Abstract
Background
Myopic eyes are longer than nonmyopic eyes and have thinner choroids. The purpose of present study was to investigate whether a thinner subfoveal choroid at 11 years of age predicted axial eye elongation and myopia during adolescence.
Methods
Longitudinal, population-based observational study. Axial length was measured using an interferometric device and choroidal thickness was measured by spectral-domain optical coherence tomography. Myopia was defined as non-cycloplegic subjective spherical equivalent refraction ≤ − 0.50 diopters.
Results
Right eyes of 714 children (317 boys) were examined at age (median (IQR)) 11.5 (0.6) years and 16.6 (0.3) years during which axial length (median (IQR)) increased by 243 (202) μm in eyes without myopia (n = 630) at baseline compared with 454 (549) μm in eyes with myopia (n = 84) at baseline, p < 0.0001. A thicker baseline subfoveal choroid was associated with increased five-year axial elongation after adjustment for baseline axial length in nonmyopic eyes (β = 27 μm/100 μm, 95%CI 6 to 48, p = 0.011) but not in myopic eyes (p = 0.34). Subfoveal choroidal thickness at 11 years of age did not predict incident myopia at 16 years of age (p = 0.11). Longer baseline axial length was associated with greater five-year axial elongation in both myopic (β = 196 μm/mm, 95%CI 127 to 265, p < 0.0001) and nonmyopic eyes (β = 28 μm/mm, 95%CI 7 to 49, p = 0.0085) and the odds for incident myopia increased with 1.57 (95%CI 1.18 to 2.09, p = 0.0020) per mm longer axial length at baseline.
Conclusion
A thin subfoveal choroid at age 11 years did not predict axial eye elongation and incident myopia from age 11 to 16 years. A longer eye at age 11 years was associated with greater subsequent axial eye elongation and with increased risk of incident myopia at age 16 years.
Keywords: Cohort study, Children, Choroidal thickness, Axial length, Incident myopia, CCC2000
Background
Myopia is predominantly caused by abnormal axial elongation of the eye [1]. In emmetropic eyes growth is believed to be guided by visual inputs to the retina with hyperopic inputs stimulating axial elongation and myopic inputs inhibiting it. This association has been found in a number of species such as chicks [2, 3], rhesus monkeys [4], marmosets [5–7] and humans [8]. Experimental settings in both animals [9–12] and humans [8] show a modulation of choroidal thickness in response to visual inputs which also leads to choroidal secretion of scleral growth regulators [13]. Consequently, the choroid is believed to play an active part in eye growth regulation [14, 15]. Eyes that develop myopia both experience a thinning of the choroid and an elongation of the eyeball [16, 17] and a study following 101 Australian children over an 18 month period found an association between choroidal thinning and increasing axial length growth [18]. Whether choroidal thinning precedes or follows axial elongation is, however, unknown. The main aim of present study was therefore to investigate whether a thinner choroid at 11 years of age predicted subsequent five-year axial eye elongation and incident myopia.
Methods
Study population
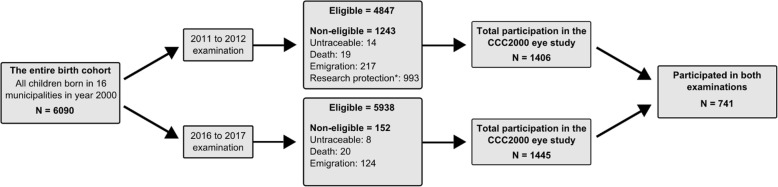
The Copenhagen Child Cohort 2000 eye study is a prospective, population-based, observational study of children born in the year 2000 in 16 municipalities of Copenhagen County, Denmark. The Copenhagen Child Cohort 2000 (CCC2000) includes 6090 children and was initiated with the main objective to study mental health and development from birth to adulthood [19]. The cohort is representative of the Danish population regarding basic child-related parameters [20]. The first eye examination was performed in year 2011 to 2012 and included 1406 participants [21]. Between August 20, 2016 and August 31, 2017, all eligible children of the cohort were invited to a new examination of which 1445 volunteered to participate. A total of 741 subjects participated in the eye examinations in both 2011–2012 and 2016–2017 (Fig. 1). A higher proportion of the girls (412/733, 56%) than the boys (329/673, 49%) from the baseline examination in 2011–2012 participated in the follow-up examination in 2016–2017. There were no differences in body height, axial length, choroidal thickness, spherical equivalent refraction or birth-related parameters (p > 0.30) between the children who attended both examinations and the children who only participated in the 11 to 12-year examination. Medical history was obtained from the participants or their parents/legal guardians.
Fig. 1.
Ascertainment of participants. Research protection (*) refers to a procedure in force until April 1, 2014 whereby citizens could opt for a general exemption from being invited to participate in research projects through the national civil registry
We excluded a total of 27 subjects. Among them, eight were due to a best corrected visual acuity < 80 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at age 16, 15 due to missing subjective refraction at age 11–12 years, two due to missing axial length measurements at age 11–12 years, and two due to missing or poor quality optical coherence tomography (OCT) scans. A total of 714 participants and their right eyes were included in the analyses.
Procedures
Eye examinations included best corrected visual acuity (BCVA), measured using ETDRS charts (4-m original series; precision-Vision, La Salle, IL, USA) as the maximal number of letters the children could read with optimal correction. Optimal correction was determined by non-cycloplegic subjective refraction guided by an autorefractor. The Retinomax K-plus (Right MFG Co., Ltd., Tokyo, Japan) was used in 2011 and the Nidek AR-660A (NIDEK CO., LTD., Gamagori, Aichi 443–0038, Japan) in 2016. The refraction suggested by the autorefractor was used in the following subjective measurement of refraction. When the autorefractor measured a negative spherical refraction, we used a 0.5 D less negative correction than suggested. Wearing this starting correction, the children were asked to read as many letters on an ETDRS chart as possible. Hereafter, positive lens power (+ 0.5 D) was added continuously until a significant blur occurred followed by the adding of − 0.25 D lenses until there was no longer a gain in letters read. We calculated the spherical equivalent refraction by adding half of the negative cylinder refraction to the spherical refraction. Eyes with a spherical equivalent refraction ≤ − 0.50 D were defined as myopic.
Axial length measurements were performed using a partial coherence interferometric device (IOL-Master, version 3.01.0294; Carl Zeiss Meditec, La Jolla, CA, USA) based on an average of at least 5 scans. The device measured axial length as the distance between cornea and retinal pigmented epithelium and thereafter automatically adjusted for the distance between pigment epithelium and inner limiting membrane. The displayed axial length values were thus the adjusted values corresponding to the distance between cornea and inner limiting membrane.
Five-year change in axial length was defined as the difference between axial length at age 11 and 16 years, divided by the follow-up time in years and then multiplied by five.
The macular choroid was imaged using enhanced depth imaging spectral domain optical coherence tomography (EDI-SD-OCT; Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany) with two scan protocols: a horizontal scan including seven scan lines within a 5° × 30° rectangle and a 4-line 30° radial scan. Each scan line consisted of an average of 25 B-scans. The horizontal scan line with the deepest foveal depression and the presence of a pronounced foveal center specular reflex was used in the evaluation of subfoveal choroidal thickness. Choroidal thickness was measured using the manufacturer’s software (Heidelberg Eye Explorer, version 1.6.1.0; Heidelberg Engineering) by manually moving the segmentation line depicting the inner limiting membrane to the choroidoscleral border. All measurements of choroidal thickness were performed by the same operator (XQL). Intragrader variability has been assessed before, showing an intraclass correlation coefficient of 0.996 [22]. We used the subfoveal choroidal thickness in all analyses which would be referred to as just choroidal thickness.
Participants’ height was measured to the nearest 0.1 cm using a wall-mounted altimeter (Height Measuring Rod 5002.02; Soehnle Professional GmbH & Co., Backnang, Germany) and body weight using an electronic scale (Exact/personal scale 6295; OBH Nordica Denmark A/S, Taastrup, Denmark) to the nearest 0.1 kg.
Statistics
The SAS® Enterprise Guide statistical software package (version 7.2; SAS Institute, Cary, NC, USA) was used for all statistical analyses. Normally distributed data were reported as mean ± standard deviation (SD) and non-normally distributed data as median ± interquartile range (IQR). The effects of baseline choroidal thickness, axial length, body height, age and sex on the five-year change in axial length were assessed in crude analyses using Student’s t-tests and linear regression. We performed a multivariate regression analysis including both baseline axial length and choroidal thickness and a multivariate analysis in a general linear model that included, baseline choroidal thickness, axial length, body height, age and sex. The odds ratios (OR) for incident myopia were calculated using logistic regression models and adjusted as aforementioned. We used the Pearson correlation coefficient to evaluate the correlation between subfoveal choroidal thickness and axial length. Body mass index was calculated as body weight in kilograms divided by the square of the body height in meters. We tested all variables for interaction with sex and baseline myopia.
Results
We analyzed the right eyes of 714 (317 boys, 397 girls) participants with a median (IQR) age at follow-up of 16.6 (0.3) years and a median (IQR) follow-up time of 5.1 (0.5) years (Table 1). The median (IQR) 5-year increase in axial length from age 11 to 16 years was 248 (225) μm (Table 1).
Table 1.
Characteristics of the 714 study participants and their right eyes at the baseline and follow-up examination
| Parameter | Baseline, year 2011–12 | Follow-up, year 2016–17 |
|---|---|---|
| Age, median (IQR), years | 11.5 (0.6) | 16.6 (0.3) |
| Follow-up time, median (IQR), years | – | 5.1 (0.5) |
| Best corrected visual acuity, mean (SD), ETDRS letters | 89 (3) | 91 (4) |
| Spherical equivalent refractive error, median (IQR) | 0.0 (0.6) | −0.125 (0.5) |
| Myopia ≤ − 0.50 D, No. (%) | 84 (12) | 174 (24) |
| Incident myopia, No. of cases/No. at risk (%) | – | 120/630 (19) |
| Axial length, mean (SD), mm | 23.2 (0.8) | 23.5 (0.9) |
| Five-year change in axial length, median (IQR), μm | – | 248 (225) |
| Subfoveal choroidal thickness, mean (SD), μm | 361 (77) | – |
| Body height, mean (SD), cm | 152 (7) | 173 (9) |
| Body weight, median (IQR), kg | 40.9 (11) | 63.4 (14) |
| Body mass index, median (IQR), kg/m2 | 17.6 (3) | 20.9 (4) |
Abbreviations: IQR interquartile range, SD Standard deviation, ETDRS Early treatment diabetic retinopathy study
Eyes with myopia at baseline (n = 84) increased more in axial length during the 5-year follow-up than eyes without myopia at baseline (454 ± 549 μm vs 243 ± 202 μm, p < 0.0001). The effects of both baseline choroidal thickness and baseline axial length on the 5-year change in axial length differed between participants with and without baseline myopia (p-values for interaction < 0.0001) and therefore the association-analyses are presented stratified on the presence of myopia at baseline.
The median (IQR) spherical equivalent refraction was − 0.75 (0.50) D in myopic eyes and 0.00 (0.63) D in nonmyopic eyes (p < 0.0001; Kruskal-Wallis test). We found no differences in baseline age (11.4 years vs 11.5 years, p = 0.067; Kruskal-Wallis Test) or sex distribution (49% vs 44% boys, p = 0.39; chi-squared test) between the myopic and nonmyopic participants.
The Pearson correlation coefficient between subfoveal choroidal thickness and axial length at age 11 was − 0.25 in nonmyopic eyes and − 0.53 in myopic eyes.
We tested all associations for interaction with sex both before and after stratifying on the presence of myopia. There were no significant interactions and hence the effect of baseline parameters on the 5-year change in axial length did not differ between sexes (all p-values for interaction > 0.53).
Five-year change in axial length in eyes without myopia at baseline
In the 630 children without myopia at baseline, the 5-year increase in axial length was 34 μm (95%CI 3 to 65, p = 0.032; Table 2) higher among girls compared with boys and the difference remained significant after adjusting for baseline axial length, choroidal thickness, body height and age (p = 0.0001, Table 2).
Table 2.
Effect of baseline parameters on the five-year axial length change (μm) from age 11 to 16 years in univariate and multivariate linear regression analyses. Stratified on the presence of baseline myopia
| Baseline parameter | Five-year change in axial length (95% CI), crude | P value | Five-year change in axial length (95% CI), multivariate* | P value |
|---|---|---|---|---|
| Eyes without myopia at baseline (n = 630) | ||||
| Subfoveal choroidal thickness, 100 μm | 19 (−2 to 39) μm/100 μm | 0.071 | 32 (12 to 53) μm/100 μm | 0.0023 |
| Axial length, mm | 28 (7 to 49) μm/mm | 0.0085 | 58 (35 to 81) μm/mm | < 0.0001 |
| Body height, cm | −3 (−5 to −1) μm/cm | 0.013 | −3 (− 5 to − 1) μm/cm | 0.0041 |
| Sex (girls vs boys), μm | 34 (3 to 65) μm | 0.032 | 64 (31 to 96) μm | 0.0001 |
| Eyes with myopia at baseline (n = 84) | ||||
| Subfoveal choroidal thickness, 100 μm | −167 (−261 to − 74) μm/100 μm | 0.0006 | −30 (− 129 to 69) μm/100 μm | 0.55 |
| Axial length, mm | 196 (127 to 265) μm/mm | < 0.0001 | 216 (129 to 302) μm/mm | < 0.0001 |
| Body height, cm | −5 (−15 to 5) μm/cm | 0.35 | −10 (− 19 to −0.3) μm/cm | 0.044 |
| Sex (girls vs boys), μm | −32 (− 198 to 134) μm | 0.70 | 107 (− 37 to 251) μm | 0.14 |
* Multivariate analysis included: subfoveal choroidal thickness, axial length, body height, sex and age at baseline
Baseline choroidal thickness was not significantly associated with the 5-year change in axial length in the crude analysis (p = 0.07, Table 2) but when adjusted for baseline axial length, a thicker choroid associated with greater axial elongation (β = 27 μm/100 μm, 95%CI 6 to 48, p = 0.011; data not tabulated) which remained significant when further adjusting for baseline body height, baseline age and sex, (p = 0.0023, Table 2).
Five-year increase in axial length was higher the longer the baseline axial length, both in the crude analysis (β = 28 μm/mm, 95%CI 7 to 49, p = 0.0085) and when adjusted for baseline choroidal thickness, baseline body height, age at baseline and sex (β = 58 μm/mm, 95%CI 35 to 81, p < 0.0001; Table 2).
Axial length increased less during the 5-year study period in children that were taller at baseline (β = − 3 μm/cm, 95%CI − 5 to − 1, p = 0.013). This remained significant when adjusting for baseline choroidal thickness, baseline axial length, sex and age at baseline (p = 0.0041, Table 2).
Five-year change in axial length in eyes with myopia at baseline
In the 84 children with myopia at baseline, there was no difference in 5-year change in axial length between girls and boys (p = 0.70, Table 2). A thicker choroid at baseline was associated with reduced axial eye elongation in the crude analysis (β = − 167 μm/100 μm, 95%CI -261 to − 74, p = 0.0006; Table 2) but not when adjusted for baseline axial length (p = 0.34, data not tabulated) or when further adjusting for baseline body height, baseline age and sex (p = 0.55, Table 2).
A longer axial length at baseline was associated with greater subsequent 5-year axial elongation (β = 196 μm/mm, 95%CI 127 to 265, p < 0.0001; Table 2), an effect that increased when adjusted for baseline choroidal thickness, baseline body height, sex and age at baseline (β = 216 μm/mm, 95%CI 129 to 302, p < 0.0001; Table 2).
Being taller at baseline had no effect on the 5-year change in axial length in the crude analysis (p = 0.35) but was associated with less axial elongation when adjusting the analysis for baseline choroidal thickness, baseline axial length, sex and age at baseline (β = − 10 μm/cm, 95%CI − 19 to − 0.3, p = 0.044; Table 2).
Incident myopia
One hundred and twenty (19%) of the 630 children without myopia at baseline developed myopia in their right eyes between age 11 and 16 years. The odds were higher for the girls compared with the boys (OR = 1.72, 95%CI 1.14 to 2.62, p = 0.011; Table 3) and the difference remained significant when adjusting for age at baseline and baseline choroidal thickness, axial length and body height (p < 0.0001, Table 3).
Table 3.
Odds ratio for incident myopia at age 16 years in 630 children without myopia at baseline
| 11-year parameter | Odds Ratio (95% CI) Crude analysis | P value | Odds Ratio (95% CI) Multivariate analysis* | P value |
|---|---|---|---|---|
| Subfoveal choroidal thickness, 100 μm | 0.80 (0.61 to 1.1) | 0.11 | 0.87 (0.65 to 1.17) | 0.37 |
| Axial length, mm | 1.57 (1.18 to 2.09) | 0.0020 | 2.10 (1.49 to 2.94) | < 0.0001 |
| Body height, cm | 1.00 (0.97 to 1.02) | 0.73 | 0.99 (0.96 to 1.02) | 0.37 |
| Sex (girls vs boys) | 1.72 (1.14 to 2.62) | 0.011 | 2.68 (1.67 to 4.29) | < 0.0001 |
* Multivariate analysis included: subfoveal choroidal thickness, axial length, body height, sex and age at baseline
The OR for incident myopia increased by 1.57 (95%CI 1.18 to 2.09, p = 0.0020; Table 3) per mm longer eye at baseline, which remained significant when adjusting for baseline choroidal thickness, baseline body height, sex and age at baseline (p < 0.0001, Table 3).
Baseline choroidal thickness and body height were not associated with incident myopia (Table 3).
Discussion
This prospective cohort study showed that a thin choroid at age 11 years did not predict the subsequent five-year axial length elongation nor did it increase the risk of incident myopia. When accounting for axial length, a thicker choroid at 11 years associated with increased eye elongation in nonmyopic eyes. A longer eye at age 11 and female sex associated with an increased five-year elongation in axial length and an increased risk of incident myopia.
The median axial length growth rate in nonmyopic eyes found in present study is comparable to a study of 41 myopic and 60 nonmyopic children aged 10 to 15 years from Australia who were followed over a period of 18-months [18]. Yet, the growth rate among myopic children was somewhat higher in their study compared with ours. Probably caused by their myopic children having a markedly more myopic mean spherical equivalent refraction [23] than the myopic children in present study. A recent three-year longitudinal study of 2408 six-year-old Dutch children found markedly higher axial length growth rates [24] which may be explained by the fact that the Dutch children were 5 years younger at baseline than the Danish children and axial eye growth speed decreases with increasing age [24–27].
In the present study, axial eye elongation increased with baseline choroidal thickness in nonmyopic eyes, whereas baseline choroidal thickness had no effect on axial length change in myopic eyes. Neither was there any effect of baseline choroidal thickness on the incidence of myopia. These findings suggest that a thin choroid does not alter the eye’s susceptibility to axial elongation and myopia development. This is consistent with studies of chicks with experimentally induced myopia where baseline choroidal thickness was found not to predict neither form-deprivation myopia [28, 29] nor myopia induced by hyperopic defocus [29]. Also, it is in line with a recent one-year longitudinal study of 118 Chinese children aged 7–12 years [30] that found choroidal thinning in concert with a myopic shift in refraction, but with no difference in baseline choroidal thickness between eyes with and without a myopic shift. The observation that baseline-nonmyopic eyes elongated more in axial length with increasing baseline choroidal thickness mainly serve to stress these findings. However, it also suggests a link between choroidal thickness and axial eye growth, which seems to disappear on the route to myopia. As this relationship between a thick baseline choroid and greater axial elongation has not been described before, additional studies are needed to confirm the association.
Longer baseline axial length was a predictor for larger subsequent five-year eye length growth in both myopic and nonmyopic eyes. Additionally, the odds of incident myopia increased with increasing baseline axial length. This suggested that children who developed myopia between age 11 and 16 years already were on a myopic track at the age of 11 years. This is consistent with a study from the US of 605 children aged 6 to 14 years [31] where eyes that eventually became myopic were longer than eyes that remained emmetropic. Consequently, children with long eyes at an early age could be a target for preventive myopia strategies such as increased time spent outdoors [32].
Taller body height at age 11 years was associated with reduced elongation of the eye. As body height was not predictive of incident myopia, this association is likely caused by the gradually decrease of axial eye growth with increasing age, and thus with body development.
Axial length increased more in girls than boys and the girls had a higher risk for incident myopia among the children without myopia at baseline. One could speculate that the general pubertal growth spurt also affects the eye. In general, girls enter puberty earlier than boys and the difference in axial eye elongation and incident myopia between sexes may thus be caused by a slightly later pubertal onset and thereby pubertal growth spurt among the boys.
The strengths of the study include its prospective, population-based cohort design and the large number of participants. Limitations include the absence of cycloplegic refraction data. Subjective refraction with fogging can only to some extend substitute cycloplegia [33–36] and it carries the risk of overestimating the prevalence and magnitude of myopia. Consequently, associations with axial length may be more reliable than associations with myopia. The relatively low number of participants with myopia further limits the power to detect associations in this subgroup of participants. No information regarding participant’s ethnicity were available. However, the cohort was found to be representative of the Danish population [37]. In 2017, the Danish population consisted of 69,260 17-years-olds of which 90% were white Caucasians who originates from Denmark (88%) or other Western Countries (2%). Five percentage originated from the Middle East and only 0.1% originated from China and less than 0.1% from other East Asian countries (Statistics Denmark). Another limitation is the limited follow-up rate (52%) and the fact that we only have data from two time points during the follow-up. Short term effects of choroidal thickness on axial elongation were thereby not observable.
Conclusion
Having a thinner choroid at age 11 years neither increased the subsequent axial eye elongation nor predicted incident myopia.
Children with longer eyes at age 11 experienced greater five-year axial elongation and a markedly higher risk of incident myopia at age 16 years.
Acknowledgements
Not applicable.
Abbreviations
- BCVA
Best corrected visual acuity
- CI
Confidence interval
- EDI-SD-OCT
Enhanced depth imaging spectral domain optical coherence tomography
- ETDRS
Early Treatment Diabetic Retinopathy Study
- IQR
Interquartile range
- OCT
Optical coherence tomography
- OR
Odds ratio
- SD
Standard deviation
Authors’ contributions
MHH examined the participants in year 2016–2017, analyzed and interpreted the data, and have drafted the manuscript. LK contributed in the interpretation of the data and substantively revised the manuscript. XQL contributed in the design of the work, examined the participants in year 2011–2012, and substantively revised the manuscript. AMS made substantial contributions to the conception of the work and substantively revised the manuscript. ML made substantial contributions to the conception and design of the work and substantively revised the manuscript. ICM made substantial contributions to the conception and design of the work, contributed in the analysis and interpretation of the data, and substantively revised the manuscript. All authors read and approved the final manuscript.
Funding
Supported by grants from Bagenkop-Nielsens Øjen-Fond who funded the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Writing of the manuscript was also supported by grants from Øjenforeningen.
Availability of data and materials
The data that support the findings of this study are available from Statistics Denmark but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Statistics Denmark in accordance with Danish regulations and laws.
Ethics approval and consent to participate
Consent from parents or legal guardians was obtained before examinations at the 11 to 12-year examination. At the follow-up examination at 16 to 17-years both the participants and the parents or legal guardians received written information regarding the examinations and the participants gave their signed informed consent. The protocol was assessed by the Regional Committee on Health Research Ethics (The Capital Region of Denmark) in both 2011 (jr.nr. H-3-2011-028) and 2016 (protocol number 16023242) that concluded that medical ethics committee approval was not required. The Danish Data Protection Agency (jr.nr. CSU-FCFS-2016-004) approved the protocol. The study was in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–48. [DOI] [PubMed]
- 2.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vis Res. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 3.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12(4):448–456. doi: 10.1111/j.1475-1313.1992.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 4.Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 5.Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vis Res. 1999;39(2):189–206. doi: 10.1016/S0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- 6.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vis Res. 2001;41(3):267–273. doi: 10.1016/S0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 7.Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optom Vis Sci. 2009;86(1):E31–E39. doi: 10.1097/OPX.0b013e318194072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read SA, Collins MJ, Sander BP. Human optical axial length and defocus. Invest Ophthalmol Vis Sci. 2010;51(12):6262–6269. doi: 10.1167/iovs.10-5457. [DOI] [PubMed] [Google Scholar]
- 9.Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, et al. Moving the retina: choroidal modulation of refractive state. Vis Res. 1995;35(1):37–50. doi: 10.1016/0042-6989(94)E0049-Q. [DOI] [PubMed] [Google Scholar]
- 10.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis Res. 1995;35(9):1175–1194. doi: 10.1016/0042-6989(94)00233-C. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46(7):2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- 12.Hung LF, Wallman J, Smith EL., 3rd Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000;41(6):1259–1269. [PubMed] [Google Scholar]
- 13.Summers JA. The choroid as a sclera growth regulator. Exp Eye Res. 2013;114:120–127. doi: 10.1016/j.exer.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read SA, Fuss JA, Vincent SJ, Collins MJ, Alonso-Caneiro D. Choroidal changes in human myopia: insights from optical coherence tomography imaging. Clin Exp Optom. 2019;102(3):270–285. doi: 10.1111/cxo.12862. [DOI] [PubMed] [Google Scholar]
- 16.Jin P, Zou H, Zhu J, Xu X, Jin J, Chang TC, et al. Choroidal and retinal thickness in children with different refractive status measured by swept-source optical coherence tomography. Am J Ophthalmol. 2016;168:164–176. doi: 10.1016/j.ajo.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Wong HB, Machin D, Tan SB, Wong TY, Saw SM. Ocular component growth curves among Singaporean children with different refractive error status. Invest Ophthalmol Vis Sci. 2010;51(3):1341–1347. doi: 10.1167/iovs.09-3431. [DOI] [PubMed] [Google Scholar]
- 18.Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015;56(5):3103–3112. doi: 10.1167/iovs.15-16446. [DOI] [PubMed] [Google Scholar]
- 19.Skovgaard AM, Olsen EM, Houmann T, Christiansen E, Samberg V, Lichtenberg A, et al. The Copenhagen County child cohort: design of a longitudinal study of child mental health. Scand J Public Health. 2005;33(3):197–202. doi: 10.1080/14034940510005662. [DOI] [PubMed] [Google Scholar]
- 20.Olsen EM, Skovgaard AM, Weile B, Jorgensen T. Risk factors for failure to thrive in infancy depend on the anthropometric definitions used: the Copenhagen County child cohort. Paediatr Perinat Epidemiol. 2007;21(5):418–431. doi: 10.1111/j.1365-3016.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 21.Li XQ, Jeppesen P, Larsen M, Munch IC. Subfoveal choroidal thickness in 1323 children aged 11 to 12 years and association with puberty: the Copenhagen child cohort 2000 eye study. Invest Ophthalmol Vis Sci. 2014;55(1):550–555. doi: 10.1167/iovs.13-13476. [DOI] [PubMed] [Google Scholar]
- 22.Li XQ, Munkholm A. Copenhagen child cohort study G, Larsen M, Munch IC. Choroidal thickness in relation to birth parameters in 11- to 12-year-old children: the Copenhagen child cohort 2000 eye study. Invest Ophthalmol Vis Sci. 2015;56(1):617–624. doi: 10.1167/iovs.14-15016. [DOI] [PubMed] [Google Scholar]
- 23.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in myopic and nonmyopic children assessed with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(12):7578–7586. doi: 10.1167/iovs.13-12772. [DOI] [PubMed] [Google Scholar]
- 24.Tideman JWL, Polling JR, Vingerling JR, Jaddoe VWV, Williams C, Guggenheim JA, et al. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018;96(3):301–309. doi: 10.1111/aos.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JS, The sagittal growth of the eye. IV Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol. 1971;49(6):873–886. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 26.Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol. 1985;103(6):785–789. doi: 10.1001/archopht.1985.01050060045020. [DOI] [PubMed] [Google Scholar]
- 27.Fledelius HC, Christensen AS, Fledelius C. Juvenile eye growth, when completed? An evaluation based on IOL-master axial length data, cross-sectional and longitudinal. Acta Ophthalmol. 2014;92(3):259–264. doi: 10.1111/aos.12107. [DOI] [PubMed] [Google Scholar]
- 28.Guggenheim JA, Chen YP, Yip E, Hayet H, Druel V, Wang L, et al. Pre-treatment choroidal thickness is not predictive of susceptibility to form-deprivation myopia in chickens. Ophthalmic Physiol Opt. 2011;31(5):516–528. doi: 10.1111/j.1475-1313.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 29.Nickla DL, Totonelly K. Choroidal thickness predicts ocular growth in normal chicks but not in eyes with experimentally altered growth. Clin Exp Optom. 2015;98(6):564–570. doi: 10.1111/cxo.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin P, Zou H, Xu X, Chang TC, Zhu J, Deng J, et al. Longitudinal changes in Choroidal and retinal thicknesses in children with myopic shift. Retina. 2019;39(6):1091–1099. doi: 10.1097/IAE.0000000000002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in primary school children. Am J Ophthalmol. 2006;142(1):68–74. doi: 10.1016/j.ajo.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 34.Jan C, Congdon N, Zhou W, Ross B, Wang N, Liang Y. The value of cycloplegia in optometric refraction of adults in a population study. Acta Ophthalmol. 2019;97(3):e484–e4e6. doi: 10.1111/aos.13933. [DOI] [PubMed] [Google Scholar]
- 35.Funarunart P, Tengtrisorn S, Sangsupawanich P, Siangyai P. Accuracy of noncycloplegic refraction in primary school children in southern Thailand. J Med Assoc Thail. 2009;92(6):806–811. [PubMed] [Google Scholar]
- 36.Hashemi H, Khabazkhoob M, Asharlous A, Soroush S, Yekta A, Dadbin N, et al. Cycloplegic autorefraction versus subjective refraction: the Tehran eye study. Br J Ophthalmol. 2016;100(8):1122–1127. doi: 10.1136/bjophthalmol-2015-307871. [DOI] [PubMed] [Google Scholar]
- 37.Olsen EM, Rask CU, Elberling H, Jeppesen P, Clemmensen L, Munkholm A, et al. Cohort profile: the Copenhagen child cohort study (CCC2000). Int J Epidemiol. 2019. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Statistics Denmark but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Statistics Denmark in accordance with Danish regulations and laws.