Abstract

The dissolution rate of nicotine in aqueous solutions of sodium chloride (NaCl) was investigated at room temperature and 70 °C by quantitatively visualizing the shrinkage rate of microscopic nicotine droplets. Four different salt concentrations were used: 15 wt % (3.0 M), 20 wt % (4.3 M), 25 wt % (5.7 M), and the saturation NaCl concentration of 26 wt % (6.0 M). These results, together with the Epstein–Plesset mathematical model, provided estimates of nicotine’s diffusion coefficient in the NaCl solutions. At room temperature, the dissolution rate of nicotine and diffusion coefficients decreased with increasing NaCl concentration, and below 15 wt %, the dissolution kinetics were too fast to measure accurately via optical microscopy. At the higher temperature of 70 °C, nicotine’s dissolution rate showed a decrease for 15 and 20% NaCl. However, at near-saturation 25% NaCl, nicotine’s dissolution rate did not exhibit significant change for the two temperatures, and for 26%, dissolution was higher at 70 °C than at room temperature.
Introduction
Nicotine (Figure 1) is widely present in tobacco products, and recently, a continuously increasing means of nicotine consumption has been in the form of electronic cigarettes (e-cigarettes; ECs).1 An extremely toxic alkaloid,2 nicotine, is completely soluble in water at temperatures that are lower than 60.8 °C3 and therefore can be easily transported from waste to groundwater, possibly compromising the environment and human health.4,5 Waste compounds from tobacco products such as nicotine and cotinine (tobacco byproduct) are found in solid-waste landfills and dumps, and these compounds have been classified as contaminants of emerging concern.6 Nicotine and related products can still persist in treated wastewater,7 and for conventional purification processes of drinking water, the removal efficiencies are 94% for cotinine and 79% for nicotine.8 Even with advanced purification treatments, these compounds cannot be completely eliminated from water,9 which means that nicotine can potentially contaminate water available for consumption. Therefore, knowledge of nicotine’s physicochemical behavior and properties will be helpful in understanding and predicting its environmental fate and transport. Related previous studies for the purification/recovery of water from nicotine have used organic solvents such as toluene, kerosene, and hexane in the widely applied liquid–liquid extraction process.10,11 To avoid the use of organic compounds, alternative methods such as adsorption,12,13 microbiological degradation,14,15 extraction by hydrophobic ionic liquids,16 and two-phase systems with hydrophilic ionic liquid and inorganic salts17 have also gained attention.
Figure 1.

Structure of nicotine.
Use of inorganic salts to separate/extract nicotine is a more sustainable treatment, and several related studies have dealt with the thermodynamics of nicotine dissolution. Davies and Gillard18 studied the solubility loop of nicotine in water, and Lopes et al.19 investigated the phase separation of nicotine from water—salting out effects—in nicotine aqueous solution in the presence of inorganic salts. In Lopes et al., sodium chloride was used as the inorganic salt, and liquid–liquid equilibria of the ternary solutions (nicotine + water + salts) were determined.19 The current paper is the first study on the kinetics of nicotine dissolution, focusing on dissolution rates of microscopic nicotine drops in aqueous solutions of different NaCl concentrations at room temperature and 70 °C.
Different approaches to quantify dissolution rates have been discussed such as measurement of the size of individual drops.20 Chen et al. used video microscopy to measure the dissolution rate of nonionic surfactant drops in water.21 In the present paper, the dissolution rate of nicotine was visualized and quantified within cylindrical, thin-walled glass capillaries microscopically. A unique capillary-pulling technique that allows very clear imaging of microscopic objects, like different kinds of droplets such as water, ethanol, and sulfuric acid undergoing reaction, has been developed in our laboratory and has elucidated several key reaction and transport processes, especially in acid neutralization by lubricant oils.22−26 Fu et al. introduced high-temperature capability to this technique by coating the capillary with an ITO (indium tin oxide) film that acted as a thermal jacket when electrical current passed through it,24 and in the present paper as well, we are using this technique for high-temperature experiments.
Results and Discussion
Nicotine was injected into the capillary that was filled with NaCl solution by using a specially pulled injection micropipette with an inside diameter of 30–40 μm. Snapshots of the dissolving drop of nicotine in 20% NaCl solution at room temperature, taken every 10 s, are depicting nicotine’s dissolution in Figure 2.
Figure 2.
Nicotine dissolution in 20% NaCl solution at room temperature. (a) 0; (b) 10; (c) 20; (d) 30; (e) 40; (f) 50; (g) 60; (h) 70; (i) 80; (j) 90; (k) 100; (l) 110; (m) 120; (n) 130; (o) 140 s.
Triplicate experiments were conducted for each salt concentration, and because the measurable horizontal and vertical dimensions of the drop are not identical, the transient characteristic drop length chosen to report is its perimeter, P(t). In Figure 2, the perimeter is shown to have shrunk from a P0 = 334 μm (average diameter = 105 μm) to a P = 89 μm (average diameter = 30 μm) within 140 s. The time dependence of the relative drop shrinkage, P/P0, for all four NaCl concentrations of the nicotine-drop dissolution experiments is shown in Figure 3.
Figure 3.
Shrinkage rates in different NaCl concentrations at room temperature.
Nicotine drop dissolution rate in deionized water was instantaneous, the reason being that, in the absence of salt, nicotine is completely soluble in water at room temperature.3 Salt concentrations lower than 15% were also tested and were found to be very fast, almost instantaneous, and therefore are not reported in Figure 3. NaCl (26.5%) is the saturation salt concentration at room temperature and thus was chosen as the highest NaCl concentration in the experiments. It should be noted that as nicotine diffused into the surrounding NaCl solution, water also diffused into the nicotine drop, much more so at the beginning of the experiment when the drop was totally devoid of water. This may explain the drop shrinkage rate being lower at the beginning and higher at later times, especially for concentration of 25 and 26.5% NaCl, when nicotine dissolution was slower. This is seen to a lesser extent for concentrations 15 and 20% in Figure 3, with slightly curved lines. In Figure 3, for nicotine drop shrinkage to P/P0 = 0.2, it took approximately 39 s in 15% saline, 145 s in 20% saline, 800 s in 25% saline, and 1000 s in 26.5%. With increasing NaCl concentration, the nicotine droplet shrinkage rate decreased, which is because of increase in interfacial tension27 and to salting out effects.28 Salting out effects play an important role in decreasing the dissolution rate and solubility of nicotine.19 Previous Fourier transform infrared studies and ab initio calculations29 have shown that when nicotine interacts with water, hydrogen bonding to the pyrrolidine nitrogen occurs, with an approximate 10:1 ratio of pyridine/pyrrolidine complex of nicotine. In the presence of NaCl, the nicotine–water hydrogen bonding is weaker than the ionic bonding of the salt ions with water, therefore when salt concentration increases, the water available to interact with and dissolve nicotine will decrease.
Dissolution rates of nicotine droplets showed a decrease at the higher temperature of 70 °C for most salt concentrations. For 20% NaCl, it took 330 s for the nicotine droplet of Figure 4 to shrink from P0 = 325 μm (average diameter = 107 μm) to P = 40 μm (average diameter = 23 μm). This was likely caused by two factors that accompany raises in temperature, one of them being decreased hydrogen bonding30 and the other a raise in interfacial tension.27
Figure 4.
Nicotine dissolution in 20% NaCl solution at 70 °C. (a) 0; (b) 30; (c) 60; (d) 90; (e) 150; (f) 210; (g) 270; (h) 330 s.
The solubility diagram of nicotine is a loop,18 which contains lower and upper critical temperatures of miscibility; Donahue and Bartell indicated that the interfacial tension of the water–nicotine system increased with increasing temperature in the region where miscibility decreased with increasing temperature.31 However, as the temperature approaches the upper critical solution temperature, the interfacial tension decreases and naturally becomes zero at the upper critical temperature.31 This explains the fact that at 70 °C, with accompanying increased interfacial tension,32 there was reduced dissolution of nicotine into the bulk aqueous solution. The results of Figure 5 show that the dissolution rate of nicotine was slower at 70 °C for NaCl concentrations of 15 and 20% than at room temperature; however, there was no sizeable difference for nicotine’s dissolution in 25% NaCl between the two temperatures. The dissolution rate in 26% NaCl was slower at the lower temperature, probably due to the fact that addition of NaCl shifts the critical temperatures,19 reinforced by ref (27) that states: “1 g mol/L of NaCl shifts critical temperature of miscibility to 22.5 °C.” A shift in the solubility loop at 26% NaCl may cause the temperature of 70 °C to be in the positive temperature coefficient region where interfacial tension decreased with increasing temperature and the dissolution rate of nicotine increased.
Figure 5.
Shrinkage rate of nicotine in different concentrations of saline at 70 °C.
Nicotine’s Diffusion Coefficients
The kinetics of nicotine dissolution can lead to estimates of diffusion coefficients of nicotine in different NaCl-concentration aqueous solutions. This can be achieved based on the Epstein–Plesset diffusion model of a liquid droplet dissolving in a secondary liquid phase33,34
| 1 |
where R = R(t) is the droplet radius at time t, D is the diffusion coefficient, C0 is the initial concentration in the bulk (r > R), Cs is the saturation concentration, and ρ is the density of the droplet. Because in our experiment t = 0 when the droplet is formed in an aqueous bulk devoid of nicotine, the initial concentration C0 is zero, and the equation can be simplified and rearranged to
| 2 |
which may be written in terms of the perimeter P = P(t) as
| 3 |
Based on eq 3, a plot of  vs
vs 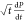 (Figures 6 and 7) with slope −4π2DCs/ρ and intercept
(Figures 6 and 7) with slope −4π2DCs/ρ and intercept 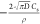 will reveal values for D.
will reveal values for D.
Figure 6.
Plot of 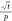 vs
vs 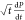 for nicotine drops in different concentrations of NaCl at room temperature.
for nicotine drops in different concentrations of NaCl at room temperature.
Figure 7.
Plot of  vs
vs  for nicotine drops in different concentrations of NaCl at 70 °C.
for nicotine drops in different concentrations of NaCl at 70 °C.
Diffusion coefficients were obtained by taking average of three independent experiments, as shown in Table 1.
Table 1. Diffusion Coefficient of Nicotine in Different Concentrations of NaCl Solution.
| concentration of NaCl (wt %) | diffusion coefficient at room temperature(1012 m2/s) | diffusion coefficient at 70 °C (1012 m2/s) |
|---|---|---|
| 15 | 437 ± 76.4 | 256.2 ± 29.2 |
| 20 | 132.3 ± 11.3 | 50.8 ± 0.7 |
| 25 | 80.9 ± 1.8 | 4.3 ± 0.6 |
| 26.5 | 24.7 ± 5.0 | 7.7 ± 0.2 |
It should be noted that diffusion coefficients decreased with increasing temperature, which appears contrary to the usual behavior of diffusion coefficients increasing with temperature, according to an Arrhenius behavior. However, temperature changes may also give rise to other factors that will have the opposite effect, for example, Guo et al. have reported that ferrocene molecules could diffuse faster in ionic liquids mixed with CO2 at lower temperatures but higher pressures because of the higher solubility of CO2.35 Based on our earlier discussion of interfacial tension effects, decreasing diffusion coefficients of nicotine may cause by increasing interfacial tension with temperature increase.31 In previous research, Bagalkot and Hamouda indicated that for CO2 diffusing into a hydrocarbon drop in a high-density CO2 system, diffusion coefficient increased with pressure because of lower interfacial tension, which assisted mass transfer of CO2.36 For the first three concentrations, the lower diffusion coefficients as temperature decreased may be explained via increases in interfacial tension increased.
It should be noted that the above analysis assumes that only the droplet dissolves into the surrounding liquid, without taking into account the diffusion of the surrounding liquid (water) into the drop (nicotine). Furthermore, the Epstein–Plesset diffusion model does not consider any phenomena other than diffusion, for example, the roles played by Laplace pressure and other components present, like NaCl in the case of our experiments. Besides, two variables (D and Cs) are unknown and affected by each other during calculation. Therefore, the extracted diffusion coefficients in this work may not be accurate. To narrow down and compare the errors, we can use the only Cs values from literature that correspond to some of our experiments: at room temperature, Cs is about 0.59 M for 15% NaCl, and at 70 °C, Cs is about 0.353 M for 15% NaCl and 0.239 M for 20% NaCl.19 Using these Cs values, diffusion coefficients can be obtained by directly solving eq 3 as: 260 × 10–12 m2/s (room temperature and 15% NaCl), 139 × 10–12 m2/s (70 °C and 15% NaCl), and 99 × 10–12 m2/s (70 °C and 20% NaCl). Comparing these diffusion coefficients with those in Table 1, differences can be seen, leading to the need for developing a more sophisticated mathematical model that will account for factors other than simple dissolution and diffusion of one phase into another.
Conclusions
Addition of inorganic salts and temperature raises being methods to separate nicotine from water, this paper has shown that nicotine’s dissolution rates decrease with increasing concentration of NaCl or temperature in the region where the temperature coefficient of miscibility is negative. However, increases in both NaCl concentration and temperature may also lead to a negative effect of separation because nicotine in a 26.5% NaCl solution had the slowest dissolution rate at room temperature but had a higher dissolution rate at 70 °C. At lower salt concentrations (15 and 20%), a slower dissolution rate was exhibited at 70 °C than at room temperature, and 25% NaCl produced similar dissolution rates at room temperature and 70 °C. Even though estimates of nicotine diffusion coefficients in different NaCl concentrations may be obtained via the Epstein–Plesset model, the latter may need to be improved upon for furnishing reliable values.
Experimental Section
Material
Nicotine (≥99%) and sodium chloride (≥98%) were purchased from Sigma-Aldrich. Deionized water was obtained from a Barnstead E-pure purifier, and the resistivity of the water was 18.2 MΩ cm. ITO, one of the most widely used transparent and conductive material,37 was purchased from Chemat technology. Microcapillaries and micropipettes were pulled from Microcaps (Drummond Scientific) using a Narashige PB-7 puller. Software XCAP was used to measure the droplet diameter.
Methods
To prepare a cylindrical, thin-walled capillary for microscopically viewing the nicotine drop inside it, a glass tube was pulled with a micropipette puller so as to achieve a middle-part diameter of ∼200 μm.26 For high-temperature experiments, sol–gel process38−40 was used to coat an electrically conductive film that acted as a thermal jacket for the capillary.24 For the experiments presented in this paper, the high-temperature capillaries were made by dipping them into the ITO precursor solution and pulling them back into the air at a rate of 7 cm/min.41 The coated substrate was heated at 530 °C for 30 min after each dipping. By repeating the above procedure until electrical resistance was below 100 kΩ, the final resistance of capillaries was reduced to 30–50 kΩ after allowing the coated capillaries to sit at room temperature overnight.
A photograph of the assembled high-temperature capillary microreactor is shown in Figure 8, with the lubricant-filled capillary fixed on a plastic holder. The two copper foils attached to the capillary served for supplying ac electrical current to the capillary-coated ITO film, which increased the temperature inside the capillary to a desired value almost instantly.
Figure 8.

Photograph of a high-temperature capillary microreactor. (Photograph courtesy of Chia-yu Chen).
A K-type fine-wire thermocouple was used to measure the temperature inside the capillary. To obtain calibration of temperature versus applied voltage, capillaries were filled with substances of very different boiling points, and voltages were recorded when boiling took place for ethanol (BP = 78 °C), water (BP = 100 °C), and n-hexadecane (BP = 287 °C). Once the thermocouple had been calibrated, the complete relationship between temperature and voltage was obtained and is shown in Figure 9.
Figure 9.
Temperature–voltage working curve (for 30–40 kΩ resistance).
Acknowledgments
Support came from Altria Client Services Regulatory Affairs as a Tobacco Product Regulatory Science Research Fellowship to Chia-Yu “Kathy” Chen.
The authors declare no competing financial interest.
References
- Ayers J. W.; Ribisl K. M.; Brownstein J. S. Tracking the Rise in Popularity of Electronic Nicotine Delivery Systems (Electronic Cigarettes) Using Search Query Surveillance. Am. J. Prev. Med. 2011, 40, 448–453. 10.1016/j.amepre.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Gosselin R. E.; Smith R. P.; Hodge H. C.. Clinical Toxicology of Commercial Products, 6th ed.; Williams & Wilkins, 1988; p 311. [Google Scholar]
- Campbell A. N.; Kartzmark E. M.; Falconer W. E. The System: Nicotine–Methylethyl Ketone–Water. Can. J. Chem. 1958, 36, 1475–1486. 10.1139/v58-218. [DOI] [Google Scholar]
- Novotny T. E.; Zhao F. Consumption and Production Waste: Another Externality of Tobacco Use. Tobac. Contr. 1999, 8, 75–80. 10.1136/tc.8.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponza D. T. Toxicity Studies in a Tobacco Industry Biological Treatment Plant. Water, Air, Soil Pollut. 2002, 134, 137–164. 10.1023/a:1014111616875. [DOI] [Google Scholar]
- Masoner J. R.; Kolpin D. W.; Furlong E. T.; Cozzarelli I. M.; Gray J. L.; Schwab E. A. Contaminants of Emerging Concern in Fresh Leachate from Landfills in the Conterminous United States. Environ. Sci.: Processes Impacts 2014, 16, 2335–2354. 10.1039/c4em00124a. [DOI] [PubMed] [Google Scholar]
- Buerge I. J.; Kahle M.; Buser H.-R.; Müller M. D.; Poiger T. Nicotine Derivatives in Wastewater and Surface Waters: Application as Chemical Markers for Domestic Wastewater. Environ. Sci. Technol. 2008, 42, 6354–6360. 10.1021/es800455q. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Tobacco and Its Environmental Impact: An Overview; World Health Organization: Printed in Switzerland, 2017. [Google Scholar]
- Boleda M. R.; Galceran M. T.; Ventura F. Behavior of Pharmaceuticals and Drugs of Abuse in a Drinking Water Treatment Plant (Dwtp) Using Combined Conventional and Ultrafiltration and Reverse Osmosis (Uf/Ro) Treatments. Environ. Pollut. 2011, 159, 1584–1591. 10.1016/j.envpol.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Maduro R. M.; Aznar M. Liquid-Liquid Equilibrium of Ternary Systems Containing Nicotine. Fluid Phase Equilib. 2007, 259, 83. 10.1016/j.fluid.2007.02.016. [DOI] [Google Scholar]
- Grozdanić N.; Calado M.; Kijevcanin M.; Serbanovic S.; Visak Z. Aqueous Nicotine Solutions: Ph-Measurements and Salting-out Effects – Analysis of the Effective Gibbs Energies of Hydration and Ionic Strengths of the Solutions. J. Serb. Chem. Soc. 2014, 79, 829–842. 10.2298/jsc130817109g. [DOI] [Google Scholar]
- Rakić V.; Damjanović L.; Rac V.; Stošić D.; Dondur V.; Auroux A. The Adsorption of Nicotine from Aqueous Solutions on Different Zeolite Structures. Water Res. 2010, 44, 2047. 10.1016/j.watres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Shin J. H.; Park S. S.; Ha C.-S. Adsorption Behavior of Nicotine on Periodic Mesoporous Organosilicas. Colloids Surf. 2011, 84, 579. 10.1016/j.colsurfb.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Wang S. N.; Liu Z.; Tang H. Z.; Meng J.; Xu P. Characterization of Environmentally Friendly Nicotine Degradation by Pseudomonas Putida Biotype a Strain S1. Microbiology 2007, 153, 1556. 10.1099/mic.0.2006/005223-0. [DOI] [PubMed] [Google Scholar]
- Wang S. N.; Xu P.; Tang H. Z.; Meng J.; Liu X. L.; Huang J.; Chen H.; Du Y.; Blankespoor H. D. Biodegradation and Detoxification of Nicotine in Tobacco Solid Waste by a Pseudomonas Sp. Biotechnol. Lett. 2004, 26, 1493. 10.1023/b:bile.0000044450.16235.65. [DOI] [PubMed] [Google Scholar]
- Freire M. G.; Teles A. R. R.; Canongia Lopes J. N.; Rebelo L. P. N.; Marrucho I. M. M.; Coutinho J. A. P. Partition Coefficients of Alkaloids in Biphasic Ionic-Liquid-Aqueous Systems and Their Dependence on the Hofmeister Series. Sep. Sci. Technol. 2012, 47, 284. 10.1080/01496395.2011.620585. [DOI] [Google Scholar]
- Freire M. G.; Neves C. M. S. S.; Marrucho I. M. M.; Canongia Lopes J. N.; Rebelo L. P. N.; Coutinho J. A. P. High-Performance Extraction of Alkaloids Using Aqueous Two-Phase Systems with Ionic Liquids. Green Chem 2010, 12, 1715. 10.1039/c0gc00179a. [DOI] [Google Scholar]
- Davies N. S. A.; Gillard R. D. The Solubility Loop of Nicotine:Water. Transition Met. Chem. 2000, 25, 628–629. 10.1023/a:1007092216627. [DOI] [Google Scholar]
- Lopes J. M.; Nunes A. V. M.; Nunes da Ponte M.; Visak Z. P.; Najdanovic-Visak V. Performance of Sodium Chloride Versus Commercial Ionic Liquid as Salting-out Media for the Separation of Nicotine from Its Aqueous Solutions. Ind. Eng. Chem. Res. 2014, 53, 9883–9888. 10.1021/ie500514y. [DOI] [Google Scholar]
- Peña A. A.; Miller C. A. Solubilization Rates of Oils in Surfactant Solutions and Their Relationship to Mass Transport in Emulsions. Adv. Colloid Interface Sci. 2006, 123–126, 241–257. 10.1016/j.cis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Chen B.-H.; Miller C. A.; Walsh J. M.; Warren P. B.; Ruddock J. N.; Garrett P. R.; Argoul F.; Leger C. Dissolution Rates of Pure Nonionic Surfactants. Langmuir 2000, 16, 5276–5283. 10.1021/la9913497. [DOI] [Google Scholar]
- Chen C.-Y.; Papadopoulos K. D. Ethanol’s Effects on Acid Neutralization by Motor Oils. Tribol. Int. 2019, 132, 24–29. 10.1016/j.triboint.2018.12.006. [DOI] [Google Scholar]
- Duan Y.; Rausa R.; Zhao Q.; Papadopoulos K. D. Neutralization Mechanism of Acetic Acid by Overbased Colloidal Nanoparticles. Tribol. Lett. 2016, 64, 8. 10.1007/s11249-016-0742-3. [DOI] [Google Scholar]
- Fu J.; Lu Y.; Campbell C. B.; Papadopoulos K. D. Optical Microscopy inside a Heating Capillary. Ind. Eng. Chem. Res. 2005, 44, 1199–1203. 10.1021/ie040083i. [DOI] [Google Scholar]
- Fu J.; Lu Y.; Campbell C. B.; Papadopoulos K. D. Acid Neutralization by Marine Cylinder Lubricants inside a Heating Capillary: Strong/Weak-Stick Collision Mechanisms. Ind. Eng. Chem. Res. 2006, 45, 5619–5627. 10.1021/ie051209u. [DOI] [Google Scholar]
- Wu R. C.; Campbell C. B.; Papadopoulos K. D. Acid-Neutralizing of Marine Cylinder Lubricants: Effects of Nonionic Surfactants. Ind. Eng. Chem. Res. 2000, 39, 3926–3931. 10.1021/ie000039c. [DOI] [Google Scholar]
- Bikerman J. J.Surface Chemistry: Theory and Application; Academic Press, Inc, 1958; pp 141–142. [Google Scholar]
- Allen A. H.Allen’s Commercial Organic Analysis Volume 6; Franklin Classics, 2018; p 238. [Google Scholar]
- Graton J.; Berthelot M.; Gal J.-F.; Laurence C.; Lebreton J.; Le Questel J.-Y.; Maria P.-C.; Robins R. The Nicotinic Pharmacophore: Thermodynamics of the Hydrogen-Bonding Complexation of Nicotine, Nornicotine, and Models. J. Org. Chem. 2003, 68, 8208–8221. 10.1021/jo035018h. [DOI] [PubMed] [Google Scholar]
- Bailey H. E.; Wang Y.-L.; Lynch S. R.; Fayer M. D. Dynamics and Microstructures of Nicotine/Water Binary Mixtures near the Lower Critical Solution Temperature. J. Phys. Chem. B 2018, 122, 9538–9548. 10.1021/acs.jpcb.8b06205. [DOI] [PubMed] [Google Scholar]
- Donahue D. J.; Bartell F. E. The Boundary Tension at Water-Organic Liquid Interfaces. J. Phys. Chem. 1952, 56, 480–484. 10.1021/j150496a016. [DOI] [Google Scholar]
- Bikerman J. J.Physical Surfaces; Academic Press, Inc, 1970; p 121. [Google Scholar]
- Duncan P. B.; Needham D. L. Microdroplet Dissolution into a Second-Phase Solvent Using a Micropipet Technique: Test of the Epstein-Plesset Model for an Aniline-Water System. Langmuir 2006, 22, 4190. 10.1021/la053314e. [DOI] [PubMed] [Google Scholar]
- Zhu P.; Harris T. V.; Driver M. S.; Campbell C. B.; Pratt L. R.; Papadopoulos K. D. Dissolution Kinetics of [Hmim][Bf4] Ionic Liquid Droplets in 1-Pentanol. J. Phys. Chem. C 2009, 113, 16458–16463. 10.1021/jp9052693. [DOI] [Google Scholar]
- Guo Y.; Kanakubo M.; Kodama D.; Nanjo H. Chronoamperometric Determination of Diffusion Coefficients of Ferrocene in Ionic Liquids Mixed with Co2 at High Pressures. J. Electroanal. Chem. 2010, 639, 109–115. 10.1016/j.jelechem.2009.11.030. [DOI] [Google Scholar]
- Bagalkot N.; Hamouda A. A. Interfacial Tension and Co2 Diffusion Coefficients for a Co2 + Water and N-Decane System at Pressures of 10 to 160 Bar. RSC Adv. 2018, 8, 38351–38362. 10.1039/c8ra03690j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T.; Matsui H.; Tanabe N. New Transparent Conductive Films: Fto Coated Ito. Thin Solid Films 2003, 445, 241–244. 10.1016/s0040-6090(03)01169-6. [DOI] [Google Scholar]
- Djaoued Y.; Vu Hong Phong V. H.; Badilescu S.; Ashrit P. V.; Girouard F. E.; Vo-Van Truong V. V. Sol-Gel-Prepared Ito Films for Electrochromic Systems. Thin Solid Films 1997, 293, 108–112. 10.1016/s0040-6090(96)09060-8. [DOI] [Google Scholar]
- Stoica T. F.; Stoica T. A.; Vanca V.; Lakatos E.; Zaharescu M. Colloidal Sol-Gel Ito Films on Tube Grown Silicon. Thin Solid Films 1999, 348, 273–278. 10.1016/s0040-6090(99)00136-4. [DOI] [Google Scholar]
- Toki M.; Aizawa M. Sol-Gel Formation of Ito Thin Film from a Sol Including Ito Powder. J. Sol-Gel Sci. Technol. 1997, 8, 717–720. 10.1007/bf02436928. [DOI] [Google Scholar]
- Ramanan S. R. Dip Coated Ito Thin-Films through SolGel Process Using Metal Salts. Thin Solid Films 2001, 389, 207–212. 10.1016/s0040-6090(01)00825-2. [DOI] [Google Scholar]









