Abstract
α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor Plasticity Sustains Severe, Fatal Status Epilepticus.
Adotevi N, Lewczuk E, Sun H, Joshi S, Dabrowska N, Shan S, Williamson J, Kapur J. Ann Neurol. 2020;87(1):84-96. doi: 10.1002/ana.25635. Epub 2019 Nov 20.
OBJECTIVE:
Generalized convulsive status epilepticus is associated with high mortality. We tested whether α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor plasticity plays a role in sustaining seizures, seizure generalization, and mortality observed during focal onset status epilepticus. We also determined whether modified AMPA receptors generated during status epilepticus could be targeted with a drug.
METHODS:
Electrically induced status epilepticus was characterized by electroencephalogram and behavior in GluA1 knockout mice and in transgenic mice with selective knockdown of the GluA1 subunit in hippocampal principal neurons. Excitatory and inhibitory synaptic transmission in CA1 neurons was studied using patch clamp electrophysiology. The dose-response of N, N, H,-trimethyl-5-([tricyclo(3.3.1.13,7)dec-1-ylmethyl]amino)-1-pentanaminiumbromide hydrobromide (IEM-1460), a calcium-permeable AMPA receptor antagonist, was determined.
RESULTS:
Global removal of the GluA1 subunit did not affect seizure susceptibility; however, it reduced susceptibility to status epilepticus. GluA1 subunit knockout also reduced mortality, severity, and duration of status epilepticus. Absence of the GluA1 subunit prevented enhancement of glutamatergic synaptic transmission associated with status epilepticus; however, γ-aminobutyric acidergic synaptic inhibition was compromised. Selective removal of the GluA1 subunit from hippocampal principal neurons also reduced mortality, severity, and duration of status epilepticus. IEM-1460 rapidly terminated status epilepticus in a dose-dependent manner.
INTERPRETATION:
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor plasticity mediated by the GluA1 subunit plays a critical role in sustaining and amplifying seizure activity and contributes to mortality. Calcium-permeable AMPA receptors modified during status epilepticus can be inhibited to terminate status epilepticus.
Excitatory GABAergic Signalling Is Associated With Benzodiazepine Resistance in Status Epilepticus
Burman RJ, Selfe JS, Lee JH, van den Berg M, Calin A, Codadu NK, Wright R, Newey SE, Parrish RR, Katz AA, Wilmshurst JM, Akerman CJ, Trevelyan AJ, Raimondo JV. Brain. 2019;142(11):3482-3501. doi:10.1093/brain/awz283.
Status epilepticus is defined as a state of unrelenting seizure activity. Generalized convulsive status epilepticus is associated with a rapidly rising mortality rate, and thus constitutes a medical emergency. Benzodiazepines, which act as positive modulators of chloride (Cl−) permeable GABAA receptors, are indicated as first-line treatment, but this is ineffective in many cases. We found that 48% of children presenting with status epilepticus were unresponsive to benzodiazepine treatment, and critically, that the duration of status epilepticus at the time of treatment is an important predictor of nonresponsiveness. We therefore investigated the cellular mechanisms that underlie acquired benzodiazepine resistance, using rodent organotypic and acute brain slices. Removing Mg2+ ions leads to an evolving pattern of epileptiform activity, and eventually to a persistent state of repetitive discharges that strongly resembles clinical electroencephalogram recordings of status epilepticus. We found that diazepam loses its antiseizure efficacy and conversely exacerbates epileptiform activity during this stage of status epilepticus-like activity. Interestingly, a low concentration of the barbiturate phenobarbital had a similar exacerbating effect on status epilepticus-like activity, while a high concentration of phenobarbital was effective at reducing or preventing epileptiform discharges. We then show that the persistent status epilepticus-like activity is associated with a reduction in GABAA receptor conductance and Cl− extrusion capability. We explored the effect on intraneuronal Cl− using both gramicidin, perforated-patch clamp recordings and Cl− imaging. This showed that during status epilepticus-like activity, reduced Cl− extrusion capacity was further exacerbated by activity-dependent Cl− loading, resulting in a persistently high intraneuronal Cl−. Consistent with these results, we found that optogenetic stimulation of GABAergic interneurons in the status epilepticus-like state, actually enhanced epileptiform activity in a GABAAR dependent manner. Together our findings describe a novel potential mechanism underlying benzodiazepine-resistant status epilepticus, with relevance to how this life-threatening condition should be managed in the clinic.
Commentary
What makes a brain stop seizing? Why does this mechanism fail in some people leading to sustained seizure activity, a clinical emergency called status epilepticus (SE)? These questions have been plaguing epilepsy researchers and clinicians for centuries. The term “status epilepticus” initially appeared in a translation of Armand Trousseau’s lectures in 1867,1 but the condition of generalized SE is known since antiquity and has first been described in writing on a Babylonian cuneiform clay tablet dated 600 to 700 bc.2 Even though SE has been appreciated for a long time, the mechanisms leading to continuous seizure activity are still poorly understood. Status epilepticus is associated with significant morbidity and mortality, and current therapies are often ineffective in ending SE. Although there is clinical evidence supporting benzodiazepines, which activate inhibitory γ-aminobutyric acid (GABA) signaling, as first-line treatment in adults and children,3 they are ineffective in about half of all cases. Likewise, the 3 most common second-line treatments, fosphenytoin, valproate, and levetiracetam, are only successful in approximately half of all cases with benzodiazepine-resistant SE.4 The low success rate of current standard therapies is not only unacceptable for clinical care but also frustrating from a scientific perspective as it illustrates the scarce knowledge about the disease mechanisms underlying SE.
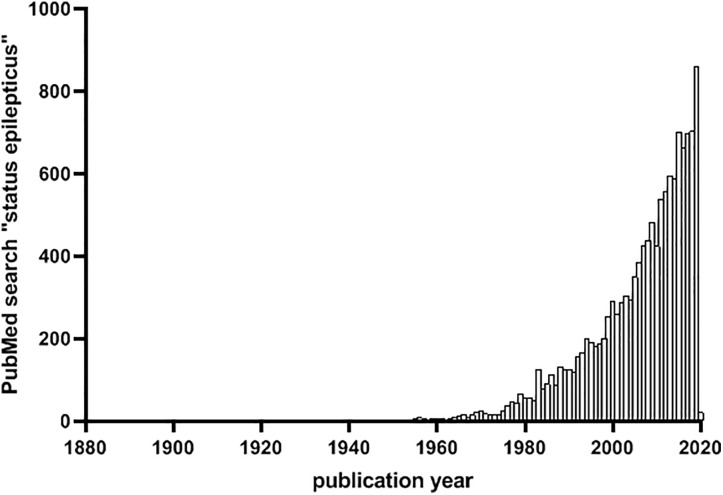
The significance of the clinical problem is reflected by the efforts in recent years to uncover etiologies and mechanisms of SE. A PubMed search with the term “status epilepticus” produces more than 13 000 results, with an exponential increase of research publications since the late 90s culminating in 859 articles in 2019 (Figure 1). There is no doubt that a better understanding of what sustains seizure activity and prevents the brain from stopping a seizure is required to develop effective disease mechanism-targeted treatments. Recent studies by Kapur’s and Raimondo’s laboratories represent steps toward this goal: they advance our understanding of underlying mechanisms and could alter treatment approaches for SE in the future. The studies address 2 different, equally pressing questions regarding SE, namely: what are the neuronal mechanisms contributing to sustained seizure activity during SE5 and why is increasing GABAergic signaling with benzodiazepines ineffective in many cases?6 Notably, both studies implicate disturbances in ion conductance in the neuronal membrane caused by a breakdown of mechanisms that normally counteract neuronal excitation.
Figure 1.
Number of PubMed abstracts with the term “status epilepticus” per year since 1880 (date of search: December 28, 2019).
The study by Adotevi et al illustrates the importance of calcium permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in maintaining SE. AMPA receptors are heterotetramers of 4 different glutamate receptor subunits. GluA1, 3, and 4 are calcium permeable; only GluA2, through posttranscriptional RNA-editing, is calcium impermeable. Usually, AMPA receptors contain at least one GluA2 subunit, and their conductance is thus limited to monovalent ions. During SE, the membrane surface expression of GluA2 is reduced, which leads to increased spontaneous excitatory postsynaptic potentials (sEPSCs) suggesting that calcium-permeable AMPA receptor-mediated synaptic plasticity contributes to sustained neuronal excitation during SE.7 The study by Adotevi et al provides several lines of evidence that the GluA1 subunit plays a crucial role in this synaptic plasticity underlying SE. Deletion of GluA1 in either global knockout mice or by postnatal GluA1 knockout in excitatory CA1 neurons using a viral strategy ablated the increase in sEPSCs during SE, reduced SE severity and mortality induced by hippocampal stimulation, and elevated the threshold to transition from discrete seizures to SE. Calcium-permeable AMPA receptors could be an effective therapeutic target, because treatment of mice with a specific inhibitor of these receptors shortened the duration of SE in mice. Calcium-permeable glutamate receptors are crucial for synaptic plasticity, including cognition,8 and caution must be taken when blocking them for longer periods of time. For transient conditions like SE, however, this treatment could still be of great value as it is expected to only be given until SE has been successfully stopped without the need for long-term treatment.
The GluA1-dependent mechanism reported by Adotevi et al does not explain why benzodiazepines, which activate inhibitory GABAergic signaling, often fail to end SE. Previous studies have suggested enhanced internalization of GABAA receptor subunits as a potential underlying mechanism reducing the efficacy of benzodiazepines. In their new study, Burman et al provide clues for another mechanism, namely the failure to maintain a normal chloride gradient across the cell membrane during prolonged seizure activity. Using 2 different in vitro hippocampal slice models of SE, they show that benzodiazepines only prevent SE-like activity if given early, before the development of late recurrent epileptiform discharges. By contrast, late application of benzodiazepines increases SE-like activity, which cannot be explained by reduced GABA receptor surface expression. Under healthy conditions, the chloride concentration is high in the extracellular space and low within the cell, leading to an influx of negatively charged chloride ions and thus hyperpolarizing inhibitory currents when GABA receptors are active. In a series of elegant electrophysiological, optogenetic, cellular imaging, and molecular experiments, the authors demonstrate that during SE-like activity in hippocampal slices, this inhibitory system collapses. Status epilepticus–like activity leads to transient but pronounced intracellular chloride ion accumulation. Consequentially, activation of GABAergic interneurons under SE conditions led to excitatory signaling and promoted epileptiform discharges in brain slices.
It remains obscure why benzodiazepines work in some patients. A possible explanation are differences in the spread of seizure activity in the brain. Probably only neuronal networks directly engaged in epileptic behavior experience the breakdown of GABAergic inhibition. If sufficiently large proportions of neurons still have an intact chloride gradient, they might suffice to confer the inhibitory SE-ending properties of benzodiazepines. Burman et al find a positive correlation between SE duration and benzodiazepine-resistance in pediatric patients, supporting this hypothesis. Administering benzodiazepine early enough before the chloride gradient is collapsed in large areas of the brain, or, alternatively, administering it locally to unaffected brain regions, may thus increase the likelihood of therapeutic success. More research is needed to test these options, and it will be important to repeat the experiments in vivo, given the potential influence of experimental artifacts affecting chloride gradients in brain slices.9 Another open question is the role of cytoplasmic impermeant anions and extracellular matrix glycoproteins, believed to play crucial roles in the neuronal chloride gradient,10 in GABAergic signaling during SE.
Considering both studies discussed here, a combinatorial treatment approach may be beneficial for SE: blocking mechanisms that promote development of SE, that is, activity of calcium-permeable AMPA receptors, together with increasing inhibition by early and focal application of benzodiazepines may be a promising future path for treatment of SE. While Raimondo’s and Kapur’s studies provide interesting novel insight advancing our understanding of mechanisms underlying SE, more research is needed to develop efficient mechanism-based treatment strategies. Most likely, many of the mechanisms contributing to SE are yet to be discovered. If PubMed searches are an indicator of research interest and activity, then there is hope that we will continue to learn more about the underlying mechanisms of SE in the coming years, which is urgently needed to develop more efficient treatment strategies.
By Christina Gross
Footnotes
ORCID iD: Christina Gross  https://orcid.org/0000-0001-6057-2527
https://orcid.org/0000-0001-6057-2527
References
- 1. Trousseau A. Lectures on Clinical Medicine. Philadelphia, USA: Lindsay & Blakiston; 1873. [Google Scholar]
- 2. Neligan A, Shorvon SD. The history of status epilepticus and its treatment. Epilepsia. 2009;50(s3):56–68. [DOI] [PubMed] [Google Scholar]
- 3. Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kapur J, Elm J, Chamberlain JM, et al. Randomized trial of three anticonvulsant medications for status epilepticus. New Engl J Med. 2019;381(22):2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adotevi N, Lewczuk E, Sun H, et al. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor plasticity sustains severe, fatal status epilepticus. Ann Neurol. 2020;87(1):84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burman RJ, Selfe JS, Lee JH, et al. Excitatory GABAergic signalling is associated with benzodiazepine resistance in status epilepticus. Brain. 2019;142(11):3482–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshi S, Rajasekaran K, Sun H, Williamson J, Kapur J. Enhanced AMPA receptor-mediated neurotransmission on CA1 pyramidal neurons during status epilepticus. Neurobiol Dis. 2017;103:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park P, Kang H, Sanderson TM, et al. On the role of calcium-permeable AMPARs in long-term potentiation and synaptic tagging in the rodent hippocampus. Front Synaptic Neurosci. 2019;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bregestovski P, Bernard C. Excitatory GABA: how a correct observation may turn out to be an experimental artifact. Front Pharmacol. 2012;3:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glykys J, Dzhala V, Egawa K, et al. Local impermeant anions establish the neuronal chloride concentration. Science. 2014;343(6171):670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]