Abstract

Safe and sustainable development of the nuclear industry has become the focus of attention, so it is important to manage byproducts of radioactive elements, such as uranium, which is inevitably discharged into water bodies. In this work, an adsorbent was fabricated by the in-site assembly of zeolitic imidazolate framework-67 (ZIF-67) on reduced graphene oxide (rGO) hydrogel. The adsorption property of the rGO/ZIF-67 aerogel toward U(VI) was studied via batch adsorption experiment. According to kinetic fitting tests, the adsorption property was in accord well with the pseudo-second-order model, revealing that the adsorption process was chemisorption; the results of the isothermal model conform to the Langmuir model, which exhibited an excellent adsorption capacity of 1888.55 mg/g. The thermodynamic parameter (ΔH° = 11.7 kJ/mol) obtained from the experimental data demonstrated that temperature rise is favorable for the adsorption. Based on the characterization of the material and results of the adsorption, the adsorption mechanism for U(VI) may be explained by surface complexation and electrostatic attraction. In general, all these results and characteristics of the adsorbent show that the rGO/ZIF-67 aerogel provides an alternative way to fabricate novel uranium adsorbent.
1. Introduction
The nuclear industry plays a significant role in the rapid development of a series of high-tech industries, especially metallurgy, chemistry, and medicine.1 Nuclear power alleviates the energy shortage caused by development.2 Uranium, as a key radioactive element, plays a crucial role in nuclear power, which forebodes the necessity for uranium growing along with the booming exploitation of nuclear power.3,4 As the nuclear energy sector advances, a series of problems brought by the development of nuclear power, such as releasing toxic and radioactive substances into the environment, cause people to worry on the ecological safety and human health. The efficient removal of radioactive uranium and other elements from the water body is one of the basic tasks for the safe and sustainable development of nuclear power.
Nowadays, several convention methods are available to eliminate uranium from aqueous solutions, including adsorption,5,6 chemical precipitation,7 electro-dialysis,8 ion exchange,9,10 and solvent extraction.11 Therein, adsorption preferred over the others for uranium removal because of its handy operation, high efficiency, and high capacity.12 To date, numerous adsorbents are applied in uranium recovery, including metal oxides,13 activated carbon,14 biopolymers,15 metal–organic frameworks,2 and magnetic nanocomposite.16 However, these adsorbents still have drawbacks. Inorganic adsorbents have high adsorption capacity but poor selectivity, while organic adsorbents have preferential adsorption for uranium, but the materials are difficult to synthesize and adsorption capacity is restricted by the number of functional groups.2 In general, composite materials, combining the common merits of inorganic and organic molecules, have a synergistic effect to remove uranium from simulated wastewater.
Metal–organic frameworks (MOFs), composed of organic ligands or molecules in addition to the metal ions through the coordination bond self-assembly,17 are regarded as neoteric materials applied in hydrogen storage,18,19 drug release,20 gas separation,21 sensors,22 and catalytic reaction fields.23,24 Zeolitic imidazolate frameworks (ZIFs) draw increasing attention due to their outstanding properties and have been verified as applicable for up-taking of organic dyes and heavy metal ions from simulated wastewater.25,26 MOFs have some attractive characteristics like porosity, large specific surface area, and the number of functional groups for ions, thus holding greater promise for capturing uranium. In recent years, various MOFs-based composites have been synthesized and applied to remove uranium from aqueous solutions. MOFs-derived porous materials, selecting MOFs as a self-sacrifice precursor by thermal calcination, have a broad prospect in the elimination of uranium from the aqueous solution because of the increase in specific surface area and porosity than their precursors.27,28 Magnetic-MOFs nanocomposite, such as ZIF-8- and ZIF-67-, were successfully combined magnetically with different MOFs nanocomposites to apply for uranium adsorption, which simplifies the separation process after adsorption. ZIF-67, with specific metal ions as well as organic ligands, has many advantages in the intent on adsorption of uranium.29,30 Note that rGO/ZIF-67 aerogel, building on ZIF-67 and using 3D rGO aerogel as a suitable carrier, offers substantial functional groups and facilitates recovery.
The rGO aerogel owns the three-dimensional structure of graphene materials and possesses remarkable mechanical properties and thermal and chemical stabilities due to its π–π conjugation structure.31 Besides, rGO aerogel possesses other advantages like homogeneous three-dimensional pore structure and high specific surface area,32 which was confirmed to be a supporter of ZIF-67 nanoparticles. Meanwhile, ZIF-67 nanoparticles were inserted into the void space of rGO aerogel to add active sites, thereby improving the capture performance. To solve the problem of separation of nanomaterials in aqueous solutions, rGO/ZIF-67 aerogel exists as the block in aqueous solutions, which is conducive to the recovery and utilization of adsorbed materials after the experiment.
In this work, first of all, we explore the reaction temperatures of rGO hydrogels to reduce the reaction time and to obtain the uniform pores of the block rGO aerogel. Second, we have combined ZIF-67 nanoparticles with rGO aerogel to synthesize a novel ZIF-based composite material for uranium adsorption from aqueous solutions. This work aims to prepare rGO/ZIF-67 aerogel by an improved method and test the removal performance of rGO/ZIF-67 aerogel for U(VI) and indicates the possible adsorption mechanisms of the rGO/ZIF-67 aerogel to remove uranium(VI) from aqueous in the simulative wastewater.
2. Material and Methods
2.1. Materials
Graphite, potassium permanganate, methanol, 2-methylimidazole, and metal nitrate were obtained from Aladdin Co. Ltd., Beijing, China. H2SO4, H2O2, HNO3, and NaOH were purchased from Beijing Chemical Works, Beijing, China. Ultrapure water was used in solution preparation and experiment. All chemicals were used as received without further purification.
2.2. Synthesis of Graphene Oxide (GO) Nanosheets
The graphene oxide (GO) nanosheets were obtained based on a modified Hummer’s method.33 The graphite powder (2 g) and NaNO3 (1 g) were mixed, and then H2SO4 (60 mL, 98%) was dropwise added to the mixture with stirring in the ice bath for 30 min. To avoid the temperature rising too fast, KMnO4 (6 g) was slowly added to the viscous liquid. The reaction stopped stirring and kept the temperature at 10 °C for 2 h. After 2 h, the temperature maintained the range of 30 to 40 °C for 24 h until the mixture became viscous. Ultra-pure water (200 mL) was added with rapid stirring followed by H2O2 (25 mL, 30%) to remove excess KMnO4. During this process, the bright yellow turbid solution appeared rapidly, and the reaction produced intense effervescence. Finally, the mixture was separated by centrifugation and washed several times with 5% HCl solution until no sulfate ion remained. The product was washed several times with distilled water until the solution contains no chloride ion, and the water was neutral. The product was dried in a vacuum oven at 60 °C.
2.3. Synthesis of rGO Hydrogel
By Adding GO nanosheets (80 mg) to ultra-pure water (40 mL) and then ultrasonic dispersion for 1 h, GO suspension solution was obtained. For preparation of procuring rGO hydrogels, ultra-pure water (2 mL), GO suspension (4 mL), and ascorbic acid solution (1 mL, 41 mg/mL) were put into a 20 mL glass container using ultrasonic dispersion to mix the solution evenly.31 Afterward, the mixture was heated at 80 °C for 90 min. At last, the rGO hydrogel was washed several times with ultra-pure water to remove the remaining ascorbic acid, where after kept in water immersed completely.
2.4. Synthesis of rGO/ZIF-67 Aerogel
Four of the prepared rGO hydrogels were placed in three-necked flask with methanol solution (100 mL) containing Co(NO3)2·6H2O (6.932 g) and heated at 60 °C for 12 h. The liquid was poured from the flask slowly, and then it was added to a methanol solution (100 mL) consisting of 2-methylimidazole (2-MIM) (15.349 g) to the hydrogel and heated at 60 °C for 12 h. Eventually, by washing the prepared rGO/ZIF-67 hydrogel with methanol solution and distilled water, rGO/ZIF-67 aerogel was obtained after freeze-dried for 24 h.
2.5. Batch Adsorption Experiment
Using batch adsorption experiment, different environmental factors, including initial pH of the solution, initial concentration, contact time, temperature, cycle index, and competing ions, were investigated for the adsorption property of the adsorbent toward uranium. Generally, a certain quantity (3.9 mg) of adsorbent was added into a 20 mL uranium(VI) solution followed by shaking for a certain period. The pH of the solution was regulated through a certain concentration of HNO3 and/or NaOH solution. After achieving the adsorption equilibrium, the liquid was separated from the adsorbent by micropore size filter. The concentration of uranium(VI) and competitive ions in the solution was detected by MP-AES (4100, Agilent). The removal rate was calculated according to eq 1:
| 1 |
The adsorption capacities at any contact time and equilibrium time were designated as qt and qe (mg/g) and were calculated according to eqs 2 and 3, respectively:
| 2 |
| 3 |
where C0, Ct, and Ce in mg/L represent the uranium(VI) concentration of original, time t, and equilibrium time, respectively; V (L) represents the volume of the solution in the adsorption experiment; and m (g) is the quantity of rGO/ZIF-7 aerogel.
2.6. Characterization Methods
The morphology and structure of rGO/ZIF-67 aerogel were observed using scanning electron microscopy (SEM; JSM-7500F, JEOL, Japan) and transmission electron microscopy (TEM; JEM-2100, JEOL, Japan), respectively. X-ray diffraction (XRD) tests were carried on an X-ray single-crystal diffractometer (Gemini A Ultra, Agilent, USA) in the 2θ range of 5–80°, step size of 0.0167°, and scan time of 10 s per step. Elemental composition and chemical state were tested by X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, ThermoFisher, USA). Specific surface areas and pore structure were calculated using Brunauer–Emmett–Teller (BET, BELSORP-max, ANKERSMID B.V., Holland). Thermogravimetric analysis (TGA) was conducted on a TG-DTA analyzer (Q600, TA, USA) at 5 °C/min under a N2 environment. The zeta potentials were measured by a Malvern Zetasizer Nano ZS90 (Malvern Instruments Ltd., UK).
3. Results and Discussion
3.1. Characterization
By using rGO hydrogel as a carrier, ZIF-67 particles were self-assembled successfully in the rGO hydrogel via the addition of Co2+ ions and 2-MIM sequentially.34 The features of the as-prepared materials were obtained by SEM and TEM. As demonstrated in Figure 1a, rGO aerogel demonstrates well-proportioned pores and three-dimensional network structure just like spongy substances. The walls are made of layered graphene and have a wrinkle on the layered graphene. As shown in Figure 1b,d, ZIF-67 is evenly distributed over the frame of rGO aerogel; meanwhile, the embedding polyhedron does not destroy the original three-dimensional network structure of rGO aerogel. After embedding ZIF-67, the wall of rGO aerogel becomes rugged because particles are formed both above and below the thin layer. From Figure 1c–f, the complete structure of ZIF-67 is displayed with hexahedron, and the average particle size of about 2 μm.
Figure 1.
SEM images of (a) rGO aerogel and (b–d) rGO/ZIF-67 aerogel. TEM images of (e, f) rGO/ZIF-67 aerogel.
The powder XRD patterns of the samples are indicated in Figure 2a. By comparing rGO aerogel with graphite, the characteristic diffraction peak is a broad peak with a 2θ value of 24.5° because GO nanosheets underwent a reduction reaction, which resulted in the loss of almost all oxygen-containing functional groups. Meanwhile, the peaks of rGO/ZIF-67 aerogel at 7.5°, 10.5°, 12.9°, 14.9°, 16.6°, 18.2°, 26.9°, and 29.9° fit with the characteristic peak of ZIF-67 at the actual XRD pattern, and the peak of rGO/ZIF-67 aerogel at 24.5° fits with the peak of rGO aerogel. As shown in Figure 2b, by comparing the information of functional groups obtained from FT-IR spectra, the successful synthetic rGO/ZIF-67 aerogel was further explained. The C=O of rGO/ZIF-67 aerogel at 1726 cm–1 mainly was disappeared, revealing the decrease of the carboxyl (−COOH) functional group, which is consistent with the result of the XRD pattern. The wavenumbers of 416, 990, and 1578 cm–1 in the rGO/ZIF-67 aerogel corresponds with the characteristic peaks of Co–N, C–N, and C=N, respectively. Eventually, rGO/ZIF-67 aerogel showed wavenumbers of 1419, 1297, 1138, and 750 cm–1, which conformed to the characteristic peak of ZIF-67 in the figure.
Figure 2.
(a) XRD pattern, (b) FT-IR spectra, and (c) TGA curve of rGO/ZIF-67 aerogel. (d) N2 adsorption–desorption isotherms of rGO/ZIF-67 aerogel.
The stability of the rGO/ZIF-67 aerogel was analyzed through thermogravimetric analysis (TGA) to obtain the regularity of material properties changing with temperature. The TGA diagram of rGO/ZIF-67 aerogel is displayed in Figure 2c. The adsorbing material first released physically adsorbed water and residual solvents, such as methanol, resulting in the foremost loss of weight (approximately 10 wt %) in the range of 70–200 °C. Within the range of 200–440 °C, rGO/ZIF-67 aerogel has lost weight (approximately 8 wt %), probably ascribed to decomposition and volatilization of the remaining bound water.34 A further weight loss (approximately 26 wt %) between 440 and 550 °C, it might be attributed to the thermal decomposition of carbon in rGO aerogel and ZIF-67 polyhedrons.2,31 Above 550 °C, the material continues to lose weight due to the slow decomposition of carbon. To study the pore size distribution and specific surface area, BET analysis was examined. As demonstrated in Figure 2d, adsorption isotherm could be classified as type I, which implies that rGO/ZIF-67 aerogel is microporous. Accordingly, the obtained pore structure parameters are as follows: surface area, 962 m2/g; pore volume, 0.452 cm3/g; and pore width, 0.714 nm, which demonstrated rGO/ZIF-67 aerogel with uniform porosity and high specific surface area.
XPS spectra measurements were performed to confirm the valence state of the atoms in the rGO/ZIF-67 aerogel from the resulted binding energies.35Figure 3a highlights the elemental valence state detection result of rGO and rGO/ZIF-67 aerogel, corresponding to C 1s, O 1s, N 1s, and Co 2p. From the high-resolution spectrum of Co 2p (Figure 3b), the characteristic peaks with the binding energies of 781.18 and 798.78 eV represent the Co 2p3/2 and Co 2p1/2, respectively. The XPS spectrum of Co 2p3/2 contains the characteristic peak of Co2+ species as a part of ZIF-67, which further proves the effective combination of rGO and ZIF-67.31,35Figure 3c shows that the C 1s peak belongs to the C–C (284.28 eV), which indicates that the reduction degree of GO nanosheets to rGO could be verified. However, C–O (285.48 eV) and C=O (287.78 eV) reveal that GO nanosheets are not completely reduced, which keeps the agreement with the FT-IR analysis conclusions. Figure 3d displays the binding energy at 532.88 eV of O 1s corresponding Co–O.34
Figure 3.
High-resolution XPS spectra of (a) survey XPS spectra, (b) Co 2p, (c) C 1s, and (d) O 1s fitting of rGO/ZIF-67 aerogel.
3.2. Effect of pH
It is well studied that the pH value of the solution affects the material’s adsorption capacity significantly. During the adsorption experiment, the effect of pH was examined over the range of pH from 1.09 to 5.99 by keeping the other conditions constant. As illustrated in Figure 4a, the removal rate of uranium(VI) showed a sharp increase over the pH range of 2.12–3.50 and then maintains the value of (97–100%) over the pH range of 3.50–5.03, finally followed by a decline at pH between 5 and 6. At pH = 4.01, the removal rate reaches a maximum of 100%. To preferably probe into the adsorption characteristics of U(VI), a subsequent adsorption test was put into practice under the optimum conditions (pH = 4.01). The trend has similarity with the result of ZIF-67 nanopowder adsorption of uranium in which the removal rate increased and reached a maximum value at a relatively low pH value.2Figure 4b demonstrates the surface charge information of rGO/ZIF-67 aerogel from zeta potential measurement. The oxygen-containing species (−OH, −COOH, and Co–OH) ensured that the changing trend of negative zeta potential is more obvious accompanied by an increase in pH. It has been found that uranium(VI) exists in the form of UO22+ at pH < 5.00.36 Electrostatic repulsion exists between UO22+ and positively charged rGO/ZIF-67 aerogel in pH less than 1.50 because rGO/ZIF-67 aerogel owns a positively charged surface, resulting in a very low removal rate of uranium by the adsorbent. When the pH exceeds 1.50, electrostatic attraction occurs between UO22+ and negatively charged rGO/ZIF-67 aerogel, resulting in a significant increase in the removal rate. Nevertheless, when the pH exceeds 5.00, the form of positively charged U(VI) becomes a negatively charged uranium(VI) category (e.g., UO2(OH)3– and (UO2)3(OH)7–) as the pH gradually increases,37 and uranium(VI) may be precipitated in the pH range of 5.00–10.0.14 The above reasons may lead to the decrease of uranium(VI) adsorption on rGO/ZIF-67 aerogel at pH exceeding 5.00. In summary, a range of 3.50–5.00 is the optimal pH for adsorbing uranium with rGO/ZIF-67 aerogel.
Figure 4.
(a) Influence of pH on the removal rate of the U(VI). (b) Zeta potential of rGO/ZIF-67 aerogel. C0(U) = 250 mg/L, V = 20 mL, mrGO/ZIF-67 aerogel = 3.9 mg, t = 48 h, and T = 298 K.
3.3. Adsorption Kinetics
The kinetics parameters play an indispensable role in assessing the material’s performance to further exploration. The contact time of the adsorbent and solution ranged from 15 min to 48 h. As displayed in Figure 5a, an increase of the adsorption capacity occurred in the contact time from 15 min to 7 h because of the availability of abundant reaction sites. As the reaction sites decreased, the adsorption capacity grows slowly after 15 h. Eventually, the adsorption process obtained the equilibrium point at 14 h. The pseudo-first-order model and pseudo-second-order model were applied in the adsorption kinetics study of rGO/ZIF-67 aerogel. The pseudo-first-order model and pseudo-second-order model are expressed by eqs 4 and 5, respectively:
| 4 |
| 5 |
where K1 (1/h) and K2 [g/(mg · h)] represent the equilibrium rate constants of the pseudo-first-order model and pseudo-second-order model, respectively.
Figure 5.
(a) Influence of contact time on U(VI) adsorption capacity. (b) Pseudo-first-order model. (c) Pseudo-second-order model. C0(U) = 300 mg/L, V = 20 mL, mrGO/ZIF-67 aerogel = 3.9 mg, T = 298 K, and pH = 4.01.
From Figure 5 and Table 1, fitting results demonstrate that the pseudo-second-order kinetic model (R2 = 0.986) preferably describes the adsorption kinetics behavior of the rGO/ZIF-67 aerogel and suggests that the adsorption process is chemisorption. From the fitting results of the pseudo-second-order kinetic model, the theoretical equilibrium uranium(VI) adsorption capacity was calculated as 1684.69 mg/g, which is similar to the equilibrium adsorption capacity calculated from the Langmuir model.
Table 1. Pseudo-First-Order Model and Pseudo-Second-Order Model Fitting Parameters for U(VI) Adsorption on rGO/ZIF-67 Aerogel.
| pseudo-first-order |
pseudo-second-order |
|||||
|---|---|---|---|---|---|---|
| kinetic parameters | K1 (h–1) | qe (mg/g) | R2 | K2 (g · mg–1 · h–1) | qe (mg/g) | R2 |
| values | 0.170 | 1379.39 | 0.984 | 1.47 × 10–4 | 1684.69 | 0.986 |
3.4. Adsorption Isotherms
From Figure 6b, within the initial concentration range of 50–440 mg/L, the adsorption capacity goes up linearly with the increase of the initial concentration and then becomes steady. By analyzing the experimental data, results indicate that the adsorption process accomplished through the transfer of uranium(VI) ions driven by the original concentration from the aqueous solution to the adsorbent until the active sites on the adsorbent approach the saturation point. Langmuir and Freundlich models were used to fit the experimental results to explore adsorption equilibrium isotherm. The Langmuir model equation is illustrated by eq 6:
| 6 |
where qm (mg/g) represents the maximum
amounts of U(VI) adsorbed
per unit mass of the rGO/ZIF-67 aerogel, and KL (L/mg) represents a constant correlated with the adsorption
energy. Through linear fitting, the plots of 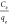 as a function of Ce, a straight line with a slope of 1/qm and intercept 1/(qm · KL) was drawn to obtain the qm and KL.
as a function of Ce, a straight line with a slope of 1/qm and intercept 1/(qm · KL) was drawn to obtain the qm and KL.
Figure 6.
(a) Adsorption isotherms model for rGO/ZIF-67 aerogel. (b) Effect of the initial concentration of U(VI). (c) Langmuir model. (d) Freundlich model. V = 20 mL, mrGO/ZIF-67 aerogel = 3.9 mg, t = 48 h, T = 298 K, and pH = 4.01.
The Freundlich model in a linear form is based on the conception that the adsorbent process on a heterogeneous surface with different adsorption energies.38 This Freundlich model is expressed in eq 7:
| 7 |
where KF represents the Freundlich constant in connection with adsorption capacity (g–1 · L1/n · mg1 – 1/n); 1/n represents the empirical parameter corresponding to surface heterogeneity or adsorption intensity.
The adsorption isotherm of rGO/ZIF-67 aerogel on uranium(VI) is indicated in Figure 6a, and the fitting results of the Langmuir and Freundlich models are shown in Figure 6c,d. As shown in Table 2, the correlation coefficient of the Langmuir model (R2 = 0.999) is greater than that of Freundlich model (R2 = 0.771). The fitting results suggest that the adsorption of uranium(VI) on the rGO/ZIF-67 aerogel is a single-layer adsorption process due to the uniform allocation of available groups on the graphene surface. The fitting results of the Langmuir model show that the maximum adsorption capacity of rGO/ZIF-67 aerogel toward uranium(VI) is 1888.55 mg/g. Compared with rGO hydrogels and ZIF-67, the maximum adsorption capacity of rGO/ZIF-67 aerogel was higher than ZIF-67 (qm = 1683.8 mg/g)2 but much higher than rGO hydrogels (qm = 134.23 mg/g).31 Compared to other types of uranium adsorbents, given in Table 3, rGO/ZIF-67 aerogel reveals that it can be applied in the removal of uranium(VI) with better adsorption capacity from aqueous solutions.
Table 2. Langmuir Model and Freundlich Model Fitting Parameters for U(VI) Adsorption on rGO/ZIF-67 Aerogel.
| Langmuir
model parameters |
Freundlich
model parameters |
|||||
|---|---|---|---|---|---|---|
| isotherms parameters | qm (mg/g) | KL (L/mg) | R2 | KF (g–1 · L1/n · mg1 – 1/n) | n | R2 |
| values | 1888.55 | 0.296 | 0.999 | 517.56 | 2.95 | 0.771 |
Table 3. Comparison of U(VI) Adsorption Capacity on Various Adsorbents.
| adsorbents | experimental conditions | qm (mg/g) | ref |
|---|---|---|---|
| ZIF-67 | pH = 4.00, T = 298 K | 1683.80 | (2) |
| Fe3O4@ZIF-8 | pH = 3.00, T = 298 K | 523.50 | (16) |
| MgO/carbon | pH = 4.00, T = 298 K | 777.51 | (27) |
| P-Al2O3 MSs | pH = 5.00, T = 298 K | 316.87 | (28) |
| rGO hydrogel | pH = 4.00, T = 298 K | 134.23 | (31) |
| Fe@ZIF-8 | pH = 4.50, T = 298 K | 277.77 | (39) |
| AT@PDA/CS aerogel | pH = 5.00, T = 328 K | 175.10 | (41) |
| PA- DFNS | pH = 8.00,T = 298 K | 1106.00 | (42) |
| PPy/ZIF-8 | pH = 3.50,T = 298 K | 534.00 | (43) |
| MF-HTC | pH = 3.00, T = 298 K | 2208.00 | (44) |
| rGO/ZIF-67 aerogel | pH = 4.01, T = 298 K | 1888.55 | this work |
3.5. Adsorption Thermodynamics
To investigate the impact of temperature on the adsorption process, a series of adsorption tests were carried out, including 293, 298, 303, 308, and 313 K. As shown in Figure 7a, the adsorption capacity of the material enhanced as the temperature increases at a constant contact time and pH value. The thermodynamic parameters, including standard enthalpy change (ΔH°), standard entropy change (ΔS°), and standard Gibbs free energy change (ΔG°), could be used to explore the relationship between the change of temperature and the uranium adsorption capacity. The distribution coefficient (Kd) was calculated by eq 8. ΔH° and ΔS° were obtained from the slope and intercept of the van’t Hoff equation as shown in eq 9. ΔG° was acquired by the Gibbs–Helmholtz equation as in eq 10:
| 8 |
| 9 |
| 10 |
where Kd is the distribution coefficient; and T and R represent the Kelvin temperature (K) and the ideal gas constant [8.314 J/(mol·K)], respectively.
Figure 7.
(a) Influence of temperature on the uranium adsorption. (b) Plot of ln Kd versus 1/T. V = 20 mL, mrGO/ZIF-67 aerogel = 3.9 mg, t = 48 h, C0 = 300 mg/L, and pH = 4.01.
The calculated values of ΔH°, ΔS°, and ΔG° were obtained by their respective equations given in Table 4. The standard enthalpy change (ΔH° = 11.7 kJ/mol) is positive and reveals that the adsorption of uranium(VI) by the adsorbent is a spontaneous endothermic process, in line with the experimental results. The standard entropy change (ΔS° = 120 J/mol·K), shown a positive value, indicates the enhanced randomness on the adsorbent–solution interface in the process of adsorbing uranium(VI) by rGO/ZIF-67 aerogel. Furthermore, the value of ΔG° is negative, proposing that the sorption of uranium(VI) on rGO/ZIF-67 aerogel is a spontaneous process under the given experimental circumstances. In the case of elevated temperature, the values of ΔG° became even more negative, meaning that increasing the temperature is advantageous to enhance the adsorption.
Table 4. Thermodynamic Parameters for the U(VI) on rGO/ZIF-67 Aerogel.
| T (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol·K) |
|---|---|---|---|
| 293 | –23.4 | 11.7 | 120 |
| 298 | –35.8 | ||
| 303 | –36.4 | ||
| 308 | –37.0 | ||
| 313 | –37.6 |
3.6. Selectivity
To evaluate the selectivity of the material toward U(VI) in the presence of other competitive ions in a solution, adsorption experiment was performed in a multi-ion solution system, including Zn(II), Ba(II), Ca(II), Al(III), K(I), Mg(II), Fe(III), Sr(II), and Cs(II). The selective adsorption coefficient (KM) was obtained through eq 11:
| 11 |
where KMU and Kd represents the U(VI) selective adsorption coefficient and distribution coefficient, respectively; KdM represents the competing metal ions distribution coefficient.
The calculated values are presented in Figure 8 and Table 5. In the presence of competing ions in the aqueous solution, rGO/ZIF-67 aerogel shows strong adsorption affinity for uranium(VI) but weak capacity to interferential ions so that the adsorption ability of uranium(VI) on rGO/ZIF-67 aerogel had no significant influence. The reason for this phenomenon may be due to the fact that the number of active sites of Co–OH and binding ability with uranium(VI) ions is higher than that of other competing ions. It also could be the diversity in the basic structure, including the difference in particle size and effective charge.31
Figure 8.

Removal rate of U(VI) and competing ions. V = 20 mL, mrGO/ZIF-67 aerogel = 3.9 mg, t = 48 h, T = 298 K, pH = 4.01, and C0 = 250 mg/L.
Table 5. Distribution Coefficient and Selective Adsorption Coefficient of rGO/ZIF-67 Aerogel.
| parameter | U(VI) | Zn(II) | Ba(II) | Ca(II) | Al(III) | K(I) | Mg(II) | Fe(III) | Sr(II) | Cs(II) |
|---|---|---|---|---|---|---|---|---|---|---|
| KdM (mL/g) | 6856 | 507 | 515 | 75 | 615 | 475 | 266 | 260 | 63 | 770 |
| KMU | 1.00 | 13.5 | 13.3 | 91.4 | 11.1 | 14.4 | 25.8 | 26.3 | 109.4 | 8.90 |
3.7. Regenerability
Taking into account the environmental safety and economic feasibility, it is crucial that an adsorbent shows a reasonable number of cycles during the adsorption. To evaluate the reusable number of times of the rGO/ZIF-67 aerogel, reusability experiments were reduplicated five times by utilizing the identical rGO/ZIF-67 aerogel. The HNO3 solution (pH = 0.20) was used as a stripping agent to release the uranium ions adsorbed by the adsorbent, and the next experiment was conducted after adsorbent freeze-drying. As illustrated in Figure 9a, after five cycles, the adsorption amount maintains 73%. The adsorbent maintains the original block structure after the 48 h oscillation as shown in Figure 9b,c. rGO/ZIF-67 aerogel has good reusability and stability for uranium(VI) adsorption, which further demonstrates that rGO/ZIF-67 aerogel has a good adsorption property for uranium(VI) in an aqueous environment.
Figure 9.

(a) Reusability of rGO/ZIF-67 aerogel in the removal of U(VI). (b) Images of rGO/ZIF-67 aerogel before the adsorption. (c) Images of rGO/ZIF-67 aerogel after 48 h oscillation. V = 20 mL, mrGO/ZIF-67 aerogel = 3.9 mg, t = 48 h, T = 298 K, pH = 4.01, and C0 = 250 mg/L.
3.8. Proposed Adsorption Mechanisms
The proposed adsorption mechanistic pathway for U(VI) is shown in Scheme 1. rGO/ZIF-67 aerogel has a large specific surface area and uniform pores inside, which is conducive to effective adsorption of uranium(VI) ions. Three main factors enable rGO/ZIF-67 aerogel to adsorb uranium(VI) efficiently. First, ZIF-67 nanoparticles were the critical element for rGO/ZIF-67 aerogel with the great sorption capacity and affinity toward uranium(VI). The uncoordinated Co in ZIF-67 is desorbed by water to produce a large number of hydroxyl groups (Co–OH), combined with uranium(VI) to form Co–O–U. Second, there was still a small amount of oxidation group (−COOH and −OH) from test results of FT-IR and XRD in rGO/ZIF-67 aerogel, which would be beneficial to increase uranium(VI) adsorption capacity.40 Finally, rGO/ZIF-67 aerogel has an electrostatic adsorption effect on uranium known from the test results of zeta potential, in which electrostatic attraction will increase the adsorption amount of uranium. Based on the above analysis, it is concluded that surface complexation and electrostatic attraction may be the possible adsorption mechanisms for U(VI).27
Scheme 1. Possible Adsorption Mechanism of U(VI) Adsorption on rGO/ZIF-67 Aerogel.
4. Conclusions
In this study, rGO aerogel decorated with ZIF-67 polyhedron is successfully fabricated, and its application for uranium(VI) elimination in an aqueous environment is assessed. The rGO/ZIF-67 aerogel owns a 3D porous network structure, which contributes to better contact with U(VI) in the solution. It is concluded that the adsorption of uranium(VI) using rGO/ZIF-67 aerogel showed the maximum removal rate in the pH range of 3.50–5.03. Adsorption kinetics study shows that uranium(VI) adsorption by rGO/ZIF-67 aerogel, in accord with the pseudo-second-order kinetic model, proposes that the chemisorption dominates the adsorption process. Based on isothermal outcomes, Langmuir is the more appropriate isothermal model for describing the uranium(VI) on rGO/ZIF-67 aerogel and further obtained that the maximum adsorbing capacity of rGO/ZIF-67 aerogel on uranium(VI) attains to 1888.55 mg/g (pH = 4.01). The value of thermodynamic calculation indicates that U(VI) removal is a spontaneous endothermic process. In addition, from the study of coexisting ions, rGO/ZIF-67 aerogel is superior in selectivity and maintains high removal efficiency.
Acknowledgments
This work was supported by the State Administration of Science, Technology and Industry for National Defence, PRC (BG17001403).
The authors declare no competing financial interest.
References
- Tan L.; Wang Y.; Liu Q.; Wang J.; Jing X.; Liu L.; Liu J.; Song D. Enhanced adsorption of uranium(VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. 10.1016/j.cej.2014.08.015. [DOI] [Google Scholar]
- Su S.; Che R.; Liu Q.; Liu J.; Zhang H.; Li R.; Jing X.; Wang J. Zeolitic imidazolate framework-67: a promising candidate for recovery of uranium (VI) from seawater. Colloids Surf., A 2018, 547, 73–80. 10.1016/j.colsurfa.2018.03.042. [DOI] [Google Scholar]
- Ma S.; Huang L.; Ma L.; Shim Y.; Islam S. M.; Wang P.; Zhao L.-D.; Wang S.; Sun G.; Yang X.; Kanatzidis M. G. Efficient uranium capture by polysulfide/layered double hydroxide composites. J. Am. Chem. Soc. 2015, 137, 3670–3677. 10.1021/jacs.5b00762. [DOI] [PubMed] [Google Scholar]
- Yue Y.; Mayes R. T.; Kim J.; Fulvio P. F.; Sun X.-G.; Tsouris C.; Chen J.; Brown S.; Dai S. Seawater uranium sorbents: preparation from a mesoporous copolymer initiator by atom-transfer radical polymerization. Angew. Chem., Int. Ed. 2013, 52, 13458–13462. 10.1002/anie.201307825. [DOI] [PubMed] [Google Scholar]
- Rout A.; Venkatesan K. A.; Srinivasan T. G.; Vasudeva Rao P. R. Liquid-liquid extraction of Pu(IV), U(VI) and Am(III) using malonamide in room temperature ionic liquid as diluent. J. Hazard. Mater. 2012, 221-222, 62–67. 10.1016/j.jhazmat.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Fryxell G. E.; Lin Y.; Fiskum S.; Birnbaum J. C.; Wu H.; Kemner K.; Kelly S. Actinide sequestration using self-assembled monolayers on mesoporous supports. Environ. Sci. Technol. 2005, 39, 1324–1331. 10.1021/es049201j. [DOI] [PubMed] [Google Scholar]
- Mellah A.; Chegrouche S.; Barkat M. The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J. Colloid Interface Sci. 2006, 296, 434–441. 10.1016/j.jcis.2005.09.045. [DOI] [PubMed] [Google Scholar]
- Djogić R.; Cuculić V.; Branica M. Precipitation of uranium(VI) peroxide (UO4) in sodium perchlorate solution. Croat. Chem. Acta 2005, 78, 575–580. [Google Scholar]
- Li Y.; Li L.; Chen T.; Duan T.; Yao W.; Zheng K.; Dai L.; Zhu W. Bioassembly of fungal hypha/graphene oxide aerogel as high performance adsorbents for U(VI) removal. Chem. Eng. J. 2018, 347, 407–414. 10.1016/j.cej.2018.04.140. [DOI] [Google Scholar]
- Saito T.; Brown S.; Chatterjee S.; Kim J.; Tsouris C.; Mayes R. T.; Kuo L.-J.; Gill G.; Oyola Y.; Janke C. J.; Dai S. Uranium recovery from seawater: development of fiber adsorbents prepared via atom-transfer radical polymerization. J. Mater. Chem. A 2014, 2, 14674–14681. 10.1039/C4TA03276D. [DOI] [Google Scholar]
- Bai J.; Yin X.; Zhu Y.; Fan F.; Wu X.; Tian W.; Tan C.; Zhang X.; Wang Y.; Cao S.; Fan F.; Qin Z.; Guo J. Selective uranium sorption from salt lake brines by amidoximated Saccharomyces cerevisiae. Chem. Eng. J. 2016, 283, 889–895. 10.1016/j.cej.2015.08.011. [DOI] [Google Scholar]
- Ren X.; Li J.; Chen C.; Gao Y.; Chen D.; Su M.; Alsaedi A.; Hayat T. Correction: Graphene analogues in aquatic environments and porous media: Dispersion, aggregation, deposition and transformation. Environ. Sci. Nano. 2018, 5, 1518–1518. 10.1039/C8EN90022A. [DOI] [Google Scholar]
- Wu S.; Li F.; Wang H.; Fu L.; Zhang B.; Li G. Effects of poly (vinyl alcohol) (PVA) content on preparation of novel thiol-functionalized mesoporous PVA/SiO2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution. Polymer 2010, 51, 6203–6211. 10.1016/j.polymer.2010.10.015. [DOI] [Google Scholar]
- Han B.; Zhang E.; Cheng G.; Zhang L.; Wang D.; Wang X. Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chem. Eng. J. 2018, 338, 734–744. 10.1016/j.cej.2018.01.089. [DOI] [Google Scholar]
- Su S.; Chen R.; Liu Q.; Liu J.; Zhang H.; Li R.; Zhang M.; Liu P.; Wang J. High efficiency extraction of U(VI) from seawater by incorporation of polyethyleneimine, polyacrylic acid hydrogel and luffa cylindrical fibers. Chem. Eng. J. 2018, 345, 526–535. 10.1016/j.cej.2018.03.164. [DOI] [Google Scholar]
- Min X.; Yang W.; Hui Y.-F.; Gao C.-Y.; Dang S.; Sun Z.-M. Fe3O4@ZIF-8: a magnetic nanocomposite for highly efficient UO22+ adsorption and selective UO22+/Ln3+ separation. Chem. Commun. 2017, 53, 4199–4202. 10.1039/C6CC10274C. [DOI] [PubMed] [Google Scholar]
- Zhou H.-C. J.; Kitagawa S. Metal organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. 10.1039/C4CS90059F. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Huang K. MOF-derived iron as an active energy storage material for intermediate-temperature solid oxide iron-air redox batteries. Chem. Commun. 2017, 53, 10564–10567. 10.1039/C7CC06131E. [DOI] [PubMed] [Google Scholar]
- Linnemann J.; Taudien L.; Klose M.; Giebeler L. Electrodeposited films to MOF-derived electrochemical energy storage electrodes: a concept of simplified additive-free electrode processing for self-standing, ready-to-use materials. J. Mater. Chem. A 2017, 5, 18420–18428. 10.1039/C7TA01874F. [DOI] [Google Scholar]
- Gao Q.; Hu J.; Li R.; Xing Z.; Xu L.; Wang M.; Guo X.; Wu G. Radiation synthesis of a new amidoximated UHMWPE fibrous adsorbent with high adsorption selectivity for uranium over vanadium in simulated seawater. Radiat. Phys. Chem. 2016, 122, 1–8. 10.1016/j.radphyschem.2015.12.023. [DOI] [Google Scholar]
- Duan J.; Higuchi M.; Zheng J.; Noro S.-i.; Chang I.-Y.; Hyeon-Deuk K.; Mathew S.; Kusaka S.; Sivaniah E.; Matsuda R.; Sakaki S.; Kitagawa S. Density gradation of open metal sites in the mesospace of porous coordination polymers. J. Am. Chem. Soc. 2017, 139, 11576–11583. 10.1021/jacs.7b05702. [DOI] [PubMed] [Google Scholar]
- Yang J.; Ye H.; Zhao F.; Zeng B. A novel CuxO Nanoparticles@ZIF-8 composite derived from core-shell metal-organic frameworks for highly selective electrochemical sensing of hydrogen peroxide. ACS Appl. Mater. Interfaces 2016, 8, 20407–20414. 10.1021/acsami.6b06436. [DOI] [PubMed] [Google Scholar]
- Yang J.; Zhang F.; Lu H.; Hong X.; Jiang H.; Wu Y.; Li Y. Hollow Zn/Co ZIF particles derived from core-shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem., Int. Ed. 2015, 54, 10889–10893. 10.1002/anie.201504242. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Tang J.; Qian H.; Wang Z.; Yamauchi Y. One-pot synthesis of zeolitic imidazolate framework 67-derived hollow Co3S4@MoS2 heterostructures as efficient bifunctional catalysts. Chem. Mater. 2017, 29, 5566–5573. 10.1021/acs.chemmater.7b00867. [DOI] [Google Scholar]
- Lin K.-Y. A.; Chang H.-A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. 10.1016/j.chemosphere.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Li X.; Gao X.; Ai L.; Jiang J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. 10.1016/j.cej.2015.03.127. [DOI] [Google Scholar]
- Lv Z.; Wang H.; Chen C.; Yang S.; Chen L.; Alsaedi A.; Hayat T. Enhanced removal of uranium(VI) from aqueous solution by a novel Mg-MOF-74-derived porous MgO/carbon adsorbent. J. Colloid Interface Sci. 2019, 537, A1–A10. 10.1016/j.jcis.2018.11.062. [DOI] [PubMed] [Google Scholar]
- Huang S.; Pang H.; Li L.; Jiang S.; Wen T.; Zhuang L.; Hu B.; Wang X. Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem. Eng. J. 2018, 353, 157–166. 10.1016/j.cej.2018.07.129. [DOI] [Google Scholar]
- Bhadra B. N.; Cho K. H.; Khan N. A.; Hong D.-Y.; Jhung S. H. Liquid-Phase Adsorption of Aromatics over a Metal–Organic Framework and Activated Carbon: Effects of Hydrophobicity/Hydrophilicity of Adsorbents and Solvent Polarity. J. Phys. Chem. C 2015, 119, 26620–26627. 10.1021/acs.jpcc.5b09298. [DOI] [Google Scholar]
- Peralta D.; Chaplais G.; Simon-Masseron A.; Barthelet K.; Pirngruber G. D. Synthesis and adsorption properties of ZIF-76 isomorphs. Microporous Mesoporous Mater. 2012, 153, 1–7. 10.1016/j.micromeso.2011.12.009. [DOI] [Google Scholar]
- He Y.-R.; Li S.-C.; Li X.-L.; Yang Y.; Tang A.-M.; Du L.; Tan Z.-Y.; Zhang D.; Chen H.-B. Graphene (rgo) hydrogel: a promising material for facile removal of uranium from aqueous solution. Chem. Eng. J. 2018, 338, 333–340. 10.1016/j.cej.2018.01.037. [DOI] [Google Scholar]
- Zhang J.-J.; Qi P.; Li J.; Zheng X.-C.; Liu P.; Guan X.-X.; Zheng G.-P. Three-dimensional Fe2O3-TiO2-graphene aerogel nanocomposites with enhanced adsorption and visible light-driven photocatalytic performance in the removal of RhB dyes. J. Ind. Eng. Chem. 2018, 61, 407–415. 10.1016/j.jiec.2017.12.040. [DOI] [Google Scholar]
- Xu Y.; Bai H.; Lu G.; Li C.; Shi G. Flexible graphene films via the filtration of watersoluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. 10.1021/ja800745y. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Lu R.; Ren S.; Chen C.; Chen Z.; Yang X. Three dimensional reduced graphene oxide/ZIF-67 aerogel: effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. 10.1016/j.cej.2018.04.176. [DOI] [Google Scholar]
- Yang Q.; Ren S.; Zhao Q.; Lu R.; Hang C.; Chen Z.; Zheng H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. 10.1016/j.cej.2017.09.099. [DOI] [Google Scholar]
- Lu X.; Zhang D.; Tesfay Reda A.; Liu C.; Yang Z.; Guo S.; Xiao S.; Ouyang Y. Synthesis of amidoxime-grafted activated carbon fibers for efficient recovery of uranium(VI) from aqueous solution. Ind. Eng. Chem. Res. 2017, 56, 11936–11947. 10.1021/acs.iecr.7b02690. [DOI] [Google Scholar]
- Li M.; Liu H.; Chen T.; Dong C.; Sun Y. Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci. Total Environ. 2019, 651, 1020–1028. 10.1016/j.scitotenv.2018.09.259. [DOI] [PubMed] [Google Scholar]
- Yuwei C.; Jianlong W. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem. Eng. J. 2011, 168, 286–292. 10.1016/j.cej.2011.01.006. [DOI] [Google Scholar]
- Zhang X.; Liu Y.; Jiao Y.; Gao Q.; Wang P.; Yang Y. Enhanced selectively removal uranyl ions from aqueous solution by Fe@ZIF-8. Microporous Mesoporous Mater. 2019, 277, 52–59. 10.1016/j.micromeso.2018.10.017. [DOI] [Google Scholar]
- Ai Y.; Liu Y.; Lan W.; Jin J.; Xing J.; Zou Y.; Zhao C.; Wang X. The effect of pH on the U(VI) sorption on graphene oxide (GO): A theoretical study. Chem. Eng. J. 2018, 343, 460–466. 10.1016/j.cej.2018.03.027. [DOI] [Google Scholar]
- Liao Y.; Wang M.; Chen D. Production of three-dimensional porous polydopamine-functionalized attapulgite/chitosan aerogel for uranium(VI) adsorption. J. Radioanal. Nucl. Chem. 2018, 316, 635–647. 10.1007/s10967-018-5816-2. [DOI] [Google Scholar]
- Yang P.; Liu Q.; Liu J.; Chen R.; Li R.; Bai X.; Wang J. Highly efficient immobilization of uranium(VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DFNS). J. Hazard. Mater. 2019, 363, 248–257. 10.1016/j.jhazmat.2018.09.062. [DOI] [PubMed] [Google Scholar]
- Li J.; Wu Z.; Duan Q.; Alsaedi A.; Hayat T.; Chen C. Decoration of ZIF-8 on polypyrrole nanotubes for highly efficient and selective capture of U(VI). J. Clean. Prod. 2018, 204, 896–905. 10.1016/j.jclepro.2018.09.050. [DOI] [Google Scholar]
- Li X.; Li Y.; Wu Q.; Zhang M.; Guo X.; Li X.; Ma L.; Li S. Ultrahigh uranium uptake by magnetic magnesium ferrite loaded hydrothermal carbon nanosheets under acidic condition. Chem. Eng. J. 2019, 365, 70–79. 10.1016/j.cej.2019.02.002. [DOI] [Google Scholar]










