Abstract
We report a direct, straightforward, and regioselective hydration of 1,4-enynes designed from Morita–Baylis–Hillman adducts. Under smooth conditions and short reaction times, gold-catalyzed hydration of internal alkynes provides synthetically useful ketones as single regioisomers in yields higher than 90%. The synthetic usefulness of this protocol was demonstrated by the conversion of selected ketones into biologically valuable α-alkylidene-γ-lactones upon reduction with sodium borohydride. In the course of the scope evaluation, we discovered that this methodology could also furnish α-arylidene-β,γ-butenolides.
Introduction
The carbonyl functional group has a central role in modern organic synthesis because its vastly developed reactivity makes it very synthetically useful. Similarly, polycarbonylated compounds are valuable motifs. In particular, 1,3- and 1,4-dicarbonyl compounds are important building blocks in the synthesis of heterocycles.1 1,4-Dicarbonyl compounds are mainly employed in the synthesis of pyrroles,2 furans,3 and thiophenes4 via the Paal–Knorr reaction (Scheme 1A–C). This framework is also present in diverse natural products and drugs, such as the cytotoxic diterpenoid maoecrystal V,5 the anticancer drug batimastat,6 and the antineoplastic food additive quassin7 (Scheme 1D).
Scheme 1. Importance of 1,4-Dicarbonyl Compounds.
Highly efficient strategies exist for achieving 1,3-dicarbonyl compounds, usually by exploiting the inherent polarity of the carbonyl group.8 On the other hand, although several methodologies helpful in achieving different levels of complexity were developed to access 1,4-dicarbonyl compounds, they are often operationally difficult (e.g., by demanding anhydrous conditions), require multistep preparation of two starting materials, or even undergo undesired homoreactions. Most of these methodologies rely on the formation of a C–C bond via enolate alkylation,9 NHC-catalyzed Stetter reaction,3c,10 and oxidative coupling reactions.11 A few examples of more straightforward strategies involving alkynes have been reported for the synthesis of 1,4-dicarbonyl compounds, including hydration of 3-alkynoates catalyzed by gold(III),12 hydration of 3-en-1-ynes catalyzed by zinc(II) and gold(I) in the presence of Selectfluor,13 hydration of alkynones catalyzed by palladium(II),14 formal hydration of 4-alkynones mediated by KOMe,15 and enantioselective formal dehydration of ynamides.8
In this scenario, gold catalysis, which allows a rapid increase in molecular complexity from alkynes as starting materials,16 is an outstanding alternative for accessing the carbonyl group because the addition of water to alkynes, when catalyzed by gold(I) and gold(III), is a mild, atom-economic, and operationally simple transformation.17 However, gold-catalyzed hydration of internal alkynes suffers from a notable lack of regioselectivity.18 In fact, to the best of our knowledge, no general method exists for achieving regioselectivity in hydration or hydroalkoxylation of internal alkynes by means of gold catalysis.19 Reported strategies rely on the presence of electron-withdrawing groups20 or, more frequently, on O- and N-based nucleophiles attached to the triple bond.21−23 These nucleophiles can act as directing groups that form cyclic cationic intermediates from intramolecular attack to the triple bond. Subsequently, water attacks a specific position on this intermediate, and the attached nucleophile becomes a leaving group. Because the formation of a cyclic intermediate is controlled by Baldwin’s rules, ketones can be obtained with great regioselectivity. This strategy of using a carbonyl group as a neighboring nucleophile was first applied by Hammond and co-workers for the synthesis of γ-keto esters (Scheme 2A).12 A similar strategy was used by Mohapatra and co-workers for the synthesis of γ-acetoxy β-keto esters (Scheme 2B).24 The acetoxy group was claimed to be responsible for the observed selectivity.
Scheme 2. Strategies for Regioselective Au-Catalyzed Alkyne Hydration.
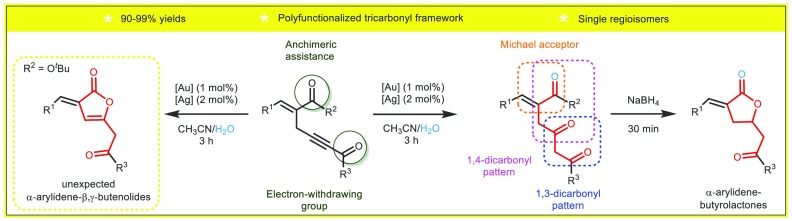
We hypothesized that 1,4-enynes (3) prepared from Morita–Baylis–Hillman (MBH) adducts could readily furnish the access to a new pattern of highly functionalized 1,4,6-tricarbonyl compounds. We explored this possibility by exploiting the exceptional ability of cationic gold(I) complexes to activate triple C≡C bonds toward nucleophilic attack under mild conditions.25 An electron-withdrawing fragment attached to the triple bond could differentiate the two C sp atoms favoring water attack at the distal carbon. Furthermore, the presence of an additional carbonyl group could speed up the reaction through anchimeric assistance. Should this assumption be correct, this strategy would allow rapid and high regioselective access to polyfunctionalized ketones (Scheme 2C).
Results and Discussion
In order to test our hypothesis, we prepared MBH adducts 1 using a methodology previously developed by our group.26 These adducts were then transformed into allylic bromides 2. Most of these allylic bromides were prepared in good yields by treatment of MBH adducts with aqueous HBr solution in the presence of concentrated H2SO4.27 However, under these conditions, we observed degradation of some starting materials. In these cases, Appel conditions furnished the desired allylic bromides.28 Alkynylation of 2 with alkyl propiolates (3a–c) or but-3-yn-2-one (3d), in presence of 0.2 equiv of CuI and 1.0 equiv of K2CO3, led to enynes 4 in 44–88% yield (Scheme 3).29 The alkynylation conditions employed to prepare 4 were adapted from the methodology described by Kim and co-workers for the synthesis of substituted naphthalenes from allylic bromides in the presence of excess of base, through propargyl–allenyl isomerization of 1,4-enynes generated in situ. Unfortunately, the naphthalenes and the 1,4-enynes have the same Rf (on TLC plates) and could not be separated by column chromatography. We therefore avoided naphthalene formation by first reducing the original amount of the base (2.0 equiv) to 1.0 equiv. Under these conditions, enynes 4aa–da and 4ae–ap were obtained in typically good yields, at 60 °C. Enynes 4ab–ad, bearing halogens in their aromatic moieties, could only be prepared at room temperature and only in moderate yields (Scheme 3).
Scheme 3. Preparation of Substrates 1,4-Enynes (3),
Reaction conditions for the alkynylation step: aMBH bromide 2 (1.0 mmol), 3 (1.2 equiv), copper iodide (0.2 equiv), potassium carbonate (1.0 equiv), and 10 mL of CH3CN (0.1 M). bIsolated yields after column chromatography. cIsolated yields based on recovery of the starting materials.
Once the substrates were prepared, enyne 4aa was chosen as a model for optimizing the reaction conditions. The key results are summarized in Table 1.
Table 1. Screening of Reaction Conditionsa.
| conversion
(%)b |
||||||
|---|---|---|---|---|---|---|
| entry | cat. (mol %) | solvent (v/v) | T (°C) | 1 h | 2 h | 3 h |
| 1 | C1 (5) | CH3CN/H2O (2/1) | Rt | 89 (83)c | ||
| 2 | C1 (5) | CH2Cl2/H2O (2/1) | Rt | 83 | ||
| 3 | C1(5) | CH3CN/H2O (1/1) | Rt | 27 | ||
| 4 | C1(5) | CH3CN/H2O (1/2) | Rt | 29 | ||
| 5 | C2 (5) | CH3CN/H2O (2/1) | Rt | 94 | ||
| 6 | C3 (5) | CH3CN/H2O (2/1) | Rt | 95 | ||
| 7 | C4 (5) | CH3CN/H2O (2/1) | Rt | 94 | ||
| 8 | C5 (5) | CH3CN/H2O (2/1) | Rt | 94 | ||
| 9 | C6 (5) | CH3CN/H2O (2/1) | Rt | 82 | ||
| 10 | C7 (5) | CH3CN/H2O (2/1) | Rt | 76 | ||
| 11 | C2 (1) | CH3CN/H2O (2/1) | Rt | 50 | 79 | 92 |
| 12 | C2 (2) | CH3CN/H2O (2/1) | Rt | 53 | 79 | 94 |
| 13 | C2 (1) | CH3CN/H2O(2/1) | 50 | 94 | 96 | >99 |
| 14 | C2 (0.2) | CH3CN/H2O (2/1) | 50 | 21 | ||
| 15d | CH3CN/H2O (2/1) | 50 | ||||
Reaction conditions: 0.2 mmol of 4aa, in 1 mL of solvent (0.2 M), [AgOTf] = 2[catalyst].
Estimated by 1H NMR of the crude reaction mixture.
Isolated yields after column chromatography.
The reaction was carried out in the absence of gold catalyst, with 10 mol % of AgOTf, only 6% of conversion after 15 h.
We first ran the hydration of 4aa in CH3CN/H2O (2/1, v/v) at room temperature, in the presence of 5 mol % of gold(I)-catalyst C1 and 10 mol % of AgOTf. To our delight, under these conditions and just after 1 h, a conversion of 89% was observed (Table 1, entry 1). The expected product 5aa was isolated in 83% yield as a single regioisomer. The structure of 5aa was confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectra as well as by high-resolution mass spectrometry (HRMS) analysis. Subsequently, CH3CN was replaced by CH2Cl2, keeping the initial proportion with water (2:1, v/v). Under these conditions, the conversion of 4aa was reduced from 89% to 83% (entry 2). Because CH3CN furnished a better result, we moved toward investigating the proportion of solvents. Whereas 4aa was converted at 89% with CH3CN/H2O (2:1, v/v), only 27% was achieved with CH3CN/H2O (1/1, v/v) (entry 3) and 29% with CH3CN/H2O (1:2, v/v) (entry 4), confirming CH3CN/H2O (2:1, v/v) as the most suitable solvent mixture for this transformation. We determined the best catalyst for this reaction by testing complexes C2–C7. Gold(I)-catalysts C2–C4 and Au(III)-catalyst C5 satisfactorily furnished conversions of 94–95% (entries 5–8). Conversely, the use of Au(III)-catalyst C6 and Au(I)-catalyst C7 yielded conversions of only 82 and 76%, respectively (entries 9 and 10). These results confirmed that this transformation was very smooth and fast; hence, we decided to investigate the possibility of reducing the catalyst loading. For this, we chose to use catalyst C2, based on its availability in our laboratory. Initially, the reaction was carried out using 1 mol % of C2; however, by 3 h, the conversion was poorer than in the previous conditions (entry 11). Similar results were observed with the use of 2 mol % of C2 (entry 12). In an attempt to improve the reactivity of 4aa, the reaction temperature was increased to 50 °C. At this temperature, using 1 mol % of C2, full conversion (>99%) of 4aa was observed in 3 h (entry 13). An additional reduction of catalyst loading to 0.2 mol % led to a conversion of only 21% (entry 14). To rule out the possibility of silver-catalysis, we run the hydration of 4aa in the presence of 10 mol % of AgOTf and in the absence of any gold catalyst. Under these conditions, a conversion of only 6% was observed after 15 h (Entry 15). Although a few examples of efficient silver(I)-catalyzed hydration of internal alkynes have been reported, they normally require harsh conditions (the use of strong acids or high temperatures).30
Having established the optimized conditions (Table 1, entry 13), we evaluated the hydration scope. The 1,4-enynes containing aliphatic, styryl, heteroaryl, and halogenated aryl substituents at R1 position and 1,4-enynes with methyl ketone and different alkyl esters directly bonded to the triple bond (R3) were all found to be suitable substrates for this transformation, readily furnishing the hydrated product. Substrates containing R2 = OEt, OMe, and Me furnished corresponding poly-carbonylated compounds 5aa–an in excellent yields (>90%) and regioselectivities (Scheme 4A). A different reactivity pattern was observed when enynes bearing a R2 = OtBu were employed. To these enynes, the tert-butyl group was lost to form unexpected α-arylidene-butyrolactones 6a and 6b. Under the optimized hydration conditions, enyne 4ao furnished cyclic enol ester 6a′ in excellent yields after filtration on a plug of Celite. However, when placed on silica, 6a′ was quantitatively converted into its endocyclic olefinic isomerized form 6a. Similar behavior was observed with enyne 4ap, although the exocyclic enolic double bond isomer could not be isolated with proper purity (Scheme 4B).
Scheme 4. Substrate scope,
Reaction conditions: 4 (0.1 mmol), Et3PAuCl (1.0 mol %), AgOTf (2.0 mol %) in CH3CN/H2O 2:1 (v/v) (0.5 mL), stirred at 50 °C for 3 h. bIsolated yields after column chromatography. cIsolated yield after filtration. dConverted from 6a′ after column chromatography.
We then endeavored to gain further insights into the mechanism and influence of substituents on regioselectivity of the hydration reaction by preparing substrate 7 and performing a control experiment under our optimized conditions (Scheme 5). Because 7 lacks one of the carbonyl moieties (the one that comes from the MBH adduct) that could work as a neighboring nucleophile, we were able to analyze the role of this carbonyl group on the rate of the hydration reaction and to determine whether the presence of the carbonyl group attached to the triple bond would be sufficient to provide selectivity on water attack, resulting in a regioselective hydration.
Scheme 5. Investigation of Carbonyl Moiety Effects on Regioselectivity and the Rate of the Hydration Reaction.
Product 8 was obtained as a single regioisomer after 3 h. In agreement with our prediction, we verified that the reaction, in the absence of the carbonyl moiety coming from MBH adduct, required a higher catalytic loading for completion in 3 h (5 mol % of Et3PhAuCl), indicating that the reaction is slower for 7 than for the other substrates.
Based on the control experiment results and preliminary works,12,21 we propose a plausible mechanism involving a cationic cyclic intermediate (Scheme 6). First, gold(I) catalyst C2 coordinates with triple bond of alkyne 4aa leading to complex A. The activated alkyne moiety, in this complex, is then attacked by the nucleophilic carbonyl group, which is placed on the substrate to increase the rate of hydration, leading to cyclic vinyl gold intermediate B or B′, in which both favored processes according to Baldwin’s rules. The B′ type intermediate was proposed by Oh and co-workers in the regioselective hydration of 1-arylalkynes through carbonyl group participation.21a Because ketone 5aa′ was not observed, 5-exo-dig cyclization should be the preferential pathway because of the presence of an electron-withdrawing group attached at the triple bond. The intermediate B is, in sequence, attacked by water to form the ring-opened enol–gold species D, which undergoes protodeauration and leads to intermediate enol E. Finally, isomerization of E affords ketone 5aa as a single product. This mechanistic proposal also accounts for the formation of 6a and 6b; upon nucleophilic attack of a water molecule, species B″ furnishes a tetrahedral intermediate C″, which then loses tBuOH to form the cyclic enol-gold ester D’’ (Scheme 6, path a). Alternatively, species D″ could be produced via direct formation of a tert-butyl cation (Scheme 6, path b). Protodeauration of D″ then generates 6a’. To investigate these mechanistic proposals, we performed the reactions to form 5aa and 6a′ using a 3:2 (v/v) mixture of H2O16/H2O18 under the optimized conditions. HRMS(ESI) analysis of 6a′ did not show any peak consistent with incorporation of O.18 This result indicates that 6a′ is formed according to path b in Scheme 6, with direct loss of a tert-butyl cation and no inclusion of water. On the other hand, HRMS (ESI) analysis of 5aa showed the presence of a peak at m/z 329.1252, two mass units more than that found for the product hydrated by H2O.16 Additionally, a quantitative NOE-decoupled 13C NMR experiment showed a new signal at δ = 167.3 ppm (see Supporting Information). Because this new signal is compatible with the chemical shift of a carboxyl carbon from an ester functional group and substantially different from the original ketone carbonyl signal (δ = 200.5 ppm), it is reasonable that the incorporation of O18 happened in the α,β-unsaturated ester moiety. Finally, the integration of the new signal is about 40% of the carboxyl carbon of the α,β-unsaturated ester (proportional to the amount of H2O18 used in the experiment). Besides supporting our mechanistic proposal of water attack on the cationic cyclic intermediate B, these results suggest that this is the only path in the reaction, without competition with a direct attack of water on the electrophilic alkyne carbon.
Scheme 6. Proposed Mechanism for the Au(I)-Catalyzed 1,4-Enyne Hydration.
We demonstrated the synthetic importance of the polyfunctionalized ketones achieved in this work by preparing a set of biologically valuable31 α-arylidene-γ-lactones 9a–g in good yields through reduction of the corresponding ketones with sodium borohydride (Scheme 7). Reduction of 5ah led to a 5:1 inseparable mixture of α-alkylidene-γ-lactone 9g and its endocyclic olefinic analogue 9g′ in 73% yield. Attempts to convert this mixture into the endocyclic regioisomer 9g′ as a single product by using rhodium(III)chloride hydrate32 was unsuccessful.
Scheme 7. Synthesis of α-Alkylidene-γ-lactones from Tricarbonyl Compounds 5.
Reaction conditions: 5 (0.1 mmol) and NaBH4 (1.2 equiv) in MeOH (1.3 mL), stirred at 0 °C for 30 min. b9g + 9g′ (0.1 mmol), RhCl3·3H2O (1 mol %) in EtOH at 80 °C.
Conclusions
In summary, we have developed an efficient and highly regioselective gold(I)-catalyzed hydration of internal alkynes in a short reaction time and with low catalytic loading. The substrates, which were designed to provide regiocontrol and acceleration of the reaction, furnished polyfunctionalized ketones 5aa–ap with a long carbon chain in excellent yields. The products are valuable intermediates in organic synthesis, as exemplified by the formation of lactones 9a–g upon reduction. In the course of the scope development, we discovered that this methodology could also give the polyfunctionalized α-arylidene-β and γ-butenolides 6a and 6b in excellent yields.
Experimental Section
General Information
Unless otherwise noted, the commercially available reagents were used without any further purification. All experiments were monitored by thin-layer chromatography (TLC) on Merck silica gel 60 F254 precoated plates. The TLC plates were visualized under UV light (254 nm) or by exposure to sulfuric vanillin/dinitrophenylhydrazine/cerium molybdate stains, followed by brief heating with a heat gun. Purification by flash chromatography was performed on silica gel (230–400 mesh). NMR spectra were obtained in CDCl3 at 25 °C using 250/400/500/600 spectrometers. For 1H NMR spectra, the peak due to residual solvent (δ 7.26 ppm) was used as the internal reference. For proton-decoupled 13C NMR spectra, the reference was the central peak of the CDCl3 signal (δ 77.16 ppm). The data are reported as follows: chemical shift in ppm (relative to TMS), multiplicity (s = singlet, brs = broad singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublets, ddd = doublet of doublet of doublets, and qd = quartet of doublets), coupling constants (J) in Hz, and integration. The absorption spectra in the infrared region (IR) were obtained with an Fourier transform infrared spectrophotometer fitted with a Ge crystal. The IR absorbance frequencies were expressed in cm–1. High resolution mass spectrometry (HRMS) was performed using electrospray ionization (ESI) (Synapt Q-TOF.). Melting points were measured and were not corrected. Compounds were named according to IUPAC rules using the MarvinSketch 15.9.21.0 program.
General Procedures for the Synthesis of Allylic Bromides 2a–l
To a stirred solution of MBH adduct 1 in CH2Cl2 (0.1 M), at 0 °C, was added dropwise 48% aqueous HBr solution (6.0 equiv) and then concentrated aqueous H2SO4 solution (5.5 equiv). After stirring overnight at room temperature, the mixture was cooled to 0 °C and diluted with H20. The aqueous phase was extracted with CH2Cl2 (3×), and the combined organic phase was washed with H2O. After removal of solvent under reduced pressure, the residue was purified by flash silica gel column chromatography using a mixture of ethyl acetate/hexane as eluent to afford the corresponding allylic bromide. The spectral data of known allylic bromides are the same as described.25
General Procedures for the Synthesis of Allylic Bromides 2m–p
To a stirred solution of MBH adduct 1 in dry CH2CI2 (0.1 M), PPh3 (2.0 equiv) and CBr4 (2.0 equiv) were added under a nitrogen atmosphere. The colored mixture was stirred at room temperature until TLC analysis showed complete consumption of the start material (1–2 h). After completion, the reaction mixture was quenched with cold water and extracted with CH2CI2 (3×). After removal of solvent under reduced pressure, the residue was purified by flash silica gel column chromatography using a mixture of ethyl acetate/hexane as eluent to afford the corresponding allylic bromide. The spectral data of known allylic bromides are the same as described.26
Ethyl (2Z)-2-(Bromomethyl)-3-(3,4,5-trimethoxyphenyl)prop-2-enoate (2f)
It was obtained as a pale yellow solid (73%): mp 64.5–66.1 °C. 1H NMR (500 MHz, CDCl3): δ 7.76 (s, 1H), 6.87 (s, 2H), 4.44 (s, 2H), 4.33 (q, J = 7.1 Hz, 2H), 3.91–3.90 (m, 9H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 166.2, 153.5 (2C), 143.2, 139.3, 129.8, 128.2, 107.2 (2C), 61.6, 61.1, 56.4 (2C), 27.7, 14.4. IR (ATR, νmax): 2956, 1713, 1253, 1122, 771. HRMS (ESI) m/z calcd for C15H20BrO5+ [M + H]+, 359.0489; found, 359.0463.
(3Z)-3-(Bromomethyl)-4-(3,4,5-trimethoxyphenyl)but-3-en-2-one (2m)
It was obtained as a white solid (80%): mp 98.6–100.3 °C. 1H NMR (250 MHz, CDCl3): δ 7.59 (s, 1H), 6.92 (s, 2H), 4.40 (s, 2H), 3.92–3.91 (m, 9H), 2.50 (s, 3H). 13C NMR (63 MHz, CDCl3): δ 197.2, 153.6 (2C), 143.5, 139.5, 136.7, 129.7, 107.3 (2C), 61.1, 56.5 (2C), 26.1, 26.1. IR (ATR, νmax): 2937, 1662, 1331, 1126, 847. HRMS (ESI) m/z calcd for C14H17BrNaO4+ [M + Na]+, 351.0202; found, 351.0201.
tert-Butyl (2Z)-2-(Bromomethyl)-3-phenylprop-2-enoate (2n)
It was obtained as pale yellow oil (62%): 1H NMR (250 MHz, CDCl3): δ 7.73 (s, 1H), 7.57–7.53 (m, 2H), 7.49–7.38 (m, 3H), 4.36 (s, 2H), 1.58 (s, 9H). 13C NMR (63 MHz, CDCl3): δ 165.4, 141.9, 134.7, 130.6, 129.6 (2C), 129.4, 129.0 (2C), 81.8, 28.2, 27.3. IR (ATR, νmax): 2970, 1704, 1365, 1138, 754, 700. HRMS (ESI) m/z calcd for C14H17BrNaO2+ [M + Na]+, 319.0304; found, 319.0321.
tert-Butyl (2Z)-2-(Bromomethyl)-3-(4-methoxyphenyl)prop-2-enoate (2o)
It was obtained as yellow oil (98%): 1H NMR (250 MHz, CDCl3): δ 7.68 (s, 1H), 7.57–7.52 (m, 2H), 7.01–6.95 (m, 2H), 4.40 (s, 2H), 3.85 (s, 3H), 1.57 (s, 9H). 13C NMR (63 MHz, CDCl3): δ 165.7, 160.7, 141.9, 131.8 (2C), 128.2, 127.2, 114.5 (2C), 81.6, 55.5, 28.3, 28.0. IR (ATR, νmax): 2974, 1700, 1603, 1512, 1255, 1141. HRMS (ESI) m/z calcd for C15H19BrNaO3+ [M + Na]+, 327.0590; found, 327.0573.
General Procedure for the Synthesis of Enynes (4aa–ap)
The corresponding MBH bromide 2 (1.0 mmol), copper iodide (0.2 mmol, 0.2 equiv), and potassium carbonate (1.0 mmol, 1.0 equiv) were added in a round-bottom flask containing a stir bar. The flask was equipped with a rubber septum and was purged with nitrogen using a needle. Dry acetonitrile (10 mL) and appropriate terminal alkyne (1.2 equiv) were then added. The reaction was stirred under a nitrogen atmosphere at the optimized temperature. After 2 or 24 h, 15 mL of saturated aqueous ammonium chloride solution was added, and the resulting mixture was extracted at room temperature with ethyl acetate (3 × 15 mL). After removal of the solvent under reduced pressure, the residue was purified by flash silica gel column chromatography, using a gradient mixture of ethyl acetate/hexane as the eluent, to afford the corresponding enyne.
1,6-Diethyl (5E)-5-(Phenylmethylidene)hex-2-ynedioate (4aa)
It was obtained after 2 h at 60 °C, as colorless oil (73%): 1H NMR (400 MHz, CDCl3): δ 7.83 (s, 1H), 7.44–7.36 (m, 5H), 4.32 (q, J = 7.1 Hz, 2H), 4.21 (q, J = 7.1 Hz, 2H), 3.52 (s, 2H), 1.37 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 166.6, 153.8, 141.8, 134.7, 129.5 (2C), 129.4, 128.9 (2C), 125.9, 86.0, 73.2, 62.0, 61.5, 18.3, 14.37, 14.1, 14.1. IR (ATR, νmax): 2982, 2235, 1704, 1246, 1201, 725. HRMS (ESI) m/z calcd for C17H18NaO4+ [M + Na]+, 309.1097; found, 309.1089.
6-Ethyl 1-Methyl (5E)-5-(phenylmethylidene)hex-2-ynedioate (4ba)
It was obtained after 2 h at 60 °C as colorless oil (75%): 1H NMR (400 MHz, CDCl3): δ 7.83 (s, 1H), 7.44–7.28 (m, 5H), 4.32 (q, J = 7.1 Hz, 2H), 3.75 (s, 3H), 3.52 (s, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 166.6, 154.2, 141.9, 134.6, 129.5 (2C), 129.4, 128.9 (2C), 125.8, 86.5, 72.9, 61.5, 52.7, 18.3, 14.4. IR (ATR, νmax): 2982, 2235, 1704, 1247, 1201, 725. HRMS (ESI) m/z calcd for C16H16NaO4+ [M + Na]+, 295.0941; found, 295.0936.
1-tert-Butyl 6-Ethyl (5E)-5-(Phenylmethylidene)hex-2-ynedioate (4ca)
It was obtained after 2 h at 60 °C as colorless oil (68%): 1H NMR (250 MHz, CDCl3): δ 7.82 (s, 1H), 7.45–7.37 (m, 5H), 4.33 (q, J = 7.1 Hz, 2H), 3.49 (s, 2H), 1.49 (s, 9H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 166.7, 152.85, 141.7, 134.7, 129.6 (2C), 129.3, 128.8 (2C), 126.1, 83.5, 83.2, 74.5, 61.5, 28.1 (3C), 18.2, 14.4. IR (ATR, νmax): 2981, 2240, 1705, 1274, 768. HRMS (ESI) m/z calcd for C19H22NaO4+ [M + Na]+, 337.1410; found, 337.1437.
Ethyl (2E)-6-Oxo-2-(phenylmethylidene)hept-4-ynoate (4da)
It was obtained after 2 h at 60 °C as colorless oil (47%): 1H NMR (250 MHz, CDCl3): δ 7.84 (s, 1H), 7.45–7.37 (m, 5H), 4.33 (q, J = 7.1 Hz, 2H), 3.56 (s, 2H), 2.31 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 184.7, 166.6, 141.7, 134.6, 129.4 (2C), 129.3, 128.8 (2C), 125.9, 90.5, 81.0, 61.4, 32.8, 18.4, 14.3. IR (ATR, νmax): 2983, 2210, 1708, 1676, 1222, 771. HRMS (ESI) m/z calcd for C16H17O3+ [M + H]+, 257.1172; found, 257.1157.
1,6-Diethyl (5E)-5-[(4-Bromophenyl)methylidene]hex-2-ynedioate (4ab)
It was obtained after 24 h at rt as a pale yellow solid (44%) (67% brsm): mp 73.0–75.0 °C. 1H NMR (500 MHz, CDCl3): δ 7.74 (s, 1H), 7.57–7.55 (2H, m), 7.31–7.30 (m, 2H), 4.31 (q, J = 7.1 Hz, 2H), 4.21 (q, J = 7.1 Hz, 2H), 3.48 (s, 2H), 1.36 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 166.4, 153.7, 140.5, 135.5, 132.1 (2C), 131.0 (2C), 126.5, 123.8, 85.4, 73.5, 62.0, 61.7, 18.2, 14.3, 14.1. IR (ATR, νmax): 2982, 2231, 1696, 812, 752, 499. HRMS (ESI) m/z calcd for C17H17BrNaO4+ [M + Na]+, 387.0202; found, 387.0229.
1,6-Diethyl (5E)-5-[(4-Chlorophenyl)methylidene]hex-2-ynedioate (4ac)
It was obtained after 24 h at rt as a pale yellow solid (57%) (>99% brsm): mp 59.0–61.0 °C. 1H NMR (500 MHz, CDCl3): δ 7.76 (s, 1H), 7.40–7.36 (m, 4H), 4.31 (q, J = 7.1 Hz, 2H), 4.21 (q, J = 7.1 Hz, 2H), 3.48 (s, 2H), 1.36 (t, J = 7.1 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 166.3, 153.6, 140.4, 135.4, 133.0, 130.8 (2C), 129.1 (2C), 126.0, 85.4, 73.4, 62.0, 61.6, 18.1, 14.3, 14.1. IR (ATR, νmax): 2982, 2231, 1696, 1253, 813, 754. HRMS (ESI) m/z calcd for C17H17ClNaO4+ [M + Na]+, 343.0708; found, 343.0713.
1,6-Diethyl (5E)-5-[(3-chlorophenyl)methylidene]hex-2-ynedioate (4ad)
It was obtained after 24 h at rt as a pale yellow solid (52%): mp 50.5–52.7 °C 1H NMR (400 MHz, CDCl3): δ 7.75 (s, 1H), 7.40–7.32 (m, 4H), 4.31 (q, J = 7.1 Hz, 2H), 4.22 (q, J = 7.1 Hz, 2H), 3.49 (2H, s), 1.37 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 166.3, 153.7, 140.2, 136.4, 134.9, 130.2, 129.5, 129.4, 127.4, 127.3, 85.4, 73.6, 62.1, 61.7, 18.2, 14.4, 14.1. IR (ATR, νmax): 2982, 2242, 1700, 1262, 797. HRMS (ESI) m/z calcd for C17H17ClNaO4+ [M + Na]+, 343.0708; found, 347.0729.
1,6-Diethyl (5E)-5-[(4-Methoxyphenyl)methylidene]hex-2-ynedioate (4ae)
It was obtained after 2 h at 60 °C as a colorless oil (87%): 1H NMR (250 MHz, CDCl3): δ 7.78, 7.45–7.42 (m, 2H), 6.98–6.95 (m, 2H), 4.31 (q, J = 7.1 Hz, 2H), 4.22 (q, J = 7.1 Hz, 2H), 3.85 (s, 3H), 3.55 (s, 2H), 1.36 (t, J = 7.1 Hz, 3H), 1.30 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 167.0, 160.6, 153.8, 141.6, 131.6 (2C), 127.1, 123.6, 114.4 (2C), 86.3, 73.1, 62.0, 61.4, 55.5, 18.3, 14.4, 14.1. IR (ATR, νmax): 2982, 2235, 1702, 1246, 828, 752. HRMS (ESI) m/z calcd for C18H20NaO5+ [M + Na]+, 339.1203; found, 339.1185.
1,6-Diethyl (5E)-5-[(3,4,5-Trimethoxyphenyl)methylidene]hex-2-ynedioate (4af)
It was obtained after 2 h at 60 °C as a pale yellow solid (88%): mp 49.0–50.2 °C. 1H NMR (500 MHz, CDCl3): δ 7.77 (s, 1H), 7.76 (s, 2H), 4.30 (q, J = 7.0 Hz, 2H), 4.19 (q, J = 7.0 Hz, 2H), 3.89–3.88 (m, 9H), 3.54 (s, 2H), 1.36 (t, J = 7.0 Hz, 3H), 1.27 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 166.6, 153.6, 153.4 (2C), 142.2, 139.0, 130.1, 125.0, 106.9 (2C), 86.1, 73.4, 62.0, 61.6, 61.0, 56.3 (2C), 18.5, 14.4, 14.1. IR (ATR, νmax): 2942, 2232, 1704, 1245, 1124, 857. HRMS (ESI) m/z calcd for C20H24NaO7+ [M + Na]+, 399.1414; found, 399.1386.
1,6-Diethyl (5E)-5-[(2E)-3-phenylprop-2-en-1-ylidene]hex-2-ynedioate (4ag)
It was obtained after 2 h at 60 °C as yellow oil (49%): 1H NMR (250 MHz, CDCl3): δ 7.54–7.32 (m, 6H), 7.16–6.94 (m, 2H), 4.28 (q, J = 7.1 Hz, 2H), 4.19 (q, J = 7.1 Hz, 2H), 3.57 (s, 2H), 1.35 (t, J = 7.1 Hz, 3H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 166.6, 153.8, 142.1, 141.1, 136.2, 129.5, 129.0 (2C), 127.6 (2C), 123.9, 122.9, 85.9, 73.1, 62.0, 61.3, 17.0, 14.4, 14.1. IR (ATR, νmax): 2983, 2237, 1706, 1251, 750. HRMS (ESI) m/z calcd for C19H20NaO4+ [M + Na]+, 335.1254; found, 335.1248.
1,6-Diethyl (5E)-5-propylidenehex-2-ynedioate (4ah)
It was obtained after 2 h at 60 °C as colorless oil (73%): 1H NMR (500 MHz, CDCl3): δ 6.91 (t, J = 7.5 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 4.20 (q, J = 7.1 Hz, 2H), 3.37 (s, 1H), 2.29 (quint, J = 7.5 Hz, 2H), 1.31 (t, J = 7.1 Hz, 3H), 1.29 (t, J = 7.5 Hz, 3H), 1.10 (t, J = 7.5 Hz). 13C NMR (126 MHz, CDCl3): δ 166.0, 153.5, 147.2, 125.2, 85.8, 72.5, 61.7, 60.9, 22.3, 16.3, 14.1, 13.9, 12.7. IR (ATR, νmax): 2980, 2236, 1706, 1247, 1074, 754. HRMS (ESI) m/z calcd for C13H18NaO4+ [M + Na]+, 261.1097; found, 261.1075.
1-Ethyl 6-Methyl (5E)-5-(phenylmethylidene)hex-2-ynedioate (4ai)
It was obtained after 2 h at 60 °C as colorless oil (72%): 1H NMR (400 MHz, CDCl3): δ 7.84 (s, 1H), 7.44–7.37 (m, 5H), 4.22 (q, J = 7.1 Hz, 2H), 3.96 (s, 3H), 3.52 (s, 2H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 167.1, 153.8, 142.2, 134.6, 129.6 (2C), 129.5, 128.9 (2C), 125.6, 85.9, 73.3, 62.0, 52.6, 18.3, 14.1. IR (ATR, νmax): 2985, 2236, 1704, 1247, 752. HRMS (ESI) m/z calcd for C16H16NaO4+ [M + Na]+, 295.0941; found, 295.0936.
1-Ethyl 6-Methyl (5E)-5-(Cyclohexylmethylidene)hex-2-ynedioate (4aj)
It was obtained after 2 h at 60 °C as yellow oil (72%): 1H NMR (250 MHz, CDCl3): δ 6.74 (d, J = 10 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.76 (s, 3H), 3.36 (s, 2H), 2.45–2.33 (m, 1H), 1.77–1.66 (m, 5H), 1.40–1.09 (m, 8H). 13C NMR (63 MHz, CDCl3): δ 167.1, 153.8, 151.1, 123.6, 86.3, 72.7, 61.9, 52.2, 38.4, 31.8 (2C), 25.8 (2C), 25.5, 16.7, 14.1. IR (ATR, νmax): 2931, 2854, 1712, 1250, 751. HRMS (ESI) m/z calcd for C16H22NaO4+ [M + Na]+, 301.1410; found, 301.1380.
1-Ethyl 6-Methyl (5E)-5-[(1,1′-Biphenyl)-4-ylmethylidene]hex-2-ynedioate (4ak)
It was obtained after 2 h at 60 °C as pale yellow oil (70%): 1H NMR (250 MHz, CDCl3): δ 7.88 (br s, 1H), 7.70–7.60 (m, 4H), 7.55–7.30 (m, 5H), 4.23 (q, J = 7.1 Hz, 2H), 3.88 (s, 3H), 3.59 (s, 2H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 167.2, 153.8, 142.3, 141.8, 140.2, 133.4, 130.2 (2C), 129.1 (2C), 128.0, 127.5 (2C), 127.2 (2C), 125.4, 85.9, 73.3, 62.1, 52.6, 18.4, 14.2.IR (ATR, νmax): 2951, 1743, 1715, 1247, 750. HRMS (ESI) m/z calcd for C22H21O4+ [M + H]+, 349.1435; found, 349.1431.
1-Ethyl 6-Methyl (5E)-5-[(2H-1,3-Benzodioxol-5-yl)methylidene]hex-2-ynedioate (4al)
It was obtained after 2 h at 60 °C as a pale yellow solid (79%): mp 80.9–83.7 °C. 1H NMR (250 MHz, CDCl3): δ 7.73 (s, 1H), 7.00–6.96 (m, 2H), 6.86 (d, J = 7.9 Hz, 1H), 6.02 (s, 2H), 4.24 (q, J = 7.1 Hz, 2H), 3.85 (s, 3H), 3.53 (s, 2H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 167.3, 153.8, 148.8, 148.2, 141.9, 128.5, 124.9, 123.8, 109.57, 108.8, 101.7, 85.8, 73.3, 62.0, 52.6, 18.3, 14.1. IR (ATR, νmax): 2995, 1743, 1714, 1236, 1036, 813. HRMS (ESI) m/z calcd for C17H16NaO6+ [M + Na]+, 339.0839; found, 339.0868.
1-Ethyl 6-Methyl (5E)-5-[(furan-2-yl)methylidene]hex-2-ynedioate (4am)
It was obtained after 2 h at 60 °C as yellow oil (69%): 1H NMR (250 MHz, CDCl3): δ 7.60 (d, J = 1.8 Hz, 1H), 7.51 (s, 1H), 6.74 (d, J = 3.5 Hz, 1H), 6.53 (dd, J = 3.5 Hz, 1.8 Hz, 1H), 4.18 (q, J = 7.2 Hz, 2H), 3.84–3.82 (m, 5H), 1.28 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 167.4, 153.9, 150.5, 145.6, 127.9, 120.9, 117.5, 112.5, 86.2, 72.5, 62.0, 52.6, 18.0, 14.2. IR (ATR, νmax): 2982, 2235, 1704, 1247, 1201, 725. HRMS (ESI) m/z calcd for C14H14NaO5+ [M + Na]+, 285.0733; found, 285.0716.
Ethyl (5E)-6-Oxo-5-[(3,4,5-trimethoxyphenyl)methylidene]hept-2-ynoate (4an)
It was obtained after 2 h at 60 °C as a yellow solid (80%): mp 92.7–94.3 °C. 1H NMR (250 MHz, CDCl3): δ 7.60 (s, 1H), 6.80 (s, 2H), 4.18 (q, J = 7.1 Hz, 2H), 390–3.89 (m, 9H), 3.52 (s, 2H), 2.48 (s, 3H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 197.5, 153.5 (2C), 143.1, 139.4, 134.2, 130.0, 107.1 (2C), 86.4, 73.2, 62.0, 61.1, 56.4 (2C), 25.8, 17.1, 14.1. IR (ATR, νmax): 2995, 2233, 1708, 1251, 1126, 752. HRMS (ESI) m/z calcd for C19H22NaO6+ [M + Na]+, 366.1309; found, 369.1290.
6-tert-Butyl 1-Ethyl (5E)-5-(Phenylmethylidene)hex-2-ynedioate (4ao)
It was obtained after 2 h at 60 °C as colorless oil (60%): 1H NMR (250 MHz, CDCl3): δ 7.93 (br s, 1H), 7.61–7.46 (m, 5H), 4.41 (q, J = 7.1 Hz, 2H), 3.66 (s, 2H), 1.76 (s, 9H), 1.49 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 165.7, 153.8, 140.9, 134.9, 129.4 (2C), 129.1, 128.8 (2C), 127.4, 86.3, 81.8, 73.1, 61.9, 28.2 (3C), 18.4, 14.1. IR (ATR, νmax): 2978, 2235, 1704, 1247, 1158, 752. HRMS (ESI) m/z calcd for C19H22NaO4+ [M + Na]+, 337.1410; found, 337.1416.
6-tert-Butyl 1-Ethyl (5E)-5-[(4-Methoxyphenyl)methylidene]hex-2-ynedioate (4ap)
It was obtained after 2 h at 60 °C as colorless oil (85%): 1H NMR (250 MHz, CDCl3): δ 7.68 (br s, 1H), 7.42–7.36 (m, 2H), 6.97–6.91 (m, 2H), 4.21 (q, J = 7.1 Hz, 2H), 3.83 (s, 3H), 3.49 (s, 2H), 1.55 (s, 9H), 1.29 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 165.9, 160.2, 153.7, 140.5, 131.2 (2C), 127.2, 125.0, 114.2 (2C), 86.4, 81.4, 72.8, 61.8, 55.3, 28.1, 18.2, 14.0. IR (ATR, νmax): 2978, 2235, 1704, 1246, 1154, 752. HRMS (ESI) m/z calcd for C20H24NaO5+ [M + Na]+, 367.1516; found, 367.1500.
Ethyl (5E)-5-Methyl-6-phenylhex-5-en-ynoate (7)
Enyne 7 was prepared according to a literature procedure:33 to an oven-dried nitrogen purged flask containing a stir bar, E-(2-methyl-pent-1-en-4-ynyl)-benzene (6.4 mmol) and dry THF (10.5 mL) were added. The system was cooled to −78 °C using a dry ice-acetone cold bath. Then n-butyllithium (2.92 mL of 2.3 M in hexanes, 1.05 equiv) was added dropwise to the solution and stirred for 30 min. Next, freshly distilled ethyl chloroformate (0.396 mL, 4.02 mmol, 1.0 equiv) was added dropwise. The reaction temperature was held at −78 °C for 6 h and allowed to warm to room temperature overnight. The reaction was quenched with saturated aqueous ammonium chloride (30 mL) and extracted with CH2Cl2 (3 × 10 mL). After removal of solvent under reduced pressure, the residue was purified by flash silica gel column chromatography using a gradient mixture of ethyl acetate/hexane as eluent to afford the desired product 7 as pale yellow oil, 51% (68% brsm). 1H NMR (250 MHz, CDCl3): δ 7.37–7.20 (m, 5H), 6.54 (br s, 1H), 4.25 (q, J = 7.1 Hz, 2H), 3.22 (d, J = 1.0 Hz, 2H), 1.94 (d, J = 1.2 Hz, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 153.9, 137.5, 131.2, 128.9 (2C), 128.2 (2C), 127.7, 126.7, 86.0, 75.5, 62.0, 29.6, 17.9, 14.2. IR (ATR, νmax): 2988, 2235, 1706, 1246, 1069, 752. HRMS (ESI) m/z calcd for C15H17O2+ [M + H]+, 229.1223; found, 229.1237.
General Procedure for the Synthesis of Hydrated Products (5aa–ap, 6a,6b)
In a 10 mL round-bottom flask containing a stir bar, enyne 4aa–ap (0.2 mmol), chloro(triethylphosphine)gold(I) (0.01 equiv), silver triflate (0.02 equiv), acetonitrile (0.33 mL), and distilled water (0.17 mL) were added. The reaction mixture was stirred at 50 °C for 3 h. After reaction time, the solvent was removed under reduced pressure. The residue was purified by flash silica gel column chromatography using a gradient mixture of ethyl acetate/hexane as eluent to afford the corresponding product.
1,6-Diethyl (2E)-2-Methoxyphenylmethylidene-4-oxohexanedioate (5aa)
It was obtained as colorless oil (93%): 1H NMR (400 MHz, CDCl3): δ 7.94 (s, 1H), 7.40–7.30 (m, 5H), 4.22 (q, J = 7.1 Hz, 2H), 4.18 (q, J = 7.1 Hz, 2H), 3.72 (s, 2H), 3.57 (s, 2H), 1.32 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 200.4, 167.3, 167.1, 142.7, 135.0, 129.1, 128.9 (2C), 128.8 (2C), 126.3, 61.5, 61.4, 49.62, 42.0, 14.3, 14.2. IR (ATR, νmax): 2982, 1745, 1704, 1260, 1026, 514. HRMS (ESI) m/z calcd for C17H20NaO5+ [M + Na]+, 327.1203; found, 327.1208.
1-Ethyl 6-Methyl (2E)-4-Oxo-2-(phenylmethylidene)hexanedioate (5ba)
It was obtained as colorless oil (90%): 1H NMR (400 MHz, CDCl3): δ 7.95 (s, 1H), 7.41–7.31 (m, 5H), 4.26 (q, J = 7.1 Hz, 2H), 3.73 (s, 3H), 3.72 (s, 2H), 3.60 (s, 2H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 200.3, 167.5, 167.3, 142.8, 135.0, 129.1, 128.9 (2C), 128.8 (2C), 126.3, 61.4, 52.5, 49.3, 42.0, 14.3. IR (ATR, νmax): 2983, 1748, 1702, 1262, 1203, 514. HRMS (ESI) m/z calcd for C16H18NaO5+ [M + Na]+, 313.1046; found, 313.1055.
6-tert-Butyl 1-Ethyl (2E)-4-Oxo-2-(phenylmethylidene)hexanedioate (5ca)
It was obtained as colorless oil (94%): 1H NMR (250 MHz, CDCl3): δ 7.94 (s, 1H), 7.42–7.29 (m, 5H), 4.25 (q, J = 7.1 Hz, 2H), 3.72 (s, 2H), 3.48 (s, 2H), 1.45 (s, 9H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 200.9, 167.3, 166.3, 142.6, 135.1, 129.0 (2C), 129.0, 128.7 (2C), 126.5, 82.1, 61.4, 51.0, 41.9, 28.1, 14.3. IR (ATR, νmax): 2981, 1714, 1264, 1142, 763. HRMS (ESI) m/z calcd for C19H24NaO5+ [M + Na]+, 355.1516; found, 355.1467.
Ethyl (2E)-4,6-Dioxo-2-(phenylmethylidene)heptanoate (5da)
It was obtained as colorless oil (91%): (keto form/enol form = 2:3.5) 1H NMR (250 MHz, CDCl3) (enol form): δ 15.34 (br s, 1H), 7.95 (br s, 1H), 7.40–7.29 (m, 5H), 5.57 (s, 1H), 4.28 (q, J = 7.1 Hz, 2H), 3.57 (s, 2H), 2.04 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H). (keto form): δ 7.98 (br s, 1H), 4.26 (q, J = 7.1 Hz, 2H), 3.70–3.68 (m, 4H), 2.26 (s, 3H). 13C NMR (126 MHz, CDCl3) (enol form): δ 194.8, 188.03 167.6, 142.5, 135.1, 129.3 (2C), 129.1, 128.8 (2C), 126.8, 99.6, 61.4, 38.1, 24.0, 14.3. IR (ATR, νmax): 2978, 1706, 1620, 1219, 1094, 706. HRMS (ESI) m/z calcd for C16H18NaO4+ [M + Na]+, 297.1097; found, 297.1074.
1,6-Diethyl (2E)-2-[(4-Bromophenyl)methylidene]-4-oxohexanedioate (5ab)
It was obtained as colorless oil (92%): 1H NMR (500 MHz, CDCl3): δ 7.86 (s, 1H), 7.52–7.50 (m, 2H), 7.21–7.19 (m, 2H), 4.26 (q, J = 7.1 Hz, 2H), 4.19 (q, J = 7.1 Hz, 2H), 3.69 (s, 2H), 3.58 (s, 2H), 1.33 (t, J = 7.1 Hz, 3H) 1.27 (s, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 200.4, 167.1, 167.0, 141.5, 133.9, 132.0 (2C), 130.5 (2), 127.0, 123.5, 61.6, 61.6, 49.7, 42.0, 14.3, 14.2. IR (ATR, νmax): 2980, 1748, 1702, 1260, 1028, 510. HRMS (ESI) m/z calcd for C17H19BrNaO5+ [M + Na]+, 405.0308; found, 405.0330.
1,6-Diethyl (2E)-2-[(4-Chlorophenyl)methylidene]-4-oxohexanedioate (5ac)
It was obtained as colorless oil (93%): 1H NMR (400 MHz, CDCl3): δ 7.88 (s, 1H), 7.36–7.30 (m, 2H), 7.27–7.25 (m, 2H), 4.25 (q, J = 7.1 Hz, 2H), 4.19 (q, J = 7.1 Hz, 2H), 3.70 (s, 2H), 3.58 (s, 2H), 1.32 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 200.4, 167.1, 167.0, 141.4, 135.2, 133.4, 130.7, 130.3 (2C), 129.0 (2C), 126.9, 61.6, 61.5, 49.6, 41.9, 14.3, 14.2. IR (ATR, νmax): 2983, 1743, 1704, 1015, 514. HRMS (ESI) m/z calcd for C17H19ClNaO5+ [M + Na]+, 361.0813; found, 361.0821.
1,6-Diethyl (2E)-2-[(3-Chlorophenyl)methylidene]-4-oxohexanedioate (5ad)
It was obtained as colorless oil (91%): 1H NMR (500 MHz, CDCl3): δ 7.86 (s, 1H), 7.32–7.20 (m, 4H), 4.26 (q, J = 7.1 Hz, 2H) 4.19 (q, J = 7.1 Hz, 2H) 3.70 (s, 2H), 3.58 (s, 2H), 1.33 (t, J = 7.1 Hz, 3H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 200.2, 167.0, 166.9, 141.1, 136.9, 134.7, 130.1, 129.1, 128.8, 127.7, 126.9, 61.7, 61.6, 49.7, 41.9, 14.3, 14.2. IR (ATR, νmax): 2982, 1745, 1704, 1089, 789. HRMS (ESI) m/z calcd for C17H19ClNaO5+ [M + Na]+, 361.0813; found, 361.0819.
1,6-Diethyl (2E)-2-[(4-Methoxyphenyl)methylidene]-4-oxohexanedioate (5ae)
It was obtained as colorless oil (95%): 1H NMR (400 MHz, CDCl3): δ 7.89 (s, 1H), 7.32–7.28 (m, 2H), 6.92–6.88 (m, 2H), 4.24 (q, J = 7.1 Hz, 2H), 4.18 (q, J = 7.1 Hz, 2H), 3.82 (s, 3H), 3.75 (s, 2H), 3.58 (s, 2H), 1.32 (t, J = 7.1 Hz, 3H) 1.26 (s, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 200.6, 167.5, 167.1, 160.3, 142.4, 130.8 (2C), 127.3, 123.9, 114.1 (2C), 61.4, 61.1, 55.3, 49.4, 42.0, 14.2, 14.1. IR (ATR, νmax): 2983, 1743, 1698, 1028, 752, 534. HRMS (ESI) m/z calcd for C18H22NaO6+ [M + Na]+, 357.1309; found, 357.1313.
1,6-Diethyl (2E)-4-Oxo-2-[(3,4,5-trimethoxyphenyl)methylidene]hexanedioate (5af)
It was obtained as pale yellow oil (93%): 1H NMR (250 MHz, CDCl3): δ 7.89 (s, 1H), 6.61 (s, 2H), 4.26 (q, J = 7.1 Hz, 2H), 4.27 (q, J = 7.1 Hz, 2H), 3.91–3.80 (m, 9H), 3.75 (s, 2H), 3.60 (s, 2H), 1.34 (t, J = 7.1 Hz, 3H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 201.2, 167.3, 167.2, 153.4 (2C), 143.2, 138.9, 130.4, 125.6, 106.2 (2C), 61.6, 61.5, 61.0, 56.3, 49.6, 42.4, 14.4, 14.2. IR (ATR, νmax): 2975, 1745, 1715, 1258, 1126. HRMS (ESI) m/z calcd for C20H26NaO8+ [M + Na]+, 417.1520; found, 417.1537.
1,6-Diethyl (2E)-4-Oxo-2-[(2E)-3-phenylprop-2-en-1-ylidene]hexanedioate (5ag)
It was obtained as yellow oil (94%): 1H NMR (250 MHz, CDCl3): δ 7.59–7.46 (m, 3H), 7.38–7.30 (m, 3H), 6.98–6.96 (m, 2H), 4.23 (q, J = 7.1 Hz, 2H), 4.21 (q, J = 7.1 Hz, 2H), 3.75 (s, 2H), 3.56 (s, 2H), 1.32 (t, J = 7.1 Hz, 3H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 13C NMR (63 MHz, CDCl3): δ 199.7, 167.4, 167.3, 142.1, 141.8, 136.2, 129.3, 128.9 (2C), 127.5 (2C), 123.9, 123.2, 61.6, 61.2, 48.8, 41.5, 14.4, 14.2. IR (ATR, νmax): 2985, 1745, 1718, 1697, 1238, 750. HRMS (ESI) m/z calcd for C19H22NaO5+ [M + Na]+, 353.1359; found, 353.1341.
1,6-Diethyl (2E)-4-Oxo-2-propylidenehexanedioate (5ah)
It was obtained as colorless oil (95%): 1H NMR (400 MHz, CDCl3): δ 6.99 (t, J = 7.5 Hz, 1H), 4.18 (quint, J = 7.1 Hz, 4H), 3.53 (s, 2H), 3.50 (s, 2H), 2.16 (quint, J = 7.5 Hz, 2H), 1.27 (t, J = 7.1 Hz, 6H), 1.05 (t, J = 7.5 Hz, H). 13C NMR (101 MHz, CDCl3): δ 199.7, 167.3, 167.0, 148.1, 124.9, 61.5, 61.0, 49.0, 40.9, 22.5, 14.3, 14.2, 13.1. IR (ATR, νmax): 2978, 1745, 1704, 1249, 1028. HRMS (ESI) m/z calcd for C13H20NaO5+ [M + Na]+, 279.1203; found, 279.1208.
6-Ethyl 1-Methyl (2E)-4-oxo-2-(phenylmethylidene)hexanedioate (5ai)
It was obtained as colorless oil (92%): 1H NMR (400 MHz, CDCl3): δ 7.95 (s, 1H), 7.40–7.30 (m, 5H), 4.19 (q, J = 7.1 Hz, 2H), 3.80 (s, 3H), 3.74 (s, 2H), 3.57 (s, 2H), 1.26 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 200.4, 167.8, 167.1, 143.0, 135.0, 129.2, 129.0 (2C), 128.8 (2C), 126.0, 61.6, 52.4, 49.6, 42.0, 14.2. IR (ATR, νmax): 2985, 1743, 1707, 1264, 1205, 512. HRMS (ESI) m/z calcd for C16H18NaO5+ [M + Na]+, 313.1046; found, 313.1046.
6-Ethyl 1-Methyl (2E)-2-(Cyclohexylmethylidene)-4-oxohexanedioate (5aj)
It was obtained as colorless oil (93%): 1H NMR (250 MHz, CDCl3): δ 6.83 (d, J = 10.1 Hz, 1H), 4.20 (q, J = 7.1 Hz, 2H), 3.72 (s, 3H), 3.54–3.53 (m, 4H), 2.27–2.19 (m, 1H), 1.71–1.56 (m, 5H), 1.36–1.10 (m, 8H). 13C NMR (63 MHz, CDCl3): δ 199.9, 167.8, 167.3, 151.7, 123.3, 61.5, 52.1, 49.2, 41.1, 38.4, 32.0 (2C), 25.9, 25.5 (2C), 14.3. IR (ATR, νmax): 2927, 2852, 1747, 1717, 1226. HRMS (ESI) m/z calcd for C16H24NaO5+ [M + Na]+, 319.1516; found, 319.1499.
6-Ethyl 1-Methyl (2E)-2-[(1,1′-Biphenyl)-4-ylmethylidene]-4-oxohexanedioate (5ak)
It was obtained as colorless oil (93%): 1H NMR (250 MHz, CDCl3): δ 8.01 (br s, 1H), 7.70–7.60 (m, 4H), 7.55–7.35 (m, 5H), 4.23 (q, J = 7.1 Hz, 2H), 3.84 (m, 5H), 3.63 (s, 2H), 1.29 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 200.4, 167.8, 167.1, 142.6, 142.0, 140.3, 133.8, 129.6 (2C), 129.0 (2C), 127.9, 127.4 (2C), 127.2 (2C), 125.8, 61.6, 52.5, 49.6, 42.2, 14.2.. IR (ATR, νmax): 2966, 1742, 1256, 1217, 753. HRMS (ESI) m/z calcd for C22H22NaO5+ [M + Na]+, 389.1360; found, 389.1349.
6-Ethyl 1-Methyl (2E)-2-[(2H-1,3-Benzodioxol-5-yl)methylidene]-4-oxohexanedioate (5al)
It was obtained as yellow oil (95%): 1H NMR (250 MHz, CDCl3): δ 7.58 (br s, 1H), 7.52 (d, J = 1.8 Hz, 1H), 6.66 (d, J = 3.5 Hz, 1H), 6.48 (dd, J = 1.8 Hz, 3.5 Hz, 1H), 4.21 (q, J = 7.1 Hz, 2H), 4.05 (s, 2H), 3.79 (s, 3H), 3.53 (s, 2H), 1.28 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 199.7, 168.1, 167.2, 151.0, 145.2, 128.5, 120.9, 117.3, 112.4, 61.5, 52.5, 49.2, 42.4, 14.2. IR (ATR, νmax): 2963, 1749, 1720, 1286, 1215, 753. HRMS (ESI) m/z calcd for C17H18NaO7+ [M + Na]+, 357.0945; found, 357.0931.
6-Ethyl 1-Methyl (2E)-2-[(Furan-2-yl)methylidene]-4-oxohexanedioate (5am)
It was obtained as yellow oil (95%): 1H NMR (250 MHz, CDCl3): δ 7.58 (br s, 1H), 7.52 (d, J = 1.8 Hz, 1H), 6.66 (d, J = 3.5 Hz, 1H), 6.48 (dd, J = 1.8 Hz, 3.5 Hz, 1H), 4.21 (q, J = 7.1 Hz, 2H), 4.05 (s, 2H), 3.79 (s, 3H), 3.53 (s, 2H), 1.28 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 199.7, 168.1, 167.2, 151.0, 145.2, 128.5, 120.9, 117.3, 112.4, 61.5, 52.5, 49.2, 42.4, 14.2. IR (ATR, νmax): 2963, 1749, 1720, 1286, 1215, 753. HRMS (ESI) m/z calcd for C14H16NaO6+ [M + Na]+, 303.0839; found, 303.0852.
Ethyl (5E)-3,6-Dioxo-5-[(3,4,5-trimethoxyphenyl)methylidene]heptanoate (5an)
It was obtained as yellow oil (92%): 1H NMR (250 MHz, CDCl3): δ 7.72 (br s, 1H), 6.64 (br s, 2H), 4.17 (q, J = 7.1 Hz, 2H), 3.88–6.86 (m, 9H), 3.70 (s, 2H), 3.62 (s, 2H), 2.46 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 201.4, 199.0, 167.3, 153.5, 144.1, 139.1, 135.6, 130.4, 106.3, 61.5, 61.1, 56.3, 41.3, 25.4, 14.2. IR (ATR, νmax): 2987, 1742, 1716, 1334, 1112. HRMS (ESI) m/z calcd for C19H24NaO7+ [M + Na]+, 387.1414; found, 387.1442.
Ethyl 2-[(2Z,4E)-5-Oxo-4-(phenylmethylidene)oxolan-2-ylidene]acetate (6a′)
It was obtained after filtration on a plug of Celite as a yellow solid (>99%): mp 171.6–173.2 °C. 1H NMR (250 MHz, CDCl3): δ 7.69 (t, J = 2.8 Hz, 1H), 7.51–7.45 (m, 5H), 5.26 (t, J = 1.6 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H), 9.93 (dd, J = 2.8 Hz, 1.6 Hz), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 168.8, 163.9, 158.6, 139.3, 133.8, 131.0, 130.5 (2C), 129.3 (2C), 118.7, 96.5, 60.5, 32.7, 14.4. IR (ATR, νmax): 2983, 1795, 1709, 1151, 765. HRMS (ESI) m/z calcd for C15H14NaO4+ [M + Na]+, 281.0784; found, 281.1171.
Ethyl 2-[(4E)-5-Oxo-4-(phenylmethylidene)-4,5-dihydrofuran-2-yl]acetate (6a)
It was obtained as yellow oil: (>99%) 1H NMR (250 MHz, CDCl3): δ 7.58–7.54 (m, 2H), 7.47–7.39 (m, 4H), 6.56 (q, J = Hz, 1.1 Hz, 1H), 4.22 (q, J = 7.1 Hz, 2H), 3.53 (t, J = 1.1 Hz, 2H), 1.30 (t, J = 4.1 Hz, 3H). 13C NMR (151 MHz, CDCl3): δ 169.2, 167.6, 153.6, 136.3, 134.9, 130.5 (2C), 130.2, 129.2 (2C), 124.8, 104.4, 61.8, 34.9, 14.2. IR (ATR, νmax): 2980, 1769, 1734, 1158, 763HRMS (ESI) m/z calcd for C15H14NaO4+ [M + Na]+, 281.0784; found, 281.0783.
Ethyl2-[(4E)-4-[(4-Methoxyphenyl)methylidene]-5-oxo-4,5-dihydrofuran-2-yl]acetate (6b)
It was obtained as a yellow solid (90%): mp 153.9–156.0 °C. 1H NMR (250 MHz, CDCl3): δ 7.55–7.52 (m, 2H), 7.33 (br s, 1H), 6.96–6.92 (m, 2H), 6.54 (br s, 1H), 4.21 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.52 (br s, 2H), 1.29 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 169.7, 167.8, 161.6, 152.5, 136.3, 132.2 (2C), 122.2, 114.8 (2C), 104.3, 61.8, 55.6, 34.9, 14.3. IR (ATR, νmax): 2924, 1776, 1715, 1151, 746. HRMS (ESI) m/z calcd for C16H16NaO5+ [M + Na]+, 311.0890; found, 311.0865.
Ethyl (5E)-5-Methyl-3-oxo-6-phenylhex-5-enoate (8)
It was obtained as colorless oil, (49%) (77% brsm) (keto form/enol form = 7.7:1) 1H NMR (250 MHz, CDCl3) (keto form): δ 7.35–7.23 (m, 5H), 6.41 (br s, 1H), 4.20 (q, J = 7.1 Hz, 2H), 3.53 (s, 2H), 3.38 (d, J = 0.9 Hz, 2H), 1.90 (d, J = 1.3 Hz, 3H), 1.28 (t, J = 7.1 Hz, 3H). (enol form): δ 12.14 (s, 1H), 5.09 (s, 1H), 3.06 (s, 2H), 1.91 (d, J = 1.3 Hz, 3H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 201.1, 167.3, 137.5, 131.5, 130.5, 129.0 (2C), 128.3 (2C), 126.8, 61.6, 54.9, 48.4, 18.3, 14.2. IR (ATR, νmax): 2982, 1745, 1719, 1316, 1255, 704. HRMS (ESI) m/z calcd for C15H18NaO3+ [M + Na]+, 269.1148; found, 269.1147.
General Procedure for the Synthesis of Lactones (9a–g)
In a 10 mL round-bottom flask containing a stir bar was added ketone 5 (0.1 mmol), methanol (1.3 mL), and sodium borohydride (0.12 mmol, 1.2 equiv). The reaction mixture was stirred for 30 min at 0 °C. After complete consumption of the standard material, the reaction was quenched with H2O (10 mL) and extracted with ethyl acetate (3 × 10 mL) to afford pure lactones 9a–g.
Ethyl 2-[(4E)-5-Oxo-4-(phenylmethylidene)oxolan-2-yl]acetate (9a)
It was obtained as colorless oil (83%): 1H NMR (500 MHz, CDCl3): δ 7.58 (t, J = 2.7 Hz, 1H), 7.49–7.39 (m, 5H), 5.00 (dddd, J = 8.0, 7.3, 6.0, 5.3 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.48 (ddd, J = 17.7, 8.0, 2.7 Hz, 1H), 2.97 (ddd, J = 17.7, 5.3, 2.7 Hz, 1H), 2.89 (dd, J = 16.3 Hz, 6.0 Hz, 1H), 2.69 (dd, J = 16.3 Hz, 7.3 Hz, 1H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 171.6, 169.6, 137.3, 134.6, 130.2 (2C), 130.1, 129.1 (2C), 123.8, 73.4, 61.2, 40.9, 33.5, 14.3. IR (ATR, νmax): 2963, 1730, 1652, 1270, 1154, 514. HRMS (ESI) m/z calcd for C15H17O4+ [M + H]+, 261.1121; found, 261.1127.
Ethyl 2-[(4E)-4-[(4-Methoxyphenyl)methylidene]-5-oxolan-2-yl]acetate (9b)
It was obtained as a pale yellow solid (75%): mp 84.5–86.2 °C. 1H NMR (500 MHz, CDCl3): δ 7.53 (t, J = 2.7 Hz, 1H), 7.46–7.44 (m, 2H), 6.96–6.95 (m, 2H), 5,00 (dddd, J = 8.2, 7.3, 6.0, 5.3 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.85 (s, 3H), 3.45 (ddd, J = 17.5, 8.2, 2.7 Hz, 1H), 2.92 (ddd, J = 17.5, 5.3, 2.7 Hz, 1H), 2.89 (dd, J = 16.3 Hz, 6.0 Hz, 1H), 2.62 (dd, J = 16.3 Hz, 7.3 Hz, 1H), 1.28 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 172.0, 169.7, 161.1, 137.0, 132.0 (2C), 127.4, 120.8, 114.6 (2C), 73.2, 61.1, 55.5, 40.9, 33.5, 14.2. IR 2967, 1726, 1650, 1259, 1171, 541. (ATR, νmax): 2 HRMS (ESI) m/z calcd for C16H19O5+ [M + H]+, 291.1227; found, 291.1220.
Ethyl 2-[(4E)-5-Oxo-4-[(3,4,5-trimethoxyphenyl)methylidene]oxolan-2-yl]acetate (9c)
It was obtained as colorless oil (74%): 1H NMR (250 MHz, CDCl3): δ 7.50 (t, J = 2.9 Hz, 1H), 6.71 (s, 2H), 5.06–4.96 (m, 1H), 4.19 (q, J = 7.2 Hz, 2H), 3.90–3.89 (m, 9H), 3.48 (ddd, J = 17.5, 8.1, 2.9 Hz, 1H), 2.97 (ddd, J = 7.5, 5.3, 2.9 Hz, 1H), 2.89 (dd, J = 16.3 Hz, 5.9 Hz), 2.70 (dd, J = 16.3 hz, 7.1 Hz, 1H), 1.28 (t. J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 171.6, 169.6, 153.6, 140.2, 137.5, 130.1, 122.7, 107.7, 73.3, 61.2, 61.2, 56.4, 40.8, 33.3, 29.8, 14.3. IR (ATR, νmax): 2920, 2849, 1741, 1651, 1199, 1127. HRMS (ESI) m/z calcd for C18H22NaO7+ [M + Na]+, 373.1258; found, 273.1264.
tert-Butyl 2-[(4E)-5-Oxo-4-(phenylmethylidene)oxolan-2-yl]acetate (9d)
It was obtained as colorless oil (78%): 1H NMR (250 MHz, CDCl3): δ 7.58 (t, J = 2.9 Hz, 1H), 7.52–7.40 (m, 5H), 4.96 (dddd, J = 8.1, 7.3, 5.8, 5.4 Hz, 1H), 3.46 (ddd, J = 17.7, 8.1, 2.9 Hz, 1H), 2.97 (ddd, J = 17.7, 5.4, 2.9 Hz, 1H), 2.82 (dd, J = 16.0 Hz, 5.8 Hz, 1H), 2.61 (dd, J = 16.0 Hz, 7.3 Hz, 1H), 1.47 (s, 9H). 13C NMR (63 MHz, CDCl3): δ 171.7, 168.9, 137.2, 134.7, 130.2 (2C), 130.1, 129.1 (2C), 124.0, 81.9, 73.6, 42.1, 33.5, 28.2 (3C). IR. (ATR, νmax): 2983, 1753, 1729, 1654, 1178, 1148. HRMS (ESI) m/z calcd for C17H21O4+ [M + H]+, 289.1434; found, 289.1407.
Ethyl 2-[(4E)-4-[(Furan-2-yl)methylidene]-5-oxooxolan-2-yl]acetate (9e)
It was obtained as colorless oil (65%): 1H NMR (500 MHz, CDCl3): δ 7.58 (d, J = 1.7 Hz, 1H), 7.32 (t, J = 2.8 Hz, 1H), 6.67 (d, J = 3.4 Hz, 1H), 6.53 (dd, J = 3.4, 1.7 Hz, 1H), 5.01 (m, 1H), 4.19 (q, J = 7.1 Hz, 3H), 3.52 (ddd, J = 18.8, 8.2, 2.8 Hz, 1H), 2.98 (ddd, J = 18.8, 5.5, 2.8 Hz, 1H), 2.87 (dd, J = 16.2, 6.1 Hz, 1H), 2.68 (dd, J = 16.2, 7.0 Hz, 1H), 1.28 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3): δ 171.5, 169.7, 151.3, 145.5, 123.36, 121.3, 116.4, 112.7, 73.6, 61.2, 41.0, 33.4, 14.3. IR (ATR, νmax): 2959, 2923, 2852, 1753, 1656, 1259, 1182, 1039. HRMS (ESI) m/z calcd for C13H14NaO5+ [M + Na]+, 273.0733; found, 273.0756.
Ethyl 2-[(4E)-5-Oxo-4-[(2E)-3-phenylprop-2-en-1-ylidene]oxolan-2-yl]acetate (9f)
It was obtained as colorless oil (73%): 1H NMR (500 MHz, CDCl3): δ 7.50–7.47 (m, 2H), 7.39–7.31 (m, 3H), 7.29–7.27 (m, 1H), 6.97 (d, J = 15.5 Hz, 1H), 6.81 (dd, J = 15.5 Hz, 11.5 Hz, 1H), 4.99 (dd, J = 8.2 Hz, J = 7.4 Hz, J = 5.9, 1H), 4.19 (q, J = 7.2 Hz, 2H), 3.33 (ddd, J = 17.8, 8.2, 2.7 Hz, 1H), 2.89 (dd, J = 16.2 Hz, 5.9 Hz, 1H), 2.81 (ddd, J = 17.8 Hz, 5.9 Hz, 2.7, 1H), 2.67 (dd, J = 16.2 Hz, 7.4 Hz, 1H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (63 MHz, CDCl3): δ 171.0, 169.7, 142.0, 136.6, 136.0, 129.6, 129.0, 127.5, 124.5, 123.8, 73.4, 61.2, 40.9, 31.8, 14.3. IR (ATR, νmax): 2983, 1743, 1640, 1188, 1027. HRMS (ESI) m/z calcd for C17H18NaO4+ [M + Na]+, 309.1097; found, 309.1068.
Acknowledgments
S.R.L. thanks the Brazilian National Council for Science and Development (CNPq) for a doctorate scholarship. F.C. thanks Fapesp (process numbers 2013/07600−3 and 2018/02611−0) and CNPq for a research fellowship (process numbers 422890/2016–2 and 301330/2018–2).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00101.
Copies of 1H and 13C NMR spectra of all of the new compounds and copies of 2D NOESY, HSQC, and HMBC correlation maps for selected compounds (PDF).
The authors declare no competing financial interest.
Supplementary Material
References
- a Geraghty N. W. A.; Morris N. M. Synthesis of 2-Alkyl-2-cyclopenten-1-ones. A Versatile Kinetic Alkylation-Ozonolysis Procedure for the Preparation of γ-Ketoaldehydes. Synthesis 1989, 1989, 603–607. 10.1055/s-1989-27330. [DOI] [Google Scholar]; b Kim D. K.; Shokova E. A.; Tafeenko V. A.; Kovalev V. V. Synthesis of 1,3-diketones from 3-(4-R-phenyl)propionic acids. Russ. J. Org. Chem. 2014, 50, 464–468. 10.1134/s1070428014040022. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Milan M.; Viktor M.; Rudolf K.; Dusan I. Preparation of Cyclic 1,3-Diketones and Their Exploitation in the Synthesis of Heterocycles. Curr. Org. Chem. 2004, 8, 695–714. 10.2174/1385272043370627. [DOI] [Google Scholar]; d Mohanty A.; Roy S. Nickel (II) Catalysed C-H Functionalization and Tandem Coupling of Terminal Alkynes with 1, 3-Dicarbonyls: Expedient Route to Functionalized Furans. Eur. J. Org. Chem. 2019, 2019, 6702–6706. 10.1002/ejoc.201901309. [DOI] [Google Scholar]
- For recent works using 1,4-dicarbonyl compounds to the synthesis of pyrroles, see:; a Rajeshkumar V.; Neelamegam C.; Anandan S. An Expedient, Direct, Three-Component Approach for the Synthesis of 4-Thioarylpyrroles. Synthesis 2019, 51, 4023–4033. 10.1055/s-0039-1690024. [DOI] [Google Scholar]; b Jiang X.; Jin H.; Wang T.; Yoo H.; Koo S. Synthesis of Phenyl-2,2′-bichalcophenes and Their Aza-Analogues by Catalytic Oxidative Deacetylation. Synthesis 2019, 51, 3259–3268. 10.1055/s-0037-1611564. [DOI] [Google Scholar]; c Fuchs P. J. W.; Zeitler K. Nitroalkenes as Latent 1,2-Biselectrophiles – A Multicatalytic Approach for the Synthesis of 1,4-Diketones and Their Application in a Four-Step One-Pot Reaction to Polysubstituted Pyrroles. J. Org. Chem. 2017, 82, 7796–7805. 10.1021/acs.joc.7b00830. [DOI] [PubMed] [Google Scholar]
- For recent works using 1,4-dicarbonyl compounds to the synthesis of furans, see:; a Parnes R.; Reiss H.; Pappo D. Cu(OTf)2-Catalyzed Pummerer Coupling of β-Ketosulfoxides. J. Org. Chem. 2018, 83, 723–732. 10.1021/acs.joc.7b02708. [DOI] [PubMed] [Google Scholar]; b Sha Q.; Liu H. De novo Synthesis of Benzofurans via Trifluoroacetic Acid Catalyzed Cyclization/oxidative Aromatization Cascade Reaction of 2-Hydroxy-1,4-diones. Org. Biomol. Chem. 2019, 17, 7547–7551. 10.1039/c9ob01422e. [DOI] [PubMed] [Google Scholar]; c Rajeshkumar V.; Neelamegam C.; Anandan S. A One-pot Metal-free Protocol for the Synthesis of Chalcogenated Furans from 1,4-Enediones and Thiols. Org. Biomol. Chem. 2019, 17, 982–991. 10.1039/c8ob03051k. [DOI] [PubMed] [Google Scholar]; d Yang Y.; Gao M.; Wu L.-M.; Deng C.; Zhang D.-X.; Gao Y.; Zhu Y.-P.; Wu A.-X. A Facile Synthesis of Indole–furan Conjugates via Integration of Convergent and Linear Domino Reactions. Tetrahedron 2011, 67, 5142–5149. 10.1016/j.tet.2011.05.058. [DOI] [Google Scholar]
- Fujieda H.; Maeda K.; Kato N. Efficient and Scalable Synthesis of Glucokinase Activator with a Chiral Thiophenyl-Pyrrolidine Scaffold. Org. Process Res. Dev. 2019, 23, 69–77. 10.1021/acs.oprd.8b00354. [DOI] [Google Scholar]
- Li S.-H.; Wang J.; Niu X.-M.; Shen Y.-H.; Zhang H.-J.; Sun H.-D.; Li M.-L.; Tian Q.-E.; Lu Y.; Cao P.; Zheng Q.-T.; Maoecrystal V. Cytotoxic Diterpenoid with a Novel C19 Skeleton from Isodon eriocalyx (Dunn.) Hara. Org. Lett. 2004, 6, 4327–4330. 10.1021/ol0481535. [DOI] [PubMed] [Google Scholar]
- Beattie G. J.; Young H. A.; Smyth J. F. Phase I Study of Intra-peritoneal Metalloproteinase Inhibitor BB-94 in Patients with Malignant Ascites. Ann. Oncol. 1994, 5, 72a. [Google Scholar]
- a Clark E. P.; Quassin I. The Preparation and Purification of Quassin and Neoquassin, with Information Concerning their Molecular Formulas. J. Am. Chem. Soc. 1937, 59, 927–931. 10.1021/ja01284a046. [DOI] [Google Scholar]; b Valenta Z.; Papadopoulos S.; Podešva C. Quassin and Neoquassin. Tetrahedron 1961, 15, 100–110. 10.1016/0040-4020(61)80013-6. [DOI] [Google Scholar]
- Kaldre D.; Klose I.; Maulide N. Stereodivergent Synthesis of 1,4-Dicarbonyls by Traceless Charge–Accelerated Sulfonium Rearrangement. Science 2018, 361, 664–667. 10.1126/science.aat5883. [DOI] [PubMed] [Google Scholar]
- a Decicco C. P.; Nelson D. J.; Corbett R. L.; Dreabit J. C. Asymmetric Synthesis of 2,3-Disubstituted Succinates via Chiral Oxazolidinone Controlled Displacement of .alpha.-Trifluoromethanesulfonate Substituted Esters. J. Org. Chem. 1995, 60, 4782–4785. 10.1021/jo00120a022. [DOI] [Google Scholar]; b Miura K.; Fujisawa N.; Saito H.; Wang D.; Hosomi A. Synthetic Utility of Stannyl Enolates as Radical Alkylating Agents. Org. Lett. 2001, 3, 2591–2594. 10.1021/ol016268s. [DOI] [PubMed] [Google Scholar]; c Esumi N.; Suzuki K.; Nishimoto Y.; Yasuda M. Synthesis of 1,4-Dicarbonyl Compounds from Silyl Enol Ethers and Bromocarbonyls, Catalyzed by an Organic Dye under Visible-Light Irradiation with Perfect Selectivity for the Halide Moiety over the Carbonyl Group. Org. Lett. 2016, 18, 5704–5707. 10.1021/acs.orglett.6b02869. [DOI] [PubMed] [Google Scholar]
- a Fang X.; Chen X.; Lv H.; Chi Y. R. Enantioselective Stetter Reactions of Enals and Modified Chalcones Catalyzed by N-Heterocyclic Carbenes. Angew. Chem., Int. Ed. 2011, 50, 11782–11785. 10.1002/anie.201105812. [DOI] [PubMed] [Google Scholar]; b Gomes R. C.; Barcelos R. C.; Rodrigues M. T. Jr.; Santos H.; Coelho F. Intermolecular Stetter Reactions on Morita-Baylis-Hillman Adducts: an Approach to Highly Functionalized 1,4-Dicarbonyl Compounds. ChemistrySelect 2017, 2, 926–930. 10.1002/slct.201602059. [DOI] [Google Scholar]; c Biju A.; Yetra R. S.; Patra A. Recent Advances in the N-Heterocyclic Carbene (NHC)-Organocatalyzed Stetter Reaction and Related Chemistry. Synthesis 2015, 47, 1357–1378. 10.1055/s-0034-1378692. [DOI] [Google Scholar]
- a Gao M.; Yang Y.; Wu Y.-D.; Deng C.; Cao L.-P.; Meng X.-G.; Wu A.-X. Formation of Unsymmetrical 1,4-Enediones via A Focusing Domino Strategy: Cross-Coupling of 1,3-Dicarbonyl Compounds and Methyl Ketones or Terminal Aryl Alkenes. Org. Lett. 2010, 12, 1856–1859. 10.1021/ol100473f. [DOI] [PubMed] [Google Scholar]; b Xu K.; Fang Y.; Yan Z.; Zha Z.; Wang Z. A Highly Tunable Stereoselective Dimerization of Methyl Ketone: Efficient Synthesis of E- and Z-1,4-Enediones. Org. Lett. 2013, 15, 2148–2151. 10.1021/ol4006344. [DOI] [PubMed] [Google Scholar]; c Zhu Y.-P.; Cai Q.; Gao Q.-H.; Jia F.-c.; Liu M.-c.; Gao M.; Wu A.-X. Target-oriented Synthesis: Miscellaneous Synthetic Routes to Access 1,4-Enediones Through the Coupling of 1,3-Dicarbonyl Compounds with Multiform Substrates. Tetrahedron 2013, 69, 6392–6398. 10.1016/j.tet.2013.05.106. [DOI] [Google Scholar]; d Selective Intermolecular Oxidative Cross-Coupling of Enolates. J. Am. Chem. Soc. 2015, 137, 10072–10075. [DOI] [PubMed] [Google Scholar]; e Robinson E. E.; Thomson R. J. A Strategy for the Convergent and Stereoselective Assembly of Polycyclic Molecules. J. Am. Chem. Soc. 2018, 140, 1956–1965. 10.1021/jacs.7b13234. [DOI] [PubMed] [Google Scholar]
- Wang W.; Xu B.; Hammond G. B. Efficient Synthesis of γ-Keto Esters through Neighboring Carbonyl Group-Assisted Regioselective Hydration of 3-Alkynoates. J. Org. Chem. 2009, 74, 1640–1643. 10.1021/jo802450n. [DOI] [PubMed] [Google Scholar]
- Jadhav A. M.; Gawade S. A.; Vasu D.; Dateer R. B.; Liu R.-S. ZnII- and AuI-Catalyzed Regioselective Hydrative Oxidations of 3-En-1-ynes with Selectfluor: Realization of 1,4-Dioxo and 1,4-Oxohydroxy Functionalizations. Chem.—Eur. J. 2014, 20, 1813–1817. 10.1002/chem.201304322. [DOI] [PubMed] [Google Scholar]
- Imi K.; Imai K.; Utimoto K. Regioselective Hydration of Alkynones by Palladium Catalysis. Tetrahedron Lett. 1987, 28, 3127–3313. 10.1016/s0040-4039(00)96302-0. [DOI] [Google Scholar]
- Bai X.-T.; Zhang Q.-Q.; Zhan S.; Chen D.-Y.; Fu J.-Y.; Zhu J.-Y.; Wang Y.-B.; Tang Y.-T. Carbonyl-Oxygen-Assisted KOMe-Mediated Formal Hydration of 4-Alkynones: Complete Regioselectivity in the One-Pot Synthesis of 1,4-Diketones under Mild Condition. Eur. J. Org. Chem. 2018, 13, 1581–1588. 10.1002/ejoc.201800024. [DOI] [Google Scholar]
- a Scully S. S.; Zheng S.-L.; Wagner B. K.; Schreiber S. L. Synthesis of Oxazocenones via Gold(I)-Catalyzed 8-Endo-Dig Hydroalkoxylation of Alkynamides. Org. Lett. 2015, 17, 418–421. 10.1021/ol503273v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cala L.; Mendoza A.; Fañanás F. J.; Rodríguez F. A Catalytic Multicomponent Coupling Reaction for the Enantioselective Synthesis of Spiroacetals. Chem. Commun. 2013, 49, 2715–2717. 10.1039/c3cc00118k. [DOI] [PubMed] [Google Scholar]; c Teng T.-M.; Das A.; Huple D. B.; Liu R.-S. Gold-Catalyzed Stereoselective Synthesis of 9-Oxabicyclo[3.3.1]nona-4,7-dienes from Diverse 1-Oxo-4-oxy-5-ynes: A Viable Formal [4 + 2] Cycloaddition on an s-Trans-Heterodiene Framework. J. Am. Chem. Soc. 2010, 132, 12565–12567. 10.1021/ja106493h. [DOI] [PubMed] [Google Scholar]; d Fang C.; Pang Y.; Forsyth C. J. Formal Total Synthesis of Okadaic Acid via Regiocontrolled Gold(I)-Catalyzed Spiroketalizations. Org. Lett. 2010, 12, 4528–4531. 10.1021/ol101833h. [DOI] [PubMed] [Google Scholar]; e Feng Y.; Jiang X.; De Brabander J. K. Studies toward the Unique Pederin Family Member Psymberin: Full Structure Elucidation, Two Alternative Total Syntheses, and Analogs. J. Am. Chem. Soc. 2012, 134, 17083–17093. 10.1021/ja3057612. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Hirano K.; Inaba Y.; Takahashi N.; Shimano M.; Oishi S.; Fujii N.; Ohno H. Direct Synthesis of Fused Indoles by Gold-Catalyzed Cascade Cyclization of Diynes. J. Org. Chem. 2011, 76, 1212–1227. 10.1021/jo102507c. [DOI] [PubMed] [Google Scholar]; g Chiba H.; Oishi S.; Fujii N.; Ohno H. Total Synthesis of (−)-Quinocarcin by Gold(I)-Catalyzed Regioselective Hydroamination. Angew. Chem., Int. Ed. 2012, 51, 9169–9172. 10.1002/anie.201205106. [DOI] [PubMed] [Google Scholar]; h Cai P.-J.; Wang Y.; Liu C.-H.; Yu Z.-X. Gold(I)-Catalyzed Polycyclization of Linear Dienediynes to Seven-Membered Ring-Containing Polycycles via Tandem Cyclopropanation/Cope Rearrangement/C–H Activation. Org. Lett. 2014, 16, 5898–5901. 10.1021/ol5028706. [DOI] [PubMed] [Google Scholar]; i Linghu X.; Kennedy-Smith J. J.; Toste F. D. Total Synthesis of (+)-Fawcettimine. Angew. Chem., Int. Ed. 2007, 46, 7671–7673. 10.1002/anie.200702695. [DOI] [PubMed] [Google Scholar]; j Lu Z.; Li Y.; Deng J.; Li A. Total Synthesis of the Daphniphyllum Alkaloid Daphenylline. Nat. Chem. 2013, 5, 679–684. 10.1038/nchem.1694. [DOI] [PubMed] [Google Scholar]; k Bellavance G.; Barriault L. Total Syntheses of Hyperforin and Papuaforins A–C, and Formal Synthesis of Nemorosone through a Gold(I)-Catalyzed Carbocyclization. Angew. Chem., Int. Ed. 2014, 53, 6701–6704. 10.1002/anie.201403939. [DOI] [PubMed] [Google Scholar]; l Jiménez-Núñez E.; Raducan M.; Lauterbach T.; Molawi K.; Solorio C. R.; Echavarren A. M. Evolution of Propargyl Ethers into Allylgold Cations in the Cyclization of Enynes. Angew. Chem., Int. Ed. 2009, 48, 6152–6155. 10.1002/anie.200902248. [DOI] [PubMed] [Google Scholar]; m Yin X.; Mato M.; Echavarren A. M. Gold(I)-Catalyzed Synthesis of Indenes and Cyclopentadienes: Access to (±)-Laurokamurene B and the Skeletons of the Cycloaurenones and Dysiherbols. Angew. Chem., Int. Ed. 2017, 56, 14591–14595. 10.1002/anie.201708947. [DOI] [PMC free article] [PubMed] [Google Scholar]; n Molawi K.; Delpont N.; Echavarren A. M. Enantioselective Synthesis of (−)-Englerins A and B. Angew. Chem., Int. Ed. 2010, 49, 3517–3519. 10.1002/anie.201000890. [DOI] [PubMed] [Google Scholar]; Yang Y.; Zhang X.; Yu B. O-Glycosylation Methods in the Total Synthesis of Complex Natural Glycosides. Nat. Prod. Rep. 2015, 32, 1331–1355. 10.1039/c5np00033e. [DOI] [PubMed] [Google Scholar]; o Kaldas S. J.; Cannillo A.; McCallum T.; Barriault L. Indole Functionalization via Photoredox Gold Catalysis. Org. Lett. 2015, 17, 2864–2866. 10.1021/acs.orglett.5b01260. [DOI] [PubMed] [Google Scholar]; p Rong Z.; Echavarren A. M. Broad Scope Gold(I)-catalysed Polyenyne Cyclisations for the Formation of up to Four Carbon–carbon Bonds. Org. Biomol. Chem. 2017, 15, 2163–2167. 10.1039/c7ob00235a. [DOI] [PubMed] [Google Scholar]; q Brandstätter M.; Freis M.; Huwyler N.; Carreira E. M. Total Synthesis of (−)-Merochlorin A. Angew. Chem., Int. Ed. 2019, 58, 2490–2494. 10.1002/anie.201813090. [DOI] [PubMed] [Google Scholar]; r Marcote D. C.; Varela I.; Fernández-Casado J.; Mascareñas J. L.; López F. Gold(I)-Catalyzed Enantioselective Annulations between Allenes and Alkene-Tethered Oxime Ethers: A Straight Entry to Highly Substituted Piperidines and aza-Bridged Medium-Sized Carbocycles. J. Am. Chem. Soc. 2018, 140, 16821–16833. 10.1021/jacs.8b10388. [DOI] [PubMed] [Google Scholar]
- For reviews of Au-catalyzed alkyne hydration, see:; a Teles J. H.Hydration and Hydroalkoxylation of CC Multiple Bonds. In Modern Gold Catalyzed Synthesis; Hashmi A. S. K., Toste F. D., Eds.; Wiley-VCH Verlag. GmbH & Co. KGaA: Weinheim, 2012; pp. 201–235. [Google Scholar]; b Brenzovich W. E. Jr. Gold in Total Synthesis: Alkynes as Carbonyl Surrogates. Angew. Chem., Int. Ed. 2012, 51, 8933–8935. 10.1002/anie.201204598. [DOI] [PubMed] [Google Scholar]
- a Mizushima E.; Sato K.; Hayashi T.; Tanaka M. Highly Efficient AuI-Catalyzed Hydration of Alkynes. Angew. Chem., Int. Ed. 2002, 41, 4563–4565. . [DOI] [PubMed] [Google Scholar]; b Marion N.; Ramón R. S.; Nolan S. P. [(NHC)AuI]-Catalyzed Acid-Free Alkyne Hydration at Part-per-Million Catalyst Loadings. J. Am. Chem. Soc. 2009, 131, 448–449. 10.1021/ja809403e. [DOI] [PubMed] [Google Scholar]; c Nun P.; Ramón R. S.; Gaillard S.; Nolan S. P. Efficient Silver-free Gold(I)-catalyzed Hydration of Alkynes at Low Catalyst Loading. J. Organomet. Chem. 2011, 696, 7–11. 10.1016/j.jorganchem.2010.08.052. [DOI] [Google Scholar]; d Casals-Cruañas E.; Gonzá-lez-Belman O. F.; Besalú-Sala P.; Nelson D. J.; Poater A. The Preference for Dual-gold(I) Catalysis in the Hydro(alkoxylation vs. phenoxylation) of Alkynes. Org. Biomol. Chem. 2017, 15, 6416–6425. 10.1039/c7ob01457k. [DOI] [PubMed] [Google Scholar]; e Ebule R. E.; Malhotra D.; Hammond G. B.; Xu B. Ligand Effects in the Gold Catalyzed Hydration of Alkynes. Adv. Synth. Catal. 2016, 358, 1478–1481. 10.1002/adsc.201501079. [DOI] [Google Scholar]; f Wang D.; Cai R.; Sharma S.; Jirak J.; Thummanapelli S. K.; Akhmedov N. G.; Zhang H.; Liu X.; Petersen J. L.; Shi X. “Silver Effect” in Gold(I) Catalysis: An Overlooked Important Factor. J. Am. Chem. Soc. 2012, 134, 9012–9019. 10.1021/ja303862z. [DOI] [PubMed] [Google Scholar]
- For a review of regioselectivity in Au-catalyzed alkyne hydration and hydroalkoxylation, see:Goodwin J. A.; Aponick A. Regioselectivity in the Au-catalyzed Hydration and Hydroalkoxylation of Alkynes. Chem. Commun. 2015, 51, 8730–8741. 10.1039/c5cc00120j. [DOI] [PubMed] [Google Scholar]
- a Kuang J.; Zhou T.; YOu T.; Chen J.; Su C.; Xia Y. Facile Access to 1,3-Diketones by Gold(I)-catalyzed Regioselective Hydration of Ynones. Org. Biomol. Chem. 2019, 17, 3940–3944. 10.1039/c9ob00494g. [DOI] [PubMed] [Google Scholar]; b Hamel J.-D.; Hayashi T.; Cloutier M.; Savoie P. R.; Thibeault O.; Beaudoin M.; Paquin J.-F. Highly Regioselective Gold-catalyzed Formal Hydration of Propargylic Gem-difluorides. Org. Biomol. Chem. 2017, 15, 9830–9836. 10.1039/c7ob02406a. [DOI] [PubMed] [Google Scholar]; c Tarigopula C.; Thota G. K.; Balamurugan R. Efficient Synthesis of Functionalized β-Keto Esters and β-Diketones through Regioselective Hydration of Alkynyl Esters and Alkynyl Ketones by Use of a Cationic NHC–AuI Catalyst. Eur. J. Org. Chem. 2016, 2016, 5855–5861. 10.1002/ejoc.201601025. [DOI] [Google Scholar]
- For examples of use of neighboring nucleophiles in Au-catalyzed hydration and hydroxylation of internal alkynes, see:; a Oh C.; Jeong J.; Ray D. Cationic Gold-Catalyzed Regioselective Hydration of 1-Arylalkynes through Carbonyl Group Participation. Synlett 2012, 23, 897–902. 10.1055/s-0031-1290619. [DOI] [Google Scholar]; b Bao M.; Lu W.; Cai Y.; Qiu L.; Xu X. Gold(I)-Catalyzed Cyclization/Carbonylation Cascade Reaction of 1,6-Diynes: An Access to β,γ-Unsaturated Ketones. J. Org. Chem. 2017, 82, 13386–13395. 10.1021/acs.joc.7b02461. [DOI] [PubMed] [Google Scholar]; c Yeom H.-S.; Lee Y.; Jeong J.; So E.; Hwang S.; Lee J.-E.; Lee S. S.; Shin S. Stereoselective One-Pot Synthesis of 1-Aminoindanes and 5,6-Fused Azacycles Using a Gold-Catalyzed Redox-Pinacol-Mannich-Michael Cascade. Angew. Chem., Int. Ed. 2010, 49, 1611–1614. 10.1002/anie.200906346. [DOI] [PubMed] [Google Scholar]; d Hashmi A. S. K.; Bührle M.; Salathé R.; Bats J. W. Gold Catalysis: Synthesis of 3-Acylindenes from 2-Alkynylaryl Epoxides. Adv. Synth. Catal. 2008, 350, 2059–2064. 10.1002/adsc.200800385. [DOI] [Google Scholar]; e Lin G.-Y.; Li C.-W.; Hung S.-H.; Liu R.-S. Diversity in Gold- and Silver-Catalyzed Cycloisomerization of Epoxide–Alkyne Functionalities. Org. Lett. 2008, 10, 5059–5062. 10.1021/ol802047g. [DOI] [PubMed] [Google Scholar]; f Ghosh N.; Nayak S.; Sahoo A. K. Gold-Catalyzed Regioselective Hydration of Propargyl Acetates Assisted by a Neighboring Carbonyl Group: Access to α-Acyloxy Methyl Ketones and Synthesis of (±)-Actinopolymorphol B. J. Org. Chem. 2011, 76, 500–511. 10.1021/jo101995g. [DOI] [PubMed] [Google Scholar]; g Körner C.; Starkov P.; Sheppard T. D. An Alternative Approach to Aldol Reactions: Gold-Catalyzed Formation of Boron Enolates from Alkynes. J. Am. Chem. Soc. 2010, 132, 5968–5969. 10.1021/ja102129c. [DOI] [PubMed] [Google Scholar]; h Pennell M. N.; Unthank M. G.; Turner P.; Sheppard T. D. A General Procedure for the Synthesis of Enones via Gold-Catalyzed Meyer-Schuster Rearrangement of Propargylic Alcohols at Room Temperature. J. Org. Chem. 2011, 76, 1479–1482. 10.1021/jo102263t. [DOI] [PubMed] [Google Scholar]; i Ghosh N.; Nayak S.; Prabagar B.; Sahoo A. K. Regioselective Hydration of Terminal Halo-Substituted Propargyl Carboxylates by Gold Catalyst: Synthesis of α-Acyloxy α′-Halo Ketones. J. Org. Chem. 2014, 79, 2453–2462. 10.1021/jo4027319. [DOI] [PubMed] [Google Scholar]
- For examples of regioselective hydration of internal alkynes catalyzed by other metals, see:; a Ahammed S.; Dey R.; Ranu B. C. Palladium and Copper Catalyzed One-pot Sonogashira Reaction of 2-Nitroiodobenzenes with Aryl Acetylenes and Subsequent Regioselective Hydration in Water: Synthesis of 2-(2-nitrophenyl)-1-aryl Ethanones. Tetrahedron Lett. 2013, 54, 3697–3701. 10.1016/j.tetlet.2013.05.021. [DOI] [Google Scholar]; b Zhang Z.; Wu L.; Liao J.; Wu W.; Jiang H.; Li J.; Li J. Amide Oxygen-Assisted Palladium-Catalyzed Hydration of Alkynes. J. Org. Chem. 2015, 80, 7594–7603. 10.1021/acs.joc.5b01178. [DOI] [PubMed] [Google Scholar]; c Vinoth P.; Nagarajan S.; Maheswari C. U.; Sudalai A.; Pace V.; Sridharan V. Palladium-Catalyzed Internal Nucleophile-Assisted Hydration–Olefin Insertion Cascade: Diastereoselective Synthesis of 2,3- Dihydro-1H-inden-1-ones. Org. Lett. 2016, 18, 3442–3445. 10.1021/acs.orglett.6b01623. [DOI] [PubMed] [Google Scholar]; d Barluenga J.; Fernández A.; Diéguez A.; Rodríguez F.; Fañanás F. J. Gold- or Platinum-Catalyzed Cascade Processes of Alkynol DerivativesInvolving Hydroalkoxylation Reactions Followed by Prins-Type Cyclizations. Chem.—Eur. J. 2009, 15, 11660–11667. 10.1002/chem.200900856. [DOI] [PubMed] [Google Scholar]; e Li D. Y.; Chen H. J.; Liu P. N. Tunable Cascade Reactions of Alkynols with Alkynes under Combined Sc(OTf)3 and Rhodium Catalysis. Angew. Chem., Int. Ed. 2016, 55, 373–377. 10.1002/anie.201508914. [DOI] [PubMed] [Google Scholar]; f Mukherjee A.; Liu R.-S. Chemoselectivities in the PlatinumCatalyzed Hydrative Carbocyclizations of Oxo-Alkyne-Nitrile Functionalities. Org. Lett. 2011, 13, 660–663. 10.1021/ol1029047. [DOI] [PubMed] [Google Scholar]; g Nishizawa M.; Takemoto T.; Sasaki I.; Nakano M.; Ho E.; Namba K.; Yamamoto H.; Imagawa H. Hg(OTf)2-Catalyzed Instantaneous Hydration of β- and δ-Hydroxy Internal Alkynes with Complete Regioselectivity. Synlett 2009, 2009, 1175–1179. 10.1055/s-0028-1088153. [DOI] [Google Scholar]
- For a recent example of acid-promoted formal hydration of internal alkyne through the participation of a neighboring nucleophile see:Zhang C.; Chang S.; Dong S.; Qiu L.; Xu X. Acid-Promoted Bicyclization of Diaryl Alkynes: Synthesis of 2H-Indazoles with in Situ Generated Diazonium Salt as Nitrogen Source. J. Org. Chem. 2018, 83, 9125–9136. 10.1021/acs.joc.8b01199. [DOI] [PubMed] [Google Scholar]
- Pradhan T. R.; Mendhekar K. L.; Mohapatra D. K. Synthesis of γ-Acetoxy β-Keto Esters Through Regioselective Hydration of γ-Acetoxy-α,β-alkynoates. J. Org. Chem. 2015, 80, 5517–5531. 10.1021/acs.joc.5b00400. [DOI] [PubMed] [Google Scholar]
- Gorin D. J.; Toste F. D. Relativistic effects in homogeneous gold catalysis. Nature 2007, 446, 395–403. 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- Coelho F.; Almeida W. P.; Veronese D.; Mateus C. R.; Silva Lopes E. C.; Rossi R. C.; Silveira G. P. C.; Pavam C. H. Ultrasound in Baylis–Hillman Reactions with Aliphatic and Aromatic Aldehydes: Scope and Limitations. Tetrahedron 2002, 58, 7437–7447. 10.1016/s0040-4020(02)00822-0. [DOI] [Google Scholar]
- Buchholz R.; Hoffmann H. M. R. α-Methylidene- and α-Alkylidene-β-lactams from Nonproteinogenic Amino Acids. Helv. Chim. Acta 1991, 74, 1213–1220. 10.1002/hlca.19910740608. [DOI] [Google Scholar]
- Das B.; Damodar K.; Bhunia N.; Shashikanth B. Mild and Practical Stereoselective Synthesis of (Z)- and (E)-Allyl Bromides from Baylis–Hillman Adducts Using Appel Agents (PPh3/CBr4): A Facile Synthesis of Semiplenamides C and E. Tetrahedron Lett. 2009, 50, 2072–2074. 10.1016/j.tetlet.2009.02.132. [DOI] [Google Scholar]
- Lim J. W.; Kim K. H.; Kim S. H.; Kim J. N. Copper-catalyzed Tandem Alkynylation, Propargyl–Allenyl Isomerization, 6π-Electrocyclization of Morita–Baylis–Hillman Adducts to Naphthalenes. Tetrahedron Lett. 2012, 53, 5449–5454. 10.1016/j.tetlet.2012.07.126. [DOI] [Google Scholar]
- a Chen Z.-W.; Ye D.-N.; Ye M.; Zhou Z.-G.; Li S.-H.; Liu L.-X. AgF/TFA-promoted highly efficient synthesis of α-haloketones from haloalkynes. Tetrahedron Lett. 2014, 55, 1373–1375. 10.1016/j.tetlet.2014.01.027. [DOI] [Google Scholar]; b Xiang J.; Yi N.; Wang R.; Lu L.; Zou H.; Pan Y.; He W. Synthesis of β-ketophosphonates via AgNO3-catalyzed hydration of alkynylphosphonates: a rate-enhancement effect of methanol. Tetrahedron 2015, 71, 694–699. 10.1016/j.tet.2014.12.001. [DOI] [Google Scholar]
- a Hwang E.-I.; Yun B.-S.; Kim Y.-K.; Kwon B.-M.; Kim H.-G.; Lee H.-B.; Jeong W.-J.; Kim S.-U. Phellinsin A, a Novel Chitin Synthases Inhibitor Produced by Phellinus sp. PL3. J. Antibiot. 2000, 53, 903–911. 10.7164/antibiotics.53.903. [DOI] [PubMed] [Google Scholar]; b Ann J.; Czikora A.; Saini A. S.; Zhou X.; Mitchell G. A.; Lewin N. E.; Peach M. L.; Blumberg P. M.; Lee J. α-Arylidene Diacylglycerol-Lactones (DAG-Lactones) as Selective Ras Guanine-Releasing Protein 3 (RasGRP3) Ligands. J. Med. Chem. 2018, 61, 6261–6276. 10.1021/acs.jmedchem.8b00661. [DOI] [PubMed] [Google Scholar]; c Naito T.; Honda Y.; Miyata O.; Ninomiya I. Total Syntheses Of (±)-Anantine and (±)-Isoanantine Via Thiyl Radical Addition-Cyclization Reaction. Chem. Pharm. Bull. 1993, 41, 217. 10.1248/cpb.41.217. [DOI] [PubMed] [Google Scholar]; d Janecki T.; Błaszczyk E.; Studzian K.; Janecka A.; Krajewska U.; Różalski M. Novel Synthesis, Cytotoxic Evaluation, and Structure-Activity Relationship Studies of a Series of α-Alkylidene-γ-lactones and Lactams. J. Med. Chem. 2005, 48, 3516–3521. 10.1021/jm048970a. [DOI] [PubMed] [Google Scholar]; e Janecka A.; Wyrębska A.; Gach K.; Fichna J.; Janecki T. Natural and Synthetic α-Methylenelactones and α-Methylenelactams with Anticancer Potential. Drug Discovery Today 2012, 17, 561–572. 10.1016/j.drudis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Fournier J.; Arseniyadis S.; Cossy J. A Modular and Scalable One-Pot Synthesis of Polysubstituted Furans. Angew. Chem., Int. Ed. 2012, 51, 7562–7566. 10.1002/anie.201202486. [DOI] [PubMed] [Google Scholar]
- Nelson A. K.; Peck C. L.; Rafferty S. M.; Santos W. L. Chemo-, Regio-, and Stereoselective Copper(II)-Catalyzed Boron Addition to Acetylenic Esters and Amides in Aqueous Media. J. Org. Chem. 2016, 81, 4269–4279. 10.1021/acs.joc.6b00648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.