Abstract
Zirconia particles are generated into a nitrile rubber (NBR) matrix via a solution sol–gel method in a controlled manner. Formation of zirconia particles from their precursor (zirconium(IV) propoxide) occurs under optimized reaction conditions. As a result, the nanoparticles are embedded and well dispersed in the NBR matrix that results in a remarkable improvement in mechanical and thermal properties of the composite. Such reinforcement is not realized when the composites are prepared following the conventional technique of filler loading by physical mixing, although the filler content remains the same. Use of a surface active coupling agent TESPT (bis-(3-triethoxysilylpropyl) tetrasulfide) in the reactive sol–gel system is found to further boost the mechanical performance of the composites. In order to ensure the practical application of the developed composites, a series of studies have been performed that consist of dynamic performance, swelling, thermal degradation, and resistance to oil, ozone, and abrasion. Analysis of the results reveals that in situ zirconia could be an excellent filler for the NBR composites to withstand in a harsh and adverse environment.
1. Introduction
Quest for novel fillers for rubber composites to meet the demand of their application in a harsh and adverse condition has produced a variety of materials of unique features. In this respect, metal oxides have become a strong contender of traditionally used carbon black since the past two to three decades. Silica is a very good example to cite in this context since it has replaced carbon black in many occasions successfully.1−4 This has been followed by application of other oxides of same class, viz., titania, zirconia, etc., to enrich the non-black filler system for rubber composites.5−10 Incorporation of such mineral oxides in rubber matrixes with a good state of dispersion, however, remains a challenging issue on account of incompatibility of inorganic fillers with organic rubber matrixes. Different methodologies and strategies have been proposed and attempted by researchers to address this issue. A sol–gel method is found to be a very effective approach in this context wherein metal oxides are directly formed inside the rubber matrix from their precursors. A lot of works on in situ silica-filled rubber composites are reported wherein very good distribution of silica in the rubber matrix with enhanced rubber–filler interaction is achieved exploiting this approach.11−15 However, this approach is limited to mostly in silica and yet to be extended in full swing for others of this category. Zirconia can be regarded as a non-black metal oxide that could be a potential filler if wisely used for rubber composites. Zirconia is well known for its valuable features like good thermal and chemical stability, superb hardness, high refractive index, and resistance to wear and tear. This is why it is of importance in the development of materials that are needed for optical, electronic, magnetic, and thermal applications. Mahmood et al. synthesized epoxidized natural rubber/zirconia hybrid films using the sol–gel technique that exhibited good optical transparency and improved thermal stability and glass transition temperature (Tg).16 Sarwar et al. synthesized transparent nanocomposites through a zirconia network from TPZ (tetrapropyl zirconate) in a glassy polyamide matrix by the sol–gel technique. It is found that incorporation of zirconia improved thermal properties.17 Preparation of epoxy/zirconia hybrid materials using bisphenol epoxy resin via in situ polymerization is reported by Ochi et al.18 Zirconia was uniformly dispersed in the epoxy matrix that exhibited excellent optical transparency and thermal resistance properties. In another work, Ma et al. prepared highly dispersive ultrafine zirconia nanoparticles by the modified sol–gel process into epoxy resin. The tensile strength and elasticity modulus of the composites were improved significantly with an increase in nanoparticle content up to 4 wt %.19 Rehman et al. synthesized the aramid–zirconia microcomposites via the sol–gel process and studied their mechanical and thermal properties.20 There are some reports that covered rubber composites filled with mixed fillers wherein zirconia is generated in situ. Wen et al. successfully synthesized mixed oxides (silica–titania, silica–zirconia, and silica–alumina) into a PDMS (poly(dimethylsiloxane)) network using the sol–gel approach. Narrow distribution of particle size in polymer matrixes offered very good mechanical properties.21 Tiankhoon et al. developed a nanocomposite solid polymer electrode using hybrid oxides of zirconia–titania with a copolymer of natural rubber and PMMA (poly(methyl) methacrylate) via the in situ sol–gel method.22
In this background, it seems that works on in situ zirconia-filled polymer composites are mostly concentrated on studies of optical and other properties. Meanwhile, reinforcement efficiency of zirconia for rubber composites has not yet been explored to that extent, and reports on this area are comparatively scanty than its congener silica or titania. Hence, we were curious to unveil the capability of zirconia in offering adequate thermal and strength-related properties to nitrile rubber (NBR), a commercially important rubber that is extensively used in petroleum and automobile industries owing to its excellent oil resistance property, cost effectiveness, and easy processibility.23 NBR is selected as the elastomer matrix since in situ titania-filled NBR composites showed superior mechanical properties in our earlier study.7,24 In this work, zirconia is incorporated in situ into the NBR matrix, for this purpose, up to 20 phr (parts per hundred parts of rubber) under controlled conditions. Thorough investigations of the zirconia-filled composites have been done, which include a series of physicochemical studies. Results of in situ zirconia-filled composites are compared with those of unfilled and externally zirconia-filled composites to assess the potential of in situ zirconia in enhancing the composite properties.
2. Results and Discussion
2.1. In Situ Generation of Zirconia in the NBR Matrix
Zirconia is grown directly in the NBR matrix from its precursor (zirconium(IV) propoxide) under designed and well-set conditions of the solution sol–gel process. In the sol–gel process, zirconium(IV) propoxide first undergoes hydrolysis to form tetrahydroxy zirconia. In the next step, polycondensation reaction generates the zirconia particles.25,26 The reactions are outlined in Scheme 1.
Scheme 1. Two Step Sol–Gel Synthesis of Zirconia.
In the present study, this sol–gel reaction is allowed to occur in the solution of NBR to form and grow zirconia particles directly into the rubber matrix. There are several crucial parameters associated with such a sol–gel process that has strong influence in controlling the percentage conversion of metal alkoxide to respective metal oxide and the size and dispersion of the generated particles. In this work, tetrahydrofuran (THF) is chosen as the solvent since, in our earlier work of in situ titania generation in NBR matrixes, this solvent was found very effective in generating titania from titanium(IV) n-butoxide owing to its polar nature. The reaction time is fixed to 60 min under a stirring condition as further continuing the reaction hardly increases the yield. The reaction is carried out at room temperature, and no catalyst is employed because the yield of zirconia is found appreciable under this reaction condition. About 70% of zirconia of nanometric dimension is produced in situ in the NBR matrix (Table 7).
Table 7. Formulation for Rubber Compounds in phr (Parts by Weight per Hundred Parts of Rubber)a.
| sample code | unfilled | In-Zr-5 | In-Zr-10 | In-Zr-20 | In-Zr-20T | Ex-Zr-20 |
|---|---|---|---|---|---|---|
| NBR | 100 | 100 | 100 | 100 | 100 | 100 |
| in situ ZrO2 | 0 | 5 | 10 | 20 | 20 | |
| external ZrO2 | 20 | |||||
| TESPT | 1 | |||||
| % of conversion | 70 | 80 | 80 | 85 |
Curing ingredients (in phr): ZnO, 4; stearic acid, 1; MBTS, 1; and Sulfur, 1.5.
X-ray diffraction patterns of some selected uncured samples show an amorphous nature of in situ zirconia that is characterized by a large hump around 2θ∼50° in the diffraction pattern (Figure 1). The amorphous nature of sol–gel-derived zirconia is well known in literature.27,28
Figure 1.
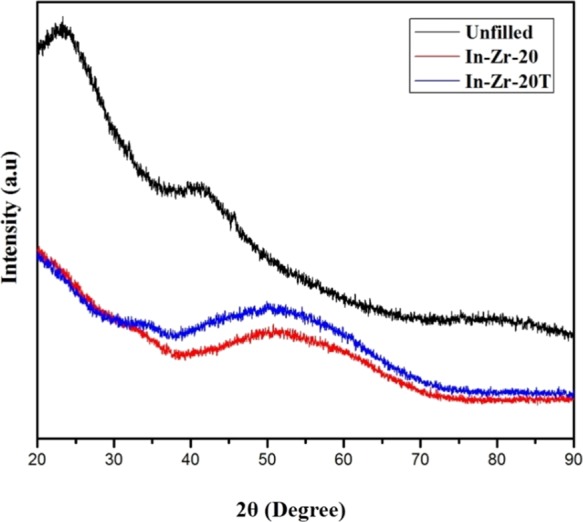
X-ray diffraction patterns of unfilled, In-Zr-20, and In-Zr-20T.
2.2. Thermogravimetric Analysis (TGA)
Thermal behavior of the composites has been studied by thermogravimetric analysis, and the results are summarized in Table 1. The analysis of the results reveals that incorporation of zirconia in NBR delivers significant improvement in the thermal behavior of the composites. The TG curves show that the weight loss of the samples occurs in three steps (Figure S1). The first one above 350 °C is due to the evaporation of residual moisture, volatilization, and thermal decomposition of organic solvents. The next one appearing between 400 and 500 °C is due to the degradation of polymer chains. The final degradation starting around 650 °C is due to the oxidation of deposited chars to produce CO2 in switching from nitrogen flow to air flow (described in the Experimental Section).
Table 1. Thermogravimetric Analysis of Unfilled and Zirconia-Filled NBR Composites.
| sample code | unfilled | In-Zr-5 | In-Zr-10 | In-Zr-20 | In-Zr-20T | Ex-Zr-20 |
|---|---|---|---|---|---|---|
| onset temp. (°C) | 408 | 409.4 | 411.9 | 413.7 | 414.4 | 411.4 |
| Tmaxa (°C) | 438.5 | 456.3 | 455.4 | 457.6 | 458.2 | 458 |
| zirconia content | 0 | 5 | 10 | 20 | 20 | 20 |
Temperature at maximum weight loss.
The increase in the onset temperature of degradation arising from combustion of polymer chains is 2–3 °C higher for zirconia-filled composites relative to the unfilled sample, while it becomes 6 °C higher in the case of TESPT-treated zirconia-filled composites. What is more, there is a significant increase in the corresponding Tmax (temperature at which the maximum weight loss occurs) that becomes 18–20 °C higher for filled composites relative to that of unfilled NBR, as noted from DTG curves. Credit for such an improvement in thermal stability goes to incorporation of zirconia, which is well known for its refractoriness.29 In addition, uniform filler dispersion and an increase in cross-linking density also restrict the movement of rubber chains that could increase the thermal stability. Among all the composites, In-Zr-20T shows the maximum thermal stability, which is correlated with its morphological and mechanical behavior (discussed in subsequent section).
2.3. Morphology
To investigate the state of dispersion and morphology of zirconia particles, an SEM study of 20 phr zirconia-filled composites was performed, and the micrographs are presented in Figure 2(a–c,f). In the images, ZrO2 and ZnO particles appear as bright spots, and the black area represents the NBR matrix. In the micrograph of externally filled composite, Ex-Zr-20 (Figure 2f), larger aggregates of zirconia (∼7–8 μm) are evident together with some finer particles of dimension ∼200 nm. On the other hand, the state of dispersion of zirconia particles is found much better in in situ filled composites without any sign of filler agglomeration. Furthermore, filler distribution and compatibility are found quite better for TESPT-treated zirconia-filled composite In-Zr-20T (∼4–5 nm for finer particles and ∼1 μm for bigger particles) than its counterpart In-Zr-20, wherein TESPT is not used (∼100 nm for finer particles and ∼1.2 μm for bigger particles). Elemental analysis by EDX of the composites was also done along with SEM to better resolve ZnO particles from ZrO2 particles. ZnO particles are brighter in appearance and much smaller than zirconia particles, as identified with EDX spectroscopy (Figure 2d,e). It should be mentioned that the zinc oxide used here are commercial types with several micrometer in particle size. However, in the compounding and vulcanizing condition, the zinc oxide reacts with stearic acid and the organic accelerator (sulfur vulcanizing agents). Thereafter, the final size of the remaining zinc oxide particles might become shorter. Nevertheless, Zn maps and Zr maps show that zinc oxide particles are uniformly distributed in all the composites. Meanwhile, zirconia particles are more uniformly dispersed in the matrix of in situ developed composites (Figure 3). In contrast, zirconia particles in the externally filled composite are aggregated or agglomerated.
Figure 2.
Scanning electron microscopy (SEM) images of (a) In-Zr-20, (b) In-Zr-20T, and (c) Ex-Zr-20. (d, e) Identification of particles by EDX spectroscopy. Darker larger particles (#1) are zirconia, and brighter smaller particles (#2) are ZnO. (f) Overview image of specimen Ex-Zr-20 showing large agglomerates of zirconia that are not properly visible in (c).
Figure 3.
SEM images, Zn maps, Zr maps, and overlaid Zn and Zr maps for (a) In-Zr-20, (b) In-Zr-20T, and (c) Ex-Zr-20.
2.4. Mechanical Characteristics
Mechanical properties of the composites are studied by stress–strain measurement, and the results are presented in Table 2 and Figure 4. A low strain modulus of elastomeric materials is very important for their dynamic performance. It is seen that the modulus at 50% and at 100% strains are much higher for all the zirconia-filled composites compared to those of unfilled one, as expected. What is more, a remarkable enhancement in moduli (two to three times higher) at the abovementioned strains is brought by in situ filled zirconia. On the other hand, it is much inferior for externally added zirconia even at the same content. The ultimate physical properties of rubber composites mostly depend on the matrix-to-filler interaction that is governed by filler dispersion, filler surface area, and finally direct chemical bonds between polymer chains and filler particles. Additionally, for a polar rubber-like NBR, the presence of polar nitrile (−CN) groups could make a hydrogen-bond type dipolar interaction with the surface hydroxyl groups of zirconia, resulting in a strong zirconia–NBR interaction (Scheme 2). Such a kind of interaction between nitrile groups of NBR and silanol groups of silica is well reported in literature30,31 Most probably, the concentration of the hydroxyl group on the sol–gel-derived zirconia particles is higher than commercially available externally added zirconia. Due to the presence of a higher number of hydroxyl groups on the in situ zirconia particles, the rubber–filler interactions become stronger that deliver superior mechanical properties to the in situ filled NBR composites. Use of TESPT as a surface modifier in the reactive sol–gel system for the highest zirconia content composition (In-Zr-20T) further enhances the mechanical properties of the composites. Dual reactivity of TESPT is believed to afford chemical bridging between zirconia and rubber matrixes that is primarily responsible for the improved mechanical property to such an extent. Acidic hydroxyl groups on the zirconia surface initiate the coupling reaction between the zirconia and hydrolyzed ethoxy groups of TESPT.32,33 Furthermore, during the vulcanization step, S–S bonds of TESPT undergo cleavage and participate in sulfur cross-links of rubber chains. Such a kind of dual activity of TESPT is very well known in silica-based rubber systems.34,35 Surface silanization of zirconia by TESPT is believed to cause an enhancement in filler dispersion, cross-linking density, and also rubber–filler interaction. This is illustrated in Scheme 2. It is also evident from Table 2 that the tensile strength of the composites shows a consistent and similar trend. Tensile strength becomes almost four-fold and six-fold higher for composites In-Zr-20 and In-Zr-20T, respectively, with respect to the unfilled NBR gum. Enhancement in tensile strength to such a great extent is attributed to the controlled incorporation of the rigid filler under optimized reaction conditions that ensure uniform dispersion of the filler in the elastomer matrix with smaller particle size. In contrast, externally zirconia-filled composite even at the same filler content (20 phr) shows very much inferior stress–strain properties. This is not surprising as poor filler dispersion in NBR matrixes is evident in the morphological study for this composite.
Table 2. Mechanical Properties of Unfilled and Zirconia-Filled Composites.
| sample code | unfilled | In-Zr-5 | In-Zr-10 | In-Zr-20 | In-Zr-20T | Ex-Zr-20 |
|---|---|---|---|---|---|---|
| σ50% (M Pa) | 0.488 ± 0.003 | 0.763 ± 0.016 | 0.921 ± 0.029 | 1.193 ± 0.028 | 1.333 ± 0.006 | 0.581 ± 0.005 |
| σ100% (M Pa) | 0.624 ± 0.003 | 0.997 ± 0.022 | 1.280 ± 0.026 | 1.725 ± 0.007 | 2.060 ± 0.050 | 0.732 ± 0.025 |
| σ200% (M Pa) | 0.724 ± 0.007 | 1.297 ± 0.032 | 2.147 ± 0.057 | 3.316 ± 0.042 | 4.223 ± 0.118 | 0.887 ± 0.050 |
| tensile strength (MPa) | 1.007 ± 0.012 | 2.673 ± 0.142 | 3.386 ± 0.083 | 4.643 ± 0.183 | 5.700 ± 0.836 | 1.652 ± 0.009 |
| elongation at break (%) | 515.67 ± 51.83 | 502.33 ± 29.01 | 294.67 ± 13.05 | 270.66 ± 13.79 | 265.33 ± 42.66 | 574.07 ± 16.51 |
| cross-linking density (υ × 10–4) | 4.05 | 6.01 | 6.25 | 7.96 | 8.25 | 5.03 |
Figure 4.

Stress–strain curves of unfilled and zirconia-filled NBR composites.
Scheme 2. Sol–Gel Synthesis of Zirconia and Its Surface Modification by TESPT and Different Interactions of Them with NBR.
To have a quantitative view of the extent of reinforcement brought by in situ zirconia, reinforcing efficiency (RE) of the composites are determined using eq 1, and the values are presented in Table 3.36 RE of the in situ zirconia is found to be ∼10 times higher than that of externally filled zirconia. The highest RE is achieved for In-Zr-20T, and this observation is consistent with the results of this composite found in other studies.
| 1 |
Table 3. Tg (°C) and Tan Delta Peak Height of Unfilled and Zirconia-Filled Composites.
| sample code | unfilled | In-Zr-10 | In-Zr-20 | In-Zr-20T | EEx-Zr-20 |
|---|---|---|---|---|---|
| Tg (°C) | –8.6 | –7.7 | –6.3 | –7.5 | –8.4 |
| tan δmax | 1.45 | 1.44 | 1.31 | 1.28 | 1.44 |
We were curious to compare the reinforcement effect of in situ zirconia found in our study with some of those reported in literature. As reported, for natural rubber–silica composites, precipitated silica increases both the σ100% and tensile strength by ∼1.1–1.2 times over the unfilled compound at 10 and 20 phr silica contents.12 Meanwhile, in our case, those parameters become 2.1 and 3.3 times higher, respectively, for 10 phr zirconia-filled composites and 2.8 and 4.5 times higher, respectively, for 20 phr zirconia-filled composites. In another study, the σ100% and tensile strength are reported to be 1.2 and 1.9 times higher, respectively, for 5 phr silica-containing NBR composites than the unfilled NBR sample.3 In the present case, at a 5 phr zirconia content, the σ100% and tensile strength are found 1.6 and 2.7 times higher, respectively, than the unfilled sample Thus, it is obvious that in situ zirconia resulted in this work is empowered with superior reinforcing capability when compared with that of silica, a standard filler used for rubber composites.
To investigate the extent of cross-linking developed in rubber matrixes, we have determined the cross-linking values of all the vulcanized sheets by a swelling method. The values are calculated from the Flory–Rhener equation, and the data are included in Table 2. It can be clearly seen here that the cross-linking density significantly increases by incorporation of zirconia itself owing to strong NBR–zirconia interaction that arises from development of hydrogen-bond–type interaction between surface hydroxyl groups of zirconia with electronegative nitrile groups present in NBR. Thus, it appears that an increase in cross-linking density also contributes toward the reinforcement effect of zirconia along with other factors.
2.5. Dynamic Mechanical Analysis (DMA)
Figure 5 represents the temperature-dependent storage modulus (E') and tan δ of NBR gum and selected zirconia-filled NBR composites. A higher storage modulus relative to the unfilled NBR gum in the rubbery region is evident for all the zirconia-filled composites. Notably, the highest zirconia-filled composites In-Zr-20 and In-Zr-20T show much higher storage moduli in the rubbery plateau region than others. On the other hand, the storage modulus of the externally filled composite Ex-Zr-20 does not improve considerably. The plot of temperature versus loss tangent (tan δ) shows a steady decrease in peak height of tan δ with an increase in zirconia (in situ) content. Additionally, the positive shift of tan δ for filled composites relative to NBR gum is also noted. All these results support the prominent reinforcement effect arising from increased cross-linking density and strong rubber–filler interaction brought by incorporation of in situ zirconia in the NBR matrix. Clearly, this effect is very inferior for externally filled NBR–zirconia composites Ex-Zr-20 that goes in same line with its stress–strain results and morphological features.
Figure 5.

(a) Temperature versus storage modules curves of unfilled and zirconia-filled composites. (b) Loss tangent (tan δ) versus temperature (°C) curves of unfilled and zirconia-filled composites; (c) Dynamic strain (Payne effect) as a function of storage modulus.
As far as the rubber-to-filler interaction is concerned, a dynamic strain sweep experiment could enlighten the physical mechanism with further details. In general, the dynamic modulus for gum compounds (a cross-linked rubber without any filler) does not depend on the dynamic strain. However, for filler-containing composites, a strong effect on the dynamic modulus can be observed with respect to dynamic strain, which is known as the Payne effect. This fact can nicely be explained in terms of filler–filler interaction operating in a soft elastomer matrix. At a lower strain, filler–filler interactions are stronger and dominate over the matrix modulus (dynamic). However, at a higher strain, filler–filler interactions are broken and a gradual decrease in the modulus becomes evident. The effect is more prominent at a higher filler loading wherein the filler content remains well above the percolation. In the present study, it is noted that the unfilled NBR gum does not show any Payne effect, while it is very prominent for composites In-Zr-20 and In-Zr-20T. Interestingly, externally filled composite Ex-Zr-20 even at the same filler content did not show a strong Payne effect. This fact can be explained in terms of strong filler–filler interaction existing in the case of the 20 phr in situ zirconia filler system. Such a strong filler–filler network for in situ zirconia is supported by its favorable morphological features. It may be recalled at this point that SEM images revealed that in situ zirconia was distributed and dispersed very evenly with much smaller particle size; whereas, even at the same loading, externally added zirconia remains in an aggregated and agglomerated structural form just like isolated islands, and therefore, they do not contribute to the modulus value at a lower strain significantly. It is also interesting to note that, after addition of TESPT, the Payne effect is reduced a bit for In-Zr-20T. It seems that, since TESPT acts as a coupling agent between the rubber and the filler, it might reduce the filler–filler interaction to some extent.
2.6. Differential Scanning Calorimetry (DSC)
The glass transition temperature (Tg) determined for selected composites from differential scanning calorimetry differs significantly from those obtained from the DMA study (Figures 5 and 6). This is not surprising as distinct principles are involved in DSC and DMA techniques. DSC measures the change in heat capacity as the rubber chain evolves from the frozen to unfrozen state, whereas DMA measures the change in mechanical response of this chain.37,38 Nevertheless, the trend of Tg along the series remains the same in both cases. Although the Tg value of In-Zr-20 is very close to that of the unfilled sample, it is raised by ∼2 °C for In-Zr-20T. Such an increment in Tg is a reflection of restriction in the segmental mobility of rubber chains that is caused by enhanced rubber–filler interaction for the latter one brought by TESPT-treated zirconia.
Figure 6.

DSC thermogram of the NBR matrix and in situ zirconia-filled composites.
To have further insight into the filler activity and the amount of rubber in the immobilized layer, the increment in heat capacity (ΔCp) of these three samples is also determined according to ASTM E1269-11 and the values are tabulated in Table 4. ΔCp values become less for both the 20 phr filled composites than that of unfilled NBR gum. This is due to the fact that the rigid filler does not contribute to the glass transition of the composite.39 Interestingly, this decrease in ΔCp values is higher for TESPT-treated zirconia-filled composites (In-Zr-20T) relative to untreated zirconia-filled composites (In-Zr-20), although both have the same filler content. To explain this decrease in ΔCp, the fraction of the immobilized rubber layer (χim) on the filler surface is calculated by following equation.40
| 2 |
where ΔCp0 is the specific heat capacity increment of the neat NBR and Wfiller is the weight fraction of zirconia.
Table 4. Glass Transition Temperature (Tg) and Heat Capacity Data (ΔCp) of Selected Composites.
| sample code | unfilled | In-Zr-20 | In-Zr-20T |
|---|---|---|---|
| Tg (°C) | –24.9 | –24.1 | –22.8 |
| ΔCp (J/(g K)) | 0.395 | 0.319 | 0.292 |
| χim | 0.00 | 0.0456 | 0.1257 |
It may be seen from Table 4 that the amount of the immobilized rubber layer on the filler surface is 4.5% for untreated zirconia-filled composites (In-Zr-20), while it becomes almost thrice (12.5%) for TESPT-treated zirconia-filled composites (In-Zr-20T). This is caused by surface treatment of zirconia by TESPT that enhances rubber–filler interaction significantly (Scheme 2). Thus, a greater fraction of the immobilized rubber layer around the filler surface in the latter case is accountable for such difference in the ΔCp value, although the filler content is the same in both cases. This is also reflected in the increment in the Tg value for In-Zr-20T relative to In-Zr-20.
2.7. Strength-Related Evaluation
Superior reinforcing ability and thermal stability delivered by in situ zirconia in the current study encouraged us to further extend their evaluation toward high-performance application. Accordingly, we have performed some industrially important testings on the prepared composites (described below) to examine their capability in withstanding a harsh and adverse environment.
2.7.1. Oil Resistance and Abrasion Resistance
NBR is very popular for its oil resistance application in industry. Therefore, we have evaluated the oil resistance property of the composites according to ASTM D471 with two standard oils, viz., IRM 901 and IRM 903. Volume change, a measure of oil resistance property, is found to decrease significantly upon incorporation of even 5 phr zirconia. Although the oil resistance characteristic does not increase proportionately with zirconia content, the maximum oil resistance property is shown by TESPT-treated zirconia-filled composite In-Zr-20T (Table 5). Strong rubber–filler interaction and an increase in cross-linking density are believed to prevent the oil molecules to permeate into the vulcanizates. Also, oil resistance property is found better for in situ filled composites than externally filled composites, as expected. Enhancement in oil resistance property could also partly be contributed by an increase in polarity of rubber matrixes by incorporation of zirconia.41,42
Table 5. Oil and Abrasion Resistance of Unfilled and Zirconia-Filled NBR Composites.
| study | sample code | unfilled | In-Zr-5 | In-Zr-10 | In-Zr-20 | In-Zr-20T | Ex-Zr-20 |
|---|---|---|---|---|---|---|---|
| oil resistance (oil-IRM 901) | volume change (%) | 2.02 | 0.79 | 0.77 | 0.72 | 0.62 | 1.57 |
| oil resistance (oil-IRM 903) | volume change (%) | 17.15 | 12.92 | 12.83 | 11.79 | 11.18 | 15.29 |
| abrasion resistance | volume loss (cc) | 0.29 | 0.20 | 0.11 | 0.12 | 0.10 | 0.35 |
| aRVL (mm3) | 270 | 185 | 99 | 115 | 93 | 327 |
RVL: relative volume loss.
Abrasion resistance of rubber composites is a measure of their capability to offer resistance to fracture or tearing.43 Abrasion loss can be expressed as volume loss and relative volume loss (RVL) with respect to standard rubber. The abrasion resistance of the zirconia-filled composites is determined by using an abrasion drum. It is found that incorporation of in situ zirconia reduces the abrasion loss of NBR composites appreciably. The improvement in the abrasion resistance property of in situ zirconia-filled composites is attributed to the increase in the cross-link density and enhancement in interfacial rubber–filler interaction.44 The maximum effect for In-Zr-20T is as much as expected from its properties found in earlier studies.
2.7.2. Ozone Resistance
Elastomers having double bonds are susceptible to attack by ozone that leads to their degradation. We have performed an ozone resistance study to check if in situ zirconia could have positive contribution in this regard. The 20 phr filled samples are selected for this purpose, and the result is compared with that of unfilled gum. Figure 7 shows the optical photographs of the surfaces of these samples taken after 24 h and 48 h. Appearance of macroscopic cracks on the surface of the samples depicts the sign of degradation. Although cracks are apparent in both the specimen, crack lengths are reduced in filled composites. Furthermore, relatively better ozone resistance is observed for TESPT-treated zirconia-filled composites. Thus, it is evident that in situ zirconia could also contribute to the ozone resistance property of the composites to some extent.
Figure 7.
Optical photographs of the surfaces of the unfilled and zirconia-filled NBR composites after ozone exposure: (a) after 24 h and (b) after 48 h.
2.8. Curing Characteristics
Curing characteristics of the composites are assessed by a rheological study, and the results are summarized in Table 6. The maximum torque increases as zirconia loading increases due to enhancement in the degree of cross-linking of rubber vulcanizates. The difference between maximum and minimum torques (Δtorque) increases with an increase in zirconia content with respect to the unfilled one. This could be attributed to the enhanced interaction between the polar acrylonitrile group present in NBR and hydroxyl groups on the zirconia surface through some dipolar interaction. The scorch time, which provides information on processing safety of the rubber compound, also increases with an increase in the zirconia content.
Table 6. Curing Characteristics of Unfilled and Zirconia-Filled Composites.
| sample code | unfilled | In-Zr-5 | In-Zr-10 | In-Zr-20 | In-Zr-20T | Ex-Zr-20 |
|---|---|---|---|---|---|---|
| minimum torque (dNm) | 1.20 | 2.22 | 2.20 | 2.9 | 2.9 | 2.87 |
| maximum torque (dNm) | 6 | 13.50 | 14.20 | 16.50 | 15.70 | 11 |
| Δtorque (dNm) | 4.80 | 11.28 | 12 | 13.6 | 12.80 | 9.13 |
| cure time, t90 (min) | 40 | 29.19 | 30 | 31.72 | 31.99 | 20 |
| scorch time (min) | 0.52 | 1.47 | 1.50 | 1.77 | 2.22 | 2.30 |
3. Conclusions
The present study demonstrates the potential of in situ zirconia as a reinforcing filler for NBR composites that could cater to high-performance application. Designed and controlled loading of zirconia, in situ into the NBR matrix, enhances thermal, mechanical, and other strength-related properties of the composites appreciably in comparison to those of unfilled NBR gum. This is achieved by a very good state of filler dispersion in NBR matrixes, improved rubber–filler interaction, and enhanced cross-linking density brought by in situ zirconia nanoparticles directly formed into NBR matrixes from their precursor. Surface modification of zirconia by an organosilane (TESPT) during its sol–gel formation is found to further strengthen the composite properties. Reinforcing efficiency of in situ zirconia toward NBR achieved in this work is found noteworthy when compared with some silica-filled rubber composites reported in literature. A series of experimental evaluation reveals that in situ zirconia-filled composites could confront an adverse atmosphere as its incorporation enhances the resistance of the NBR composites toward oil, ozone, and abrasion. This piece of study therefore unfolds potential of in situ zirconia for the preparation of high-performance rubber composites. Finally, it must be stated that a lot of scope still remains there to further explore the area considering varying processing conditions, different types of rubber, and different kinds of surface modifiers and their optimal dose.
4. Experimental Section
4.1. Materials
Nitrile rubber (KNB 35L) (acrylonitrile content, 34%) was collected from Heritage Rubber (Nagpur, India). Zirconium(IV) propoxide solution (70% in 1-propanol) and mercaptobenzothiazole disulfide (97%) (MBTS) were bought from Sigma-Aldrich (India). Zirconium dioxide (98%) of particle size 11–39 μm and rhombic sulfur (99.8%) was purchased from Loba Chemie (India). Bis-(3-triethoxysilylpropyl) tetrasulfide (TESPT) was purchased from Evonik Degussa (Thailand). Tetrahydrofuran (THF) (purity, ≥99.0), zinc oxide (99%), and stearic acid (90%) were purchased from Fisher Scientific Ltd. (India). Mercaptobenzothiazole disulfide (MBTS) was provided by Sara polymer Pvt. Ltd. (Nagpur India).
4.2. In Situ Synthesis of Zirconia into NBR by the Solution Sol–Gel Method
Zirconia particles were grown in situ into the NBR matrix as follows: 50 g of small pieces of NBR was dissolved in 400 mL of tetrahydrofuran (THF) in a stirring condition. Then, the calculated amount of zirconium(IV) propoxide was added into the homogeneous rubber solution to prepare composites In-Zr-5, In-Zr-10, In-Zr-20, and In-Zr-20T (e.g., in the preparation of In-Zr-5, 9 mL of zirconium(IV) propoxide was added). Next, water was added (mole ratio of zirconium(IV) propoxide and water = 1:2), and stirring was continued for another 1 h. For the preparation of composite In-Zr-20T, 0.9 mL of TESPT was added in rubber solution in addition to the abovementioned reagents. The solution was subjected to 4 day gelation at ambient temperature. Finally, the gel was dried at 80 °C under vacuum till the constant weight is reached.
4.3. Formulation and Compounding of Rubber
On a two-roll mill, zirconia-filled rubber sheets were compounded. Cross-linking ingredients, viz., zinc oxide, stearic acid, mercaptobenzothiazole disulfide (MBTS), and sulfur, were added one by one and mixed well till the rubber mix became homogeneous. The recipe of composite preparation is presented in Table 6. Here, zinc oxide was used as an activator along with stearic acid. A combination of zinc oxide and stearic acid is well known to produce satisfactory results. MBTS was used as an accelerator and sulfur as the cross-linking agent. One unfilled sample was prepared for reference following the same procedure except that unfilled NBR was taken. Composite Ex-Zr-20 was prepared by mixing raw NBR, zirconium dioxide powder, and other cross-linking ingredients. Each compounded mix was subjected to compression molding at 160 °C for the duration of their respective cure time (t90) for vulcanization.
5. Characterizations
5.1. Thermogravimetric Analysis (TGA)
A thermal analyzer TG-DTA 7200 (Hitachi, Japan) was used to evaluate the thermal stability of the NBR composites by thermogravimetric analysis (TGA) and derivative of thermogravimetry (DTG). Temperature interval was kept between 40 and 800 °C with a heating rate of 10 °C/min. The thermal scan was started under nitrogen flow, and it was switched to air flow at 600 °C.
The zirconia content was calculated using eq 3
| 3 |
where W is the weight of ashes remaining after heating at 800 °C.
Percent conversion of zirconium(IV) propoxide to ZrO2 was calculated using eq 4
| 4 |
where W′ is the calculated amount of zirconia assuming a full conversion of zirconium(IV) propoxide into zirconia.
5.2. X-ray Diffraction Study (XRD)
The XRD patterns of the selected samples were determined using a Rigaku SmartLab diffractometer with Cu target (Kα wavelength of 1.542 Å) in a 2θ range of 15°–90°.
5.3. Morphological Study
Scanning electron microscopy (SEM) images were recorded with a Zeiss Ultra Plus field emission scanning electron microscope operated at an acceleration voltage of 3 kV. Small pieces (ca. 10 mm × ca. 2 mm × ca. 2 mm) of the rubber composites were cryo-fractured in liquid nitrogen, glued on scanning electron microscope holders, and coated with 20 nm carbon (Leica SCD500 sputter coater) to prevent charging in the electron beam. EDX spectra and EDX maps (EDX = energy-dispersive X-ray spectroscopy) were recorded in the same scanning electron microscope at an acceleration voltage of 6 kV with a Bruker X Flash 5060F spectrometer.
5.4. Mechanical Characteristics
Dumbbell-shaped specimens of vulcanized rubber sheets were subjected to a stress–strain study using a Universal Testing Machine (UTM) (H 10 KT Tinus Olsen, UK). ISO 527 standard was followed using a load cell of 500 N with a crosshead speed of 200 mm/min at room temperature. Stress–strain measurement was carried out three times for each sample, and the average value was tabulated. Error analysis of mechanical properties was performed using the analysis of variance (ANOVA) test by using the origin pro 2018 software.
5.5. Dynamic Mechanical Analysis (DMA)
Viscoelastic behavior of the samples was evaluated with the help of an Eplexor 2000 N dynamic mechanical analyzer (Gabo Qualimeter, Ahlden, Germany) at tension mode. The temperature range of measurement was −50 to 60 °C with a heating rate of 2 °C min–1. The frequency was kept constant at 10 Hz.
5.6. Differential Scanning Calorimetry
The glass transition temperature (Tg) and specific heat capacity (Cp) of selected samples were determined using a DSC 3 (Mettler Toledo, Switzerland) instrument with nitrogen flow at the rate of 40 mL/min. Samples were heated up to 200 °C at the rate of 40 °C min–1 to remove the volatile impurities. Then, the temperature is quenched to −70 °C. Finally, DSC scan was performed from −70 °C to 70 °C at a heating rate of 5 °C min–1. The specific heat capacity of the samples was determined according to ASTM E1269-11.
5.7. Curing Study
The cure characteristics of the composites were assessed using a moving die rheometer MDR Xgen100 (Future Foundation, India) at 160 °C for an hour with a frequency of 1.66 Hz.
5.8. Studies of Resistance to Oil, Ozone, and Abrasion
Oil resistance was evaluated according to ASTM D471 standard. A sample of one square inch dimension was cut, and the initial volume was calculated from its weight and specific gravity. The rubber specimens were immersed in standard oils (IRM 901 and IRM 903) for 70 h at 100 °C temperature. After that, the rubber samples were removed from the oil and were cleaned with tissue paper. The final volume was calculated by determining the final weight. The swelling percentage of oil was calculated from following formula.
| 5 |
Ozone resistance was checked according to ASTM D1149. A sample was stretched up to 20% and exposed to an ozone atmosphere in an ozone chamber of 50 pphm (parts per hundred milligram) dose for 24 h and 48 h separately. Then, the samples were removed, and the cracks perpendicular to the strain direction were examined.
For determination of abrasion resistance, 1.5–2.0 g of rubber sample was abraded on an abrasion drum at 84 rpm and the distance covered was 40 m. After that, the abraded weight was taken and divided by specific gravity to get the volume loss.
5.9. Cross-linking Density Measurement
We have determined the degree of cross-linking of all the vulcanized sheets by a swelling method. Cross-linking densities are calculated using the Flory–Rehner equation (given below)45
| 6 |
where Vs is the molar volume of toluene (106.2), Vr is the volume fraction of rubber in the swollen gel, and χ is the Flory–Huggins rubber–solvent interaction parameter.
Acknowledgments
Financial support received from IRMRA, Thane, to carry out this work is sincerely acknowledged (project code: IRMRA/R&D/16-17/EXT-VNIT/03). S.C.A. thanks VNIT for providing the research fellowship. Characterization support received from DST-FIST sponsored research facilities (SR/FST/CSI-279/2016 (c)) at Chemistry Department, VNIT, Nagpur, is acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03495.
Thermo gravimetric curves of the unfilled and zirconia-filled composites (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kang S.; Hong S. I.; Choe C. R.; Park M.; Rim S.; Kim J. Preparation and characterization of epoxy composites filled with functionalized nanosilica particles obtained via sol–gel process. Polymer 2001, 42, 879–887. 10.1016/S0032-3861(00)00392-X. [DOI] [Google Scholar]
- Ikeda Y.; Poompradub S.; Morita Y.; Kohjiya S. Preparation of high performance nanocomposite elastomer: effect of reaction conditions on in situ silica generation of high content in natural rubber. J. Sol-Gel Sci. Technol. 2008, 45, 299–306. 10.1007/s10971-008-1682-7. [DOI] [Google Scholar]
- Kapgate B. P.; Das C.; Basu D.; Das A.; Heinrich G.; Reuter U. Effect of silane integrated sol–gel derived in situ silica on the properties of nitrile rubber. J. Appl. Polym. Sci. 2014, 131, 40531. 10.1002/app.40531. [DOI] [Google Scholar]
- Vaikuntam S. R.; Stöckelhuber K. W.; Subramani Bhagavatheswaran E.; Wießner S.; Scheler U.; Saalwächter K.; Formanek P.; Heinrich G.; Das A. Entrapped styrene butadiene polymer chains by sol–gel-derived silica nanoparticles with hierarchical raspberry structures. J. Phys. Chem. B 2018, 122, 2010–2022. 10.1021/acs.jpcb.7b11792. [DOI] [PubMed] [Google Scholar]
- Alexandru M.; Cazacu M.; Nistor A.; Musteata V. E.; Stoica I.; Grigoras C.; Simionescu B. C. Polydimethylsiloxane/silica/titania composites prepared by solvent-free sol–gel technique. J. Sol-Gel Sci. Technol. 2010, 56, 310–319. 10.1007/s10971-010-2307-5. [DOI] [Google Scholar]
- Yang D.; Huang S.; Wu Y.; Ruan M.; Li S.; Shang Y.; Cui X.; Wang Y.; Guo W. Enhanced actuated strain of titanium dioxide/nitrile-butadiene rubber composite by the biomimetic method. RSC Adv. 2015, 5, 65385–65394. 10.1039/C5RA12311A. [DOI] [Google Scholar]
- Das C.; Bansod N. D.; Kapgate B. P.; Rajkumar K.; Das A. Incorporation of titania nanoparticles in elastomer matrix to develop highly reinforced multifunctional solution styrene butadiene rubber composites. Polymer 2019, 162, 1–10. 10.1016/j.polymer.2018.12.022. [DOI] [Google Scholar]
- Selyanchyn R.; Fujikawa S. Molecular hybridization of polydimethylsiloxane with zirconia for highly gas permeable membranes. ACS Appl. Polym. Mater. 2019, 1165–1174. 10.1021/acsapm.9b00178. [DOI] [Google Scholar]
- Huang Y.; Feng Y.; Sun X.; Han Y.; Liu D.; Tan X. Preparation of ZrO2/silicone hybrid materials for LED encapsulation via in situ sol-gel reaction. Polym. Adv. Technol. 2019, 1818. 10.1002/pat.4614. [DOI] [Google Scholar]
- Chung P.-T.; Chiou S.-H.; Tseng C.-Y.; Chiang A. S.-T. Preparation and evaluation of a zirconia/oligosiloxane nanocomposite for LED encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 9986–9993. 10.1021/acsami.6b02082. [DOI] [PubMed] [Google Scholar]
- Messori M.In situ synthesis of rubber nanocomposites. Recent Advances in Elastomeric Nanocomposites; Springer, 2011, 57–85. [Google Scholar]
- Ikeda Y.; Kameda Y. Preparation of “green” composites by the sol-gel process: in situ silica filled natural rubber. J. Sol-Gel Sci. Technol. 2004, 31, 137–142. 10.1023/B:JSST.0000047975.48812.1b. [DOI] [Google Scholar]
- Bansod N. D.; Kapgate B. P.; Das C.; Basu D.; Debnath S. C.; Roy K.; Wiessner S. Controlled growth of in situ silica in a NR/CR blend by a solution sol–gel method and the studies of its composite properties. RSC Adv. 2015, 5, 53559–53568. 10.1039/C5RA08971A. [DOI] [Google Scholar]
- Bansod N. D.; Kapgate B. P.; Das C.; Das A.; Basu D.; Debnath S. C. Compatibilization of natural rubber/nitrile rubber blends by sol–gel nano-silica generated by in situ method. J. Sol-Gel Sci. Technol. 2016, 80, 548–559. 10.1007/s10971-016-4114-0. [DOI] [Google Scholar]
- Scotti R.; Wahba L.; Crippa M.; D’Arienzo M.; Donetti R.; Santo N.; Morazzoni F. Rubber–silica nanocomposites obtained by in situ sol–gel method: particle shape influence on the filler–filler and filler–rubber interactions. Soft Matter 2012, 8, 2131–2143. 10.1039/c1sm06716h. [DOI] [Google Scholar]
- Mahmood W. A. K.; Khan M. M. R.; Azarian M. H. Sol-gel synthesis and morphology, thermal and optical properties of epoxidized natural rubber/zirconia hybrid films. J. Non-Cryst. Solids 2013, 378, 152–157. 10.1016/j.jnoncrysol.2013.07.002. [DOI] [Google Scholar]
- Sarwar M. I.; Zulfiqar S.; Ahmad Z. Properties of polyamide-zirconia nanocomposites prepared from sol-gel technique. Polym. Compos. 2009, 30, 95–100. 10.1002/pc.20538. [DOI] [Google Scholar]
- Ochi M.; Nii D.; Harada M. Preparation of epoxy/zirconia hybrid materials via in situ polymerization using zirconium alkoxide coordinated with acid anhydride. Mater. Chem. Phys. 2011, 129, 424–432. 10.1016/j.matchemphys.2011.04.034. [DOI] [Google Scholar]
- Ma X.; Peng C.; Zhou D.; Wu Z.; Li S.; Wang J.; Sun N. Synthesis and mechanical properties of the epoxy resin composites filled with sol– gel derived ZrO2 nanoparticles. J. Sol-Gel Sci. Technol. 2018, 88, 442–453. 10.1007/s10971-018-4827-3. [DOI] [Google Scholar]
- Rehman H. U.; Sarwar M. I.; Ahmad Z.; Krug H.; Schmidt H. Synthesis and characterization of novel aramid-zirconium oxide micro-composites. J. Non-Cryst. Solids 1997, 211, 105–111. 10.1016/S0022-3093(96)00614-X. [DOI] [Google Scholar]
- Wen J.; Mark J. E. Synthesis, structure, and properties of poly (dimethylsiloxane) networks reinforced by in situ-precipitated silica–titania, silica–zirconia, and silica–alumina mixed oxides. J. Appl. Polym. Sci. 1995, 58, 1135–1145. 10.1002/app.1995.070580707. [DOI] [Google Scholar]
- TianKhoon L.; Hassan N. H.; Rahman M. Y. A.; Vedarajan R.; Matsumi N.; Ahmad A. One-pot synthesis nano-hybrid ZrO2–TiO2 fillers in 49% poly (methyl methacrylate) grafted natural rubber (MG49) based nano-composite polymer electrolyte for lithium ion battery application. Solid State Ionics 2015, 276, 72–79. 10.1016/j.ssi.2015.03.034. [DOI] [Google Scholar]
- Nihmath A.; Ramesan M. T. Synthesis, characterization, processability, mechanical properties, flame retardant, and oil resistance of chlorinated acrylonitrile butadiene rubber. Polym. Adv. Technol. 2018, 2165–2173. 10.1002/pat.4324. [DOI] [Google Scholar]
- Das C.; Bansod N. D.; Kapgate B. P.; Reuter U.; Heinrich G.; Das A. Development of highly reinforced acrylonitrile butadiene rubber composites via controlled loading of sol-gel titania. Polymer 2017, 109, 25–37. 10.1016/j.polymer.2016.12.018. [DOI] [Google Scholar]
- Zhao J.; Fan W.; Wu D.; Sun Y. Synthesis of highly stabilized zirconia sols from zirconium n-propoxide-diglycol system. J. Non-Cryst. Solids 2000, 261, 15–20. 10.1016/S0022-3093(99)00651-1. [DOI] [Google Scholar]
- Tyagi B.; Sidhpuria K.; Shaik B.; Jasra R. V. Synthesis of Nanocrystalline Zirconia Using Sol-Gel and Precipitation Techniques. Ind. Eng. Chem. Res. 2006, 45, 8643–8650. 10.1021/ie060519p. [DOI] [Google Scholar]
- Shukla S.; Seal S.; Vanfleet R. Sol-gel synthesis and phase evolution behavior of sterically stabilized nanocrystalline zirconia. J. Sol-Gel Sci. Technol. 2003, 27, 119–136. 10.1023/A:1023790231892. [DOI] [Google Scholar]
- Gómez R.; López T.; Bokhimi X.; Muñoz E.; Boldú J. L.; Novaro O. Dehydroxylation and the crystalline phases in sol-gel zirconia. J. Sol-Gel Sci. Technol. 1998, 11, 309–319. 10.1023/A:1008666531404. [DOI] [Google Scholar]
- Karaulov A. G.; Piskun T. V.; Kvasman N. M. Physical and mechanical properties of zirconium dioxide refractories and their resistance to attack by molten metals. Refractories 1993, 34, 367–375. 10.1007/BF01602699. [DOI] [Google Scholar]
- Suzuki N.; Ito M.; Ono S. Effects of Rubber/Filler Interactions on the Structural Development and Mechanical Properties of NBR/Silica Composites. J. Appl. Polym. Sci. 2005, 95, 74–81. 10.1002/app.20800. [DOI] [Google Scholar]
- Mohamed R. M.; Khattab M. M.; Abdel-Aziz M. M. Effect of Gamma-Irradiation on the Physicomechanical Properties of Synthetic Rubber–Based Silica Composites. Adv. Polym. Technol. 2011, 30, 301–311. 10.1002/adv.20225. [DOI] [Google Scholar]
- Wisser F. M.; Abele M.; Gasthauer M.; Müller K.; Moszner N.; Kickelbick G. Detection of surface silanol groups on pristine and functionalized silica mixed oxides and zirconia. J. Colloid Interface Sci. 2012, 374, 77–82. 10.1016/j.jcis.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Lavalley J. C.; Bensitel M.; Gallas J. P.; Lamotte J.; Busca G.; Lorenzelli V. FT-IR study of the δ (OH) mode of surface hydroxy groups on metal oxides. J. Mol. Struct. 1988, 175, 453–458. 10.1016/S0022-2860(98)80119-1. [DOI] [Google Scholar]
- De D.; Das A.; De D.; Panda P. K.; Dey B.; Roy B. C. Reinforcing Effect of Silica on the Properties of Styrene Butadiene Rubber–Reclaim Rubber Blend System. J. Appl. Polym. Sci. 2006, 99, 957–968. 10.1002/app.22379. [DOI] [Google Scholar]
- Goerl U.; Hunsche A.; Mueller A.; Koban H. G. Investigations into the Silica/Silane Reaction System. Rubber Chem. Technol. 1997, 70, 608–623. 10.5254/1.3538447. [DOI] [Google Scholar]
- Das A.; Jurk R.; Werner Stöckelhuber K.; Heinrich G. Silica-ethylene propylene diene monomer rubber networking by in situ sol-gel method. J. Macromol. Sci., Part A: Pure Appl.Chem. 2007, 45, 101–106. 10.1080/10601320701683447. [DOI] [Google Scholar]
- Kader M. A.; Kim W. D.; Kaang S.; Nah C. Morphology and dynamic mechanical properties of natural rubber/nitrile rubber blends containing trans-polyoctylene rubber as a compatibilizer. Polym. Int. 2005, 54, 120–129. 10.1002/pi.1655. [DOI] [Google Scholar]
- Heinrich G.; Klüppel M. In Filled Elastomers Drug Delivery Systems; Springer: Berlin Heidelberg, 2002, 1–44. [Google Scholar]
- Sargsyan A.; Tonoyan A.; Davtyan S.; Schick C. The amount of immobilized polymer in PMMA SiO2 nanocomposites determined from calorimetric data. Eur. Polym. J. 2007, 43, 3113–3127. 10.1016/j.eurpolymj.2007.05.011. [DOI] [Google Scholar]
- Fragiadakis D.; Bokobza L.; Pissis P. Dyanamics near the filler surface in natural rubber-silica nanocomposites. Polymer 2011, 52, 3175–3182. 10.1016/j.polymer.2011.04.045. [DOI] [Google Scholar]
- Jasna V. C.; Anilkumar T.; Naik A. A.; Ramesan M. T. Chlorinated styrene butadiene rubber/ zinc sulfide: novel nanocomposites with unique properties- structural, flame retardant, transport and dielectric properties. J. Polym. Res. 2018, 25, 144. 10.1007/s10965-018-1536-0. [DOI] [Google Scholar]
- Meng Y.; Chu J.; Liu C.; Wei Z.; Zhang L. Oil Resistance and Mechanical Properties of Polysiloxane Nanocomposites Prepared by In Situ Reaction of Reactive Polar Monomers. J. Appl. Polym. Sci. 2014, 10.1002/app.40983. [DOI] [Google Scholar]
- Buitrago-Suescún O.; Monroy M. Maleated polyethylene as a compatibilizing agent in cannabis indicastem’s flour-reinforced composite materials. Iran. Polym. J. 2018, 819–827. 10.1007/s13726-018-0656-z. [DOI] [Google Scholar]
- Thepsuwan U.; Sae-oui P.; Sirisinha C.; Thaptong P. Influence of Halloysite Nanotube on Properties of Tire Tread Compounds Filled with Silica and Carbon Black Hybrid Filler. J. Appl. Polym. Sci. 2018, 46987. 10.1002/app.46987. [DOI] [Google Scholar]
- Sperling L. H.Introduction to Physical Polymer Science; John Wiley & Sons, 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








