Abstract
Glia-Neuron Interactions Underlie State Transitions to Generalized Seizures
Diaz Verdugo C, Myren-Svelstad S, Aydin E, et al. Nat Commun. 2019;10:3830. doi:10.1038/s41467-019-11739-z.
Brain activity and connectivity alter drastically during epileptic seizures. The brain networks shift from a balanced resting state to a hyperactive and hypersynchronous state. It is, however, less clear which mechanisms underlie the state transitions. By studying neural and glial activity in zebrafish models of epileptic seizures, we observe striking differences between these networks. During the preictal period, neurons display a small increase in synchronous activity only locally, while the gap-junction-coupled glial network was highly active and strongly synchronized across large distances. The transition from a preictal state to a generalized seizure leads to an abrupt increase in neural activity and connectivity, which is accompanied by a strong alteration in glia-neuron interactions and a massive increase in extracellular glutamate. Optogenetic activation of glia excites nearby neurons through the action of glutamate and gap junctions, emphasizing a potential role for glia–glia and glia–neuron connections in the generation of epileptic seizures.
Commentary
Hypersynchronous neuronal activity is the defining feature of epileptic seizures, and almost all of our strategies for recording ictal brain states focus on identifying signatures of this neuronal dysfunction. But it is not all about the neurons: astrocytes have recently achieved due recognition as more than just “support crew” for the more flashy neurons; they actively participate in brain homeostasis, signal processing, and dynamic regulation of brain states.1 And as Diaz Verdugo and colleagues elegantly demonstrate in zebrafish, a gap junction coupled network of astrocytes also seems to play a key role in the ictogenesis of generalized seizures.
Although zebrafish are distant cousins of ours on the phylogenetic tree, they share key features in brain organization, function, and pathology with human. This includes a propensity for epileptic seizures when exposed to chemoconvulsants or in the context of certain gene mutations.2 This can be demonstrated even in very early larval stages, mere days after fertilization. Furthermore, the larvae’s small size coupled with advances in microscopy now allow studies that are impossible in any other vertebrates. As shown elegantly by Diaz Verdugo and colleagues, with calcium imaging in larval zebrafish, we can now track changes in brain-wide activity at single-cell resolution during the transition into epileptic seizures.
Acute epileptic seizures induced by the γ-aminobutyric acid (GABA) blocking chemoconvulsant pentylenetetrazole (PTZ) had already been shown to produce seizure-like discharges and network connectivity changes.3 But we can now move beyond this observation and start dissecting how different cell populations contribute to the brain-wide dynamics. Using a range of specifically engineered zebrafish lines with cell-type specific expression of fluorescent calcium indicators, Diaz Verdugo and colleagues demonstrate the dynamics of both neurons and glial cells during PTZ-induced seizures. They show that early after PTZ exposure, glial cells are already more synchronized than neurons. Synchronous activity of glial cells in this early stage is associated with a dampening of neuronal activity—suggesting, perhaps, that this glial cell activity dampens runaway neuronal activity bursts in the early phase following PTZ. However, this relationship drastically changes when the ictal activity generalizes. On generalization of the seizure, synchronized bursts of astrocytic activity precede neuronal ictal bursts by seconds. This temporal relationship, and some additional optogenetic validation experiments indeed suggest that synchronized bursts of glial cell activity may play a causative role in the transition toward a generalized epileptic seizure in this model.
Glial cells—in particular mammalian astrocytes—have long been acknowledged for their key role in acquired epilepsies. Glial cell changes in metabolic regulation and neurotransmitter homeostasis for example is a key step in epileptogenesis in a number of chronic pharmacologically induced epilepsy models.4 But studies like the one by Diaz Verdugo et al now point to a much more dynamic role of glial cells in the generation and evolution of individual seizures. Astrocytes have been previously shown to respond to neuronal input, and be able to generate intracellular Ca2+-waves, which in turn can affect neuronal function and thus play an active role in information processing. This causal role in information processing has most recently also been impressively demonstrated by Mu and colleagues in zebrafish using a virtual reality setup.5 Thus, even in this most simple of vertebrate models, a dynamic coupling between glial cells and neurons is present during normal brain function, and may thus go awry during pathological states such as epileptic seizures.
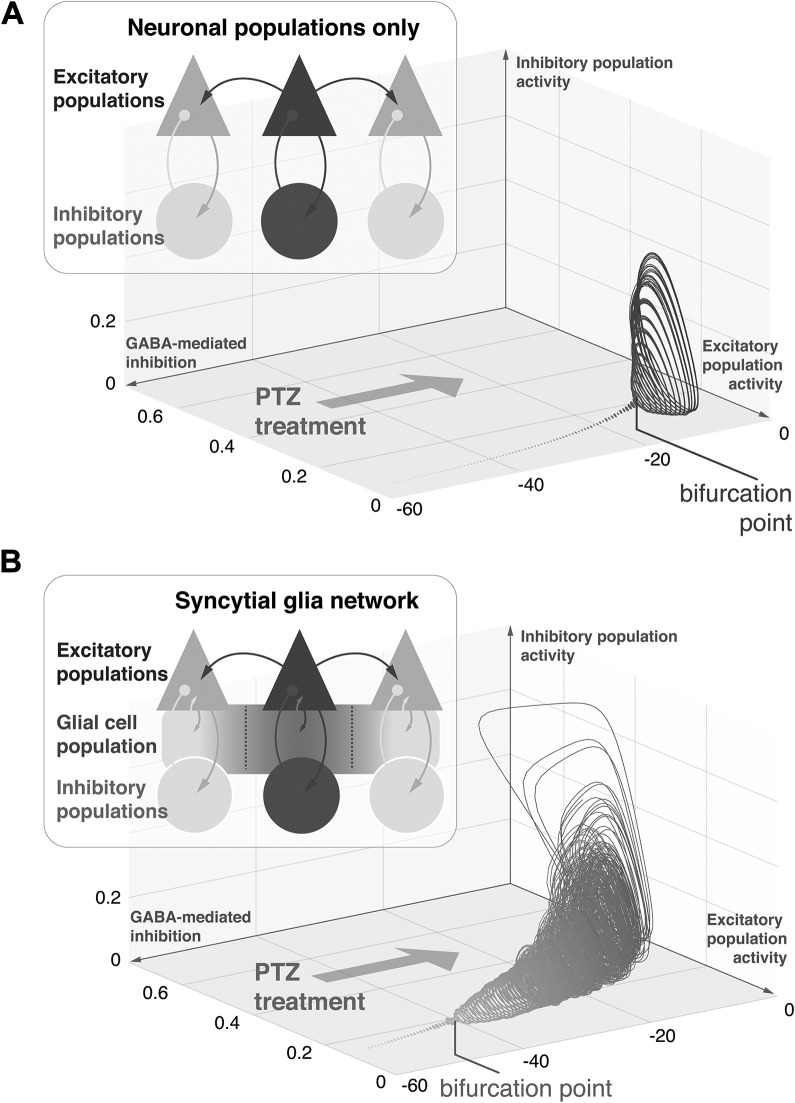
But how does this novel appraisal of glial cells’ role sit with our existing models of brain dynamics in epileptic seizures? Decades of theoretical work in epilepsy have focused on how disturbances in the intricate balance between coupled excitatory and inhibitory neuronal populations allow for transitions of brain dynamics into pathological, epileptic regimes. In fact many key features of epileptic seizures—such as their sudden emergence from resting brain activity, the widespread synchronization across brain areas, or the sudden seizure offset—can be explained with simple models of coupled excitatory and inhibitory neuronal populations.6 An example toy model consisting of coupled excitatory and inhibitory populations is shown in Figure 1A. Here the effect of an acute reduction in inhibitory transmission (as could be induced by PTZ) leads to the emergence of synchronized large amplitude oscillations, after a “threshold” is crossed.
Figure 1.
Glial cell networks modulate excitation-inhibition balance during epileptic seizures. A, A model of connected excitatory and inhibitory neuronal populations shows high amplitude oscillations when GABAergic inhibition is reduced (simulating the effect of PTZ treatment). This simulated seizure activity represents coordinated oscillations of excitatory and inhibitory populations, which when plotted against each other form so-called limit cycles. The value of GABAergic inhibition at which stationary activity transitions into the high amplitude limit cycle is known as bifurcation point. B, Here a syncytial network of glial cells is added to the model above with other parameters unchanged. The syncytium allows for lateral spread of activity and is recurrently coupled to excitatory cells, reproducing calcium mediated glial glutamate release. Without changing neuronal parameters from (A), the glial cells alter the response of the whole system. Specifically, much smaller reductions of GABAergic inhibition are sufficient to push the system into the high-amplitude, limit-cycle regime. Glial cells can therefore contribute to the emergence of generalized seizure-like dynamics in simple network models of neuronal population activity. PTZ indicates pentylenetetrazole.
What contributions then can a glial cell network—like the one described by Diaz Verdugo and colleagues from their zebrafish data—make to the dynamics of an epileptic seizure? We can explore this by adding to our toy model a simple syncytium of glial cells—here the glial syncytium is recurrently coupled to the excitatory cell population, and when activated will excite the excitatory neurons. This allows for fast lateral spread of activity, amplification of neuronal signals, and some degrees of temporal summation that alter the dynamics of the neuronal populations that were otherwise unchanged (Figure 1B). In this case, PTZ still induces “epileptic” oscillations, but the bifurcation point or “threshold” occurs sooner (ie, with more intact inhibition) and responses at each stage are greatly exaggerated compared in the neuron-only model. Thus in this toy model, glial cells shape the dynamic regime under which the neuronal oscillations operate and thus sensitize the system to blockade of inhibition.
The mechanisms by which glial cells could amplify neuronal signals remain to be seen, but potential mechanisms include modulation of neuronal glutamate excitation, alteration in extracellular K+ buffering, release of “gliotransmitters,” and more. Diaz Verdugo and colleagues’ work highlights the role of calcium mediated glial glutamate release that has been described previously in rodents.7 But there are other mechanisms through which glial cells shape neuronal dynamics—including regulation of extracellular potassium concentrations, pH, and neuronal energy metabolism.8 Biophysically inspired computational models of varying complexity have been used to expand the view on glial cell contribution to ictal dynamics,9 and more theoretical models have long emphasized the role of non-neuronal dynamics in seizures. But, so far compelling whole-brain data on which to test some of the resultant prediction has been scarce. However, this is about to change with many new zebrafish lines of genetic epilepsies arriving on the scene,10 and the incredible detail with which we can now image their brain dynamics across neurons and glial cells alike.
By Richard E. Rosch and Chris G. Dulla
Footnotes
ORCID iDs: Richard E. Rosch  https://orcid.org/0000-0002-0316-5818
https://orcid.org/0000-0002-0316-5818
Chris G. Dulla  https://orcid.org/0000-0002-6560-6535
https://orcid.org/0000-0002-6560-6535
References
- 1. Seifert G, Steinhäuser C. Neuron-astrocyte signaling and epilepsy. Exp Neurol. 2013;244:4–10. [DOI] [PubMed] [Google Scholar]
- 2. Grone BP, Baraban SC. Animal models in epilepsy research: legacies and new directions. Nat Neurosci. 2015;18(3):339–343. [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Baraban SC. Network properties revealed during multi-scale calcium imaging of seizure activity in zebrafish. Eneuro. 2019;6(1): ENEURO.0041-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hadera MG, Eloqayli H, Jaradat S, Nehlig A, Sonnewald U. Astrocyte-neuronal interactions in epileptogenesis. J Neurosci Res. 2015;93(7):1157–1164. [DOI] [PubMed] [Google Scholar]
- 5. Mu Y, Bennett DV, Rubinov M, et al. Glia accumulate evidence that actions are futile and suppress unsuccessful behavior. Cell. 2019;178(1):27–43. e19. [DOI] [PubMed] [Google Scholar]
- 6. da Silva FHL, Blanes W, Kalitzin SN, Parra J, Suffczynski P, Velis DN. Dynamical diseases of brain systems: different routes to epileptic seizures. IEEE Trans Biomed Eng. 2003;50(5):540–548. [DOI] [PubMed] [Google Scholar]
- 7. Tian GF, Azmi H, Takano T, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11(9):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glia and epilepsy: excitability and inflammation | Elsevier Enhanced Reader [Internet]. https://reader.elsevier.com/reader/sd/pii/S0166223612002056?token=824A9DF751D5ECD2D208EC131887B9E0C58AA6EE38146C27131B5CA537C239B3C0C9AC355C66C30CE3FB04A8DE27474C. Accessed January 07, 2020.
- 9. Volman V, Bazhenov M, Sejnowski TJ. Computational models of neuron-astrocyte interaction in epilepsy. Front Comput Neurosci. 2012;6:58 https://www.frontiersin.org/articles/10.3389/fncom.2012.00058/full. Accessed January 07, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosch R, Burrows DRW, Jones LB, Peters CH, Ruben P, Samarut É. Functional genomics of epilepsy and associated neurodevelopmental disorders using simple animal models: from genes, molecules to brain networks. Front Cell Neurosci. 2019;13:556 https://www.frontiersin.org/articles/10.3389/fncel.2019.00556/full. Accessed January 07, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]