Abstract
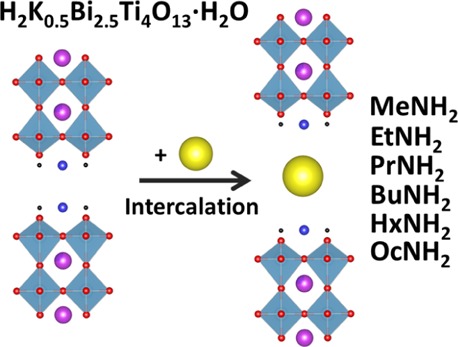
New organic–inorganic hybrids have been synthesized by the intercalation of n-alkylamines (methylamine, ethylamine, n-propylamine, n-butylamine, n-hexylamine, and n-octylamine) into the structure of the protonated and hydrated form of the perovskite-like layered titanate H2K0.5Bi2.5Ti4O13·H2O (HKBT4·H2O). The possibility of the synthesis of the hybrid materials was studied in a wide range of conditions. It was found that interlayer water plays a crucial role in the formation of intercalated hybrids. The obtained compounds were characterized with powder X-ray diffraction analysis; Raman, IR, and NMR spectroscopies; thermogravimetry (TG), TG coupled with mass spectrometry, and CHN analyses; and scanning electron microscopy. It was suggested that the intercalated n-alkylamines exist in the form of alkylammonium ions forming a paraffin-like bilayer with an average tilting angle of ∼77.5°. The obtained HKBT4×RNH2 compounds contain 0.4–0.7 n-alkylamine molecules per formula unit as well as the varied amount of intercalated water. By gentle heating, they can be obtained as dehydrated forms, which are thermally stable up to 250 °C.
1. Introduction
Layered perovskite-like oxides are mixed oxide ceramics formed by perovskite slabs interleaved with cations and structural units. Perovskites are widely studied as perspective materials for a wide range of applications because of their physical and chemical properties such as superconductivity,1,2 colossal magnetoresistance,3 and catalytic and photocatalytic activity.4−7 Among them, bismuth-containing structures draw special attention. They are considered as a lead-free ferroelectric8−10 and multiferroic materials.11 They may perform as effective photoanode materials for photoelectrochemical water splitting,12 and Bi3+/Mn4+ codoping has been recently applied as an effective approach for the preparation of red-emitting phosphors for white light-emitting diodes.13,14
One of the unique features of layered compounds is the possibility of the introduction of molecules into the interlayer space.15 In the case of inorganic layered compounds, the most interesting process is the intercalation of organic molecules into the inorganic matrix, resulting in new organic–inorganic hybrid materials.16−20 Such compounds combine physicochemical properties that are a superposition of the original inorganic compound and the introduced organic matter. According to the type of chemical bond between inorganic and organic parts, there are two main ways of formation hybrids with layered compounds: intercalation and grafting.21 Intercalation is a reversible noncovalent introduction of organic molecules into the interlayer space proceeding usually by the acid–base or ion-exchange mechanism.15,22,23 Covalent inorganic–organic hybrids may be produced by grafting reactions—intercalation of appropriate organic substances accompanied by the formation of covalent bonds.24−26 Recently, a large number of works have been devoted to the study of metal halide inorganic–organic layered perovskite hybrids exhibiting interesting optoelectrical properties,27,28 while much less attention has been paid to hybrids based on layered perovskite-like oxides.
Ion-exchangeable layered perovskite-like oxides are solid crystalline substances with a block-type structure in which perovskite slabs with a thickness of n-octahedra BO6 (B = Ti, Nb, Ta, etc.) alternate with the so-called interlayer spaces containing alkali cations.29 Such compounds have the ability to replace the interlayer cations of alkali metals by ion exchange with other cations, in particular, cations of other metals, complex cationic units, or protons, to obtain the so-called protonated forms. One of the significant features of protonated forms of perovskite-like oxides is their acidic properties, which allows the intercalation of organic bases into their interlayer space forming organic–inorganic hybrids. Such compounds can be interesting both on their own because of their physicochemical properties, such as photocatalytic activity,30−32 and can be the basis for the creation of new materials by liquid-phase exfoliation.7,33,34
Among ion-exchangeable layered perovskites, the most widely studied compounds amenable to intercalation of organic molecules are protonated derivatives of Dion–Jacobson (DJ), Ruddlesden–Popper (RP), and Aurivillius phases.35 The most thoroughly studied among others in terms of intercalation and grafting of organic molecules are HLaNb2O7,18,20,36−39 HCa2Nb3O10,24,40 H2CaTa2O7,39,41 and H2La2Ti3O1042 compounds. Although the number of obtained protonated forms of layered perovskite-like oxides is limited, new such compounds are synthesized regularly.
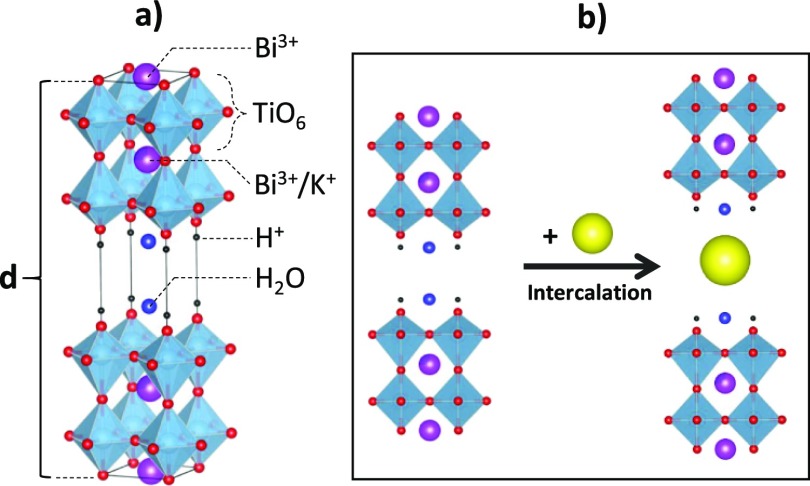
Recently, Liu et al.43 have synthesized a new n = 4 layered RP phase K2.5Bi2.5Ti4O13 (KBT4) with mixed K/Bi co-occupancy on the perovskite A site showing stoichiometric hydration and amenable to form the protonated compound H2K0.5Bi2.5Ti4O13·yH2O44,45 by substitution of K+ by H+. The H2K0.5Bi2.5Ti4O13·H2O (HKBT4·H2O) structure can be described as an alternation of perovskite slabs (K0.5Bi2.5Ti4O13) with interlayer protons and water molecules (Figure 1a).
Figure 1.
Schematic representation of HKBT4·H2O structure (a) and intercalation process (b). The d-value represents the basal spacing (interlayer distance along the c-axis).
This work presents a complex study on the behavior of protonated and hydrated forms of H2K0.5Bi2.5Ti4O13·yH2O obtained from K2.5Bi2.5Ti4O13 toward the intercalation (Figure 1b) of n-alkylamines with different numbers of carbon atoms (nc) in alkyl chain, namely, methylamine, ethylamine, n-propylamine, n-butylamine, n-hexylamine, and n-octylamine, and the characterization of obtained hybrids in terms of their composition, structure, and thermal stability.
The previously studied hosts belonging to the RP phases (e.g., H2La2Ti3O10,42 H2CaTa2O7,41 and HLnTiO446) and DJ phases (HCa2Nb3O1040 and HLaNb2O739) are known to intercalate n-amines in solvothermal or microwave-assisted solvothermal conditions. In this work, various approaches have been considered in order to determine the optimal experimental conditions of amine intercalation into the interlayer space of H2K0.5Bi2.5Ti4O13·yH2O.
There are two main factors that are reported to strongly affect the intercalation reactivity of protonated forms obtained from layered perovskites. The first one is the Brønsted acidity of interlayer protons, which is associated with the interlayer charge densities and depends on the nature of B-site and A-site cations. In addition, the second one is the sterical accessibility of the interlayer space,47 which could be theoretically “tailored” by the preparation of various hydrated forms. As is already known, some phases show poor or no reactivity in intercalation reactions compared to other ones,39,48,49 which is assumed to be the result of their irreversible dehydration. Considering the possibility of a strong influence of the interlayer water on the occurrence of amine intercalation reactions, both the as-prepared hydrated phase (H2K0.5Bi2.5Ti4O13·H2O) and partly and fully dehydrated phases H2K0.5Bi2.5Ti4O13·0.5H2O and H2K0.5Bi2.5Ti4O13 have been used for the experiments.
2. Results and Discussion
2.1. Synthesis
2.1.1. Protonation
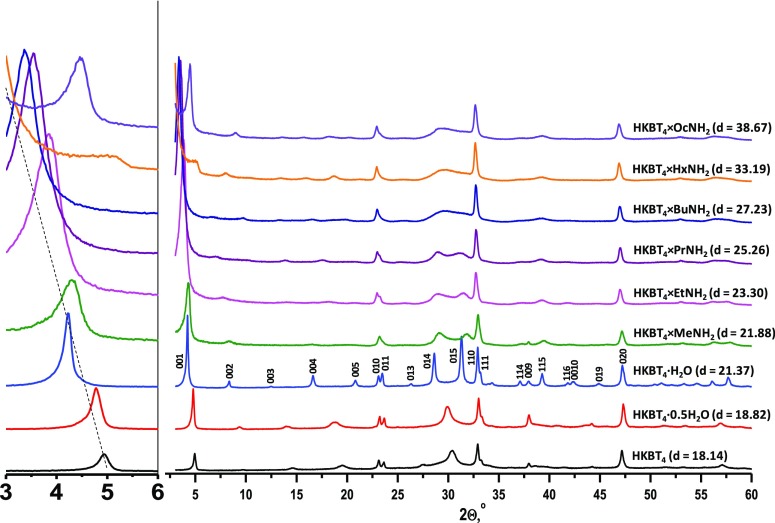
The protonated hydrated compound H2K0.5Bi2.5Ti4O13·H2O (HKBT4·H2O) was successfully prepared from K2.5Bi2.5Ti4O13. The amount of intercalated water and the substitution degree of the as-prepared protonated form calculated from thermogravimetry (TG) data, energy-dispersive X-ray, and inductively coupled plasma (ICP) elemental analyses confirm the complete substitution of interlayer K+ by H+, and the amount of intercalated water was found to be ∼1 H2O molecule per formula unit. Partially (HKBT4·0.5H2O) and fully (HKBT4) dehydrated forms were prepared by subsequent heating of HKBT4·H2O. Figure 2 shows X-ray diffraction (XRD) data for the as-prepared HKBT4·H2O sample and dehydrated samples obtained by heat treatment at 90 °C for HKBT4·0.5H2O and 160 °C for HKBT4. Their XRD patterns and lattice parameters are in accordance with previously reported data.45
Figure 2.
XRD patterns of hydrated and dehydrated forms of HKBT4 and obtained n-alkylamine intercalated compounds HKBT4×RNH2 together with calculated interlayer distances.
2.1.2. Intercalation of Amines
A series of experiments of amine intercalation with varying conditions have been first carried out for three amines (methylamine, n-butylamine, and n-octylamine) (Table 3). Then, the optimal conditions with respect to the phase purity of the obtained samples, amine loading, and reaction time have been chosen and applied for the remaining three amines (ethylamine, n-propylamine, and n-hexylamine).
Table 3. Reaction Conditions Examined for the Preparation of Organic–Inorganic Hybrids.
| precursor | reaction medium | temperature, °C | duration |
|---|---|---|---|
| Intercalation of Methylamine | |||
| HKBT4·H2O | 38% solution in water | 20 | 24 h–2 weeks |
| 60 | 24 h–1 week | ||
| HKBT4·0.5H2O | 38% solution in water | 20 | 24 h–1 week |
| 60, 80 | |||
| HKBT4 | 38% solution in water | 20 | 24 h–1 week |
| 60, 80 | |||
| Intercalation of Ethylamine | |||
| HKBT4·H2O | 50% solution in water | 60 | 24 h |
| Intercalation of n-Propylamine | |||
| HKBT4·H2O | 50% solution in water | 60 | 24 h |
| Intercalation of n-Butylamine | |||
| HKBT4·H2O | 10, 50, 90% solution in water, 100% n-butylamine | 20, 60 | 24 h–1 week |
| 50% solution in water | 60 | 24–72 h | |
| HKBT4·0.5H2O | 50% solution in water | 20 | 24 h–1 week |
| 60, 80 | |||
| HKBT4 | 50% solution in water | 20 | 24 h–1 week |
| 60, 80 | |||
| Intercalation of n-Hexylamine | |||
| HKBT4·H2O | 50% solution in n-heptane | 80 | 24 h |
| Intercalation of n-Octylamine | |||
| HKBT4·H2O | 100%; 50% solution in n-heptane | 20, 60 | 24 h–1 week |
| 50% solution in n-heptane | 80 | 24 h–1 week | |
In the case of methylamine, the phase pure samples could be obtained in all tested conditions; however, intercalation does not lead to any significant changes in the interlayer space. TG measurements have been employed in order to estimate the amount of intercalated organic molecules in different conditions. It was found that methylamine is intercalated into HKBT4·H2O at 25 °C after 24 h of stirring, but the highest mass loss is achieved after 14 days of stirring. Similar mass losses could be reached, though, using heating at 60 °C for 24 h in order to optimize the reaction time, so the sample obtained in these conditions was used for further characterization.
In the case of n-butylamine, the intercalation leads to a significant change in the XRD pattern of the sample corresponding to the interlayer distance change. Reactions with concentrated n-butylamine (90–100%) may cause the formation of a side product surely resulted by dehydration of the initial form, while using 10 and 50% water solutions both leads to the formation of single-phase products. However, using 10% concentrated n-butylamine requires longer reaction time compared to 50% solutions. Unlike methylamine, the maximum amount of n-butylamine is readily intercalated into HKBT4·H2O by facile stirring at 25 °C in the shortest reaction time tested (24 h) using 50% water solution. The sample obtained at these reaction conditions has been used for further characterization.
In the case of n-octylamine, no water was added into suspension because of its low solubility in water. The attempts of direct intercalation of pure n-octylamine into hydrated HKBT4·H2O at room temperature have led to its dehydration. Considering the experiments with intercalation of n-butylamine into dehydrated forms (described later), it was assumed that once dehydrated in the presence of n-octylamine, HKBT4·H2O would not be able to intercalate it. The experiments with the n-octylamine solution in heptane showed that the single-phase sample could be obtained after 24 h of treatment at 80 °C. Additionally, longer reaction time (1 week) leads to the formation of the same phase with similar amine loading.
2.2. Characterization
The detailed characterization of organic–inorganic hybrids was carried out for six samples obtained by optimized methods. Namely, methylamine, ethylamine, and n-propylamine derivatives were obtained at 60 °C within 24 h. The n-butylamine derivative was prepared by facile suspending and stirring the as-prepared hydrated HKBT4·H2O powder in 50% water solution of n-butylamine at 25 °C for 24 h. Finally, hybrids with n-hexylamine and n-octylamine were prepared by 24 h treatment with 50% solution in n-heptane at 80 °C.
2.2.1. Powder XRD Analysis
As displayed in Figure 2, the XRD patterns show that because of the small molecular size, the intercalation of methylamine does not strongly affect the lattice parameters of the compound in respect to HKBT4·H2O, and the slight changes obtained in the XRD patterns could be associated with the intercalation of methylamine or possible formation of different hydrated HKBT4 compounds, so such changes cannot prove intercalation of methylamine without other characterization methods. However, in the case of nc = 2–8 amines, the XRD patterns clearly indicate major structural changes that could be associated with the intercalation of big molecules into interlayer space.
Despite the fact that the obtained organic–inorganic samples show low crystallinity, the obtained n-alkylamine intercalated compounds could be indexed based on the index of the starting HKBT4·H2O compound, which can be well indexed in the primitive tetragonal cell with lattice parameters a, b = 3.85 Å and c = 18.14 Å (although a slightly better XRD pattern fit can be obtained using an orthorhombic C222 group or a P4 space group with a doubled c lattice parameter45). After the reaction of HKBT4·H2O with n-alkylamines, the (00l) reflection shifts to a lower 2-theta value, indicating the increase in interlayer distance and shows a gradual increase as larger amines are intercalated; the calculated interlayer distances are presented in Figure 2. Widening and low intensities of observed reflections are typical for such intercalated compounds and could be explained by the weakening of bonding between layers resulting in possible turbostratic stacking and interlayer distance deviations.15,22 However, the preservation of sharp, intense reflections with indexes 010, 110, and 020 for the starting protonated form (without the contribution of c in hkl) indicates no significant structural changes in the perovskite slabs.
2.2.2. IR and Raman Spectroscopy
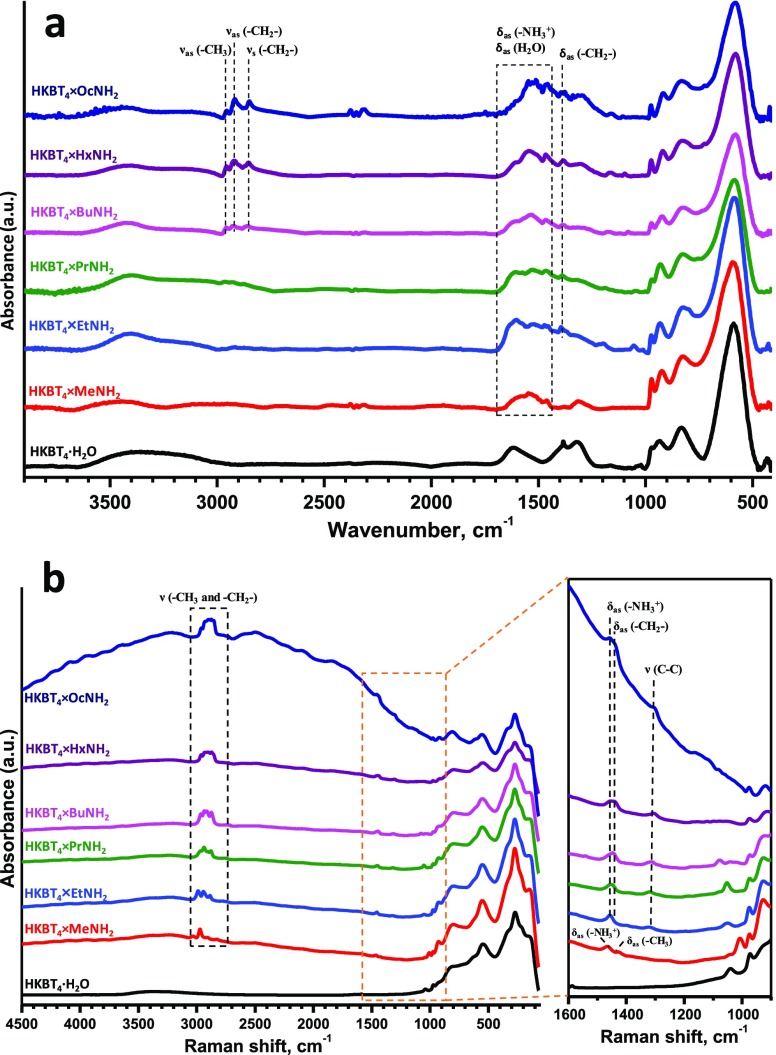
The Fourier transform infrared (FT-IR) and Raman spectra of the protonated hydrated form and organic–inorganic hybrids are shown in Figure 3. The appearance of characteristic bands at 1400–1600 and 2800–3000 cm–1 corresponding to NH3+ and C–H vibrations confirms the formation of organic–inorganic hybrids in all of the cases (including methylamine). In particular, the bands at the range 2800–3000 cm–1 are attributed to CH2 symmetric stretching νs (−CH2−), and the lower intensity bands at 1400–1550 cm–1 correspond to CH2 asymmetric bending δas (−CH2−) and NH3+ asymmetric bending δas (−NH3+).50 The presence of NH3+ bands (and also the absence of characteristic to NH2 group νs and νas bands in the region 3500–3300 cm–1) implies the acid–base mechanism of amine intercalation into this protonated form, leading to the formation of cationic forms of amines in the interlayer space. In the IR spectra of HKBT4×RNH2 derivatives, where R = Bu, Hx, and Oc, two major bands in the C–H stretching region were clearly exhibited, namely, the νas (−CH2−) (∼2918 cm–1) and the νs (−CH2−) (∼2854 cm–1) of CH2 groups and also νas (−CH3) (∼2956 cm–1) are visible. Because the frequency and width of νas (−CH2−) are sensitive to the gauche/trans conformer ratio and the packing density of methylene chains, the appearance of sharp νas (−CH2−) band and its frequency points to the all-trans conformation of n-alkyl chains in the interlayer space.51 The broad band occurring in the 3100–3500 cm–1 range is produced by O–H stretching vibration, and the broad band near 1600 cm–1 is due to the bending vibration of H2O molecule, suggesting the existence of interlayer water in the samples.
Figure 3.
FT-IR (a) and Raman (b) spectra of obtained n-alkylamine intercalated compounds HKBT4×RNH2 in comparison with the initial HKBT4·H2O compound.
It should also be noted that the Raman spectrum of the n-octylamine intercalated sample shows a significantly enhanced and reproducible fluorescence compared to other samples at measurement conditions used. This motivated us to additionally examine the optical properties of the samples. As shown in Table 1 containing optical band gaps (Eg) of the samples, calculated from their diffuse reflectance spectra (Figure S1), intercalation of amines does not significantly affect the Eg and the corresponding maximum wavelengths λmax of absorbed light. There is only a slight decrease in Eg values and a slight increase in the wavelengths λmax of absorbed light for the hybrids in comparison with the protonated form, and in general, spectra do not differ from each other, which indicates that the enhanced fluorescence of HKBT4×OcNH2 is not related to the optical properties of the sample and should be explained by other reasons.
Table 1. Absorption Characteristics of Initial Protonated Form and Inorganic–Organic Hybrids.
| sample | Eg, eV | λmax, nm | sample | Eg, eV | λmax, nm |
|---|---|---|---|---|---|
| HKBT4·H2O | 3.29 | 377 | HKBT4×BuNH2 | 3.17 | 391 |
| HKBT4×MeN2 | 3.17 | 391 | HKBT4×HxNH2 | 3.15 | 394 |
| HKBT4×EtNH2 | 3.19 | 389 | HKBT4×OcNH2 | 3.15 | 394 |
| HKBT4×PrNH2 | 3.19 | 389 |
2.2.3. 13C NMR-MAS Spectroscopy
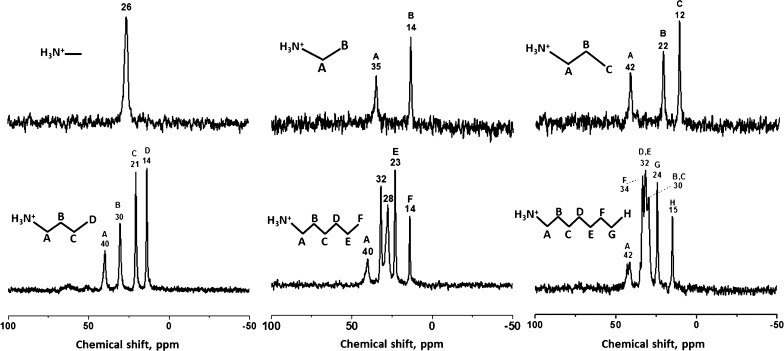
The solid-state 13C MAS-NMR spectra of six hybrids are shown in Figure 4. The characteristic peak at 26 ppm for the methylamine-intercalated compound is shifted upfield compared to the raw methylamine (28 ppm), which indicates that methylamine is presented in the cationic form and the interaction between the inorganic matrix and methylamine takes place.52 A similar shift of (A) and (B) carbon peak position is also observed for ethylamine (36.7 → 35, 18.8 → 14), n-propylamine (44.4 → 42, 27.3 → 22), n-butylamine (41.9 → 40, 36 → 30), n-hexylamine (42.4 → 40, 34.9 → 32), and n-octylamine (42.35 → 42, 34 → 30), which is in good agreement with the13 C NMR spectra of the corresponding alkylammonium salts. In the case of n-butylamine hybrid, four signals could be attributed to four carbon atoms in different environments NH3+–CH2(A)–CH2(B)–CH2(C)–CH3(D) at 40(A), 30(B), 21(C), and 14(D) ppm. These results correlate well with previously presented results for n-butylamine-intercalated RP titanate H2La2Ti3O1042 and Aurivillius-based tantalate H2Bi0.1Sr0.85Ta2O7.53 The spectrum of n-octylamine hybrid shows a multiplet in the range 34–30 ppm, which could be assigned to methylene units in the middle of the carbon chain [B,C (30); D,E (32); and F (34)]. Single peaks at 15 and 24 ppm account to methyl (−CH3 (H)) and methylene (−CH2 (G)) groups. The signal at ∼42 ppm corresponds to the CH2 group in A position with respect to the—NH3+ group.54 The splitting of this signal may be due to the presence of some part of n-octylamine in both cationic and neutral forms as well as because of different alkylammonium cation surroundings. It should be underlined that the MAS-NMR results confirm the assumption of the acid–base mechanism of the intercalation reaction, showing that amines are mostly presented in the interlayer space in their cationic forms which correlate well with the results of Raman and IR spectroscopies.
Figure 4.
13C MAS-NMR spectroscopy for the obtained n-alkylamine intercalated compounds HKBT4×RNH2.
2.2.4. TG and CHN Analysis
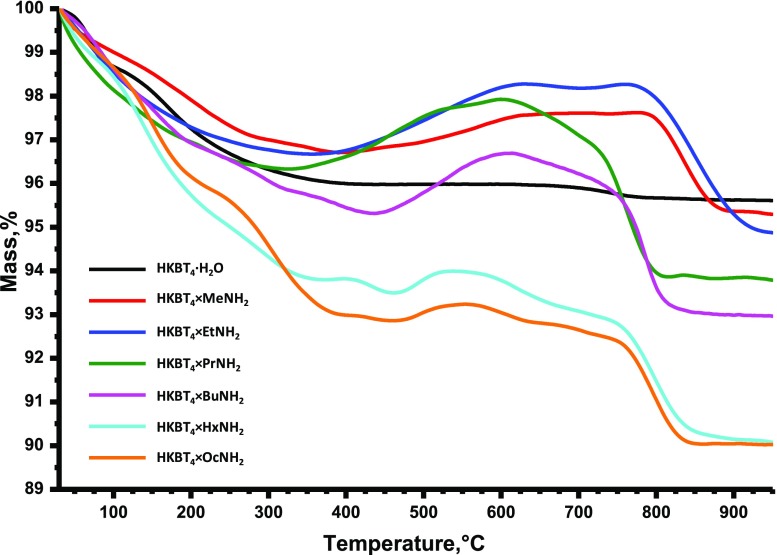
TG analysis additionally confirms the intercalation of n-amines, as the total mass loss for all of the samples is higher than that for an initial protonated form (Figure 5). The total mass losses and compositions estimated using CHN and TG analyses are presented in Table 2. The amounts of amines intercalated in the compound were estimated from the carbon and nitrogen content obtained by CHN analysis, and the residual water content was calculated from TG analysis. As can be seen, the calculated compositions correspond to only 20–35% of the theoretical cation-exchange capacity (interlayer proton/amine ratio = 1:1), which is lower than for other reported n-alkylamine derivatives of RP titanates.42,55 The absence of interlayer water in the octylamine sample, according to the calculation data, may explain the luminescence observed in this case during the measurements of Raman spectra (Figure 3).
Figure 5.
TG curves of the obtained n-alkylamine intercalated compounds HKBT4×RNH2 in the air atmosphere in comparison with the initial HKBT4·H2O compound.
Table 2. Experimental and Calculated Composition Data of the Obtained Intercalated Compounds.
| experimental |
calculated |
||||||
|---|---|---|---|---|---|---|---|
| sample | Δm, % | C, % | N, % | C/N | estimated composition | C, % | N, % |
| HKBT4×MeNH2 | 4.7 | 0.6 | 0.85 | 0.8:1 | H2K0.5Bi2.5Ti4O13·0.6MeNH2·0.6H2O | 0.75 | 0.85 |
| HKBT4×EtNH2 | 5.1 | 0.9 | 0.55 | 2.0:1 | H2K0.5Bi2.5Ti4O13·0.4EtNH2·0.9H2O | 1.0 | 0.55 |
| HKBT4×PrNH2 | 6.3 | 1.5 | 0.6 | 2.9:1 | H2K0.5Bi2.5Ti4O13·0.4PrNH2·1.1H2O | 1.45 | 0.6 |
| HKBT4×BuNH2 | 7.1 | 2.6 | 0.7 | 4.4:1 | H2K0.5Bi2.5Ti4O13·0.5BuNH2·0.9H2O | 2.4 | 0.7 |
| HKBT4×HxNH2 | 9.9 | 4.7 | 0.7 | 7.4:1 | H2K0.5Bi2.5Ti4O13·0.5HxNH2·1.0H2O* | 3.55 | 0.7 |
| HKBT4×OcNH2 | 10.0 | 6.5 | 0.95 | 8.3:1 | H2K0.5Bi2.5Ti4O13·0.7OcNH2 | 6.5 | 0.95 |
Relatively high carbon content can be explained by the presence of residual solvent (approximately 0.1 molecules of n-heptane per formula unit).
In the case of all obtained hybrids, the thermal decomposition process is complex and includes three main stages. The first mass loss stage at temperature up to 350–400 °C most probably associated with the deintercalation and vaporization of water and organic molecules, as well as the decomposition of the protonated form itself. The samples heated up to 350–400 °C change their color from white to yellowish-brown probably because of the reduction of partial inorganic part by intercalated amines. At the second stage, a significant mass gain occurs apparently associated with oxidation processes and can be explained by the oxidation of the remaining organic part or as oxidation of reduced inorganic part (associated most probably with Ti4+/Ti3+ or Bi3+/Bi0 redox transition). At the third stage, the final mass loss can be associated with decomposition of the remaining oxidized organic compounds and/or with their “burning” in an oxidative atmosphere. Despite the fact that the difference in the behavior of the samples during thermal decomposition is obvious, there is no single explanation for this based only on the composition data or synthetic conditions of the samples.
2.2.5. TG Coupled with Mass Spectrometry
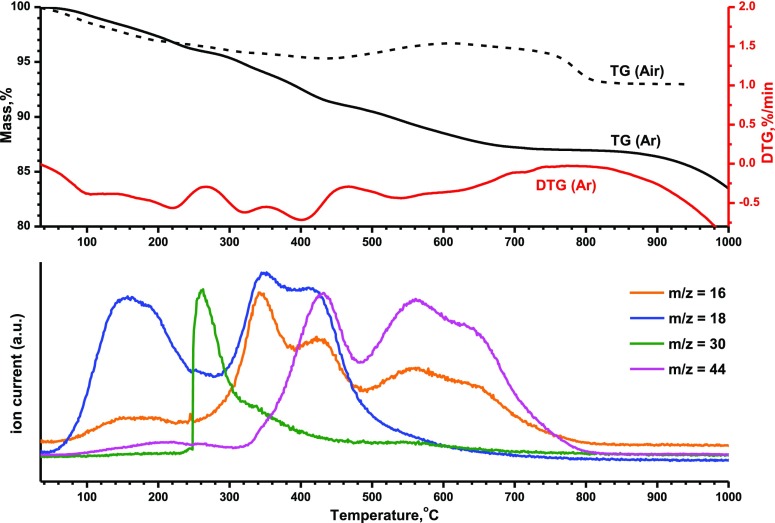
The mechanism of dehydration and decomposition and the thermal stability of organic–inorganic composites were studied by TG coupled with mass spectrometry of eliminated gases in an argon atmosphere (Figure 6). The results suggest that unlike H2La2Ti3O10-based amine hybrids,55 the n-butylamine-intercalated HKBT4 is rather stable under heat treatment. Its thermal decomposition may be separated into two main stages: elimination of water at low temperatures and decomposition of n-butylamine at higher temperatures. The first stage at 30–230 °C shows the elimination of water (major ion current at this range is m/z = 18), which is the result of dehydration. The further heating from 230 to 450 °C first leads to the detection of ion current m/z = 30 in evolved gases associated with elimination of intercalated amine (CH2NH2+) and then (starting from temperatures about 300 °C) the increase in the contribution of m/z = 18 and 16 is detected, which could be attributed to water elimination. It should be noted that the ion current m/z = 16 is too high at this stage to be explained only as water elimination. At the same time, it could not be associated with CO2 elimination because the related ion current with m/z = 44 is negligible at this stage. A possible explanation of this phenomenon could be related to the elimination of lattice oxygen or the elimination of NH3 (associated with m/z = 16—NH2+). Heating the sample at temperatures higher than 350 °C leads to the elimination of CO2 (m/z = 44) and water (m/z = 18), which continues up to ∼500 °C; after that, the mass loss on TG is mainly associated with CO2 elimination (m/z = 44, 16). The last two processes in Ar atmosphere could be explained as the reaction between organic and inorganic parts of the hybrid material accompanied by the reduction of metal (Bi3+ and Ti4+) cations; hence, the mass loss at temperature higher 800 °C (without detection of ion currents) can be associated with metallic Bi evaporation (attention! A repetition of such TG coupled with mass spectrometry (TG-MS) experiment in an inert atmosphere is strongly discouraged; the precipitated bismuth can cause damage to the platinum parts of the device!).
Figure 6.
Results of TG-MS analysis of evolved gases during HKBT4×BuNH2 heating in the Ar atmosphere.
To sum up, dehydration, which is expected to be similar in Ar and Air atmospheres, is completed at 230 °C, and the further mass loss at higher temperatures may be attributed to the elimination of n-butylamine and decomposition of the protonated form itself.
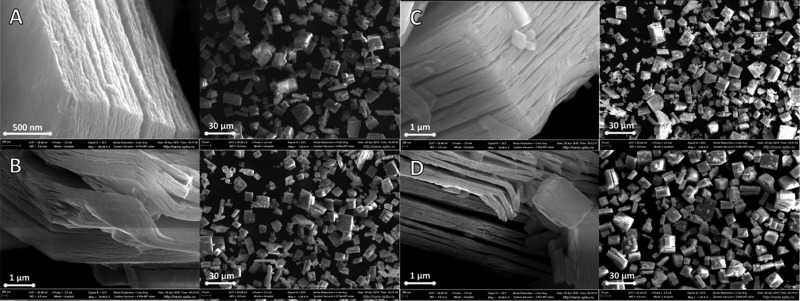
2.2.6. Scanning Electron Microscopy
The morphologies of the obtained protonated and intercalated forms were studied by scanning electron microscopy (SEM) (Figure 7). The as-prepared protonated forms have a plate-like shape, which is typical for layered compounds. The obtained microcrystals have relatively big sizes of 5–10 μm in two directions and partly split into thin stacked sheets in the third direction. The intercalation of amines leads to the further splitting and swelling of the platelets, though, the major shape and sizes have remained. Such behavior could explain the observed peak widening on the XRD patterns of the obtained hybrid compounds.
Figure 7.
SEM images for the (a) protonated form of HKBT4·H2O and organic–inorganic hybrids (b) HKBT4×MeNH2, (c) HKBT4×BuNH2, and (d) HKBT4×OcNH2.
2.3. Influence of Interlayer Water on Hybrid Formation
The reported structural characterization of HKBT4·H2O showed that it may be indexed in tetragonal P4. The partial dehydration at 90 °C leads to the decrease of c parameter (and therefore the interlayer distance), accompanied by displacement of perovskite blocks in a and b directions, leading to I-type stacking sequence. Both of these factors are likely to result in lower proton accessibility and therefore lower reactivity in intercalation reactions. The complete dehydration at 160 °C and formation of HKBT4 leads to a further decrease in the interlayer distance with the displacement of perovskite blocks in the c direction as implied by calculated lattice parameters.45
In order to examine the influence of interlayer water in the host compound on its activity in intercalation reactions, a partly dehydrated HKBT4·0.5H2O and fully dehydrated HKBT4 samples have been used for intercalation reactions with methylamine and n-butylamine. The conditions tested are presented in Table 3. The treatment of both dehydrated samples with 50% water solution of n-butylamine at room temperature and under heating (60–80 °C) did not show any changes in the XRD patterns, which indicate that no reaction takes place. These results are in accordance with the previously reported assumptions.48,49 Methylamine has a smaller molecular size, and as reported earlier, it could be directly intercalated into a three-layered RP titanate H2La2Ti3O10 with a I-type stacking sequence,55 which allows the assumption of the possibility of its intercalation in partly or fully dehydrated protonated forms HKBT4·0.5H2O and HKBT4. However, the experiments showed that it could not be intercalated into these phases nor in room temperature conditions, nor upon heating.
2.4. Discussion
Intercalated organic derivatives HKBT4×RNH2 can be synthesized using a hydrated and protonated RP compound HKBT4·H2O as the starting material but not its partially (HKBT4·0.5H2O) or completely dehydrated (HKBT4) forms, suggesting a strong influence of interlayer water molecules on chemical activity upon intercalation. Despite the attempts to optimize the synthetic procedure, we were unable to obtain compounds with an organic content higher than 0.35 per proton of the initial compound. This is far less than the theoretic capacity equal of approximately one amine molecule per proton limited by steric factors as opposed to previously obtained results for other RP titanates.42,46,55 The other difference between the obtained HKBT4×RNH2 compounds and the previously reported n-alkylamine derivatives of perovskite-like layered compounds is the thermal decomposition behavior. First of all, because of the high thermal stability of its organic parts, synthesized hybrids, apparently, may be obtained in an anhydrous (dehydrated) form.
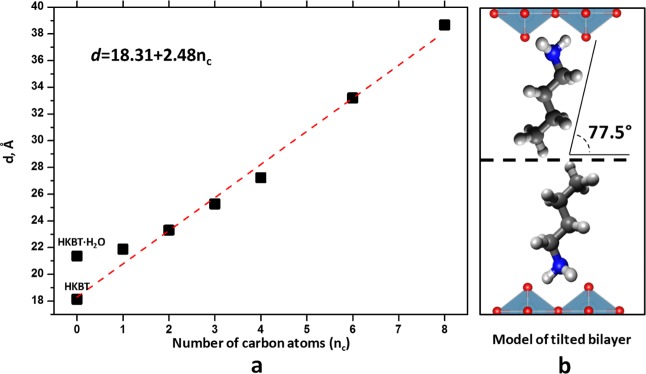
Comparing the interlayer distance change between HKBT4 and the obtained n-amine-intercalated compounds HKBT4×RNH2 with nc = 1, 2, 3, 4, 6, and 8 (Δd = 3.74, 5.16, 7.12, 9.09, 15.11, and 20.53 Å correspondingly) with the linear size of the intercalated amine molecules (2.94, 4.16, 5.44, 6.69, 9.22, and 11.75 Å correspondingly), we can see that the calculated distance changes are higher than the linear size of the single amine molecule. Because several types of linear amine arrangements are known for layered structures,22 there could be different explanations of such interlayer space widening. Taking into account the results of Raman, IR, and 13C NMR spectroscopy characterization, suggesting the all-trans conformation of the n-alkyl chains, we can assume a bilayer arrangement model for intercalated amines. Because the length of n-alkyl chains in the all-trans conformation increases by 0.127 nm per CH2 unit, the calculated linear relationship between the interlayer distance and the number of carbon atoms in n-alkylamine (Figure 8a) can be explained by a tilted bilayer arrangement of n-alkyl chains in the interlayer with a tilt angle of ∼77.5°. This value is much higher than that previously observed for the n-alkylamine derivatives of RP phase H2SrTa2O7 (51°) with a similar amount of intercalated molecules (∼25% of the theoretical cation-exchange capacity) and bilayer chain arrangement,56 but it can be compared to n-alkylamine-intercalated RP H2W2O757 (∼71.6°), H1.8[Sr0.8Bi0.2Ta2O7]58 (∼60°), DJ niobate HCa2Nb3O1040 (∼62.3°), and also to n-alcohol-grafted derivatives of RP tantalate H2CaTa2O7 (∼70°)41 and titanate H2La2Ti3O10 (∼75°).42
Figure 8.
(a) Correlation between interlayer distance and the number of carbon atoms (nc) in n-alkylamine and (b) schematic representation of the proposed HKBT4×RNH2 alkyl chain arrangement (on the example of HKBT4×BuNH2).
Taking into account the n-alkylamine chain size and an assumption that during intercalation of n-alkylammonium cations occupy cavities on the (100) surface of the perovskite-like slabs as it was previously reported for other layered perovskite compounds,57,58 a simple structural model with tilted bilayer arrangement of alkyl chains can be proposed (Figure 8b).
The nature of enhanced and reproducible fluorescence of the HKBT4×OcNH2 sample is not clear. As we have shown, it is not related to the differences in the optical properties of the samples and should be explained by other reasons. We suppose that one of the possible reasons may be the presence of a higher organic carbon content in this sample, as it is known that the organic substance fluorescence is one of the limitations of the Raman spectroscopy method, and typically, the longer-wavelength lasers are used for organic substances (NIR and IR lasers) in order to reduce the fluorescence. From the other side, bismuth-containing compounds, including complex oxides and layered oxides, are already known to exhibit luminescent properties,59 so the observed luminescence may not be directly connected to the organic content and type. We assume that it may actually be in one way or another connected with changes in the composition (organic content and type and water content), structure (change in the interlayer distance), or morphology of the sample (in particular, partial splitting or presence of an exfoliated phase). However, more complex research is needed for rigorous conclusions.
3. Conclusions
Intercalation reactions of a new RP-layered perovskite-like bismuth titanate protonated form with n-alkylamines have been studied for the first time. It was shown that it readily undergoes the intercalation of n-alkylamines in moderate and enhanced temperatures. The optimal conditions for the preparation of organic–inorganic hybrids have been determined. It was shown that intercalated amines are mainly presented in the interlayer space in cationic forms. The study of the impact of interlayer water on intercalation reactivity showed that it is a crucial factor for proceeding intercalation reactions.
4. Experimental Section
4.1. Materials
KNO3 (Vekton, 99.9%), Bi2O3 (Vekton, 99.9%), and TiO2 (Vekton, 99.9%) were dried at 200, 600, and 1000 °C, respectively. Methylamine (38% water solution, Chemical line), n-ethylamine (70% water solution, Merck), n-propylamine (Sigma-Aldrich, 98%), n-butylamine (99.9%, Chemical line), n-hexylamine (Sigma-Aldrich, 99.9%), n-octylamine (Vekton, 99.9%), and n-heptane (ECOS, 99.9%) were used as received.
4.2. Preparation of Inorganic Hosts
The starting oxide K2.5Bi2.5Ti4O13 and its protonated forms were obtained according to the previously described procedures. K2.5Bi2.5Ti4O13 host was synthesized by conventional ceramic technique from Bi2O3, TiO2, and KNO3.43 The amounts of oxides were taken in stoichiometric molar ratios, and KNO3 was taken with 20% excess to compensate for its loss due to the volatilization. The mixture was ball milled at 600 rpm for 100 min, pelletized into ∼2 g tablets, and calcined at 750 °C for 8 h twice with intermediate regrinding. The protonated hydrated form H2K0.5Bi2.5Ti4O13·H2O (HKBT4·H2O) was obtained by suspending 2 g of alkali form in 40 mL of 1 M HNO3 and stirring it at 25 °C for 1 week.44 Then, the obtained suspension was centrifuged, washed with distilled water, and dried at room temperature over CaO. The partly and fully dehydrated forms H2K0.5Bi2.5Ti4O13·0.5H2O (HKBT4·0.5H2O) and H2K0.5Bi2.5Ti4O13 (HKBT4) were prepared by heat treatment of the as-prepared hydrated form at 90 and 160 °C for 30 min correspondingly.45
4.3. Reactions with n-Amines
In order to determine the optimal conditions for the preparation of amine-intercalated hybrids (HKBT4×RNH2), the series of experiments in various conditions were carried out, including varying the reaction temperature, time, and medium. The examined experimental conditions are presented in Table 3. Room-temperature reactions were carried out by suspending the HKBT4 powder in a solution of amines and stirring at 25 °C for 1–14 days in sealed glass vessels. Reactions with heating were carried out at 60/80 °C for 1–7 days using ∼50 mL Teflon-lined vessels inserted in stainless steel autoclaves. The volumes of suspensions were taken according to the 80% occupancy rate of the vessel. The amine-treated powders were collected by filtration (poly(tetrafluoroethylene) membrane filters, 0.20 μm porosity diameter) and washed with acetone (in case of amines with nc = 1–4) and hexane (in case of amines with nc = 6 and 8).
4.4. Instrumentation
The formation of new phases was observed by powder XRD analysis with a Rigaku MiniFlex II diffractometer (Cu Kα radiation, λ = 0.15406 nm, 2θ = 3 – 60°, and scan speed = 10° min–1). The intercalation of organic molecules into interlayer space was confirmed by Raman and FT-IR-spectroscopies and MAS-NMR spectroscopy. The FT-IR spectra of the samples were recorded with an FT-IR spectrometer IR Prestige-21, with a resolution of 1 cm–1 in the range of 4000–400 cm–1, using the KBr pellet technique. Raman spectra were collected by a Bruker SENTERRA spectrometer in the range of 100–4000 cm–1 using a 488 nm 4 mW laser with a 25 μm aperture, and the spectrum accumulation time was 60 s. In order to control the possible decomposition of the samples influenced by the laser beam, the measurements have been repeated six times in one point. Solid-state NMR spectroscopy was performed by a Bruker AVANCE III 400 WB spectrometer at operating frequencies 100.64 MHz (for13 C). Powder samples were placed in a zirconium oxide rotor with an external diameter of 4 mm and rotated at a frequency of 12.5 kHz at a magic angle to the direction of a constant magnetic field. To register the spectra on 13C nuclei, a cross-polarization excitation pulse sequence was used (CP/MAS technique). For the CP/MAS technique, the contact time was 2 ms, the relaxation delay time was 5 s, and the number of accumulations was 12,000. Tetramethylsilane was used as an external reference. The investigation of dehydration and decomposition of organic–inorganic hybrids were performed by means of TG analysis (TG 209 F1 Libra, Netzsch) in the flow of synthetic dry air (50 mL/min) at a heating rate of 10°C/min. The quantitative composition of the obtained hybrids was estimated from the results of TG analysis and CHN analysis. The mechanism of dehydration and decomposition of the samples was estimated using the results of TG–MS in an argon atmosphere (STA 449 F1 Jupiter, Netzsch and QMS 403C Aëolos), and studies were performed in the flow of argon (50 mL/min) at a heating rate of 20°C/min. The CHN analysis was performed using a Euro EA3028-HT analyzer.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (grant no. 18-03-00915). I.A.M. also thank the Russian Foundation for Basic Research for its financial support (grant no. 19-33-90050). The authors are also grateful to Saint Petersburg State University Research Park: Center for X-ray Diffraction Studies, Center for Optical and Laser Materials Research, Center for Chemical Analysis and Materials Research, Center for Thermal Analysis and Calorimetry, Magnetic Resonance Research Centre, Interdisciplinary Center for Nanotechnology, and SDBSWeb: https://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00424.
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Liu Y.; Mao Z.-Q. Unconventional Superconductivity in Sr2RuO4. Phys. C 2015, 514, 339–353. 10.1016/j.physc.2015.02.039. [DOI] [Google Scholar]
- Ogino H.; Sato S.; Kishio K.; Shimoyama J.-i. Relationship between Crystal Structures and Physical Properties in Iron Arsenides with Perovskite-Type Layers. Phys. Procedia 2012, 36, 722–726. 10.1016/j.phpro.2012.06.274. [DOI] [Google Scholar]
- Moritomo Y.; Asamitsu a.; Kuwahara H.; Tokura Y. Giant Magnetoresistance of Manganese Oxides with a Layered Perovskite Structure. Nature 1996, 380, 141–144. 10.1038/380141a0. [DOI] [Google Scholar]
- Takata T.; Furumi Y.; Shinohara K.; Tanaka A.; Hara M.; Kondo J. N.; Domen K. Photocatalytic Decomposition of Water on Spontaneously Hydrated Layered Perovskites. Chem. Mater. 1997, 9, 1063–1064. 10.1021/cm960612b. [DOI] [Google Scholar]
- Rodionov I. A.; Zvereva I. A. Photocatalytic Activity of Layered Perovskite-like Oxides in Practically Valuable Chemical Reactions. Russ. Chem. Rev. 2016, 85, 248–279. 10.1070/RCR4547. [DOI] [Google Scholar]
- Oshima T.; Yokoi T.; Eguchi M.; Maeda K. Synthesis and Photocatalytic Activity of K2CaNaNb3O10, a New Ruddlesden–Popper Phase Layered Perovskite. Dalton Trans. 2017, 46, 10594–10601. 10.1039/C6DT04872B. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Mao L.; Guan X.; Tucker K. A.; Xie H.; Wu X.; Shi J. Layered Perovskite Oxides and Their Derivative Nanosheets Adopting Different Modification Strategies towards Better Photocatalytic Performance of Water Splitting. Renewable Sustainable Energy Rev. 2020, 119, 109527. 10.1016/j.rser.2019.109527. [DOI] [Google Scholar]
- Badge S. K.; Deshpande A. V. Study of Dielectric and Ferroelectric Properties of Bismuth Titanate (Bi4Ti3O12) Ceramic Prepared by Sol-Gel Synthesis and Solid State Reaction Method with Varying Sintering Temperature. Solid State Ionics 2019, 334, 21–28. 10.1016/j.ssi.2019.01.028. [DOI] [Google Scholar]
- Chen C.; Ning H.; Lepadatu S.; Cain M.; Yan H.; Reece M. J. Ferroelectricity in Dion–Jacobson ABiNb2O7 (A = Rb, Cs) Compounds. J. Mater. Chem. C 2015, 3, 19–22. 10.1039/C4TC02136C. [DOI] [Google Scholar]
- Gomah-Pettry J. Sodium-Bismuth Titanate Based Lead-Free Ferroelectric Materials. J. Eur. Ceram. Soc. 2004, 24, 1165–1169. 10.1016/S0955-2219(03)00473-4. [DOI] [Google Scholar]
- Kuo C.-Y.; Hu Z.; Yang J. C.; Liao S.-C.; Huang Y. L.; Vasudevan R. K.; Okatan M. B.; Jesse S.; Kalinin S. V.; Li L.; et al. Single-Domain Multiferroic BiFeO3 Films. Nat. Commun. 2016, 7, 12712. 10.1038/ncomms12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng B.; Grice C. R.; Ge J.; Poudel T.; Deng X.; Yan Y. Barium Bismuth Niobate Double Perovskite/Tungsten Oxide Nanosheet Photoanode for High-Performance Photoelectrochemical Water Splitting. Adv. Energy Mater. 2018, 8, 1701655. 10.1002/aenm.201701655. [DOI] [Google Scholar]
- Xing G.; Feng Y.; Pan M.; Wei Y.; Li G.; Dang P.; Liang S.; Molokeev M. S.; Cheng Z.; Lin J. Photoluminescence Tuning in a Novel Bi3+/Mn4+ Co-Doped La2ATiO6 :(A = Mg, Zn) Double Perovskite Structure: Phase Transition and Energy Transfer. J. Mater. Chem. C 2018, 6, 13136–13147. 10.1039/C8TC05171B. [DOI] [Google Scholar]
- Huang D.; Dang P.; Lian H.; Zeng Q.; Lin J. Luminescence and Energy-Transfer Properties in Bi3+/Mn4+-Codoped Ba2GdNbO6 Double-Perovskite Phosphors for White-Light-Emitting Diodes. Inorg. Chem. 2019, 58, 15507–15519. 10.1021/acs.inorgchem.9b02558. [DOI] [PubMed] [Google Scholar]
- Lerf A. Storylines in Intercalation Chemistry. Dalton Trans. 2014, 43, 10276–10291. 10.1039/C4DT00203B. [DOI] [PubMed] [Google Scholar]
- Wang C.; Tang K.; Wang D.; Liu Z.; Wang L.; Zhu Y.; Qian Y. A New Carbon Intercalated Compound of Dion-Jacobson Phase HLaNb2O7. J. Mater. Chem. 2012, 22, 11086–11092. 10.1039/c2jm14902h. [DOI] [Google Scholar]
- Uma S.; Gopalakrishnan J.; State S.; Unit S. C. Polymerization of Aniline in Layered Perovskites. Mater. Sci. 1995, 34, 175–179. 10.1016/0921-5107(95)01235-4. [DOI] [Google Scholar]
- Takeda Y.; Suzuki H.; Notsu K.; Sugimoto W.; Sugahara Y. Preparation of a Novel Organic Derivative of the Layered Perovskite Bearing HLaNb2O7·nH2O Interlayer Surface Trifluoroacetate Groups. Mater. Res. Bull. 2006, 41, 834–841. 10.1016/j.materresbull.2005.10.004. [DOI] [Google Scholar]
- Guo T.; Wang L.; Evans D. G.; Yang W. Synthesis and Photocatalytic Properties of a Polyaniline-Intercalated Layered Protonic Titanate Nanocomposite with a P–n Heterojunction Structure. J. Phys. Chem. C 2010, 114, 4765–4772. 10.1021/jp9055413. [DOI] [Google Scholar]
- Takeda Y.; Momma T.; Osaka T.; Kuroda K.; Sugahara Y. Organic Derivatives of the Layered Perovskite HLaNb2O7·xH2O with Polyether Chains on the Interlayer Surface: Characterization, Intercalation of LiCl, and Ionic Conductivity. J. Mater. Chem. 2008, 18, 3581. 10.1039/b802003e. [DOI] [Google Scholar]
- Ranmohotti K. G. S.; Josepha E.; Choi J.; Zhang J.; Wiley J. B. Topochemical Manipulation of Perovskites: Low-Temperature Reaction Strategies for Directing Structure and Properties. Adv. Mater. 2011, 23, 442–460. 10.1002/adma.201002274. [DOI] [PubMed] [Google Scholar]
- Lagaly G. Interaction of Alkylamines with Different Types of Layered Compounds. Solid State Ionics 1986, 22, 43–51. 10.1016/0167-2738(86)90057-3. [DOI] [Google Scholar]
- Constantino V. R. L.; Barbosa C. A. S.; Bizeto M. A.; Dias P. M. Intercalation Compounds Involving Inorganic Layered Structures. An. Acad. Bras. Cienc. 2000, 72, 45–50. 10.1590/S0001-37652000000100006. [DOI] [PubMed] [Google Scholar]
- Tahara S.; Sugahara Y. Interlayer Surface Modification of the Protonated Triple-Layered Perovskite HCa2Nb3O10 · xH2O with n-Alcohols. Langmuir 2003, 19, 9473–9478. 10.1021/la0343876. [DOI] [Google Scholar]
- Aznar A. J.; Sanz J.; Ruiz-Hitzky E. Mechanism of the Grafting of Organosilanes on Mineral Surfaces. IV. Phenylderivatives of Sepiolite and Poly (Organosiloxanes). Colloid Polym. Sci. 1992, 270, 165–176. 10.1007/BF00652183. [DOI] [Google Scholar]
- Kimura N.; Kato Y.; Suzuki R.; Shimada A.; Tahara S.; Nakato T.; Matsukawa K.; Mutin P. H.; Sugahara Y. Single- and Double-Layered Organically Modified Nanosheets by Selective Interlayer Grafting and Exfoliation of Layered Potassium Hexaniobate. Langmuir 2014, 30, 1169–1175. 10.1021/la404223x. [DOI] [PubMed] [Google Scholar]
- Yang J.-M.; Luo Y.; Bao Q.; Li Y.-Q.; Tang J.-X. Recent Advances in Energetics and Stability of Metal Halide Perovskites for Optoelectronic Applications. Adv. Mater. Interfaces 2019, 6, 1801351. 10.1002/admi.201801351. [DOI] [Google Scholar]
- Kamminga M. E.; Stroppa A.; Picozzi S.; Chislov M.; Zvereva I. A.; Baas J.; Meetsma A.; Blake G. R.; Palstra T. T. M. Polar Nature of (CH3NH3)3Bi2I9 Perovskite-Like Hybrids. Inorg. Chem. 2017, 56, 33–41. 10.1021/acs.inorgchem.6b01699. [DOI] [PubMed] [Google Scholar]
- Schaak R. E.; Mallouk T. E. Perovskites by Design: A Toolbox of Solid-State Reactions. Chem. Mater. 2002, 14, 1455–1471. 10.1021/cm010689m. [DOI] [Google Scholar]
- Machida M.; Mitsuyama T.; Ikeue K.; Matsushima S.; Arai M. Photocatalytic Property and Electronic Structure of Triple-Layered Perovskite Tantalates, MCa2Ta3O10 (M = Cs, Na, H, and C6H13NH3). J. Phys. Chem. B 2005, 109, 7801–7806. 10.1021/jp044833d. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang C.; Wang L.; Hao Q.; Zhu X.; Chen X.; Tang K. Preparation of Interlayer Surface Tailored Protonated Double-Layered Perovskite H2CaTa2O7 with n-Alcohols, and Their Photocatalytic Activity. RSC Adv. 2014, 4, 4047–4054. 10.1039/C3RA44623A. [DOI] [Google Scholar]
- Rodionov I. A.; Maksimova E. A.; Pozhidaev A. Y.; Kurnosenko S. A.; Silyukov O. I.; Zvereva I. A. Layered Titanate H2Nd2Ti3O10 Intercalated with N-Butylamine: A New Highly Efficient Hybrid Photocatalyst for Hydrogen Production from Aqueous Solutions of Alcohols. Front. Chem. 2019, 7, 863. 10.3389/fchem.2019.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolosi V.; Chhowalla M.; Kanatzidis M. G.; Strano M. S.; Coleman J. N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. 10.1126/science.1226419. [DOI] [Google Scholar]
- Ida S.; Ogata C.; Eguchi M.; Youngblood W. J.; Mallouk T. E.; Matsumoto Y. Photoluminescence of Perovskite Nanosheets Prepared by Exfoliation of Layered Oxides, K2Ln2Ti3O10, KLnNb2O7, and RbLnTa2O7 (Ln: Lanthanide Ion). J. Am. Chem. Soc. 2008, 130, 7052–7059. 10.1021/ja7114772. [DOI] [PubMed] [Google Scholar]
- Uppuluri R.; Sen Gupta A.; Rosas A. S.; Mallouk T. E. Soft Chemistry of Ion-Exchangeable Layered Metal Oxides. Chem. Soc. Rev. 2018, 47, 2401–2430. 10.1039/c7cs00290d. [DOI] [PubMed] [Google Scholar]
- Takahashi S.; Nakato T.; Hayashi S.; Sugahara Y.; Kuroda K. Formation of Methoxy-Modified Interlayer Surface via the Reaction between Methanol and Layered Perovskite HLaNb2O7·xH2O. Inorg. Chem. 1995, 34, 5065–5069. 10.1021/ic00124a023. [DOI] [Google Scholar]
- Shimada A.; Yoneyama Y.; Tahara S.; Mutin P. H.; Sugahara Y. Interlayer Surface Modification of the Protonated Ion-Exchangeable Layered Perovskite HLaNb2O7·xH2O with Organophosphonic Acids. Chem. Mater. 2009, 21, 4155–4162. 10.1021/cm900228c. [DOI] [Google Scholar]
- Suzuki H.; Notsu K.; Takeda Y.; Sugimoto W.; Sugahara Y. Reactions of Alkoxyl Derivatives of a Layered Perovskite with Alcohols: Substitution Reactions on the Interlayer Surface of a Layered Perovskite. Chem. Mater. 2003, 15, 636–641. 10.1021/cm0200902. [DOI] [Google Scholar]
- Akbarian-Tefaghi S.; Teixeira Veiga E.; Amand G.; Wiley J. B. Rapid Topochemical Modification of Layered Perovskites via Microwave Reactions. Inorg. Chem. 2016, 55, 1604–1612. 10.1021/acs.inorgchem.5b02514. [DOI] [PubMed] [Google Scholar]
- Jacobson A. J.; Johnson J. W.; Lewandowski J. T. Intercalation of the Layered Solid Acid HCa2Nb3O10 by Organic Amines. Mater. Res. Bull. 1987, 22, 45–51. 10.1016/0025-5408(87)90148-6. [DOI] [Google Scholar]
- Wang Y.; Wang C.; Wang L.; Hao Q.; Zhu X.; Chen X.; Tang K. Preparation of Interlayer Surface Tailored Protonated Double-Layered Perovskite H2CaTa2O7 with n-Alcohols, and Their Photocatalytic Activity. RSC Adv. 2014, 4, 4047–4054. 10.1039/C3RA44623A. [DOI] [Google Scholar]
- Tahara S.; Ichikawa T.; Kajiwara G.; Sugahara Y. Reactivity of the Ruddlesden–Popper Phase H2La2Ti3O10 with Organic Compounds: Intercalation and Grafting Reactions. Chem. Mater. 2007, 19, 2352–2358. 10.1021/cm0623662. [DOI] [Google Scholar]
- Liu S.; Avdeev M.; Liu Y.; Johnson M. R.; Ling C. D. A New n = 4 Layered Ruddlesden–Popper Phase K2.5Bi2.5Ti4O13 Showing Stoichiometric Hydration. Inorg. Chem. 2016, 55, 1403–1411. 10.1021/acs.inorgchem.5b01913. [DOI] [PubMed] [Google Scholar]
- Silyukov O. I.; Minich I. A.; Zvereva I. A. Synthesis of Protonated Derivatives of Layered Perovskite-like Bismuth Titanates. Glas. Phys. Chem. 2018, 44, 115–119. 10.1134/S1087659618020153. [DOI] [Google Scholar]
- Minich I. A.; Silyukov O. I.; Kulish L. D.; Zvereva I. A. Study on Thermolysis Process of a New Hydrated and Protonated Perovskite-like Oxides H2K0.5Bi2.5Ti4O13·yH2O. Ceram. Int. 2019, 45, 2704–2709. 10.1016/j.ceramint.2018.10.004. [DOI] [Google Scholar]
- Silyukov O. I.; Kurnosenko S. A.; Zvereva I. A. Intercalation of Methylamine into the Protonated Forms of Layered Perovskite-Like Oxides HLnTiO4 (Ln = La and Nd). Glas. Phys. Chem. 2018, 44, 428–432. 10.1134/S1087659618050176. [DOI] [Google Scholar]
- Gopalakrishnan J.; Uma S.; Bhat V. Synthesis of Layered Perovskite Oxides, ACa2-XLaxNb3-XTixO10 (A = K, Rb, Cs) and Characterization of New Solid Acids, HCa2-XLaxNb3-XTixO10 (0 < x ≤ 2), Exhibiting Variable Bronsted Acidity. Chem. Mater. 1993, 5, 132–136. 10.1021/cm00025a025. [DOI] [Google Scholar]
- Nakato T.; Nakade M.; Kuroda K.; Kato C.. Intercalation of N-Alkylamines into Perovskite-Related Layered Niobic Acid HBiNb2O7·nH2O. Studies in Surface Science and Catalysis; Elsevier, 1994; Vol. 90, pp 285–290. [Google Scholar]
- Ida S.; Okamoto Y.; Matsuka M.; Hagiwara H.; Ishihara T. Preparation of Tantalum-Based Oxynitride Nanosheets by Exfoliation of a Layered Oxynitride, CsCa2Ta3O10–xNy, and Their Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 15773–15782. 10.1021/ja3043678. [DOI] [PubMed] [Google Scholar]
- Xie L.-Q.; Zhang T.-Y.; Chen L.; Guo N.; Wang Y.; Liu G.-K.; Wang J.-R.; Zhou J.-Z.; Yan J.-W.; Zhao Y.-X.; et al. Organic-Inorganic Interactions of Single Crystalline Organolead Halide Perovskites Studied by Raman Spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 18112–18118. 10.1039/c6cp01723a. [DOI] [PubMed] [Google Scholar]
- Vaia R. A.; Teukolsky R. K.; Giannelis E. P. Interlayer Structure and Molecular Environment of Alkylammonium Layered Silicates. Chem. Mater. 1994, 6, 1017–1022. 10.1021/cm00043a025. [DOI] [Google Scholar]
- Ai-Jie H.; Yu Z.; Juan G.; Yue-Fang H.; He-Yong H.; Ying-Cai L. Interaction of Methylamine with Highly Siliceous MFI, FAU and FER-Type Zeolites. Chin. J. Chem. 2005, 23, 413–417. 10.1002/cjoc.200590413. [DOI] [Google Scholar]
- Wang Y.; Nikolopoulou M.; Delahaye E.; Leuvrey C.; Leroux F.; Rabu P.; Rogez G. Microwave-Assisted Functionalization of the Aurivillius Phase Bi2SrTa2O9: Diol Grafting and Amine Insertion: vs. Alcohol Grafting. Chem. Sci. 2018, 9, 7104–7114. 10.1039/c8sc01754a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A. S.; Ferrara C.; Marculescu A. M.; Giannici F.; Martorana A.; Mustarelli P.; Tealdi C. Solid-State NMR Characterization of the Structure and Thermal Stability of Hybrid Organic–Inorganic Compounds Based on a HLaNb2O7 Dion–Jacobson Layered Perovskite. Phys. Chem. Chem. Phys. 2016, 18, 21903–21912. 10.1039/C6CP02943D. [DOI] [PubMed] [Google Scholar]
- Kurnosenko S. A.; Silyukov O. I.; Mazur A. S.; Zvereva I. A. Synthesis and Thermal Stability of New Inorganic-Organic Perovskite-like Hybrids Based on Layered Titanates HLnTiO4 (Ln = La, Nd). Ceram. Int. 2020, 46, 5058–5068. 10.1016/j.ceramint.2019.10.249. [DOI] [Google Scholar]
- Shimizu K.-i.; Itoh S.; Hatamachi T.; Kitayama Y.; Kodama T. Pillaring of Ruddlesden–Popper Perovskite Tantalates, H2ATa2O7 (A = Sr or La2/3), with n-Alkylamines and Oxide Nanoparticles. J. Mater. Chem. 2006, 16, 773. 10.1039/b514066h. [DOI] [Google Scholar]
- Wang B.; Dong X.; Pan Q.; Cheng Z.; Yang Y. Intercalation Behavior of N-Alkylamines into an A-Site Defective Layered Perovskite H2W2O7. J. Solid State Chem. 2007, 180, 1125–1129. 10.1016/j.jssc.2007.01.009. [DOI] [Google Scholar]
- Tsunoda Y.; Sugimoto W.; Sugahara Y. Intercalation Behavior of N-Alkylamines into a Protonated Form of a Layered Perovskite Derived from Aurivillius Phase Bi2SrTa2O9. Chem. Mater. 2003, 15, 632–635. 10.1021/cm0200893. [DOI] [Google Scholar]
- Ida S.; Ogata C.; Unal U.; Izawa K.; Inoue T.; Altuntasoglu O.; Matsumoto Y. Preparation of a Blue Luminescent Nanosheet Derived from Layered Perovskite Bi2SrTa2O9. J. Am. Chem. Soc. 2007, 129, 8956–8957. 10.1021/ja073105b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.