Abstract

Superhydrophobicity is of interest for practical applications such as water repellency, self-cleaning, stain resistance, antibacterial properties, and oil–water separation. In this work, a superhydrophobic coating on cotton fabric is prepared by simple immersion in TiO2 nanoparticles and perfluorodecyltriethoxysilane solution. Its antiwetting properties, surface morphology, and functionality are characterized. The cotton fabric shows superhydrophobicity with a water static contact angle of 169.3 ± 2.1° and tilt angle of 6.3 ± 2.0°. The coating is also characterized by performing stability tests, and it shows excellent mechanical durability, chemical stability, and thermal stability. Additionally, the water droplet dynamic on the coated surface is also studied. The coated cotton fabric exhibits excellent self-cleaning, stain resistance, rust stain resistance, anti-water absorption, and antibacterial properties. It can also be used in oil–water separation with a high separation efficiency and excellent reusability.
1. Introduction
Recently, the development of superhydrophobic coatings on cotton fabric has shown great interest because of its self-cleaning, antibacterial, antifungal, antistain, and other properties.1,2 Superhydrophobicity is a phenomenon where water droplets make a contact angle higher than 150 ± 1°, and it can be seen in naturally found materials such as lotus leaf, rose petals, wings of a butterfly, and water strider. Many attempts have been made to make superhydrophobic coatings on cotton fabric and textiles. For instance, Huang et al.3 fabricated a superhydrophobic cotton fabric using TiO2 nanoparticles and perfluorodecyltriethoxysilane (PFDTS) with a contact angle of 160 ± 1°. Li et al.4 developed a robust flowerlike TiO2@cotton fabric for self-cleaning and oil–water separation. Xu et al.5 fabricated a superhydrophobic cotton surface with a contact angle of 161 ± 2° using a ZnO nanorod and dodecyltrimethoxysilane (DTMS). Xue et al.6 fabricated a superhydrophobic surface with amino- and epoxy-functionalized silica nanoparticles with a combination of stearic acid and 1H,1H,2H,2H-perfluorodecyltrichlorosilane. Bae et al.7 prepared superhydrophobic cotton fabric using silica nanoparticles and tetraethyl orthosilicate (TEOS). Ivanova and Zaretskaya8 fabricated superhydrophobic cotton with fluoroalkylsiloxane. Shirgholami et al.9 fabricated superhydrophobic cotton using polymethylsilsesquioxane by the immersion process. Du et al.10 developed superhydrophobic fabric using SiO2 nanoparticles and poly(dimethylsiloxane) for oil–water separation. Nguyen-Tri et al.11 fabricated superhydrophobic cloth using SiO2 and tetraethyl orthosilicate with a water contact angle (WCA) of 173°. Xiao et al.12 made switchable superhydrophobic cotton using poly(3-triisopropyloxysilylpropyl methacrylate), poly(dimethylsiloxane), and poly(N,N-dimethylaminoethyl methacrylate) for oil–water separation. Lin et al.13 prepared superhydrophobic polyester fabric via controlled hydrolysis of methyltrimethoxysilane.
Pan et al.14 fabricated superhydrophobic cotton fabric with aluminum nitrate and sodium stearate. Xue et al.15 made superhydrophobic cotton fabric using silver nanoparticles and hexadecyltrimethoxysilane. Zhao et al.16 reported a superhydrophobic coating on cotton fabric using a hybrid layered double-hydroxide nanoplatelet intercalated with 2-hydroxy- 4-methoxybenzophenone-5-sulfonic acid molecules and an electrostatic layer-by-layer assembly technique. Liu et al.17 fabricated superhydrophobic cotton fabric using SiO2 nanoparticles and octadecyltrichlorosilane for oil–water separation. Wang et al.18 prepared superhydrophobic cotton fabric using silica nanoparticles and polydopamine adhesion and dodecyltrimethoxysilane. Pi et al.19 fabricated superhydrophobic cotton fabric using a cross-linkable polyhedral oligomeric silsesquioxane (POSS)-containing fluorinated copolymer. Lei et al.20 made superhydrophobic cotton fabric for oil–water separation using diluted poly(tetrafluoroethylene) (PTFE) dispersion. Tudu et al.21 fabricated superoleophobic cotton fabric using both TEOS and PFDTS. Pan et al.22 prepared superhydrophobic cotton fabric using tetraethyl orthosilicate (TEOS) and dodecyltrimethoxysilane (DTMS). Foorginezhad et al.23 reported superhydrophobic cotton fabric using octa vinyl polyhedral oligomeric silsesquioxane/poly(dimethylsiloxane) nanocomposite. Chauhan et al.24 prepared superhydrophobic cotton using hexadecyltrimethoxysilane with antibacterial ability. Cheng et al.25 developed superhydrophobic cotton fabric with the help of enzyme etching and sebacic acid, as an eco-friendly method for application in oil–water separation. Although superhydrophobic surfaces could be prepared by the above-mentioned studies, poor durability can limit their applications. Further investigation on the durability of the surface is to be carried out.
The typical strategy for the fabrication of a superhydrophobic surface is the use of a hydrophobic material having a low surface tension along with nanoparticles. For superhydrophobicity, many nanoparticles like TiO2,26,27 SiO2,28−32 CuS,33 FeCl2, carbon nanotubes,35 etc. have been used to create roughness on the surface, which later coated with hydrophobic materials. The surface tension of these hydrophobic materials depends on the end group present in the substances, and it follows the following order: CF3 (15 dyn/cm) < CF2 (23 dyn/cm) < CH3 (30 dyn/cm) < CH2 (36 dyn/cm), which can explain the extensive use of fluoroalkylsilanes.36 Among the different fluoroalkylsilanes, perfluorodecyltriethoxysilane (PFDTS) has been extensively used to achieve superhydrophobicity on metal,37 paper,38 glass,39 ceramic substrates,40 and silica membrane.41 PFDTS-based coating can repel both water and oil due to its long chain of fluorocarbon. Additionally, it can form self-assembled monolayers rapidly during functionalization of hydrophilic surfaces.42 Further, Raza et al. demonstrated that besides providing low surface energy, PFDTS could increase the surface roughness.43 PFDTS-based superhydrophobic coatings on different substrates have been reported for several applications such as self-cleaning and oil–water separation.37−41 However, the wetting stability of coatings has rarely been evaluated. The durability of the wetting properties of the superhydrophobic surface is one of the critical parameters toward its use in the industry. Therefore, the preparation of a durable superhydrophobic surface using PFDTS is desirable.
In this work, a superhydrophobic coating on the cotton fabric was created using a mixture of TiO2 nanoparticles and PFDTS. TiO2 nanoparticles have been chosen as they provide nanoroughness to the surface and also enhance the antibacterial activities44 due to their photocatalytic activity. Superhydrophobic cotton samples were characterized by different techniques. Further, superhydrophobic cotton fabric was examined under a harsh environment like exposure to high temperature, highly corrosive chemicals, and mechanical disturbances. The dynamic behavior of water droplets on coated cotton fabric was also studied. Additionally, self-cleaning, stain resistance, rust stain resistance, anti-water absorption, oil–water separation, and antibacterial characteristics of coated cotton fabric were examined.
2. Results and Discussion
Superhydrophobicity was achieved in cotton fabric by immersing the sample in a solution of TiO2 nanoparticles and PFDTS. The wettability, morphology, and droplet dynamics behavior of superhydrophobic cotton fabric have been discussed along with mechanical, chemical, and thermal stabilities. Further rust stain resistance, stain resistance, self-cleaning, antibacterial, anti-water absorption, and oil–water separation activities using coated cotton fabric have been discussed (Figure 1).
Figure 1.

Schematic diagram of the preparation of superhydrophobic cotton fabric using PFDTS and TiO2 nanoparticles in toluene.
The superhydrophobic coating on cotton fabric was prepared by varying molar ratios of PFDTS and TiO2. The deferent molar ratio has been obtained by changing the PFDTS amount for a fixed amount of TiO2. Water contact angle versus molar ratio of PFDTS and TiO2 is presented in Figure S1a. The results indicated that the contact angle increases with increasing the molar ratio up to 0.38. No significant change is observed on further increase in molar ratio. The results may be explained as the molar ratio increases the concentration of PFDTS as well as superhydrophobicity and reaches the optimized point at 0.024 mol/L. Further, the coating was prepared by varying the concentration at the optimized molar ratio of PFDTS and TiO2, i.e., 0.38. The concentration of TiO2 was studied from 0.05 to 0.125 mol/L, and that of PFDTS was studied from 0.19 to 0.48 mol/L. Figure S1b shows that the change in the concentration has no significant effect on water contact angle. However, it shows a slight tendency to decrease the contact angle at lower (0.019 mol/L) and higher (0.048 mol/L) concentrations. The results suggest that at an optimized molar ratio, the fictionalization of TiO2 by PFDTS has been completed, yielding a maximum water contact angle. The increase in concentration can provide advantages in different properties such as UV-blocking and antibacterial activity. However, higher concentrations of TiO2 and PFDTS also added some disadvantages such as poor mechanical stability, alteration of textural properties, and cost. Therefore, further study has been performed with the PFDTS concentration of 0.024 mol/L and TiO2 concentration of 0.0625 mol/L, i.e., optimized molar ratio, 0.38.
To analyze the surface morphology, scanning electron microscopy (SEM) images of different magnifications were taken for both coated and uncoated samples. Figure 2a,b demonstrates the SEM images of both coated and uncoated samples along with the water static contact and sliding angles. The uncoated specimen exhibits the hydrophilic nature with a water static contact angle of 64 ± 2°. SEM images of uncoated samples show the presence of a large number of symmetrical valleys on cotton fabrics. Fabric threads of cotton create microstructures on the surface, which implies the existence of inherent roughness on the surface, and the water fills up the valley and gaps present on the surface. Further, energy-dispersive spectrometry (EDS) analysis was performed for coated cotton fabric. The EDS spectra (Figure 2e) reveal the presence of elements like C, Si, Ti, O, and F on the coated cotton. The results suggest the uniform coating of PFDTS and TiO2 over cotton fabric.
Figure 2.

SEM images of (a, b) coated and (c, d) uncoated cotton fabric with static contact angle of water (inset). (e) EDS spectra of the coated sample displaying the presence of C, Ti, O, F, and Si elements.
After modification, coated cotton shows extreme water-repellent nature with a water static contact angle of 169.3 ± 2.1° and a tilting angle of 6.3 ± 2.0°. The contact angles against other water-based solutions (tea, turmeric water, milk, mud water, urine, and blood) and liquids of lower surface tension (glycerol and ethylene glycol) were measured, and the results are tabulated in Table 1. It is found that coated cotton fabric shows the excellent repellency of the aforesaid water-based solution and liquid having a surface tension of more than 47.70 mN/m. SEM analysis shows that the surface morphology of cotton fabric changed after modification (Figure 2c,d).
Table 1. Static Contact Angle of Different Liquids on Uncoated and Coated Cotton Fabric.
The coated sample was further characterized using Fourier transform infrared (FTIR) spectroscopy to examine the presence of a functional group on the surface, which assists superhydrophobicity. Figure 3 represents the FTIR analysis of the coated sample. The FTIR graph shows several peaks at different wavenumbers. The peak at 1068 cm–1 represents the presence of the C functional group on the surface. The peaks at 1145 and 1199 cm–1 correspond to −CF– functional groups. These −SiO– and −CF– functional groups indicate that the hydrophobic tail of PFDTS is attached to the surface. The inherent surface roughness of fabric creates air pockets beneath the water droplets. These air pockets can further increase water contact angle, and thus, superhydrophobicity is achieved.48
Figure 3.

FTIR spectra of coated cotton fabric showing the presence of −CF– and −SiO– functional groups at 1068 and 1145 cm–1, respectively; 1199 cm–1 represents the hydrophobic tail of PFDTS.
2.1. Stability Characterization of Coated Cotton
Mechanical durability of coated cotton has been examined by performing water jet and washing tests. Figure 4a presents the water jet test on both uncoated and coated cotton fabric. When the water jet impacts on uncoated cotton fabric surface, it immediately sticks to the surface. When the water jet impacts on coated cotton fabric at a certain angle, it bounces in the opposite direction. This is due to the water repellency property of coated cotton fabric. A water jet is continuously injected for 2 min on the coated surface, and it is found bouncing without any discontinuity, showing its excellent durability of the coating. The coated sample was washed with hot water (∼90 °C), detergent water, toluene, acetone, and benzene to check the durability of the coating. Table 2 shows the water static contact angle of the coated sample after 1 h washing. The results show that the water static contact angle remains higher than 150 ± 2°. It confirms that washing does not affect the superhydrophobicity of the coating.
Figure 4.

(a) Optical image of water jet test on uncoated cotton fabric displaying accumulation of water on the surface. Optical image of water jet test on coated cotton fabric revealing bouncing of water in the opposite direction, showing its superhydrophobic nature (water jet angle is 20°). (b) Immersion time and water contact angle vs pH plot displaying the retention of superhydrophobicity of coated cotton fabric in different pH solutions (HCl for acid solution and NaOH for base solution). (c) Contact angle vs annealing temperature plot displaying thermal stability of superhydrophobic cotton fabric.
Table 2. Water Static Contact Angle of Water before and after 1 h Washing with Different Solvents.
| water static contact angle (deg) |
sliding angle (deg) |
|||
|---|---|---|---|---|
| solvent | before | after 1 h washing | before | after 1 h washing |
| detergent water | 169.3 ± 2.1 | 155.2 ± 1.0 | 6.3 ± 2.0 | 6.3 ± 2.0 |
| hot water (∼90 °C) | 169.3 ± 2.1 | 157.3 ± 2.2 | 6.3 ± 2.0 | 6.3 ± 2.0 |
| toluene (28.40)a | 169.3 ± 2.1 | 153.2 ± 1.0 | 6.3 ± 2.0 | 8.2 ± 2.0 |
| acetone (25.20)a | 169.3 ± 2.1 | 150.4 ± 1.0 | 6.3 ± 2.0 | 8.1 ± 2.0 |
| benzene (28.88)a | 169.3 ± 2.1 | 152.1 ± 2.1 | 6.3 ± 2.0 | 8.2 ± 2.0 |
The value provided in parentheses is the surface tension of the corresponding liquid.
Additionally, the durability against abrasion was investigated on the coated sample. Figure S2 shows the abrasion test results on the cotton sample. At 35 cycles, the contact angle reduces to nearly 150.3 ± 2.1° and the sliding angle reduces to 8.2 ± 2.1°. Further, after 60 cycles, the contact angle reduces to 135.6 ± 2.3° and sliding angle increases to 20.4 ± 2.2°. The abrasion leads to the breaking of covalent bonds between cotton fabric and PFDTS monomer/TiO2, as well as loss of superhydrophobicity.
Further, the laundering effect was assessed on the coated cotton fabric (Figure S3). The prepared cloth exhibited excellent resistance to laundering. The superhydrophobicity (WCA = 169.3 ± 2.1°) remains intact for the first five cycles. After that, the WCA decreases and the sliding angle increases. However, it shows superhydrophobicity (WCA = 152.4 ± 2.1°) up to 20 cycles. The loss of superhydrophobicity can be correlated to the destruction of covalent bonds between cloth and coating due to friction with water during the laundering process.
The chemical stability of the coating was tested by keeping the coated sample in inorganic solvents (pH solution). Figure 4b represents the immersion time of coated sample in each solvent. It shows the longevity of superhydrophobicity of coated cotton with different pH solutions. In pH 2 and pH 13 solutions, coated cotton loses its superhydrophobicity within 20 and 10 h, respectively. The reason behind this is the attack of the Cl– ion of the pH 2 solution on the sample. Similarly, in the case of pH 13, the OH– ion may attack the coated cotton fabric. In the case of pH 5, 8, and 11, the superhydrophobicity of the sample remains unaffected even after 50 days, showing its high chemical durability.
The thermal stability of coated cotton fabric was investigated by annealing it at different temperatures ranging from 50 to 300 °C under ambient atmosphere. Before the test, the oven was left for 30 min at an elevated temperature to remove moisture from inside. The water contact angle of each sample after annealing was measured. Figure 4c represents the water static contact angle against the annealing temperature. The water static contact angle is found to be above 150.6 ± 2.0° for all samples; those are annealed at a temperature range of 50–200 °C, i.e., superhydrophobicity remains unchanged. There is a slight reduction in water static contact angle after annealing at a temperature range of 200–250 °C, but the water static contact angle remains above 150.6 ± 2.0°. After annealing at 300 °C, the coated sample shows a tremendous reduction in water static contact angle from 169.3 ± 2.1 to 50 ± 2°. The possible reason for the decrease in superhydrophobicity is the devastation of coating due to the decomposition of PFDTS as the boiling temperature of PFDTS is between 209 and 230 °C. At temperatures above 250 °C, the CH2 bond in PFDTS gets disordered and the molecular structure collapses that leaves Si–O–Si species over the cotton surface.48
2.2. Droplet Impact Behavior
Water droplet dynamic behavior was studied on superhydrophobic cotton fabric in two ways: impacting of water droplet on coated cotton fabric placed on the solid surface and hanging in air. Figure 5 shows the droplet impact behavior on coated cotton surface at different impact velocities. Water droplets at different velocities show different impact behavior. At low impact velocities of 0.54 and 0.61 m/s, water droplets form pancake-like shapes on the coated cotton fabric placed on a solid surface and hanged in air, respectively, and they bounce back without changing the volume. When a water droplet hits the superhydrophobic surface, its shape deforms, but it restores the kinetic energy and subsequently bounces back. This is due to the barrier formed by air pockets that are present on the coated surface.
Figure 5.
Dynamics behavior of water droplets on superhydrophobic cotton fabric placed on a solid surface and hanged in air (i.e., no solid surface in contact). Bouncing, pinning, and splashing of water droplets are observed at different impact velocities.
At medium impact velocities of 0.76 and 0.82 m/s, water droplets bounce upward and hit on coated cotton fabric placed on a solid surface and hanged in air, respectively, and are overextended vertically. Water droplets break into two parts, and the bottom parts remain sticky on the surface, as shown in Figure 5. This phenomenon is known as pinning. This shows the transition of the Cassie state to the Wenzel state,49 where air pockets on the surface begin to be replaced by water.
On the other hand, at much higher impact velocities of 0.99 and 1.03 m/s on coated cotton fabric placed on a solid surface and hanged in air, respectively, water droplets spread and break into several small water droplets on the surface. This phenomenon is called splashing, where liquid droplets with a high impact velocity break into many tiny droplets during the spreading and retracting stage. To further understand, the Reynold’s and Weber numbers of the water droplet were calculated and are tabulated in Table 3. It is clear that with an increase in impact velocity, the Reynolds and Weber numbers also increase.
Table 3. Dynamic Behavior of Water Droplets on Superhydrophobic Cotton Fabric Placed on Solid Surface and Hanged in Air.
| impact velocities | Reynolds no. | Weber no. | dynamic behavior |
|---|---|---|---|
| Superhydrophobic Cotton Fabric Surface Placed on Solid Surface | |||
| 0.54 | 2430 | 16 | bouncing |
| 0.76 | 3420 | 32 | pinning |
| 0.99 | 4450 | 55 | splashing |
| Superhydrophobic Cotton Fabric Surface Hanged in Air (i.e., No Solid Surface in Contact) | |||
| 0.61 | 2740 | 21 | bouncing |
| 0.82 | 3720 | 38 | pinning |
| 1.03 | 4630 | 59 | splashing |
2.3. Applications of Coated Cotton Fabric
Figure 6a demonstrates the self-cleaning property of both coated and uncoated cotton fabric. Both samples were covered with sand dust particles, and water was dropped on dust-containing surfaces. The sand dust particle size was between 5 and 20 μm, as shown in Figure S4. The particle density on the coated sample is 5.1 mg/cm2, and after self-cleaning, the particle density on the coated sample is 0.4 mg/cm2. In the self-cleaning test, the sand particles are spread over on both coated and uncoated samples by blowing from the top. When the water droplet falls on the uncoated sample, it sticks to it and does not move further. On further flowing water, it starts accumulating on the surface. Eventually, it leaves as muddy water, but the surface remains dirty. On the other hand, when the water droplet falls on the coated surface, it immediately rolls. While rolling downward, it collects the dust particle and moves downward, leaving a cleaned surface. This shows the excellent self-cleaning property of the coated surface.
Figure 6.
(a) Optical images of uncoated cotton fabric having no self-cleaning property. Optical images of coated cotton fabric with a thick path of water drop showing self-cleaning property of the superhydrophobic surface. (b) Optical images of color water droplets on uncoated and coated cotton fabric before and after annealing. No stain on coated cotton fabric displays the stain resistance property of coating.
Stain is becoming a significant problem in cloth and textiles. It comes from the contact of dirt and several types of liquids such as color water, food product, etc. Although many liquids can be easily removed from cloth, they leave some spots on the cloth. Therefore, it is necessary to examine the stain resistivity of the aforesaid coated cloth. Figure 6b demonstrates the stain resistance property of the coated cotton fabric. In this experiment, colored water droplets were kept both uncoated and coated and left for drying in air. It was observed that the water droplet is immediately soaked on the uncoated surface, whereas it makes a spherical shape on the coated sample. Both samples were left for 1 day drying in air. It is noted that water is evaporated from the surface. It is observed that a large area of stain marks is formed on the uncoated surface. In the case of the coated sample, the water droplet is evaporated, and it leaves some colored particles on the surface. However, after rolling a water droplet on it, colored particles are removed from the surface, and eventually, the coated sample looks clean as previously.
When the cloth comes in contact with metallic bodies, it quickly catches rust and makes a permanent stain. It is therefore desirable to have a fabric that rejects rust forms on the metal surface. The prepared cotton fabrics have been examined by dipping in iron-rusted water. Figure 7 demonstrates both uncoated and coated cotton fabric after 48 h immersion in rusted water. It is observed that uncoated cotton fabric is completely covered with marks of rust particles. On the other hand, only a negligible amount of rust particles is found on the coated surface due to its excellent water-repellent nature.
Figure 7.
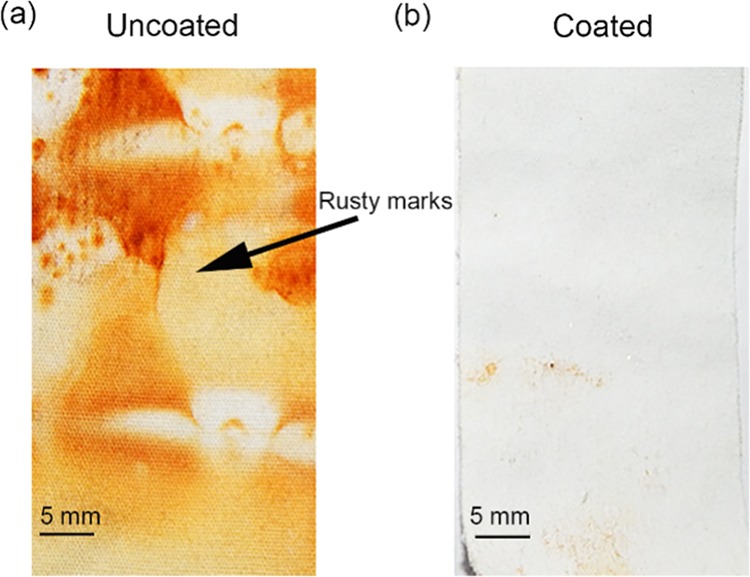
Optical images of uncoated and coated cotton fabric after immersing in iron-rusted water for 48 h. No rust stain on coated cotton fabric displaying the rust stain resistance property.
Most of the cellulosic materials easily catch the atmospheric moisture and degrade the quality of the materials. It is highly recommended to fabricate low-water-absorbing cellulose materials. In the current work, a water absorption test on coated cotton fabric was also performed. In this test, coated and uncoated cotton fabric was kept on top of a hot water (∼90 °C) beaker. Table 4 presents the weights of both uncoated and coated cotton fabric before and after 15 min of treatment. It is observed that uncoated cotton fabric absorbs 0.47% of its body weight amount of moisture, whereas coated cotton fabric absorbs only 0.03% of its body weight amount of moisture due to excellent water-repellent nature. This difference gives a clear indication of the anti-water absorption ability of coated cotton fabric.
Table 4. Weight of Both Uncoated and Coated Cotton Fabric before and after Immersion in Water, and % Absorption of Water.
| samples | initial weight (mg) | weight after immersion in water (mg) | water absorption (%) |
|---|---|---|---|
| uncoated cotton fabric | 2352.4 | 2363.6 | 0.47 |
| coated cotton fabric | 2524.0 | 2525.0 | 0.03 |
The coating was also examined for UV-blocking ability under UV-A and UV-C light. Figure S5 displays the UV resistance of coatings with different molar ratios of PFDTS and TiO2 under UV-A and UV-C light. The UV-A and UV-C transmittances for a 0.38 molar ratio of PFDTS/TiO2 are 64.56 and 65%, respectively. Further increasing TiO2 can reduce the UV transmittance, but it will decrease the superhydrophobicity of the sample.
Due to the presence of selective superoleophilic and superhydrophobic properties, the present coated cotton fabric can be used for oil–water separation. To confirm this application, separation of oil from its water mixture and emulsion was studied. Figure 8a shows the separation process of oil–water mixture. It shows that on pouring an oil–water mixture on coated cotton, oil simply passes through due to its superoleophilic nature, whereas water remains on the surface of coated cotton due to its superhydrophobic nature. In this way, 99% of oil can be separated easily with a simple pouring method. Figure 8b displays the oil separation efficiency of the oil–water mixture. The separation process was repeatedly performed more than 100 times, and it shows 99% oil separation without any discontinuity. After 140 cycles, the separation efficiency starts decreasing very slowly, and after 350 cycles, the separation efficiency is found to be 80%.
Figure 8.

(a) Optical image demonstrating oil–water separation of superhydrophobic cotton fabric. (b) Separation efficiency vs no. of cycles representing the durability of superhydrophobic cotton after several uses.
In another set of experiments, a surfactant-free oil–water emulsion was prepared with a low-surface-tension oil (n-hexane) and distilled water using 5 h of sonication and 15 h of continuous stirring followed by 5 h of sonication. The oil–water emulsion was prepared using a 1:1 ratio of oil and water. The oil-in-water emulsion contains a large number of tiny oil droplets separated by a water layer around each other. This emulsion was poured on the coated cotton fabric as in the separation of the oil–water mixture. Part of oil passes through the coated cotton fabric. The emulsion size distribution of both emulsion feed and filtrate water was qualitatively analyzed by Zetasizer. Figure 9 represents the optical images and size distribution of micelles present in feed and water filtrate. The optical microscopy images show that a large number of oil droplets are present in feed emulsion and a small number of oil droplets are in the filtrate. This implicates that the quantity of oil droplets is higher in the feed. The presence of less amount of oil droplets confirms that water filtrate still contains less amount of oil. The oil separation efficiency is found to be approximately 88%.
Figure 9.
Optical image of micelles present in (a) feed and (b) water filtrate. Size distribution of micelles present in (c) feed and (d) water filtrate.
In general, cellulosic materials are prone to bacterial growth, which leads to the degradation of the materials and limits the applications. Therefore, it is always desirable to fabricate antibacterial cloth. In this work, qualitative analysis of the growth of Escherichia coli bacteria around the coated cotton fabric was done to examine its antibacterial activity. Figure 10 shows the optical images of bacterial growth around the uncoated and coated samples. Due to the presence of air, bacteria start growing near the examples and also spread all over the Petri dish. After 24 h of incubation, a negligible amount of bacteria growth around the uncoated sample is visible, whereas no bacteria growth is noted for coated samples. Further, the Petri dishes were left in the incubator for 48 h. A definite inhibition zone was observed on the coated sample. The size of the inhibition zone is 2.5 cm. Further, the experiment is continued for 72 h (Figure S6). After 72 h, a high growth of bacteria is found around the uncoated sample. The inhibition zone is still 2.5 cm on coated sample, which suggested that the grown bacteria cannot penetrate the inhibition zone. TiO2 nanoparticles are well known for their antibacterial properties, which may reduce bacterial growth on the coated sample.50 This inhibition of bacterial growth may be due to the electron/hole (e–/h+) generated upon light irradiation. The h+ present in the valence band of TiO2 acts as oxidizing agents that degrade the protein of the bacteria and lead to the inhibition of the growth of the organism. Therefore, the coated fabric exhibited antibacterial activity due to the presence of TiO2 nanoparticles.51
Figure 10.
Optical images of uncoated and coated cotton fabric before and after 48 h incubation with E. coli bacteria at 37 °C. Inhibition zone (no growth of bacteria) near the coated fabric shows its antibacterial property.
3. Conclusions
The superhydrophobic cotton fabric was prepared by a simple immersing technique with a mixture of PFDTS and TiO2 nanoparticles. Coated fabric shows a static water contact angle of 169.3 ± 2.1° and a tilting angle of 6.3 ± 2.0°. To assess the wetting stability, coated cotton fabric underwent several harsh conditions, and as a result, the coating shows promising outcomes in mechanical, chemical, and thermal stabilities. The prepared cotton fabric shows great self-cleaning, stain resistance, rust stain resistance, and anti-water absorption ability. Additionally, superhydrophobic cotton fabric shows a reliable antibacterial property. Coated cotton fabric can also be used for oil–water separation with a high separation efficiency. Thus, multiple applications make the prepared coated fabric useful for industrial and domestic purposes.
4. Experimental Section
4.1. Preparation of Superhydrophobic Fabric
PFDTS (molecular weight: 610.38, purity: 97%) was purchased from Sigma-Aldrich. TiO2 nanoparticles (molecular weight: 79.87, purity: 98%, average particle size: 50 nm) were purchased from SRL Chem., India. Cotton fabric was purchased from a local vendor. NaOH, HCl, toluene, ethylene glycol, glycerol, and other chemicals were purchased from Merck Specialities Private Limited, India. E. coli bacteria were purchased from Microbial Type Culture Collection and Gene Bank, India.
The cotton fabric was cut into 5 × 2 cm2 dimensions and also in different shapes and sizes. As shown in Figure 1, the cotton substrate was properly cleaned with acetone and water using the sonication method. Then, the cotton fabrics were kept in a hot-air oven at 60 °C for 1 h. The desired amount of PFDTS (0.0095 mol) was added in 40 mL of toluene under continuous stirring. After 15 min, 200 mg of TiO2 (0.0025 mol) was added in the mixture and stirred for another 15 min. Then, the prepared mixture was sonicated for 15 min. After sonication, the cleaned cotton fabric was immersed in the solution for 2 h. Then, the samples were kept in a hot air oven at 120 °C for 1.5 h.
4.2. Characterization of Superhydrophobic Fabric
Coated and uncoated samples were investigated for their wettability using water droplets with a size of about 3 mm by a contact angle measuring instrument (drop shape analyzer DSA25, Krüss Optronics, Germany). The morphological study of samples was done using a scanning electron microscope (SEM-EDS, JEOL JSM-5600, Japan). The Fourier transform infrared (FTIR) spectroscopy (Cary 660, Agilent Technologies) analysis gives information of the functional groups present on the surface.
The mechanical stability was achieved by regular washing with different solvent solutions and detergent water using the sonication method for 1 h. After that, the coated samples were dried, and the wettability was also checked. Mechanical durability of coated fabric was also examined by spraying high-speed water jet. The abrasion test was performed by rubbing the coated sample against sandpaper by applying 20 g weight. During this experiment, both water contact and sliding angles were measured after each cycle of abrasion. Additionally, a laundering test was performed by washing the coated sample in water for several cycles for 1 h. During the process, the water contact angle and tilting angle were measured to assess the effect of laundering on wettability. To mimic harsh corrosive environmental conditions, the coated samples were dipped in different solutions with pH ranging from 2 to 13. The wettability of each coated sample was checked after a regular interval of immersion. The coated fabric was kept at different annealing temperatures ranging from 50 to 300 °C to examine its stability at elevated temperatures. After 1 h of annealing, the sample was cooled at room temperature and then checked for any physical changes like color change and powder coming out from the surface. After that, the water contact angle of the samples was also measured.
The droplet dynamics was studied on prepared superhydrophobic cotton fabric by impacting water droplets at different impact velocities. The droplet behaviors were recorded with a video camera (480 FPS, Exmor, Sony). The Weber number (We = ρDv2/σ, the ratio of kinetic energy to surface energy) and Reynolds number (Re = ρDv/μ, the ratio of inertial force to viscous force) were also calculated to study the impact dynamics, where ρ, D, μ, and σ are the density, diameter of the droplet, dynamic viscosity, and surface tension of the liquid, respectively.
All of the optical images were taken using a DSLR camera (Nikon D3500) by one of the authors of the manuscript.
4.3. Applications of Superhydrophobic Fabric
The self-cleaning property of the prepared coated sample was tested by spreading dust particles on the surface, followed by spraying water. To examine the stain resistance property of the prepared coated sample, a color water drop was kept on it, and after a few hours, the color water drop evaporated leaving a stain on the surface. Later, the size of stain-affected area was measured. When the cloth was hanged on a metal wire, it gets stained due to rust. The rust stain resistance property of the coated fabric was examined by immersing in iron-rusted water for 48 h. The above experiments were also done for uncoated fabric to compare the results from the coated sample.
UV light is hazardous for humans. Depending on the amount of exposure, it can cause sunburn, erythema, and damage to the eyes. Further, upon UV light exposure, the fabric is also wrecked quickly. As UV light was not transmitted through the cloth samples, the UV-blocking ability of the coating was measured via replicating the coating on quartz slides. The quartz slides were checked before and after coating for transmittance of UV light using a UV light meter (Lutron Electronics, Inc.).
For the anti-water absorption test, both coated and uncoated cotton fabric samples were placed on top of a water beaker. Then, the beaker was heated at 70 °C for 15 min. The weight of both the samples was measured before and after treatment. The antibacterial ability of both uncoated and coated cotton fabric samples was investigated with the E. coli bacteria. First, precisely 20 mL of sterilized nutrient agar was poured into a Petri dish and allowed for 30 min to get solidified before inoculation. Exactly 500 μL of the bacterial suspension with a concentration of 1 × 105 colony-forming units/mL was spread all over the surface of each agar-prepared Petri dish. Then, cavities were made and both uncoated and coated samples were placed inside these cavities. Afterward, the prepared Petri dishes were placed in an incubator at 37 °C for 48 h for bacterial growth. After 48 h, the growth of bacteria was checked in each Petri dish. Further, the test was performed for up to 72 h to check the growth of bacteria.
In the oil–water separation experiment, a mixture of n-hexane and distilled water (1:1 ratio) was poured on a coated fabric sample, which was placed on a funnel. The filtrate was collected using a beaker. The above experiment was also repeated for a surfactant-free oil-in-water emulsion. The oil–water emulsion was made by mixing a 1:1 ratio of n-hexane and distilled water using 15 h of continuous stirring followed by 5 h of sonication. The dispersion images of the oil-in-water emulsions were taken using an optical microscope, and the emulsion particle size distribution was analyzed with Zetasizer. For further investigation, the oil–water separation efficiency, η = V/V0 × 100, was calculated, where V is the volume of oil collected and V0 is the initial volume of oil used for mixing.
Acknowledgments
A.S. acknowledges DST, India, for INSPIRE FACULTY program.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04067.
Results for change in wettability of coated sample, abrasion test, laundering test, optical microscopic and particle size distribution of dust particles, UV-blocking property, and antibacterial property of superhydrophobic cotton (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bhushan B.Biomimetics: Bioinspired Hierarchical-Structured Surfaces for Green Science and Technology, 3rd ed.; Springer International: Cham, Switzerland, 2018. [Google Scholar]
- Zhang C.; Liang F.; Zhang W.; Liu H.; Ge M.; Zhang Y.; Dai J.; Wang H.; Xing G.; Lai Y.; Tang Y. Constructing Mechanochemical Durable and Self-Healing Superhydrophobic Surfaces. ACS Omega 2020, 5, 986–994. 10.1021/acsomega.9b03912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. Y.; Li S. H.; Ge M. Z.; Wang L. N.; Xing T. L.; Chen G. Q.; Liu X. F.; Al-Deyab S. S.; Zhang K. Q.; Chen T.; Lai Y. K. Robust superhydrophobic TiO2@fabric for UV shielding, self-cleaning and oil-water separation. J. Mater. Chem. A 2015, 3, 2825–2832. 10.1039/C4TA05332J. [DOI] [Google Scholar]
- Li S.; Huang J.; Ge M.; Cao C.; Deng S.; Zhang S.; Chen G.; Zhang K.; Al-Deyab S. S.; Lai Y. Robust Flower-Like TiO2@Cotton Fabrics with Special Wettability for Effective Self-Cleaning and Versatile Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1500220 10.1002/admi.201500220. [DOI] [Google Scholar]
- Xu B.; Cai Z. Fabrication of a superhydrophobic ZnO nanorod array film on cotton fabrics via a wet chemical route and hydrophobic modification. Appl. Surf. Sci. 2008, 254, 5899–5904. 10.1016/j.apsusc.2008.03.160. [DOI] [Google Scholar]
- Xue C. H.; Jia S. T.; Zhang J.; Tian L. Q. Superhydrophobic surfaces on cotton textiles by complex coating of silica nanoparticles and hydrophobization. Thin Solid Films 2009, 517, 4593–4598. 10.1016/j.tsf.2009.03.185. [DOI] [Google Scholar]
- Bae G. Y.; Min B. G.; Jeong Y. G.; Lee S. C.; Jang J. N.; Koo G. H. Superhydrophobicity of cotton fabrics treated with silica nanoparticles and water-repellent agent. J. Colloid Interface Sci. 2009, 337, 170–175. 10.1016/j.jcis.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Ivanova N. A.; Zaretskaya A. K. Simple treatment of cotton textile to impart high water repellent properties. Appl. Surf. Sci. 2010, 257, 1800–1803. 10.1016/j.apsusc.2010.09.021. [DOI] [Google Scholar]
- Shirgholami M. A.; Khalil-Abad M. S.; Khajavi R.; Yazdanshenas M. E. Fabrication of superhydrophobic polymethylsilsesquioxane nanostructures on cotton textiles by a solution–immersion process. J. Colloid Interface Sci. 2011, 359, 530–535. 10.1016/j.jcis.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Du B.; Chen F.; Luo R.; Li H.; Zhou S.; Liu S.; Hu J. Superhydrophobic surfaces with pH-induced switchable wettability for oil–water separation. ACS Omega 2019, 4, 16508–16516. 10.1021/acsomega.9b02150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Tri P.; Altiparmak F.; Nguyen N.; Tuduri l.; Ouellet-plamondon C. M.; Prud’homme R. E. Robust superhydrophobic cotton fibers prepared by simple dip-coating approach using chemical and plasma-etching pretreatments. ACS Omega 2019, 4, 7829–7837. 10.1021/acsomega.9b00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M.; Huang Y.; Xu A.; Zhang T.; Zhan C.; Hong L. On-Demand oil–water separation by environmentally responsive cotton fabrics. ACS Omega 2019, 4, 12333–12341. 10.1021/acsomega.9b01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.; Rosu C.; Jiang L.; Sundar V. A.; Breedveld V.; Hess D. W. Nonfluorinated superhydrophobic chemical coatings on polyester fabric prepared with kinetically controlled hydrolyzed methyltrimethoxysilane. Ind. Eng. Chem. Res. 2019, 58, 15368–15378. 10.1021/acs.iecr.9b02471. [DOI] [Google Scholar]
- Pan C.; Shen L.; Shang S.; Xing Y. Preparation of superhydrophobic and UV blocking cotton fabric via sol–gel method and self-assembly. Appl. Surf. Sci. 2012, 259, 110–117. 10.1016/j.apsusc.2012.07.001. [DOI] [Google Scholar]
- Xue C. H.; Chen J.; Yin W.; Jia S. T.; Ma J. Z. Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Appl. Surf. Sci. 2012, 258, 2468–2472. 10.1016/j.apsusc.2011.10.074. [DOI] [Google Scholar]
- Zhao Y.; Xu Z.; Wang X.; Lin T. Superhydrophobic and UV-blocking cotton fabrics prepared by layer-by-layer assembly of organic UV absorber intercalated layered double hydroxides. Appl. Surf. Sci. 2013, 286, 364–370. 10.1016/j.apsusc.2013.09.092. [DOI] [Google Scholar]
- Liu F.; Ma M.; Zang D.; Gao Z.; Wang C. Fabrication of superhydrophobic-superoleophilic cotton for application in the field of water/oil separation. Carbohydr. Polym. 2014, 103, 480–487. 10.1016/j.carbpol.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Wang J.; Geng G.; Wang A.; Liu X.; Du J.; Zou Z.; Zhang S.; Han F. Double biomimetic fabrication of robustly superhydrophobic cotton fiber and its application in oil spill cleanup. Ind. Crops Prod. 2015, 77, 36–43. 10.1016/j.indcrop.2015.08.044. [DOI] [Google Scholar]
- Pi P.; Hou K.; Wen X.; Xu S.; Cheng J.; Xu G.; Wang S. A facile one-step fabrication of robust superhydrophobic/superoleophilic cotton fabric using a crosslinkable POSS-containing fluorinated copolymer. Prog. Org. Coat. 2016, 101, 522–529. 10.1016/j.porgcoat.2016.09.023. [DOI] [Google Scholar]
- Lei S.; Shi Z.; Ou J.; Wang F.; Xue M.; Li W.; Qiao G.; Guan X.; Zhang J. Durable superhydrophobic cotton fabric for oil/water separation. Colloid Surf., A 2017, 533, 249–254. 10.1016/j.colsurfa.2017.08.012. [DOI] [Google Scholar]
- Tudu B. K.; Kumar A.; Bhushan B. Fabrication of superoleophobic cotton fabric for multi-purpose applications. Philos. Trans. R. Soc., A 2019, 377, 20190129 10.1098/rsta.2019.0129. [DOI] [PubMed] [Google Scholar]
- Pan G.; Xiao X.; Yu N.; Ye Z. Fabrication of superhydrophobic coatings on cotton fabric using ultrasound-assisted in-situ growth method. Prog. Org. Coat. 2018, 125, 463–471. 10.1016/j.porgcoat.2018.09.026. [DOI] [Google Scholar]
- Foorginezhad S.; Zerafat M. M. Fabrication of superhydrophobic coatings with self-cleaning properties on cotton fabric based on octa vinyl polyhedral oligomeric silsesquioxane-polydimethylsiloxane (OV-POSS/PDMS) nanocomposite. J. Colloid Interface Sci. 2019, 540, 78–87. 10.1016/j.jcis.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Chauhan P.; Kumar A.; Bhushan B. Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique. J. Colloid Interface Sci. 2019, 535, 66–74. 10.1016/j.jcis.2018.09.087. [DOI] [PubMed] [Google Scholar]
- Cheng Q.-Y.; Zhao X.-L.; Weng Y.-X.; Li Y.-D.; Zeng J-B. Fully Sustainable, Nanoparticle-Free, Fluorine-Free, and Robust Superhydrophobic Cotton Fabric Fabricated via an Eco-Friendly Method for Efficient Oil/Water Separation. ACS Sustainable Chem. Eng. 2019, 7, 15696–15705. 10.1021/acssuschemeng.9b03852. [DOI] [Google Scholar]
- Xiong J.; Sarkar D. K.; Chen X. G. Ultraviolet-durable superhydrophobic nanocomposite thin films based on cobalt stearate-coated TiO2 nanoparticles combined with polymethylhydrosiloxane. ACS Omega 2017, 2, 8198–8204. 10.1021/acsomega.7b01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.; Guo N.; Wang C.; Rao Q. Alterable Superhydrophobic–Superhydrophilic Wettability of Fabric Substrates Decorated with Ion–TiO2 Coating via Ultraviolet Radiation. Ind. Eng. Chem. Res. 2014, 53, 14322–14328. 10.1021/ie502338y. [DOI] [Google Scholar]
- Meena M. K.; Sinhamahapatra A.; Kumar A. Superhydrophobic polymer composite coating on glass via spin coating technique. Colloid Polym. Sci. 2019, 297, 1499–1505. 10.1007/s00396-019-04560-z. [DOI] [Google Scholar]
- Han S.-W.; Kim H.-J.; Woo T.-G.; Jeong J.-W.; Cha B. J.; Kim Y. D. Superhydrophobic Fabric Resistant to an Aqueous Surfactant Solution as Well as Pure Water for the Selective Removal of Spill Oil. ACS Appl. Nano Mater. 2018, 1, 5158–5168. 10.1021/acsanm.8b01223. [DOI] [Google Scholar]
- Lahiri S. K.; Zhang P.; Zhang C.; Liu L. Robust Fluorine-Free and Self-Healing Superhydrophobic Coatings by H3BO3 Incorporation with SiO2–Alkyl-Silane@PDMS on Cotton Fabric. ACS Appl. Mater. Interfaces 2019, 11, 10262–10275. 10.1021/acsami.8b20651. [DOI] [PubMed] [Google Scholar]
- Cai R.; Glinel K.; Smet D. D.; Vanneste M.; Mannu N.; Kartheuser B.; Nysten B.; Jonas A. M. Environmentally Friendly Super-Water-Repellent Fabrics Prepared from Water-Based Suspensions. ACS Appl. Mater. Interfaces 2018, 10, 15346–15351. 10.1021/acsami.8b02707. [DOI] [PubMed] [Google Scholar]
- Chen J.; Liu Z.; Wen X.; Xu S.; Wang F.; Pi P. Two-Step Approach for Fabrication of Durable Superamphiphobic Fabrics for Self-Cleaning, Antifouling, and On-Demand Oil/Water Separation. Ind. Eng. Chem. Res. 2019, 58, 5490–5500. 10.1021/acs.iecr.9b00049. [DOI] [Google Scholar]
- Xu L.; Zhang X.; Shen Y.; Ding Y.; Wang L.; Sheng Y. Durable Superhydrophobic Cotton Textiles with Ultraviolet-blocking Property and Photocatalysis Based on Flower-Like Copper Sulfide. Ind. Eng. Chem. Res. 2018, 57, 6714–6725. 10.1021/acs.iecr.8b00254. [DOI] [Google Scholar]
- Zhou X.; Zhang Z.; Xu X.; Guo F.; Zhu X.; Men X.; Ge B. Robust and Durable Superhydrophobic Cotton Fabrics for Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 7208–7214. 10.1021/am4015346. [DOI] [PubMed] [Google Scholar]
- Jung K. K.; Jung Y.; Choi C. J.; Ko J. S. Highly reliable superhydrophobic surface with carbon nanotubes immobilized on a PDMS/Adhesive multilayer. ACS Omega 2018, 3, 12956–12966. 10.1021/acsomega.7b01872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Ober C. K. Self-organizing materials with low surface energy: the synthesis and solid-state properties of semifluorinated side-chain ionenes. Macromolecules 1997, 30, 7560. 10.1021/ma9700901. [DOI] [Google Scholar]
- Yu H.; Lian Z.; Wan Y.; Weng Z. K.; Xu J.; Yu Z. Fabrication of durable superamphiphobic aluminum alloy surfaces with anisotropic sliding by HS-WEDM and solution immersion processes. Surf. Coat. Technol. 2015, 275, 112–119. 10.1016/j.surfcoat.2015.05.032. [DOI] [Google Scholar]
- Oh M. J.; Lee S. Y.; Paik K. H. Preparation of hydrophobic self-assembled monolayers on paper surface with silanes. J. Ind. Eng. Chem. 2011, 17, 149–153. 10.1016/j.jiec.2010.12.014. [DOI] [Google Scholar]
- Xu M.; Feng Y.; Li Z.; Wang X.; Li C.; Jiang H.; Chen Y. A novel, efficient and cost-effective synthesis technique for the development of superhydrophobic glass surface. J. Alloys Compd. 2019, 781, 1175–1181. 10.1016/j.jallcom.2018.12.084. [DOI] [Google Scholar]
- Wei X. L.; Li N.; Yi W. J.; Li L. J.; Chao Z. S. High performance super-hydrophobic ZrO2-SiO2 porous ceramics coating with flower-like CeO2 micro/nano-structure. Surf. Coat. Technol. 2017, 325, 565–571. 10.1016/j.surfcoat.2017.06.004. [DOI] [Google Scholar]
- Wei Q.; Ding Y. L.; Nie Z. R.; Liu X. G.; Li Q. Y. Wettability, pore structure and performance of perfluorodecyl-modified silica membranes. J. Membr. Sci. 2014, 466, 114–122. 10.1016/j.memsci.2014.04.036. [DOI] [Google Scholar]
- Burton Z.; Bhushan B. Hydrophobicity, adhesion, friction properties of nanopatterned polymers and scale dependence for micro- and nanoelectromechanical systems. Nano Lett. 2005, 5, 1607–1613. 10.1021/nl050861b. [DOI] [PubMed] [Google Scholar]
- Raza M. A.; Kooij E. S.; van Silfhout A.; Poelsma B. Superhydrophobic surfaces by anomalous fluoroalkylsilane self-assembly on silica nanosphere arrays. Langmuir 2010, 26, 12962–12972. 10.1021/la101867z. [DOI] [PubMed] [Google Scholar]
- Wanag A.; Rokicka P.; Nejman E. K.; Kozar J. K.; Wrobel R. J.; Szczupak A. M.; Morawski A. W. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. 10.1016/j.ecoenv.2017.09.039. [DOI] [PubMed] [Google Scholar]
- Haynes W. M.CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press: Boca Raton, FL, 2014. [Google Scholar]
- Chandan R.Dairy Based Ingredients; Eagan Press: St. Paul, MN, 1997. [Google Scholar]
- Takamura K.; Fisher H.; Morrow N. R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. 10.1016/j.petrol.2012.09.003. [DOI] [Google Scholar]
- Prakash P.; Satheesh U.; Devaprakasam D.. Study of High Temperature Thermal Behavior of Alkyl and Perfluoroalkylsilane Molecules Self-Assembled on Titanium Oxide Nanoparticles. 2014, arXiv:1409.6823. arXiv.org e-Print archive. https://arxiv.org/abs/1409.6823.
- Cassie A. B. D.; Baxter S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. 10.1039/tf9444000546. [DOI] [Google Scholar]
- Hebeish A. A.; Abdelhady M. M.; Youssef A. M. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohydr. Polym. 2013, 91, 549–559. 10.1016/j.carbpol.2012.08.068. [DOI] [PubMed] [Google Scholar]
- Riaz S.; Ashraf M.; Hussain T.; Hussain M. T.; Younus A. Fabrication of robust multifaceted textiles by application of functionalized TiO2 nanoparticles. Colloids Surf., A 2019, 581, 123799 10.1016/j.colsurfa.2019.123799. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






