Abstract

Continuous hydrogenation of aqueous furfural (4.5%) was studied using a monolith form (ACM) of an activated carbon Pd catalyst (∼1.2% Pd). A sequential reaction pathway was observed, with ACM achieving high selectivity and space time yields (STYs) for furfuryl alcohol (∼25%, 60–70 g/L-cat/h, 7–15 1/h liquid hourly space velocity, LHSV), 2-methylfuran (∼25%, 45–50 g/L-cat/h, 7–15 1/h LHSV), and tetrahydrofurfuryl alcohol (∼20–60%, 10–50 g/L-cat/h, <7 1/h LHSV). ACM showed a low loss of activity and metal leaching over the course of the reactions and was not limited by H2 external mass transfer resistance. Acetic acid (1%) did not significantly affect furfural conversion and product yields using ACM, suggesting Pd/ACM’s potential for conversion of crude furfural. Limited metal leaching combined with high metal dispersion and H2 mass transfer rates in the composite carbon catalyst (ACM) provides possible advantages over granular and powdered forms in continuous processing.
1. Introduction
The negative economic and environmental impact of petroleum utilization in the chemical industry has been a concern for a long time. Issues such as toxicity, climate change, and acid rain are some of the unavoidable impacts of petroleum carbon sources. Therefore, finding suitable biorenewable replacements for petroleum-based resources is one of the most important aims of the chemical industry. Furfural (FUR), an aldehyde of furan, is a biomass-derived chemical that can be generated by a two-step acid hydrolysis of hemicellulose-rich biomasses such as corn stover, wheat bran, or corncobs. The first step generates xylose, and in the second step catalytic dehydration of xylose yields furfural. The global production of furfural is ∼300 000 tons per year,1 with a market price of 1000 U.S. dollars per ton.2 Furfural can undergo different reactions, such as C–C dissociation, C–O hydrogenolysis, C=O hydrogenation, furan ring rearrangement, furan ring hydrogenation, and polymerization.3 Among these reactions, hydrogenation of furfural can result in a series of valuable upgraded products such as furfuryl alcohol (important monomer for furan resins), tetrahydrofurfuryl alcohol (solvent for agricultural, cleaning, coating, and paint stripper formulations), 2-methyltetrahydrofuran and 2-methylfuran (promising alternative fuels), and cyclopentanone (precursor for aviation fuels, rubber chemicals, and pharmaceuticals). Several research groups have investigated hydrogenation of furfural in both vapor and liquid phases. Examples of furfural hydrogenation in the vapor phase to furfuryl alcohol and 2-methylfuran include the use of a carbon-supported copper catalyst at temperatures of 200–300 °C, silica-supported copper at temperatures of 230–290 °C, Cu/Zn/Al/Ca/Na (59:33:6:1:1atomic ratio) and Cu/Cr/Ni/Zn/Fe (43:45:8:3:1, atomic ratio) at temperatures of 200–300 °C, and SBA-15 silica-supported copper at temperatures of 170–270 °C.4−6 Examples of liquid-phase hydrogenation of furfural to furfuryl alcohol and 2-methylfuran include carbon-supported ruthenium at temperatures of 120–200 °C using 2-propanol as hydrogen donor under 2.04 MPa nitrogen pressure, over Ni–Co–B amorphous alloy catalyst at 100 °C and 1 MPa (in ethanol), and metal oxide-supported platinum at temperatures of 50–70 °C (in methanol and n-butanol).7−10 Hydrogenation of furfural to cyclopentanone involves a fast hydrogenation step to furfuryl alcohol followed by an acid-catalyzed rearrangement of the furan ring, also known as Piancatelli rearrangement, to 4-hydroxy-2-cyclopentenone. In 1976, Giovanni Piancatelli and co-workers discovered the rearrangement of 2-furylcarbinols into 4-hydroxycyclopentenones in the presence of acid catalysts (2:1 acetone–water mixture, with the type of acid not reported).11,12 Piancatelli et al. observed that for more reactive reaction substrates weaker Lewis acids are required. After furfuryl alcohol rearrangement, 4-hydroxy-2-cyclopentenone undergoes fast hydrogenation and dehydration steps to form cyclopentanone (due to high reactivity). Further hydrogenation of cyclopentanone can result in the formation of cyclopentanol. Hronec et al. investigated the rearrangement of furfuryl alcohol to 4-hydroxy-2-cyclopentenone in water at temperatures of 110–200 °C and reported that the yield of 4-hydroxy-2-cyclopentenone increased with increasing temperature.13 They suggested that due to the presence of hydrogen ions resulting from the dissociation of water to hydrogen and hydroxyl ions at high temperatures the aqueous medium acted as an acid catalyst for the rearrangement step. In another study, Hronec et al. investigated the effect of solvent type on furfural rearrangement, in which N-decanol, water, and 2-propanol were tested in the presence of precious metal catalysts at temperatures of 160–175 °C.14 Only in the presence of water were high cyclopentanone yields reported. Use of alcohols as the solvent increased selectivity toward 2-methylfuran and tetrahydrofurfuryl alcohol, which confirms the key role of water in the ring rearrangement leading to cyclopentanone. Further hydrogenation of all of the mentioned products can lead to the production of long-chain alcohols.
Activated carbon (AC) is often used as a support in catalytic hydrogenation, since it can be synthesized from renewable carbon sources and is stable in acidic, basic, and aqueous environments.15−17 Often, powdered forms are used in batch slurry reactors or granular forms are used in trickle bed reactors, but these forms are difficult to use in continuous processing.15 There is much interest in continuous processing since it can reduce the costs, relative to batch processes.18 The powdered forms can undergo attrition and are difficult to recover and reuse continuously.15,18 High flow rates in trickle bed systems can cause large pressure drops, and H2 external and internal mass transfer can be rate-limiting if a large particle size is used to minimize the pressure drop. Activated carbon formed into monoliths used as supports can overcome the problems in using powdered and granular forms in continuous processing.
Monolith catalysts are honeycomb-shaped structures that have been mainly used in the automotive and environmental industry for air pollution control. The use of monolith catalysts in the automotive industry dates back to the mid-1970s when monolith catalysts were applied as catalytic converters for the reduction of nitric oxides in exhaust gas.19 Monolith structures, commonly fabricated using ceramic and metallic materials, provide a group of uniform straight channels that are separated by thin walls. For catalytic purposes, these ceramic or metallic structures are coated with a layer of catalyst, such as palladium and platinum, or catalyst support, such as carbon, zeolites, and silica. To assure the best adherence of the catalyst coat to the monolith structure, binders, additive, chemical, and heat pretreatment are applied.20 Compared to conventional packed bed reactors, monolithic reactors provide lower pressure drop, high surface-area-to-volume ratio, high mass transfer rates, and easy scale-up and filtration.20 Activated carbon is a promising catalyst support that provides well-developed porosity and high surface area for catalyst particles. Activated carbon-coated ceramic and metallic monolith structures have been employed as supports for catalysts such as precious metals. However, there are some disadvantages associated with using activated carbon-coated ceramic or metal catalyst support, such as durability and inertness. One solution to overcome these issues is to use renewable integrated carbon materials. In addition to solving durability and inertness issues, employing activated carbon monolith (ACM) catalysts as a support for precious metals offers easy recovery of the metal by a combustion step.
This work focuses on continuous aqueous phase hydrogenation of furfural using metal-supported activated carbon monolith catalysts compared to traditional granular and fine powdered carbons. Activated carbon monolith (ACM) catalysts, derived from woody biomass, are impregnated with precious metals and employed for furfural hydrogenation reactions. The advantages of using ACM catalysts, in addition to their low pressure drop, high mass transfer rates, high surface-area-to-volume ratios, and easy scale-up, are that the monolith is made from renewable carbon sources and that they are highly stable in aqueous/acid/base reaction medium. The most novel aspects of this work were the use of activated carbon monolith catalysts for continuous furfural hydrogenation in an aqueous and acidic environment and the low metal loadings used to achieve high conversions and space time yield (STY) with limited leaching. Although activated carbon monoliths have distinct advantages as a catalyst support, there is little information on the use of these structured catalysts highlighting their advantages.
2. Results and Discussion
2.1. Catalyst Characterization
Untreated Pd/ACM (0.8 wt % Pd) catalyst had a surface area and pore volume similar to those of ACM only, but there were differences between the powdered and granular forms of activated carbon (Table 1, Figures S1 and S2). The surface area and pore volume for Pd/ACM were lower than those of Pd/GAC, but similar to those of Pd/powder C (Table 1). These data do suggest that the surface properties of the activated carbon were changed and the resultant material differentiated (Pd/ACM) by processing and extrusion into monolith supports. Most notably, the Pd/ACM did have a significantly smaller micropore volume, higher Pd dispersion, and resultant smaller active particle size compared to the GAC and powder supports (Table 1, Figures S1 and S2).
Table 1. Physical Properties of the Carbon Catalysts.
| catalyst properties | Pd/ACM (fresh) | Pd/ACM (spent)d | Pd/GAC (fresh) | Pd/powder C (fresh) |
|---|---|---|---|---|
| metal loading (wt %) | 1.2 | 0.95 (21%)e | 0.56 | 4.8 |
| bulk density (g/cm3) | 0.28 | NP | 0.22 | 0.25 |
| surface area (m2/g) | 608 | 470 | 805–914 | 686 |
| pore volume (cm3/g) | 0.45 | 0.46 | 0.43 | 0.47 |
| average pore size (radius, Å) | 29.8 | 39.3 | 21.6 | 27.5 |
| Pd dispersion (%) (μmoles CO/g)b | 47 (53.2) | NP (7.8) | 39 (20.5) | 17.7 (79.8) |
| Pd particle size (nm)b | 2.4 | NP | 2.8 | 6.3 |
| micropore area (m2/g)c | 14 | 0.0 | 625–737 | 349 |
| micropore volume (cm3/g)c | 0.005 | 0.0 | 0.33 | 0.18 |
| weak acid sitesf (μmoles NH3/g)—155 °C | 0.0 | NP | 0.0 | 86 |
| strong acid sitesf (μmoles NH3/g)—390 °C | 239 | NP | 0.0 | 0.0 |
CO pulse titration, 1:1 stoichiometry assumed, reduced with 100% H2 at 250 °C for 2 h. Particle size and dispersion calculations were not performed (NP) for the spent catalysts due to possible coking effect on CO uptake.
Particle size estimated from dispersion via CO pulse titration.
Estimated from t-plot analysis.
Spent catalysts from furfural hydrogenation.
% Pd loss via leaching in brackets.
Estimated from NH3 temperature-programmed desorption (TPD) for H2-reduced catalysts, estimated peak desorption temperature. ACM is activated carbon monolith, GAC is granular activated carbon, powder is from Alfa Aesar, NP—not performed.
Reducibility of the metal catalysts was determined from the hydrogen consumption of the catalyst versus temperature using temperature-programmed reduction (TPR) analysis (Figure S3A). All Pd catalysts showed a negative peak between 45 and 75 °C (Figure SI3B). These peaks are indicative of PdHx (Pd hydride) decomposition due to freely available PdO and have been reported for Pd-supported catalysts in the range from 60 to 80 °C.21−23 The ACM support (without Pd) did show a negative peak as well at 48 °C, which we attribute to an unknown off-gas component potentially due to the binder.
TPR analysis of the 0.8% Pd/ACM catalyst demonstrates two additional peaks at temperatures of 205 and 287 °C, which is an indicator for the reduction of Pd oxide to Pd metal (Figure S3A). Pd2+ reduction to Pd° has been shown to range from 130 to 227 °C on activated carbons and can depend on the pretreatment method.21 The peak at 287 °C could be due to Pd interaction with the binder component alumina. For example, TPR analysis of Pd/Al2O3 catalysts has indicated the reduction of PdO at 295 and 350 °C (prepared from H2PdCl4 or Pd(NO3)2).23,24 We attribute the peak at ∼470 °C to hydrogen’s reaction with oxygen functional groups on the carbon surface or a gasification reaction with the carbon. CO and CO2 TPD analyses of activated carbons in helium suggest that peak evolution at temperatures >300 °C can occur due to decomposition of oxygen functional groups.25
Pd/powder C showed three additional peaks, one small peak at 75 °C after the negative peak (Figure SI3B) and two broad peaks (relative to Pd/ACM) centered at around 350 °C (Figure S3A) and 560 °C. The positive peak at 75 °C is indicative of further PdO reduction and has been reported on activated carbon and Al2O3 supports.22,23,26 Similar to Pd/ACM analysis, we attribute the peaks at 350 and 560 °C to hydrogen’s reaction with oxygen functional groups on the carbon surface or a gasification reaction with the carbon.
After the negative peak at 50 °C, Pd on granular activated carbon (GAC) demonstrated two small broad peaks at temperatures of 125 and 350 °C (attributed to thermal reactions with carbon). Compared to the Pd/GAC, the Pd/ACM appeared to have a higher peak hydrogen reduction temperature (205 and 287 versus 125 °C) and higher H2 consumption (Figure S3A). The peak at 125 °C for Pd/GAC is at the lower end of the range reported for PdO reduction on many activated carbons (127–227 °C).21 Some Pd on activated carbon materials only show the low-temperature negative peak (50–80 °C) with the associated H2 consumption peak (75–100 °C), which has been suggested to be due to a weak interaction of PdO with carbon.22,23
These differences may be attributed to different metal support interactions in ACM from the other two catalysts since the ACM support is manufactured from activated carbon, binders, and other additives. Scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDS) analysis indicates some co-location of Pd with Al and Si, suggesting interaction with a ceramic binder, but much of the Pd appears to be distributed on carbon (Figure S4). TPR analysis of the ACM only (no Pd present) did indicate a small background H2 consumption over the entire temperature range, possibly due to the metal oxides (e.g., Al2O3) present in the binder (Figure S3A). However, we acknowledge that the background H2 consumption could also be due to the carbon reacting with H2 as well.
A shift to higher peak hydrogen temperatures and higher H2 consumption in the Pd/ACM could be due to interaction with the binder in the ACM and its higher Pd dispersion than the other two carbon catalysts (Table 1). It is generally recognized that oxygen functional groups (e.g., carboxylic, phenolic, and laconic groups) in activated carbon can provide nucleation sites for metallic crystallites.17 Increased oxygen functional group density reportedly leads to increased metal dispersion, a reduction in particle size, and resistance to sintering.27 Thus, smaller particle size or higher dispersion could shift the TPR peaks to higher temperatures. Smaller particles could have increased interaction with the support in the Pd/ACM catalyst, resulting in a higher dispersion and thus significant change in the TPR curves. This effect has been noted for Pd/Al2O3 and indicated for Pd on carbon catalysts.23,28,29 For example, higher Pd dispersion on Al2O3 due to stronger interaction with the support leads to a TPR peak at 350 °C.3 Calcination of the Pd/Al2O3 in air eliminated this TPR peak (350 °C), which was indicated to be due to enlargement of the PdO particles and reduced interaction with the support, allowing PdO reduction at room temperature.23 Also, such a peak (350 °C) was not observed for Pd/SiO2 and Pd/C in this work and was suggested to be due to a lack of interaction with these supports.23 Similarly, increased oxygen groups in carbon, which provide anchoring/adsorption sites for Pd (via C–O groups), lead to an increase in the H2-TPR peak (∼183 °C), increased Pd interaction with the support, and increased Pd dispersion, compared to nonfunctionalized carbon (∼137 and ∼175 °C, maximum peak).28,29
Additionally, such shifts in TPR profiles have been observed in carbon-encapsulated metal oxide supports (e.g., Al2O3, ZrO2) when active metals have been deposited.30,31 For example, Ni on carbon-covered Al2O3 showed a shift in peak hydrogen consumption from ∼250 to ∼400 °C, relative to Ni on AC, and better stability toward hydrogenation of nitrobenzene to aniline.30 Carbon-encapsulated ZrO2 deposited with Ru was shown to increase the metal support strength, minimizing Ru leaching in aqueous, acidic environments, while hydrogenating levulinic acid to γ-valerolactone.31 Again, a shift to higher peak hydrogen consumption temperatures was observed for the Ru/carbon-encapsulated ZrO2 versus Ru on AC.31
Ammonia-TPD analysis of the unreduced Pd/ACM and Pd/powder C catalysts demonstrated a peak at 200–260 °C, suggesting the presence of weak acid sites, potentially due to metal oxides in the carbon or binders used in the ACM (Figure 1). The Pd/GAC catalyst did not show any peaks during ammonia desorption (Figure 1). Upon H2 reduction of the Pd/ACM, the weak acid sites disappeared, suggesting that metal oxides acting as weak acid sites were reduced during the pretreatment step to generate the Pd metal (Figure 1). A lower amount of strong acid sites was observed in the Pd/ACM (300–500 °C) upon H2 reduction, again probably due to the binder in the ACM, since the GAC that was used in the synthesis of the ACM showed no observable peaks. The TPD for H2-reduced Pd/GAC was the same as that for the unreduced Pd/GAC catalyst (Figure 1). Upon H2 reduction, the Pd/powder C did show a small weak acid site, which shifted in desorption from 200–260 to 100–200 °C. When comparing TPD analysis of the three H2-reduced catalysts, the presence of this weak acid site in the Pd/powder C and strong acid site in the Pd/ACM was the most noticeable difference (bottom plot, Figure 1).
Figure 1.
Ammonia-TPD analysis of Pd on carbon catalysts prereduced with H2 (100% H2 for 2 h at 250 °C) and without H2 reduction. A moving average is reported for the NH3-TPD.
X-ray diffraction (XRD) analysis indicated the presence of graphite with sharp peaks at 2θ of 21° and 26° for both powdered and monolith forms of catalyst, whereas GAC indicated broader peaks implying a more amorphous carbon structure (Figure S5). The Pd/powder C XRD did indicate a peak at 40° (2θ), suggesting Pd(111). XRD results for GAC and ACM before and after impregnation with Pd did not indicate a significant change, which suggests that Pd is well dispersed on both GAC and ACM. The lack of Pd or PdO peaks for Pd/ACM and GAC was probably due to the low Pd loading and high dispersion. We also performed XRD on the spent Pd/ACM catalyst (prereduced with H2 and reacted) and observed little difference (Figure S5). Overall, the XRD analysis indicates no change in the Pd/ACM catalyst. The XRD analysis of the Pd/ACM and Pd/GAC was indicative of low Pd loading and high dispersion.
2.2. Temperature and Pressure Effects
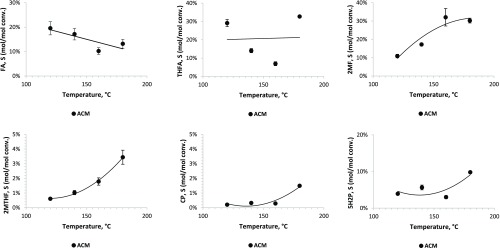
Since little work has been conducted on continuous hydrogenation of furfural for the synthesis of furfuryl alcohol, tetrahydrofurfuryl alcohol, and cyclopentanone, using activated carbon monoliths, we wanted to determine the effect of temperature and pressure on selectivity and space time yields or conversion. Thus, in a set of experiments, Pd/ACM was employed to determine the effect of temperature on furfural hydrogenation. At all temperatures, furfural conversion was higher than 90% for ACM. The highest carbon closure for ACM was achieved at 180 °C (∼90%, Figure S6). Increasing temperatures reduced furfuryl alcohol (FA) selectivity and conclusively increased the selectivity for all products except tetrahydrofurfuryl alcohol (THFA) (Figure 2).
Figure 2.
Effect of reaction temperature on product selectivity (FA, furfuryl alcohol; THFA, tetrahydrofurfuryl alcohol; 2MF, 2-methylfuran; 2MTHF, 2-methyltetrahydrofuran; 5H2P, 5-hydroxy-2-pentanone; CP, cyclopentanone). All reactions were conducted at 180 °C, 300 psig (2.04 MPa) and 1.32 1/h liquid hourly space velocity (LHSV) with 0.8% Pd on ACM.
After a series of reaction studies, varying temperature indicated that a temperature of 180 °C would generate high furfural conversions, high carbon closures, and high product selectivity, and the effect of pressure was studied at this temperature. For the Pd/ACM catalyst, FA, THFA, and 2MF selectivities increased with pressure and were significantly higher at 300 psig (Figure 3). 2MTHF selectivity declined with increasing pressure (Figure 3). The selectivity of 5H2P also increased with pressure, and the cyclopentanone (CP) levels were low under all conditions (Figure 3). As discussed later, the type and level of acid sites in the catalysts combined with pressure may have played a role in 5H2P production and selectivity over CP formation. It has been suggested that water, under subcritical conditions, and Lewis acids act as catalysts in promoting a Piancatelli rearrangement to 5H2P or CP from furfuryl alcohol.11−13 Since reaction rates were higher at 180 °C and the selectivity of the Piancatelli rearrangement product, 5H2P, the highest at 300 psig, it was decided to conduct future reaction experiments under these conditions (180 °C, 300 psig or 2.1 MPa).
Figure 3.
Effect of reaction pressure on product selectivity (FA, furfuryl alcohol; THFA, tetrahydrofurfuryl alcohol; 2MF, 2-methylfuran; 2MTHF, 2-methyltetrahydrofuran; 5H2P, 5-hydroxy-2-pentanone; CP, cyclopentanone). All reactions were performed at 180 °C and 1.32 1/h LHSV using Pd/ACM.
2.3. Liquid Hourly Space Velocity Effect
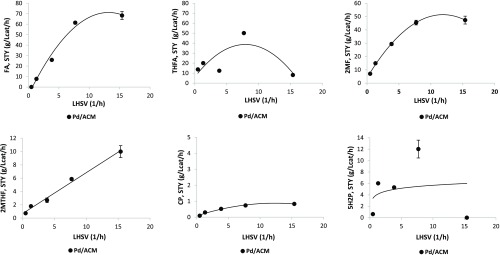
In the next set of experiments, the effect of liquid residence time on furfural hydrogenation over 0.8% Pd/ACM was determined (the H2 gas flow was held constant at 100 mL/min). Furfural conversions declined with increasing LHSV or shorter residence time, and carbon closure ranged from 74 to 100% (Figure S7). With increasing LHSV (higher flow rates or shorter contact time), the selectivity of FA increased and reached a plateau, THFA declined significantly from 60%, 2MF declined from 38 to 18%, and 5H2P declined as well, and 2MTHF selectivity changed little with LHSV (Figure 4). These same trends were observed for the space time yield or STY of products; i.e., the ACM catalyst generated the highest FA, 2MF, and THFA space time yields at residence times between 0.07 and 0.53 h (Figure 5). The selectivity and STY plots demonstrate that at short contact time or high flow rates the primary products of the reactions in the presence of the Pd/ACM catalysts (180 °C, 300 psig) are furfuryl alcohol and 2-methylfuran (Figures 4 and 5). A high furfuryl alcohol STY at short contact time indicates that the furfural to furfuryl alcohol (FA) reaction occurs at a fast rate. In a second fast hydrogenolysis step furfuryl alcohol forms 2-methylfuran (2MF), and in a further hydrogenation step 2-methyltetrahydrofuran (2MTHF) is formed from 2MF. Interestingly, as LHSV decreased, there was an increase in the 5-hydroxy-2-pentanone (5H2P) selectivity and then a decline at very long residence times (Figure 5). 5H2P STY was significantly higher between a 0.16 and 0.3 h liquid residence time. The results from the temperature, pressure, and liquid residence time effect studies suggest that Pd/ACM primarily promoted the formation of FA and 2MF from furfural and minor formation of CP and 5H2P via a speculative reaction pathway, requiring acid catalysis for the ketone(s) formation (Figure 6).
Figure 4.
Effect of liquid hourly space velocity (LHSV, 1/h) on product selectivity using Pd/ACM at 180 °C and 300 psig (FA, furfuryl alcohol; THFA, tetrahydrofurfuryl alcohol; 2MF, 2-methylfuran; 2MTHF, 2-methyltetrahydrofuran; 5H2P, 5-hydroxy-2-pentanone; CP, cyclopentanone).
Figure 5.
Effect of liquid residence time on space time yields (STYs, g/L-cat/h) using Pd/ACM at 180 °C and 300 psig (FA, furfuryl alcohol; THFA, tetrahydrofurfuryl alcohol; 2MF, 2-methylfuran; 2MTHF, 2-methyltetrahydrofuran; 5H2P, 5-hydroxy-2-pentanone; CP, cyclopentanone).
Figure 6.
Speculative pathway for furfural (FUR) hydrogenation, dehydration, and Piancatelli rearrangement to 2-methylfuran (2MF), cyclopentanone (CP), and 5-hydroxy-2-pentanone (5H2P) using Pd on carbon catalysts. FA, furfuryl alcohol; THFA, tetrahydrofurfuryl alcohol; 2MTHF, 2-methyltetrahydrofuran; 2CP, 2-cyclopentenone; 4HCP, 4-hydroxy-2-cyclopentenone; 1,4-PD, 1,4-pentanediol.40,46 [ ] indicates a possible short-lived intermediate; { } indicates an anticipated product or intermediate not observed.
2.4. Palladium Loading Effect
In reactions with multiple products and side reactions, the size of the active metal particle can affect product selectivity. Past work on furfural hydrogenation using palladium catalysts suggested that the product distribution can be controlled by particle size and differences in product selectivity can be attributed to metal dispersion.32 Given the possibility that Pd particle size may have affected product selectivity, a series of catalysts were prepared with higher Pd loading, lower dispersion, and thus larger particle size. Originally, it was considered to change the Pd particle size by increasing the H2 reduction temperature, anticipating a reduction in dispersion and an increase in particle size. However, a series of CO pulse titrations with increasing H2 reduction temperature had little effect on the 0.8 wt % Pd on ACM, except at temperatures greater than 350 °C (Figure S8). Thus, it was decided to increase the Pd loading and reduce H2 at 250 °C. These catalysts were prepared by crushing the base ACM support and loading Pd via wet impregnation. Our rationale for this preparation method was that in future experiments it was anticipated to develop bimetal/bifunctional catalysts and felt that this technique would be easier to use for the ACM material to screen activity (a very limited supply of the monolith cores was available). Subsequently, once an optimum metal loading and bimetal ratio were determined, the monolith structure could be synthesized and tested. As indicated in Table 2, increasing the Pd loading did reduce the surface area, pore volume, and metal dispersion. The most significant observed effects were for THFA and 2MTHF; THFA selectivity and reaction rate declined with increased Pd loading and 2MTHF selectivity, and reaction rate increased with increased Pd loading (Figure S9, data not shown for rates [both r, mmol/g-cat/h and STY, g/L-cat/h]). These results suggest that Pd loading had limited effect on CP and 5H2P formation and the hydration/dehydration/rearrangement of FA.
Table 2. Physical Properties of Carbon Catalysts with Different Palladium Loadings Using Crushed ACMa.
| catalyst properties | 0.8Pd/cACM (fresh) | 2.5Pd/cACM (fresh) | 5.0Pd/cACM (fresh) |
|---|---|---|---|
| metal loading (wt %) | 0.3 | 2.4 | 5.6 |
| bulk density (g/cm3) | 0.28 | 0.28 | 0.28 |
| surface area (m2/g) | 844 | 761 | 628 |
| micropore area (m2/g)b | 33.6 | 44.7 | 37.5 |
| pore volume (cm3/g)c | 0.732 | 0.659 | 0.545 |
| average pore size (radius, Å) | 17.34 | 17.32 | 17.34 |
| Pd dispersion (%)d | 72 | 26 | 8 |
| Pd particle size (Å)e | 15.6 | 42.4 | 145.5 |
cACM is crushed activated carbon monolith.
Estimated using the de Boer equation.
Total pore volume at P/P0 = 0.992.
CO pulse titration, 1:1 stoichiometry assumed, reduced with 100% H2 at 250 °C for 2 h.
Particle size estimated from dispersion via CO pulse titration.
2.5. External Weak Acid Effect
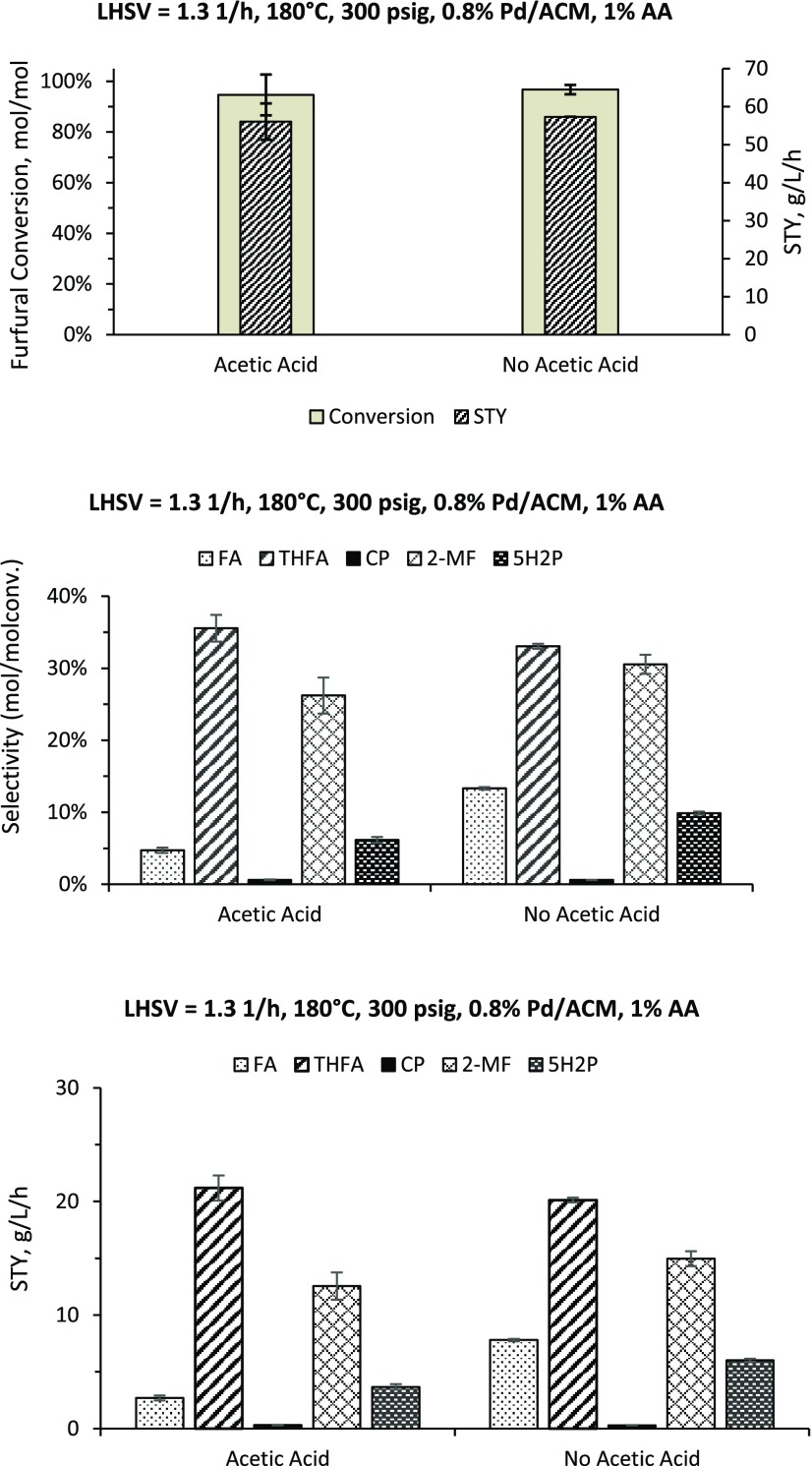
Industrial production of furfural involves acid hydrolysis of biomass followed by a dehydration step in the presence of an acid catalyst at temperatures of 200–240 °C. After several steam-stripping steps, this process can result in furfural concentrations of up to 99%. These purifying steps are high in energy consumption and are not cost-effective. Therefore, one approach to reducing the process cost is to use crude furfural (∼5 wt % before purification) with impurities or a feed generated from a single distillation step.33 The impurities associated with crude furfural are water and carboxylic acids, such as acetic acid. The presence of these impurities in the reaction medium can have an impact on catalyst activity, selectivity, and reaction pathways. In this work, the effect of acetic acid on product distribution was determined for the ACM support (Figure 7). Adding acetic acid did not significantly affect furfural conversion and 2MF and THFA selectivities (the primary products of the reaction) using Pd/ACM, but it did lower FA selectivity. The presence of acetic acid did lower the 5H2P selectivity when using Pd/ACM. Acetic acid was converted using Pd/ACM (63%). The products of acetic acid transformation could not be confirmed, and ethanol, a possible direct reduction product, was not observed.
Figure 7.
Effect of acetic acid (1 wt %) on product selectivity and space time yield using Pd/ACM (FA, furfuryl alcohol; THFA, tetrahydrofurfuryl alcohol; 2MF, 2-methylfuran; 2MTHF, 2-methyltetrahydrofuran; 5H2P, 5-hydroxy-2-pentanone; CP, cyclopentanone). Reactions were performed at 180 °C, 300 psig (2.07 MPa), and LHSV 1.3 h–1.
2.6. Catalyst Coking and Metal Leaching
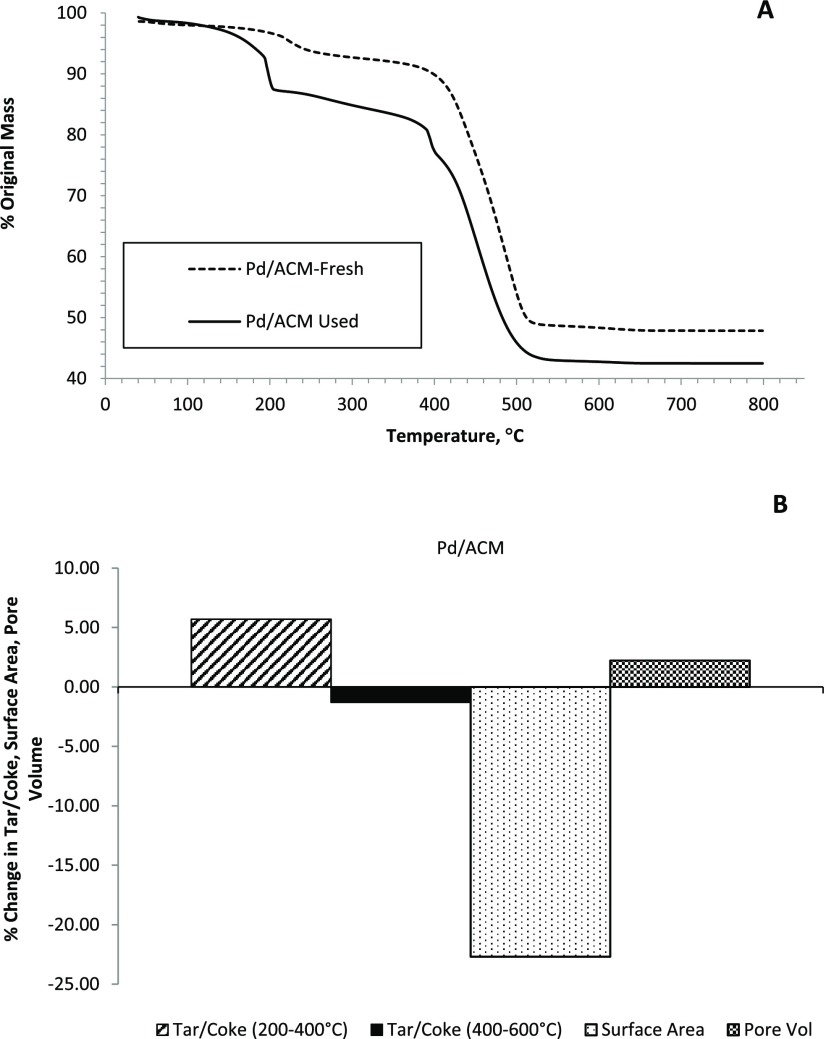
After the furfural hydrogenation experiments, Pd/ACM catalyst was collected and analyzed for changes in surface properties. Time-on-stream (TOS) analysis indicated high furfural conversions during the course of reactions, although conversion is not a good indicator of catalyst activity (Figure S10). Analyzing the activity data, there was a small reduction in surface area, pore volume, and micropore area for Pd/ACM (Table 1). After the reactions, the surface area of Pd/ACM decreased by 23% (Table 1), and there was a reduction in micropore area, which was most noticeable when comparing t-plots between fresh and used catalysts (Figure S11).
Since surface area declined, thermogravimetric analysis (TGA) was also performed to estimate tar/coke levels (Figure 8). Compared to its fresh counterpart over the 200–400 °C range, Pd/ACM only had a 5.7% mass loss, and the % mass loss over the 400–600 °C range was negligible for the Pd/ACM (0%). It is speculated that the coke formed on the Pd/ACM may have deposited in/on an area that did not affect furfural or H2 chemisorption on the Pd metal particles, potentially the ceramic binder. Finally, elemental analysis of the spent catalyst indicated limited leaching of Pd from the ACM (20%, Table S1). We acknowledge that longer and more detailed time-on-stream studies are needed to determine the time effect on Pd leaching, product selectivity, and reaction rates, and to develop regeneration methods for the Pd/ACM.
Figure 8.
TGA analysis (A) of spent catalysts compared to unreacted materials for Pd/ACMC (% original mass is % loss in mass relative to the starting mass) and the change in tar/coke relative to the fresh catalyst, surface area, and pore volume (B) of the spent catalysts relative to the fresh catalyst.
2.7. Mass Transfer Effect
Our results suggest that the higher mass transfer rates of H2 possibly account for the low coke levels, high STYs, and product selectivity for FA, THFA, and 2MF using the Pd/ACM catalyst. Calculating the gas/liquid velocity (Qg/Ql) ratios in this work (6.3–200) indicated film or annular flow (liquid superficial velocity varied from 1.6 to 53 × 10–5 m/s and the gas velocity was 3.3 × 10–3 m/s). Film or annular flow occurs from a Qg/Ql of 6 to 200, and Qg/Ql ratios of 0.2–2 are in Taylor flow for monoliths.34,37 Monolith reactors have three mass transfer steps—gas to solid through a very thin film, gas to a liquid slug with circulating eddies, and from the liquid slug to the solid surface.35,36 Given our Qg/Ql ratio, film flow was assumed, and H2 transport was considered from the gas phase to the solid through a thin film. For the monolith reactions, mass transfer coefficients were estimated using the methods described in the Supporting Information (SI) based on the assumption of film flow.38 The Mears criterion was used to estimate the external mass transfer resistance, and the Wiesz–Prater criterion was calculated to estimate the intraparticle resistance (details in SI). Mears criterion calculations for the Pd/ACM suggest that H2 external mass transfer was not rate-limiting (Table S2). For example, the H2 Mears criterion ranged from 2 to 4 × 10–5 (assuming a second-order reaction) as LHSV increased from 1.3 to 15 h–1 for Pd/ACM, where a value less than 0.15 indicates that external mass transfer is not rate-limiting.29 The estimated Wiesz–Prater criterion was lower than 1, suggesting that our reactions using Pd/ACM were not internally mass-transfer-limited (Table SI3).39 However, we acknowledge that at higher LHSV and lower Qg/Ql ratio CWP ranged from 0.2 to 0.25, indicating possible mass transfer limitation and the need to explore operating at higher Qg/Ql ratio (Table S3).
3. Comparison to Literature
As mentioned earlier, little work has been performed on continuous furfural hydrogenation using palladium catalysts, especially in aqueous systems using a honeycombed activated carbon monolith. One recent work using 5-hydroxymethyl furfural (HMF) is included, which is analogous to furfural.44 No work on the use of activated carbon monolith catalysts for furfural hydrogenation could be found. Table 3 demonstrates a comparison of selectivity and space time yield of furfural hydrogenation products between this work and previously reported literature. All of the reported works in Table 3 employed a Pd on activated carbon or carbon catalyst for furfural or HMF hydrogenation. Batch processing at high pressures (≥30 bar) and long residence times in water produced Piancatelli rearrangement products (CP and 5H2P, entries A, G, H). Using isopropanol (as an organic solvent) eliminated the Piancatelli rearrangement products, resulting in high selectivity toward FA and THFA (entry F, Table 3). In continuous processing, little Piancatelli rearrangement products were produced regardless of the solvent used. For example, using ethyl acetate at 150 °C produced a high THFA selectivity (entry C, Table 3). Using cyclopentyl methyl ether as a solvent at the same temperature gave predominately FA and THFA (entry D, Table 3). When using water in continuous processing, the products were predominately FA, THFA, and 2MF (entry E). Continuous hydrogenation of HMF using a Pd/GAC catalyst produced primarily DMTHF (analogues to FA) and DHMTHF (analogues to THFA) at similar STYs compared to furfural hydrogenation, and no Piancatelli rearrangement products were observed (entry I, Table 3). Comparing our results with an earlier work by Resasco et al. indicates how the support may significantly affect the furfural hydrodeoxygenation pathway.45 In this work, Pd/SiO2 was tested for continuous hydrogenation of furfural (no solvent, vapor phase, 210–250 °C) and resulted in the formation of furan through a decarbonylation step followed by hydrogenation steps to form minor levels of tetrahydrofuran, FA, and THFA (in order of decreasing yield).45 As the temperature was increased, decarbonylation selectivity increased and hydrogenation product selectivity decreased.45 In our work, we did not measure furan formation but observed an increase in the hydrogenolysis step with increasing temperature (Figure 2, increase in 2MF selectivity). We also formed higher levels of THFA (hydrogenation of FA) and formed CP and 5H2P, probably due to the presence of water (rearrangement of FA).
Table 3. Comparison of Selectivity and Space Time Yield for Products of Furfural Hydrogenation Reactions Using Carbon-Supported Catalystsa.
| selectivity
(%) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| entry | Pd catalyst | reactor mode | solvent | T (°C) | P (bar) | conversion (%) | FA | THFA | 2MF | 2MTHF | CP | 5H2P | STYb (g/(L h)) | reference |
| A | 1% Pd/CNT | B | water | 150 | 30 | 98 | 0 | 1.7 | NR | 5.7 | 49.4 | 3.1 | Mironenko et al.40 | |
| B | 5% Pd/AC | C | EtOAc | 90 | 50 | 99 | 0 | 0 | 75 | NR | NR | NR | Garcia-Olmo et al.41 | |
| C | 10% Pd/AC | C | EtOAc | 150 | 50 | 99 | 0 | 75 | NR | NR | NR | NR | Ouyang et al.42 | |
| D | 3% Pd/AC | C | CPME | 150 | 50 | 97 | 25 | 56 | 5 | <10 | NR | NR | Wang et al.43 | |
| E | 0.8% Pd/ACM | C | water | 180 | 20 | 97 | 25 | 20 | 22 | 3 | 0.5 | 5 | 61, ∼40 | this work |
| F | 3% Pd/AC | B | i-PrOH | 200 | 30 | 47 | 33 | 52 | 5 | NR | NR | NR | Wang et al.43 | |
| G | 5% Pd/C | B | water | 160 | 30 | 97.8 | 0 | 2.1 | 2.4 | 6 | 69 | NR | Hronec et al.14 | |
| H | 5% Pd/C | B | water | 175 | 80 | 100 | 0 | 36 | 3 | 10 | 39 | NR | Hronec et al.14 | |
| I | 10% Pd/C (HMF) | C | water | 90 | 90 | 100 | 21 (DMTHF) | 17 (DHMTHF) | 33, 30 | Lima et al.44 | ||||
NR: not reported; C: continuous; B: batch; HMF: hydroxymethyl furfural. FA: furfuryl alcohol; THFA: tetrahydrofurfuryl alcohol; 2MF: 2-methylfuran; 2MTHF: 2-methyltetrahydrofuran; CP: cyclopentanone; 5H2P: 5-hydroxy-2-pentanone. CPME: cyclopentyl methyl ether; EtOAc: ethyl acetate; i-PrOH: isopropanol. DMTHF: 2,5- dihydroxymethylfuran; DHMTHF: 2,5-bis(hydroxymethyl) tetrahydrofuran.
Space time yield for FA or DMTHF and THFA or DHMTHF, respectively, at LHSV of 8 1/h.
Taken together, this analysis suggests that water under subcritical conditions can act as a weak acid and catalyze the Piancatelli rearrangement of FA to CP or 5H2P if given long enough residence times. In continuous processing, it is possible that a higher number of weak acid or Lewis acid sites are needed, especially on the catalyst surface, to promote the Piancatelli rearrangement in shorter process times. Thus, the low cyclopentanone or 5H2P yields using Pd/ACM might be attributed to a lack of weak acid sites on the Pd/ACM catalyst (only a strong acid site was measured) and a much shorter contact time for furfuryl alcohol ring rearranging to CP or 5H2P. Comparing the results of the Pd/ACM with the literature suggests that the higher 5H2P and CP yields reported in the literature are possibly due to the presence of weak acid sites, contrary to the Pd/ACM. Liu et al. suggest weak acid sites as a reason for the formation of 1,4-pentanediol (through 5-hydroxy-2-pentanone) from furfural in water using Ru on mesoporous carbon.46
Overall, the Pd/ACM generated high STYs of FA, THFA, and 2MF from aqueous furfural with very low Pd loading compared to previous work (Table 3). The absence of furfural and H2 external mass transfer resistance and a possible stronger metal support interaction in Pd/ACM resulted in low loss of activity and coke formation. Despite the previously reported works, where high yields of 2MF and THFA were achieved in the presence of organic solvents, the results of using activated carbon monolith catalyst in aqueous furfural hydrogenation indicate that this catalyst was able to achieve a high yield of 2MF and THFA (and FA at lower LHSVs) using only water, an inexpensive, environmentally friendly, and safe solvent. Comparing the NH3-TPD analysis and product selectivity between the Pd/ACM and the literature does suggest that it would be fruitful to add/increase the number of weak acid sites, especially Lewis acid sites, to Pd/ACM. Since Lewis acids are known to promote the Piancatelli rearrangement of furfuryl alcohol (FA)11,12 and ours and other work indicate that furfural conversion to FA is fast, it is theorized that Lewis acid sites on Pd/ACM would overcome the potentially rate-limiting Piancatelli rearrangement step in continuous processing and generate higher selectivity of 5H2P or CP. If the Piancatelli step is promoted (via Lewis acid sites) and overhydrogenation to 2MF and THFA reduced (potentially by adding a second metal to reduce H2 chemisorption), one may successfully increase 5H2P or CP selectivity and STYs from furfural in continuous processing.
Since activated carbon does not have strong acid or Bronsted acid sites, without treatment, we assume that the strong acid sites observed in the Pd/ACM are due to alumina in the binder (Table 1). As noted in our discussion, the general consensus in the literature is that Lewis acid sites or weak acid sites promote the Piancatelli rearrangement of furfural alcohol (generated from furfural) in the presence of water.11,12,47 When stronger acids are used, resinification of the furfural occurs. Thus, the strong acid sites present in the Pd/ACM could have induced oligomerization/resinification of furfural, leading to the coke formation. Substituted furans such as 5-hydroxy-methyl furfural require Bronsted acid or moderate to strong Lewis acid sites to promote the Piancantelli rearrangement.47 Blocking the strong acid sites in the Pd/ACM and adding weak Lewis acid sites should promote the formation of Piancantelli rearrangement products.
4. Conclusions
Comparing the results with the literature, the Pd on activated carbon monolith showed high selectivity toward FA, THFA, and 2MF, resulting in the high STYs of these products, along with a low percentage of metal leaching and activity loss, which makes this catalyst a promising alternative to other forms of carbon catalysts. The activated carbon monolith did not show an external mass transfer resistance for hydrogen, which contributed to its lower level of coking. The presence of acetic acid in the reaction medium did not affect the furfural conversion using Pd/ACM, suggesting that Pd/ACM can be used to process crude furfural. Using water as a safe alternative to organic solvents resulted in high yields and selectivity of FA, THFA, and 2MF using the activated monolith carbon catalyst. Finally, compared to most Pd/GAC catalysts used in the literature to process furfural, the Pd/ACM accomplished these results at much lower Pd loadings (∼1 wt % versus 3–10 wt %).
In general, this work demonstrated the prolonged hydrogenation of furfural using Pd/ACM catalyst in an aqueous and acidic environment with low metal loadings and high space time yields of furfuryl alcohol and 2-methylfuran, due to limited Pd leaching and higher H2 mass transfer rates. It should also be noted that these results with the metal/ACM have implications for a wider range of reactants (or substrates) generated in microbial fermentations or acid hydrolysis of biomass requiring catalytic upgrading, e.g., hydrogenation of 5-hydroxymethyl furfural, xylose to xylitol, glucose to sorbitol, succinic acid to butanediol, levulinic acid to γ-valerolactone, and muconic acid to adipic acid.
5. Experimental Section
5.1. Materials and Catalysts
Furfural was purchased from Sigma-Aldrich (99%). Desired concentrations of furfural solution were prepared using deionized water. Activated carbon monolith (ACM) and Pd on granular activated carbon or GAC (∼3 mm particle size) were provided by Applied Catalysts (Laurens, SC). The GAC was wood-based, and the same GAC was used to produce the Pd/ACM. 5% Pd on powder-activated carbon (Pd/powder C, ∼0.25 mm particle size) was purchased from Alfa Aesar (type 87L, Dry) and was selected since this catalyst type is commonly used in hydrogenations in the literature.14 The 3 mm particle size of the GAC was selected since this size is typically used in industrial-scale trickle beds to minimize pressure drop and promote heat transfer for hydrogenations.48 The Pd/GAC and Pd/powder C were characterized for comparison purposes with the Pd/ACM. The ACM and GAC forms were designed to have 0.8% Pd and were thus labeled as 0.8% Pd/ACM and 0.8% Pd/GAC, respectively. ACM is manufactured by the coextrusion of 50% activated carbon and 50% ceramic binder and has recently been used for the hydrogenation of nitrobenzene to aniline and ketones to alcohols.49,50 Images of the ACM cores used in this work can be seen in previous literature50 and the graphical abstract. The monolith properties (1 in × 1 in cores) have been reported previously, and the surface area of the blank ACM is 598 m2/g with a pore volume of 0.45 cm3/g.50 For the effect of metal loading studies (0.8, 2.5, and 5 wt % Pd), different concentrations of palladium(II) nitrate dihydrate (purchased from Sigma-Aldrich, 40% Pd basis) were prepared using deionized water and added to crushed monolith catalysts or cACM (particle size of 0.5–1 mm), via a wet impregnation method (a defined mass of palladium(II) nitrate dihydrate was dissolved in 5 mL of DI water and then added dropwise to 5.5 g of cACM). The mixture was dried at 120 °C for 2 h followed by H2 reduction at 250 °C for 4 h. The palladium catalysts were reduced in 100 mL/min flowing H2 (100%) at 250 °C for 4 h inside the packed bed reactor (PBR) prior to the reaction. It should be noted that elemental analysis of the catalysts indicated Pd loadings different from original designs and is reported in the text and tables (Table SI1).
5.2. Catalyst Characterization
Surface area analysis and pore size analysis were performed as previously described.50,51 Micropore analysis was performed using the t-method of de Boer52 (t is the statistical thickness of an adsorbed film [t(Å) = [13.99/log(P0/P) + 0.034]1/2]) and the BET surface area data extended to higher pressures (Quantachrome, AUTOSORB-1C; Boynton Beach, Florida). Scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDS) was performed using FEI TENEO with a 150 mm Oxford XMaxN detector at 10 kV. X-ray diffraction (XRD) was performed on a PANalytical X’Pert PRO using a Cu Kα radiation source (l = 1.5418 Å) with a step size of 0.02° and 2θ range of 15–80°. Ammonia-TPD analysis was performed to determine the quantity and strength of acid sites as previously described.49 It should be noted that, to obtain strong acid site density for Pd/ACM (Table 1), the peak area of a Pd/GAC desorption curve (same GAC used to make the ACM) from 300 to 500 °C was subtracted from the Pd/ACM peak area over the same range. TPR analysis was performed to determine the reducibility of the catalyst, and CO pulse titration was performed to determine the dispersion and particle size of the metal on the fresh catalysts.51 A 1:1 CO to Pd stoichiometry was assumed in calculating dispersion. Dispersion was estimated from the pulse titration data as described in past work.50 The average particle size of the active metal for a fresh catalyst was estimated from CO pulse titration using the equation below
where d is the average crystallite size (Å), ASA is the active metal surface area (m2/g), ρ is the metal density, and f is the shape factor (6 was used assuming spherical particles).
TGA analysis in air was used to estimate tar and coke formation on the catalysts (Discovery TGA from TA Instruments). The airflow over the sample (10–25 mg in ceramic pans) was set at 25 mL/min with a balance flow rate of 10 mL/min (N2). The temperature of the sample was equilibrated at 40 °C before ramping at a rate of 10 °C/min to 800 °C. Elemental analysis of fresh and spent catalysts was performed following the Environmental Protection Agency (EPA) ICP method 200.8. Concentrated HNO3 was added (5 mL) to the sample (∼ 0.1g) for microwave digestion following protocols listed in EPA method 3051A. Digested solutions were analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES, Spectro Arcos FHS16 AMETEK ICP-OES).
5.3. Analytical
Once the liquid sample was collected from the reactor, it was analyzed in triplicate using gas chromatography with flame ionization detection (GC/FID, HP 5890 Series II) with a HP Innowax column (30 m × 0.25 mm × 0.25 mm). The GC/FID was operated with an inlet temperature of 230 °C, a detector temperature of 240 °C, and an initial oven temperature of 45 °C for 2.5 min followed by a ramp of 10 °C/min for 15.5 min and then held at 200 °C for 3 min. A sample of 1 μL was added to the GC/FID in triplicate. The concentrations of furfural (FUR, 99%), furfuryl alcohol (FA, 98%), tetrahydrofurfuryl alcohol (THFA, 98%), 2-methylfuran (2MF, 99%), 2-methyltetrahydrofuran (2MTHF, 99%), cyclopentanol (CPO, 99%), cyclopentanone (CP, 95%), 5-hydroxy-2-pentanone (5H2P, 95%), and 1,4-pentanediol (1,4-PD, 99%) were determined using 4-point standard curves (chemicals purchased from Sigma-Aldrich, each point run in triplicate). All standards were prepared in DI water, except for 2MF, which was prepared with ethanol as the solvent. The presence of all intermediates and products was confirmed using gas chromatography–mass spectrometry (GC/MS) (HP-6890 with a HP Innowax column, the same method as for GC/FID, 1 μL injection volume, 25:1 split ratio, 0.8 mL/min, 10–500 mass units, MSD ChemStation D.03.00.611 with NIST 2008 database for identification). Neat compounds identified by GC/MS were purchased (standards) and then matched with retention times of our products on a GC/FID. A typical progression of reactant conversion and product formation is shown in a series of GC/FID chromatograms in the Supporting Information.
5.4. Catalytic Reactions
Furfural hydrogenation reactions were performed in a continuous reactor system, designed by Parr Instrument Company. Liquid (0.5–16 mL/min) and gas (100 mL/min) were flowed through the stainless-steel tube (1 in inner diameter) downwards and concurrently through a T-junction. Gas flow rate was controlled by a Brooks Delta II Smart Mass Flow Controller. Liquid feedstock was pumped into the reactor using a Scientific Systems LD-Class HPLC pump. The liquid product was collected in a stainless-steel vessel with a cooling jacket attached to a Brookfield TC-602 water bath at 7 °C. Reactor temperature was controlled by a Thermcraft Lab-Temp 1760 watt furnace and a Parr 4875 power controller. Reactor pressure was controlled using a TESCOM backpressure regulator. Cores of activated carbon monolith catalyst (4 cores) were wrapped in Teflon tape and loaded into the center of the reactor. These four ACM cores were 4 inches (10 cm) in packing height at a total weight of 15 g. To determine the optimum conditions for furfural hydrogenation in a continuous reactor system, a series of experiments at temperatures ranging from 120 to 180 °C, an atmospheric pressure of 300 psi (0.1–2.1 MPa), and liquid flow rates of 0.5–16 mL/min were performed. The order of performing reactions to determine the effect of different reaction parameters on the product distribution for ACM was as follows: (1) testing varying liquid flow rates, (2) testing varying pressures, (3) testing varying temperatures, and (4) the effect of acetic acid. The timing of these events and chronological use of the catalysts are shown in a time-on-stream (TOS) plot in the Supporting Information (Figure SI10). Finally, it should be noted that, given the limited supply of Pd/ACM, we selected to use the same set of Pd/ACM cores for all runs.
Key kinetic parameters were calculated in the following manner. Conversion (X), yield (Y), selectivity (S), weighted hourly space velocity (WHSV), liquid hourly space velocity (LHSV), space time yield (STY), and the catalyst to mass rate ratio (W/F) were calculated using the following equations: XA = 1 – FAout/FAin, where FA is the molar rate for species A (e.g., FAout = CAoutQout; CAout is the measured concentration and the measured volumetric flow rate), YA = FAout/FTin, where FTin = ∑Fi and i is the species, and SA = FAout/(FTin – FTout). WHSV was calculated as [MWA*FAin]/W, where W is the catalyst mass and MW is the molecular weight. LHSV was calculated as [Qin*ρcat]/W and GHSV as [Qgas,in*ρcat]/W, where ρcat is the bulk density of the catalyst. STY was calculated as FAoutρcatMWA/W (g/L-cat/h).
Acknowledgments
Support for this research and Maryam Pirmordi’s PhD in Biochemical Engineering, and support for Nida Janulaitis’s undergraduate research were provided by a USDA-NIFA Grant (Carbon Monolith Catalysts from Wood for Biobased Platform Chemicals: 2017-67021-26136). The authors acknowledge and thank Dr. Yiping Zao, Sarada Sarapida, and Yanjun Yang for their contribution in performing XRD analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04010.
Results are presented: (1) additional catalyst characterization results, (2) effect of temperature on furfural conversions, (3) effect of liquid residence times on furfural conversions, (4) effect of H2 reduction temperature on Pd dispersion, (5) effect of Pd loading on selectivity, (6) SEM–EDS pictures, (7) XRD analysis, (8) GC/FID analysis of a product stream, (9) time-on-stream (TOS) plot, and (10) description of external and internal (or intraparticle) mass transfer calculations (DOCX)
The authors declare no competing financial interest.
Supplementary Material
References
- Dashtban M.; Gilbert A.; Fatehi P. Production of furfural: overview and challenges. J. Sci. Technol. For. Prod. Processes 2012, 2, 44–53. [Google Scholar]
- Machado G.; Leon S.; Santos F.; Lourega R.; Dullius J.; Mollmann M. E.; Eichler P. Literature review on furfural production from lignocellulosic biomass. Nat. Resour. 2016, 7, 115–129. 10.4236/nr.2016.73012. [DOI] [Google Scholar]
- Nakagawa Y.; Tamura M.; Tomishige K. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACS Catal. 2013, 3, 2655–2668. 10.1021/cs400616p. [DOI] [Google Scholar]
- Rao R. S.; Baker R. T. K.; Vannice M. A. Furfural hydrogenation over carbon-supported copper. Catal. Lett. 1999, 60, 51–57. 10.1023/A:1019090520407. [DOI] [Google Scholar]
- Sitthisa S.; Sooknoi T.; Ma Y.; Balbuena P. B.; Resasco D. E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. 10.1016/j.jcat.2010.10.005. [DOI] [Google Scholar]
- Zheng H. Y.; Zhu Y. L.; Teng B. T.; Bai Z. Q.; Zhang C. H.; Xiang H. W.; Li Y. W. Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J. Mol. Catal. A: Chem. 2006, 246, 18–23. 10.1016/j.molcata.2005.10.003. [DOI] [Google Scholar]
- Vargas-Hernández D.; Rubio-Caballero J. M.; Santamaría-González J.; Moreno-Tost R.; Mérida-Robles J. M.; Pérez-Cruz M. A.; Jiménez-López A.; Hernández-Huesca R.; Maireles-Torres P. Furfuryl alcohol from furfural hydrogenation over copper supported on SBA-15 silica catalysts. J. Mol. Catal. A: Chem. 2014, 383, 106–113. 10.1016/j.molcata.2013.11.034. [DOI] [Google Scholar]
- Panagiotopoulou P.; Vlachos D. G. Liquid phase catalytic transfer hydrogenation of furfural over a Ru/C catalyst. Appl. Catal., A 2014, 480, 17–24. 10.1016/j.apcata.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Luo H.; Li H.; Zhuang L. Furfural hydrogenation to furfuryl alcohol over a novel Ni–Co–B amorphous alloy catalyst. Chem. Lett. 2001, 30, 404–405. 10.1246/cl.2001.404. [DOI] [Google Scholar]
- Taylor M. J.; Durndell L. J.; Isaacs M. A.; Parlett C. M.; Wilson K.; Lee A. F.; Kyriakou G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal., B 2016, 180, 580–585. 10.1016/j.apcatb.2015.07.006. [DOI] [Google Scholar]
- Piancatelli G.; Scettri A.; Barbadoro S. A useful preparation of 4-substituted 5-hydroxy-3-oxocyclopentene. Tetrahedron Lett. 1976, 17, 3555–3558. 10.1016/S0040-4039(00)71357-8. [DOI] [Google Scholar]
- Piutti C.; Quartieri F. The piancatelli rearrangement: New applications for an intriguing reaction. Molecules 2013, 18, 12290–12312. 10.3390/molecules181012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hronec M.; Fulajtárová K.; Soták T. Kinetics of high temperature conversion of furfuryl alcohol in water. J. Ind. Eng. Chem. 2014, 20, 650–655. 10.1016/j.jiec.2013.05.029. [DOI] [Google Scholar]
- Hronec M.; Fulajtarová K.; Liptaj T. Effect of catalyst and solvent on the furan ring rearrangement to cyclopentanone. Appl. Catal., A 2012, 437, 104–111. 10.1016/j.apcata.2012.06.018. [DOI] [Google Scholar]
- Bartholomew C. H.; Farrauto R. J.. Fundamentals of Industrial Catalytic Processes, 2nd ed.; John Wiley & Sons, 2011; pp 120–200. [Google Scholar]
- Bravo-Suárez J. J.; Chaudhari R. V.; Subramaniam B.. Design of Heterogeneous Catalysts for Fuels and Chemicals Processing: An Overview. In Novel Materials for Catalysis and Fuels Processing; American Chemical Society, 2013; Vol. 1132, pp 3–68. [Google Scholar]
- Auer E.; Freund A.; Pietsch J.; Tacke T. Carbons as supports for industrial precious metal catalysts. Appl. Catal., A 1998, 173, 259–271. 10.1016/S0926-860X(98)00184-7. [DOI] [Google Scholar]
- Amara Z.; Poliakoff M.; Duque R.; Geier D.; Franciò G.; Gordon C. M.; Meadows R. E.; Woodward R.; Leitner W. Enabling the scale-up of a key asymmetric hydrogenation step in the synthesis of an API using continuous flow solid-supported catalysis. Org. Process Res. Dev. 2016, 20, 1321–1327. 10.1021/acs.oprd.6b00143. [DOI] [Google Scholar]
- Boger T.; Heibel A. K.; Sorensen C. M. Monolithic catalysts for the chemical industry. Ind. Eng. Chem. Res. 2004, 43, 4602–4611. 10.1021/ie030730q. [DOI] [Google Scholar]
- Tomašić V.; Jović F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal., A 2006, 311, 112–121. 10.1016/j.apcata.2006.06.013. [DOI] [Google Scholar]
- Gurrath M.; Kuretzky T.; Boehm H. P.; Okhlopkova L. B.; Lisitsyn A. S.; Likholobov V. A. Palladium catalysts on activated carbon supports: Influence of reduction temperature, origin of the support and pretreatments of the carbon surface. Carbon 2000, 38, 1241–1255. 10.1016/S0008-6223(00)00026-9. [DOI] [Google Scholar]
- Biniak S.; Diduszko R.; Gac W.; Pakuła M.; S′wiatkowski A. Reduction and oxidation of a Pd/activated carbon. catalyst: evaluation of effects. React. Kinet., Mech. Catal. 2010, 101, 331–342. 10.1007/s11144-010-0233-8. [DOI] [Google Scholar]
- Pinna F.; Menegazzo F.; Signoretto M.; Canton P.; Fagherazzi G.; Pernicone N. Consecutive hydrogenation of benzaldehyde over Pd catalysts. Influence of supports and sulfur poisoning. Appl. Catal., A 2001, 219, 195–200. 10.1016/S0926-860X(01)00685-8. [DOI] [Google Scholar]
- Yang Q.; Hou R.; Sun K. Tuning butene selectivities by Cu modification on Pd-based catalyst for the selective hydrogenation of 1,3-butadiene. J. Catal. 2019, 374, 12–23. 10.1016/j.jcat.2019.04.018. [DOI] [Google Scholar]
- Salame I. I.; Bandosz T. J. Surface Chemistry of Activated Carbons: Combining the results of temperature-programmed desorption, Boehm, and potentiometric titrations. J. Colloid Interface Sci. 2001, 240, 252–258. 10.1006/jcis.2001.7596. [DOI] [PubMed] [Google Scholar]
- Martin-Martinez M.; Gómez-Sainero L. M.; Bedia J.; Arevalo-Bastante A.; Rodriguez J. J. Enhanced activity of carbon-supported Pd–Pt catalysts in the hydrodechlorination of dichloromethane. Appl. Catal., B 2016, 184, 55–63. 10.1016/j.apcatb.2015.11.016. [DOI] [Google Scholar]
- Prado-Burguete C.; Linares-Solano A.; Rodriguez-Reinoso F.; De Lecea C. S. M. The effect of oxygen surface groups of the support on platinum dispersion in Pt/carbon catalysts. J. Catal. 1989, 115, 98–106. 10.1016/0021-9517(89)90010-9. [DOI] [Google Scholar]
- Xu T.; Zhang Q.; Cen J.; Xiang Y.; Li X. Selectivity tailoring of Pd/CNTs in phenol hydrogenation by surface modification: Role of C-O oxygen species. Appl. Surf. Sci. 2015, 324, 634–639. 10.1016/j.apsusc.2014.10.165. [DOI] [Google Scholar]
- Yang G.; Zhang J.; Jiang H.; Liu Y.; Chen R. Turning surface properties of Pd/N-doped porous carbon by trace oxygen with enhanced catalytic performance for selective phenol hydrogenation to cyclohexanone. Appl. Catal., A 2019, 588, 117306 10.1016/j.apcata.2019.117306. [DOI] [Google Scholar]
- Venkateshwarlu V.; Mohan V.; Rao M. V.; Nagaiah P.; Raju B. D.; Rao K. R. Advantage of carbon coverage over Al2O3 as support for Ni/C-Al2O3 catalyst in vapour phase hydrogenation of nitrobenzene to aniline. Catal. Commun. 2016, 86, 1–4. 10.1016/j.catcom.2016.07.026. [DOI] [Google Scholar]
- Cao W.; Luo W.; Ge H.; Su Y.; Wang A.; Zhang T. UiO-66 derived Ru/ZrO 2@C as a highly stable catalyst for hydrogenation of levulinic acid to γ-valerolactone. Green Chem. 2017, 19, 2201–2211. 10.1039/C7GC00512A. [DOI] [Google Scholar]
- Date N. S.; Biradar N. S.; Chikate R. C.; Rode C. V. Effect of Reduction Protocol of Pd Catalysts on Product Distribution in Furfural Hydrogenation. ChemistrySelect 2017, 2, 24–32. 10.1002/slct.201601790. [DOI] [Google Scholar]
- Mariscal R.; Maireles-Torres P.; Ojeda M.; Sádaba I.; López Granados M. Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. 10.1039/C5EE02666K. [DOI] [Google Scholar]
- Bauer T.; Guettel R.; Roy S.; Schubert M.; Al-Dahhan M.; Lange R. Modelling and simulation of the monolithic reactor for gas–liquid–solid reactions. Chem. Eng. Res. Des. 2005, 83, 811–819. 10.1205/cherd.04335. [DOI] [Google Scholar]
- Kawakami K.; Kawasaki K.; Shiraishi F.; Kusunoki K. Performance of a honeycomb monolith bioreactor in a gas-liquid-solid three-phase system. Ind. Eng. Chem. Res. 1989, 28, 394–400. 10.1021/ie00088a003. [DOI] [Google Scholar]
- Mogalicherla A. K.; Kunzru D. Effect of gas and liquid superficial velocities on the performance of monolithic reactors. Ind. Eng. Chem. Res. 2010, 49, 1631–1641. 10.1021/ie901442d. [DOI] [Google Scholar]
- Han Y.; Shikazono N. Measurement of the liquid film thickness in micro tube slug flow. Int. J. Heat Fluid Flow 2009, 30, 842–853. 10.1016/j.ijheatfluidflow.2009.02.019. [DOI] [Google Scholar]
- Fogler H. S.Elements of Chemical Reaction Engineering, 5th ed.; Prentice-Hall: New Jersey, 1986; pp 600–650. [Google Scholar]
- Mukherjee S.; Vannice M. A. Solvent effects in liquid-phase reactions. I. Activity and selectivity during citral hydrogenation on Pt/SiO2 and evaluation of mass transfer effects. J. Catal. 2006, 243, 108–130. 10.1016/j.jcat.2006.06.021. [DOI] [Google Scholar]
- Mironenko R. M.; Talsi V. P.; Gulyaeva T. I.; Trenikhin M. V.; Belskaya O. B. Aqueous-phase hydrogenation of furfural over supported palladium catalysts: effect of the support on the reaction routes. React. Kinet., Mech. Catal. 2019, 126, 811–827. 10.1007/s11144-018-1505-y. [DOI] [Google Scholar]
- Garcia-Olmo A. J.; Yepez A.; Balu A. M.; Romero A. A.; Li Y.; Luque R. Insights into the activity, selectivity and stability of heterogeneous catalysts in the continuous flow hydroconversion of furfural. Catal. Sci. Technol. 2016, 6, 4705–4711. 10.1039/C6CY00249H. [DOI] [Google Scholar]
- Ouyang W.; Yepez A.; Romero A. A.; Luque R. Towards industrial furfural conversion: Selectivity and stability of palladium and platinum catalysts under continuous flow regime. Catal. Today 2018, 308, 32–37. 10.1016/j.cattod.2017.07.011. [DOI] [Google Scholar]
- Wang Y.; Prinsen P.; Triantafyllidis K. S.; Karakoulia S. A.; Trikalitis P. N.; Yepez A.; Len C.; Luque R. Comparative study of supported monometallic catalysts in the liquid-phase hydrogenation of furfural: Batch versus continuous flow. ACS Sustainable Chem. Eng. 2018, 6, 9831–9844. 10.1021/acssuschemeng.8b00984. [DOI] [Google Scholar]
- Lima S.; Chadwick D.; Hellgardt K. Towards sustainable hydrogenation of 5-(hydroxymethyl) furfural: a two-stage continuous process in aqueous media over RANEY catalysts. RSC Adv. 2017, 7, 31401–31407. 10.1039/C7RA03318D. [DOI] [Google Scholar]
- Sitthisa S.; Resasco D. E. Hydrodeoxygenation of furfural over supported metal catalysts: a comparative study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. 10.1007/s10562-011-0581-7. [DOI] [Google Scholar]
- Liu F.; Liu Q.; Xu J.; Li L.; Cui Y. T.; Lang R.; Su Y.; Miao S.; Sun H.; Qiao B. Catalytic cascade conversion of furfural to 1, 4-pentanediol in a single reactor. Green Chem. 2018, 20, 1770–1776. 10.1039/C8GC00039E. [DOI] [Google Scholar]
- Ohyama J.; Satsuma A.. Reductive Conversion of 5-Hydroxymethylfurfural in Aqueous Solutions by Furan Ring Opening and Rearrangement. In Production of Biofuels and Chemicals with Bifunctional Catalysts; Springer: Singapore, 2017. [Google Scholar]
- Azarpour A.; Zahedi G. Performance analysis of crude terephthalic acid hydropurification in an industrial trickle-bed reactor experiencing catalyst deactivation. Chem. Eng. J. 2012, 209, 180–193. 10.1016/j.cej.2012.07.140. [DOI] [Google Scholar]
- Gulotty R. J. Jr.; Rish S.; Boyd A.; Mitchell L.; Plageman S.; McGill C.; Keller J.; Starnes J.; Stadalsky J.; Garrison G. Run Parameters for a Continuous Hydrogenation Process Using ACMC-Pd To Replace Commercial Batch Reactor Processes. Org. Process Res. Dev. 2018, 22, 1622–1627. 10.1021/acs.oprd.8b00286. [DOI] [Google Scholar]
- Weber J.; Thompson A.; Wilmoth J.; Gulotty R. J. Jr.; Kastner R. J. Coupling red-mud ketonization of a model bio-oil mixture with aqueous phase hydrogenation using activated carbon monoliths. Energy Fuels 2017, 31, 9529–9541. 10.1021/acs.energyfuels.7b01500. [DOI] [Google Scholar]
- Weber J.; Thompson A.; Wilmoth J.; Batra S. B.; Janulaitis N.; Kastner J. R. Effect of metal oxide redox state in red mud catalysts on ketonization of fast pyrolysis oil derived oxygenates. Appl. Catal., B 2019, 241, 430–441. 10.1016/j.apcatb.2018.08.061. [DOI] [Google Scholar]
- De Boer J. H.; Linsen B. G.; Van der Plas T.; Zondervan G. J. Studies on pore systems in catalysts: VII. Description of the pore dimensions of carbon blacks by the t method. J. Catal. 1965, 4, 649–653. 10.1016/0021-9517(65)90264-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.