Abstract
For decades, titanium and its alloys have been established as a biocompatible material for cardiovascular medical devices such as heart valves, stents, vascular grafts, catheters, etc. However, thrombosis is one of the reasons for implant failure, where blood clot forms on the implant surface, thus obstructing the flow of the blood and that leads to some serious complications. Various surface modification techniques such as heparin modification, albumin coating, surface anodization, plasma etching, and hydrothermal treatments have been explored to improve the hemocompatibility of titanium-based materials. However, there are several limitations related to the robustness of the surfaces and long-term efficacy in vivo. In this study, titanium and its alloy Ti–6Al–4V were hydrothermally treated to form nanostructured surfaces with the aim to enhance their hemocompatibility. These modified surfaces were characterized for their wettability, surface morphology, surface chemistry, and crystallinity. The hemocompatibility of these surfaces was characterized by evaluating blood plasma protein adsorption, platelet adhesion and activation, platelet–leukocyte complex formation, and whole blood clotting. The results indicate lower fibrinogen adsorption, cell adhesion, platelet activation, and whole blood clotting on hydrothermally treated surfaces. Thus, these surfaces may be a promising approach to prevent thrombosis for several titanium blood-contacting medical devices.
1. Introduction
Different types of blood-contacting medical devices such as mechanical heart valves, stents, vascular grafts, catheter, etc. are implanted in patients worldwide to treat cardiovascular diseases. It is estimated that the number of coronary procedures carried out in the United States to implant these medical devices in the year 2019 is approximately 1 055 000, out of which 335 000 (approximately 31%) are recurring procedures.1 Most of the recurring procedures are due to inappropriate interaction of the implant material with blood and its components, which results in infection, inflammation, and thrombosis.2,3 Thrombosis is one of the reasons for implant failure, where a blood clot forms on the implant surface, thus obstructing the flow of the blood. Fibrinogen is adhered on the surface immediately after blood comes in contact with the surface. Simultaneously, factor XII also adsorbs to the surface and gets autoactivated resulting in conversion of prekallikrein to kallikrein, thus initiating coagulation and thrombin formation on the surface.4 Fibrinogen reacts with thrombin and gets converted to fibrin and traps activated platelets and red blood cells to form blood clots. Hence, it is important to understand the blood–implant surface interactions and modify the implant surface characteristics to improve its compatibility with blood and its components, i.e., hemocompatibility.5
Several clinical approaches have been employed to prevent blood clotting on implant surfaces. The most common approach is to prescribe patients with blood thinners such as aspirin, clopidogrel, and vorapaxar to avoid clotting of the blood.6 However, there are significant risks associated with internal bleeding and weakened immune response that may compromise the overall health of these patients.7 Implants coated with anticoagulants such as warfarin and heparin have also been widely used;8−10 however, there are risks associated with a decrease in plate count, body pain, and internal bleeding.11 Studies have also shown that the presence of nitric oxide reduces thrombosis as NO is a potent inhibitor of platelet function.12,13 Several other research approaches have also been investigated to the coating of implant surfaces with antifouling agents like poly(ethylene oxide) and albumin protein to inhibit adsorption of protein to the surface.14,15 However, the results show that there is no significant difference in blood clotting compared to unmodified surfaces.2,10,16,17 Other studies of different surface nanofeatures with varied roughness and wettability have also been investigated, and the results have shown significant differences in blood plasma protein adsorption.18−20 However, the effect of the size and shape of nanofeatures on protein adsorption and whole blood clotting is not well established. Different approaches on modifying the surface chemistry and topography combined have also been investigated. For example, superhydrophobic surfaces have shown a significant reduction in adsorption of blood plasma proteins and whole blood clotting.21−23 Nevertheless, these surfaces may not be stable for prolonged duration when exposed to blood. Also, superhydrophobic surfaces will not adhere endothelial cells, which plays a major role in the integration of many blood-contacting medical devices with the native tissue.23 Thus, there is a need to develop robust surfaces that prevents fibrinogen adsorption, controls blood clotting, and also have the potential to interact appropriately with the native tissue without compromising the implant integration in clinical environments.
Titanium (Ti) and its alloys have been established as a material of choice for different implantable medical devices such as heart valves, stents, dental, and orthopedic implants mainly due to its high specific strength to weight ratio, low modulus of elasticity and titanium oxide layers are biological inert.24−26 Furthermore, titanium is also considered to be biocompatible because of its low electrical conductivity, which leads to stable oxide layers. These oxide layers protect the implant from further corrosion.27 Surface modification on titanium surfaces has shown improved cytocompatibility with different cells.18−22 Various nanostructures such as nanowires, nanotubes, and nanopores have been developed on titanium surfaces using different techniques such as anodization,28,29 micromachining,30 and etching process25 where the results show improved cell adhesion. Even though titanium implants interact appropriately with the native tissue, they are prone to thrombosis when in contact with blood. In this study, titanium and its alloy Ti–6Al–4V were hydrothermally treated to form nanostructured surfaces with the aim to enhance their hemocompatibility. The hydrothermal treatment modified the surface of titanium and Ti–6Al–4V with different nanotopographies and wettability properties. Previous studies have shown that nanostructured titanium surfaces have led to better hemocompatibility when compared to nontextured surfaces.25,26,31,32 However, it is not well established whether it is the alteration of surface wettability or the nanostructure that majorly contributes to the difference in hemocompatible response. In this study, we have developed three different types of nanostructured surfaces by hydrothermal treatment on titanium and Ti–6Al–4V. The surfaces were characterized for their wettability, surface morphology, surface chemistry, and crystallinity. The hemocompatibility of these surfaces was characterized by evaluating blood plasma protein adsorption, platelet adhesion and activation, platelet–leukocyte complex formation, and whole blood clotting. The results indicate that surface topography and the size of the nanostructure play a major role in determining the hemocompatible response on hydrothermally treated surfaces and may have long-term implications in blood-contacting medical devices.
2. Results and Discussion
Despite extensive research carried out to understand the interaction of blood and its components with the surface of implantable medical devices,33−37 implants fail majorly due to blood clotting. This is a major concern with blood-contacting biomaterials where protein adsorption, platelet adhesion and activation, which cause blood clotting, could be fatal for patients. In this study, titanium and its alloy, Ti–6Al–4V, were exposed to an alkaline solution (NaOH), which leads to a corrosive reaction on the surface along with oxidization of the element(s) present (eq 1). Various permutations and combinations of variables such as NaOH concentration, temperature, and time for treatment were used to determine the optimal conditions for fabrication that resulted in uniform and repeatable nanostructures on different surfaces.
| 1 |
The rate of the reaction is influenced by various factors such as the concentration of NaOH, reaction temperature, and time.38 These corrosive reactions led to unique nanostructures on the material surface.39 The modified surfaces were characterized for surface morphology, surface energy, surface chemistry, and crystal structure. The possible oxides states of titanium formed during the reaction in a NaOH solution according to the Pourbaix diagram40 for titanium are TiO241 and Ti2O3 (Ti1, Ti2, A1, A2). These titanium oxides when further treated with HCl (Ti3, A3) develop titanate structures (eq 2), and this modification led to unique surface topography that is different than the treatment with just NaOH.
| 2 |
In this study, hemocompatibility of the treated surfaces was characterized by protein adsorption, cell cytotoxicity, cell adhesion, platelet adhesion and platelet activation, and whole blood clotting. Improved hemocompatibility may lead to fewer implant failures, thus increasing the implant life and decreasing recurring procedures in patients.
The morphology of different surfaces was visualized using scanning electron microscope (SEM). The results indicate that the unmodified surfaces as expected do not have any unique surface features. Whereas after the treatment with NaOH, the surfaces have developed unique nanostructures. Ti1 and A1 when exposed to 1 M NaOH for 4 h have developed nanoporous surfaces (Figure 1). There are also nanosized protrusions present on A1 with the length of single protrusions approximately 250–430 nm. These protrusions are not present on Ti1. Ti2 and A2 when exposed to 5 M NaOH for 24 h have developed interconnected web-like nanoporous surfaces. The grain boundaries are also visible on the surface since they get etched faster than the rest of the surface. The web-like nanoporous architecture is more clearly visible on the A2 (approximate pore size 150–280 nm) as compared to that on Ti2 (approximate pore size 70–170 nm). Ti3 and A3 were exposed to 1 M NaOH for 2.5 h and later were further exposed to 0.6 M HCl for 1 h. This led to a dramatically different nanostructured surface, almost similar to nanosized granules. The average size of granules is approximately 160–220 nm for Ti3 and 90–120 nm for A3. All of the surfaces fabricated had respective uniform morphology (Figure 1). The mechanism for the formation of different nanostructures on surfaces is not well established. However, it is likely that titanium when exposed to NaOH solution at specific temperatures will start reacting to form TiO2 and Na+–O–Ti. The charge repulsion within Na+–O–Ti leads to protrusions in Ti1 and A1 (Figure 1). When the reaction time is significantly increased (Ti2 and A2), the TiO2 and Na+–O–Ti are denser on the surfaces and hence result in the absence of protrusions but a nanoporous surface. When the NaOH-treated surfaces are exposed to HCl, the surface becomes neutral due to the absence of Na+–O–Ti, resulting in formation of denser granule-like structures on the surface (Figure 1).
Figure 1.
Representative SEM images of Ti and A surfaces treated by the hydrothermal treatment. Images were taken at two different magnifications (500× and 15 000×).
The contact angle on different surfaces was measured using a goniometer. There are two different configurations that define the contact angle on a nanostructured surface, namely, Wenzel and Cassie–Baxter.42 In the Wenzel state, there is a complete wetting of the surface. Whereas, Cassie–Baxter is a metastable state where air is trapped in between the surface and the liquid. Therefore, the contact angle is influenced by the area fraction of the liquid in contact with the solid and air. The apparent contact angles (θ*) for different surfaces were measured with deionized (DI) water, blood, and platelet-rich plasma (PRP) (Table 1). DI water is commonly used to characterize the surface wettability.43 However, the goal in this study is to understand how blood and its components interact with different surfaces, therefore contact angles of blood and PRP on different surfaces were also measured. Blood is a very viscous fluid that includes PRP44 (water, proteins, platelets, white blood cells, other biological factors) and red blood cells. However, when PRP is separated from blood, it is less viscous than blood, almost similar to DI water. All modified surfaces were hydrophilic (θ* < 90°) with DI water (Table 1). The following trend for θ* was observed: Ti > A > Ti3 > A3 > A1 > Ti1 > A2 > Ti2. Ti2 and A2 are more hydrophilic compared to all of the other surfaces, almost close to being superhydrophilic (θ* < 10°). This is mainly due to the etched grain boundaries that increase the surface area and thus more wetting on the surface. A1 and A3 had almost a similar contact angle despite different surface features and this could be due to the difference in surface chemistries that lead to similar polar interactions. Ti3 was least hydrophilic compared to all modified surfaces. In summary, titanium surfaces Ti, Ti1, and Ti2 are more hydrophilic than alloy surfaces A, A1, and A2, whereas A3 is more hydrophilic than Ti3 (Table 1). The surface wettability with PRP and blood is more influential for blood clotting.45 All of the modified surfaces are hydrophilic/hemophilic (θ* < 90°) with PRP (Table 1). The following trend for θ* was observed: Ti > A > Ti2 > A3 > A2 > Ti3 > Ti1 > A1. Further, surface wettability characteristics were completely different from blood when compared to DI water and PRP (Table 1). The following trend for θ* was observed: A > Ti > Ti3 > Ti2 > A3 > A2 > Ti1 > A1. This could be due to the difference in the liquid properties specifically the viscosity and surface tension.46−49 The results indicate that the interactions between different surfaces with different liquids were always in the Wenzel state, which is more stable than the Cassie–Baxter state.50
Table 1. Apparent Contact Angles of DI Water, Blood, and PRP on Different Surfacesa.
| DI water | blood | PRP | |
|---|---|---|---|
| Ti | 86 | 82 | 96 |
| A | 79 | 87 | 94 |
| Ti1 | 32 | 25 | 12 |
| A1 | 46 | 22 | 8 |
| Ti2 | 13 | 41 | 29 |
| A2 | 17 | 38 | 18 |
| Ti3 | 64 | 47 | 16 |
| A3 | 46 | 39 | 28 |
The values were rounded off to zero decimal places. There is a significant difference in the apparent contact angle (p < 0.05) between all surfaces except A1 and A3 and Ti and Ti3 (statistical differences are not shown in the table).
The advancing contact angle (θadv) for different surfaces with DI water (polar) and hexadecane (nonpolar) was measured (Table 2). The trend for θadv with DI water was similar to θ*. The θadv values were used to further calculate the surface energy using the Owens–Wendt equation. Higher surface energy relates to higher hydrophilicity, whereas lower surface energy relates to higher hydrophobicity of the surface. The following trend for surface energy was observed: Ti2 > A2 > Ti1 > A1 > Ti3 > A3 > Ti > A. Ti and A have non zero θadv with hexadecane unlike all of the modified surfaces. This is because of their low surface energy compared to other surfaces.
Table 2. Advancing Contact Angle of DI Water and Hexadecane on All Surfacesa.
| DI water | hexadecane | surface energy (mJ/m2) | |
|---|---|---|---|
| Ti | 92 | 19 | 27.75 |
| A | 98 | 24 | 25.85 |
| Ti1 | 40 | 0 | 57.56 |
| A1 | 63 | 0 | 42.07 |
| Ti2 | 18 | 0 | 69.36 |
| A2 | 30 | 0 | 63.07 |
| Ti3 | 76 | 0 | 34.70 |
| A3 | 65 | 0 | 40.81 |
The values were rounded off to zero decimal places. There is a significant difference in surface energy (p < 0.05) between all surfaces except A1 and A3 and Ti and Ti3.
The surface chemistry was analyzed using X-ray photoelectron spectroscopy (XPS). Survey scans were obtained and processed using MultiPak and Origin software (Figure 2). All of the surfaces showed peaks for O 1s (529–530 eV for metal oxides), Ti 2p (458.5 eV for TiO2), and C 1s (284.8 eV). Carbon is present on the surface due to impurities in the XPS chamber or on the surface. Ti1, Ti2 and A1, A2 have lower C 1s peaks compared to Ti and A, respectively (Table 3), indicating that the NaOH treatment removes some of the carbon impurities from the surfaces. Ti1 and A1 have higher Ti 2p peaks compared to Ti and A, respectively. This is due to the etching process that exposes more surface titanium. However, Ti2 and A2 have lower Ti 2p peaks compared to A1 and Ti1, respectively, due to the longer etching process that oxidizes the titanium on the surface. In contrast, Ti3 and A3 have higher Ti 2p peaks compared to all of the other surfaces due to the acidic environment that etches the oxide exposing more surface titanium (Table 3).
Figure 2.
XPS survey scans for different surfaces. Survey spectra were collected from 0 to 1100 eV with a pass energy of 187.85 eV.
Table 3. XPS Elemental Composition Calculated from Survey Scans for Different Surfaces.
| O 1s | Ti 2p | C 1s | |
|---|---|---|---|
| Ti | 20.8 | 2.8 | 76.4 |
| A | 39.0 | 7.0 | 54.0 |
| Ti1 | 52.1 | 16.1 | 31.8 |
| A1 | 51.5 | 16.8 | 31.7 |
| Ti2 | 45.2 | 12.2 | 42.4 |
| A2 | 50.3 | 12.1 | 37.6 |
| Ti3 | 37.1 | 17.7 | 45.2 |
| A3 | 38.9 | 20.1 | 41.0 |
High-resolution O 1s scans were obtained to evaluate the oxidation of titanium on different surfaces (Figure 3). The O–Ti and O–H peaks were present on all surfaces and H2O peaks were present only on Ti1 and A1. Ti1 and A1 surfaces have higher O–Ti when compared to Ti and A, respectively. However, with longer exposure to NaOH, Ti2 and A2 have no presence of H2O, lower O–H peaks, and higher O–Ti peaks compared to Ti and A, respectively, due to an increase in oxidation on the surface. In contrast, Ti3 and A3 have higher O–H peaks and lower O–Ti peaks when compared to all of the other substrates as the acid etches the oxide formed on the surface.
Figure 3.
XPS high-resolution O 1s scans for different surfaces. Survey spectra were collected from 520 to 550 eV with a pass energy of 187.85 eV.
Surface crystallinity of different surfaces was analyzed using X-ray diffraction (XRD) (Figure 4). Different peaks relevant to surfaces here are as follows:
Intensity peaks at 24° (101) and 62° (204) correspond to the anatase phase (JCPDS no. 21-1272).
Intensity peaks at 27° (110) and 76° (110) correspond to the rutile phase of TiO2 (JCPDS no. 21-1276).
Intensity peaks at 38.1° (002) and 39.9° (101) planes correspond to metallic Ti (JCPDS no. 89-5009).
Intensity peak at 48.7° (030) corresponds to the titanate structure (JCPDS no. 37-951), which suggests the presence of Na2Ti3O7.
Figure 4.
XRD scans for different surfaces. XRD scans were collected at θ = 1.5° and 2θ ranges were chosen based on significant peak intensities. Detector scans were run at a step size of 0.01 with a time per step of 1 s.
Ti1 and A1 show similar results with lower metallic Ti peaks compared to Ti and A, respectively, since both of these surfaces are oxidized. Ti2 and A2 also have higher TiO2 rutile and metallic Ti peaks compared to Ti1 and A1 due to higher oxidation and etching during prolonged exposure to NaOH. Ti3 and A3 have higher TiO2 rutile and Ti compared to all of the surfaces.28 A3 also showed a titanate peak.
Protein adsorption was characterized on different surfaces using a micro-bicinchoninic acid (BCA) assay (Figure 5). Protein adsorption to a surface is influenced by surface properties such as chemistry, topography, charge, and roughness.51−53 Fibrinogen is an inflammatory protein that gets converted to fibrin in the presence of thrombin and directly influences the platelet adhesion and activation. It is excreted by the liver and is present in blood. It is a planar protein that has a trinodular structure linked by two coiled-coil regions with a molecular weight of 340 kDa.54 When factor XII zymogen interacts with an unfamiliar surface (e.g., implant surface), it converts prothrombin to thrombin and in the presence of thrombin, fibrinogen gets converted to fibrin and drives the kinetics of thrombin formation.55 Hence, it is important to understand the amount of protein adsorbed to the surface. Albumin is the most abundant protein in the blood. It is a passivating protein that prevents blood thrombosis.56,57 It is a globular protein with a molecular weight 66.5 kDa.58 However, more albumin does not directly correlate to less blood clotting as it is just one of many factors.59 In this study, all modified surfaces are more hydrophilic than unmodified surfaces. More hydrophilicity is equated with less protein adsorption on the surface due to the energy barrier created by the liquid adsorbed to the surface.32,60 The results for fibrinogen adsorption follow the following trend (Figure 5a): A3 > A1 > A2 > Ti3 > Ti2 > Ti > A > Ti2, which does not follow the apparent contact angle trend (Table 1). Ti2 has significantly lower fibrinogen adsorption compared to all of the other surfaces since it is the most hydrophilic and has the densest porous structure compared to other surfaces (Figure 1). However, A2 with almost a similar contact angle and less dense porous structure has significantly higher fibrinogen adsorption compared to Ti2. Further, Ti1, Ti2, and Ti3 have lower protein adhesion compared to A1, A2, and A3, respectively, in spite of similar nanostructures but different feature sizes. This is because the protein adsorption is also not merely influenced by the contact angle but also majorly by surface topography.61 Other statistical differences between different surfaces are included in Table 4. In contrast, the results for albumin adsorption follows the following trend (Figure 5b): A2 > Ti1 > A1 > Ti3 > Ti > A > A3 > Ti2. Similar to fibrinogen adsorption, Ti2 has significantly lower albumin adsorption compared to all of the others. Further, there are no significant differences in albumin adsorption on other surfaces irrespective of changes in the contact angle and surface topography. The surface interaction with albumin is different than that of fibrinogen due to its globular shape and different charge.29
Figure 5.
(a) Fibrinogen adsorption on different surfaces measured using the micro-BCA assay. The results indicate significantly lower fibrinogen adsorption on Ti2 compared to all of the other surfaces (*p < 0.05). Other statistical differences are included in Table 4. The error bar represents the standard deviation. (b) Albumin adsorption on different surfaces measured using the micro-BCA assay. The results indicate significantly lower albumin adsorption on Ti2 compared to all of the other surfaces (*p < 0.05). The error bar represents the standard deviation.
Table 4. Statistical Comparison of Different Results on Unmodified and Modified Surfacesa.
Fibrinogen adhesion by micro-BCA, fibrinogen binding by enzyme-linked immunosorbent assay (ELISA), cell adhesion by fluorescence microscopy, and whole blood clotting after 45 min. (≅p > 0.05) indicating no significant difference between the compared set (*p > 0.05), indicating that there is a significant difference between the compared set.
Cytotoxicity of different surfaces was evaluated using the lactate dehydrogenase (LDH) assay. The surface modification changes the topography and chemistry, and it is important to evaluate if these changes induce any toxicity to the cells in contact with the surface. Cells when influenced by any toxic element stop growing and eventually die. When a cell begins to breakdown, they lose membrane integrity and release components from the cytoplasm into the medium and one of the stable enzymes excreted during this process is LDH.62 Hence, the presence of this LDH enzyme is a marker for cytotoxicity.63 The host body should be able to tolerate the implant while maintaining stability, without any exclusion and destruction.64 The results indicate that all of the substrates have similar amount of LDH expression compared to the positive control (100% live cells), indicating that none of the surfaces is inducing toxicity to the cells present in the PRP (Figure 6). LDH expression from the negative control (100% dead cells) was significantly higher when compared to all of the surfaces and the positive control (Figure 6). Thus, the results indicate that none of the surfaces demonstrates short-term cytotoxic effects on the cells present in the PRP.
Figure 6.
Cell cytotoxicity for PRP exposed to different surfaces measured using the LDH assay. The results indicate no significant differences in the LDH activity on all of the surfaces and positive control (100% live cells), whereas the LDH activity for the negative control (100% dead cells) was significantly different than all of the other surfaces (*p < 0.05). The error bar represents the standard deviation.
Fibrinogen binding from PRP on different surfaces was evaluated using an ELISA assay. Fibrinogen binding from PRP is a realistic environment since it is also influenced by other components that are present in PRP (other proteins, platelets, thrombin, leukocytes, etc.). Fibrinogen when in contact with thrombin gets converted to fibrin. This fibrin fibers form the blood clot on the surface. Hence, it is important to evaluate fibrinogen binding directly from the PRP on different surfaces. The surface-exposed PRP was assayed to measure the indirect amount of fibrinogen that was adsorbed on the surface. Higher fibrinogen in the surface-exposed PRP indicates less fibrinogen adsorbed on the surface. The results indicate that all of the surfaces have a significantly higher amount of fibrinogen in the surface-exposed PRP, indicating lower fibrinogen adsorption on the surface when compared to the Ti and A (Figure 7). Further, Ti2 has significantly higher amount of fibrinogen in the surface-exposed PRP, indicating significantly lower fibrinogen adsorption on the surface when compared to all surfaces except Ti1. This is because Ti1 and Ti2 have similar nanostructures; however, Ti1 has a less dense porous structure than Ti2 and when these surfaces are in contact with PRP, the fibrinogen binding may be influenced. Other statistical differences between different surfaces are included in Table 4. These results are similar to that of fibrinogen adsorption measured by the micro-BCA assay.
Figure 7.
Fibrinogen binding from PRP on different surfaces measured using ELISA. The results indicate a significant reduction in fibrinogen binding on Ti2 when compared to all of the surfaces except Ti1 (*p < 0.05). Other statistical differences are included in Table 4. The error bar represents the standard deviation.
Cell adhesion from PRP (platelet and leukocyte adhesion) on different surfaces was evaluated by fluorescence microscopy (Figure 8a). The surfaces with adhered cells were stained with calcein-AM.65 Platelet and leukocyte adhesion plays a vital role in the stimulation of the coagulation factors for hemostasis.66 Leukocytes can impact coagulation directly by producing anticoagulant molecules or procoagulants or indirectly acting on platelets, other leukocytes, or endothelial cells.67 Hence, it is important to understand the influence of surface modification on cell adhesion. The fluorescence microscopy images show that Ti and A have higher cell adhesion when compared to all of the other surfaces (Figure 8a). The area covered by the cells adhered on the surface was calculated using ImageJ (Figure 8b). The results indicate a significant decrease in cell adhesion on the modified surfaces when compared to the Ti and A (Figure 8b), with Ti1 and Ti2 having the least cell adhesion. Further, Ti1, Ti2, and Ti3 showed less cell adhesion when compared to A1, A2, and A3, respectively. The surface area is a key factor that influences cell adhesion. In general, higher surface area results in higher cell adhesion.68 However, nanotopography of surfaces also influences cell adhesion. In porous surfaces like Ti2, the dense porous structure majorly influences cell adhesion.69,70 The results indicate lower cell adhesion on Ti2 due to its dense porous structure that drastically reduces the area of contact with the cells. Cell adhesion through penetration into the porous structure is also not possible as the size of the pores is smaller than the size of the cells.70 Other statistical differences between different surfaces are included in Table 4.
Figure 8.

(a) Representative fluorescence microscope images of adhered live cells (platelets and leukocytes) stained with calcein-AM stain on different surfaces. The results indicate lower cell adhesion on all modified surfaces when compared to unmodified surfaces. (b) Percentage of the area covered by the cells adhered to the surface calculated using ImageJ. The results indicate that there is significantly lower cell adhesion on all modified surfaces when compared to unmodified surfaces (*p < 0.05). Other statistical differences are included in Table 4. The error bar represents the standard deviation.
Identification of different cell types (platelets and leukocytes) adhered on the surface from PRP was evaluated by fluorescence microscopy (Figure 9a). The surfaces with adhered cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine–phalloidin. DAPI stains the nucleus of the cells, whereas rhodamine–phalloidin stains the cytoskeleton of the cells. Since platelets do not have a nucleus, they will not stain positive for DAPI, whereas both platelet and leukocytes will stain positive of rhodamine–phalloidin. Thus, by staining the cells with DAPI and rhodamine–phalloidin, leukocytes adhered on the surface can be identified. During thrombosis, once the platelets are activated, they interact with leukocytes and further enhance the platelet activation rate.71,72 Hence, it is important to identify and evaluate platelet and leukocyte adhesion on the surface. The fluorescence microscopy images show that Ti and A have higher leukocytes and platelets adhered on the surface when compared to all of the other surfaces (Figure 9a). The area covered by the leukocytes and platelets on the surface was calculated using ImageJ. The results from DAPI images indicate a significant decrease in leukocyte adhesion on all of the modified surfaces (Figure 9b), except Ti3, when compared to Ti and A. The results from rhodamine–phalloidin images indicate that platelet and leukocyte counts from rhodamine–phalloidin stains (Figure 9c) are significantly lower on all modified surfaces when compared to Ti and A, with Ti1 and Ti2 having least cell adhesion. Further, Ti1, Ti2, and Ti3 showed less cell adhesion when compared to A1, A2, and A3, respectively. Other statistical differences between different surfaces are included in Table 4. Similar trends as to that of the cell adhesion study were observed in this study.
Figure 9.

(a) Representative fluorescence microscope images of adhered platelets and leukocytes stained with DAPI and rhodamine–phalloidin on different surfaces. The results indicate lower cell adhesion on all modified surfaces when compared to unmodified surfaces. (b) Percentage of the area covered by the cells adhered to the surface stained with DAPI calculated using ImageJ. The results indicate significantly lower cell adhesion on all modified surfaces when compared to unmodified surfaces (*p < 0.05), except Ti3 (#p > 0.05). The error bar represents the standard deviation. (c) Percentage of the area covered by the cells adhered to the surface stained with rhodamine–phalloidin calculated using ImageJ. The results indicate significantly lower cell adhesion on all modified surfaces when compared to unmodified surfaces (*p < 0.05). Other statistical differences are included in Table 4. The error bar represents the standard deviation.
Platelet activation and platelet–leukocyte complex formation on different surfaces were visualized using SEM. Platelets when activated change shapes, form dendrites, and start aggregating.26 The activated platelet may assist leukocyte localization during thrombosis and modulate their function.67 The leukocyte–platelet interaction thus initiates biosynthesis of cytokines and inflammatory reactions, which leads to several heart conditions.73 Therefore, it is crucial to evaluate if the surfaces induce platelet activation and platelet–leukocyte complex formation. The SEM images show activated platelets with dendrites on Ti and A (Figure 10). Further, leukocyte–platelet interactions are also observed on Ti and A (Figure 10, dotted circle). However, there is a drastic reduction in platelet activation and the absence of leukocyte–platelet interactions on all of the other surfaces. Further, platelets seem to aggregate on modified surfaces. However, the platelets are not activated as no dendrite extensions are present.
Figure 10.
Representative SEM images of adhered platelets and leukocytes on different surfaces. Images show a higher degree of platelet activation and platelet–leukocyte complex formation on unmodified surfaces when compared to modified surfaces. Images were taken at two different magnifications (500× and 15 000×).
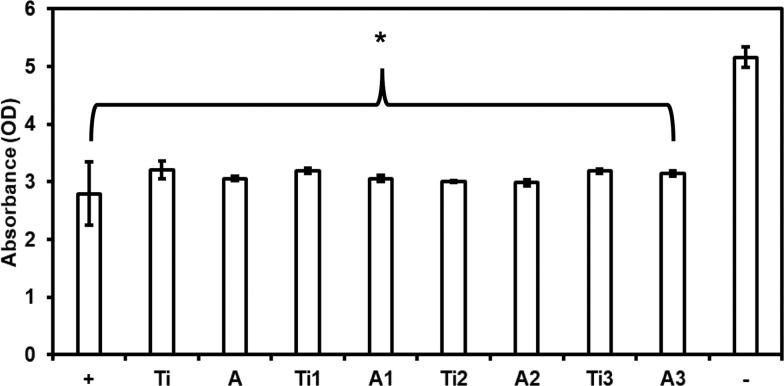
Whole blood clotting was evaluated on different surfaces by measuring the free hemoglobin present on the surface that was in contact with blood. The unclotted blood when diluted with DI water results in lysis of red blood cells and thus releasing hemoglobin. Hence, higher hemoglobin in the diluted solution indicates less blood clotting on the surface. The blood was allowed to clot for 45 min on all of the surfaces, and the amount of free hemoglobin was measured every 15 min (Figure 11). During the three-time points evaluated, the blood clotting was significantly lower on all of the modified surfaces when compared to Ti and A. The amount of free hemoglobin on Ti and A decreased drastically from 15 to 45 min, indicating that the blood is clotting on the surface. However, Ti1, A1, Ti2, and A2 surfaces did not show a significant reduction in free hemoglobin from 15 to 45 min. Further, Ti3 and A3 did not show significant differences in free hemoglobin after 15 min, however showed a significant reduction in free hemoglobin after 30 and 45 min. After 45 min, Ti2 and A2 showed the highest free hemoglobin when compared to all surfaces, indicating minimum blood clotting. Other statistical differences between different surfaces are included in Table 4. In summary, Ti1, Ti2, and Ti3 showed less blood clotting when compared to A1, A2, and A3, respectively, at all time points.
Figure 11.
Whole blood clotting on different surfaces for up to 45 min. The dotted line represents the absorbance of free hemoglobin in unclotted blood. Statistics not shown in the graph. There is a significant increase in free hemoglobin present on all modified surfaces when compared to the unmodified surface at respective time intervals. Ti2 has the highest free hemoglobin after 45 min compared to all surfaces. Other statistical differences for 45 min study are included in Table 4. The error bar represents the standard deviation.
3. Conclusions
Various permutations and combinations of variables such as NaOH concentration, temperature, and time for treatment were used to determine the optimal conditions for fabrication that resulted in uniform and repeatable nanostructures on different surfaces. The results for cytotoxicity indicate that there were no short-term cytotoxic effects on the cell present in the PRP due to the surface modification. The results for fibrinogen binding from PRP and fibrinogen adsorption from the solution indicate that the Ti2 had significantly lower fibrinogen on the surface when compared to all surfaces. This reduction of protein adsorption influenced cell adhesion (platelet and leukocytes), leading to reduced blood clotting. Thus, the results indicate that Ti2 with a small-sized dense porous structure is the most hemocompatible surface when compared to all modified and unmodified surfaces. Final analyses and comparisons of different experiments indicate three main conclusions. Pure titanium is more hemocompatible than the Ti–6Al–4V alloy. Ti and Ti3 have similar surface energy (nonsignificant difference). The results show a significant reduction in protein and platelet adhesion on Ti3 when compared to Ti. Hence, surface morphology plays a major role in cell and protein interaction despite similar surface energy. Porous surfaces (Ti2) are more hemocompatible than granular surfaces (Ti3). The pore size majorly influences cell and platelet adhesion. For example, Ti1 and Ti2 are both porous, but the Ti2 pores are smaller and denser than Ti1 and that changes the wettability and hemocompatibility significantly.
4. Experimental Section
4.1. Fabrication of Nanostructures on the Different Surfaces
The materials used in this study were sheets of 0.5 mm thick commercially pure titanium (grade 2) and the Ti–6Al–4V alloy. Square substrates of dimension 6 mm × 6 mm were cut from the sheets. The surfaces were polished using SiC abrasive sheets up to grade 1400. Polished substrates were then cleaned ultrasonically with acetone and deionized (DI) water for 10 min each. Three different fabrication processes were used to hydrothermally treat the substrates to form nanostructures on the surface and are explained below:
-
1.
Substrates were immersed in 75 mL of 1 M NaOH solution inside an inert poly(tetrafluoroethylene) (PTFE) bottle and hydrothermally treated for 4 h at 200 °C inside an oven. The treated substrates were rinsed with DI water and were annealed for 1 h at 300 °C.
-
2.
Substrates were immersed in 50 mL of 5 M NaOH solution inside an inert PTFE bottle and hydrothermally treated for 24 h at 60 °C inside an oven. The treated substrates were rinsed with DI water and were annealed for 1 h at 300 °C.
-
3.
Substrates were immersed in 75 mL of 1 M NaOH solution inside an inert PTFE bottle and hydrothermally treated for 2.5 h at 200 °C inside an oven. The treated substrates were rinsed with DI water and were annealed for 1 h at 300 °C. After annealing, they were again rinsed with DI water and immersed in 50 mL of 0.6 M HCl solution inside an inert PTFE bottle for 1 h inside an oven. The treated substrates were rinsed with DI water and were annealed for 2 h at 600 °C.
After the treatments, all substrates were ultrasonically cleaned with DI water for 10 min and dried using nitrogen gas.
The following notation will be used in the manuscript for different surfaces: Ti for unmodified titanium, A for unmodified Ti–6Al–4V. The modified surfaces are abbreviated as Ti1, Ti2, Ti3, A1, A2, and A3, where the number corresponds to the hydrothermal treatment used.
4.2. Characterization of Different Surfaces
The modified surfaces were characterized using different techniques to evaluate their surface energy, morphology, chemistry, and crystal structure.
4.2.1. Surface Morphology
The surface morphology was characterized using a JEOL 6500 field emission scanning electron microscope (SEM), operated at an accelerating voltage of 15 kV. The surfaces were coated with 5 nm gold before imaging. The surfaces were imaged at magnifications of 5000× and 30 000×. The images were processed using the ImageJ software.
4.2.2. Contact Angle Measurements and Surface Energy Calculations
The surface hydrophobicity/hydrophilicity was characterized by measuring the apparent contact angle (θ*) and advancing contact angle (θadv) at room temperature using the sessile drop method (Ramé-hart 260F4 goniometer). Images were captured 3 s after 5 μL droplet of DI water (polar) and hexadecane (nonpolar) was placed on the substrate. The errors in apparent and advancing contact angles were ±4 and ±3°, respectively. The images were analyzed using the manufacturer-provided software to measure the contact angles. Apparent contact angles for blood and platelet-rich plasma (PRP) (see Section 4.5 for isolation of blood and PRP) were also measured using the same method.
The advancing contact angles for water and hexadecane were used to calculate the solid–vapor surface energy. The polar and dispersive components of the surface energy were calculated using Young’s equation (eq 3) and Owens–Wendt equation (eq 4).
| 3 |
| 4 |
where γlν is the liquid (water)–vapor interfacial tension (72.8 mN/m) and hexadecane (27.5 mN/m), θ is the advancing contact angle, γsν is the solid surface free energy, γsl is the solid–liquid interfacial free energy, γsνd and γlν are the dispersive components, and γsνp and γlν are the polar components of the solid and liquid-free surface energy, respectively. The above two equations were solved to get the final equations to calculate the dispersive (eq 5) and polar (eq 6) components of the solid–vapor surface energy. The overall solid–vapor surface energy is the sum of the dispersive and polar components (eq 7).74
| 5 |
| 6 |
| 7 |
4.2.3. Surface Chemistry
The surface chemistry was characterized using a PE-5800 X-ray photoelectron spectrometer. Survey spectra were collected from 0 to 1100 eV with a pass energy of 187.85 eV. High-resolution spectra were collected for oxygen (O 1s) using a pass energy of 10 eV. The surface elemental composition was calculated using peak fit analysis in the Multipack and Origin software.
4.2.4. Surface Crystal Structure
The presence of anatase and rutile crystal phases was characterized by Bruker D8 glancing angle X-ray diffraction (GAXRD). XRD scans were collected at θ = 1.5°, and 2θ ranges were chosen based on significant peak intensities. Detector scans were run at a step size of 0.01 with a time per step of 1 s. Peaks were filtered and correlated to crystal structures using the DIFFRACT.EVA software.
4.3. Surface Preparation Prior to Biological Studies
Prior to biological studies, the surfaces were sonicated in acetone for 10 min followed by rinsing with DI water and phosphate-buffered saline (PBS) solution. The surfaces were sterilized by exposure to UV light for 15 min inside a biosafety cabinet.
4.4. Protein Adsorption on the Different Surfaces
Albumin and fibrinogen adsorptions were characterized using a micro-BCA assay. The surfaces were incubated for 2 h in a 48-well plate on a horizontal shaker (100 rpm) at 37 °C and 5% CO2 with 300 μL of protein solution (concentration 100 μg/mL in PBS). After 2 h, the protein solution was aspirated, and surfaces were rinsed three times with PBS. To measure the amount of protein adhered on different surfaces, the substrates are incubated for 1 h in a 48-well plate on a horizontal shaker (100 rpm) at 37 °C and 5% CO2 with 200 μL of 1% sodium dodecyl sulfate (SDS). This was repeated twice, and the SDS solution with the desorbed protein was pooled. The pooled SDS was used with the micro-BCA assay to measure the absorbance of the resulting solution at a wavelength of 562 nm using a plate reader. The protocol given by the manufacturer was followed to plot the standard curve, and the protein concentration from the absorbance values was calculated.
4.5. Platelet-Rich Plasma (PRP) Isolation from Whole Blood
Whole blood was isolated through venipuncture from healthy donors, who refrained from having drugs that may have affected their blood. The isolation procedure was in accordance with the protocol approved by the Colorado State University Institutional Review Board. Procedures were performed in compliance with the National Institutes of Health’s “Guiding Principles for Ethical Research”. Informed consents were obtained from human participants prior to enrolling in this study. The blood was collected in 6 mL tubes coated with the anticoagulant, ethylenediaminetetraacetic acid (EDTA). The first tube of blood was discarded to account for the platelet plug and locally activated platelets resulting from the needle insertion. The PRP was isolated by centrifuging the blood tubes at 150g for 15 min. The centrifugation results into two layers, the top layer, which is PRP, and the bottom layer, which is the red blood cells. After centrifugation, the tubes were let to rest for another 15 min before the PRP was used. All of the biological studies were repeated at least three times with blood drawn from at least three different donors. However, for each experiment, the PRP was only pooled from the same donor. This is because there is donor-to-donor variability in the number of platelets and it is not possible to compare the absolute values from different donors.
4.6. Cytotoxicity of Different Surfaces
Cytotoxicity of different surfaces was evaluated using a commercially available lactate dehydrogenase (LDH) assay kit. The surfaces along with positive (provided with the assay) and negative controls (PRP treated with Triton-X provided with the assay) were incubated with 300 μL of PRP in a 48-well plate on a horizontal shaker (100 rpm) for 2 h at 37 °C and 5% CO2. After the incubation, the PRP solution was aspirated and surfaces were rinsed three times with PBS to remove any nonadherent cells. One hundred microliter of surface-exposed PRP was pipetted to the 96-well plate, and the protocol provided by the manufacturer was followed. The absorbance of the resulting solution was measured at a wavelength of 490 nm using a plate reader.
4.7. Fibrinogen Binding from PRP on Different Surfaces
Fibrinogen binding was measured using a commercially available ELISA kit for human fibrinogen. The surfaces were incubated in 300 μL of PRP in a 48-well plate on a horizontal shaker (100 rpm) for 2 h at 37 °C and 5% CO2. The surface-exposed PRP was then diluted to 1/10 000 with the diluent provided with the ELISA kit. This diluted PRP was pipetted to the 96-well plate, and the protocol provided by the manufacturer was followed. The absorbance of the resulting solution was measured at a wavelength of 450 nm using a plate reader.
4.8. Cell Adhesion on Different Surfaces
Cell adhesion on different surfaces was imaged by fluorescence microscopy. The surfaces were incubated in 300 μL of PRP in a 48-well plate on a horizontal shaker (100 rpm) for 2 h at 37 °C and 5% CO2. After the incubation, the PRP was aspirated and surfaces were rinsed three times with PBS to remove any unadhered cells. The surfaces were then incubated in dark with 1 mL of 5% calcein-AM solution in PBS for 20 min at room temperature. The stain solution was aspirated, and the surfaces were rinsed two times with PBS. The surfaces were imaged using a fluorescence microscope (Zeiss AxioVision) at 20×. All images were further processed using ImageJ to calculate the surface coverage of cells.
4.9. Identification of Platelets and Leukocytes Adhered on Different Surfaces
Identification of platelets and leukocytes adhered on different surfaces was imaged by fluorescence microscopy. The surfaces were incubated in 300 μL of PRP in a 48-well plate on a horizontal shaker (100 rpm) for 2 h at 37 °C and 5% CO2. After the incubation, the PRP was aspirated and surfaces were rinsed three times with PBS to remove any unadhered cells. The surfaces were fixed in a 3.7% formaldehyde solution diluted with PBS for 15 min. The fixed surfaces were rinsed twice in PBS for 5 min each. Subsequently, the surfaces were incubated in a solution of 1% Triton-X diluted with PBS for 3 min. The surfaces were again rinsed twice in PBS and moved to a new 48-well plate. Two hundred microliter of 0.05% rhodamine–phalloidin (actin) solution in PBS was added to each well and incubated for 25 min. Twenty one microliter of 3% 4′,6-diamidino-2-phenylindole (DAPI) stain stock solution was added to each well and incubated for 5 more minutes. The surfaces were then rinsed twice in PBS and imaged using a fluorescence microscope. ImageJ was used to calculate the actin cell coverage and number of nuclei on the substrates. The DAPI will stain the nucleus of leukocytes blue, whereas the rhodamine–phalloidin will stain the cytoskeleton of both the platelets and leukocytes red.
4.10. Platelet Activation on Different Surfaces
Platelet activation on different surfaces was characterized using SEM. The surfaces were incubated in 300 μL of PRP in a 48-well plate on a horizontal shaker (100 rpm) for 2 h at 37 °C and 5% CO2. After the incubation, the PRP was aspirated and surfaces were rinsed three times with PBS to remove any unadhered cells. The cells adhered to the surface were fixed by incubating the surfaces in a solution containing 6% glutaraldehyde, 0.1 M sodium cacodylate, and 0.1 M sucrose in DI water for 45 min. The surfaces were then incubated in a buffer solution containing 0.1 M sodium cacodylate and 0.1 M sucrose for 10 min. This was followed by incubating the surfaces in 35, 50, 70 and 100% ethanol for 10 min each. The surfaces were air-dried and imaged using a SEM, as discussed in Section 4.2.1.
4.11. Whole Blood Clotting on Different Surfaces
Whole blood clotting on different surfaces was characterized by indirectly measuring the amount of free hemoglobin in unclotted blood after exposure of surfaces to whole human blood using a plate reader.75−77 For this study, blood was drawn in a vacuum tube without any anticoagulant and was used immediately after drawing. Five microliter of blood was pipetted on top of different surfaces in a 24-well plate, and the blood was allowed to clot for up to 45 min. After every 15 min, the surfaces were evaluated for the presence of free hemoglobin. Five hundred microliter of DI water was added to the surfaces and gently shaken for 30 s to lyse red blood cells that were not trapped in the clot on the surface. The absorbance of free hemoglobin released by the lysed red blood cells was measured at a 540 nm wavelength using a plate reader.78
4.12. Statistical Analysis
Surface characterization was repeated for at least three different samples of each surface. SEM images and contact angle measurements were taken at three different locations on each sample (nmin = 9). Protein adsorption was carried out on at least three different samples of each surface and was repeated at least three times (nmin = 9). The LDH assay, fluorescence microscopy, platelet activation, and whole blood clotting were repeated at least two times (with PRP from different donors) with at least three different samples of each surface (nmin = 6). The quantitative results were analyzed using a two-way analysis of variance (ANOVA) test using the R software. The results were considered statistically significant with a p-value <0.05. The data presented (i.e., the arithmetic mean and standard deviation) is only from one donor as it is not appropriate to compare values between different donors due to the variability in the platelet counts for each donor. However, similar trends were observed for blood from different donors for all of the results presented, indicating the reproducibility of the data.
Acknowledgments
Research reported in this publication was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL135505 and R21HL139208. The authors acknowledge Patrick McCurdy from CIF CSU for his training with SEM and XPS, Roberta Maia Sabino from CSU for her training with biological experiments, Paulo Soares from PUCPR, Brazil for his assistance with XRD, and all of the people who donated blood for these experiments.
The authors declare no competing financial interest.
References
- Benjamin E. J.; Muntner P.; Alonso A.; Bittencourt M. S.; Callaway C. W.; Carson A. P.; Chamberlain A. M.; Chang A. R.; Cheng S.; Das S. R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Gorbet M. B.; Sefton M. V. Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 5681–5703. 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Nezafati M.Analyzing Biomaterial Surfaces and Blood–Surface Interactions. Hemocompatibility of Biomaterials for Clinical Applications: Blood–Biomaterials Interactions; Elsevier Inc., 2018; pp 107–117. [Google Scholar]
- Jaffer I. H.; Fredenburgh J. C.; Hirsh J.; Weitz J. I. Medical Device-Induced Thrombosis: What Causes It and How Can We Prevent It?. J. Thromb. Haemostasis 2015, S72–S81. 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- Siedlecki C. A.Hemocompatibility of Biomaterials for Clinical Applications: Blood–Biomaterials Interactions; Elsevier, 2017. [Google Scholar]
- Jneid H.; Bhatt D. L.; Corti R.; Badimon J. J.; Fuster V.; Francis G. S. Aspirin and Clopidogrel in Acute Coronary Syndromes: Therapeutic Insights from the CURE Study. Arch. Intern. Med. 2003, 1145–1153. 10.1001/archinte.163.10.1145. [DOI] [PubMed] [Google Scholar]
- Torosian M.; Michelson E. L.; Morganroth J.; MacVaugh H. III Aspirin- and Coumadin -Related Bleeding after Coronary-Artery Bypass Graft Surgery. Ann. Intern. Med. 1978, 89, 325–328. 10.7326/0003-4819-89-3-325. [DOI] [PubMed] [Google Scholar]
- Callahan B. C.; Lisecki E. J.; Banks R. E.; Dalton J. E.; Cook S. D.; Wolff J. D. The Effect of Warfarin on the Attachment of Bone to Hydroxyapatite-Coated and Uncoated Porous Implants. J. Bone Jt. Surg. 1995, 77, 225–230. 10.2106/00004623-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Wang A.; McAllister J. P.; Finlayson P.; Li J.; Brabant K.; Tang H.; Black C.; Cao T.; Liang X.; Salley S. O.; et al. Short-and Long-Term Neural Biocompatibility of Heparin Coated Sapphire Implants. Mater. Sci. Eng., C 2007, 27, 237–243. 10.1016/j.msec.2006.05.011. [DOI] [Google Scholar]
- Levy M.; Hartman A. R. Heparin-Coated Bypass Circuits in Cardiopulmonary Bypass: Improved Biocompatibility or Not. Int. J. Cardiol. 1996, 53, S81–S87. 10.1016/0167-5273(96)02611-3. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy M.; Freedman M. L. Complications of Anticoagulation with Heparin. AMA J. Ethics 2005, 7, 297–300. 10.1001/virtualmentor.2005.7.4.cprl1-0504. [DOI] [PubMed] [Google Scholar]
- Reynolds M. M.; Annich G. M. The Artificial Endothelium. Organogenesis 2011, 42–49. 10.4161/org.7.1.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Walker R.; Romero R.; Staver J. M.; Zang Y.; Reynolds M. M.; Popat K. C.; Kipper M. J. Glycocalyx-Inspired Nitric Oxide-Releasing Surfaces Reduce Platelet Adhesion and Activation on Titanium. ACS Biomater. Sci. Eng. 2017, 68. 10.1021/acsbiomaterials.6b00572. [DOI] [PubMed] [Google Scholar]
- Goh S. C.; Luan Y.; Wang X.; Du H.; Chau C.; Schellhorn H. E.; Brash J. L.; Chen H.; Fang Q. Polydopamine-Polyethylene Glycol-Albumin Antifouling Coatings on Multiple Substrates. J. Mater. Chem. B 2018, 6, 940–949. 10.1039/C7TB02636F. [DOI] [PubMed] [Google Scholar]
- Sommakia S.; Rickus J. L.; Otto K. J. In Effects of Adsorbed Proteins, an Antifouling Agent and Long-Duration DC Voltage Pulses on the Impedance of Silicon-Based Neural Microelectrodes, Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC 2009; IEEE Computer Society, 2009; pp 7139–7142. [DOI] [PubMed]
- Defraigne J. O.; Pincemail J.; Larbuisson R.; Blaffart F.; Limet R. Cytokine Release and Neutrophil Activation Are Not Prevented by Heparin- Coated Circuits and Aprotinin Administration. Ann. Thorac. Surg. 2000, 69, 1084–1091. 10.1016/S0003-4975(00)01093-6. [DOI] [PubMed] [Google Scholar]
- Sheppard J. I.; McClung W. G.; Feuerstein I. A. Adherent Platelet Morphology on Adsorbed Fibrinogen: Effects of Protein Incubation Time and Albumin Addition. J. Biomed. Mater. Res. 1994, 28, 1175–1186. 10.1002/jbm.820281008. [DOI] [PubMed] [Google Scholar]
- Rechendorff K.; Hovgaard M. B.; Foss M.; Zhdanov V. P.; Besenbacher F. Enhancement of Protein Adsorption Induced by Surface Roughness. Langmuir 2006, 22, 10885–10888. 10.1021/la0621923. [DOI] [PubMed] [Google Scholar]
- Akkas T.; Citak C.; Sirkecioglu A.; Güner F. S. Which Is More Effective for Protein Adsorption: Surface Roughness, Surface Wettability or Swelling? Case Study of Polyurethane Films Prepared from Castor Oil and Poly(Ethylene Glycol). Polym. Int. 2013, 62, 1202–1209. 10.1002/pi.4408. [DOI] [Google Scholar]
- Cai N.; Wong C. C.; Gong Y. X.; Tan S. C. W.; Chan V.; Liao K. Modulating Cell Adhesion Dynamics on Carbon Nanotube Monolayer Engineered with Extracellular Matrix Proteins. ACS Appl. Mater. Interfaces 2010, 2, 1038–1047. 10.1021/am9008117. [DOI] [PubMed] [Google Scholar]
- Khanmohammadi Chenab K.; Sohrabi B.; Rahmanzadeh A. Superhydrophobicity: Advanced Biological and Biomedical Applications. Biomater. Sci. 2019, 3110–3137. 10.1039/c9bm00558g. [DOI] [PubMed] [Google Scholar]
- Neto A. I.; Levkin P. A.; Mano J. F. Patterned Superhydrophobic Surfaces to Process and Characterize Biomaterials and 3D Cell Culture. Mater. Horiz. 2018, 379–393. 10.1039/c7mh00877e. [DOI] [Google Scholar]
- Falde E. J.; Yohe S. T.; Colson Y. L.; Grinstaff M. W. Superhydrophobic Materials for Biomedical Applications. Biomaterials 2016, 87. 10.1016/j.biomaterials.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner B. D.A Perspective on Titanium Biocompatibility. Titanium in Medicine. Engineering Materials; Springer: Berlin, Heidelberg, 2001; pp 1–12. [Google Scholar]
- Vishnu J.; Manivasagam V. K.; Gopal V.; Garcia C. B.; Hameed P.; Manivasagam G.; Webster T. J. Hydrothermal Treatment of Etched Titanium: A Potential Surface Nano-Modification Technique for Enhanced Biocompatibility. Nanomedicine 2019, 20, 102016 10.1016/j.nano.2019.102016. [DOI] [PubMed] [Google Scholar]
- Bartlet K.; Movafaghi S.; Kota A.; Popat K. C. Superhemophobic Titania Nanotube Array Surfaces for Blood Contacting Medical Devices. RSC Adv. 2017, 35466. 10.1039/c7ra03373g. [DOI] [Google Scholar]
- Greger M.; Widomská M.; Snášel V. In Structure and Properties of Dental Implants, Metal 2012, Conference Proceedings, 2012.
- Kasuga T.; Hiramatsu M.; Hoson A.; Sekino T.; Niihara K. Titania Nanotubes Prepared by Chemical Processing. Adv. Mater. 1999, 11, 1307–1311. . [DOI] [Google Scholar]
- Kulkarni M.; Mazare A.; Park J.; Gongadze E.; Killian M. S.; Kralj S.; von der Mark K.; Iglič A.; Schmuki P. Protein Interactions with Layers of TiO2 Nanotube and Nanopore Arrays: Morphology and Surface Charge Influence. Acta Biomater. 2016, 45, 357–366. 10.1016/j.actbio.2016.08.050. [DOI] [PubMed] [Google Scholar]
- Domanski M.; et al. Submicron-Patterning of Bulk Titanium by Nanoimprint Lithography and Reactive Ion Etching Related Content. Nanotechnology 2012, 065306 10.1088/0957-4484/23/6/065306. [DOI] [PubMed] [Google Scholar]
- Leszczak V.; Popat K. C. Improved in Vitro Blood Compatibility of Polycaprolactone Nanowire Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 15913–15924. 10.1021/am503508r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinow B. E.; Ding Y. S.; Qin C.; McHalsky M. L.; Schneider J. H.; Ashline K. A.; Shelbourn T. L.; Albrecht R. M. Biomaterials with Permanent Hydrophilic Surfaces and Low Protein Adsorption Properties. J. Biomater. Sci., Polym. Ed. 1995, 6, 91–109. 10.1163/156856295X00788. [DOI] [PubMed] [Google Scholar]
- Seyfert U. T.; Biehl V.; Schenk J. In Vitro Hemocompatibility Testing of Biomaterials According to the ISO 10993-4. Biomol. Eng. 2002, 19, 91–96. 10.1016/S1389-0344(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Tanzi M. C. Bioactive Technologies for Hemocompatibility. Expert Rev. Med. Devices 2005, 473–492. 10.1586/17434440.2.4.473. [DOI] [PubMed] [Google Scholar]
- Werner C.; Maitz M. F.; Sperling C. Current Strategies towards Hemocompatible Coatings. J. Mater. Chem. 2007, 17, 3376–3384. 10.1039/b703416b. [DOI] [Google Scholar]
- Fischer M.; Maitz M. F.; Werner C.. Coatings for Biomaterials to Improve Hemocompatibility. Hemocompatibility of Biomaterials for Clinical Applications: Blood–Biomaterials Interactions; Elsevier Inc., 2018; pp 163–190. [Google Scholar]
- Huang N.; Yang P.; Leng Y. X.; Chen J. Y.; Sun H.; Wang J.; Wang G. J.; Ding P. D.; Xi T. F.; Leng Y. Hemocompatibility of Titanium Oxide Films. Biomaterials 2003, 24, 2177–2187. 10.1016/S0142-9612(03)00046-2. [DOI] [PubMed] [Google Scholar]
- Mentus S.; Pjescic J.; Blagojevic N. Investigation of Titanium Corrosion in Concentrated NaOH Solutions. Mater. Corros. 2002, 53, 44–50. . [DOI] [Google Scholar]
- Guo Z.; Jiang N.; Chen C.; Zhu S.; Zhang L.; Li Y. Surface Bioactivation through the Nanostructured Layer on Titanium Modified by Facile HPT Treatment. Sci. Rep. 2017, 7, 4155 10.1038/s41598-017-04395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbaix M.Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, 1974; pp 214–218. [Google Scholar]
- Wang P.; Yi X.; Lu Y.; Yu H.; Yu J. In-Situ Synthesis of Amorphous H2TiO3-Modified TiO2 and Its Improved Photocatalytic H2-Evolution Performance. J. Colloid Interface Sci. 2018, 532, 272–279. 10.1016/j.jcis.2018.07.139. [DOI] [PubMed] [Google Scholar]
- Giacomello A.; Meloni S.; Chinappi M.; Casciola C. M. Cassie-Baxter and Wenzel States on a Nanostructured Surface: Phase Diagram, Metastabilities, and Transition Mechanism by Atomistic Free Energy Calculations. Langmuir 2012, 28, 10764–10772. 10.1021/la3018453. [DOI] [PubMed] [Google Scholar]
- Pereira M. M.; Kurnia K. A.; Sousa F. L.; Silva N. J. O.; Lopes-Da-Silva J. A.; Coutinho J. A. P.; Freire M. G. Contact Angles and Wettability of Ionic Liquids on Polar and Non-Polar Surfaces. Phys. Chem. Chem. Phys. 2015, 17, 31653–31661. 10.1039/C5CP05873B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell S. G.; Cole B. J.; Sundman E. A.; Karas V.; Fortier L. A. Platelet-Rich Plasma: A Milieu of Bioactive Factors. Arthrosc.: J. Arthrosc. Relat. Surg. 2012, 28, 429–439. 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Carter A. M.; Standeven K. F.; Grant P. J.. Common Genetic Determinants of Coagulation and Fibrinolysis. Emery and Rimoin’s Principles and Practice of Medical Genetics; Elsevier Ltd., 2013; pp 1–20. [Google Scholar]
- Pirofsky B. The Determination of Blood Viscosity in Man by a Method Based on Poiseuille’s law. J. Clin. Invest. 1953, 292–298. 10.1172/JCI102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrnčíř E.; Rosina1 J. Surface Tension of Blood. Physiol. Res. 1997, 46, 319. [PubMed] [Google Scholar]
- Vargaftik N. B.; Volkov B. N.; Voljak L. D. International Tables of the Surface Tension of Water. J. Phys. Chem. Ref. Data 1983, 12, 817. 10.1063/1.555688. [DOI] [Google Scholar]
- Krishnan A.; Wilson A.; Sturgeon J.; Siedlecki C. A.; Vogler E. A. Liquid-Vapor Interfacial Tension of Blood Plasma, Serum and Purified Protein Constituents Thereof. Biomaterials 2005, 26, 3445–3453. 10.1016/j.biomaterials.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Giacomello A.; Meloni S.; Chinappi M.; Casciola C. M. Cassie-Baxter and Wenzel States on a Nanostructured Surface: Phase Diagram, Metastabilities, and Transition Mechanism by Atomistic Free Energy Calculations. Langmuir 2012, 28, 10764–10772. 10.1021/la3018453. [DOI] [PubMed] [Google Scholar]
- Michiardi A.; Aparicio C.; Ratner B. D.; Planell J. A.; Gil J. The Influence of Surface Energy on Competitive Protein Adsorption on Oxidized NiTi Surfaces. Biomaterials 2007, 28, 586–594. 10.1016/j.biomaterials.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Wahlgren M.; Arnebrant T. Protein Adsorption to Solid Surfaces. Trends Biotechnol. 1991, 9, 201–208. 10.1016/0167-7799(91)90064-O. [DOI] [PubMed] [Google Scholar]
- Jansen B.; Ellinghorst G. Modification of Polyetherurethane for Biomedical Application by Radiation Induced Grafting. II. Water Sorption, Surface Properties, and Protein Adsorption of Grafted Films. J. Biomed. Mater. Res. 1984, 18, 655–669. 10.1002/jbm.820180607. [DOI] [PubMed] [Google Scholar]
- Matsuda M.; Sugo T. Structure and Function of Human Fibrinogen Inferred from Dysfibrinogens. Int. J. Hematol. 2002, 352–360. 10.1007/BF03165284. [DOI] [PubMed] [Google Scholar]
- Blombäck B.; Hessel B.; Hogg D.; Therkildsen L. A Two-Step Fibrinogen–Fibrin Transition in Blood Coagulation. Nature 1978, 275, 501–505. 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Mulvihill J. N.; Faradji A.; Oberling F.; Cazenave J.-P. Surface Passivation by Human Albumin of Plasmaperesis Circuits Reduces Platelet Accumulation and Thrombus Formation. Experimental and Clinical Studies. J. Biomed. Mater. Res. 1990, 24, 155–163. 10.1002/jbm.820240203. [DOI] [PubMed] [Google Scholar]
- Sweryda-Krawiec B.; Devaraj H.; Jacob G.; Hickman J. J. A New Interpretation of Serum Albumin Surface Passivation. Langmuir 2004, 20, 2054–2056. 10.1021/la034870g. [DOI] [PubMed] [Google Scholar]
- Abildgaard U. Purification of Two Progressive Antithrombins of Human Plasma. Scand. J. Clin. Lab. Invest. 1967, 19, 190–195. 10.3109/00365516709093501. [DOI] [Google Scholar]
- Niemi T. T.; Suojaranta-Ylinen R. T.; Kukkonen S. I.; Kuitunen A. H. Gelatin and Hydroxyethyl Starch, but Not Albumin, Impair Hemostasis After Cardiac Surgery. Anesth. Analg. 2006, 102, 998–1006. 10.1213/01.ane.0000200285.20510.b6. [DOI] [PubMed] [Google Scholar]
- Vogler E. A. Protein Adsorption in Three Dimensions. Biomaterials 2012, 33, 1201–1237. 10.1016/j.biomaterials.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. J.; Clegg R. E.; Leavesley D. I.; Pearcy M. J. Mediation of Biomaterial-Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 1–18. 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- Korzeniewski C.; Callewaert D. M. An Enzyme-Release Assay for Natural Cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Legrand C.; Bour J. M.; Jacob C.; Capiaumont J.; Martial A.; Marc A.; Wudtke M.; Kretzmer G.; Demangel C.; Duval D.; et al. Lactate Dehydrogenase (LDH) Activity of the Number of Dead Cells in the Medium of Cultured Eukaryotic Cells as Marker. J. Biotechnol. 1992, 25, 231–243. 10.1016/0168-1656(92)90158-6. [DOI] [PubMed] [Google Scholar]
- Kunzmann A.; Andersson B.; Thurnherr T.; Krug H.; Scheynius A.; Fadeel B. Toxicology of Engineered Nanomaterials: Focus on Biocompatibility, Biodistribution and Biodegradation. Biochim. Biophys. Acta, Gen. Subj. 2011, 361–373. 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Zan X.; Fang Z.; Wu J.; Xiao F.; Huo F.; Duan H. Freestanding Graphene Paper Decorated with 2D-Assembly of Au@Pt Nanoparticles as Flexible Biosensors to Monitor Live Cell Secretion of Nitric Oxide. Biosens. Bioelectron. 2013, 49, 71–78. 10.1016/j.bios.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Periayah M. H.; Halim A. S.; Saad A. Z. M. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol.—Oncol. Stem Cell Res. 2017, 319–327. [PMC free article] [PubMed] [Google Scholar]
- Elstad M. R.; McIntyre T. M.; Prescott S. M.; Zimmerman G. A. The Interaction of Leukocytes with Platelets in Blood Coagulation. Curr. Opin. Hematol. 1995, 2, 47–54. 10.1097/00062752-199502010-00007. [DOI] [PubMed] [Google Scholar]
- Chen H.; Song W.; Zhou F.; Wu Z.; Huang H.; Zhang J.; Lin Q.; Yang B. The Effect of Surface Microtopography of Poly(Dimethylsiloxane) on Protein Adsorption, Platelet and Cell Adhesion. Colloids Surf., B 2009, 71, 275–281. 10.1016/j.colsurfb.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Ferraz N.; Ott M. K.; Hong J. Time Sequence of Blood Activation by Nanoporous Alumina: Studies on Platelets and Complement System. Microsc. Res. Tech. 2010, 73, 1101–1109. 10.1002/jemt.20854. [DOI] [PubMed] [Google Scholar]
- Asthana A.; White C. M.; Douglass M.; Kisaalita W. S. Evaluation of Cellular Adhesion and Organization in Different Microporous Polymeric Scaffolds. Biotechnol. Prog. 2018, 34, 505–514. 10.1002/btpr.2627. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Adhesive Interactions of Leukocytes, Platelets, and the Vessel Wall during Hemostasis and Inflammation. Thromb. Haemostasis 2001, 746–756. 10.1055/s-0037-1616128. [DOI] [PubMed] [Google Scholar]
- Ogura H.; Kawasaki T.; Tanaka H.; Koh T.; Tanaka R.; Ozeki Y.; Hosotsubo H.; Kuwagata Y.; Shimazu T.; Sugimoto H. Activated Platelets Enhance Microparticle Formation and Platelet-Leukocyte Interaction in Severe Trauma and Sepsis. J. Trauma: Inj., Infect., Crit. Care 2001, 50, 801–809. 10.1097/00005373-200105000-00005. [DOI] [PubMed] [Google Scholar]
- Cerletti C.; Tamburrelli C.; Izzi B.; Gianfagna F.; De Gaetano G. Platelet-Leukocyte Interactions in Thrombosis. Thromb. Res. 2012, 263–266. 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Annamalai M.; Gopinadhan K.; Han S. A.; Saha S.; Park H. J.; Cho E. B.; Kumar B.; Patra A.; Kim S.-W.; Venkatesan T. Surface Energy and Wettability of van Der Waals Structures. Nanoscale 2016, 8, 5764–5770. 10.1039/C5NR06705G. [DOI] [PubMed] [Google Scholar]
- Ogawa M.; Matsuda Y.; Kobayashi A.; Mitani M.; Makino Y.; Matsui H. Plasma Antithrombin Levels Correlate with Albumin and Total Protein in Gestational Hypertension and Preeclampsia. Pregnancy Hypertens. 2014, 4, 174–177. 10.1016/j.preghy.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Damodaran V. B.; Leszczak V.; Wold K. A.; Lantvit S. M.; Popat K. C.; Reynolds M. M. Antithrombogenic Properties of a Nitric Oxide-Releasing Dextran Derivative: Evaluation of Platelet Activation and Whole Blood Clotting Kinetics. RSC Adv. 2013, 3, 24406–24414. 10.1039/c3ra45521a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczak V.; Smith B. S.; Popat K. C. Hemocompatibility of Polymeric Nanostructured Surfaces. J. Biomater. Sci., Polym. Ed. 2013, 24, 1529–1548. 10.1080/09205063.2013.777228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino R.; Popat K. Evaluating Whole Blood Clotting in Vitro on Biomaterial Surfaces. Bio-Protoc. 2020, 10, e3505 10.21769/BioProtoc.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]