Abstract
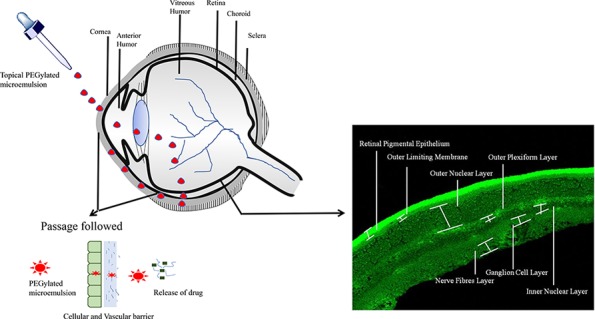
Present work investigates the possibility of a polyethyleneglycolylated (PEGylated) microemulsion (ME) to deliver drug to the posterior segment of eye. Triamcinolone acetonide (TA), a widely used drug in intraocular diseases, was selected as the model drug. Based on solubility and emulsification capacity, components of microemulsion were selected and optimum formulation was obtained using a pseudoternary phase diagram. The optimized ratio of Capmul MCM C8 (oil): AccononMC8-2 (surfactant): Transcutol (cosurfactant): deionized water was 5:35.5:4.5:55. This was further PEGylated using 1,2-distearoylphosphatylethanolamine-polyethyleneglycol 2000 (DSPE-PEG 2000). This PEGylated ME loaded with TA was characterized and evaluated in vitro, ex vivo, and in vivo for topical ocular use. The developed PEGylated ME loaded with TA was homogenous, stable, and nonirritable to eye and had the ability to reach the posterior segment of eye on topical instillation.
Introduction
The posterior segment of eye comprises of vitreous humor, retina, choroid, and sclera. Certain internal (ageing, hormonal imbalance, alteration in homeostasis, etc.) and external (injury, infection, etc.) factors harm this part of eye and manifest in conditions or diseases which are not only chronic but impede the quality of life by impairing vision.1,2 The diseases range from diabetic macular edema, glaucoma, age-related macular degeneration, diabetic retinopathy, choroidal neovascularization, to endophthalmitis, retinitis, and other infectious diseases.3,4 As the exact aetiology of these diseases is not certain, treatment involves only symptomatic relief in the form of antivascular endothelial growth factor and anti-inflammatory drugs/biological molecules.5−7 One amongst such medicaments is triamcinolone acetonide (TA) which is frequently utilized as intraocular injection and implant.8−10 Some marketed formulations are KENALOG-40,4,11,12 Triesence,7,13,14 Transton,15 Trivaris (TA injectable suspension 80 mg/mL),16 I-vation,14,17 and so forth. These formulations are injected intraocular which is local therapy but invasive in nature. These are quite effective in delaying the progression of diseases but considering chronic nature of these diseases; frequent administration certainly harm the eye and cause more severe conditions than earlier ones. Route of administration being used can result into hazardous impact on ocular health rather than drug being used on long term.18−21 Better alternative for such situation is topical ocular drug delivery of TA which is not only noninvasive and patient compliant but also exhibit minimal side effects on chronic use. It is also better than systemic and oral routes as these routes unnecessarily expose other organs with dose.22,23 Thus, the present work involves the development of topical ocular formulation which can deliver TA to posterior segment without causing side effects. It is well known that simple drug solution as topical eye drops are not able to reach posterior segment of eye in significant amount. To overcome this, topical polyethyleneglycolated (PEGylated) microemulsion (ME) was devised. ME being in nano-range and having cell membrane-like architecture allows it to cross membranous barriers of eye-like cornea, conjunctiva, sclera and so forth,24 while PEGylation on its interface helps it to avoid opsonization and ensure its circulation in fluidic barrier of eye-like tear, choroid, and anterior and vitreous humor.25−27 PEGylation on formulation also aids in crossing multiple layer. It was also noted that PEG can remain in vitreous humor for long duration.28,29 Among various PEGylating agents, 1,2-distearoylphosphatylethanolamine-polyethyleneglycol 2000 (DSPE-PEG 2000) was chosen as it possesses long circulatory property in fluid, is biocompatible, has surfactant property, and is required in small amount.30−32 Docetaxel,33 paclitaxel,34 ascorbyl 2,6-dipalmitate,35 trans resveratol,36 piplartine,37 amphotericin B,38 and vincristine39 were amongst some of the drugs which when utilized with DSPE-PEG 2000 or in formulation PEGylated with the same showed enhanced availability at site of action in ex vivo or in vivo pharmacokinetic/dynamic studies in animals/tissues/organs. PEGylation with such phospholipid also helped in diagnostic imaging purposes.40 Along with these, PEGylated phospholipids have shown efficiency in overcoming multidrug resistance.41,42 PEGylated phospholipid-based MEs were prepared and utilized for various purposes. For example, shikonin and docetaxel-loaded parenteral ME using DSPE-PEG 2000 was developed and evaluated for antiglioma therapy. This ME was not only capable to cross blood brain barrier but also have the ability to prolong the therapeutic effect.43 Similarly, indinavir-loaded PEGylated ME using DSPE-PEG 2000 was also reported which showed brain specificity when administered intravenously.44 Using PEGylated ME approach, retinoids were also delivered specifically to cancer cells.45 PEGylated MEs are reported to be significant in crossing membranous barrier (with tight junctions like brain blood barrier43) and being able to remain in fluidic barrier.
Thus, this approach was attempted to deliver drugs to posterior segment of eye via the topical ocular route. DSPE-PEG 2000 PEGylated ME loaded with TA was developed and characterized based on size, homogeneity, and stability. It was further evaluated for ocular irritancy, sterility, and isotonicity. The in vivo pharmacokinetic study was performed on Sprague Dawley rats.
Materials
Capmul MCM C8, Capmul MCM EP, Captex, and Acconon MC8-2 were procured from Abitec Corporation, Mumbai. Tween 80 was purchased from Sigma-Aldrich, and Kolliphor RH40 was a gift sample from BASF, Mumbai. Labrasol and transcutol HP were provided from Gottefosse, Germany as gift samples. Nonoxynol-9 and Octoxynol-10 were procured from Dhiren Chemicals, Vadodara, as a gift sample. TA was obtained as a gift sample from Maharshi Pharma Chem Pvt. Ltd., Ahmedabad, India. DSPE-PEG 2000 was purchased from Lipoid AG, Switzerland. High-performance liquid chromatography (HPLC) grade acetonitrile (ACN) and methanol were purchased from Fisher Scientific. Rest of the chemicals used were of analytical grade and utilized without any further processing.
Methods
Analytical Method Revalidation
The HPLC method was revalidated for TA. For the preparation of stock solution, 1 mg of TA was dissolved in 1 mL of methanol and different concentrations like 0.25, 0.5, 1, 2, 5, 10, and 20 μg/mL were prepared from this stock solution by serial dilution with methanol. Kromasil C-18 column used for HPLC as TA is lipophilic drug. The isocratic HPLC method involved ACN and deionized water (pH 4 adjusted with glacial acetic acid) in 50:50 ratio as mobile phase with a flow rate of 1 mL/min. λmax used was 240 nm and was screened using an ultraviolet (UV)–visible (vis) spectrophotometric method by a Shimadzu UV-1800 UV–vis spectrophotometer.46,47 The method was revalidated on the basis of accuracy and precision. A linear calibration curve was also obtained with the UV–vis spectrophotometric method for drug content analysis.
Compatibility Study
Compatibility between DSPE-PEG 2000 and TA was determined using a differential scanning calorimeter (DSC) by DSC 214 PolymaNetzsch and Fourier transform infrared (FT-IR) by Alpha FT-IR Bruker. For this, individual chemical and 1:1 mix of both prepared by simple physical mixing was subjected to DSC and FT-IR analysis.48
Screening of Vehicles
ME consists of oil, surfactant, cosurfactant, and water. For the production of ocular ME of TA, it is prerequisite that components selected must be nontoxic to eye and can hold large amount of TA. Thus, oil, surfactant, and cosurfactant were screened on the basis of solubility of TA and their emulsification capacity. Oils screened were isopropyl myristate, Capmul MCM C8, Lauroglycol 90, Captex 300 EP, Paceol, Capmul MCM EP, Maisine 35-1,49 and Capryol 90. Labrafil M2125, span 60, Transcutol HP,50,51 Tween 80, Nonoxynol 9, Kolliphor RH 40,52,53 Labrasol,54 and Acconon MC8-2 EP55 were among the surfactants and cosurfactants screened. First, TA in excess was dissolved in fixed amount of vehicle by vortexing, heating, and sonication in a bath sonicator until TA started to precipitate. The mixture was then transferred to an orbital shaker incubator set at 37 °C with 100 rpm for achieving equilibration for 72 h. After 72 h, the supernatant was removed and centrifuged at 10,000 rpm for 30 min. From this centrifuged sample, 100 mg of the supernatant was taken and dissolved with the help of ethyl acetate and further diluted with HPLC grade methanol. These samples were then subjected to revalidated HPLC analysis in methanol. From this, solubility of TA in individual vehicle was determined. From the ones with highest solubility of TA, placebo MEs were prepared and checked for emulsification capacity. The ones which formed a single-phase system were finally selected.
Development and Characterization of ME
To determine the concentration ratio of different components selected from the previous step, a pseudoternary phase diagram was constructed. As most of the ocular formulations are aqueous based owing to improved patient compliance and comfort associated with it, oil in water (o/w) ME was prepared using a water titration preparation method. For this, surfactant and cosurfactant in four levels of different ratios were mixed together, namely, 1:1, 2:1, 4:1, and 8:1 (Smix). Further, oil and Smix were mixed in different ratios ranging from 1:9 to 9:1 and then titrated against deionized water until system became turbid. The obtained weight ratios of these components were plotted in freely available software; Triplot software (product by Todd Thompson) and 4 pseudoternary phase diagrams were obtained. The phase diagram with highest ME area was chosen for ME preparation.
For estimation of the concentration of DSPE-PEG 2000 needed to be used in ME, three levels (0.2, 0.5, and 1% of total volume of ME) were selected based on the available literature. Considering the water miscibility of DSPE-PEG 2000, it was mixed in deionized water which was then used to titrate against optimized mixture of oil, surfactant, and cosurfactant. The concentration of DSPE-PEG 2000 at which prepared ME resulted into the smallest size, homogeneity, and greater stability was selected as optimum for the development of ME.
The optimized PEGylated ME (PTA) and non-PEGylated ME (NTA) were then characterized for various physicochemical parameters which included size, homogeneity, and zeta potential determination by Zetasizer (Malvern Zetasizer Nano ZS), morphology by transmission electron microscopy (TEM, Tecnai 20, Philips), % transmittance by UV–vis spectrophotometry at 650 nm (with deionized water as reference), and drug content analysis by the UV–vis spectrophotometric method.
Stability was confirmed by the centrifugation test, freeze–thaw cycle, and storing formulation at 4 °C for 3 months. In the centrifugation test, the formulations were centrifuged at 30,000 rpm for 30 min at room temperature and observed for any precipitation and phase separation. Freeze–thaw cycle was performed by subjecting formulations to 4 °C for 48 h and then room temperature for 48 h. The cycle was repeated thrice and evaluated for any phase separation. Samples were periodically withdrawn at definite time intervals (15, 30, 60, and 90 days) and were checked for alteration in size, zeta potential, PDI, pH, % transmittance, and drug content.
In Vitro TA Release Study
Both the formulations (PTA and NTA) along with TA solution were evaluated for TA release in physiological conditions. For this, 1 mL of formulation and TA solution having concentration of 1 mg/mL was sealed in dialysis membrane (12,000 Da). These were then immersed inside vials filled with 15 mL phosphate buffer saline pH 7.4 (PBS) and maintained at temperature 37 °C. This assembly was kept inside an orbital shaker incubator preconditioned with 37 °C temperature and 100 rpm mimicking ocular conditions. Approximately, 500 μL of samples from each assembly was removed and replenished with fresh PBS at definite time intervals of 0.5, 1, 2, 4, 6, 8, and 10 h. These samples were appropriately diluted with PBS and analyzed using a revalidated HPLC analytical method.
Sterility, Isotonicity, and Ex Vivo Ocular Irritation Study
Topical ocular formulations are expected to possess sterility, isotonicity, and nonirritancy to be acceptable for instillation on ocular surface. As developed MEs were intended for topical ocular use, these must be screened for the same. For sterility testing, luria agar broth was prepared inside biosafety cabinet class II and poured in Petri dishes. These Petri dishes were then allowed to settle for some time inside the same sterile environment of biosafety cabinet class II. Further, 1 mL of developed MEs, 0.9% weight/volume (w/v) NaCl solution (as negative control), and bacterial culture (as positive control) were spread over the surface of settled luria agar broth which were then covered and sealed with a paraffin film. These were then incubated inside an incubator at 37 °C and photographed at different time points. The absence of any contamination and/or microbial growth would confirm the sterility of MEs.
Lachrymal fluid and blood have similar osmolarity. As red blood cells (RBCs) can maintain their morphology in flowing blood and lachrymal fluid, topical ocular formulations should be able to elicit the same effect. Thus, RBCs were utilized for the isotonicity test. For this, equal volumes of RBCs and MEs, isotonic solution (0.9% w/v NaCl solution), hypotonic (0.45% w/v NaCl) solution, and hypertonic (1.5% w/v NaCl) solution were incubated individually. Then, samples were spread over glass slides which were observed for morphological changes in RBCs under an optical microscope (Zeiss Axio Imager M2m). Alteration in the morphology of RBCs would indicate the nonisotonicity of MEs with ocular fluid.
On topical ocular instillation of the dosage form, they first encounter cornea (has tightly packed cells) and vascular conjunctiva. These must be nonirritant to both. For this, cornea hydration test, hen’s egg test chorioallantoin membrane (HET-CAM), and hematoxylin and eosin (H and E) staining were employed. In cornea hydration test, excised goat corneas were procured from local slaughter house and weighed. Then, these were placed in between an upper donor and bottom receptor chamber of Franz diffusion assembly. Lower chamber was filled with PBS while 1 mL of developed MEs was poured in an upper chamber. Saline solution was taken as control. After 1 h, the cornea were removed and weighed again. Weight variation was evaluated. Significant deviation in weights of corneas would be indication for possible edema instilled dosage form can cause on topical ocular use.
A similar assembly was set up for H and E staining. Goat corneas were placed in Franz diffusion assembly and 1 mL of developed MEs was poured in a donor chamber. As a positive control, 0.9% w/v NaCl solution was taken while for negative control, 1 normal (N) NaOH solution was chosen. These assemblies were maintained at 37 °C with 35 rpm inside an orbital shaker incubator for 1 h. After this, corneas were removed from assemblies, washed with PBS, and immersed in 4% para-formaldehyde at 4 °C for 24 h followed by subsequent infiltration with 5 and 10% w/v sucrose solution. Finally, these were transferred into 30% w/v sucrose solution for overnight. Then, these were then casted into moulds using OCT (optimum cutting temperature solution) and subjected to sectioning in 20 μm thick sections using Cryostat (CryoStar NX70 Cryostat, Thermo Scientific). These sections were then dipped into xylene for some time to remove extra OCT and subjected to H and E staining protocol. The obtained slides were observed under an optical microscope for alteration in morphology of cornea.
The HET-CAM test was aimed to elucidate the impact of developed MEs on the vascular structure of ocular surface on administration. For this, fertilized hen’s eggs were obtained from a local poultry farm. After swabbing with 70% isopropyl alcohol, these were placed in an incubator preset at 37 °C. For complete circumferential formation of membrane, eggs were rotated at every 12 h.
Candling was also performed frequently to remove decayed eggs. On third day, eggs were broken at their tapered ends from where about 1 mL volume of albumin was sucked. These pointed ends were covered with a paraffin film and eggs were again placed inside an incubator. On day 5, after candling, 1 mL of developed MEs, 1 N NaOH (positive control) and 0.9% w/v NaCl solution (negative control) were introduced in respective eggs and photographed at definite time intervals to observe any changes in vasculature. Formulation which would elicit negligible alteration in vasculature will be referred as a nonirritant for topical ocular administration.
In Vitro Cell Line Studies
In vitro cell line studies were carried out here for two purposes; first being to evaluate the cytotoxicity of developed formulation and second to check ability of these to affect tight junctions prevalent in ocular epithelial cells. A major membranous barrier for passive diffusion of drug to the posterior eye through a topical route is the corneal epithelium and retinal pigmental epithelium (RPE) because both possess tight junctions between cells marring paracellular transport. To mimic these in in vitro cell line studies, two cell lines SIRC (Statens Seruminstitut rabbit cornea) and ARPE-19 (adult RPE cell) were employed. Both were purchased from American type culture collection (ATCC, USA). ARPE-19 cell line was grown in DMEM-F12 (Dulbecco’s modified Eagle medium/nutrient mixture F-12) while SIRC cell line was nourished in MEM with NEAA (minimum essential medium with non-essential amino acids). Penicillin G/streptomycin and 10% fetal bovine serum were added in both media prior to use in in vitro cell line studies. Cells were grown on a 25T flask by reviving cryovials containing 1 × 106 cells each with respective media in an incubator maintained at 37 °C and 5% CO2 supply. On attainment of confluence in flasks, cells were detached using trypsin ethylenediaminetetraacetic acid after removing media. The detached cells were then diluted with media and centrifuged at 200g (relative centrifugal force) for 7 min. The precipitate of cells obtained was then redispersed with media and cells were counted using trypan blue and a neubauer chamber. Cells in appropriate number were seeded in well plates and allowed to adhere at bottom. Further, they were treated with developed formulations followed by cell assays. Two kind of cell assays were followed here: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and transepithelium electrical resistance (TEER) value determination assay. SIRC cell line utilized here belonged to passage number 20–25 and for ARPE-19, it was 5–10.
MTT Assay
MTT assay is one of the most common assays to be utilized for evaluating cell viability. Here, it was used to check that cells can survive in the environment of developed MEs with comparison of TA solution. For this, 10,000 cells were plated and allowed to get attached at bottom of 96-well plates for 24 h in an incubator maintained at 37 °C and 5% CO2. Post 24 h, cells were treated with predefined concentrations of NTA, PTA, and TA solution and then again subjected to an incubator for 1 h. After 1 h, wells were introduced with 20 μL of MTT solution in the concentration of 5 mg/mL under dark conditions and again incubated for 4 h. Post this duration, wells were emptied by removing contents using a micropipette, and 100 μL of dimethylsulfoxide was instilled in each well. This was then subjected to analysis at 575 nm by a UV plate reader (Multiskan FC microplate photometer, Thermo Scientific). Wells without any cells filled with only media were considered blank, whereas cells without any treatment were taken as control.
TEER Value Determination
An epithelial cellular layer of corneal origin has the property of forming tight junctions in between them which makes them unique. The presence of tight junctions creates electrical resistance in the cell layer. Alteration in this would give information about intactness of these tight junctions. Cells (20,000) were plated in each transwell insert and kept inside 12 well plates. Media (500 μL) was filled in inserts and 600 μL media in each well. Blanks were also maintained in which there were no cells. The TEER value was recorded on every alternate day. Media was also changed frequently. It was pursued until a stable TEER value was obtained. On achieving a stable TEER value, PTA, NTA, and TA solution in 10 μM concentrations were introduced in inserts and at predefined time intervals; 0.5, 1, 2, and 3 h, TEER values were recorded. Same was also noted for both control group and blank.
In Vivo Pharmacokinetic Study
For the assessment of the capability of non-PEGylated and PEGylated MEs to outreach rat retina via topical ocular administration, an in vivo pharmacokinetic study was performed on Sprague Dawley rats. For this study, TA was replaced with fluorescent dye; coumarin 6 in MEs and simple solution. Male sprague dawley rats with weights in the range of 220–240 g were obtained from animal facility of Zydus Research Centre, Ahmedabad, and accommodated in animal house of NIPER, Ahmedabad. These rats were maintained in a controlled environment of 60 ± 5% relative humidity and 25 ± 3 °C room temperature and given ad libitum access to food as well as water. The protocol for in vivo experiments on rats was approved by Institutional Animal Ethics Committee (IAEC) with registration number NIPER A/IAEC/2018/008 [under The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Delhi, India]. To acclimatize the rats for topical ocular administration, saline solution in the volume of 5 μL was instilled in both eyes for 7 days. For this study, there were three groups, namely, C6 solution, C6 loaded non-PEGylated ME (CNP), and C6-loaded PEGylated ME (CP). Further segregation was done on the basis of time intervals (0.5, 1, 2, 4, and 6 h). After acclimatization for 1 week, approximately 5 μL of C6, CP, and CNP was topically instilled in right rat eye of the respective group while equal volume of saline solution was administered in contralateral (left) rat eye (as control rat eye). Dark condition was maintained throughout the experiment to limit quenching of C6. As per the subdivisions, rats were sacrificed at predefined time points according to protocol (anaesthesia using isoflurane followed by cervical dislocation). Their eyes were removed using scissors followed by washing with PBS and immersion in 4% w/v para-formaldehyde at 4 °C for 24 h. Surrounding fats, cornea and lens were separated from eyes. These eye cups were then kept in a biopsy cassette and subsequently infiltrated in different solvents for a certain time. First, they were immersed in acetone for 4 h followed by in xylene for 3 h and then in paraffin wax maintained at 55 °C for 4 h. At the end, paraffin blocks were prepared by casting these eye cups in moulds. From these paraffin blocks, thin sections of 5 μm on glass slides were obtained using microtome (Leica RM 2125 RT). These glass slides were then warmed for fraction of time to fix tissue on it. Extra paraffin was removed by dipping these slides into xylene for 2 min. The slides were then observed under a confocal laser scanning microscope (CLSM, Leica TCS SP8) at 458 nm laser. The images were analyzed using ImageJ software.
Statistical Analysis
Statistical analysis of all outcomes of experiments was achieved using Graph Pad Prism version 6.01 (Graph Pad Software Inc., San Diego, CA). All data were reported as mean ± standard deviation (SD) (n = 3). A student t-test was used for testing the difference between two groups and one-way analysis of variance (ANOVA) was for comparing more than two groups. The P value of P < 0.05 was considered as the level of significance.
Results and Discussion
Analytical Method Revalidation
Linear correlated calibration curves were obtained using both UV–vis spectrophotometer and HPLC with correlation coefficient 0.999. λmax obtained was 240 nm. The HPLC method was revalidated and found to be accurate and precise for use. For the determination of the drug content, the UV–vis method was utilized, whereas the HPLC method was used for solubility determination and in vitro TA release pattern determination (SD 1).
Compatibility Study
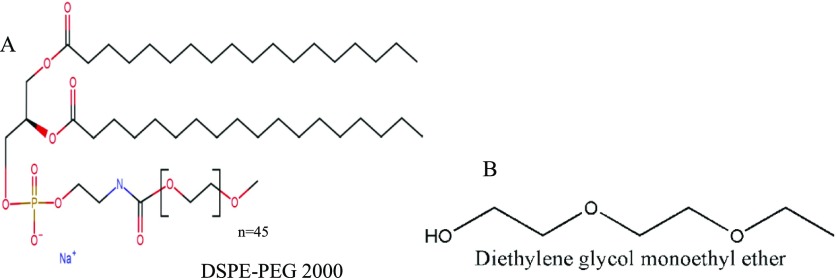
The chemical structure of DSPE-PEG 2000 is shown in Figure 1A. Both TA and DSPE-PEG 2000 were found to be compatible with each other (as evident from SD 2). DSC and FT-IR data did not show any kind of overlapping of peaks (endothermic peak of melting in DSC and functional groups peaks in FT-IR). Although a shift in the endothermic peak of melting of TA was observed, it may be attributed to mixing of lipophilic drug into lipophilic portion of PEGylated phospholipid.56−59 As there was no overlapping in FT-IR data, it confirmed that there was no chemical change on using them together. Thus, both can be utilized in same formulation (SD 2).
Figure 1.
Structure of (A) DSPE-PEG 2000 and (B) transcutol HP (diethylene glycol monoethyl ether).
Screening of Vehicles
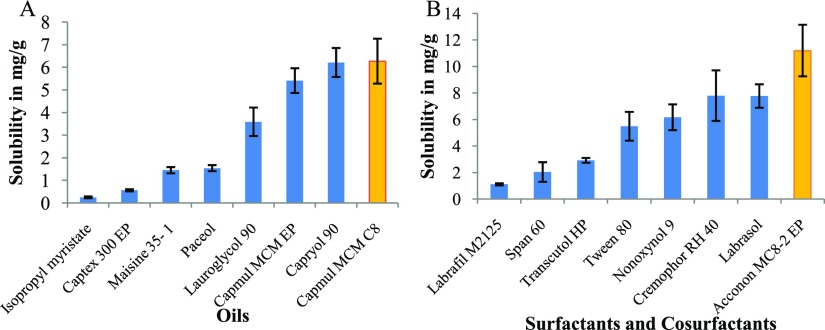
Oil, surfactant, and cosurfactant were screened based on solubility of TA and their emulsification capacity. Figure 2 shows the solubility data. It revealed approximately similar solubility of TA in two oils: Capryol 90 and Capmul MCM C8. Among surfactants, maximum solubility was achieved in Acconon MC8-2 EP and similarly, highest solubility was obtained in transcutol HP (chemical structure shown in Figure 1B) among cosurfactants. These two oils were further screened for emulsification capacity by preparing dummy MEs. Capmul MCM C8 stood out for having better emulsification capacity. Thus, it was selected as oil for ME preparation. Acconon MC8-2 EP and transcutol HP were selected as the surfactant and cosurfactant, respectively. All three have been previously utilized for topical ocular formulations55,60 and even transcutol HP was reported to possess permeation enhancing property.51
Figure 2.
Solubility of TA in (A) oils and (B) surfactants and cosurfactants. Values are in mean ± SD (n = 3).
Development and Characterization of ME
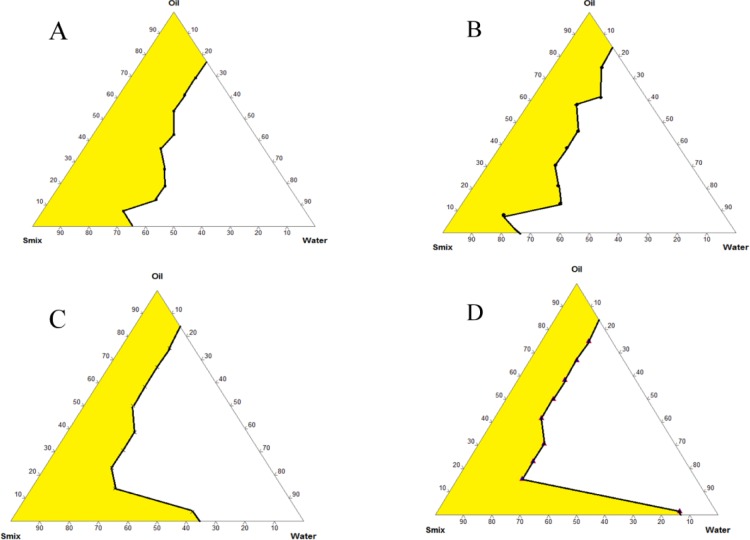
Pseudoternary phase diagrams developed for determining relative weight ratio of all of the constituents of ME are shown in Figure 3 which clearly indicated that the Smix ratio of 8:1 gave the highest ME region. Thus, this pseudoternary phase diagram was utilized for obtaining optimum formulation. Different weight % points from this ME region was selected, and placebo MEs were prepared. These were observed for size, PDI, and stability for 7 days (SD 3). It was found that 5% w/w Capmul MCM C8, 40% w/w Smix [Acconon MC8-2 EP (8): Transcutol HP (1)], and 55% w/w deionized water gave reproducible and stable ME. Thus, this ratio was selected as the final composition.
Figure 3.
Pseudoternary phase diagram using Capmul MCM C8 as oil and mixture of Acconon MC8-2 EP and transcutol HP (Smix) in different ratios; (A) 1:1, (B) 2:1, (C) 4:1, and (D) 8:1.
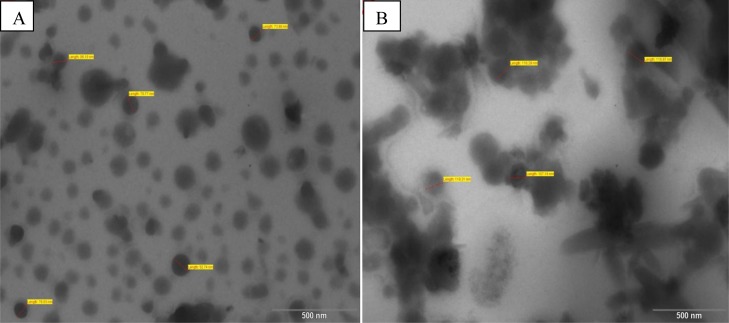
For the determination of amount of DSPE-PEG 2000 optimum for use in ME, three levels were screened amongst which 0.2% was selected (10 mg in 5 mL of ME) as smallest size, good homogeneity and transparency of ME was achieved with this. The average size obtained was 157.720 ± 17.85 nm for NTA and 131.57 ± 0.76 nm for PTA (SD 4). This was reconfirmed by TEM (Figure 4). The size of PTA was smaller than NTA which was most likely as PEG units of PEGylated phospholipid enhanced the curvature at the interface of the ME droplet. On account of the amphiphilic nature of DSPE-PEG 2000, it got accommodated at the interface of the droplet. Its lipidic phospholipid portion embedded in the lipophilic part (discontinuous phase) of ME droplet, whereas the PEG portion spanned toward the continuous aqueous phase. These PEG units have the tendency to form a hydrogel-like layer with an adjacent PEG unit which helped to constrict the curvature of droplet thus the decreased size also.25,30,35 Hydrogel-like consistency at the interface of ME droplet also decreased PDI and gave stability to ME. The pH observed was 5.6 ± 0.54 which is suitable for topical ocular use. The drug contents observed were 99.32 ± 3.214% and for NTA and 99.57 ± 2.141% PTA, respectively. % Transmittance recorded were 95.21 ± 2.364 and 98.12 ± 1.014%, respectively. Smaller size was also the reason of higher % transmittance of PTA.
Figure 4.
TEM images of (A) NTA and (B) PTA.
MEs are thermodynamically stable systems with the ability to maintain physicochemical integrity for longer duration. It was also apparent from the other stability tests. The centrifugation test and freeze–thaw cycle did not manifest into phase separation or any form of precipitation. Dilution with dispersion media till 1000 times did not significantly alter the ME size. Stability study at 4 °C till 3 months also did not present significant alteration in size and PDI confirming the stability of NTA and PTA as evident from Table 1.
Table 1. Stability Data of PTA over the Period of 3 Months at 4 °Ca.
| parameters | day of preparation | post 15 days | post 30 days | post 60 days | post 90 days |
|---|---|---|---|---|---|
| size (nm) | 131.57 ± 0.760 | 154.79 ± 8.902 | 189.46 ± 14.738 | 174.89 ± 1.583 | 204.65 ± 2.028 |
| PDI | 0.208 ± 0.013 | 0.238 ± 0.047 | 0.296 ± 0.043 | 0.121 ± 0.012 | 0.098 ± 0.031 |
| zeta potential (mV) | 0.076 ± 0.016 | 0.091 ± 0.023 | 0.069 ± 0.199 | 0.156 ± 0.146 | –0.105 ± 0.329 |
Values were in mean ± SD, n = 4.
In Vitro TA Release Study
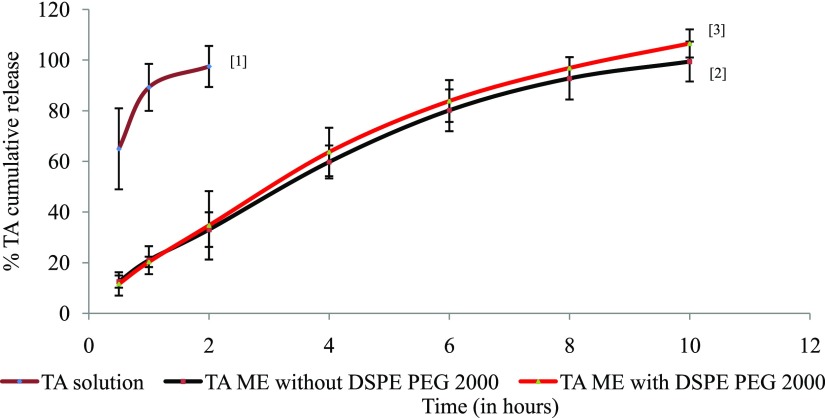
In vitro TA release study using the dialysis membrane revealed that plain TA solution immediately started releasing TA and in first 2 h, all was released in dispersion media of PBS, whereas other two NTA and PTA did not immediately release the entrapped TA but released it in slow and steady manner (Figure 5). Both NTA and PTA released almost 12% in first 0.5 h which progressively stretched to approximately 60% in 4 h. It took 10 h for complete TA release from both NTA and PTA. Release patterns of NTA and PTA were almost linear and similar which indicated that the presence of PEG on the interface did not affect the release pattern although it decreased size of ME. The linear pattern indicates zero-order kinetics which would help in maintaining constant drug concentration at site.
Figure 5.
In vitro TA release pattern from TA solution[1], NTA[2], and PTA[3]. Values were in mean ± % RSD (n = 3).
Sterility, Isotonicity, and Ex Vivo Ocular Irritation Studies
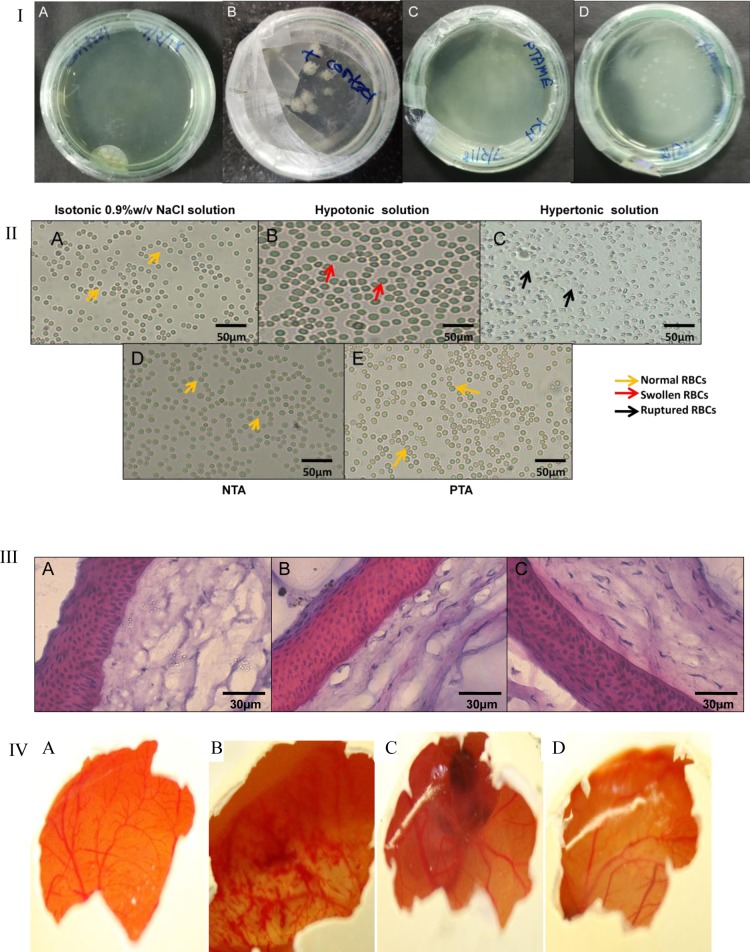
The outcomes of sterility and isotonicity test were illustrated in Figure 6. No contamination or microbial growth was found in any Petri dish even after 1 week. This study clearly indicated that aseptically prepared MEs do not support growth of microbes. Thus, these were suitable for use via a topical ocular route (Figure 6I). Results of the isotonicity test are presented in Figure 6II(A–E). RBCs swell in hypotonic solution and get ruptured/constricted in hypertonic solution; meanwhile, in isotonic solution, the architecture of RBCs was maintained. Blood and tear fluid almost have similar osmolarity; thus, RBCs were utilized for the isotonicity test. NTA and PTA both did not distort the RBCs. This confirmed the isotonicity with ocular fluid. Cornea hydration test and H and E staining both showed nonirritancy of NTA and PTA. The nucleus was stained by hematoxylin stain, whereas cytoplasm was stained by eosin stain. As evident from Figure 6III, nuclei of cornea incubated with NTA and PTA were undamaged assuring no hazardous effect with them. No significant disparity in weights of corneas was observed pre- and post-incubation with MEs erasing the possibility of edema. H and E staining and the cornea hydration test both are indicators for irritancy to ocular membranes. For illustration of the irritant impact on vascular structures of conjunctiva and other parts on dosage instillation on ocular surface, the HET-CAM test played a significant role. Outcomes of HET-CAM are showed in Figure 6IV which reconfirmed the nonirritancy of NTA and PTA.
Figure 6.
(I) Sterility test; images of culture plates in incubation with (A) saline solution, (B) positive control, (C) PTA and (D) NTA. (II) Isotonicity test with RBCs treated with (A) saline solution, (B) hypotonic solution, (C) hypertonic solution, (D) NTA, and (E) PTA observed under a microscope, (III) H and E staining on corneal sections treated with (A) saline solution, (B) NTA and (C) PTA; observed under a microscope. (IV) Images after 3 h of HET-CAM test on hen’s eggs treated with (A) saline solution, (B) NaOH solution, (C) NTA, and (D) PTA.
In Vitro Cell Line Studies
MTT Assay
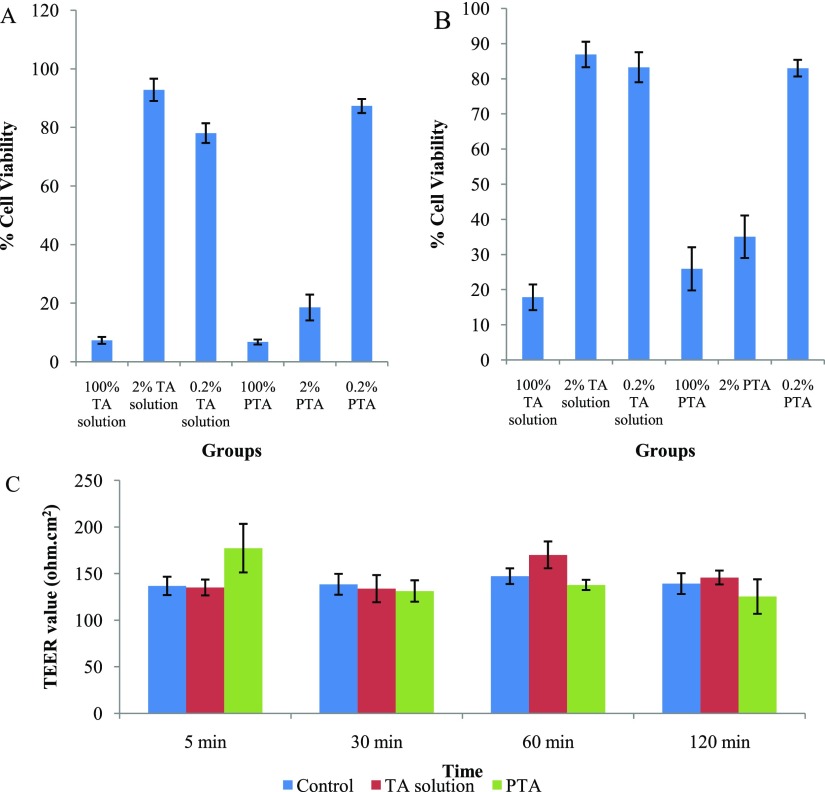
Figure 7A,B shows that simple TA solution and PTA were cytotoxic, but in diluted form (2 and 0.2%), they were less toxic on SIRC and ARPE-19 cells. PTA (0.2%) was found showing nontoxicity on cell lines, whereas below 40% cell viability was observed with 2% PTA. Although the data were indicating nontoxicity only at higher dilution, some points are important to be considered that topical ocular formulations get diluted on instillation by tear and not whole dose would enter the eye at once. Second, the number of cells coming in contact with dose in in vitro studies was 10,000 while in case of in vivo situation; it would extend up to number of millions of cells. Apart from this, it should also be noted that SIRC cells were more sensitive to PTA as compared ARPE-19 cells. As we know from existing research, topical ocular formulation can take any of two routes to have access to retina: corneal and noncorneal route. Later being more preferable route for retinal drug delivery systems, less cytotoxic impact on ARPE-19 cell line would be considered positive for this purpose.61
Figure 7.
MTT assay with PTA at different dilutions on (A) SIRC and (B) ARPE-19 cell lines with comparison to TA solution. (C) TEER value determination of SIRC cell line on instillation of TA solution, and PTA at different time points (5 min, 0.5, 1 and 2 h) with comparison to control group (cells without any treatment). Values were in mean ± SD, n = 3.
TEER Value Determination
Figure 7C displays the results from the experiment where SIRC cell line was made to come in contact with diluted form (0.2%) of developed formulation and impact on their tight junctions were noted as changes in the TEER value. Although a decline in the TEER value with time was observed on comparison with the control group, it was not in appreciable magnitude, indicating that the major mechanism of permeation through epithelium cells was not breakage of tight junction. Higher TEER value indicates integrity of tight junctions amongst epithelial cells of SIRC monolayer. Before experimentation, cells were allowed to form tight junctions in between them, leading to monolayer construction. After this only, the TEER value became constant. On treatment with samples, if the TEER value decreases, it suggested distortion of tight junctions and passage of drug or delivery system through it. A comparable TEER outcome of TA solution and diluted PTA nullified the probable negative impact. Major mechanism of drug passage through cornea would not be breakage of tight junctions. It might be passive diffusion of whole droplet of ME.
In Vivo Pharmacokinetic Study
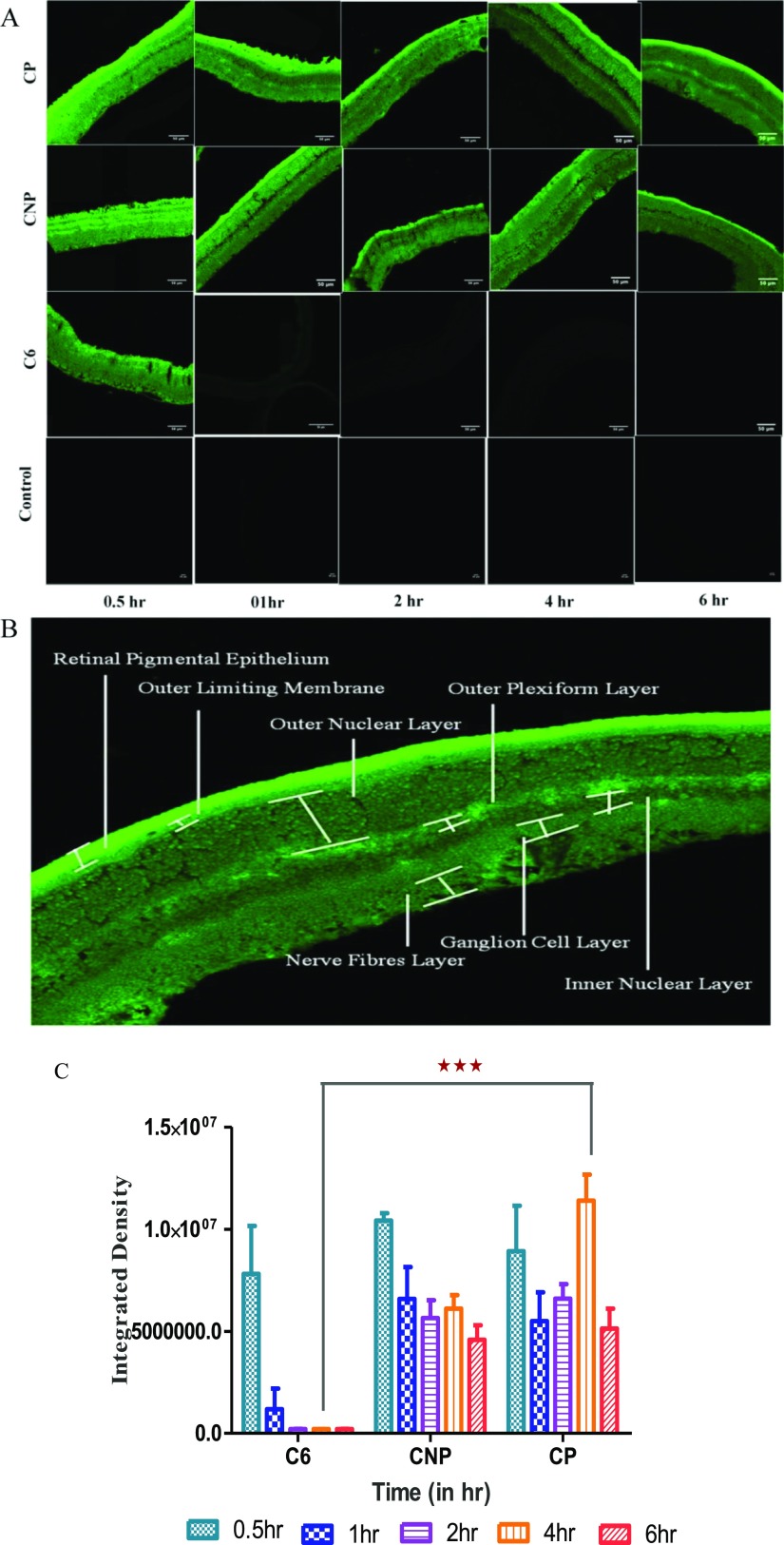
The purpose of in vivo pharmacokinetic study on Sprague Dawley rats was to know whether or not the developed PEGylated ME can have access to retina via topical ocular route as compared to solution and non-PEGylated ME. Figure 8A shows the representative images of the retina of the left eye of rats treated with respective solution, CNP and CP at different time intervals, and Figure 8B shows the enlarged image of retina of right eye of rat treated with PEGylated ME (CP). No fluorescence was observed in contralateral eye suggesting that instilled dose of samples did not move to left eye via a systemic pathway in all cases even with C6 solution. Figure 8B displays fluorescence in all the retinal layers. From Figure 8A, it was found that C6 solution reached the eye within 0.5 h but subsequently depleted with time owing to eliminatory pathways present in intraocular tissue but in case of CNP and CP, fluorescence was observed in retina even after 6 h. This suggested that MEs are obviously more capable to reach and stay at the desired site. They crossed not only the membranous barrier (corneal epithelium, RPE) but also the fluidic barrier (tear film, aqueous humor, choroid, and vitreous humor). The presence of fluorescence in retina for such a long time establishes the utility of ME in the topical ocular drug delivery system for the posterior segment of eye. When the observed fluorescence was compared using ImageJ software, the resultant was Figure 8C. It showed the comparison as a graph between time and integrated density. It was very clear and can be synchronized with Figure 8A. For C6 solution, the level of fluorescence was higher in first 0.5 h, and then, it decreased with time, being almost negligible. In case of CNP, fluorescence was present and higher than that in the C6 solution. However, the descending trend in fluorescence was observed post 0.5 h which subsequently became quite constant. It clearly states that non-PEGylated ME could arrive at retina, but they also fall prey of eliminatory pathways of eye. It can be assumed that the rate of access to retina and elimination was almost similar, leading to constant fluorescence after 0.5 h. The major passage pathway is passive diffusion. In case of CP, fluorescence was present for up to 6 h. An ascending trend in fluorescence followed till 4 h, and then, it descended. As the increase in fluorescence was gradual till 4 h, it can be estimated that CP was present in the vicinity to retina but did not reach directly to the retina. It stayed in intraocular tissue probably retained in the fluidic barrier of posterior segment and as time passes, the one which already reached to retina, gets eliminated from retina by obvious pathways, and the one present in fluidic barrier moves toward the retina owing the concentration gradient. As it was a single-dose administration study, the dose decreased after 4 h, and lower fluorescence was observed at 6th hour. CP was capable to be in the fluidic barrier for longer time. It retained there and slowly approaches to retina on account of passive diffusion. Thus, PEGylation also worked in case of ME to enhance the circulation time of ocular formulation and was beneficial for drug delivery to posterior segment of eye.
Figure 8.
In vivo pharmacokinetic study on Sprague Dawley rats. (A) Images (scale bar 50 μm) of retina of rat eye by a CLSM after definite time duration of topical instillation of 5 μL of C6 solution, CNP, CP as compared to control eye (untreated contralateral eye) (in images of C6, CNP, and CP groups, zoom factor 3 was applied to make retinal layers more distinguishable while same was not applied in control group) (B) representative image of retina observed under a CLSM showing different layers and (C) graph showing comparison of raw integration density observed after instillation of different formulation (CNP and CP) and C6 solution at definite time points. Values were obtained using ImageJ software and were in mean ± SD, n = 3. ***Indicates the significant difference in fluorescence observed at 4 h of instillation of topical dosage of CP as compared to other groups (P < 0.001).
Conclusions
It was a known and accepted fact that PEGylation always enhances the retention/residence time of ligand/molecule/nanoformulation to which it is attached, in fluidic medium whether oral or parenteral route were considered. Drug delivery to retina is hamstrung by two kinds of barriers: membranous and fluidic. The ME system was well reported to overcome the membranous barrier. To surpass the fluidic barrier, these MEs were PEGylated using PEGylated phospholipid. This adjuvant is biodegradable and nontoxic and can attach to interface of ME leaving its PEG chain in aqueous dispersion phase of ME for eliciting its longer circulatory effect in fluids. Developed PEGylated ME in present investigation was capable enough to maintain circulation of loaded dye up to 6 h and that too, in higher amount as compared to plain dye solution and non-PEGylated ME. PEGylation was also proven true for its utility in topical ocular ME for retinal drug delivery.
Acknowledgments
The corresponding author would like to acknowledge the Department of Science and Technology and SERB (INSPIRE grant no: IFA-LSBM-13 and EMR/2016/007966/HS) for project funds. All other authors are grateful to the Ministry of Chemicals and Fertilizers, Government of India for providing fellowship to conduct research at NIPER Ahmedabad.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04244.
Analytical data; compatibility study between TA and DSPE-PEG 2000 by DSC and FT-IR; formulation optimization; hydrodynamic size and zeta potential of PTA and NTA; and in vivo pharmacokinetic study (PDF)
Author Present Address
† Scientist B, B V Patel PERD centre, Off SG Highway, Ahmedabad—380054.
The authors declare no competing financial interest.
Supplementary Material
References
- Nayak K.; Misra M. A Review on Recent Drug Delivery Systems for Posterior Segment of Eye. Biomed. Pharmacother. 2018, 107, 1564–1582. 10.1016/j.biopha.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Nayak K.; Misra M. Cow ghee fortified ocular topical microemulsion; in vitro, ex vivo, and in vivo evaluation. J. Microencapsulation 2019, 36, 603–621. 10.1080/02652048.2019.1662121. [DOI] [PubMed] [Google Scholar]
- Varma R.; Bressler N. M.; Doan Q. V.; Gleeson M.; Danese M.; Bower J. K.; Selvin E.; Dolan C.; Fine J.; Colman S.; et al. Prevalence of and Risk Factors for Diabetic Macular Edema in the United States. JAMA Ophthalmol. 2014, 132, 1334–1340. 10.1001/jamaophthalmol.2014.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangueiro J. F.; Silva A. M.; Garcia M. L.; Souto E. B. Current Nanotechnology Approaches for the Treatment and Management of Diabetic Retinopathy. Eur. J. Pharm. Biopharm. 2015, 95, 307–322. 10.1016/j.ejpb.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Velpandian T.Pharmacology of Ocular Therapeutics; Velpandian T., Ed.; Springer International Publishing, 2016. [Google Scholar]
- Yasukawa T.; Ogura Y.; Tabata Y.; Kimura H.; Wiedemann P.; Honda Y. Drug Delivery Systems for Vitreoretinal Diseases. Prog. Retinal Eye Res. 2004, 23, 253–281. 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Rivers H. M.; Ray Chaudhuri S.; Shah J. C.; Mittal S. A New Vision for the Eye: Unmet Ocular Drug Delivery Needs. Pharm. Res. 2015, 32, 2814–2823. 10.1007/s11095-015-1717-z. [DOI] [PubMed] [Google Scholar]
- Kuno N.; Fujii S. Biodegradable Intraocular Therapies for Retinal Disorders. Drugs Aging 2010, 27, 117–134. 10.2165/11530970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kuno B. N.; Fujii S. Ocular Drug Delivery Systems for the Posterior Segment: A Review. Retin. Today 2012, 5, 54–59. [Google Scholar]
- del Amo E. M.; Rimpelä A.-K.; Heikkinen E.; Kari O. K.; Ramsay E.; Lajunen T.; Schmitt M.; Pelkonen L.; Bhattacharya M.; Richardson D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retinal Eye Res. 2017, 57, 134–185. 10.1016/j.preteyeres.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Kompella U. B.; Edelhauser H. F.. Drug Product Development for the Back of the Eye; Kompella U. B., Edelhauser H. F., Eds.; Springer International Publishing, 2011. [Google Scholar]
- Hazirolan D.; Pleyer U. Think Global – Act Local: Intravitreal Drug Delivery Systems in Chronic Noninfectious Uveitis. Ophthalmic Res. 2013, 49, 59–65. 10.1159/000345477. [DOI] [PubMed] [Google Scholar]
- Agrahari V.; Mandal A.; Agrahari V.; Trinh H. M.; Joseph M.; Ray A.; Hadji H.; Mitra R.; Pal D.; Mitra A. K. A Comprehensive Insight on Ocular Pharmacokinetics. Drug Delivery Transl. Res. 2016, 6, 735–754. 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Mieler J. J.; Dosmar E.; Liu W.; Mieler W. F. Extended Ocular Drug Delivery Systems for the Anterior and Posterior Segments: Biomaterial Options and Applications. Expert Opin. Drug Delivery 2017, 14, 611–620. 10.1080/17425247.2016.1227785. [DOI] [PubMed] [Google Scholar]
- Li X.; Wang Y.; Yang C.; Shi S.; Jin L.; Luo Z.; Yu J.; Zhang Z.; Yang Z.; Chen H. Supramolecular Nanofibers of Triamcinolone Acetonide for Uveitis Therapy. Nanoscale 2014, 6, 14488–14494. 10.1039/c4nr04761c. [DOI] [PubMed] [Google Scholar]
- Shen H.-H.; Chan E. C.; Lee J. H.; Bee Y.-S.; Lin T.-W.; Dusting G. J.; Liu G.-S. Nanocarriers for treatment of ocular neovascularization in the back of the eye: new vehicles for ophthalmic drug delivery. Nanomedicine 2015, 10, 2093–2107. 10.2217/nnm.15.47. [DOI] [PubMed] [Google Scholar]
- Yasukawa T.; Tabata Y.; Kimura H.; Ogura Y. Recent Advances in Intraocular Drug Delivery Systems. Recent Pat. Drug Delivery Formulation 2011, 5, 1–10. 10.2174/187221111794109529. [DOI] [PubMed] [Google Scholar]
- Urtti A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Delivery Rev. 2006, 58, 1131–1135. 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Bansal P.; Garg S.; Sharma Y.; Venkatesh P. Posterior Segment Drug Delivery Devices: Current and Novel Therapies in Development. J. Ocul. Pharmacol. Ther. 2016, 32, 135–144. 10.1089/jop.2015.0133. [DOI] [PubMed] [Google Scholar]
- Parmar K.; Patel J. K.; Bhatia D.; Pathak Y. V.. Drug Delivery for the Retina and Posterior Segment Disease. Drug Delivery for the Retina and Posterior Segment Disease; Springer International Publishing, 2018; pp 397–409. [Google Scholar]
- Rowe-Rendleman C. L.; Durazo S. a.; Kompella U. B.; Rittenhouse K. D.; Di Polo A.; Weiner A. L.; Grossniklaus H. E.; Naash M. I.; Lewin A. S.; Horsager A.; et al. Drug and Gene Delivery to the Back of the Eye: From Bench to Bedside. Investig. Opthalmology Vis. Sci. 2014, 55, 2714–2730. 10.1167/iovs.13-13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholkar K.; Gunda S.; Earla R.; Pal D.; Mitra A. K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech 2014, 16, 610–622. 10.1208/s12249-014-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikamura Y.; Taylor P.; Yamazaki Y.; Matsunaga T.; Sato T.; Ohtori A. Hydrogel Ring for Topical Drug Delivery to the Ocular Posterior Segment Hydrogel Ring for Topical Drug Delivery to the Ocular Posterior Segment. Curr. Eye Res. 2016, 41, 653–661. 10.3109/02713683.2015.1050738. [DOI] [PubMed] [Google Scholar]
- Gautam N.; Kesavan K. Development of Microemulsions for Ocular Delivery. Ther. Delivery 2017, 8, 313–330. 10.4155/tde-2016-0076. [DOI] [PubMed] [Google Scholar]
- Suk J. S.; Xu Q.; Kim N.; Hanes J.; Ensign L. M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Delivery Rev. 2016, 99, 28–51. 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-López E.; Egea M. A.; Cano A.; Espina M.; Calpena A. C.; Ettcheto M.; Camins A.; Souto E. B.; Silva A. M.; García M. L. PEGylated PLGA Nanospheres Optimized by Design of Experiments for Ocular Administration of Dexibuprofen-in Vitro, Ex Vivo and in Vivo Characterization. Colloids Surf., B 2016, 145, 241–250. 10.1016/j.colsurfb.2016.04.054. [DOI] [PubMed] [Google Scholar]
- Eriksen A. Z.; Brewer J.; Andresen T. L.; Urquhart A. J. The Diffusion Dynamics of PEGylated Liposomes in the Intact Vitreous of the Ex Vivo Porcine Eye: A Fluorescence Correlation Spectroscopy and Biodistribution Study. Int. J. Pharm. 2017, 522, 90–97. 10.1016/j.ijpharm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Liu M.; Hu H.; Liu D.; Zhou S. Development, Optimization, and Characterization of PEGylated Nanoemulsion of Prostaglandin E1 for Long Circulation. AAPS PharmSciTech 2016, 17, 409–417. 10.1208/s12249-015-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.-Z.; Jiang S. P.; He S. N.; Li Y. L.; Feng D. L.; Lu X. Y.; Yu H. Y.; Hu F. Q.; Yuan H. Preparation and Characteristics of Lipid Nanoemulsion Formulations Loaded with Doxorubicin. Int. J. Nanomed. 2013, 8, 3141–3150. 10.2147/IJN.S47708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.; Tang W.; Song Y.; Wang C.; Tian Q.; Wang X.; Quan J.; Li B.; Wang S.; Deng Y. Mixed PEGylated Surfactant Modifying System Decrease the Accelerated Blood Clearance Phenomenon of Nanoemulsions in Rats. Asian J. Pharm. Sci. 2017, 12, 28–36. 10.1016/j.ajps.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J.; Giasson S.; Khalid M. N.; Delmas P.; Allen C.; Leroux J.-C. Long-Circulating Poly(ethylene Glycol)-Coated Emulsions to Target Solid Tumors. Eur. J. Pharm. Biopharm. 2007, 67, 329–338. 10.1016/j.ejpb.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Kwasigroch B.; Escribanoa E.; Carmen M. M.; Queralt J.; Busquetsa M. A.; Estelricha J. Oil-in-Water Nanoemulsions Are Suitable for Carrying Hydrophobic Compounds: Indomethacin as a Model of Anti-Inflammatory Drug. Int. J. Pharm. 2016, 515, 749–756. 10.1016/j.ijpharm.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Afzal S. M.; Shareef M. Z.; Dinesh T.; Kishan V. Folate-PEG-Decorated Docetaxel Lipid Nanoemulsion for Improved Antitumor Activity. Nanomedicine 2016, 11, 2171–2184. 10.2217/nnm-2016-0120. [DOI] [PubMed] [Google Scholar]
- Zeng N.; Hu Q.; Liu Z.; Gao X.; Hu R.; Song Q.; Gu G.; Xia H.; Yao L.; Pang Z.; et al. Preparation and Characterization of Paclitaxel-Loaded DSPE-PEG-Liquid Crystalline Nanoparticles (LCNPs) for Improved Bioavailability. Int. J. Pharm. 2012, 424, 58–66. 10.1016/j.ijpharm.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Higashi K.; Mibu F.; Saito K.; Limwikrant W.; Yamamoto K.; Moribe K. Composition-Dependent Structural Changes and Antitumor Activity of ASC-DP/DSPE-PEG Nanoparticles. Eur. J. Pharm. Sci. 2017, 99, 24–31. 10.1016/j.ejps.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M. R.; Kosuru R.; Vuddanda P. R.; Singh S. K.; Singh S. Trans Resveratrol Loaded DSPE PEG 2000 Coated Liposomes: An Evidence for Prolonged Systemic Circulation and Passive Brain Targeting. J. Drug Delivery Sci. Technol. 2016, 33, 125–135. 10.1016/j.jddst.2016.02.009. [DOI] [Google Scholar]
- Fofaria N. M.; Qhattal H. S. S.; Liu X.; Srivastava S. K. Nanoemulsion formulations for anti-cancer agent piplartine-Characterization, toxicological, pharmacokinetics and efficacy studies. Int. J. Pharm. 2016, 498, 12–22. 10.1016/j.ijpharm.2015.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani P.; Patil A.; Wu K.-W.; Sweeney C.; Tripathi S.; Avula B.; Taskar P.; Khan S.; Majumdar S. Optimization, Stabilization, and Characterization of Amphotericin B Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery. Int. J. Pharm. 2019, 572, 118771. 10.1016/j.ijpharm.2019.118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.-l.; Liu S.; Jiang Y.; Gu L.-y.; Xiao Y.; Wang X.; Cheng L.; Li X.-t. Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG 2000 -transferrin in treatment of brain glioma. Eur. J. Pharm. Sci. 2017, 96, 129–140. 10.1016/j.ejps.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Tong S.; Bao G.; Gao C.; Dai Z. Indocyanine Green Loaded SPIO Nanoparticles with Phospholipid-PEG Coating for Dual-Modal Imaging and Photothermal Therapy. Biomaterials 2013, 34, 7706–7714. 10.1016/j.biomaterials.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Ganta S.; Singh A.; Rawal Y.; Cacaccio J.; Patel N. R.; Kulkarni P.; Ferris C. F.; Amiji M. M.; Coleman T. P. Formulation Development of a Novel Targeted Theranostic Nanoemulsion of Docetaxel to Overcome Multidrug Resistance in Ovarian Cancer. Drug Delivery 2014, 7544, 1–13. 10.3109/10717544.2014.923068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganta S.; Amiji M. Coadministration of Paclitaxel and Curcumin in Nanoemulsion Formulations to Overcome Multidrug Resistance in Tumor Cells. Mol. Pharm. 2009, 6, 928–939. 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- Wang H.; Zhu Z.; Zhang G.; Lin F.; Liu Y.; Zhang Y.; Feng J.; Chen W.; Meng Q.; Chen L. AS1411 Aptamer/Hyaluronic Acid-Bifunctionalized Microemulsion Co-Loading Shikonin and Docetaxel for Enhanced Antiglioma Therapy. J. Pharm. Sci. 2019, 108, 3684–3694. 10.1016/j.xphs.2019.08.017. [DOI] [PubMed] [Google Scholar]
- Kandadi P.; Syed M. A.; Goparaboina S.; Veerabrahma K. Brain Specific Delivery of Pegylated Indinavir Submicron Lipid Emulsions. Eur. J. Pharm. Sci. 2011, 42, 423–432. 10.1016/j.ejps.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Kim S.; Kim J. K.; Lim S. J.; Park J. S.; Lee M. K.; Kim C. K. Folate-Tethered Emulsion for the Target Delivery of Retinoids to Cancer Cells. Eur. J. Pharm. Biopharm. 2008, 68, 618–625. 10.1016/j.ejpb.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Matysová L.; Hájková R.; Šícha J.; Solich P. Determination of Methylparaben, Propylparaben, Triamcinolone Acetonide and Its Degradation Product in a Topical Cream by RP-HPLC. Anal. Bioanal. Chem. 2003, 376, 440–443. 10.1007/s00216-003-1756-x. [DOI] [PubMed] [Google Scholar]
- Nastiti C.; Ponto T.; Abd E.; Grice J. E.; Benson H. A. E.; Roberts M. S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. 10.3390/pharmaceutics9040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandagi P.; Kerur S.; Mastiholimath V.; Gadad A.; Kulkarni A. Polymeric Ocular Nanosuspension for Controlled Release of Acyclovir: In Vitro Release and Ocular Distribution.. Iran. J. Pharm. Res. 2009, 8, 79–86. [Google Scholar]
- Prajapati H. N.; Dalrymple D. M.; Serajuddin A. T. M. A Comparative Evaluation of Mono-, Di- and Triglyceride of Medium Chain Fatty Acids by Lipid/surfactant/water Phase Diagram, Solubility Determination and Dispersion Testing for Application in Pharmaceutical Dosage Form Development. Pharm. Res. 2012, 29, 285–305. 10.1007/s11095-011-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadzadeh Y.; Adibkia K.; Hamishekar H.. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Modification of the Stratum Corneum; Dragicevic N., Maibach H. I., Eds.; Springer-Verlag Berlin Heidelberg, 2015; pp 195–205. [Google Scholar]
- Liu Z.; Nie S.; Guo H.; Pan W.; Li J. Effects of Transcutol P on the Corneal Permeability of Drugs and Evaluation of Its Ocular Irritation of Rabbit Eyes. J. Pharm. Pharmacol. 2006, 58, 45–50. 10.1211/jpp.58.1.0006. [DOI] [PubMed] [Google Scholar]
- Jiao J. Polyoxyethylated Nonionic Surfactants and Their Applications in Topical Ocular Drug Delivery. Adv. Drug Delivery Rev. 2008, 60, 1663–1673. 10.1016/j.addr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Zeng L.; Xin X.; Zhang Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017, 7, 19815–19827. 10.1039/c6ra27096d. [DOI] [Google Scholar]
- Liu Z.; Zhang X.; Li J.; Liu R.; Shu L.; Jin J. Effects of Labrasol on the Corneal Drug Delivery of Baicalin. Drug Delivery 2009, 16, 399–404. 10.1080/10717540903126165. [DOI] [PubMed] [Google Scholar]
- Elkasabgy N. A. Ocular Supersaturated Self-Nanoemulsifying Drug Delivery Systems (S-SNEDDS) to Enhance Econazole Nitrate Bioavailability. Int. J. Pharm. 2014, 460, 33–44. 10.1016/j.ijpharm.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Alshahrani S. M.; Lu W.; Park J.-B.; Morott J. T.; Alsulays B. B.; Majumdar S.; Langley N.; Kolter K.; Gryczke A.; Repka M. A. Stability-enhanced Hot-melt Extruded Amorphous Solid Dispersions via Combinations of Soluplus and HPMCAS-HF. AAPS PharmSciTech 2015, 16, 824–834. 10.1208/s12249-014-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F.; Trivino A.; Prasad D.; Chauhan H. Investigation and Correlation of Drug Polymer Miscibility and Molecular Interactions by Various Approaches for the Preparation of Amorphous Solid Dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. 10.1016/j.ejps.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Bao J.; Xue X.; Li K.; Chang X.; Xie Q.; Yu C.; Pan P. Competing Stereocomplexation and Homocrystallization of Poly(l-lactic acid)/Poly(d-lactic acid) Racemic Mixture: Effects of Miscible Blending with Other Polymers. J. Phys. Chem. B 2017, 121, 6934–6943. 10.1021/acs.jpcb.7b03287. [DOI] [PubMed] [Google Scholar]
- Pan P.; Bao J.; Han L.; Xie Q.; Shan G.; Bao Y. Stereocomplexation of High-Molecular-Weight Enantiomeric Poly(lactic Acid)s Enhanced by Miscible Polymer Blending with Hydrogen Bond Interactions. Polymer 2016, 98, 80–87. 10.1016/j.polymer.2016.06.014. [DOI] [Google Scholar]
- Anjana D.; Nair K. A.; Somashekara N.; Venkata M.; Sripathy R.; Yelucheri R.; Parmar H.; Upadhyay R.; Verma S. R.; Ramchand C. N. Development of curcumin based ophthalmic formulation. Am. J. Infect. Dis. 2012, 8, 41–49. [Google Scholar]
- Tahara K.; Karasawa K.; Onodera R.; Takeuchi H. Feasibility of drug delivery to the eye’s posterior segment by topical instillation of PLGA nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. 10.1016/j.ajps.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.