arm Entries interaction (χ2(5) = 6.05, p < 0.05). Additional comparisons tests across the Open Arm Entries spectrum did not reveal dose-specific significant effects.
3.7. Extinction
Active nosepoke entries decreased from the mean of 150.66 on session 1 to the mean of 47.44 on session 7 of the extinction phase (3.17-fold decrease; Fig. 5A). Individual demand for nicotine (EV) significantly predicted total active nosepoke entries in extinction [χ2(1) = 11.15, p < 0.001; Fig. 5B). Rats with higher demand for nicotine had higher nicotine seeking in extinction.
Fig. 5.

Grouped or individual active nosepoke entries during extinction (A-B; N = 18) and reinstatement (C-D; N = 17). The effect of nicotine (C), yohimbine (D), or non-contingent cue presentation (E) on active nosepoke entries in extinction. *Indicates significant difference from responding after pretreatment with saline (0 mg/kg).
3.8. Reinstatement
3.8.1. Nicotine-triggered reinstatement: grouped effects
Active nosepoke entries during nicotine-triggered reinstatement tests did not significantly differ depending on the nicotine Dose [χ2(2) = 5.73, p = 0.057]. Because this effect was trending towards significance, we performed additional pairwise comparisons. These comparisons showed that responding following pretreatment with 0.3 mg/kg nicotine was significantly higher than after pretreatment with 0 mg/kg (b = 12.41, t(32) = 2.42, p < 0.05; Fig. 5C).
3.8.2. Yohimbine-triggered reinstatement: grouped effects
Active nosepoke entries during yohimbine-triggered reinstatement tests significantly differed depending on the yohimbine Dose [χ2(2) = 13.32, p < 0.01]. Responding following pretreatment with 1.0 (b = 16.41, t(32) = 3.24, p < 0.01;) or 2.5 mg/kg (b = 17.82, t (32) = 3.52, p < 0.01;) was significantly higher than after pretreatment with 0 mg/kg (Fig. 5D).
3.8.3. Cue-triggered reinstatement: grouped effects
Non-contingent presentation of cues did not reinstate nicotine seeking in extinction (Fig. 5E).
3.8.4. Nicotine-triggered reinstatement: individual effects
Assessment of nicotine-triggered responding over a spectrum of individual demand for nicotine showed that responding on the active nosepokes did not significantly differ depending on the nicotine Dose (p = 0.057) but did depending on the Demand for nicotine (χ2(3) = 3.91, p < 0.05). There was no Dose × Demand interaction (p = 0.71). Rats with higher demand for nicotine had higher responding during nicotine-triggered reinstatement tests (Fig. 6A).
Fig. 6.

Assessment of nicotine- (A) or yohimbine-triggered (B) responding in extinction over a spectrum of individual demand for nicotine (N = 17). EV -Essential Value.
3.8.5. Yohimbine-triggered reinstatement: individual effects
Assessment of yohimbine-triggered responding over a spectrum of individual demand for nicotine showed that responding on the active nosepokes significantly differed depending on the nicotine Dose (χ2(2) = 13.32, p < 0.01) and Demand for nicotine (χ2(3) = 4.03, p < 0.05). There was no Dose × Demand interaction (p = 0.24). Rats with higher demand for nicotine had higher responding during yohimbine-triggered reinstatement tests (Fig. 6B).
4. Discussion
The majority of previous reports show that bupropion or varenicline are effective at decreasing nicotine self-administration in rats [12–17,20,21,33,34,45,46]. However, most of the studies investigating the effects of bupropion or varenicline on responding for nicotine adopted short-access model of nicotine self-administration to test these effects. Although most of the preclinical studies demonstrate that both bupropion and varenicline are effective at decreasing nicotine intake, reports from clinical studies suggest that these pharmacological treatments are only marginally effective at increasing cessation rates when compared to placebo [5]. We previously demonstrated that rats vary in their individual economic demand for self-administered nicotine using short-access model of nicotine use [17]. In that study we also showed that the individual demand for nicotine can predict response to the acute pretreatment with bupropion or varenicline, responding in extinction, and a magnitude of response during modeled relapse. With this in mind, the goal of this study was to test the effects of bupropion or varenicline in a long-access model of nicotine self-administration using a population of rats that we know very little about - female rats. To achieve this goal, we designed a study where in addition to testing the effects of bupropion or varenicline on nicotine intake we sampled a variety of behavioral responses before and after repeated access to nicotine to improve our understanding of individual characteristics associated with variance in economic demand for nicotine, response to a treatment or response during a simulated relapse. We found that rats in the present study that self-administered nicotine for 12 h a day had significantly higher economic demand for nicotine than rats in our previous study that self-administered nicotine for 2 h a day. We also found that neither bupropion nor varenicline decreased responding for nicotine on test days. On the contrary, a moderate dose of bupropion (30 mg/kg) significantly increased active nosepoke entries on a PR schedule of reinforcement. In addition, we show that rats with higher demand for nicotine were more sensitive to pretreatment with yohimbine as they worked more for nicotine after pretreatment with yohimbine during the dose-effect tests. Finally, we show that rats that had higher demand for nicotine also were more persistent in seeking nicotine during extinction and reinstatement tests. Overall, our findings suggest that long-access self-administration protocol may significantly increase economic demand for nicotine when compared to short-access protocols and that this increase in nicotine demand may explain the lack of treatment effects in some individuals.
Males and females are different in their reproductive functions, neurobiological mechanisms, and their response to drugs of abuse [47,48]. These differences in behavioral and neurobiological mechanisms result in complex interactions with cultural, social, and genetic factors that form substance use phenotype. Most of the previous preclinical research focusing on understanding behavioral and biological mechanisms underlying nicotine use involved male subjects [48,49]. As a result, our understanding of female responses to nicotine are limited [50]. The few studies that included female subjects point out important differences between males and females across various modalities of nicotine reinforcement. Clinical studies show that women smoke less, find nicotine less reinforcing, but find conditioned stimuli associated with nicotine more reinforcing than men [49]. Preclinical studies show similar acquisition rates for nicotine self-administration but do show that female rats self-administer more nicotine on a PR schedule of reinforcement and respond more for nicotine paired with visual stimuli [49,51–53]. For example, Feltensteine et al. [53] showed no differences in nicotine self-administration between males and females in a study where rats had 2 h access to nicotine across a range of doses. Furthermore, Donny et al. [52] showed that when rats were trained to self-administer nicotine using 4 h daily access there was no differences in acquisition of nicotine self-administration between males and females on FR schedules of reinforcement (FR1-FR5), no differences in plasma nicotine concentrations, and there was no effect of estrous cycle on responding across a range of doses and schedules of reinforcement. However, the same study showed that when rats were challenged with PR schedule of reinforcement, female rats had higher break points which suggests that female rats may have higher motivation for reinforcement with nicotine. A followup study confirmed that there are no major differences in acquisition of nicotine self-administration between male and females but did show that female rats respond more for nicotine in a presence of a visual stimulus than for nicotine alone [51]. Finally, a recent study examining sex differences in economic demand for nicotine showed no differences in acquisition of nicotine self-administration under FR1 schedule of reinforcement and less elasticity (higher demand) in males but not female rats [54]. Overall, these findings suggest that there are some notable differences between males and females in nicotine reinforcement and that there is limited understanding about the mechanisms underlying these effects. With this in mind, our study was designed to continue expanding our understanding of female responses across nicotine use cycle with a special emphasis on individual differences in reinforcing effects of nicotine, response to treatment, nicotine seeking in extinction or during modeled relapse.
We here show that bupropion administered prior to nicotine self-administration session did not decrease nicotine self-administration during the 2 h test period where rats were responding on the PR schedule of reinforcement. On the contrary, pretreatment with 30 mg/kg of bupropion significantly increased active nosepoke entries during the 2 h test. Our findings are in contrast to those previously reported in studies using short access nicotine self-administration protocols [13–17]. In these previous reports, pretreatment with a range of doses, comparable to those used in the present study, significantly decreased rates of nicotine self-administration. Various methodological factors may explain this discrepancy. However, one of the most salient differences between previous reports and present study is the difference in the length of access to a nicotine throughout the day. While previous studies were investigating the effects of bupropion in rats with limited daily access to nicotine (1 or 2 h/day), the present study tested the effects of acute bupropion treatment in rats with extended daily access to nicotine (12 h/day). This long access protocol more closely resembles daily nicotine intake in clinical populations by incorporating long daily access to nicotine and long daily abstinence from nicotine. It is likely that there are some distinct behavioral and neural adaptations evoked by repeated long-access to nicotine self-administration when compared to limited daily access and that these adaptations may be responsible for the conflicting results between the present study and previous reports. On the other hand, our findings support a small subset of studies showing no effect [10,11] or increase of nicotine self-administration [12] after bupropion treatment. For example, rats in Rauhut et al. [12], study were trained to self-administer nicotine (0.01 or 0.02 mg/kg/infusion) over 1 h daily sessions. In that study, pretreatment with lower bupropion doses (9 or 15 mg/kg) significantly increased nicotine self-administration while a higher dose (78 mg/kg) significantly decreased nicotine self-administration. A similar trend of responding can be observed in the Shoaib et al. [11] study where pretreatment with 30 mg/kg of bupropion increased responding for nicotine but did not reach statistical significance. One of the factors that may explain increased responding for nicotine after pretreatment with 30 mg/kg of bupropion in our study is the locomotor enhancing effects evoked by this dose [55–57]. Although our analysis indicates no effect of Locomotion on active nosepoke entries during dose-effect tests (likely because of the large variance across doses), locomotor activity after pretreatment with 30 mg/kg bupropion increased from the mean of 100.57 beam breaks following pretreatment with 0 m/kg to the mean of 322.05 (3.2 folds increase). In contrast, our previous study that closely resembles methodological procedures used in this study, including bupropion doses and testing procedures, showed a decrease in responding for nicotine that was associated with a significant increase in bupropion-evoked locomotion [17]. The major difference between our previous report and the present study is the length of access to nicotine and the sex of rats. It is possible that either or both of these factors contribute to the effects we show in this study. Additional parametric studies with both sexes are warranted to better understand the underlying nature of these effects.
Previous reports using short-access to nicotine self-administration protocols (1–2 h) show that varenicline consistently decreases responding for nicotine [13,17,21,34,45,46]. Our results show that in a long-access nicotine self-administration model pretreatment with varenicline had no effect on responding during dose-effect tests. Once again, the major difference between the present study and previous reports is the length of access to nicotine and the sex of subjects. Although it is possible that the effect that we show here can be explained by the differences in sex of subjects, one previous study suggests that the length of access is likely to be relevant to the lack of effect of varenicline on responding for nicotine. George et al. [20] compared the effects of varenicline in rats with short- (1 h/day) or long-access (23 h/day) to nicotine. In that study, a high dose of varenicline (3 mg/kg) decreased responding of rats for nicotine in both short- and long-access conditions but the decrease of responding was much more pronounced in rats with a history of short-access. For example, rats decreased active lever presses in short-access condition from approximately 9.2–3 while rats in the long-access condition only decreased active lever presses from approximately 3.5 to 1. Findings from George et al. [20] suggest that the length of access to nicotine may differentially affect response to varenicline treatment.
In contrast to previous reports, our results show that neither bupropion nor varenicline decreased responding for nicotine in female rats with a history of long-access to nicotine. Our recently published study [17] provides an opportunity for closer examination of possible factors involved in this discrepancy as its methodology closely resembles the one used in the present study with few exceptions. In that recent report [17], we used male rats to access the bupropion and varenicline on responding for nicotine but the nicotine, bupropion, and varenicline doses were identical to those used in the present study with the identical methodology to assess individual demand for nicotine. The similarities in the way we acquired economic demands in these studies allow for their comparison. Prior to this comparison, we hypothesized that the limited effect of bupropion or varenicline treatment in this study may be explained by the higher economic demand for nicotine when rats were allowed to self-administer nicotine for 12 h rather than for 2 h a day. The comparison of economic demands from rats with short- or long-access to nicotine has not previously been reported. With these considerations in mind, we here show that the overall economic demand for nicotine was significantly increased when rats were allowed self-administer nicotine over 12 h instead of 2 h a day (Fig. 2). This increase in demand was evident across all major parameters that were derived from the economic demand model. Most importantly, rats with a history of long-access to nicotine showed less elasticity in their demand curves (Fig. 2A and C) and had significantly higher EV - a value derived from the elasticity parameter (ɑ) that answers the general question of how hard rats are willing to work for a reinforcer. These results suggest that economic demand for nicotine may be one of the critical factors explaining the response to treatments like bupropion or varenicline but additional studies comparing these effects across both sexes are needed to confirm this assumption.
One of the goals of this study was to expand our understanding of prognostic and predictive markers associated with modeled nicotine use and response to treatment. Prognostic marker is a biological or behavioral characteristic that provides information about the likelihood of a clinical event. Predictive markers are used to identify individuals who are more likely to experience favorable or unfavorable effects from the treatment. In the present study, we elected to use non-invasive tests like open field and elevated plus maze to assess individual performance prior to nicotine access and during withdrawal from nicotine to attempt to explain individual demand for nicotine or response to treatment. We also tested all rats for their response to a pharmacological stressor yohimbine in dose-effect and reinstatement tests with same goals in mind. We found that yohimbine (1.0 mg/kg) increased responding for nicotine and that rats with a higher nicotine demand had a more pronounced response to yohimbine pretreatment (Fig. 3E–F). These findings support previous reports showing this effect in both male and female rats. For example, Li et al. [58] showed that pretreatment with yohimbine increased nicotine intake and increased PR breakpoints in both male and female adolescent rats. We are here extending these findings to a long-access model of nicotine self-administration and by providing additional individual characteristics of rats more sensitive to these treatments. When comparing the individual response to bupropion and yohimbine on dose-effect tests it is evident that rats with higher economic demand for nicotine were more sensitive to both pretreatments. This finding suggests that the effect of bupropion in some individuals may be driven in part by the response to stress or sensitivity of a stress system. Furthermore, we found that the individual demand for nicotine predicts distance traveled in withdrawal during the open field test. We also found that additional behavioral measures from the open field or elevated plus maze tests could be used to explain the yohimbine-evoked response for nicotine. These findings suggest that the individual economic demand for nicotine can also be used to predict some behaviors in withdrawal and that individual sensitivity to stressors can be used to explain nicotine taking. Although our findings are correlational in nature they provide a framework for identification of individuals that may be more vulnerable to modeled nicotine use or may be more or less responsive to treatment protocols. The ability to predict individuals vulnerable to nicotine use and those more likely to experience favorable or unfavorable treatment outcomes is the initial step towards designing more efficacious prevention and treatment strategies.
A breakthrough in treatment strategies for substance use is likely to come from a better understanding of behavioral and neural processes during abstinence and relapse. Substance users that seek cessation treatments are coming to practitioners with a variable history of substance use and are seeking therapies to ease the transition to abstinence and to minimize the chances of relapse. We previously showed that the economic demand for nicotine is a strong predictor of nicotine seeking in extinction [17]. We are further extending these results to a long-access model where we show a comparable effect (Fig. 5B). We are also showing that nicotine and yohimbine reinstate nicotine seeking in extinction after a period of abstinence and that rats with higher demand for nicotine or higher nicotine seeking in extinction show a higher magnitude of reinstatement following both of these triggers. Our results support previous reports demonstrating the ability of nicotine or yohimbine to reinstate nicotine seeking in extinction [17,21,53,59,60]. We are further expanding previous findings by demonstrating how these effects vary on the individual level. For example, we are showing that rats with a higher magnitude of nicotine- or yohimbine-triggered reinstatement work harder for nicotine throughout the study (higher economic demand), more persistent seekers of nicotine in extinction, and are more sensitive to yohimbine during dose-effect tests. Findings from our study also suggest that the length of access to nicotine self-administration may play an important role in behavioral or neural mechanisms associated with nicotine use. We also show that behaviors across different phases of nicotine self-administration vary on an individual level and that some of these behaviors can be used to better explain response in the dose-effect tests, modeled abstinence or relapse. Understanding individual variability across the addiction cycle can inform future studies focusing on underlying neural mechanisms in individuals with different histories of nicotine use or focusing on the development of individualized treatment strategies.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences (GM113131).
Footnotes
Declaration of Competing Interest
Authors report no conflicts of interest.
Disambiguation: “nosepoke” - a manipulandum used in the operant chambers; “nosepoke entry” - an act of inserting a rat’s snout into a nosepoke hole.
References
- [1].WHO, Tobacco, (2019) (Accessed October 28, 2019), https://www.who.int/en/news-room/fact-sheets/detail/tobacco.
- [2].CDC’s Office on Smoking, Health, Smoking and Tobacco Use, 50th Anniversary Surgeon General’s Report, (2018) (Accessed August 6, 2018), http://www.cdc.gov/tobacco/data_statistics/sgr/2012/.
- [3].CDCTobaccoFree, Economic Trends in Tobacco, Centers for Disease Control and Prevention, 2019. (Accessed October 28, 2019), https://www.cdc.gov/tobacco/data_statistics/fact_sheets/economics/econ_facts/index.htm.
- [4].Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF, Annual healthcare spending attributable to cigarette smoking: an update, Am. J. Prev. Med 48 (2015) 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cahill K, Stevens S, Perera R, Lancaster T, Pharmacological interventions for smoking cessation: an overview and network meta-analysis, Cochrane Database Syst. Rev (2013) CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Santamaría A, Arias HR, Neurochemical and behavioral effects elicited by bupropion and diethylpropion in rats, Behav. Brain Res 211 (2010) 132–139. [DOI] [PubMed] [Google Scholar]
- [7].Richmond R, Zwar N, Review of bupropion for smoking cessation, Drug Alcohol Rev 22 (2003) 203–220. [DOI] [PubMed] [Google Scholar]
- [8].Ascher JA, Cole JO, Colin J-N, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, Bupropion: a review of its mechanism of antidepressant activity, J. Clin. Psychiatry 56 (1995) 395–401. [PubMed] [Google Scholar]
- [9].Dale LC, Hurt RD, Hays JT, Drug therapy to aid in smoking cessation. Tips on maximizing patients’ chances for success, Postgrad. Med 104 (1998) 75–78 83–4. [DOI] [PubMed] [Google Scholar]
- [10].Paterson NE, Balfour DJK, Markou A, Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats, Nicotine Tob. Res 10 (2008) 995–1008. [DOI] [PubMed] [Google Scholar]
- [11].Shoaib M, Sidhpura N, Shafait S, Investigating the actions of bupropion on dependence-related effects of nicotine in rats, Psychopharmacology 165 (2003) 405–412. [DOI] [PubMed] [Google Scholar]
- [12].Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT, Effect of bupropion on nicotine self-administration in rats, Psychopharmacology 169 (2003) 1–9. [DOI] [PubMed] [Google Scholar]
- [13].Hall BJ, Slade S, Wells C, Rose JE, Levin ED, Bupropion-varenicline interactions and nicotine self-administration behavior in rats, Pharmacol. Biochem. Behav 130 (2015) 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF, Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose, Psychopharmacology 196 (2008) 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rauhut AS, Dwoskin LP, Bardo MT, Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration, Nicotine Tob. Res 7 (2005) 901–907. [DOI] [PubMed] [Google Scholar]
- [16].Stairs DJ, Dworkin SI, Rate-dependent effects of bupropion on nicotine self-administration and food-maintained responding in rats, Pharmacol. Biochem. Behav 90 (2008) 701–711. [DOI] [PubMed] [Google Scholar]
- [17].Kazan T, Charntikov S, Individual differences in responding to bupropion or varenicline in a preclinical model of nicotine self-administration vary according to individual demand for nicotine, Neuropharmacology 148 (2019) 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rollema H, Hajós M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE, Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition, Biochem. Pharmacol 78 (2009) 813–824. [DOI] [PubMed] [Google Scholar]
- [19].Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD III, Williams KE, Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid, Neuropharmacology 52 (2007) 985–994. [DOI] [PubMed] [Google Scholar]
- [20].George O, Lloyd A, Carroll FI, Damaj MI, Koob GF, Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration, Psychopharmacology 213 (2011) 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O’Connor EC, Parker D, Rollema H, Mead AN, The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats, Psychopharmacology 208 (2010) 365–376. [DOI] [PubMed] [Google Scholar]
- [22].Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, Charntikov S, Disentangling the nature of the nicotine stimulus, Behav. Processes 90 (2012) 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Charntikov S, deWit NR, Bevins RA, Interoceptive conditioning with nicotine using extinction and re-extinction to assess stimulus similarity with bupropion, Neuropharmacology 86 (2014) 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Glennon RA, Young R, Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies, John Wiley & Sons, New Jersey, 2011. [Google Scholar]
- [25].Garcia-Rivas V, Fiancette J-F, Cannella N, Carbo-Gas M, Renault P, Tostain J, Deroche-Gamonet V, Varenicline targets the reinforcing-enhancing effect of nicotine on its associated salient cue during nicotine self-administration in the rat, Front. Behav. Neurosci 13 (2019) 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garcia-Rivas V, Deroche-Gamonet V, Not all smokers appear to seek nicotine for the same reasons: implications for preclinical research in nicotine dependence, Addict. Biol (2018), 10.1111/adb.12607. [DOI] [PubMed] [Google Scholar]
- [27].Alpert HR, Connolly GN, Biener L, A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation, Tob. Control 22 (2013) 32–37. [DOI] [PubMed] [Google Scholar]
- [28].Le Strat Y, Rehm J, Le Foll B, How generalisable to community samples are clinical trial results for treatment of nicotine dependence: a comparison of common eligibility criteria with respondents of a large representative general population survey, Tob. Control 20 (2011) 338–343. [DOI] [PubMed] [Google Scholar]
- [29].Le Foll B, Pushparaj A, Pryslawsky Y, Forget B, Vemuri K, Makriyannis A, Trigo JM, Translational strategies for therapeutic development in nicotine addiction: rethinking the conventional bench to bedside approach, Prog. Neuropsychopharmacol. Biol. Psychiatry 52 (2014) 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stafford NP, Kazan TN, Donovan CM, Hart EE, Drugan RC, Charntikov S, Individual vulnerability to stress is associated with increased demand for intravenous heroin self-administration in rats, Front. Behav. Neurosci 13 (2019) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hursh SR, Roma PG, Behavioral economics and the analysis of consumption and choice: BEHAVIORAL ECONOMICS ANALYSIS, Manage. Decis. Econ 37 (2016) 224–238. [Google Scholar]
- [32].Hursh SR, Silberberg A, Economic demand and essential value, Psychol. Rev 115 (2008) 186–198. [DOI] [PubMed] [Google Scholar]
- [33].Bruijnzeel AW, Markou A, Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats, Synapse 50 (2003) 20–28. [DOI] [PubMed] [Google Scholar]
- [34].Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer ANM, Pattij T, De Vries TJ, Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control, Psychopharmacology 216 (2011) 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Charntikov S, Pittenger ST, Pudiak CM, Bevins RA, The effect of N-acetylcysteine or bupropion on methamphetamine self-administration and methamphetamine-triggered reinstatement of female rats, Neuropharmacology 135 (2018) 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, Fleckenstein AE, Hu G, Li M, Bevins RA, Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine, Neuropharmacology 75 (2013) 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW, Parameters of self-administration of cocaine in rats under a progressive-ratio schedule, Pharmacol. Biochem. Behav 45 (1993) 539–548. [DOI] [PubMed] [Google Scholar]
- [38].Hursh SR, Behavioral economics and the analysis of consumption and choice, in: McSweeney FK, Murphy ES (Eds.), The Wiley Blackwell Handbook of Operant and Classical Conditioning, John Wiley & Sons, Ltd, Oxford, UK, 2014, pp. 275–305. [Google Scholar]
- [39].R Core Team, R: A Language and Environment for Statistical Computing, (2019) https://www.R-project.org/.
- [40].Pinheiro J, Bates D, DebRoy S, Sarkar D, (R CoreTeam), {nlme}: Linear and Nonlinear Mixed Effects Models 3 (2017), pp. 1–96 http://cran.r-project.org/package=nlme. [Google Scholar]
- [41].Charntikov S, Falco AM, Fink K, Dwoskin LP, Bevins RA, The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats, Neuropharmacology 113 (2017) 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hursh SR, Galuska CM, Winger G, Woods JH, The economics of drug abuse: a quantitative assessment of drug demand, Mol. Interv 5 (2005) 20–28. [DOI] [PubMed] [Google Scholar]
- [43].Ko MC, Terner J, Hursh S, Woods JH, Winger G, Relative reinforcing effects of three opioids with different durations of action, J. Pharmacol. Exp. Ther 301 (2002) 698–704, 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- [44].Winger G, Hursh SR, Casey KL, Woods JH, Relative reinforcing strength of ThreeN-Methyl-d-aspartate antagonists with different onsets of action, J. Pharmacol. Exp. Ther 301 (2002) 690–697. [DOI] [PubMed] [Google Scholar]
- [45].Funk D, Lo S, Coen K, Lê AD, Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse, Behav. Brain Res 296 (2016) 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I, Yan Y, Khaled M, Goldberg SR, Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used, Int. J. Neuropsychopharmacol 15 (2012) 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pogun S, Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning, Int. J. Psychophysiol 42 (2001) 195–208. [DOI] [PubMed] [Google Scholar]
- [48].Pogun S, Yararbas G, Sex differences in nicotine action, Handb. Exp. Pharmacol 192 (2009) 261–291. [DOI] [PubMed] [Google Scholar]
- [49].Perkins Ka., Donny E, Caggiula AR, Sex differences in nicotine effects and self-administration: review of human and animal evidence, Nicotine Tob. Res 1 (1999) 301–315. [DOI] [PubMed] [Google Scholar]
- [50].Bevins RA, Charntikov S, We know very little about the subjective effects of drugs in females, ACS Chem. Neurosci 6 (2015) 359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA, Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats, Psychopharmacology 180 (2005) 258–266. [DOI] [PubMed] [Google Scholar]
- [52].Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S, Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding, Psychopharmacology 151 (2000) 392–405. [DOI] [PubMed] [Google Scholar]
- [53].Feltenstein MW, Ghee SM, See RE, Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats, Drug Alcohol Depend 121 (2012) 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chellian R, Wilson R, Polmann M, Knight P, Behnood-Rod A, Bruijnzeel AW, Evaluation of sex differences in the elasticity of demand for nicotine and food in rats, Nicotine Tob. Res (2019), 10.1093/ntr/ntz171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Nielsen JA, Shannon NJ, Bero L, Moore KE, Effects of acute and chronic bupropion on locomotor activity and dopaminergic neurons, Pharmacol. Biochem. Behav 24 (1986) 795–799. [DOI] [PubMed] [Google Scholar]
- [56].Wilkinson JL, Palmatier MI, Bevins Ra., Preexposure to nicotine alters the subsequent locomotor stimulant effects of bupropion in rats, Nicotine Tob. Res 8 (2006) 141–146. [DOI] [PubMed] [Google Scholar]
- [57].Sidhpura N, Redfern P, Rowley H, Heal D, Wonnacott S, Comparison of the effects of bupropion and nicotine on locomotor activation and dopamine release in vivo, Biochem. Pharmacol 74 (2007) 1292–1298. [DOI] [PubMed] [Google Scholar]
- [58].Li S, Zou S, Coen K, Funk D, Shram MJ, Lê AD, Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats, Addict. Biol 19 (2014) 156–164. [DOI] [PubMed] [Google Scholar]
- [59].Macnamara CL, Holmes NM, Westbrook RF, Clemens KJ, Varenicline impairs extinction and enhances reinstatement across repeated cycles of nicotine self-administration in rats, Neuropharmacology 105 (2016) 463–470. [DOI] [PubMed] [Google Scholar]
- [60].Shaham Y, Adamson LK, Grocki S, Corrigall WA, Reinstatement and spontaneous recovery of nicotine seeking in rats, Psychopharmacology 130 (1997) 396–403. [DOI] [PubMed] [Google Scholar]


