Abstract
Phosphatidylserine (PS) is a major anionic phospholipid constituent of membrane bilayers, which is specifically enriched in the cytoplasmic leaflet, has functions of regulating the intracellular signaling pathways of neuronal survival and differentiation, and acts as a neurotransmitter to control the activity of neurons. Oil-in-water (O/W) emulsions could improve the bio-availability of PS. Thus, there is a high level of interest in PS emulsion because of its purported health benefits. However, because of high viscosity and poor fluidity, it remains difficult to make the emulsion. A detailed analysis with suited biophysical methods would help to better understand the processes on a molecular level. Therefore, the main aim of the present study was to engineer and characterize a stable O/W phosphatidylserine emulsion. Furthermore, the effect of emulsifiers mixture, whey protein isolate (WPI), and Tween 80 (T80), as well as the oil phase was systematically evaluated. The key parameters were the chain length and the degree of nonsaturation (sunflower oil, a long-chain triglycerides [LCTs] or a medium-chain triglycerides [MCTs]). Small droplets of emulsions could be obtained by adjusting the type of emulsifier and the LCT/MCT ratio. A stable PS emulsion characterized by a smaller droplet size, higher negative zeta-potential, lower centrifugal stability constant, and longer storage time was produced by MCTs T80 (2.0%, w/w) with T80 (2.0%, w/w) as the emulsifier, and by LCTs with the WPI (0.5%, w/w)—T80 (1.5%, w/w) as the emulsifier, respectively. The PS emulsion with LCTs exhibited higher viscosity, when compared to the emulsion made by MCT at the same emulsifier concentration, while all emulsions exhibited a shear thinning behavior. The microstructure images revealed that the PS emulsions produced by MCTs and T80 (2.0%, w/w) or WPIs (0.5%, w/w)—T80 (1.5%, w/w) mixed with LCTs can form specific uniform networks, in order to prevent flocculation. After 28 days of storage, no visual phase separation was observed in the emulsions, except for the PS emulsion with the WPI (2.0%, w/w). It was concluded that the characteristics of the interfacial layer of particles in the PS emulsion system were not only dependent on the proportion of the applied emulsifiers, but also dependent on the oily phase features. These findings may provide indications for choosing the suitable process parameters when a stable PS emulsion is produced.
1. Introduction
Phosphatidylserine (PS) is a natural phospholipid component of the cell membranes in the human brain.1 The regulation of the PS level in a neuron plasma membrane has significant effects on protein kinase C (pkc), actin-associating protein kinase (Akt), and signal transduction, thereby, promoting axon differentiation and supporting neuron survival.2−6 Brose and Augustine. reported that PS regulates the function of prominent receptors and the release of neurotransmitters.7−10 Furthermore, the supplementation of PS can improve some memory functions in cognitive-impaired subjects.11 These functions of PS in neurophysiology and neuropathology have aroused renewed interest in the PS production. The PS production has been certified by the Food and Drug Administration of many countries. In China, PS was added to the new resource food catalogue on October 21, 2010 by the former Chinese Ministry of Health (now renamed as the National Health and Family Planning Commission), allowing it to be used as a new resource food, with a recommended consumption of ≤600 mg/day.12 However, when PS is added to the product as a functional food ingredient, the stability of PS would influence the production of food, which in turn makes the production process difficult, resulting in raw material losses. Hence, it is crucial to find a way to dissolve PS, in order to improve its stability. Therefore, the main aim of the present study was to utilize ingredients to create an emulsion that would improve the stability of PS during food processing.
Oil-in-water (O/W) emulsions are commonly used in food chemistry and in biotechnological applications, allowing the lipid absorption control and increasing the bio-accessibility of active ingredients.13 However, the preparation of a stable PS emulsion in industrial production remains challenging. Hence, it is necessary to determine the proper emulsion composition and interaction relationship between the components in the emulsion.14−20 The important types of surface-active materials in food are low molecular weight surfactants and proteins.21 Among these proteins, whey protein has been largely used as a food ingredient in the food industry. Whey protein is the principal protein component of milk. In its structure, there are polar regions, nonpolar regions, and ions. These structural properties of proteins play a crucial role in determining the stability and other physicochemical properties of emulsions, such as texture and viscosity.22,23 Indeed, the characteristics show that this natural dairy product makes whey protein a good emulsifier for food emulsions.
Tween 80 (T80) is a nonionic semisynthetic molecule derived from polyethoxylated sorbitol anhydride and oleic acid, which is often used in food products. This can rapidly and effectively reduce the interfacial tension, promote the emulsification process, and produce a more stable emulsion with emulsification. Therefore, it was hypothesized that whey protein isolate (WPI) and T80 can complement each other to improve the long-term and short-term stability of the emulsion. The use of an emulsifier mixture is a good strategy to reduce the amount of chemical substances in food product formulations, especially when natural ingredients are used, meeting the demands of the consumers for clean labeling.24
Few studies correlated to the interaction between the ingredients and how the emulsifier affects the stability of the PS emulsion have been published. Hence, further research in this area is required. In the present study, the utilization of WPI-T80 mixtures and different oil phases, such as long chain triglycerides (LCTs) and medium chain triglycerides (MCTs), was proposed, with the aim to understand how the nature of these ingredients affect the stability of PS emulsions. For this reason, the present study focused on the preparation and characterization of PS emulsions prepared with a blend of whey protein with the nonionic surfactant T80. The stability was assessed through the measurements of the size, zeta-potential, centrifugal stability constant, microstructure, viscosity, and creaming index. The knowledge gained from the present study would be beneficial for the development of an optimal PS emulsion formulation for applications in health care and the food industry.
2. Results and Discussion
2.1. Influences of Emulsifier Types and Concentrations on the Droplet Size of PS Emulsions
The different oil types and concentrations of emulsifiers noticeably influenced the characteristics of the PS emulsions. In the present study, 0.6% (w/w) of PS emulsions stabilized by T80, WPI, and a blend of emulsifiers in various ratios were analyzed and compared. The overall amount of surface-active molecules varied within the range of 0–2.0% (w/w). Henceforth, this was referred to as T80-O/W (PS emulsion stabilized by T80, 2.0%, w/w), WPI-O/W (PS emulsion stabilized by whey protein, 2.0%, w/w), and mix-O/W (PS emulsions stabilized by WPIs and T80 as emulsifiers, 2.0%, w/w). The droplet size of the PS emulsions is presented in Table 1. The data indicates that PS emulsions with MCTs have a smaller mean droplet size, when compared with emulsions stabilized by LCTs. This could be correlated to the viscosity of the oil phases. The viscosity of the MCT oil is lower (approximately 25 mPa·s) than the corn oil (approximately 60 mPa·s), which allows the droplets to break up into smaller sizes in the system of MCT oil.26−28 In addition, if the emulsion is produced by LCTs or MCTs, the size of the droplet tends to gradually decrease with the increase in the T80 content in the emulsifier. Among these emulsions, the smallest droplet size was 175.83 ± 0.99 nm, which was produced by the MCT, with T80 (2.0% w/w) as the emulsifier. This might be due to the competition and/or combination of the WPI and T80 adsorption onto the droplet surface during emulsification, and the rate of change of the interfacial tension.29,30 During the process of emulsification, systems with higher T80 concentrations were capable of accelerating the decrease in the interfacial tension, forming smaller droplets. Compared to surfactants, proteins are present with low efficiency in decreasing the interfacial tension, and this is due to the slower diffusion and strong dependence on the environmental conditions (such as pH, temperature and ionic strength), thereby restricting their application in colloidal systems.18 Therefore, it can be concluded that small molecule surfactants can be more rapidly adsorbed onto the droplet surface of PS emulsions during emulsification because these are more flexible and smaller in size, when compared to large globular proteins, such as WPIs (Figure 1).
Table 1. Mean Droplet Diameter (nm) and Polymer Disperse Index (PDI) of the Oil in Water PS Emulsions Produced by LCT or MCT as the Oily Phase (Mean ± SD)a.
| LCT | MCT | |||
|---|---|---|---|---|
| emulsifiers mixture (WPI/T80, % w/w) | LCT (nm) | MCT (nm) | PDI | PDI |
| H2.0/0 | 317.10 ± 3.48Aa | 242.10 ± 0.91Ab | 0.301 ± 0.011 | 0.296 ± 0.006 |
| 1.5/0.5 | 266.65 ± 1.57Ba | 219.41 ± 0.56Bb | 0.300 ± 0.008 | 0.281 ± 0.010 |
| 1/1 | 269.89 ± 1.49ACa | 223.51 ± 0.60Cb | 0.342 ± 0.019 | 0.376 ± 0.005 |
| 0.5/1.5 | 208.89 ± 4.01Da | 234.52 ± 0.50Db | 0.280 ± 0.006 | 0.360 ± 0.006 |
| 0/2.0 | 229.04 ± 0.91Ea | 175.83 ± 0.99Eb | 0.298 ± 0.005 | 0.227 ± 0.007 |
The different letters indicate significant difference, P < 0.05. Capital letters: the differences in the same column. Small letters: the differences between the oily phase composition at the same ratio of emulsifier mixture.
Figure 1.
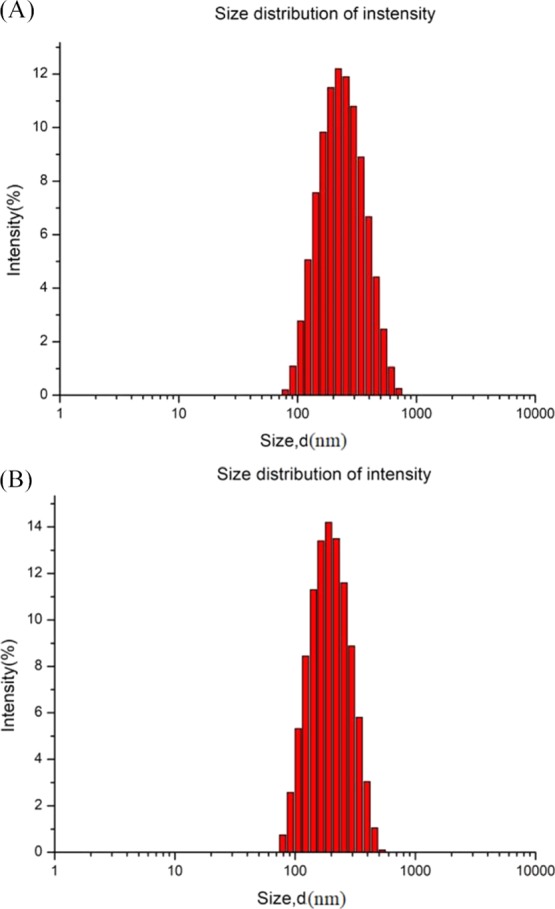
Size distribution of the PS emulsions. (A) PS emulsion produced by LCTs, with the WPI (0.5%, w/w)-T80 (1.5%, w/w) as the emulsifier; (B) PS emulsion produced by MCTs, with T80 (2.0%, w/w) as the emulsifier.
The change in the particle size (mean ± SD) of the samples over a period of 21 days stored at 4 °C was also investigated, and this is shown in Figure 2. Figure 2A,B shows that the size at the dispersed phase for all PS emulsions was approximately close to each other for all the analyzed systems. The analysis of variance (ANOVA) statistical results revealed no significant differences among the droplet sizes for all emulsions.
Figure 2.
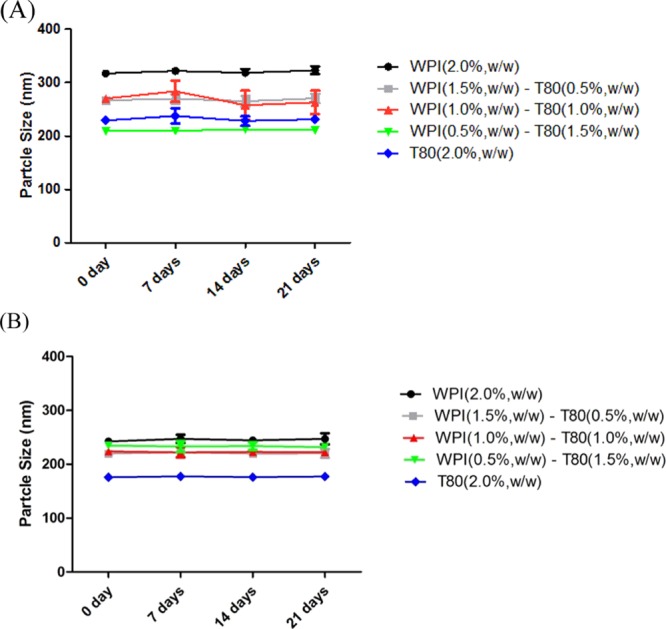
Particle size of PS emulsions with different ratios of the emulsifier mixture produced by different types of oil at 0, 7, 14, and 21 days. (A) PS emulsions produced by LCTs; (B) PS emulsions produced by MCTs.
2.2. Influences of Emulsifier Types and Concentrations on the Zeta-Potential of the Emulsions
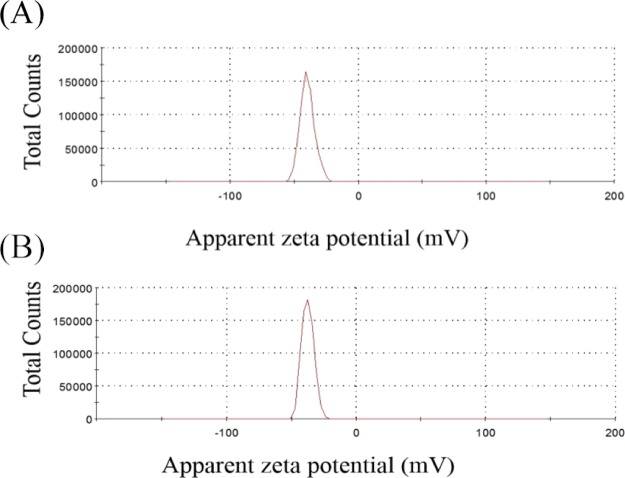
The zeta-potential of the LCT and MCT oil droplets emulsified by T80 was approximately −38 mV (Table 2), and the negative charge was significantly higher than that of the droplets with other emulsifiers, regardless of the oil type. T80 is a nonionic surfactant. The negative charge of these droplets arose from the presence of minority molecules adsorbed onto the interface, such as OH– species from the aqueous phase and possibly the negative charge from the oil (e.g. free fatty acids).31,32 The zeta-potential distribution of the PS emulsion produced by LCTs, with the concentrations of the WPI (0.5%, w/w)-T80 (1.5%, w/w) as the emulsifier, is shown in Figure 3A, while the zeta-potential distribution of the PS emulsion formed by MCTs, with the concentrations of T80 (2.0%, w/w) as the emulsifier, is shown in Figure 3B. It was observed that the zeta-potential of the LCT droplets were significantly higher than that of the MCT droplets, which was possibly due to the different states of the WPI-/T80-adsorbed layer on the LCT and MCT oil droplet surfaces.18
Table 2. Mean Zeta-Potential of PS Emulsions Prepared with Different Oil Types and Concentrations of Emulsifiers at an Oil-to-Aqueous Phase Ratio of 10:90 (Mean ± SD)a.
| emulsifiers mixture (WPI/T80, % w/w) | LCT (mV) | MCT (mV) |
|---|---|---|
| 2.0/0 | –32.56 ± 0.57Aa | –29.92 ± 0.50Ab |
| 1.5/0.5 | –33.88 ± 0.60Ba | –32.19 ± 0.16Bb |
| 1/1 | –35.82 ± 0.63Ca | –33.99 ± 0.28Cb |
| 0.5/1.5 | –37.39 ± 0.68Da | –35.81 ± 0.33Db |
| 0/2.0 | –38.67 ± 0.95Ea | –36.78 ± 0.40Eb |
The different letters indicate the significant difference, P < 0.05. capital letters: The differences in the same column. Small letters: The differences between oily phase compositions at the same ratio of the emulsifier mixture.
Figure 3.
Zeta potential distribution of PS emulsions. (A) PS emulsion produced by LCTs, with the concentration of WPI (0.5%, w/w)-T80 (1.5%, w/w) as the emulsifier; (B) PS emulsion produced by MCTs, with the concentration (2.0%, w/w) of T80 as the emulsifier.
The increase in the amount of WPIs in the mixture of emulsifiers promoted a slight decrease in the negative charge, with a value of approximately −30 mV in the PS emulsions. These results may correlate to the different states of the WPI adsorbed on the droplet surfaces of PS emulsions produced by the LCT/MCT oil. In addition, the zeta-potential provides information on the emulsion stability, and the surface charge of the protein is influenced by the pH. When the pH value was lower than the isoelectric point (pI) value of the WPI (approximately pH 4.9), the surface became positively charged. This approached the isoelectric point value of the surface charge, which was close to zero. With a higher pH, the zeta-potential value was more negative.33 Combined with the benefits of the use of WPI, the issue related to the effects induced by the pH fluctuation of the medium should be handled with utmost care. The WPI-stabilized emulsions were unstable in terms of the droplet aggregation at a pH near the pI, and this could pose a problem for WPI-stabilized emulsions in food systems with pH values of approximately 4.9. In these conditions, the WPI loses the repulsive forces that support the protein solubilization, and as a consequence, this precipitates.23 Therefore, in the present study, the neutral pH (7.0) was used. This reduced the flocculation when the WPI was used as the emulsion stabilizer. These findings indicate that the zeta-potential values are slightly influenced by the conformational change in the emulsifier, pH, and concentration of each emulsifier on the interfacial layer.
2.3. Influences of Emulsifier Types and Concentrations on the Centrifugal Stability Constant of Emulsions
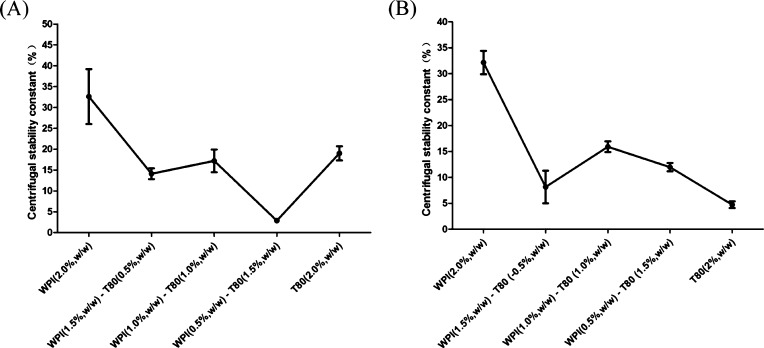
Previous studies have suggested that the centrifugal stability constant should be used as the identification index to evaluate the stability of emulsions. Xie et al. reported that the evaluation of emulsions with lower interfacial tension between oil and water, and those with smaller centrifugal stability constants exhibit better stability.34 As shown in Figure 4A, the PS emulsion produced by LCTs that contain WPIs (0.5%, w/w)-T80 (1.5%, w/w) as the emulsifier had smaller centrifugal stability constants. Figure 4B shows that the centrifugal stability constant of the PS emulsion produced by MCTs emulsified with 2.0% (w/w) T80 was smaller, when compared to other combinations, indicating better stability. The centrifugal stability constant was slightly smaller when the T80 dosage was increased. This may be due to the T80, which plays a beneficial role. T80 can provide steric stabilization, increase the repulsion between droplet particles, and prevent the flocculation of droplets during emulsion formation.35 Thus, it was inferred that different ratios of WPI-T80 as the emulsifier with different oil types might arrange into a stable emulsion membrane. It was estimated that in the O/W emulsions, relatively large hydrophilic groups are adsorbed between the molecules, and the surfactant may be inserted into the droplet interface to protect the droplet interface, preventing protein precipitation and emulsion instability.
Figure 4.
Centrifugal stability constant of PS emulsions produced by LCTs (A) and MCTs (B) at different concentrations of WPI/T80 as emulsifiers.
2.4. Influences of Emulsifier Types and Concentrations on the Viscosity of Emulsions
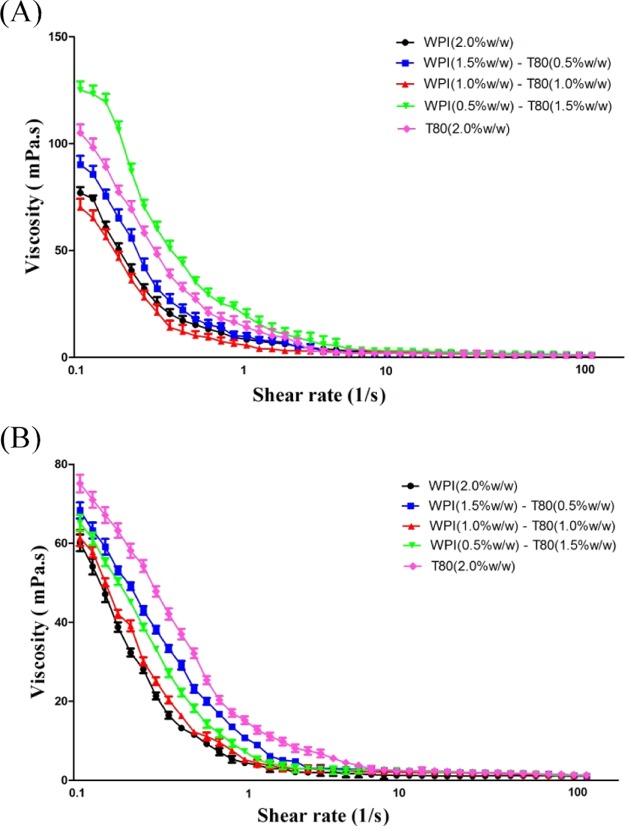
The viscosity of the emulsion is affected by many factors, such as the droplet size, the repulsive colloidal interactions between droplets, the presence of charged droplets in the system, and the viscosity in the dispersed and continuous phase.36 The viscosity values, which is a function of the shear rate (0.1–100.0 S–1) of PS emulsions that contain WPIs, T80 and WPI/T80, are shown in Figure 5. The apparent viscosity values at a shear rate of 100 S–1 were compared. PS emulsions with LCTs had the highest viscosity, when compared to emulsions with MCTs, at the same WPI/T80 concentration. This may be due to the corn oil, which is quite viscous. In PS emulsions with LCTs, the apparent viscosity increases with the concentration of the WPI (0.5%, w/w)-T80 (1.5%,w/w). This viscosity could be associated with the droplet size, steric hindrance, and charge of the droplet.35 Because of the large molecular size, when proteins cover the droplets, higher steric hindrances are formed. These prevent the droplets from being so close to each other, thereby resulting in a high viscosity.35 In addition, for PS emulsions produced by MCTs, the viscosity increased with the increase in the concentration of T80. Such a behavior could be correlated to the droplet size reduction because most of the interface was covered by T80. This led to the increase in hydrodynamic interactions between droplets, and consequently, a higher viscosity.
Figure 5.
Viscosity presented as a function of the shear rate of PS emulsions with different type oils. (A) PS emulsions with the LCT oil; (B) PS emulsions with the MCT oil.
All PS emulsions behaved similar to a non-Newtonian fluid (shear thinning), irrespective of the oil type and the concentration used in the emulsions. With the increase in the shear rate, the viscosity of the emulsions decreased. This shear thinning behavior can be explained by the following mechanism: when the shear rate sufficiently increases to overcome the Brownian motion, the emulsion droplets become more orderly along the flow field and have less resistance to the flow. Therefore, the viscosity of the PS emulsions was lower.37,38 The PS emulsion has a smaller droplet size (<1 μm), which is beneficial for the droplet–droplet interaction. This weak interaction can be easily destroyed by the increase in the shear rate. These results suggest that the properties of the colloidal interaction in emulsions play an important role in the rheology of suspensions.
2.5. Effect on the Microstructure of Emulsions
The images present the differences in the microstructures of emulsions with different oil type/emulsifier concentrations. The PS emulsions are shown in Figure 6A–D. The microstructures in these images demonstrate that droplet structures were formed in PS emulsions. These emulsions have a special particulate network, and emulsion droplets of different sizes were observed. Figure 6A,B shows that the droplets are larger when aggregation occurs with LCT-PS/MCT-PS at the WPI 2.0% (w/w) concentration level. Furthermore, it can be observed that more empty spaces appeared between the droplets. This means that the droplets aggregated together and formed in flocculation. These results show that the formation of unstable regions of the PS emulsion was due to the large droplet aggregates. In addition, Figure 6C,D shows that the PS emulsions produced by WPIs (0.5%, w/w) with T80 (1.5%, w/w) mixed with LCTs and T80 (2.0%, w/w) mixed with MCTs presented with a small particle size and uniform distributions. This suggests that this emulsification system inhibited the droplet flocculation. It was speculated that the stable emulsion is due to the highly viscous networks formed by the mutual combination of LCT/MCT and WPI/T80, which prevented the droplet aggregation. This microstructure further demonstrates that emulsions with different oil types/emulsifier concentrations had improved physical stability. In addition, the microstructure analysis was consistent with the above particle size, viscosity, and centrifugal stability constant.
Figure 6.
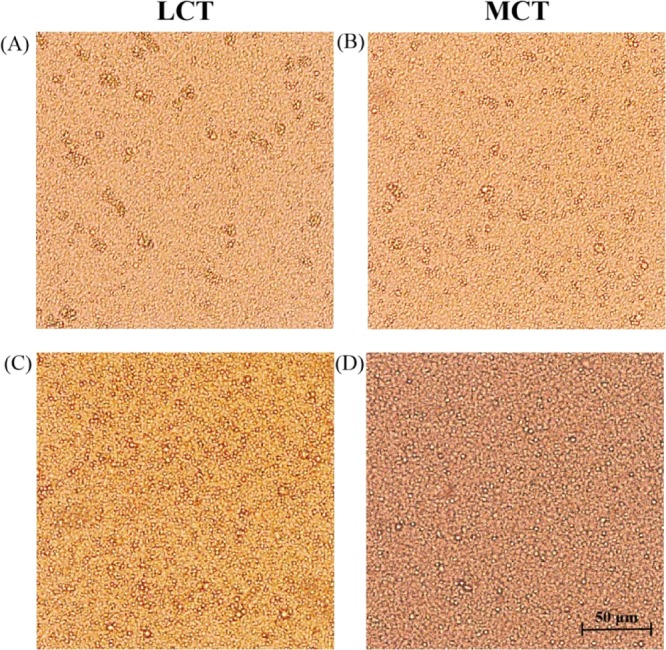
Microstructures of the PS emulsions: (A) PS emulsion produced by LCTs at the WPI (2.0%, w/w) concentration; (B) PS emulsion produced by MCTs at the WPI (2.0%, w/w) concentration; (C) PS emulsion produced by LCTs at the WPI (0.5%, w/w) and T80 (1.5%, w/w) concentration; (D) PS emulsion produced by MCTs at the T80 (2.0%, w/w) concentration.
2.6. Influences of Emulsifier Types and Concentrations on the Creaming Stability of Emulsions
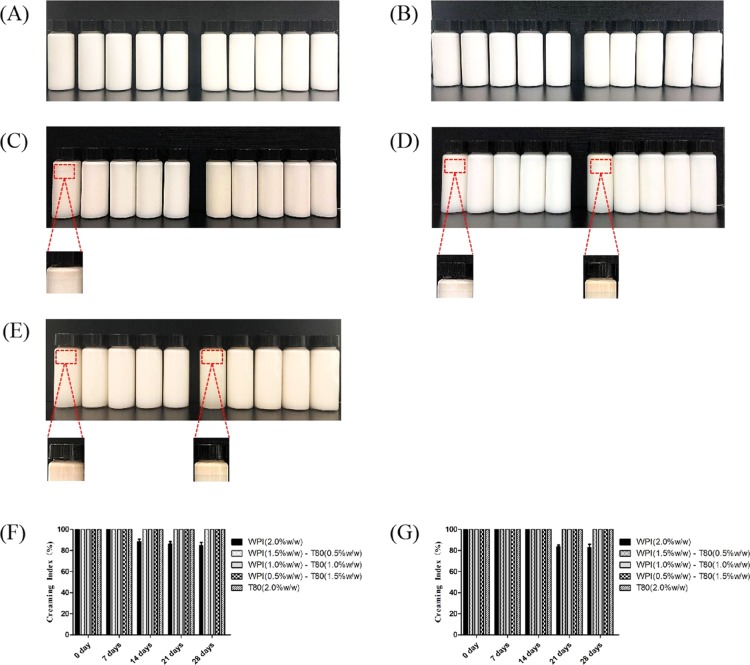
The creaming behavior was determined on the 28th day of storage, and is shown in Figure 7. Creaming index measurements were conducted to evaluate the physical stability of O/W PS emulsions. As shown in Figure 7, it is clear that the storage time affected the creaming index of the PS emulsions produced by LCTs and MCTs, respectively. The separation of layers was measured soon after the emulsion preparation, and on the 7th, 14th, 21st, and 28th day of storage, emulsions with the Mix-O/W mixtures were more stable. The PS emulsion that contained only WPIs (2.0%, w/w) exhibited a rapid phase separation.
Figure 7.
Creaming stability of PS emulsions after a storage time of 28 days. (A) Fresh emulsion, PS emulsions produced by LCTs and MCTs, and the emulsifier from left to right is as follows: LCT with WPI (2.0%, w/w), LCT with WPI (1.5%, w/w)-T80 (0.5%, w/w), LCT with WPI (1.0%, w/w)-T80 (1.0%, w/w), LCT with WPI (0.5%, w/w)-T80 (1.5%, w/w), LCT with T80 (2.0%, w/w), MCT with WPI (2.0%, w/w), MCT with WPI (1.5%, w/w)-T80 (0.5%, w/w), MCT with WPI (1.0%, w/w)-T80 (1.0%, w/w), MCT with WPI (0.5%, w/w)-T80 (1.5%, w/w), and MCT with T80 (2.0%, w/w). (B–E) PS emulsions with the same concentration emulsifier as (A), in different storage times of 7, 14, 21, and 28 days. (F) PS emulsions produced by LCTs and the creaming index of emulsions at different storage times. (G) PS emulsions produced by MCTs, and the creaming index of emulsions at different storage times.
The visual observation of creaming boundaries indicated that the PS emulsion that contained WPIs (2.0%, w/w) in LCTs alone has the highest phase separation among all emulsions at the 14-, 21-, and 28-day periods. No significant change in the creaming was observed between emulsions that contained the T80-O/W and Mix-O/W mixtures at all concentration levels. The PS emulsion produced by MCTs and the highest concentration (2.0%, w/w) of the WPI exhibited a phase separation after 21 days of storage. However, PS emulsions that contained the T80-O/W and mix-O/W mixtures did not present with a phase separation at any of the concentration levels. By comparing these outcomes with those of the samples stored at 24 °C, it is clear that higher WPI protein concentrations destabilized the PS emulsions. When the concentration of the WPI increased, more WPIs were adsorbed on the oil droplets interface to form a protective layer, thereby promoting repulsion between charged droplets, and increasing the emulsion stability to the creaming process.39−41 However, the emulsion was formed during the high press homogenization. The heat energy released during the process could be associated to the arrangement and adsorption of the WPI aggregates to a droplet surface. Furthermore, the protein unfolding, exposure, and interaction between hydrophobic groups could also result in a decrease in the emulsifying capacity. Whey proteins are classified as globular proteins that mostly consist of β-lactoglobulin and α-lactalbumin. Heating may cause protein unfolding, and expose the amino acid with disulfide-bond and nonpolar fragments to the aqueous phase. The exposure of these active amino acids may destabilize the emulsion, leading to bridging flocculation.42 Thus, it was considered that the destabilization of emulsions was due to the increase in the WPI adsorbed onto the interface in the mixture of emulsifiers in the LCT and MCT systems. Compared to WPI-O/W with MCTs, WPI-O/W in LCTs exhibited more instability. This may be due to the corn oil that comprised of long-chain fatty acids, which decreased its solubility in water and facilitated the phase separation. Therefore, it can be deduced that the interface composition is the key factor in stability mechanisms, and consequently, on the creaming process.
3. Conclusions
These present findings indicate that a stable PS emulsion is not only dependent on the ratio of WPI/T80 emulsifiers in the bulk phase but also on the characteristics of the oil phase. More stable emulsions were produced with the addition of T80. However, the presence of the WPI allowed for the formation of a viscoelastic interface, which promoted the stabilization of emulsions. Compared to MCTs, unsaturated LCTs can more likely interact with WPIs, which attributed to the hydrophobic interactions between the oil and emulsifier. However, the most stable PS emulsion was produced with the saturated MCT with T80. The presence of T80 ensured the steric stabilization, promoting the stability of the PS emulsions.
The combination of the surfactant/protein and the oil phase can be used to obtain systems with specific characteristics. Thus, these obtained results can provide additional information for the development of emulsified products, in an effort to design structured systems with specific functional performances. The prepared PS emulsions can dissolve in the production process of food ingredients and improve its utilization rate. It was found that WPI/T80 as the emulsifier has the best effect on the stability of PS emulsions, and this could dissolve PS without precipitation. The addition of WPI/T80 can improve the fluidity of PS emulsions and facilitate the processing and production of products. Therefore, these results demonstrate that the oil type and emulsifier concentration are important for producing stable PS emulsions under different environmental conditions.
4. Experimental Section
4.1. Materials
The ingredients used to prepare the emulsions were polyoxyethylene sorbitan monooleate (Tween 80; Damao Chemical Reagent Co., Ltd., Tianjin, China), phosphatidylserine (Lipogen Co., Ltd., Haifa, Israel), WPI (Agropur, Canada), corn oil (LCT; Carrefour Food Co., Ltd., Jilin, China), and MCT (Guangdong Shengtong Trading Co., Ltd., Guangzhou, China). The main fatty acid composition was 60.8%, caprylic acid was 8:0, and the 39.0% capriacid was 10:0. The ultrapure water was purified by Milli-Q apparatus (Millipore, Billerica, USA) and was used to prepare all the solutions.
4.2. Preparation of PS Emulsions
In this method, two different phases, denoted as the oil phase and aqueous phase, were initially separately prepared, and mixed at an oil phase and aqueous phase ratio of 10:90. The PS was dissolved in the LCT or MCT by stirring for 10 h at 50 °C and subsequently for 1 h at room temperature, in order to ensure the solubilization. Then, the WPI or T80 was dispersed in 10 mM of phosphate buffer (PB) to ensure the full dissolution and hydration, and the samples were stirred overnight at 4 °C. The PS (0.6%, w/w) crude emulsions of 10% (w/w) of the LCT or MCT solution in the WPI/T80 10 mM PB (pH = 7.0) solution was prepared by high-speed homogenization for 15 min at 4000 rpm (L5M-A, Silverson Ltd., UK). The total amount of mixture emulsifier at the crude emulsions was equal to 2.0% (w/w), and the mixture of emulsifiers comprised of a WPI-T80 ratio of 2.0–0.0, 1.5–0.5, 1.0–1.0, 0.5–1.5, and 0.0–2.0% (w/w). The coarse emulsion was passed through a high-pressure homogenizer (LLCD-3L, PhD Technology LLC, USA) for four times at 10, 150 psi (70 MPa) to produce a fine emulsion. The final composition of the emulsion was 600 mg PS/100 mL. After the preparation, 0.02% (w/w) sodiumazide was added to prevent microbial growth.
4.3. Characterization of PS Emulsions
4.3.1. Particle Size and Size Distribution
The particle size and size distribution of emulsions were measured by the dynamic light scattering technique using a Malvern Zetasizer Nano ZSP (Malvern Instruments Ltd., Worcestershire, UK). The particle size distribution and stability were analyzed after the sample preparation, and the measurements were performed after 7, 14, and 21 days of storage at 4 °C.
4.3.2. Zeta-Potential
The zeta-potential of the PS emulsions was determined by dynamic light scattering (DLS) using a Zetasizer Nano ZS analyzer (Malvern Instruments Ltd., Worcestershire, UK). The samples were diluted in Milli-Q water, and the particle charge data were collected over three continuous readings.
4.3.3. Centrifugal Stability Constant Measurement
A certain amount of an emulsion was diluted (1:400) with distilled water in a 10 mL brown volumetric flask, and the absorbance value (A) was measured at a wave length of 500 nm after mixing using an ultraviolet light-visible spectrophotometer (U-3900H; Hitachi Corporation, Japan). Then, 1.5 mL aliquot of each of the prepared emulsions was transferred into a 2 mL centrifuge tube and centrifuged at 4000 rpm for 15 min in a high-speed centrifuge (5804R; Eppendorf Corporation, Germany). Afterward, a certain amount of subnatant was diluted (1:400) with distilled water in a 10 mL brown volumetric flask, and the absorbance value (A0) was measured at a wavelength of 500 nm after the mixing. The centrifugal stability constant (Ke) was calculated using the eq 1
| 1 |
4.3.4. Viscosity Measurements
The PS emulsion viscosity against the shear rate were measured using a discovery hybrid rheometer (TA Instruments, UK), which was equipped with a cone and plate geometry (a diameter of 40 mm, an angle of 4°, and a 1 mm gap). Temperature control was performed using a Parr plate (25.00 ± 0.05 °C). The shear rate range was 0.1–100.0 S–1. All samples were measured in duplicate.
4.3.5. Microscopy Observation
The microstructures of the emulsions were observed using a Zeiss AxioLab.A1 microscope observer (Zeiss, Inc., Germany), which was equipped with an AxioCam MRc5 digital camera. A 20 μL freshly made emulsion sample was placed on a microscope slide, and a cover slip was placed to ensure that no air bubbles would appear. The objective magnification used was 50×.
4.3.6. Creaming Index
The creaming stability was investigated to evaluate the relative stability of the PS O/W emulsions. The creaming index can provide indirect information about the extent of the droplet aggregation in the emulsion: the more the aggregation, the larger the flocs, and the faster the creaming. The measurements of the storage stability and the creaming index of the emulsion were performed according to the study of Piriyaprasarth, Juttulapa, and Sriamornsak.25 Immediately after preparation, 28 mL of emulsions were transferred to 30 mL clear glass vials, and these vials were sealed with caps to prevent evaporation. All emulsions were stored at 24 °C for 7, 14, 21, and 28 days. Next, the emulsions separated into the “cream layer” at the top and the “serum layer” at the bottom. The PS emulsions were photographed on day 7, 14, 21, and 28 using a Canon IXUS 320 (Canon Co., Ltd., Beijing, China). The creaming index (CI) was calculated as follows
| 2 |
The total emulsion height (HT) and the bottom serum layer (HS) height were measured in millimeters using a precise ruler.
4.4. Statistical Design and Analysis
At least two replicate determinations with three freshly prepared subsamples were used for each measurement, and the results were presented as a mean ± standard deviation (SD). The experimental data were analyzed using SPSS 17.0 (SAS Institute Inc., Carry, NC, USA). The significant differences between the means were detected using ANOVA, followed by Bonferroni post hoc comparisons tests. A P-value of <0.05 indicated a significant difference.
Acknowledgments
This work was supported by the Open Project of Shandong Collaborative Innovation Center for Antibody Drugs (nos. CIC-AD1829, CIC-AD1834, and CIC-AD1839), the Doctoral Foundation of Liaocheng University (nos. 318051738 and 318051827), the Foundation of Liaocheng University (no. 318011907), the Shandong Provincial Medical Food Research Plan (2018YYP008), and the Taishan Scholar Research Foundation.
The authors declare no competing financial interest.
References
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968, 9, 570–579. [PubMed] [Google Scholar]
- Akbar M.; Calderon F.; Wen Z.; Kim H.-Y. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 10858–10863. 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B. X.; Akbar M.; Kevala K.; Kim H.-Y. Phosphatidylserine is a critical modulator for Akt activation. J. Cell Biol. 2011, 192, 979–992. 10.1083/jcb.201005100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-Y.; Akbar M.; Lau A.; Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3), Role of phosphatidylserine in antiapoptotic effect. J. Biol. Chem. 2000, 275, 35215–35223. 10.1074/jbc.m004446200. [DOI] [PubMed] [Google Scholar]
- Kim H.-Y. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem. 2007, 282, 18661–18665. 10.1074/jbc.r700015200. [DOI] [PubMed] [Google Scholar]
- Kim H.-Y.; Akbar M.; Kim Y.-S. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins, Leukotrienes Essent. Fatty Acids 2010, 82, 165–172. 10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N.; Petrenko A.; Sudhof T.; Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 1992, 256, 1021–1025. 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Augustine G. J. How does calcium trigger neurotransmitter release?. Curr. Opin. Neurobiol. 2001, 11, 320–326. 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Tucker W. C.; Weber T.; Chapman E. R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 2004, 304, 435–438. 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- Dennison S. M.; Bowen M. E.; Brunger A. T.; Lentz B. R. Neuronal SNAREs do not trigger fusion between synthetic membranes but do promote PEG-mediated membrane fusion. Biophys. J. 2006, 90, 1661–1675. 10.1529/biophysj.105.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader Lange M. L.; Cenini G.; Piroddi M.; Mohmmad Abdul H.; Sultana R.; Galli F.; Memo M.; Butterfield D. A. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol. Dis. 2008, 29, 456–464. 10.1016/j.nbd.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Announcement on Approving of the Three Kinds of New Resource Foods Including Sucrosepolyester, Corn Oligopeptide Powder, Phosphatidylserine and So On. Publication of the Ministry of Health of the People’s Republic of China; No. 15 of 2010. China Food Additives, 2010;Vol. 6, pp 232–233.
- Gomes A.; Costa A. L. R.; Cunha R. L. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: Adsorption, interfacial rheology and emulsion features. Colloids Surf., B 2018, 164, 272–280. 10.1016/j.colsurfb.2018.01.032. [DOI] [PubMed] [Google Scholar]
- McClements D. J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. 10.1016/j.cocis.2004.09.003. [DOI] [Google Scholar]
- Tadros T. F.Emulsion Science and Technology: A General Introduction. Emulsion Science and Technology; Wiley, 2009; pp 1–56. [Google Scholar]
- Wan Z.-L.; Wang L.-Y.; Wang J.-M.; Zhou Q.; Yuan Y.; Yang X.-Q. Synergistic interfacial properties of soy protein-stevioside mixtures: Relationship to emulsion stability. Food Hydrocolloids 2014, 39, 127–135. 10.1016/j.foodhyd.2014.01.007. [DOI] [Google Scholar]
- Zare D.; Allison J. R.; McGrath K. M. Molecular Dynamics Simulation of β-Lactoglobulin at Different Oil/Water Interfaces. Biomacromolecules 2016, 17, 1572–1581. 10.1021/acs.biomac.5b01709. [DOI] [PubMed] [Google Scholar]
- Grigoriev D.; Derkatch S.; Kragel J.; Miller R. Relationship between structure and rheological properties of mixed BSA/Tween 80 adsorption layers at the air/water interface. Food Hydrocolloids 2007, 21, 823–830. 10.1016/j.foodhyd.2006.08.018. [DOI] [Google Scholar]
- Mao Y.; Dubot M.; Xiao H.; McClements D. J. Interfacial Engineering Using Mixed Protein Systems: Emulsion-Based Delivery Systems for Encapsulation and Stabilization of β-Carotene. J. Agric. Food Chem. 2013, 61, 5163–5169. 10.1021/jf401350t. [DOI] [PubMed] [Google Scholar]
- McClements D. J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. 10.1146/annurev.food.080708.100722. [DOI] [PubMed] [Google Scholar]
- Dickinson E.Introduction to Food Colloids; Oxford University Press, 1992; Vol. 36, p 514. [Google Scholar]
- Zhai J.; Wooster T. J.; Hoffmann S. V.; Lee T.-H.; Augustin M. A.; Aguilar M.-I. Structural Rearrangement of β-Lactoglobulin at Different Oil-Water Interfaces and Its Effect on Emulsion Stability. Langmuir 2011, 27, 9227–9236. 10.1021/la201483y. [DOI] [PubMed] [Google Scholar]
- Teo A.; Goh K. K. T.; Wen J.; Oey I.; Ko S.; Kwak H.-S.; Lee S. J. Physicochemical properties of whey protein, lactoferrin and Tween 20 stabilised nanoemulsions: Effect of temperature, pH and salt. Food Chem. 2016, 197, 297–306. 10.1016/j.foodchem.2015.10.086. [DOI] [PubMed] [Google Scholar]
- Gülseren İ.; Corredig M. Interactions between polyglycerol polyricinoleate (PGPR) and pectins at the oil-water interface and their influence on the stability of water-in-oil emulsions. Food Hydrocolloids 2014, 34, 154–160. 10.1016/j.foodhyd.2012.11.015. [DOI] [Google Scholar]
- Piriyaprasarth S.; Juttulapa M.; Sriamornsak P. Stability of rice bran oil-in-water emulsions stabilized by pectin-zein complexes: Effect of composition and order of mixing. Food Hydrocolloids 2016, 61, 589–598. 10.1016/j.foodhyd.2016.06.015. [DOI] [Google Scholar]
- Brock J.; Nogueira M. R.; Zakrzevski C.; de Castilhos Corazza F.; Corazza M. L. Determinação experimental da viscosidade e condutividade térmica de óleos vegetais. Ciência Tecnol. Aliment. 2008, 28, 564–570. 10.1590/s0101-20612008000300010. [DOI] [Google Scholar]
- Oliveira E. A.; Maciel Filho R.; Wof Maciel M. R.; Rios H. I.; Perez H. I. Q. Palm oil carotenoids extraction: preparation process optimization. Chem. Eng. Trans. 2009, 17, 1341–1346. 10.3303/CET0917224. [DOI] [Google Scholar]
- Nogueira C. A.; Nogueira V. M.; Santiago D. F.; Machado F. A.; Fernandes F. A. N.; Santiago-Aguiar R. S.; de Sant’Ana H. B. Density and Viscosity of Binary Systems Containing (Linseed or Corn) Oil, (Linseed or Corn) Biodiesel and Diesel. J. Chem. Eng. Data 2015, 60, 3120–3131. 10.1021/acs.jced.5b00289. [DOI] [Google Scholar]
- Jafari S. M.; Beheshti P.; Assadpoor E. Rheological behavior and stability of D-limonene emulsions made by a novel hydrocolloid (Angum gum) compared with Arabic gum. J. Food Eng. 2012, 109, 1–8. 10.1016/j.jfoodeng.2011.10.016. [DOI] [Google Scholar]
- Wilde P. J. Interfaces: their role in foam and emulsion behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. 10.1016/s1359-0294(00)00056-x. [DOI] [Google Scholar]
- Sakuno M. M.; Matsumoto S.; Kawai S.; Taihei K.; Matsumura Y. Adsorption and Structural Change of β-Lactoglobulin at the Diacylglycerol–Water Interface. Langmuir 2008, 24, 11483–11488. 10.1021/la8018277. [DOI] [PubMed] [Google Scholar]
- Mun S.; Decker E. A.; McClements D. J. Influence of emulsifier type on in vitro digestibility of lipid droplets by pancreatic lipase. Food Res. Int. 2007, 40, 770–781. 10.1016/j.foodres.2007.01.007. [DOI] [Google Scholar]
- Anema S. G.The Whey Proteins in Milk: Thermal Denaturation, Physical Interactions, and Effects on the Functional Properties of Milk. In Milk Protein, 2nd ed.; AcademicPress, 2014, pp 269–318. [Google Scholar]
- Xie Y.; Chen J.; Zhang S.; Fan K.; Chen G.; Zhuang Z.; Zeng M.; Chen D.; Lu L.; Yang L.; Yang F. The research about microscopic structure of emulsion membrane in O/W emulsion by NMR and its influence to emulsion stability. Int. J. Pharm. 2016, 500, 110–119. 10.1016/j.ijpharm.2016.01.032. [DOI] [PubMed] [Google Scholar]
- Gomes A.; Costa A. L. R.; Cunha R. L. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: Adsorption, interfacial rheology and emulsion features. Colloids Surf., B 2018, 164, 272–280. 10.1016/j.colsurfb.2018.01.032. [DOI] [PubMed] [Google Scholar]
- McClements D. J.Food Emulsions: Principles, Practice and Techniques: CRC Press, 2005; p 714. [Google Scholar]
- Sun C.; Gunasekaran S.; Richards M. P. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocolloids 2007, 21, 555–564. 10.1016/j.foodhyd.2006.06.003. [DOI] [Google Scholar]
- Pal R. Shear Viscosity Behavior of Emulsions of Two Immiscible Liquids. J. Colloid Interface Sci. 2000, 225, 359–366. 10.1006/jcis.2000.6776. [DOI] [PubMed] [Google Scholar]
- Day L.; Zhai J.; Xu M.; Jones N. C.; Hoffmann S. V.; Wooster T. J. Conformational changes of globular proteins adsorbed at oil-in-water emulsion interfaces examined by Synchrotron Radiation Circular Dichroism. Food Hydrocolloids 2014, 34, 78–87. 10.1016/j.foodhyd.2012.12.015. [DOI] [Google Scholar]
- Pugnaloni L. A.; Dickinson E.; Ettelaie R.; Mackie A. R.; Wilde P. J. Competitive adsorption of proteins and low-molecular-weight surfactants: computer simulation and microscopic imaging. Adv. Colloid Interface Sci. 2004, 107, 27–49. 10.1016/j.cis.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hu M.; McClements D. J.; Decker E. A. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. 10.1021/jf020952j. [DOI] [PubMed] [Google Scholar]
- Kim H.J.; Decker E.A.; McClements D.J. Role of Postadsorption Conformation Changes of β-Lactoglobulin on Its Ability To Stabilize Oil Droplets against Flocculation during Heating at Neutral pH. Langmuir 2002, 18, 7577–7583. 10.1021/La020385u. [DOI] [Google Scholar]