Abstract
Spontaneous and radiation-induced mutants of soybean, despite loss of abundant seed proteins, have been reported to grow and reproduce normally without any apparent physiological abnormalities. Here, we report the development and characterization of a soybean line (BSH-2) that lacks several abundant seed storage proteins. One-dimensional and high-resolution two-dimensional gel electrophoresis revealed the absence of the α′ and α subunits of β-conglycinin and G1, G2, G4, and G5 glycinin in the newly developed mutant line (BSH-2). Like our earlier developed soybean mutant line (BSH-3), the seeds of BSH-2 also accumulated high levels of free amino acids as compared with wild-type DN47 seeds. An examination of the germination rates revealed that both BSH-2 and BSH-3 had significantly lower germination rates compared with the parent line DN47. Two-dimensional gel electrophoresis analysis demonstrated that these mutants had slower rates of mobilization of seed storage proteins. The delayed mobilization of storage proteins in BSH-2 and BSH-3 seeds was also correlated with a delayed induction of proteolytic activity in the mutants when compared to DN47. Similarly, qRT-PCR analysis revealed distinct expression pattern of genes involved in proteolytic pathway in the mutants when compared to DN47. Transmission electron microscopy observation of soybean seeds at two germination stages revealed striking differences in the breakdown of protein storage vacuoles and lipid bodies in the mutants. Our study demonstrates that BSH-2 and BSH-3 are compromised in mobilization of storage reserves and the absence of abundant storage proteins may affect the seed germination efficiency and post-germinative seedling establishment.
Introduction
Soybean is a high-quality plant protein source for feed, food, and industrial applications. In several regions of the world, soybean is the single most dominant protein source in many animal diets due its high nutritive value and relative lower cost to obtain compared with other protein sources. Typically, commercial soybean cultivars contain about 36–38% protein on a dry weight basis. The abundant seed storage proteins of soybean, the salt-soluble globulins, is grouped into 7S (vicilin type) and 11S (legumin type).1 The 7S globulins are coined as β-conglycinin, and the 11S globulins are called glycinins. On the basis of their sequence homology and size, the glycinins are classified into two groups, group-I (A1aB2, A1bB1b, and A2B1a) and group-II (A3B4 and A5A4B3).2,3 Additionally, the group-II has been subdivided into IIa (A5A4B3) and IIb (A3B4).4 In contrast to glycinins, the β-conglycinins are glycoproteins composed of α′, α, and β-subunits.5 The glycinins and β-conglycinins account for about 70% of the total seed protein content of soybean.6,7 Due to their abundance, these proteins are primarily responsible for the nutritive value of soybean. In addition, soybean also accumulate several other moderately abundant proteins such as the Kunitz and Bowman-Birk trypsin inhibitors, lectin, β-amylase, sucrose binding protein, seed maturation proteins, urease, oleosins, and numerous low abundance proteins.8−10
Even though soybean seed is a good source of protein, its amino acid composition is deficient in sulfur-containing amino acids, cysteine and methionine.11,12 This presents a problem since monogastric animals cannot synthesize essential amino acids and are dependent on food they consume for the essential amino acids. On account of deficiency in sulfur-containing amino acids, efforts have been made to improve the overall sulfur amino acid content of soybean seeds.12 One approach to improve the sulfur amino acid content of soybean is by altering the ratio of 7S β-conglycinin and 11S glycinin. The 11S glycinins are relatively rich, while the 7S β-conglycinins are deficient in sulfur amino acids.11 In particular, the mature β-subunit of β-conglycinin is totally devoid of methionine, and it has been proposed that by suppressing or eliminating the accumulation of this subunit one can improve the nutritive value of soybean protein.13
Both spontaneous mutants and radiation-induced mutants that lack different subunits of glycinin and β-conglycinin have been described in the literature.14,15 A soybean breeding line that is deficient in all group-I subunits (A1aB2, A1bB1b, and A2B1a), A5A4B3 (G5) subunit, and A3B4 (G4) subunit has been reported.4 Interestingly, a mutant soybean line that lacks all glycinin and β-conglycinin subunits has been developed by crossbreeding of the corresponding null mutants.16 In spite of loss of abundant seed proteins, these mutants have been reported to grow and reproduce normally without any apparent physiological abnormalities.17,18 Strikingly, the nitrogen content of mutant seeds were very similar to wild-type cultivars due to a drastic increase in the accumulation of free amino acids and large-scale proteome rebalancing.16
The major storage reserves of soybean, protein, and oil are mobilized during germination and seedling growth. These storage reserves provide the necessary energy and metabolic intermediates needed by the growth of seedling. Therefore, it is logical to speculate that lack of storage proteins will have a deleterious effect on soybean seed germination. The availability of seed storage protein mutants offers an attractive system to study the role of seed proteins during germination and seedling growth. Here, we describe the development of two Chinese soybean mutant lines that are deficient in several abundant seed storage proteins. Characterization of these mutant lines demonstrates that mutant lines lacking major storage proteins are affected in mobilization of seed reserves.
Results
BSH-2 Fail to Accumulate Several Abundant Seed Storage Proteins
Previously, we described the development of a soybean mutant line (BSH-3) that fails to accumulate the α′ subunit of β-conglycinin and G1 (A1aB2), G2 (A2B1a), and G4 (A5A4B3) glycinin subunits.19 This mutant line was obtained in a backcross breeding program using HS99B, a soybean line lacking several subunits of both 7S β-conglycinin and the 11S glycinin as male parent, and DN47, a high-oil elite Chinese cultivar, as a female parent. During this breeding process, we also obtained an additional mutant line (BSH-2) that lacked different subunits of β-conglycinin and 11S glycinins. These soybean lines were grown in the field to evaluate their growth and reproductive characteristics. The vegetative growth performance of both the mutant lines BSH-2 and BSH-3 were very similar to that of DN47 (Figure S1). However, BSH-2 and BSH-3 required a longer duration to attain maturity. Additionally, the mutant lines were significantly taller than the DN47, though the seed yield was not significantly different (Figure S1).
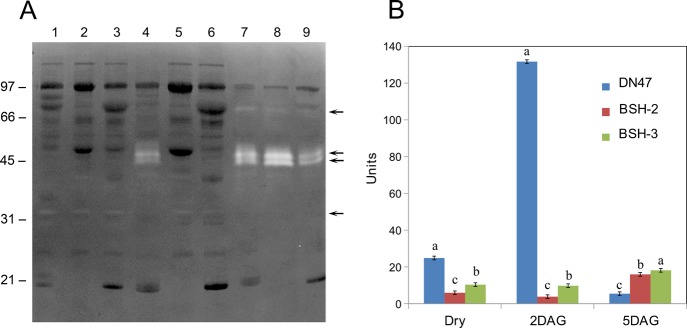
The seed protein profile of DN47, BSH-2, and BSH-3 was first examined by 1D-SDS-PAGE (Figure 1). An examination of the seed protein profile of DN 47 revealed several distinct protein bands that ranged in molecular weight from 100 to 14 kDa. Prominent among them were the lipoxygenase (97 kDa), α′-subunit of β-conglycinin (78 kDa), α-subunit of β-conglycinin (72 kDa), β-subunit of β-conglycinin (52 kDa), acidic subunits (34–36 kDa), and basic subunits of glycinin (21–23 kDa). These abundant proteins represent about 70% of the total seed proteins. In contrast, the mutant line BSH-2 revealed a distinct protein profile (Figure 1). Mutant BSH-2 lacked both the α′-subunit (78 kDa) and α-subunit of β-conglycinin (72 kDa). In addition, it also accumulated drastically lower amounts of the acidic subunits (34–36 kDa) and basic subunits of glycinin (21–23 kDa) when compared to wild-type DN47 (Figure 1). Interestingly, the absence of some of the abundant seed proteins resulted in preferential accumulation few other proteins. For example, BSH-2 accumulated higher amounts of β-subunit of β-conglycinin (52 kDa) when compared to the wild-type seeds (Figure 1). These differences in the protein profiles were further examined by high-resolution 2D SDS-PAGE. This procedure resolved the seed proteins of DN47 into greater than 100 discrete protein spots. These 2D resolved protein spots have been previously identified by mass spectrometry.26,27 The absence of several protein spots in the mutant lines was verified by overlaying the DN47 with BSH-2 2D gel protein profiles using Delta2D software (Figure S2). Based on this analysis, it is evident that BSH-2 failed to accumulate the α′ and α subunits of β -conglycinin, glycinin G1, G2, G4, and G5.
Figure 1.

SDS-PAGE analysis of soybean seed proteins. Total seed proteins extracted from DN47 (lane 1), BSH-2 (lane 2), and BSH-3 (lane 3) were separated by 13.5% SDS-PAGE gel. Resolved proteins were detected by staining the gel with Coomassie Blue. Sizes of molecular weight markers in kDa are shown on the left side of the figure.
Protein, Oil, and Fatty Acid Profile of DN-47 and BSH-2 Seeds
In our earlier study, we reported that the BSH-3, a mutant line that failed to accumulate the α′ subunit of β -conglycinin, glycinin G1, G2, and G4, had a higher protein and lower oil content than DN47.19 To investigate if the newly developed seed storage mutant BSH-2 also showed a similar trend as BSH-3, we determined the protein and oil content of their seeds. Measurement of the total nitrogen/protein content by a LECO nitrogen analyzer revealed that BSH-3 seeds had 37 ± 0.13% protein, which is slightly higher than DN47 (36 ± 0.28%). Determination of oil content with near-infrared reflectance spectroscopy showed that BSH-2 and DN47 had 19 ± 0.49 and 18.1 ± 0.03%, respectively. We also analyzed the five major fatty acids of BSH-2 and DN47 seeds by gas chromatography. This analysis indicated that there are no significant differences in palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linolenic acid (18:3) between DN47 and BSH-2.
Loss of Abundant Seed Storage Proteins Has No Significant Effect on the Overall Methionine and Cysteine Content of BSH-2 Seeds
Earlier studies have examined the amino acid profiles of several seed storage protein mutants. Such mutants have been shown to accumulate high levels of free amino acids.16 To examine if a similar situation also occurred in BSH-2, we determined the total and free amino acid content of the seeds (Table 1). A comparison of the total amino acid composition revealed significant increases in several of the essential amino acids (Thr, Val, Leu, Phe, and Lys) in BSH-2 when compared with that of DN47 (Table 1). However, the levels of the other two essential amino acids (Met and Ile) were not significantly altered. Similarly, the levels of several other non-essential amino acids (Asp, Ser, Glu, Gly, Ala, His, Arg, and Pro) were also significantly higher in the BSH-2 seeds when compared to that of DN47 (Table 1). The overall total sulfur amino acid content (Met + Cys) was not significantly altered between BSH-2 and DN47 seeds. In contrast, a comparison of the free amino acid content revealed that the overall free amino acid content was almost three times higher in BSH-2 seeds when compared to that of DN47 (Table 1). The elevated levels of free amino acids in the BSH-2 seeds was mainly due to an increase in three of the essential amino acids (Thr, Ile, and Leu) and nine of the non-essential amino acids (Asp, Ser, Glu, Gly, Ala, Cys, Tyr, His, and Arg). Especially, the level of arginine was several folds higher in BSH-2 seeds (Table 1 and Figure S3).
Table 1. Protein-Bound and Free Amino Acid Content of DN47 and BSH-2a.
| AA |
FAA |
|||
|---|---|---|---|---|
| type | DN47 | BSH-2 | DN47 | BSH-2 |
| Essential amino acid | ||||
| Thr | 1.343 ± 0.049 | 1.603 ± 0.015** | 0.232 ± 0.003 | 0.327 ± 0.021* |
| Val | 1.503 ± 0.051 | 1.740 ± 0.053* | 0.113 ± 0.004 | 0.130 ± 0.004 |
| Met | 0.353 ± 0.038 | 0.440 ± 0.000 | 0.068 ± 0.002 | 0.071 ± 0.002 |
| Ile | 1.513 ± 0.137 | 1.683 ± 0.133 | 0.100 ± 0.003 | 0.096 ± 0.003* |
| Leu | 2.717 ± 0.042 | 2.967 ± 0.031** | 0.186 ± 0.008 | 0.151 ± 0.004* |
| Phe | 1.817 ± 0.021 | 1.977 ± 0.029* | 0.186 ± 0.005 | 0.250 ± 0.033 |
| Lys | 2.277 ± 0.021 | 2.367 ± 0.012** | 0.233 ± 0.006 | 0.263 ± 0.009 |
| Nonessential amino acid | ||||
| Asp | 3.860 ± 0.121 | 4.263 ± 0.055* | 0.667 ± 0.041 | 1.028 ± 0.046* |
| Ser | 1.780 ± 0.035 | 1.940 ± 0.046** | 0.091 ± 0.002 | 0.129 ± 0.009* |
| Glu | 6.050 ± 0.108 | 5.830 ± 0.040* | 0.422 ± 0.037 | 1.039 ± 0.040** |
| Gly | 1.467 ± 0.006 | 1.643 ± 0.023** | 0.053 ± 0.001 | 0.088 ± 0.007* |
| Ala | 1.463 ± 0.021 | 1.640 ± 0.035** | 0.129 ± 0.002 | 0.236 ± 0.013** |
| Cys | 0.503 ± 0.055 | 0.543 ± 0.021 | 0.164 ± 0.001 | 0.180 ± 0.003* |
| Tyr | 1.090 ± 0.079 | 1.200 ± 0.121 | 0.110 ± 0.003 | 0.153 ± 0.011* |
| His | 0.906 ± 0.031 | 1.097 ± 0.012* | 0.096 ± 0.013 | 0.314 ± 0.026** |
| Arg | 2.397 ± 0.129 | 3.030 ± 0.010* | 1.181 ± 0.046 | 6.526 ± 0.774** |
| Pro | 1.846 ± 0.029 | 1.923 ± 0.035* | 0.164 ± 0.004 | 0.175 ± 0.011 |
| T.S.A.A. | 0.857 ± 0.093 | 0.983 ± 0.021 | 0.233 ± 0.003 | 0.250 ± 0.025 |
| T.A.A | 32.887 ± 0.544 | 35.887 ± 0.169** | 4.173 ± 0.105 | 11.112 ± 0.776** |
Dry seed powder from triplicate samples were analyzed by HPLC. Significantly different means are indicated by * (p < 0.05) or ** (p < 0.01). T.S.A.A.: total sulfur amino acids; T.A.A: total amino acids.
Soybean Storage Protein Mutants Reveal Slower Germination Rates
To examine if the loss of abundant seed proteins affected seed germination, we performed experiments to monitor their germination rates (Figure 2A). For this purpose, we also included another seed storage protein mutant, BSH-3 for comparison. Germination of DN47 seeds could be detected as early as 24 h after imbibition. At this point, radicles were seen protruding from the seed coat. In contrast to DN47, the appearance of radicles was not observed in the mutants even after 36 h after imbibition. The germination rate of DN 47, BSH-2, and BSH-3 after 2 DAG were 90, 38, and 66%, respectively. Even though the germination rate of BSH-2 and BSH-3 seeds gradually improved over a period, the overall germination rate of these mutant seeds even at 5 DAG lagged beyond that of DN47 (Figure 2B). We also measured the length of the radicles and found that DN47 had a significantly greater radicle length (4.6 ± 0.86 cm) after 2 DAG when compared to that of BSH-2 (1.44 ± 0.85 cm) and BSH-3 (1.79 ± 1.2 cm).
Figure 2.
Soybean seed germination measurement. (A) DN47, BSH-2, and BSH-3 seeds were germinated in a 30 °C incubator, and the germination rate was recorded at 2 and 5 days after imbibition. Values are presented as mean ± SE. (B) Representative photograph of 5 DAG seedlings of DN47, BSH-2, and BSH-3. DAG, days after germination; Means followed by the same letter are not significant at the 5% significance level by the least significant difference test (LSD = 0.05). Bars indicate standard error of the mean.
Storage Protein Mutants Reveal a Slower Rate of Mobilization of Seed Reserves during Germination
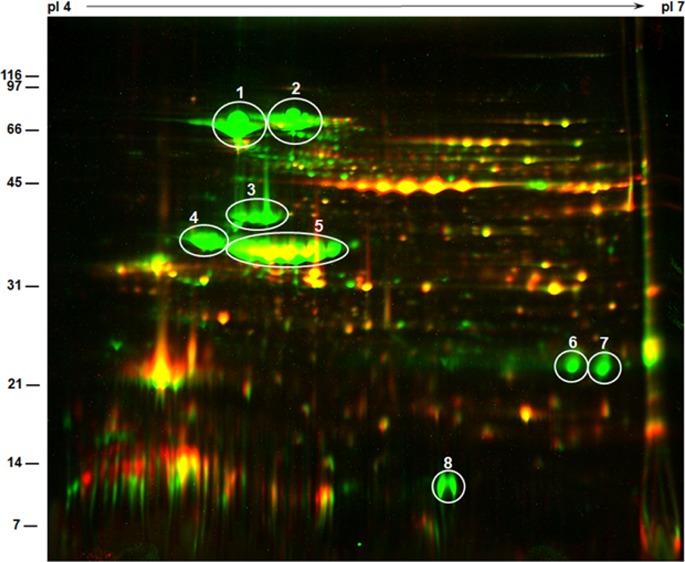
In order to examine if the slower seed germination rate observed with mutant lines can be correlated with a lower rate of mobilization of seed storage proteins, we compared the protein profile of seed proteins isolated from 0 DAG and 5 DAG seeds by high-resolution 2D SDS-PAGE (Figure 3). Proteins isolated from 0 DAG DN47 seeds were resolved into several discrete spots, the predominant among them were earlier identified as the α′-subunit of β-conglycinin (78 kDa), α-subunit of β-conglycinin (72 kDa), β-subunit of β-conglycinin (52 kDa), acidic subunits (34–36 kDa), and basic subunits of glycinin (21–23 kDa) (Figure 3A). By 5 DAG, this protein profile has dramatically changed with most of the prominent spots undergoing proteolytic digestion resulting in the appearance of several intermediate protein spots (Figure 3B). Interestingly, protein spots corresponding to Kunitz trypsin inhibitors, basic subunits of glycinin, and the small subunit of G4 remained unchanged (Figure 3B). In contrast, a comparison of the 2D gel protein profile of BSH-2 seeds at 0 DAG (Figure 3C) and 5 DAG (Figure 3D) showed only small changes. Remarkably, the β-subunits of β-conglycinin were unaffected even after 5 DAG (Figure 3D). Even the acidic subunits of glycinin were not degraded during seed germination. However, few new protein spots suggesting protein mobilization was also evident. The most abundant protein spots of BSH-3 seeds at 0 DAG were the α-subunit of β-conglycinin, the β-subunits of β-conglycinin, and G5 (Figure 3E). At 5 DAG, these major protein spots have mostly degraded, and several intermediate proteolytic digestion products were also observed (Figure 3F). A side–side comparison of the protein profiles indicates that the seed proteins of BSH-2 and BSH-3 seeds were not degraded as efficiently as in DN47 seeds.
Figure 3.
Two-dimensional gel electrophoresis of soybean seed proteins. Seed proteins (300 μg) were separated by isoelectric focusing on pI 4–7 strips followed by SDS-PAGE on 15% gels. The gels were stained with Colloidal Coomassie Blue G-250. The position and sizes of protein markers in kDa are shown on the left side of the figures. Circles designated as 1, 2, and 3 represent the α′, α, and β subunits of β-conglycinin, while those designated as 4 and 5 represent the acidic and basic subunits of glycinin, respectively. Circles 6 and 7 represents the KTi and A5 glycinin subunit, respectively. (A) DN47 dry seeds. (B). DN47 5 DAG. (C) BSH-2 dry seeds. (D). BSH-2 5 DAG. (E) BSH-3 dry seeds. (F) BSH-3 5 DAG.
Induction of Protease Activity Is Delayed in Storage Protein Mutants
During germination, the storage proteins are subjected to proteolytic cleavage by proteases. Several soybean proteases have been identified, and some of these proteases are responsible for the degradation of the 7S and 11S proteins.28,29 The delayed mobilization of storage proteins in BSH-2 and BSH-3 seeds when compared to DN47 promoted us to examine if there are any differences in the onset of protease activity. For this purpose, we employed zymogram gels that utilized gelatin as a substrate. The proteolytic digestion of the gelatin resulted in the appearance of white bands against a dark background (Figure 4A). In dry seed extracts, a faint protease activity corresponding to a 33 kDa polypeptide was identified. In 2 DAG seeds, two polypeptides (45 and 47 kDa) with pronounced protease activity was detected in DN47 seeds but were not seen in the seeds of BSH-2 and BSH-3 (Figure 4A). However, this protease activity was prominently seen in all three seed samples at 5 DAG (Figure 4A). In addition, a higher-molecular-weight protease (68 kDa) was also seen at 5 DAG seeds. Our zymogram gel analysis indicates a temporal delay in the appearance of some proteolytic activity may be responsible for the differential seed protein mobilization seen in these mutant seeds during germination.
Figure 4.
Visualization and measurement of proteolytic activity. (A) Soybean seed proteins were separated on a SDS-PAGE gel that contained gelatin (0.04%) in the resolving gels. After electrophoresis, the gels were incubated in 0.1 M citrate–phosphate buffer, pH 5.5, containing 0.18 M NaCl and 0.002 M dithiothreitol overnight in a 30 °C incubator. The gels were stained in Coomassie Blue R-250 and destained briefly to visualize protease activity (pointed out by arrows), which appeared as white bands. The numbers on the left side of the figure indicate the sizes of protein standards in kDa. (B) Protease activity in soybean seed extracts was measured following Sigma’s non-specific protease activity assay using casein as the substrate. A unit of protease activity is defined as the amount in micromoles of tyrosine equivalents released from casein per minute. Different letters on the top of the columns indicate significant differences between mean. Means followed by the same letter are not significant at the 5% significance level by the least significant difference test (LSD = 0.05). Bars indicate standard error of the mean.
We also measured the protease enzyme activity in BSH-2, BSH-3, and DN47 seeds at different stages of seed development (Figure 4B) by performing Sigma’s protease activity assay utilizing casein as the non-specific substrate. Protease activity was readily detected in the dry seeds, but a relatively higher activity levels was found in DN47 dry seeds when compared with that of BSH-2 and BSH-3 dry seeds (Figure 4B). By 2 DAG, there was a drastic increase in protease activity in DN47 seeds, while the protease activity in BSH-2 and BSH-3 seeds remained almost unchanged (Figure 4B). Interestingly after 5 DAG the protease activity in DN47 seeds dropped to levels that was even lower than values found even in dry seeds. In contrast, a slight increase in protease activity was detected in both BSH-2 and BSH-3 5 DAG seed extracts (Figure 4B).
Genes Encoding Proteolytic Enzymes Are Differentially Expressed in Seed Storage Protein Mutant Seeds
To monitor differences in the expression of genes encoding hydrolytic enzymes in DN47, BSH-2 and BSH-3 seeds during germination, we examined the transcript levels of 15 cDNA clones belonging to cysteine proteases, ubiquitinyl hydrolases, and cysteine proteinase inhibitors by qRT-PCR (Figure 5). The transcript levels of ubiquitin hydrolase (Glyma.15G010400, Glyma.20G19500, Glyma.10G0170500, Glyma.13G248100, and Glyma.03G122400) were low in the dry seeds and increased during seed germination with a maximum at 2 DAG (Figure 5). In contrast, transcripts encoded by cDNA clones Glyma.03G122400 and Glyma.03G122400 were readily detected even in dry seeds. However, the temporal pattern of accumulation of transcripts encoding ubiquitin hydrolases was markedly different in DN47 and BSH-2 and BSH-3 seeds. Two cysteine protease cDNAs (Glyma.01G110900 and Glyma.11G181500) was expressed at the highest level in BSH-2 mutant seeds even in dry seeds, while its transcripts could be detected in BSH-3 seeds only at 2 and 5 DAG. Interestingly, the accumulation of these two cysteine protease transcripts was greater in the mutant seeds when compared to the wild-type DN47 (Figure 5). Similarly, the expression levels of cDNA clones encoding cysteine proteinase inhibitors (Glyma.11G253300, Glyma.18G003700, Glyma.14G038200, and Glyma.05G149800) was also found to be much higher in BSH-2 when compared to that of DN47 and BSH-3 seeds (Figure 5). In contrast, the expression of another cysteine proteinase inhibitor (Glyma.14G038500) was higher in DN47 seeds at 2 and 5 DAG when compared to the mutants (Figure 5).
Figure 5.
qRT-PCR analysis of genes encoding hydrolytic enzymes in DN47, BSH-2, and BSH-3 seeds during germination. Selected genes involved in proteolytic pathway and primers employed for their amplification are listed as Table S1. Different letters (a, b, and c) on the top of the columns are statistically significant at the 5% probability level. Bars indicate standard error of the mean.
Transmission Electron Microscopy of Seed Storage Protein Mutant Seeds Reveal a Delay in Seed Reserve Mobilization
Since SDS-PAGE analysis reveal that the mobilization of storage proteins in mutants BSH-2 and BSH-3 during germination are compromised when compared to the wild-type plants, we wanted to examine the structure and morphology of protein storage vacuoles during germination. Transmission electron micrographs of mature dry seeds of DN47, BSH-2, and BSH-3 revealed that storage parenchyma cells in the cotyledons were filled with numerous storage protein vacuoles (PSV) of different shapes and sizes (Figure 6A,C,E). Previous immunocytochemical studies have demonstrated that the PSVs are the site where the 11S glycinin and 7S β-conglycinin are deposited. Dispersed within these PSVs are phytate crystals, which appear as small holes within the PSVs, which were also observed. In contrast, an examination of 5 DAG DN47 seed showed a prominent large central vacuole (Figure 6B). Unlike the dry seeds where the lipid bodies were dispersed throughout the cytoplasm, at 5 DAG, the lipid bodies were seen closer to the cell walls (Figure 6B). The numerous PSV seen in dry seeds appear to have merged during seed germination resulting in the formation of large central vacuole. In contrast to DN47, these large central vacuoles were not seen in the 5 DAG seeds of mutants BSH-2 and BSH-3 (Figure 6D,F). The appearance of PSVs in the seeds of BHS-2 even at 5 DAG was very similar to that seen in dry seeds, suggesting that the mobilization of storage reserves have been drastically affected in this mutant. The appearance of PSVs in the seeds of BSH-3 was intermediate between that observed between DN47 and BSH-2. In the case of BSH-3, several PSVs have merged together, leading to the formation of bigger vacuoles (Figure 6F). Interestingly, several large-sized lipid bodies and prominent starch grains were also seen within these cells (Figure 6F). The intact morphology of PSV in the seeds of the mutants even after 5 DAG indicates that the protein hydrolysis in these seeds occur at a much slower rate than that observed in DN 47.
Figure 6.
Transmission electron micrographs of soybean seeds. Thin sections of dry and 5 days after imbibition seeds (DAG) of (A, B) DN47, (C, D) BSH-2, and (E, F) BSH-3 were viewed by electron microscopy. Note the presence of a large central vacuole (V) in 5 DAG seeds of DN47 and the disappearance of protein storage vacuoles (PSV). Almost intact protein storage vacuoles are seen even in 5 DAG seeds of (D) BSH-2 seeds, while in (F) BSH-3 seeds, prominent amyloplasts (A) and large lipid bodies (LB) are seen. CW, cell wall.
Discussion
The seed storage protein mutants employed in this study, BSH-2 and BSH-3, are lacking a majority of the 7S β-conglycinin and 11S glycinin. Earlier studies have demonstrated that soybean seed storage protein mutants developed either by mutagenesis or biotechnology reveal proteome rebalancing. The loss of abundant seed proteins results in elevation of less abundant proteins in order to maintain the overall nitrogen content of the seeds. In BSH-2 seeds, proteome rebalancing is reflected in preferential accumulation of the β-subunit of β-conglycinin and an overall increase in the abundance of several other proteins. Interestingly, the overall nitrogen content of the mutant seeds is almost like that of wild-type seeds. Our observation is consistent with other previous reports where the loss of abundant seed proteins had little effect on the overall protein content.14,15 It has been suggested that the loss of storage proteins in these mutants is compensated by a drastic increase in the accumulation of free amino acids.16 The role of these free amino acids in seed germination is not clear. Free amino acids have been shown to play an important role in seeds metabolic network and act as precursors for the synthesis of several primary and secondary metabolites.30 In addition to contributing to the synthesis of seed storage proteins during seed development, they can also serve as an alternative source of accessible energy during the early germination process. However, the levels of FAAs in soybean are significantly lower when compared to those that are incorporated into 7S and 11S globulins. Thus, the role of the high levels of free amino acids in the BSH-2 and BSH-3 seeds needs further investigation.
In contrast to our study, earlier studies reported that the seed protein mutants grew and reproduced normally without any apparent physiological abnormalities.17,18 It is likely that these conclusions were made based on the growth of mature plants in either growth chamber or in the field. In fact, the performance of BSH-2 and BSH-3 was indistinguishable from the DN47 when mature plants were evaluated. However, our studies clearly establish that the loss of abundant seed proteins affect the germination rate and could affect seedling growth during the early stages of soybean growth. Several investigations on the mobilization of soybean seed proteins during germination have been conducted.28,29,31,32 Based on these studies, it is evident that glycinin and β-conglycinin undergo limited proteolysis during germination, resulting in the appearance of intermediate protein products, which are eventually degraded during seedling growth. With regard to 7S β-conglycinin, the α′ and α subunits are degraded at a faster rate than the β subunit. Similarly, the degradation of the acidic subunits of glycinin is initiated much earlier than the basic subunits. A serine protease is responsible for the degradation of the α′ and α subunits of β-conglycinin, while a cysteine endopeptidase, protease C2, is responsible for the cleavage of the β subunits of β-conglycinin.31,32 In this study, we found that seed storage protein mutants lacking both the α′ and α subunits (BSH-2) or just the α subunit (BSH-3) of β-conglycinin exhibited slower germination rate compared to that of DN47. Our observation indicates a potential role for the breakdown products of the α′ and α subunits of β-conglycinin as a source of accessible energy during the initial stages of seed germination.
The different rates of lipid mobilization in BSH-2 and BSH-3 mutant seeds compared to DN47 are also intriguing. Lipids, which are utilized to nourish the developing seedling, hve been shown to be mobilized by lipoxygenase (LOXs). At least six different lipoxygenases have been reported in soybean, three of them (LOX1, LOX2, and LOX3) expressed during seed development and another three (LOX4, LOX5, and LOX6) during seed germination.33 The highest accumulation of LOX4, LOX5, and LOX6 occurs in 3 day old germinating soybean seeds at which stage the oil bodies start to disappear. This observed correlation led to the suggestion that LOX4, LOX5, and LOX6 may play a role in lipid mobilization.34 Since the mobilization of lipids in BSH-2 and BSH-3 is impaired, it can be speculated that the accumulation of LOX4, LOX5, and LOX6 may be comprised in these mutants compared to that of wild-type seeds. However, subcellular fractionation and imunocytochemical localization studies have shown that LOX1, LOX2, LOX3, LOX4, LOX5, and LOX6 isozymes were not associated with purified oil bodies but mostly found in the soluble fraction.33 Additionally, degradation rates of total lipids and triacylglycerols between the wild-type and LOX1/2/3 triple null mutant (L0) L0 were not significantly different. These observations indicated that LOX1, LOX2, LOX3, LOX4, LOX5, and LOX6 are not directly involved in reserve lipid mobilization during soybean germination.33
Temperature, light, salinity, and hormones have a pronounced effect on seed germination.35 Environmental factors such as light and temperature can regulate seed germination by regulating the biosynthesis and catabolism of gibberellic acid and abscisic acid. A transcriptome sequencing study of embryonic axis during soybean germination uncovered several regulatory and metabolic aspects of seed germination.36 The authors reported a strong preferential activation of transcription factors (TFs) and protein kinases during the onset of germination, early activation of glycolysis, Krebs cycle, and cell wall remodeling. Additionally, a preferential upregulation of protein kinases, transcription factors, genes involved in lipid mobilization, and hormone signaling pathways was also reported.36 Transcription factors such as Dof proteins have been shown to play important roles in seed germination.37 Dof genes, which have been identified in both monocots and dicots, participate in the regulation of genes that encode storage proteins, carbon metabolism, and stress response and in the control of seed germination.38−40 In Arabidopsis, two Dof genes DAG1 (Dof Affecting Germination 1) and DAG2 have been shown to play opposite regulatory roles in the control of seed germination.41 Currently, we know very little if the delayed germination observed in the mutants BSH-2 and BSH-3 is mediated by loss of specific interactions of Dof proteins with other regulatory genes. Since both BSH-2 and BSH-3 are lacking several abundant seed proteins and show delayed seed germination, it will be interesting to examine the role of Dof transcription factors in these physiological processes.
Materials and Methods
Plant Materials
The strategy for the generation of seed storage mutants involved the use of a Chinese high-oil elite soybean cultivar “Dongnong47” (DN47) as a recurrent parent. A soybean line (“HS99B”) that lacks α′ and α subunits of β-conglycinin and some components of group I and IIa glycinin was used as an introgression line. For breeding purposes, DN47 was used as the female parent and “HS99B”as the male parent. Steps involved in the development of a soybean mutant line have been described earlier.19 A new mutant (BSH-2) that lacks the α′ and α subunits of β-conglycinin and G1 (A1aB2), G2 (A2B1a), G4 (A3B4), and G5 (A5A4B3) glycinin was identified and designated as BSH-2. This mutant and a previously characterized mutant BSH-319 along with DN47 were grown in a field at the soybean experimental plot at the Bradford Research Center (Columbia, MO, USA, latitude 38.893990, longitude 92.196405, Mexico silt loam soil). Seeds were planted in 15 ft rows with a 3 ft gap between plots, with a spacing of about 2 inches between plants and a row spacing of 30 inches between rows with three replications. At maturity, each plot was harvested and stored at 4 °C and 39% relative humidity.
Seed Protein, Oil, and Fatty Acid Profile
Mature seeds of DN47, BSH-2, and BSH-3 were harvested by hand and stored at room temperature in a low humidity environment. Seeds from 10 individual plants were pooled for analysis and were ground to a fine powder using a milling machine. These combined seed samples were utilized for both the protein and amino acid analyses. Total nitrogen (protein content) of these soybean seed samples was measured using a CHNS-O Elemental Combustion System (NC Technologies, Milan, Italy). The protein content of seeds was determined using the total nitrogen content value along with a conversion factor of 6.25. Each experimental line was analyzed with three biological replicates. The overall oil content was quantified using near-infrared reflectance (NIR) spectroscopy (Foss-Tecator AB, Hoganas, Sweden), and the fatty acid profiles were determined with an Agilent 6890 capillary gas chromatograph (Agilent, Santa Clara, CA, USA). Briefly, crushed seeds were extracted overnight with 5 mL of chloroform/hexane/methanol (8:5:2, v/v/v). Fatty acids from 100 μL aliquots of the extract were methylated with 75 μL of methanolic sodium methoxide/petroleum ether/ethyl ether (1:5:2, v/v/v). Fatty acids were separated with an AT-Silar capillary column (Alltech Associates, Deerfield, IL, USA). For determining relative amounts of each fatty acid, a standard containing known amounts of a variety of fatty acids was used.
Seed Amino Acid Analysis
Total amino acid content was determined using 6 N HCl hydrolysis of soybean seed powder in sealed, evacuated tubes at a constant temperature of 110 °C for 22 h. A Hitachi L-8000 amino acid analyzer (Hitachi, Tokyo, Japan) was used to determine the amino acid composition of the hydrolysates. For quantification of methionine and cysteine, the samples were first subjected to an initial oxidation step using performic acid prior to 6 N HCl hydrolysis. All samples were run in triplicate and subjected to appropriate statistical analysis. Free amino acid content was measured as described earlier.20
Germination Assays
DN47, BSH-2, and BSH-3 seeds were briefly sterilized with 30% bleach (2% sodium hypochlorite) for 5 min and rinsed with distilled water three times (2 min each rinse). Seeds were then incubated in 9 cm Petri dishes on two layers of sterile filter paper. Ten seeds were placed in each dish, and 12 mL of sterile water was added, using three replicates for each seed variety. Seeds were then incubated in a 30 °C under dark conditions. The germination percentages were calculated and recorded at 2 and 5 days after imbibition according to the particular experiment. Post-germination data include radicle length and fresh weight of germinated seeds. For each germination test, three experimental replications were performed, and the average germination percentage ± SE (standard error) of experiments was calculated.
Protein Isolation and SDS-PAGE Analysis
Mature dry seeds of DN47, BSH2, and BSH3 were hand harvested from the field and stored at room temperature. Seeds from five plants were pooled and ground to a powder using a Hamilton Beach Coffee Grinder. Then, 10 mg of seed powder was transferred to a 2 mL microcentrifuge tube, and 1 mL of sodium dodecyl sulfate (SDS)-sample buffer (0.06 M Tris-HCl, pH 6.8, 2% SDS (w/v), 10% glycerol (v/v), and 5% 2-mercaptoethanol (v/v)) was added. The contents of each tube were vigorously vortexed for 10 min at room temperature followed by boiling for 5 min. Each sample was centrifuged at 15,800g for 5 min, and the clear supernatant collected in a fresh tube. Seed proteins were resolved using a Hoeffer SE250 Mini-Vertical one-dimensional SDS-PAGE electrophoresis apparatus (GE Healthcare, Pittsburg, PA, USA). Approximately 50 μg of seed proteins was loaded onto 13.5% acrylamide gel. Separation of proteins was achieved with a constant of 20 mA/gel until the tracking dye reached the bottom of the gel. Gels were then removed from the cassette and stained overnight with Coomassie Blue R-250.
For two-dimensional electrophoretic analysis, proteins were isolated from 250 mg of finely ground seed using 5 mL of 0.1 M Tris-Cl, pH 8.8, containing 0.9 M sucrose, 0.25 M NaCl, 0.4% w/v β-mercaptoethanol, and plant protease inhibitor cocktail. To this was added 5 mL of saturated phenol, pH 4.3. This was continuously mixed for 30 min at room temperature, and the phenolic upper phase obtained by centrifugation at 5000g for 20 min at 23 °C in a swing-bucket rotor. The phenolic phase was removed and added to 10 volumes of 100% methanol with 0.1 M ammonium acetate, and proteins were precipitated for 2 h at −80 °C. This was followed by centrifugation at 12,000g for 15 min at 4 ° C, washing of protein pellet twice more in a solution of 100% methanol, 0.1 M ammonium acetate, and 0.01 M DTT, and washing of protein pellet three times more using a solution of 100% acetone and 0.01 M DTT. Following the final wash, the protein pellet was allowed to air dry slightly and then dissolved in a small volume of isoelectric focusing (IEF) resuspension buffer (7 M urea, 2 M thiourea, 1% w/v CHAPS, and 2% w/v C7BzO).
For IEF, 300 μg of protein sample was loaded per strip using overnight in-gel rehydration. Linear gradient, 13 cm 4–7 IPG strips (GE Healthcare) were brought to a rehydration volume of 250 μL using the IEF buffer containing sample with final concentrations of the following: 5% v/v glycerol, 2.2% v/v 2-HED, and 0.06 M DTT.21 Strips were then passively rehydrated at 25 °C for 15 h prior to focusing. IEF was performed with a BioRad Protean II apparatus (Hercules, CA, USA). Prior to the second dimension, strips were equilibrated with 5% w/v SDS in a urea-based solution (0.05 M Tris-Cl, pH 8.8, 6 M urea, 30% v/v glycerol, and 0.01% w/v bromophenol blue) for 15 min each incubation. The first equilibration solution contained 2% w/v DTT, and the second 2.5% w/v IAA. IEF strips were carefully placed onto a 12–16%T vertical second dimension gradient gel with an upper 5% stacking gel and secured into place with a warm 1% w/v agarose SDS-PAGE running buffer solution (0.2% w/v SDS). Gels were run at an initial 20 mA/gel for 1 h followed by 50 mA/gel for completion using a Hoeffer SE600 apparatus (GE Healthcare). After electrophoresis, gels were immediately removed and fixed in a 5:4:1 (methanol:water:acetic acid) solution for 1 h followed by two brief rinses in ultrapure water and then stained in a Coomassie G-250 solution overnight.
Image Acquisition and Analysis
One- and two-dimensional electrophoresis (1DE and 2DE) Coomassie stained gels were destained with multiple changes of ultrapure H2O to remove background. All gels were scanned separately using an Epson V700 Perfection scanner controlled through Adobe Photoshop.
Detection of Proteases in Polyacrylamide Gels
Detection of proteases in polyacrylamide gels was performed following the procedure outlined previously.22 Briefly, soybean seed protein samples were prepared as described above except the samples were not boiled before loading on SDS-PAGE gels. The protease substrate gelatin (0.04%) was incorporated into the resolving gels. After electrophoresis, the gels were washed several times in distilled water and incubated in 0.1 M citrate–phosphate buffer, pH 5.5 containing 0.18 M NaCl and 0.002 M dithiothreitol overnight in a 30 °C incubator. The gels were stained in Coomassie Blue R-250 for 2 h and destained briefly to visualize protease activity, which appeared as white bands.
Measurement of Protease Activity
Protease activity assays were performed following Sigma’s non-specific protease activity protocol.23 This assay is based on the fact that the tyrosine liberated by the protease digestion of casein can react with Folin’s reagent to produce a blue colored chromophore, which can be quantified and measured with the help of a spectrophotometer. The absorbance values generated by the activity of the soybean seed extracts were compared to a standard curve that was generated by reacting known quantities of tyrosine with the Folin’s reagent. A unit of protease activity is defined as the amount in micromoles of tyrosine equivalents released from casein per minute.
qRT-PCR Analysis
Seed samples collected at 0, 2, and 5 days after germination were frozen immediately in liquid nitrogen and stored at −80 °C until used. Total RNA was extracted using TRIzol RNA extraction kit (ThermoFisher, Waltham, MA, USA), and 1 μg of RNA was used for the first strand cDNA synthesis using a TIANScript RT Kit (TIANGEN, Beijing, China). Specific primers (Table S1) for each candidate gene were designed using Primer 5 software. The qRT-PCR reactions were conducted with three technical replications in 20 μL reactions for each biological replicate using the SuperReal PreMix Plus (SYBR Green) kit (Tiangen Biotech, Beijing, China). The soybean housekeeping gene GmActin4 (GenBank accession no. AF049106) was used as the internal control. Quantification of gene expression was performed using the relative 2–ΔΔCt method.24
Transmission Electron Microscopy
Dry seeds of DN47, BSH-2, and BSH-3 were germinated on 1% water agar plates in a 30 °C incubator. Seeds at 0 and 5 days after germination were dissected into small cubes (2–4 mm) with a scalpel and immediately fixed in 2.5% glutaraldehyde buffered at pH 7.2 with 0.05 M sodium phosphate for 4 h at 23 °C. Following the primary fixation, the seed samples were rinsed several times in distilled water and post-fixed for 1 h with 1% aqueous osmium tetroxide. The seed tissue was successively washed four times (15 min intervals) in distilled water and dehydrated in a graded acetone series and infiltrated with Spurr’s resin as described earlier.25 Thin sections of the seed tissue were cut, collected on copper grids, and stained with 0.5% uranyl acetate and 0.4% lead citrate. The stained sections were examined at 100 kV under an FEI Tecnai F30 Twin 300 kV (FEI Company, Japan) transmission electron microscope.
Acknowledgments
This research was supported by the USDA-Agricultural Research Service and a State Scholarship Fund provided to Xiaoshuang Wei by the China Scholarship Council. Mention of a trademark, vendor, or proprietary product does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00128.
Field growth characteristics of DN47, BSH-2, and BSH-3; overlay of 2D gels of DN47 and BSH-2 seed proteins using Delat2D software; protein-bound and free amino acid content of DN47 and BSH-2; and primer sequences of genes used for qRT-PCR (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wolf W. J. Soybean protein nomenclature: progress report. Cereal Science Today 1969, 14, 75–78. [Google Scholar]
- Nielsen N. C.Soybean Seed Composition. In Soybean: Genetics, Molecular Biology and Biotechnology; Verma D. P. S., Shoemaker R. C., Eds.; Centre for Agriculture and Bioscience International: Wallingford, U.K., 1996. [Google Scholar]
- Nielsen N. C.; Dickinson C. D.; Cho T. J.; Thanh V. H.; Scallon B. J.; Fischer R. L.; Sims T. L.; Drews G. N.; Goldberg R. B. Characterization of the glycinin gene family in soybean. Plant Cell 1989, 1, 313–328. 10.1105/tpc.1.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagasaki K.; Kalzuma N.; Kitamura K. Inheritance of glycinin subunits and characterization of glycinin molecules lacking the subunits in soybean (Glycine max (L.) Merr.). J. Jpn. Breed. 1996, 46, 11–15. 10.1270/jsbbs1951.46.11. [DOI] [Google Scholar]
- Thanh V. H.; Shibasaki K. β-Conglycinin from soybean proteins. Isolation and immunological and physicochemical properties of the monomeric forms. Biochim. Biophys. Acta, Protein Struct. 1977, 490, 370–384. 10.1016/0005-2795(77)90012-5. [DOI] [PubMed] [Google Scholar]
- Koshiyama I. Chemical and physical properties of a 7S protein in soybean globulins. Cereal Chem. 1968, 45, 394–404. [Google Scholar]
- Thanh V. H.; Shibasaki K. Major proteins of soybean seeds: a straightforward fractionation and their characterization. J. Agric. Food Chem. 1976, 24, 1117–1121. 10.1021/jf60208a030. [DOI] [PubMed] [Google Scholar]
- Krishnan H. B. Biochemistry and molecular biology of soybean seed storage proteins. J. New Seeds 2001, 2, 1–25. 10.1300/J153v02n03_01. [DOI] [Google Scholar]
- Natarajan S. S.; Krishnan H. B.; Lakshman S.; Garrett W. M. An efficient extraction method to enhance analysis of low abundant proteins from soybean seed. Anal. Biochem. 2009, 394, 259–268. 10.1016/j.ab.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Herman E. M.; Burks A. W. The impact of plant biotechnology on food allergy. Curr. Opin. Biotech. 2011, 22, 224–230. 10.1016/j.copbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Krishnan H. B. Engineering soybean for enhanced sulfur amino acid content. Crop Sci. 2005, 45, 454–461. 10.2135/cropsci2005.0454. [DOI] [Google Scholar]
- Krishnan H. B.; Jez J. M. Review: the promise and limits for enhancing sulfur-containing amino acid content of soybean seed. Plant Sci. 2018, 272, 14–21. 10.1016/j.plantsci.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Kwanyuen P.; Pantalone V. R.; Burton J. W.; Wilson R. F. A new approach to genetic alteration of soybean protein composition and quality. J. Am. Oil Chem. Soc. 1997, 74, 983–987. 10.1007/s11746-997-0015-2. [DOI] [Google Scholar]
- Kitamura K.; Kaizuma N. Mutant strains with low level of subunits of 7S globulin in soybean (Glycine max Merr.) seed. J. Jpn. Breed. 1981, 31, 353–359. 10.1270/jsbbs1951.31.353. [DOI] [Google Scholar]
- Takahashi K.; Banba H.; Kikuchi A.; Ito M.; Nakamura S. An induced mutant line lacking the α-subunit of β-conglycinin in soybean (Glycine max (L.) Merrill). J. Jpn. Breed. 1994, 44, 65–66. 10.1270/jsbbs1951.44.65. [DOI] [Google Scholar]
- Takahashi M.; Uematsu Y.; Kashiwaba K.; Yagasaki K.; Hajika M.; Matsunaga R.; Komatsu K.; Ishimoto M. Accumulation of high levels of free amino acids in soybean seeds through integration of mutations conferring seed protein deficiency. Planta 2003, 217, 577–586. 10.1007/s00425-003-1026-3. [DOI] [PubMed] [Google Scholar]
- Hajika M.; Takahashi M.; Sakai S.; Matsunaga R. Dominant inheritance of a trait lacking β-conglycinin detected in a wild soybean line. J. Jpn. Breed. 1998, 48, 383–386. 10.1270/jsbbs1951.48.383. [DOI] [Google Scholar]
- Teraishi M.; Takahashi M.; Hajika M.; Matsunaga R.; Uematsu Y.; Ishimoto M. Suppression of soybean β-conglycinin genes by a dominant gene, Scg-1. Theor. Appl. Genet. 2001, 103, 1266–1272. 10.1007/s001220100702. [DOI] [Google Scholar]
- Song B.; Oehrle N. W.; Liu S.; Krishnan H. B. Development and Characterization of a Soybean Experimental Line Lacking the α′ Subunit of β-Conglycinin and G1, G2, and G4 Glycinin. J. Agric. Food Chem. 2018, 66, 432–439. 10.1021/acs.jafc.7b05011. [DOI] [PubMed] [Google Scholar]
- Song B.; An L.; Han Y.; Gao H.; Ren H.; Zhao X.; Wei X.; Krishnan H. B.; Liu S. Transcriptome profile of near-isogenic soybean lines for β-conglycinin α-subunit deficiency during seed maturation. PLoS One 2016, 11, e0159723 10.1371/journal.pone.0159723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma A. D.; Oehrle N. W.; Emerich D. W. Plant protein isolation and stabilization for enhanced resolution of two-dimensional polyacrylamide gel electrophoresis. Anal. Biochem. 2008, 379, 192–195. 10.1016/j.ab.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Becker C.; Shutov A. D.; Nong V. H.; Senyuk V. I.; Jung R.; Horstmann C.; Fischer J.; Nielsen N. C.; Muntz K. Purification, cDNA cloning and characterization of proteinase B, an asparagine-specific endopeptidase from germinating vetch (Vicia sativa L.) seeds. Eur. J. Biochem. 1995, 228, 456–462. 10.1111/j.1432-1033.1995.tb20284.x. [DOI] [PubMed] [Google Scholar]
- Cupp-Enyard C. Sigma’s Non-specific Protease Activity Assay - Casein as a Substrate. J. Visualized Exp. 2008, 19, e899 10.3791/899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J.; Schmittgen T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Krishnan H. B. Preparative procedures markedly influence the appearance and structural integrity of protein storage vacuoles in soybean seeds. J. Agric. Food Chem. 2008, 56, 2907–2912. 10.1021/jf0735228. [DOI] [PubMed] [Google Scholar]
- Mooney B. P.; Krishnan H. B.; Thelen J. J. High-throughput peptide mass fingerprinting of soybean seed proteins: automated workflow and utility of UniGene expressed sequence tag databases for protein identification. Phytochemistry 2004, 65, 1733–1744. 10.1016/j.phytochem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hajduch M.; Ganapathy A.; Stein J. W.; Thelen J. J. A Systematic proteomic study of seed filling in soybean. Establishment of high resolution two-dimensional reference maps, expression profiles, and an interactive proteome database. Plant Physiol. 2005, 137, 1397–1419. 10.1104/pp.104.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. A.; Rightmire B. R.; Chen J. C.; Tan-Wilson A. L. Differential proteolysis of glycinin and β-conglycinin polypeptides during soybean germination and seedling growth. Plant Physiol. 1986, 82, 71–76. 10.1104/pp.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. A.; Papastoitsis G.; Hartl P.; Tan-Wilson A. L. Survey of the proteolytic activities degrading the Kunitz trypsin inhibitor and glycinin in germinating soybeans (Glycine max). Plant Physiol. 1988, 88, 355–360. 10.1104/pp.88.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R.; Galili G.; Cohen H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. 10.1016/j.plantsci.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Qi X.; Chen R.; Wilson K. A.; Tan-Wilson A. L. Characterization of a soybean [beta]-conglycinin-degrading protease cleavage site. Plant Physiol. 1994, 104, 127–133. 10.1104/pp.104.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.; Tan-Wilson A.; Wilson K. A. Protease C2, a cysteine endopeptidase involved in the continuing mobilization of soybean β-conglycinin seed proteins. Biochim. Biophys. Acta 2001, 1545, 192–206. 10.1016/S0167-4838(00)00277-6. [DOI] [PubMed] [Google Scholar]
- Wang C.; Croft K. P. C.; Järlfors U.; Hildebrand D. F. Subcellular localization studies indicate that lipoxygenases 1 to 6 are not involved in lipid mobilization during soybean germination. Plant Physiol. 1999, 120, 227–236. 10.1104/pp.120.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.; Love M. H.; Murphy P. Subcellular localization of lipoxygenase-1 and -2 in germinating soybean seeds and seedlings. J. Am. Oil Chem. Soc. 1990, 67, 961–965. 10.1007/BF02541858. [DOI] [Google Scholar]
- Rajjou L.; Duval M.; Gallardo K.; Catusse J.; Bally J.; Job C.; Job D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. 10.1146/annurev-arplant-042811-105550. [DOI] [PubMed] [Google Scholar]
- Bellieny-Rabelo D.; De Oliveira E. A. G.; da Silva Ribeiro E.; Costa E. P.; Oliveira A. E. A.; Venancio T. M. Transcriptome analysis uncovers key regulatory and metabolic aspects of soybean embryonic axes during germination. Sci. Rep. 2016, 6, 36009. 10.1038/srep36009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X.; Zhang Y.; Zhang C. Genome-wide analysis of plant-specific Dof transcription factor family in tomato [J]. J. Integr. Plant Biol. 2013, 55, 552–566. 10.1111/jipb.12043. [DOI] [PubMed] [Google Scholar]
- Mena M.; Vicente-Carbajosa J.; Schmidt R. J.; Carbonero P. An endosperm-specific Dof protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native B-hordein promoter in barley endosperm. Plant J. 1998, 16, 53–62. 10.1046/j.1365-313x.1998.00275.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S. Dof1 and Dof2 transcription factors are associated with expression of multiple genes involved in carbon metabolism in maize. Plant J. 2000, 21, 281–288. 10.1046/j.1365-313x.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- Papi M.; Sabatini S.; Bouchez D.; Camilleri C.; Costantino P.; Vittorioso P. Identification and disruption of an Arabidopsis zinc finger gene controlling seed germination. Genes Dev. 2000, 14, 28–33. [PMC free article] [PubMed] [Google Scholar]
- Gualberti G.; Papi M.; Bellucci L.; Ricci I.; Bouchez D.; Camilleri C.; Costantino P.; Vittorioso P. Mutations in the Dof zinc finger genes DAG2 and DAG1 influence with opposite effects the germination of Arabidopsis seeds. Plant Cell 2002, 14, 1253–1263. 10.1105/tpc.010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.