Abstract
Objective
The purpose of this study was to explore the prognostic role of c-MYC amplification in colorectal cancer, particularly in schistosomiasis-associated colorectal cancer.
Methods
Three hundred and fifty four cases of colorectal cancer, which were from Qingpu Branch of Zhongshan Hospital affiliated to Fudan University, were retrospectively analyzed in a tissue microarray (TMA) format, with fluorescence in situ hybridization (FISH) assay and immunohistochemistry (IHC).
Results
c-MYC gene amplification was found in 14.1% (50 out of 354) of patients with colorectal cancer and was correlated with old age (P = 0.028), positive lymph node metastasis (P = 0.004) and advanced stage tumors (P = 0.002). The overexpression of c-MYC was closely associated with the amplification status (P = 0.023). Kaplan–Meier survival curves for overall survival (OS) showed a statistically significant difference for patients with c-MYC amplification in full cohort of colorectal cancer, stage III-IV set and patients with lymph node metastasis (P = 0.002, 0.034, 0.012, respectively). Further analysis found c-MYC amplification associated with poorer survival in the subgroup of colorectal cancer with schistosomiasis (CRC-S, P < 0.001), but not in colorectal cancer without schistosomiasis (CRC-NS, P = 0.155). By multivariate analysis, c-MYC amplification was an independent poor-prognostic factor in CRC-S set (P = 0.046).
Conclusions
Our study firstly found c-MYC amplification could predict poor prognosis in schistosomiasis-associated colorectal cancer, but not in colorectal cancer without schistosomiasis.
Keywords: c-MYC, colorectal cancer, gene amplification, prognosis, Schistosoma japonicum
Our study firstly found c-MYC amplification was an independently poor-prognostic factor in schistosomiasis-associated colorectal cancer, but not in colorectal cancer without schistosomiasis.
Introduction
Colorectal cancer is the fourth most common cancer and the second leading cause of cancer deaths in the world (1). An estimated 1.09 million new colorectal cancer cases and 551 269 colorectal cancer deaths occurred in 2018 (1). Approximately 25% of patients have metastatic disease at diagnosis and ~50–60% of patients diagnosed with colorectal cancer go on to develop metastatic disease (2). Although the advance of surgery, radiotherapy and chemotherapy has improved the survival of colorectal cancer patients in recent years, the 5-year survival in patients with stage IV disease is 14% (3). Response to treatment and patients’ survival were variable among different population. It was known that tumor heterogeneity is a potential cause for these varied clinical outcomes (4). Since colorectal cancer is known to be a heterogeneous disease with diverse molecular alterations, which involve in biological tumor progression. Thus, a precise molecular marker could be used to predict patients’ survival or monitor cancer recurrence, which is urgently needed.
c-MYC, a proto-oncogene located on chromosome 8q24, is involved with regulating cell proliferation, differentiation and apoptosis (5). In solid tumors, such as breast, ovary and prostate, c-MYC amplification has been documented to be related to lymph node metastasis, recurrence and disease progression to a variable degree (6–9). These results manifested the possibility of c-MYC as a clinically useful indicator in the prognosis of cancer. However, the criteria for c-MYC amplification in colorectal cancer have not been unified, and whether it could be an independent prognostic factor in colorectal cancer that has been scarcely investigated. And recent studies provided inconsistent conclusions (10–13). Some early studies revealed the incidence of c-MYC amplification in colorectal cancer and found it was associated with tumor invasion and poor prognosis (14,15), but a recent study showed c-MYC amplification was unrelated with clinicopathologic features and clinical outcomes (12). Therefore, further detailed analysis is needed to confirm the prognostic significance of c-MYC amplification in colorectal cancer.
Intriguingly, we observed schistosome eggs under microscope in hematoxylin and eosin (HE) stained slides from our cohort. Qingpu District used to be schistosomiasis endemic areas and majorly infected with Schistosoma japonicum. In endemic areas, it is not uncommon to detect schistosome eggs in the intestines of colorectal cancer patients, but the relationship between schistosomiasis and colorectal cancer remains controversial. In Egypt, the reports tend to deny any association of S. mansoni and colorectal cancer (16). In Asia, S. japonicum infection is considered a risk factor for colorectal cancer (17). This may be due to the higher egg production of S. japonicum female worms and that the eggs are laid in large aggregates that induce intensive tissue reactions in host organs (18).
Here, we analyzed c-MYC amplification status in 354 colorectal cancer patients using tissue microarrays (TMA) by FISH, and compared its amplification in patients with schistosomiasis and without schistosomiasis groups. Besides, we also compared c-MYC amplification status in different stage and different state of lymph node metastasis. We investigated correlations between c-MYC amplification status and prognosis in colorectal cancer.
Materials and methods
Patients and samples
The whole cohort was consisted by 354 colorectal cancer patients who underwent surgical resection from Qingpu Branch of Zhongshan Hospital affiliated to Fudan University, from January 2008 to August 2016. None of them received preoperative chemotherapy or radiation therapy. Clinical follow-up data and clinicopathological characteristics, such as age, gender, tumor site, clinical stage, were obtained from medical records and pathologic reports. Two expert pathologists reviewed HE-stained slides to determine the diagnosis and to restage the tumors according to the eighth edition of American Joint Committee on Cancer (AJCC). The diagnosis of schistosomiasis was done by finding schistosome eggs in HE-stained slides.
The present study has been carried out in accordance with the Declaration of Helsinki and was approved by the local institution’s Human Research Ethics Committee. Prior written informed consent was obtained from all patients.
Tissue microarrays
The TMA blocks were manufactured from the most representative areas of individual paraffin blocks, as previously described (19). Briefly, reviewed HE-stained slides and marked the represented areas in tumor tissues, and the single core (2-mm wide and 6-mm long) for each case was precisely arrayed into a new recipient paraffin block. The cores containing >20% tumor cells were considered as valid cores.
Fluorescence in situ hybridization (FISH)
FISH for c-MYC amplification was performed on the TMA sections of 4-μm thickness by using commercial available probe (MYC (8q24) Probe, lot: 201812001, LBP Medicine Science and Technology Company, LTD, Guangzhou, China). c-MYC probe would hybridize to the band 8q with Spectrum Red signal, CEP8 probe would hybridize to the centromeric region of chromosome 8 with Spectrum Green signal.
The FISH slides were interpreted by two experienced evaluators with a fluorescence microscope (Olympus BX43, Olympus Optical Company, LTD, Tokyo, Japan) (Fig. 1A and B). A ratio of the total number of c-MYC signals to the total number of CEP8 signals in at least 60 non-overlapping tumor nuclei was determined. Cells with no signals or with signals of only one color were disregarded. When the red c-MYC signals were clearly amplified (large clouds of amplification), we assigned 20 red signals and counted the green CEP8 signals. For such cases, the ratio was defined as 20 divided by the average number of green signals per cell. c-MYC:CEP8 ratio ≥1.8 was considered as the criterion for gene amplification (20).
Figure 1.
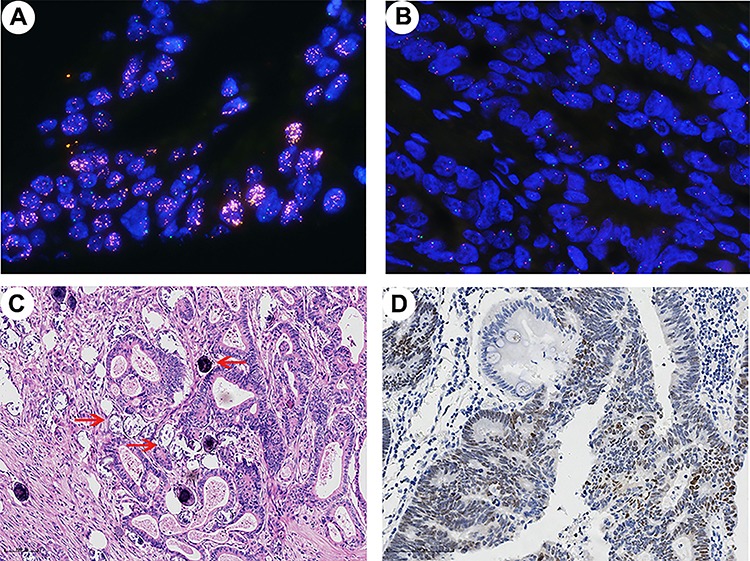
(A-B) Representative patterns of c-MYC gene by FISH (oil immersion, ×1000). (A) c-MYC amplification (c-MYC:CEP8 ratio = 9.03). (B) c-MYC gene disomy (c-MYC:CEP8 ratio = 0.97). (C) Typical sample of schistosomiasis-associated colorectal cancer, the red arrows indicate schistosome eggs (HE, ×100). (D) Positive staining for c-MYC showed frequent nuclear expression (×200).
Immunohistochemistry (IHC)
IHC labeling was performed as previously described (21) by Ascend Aliya autostainer (Ascend microsystems, Guangzhou, China), using a commercially available rabbit monoclonal c-MYC antibody (clone EP121, lot: 180712803C1, MXB Biotechnologies, Fuzhou, China). c-MYC expression was evaluated by two pathologists independently, who were blinded to clinic data. The percentage of positively stained cells and staining intensity were all evaluated. When nucleus of strong and moderate staining in >10% of the neoplastic cells, it was regarded as positive. Otherwise, the results were recorded as negative (10).
Statistical analysis
The association between c-MYC status and clinicopathological characteristics was evaluated by using the Chi square and Fisher’s exact tests. Overall survival (OS) was defined as the time of surgery to death. Kaplan–Meier curves with log-rank tests were used to determine the prognostic significance for OS, and multivariate Cox proportional hazard regression analysis was used to identify the independent prognostic factors. All statistical analyses were performed by using SPSS version 20.0 and GraphPad prism 7.0. P values <0.05 were considered statistically significant.
Results
Patient characteristics
The clinicopathologic characteristics of the study cohort are summarized in Table 1. Among these patients, 39.0% (138 out of 354) had schistosomiasis (Table 1 and Fig. 1C). The median age of patients with schistosomiasis was 74 years (CRC-S, range 54–91), and age of patients without schistosomiasis was 64 years (CRC-NS, range 33–90). The differences of clinicopathologic characteristics between CRC-S set and CRC-NS set were summarized in Supplementary Table S1. There was no magnificent difference between two sets except for age (P < 0.001, Supplementary Table S1).
Table 1.
The association between clinicopathological characteristics and c-MYC status in full cohort of colorectal cancer patients (N = 354)
| Characteristics | All patients | c-MYC amplification | P values | c-MYC IHC | P values | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No | Yes | Neg | Pos | |||
| Age | 0.028 | 0.627 | ||||||
| <60 | 84 | 23.7 | 66 | 18 | 25 | 59 | ||
| ≥ 60 | 270 | 76.3 | 238 | 32 | 88 | 182 | ||
| Gender | 0.775 | 0.036 | ||||||
| Female | 141 | 39.8 | 122 | 19 | 36 | 105 | ||
| Male | 213 | 60.2 | 182 | 31 | 77 | 136 | ||
| Tumor site | 0.077 | 0.460 | ||||||
| Rectum | 96 | 27.1 | 80 | 16 | 28 | 68 | ||
| Left-sided | 115 | 32.5 | 94 | 21 | 34 | 81 | ||
| Right-sided | 143 | 40.4 | 130 | 13 | 51 | 92 | ||
| Tumor sizea | 0.075 | 0.269 | ||||||
| <5 cm | 174 | 49.2 | 144 | 30 | 50 | 124 | ||
| ≥5 cm | 154 | 43.5 | 138 | 16 | 53 | 101 | ||
| Differentiation | 0.756 | 0.165 | ||||||
| Low | 84 | 23.7 | 73 | 11 | 81 | 189 | ||
| High | 270 | 76.3 | 231 | 39 | 32 | 52 | ||
| Invasive depth | 0.211 | 0.033 | ||||||
| I + II | 81 | 22.9 | 73 | 8 | 18 | 63 | ||
| III | 273 | 77.1 | 231 | 42 | 95 | 178 | ||
| Lymph node metastasis | 0.004 | 0.747 | ||||||
| No | 208 | 58.8 | 188 | 20 | 65 | 143 | ||
| Yes | 146 | 41.2 | 116 | 30 | 48 | 98 | ||
| Clinical stage | 0.002 | 0.889 | ||||||
| I + II | 193 | 54.5 | 176 | 17 | 61 | 132 | ||
| III + IV | 161 | 45.5 | 128 | 33 | 52 | 109 | ||
| Schistosomiasis | 0.878 | 0.344 | ||||||
| No | 216 | 61.0 | 185 | 31 | 73 | 143 | ||
| Yes | 138 | 39.0 | 119 | 19 | 40 | 98 | ||
a: Missing data.
Abbreviation: IHC, immunohistochemistry; Pos, positive; Neg, negative.
Invasive depth I = confined to submucosal layer; Invasive depth II = invasion of muscular layer; Invasive depth III = beyond the adventitia. P values are calculated by using the Chi square and Fisher’s exact test.
c-MYC status and correlation with clinicopathologic features
The median of c-MYC:CEP8 ratio identified by FISH was 1.20 (range of 0.83–9.03). In present study, results demonstrated that c-MYC amplification was detected in 14.1% (50 out of 354) of patients (Fig. 1A) by defining the c-MYC amplification as c-MYC:CEP8 ≥1.8. There was no difference between CRC-S set and CRC-NS set in the distribution of c-MYC:CEP8 ratio (Fig. 2A). Table 1 showed the correlation between c-MYC amplification status and clinicopathologic features in total of 354 patients. Briefly, c-MYC amplification was linked with young age (P = 0.028), positive lymph node metastasis (P = 0.004) and advanced stage (P = 0.002). Overexpression of c-MYC was observed in 68.1% (241 out of 354) of full cohort (Fig. 1D) and associated with male (P = 0.036) and deeper invasive depth (P = 0.033). There were 82% (41 out of 50) c-MYC amplification samples showed strong nuclear protein expression, by statistical analysis, the nuclear expression of c-MYC was significantly related with gene amplification (P = 0.023) (data not showed).
Figure 2.
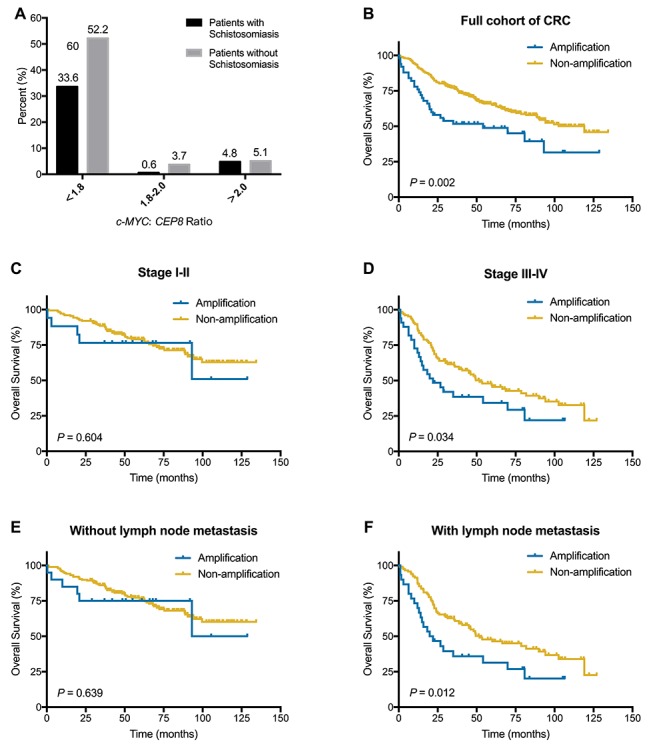
(A) Distribution of c-MYC:CEP8 ratio in CRC-NS set. (B–F) Kaplan–Meier survival curves illustrating prognostic effects of c-MYC amplification in CRC. (B) c-MYC amplification for OS in full cohort. (C) c-MYC amplification for OS in stage I-II set. (D) c-MYC amplification for OS in stage III-IV set. (E) c-MYC amplification for OS in patients without lymph node metastasis. (F) c-MYC amplification for OS in patients with lymph node metastasis.
Survival analyses in full cohort of patients with colorectal cancer
The median follow-up times were 62.4 months (range from 0.4 to 134.4 months). During the follow up, there were 41.8% (148 out of 354) patients died. Mean and median times to OS were 62.63 and 62.49 months, respectively.
A Kaplan–Meier curve for OS showed c-MYC amplification was significantly associated with poor survival in total colorectal cancer patients (P = 0.002) (Fig. 2B). Univariate analyses involving Cox proportional hazards models showed that age, gender, invasive depth, lymph node metastasis, clinical stage, differentiation, schistosomiasis and c-MYC amplification had association with OS (Table 2). In multivariate analysis for OS, age, gender, clinical stage, differentiation and c-MYC amplification were identified as independent poor prognostic factors.
Table 2.
Univariate and multivariate survival analyses of clinicopathological and molecular features for overall survival (OS)
| Variable | All patients | Patients with stage I-II disease | Patients with stage III-IV disease | Patients with lymph node metastasis | Patients without lymph node metastasis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| P values | Hazard ratio (95%CI) | P values | Hazard ratio (95%CI) | P values | Hazard ratio (95%CI) | P values | Hazard ratio (95%CI) | P values | Hazard ratio (95%CI) | |
| Univariate analysis | ||||||||||
| Age | 0.006 | 1.828(1.188–2.814) | 0.008 | 3.516(1.397–8.847) | 0.167 | 1.419(0.864–2.328) | 0.074 | 1.624(0.953–2.765) | 0.021 | 2.397(1.138–5.047) |
| Gender | 0.009 | 1.582(1.119–2.237) | 0.071 | 1.761(0.954–3.252) | 0.011 | 1.735(1.136–2.649) | 0.012 | 1.787(1.137–2.809) | 0.142 | 1.501(0.873–2.581) |
| Tumor site | ||||||||||
| Rectum | Reference | Reference | Reference | Reference | Reference | |||||
| Left colon | 0.931 | 1.019(0.674–1.540) | 0.672 | 1.167(0.571–2.383) | 0.863 | 0.956(0.575–1.590) | 0.955 | 0.985(0.581–1.670) | 0.940 | 1.026(0.527–1.996) |
| Right colon | 0.531 | 0.879(0.589–1.314) | 0.991 | 1.004(0.503–2.006) | 0.457 | 0.827(0.502–1.363) | 0.265 | 0.739(0.434–1.258) | 0.868 | 1.054(0.565–1.965) |
| Tumor size | 0.550 | 1.108(0.792–1.549) | 0.954 | 1.017(0.573–1.804) | 0.389 | 1.199(0.793–1.813) | 0.165 | 1.359(0.881–2.098) | 0.627 | 0.876(0.515–1.492) |
| Invasive depth | <0.001 | 2.628(1.585–4.357) | 0.648 | 1.150(0.631–2.097) | 0.005 | 7.496(1.846–30.445) | 0.007 | 6.922(1.702–28.156) | 0.276 | 1.383(0.772–2.478) |
| Lymph node metastasis | <0.001 | 2.717(1.956–3.774) | 0.048 | 4.184(1.010–17.335) | 0.562 | 0.830(0.442–1.558) | — | — | — | — |
| Clinical stage | <0.001 | 3.109(2.215–4.365) | — | — | — | — | 0.828 | 0.856(0.210–3.484) | <0.001 | 4.110(2.121–7.967) |
| Differentiation | 0.001 | 1.846(1.305–2.611) | 0.897 | 1.051(0.495–2.233) | 0.012 | 1.690(1.123–2.541) | 0.128 | 1.402(0.907–2.167) | 0.086 | 1.687(0.929–3.066) |
| Schistosomiasis | 0.041 | 1.402(1.014–1.940) | 0.405 | 1.262(0.730–2.182) | 0.020 | 1.627(1.080–2.452) | 0.020 | 1.663(1.083–2.555) | 0.345 | 1.276(0.770–2.115) |
| c-MYC amplification | 0.002 | 1.912(1.266–2.887) | 0.604 | 1.276(0.507–3.210) | 0.036 | 1.653(1.032–2.646) | 0.014 | 1.839(1.132–2.990) | 0.640 | 1.223(0.526–2.843) |
| c-MYC IHC | 0.064 | 0.732(0.526–1.019) | 0.400 | 0.786(0.449–1.376) | 0.079 | 0.692(0.459–1.043) | 0.130 | 0.717(0.467–1.103) | 0.330 | 0.773(0.460–1.298) |
| Multivariate analysis | ||||||||||
| Age | 0.020 | 2.021(1.297–3.147) | 0.005 | 3.874(1.519–9.883) | — | — | — | — | 0.022 | 2.390(1.133–5.041) |
| Gender | 0.008 | 1.603(1.128–2.277) | — | — | 0.032 | 1.592(1.041–2.435) | 0.048 | 1.586(1.004–2.503) | — | — |
| Invasive depth | — | — | — | — | 0.006 | 7.271(1.783–29.647) | 0.010 | 6.293(1.543–25.668) | — | — |
| Lymph node metastasis | — | — | 0.011 | 6.607(1.541–28.326) | — | — | — | — | — | — |
| Clinical stage | <0.001 | 2.639(1.849–3.765) | — | — | — | — | — | — | <0.001 | 4.105(2.111–7.982) |
| Differentiation | 0.006 | 1.664(1.161–2.384) | — | — | 0.003 | 1.900(1.245–2.901) | — | — | — | — |
| Schistosomiasis | — | — | — | — | 0.011 | 1.701(1.128–2.565) | 0.025 | 1.634(1.064–2.510) | — | — |
| c-MYC amplification | 0.002 | 1.966(1.278–3.027) | — | — | 0.018 | 1.790(1.107–2.896) | 0.021 | 1.775(1.089–2.893) | — | — |
—, not applicable
Abbreviation: CI, confidence interval.
Survival analyses based on clinical stage
In stage I-II set (n = 193), no correlation was found between c-MYC amplification and prognosis (P = 0.604) (Fig. 2C). However, in stage III-IV set (n = 161), c-MYC amplification was correlated with poor survival (P = 0.034) (Fig. 2D). In univariate analyses for OS, gender, invasive depth, differentiation, schistosomiasis and c-MYC amplification were significant prognostic factors. In multivariate analysis with c-MYC amplification and conventional significant variables, c-MYC amplification was a significant prognostic factor for OS (P = 0.018, HR = 1.790, 95%CI, 1.107–2.896) (Table 2).
Survival analyses based on lymph node metastasis status
In patients without lymph node metastasis, c-MYC amplification was not associated with OS (Table 2 and Fig. 2E). In patients with lymph node metastasis (n = 146), c-MYC amplification was observed in 20.5% (30 out of 146) and associated with poor survival (P = 0.012) (Table 1 and Fig. 2F). By using univariate and multivariate analysis, c-MYC amplification was independently prognostic factor in this subgroup (P = 0.021, HR = 1.775, 95%CI, 1.089–2.893) (Table 2).
Survival analyses based on schistosomiasis status
During the follow up, there was 47.8% (66 out of 138) patients died in CRC-S set, 38.0% (82 out of 216) patients died in CRC-NS set. Mean and median times to OS in CRC-S set were 56.49 and 49.97 months, respectively. The CRC-NS set were 66.45 and 67.93 months, respectively.
In CRC-S set, Kaplan–Meier curve for OS showed c-MYC amplification was correlated with poor survival (P < 0.001) (Fig. 3A). Univariate analyses showed that lymph node metastasis, clinical stage and c-MYC amplification were associated with OS (Table 3). Owing to age as a known factor was associated with survival or prognosis, and most patients’ age were over 60 years old (133 out of 138), age was not included in univariate analysis. In multivariate analysis for OS, clinical stage and c-MYC amplification were the only factors manifested statistical significance (P < 0.001, HR = 3.640, 95%CI, 2.143–6.183; P = 0.046, HR = 1.861, 95%CI, 1.012–3.419, respectively) (Table 3). Further analyses in CRC-S subsets stratified by clinical stage and lymph node metastasis showed that patients with c-MYC amplification tend to be poor prognosis in stage III-IV set and patients with lymph node metastasis (P = 0.068, 0.024, respectively) (Fig. 3B–E), which were similar with the results in full cohort.
Figure 3.
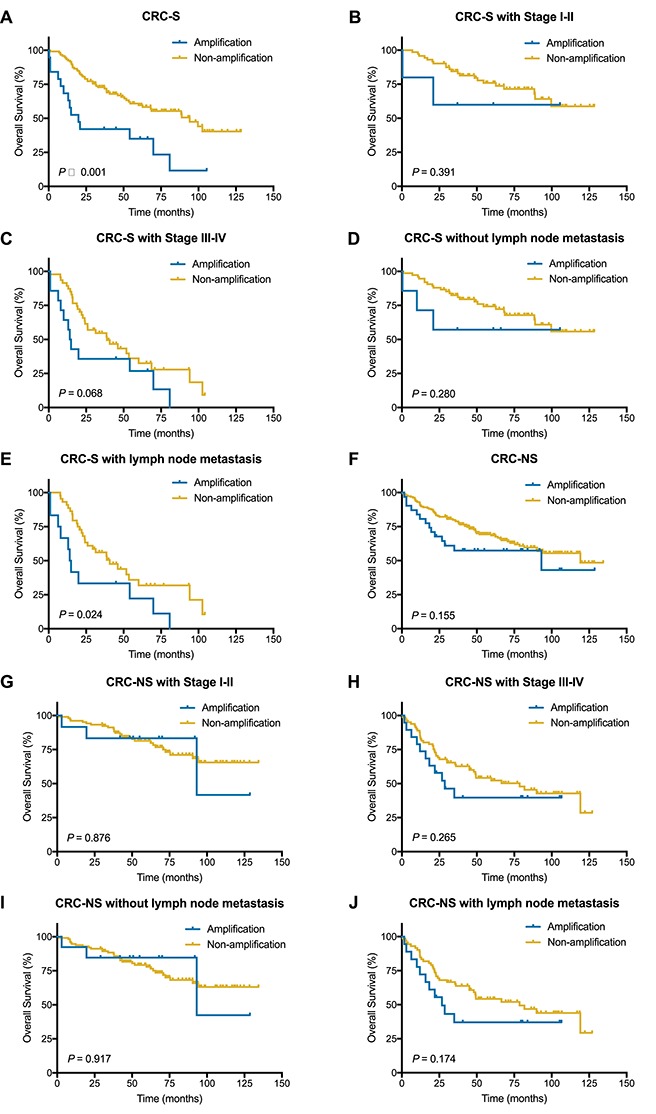
Kaplan–Meier survival curves illustrating prognostic effects of c-MYC amplification in CRC-S set and CRC-NS set. (A and F) c-MYC amplification for OS in CRC-S set and CRC-NS set. (B and C) c-MYC amplification for OS in CRC-S with stage I-II or stage III or IV. (D and E) c-MYC amplification for OS in CRC-S without or with lymph node metastasis. (G and H) c-MYC amplification for OS in CRC-NS with stage I-II or stage III-IV. (I and J) c-MYC amplification for OS in CRC-NS without or with lymph node metastasis.
Table 3.
Univariate and multivariate survival analyses for OS in CRC-S set and CRC-NS set
| Variable | Patients with schistosomiasis | Patients without schistosomiasis | ||
|---|---|---|---|---|
| P values | Hazard ratio (95%CI) | P values | Hazard ratio (95%CI) | |
| Univariate analysis | ||||
| Age | — | — | 0.090 | 1.506(0.939–2.416) |
| Gender | 0.392 | 1.251(0.749–2.088) | 0.011 | 1.839(1.148–2.945) |
| Tumor site | ||||
| Rectum | Reference | Reference | ||
| Left colon | 0.487 | 1.261(0.656–2.4223) | 0.653 | 0.884(0.515–1.516) |
| Right colon | 0.104 | 1.687(0.898–3.168) | 0.050 | 0.587(0.345–1.000) |
| Tumor size | 0.072 | 1.574(0.960–2.582) | 0.578 | 0.876(0.549–1.397) |
| Invasive depth | 0.079 | 1.882(0.930–3.805) | 0.001 | 3.401(1.640–7.056) |
| Lymph node metastasis | <0.001 | 3.391(2.055–5.595) | <0.001 | 2.441(1.572–3.790) |
| Clinical stage | <0.001 | 3.998(2.384–6.704) | <0.001 | 2.753(1.749–4.335) |
| Differentiation | 0.066 | 1.627(0.968–2.734) | 0.004 | 1.985(1.246–3.164) |
| c-MYC amplification | 0.001 | 2.719(1.504–4.918) | 0.158 | 1.515(0.852–2.694) |
| c-MYC IHC | 0.454 | 0.824(0.496–1.368) | 0.062 | 0.659(0.425–1.022) |
| Multivariate analysis | ||||
| Gender | — | — | 0.009 | 1.875(1.167–3.012) |
| Invasive depth | — | — | 0.017 | 2.477(1.177–5.216) |
| Clinical stage | <0.001 | 3.640(2.143–6.183) | <0.001 | 2.527(1.588–4.019) |
| Differentiation | — | — | — | — |
| c-MYC amplification | 0.046 | 1.861(1.012–3.419) | — | — |
—, not applicable
Abbreviation: CI, confidence interval.
However, in CRC-NS set (n = 216), no correlation was found between c-MYC amplification and prognosis in total or in the subsets stratified by clinical stage or lymph node metastasis status (Table 3, Fig. 3F–J).
Discussion
The c-MYC gene encodes nuclear DNA binding proteins that regulate the expression of a variety of genes implicated in cell proliferation, apoptosis, metabolism, stemness, invasiveness and inhibition of differentiation (22,23). In general, c-MYC dysregulation in lymphoma is usually caused by chromosome translocation and is typically associated with aggressive clinical behavior. Similarly, c-MYC amplification is clearly correlated with adverse biological features of the tumors. A previous study of chondrosarcoma showed that c-MYC amplification was prognostic markers of poor outcome for chondrosarcomas of grade 2 or higher (10). Another study found an association between c-MYC amplification and disease progression in prostate cancer (7).
However, the research about association between c-MYC amplification and colorectal cancer was really rare. In our study, we found that c-MYC amplification was detected in 14.1% (50 out of 354) of patients with colorectal cancer, and c-MYC amplification was related to poor prognosis in full cohort. Further study showed that c-MYC amplification was also a poor predictor in schistosomiasis-associated colorectal cancer, but was not in colorectal cancer without schistosomiasis.
According to previously published reports, the frequency of c-MYC amplification in colorectal cancer was ~8–14% (12,24), this was consistent with our result, which was 14.1%. Masramon’s results showed that c-MYC amplification was correlated with shorter disease-free survival (14), whereas the other studies showed that not c-MYC amplification (12) but c-MYC copy number gain can be a poor prognostic factor in colorectal cancer (13,25). In our study, c-MYC amplification was correlated with poor prognosis in the whole cohort (Fig. 2B), which was inconsistent with previous reports. The unexpectedly discovered schistosome eggs reminded us that this may contributed to the inconsistence. Hence, further subgroups were generated based on schistosomiasis, the total cohort was divided into two groups: CRC-S and CRC-NS. Interestingly, we found that c-MYC amplification could predict poor prognosis in schistosomiasis-associated colorectal cancer, but not in colorectal cancer without schistosomiasis (Fig. 3A). These findings, therefore, suggest that c-MYC amplification may involve in the pathogenesis and mechanism of schistosomiasis-associated colorectal cancer. The overexpression of c-MYC was not associated with OS in CRC-S set or CRC-NS set (Table 3). Although there was a weak correlation between c-MYC protein overexpression and c-MYC amplification, c-MYC amplification was not detected in most c-MYC protein overexpression cases, suggesting that there are alternative mechanisms responsible for c-MYC protein overexpression, rather than just gene amplification. The potential mechanisms including single nucleotide polymorphism in regulatory regions, mutation of upstream signaling pathways and mutations that enhance the stability of the protein (26–28). Further research is needed to explore the association of c-MYC overexpression and gene amplification in schistosomiasis-associated colorectal cancer.
Schistosoma haematobium, S. mansoni and S. japonicum are three main species of schistosomes that infect human beings. In China, the majority of schistosomes that infect human are S. japonicum. Historically, Qingpu District was one of serious schistosomiasis endemic areas between 1940s and 1960s, 154 767 of the 390 000 people in Qingpu District were suffering from schistosomiasis, with an infection rate of ~39% (29). Although through effective prevention and treatment, Qingpu District had reached the standard of schistosomiasis elimination in 1983, the effects of schistosomiasis still exist. All evidence suggests that schistosome eggs, and not adult worms, induce the host’s immune response and the granulomatous reaction (30). Many eggs permanently deposit in the intestines or liver (for S. mansoni and S. japonicum) or in the bladder and urogenital system (for S. haematobium). At present, there is solid evidence to confirm that S. haematobium is strongly associated with squamous cell carcinoma of the bladder (31). The International Agency for Research on Cancer (IARC) has regarded the infection with S. haematobium as Group 1 carcinogen (32). Similarly, other macroparasites such as the liver flukes Opisthorchis viverrini and Clonorchis sinensis have a role in causing some type of cholangiocarcinoma (32). Direct and indirect mechanisms may cause these parasites to be associated with specific tumors (33). The relationship between S. japonicum and colorectal cancer remains controversial. Although many studies showed that there is a strong association between S. japonicum and colorectal cancer, there is no definite evidence that S. japonicum is a causative agent in the development of colorectal cancer (34,35).
After infection with S. japonicum, schistosome eggs will deposit in the digestive tract, release of egg antigen, and the process of granulomas formation will be accompanied by chronic inflammation (36). The vast majority of the burden of disease due to S. japonicum appears to be caused by chronic inflammation (37). As a hallmark of cancer, inflammation may cause the formation of tumor. During the process of schistosomal infection, inflammatory cells can generate potential genotoxic mediators such as reactive oxygen and nitrogen species and proinflammatory cytokines, which induce genomic instability and dysregulation of oncogenes and tumor-suppressor genes (32,38). Chromosome region 8q24 including c-MYC and PRL-3 loci, as one category of genomic instability (39), is the most commonly amplified region in multiple cancer types, including colorectal cancer (7,40–42). When chronic inflammation and gene amplification co-exist in CRC-S set, we speculate that the accumulation of molecular disturbance may drive the progression toward dysplasia and carcinoma, even leading to a worse prognosis.
Besides, c-MYC is frequently dysregulated in inflammation and overexpressed in both sporadic and colitis-associated colon adenocarcinomas. Some studies revealed that c-MYC dysregulation functionally contributes to colitis-associated cancer progression (43,44). In inflammatory bowel disease (IBD)-associated intestinal adenocarcinoma, the frequency of c-MYC amplification increases to 26–33% (45,46). Yaeger et al. hypothesized that the infrequent WNT pathway activation in IBD-associated intestinal adenocarcinomas provides a selective drive for c-MYC gene amplification (45). Besides, recent research indicates a tight junction-associated protein, blood vessel epicardial substance (BVES), which promotes inflammatory tumorigenesis through dysregulation of WNT pathway and the oncogene c-MYC (47). To fully elucidate the association between c-MYC and schistosomiasis-associated colorectal cancer, it is essential to further investigate the roles of c-MYC, its regulators, its downstream effectors and the relationship between inflammation and schistosomiasis-associated colorectal cancer.
As the first limitation of the present study, we need more bench to lab work to validate the relationship between c-MYC and schistosomiasis and to interpret how schistosomiasis exert impact on c-MYC amplification. Second, the criterion of c-MYC amplification was diverse in different tumors (7,10,20). The optimal c-MYC amplification cutoff value for the prediction of prognosis remains to be established. Third, the proportion of schistosomiasis-associated colorectal cancer patients analyzed herein is <40%, so that the number of patients in subgroup was too small to draw a definitive conclusion. Therefore, we will increase sample size to validate the clinical meaning of c-MYC amplification in further study.
In summary, we first found c-MYC amplification was an adverse prognostic factor in schistosomiasis-associated colorectal cancer. These findings might shed light on detailed risk stratification in patients with colorectal cancer and provide an insight into pathogenesis and mechanism of progression in schistosomiasis-associated colorectal cancer.
Supplementary Material
Conflict of interest statement
None declared.
Funding
This work was supported by Qingpu Commission of Science & Technology Development Foundation [QKY2018–05] and Health System Key Specialist Fund of Qingpu District [WZ2015–03].
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Oliveira J, Group EGW . Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20Suppl 4:61–3. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Baldus SE, Schaefer KL, Engers R, et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res 2010;16:790–9. [DOI] [PubMed] [Google Scholar]
- 5. Li Z, Owonikoko TK, Sun SY, et al. C-Myc suppression of DNA double-strand break repair. Neoplasia 2012;14:1190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darcy KM, Brady WE, Blancato JK, et al. Prognostic relevance of c-MYC gene amplification and polysomy for chromosome 8 in suboptimally-resected, advanced stage epithelial ovarian cancers: a Gynecologic Oncology Group study. Gynecol Oncol 2009;114:472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fromont G, Godet J, Peyret A, et al. 8q24 amplification is associated with Myc expression and prostate cancer progression and is an independent predictor of recurrence after radical prostatectomy. Hum Pathol 2013;44:1617–23. [DOI] [PubMed] [Google Scholar]
- 8. Ghadimi BM, Grade M, Liersch T, et al. Gain of chromosome 8q23-24 is a predictive marker for lymph node positivity in colorectal cancer. Clin Cancer Res 2003;9:1808–14. [PubMed] [Google Scholar]
- 9. Perez EA, Jenkins RB, Dueck AC, et al. C-MYC alterations and association with patient outcome in early-stage HER2-positive breast cancer from the north central cancer treatment group N9831 adjuvant trastuzumab trial. J Clin Oncol 2011;29:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morrison C, Radmacher M, Mohammed N, et al. MYC amplification and polysomy 8 in chondrosarcoma: array comparative genomic hybridization, fluorescent in situ hybridization, and association with outcome. J Clin Oncol 2005;23:9369–76. [DOI] [PubMed] [Google Scholar]
- 11. Obara K, Yokoyama M, Asano G, Tanaka S. Evaluation of myc and chromosome 8 copy number in colorectal cancer using interphase cytogenetics. Int J Oncol 2001;18:233–9. [DOI] [PubMed] [Google Scholar]
- 12. Al-Kuraya K, Novotny H, Bavi P, et al. HER2, TOP2A, CCND1, EGFR and C-MYC oncogene amplification in colorectal cancer. J Clin Pathol 2007;60:768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee KS, Kwak Y, Nam KH, et al. C-MYC copy-number gain is an independent prognostic factor in patients with colorectal cancer. PLoS One 2015;10:e0139727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masramon L, Arribas R, Tortola S, Perucho M, Peinado MA. Moderate amplifications of the c-myc gene correlate with molecular and clinicopathological parameters in colorectal cancer. Br J Cancer 1998;77:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kozma L, Kiss I, Szakall S, Ember I. Investigation of c-myc oncogene amplification in colorectal cancer. Cancer Lett 1994;81:165–9. [DOI] [PubMed] [Google Scholar]
- 16. Cheever AW, Kamel IA, Elwi AM, et al. Schistosoma mansoni and S. haematobium infections in Egypt. III. Extrahepatic pathology. Am J Trop Med Hyg 1978;27:55–75. [DOI] [PubMed] [Google Scholar]
- 17. Ye C, Tan S, Jiang L, et al. Endoscopic characteristics and causes of misdiagnosis of intestinal schistosomiasis. Mol Med Rep 2013;8:1089–93. [DOI] [PubMed] [Google Scholar]
- 18. Ishii A, Matsuoka H, Aji T, et al. Parasite infection and cancer: with special emphasis on Schistosoma japonicum infections (Trematoda). Rev Mutat Res 1994;305:273–81. [DOI] [PubMed] [Google Scholar]
- 19. Shi Y, He D, Hou Y, et al. An alternative high output tissue microarray technique. Diagn Pathol 2013;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blancato J, Singh B, Liu A, Liao DJ, Dickson RB. Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses. Br J Cancer 2004;90:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang D, Song Q, Wang H, et al. Independent prognostic role of PD-L1expression in patients with esophageal squamous cell carcinoma. Oncotarget 2017;8:8315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hynes NE, Stoelzle T. Key signalling nodes in mammary gland development and cancer: Myc. Breast Cancer Res 2009;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dang CV, O'Donnell KA, Zeller KI, et al. The c-Myc target gene network. Semin Cancer Biol 2006;16:253–64. [DOI] [PubMed] [Google Scholar]
- 24. Rochlitz CF, Herrmann R, de Kant E. Overexpression and amplification of c-myc during progression of human colorectal cancer. Oncology 1996;53:448–54. [DOI] [PubMed] [Google Scholar]
- 25. Kwak Y, Yun S, Nam SK, et al. Comparative analysis of the EGFR, HER2, c-MYC, and MET variations in colorectal cancer determined by three different measures: gene copy number gain, amplification status and the 2013 ASCO/CAP guideline criterion for HER2 testing of breast cancer. J Transl Med 2017;15:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pomerantz MM, Ahmadiyeh N, Jia L, et al. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet 2009;41:882–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wright JB, Brown SJ, Cole MD. Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol Cell Biol 2010;30:1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer 2008;8:976–90. [DOI] [PubMed] [Google Scholar]
- 29. Yu S. Schistosomiasis must be eradicated: a review of fighting schitosomiasis in Qingpu, Shanghai. Chin J Epidemiol 2016;37:1044–6. [DOI] [PubMed] [Google Scholar]
- 30. Burke ML, Jones MK, Gobert GN, et al. Immunopathogenesis of human schistosomiasis. Parasite Immunol 2009;31:163–76. [DOI] [PubMed] [Google Scholar]
- 31. Honeycutt J, Hammam O, Fu CL, Hsieh MH. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol 2014;30:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological agents. Volume100B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 33. Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol 2009;31:686–96. [DOI] [PubMed] [Google Scholar]
- 34. Mei J, Hong H, Ding Y, Fang Y. Clinic pathological characteristics of chronic schistosomiasis concurrent colorectal cancer. Chin J Dig Endosc 2004;1: 50-1(in Chinese). [Google Scholar]
- 35. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Infection with schistosomes (Schistosoma haematobium, Schistosoma mansoni and Schistosoma japonicum). IARC Monogr Eval Carcinog Risks Hum 1994;61:45–119. [PMC free article] [PubMed] [Google Scholar]
- 36. Peterson WP, Von Lichtenberg F. Studies on granuloma formation. IV. In vivo antigenicity of schistosome egg antigen in lung tissue. J Immunol 1965;95:959–65. [PubMed] [Google Scholar]
- 37. Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol 2014;36:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soliman NA, Keshk WA, Shoheib ZS, Ashour DS, Shamloula MM. Inflammation, oxidative stress and L-fucose as indispensable participants in schistosomiasis-associated colonic dysplasia. Asian Pac J Cancer Prev 2014;15:1125–31. [DOI] [PubMed] [Google Scholar]
- 39. Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 2008;135:1079–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown JR, Hanna M, Tesar B, et al. Integrative genomic analysis implicates gain of PIK3CA at 3q26 and MYC at 8q24 in chronic lymphocytic leukemia. Clin Cancer Res 2012;18:3791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bardelli A, Saha S, Sager JA, et al. PRL-3 expression in metastatic cancers. Clin Cancer Res 2003;9:5607–15. [PubMed] [Google Scholar]
- 42. Saha S, Bardelli A, Buckhaults P, et al. A phosphatase associated with metastasis of colorectal cancer. Science 2001;294:1343–6. [DOI] [PubMed] [Google Scholar]
- 43. Brentnall TA, Pan S, Bronner MP, et al. Proteins that underlie neoplastic progression of ulcerative colitis. Proteomics Clin Appl 2009;3:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ciclitira PJ, Macartney JC, Evan G. Expression of c-myc in non-malignant and pre-malignant gastrointestinal disorders. J Pathol 1987;151:293–6. [DOI] [PubMed] [Google Scholar]
- 45. Yaeger R, Shah MA, Miller VA, et al. Genomic alterations observed in colitis-associated cancers are distinct from those found in sporadic colorectal cancers and vary by type of inflammatory bowel disease. Gastroenterology 2016;151:278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hartman DJ, Binion DG, Regueiro MD, et al. Distinct histopathologic and molecular alterations in inflammatory bowel disease-associated intestinal adenocarcinoma: C-MYC amplification is common and associated with mucinous/signet ring cell differentiation. Inflamm Bowel Dis 2018;24:1780–90. [DOI] [PubMed] [Google Scholar]
- 47. Parang B, Kaz AM, Barrett CW, et al. BVES regulates c-Myc stability via PP2A and suppresses colitis-induced tumourigenesis. Gut 2017;66:852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


