Abstract

The physicochemical properties and the synthesis of four α-pinene oxidation products, terebic acid, 3-methyl-1,2,3-butanetricarboxylic acid (MBTCA), diaterpenylic acid acetate (DTAA), and pinanediol, are presented in this study. The physicochemical properties encompass thermal properties, solubility in water, and dissociation constant (pKa) for the investigated compounds. It was found that terebic acid exhibits a relatively high melting temperature of 449.29 K, whereas pinanediol revealed a low melting temperature of 329.26 K. The solubility in water was determined with the dynamic method and the experimental results were correlated using three different mathematical models: Wilson, NRTL, and UNIQUAC equations. The results of the correlation indicate that the Wilson equation appears to work the best for terebic acid and pinanediol. The calculated standard deviation was for 3.79 for terebic acid and 1.25 for pinanediol. In contrast, UNIQUAC was the best mathematical model for DTAA and MBTCA. The calculated standard deviation was 0.57 for DTAA and 2.21 for MBTCA. The measured water solubility increased in the following order: pinanediol > DTAA ≥ MBTCA > terebic acid, which affects their multiphase aging chemistry in the atmosphere. Moreover, acidity constants (pKa) at 298, 303, and 308 K were determined for DTAA with the Bates–Schwarzenbach spectrophotometric method. The pKa values obtained at 298, 303, and 308 K were found to be 3.76, 3.85, and 3.88, respectively.
1. Introduction
Atmospheric aerosol is a highly variable and complex chemical mixture containing organic and inorganic compounds. It is estimated that about 2000 Tg/year of volatile organic compounds (VOCs) are emitted into the atmosphere as gases and 300 Tg/year as suspended particulate matter (aerosol).1−3 A significant portion of tropospheric aerosol is represented by secondary organic aerosol (SOA), which formed chemical reactions of VOCs followed by heterogenous and/or multiphase chemistry. α-Pinene (C10H16) is considered as a one of the most important global SOA sources.4 α-Pinene is the second foremost released nonmethane VOC with an estimated yearly emission rate of about 70 Tg C year–1. A row of intense studies on α-pinene photo-oxidation were conducted, which allowed to unveil the chemical composition of α-pinene SOA.5−9 These studies revealed a suit of hydrophilic and polar organic compounds bearing one or more functional groups in α-pinene-derived skeleton, such as ketone (−C(=O)−), alcohol (−OH), aldehyde (−C(=O)H), or carboxylic acids (−COOH).1,2,4,8,10 It was also shown that numerous α-pinene SOA products with an increased oxygen-to-carbon ratio are sufficiently water-soluble to undergo aqueous-phase processing.4
A large amount of water dispersed in the atmosphere (i.e., cloud, fog, and aerosol liquid water) makes it an important reaction medium for the further processing of α-pinene oxidation products.11−13 In recent years, the atmospheric aqueous-phase has been recognized as an important reaction media for the processing of water-soluble organic compounds.14−16 Atmospheric models suggest that through these processes contribute to global annual SOA production with 20 to 30 Tg year–1.3,5,11,17−19 In contrast to the gas-phase processes, aqueous-phase reactions leading to SOA are vaguely recognized, posing a great challenge for researchers in recent years.13
In our previous studies, we have reported on physicochemical properties (i.e., thermal properties, solid–liquid phase equilibria, and dissociation constants) of four α-pinene/β-pinene oxidation products, i.e., cis-pinic acid, cis-pinonic acid, cis-norpinic acid, and cis-norpinonic acid.20 It was proved that the water solubility of the studied compounds increases in the following order: cis-pinic acid > cis-norpinic acid > cis-pinonic acid > cis-norpinonic acid. Moreover, the experimental value of pKa for cis-norpinonic acid was measured to be 4.56 at 298.15 K and 4.75 at 310.15 K, whereas for cis-pinonic acid 5.19 at 298.15 K and 5.25 at 310.15 K. The measured physicochemical parameters for monoterpene SOA aging products are needed to assist experimentation but also for better modeling of multiphase processes in atmospheric chemistry. The measured physicochemical parameters are a relevant factor to improve the description of the partitioning processes of a given oxidized monoterpene-derived compound from the gas phase to aerosol particle/water-droplet and thus could be implemented by both atmospheric chemistry and air quality models, such as the Community Multiscale Air Quality (CMAQ) or the Chemical Aqueous Phase Radical Mechanism (CAPRAM) models.43
Therefore, to extend an useful physicochemical database for the atmospheric community, herein we decided to report the solid–liquid phase equilibria (solubility in water) and thermal properties of methyl-1,2,3-butanetricarboxylic acid (MBTCA), diaterpenylic acid acetate (DTAA), terebic acid (TER), as well as pinanediol (PD). These compounds are important tracers for simple monoterpenes in the atmosphere, including mainly α-pinene.25 They are formed through the gas-phase oxidation of α-pinene and leading to an array of highly oxidized products such as MBTCA.1,22 The latter was proposed as the most relevant tracer compound for atmospheric monoterpene SOA by Szmigielski et al.21 A lactone ring-containing species such as TER, but also DTAA and PD, were considered as early-stage oxidation products formed via the photooxidation and/or ozonolysis of α-pinene.22−24 Moreover, these compounds were found at elevated concentrations in the continental aerosol samples.1,25 Owing to their enhanced polarities, they can also be expected to sufficiently partition into the aqueous phase in cloud droplets or into the surface of deliquescent particles.26−28 Taking this into account, the objective of this study was to determine the solid–liquid phase equilibria (i.e., solubility in water) for relevant α-pinene oxidation products TER, MBTCA, DTAA, and PD, to the best of our knowledge for the first time. The experimental data, such as the dissociation constant (pKa) for DTAA, are also reported.
2. Results and Discussion
The 1H NMR and 13C NMR spectra obtained for each α-pinene SOA product supported by differential scanning calorimetry (DSC) technique measurements have shown a high purity of synthesized compounds (≥99%). Using DSC, their basic thermal properties have been determined, including the temperature of fusion (Tfus,1), the enthalpy of fusion (ΔfusH1), the temperature of transition (Ttr,1), and the enthalpy of transition (ΔtrH1). The representative thermograms for each investigated α-pinene SOA oxidation product clearly reveal the effect of a heat flow on the temperature (Figure 1).
Figure 1.
DSC thermograms recorded for investigated α-pinene SOA products.
The thermographs measured for studied α-pinene SOA aging products exhibit melting temperatures from 449.28 K for TER to 329.26 K for PD. Temperature of melting for MBTCA was found to be 422.74 K, whereas for DTAA it was 394.28 K. The measured thermal properties included Tfus,1, ΔfusH1, Ttr,1, and ΔtrH1, and are presented in Table 1.
Table 1. Physicochemical Characteristics of Selected α-Pinene SOA Aging Products: Tfus,1, ΔfusH1, Heat Capacity Changes at the Fusion Temperature, ΔCp(fus),1, Ttr,1, ΔtrH1, and the Molar Volume Vm293.15K.
| compound | Tfus,1/K | ΔfusH1/kJ·mol–1 | Ttr,1/K | ΔtrH1/kJ·mol–1 | ΔCp(fus),1b/J·mol–1·K–1 | <keep-together>Vm293.15Ka/cm3·mol–1</keep-together> | <keep-together>Tfus,1lit/K</keep-together> |
|---|---|---|---|---|---|---|---|
| MBTCA | 422.74 | 85.17 | 201.48 | 160.4 | 425.99 | ||
| DTAA | 394.28 | 7.12 | 387.40 | 22.98 | 18.06 | 195.5 | |
| TER | 449.28 | 30.00 | 66.76 | 129.4 | 446.65 | ||
| PD | 329.26 | 15.85 | 48.15 | 133.3 | 329.15 |
Calculated according to Barton’s group contribution method.29
Calculated with Tfus,1 and ΔfusH1. Standard uncertainties u are as follows: u(Tfus,1) = ±0.1 K, u(ΔfusH1) = ±0.1 kJ·mol–1.
The melting temperature for MBTCA reported in earlier studies to be 425.99 K30 differs about 3 K from the value obtained in the presented measurements. The melting temperature in the literature reported temperature for TER is 446.65 K, which differs also by about 3 K from the value obtained in this study.31 PD is a commercially available compound with the melting point of 329.15 K, which is in line with the value retrieved in this study.32 The enthalpy of fusion for MBTCA was calculated to be 85.17 kJ·mol–1, which reaches the highest value of all investigated α-pinene SOA products. It was found that the enthalpy of fusion of TER is 30 kJ·mol–1, whereas for PD it is 15.85 kJ·mol–1. Interestingly, for DTAA a solid–solid phase transition was observed (Ttr,1 = 387.4 K, ΔtrH1 = 22.98 kJ·mol–1) in proximity of its melting temperature (Tfus,1 = 394.28 K). The enthalpy of transition greatly exceeds the enthalpy of fusion for this compound (7.12 kJ·mol–1). The same trend between the enthalpy of transition and enthalpy of fusion was observed for other α-pinene SOA products, including cis-norpinic acid as it was stated in our previous report.20 The PD exhibits the lowest melting temperature, whereas for TER the melting temperature is the highest. More stable crystal structures will require higher temperatures to melt in order to overcome the thermodynamic stability in its structure. The thermal measurements have shown that TER is a more stable compound than PD, which can also influence the solubility properties of the examined compounds.
The solubility for MBTCA, DTAA, TER, and PD have been determined in water to expand a useful database of solubility parameters of the SOA products. Four binary systems {that is, MBTCA (1) + water (2), DTAA (1) + water (2), TER (1) + water (2), PD (1) + water (2)} have been measured using a dynamic method. Experimental solubilities for all binary systems in mole fraction and calculated activity coefficients have been summarized in Table 2 and in the Supporting Information in Figures S4–S7. The values of the activity coefficients were retrieved from the correlation equations, which could be used for a large number of experimental points. In this study, three methods were implemented to fit the solute activity coefficients, to the so-called correlation equations that describe the Gibbs excess energy, GE: Wilson, UNIQUAC, and NRTL models. The exact mathematical forms of the equations have been presented in our previous paper.20
Table 2. Experimental Solubility for the {α-Pinene-Derived Product (1) + Water (2)} Binary System in Mole Fraction, x1 vs Equilibrium Temperature T at Saturated Solution at p = 101.3 kPad and Calculated Activity Coefficients, γ1.
| x1b | Tc/K | γ1a | x1b | Tc/K | γ1a |
|---|---|---|---|---|---|
| MBTCA | |||||
| 0.0400 | 308.15 | 0.003 | 0.1037 | 335.75 | 0.018 |
| 0.0666 | 318.55 | 0.005 | 0.1131 | 340.65 | 0.026 |
| 0.0798 | 324.35 | 0.008 | 0.1361 | 351.35 | 0.053 |
| 0.0832 | 326.35 | 0.009 | 1.0000 | 422.74 | 1.00 |
| 0.0877 | 328.85 | 0.011 | |||
| DTAA | |||||
| 0.0314 | 303.65 | 2.76 | 0.1051 | 319.35 | 1.39 |
| 0.0382 | 306.65 | 2.55 | 0.1373 | 324.35 | 1.30 |
| 0.0550 | 309.85 | 2.01 | 0.1665 | 328.15 | 1.18 |
| 0.0739 | 312.95 | 1.67 | 1.0000 | 394.28 | 1.00 |
| 0.0914 | 315.75 | 1.50 | |||
| TER | |||||
| 0.0016 | 320.35 | 24.23 | 0.0130 | 339.65 | 5.75 |
| 0.0029 | 322.95 | 15.01 | 0.0160 | 344.25 | 5.40 |
| 0.0044 | 326.35 | 10.94 | 0.0191 | 349.55 | 5.31 |
| 0.0059 | 328.85 | 8.95 | 1.0000 | 449.28 | 1.00 |
| 0.0092 | 334.35 | 6.89 | |||
| PD | |||||
| 0.0724 | 304.35 | 8.60 | 0.2796 | 316.65 | 2.84 |
| 0.1163 | 307.70 | 5.73 | 1.0000 | 329.26 | 1.00 |
| 0.1615 | 311.00 | 4.41 | |||
| 0.2014 | 312.97 | 3.67 | |||
| 0.2461 | 315.25 | 3.14 | |||
Standard uncertainties u are as follows: calculated from the UNIQUAC equation for MBTCA as well as DTAA and Wilson equation for TER as well as PD.
u(x1water) = ±0.005
u(T/K) = ±0.1
u(p/kPa) = ±2.
It was found that PD, which contains two hydroxyl groups (−OH), together with the lowest melting point (329.26 K), revealed the high solubility in water. This result was found interesting considering the low O/C ratio (0.2) of the discussed compound. Furthermore, TER—a compound with a lactone moiety (−(C=O)–O−) in the structure, the highest melting point (449.28 K) and O/C ratio—0.57 PD revealed very low solubility in water. Two more polar substances, that is, MBTCA and DTAA, together with O/C ratio 0.75 and 0.6, respectively, exhibit very good solubility in water. It was also found that the solubility of the tested compounds in water increases in the order PD > MBTCA ≥ DTAA > TER. The combined experimental data for a better comparison of reported results are presented in Figure 2.
Figure 2.

Experimental solubility of {α-pinene-derived acid (1) + water (2)} binary systems; (□) MBTCA, (○) DTAA, (△) TER and (◊) PD.
The experimental points obtained for four binary systems were subsequently correlated with the use of the three following thermodynamic models reported elsewhere: Wilson, NRTL, and UNIQUAC equations.33−35 The parameters of the correlation and standard deviations are listed in Table S4. The low standard deviation values δT suggest a good fit between the experimental data and the mathematical models used. The standard deviation for MBTCA was set at around 2.5 for each equation; however, the lowest value of 2.21 was obtained with an UNIQUAC equation. The ideal solubility calculated for MBTCA is higher than the experimental value in a measured concentration range. A UNIQUAC equation was also found to be the best for the DTAA solubility correlation with a standard deviation of 0.57. Interestingly, the calculated ideal solubility for this compound is lower than the experimental solubility to the value around x1 = 0.2 and after this point remains unchanged. A Wilson equation was found to be the best to correlate the experimental points obtained for TER. A standard deviation value δT of 3.79 for this binary system was found, which is due to the low solubility of the compound investigated and the measurements constraints in a narrow range of mole fraction, x1. The ideal solubility calculated for the TER system is lower than the experimental solubility in a measured concentration ranges. For PD, Wilson equation was demonstrated to obtain the best correlation with the lowest standard deviation values (1.25). The ideal solubility calculated for this compound is significantly lower than the experimental solubility.
The pKa studies for DTAA were conducted by a spectrophotometric Bates–Schwarzenbach method. The measurements were obtained at three temperatures: 298.15, 303.15, and 308.15 K. However, owing to the chromophore inactivity, we could not pursue the measurements of pKa for MBTCA, TER, and PD. The measurement of the absorbance of DTAA solution as a function of the wavelength in three solutions of 0.2 M NaOH, 0.2 M HCl, and in buffer were performed. The wavelengths were selected for a maximum distance of the border absorbance. The determination of the concentration ratio in the spectrophotometric measurements was possible by assumption of the absorbance additivity law and the Bouguer–Lambert–Beer law for different forms of DTAA existing in the solution. The comparison of the experimental results obtained for DTAA with those available in the literature are summarized in Table 3. The UV–vis spectra acquired for DTAA at the three temperatures are presented in the Supporting Information in Figures S1–S3. The experimental value of pKa for DTAA obtained with a Bates–Schwarzenbach method is 3.76 at 298.15 K, 3.85 at 303.15 K and at 308.15 K the value is 3.88, respectively. The experimental value measured at 298.15 K turned out to be slightly lower than that reported in the literature at the same temperature (pKalit = 3.93). In contrast, the pKa values at 305.15 K and at 308.15 K are reported for the first time. It is worth noting that literature pKa values for either compound were only predicted (not experimentally derived) based on the molecular structure algorithm (ACD/Labs).
Table 3. Experimental and Literature Values of pKa Determined at 298.15, 303.15, and 308.15 K for DTAA.
| compound | pKalit | pKaexp | pKaexp | pKaexp |
|---|---|---|---|---|
| 298.15 K | 298.15 K | 303.15 K | 308.15 K | |
| DTAA | 3.93 ± 0.10a | 3.76 ± 0.15 | 3.85 ± 0.31 | 3.88 ± 0.39 |
Advanced Chemistry Development (ACD/Labs)
In this paper, we have reported for the first time the physicochemical properties together with the solubility properties for four important α-pinene SOA aging products—MBTCA, DTAA, TER, and PD. The comparison of results obtained with those published in our previous paper exhibits that the selected α-pinene SOA products revealed significant differences in the physicochemical properties and in the solubility. The eight binary systems were investigated so far, including only important α-pinene SOA products, that is, PD, TER, MBTCA, DTAA, cis-pinic acid, cis-pinonic acid, cis-norpinic acid, cis-norpinonic acid.17Figure 3 shows the combined melting temperatures for the eight investigated compounds. These products exhibit a melting temperature ranging from 329.26 K for PD to 449.28 K for TER. The results obtained clearly demonstrate that cis-pinic acid and cis-norpinic acid, which both are dicarboxylic acids with a similar core structure, reveal a significant difference in the melting points. Namely, for cis-norpinic acid the melting temperature was found to be 440.68 K, whereas for cis-pinonic acid it was 376.68 K, which differs by about 64 K. Interestingly, a similar difference was also reported for cis-pinonic acid and cis-norpinonic acid, the homological compounds, where the difference of the melting temperature was found to be about 40 K.
Figure 3.
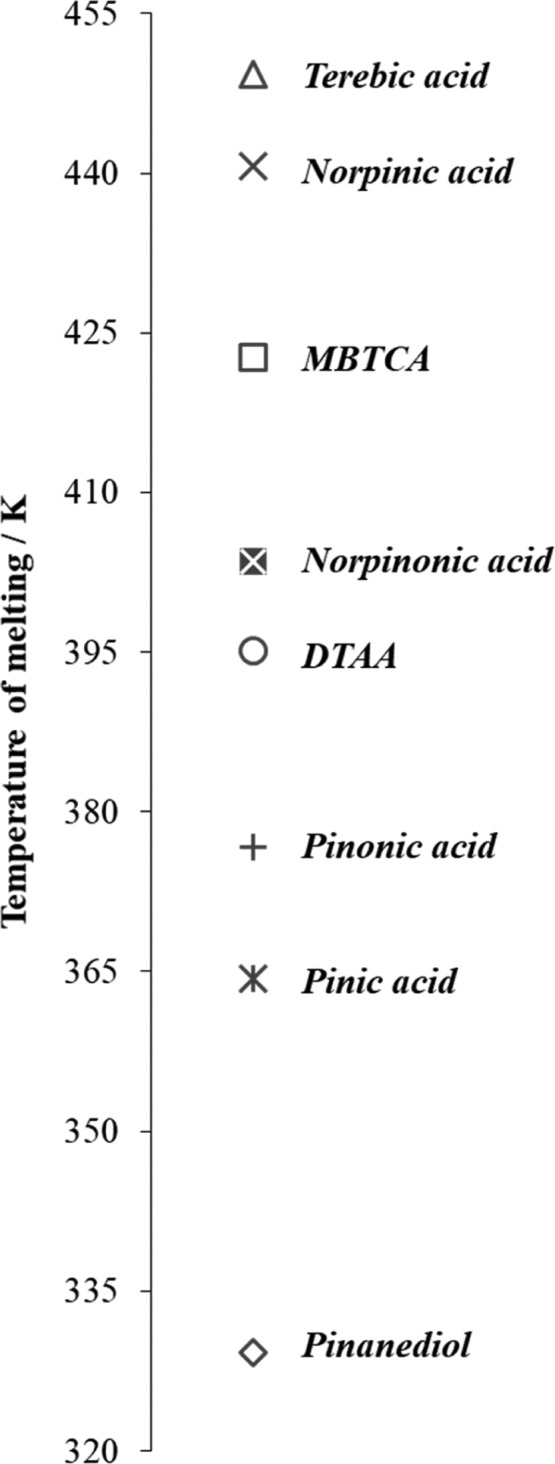
Experimental melting temperatures of α-pinene SOA products (both obtained here and elsewhere20). Reproduced from ref (20). Copyright 2019 American Chemical Society.
The comparison of the solubility data has shown that PD along with cis-norpinic acid reveal the highest solubility in water. It was found that TER, cis-pinonic acid, as well as cis-norpinonic acid revealed very low solubility in water. The analysis of the solubility properties has shown that the solubility of α-pinene SOA products in water increases in the order PD > cis-pinic acid > cis-norpinic acid ≥ DTAA ≥ MBTCA > cis-pinonic acid > cis-norpinonic acid > TER.
3. Conclusions
In summary, this paper provides for the first time the determination of the physicochemical properties, that is, thermal properties, solid–liquid phase equilibria, and dissociation constant (pKa) for relevant α-pinene SOA oxidation products: PD, TER, MBTCA, as well as DTAA along with their improved synthesis. The DSC was used to measure thermal properties, that is, temperatures of fusion (Tfus,1), enthalpy of fusion (ΔfusH1), temperatures of transition (Ttr,1), and enthalpy of transition (ΔtrH1). The thermograms acquired revealed melting temperatures of all investigated products, which a range from 329.26 K for PD to 449.28 K for TER. The enthalpies of fusion varied from 7.12 kJ·mol–1 for DTAA to 85.17 kJ·mol–1 for MBTCA. It is noteworthy that, only DTAA revealed the solid–solid phase transition. In this work, the four binary systems {SOA component (1) + water (2)} were tested. The solubility was determined for all the systems for which the visual method was possible to use. The phase diagrams of the solid–liquid phase equilibria derived experimentally were subsequently correlated with the use of the three thermodynamic models: Wilson, NRTL, and UNIQUAC equations. The measured water solubility was evidenced to increase in the following order: PD > DTAA ≥ MBTCA > TER, which may affect their multiphase aging chemistry in the atmosphere. The results obtained were also compared with the literature data for different α-pinene SOA products. It was found that an early-stage oxidation products of α-pinene, that is, cis-norpinonic acid and cis-pinic acid, are of lower solubility in water than secondary products of α-pinene oxidation, that is, DTAA, MBTCA, cis-norpinic acid. A determined set of physicochemical parameters for key α-pinene SOA aging products could serve as a useful database for the atmospheric community for better modeling of multiphase processes in the atmospheric chemistry. It is planned to extend this database of the physicochemical parameters over other important aerosol products, including these originated from the oxidation of isomeric monoterpenes and sesquiterpene.
4. Experimental Section
4.1. Materials and Methods
Selected α-pinene oxidation products MBTCA, DTAA, and TER were synthesized in the Laboratory of the Environmental Chemistry in the Institute of Physical Chemistry PAS. Details of the synthesis are provided in Section 4.2. The names, abbreviations, and structural formulas are given in Table 4. However, general information about chemicals used during synthesis, yields, molecular weights, ESI-HR-MS, IR, 13C NMR and 1H NMR analysis of the synthesized compounds are provided in the Supporting Information (Tables S1–S2).
Table 4. Names, Abbreviations, and Structures of the Investigated Compounds.
4.2. Synthesis of MBTCA, DTAA, and TER
4.2.1. Synthesis of MBTCA
MBTCA was synthesized following a two-stage protocol reported by Kostenidou et al. (see Figure 4).36 A first step was carried out under anhydrous conditions in the argon atmosphere. A solution of ethyl isobutyrate (6.6 mL, 49 mmol) in anhydrous tetrahydrofuran (12 mL) was added slowly over the period of 20 min to the solution of lithium diisopropylate in THF (1 M, 54 mL, 54 mmol) at −78 °C; then the solution of diethyl fumarate (8 mL, 49 mmol) in anhydrous THF (12 mL) was added dropwise within the next 15 min. The color of the reaction solution turned from transparent to yellow. The reaction mixture was stirred for another 30 min at −78 °C and then poured into 1 M HCl (100 mL) under ice bath cooling. An organic phase was separated and an aqueous layer was extracted with diethyl ether (3 × 20 mL). Organic layers were combined and dried over sodium sulfate. The solvent was removed using a rotary evaporator and an oily residue was distilled at 0.7 mbar to obtain an MBTCA triester derivative as a yellow oil (bp 114 °C; 81%). In the second stage, the isolated triester was added to the aqueous HCl (6 M, 30 mL) and the mixture was heated up under reflux for 9 h. After cooling to the room temperature, the aqueous phase was removed in vacuo to yielding MBTCA as a white powder (yield: 93%).
Figure 4.

Synthetic approach for MBTCA.
4.2.2. Synthesis of DTAA
The synthesis of DTAA encompasses three steps. A first stage was the Grignard reaction, the second was the acylation reaction of the alcohol formed in the first step. In turn, the third step was an oxidative C=C bond cleavage leading to the final product (Figure 5). The first reaction was performed in an argon atmosphere under anhydrous conditions. A solution of the methylmagnesium bromide in diethyl ether (3 M, 15 mL, 36.5 mmol) was added dropwise to the solution of the methyl cyclopent-3-ene-1-carboxylate (2 g, 16 mmol) in anhydrous tetrahydrofuran (25 mL) at −78 °C. The resulting mixture was stirred overnight at room temperature. Then, a saturated ammonium chloride solution was added slowly. The organic phase was separated and the aqueous phase was extracted with diethyl ether (3 × 20 mL). Organic layers were combined and dried over sodium sulfate. The solvent was removed by the rotary evaporation to yield the tertiary alcohol, which was used in the next step without further purification.
Figure 5.

Synthetic approach for DTAA.
The second step was executed according to the procedure described by Höfle et al.37 A mixture of the tertiary alcohol (1 g, 8 mmol), triethylamine (1.75 mL, 12 mmol), acetic anhydride (1.2 g, 12 mmol), and 4-dimethylaminopyridine (DMAP) (1.2 g, 12 mmol) was stirred for 10 h at room temperature. Next, the solution was partitioned between ether (30 mL) and 2 M HCl (30 mL). The organic phase was washed out with saturated NaHCO3 solution (20 mL), dried over sodium sulfate, and the solvent was removed by the rotary evaporation. The final product was isolated after chromatography on silica gel (90:10 → 70:30 hexanes/EtOAc) as a brownish oil (yield: 93%).
The third step followed the procedure described by Moglioni et al.38 To a stirred solution of the 2-(cyclopent-3-en-1-yl)propan-2-yl acetate (1 g, 8 mmol) in 2:2:3 carbon tetrachloride-acetonitrile-water (65 mL), catalytic RuCl3 hydrate (40 mg) and NaIO4 (5.2 g, 31.5 mmol) were added. The resulting mixture was stirred at room temperature overnight, then ether (50 mL) was added and the mixture was stirred for 5 min, and extracted with ether (3 × 50 mL). The combined organic extracts were dried (MgSO4) and concentrated under reduced pressure. DTAA was isolated as white crystals (yield: 83%) after recrystallization from methylene chloride/pentane solution.
4.2.3. Synthesis of TER
TER was synthesized through a two-step protocol. A first stage was the nucleophilic addition of the Grignard reagent into diethyl acetylsuccinate, whereas the second stage was hydrochloric acid hydrolysis of the product formed (Figure 6). The first reaction was performed in the argon atmosphere under anhydrous conditions. In this reaction solution of diethyl acetylsuccinate (2.5 g, 10.6 mmol) in dry THF (20 mL) was added slowly a stirred solution of a methylmagnesium bromide in diethyl ether (3 M, 45 mmol) at −78 °C. The resulting mixture was stirred overnight at room temperature. Then, a saturated ammonium chloride solution was slowly added. The organic phase was separated and the aqueous phase was extracted twice with diethyl ether. Organic layers were combined and dried over sodium sulfate. The solvent was removed in vacuo to yield the tertiary alcohol after distillation at the reduced pressure (145–147 °C, 20 mbar). In the second stage, the isolated product was treated with the aqueous HCl (6 M, 40 mL) and the mixture was heated up under reflux for 5 h. After cooling to room temperature, the hydrochloric acid was evaporated. TER was isolated after recrystallization from water (yield: 89%).
Figure 6.

Synthetic approach for TER.
4.3. Differential Scanning Microcalorimetry
The DSC technique was used to measure the basic thermal properties of the studied compound, that is, the temperature of fusion (Tfus,1), the enthalpy of fusion (ΔfusH1), the temperature of transition (Ttr,1), and the enthalpy of transition (ΔtrH1). The DSC 1 STAR System (Mettler Toledo) calorimeter equipped with the liquid nitrogen cooling system and operating in a heat-flux mode was used for these measurements. The amount of each compound used for the determination of thermal properties was about 10 mg. Each sample was held for 1 min at 253.15 K and then was scanned with a scan rate of 10 K·min–1 with power and recorder sensitivities of 16 mJ·s –1 and 5 mV, respectively. The apparatus was calibrated with a 0.999999 mol fraction purity indium sample. The uncertainty of the fusion temperature (Tfus,1) was ±0.1 K and that of the enthalpy of fusion (ΔfusH1) was ±0.05 kJ·mol–1. The thermos-physical characteristic was analyzed using STAR software. The molar volumes of each studied compound were calculated using Barton’s method.29
4.4. (Solid + Liquid) Phase Equilibria
Solid–liquid equilibrium temperatures were determined using a dynamic method, reported also previously.20,39−41 In the presented paper, we measured four binary systems: {i.e., MBTCA (1) + water (2), DTAA (1) + water (2), TER (1) + water (2), PD (1) + water (2)}. The samples were prepared by weighing the pure compound and water with the uncertainty of 1 × 10–4 g. The experimental points have been determined from 303.15 K to water boiling point. Each sample was heated gradually (5 K·min–1) with continuous stirring inside a Pyrex glass cell placed in a thermostated water bath. Temperatures of crystal disappearance were measured with an electronic thermometer P 550 (Dostmann Electronic GmbH), and detected visually. When the sample was in the vicinity of the phase transition temperature, the heating rate was decreased to about 0.2 K·min–1. All mixtures were measured by mass, and errors did not exceed 5 × 10–4 in mole fraction, whereas for the uncertainties of the temperature measurements were determined to be ±0.1 K. The repeatability of the SLE experimental points was ±0.1 K.
4.5. pKa Measurements
The pKa measurements were performed with the Bates–Schwarzenbach method using an UV–vis spectrophotometer (PerkinElmer Life and Analytical Sciences Lambda 35, Shelton USA). The measurements were conducted at the three temperatures: 298.15, 303.15, and 308.15 K. Solution in water of tested compound were prepared with a mol concentration of 1 × 10–2 mol·dm–3. The buffer was selected (mol concentration) that is, formic acid (0.017136), potassium formate (0.021363), and potassium chloride (0.018488).42 The buffer was chosen on the basis of the literature value of pKa of investigated compounds. The values of pH, acidity function (p(αHγCl)), and the ionic strength (I) for the used buffer are presented in the Supporting Information in Table S3. For the compounds to be tested, three samples were prepared: in 0.2 M hydrochloric acid, in 0.2 M sodium hydroxide, and in a buffer solution. Samples were scanned with 0.2 M water–acid, 0.2 M water–base and water–buffer solutions as a reference, respectively, with a scan step of 1 nm from 320 to 190 nm. The pKa values have been calculated by the following equation
where pKa is the acidity constant, p(αHγCl) is the acidity function, DHA, DA–, DBUF are absorbance values in acidic, basic and buffered solutions, respectively. The measurement error was calculated as a standard deviation for the wavelength range.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04231.
Analytical data and copies of 1H and 13C NMR spectra of all compounds; phase diagrams of MBTCA, DTAA, TER, and PD in water obtained by dynamic method; and pKa measurements obtained by the Bates–Schwarzenbach method (PDF)
Funding for this research was provided by the Polish National Science Centre grant ETIUDA6 2018/28/T/ST4/00036 and by the Warsaw University of Technology, Warsaw, Poland.
The authors declare no competing financial interest.
Supplementary Material
References
- Hallquist M.; Wenger J. C.; Baltensperger U.; Rudich Y.; Simpson D.; Claeys M.; Dommen J.; Donahue N. M.; George C.; Goldstein A. H.; Hamilton J. F.; Herrmann H.; Hoffmann T.; Iinuma Y.; Jang M.; Jenkin M. E.; Jimenez J. L.; Kiendler-Scharr A.; Maenhaut W.; McFiggans G.; Mentel T. F.; Monod A.; Prévôt A. S. H.; Seinfeld J. H.; Surratt J. D.; Szmigielski R.; Wildt J. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. 10.5194/acp-9-5155-2009. [DOI] [Google Scholar]
- Pathak R. K.; Presto A. A.; Lane T. E.; Stanier C. O.; Donahue N. M.; Pandis S. N. Ozonolysis of α-pinene: Parameterization of secondary organic aerosol mass fraction. Atmos. Chem. Phys. 2007, 7, 3811–3821. 10.5194/acp-7-3811-2007. [DOI] [Google Scholar]
- Müller L.; Reinnig M.-C.; Naumann K. H.; Saathoff H.; Mentel T. F.; Donahue N. M.; Hoffmann T. Formation of 3-methyl-1,2,3-butanetricarboxylic acid via gas phase oxidation of pinonic acid – a mass spectrometric study of SOA aging. Atmos. Chem. Phys. 2012, 12, 1483–1496. 10.5194/acp-12-1483-2012. [DOI] [Google Scholar]
- Kroll J. H.; Seinfeld J. H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008, 42, 3593–3624. 10.1016/j.atmosenv.2008.01.003. [DOI] [Google Scholar]
- Aljawhary D.; Zhao R.; Lee A. K. Y.; Wang C.; Abbatt J. P. D. Kinetics, Mechanism, and Secondary Organic Aerosol Yield of Aqueous Phase Photo-oxidation of α-Pinene Oxidation Products. J. Phys. Chem. A 2016, 120, 1395–1407. 10.1021/acs.jpca.5b06237. [DOI] [PubMed] [Google Scholar]
- Christoffersen T. S.; Hjorth J.; Horie O.; Jensen N. R.; Kotzias D.; Molander L. L.; Neeb P.; Ruppert L.; Winterhalter R.; Virkkula A.; Wirtz K.; Larsen B. R. cis-Pinic Acid, a Possible Precursor for Organic Aerosol Formation from Ozonolysis of α-Pinene - effect of HCHO and HCOOH addition on product and aerosol formation. Atmos. Environ. 1998, 32, 1657–1661. 10.1016/s1352-2310(97)00448-2. [DOI] [Google Scholar]
- Claeys M.; Szmigielski R.; Kourtchev I.; Van der Veken P.; Vermeylen R.; Maenhaut W.; Jaoui M.; Kleindienst T. E.; Lewandowski M.; Offenberg J. H.; Edney E. O. Hydroxydicarboxylic Acids: Markers for Secondary Organic Aerosol from the Photooxidation of α-Pinene. Environ. Sci. Technol. 2007, 41, 1628–1634. 10.1021/es0620181. [DOI] [PubMed] [Google Scholar]
- Feltracco M.; Barbaro E.; Contini D.; Zangrando R.; Toscano G.; Battistel D.; Barbante C.; Gambaro A. Photo-oxidation products of α-pinene in coarse, fine and ultrafine aerosol: A new high sensitive HPLC-MS/MS method. Atmos. Environ. 2018, 180, 149–155. 10.1016/j.atmosenv.2018.02.052. [DOI] [Google Scholar]
- Neuenschwander U.; Guignard F.; Hermans I. Mechanism of the Aerobic Oxidation of α-Pinene. ChemSusChem 2010, 3, 75–84. 10.1002/cssc.200900228. [DOI] [PubMed] [Google Scholar]
- Zhang X.; McVay R. C.; Huang D. D.; Dalleska N. F.; Aumont B.; Flagan R. C.; Seinfeld J. H. Formation and evolution of molecular products in α-pinene secondary organic aerosol. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 14168–14173. 10.1073/pnas.1517742112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen N.; Carlton A. G.; Surratt J. D.; Gold A.; Lee B.; Lopez-Hilfiker F. D.; Mohr C.; Thornton J. A.; Zhang Z.; Lim Y. B.; Turpin B. J. Identifying precursors and aqueous organic aerosol formation pathways during the SOAS campaign. Atmos. Chem. Phys. 2016, 16, 14409–14420. 10.5194/acp-16-14409-2016. [DOI] [Google Scholar]
- Zhao R.; Aljawhary D.; Lee A. K. Y.; Abbatt J. P. D. Rapid Aqueous-Phase Photooxidation of Dimers in the α-Pinene Secondary Organic Aerosol. Environ. Sci. Technol. Lett. 2017, 4, 205–210. 10.1021/acs.estlett.7b00148. [DOI] [Google Scholar]
- Herrmann H.; Schaefer T.; Tilgner A.; Styler S. A.; Weller C.; Teich M.; Otto T. Tropospheric Aqueous-Phase Chemistry: Kinetics, Mechanisms, and Its Coupling to a Changing Gas Phase. Chem. Rev. 2015, 115, 4259–4334. 10.1021/cr500447k. [DOI] [PubMed] [Google Scholar]
- Gray Bé A.; Upshur M. A.; Liu P.; Martin S. T.; Geiger F. M.; Thomson R. J. Cloud Activation Potentials for Atmospheric α-Pinene and β-Caryophyllene Ozonolysis Products. ACS Cent. Sci. 2017, 3, 715–725. 10.1021/acscentsci.7b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski B.; Al-sharafi M.; Gierczak T. Ozonolysis of β-Caryophyllonic and Limononic Acids in the Aqueous Phase: Kinetics, Product Yield, and Mechanism. Environ. Sci. Technol. 2019, 53, 8823–8832. 10.1021/acs.est.9b02471. [DOI] [PubMed] [Google Scholar]
- Witkowski B.; Al-sharafi M.; Gierczak T. Kinetics and products of the aqueous-phase oxidation of β-caryophyllonic acid by hydroxyl radicals. Atmos. Environ. 2019, 213, 231–238. 10.1016/j.atmosenv.2019.06.016. [DOI] [Google Scholar]
- McNeill V. F. Aqueous Organic Chemistry in the Atmosphere: Sources and Chemical Processing of Organic Aerosols. Environ. Sci. Technol. 2015, 49, 1237–1244. 10.1021/es5043707. [DOI] [PubMed] [Google Scholar]
- Witkowski B.; Gierczak T. cis-Pinonic Acid Oxidation by Hydroxyl Radicals in the Aqueous Phase under Acidic and Basic Conditions: Kinetics and Mechanism. Environ. Sci. Technol. 2017, 51, 9765–9773. 10.1021/acs.est.7b02427. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Chen Z.; Wang H.; He S.; Huang D. An important pathway for ozonolysis of alpha-pinene and beta-pinene in aqueous phase and its atmospheric implications. Atmos. Environ. 2009, 43, 4465–4471. 10.1016/j.atmosenv.2009.06.028. [DOI] [Google Scholar]
- Kołodziejczyk A.; Pyrcz P.; Pobudkowska A.; Błaziak K.; Szmigielski R. Physicochemical Properties of Pinic, Pinonic, Norpinic, and Norpinonic Acids as Relevant α-Pinene Oxidation Products. J. Phys. Chem. B 2019, 123, 8261–8267. 10.1021/acs.jpcb.9b05211. [DOI] [PubMed] [Google Scholar]
- Szmigielski R.; Surratt J. D.; Gómez-González Y.; Van der Veken P.; Kourtchev I.; Vermeylen R.; Blockhuys F.; Jaoui M.; Kleindienst T. E.; Lewandowski M.; Offenberg J. H.; Edney E. O.; Seinfeld J. H.; Maenhaut W.; Claeys M. 3-methyl-1,2,3-butanetricarboxylic acid: An atmospheric tracer for terpene secondary organic aerosol. Geophys. Res. Lett. 2007, 34, L24811. 10.1029/2007gl031338. [DOI] [Google Scholar]
- Claeys M.; Iinuma Y.; Szmigielski R.; Surratt J. D.; Blockhuys F.; Van Alsenoy C.; Böge O.; Sierau B.; Gómez-González Y.; Vermeylen R.; Van der Veken P.; Shahgholi M.; Chan A. W. H.; Herrmann H.; Seinfeld J. H.; Maenhaut W. Terpenylic Acid and Related Compounds from the Oxidation of α-Pinene: Implications for New Particle Formation and Growth above Forests. Environ. Sci. Technol. 2009, 43, 6976–6982. 10.1021/es9007596. [DOI] [PubMed] [Google Scholar]
- Iinuma Y.; Keywood M.; Gnauk T.; Herrmann H.; Herrmann H. Diaterebic Acid Acetate and Diaterpenylic Acid Acetate: Atmospheric Tracers for Secondary Organic Aerosol Formation from 1,8-Cineole Oxidation. Environ. Sci. Technol. 2009, 43, 280–285. 10.1021/es802141v. [DOI] [PubMed] [Google Scholar]
- Ye P.; Zhao Y.; Chuang W. K.; Robinson A. L.; Donahue N. M. Secondary organic aerosol production from pinanediol, a semi-volatile surrogate for first-generation oxidation products of monoterpenes. Atmos. Chem. Phys. 2018, 18, 6171–6186. 10.5194/acp-18-6171-2018. [DOI] [Google Scholar]
- Nozière B.; Kalberer M.; Claeys M.; Allan J.; D’Anna B.; Decesari S.; Finessi E.; Glasius M.; Grgić I.; Hamilton J. F.; Hoffmann T.; Iinuma Y.; Jaoui M.; Kahnt A.; Kampf C. J.; Kourtchev I.; Maenhaut W.; Marsden N.; Saarikoski S.; Schnelle-Kreis J.; Surratt J. D.; Szmigielski R.; Wisthaler A.; Wisthaler A. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev. 2015, 115, 3919–3983. 10.1021/cr5003485. [DOI] [PubMed] [Google Scholar]
- O’Dowd C. D.; Aalto P.; Hmeri K.; Kulmala M.; Hoffmann T. Atmospheric particles from organic vapours. Nature 2002, 416, 497–498. 10.1038/416497a. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Jimenez J. L.; Canagaratna M. R.; Allan J. D.; Coe H.; Ulbrich I.; Alfarra M. R.; Takami A.; Middlebrook A. M.; Sun Y. L.; Dzepina K.; Dunlea E.; Docherty K.; DeCarlo P. F.; Salcedo D.; Onasch T.; Jayne J. T.; Miyoshi T.; Shimono A.; Hatakeyama S.; Takegawa N.; Kondo Y.; Schneider J.; Drewnick F.; Borrmann S.; Weimer S.; Demerjian K.; Williams P.; Bower K.; Bahreini R.; Cottrell L.; Griffin R. J.; Rautiainen J.; Sun J. Y.; Zhang Y. M.; Worsnop D. R. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 2007, 34, L13801. 10.1029/2007gl029979. [DOI] [Google Scholar]
- Jimenez J. L.; Canagaratna M. R.; Donahue N. M.; Prevot A. S. H.; Zhang Q.; Kroll J. H.; DeCarlo P. F.; Allan J. D.; Coe H.; Ng N. L.; Aiken A. C.; Docherty K. S.; Ulbrich I. M.; Grieshop A. P.; Robinson A. L.; Duplissy J.; Smith J. D.; Wilson K. R.; Lanz V. A.; Hueglin C.; Sun Y. L.; Tian J.; Laaksonen A.; Raatikainen T.; Rautiainen J.; Vaattovaara P.; Ehn M.; Kulmala M.; Tomlinson J. M.; Collins D. R.; Cubison M. J.; Dunlea J.; Huffman J. A.; Onasch T. B.; Alfarra M. R.; Williams P. I.; Bower K.; Kondo Y.; Schneider J.; Drewnick F.; Borrmann S.; Weimer S.; Demerjian K.; Salcedo D.; Cottrell L.; Griffin R.; Takami A.; Miyoshi T.; Hatakeyama S.; Shimono A.; Sun J. Y.; Zhang Y. M.; Dzepina K.; Kimmel J. R.; Sueper D.; Jayne J. T.; Herndon S. C.; Trimborn A. M.; Williams L. R.; Wood E. C.; Middlebrook A. M.; Kolb C. E.; Baltensperger U.; Worsnop D. R. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- Barton A. F. M.CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, 1983; p 64. [Google Scholar]
- Dette H. P.; Qi M.; Schröder D. C.; Godt A.; Koop T. Glass-Forming Properties of 3-Methylbutane-1,2,3-tricarboxylic Acid and Its Mixtures with Water and Pinonic Acid. J. Phys. Chem. A 2014, 118, 7024–7033. 10.1021/jp505910w. [DOI] [PubMed] [Google Scholar]
- Fukuyama T.; Yamada K.; Nishikawa T.; Ravelli D.; Fagnoni M.; Ryu I. Site-selectivity in TBADT-photocatalyzed C(sp3)–H Functionalization of Saturated Alcohols and Alkanes. Chem. Lett. 2017, 47, 207–209. 10.1246/cl.171068. [DOI] [Google Scholar]
- Matteson D. S.; Ray R. Directed chiral synthesis with pinanediol boronic esters. J. Am. Chem. Soc. 1980, 102, 7590–7591. 10.1021/ja00545a046. [DOI] [Google Scholar]
- Wilson G. M. Vapor-Liquid Equilibrium. XI. A New Expression for the Excess Free Energy of Mixing. J. Am. Chem. Soc. 1964, 86, 127–130. 10.1021/ja01056a002. [DOI] [Google Scholar]
- Abrams D. S.; Prausnitz J. M. Statistical Thermodynamics of Liquid Mixtures: A New Expression for the Excess Gibbs Energy of Partly or Completely Miscible Systems. AIChE J. 1975, 21, 116–128. 10.1002/aic.690210115. [DOI] [Google Scholar]
- Renon H.; Prausnitz J. M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. 10.1002/aic.690140124. [DOI] [Google Scholar]
- Kostenidou E.; Karnezi E.; Kolodziejczyk A.; Szmigielski R.; Pandis S. N. Physical and Chemical Properties of 3-Methyl-1,2,3-butanetricarboxylic Acid (MBTCA) Aerosol. Environ. Sci. Technol. 2018, 52, 1150–1155. 10.1021/acs.est.7b04348. [DOI] [PubMed] [Google Scholar]
- Höfle G.; Steglich W.; Vorbrüggen H. 4-Dialkylaminopyridines as Highly Active Acylation Catalysts. Angew. Chem., Int. Ed. 1978, 17, 569–583. 10.1002/anie.197805691. [DOI] [Google Scholar]
- Moglioni A. G.; García-Expósito E.; Aguado G. P.; Parella T.; Branchadell V.; Moltrasio G. Y.; Ortuño R. M. Divergent Routes to Chiral Cyclobutane Synthons from (−)-α-Pinene and Their Use in the Stereoselective Synthesis of Dehydro Amino Acids. J. Org. Chem. 2000, 65, 3934–3940. 10.1021/jo991773c. [DOI] [PubMed] [Google Scholar]
- Pobudkowska A.; Ràfols C.; Subirats X.; Bosch E.; Avdeef A. Phenothiazines solution complexity - Determination of pKa and solubility-pH profiles exhibiting sub-micellar aggregation at 25 and 37°C. Eur. J. Pharm. Sci. 2016, 93, 163–176. 10.1016/j.ejps.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Królikowska M.; Karpińska M.; Zawadzki M. Phase Equilibria Study of the Binary Systems (N-Hexylisoquinolinium Thiocyanate Ionic Liquid + Organic Solvent or Water). J. Phys. Chem. B 2012, 116, 4292–4299. 10.1021/jp301081b. [DOI] [PubMed] [Google Scholar]
- Halayqa M.; Pobudkowska A.; Domańska U.; Zawadzki M. Studying of drug solubility in water and alcohols using drug-ammonium ionic liquid-compounds. Eur. J. Pharm. Sci. 2018, 111, 270–277. 10.1016/j.ejps.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Bates R. G.; Gary R. Acidity functions. Values of the quantity p(aHγCl) for buffer solutions from 0 to 95°C. J. Res. Natl. Bur. Stand., Sect. A 1961, 65, 495–505. 10.6028/jres.065a.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnezhad A.; Afshar O. A.; Khansary M. A.; Shirazian S.; Ghadiri M. Correlation of interaction parameters in Wilson, NRTL and UNIQUAC models using theoretical methods. Fluid Phase Equilib. 2016, 417, 181–186. 10.1016/j.fluid.2016.02.041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




