Abstract
From a clinical perspective, prostate-specific membrane antigen (PSMA) is a valuable target for both diagnosis and radioligand therapy (RLT) of prostate cancer. The term ‘specific’ has been used to characterize a histologic hallmark of overexpression in the membrane of most prostate cancer. Many PSMA ligands have been developed since the previous decade and have been used in several clinical trials and clinical studies. However, procedure, specification, protocol, interpretation criteria, radiation dose, and cost-effectiveness of PSMA ligands have not been fully explained. Regardless of worldwide use of promising PSMA-ligand PET and RLT, it has not been approved in Japan. Expedited introduction of PSMA-ligand PET and RLT to Japan and implementation of clinical study are eager for many patients with prostate cancer.
Keywords: PET, PET/CT, PSMA, prostate cancer, prostate-specific membrane antigen
PSMA-ligand PET is a promising modality for initial staging, secondary staging, detection of biochemical recurrence, treatment planning, and response evaluation in prostate cancer. Expedited implementation of clinical study in Japan is eager for many patients with prostate cancer.
Introduction
From the clinical perspective, prostate cancer shows the first prevalence in most countries and has been a global concern. The disease extent of prostate cancer at the time of initial diagnosis is mostly limited—that is, the presence of disease is suspected when the serum prostatic specific antigen (PSA) is elevated and the symptoms are present when the disease is already at an advanced stage after initial treatment—and as a rule there are no specific imaging methods or biomarkers to diagnose extent of disease except for PSA. Although treatment for patients with castration-resistant prostate cancer (CRPC) with more recently developed oral agents has improved quality of life, the group of CRPC has remained fatal after long duration of disease whether due to progression of systemic metastasis including the lymph node, bone, liver, and lungs. Prostatic specific membrane antigen (PSMA) is a valuable target for both diagnosis and therapy of prostate cancer. A series of PSMA ligands has been developed since previous decade.
PSMA ligands
Since PSMA is overexpressed on the surface of prostate cancer, various targeting PSMA ligands have been developed (Table 1). PSMA monoclonal antibodies with different radionucleotides were introduced both for intracellular and extracellular domains of PSMA (1), (Fig. 1). To obtain fast blood clearance and specific accumulation, PSMA antibody fragments were also developed as PSMA–PET ligands; however, significant kidney uptake and retention were also found to be a limitation for clinical use. Small molecular inhibitors of PSMA have been introduced to be recognizing enzymatic site and has been developed for clinical studies. There are three types of small molecular inhibitors based on the zinc-binding portions: phosphorous-based, thiol-based, and urea-based (2). The phosphorous-based type is considered gold standard binding phosphonate core to two zinc ions located in the active domain of PSMA. The difference between phosphorous-based type and thiol-based type depends on polarity. On the other hand, the urea-based type is internalized into cell after binding to the active domain of PSMA.
Table 1.
Current diagnostic PSMA ligand
| Isotope | Target | Imaging agent |
|---|---|---|
| 89Zr | ||
| Monoclonal antibody | 89Zr-DFO-7E11 | |
| Monoclonal antibody | 89Zr-DFO-J591 | |
| Antibody fragment | 89Zr-Cys-Db | |
| 64Cu | ||
| Monoclonal antibody | 64Cu-DOTA-3/A12 | |
| Monoclonal antibody | 64Cu-DOTA-3/F11 | |
| Monoclonal antibody | 64Cu-DOTA-3/E7 | |
| 111In | ||
| Antibody fragment | 111In-JVZ007-cys | |
| 99mTc | ||
| Antibody fragment | 99mTc-J591Cdia | |
| Small molecule inhibitor | 99mTc-MIP-1404 | |
| Small molecule inhibitor | 99mTc-MIP-1405 | |
| Small molecule inhibitor | 99mTc-DUPA | |
| 68Ga | ||
| Antibody fragment | 68Ga-THP-scFv | |
| Small molecule inhibitor | 68Ga-rhPSMA | |
| Small molecule inhibitor | 68Ga-THP-PSMA | |
| Small molecule inhibitor | 68Ga-PSMA-11 | |
| Small molecule inhibitor | 68Ga-PSMA-I&T | |
| 18F | ||
| Small molecule inhibitor | 18F-SFB | |
| Small molecule inhibitor | 18F-CTT-1298 | |
| Small molecule inhibitor | 18F-CTT-1057 | |
| Small molecule inhibitor | 18F-DCFBC | |
| Small molecule inhibitor | 18F-DCFPyL | |
| Small molecule inhibitor | 18F-YC-88 | |
| Small molecule inhibitor | 18F-PSMA-1007 | |
| Small molecule inhibitor | 18F-rhPSMA-7.3 | |
| Small molecule inhibitor | 18F-FSU-880 | |
Figure 1.
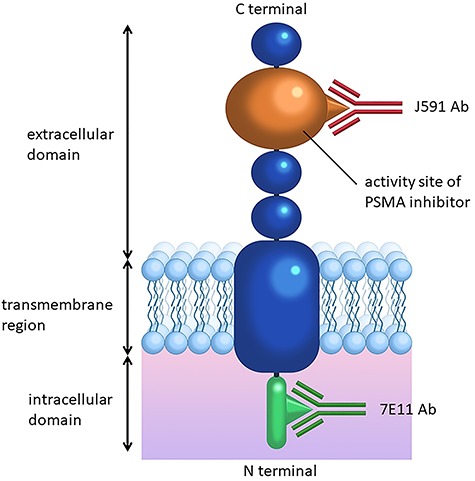
Molecular structure of prostate-specific membrane antigen (PSMA). Prostate-specific membrane antigen (PSMA) monomer has three domains. J591 antibody binds to activity site of extracellular domain which has 707 amino acids. 7E11 antibody binds to intracellular domain which has 19 amino acids. The homodimeric form of PSMA has enzymatic activity as glutamate carboxypeptidase II or folate hydrolase.
Positron emission tomography–computed tomography (PET/CT) imaging of prostate cancer
Recent advance of PET tracers using PSMA provides us a more accurate diagnosis of prostate cancer both at staging and in biochemical recurrence after radical prostatectomy or radiation therapy (Table 2). Detection of tumor regions with PSMA–PET/CT shows higher detection rate compared to other conventional imaging modalities. Several PSMA–PET tracers have been developed to date demonstrating significant detection rate of prostate cancer. They have different chemical structures and radiolabeled with many different radioisotopes including 11C, 18F, 123I, 124I, 125I, 131I, 99mTc, 68Ga, 177Lu, 44Sc, 64Cu, 111In, 86Y, 90Y, 225Ac, 213Bi, and 211At. At first, 68Ga was introduced only available for generator use. However, technical advance enables us to produce this under cyclotron use. 18F and 68Ga are major radioisotopes for PSMA tracers. 18F has 110 min half-life and is suitable for in-house or delivery setting, but 68Ga has 68 min half-life and can be available for generator use in most institutions.
Table 2.
Clinical application of PSMA–PET/CT
| Primary staging | Comparison with mpMRI is preferable because low spatial resolution and artifact given by excreted tracer |
| Secondary staging | Accuracy depends on serum PSA level |
| Diagnosis of biochemical recurrence | Accuracy depends on serum PSA level, Biochemical progression-free survival |
| Treatment planning | Delineation of CTV to include potential occult tumor for SRT |
| Response evaluation | RLT with alpha- or beta-emitting radionucleotides |
Abbreviations: PSMA, prostatic specific membrane antigen; mpMRI, multiparametric magnetic resonance imaging; PSA, prostate specific antigen; CTV, clinical target volume; SRT, stereotactic radiotherapy; RLT, radioligand therapy.
18F-labeled PSMA–PET ligands
Fluorine-18 is the most prevailing imaging isotope with positron emission yield of 97%. In 2008, the first 18F-labeled ligand N-[N-(S)-1,3-dicarboxypropyl]carbamol]-4-[18F]fluorobenzyl-L-cysteine (18F-DCFBC) (Fig. 2) was introduced (3). 18F-DCFPyL (Fig. 3) was developed as second generation demonstrating five times higher Ki than 18F-DCFBC (4). The biodistribution was noted with 36.6%ID/g after 4 h in PC-3 PIP mice. Chen and the colleagues introduced 18F-YC-88 which exhibited favorable kidney uptake of 47.6%ID/g compared to 18F-DCFPyL (5). 18F-labeled to DKFZ-PSMA-617 which was originally developed for 68Ga-ligand was introduced as 18F-PSMA-1007 (Fig. 4) (6). Biodistribution was 8.0%ID/g for tumor at 1 h in LNCaP tumor-bearing mice. The comparability of 177Lu-PSMA-617 and 18F-PSMA-1007 in tumor and normal organ uptake was noted. Kelly and the colleagues developed 18F-labeled ligands as lead compounds of RPS-040 and RPS-041 demonstrating biodistribution of 14.3%ID/g in tumor (7). Behr and the colleagues developed 18F-ligand targeting phospharamidate core as 18F-CTT1057 showing the same biodistribution to urea-based PSMA-targeted ligands with low exposure to the kidneys and salivary glands (8). Radiohybrid PSMA (rhPSMA) ligands were developed as theranostic agents with fast 18F synthesis and labeling with radiometals. 18F-rhPSMA-7.3 demonstrated high tumor uptake and low kidney uptake in human study (9). Saga and the colleagues developed 18F-FSU-880 sharing the same binding moiety as 68Ga-PSMA-11 (10).
Figure 2.

Chemical structure of 18F-DCFBC.
Figure 3.
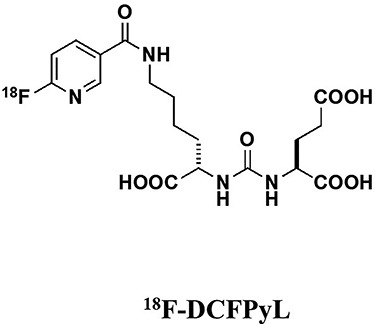
Chemical structure of 18F-DCFPyL.
Figure 4.

Chemical structure of 18F-PSMA-1007.
68Ga-labeled PSMA–PET ligands
68Ga-labeled inhibitor of PSMA of urea-based ligands having DOTA chelator was firstly introduced. Tumor uptake in biodistribution demonstrated 3.78%ID/g at 30 min post injection. Eder and the colleagues developed 68Ga-PSMA-HBED-CC which possessed both DOTA and HBED-CC chelating moieties having a tumor uptake of 7.70%ID/g in biodistribution (11). Consequently, they identified higher tumor uptake of 8.22%ID/g with dimerizated HBED-CC chelators (12). 68Ga-PSMA-HBED-CC and 68Ga-PSMA-11 are the same material. Weineisen and the colleagues introduced coupled chelators with DOTAGA and DOTA demonstrating tumor biodistribution of 4.95%ID/g as 68Ga-PSMA I&T and 7.96%ID/g as 177Lu-PSMA I&T 1 h after injection (13). PSMA-617, which contains a urea-binding motif coupled to a DOTA chelator, was developed for imaging and therapy (6). Tumor uptake value was 8.47%ID/g for 68Ga-PSMA-617 and 11.2%ID/g for 177Lu-PSMA-617, respectively (14). Gourni and the colleagues developed another ligand using NODAGA chelating 68Ga, 64Cu, and 111In (15).
Comparison of 18F-labeled PSMA–PET ligands
Treglia and the colleagues described the results of systematic review and meta-analysis based on six studies (16–22). They compared three different fluorinated PSMA–PET ligands for detection of biochemical recurrent prostate carcinoma. On the basis of a sub-group analysis, the pooled detection rate of F-PSMA-1007, F-DCFPyL, and F-DCFBC were 89% (95% CI: 72–98%), 81% (74–87%), and 60% (48–72%), respectively. F-PSMA-1007 demonstrates highest detectability of these three ligands. However, there is no study to compare the detection rate directly between three PET tracers to date. The authors also suggested that limitations contained availability of only six studies, and their results were lack of verification by histologic confirmation. F-PSMA-1007 and F-DCFPyL are developed under clinical trials in Europe and the USA, respectively. Other fluorinated PSMA–PET ligands are also under clinical phase I study, and the results will be out in the near future (Table 3).
Table 3.
Clinical use of PSMA ligands (Oct 2019)
| 18F-PSMA ligands | Organization or company | Clinical phase | |
| 1 | 18F-DCFPyL | Progenics Pharmaceuticals | Phase 3 |
| 2 | 18F-PSMA-1007 | ABX | Clinical study |
| 3 | 18F-CTT1057 | Novartis (AAA) | Phase 1 |
| 4 | 18F-rhPSMA-7.3 | Blue Earth Diagnostics | Phase 1 |
| 5 | 18F-FSU-880 | Kyoto University | Phase 2 |
| 68 Ga-PSMA ligands | Organization or company | Clinical phase | |
| 1 | 68Ga-PSMA-11 | Telix Pharmaceuticals | Phase 3 |
| 2 | 68Ga-PSMA-617 | Novartis (Endocyte) | Clinical study |
| 3 | 68Ga-PSMA-I&T | Technical University of Munich | Clinical study |
| PSMA ligands for RLT | Organization or company | Clinical phase | |
| 1 | 177Lu-PSMA-617 | Novartis (Endocyte) | Phase 3 |
| 2 | 225Ac-PSMA-617 | Novartis (Endocyte) | Phase 1 |
| 3 | 177Lu-TX591 | Telix Pharmaceuticals | Phase 2 |
| 4 | 177Lu-PSMA-R2 | Novartis (AAA) | Phase 1/2 |
| 5 | 227Th-PSMA-TTC | Bayer | Phase 1 |
Abbreviation: RLT, radioligand therapy.
Comparison of 68Ga-labeled PSMA–PET ligands
There were no evidence demonstrating the results of direct comparisons between 68Ga-labeled PSMA–PET ligands. 68Ga-PSMA-HBED-CC is the most widely used small molecular inhibitor, and evidence supporting its use in CRPC or advanced PC has been described to date. Perera and the colleagues conducted a meta-analysis to demonstrate updated data based on a total of 37 articles including 4790 patients ((23), Table 4). Diagnostic accuracy of 68Ga-labeled PSMA–PET/CT depends on serum PSA level. The pooled estimate positivity was 33% (confidence interval [CI], 16–51%) for prescan PSA of <0.2 ng/ml, 45% (39–52%) for 0.20–0.49 ng/ml, 59% (50–68%) for 0.50–0.99 ng/ml, 75% (66–84%) for 1.00–1.99 g/ml, and 95% (92–97%) for >2.00 ng/ml. On the basis of secondary staging purpose, overall estimates of positivity comprised of prostatic bed (28%), pelvic lymph nodes (38%), extrapelvic lymph nodes (13%), bone (22%), and distant viscera (5%). The detectability of recurrent tumor in prostatic bed was significantly higher in patients who underwent radiotherapy (52%) compared to prostatectomy (22%). On the pathologic basis of lesion by lesion, the summary sensitivity and specificity to detect primary tumor within prostate were 75 and 99%, respectively.
Table 4.
Large-scale clinical studies of PSMA–PET/CT
| References | Year | Study design | Type of patients evaluated | Tracer | Study objectives | Study results |
|---|---|---|---|---|---|---|
| Treglia et al. (16) | 2019 | Meta-analysis of 6 studies | BRPCa (n = 645) | 18F-PSMA | Perform a meta-analysis about the DR of 18F-PSMA–PET/CT in BRPCa patients | DR 81% (per patient analysis) 86% for PSA ≥ 0.5 ng/ml 49% for PSA < 0.5 ng/ml |
| Perera et al. (23) | 2019 | Systematic review of 37 studies | Advanced prostate cancer (n = 4790) | 68Ga-PSMA | Provide updated data on the predictors of a positive 68Ga-PSMA–PET with sensitivity and specificity and additionally to identify locational patterns of PSMA-avid lesions in the setting of prostate cancer staging in both primary and biochemical recurrence situations | Positive 68Ga-PSMA–PET in BRPCa patients 33% for PSA 0.0–0.19 ng/ml 45% for PSA 0.2–0.49 ng/ml 59% for PSA 0.5–0.99 ng/ml 75% for PSA 1.0–1.99 ng/ml 95% for PSA ≥2 ng/ml NSD: Gleason sums ≤7 and ≥ 8 Primary staging (per node analysis): sensitivity 75% and specificity 99% |
Abbreviations: BRPCa, biochemical recurrent prostate cancer; DR, detection rate; NSD, no significant difference.
Clinical utility
Biochemical recurrence
PSMA–PET/CT is highly sensitive for detecting regional and distant metastases of prostate cancer at low serum PSA level. For patients with serum PSA level lower than 1 ng/ml, standard care imaging is insensitive for detecting recurrence. Detection of focus in biochemical recurrence after prostatectomy offers long-term biochemical control after introduction of salvage radiotherapy. Calais and the colleagues conducted a randomized phase III trial of salvage radiotherapy with or without PSMA–PET/CT investigating its potential benefit on clinical outcome (24). Their hypothesis was that the incorporation of PSMA–PET/CT to salvage radiotherapy can improve a 5-year progression-free survival by 20%. PSMA–PET/CT can offer precise patient selection for salvage radiotherapy by improving the coverage of the recurrent lesions in the pelvic radiation field and by excluding patients with metastasis where salvage radiotherapy would not be curative. Calais and the colleagues will randomize a total of 193 patients for control arm with standard salvage radiotherapy and for intervention arm with PSMA–PET/CT prior to salvage radiotherapy planning: NCT03582774 (24). The primary endpoint is the success rate of salvage radiotherapy measured as biochemical progression-free survival. The results of the study will lead potential benefit of PSMA–PET/CT for the management of biochemical recurrence in prostate cancer. Recent advance in kit-based 68Ga-labeling, 68Ga-THP-PSMA was introduced to be suitable tracer for patients with biochemical recurrence greater than serum PSA 2.0 ng/ml (25).
Diagnostic accuracy of PSMA–PET and bone scan index
Bone is the common site of distant metastasis in patients with prostate cancer of advanced stage. Bone scintigraphy (BS) using 99mtechnetium–methylene diphosphate (99mTc-MDP) or 99mtechnetium-hydroxymethylene diphosphate (99mTc-HMDP) plays an important role to detect bone metastasis in CRPC. Zacho and the colleagues demonstrated that bone scan index (BSI) was an independent risk factor for the time from initiation of androgen deprivation therapy to CRPC and one of the prognostic factors (26). However, prognostic evaluation by BS alone is not satisfactory because of the presence of extraosseous metastases and limited spatial resolution. Anand and colleagues observed BSI reproducibility and dependence on the scanning speed of bone scan and suggested that standardization of scanning speed was needed to obtain image counts above 1.5 million prior to prospective BSI studies (27). However, standardization has not been applied for prospective studies to date. PSMA–PET/CT doesn’t depend on four different types of bone metastases: lytic/lucent, sclerotic, mixed and without any morphologic abnormalities which often complicate assessment of bone metastasis by BS. It has been proposed that in patients with CRPC of advance stage, BS has a lower sensitivity for detecting distant metastasis when compared with PET/CT using PSMA ligands.
Diagnosis accuracy of PSMA–PET/CT and PSMA–PET/MRI
PSMA–PET/CT has been shown to be more accurate than conventional imaging to assess both osseous and extraosseous metastasis in CRPC. Accurately co-registered functional and morphologic data sets generated by integrated imaging systems provide us accurate diagnosis of metastasis in CRPC. The integrated whole-body PET/MRI also enables us to perform a functional and morphologic imaging for CRPC. From initial report describing comparison between PSMA–PET/MRI and PSMA–PET/CT, PSMA–PET/MRI was more accurate than PSMA–PET/CT in patients with local recurrence (28). With regard to metastatic CRPC, PSMA–PET/MRI does not seem to provide a considerable benefit as compared with PSMA–PET/CT (29). Although small nodal or bone metastasis can be easily detected by PSMA–PET/MRI, the clinical utility of PSMA–PET/MRI with comparison to PSMA–PET/CT in metastatic CRPC has not been fully elucidated.
Radioligand therapy (RLT)
Response and safety of RLT using urea-based PSMA inhibitors has demonstrated the potential for expanded therapeutic options for metastatic CRPC. Baum and the colleagues reported response and safety in 56 metastatic CRPC using 177Lu-PSMA-617 (Fig. 5) (30). The response to 177Lu-PSMA-617 demonstrated 80.4% decline of serum PSA levels and median progression-free survival of 13.7 months. Heck and colleagues described the efficacy of 177Lu-PSMA-I&T (Fig. 6) in patients with metastatic CRPC (31). The response to 177Lu-PSMA-I&T demonstrated over 30% decline of serum PSA levels in 47% of patients and progression-free survival of 4.1 months. Although high accumulation of kidney and parotid glands is noted in these agents, overall the use is safe and effective. The results of large scale clinical studies are summarized in Table 5. Kratochwil and colleagues described response and safety of 225Ac-PSMA-617 for metastatic CRPC (32). The results demonstrated PSA decline in 87% of patients after 3 cycles. 213 Bi and 211At using urea-based PSMA inhibitors have been also developed. Sathekge and colleagues reported therapeutic efficacy in patients with metastatic CRPC with 213 Bi-PSMA-617 (33). 211At is a favorable pure alpha-emitting radionucleotide because its daughter gives excessive dose with 7.2-h half-life. Kiess and colleagues developed 211At-DCAtBzL which improved survival of tumor bearing mice (34).
Figure 5.

Chemical structure of PSMA-617. 68Ga and 177Lu can bind to PSMA-617.
Figure 6.
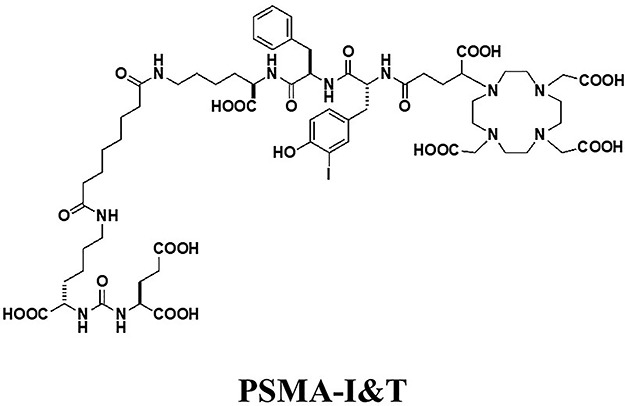
Chemical structure of PSMA-I&T. 68Ga and 177Lu can bind to PSMA-I&T.
Table 5.
Summary of efficacy of 177Lu-PSMA RLT in large-scale clinical studies
| References | Year | Study design | Radioligand | Patient characteristics | TEAE | Response | Survival |
|---|---|---|---|---|---|---|---|
| Rahbar et al (20) | 2017 | Retrospective, 12 centers, Germany |
177Lu- PSMA-617 2–8 GBq/cycle Total 248 cycles |
PSMA-avid mCRPC, 145 patients (median age 73 years) Prior Tx: AA 64%, ENZ 52%, CTx 54%, 223Ra 17% Mets: bone 87%, LN 77%, liver 20%, lung 14% |
Grade 1/2 Xerostomia 8%, Nausea 6% Grade 3/4 Anemia 10%, Thrombocytopenia 4% Leukopenia 3% |
PSA decline ≥50%, 45% PSA response Good: ≥3 cycles Bad: visceral mets, elevated ALP |
NA |
| Heck et al. (31) | 2019 | Retrospective, single-center, Germany |
177Lu- PSMA-I&T 7.4 GBq/cycle Every 6–8 weeks Total 319 cycles |
PSMA-avid mCRPC, 100 patients Prior Tx ≥3 regimens 57% Mets: bone 96%, LN 87%, viscera 35% |
Grade 1/2 Xerostomia 24% Fatigue 20% Loss of appetite 10% Diarrhea 7% Grade 3/4 Anemia 9% Thrombocytopenia 4% Neutropenia 6% |
PSA decline ≥30%, 47% ≥50%, 38% ≥90%, 11% |
Median PFS 4.1 months Median OS 12.9 months Prognostic factors Good: PSA decline≥50% Bad: visceral mets, rising LDH |
Abbreviations: PSMA, prostate-specific membrane antigen; RLT, radioligand therapy; TEAE, treatment-emergent adverse events; mCRPC, metastatic castration-resistant prostate cancer; Tx, therapy; AA, abiraterone acetate; ENZ, enzalutamide; CTx, chemotherapy; mets, metastases; LN, lymph node; ALP, alkaline phosphatase; NA, not available; PFS, progression-free survival; OS, overall survival.
Pitfalls of PSMA–PET/CT
PSMA–PET/CT is promising for the management of prostate carcinoma; however, it also has pitfalls for clinical applications. False positive findings are noted in up to 10% of patients in which etiology is non-specific or unclear (35). False negative findings are known as tumors less than spatial resolution of PET/CT (5 mm). Neuroendocrine prostate cancer can’t be identified on PSMA–PET/CT (36). Furthermore, most PSMA–PET tracers show high specificity for lesion detection, while their sensitivity depends on serum PSA level. Benign pathologies identified on PSMA–PET/CT comprised of granulomatous diseases, benign bone disorders, benign tumors, and soft-tissue tumors (36) (Table 6). PSMA is type II glycoprotein originally identified in prostatic epithelium and overexpressed in the surface of prostate carcinoma cells. This glycoprotein is encoded by the folate hydrolase 1 (FOLH1) gene. However, this antigen also exists in other human tissues and carcinoma cells (37). Regardless of being ‘prostate-specific’, there are many malignant diseases which demonstrate positive PSMA–PET/CT (Table 4). Tumors exhibiting PSMA overexpression are associated with microenvironment of neovascularization. Since PSMA functions as folate hydrolase 1, these tumors are activated in folate metabolism. Actually, these tumors are also possible indications of another PSMA theranostics because insufficient information exists to recommend these situations.
Table 6.
False-positive conditions of PSMA–PET in the diagnosis of prostate cancer
| Physiological uptake | |
| Head and neck | Lacrimal gland, parotid gland, submandibular gland |
| Abdomen | Liver, spleen, kidney, small bowel, ureter |
| Pelvis | Urinary bladder, myometrium |
| Other | Sympathetic ganglia (cervical, celiac, sacral ganglia) |
| Benign condition or disease | |
| Granuloma | Granulation tissue, keroid, sarcoidosis, active tuberculosis, anthracosis |
| Inflammation | Nodular fasciitis, bronchiectasis |
| Bone disorder | Paget’s disease, fibrous dysplasia, healing bone fracture |
| Benign tumor | Meningioma, neurogenic tumor (schwannoma), hemangioma of liver, hemangioendothelioma of liver, adrenal adenoma, pancreatic serous cystadenoma, pancreatic neuroendocrine tumor |
| Soft tissue tumor | desmoid tumor, intramuscular myxoma |
| Other | Amyloidosis |
| Malignant tumor | |
| Brain | Glioblastoma multiforme |
| Head and neck | Salivary gland ductal carcinoma, squamous cell carcinoma of oropharynx, thyroid carcinoma |
| Chest | Pulmonary adenocarcinoma, breast carcinoma |
| Abdomen | Hepatocellular carcinoma, renal cell carcinoma, gastrointestinal stromal tumor, urothelial carcinoma, colorectal carcinoma |
| Hematologic malignancy | Malignant lymphoma, multiple myeloma |
Conclusions
PSMA-ligand PET can contribute diagnosis of prostate cancer in several clinical applications including initial staging, secondary staging, detection of biochemical recurrence, treatment planning, and response evaluation. To this end, further research is required to determine diagnostic utility with PSMA-ligand PET for prostate cancer with consideration given to the imaging study procedure, imaging protocol, image interpretation criteria, radiation dose, and cost-effectiveness against the yield of information. Regardless of worldwide use of promising PSMA-ligand PET, it has not been approved in Japan. Expedited introduction of PSMA-ligand PET to Japan and implementation of clinical study are eager for many patients with prostate cancer.
Conflict of interest statement
None declared.
Funding
This study was grated for the budget for promotion of strategic international standardization given by the Ministry of Economy, Industry, and Trade (METI).
References
- 1. Grauer LS, Lawler KD, Marignac JL, et al.. Identification, purification, and subcellular localization of prostate-specific membrane antigen PSMA protein in the LNCaP prostatic carcinoma cell line. Cancer Res 1998;58:4787–9. [PubMed] [Google Scholar]
- 2. Tsukamoto T, Wozniak KM, Slusher BS. Progress in the discovery and development of glutamate carboxypeptidase II inhibitors. Drug Discov Today 2007;12:767–76. [DOI] [PubMed] [Google Scholar]
- 3. Mease RC, Dusich CL, Foss CA, et al.. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: A new imaging probe for prostate cancer. Clin Cancer Res 2008;14:3036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y, Pullambhatla M, Foss CA, et al.. 2-(3-1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA based PET imaging agent for prostate cancer. Clin Cancer Res 2011;17:7645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Lisok A, Chatterjee S, et al.. [(18)F]fluoroethyl triazole substituted PSMA inhibitor exhibiting rapid normal organ clearance. Bioconjug Chem 2016;27:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benešová M, Schäfer M, Bauder-Wüst U, et al.. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med 2015;56:914–20. [DOI] [PubMed] [Google Scholar]
- 7. Kelly J, Amor-Coarasa A, Nikolopoulou A, et al.. Synthesis and pre-clinical evaluation of a new class of high-affinity 18F labeled PSMA ligands for detection of prostate cancer by PET imaging. Eur J Nucl Med Mol Imaging 2017;44:647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behr SC, Aggarwal R, VanBrocklin HF, et al.. Phase I study of CTT1057, an 18F-Labeled imaging agent with Phosphoramidate Core targeting prostate-specific membrane antigen in prostate cancer. J Nucl Med 2019;60:910–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oh SW, Wurzer A, Yusufi N, et al.. Preclinical dosimetry and human biodistribution of 18F-rhPSMA-7 and 18F-rhPSMA-7.3. J Nucl Med 2019;60:1635.31076502 [Google Scholar]
- 10. Saga T, Nakamoto Y, Ishimori T, et al.. Initial evaluation of PET/CT with 18F-FSU-880 targeting prostate-specific membrane antigen in prostate cancer patients. Cancer Sci 2019;110:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eder M, Schafer M, Bauder-Wust U, et al.. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012;23:688–97. [DOI] [PubMed] [Google Scholar]
- 12. Schäfer M, Bauder-Wüst U, Leotta K, et al.. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res 2012;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weineisen M, Simecek J, Schottelius M, et al.. Synthesis and preclinical evaluation of DOTAGA conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res 2014;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benešová M, Bauder-Wüst U, Schäfer M, et al.. Linker modification strategies to control the prostate-specific membrane antigen (PSMA)—Targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem 2016;59:1761–75. [DOI] [PubMed] [Google Scholar]
- 15. Gourni E, Canovas C, Goncalves V, et al.. (R)-NODAGA-PSMA: A versatile precursor for radiometal labeling and nuclear imaging of PSMA-positive tumors. PLoS One 2015;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Treglia G, Annunziata S, Pizzuto DA, et al.. Detection rate of 18F-Labeled PSMA PET/CT in biochemical recurrent prostate cancer: A systematic review and a meta-analysis. Cancers 2019;11:E710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dietlein F, Kobe C, Neubauer S, et al.. PSA-stratified performance of (18)F- and (68)Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med 2017;58:947–52. [DOI] [PubMed] [Google Scholar]
- 18. Mena E, Lindenberg ML, Shih JH, et al.. Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging 2018;45:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giesel FL, Will L, Kesch C, et al.. Biochemical recurrence of prostate cancer: Initial results with [(18)F]PSMA-1007 PET/CT. J Nucl Med 2018;59:632–5. [DOI] [PubMed] [Google Scholar]
- 20. Rahbar K, Afshar-Oromieh A, Seifert R, et al.. Diagnostic performance of (18)F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2018;45:2055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rousseau E, Wilson D, Lacroix-Poisson F, et al.. A prospective study on (18)F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J Nucl Med 2019;pii:jnumed.119.226381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giesel FL, Knorr K, Spohn F, et al.. Detection efficacy of (18)F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med 2019;60:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perera M, Papa N, Roberts M, et al.. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: A systematic review and meta-analysis. Eur Urol 2019;pii:S0302-2838(19)30095-8. [DOI] [PubMed] [Google Scholar]
- 24. Calais J, Czernin J, Fendler WP, et al.. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 2019;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Derlin T, Schmuck S, Juhl C, et al.. PSA-stratified detection rates for [68Ga]THP-PSMA, a novel probe for rapid kit-based 68Ga-labeling and PET imaging, in patients with biochemical recurrence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging 2018;45:913–22. [DOI] [PubMed] [Google Scholar]
- 26. Zacho HD, Gade M, Mortensen JC, et al.. Bone scan index is an independent predictor of time to castration-resistant prostate cancer in newly diagnosed prostate cancer: A prospective study. Urology 2017;108:135–41. [DOI] [PubMed] [Google Scholar]
- 27. Anand A, Morris MJ, Kaboteh R, et al.. A Preanalytic validation study of automated bone scan index: Effect on accuracy and reproducibility due to the procedural Variabilities in bone scan image acquisition. J Nucl Med 2016;57:1865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Afshar-Oromieh A, Haberkorn U, Schlemmer HP, et al.. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: Initial experience. Eur J Nucl Med Mol Imaging 2014;41:887–97. [DOI] [PubMed] [Google Scholar]
- 29. Freitag MT, Kesch C, Cardinale J, et al.. Simultaneous whole-body 18F-PSMA-1007-PET/MRI with integrated high-resolution multiparametric imaging of the prostatic fossa for comprehensive oncological staging of patients with prostate cancer: A pilot study. Eur J Nucl Med Mol Imaging 2018;45:340–7. [DOI] [PubMed] [Google Scholar]
- 30. Baum RP, Kulkarni HR, Schuchardt C, et al.. 177Lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med 2016;57:006–1013. [DOI] [PubMed] [Google Scholar]
- 31. Heck MM, Retz M, D’Alessandria C, et al.. Systemic radioligand therapy with (177)Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol 2016;196:382–91. [DOI] [PubMed] [Google Scholar]
- 32. Kratochwil C, Bruchertseifer F, Rathke H, et al.. Targeted a-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry estimate and empiric dose finding. J Nucl Med 2017;58:1624–31. [DOI] [PubMed] [Google Scholar]
- 33. Sathekge M, Knoesen O, Meckel M, et al.. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:1099–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiess AP, Minn I, Vaidyanathan G, et al.. (2S)-2-(3-(1-Carboxy-5-(4-211At-astatobenzamido)pentyl)Ureido)-pentanedioic acid for PSMA targeted alpha-particle radiopharmaceutical therapy. J Nucl Med 2016;57:1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maurer T, Gschwend JE, Rauscher I, et al.. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016;195:1436–43. [DOI] [PubMed] [Google Scholar]
- 36. Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al.. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging 2017;44:2117–36. [DOI] [PubMed] [Google Scholar]
- 37. Krohn T, Verburg FA, Pufe T, et al.. [(68)Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur J Nucl Med Mol Imaging 2015;42:210–4. [DOI] [PubMed] [Google Scholar]


