Abstract

Methylation of cytosine has been known to play a significant role in epigenetic regulation. 5-Methylcytosine was among the first base modification that was discovered for the capability to facilitate B/Z-DNA transition as observed in CG repeated tracks. A study on gene repression by Z-DNA prone sequence as in ADAM-12 has ignited our research interest for the Z-DNA role in epigenetics. Ten eleven translocation family proteins are responsible to catalyze 5-methylcytosine to produce oxidative products including 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine, which each may have unique function rather than the sole purpose of 5-methylcytosine clearance. Although the Z-DNA-promoting effect of 5-methylcytosine was well established, the effect of its oxidative products on Z-DNA remain unknown. In this study, the Z-DNA-promoting effect of 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine on the CG decamer model were investigated along with known Z-DNA stabilizers, 5-methylcytosine and 8-oxoguanine. Experimental results from circular dichroism (CD) and NMR indicates that all oxidative products of 5-methylcytosine hinder B/Z-DNA transition as high salt concentration suitable to stabilize and convert unmodified CG decamer to Z-DNA conformation is insufficient to facilitate the B/Z-DNA transition of CG decamer containing 5-hydroxymethylcytosine, 5-formylcytosine, or 5-carboxycytosine. Molecular dynamic simulation and free energy calculation by MM-PBSA are in agreement with the experimental finding that 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine destabilize Z-DNA conformation of CG decamer, in contrast to its precursor. Investigation of Z-DNA switch-on/switch-off regulated by 5-methylcytosine and its oxidative products is a further step to elucidate the potential of epigenetic regulated via Z-DNA.
Introduction
Several base modifications have been found to be endogenous products of chemical or biological processes in a living system and they may play a significant role in regulating the cellular function or pathogenesis. A number of nonconventional nucleotide bases have been investigated and identified as epigenetic marks. Methylation of cytosine at C5 position by DNA methyltransferase enzyme (DNMT)1 produces 5-methylcytosine (5mC), the “fifth base” that has long been known to play a central role in gene repression via epigenetic regulation.2,3 Oxidative products of mC catalyzed by ten eleven translocation (TET) family proteins (Figure 1, panel A), 5-hydroxymethylcytosine (5hmC),4,5 5-formylcytosine (5fC),6 and 5-carboxylcytosine (5caC)6 were established as intermediates in the reversal process of 5mC-mediated gene repression by converting 5mC back to unmodified cytosine. In addition to cytosine modification, recent studies on 8-oxoguanine (8oxG) distribution in genome7,8 and 8oxG involvement in gene regulation9−12 have proposed a role of modified guanosine as an epigenetic mark in addition to its well-known trait as a hallmark of oxidative DNA damage. Research studies and new findings in the field of epigenetics have surged during the last decade, especially analytical works to identify, map,13 and quantify14,15 the level of the epigenetic mark in genome and tissue as well as elucidate these modifications role in epigenetics. Further investigation on the structural biology of the epigenetic marked DNA may shed some light to explain how these modified bases orchestrate gene regulation.
Figure 1.
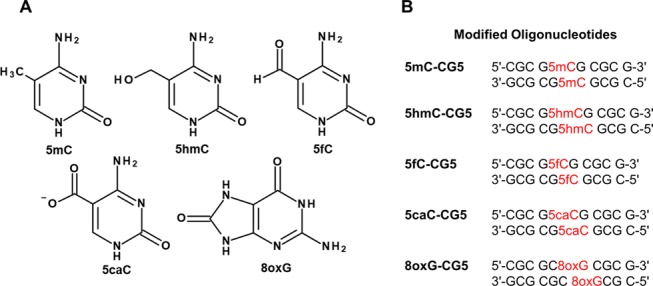
(A) Chemical structures of epigenetic bases and (B) epigenetic base containing CG decamers used in this study.
Z-DNA, the noncanonical left-handed structure of DNA, has been shown to play a role in gene regulation as previously demonstrated by the activation of human colony-stimulating 1 (CSF1) gene16 and upregulation of c-myc(17) through Z-DNA formation located near the promoter region. The epigenetic role of Z-DNA has recently been investigated in the regulation of disintegrin and metalloprotease domain-containing protein 12 (ADAM-12) gene.18 The Z-DNA-forming sequence conserved in the negative regulatory element (NRE) has been demonstrated to be crucial in the ADAM-12 gene regulation. Interaction between the Z-DNA-forming sequence in NRE of ADAM-12 and methyl-CpG-binding protein (MBP), MeCP2, is found to be involved in the cross-talk between MeCP2 and transcription factor,19 hence suggesting the role of Z-DNA in epigenetics. A number of factors have been shown to promote canonical B-DNA conversion to a less thermodynamic stable Z-DNA including pyrimidine purine track as in the CpG repeated sequences, monovalent cations,20 bivalent cations,21−23 polyamines,24−28 Z-DNA binding proteins,29,30 and base modifications.31−33 Covalent modifications of nucleotide bases have been linked to the alteration of the Watson–Crick structure of DNA such that they may affect the canonical base pairing and destabilize the B-DNA structure, while, in contrast, they may stabilize and promote the formation of unique secondary structures. Z-DNA stabilization by 5mC in CG repeated sequences34,35 is one of early finding on how the base modification affects the DNA structure, which, in turn, conceives the biological effect including recognition by IgG36 and M*HhaI enzyme.37 Several modifications on the C8 position of guanine such as 8-methyl,31 8-aryl,32,33 and 8-oxo38 have been associated with Z-DNA promotion through steric hindrance that causes unfavorable energy to anti-conformation of the purine nucleosides in B-DNA.
With cumulative findings that prerequisite of some epigenetic events involves Z-DNA-promoting factors, i.e., repeated CpG sequence and Z-DNA-promoting epigenetic bases, for instance, 5mC and 8oxG, investigating the potential of Z-DNA connection in epigenetic regulation is an interesting idea to shed some light on the mechanistic process of epigenetics. Enzymatic metabolism of 5mC from TET produces 5hmC, 5fC, and 5caC, which have been shown to play an important part in epigenetic regulation. Recent studies on 5hmC, 5fC, and 5caC have reported no global effect of CG hexamer39 and CG-containing dodecamer on B-DNA conformation,40 while 5fC has been shown to promote the formation of F-DNA.40 Although the previous study reported no sign of Z-DNA formation characterized for 5hmC-, 5fC-, and 5caC-containing oligos,40 the experiment was performed at a relatively low salt condition where Z-DNA formation is restricted due to the limit of the stabilizing effect of a cation. Therefore, to clearly answer how the TET-catalyzed products of 5mC affect Z-DNA, our study was performed in conditions that allow seeing B-DNA-to-Z-DNA conversion in comparison with 5mC and 8oxG, the well-established epigenetic bases that promote Z-DNA formation.
Circular dichroism (CD) and nuclear magnetic resonance (NMR) were the main techniques used to study and characterize the conformation of Z-DNA-prone sequence CG decamers that contain epigenetic bases. Molecular dynamic (MD) simulation and free energy calculation of CG decamers containing 5mC, 5hmC, 5fC, 5caC, and 8oxG (Figure 1, panel B) were conducted to complement the experimental results and provide further detail on the DNA structure including the global and local effects of the epigenetic bases on the CG repeated sequences. The effect of 5mC products on Z-DNA formation will be an important piece of information to help elucidate and envision the role of Z-DNA in epigenetics.
Results and Discussion
CD Studies to Examine B-DNA-to-Z-DNA Transition of 5mC-, 5hmC-, 5fC-, 5caC-, and 8oxG-Containing CG Decamers
Spectrophotometric analysis has long been used to study the secondary structure of nucleic acids. The CD experiment provides the spectra that corresponded to the DNA conformation of our interest, B-DNA and Z-DNA. The spectra of B-DNA give negative ellipticity around 250 nm and positive ellipticity around 280 nm, which is in contrast to that of Z-DNA, which has a unique negative ellipticity of around 290 nm and a positive one of around 250 nm. The effect of 5mC on Z-DNA promotion has been studied extensively. It is well-known that the methylation on C5 of cytosine can promote the transition of B-DNA to Z-DNA in short CG repeated sequences34,41,42 and poly CG.37 Higher degree of methylation has the additional effect of promoting Z-DNA formation, as has been demonstrated in our previous work.42 In addition, a fully methylated CG is more prone to B-DNA-to-Z-DNA transition when compared to hemimethylated CG. The objective of including 5mC in this study is to use it as a reference point to see the effect of TET-catalyzed 5mC products on the B-DNA-to-Z-DNA transition. The oxidative damage of DNA adduct, 8oxG, has also recently been shown to have the ability to promote the Z-DNA formation in the CG hexamers and dodecamers.38 Therefore, 8oxG–CG5 was studied to gain further insight into how 8oxG affects the B-DNA-to-Z-DNA transition of the CG decamer, in a setup similar to that of other epigenetic bases in this study. Due to the narrow phosphate backbone of Z-DNA, the left-handed conformation is energy unfavorable through electrostatic repulsion between negatively charged phosphates in close proximity in the duplex. The addition of cations into the sample solution will help screen the negative charge of phosphates and, in turn, facilitate the formation of Z-DNA. Therefore, salt was gradually titrated to oligo samples in our study to investigate the effect of epigenetic bases on the B-DNA-to-Z-DNA transition. For unmodified CG decamer, a full transition to Z-DNA can be observed in the presence of a salt concentration of 4 M. Transition concentration, which is the required concentration of salt for oligo to adopt equimolar of B-DNA and Z-DNA, of the unmodified CG decamer was calculated to be around 2544 mM at 283 K.33 As expected from 5mC- and 8oxG-containing CG decamers, lower salt concentrations were required for modified duplexes to adopt Z-DNA. Similar to several adducts on the C8 position of guanine that have been associated with Z-DNA promotion, 8oxG also showed the ability to promote B-DNA-to-Z-DNA formation. The addition of carbonyl group to C8 of guanine causes steric hindrance to anti conformation of dG residue, which is the glycosidic base conformation present in B-DNA, hence destabilizing the right-handed helix. On the other hand, syn dG adopted in Z-DNA is not sterically affected by modification on C8 of guanine. Single 8oxG on both strands of CG decamer renders the salt concentration required for full Z-DNA formation to 2 M as observed in Figure 2 and reduces the transition concentration of 8oxG–CG5 to 1132 mM at 283 K. The result of 8oxG-containing CG decamer was well aligned with a previous study on 8oxG-containing CG hexamers and dodecamers.38 5mC–CG5 has been shown to be able to form Z-DNA more easily than the unmodified counterpart. As shown in Figure 2, the transition of B-DNA to Z-DNA in 5mC–CG5 has been observed in the CD spectrum of the sample containing 2 M NaCl with the characteristic negative ellipticity at 295 nm and positive ellipticity at 265 nm, while a similar level of salt cannot facilitate the formation of Z-DNA of unmodified CG decamer. Cytosine methylation has been extensively studied for the scope of its effect on the structure of Z-DNA. Somewhat different from the steric effect of C8-guanosine modification, the promoting effect of 5mC on Z-DNA was explained by hydrophobic interaction between the methyl adduct, which tucked inward the duplex and stacking guanine bases closed to the modified cytosine that help stabilize Z-DNA conformation.34 The same study has also discussed that a more solvent accessible methyl group of cytosine in B-DNA causes unfavorable contact with water molecules, which, in turn, destabilize the right-handed DNA. Moreover, with a recent report on MD simulation study of methylated CG pentadecamer in B-DNA conformation,43 steric clash between the methyl and 5′-sugar, i.e., C2′ and its protons, suggests a major contribution to B-DNA destabilization. For the TET-catalyzed 5mC products, namely, 5hmC, 5fC, and 5caC, their effects on B-DNA-to-Z-DNA transition were not observed (Figure 2) in CD experiment. A slight drop in negative ellipticity around 295 nm was observed in 5hmC–CG5, with a salt concentration equal to or above 4 M suggesting partial conformation transition. Near saturation of salt at 5 M has a negligible effect on the Z-DNA formation of 5fC–CG5 and 5caC–CG5. The results from the CD show that there was no Z-DNA-promoting effect of 5hmC, 5fC, and 5caC. Compared with the unmodified CG decamer, which fully converts to Z-DNA in the presence of 4 M NaCl, the downstream oxidative products of 5mC seem to impede the formation of Z-DNA as opposed to their Z-DNA-promoting predecessor.
Figure 2.
CD spectra of the unmodified and modified oligomers containing 5mC, 5hmC, 5fC, 5caC, and 8oxG. B-DNA-to-Z-DNA conversion with increased salt concentration was observed with CG5, 5mC-, and 8oxG-containing CG5, while no Z-DNA formation was observed with 5hmC-, 5fC-, and 5caC-containing CG5.
NMR Studies of 5mC-, 5hmC-, 5fC-, 5caC-, and 8oxG-Containing CG Decamers
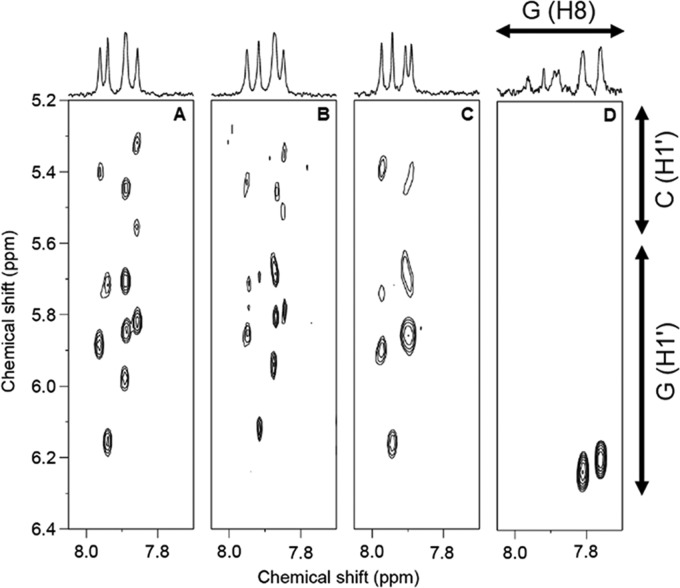
To firmly verify what has been discovered in the CD experiment, one-dimensional (1D) and two-dimensional (2D) 1H NMR including nuclear Overhauser effect spectroscopy (NOESY) and homonuclear Hartmann–Hahn spectroscopy (HOHAHA) were used to investigate the conformation of oligos. Changes in the chemical shift of protons in the 1D NMR spectrum reflect an alteration in the structure of oligos. In our NMR experiment, Na+ in the form of salt was titrated to an oligo sample ranging from 50 mM to 1.5 M. Higher salt concentration more than 1.5 M should be avoided due to the hardware limitation. This post limit to NMR study to be unable to obtain useful NMR spectrum at high salt. All NMR spectra of oligos used in this study except for 8oxG–CG5 have shown no significant change with any level of NaCl in the sample. Imino proton signals of 8oxG–CG5 observed with 50 mM NaCl sample shifted with increasing NaCl concentration (see the Supporting Information). A similar trend was also observed with G-H8 and C-H6 (Figure 3), indicating the conformational change to Z-DNA. Full conversion to Z-DNA was observed with the sample containing 1.5 M NaCl, as confirmed with correlation signals from 2D NOESY experiments (Figure 4). Sequential signal analysis of 2D NMR comprising NOESY and correlation spectroscopy,44 which has been proved to be informative in assigning conformation of B-DNA and Z-DNA, was performed to identify DNA conformation. For unmodified CG5, 5mC–CG5, 5hmC–CG5, 5fC–CG5, and 5caC–CG5, B-DNA was fully adopted in all samples. Different from the rest, 8oxG–CG5 adopted B-DNA conformation at 50 mM NaCl, while an increase in salt concentration led to B-DNA-to-Z-DNA transition, which was observed from a strong intraresidual NOE correlation between G-H8 and G-H1′ due to syn-conformation of dG in Z-DNA. Full transition to Z-DNA of 8oxG–CG5 in 1.5 M NaCl was confirmed by strong NOE of G-H8/G-H1′ correlation from Z-DNA and the absence of interresidual NOE between G-H8 and C-H1′ of dC in B-DNA conformation. Experimental results from CD and NMR studies are aligned well together. For the case of 8oxG-containing CG decamer with salt at 1 M, the level around transition concentration, NMR spectrum shows the presence of both DNA conformations. For 5mC–CG5, the NMR spectra from the samples with salt up to 1.5 M indicate no Z-DNA formation, which corresponded to the CD experiment that B-DNA-to-Z-DNA transition has been shown at the earliest with 2 M NaCl. The rest of the epigenetic base containing CG decamers, CD and NMR experiments are in agreement that 5hmC, 5fC, and 5caC do not facilitate Z-DNA formation. On the other hand, if compared with unmodified CG decamer, these methylcytosine oxidative products hinder the B-DNA-to-Z-DNA transition. This finding has attracted our attention on how the TET-catalyzed 5mC oxidative products possess different effects contrasted to their precursor. If we consider the way 5mC facilitates B-DNA-to-Z-DNA transition based on Z-DNA stabilization through van der Waals interaction between the methyl group and hydrophobic patch of stacking dG and in turn undermining the B-DNA stability via exposing hydrophobic methyl group to the surrounding water molecules, the opposite effect may occur for 5hmC, 5fC, and 5caC, which, by nature of substitutions, hydroxymethyl, formyl, and carboxyl, are more polar than methyl. To further investigate and gain more detail on how epigenetic bases affect B-DNA and Z-DNA conformations, MD simulation has been conducted with modified CG decamers.
Figure 3.
Proton NMR spectra of modified oligomers containing 5mC, 5hmC, 5fC, 5caC, and 8oxG. Change in G-H8 chemical shift was observed in 8oxG-containing CG5, indicating the conformation change from B-DNA to Z-DNA, while the formation of Z-DNA was not observed with epigenetic cytosine-containing CG5.
Figure 4.
NOESY spectra of selected CG decamers in 20 mM phosphate buffer of pH 6.5 and 5% v/v D2O (A) 5mC–CG5 with 50 mM NaCl, (B) 5mC–CG5 with 1.5 M NaCl, (C) 8oxG–CG5 with 50 mM NaCl, and (D) 8oxG–CG5 with 1.5 M NaCl.
MD Simulations of CG Decamers Containing Epigenetic Bases
MD simulations were performed under AMBER 12 on the unmodified and modified CG decamers in both B-DNA and Z-DNA conformations to gain insight into how epigenetic base modification affects the B-DNA-to-Z-DNA transition, such as we observed with CD and NMR studies. The cluster analysis on each MD run has been performed and visualized using UCSF Chimera suit.45 From highly populated clusters, representative frames have been analyzed for potential interaction, especially from modified residues, that may support experimental results. Steric from C8 substitution of dG in anti conformation of B-DNA is recognized to be the main force to drive B-DNA-to-Z-DNA conversion, which also seems to be the case for 8oxG-containing CG decamer. From the simulation, 8-oxo has been shown to be in close proximity to H2′/H2″ of the 5′-adjacent C residue, which may cause a steric effect to the B-DNA structure. This is not the case for Z-DNA, explaining the effect of 8oxG in promoting Z-DNA formation. Cytosine methylation has been reported to destabilize the B-DNA conformation by placing hydrophobic methyl accessible to water.34 In the case of 5hmC, 5fC, and 5caC, which contain hydroxymethyl, formyl, and carboxyl, respectively, the effect of methyl would, in turn, be opposite, as these polar substitutions are observed in major groove of B-DNA and can form H-bonding with surrounding water molecules. Clash between H2′/H2″ of the 5′-adjacent G residue and the methyl of 5mC also contributes to B-DNA destabilization,43 which also poses similar B-DNA destabilization of 5hmC-, 5fC-, and 5caC-containing CG decamers due to steric 5 position substitution on cytosine. For Z-DNA, hydrophobic contact between methyl and purine ring of the 5′-adjacent G residue help increase the stability of the stacking base pair,34 hence stabilizing the Z-DNA of 5mC–CG5. In the case of TET-catalyzed oxidative products of 5mC, analyses of the representative frames of Z-DNA have shown general trend of the adducts such that they are also in close proximity to H2′/H2″, which can contribute to the steric destabilization of the left-handed conformation. Furthermore, stability penalty from electrostatic repulsion between oxygen atoms of cytosine modifications and nearby electronegative nuclei in Z-DNA conformation could impede B-DNA-to-Z-DNA conversion. In the MD analysis of Z-DNA, hydroxy oxygen of 5hmC appeared close to N7 and N9 of the 5′-adjacent G residue as well as O6 of the 3′-adjacent G residue with distance close to 4 Å (Figure 5), which is not observed in B-DNA model. In addition to what we observed in the model of 5hmC–CG5, due to sliding of base pairs toward axial of Z-DNA structure, carbonyl oxygens from formyl of 5fC of both strands are pushed close together in the range of 3–4 Å, which may undermine the stability of the duplex as shown in Figure 5. Comparable to 5fC, negatively charged carboxyl of 5caC in both strands of 5caC–CG5 duplex is moved in close proximity, hence destabilizing the Z-DNA conformation through electrostatic repulsion between negatively charged substitutions. Observation from the MD simulation of the epigenetic base containing CG decamers helps explain the effect of 5hmC, 5fC, and 5caC on hampering the Z-DNA formation.
Figure 5.
Partial structure of 5hmC–CG5, 5fC–CG5, and 5caC–CG5 (modified C and adjacent 5′ and 3′ G) from MD simulations in B-DNA (top) and Z-DNA (bottom) conformations. The modified cytosine residues are shown with colored heteroatoms. The red dash lines in Z-DNA models indicate a potential clash between cytosine modifications and neighboring bases.
Free Energy Comparison between B-DNA and Z-DNA of CG Decamers Containing Epigenetic Bases
To gain more insight into the energy terms of both B-DNA and Z-DNA of 5mC–CG5, 5hmC–CG5, 5fC–CG5, 5caC–CG5, and 8oxG–CG5, MM-PBSA analysis was performed to calculate the free energies of the modified oligos in both conformations along with unmodified CG5. The energy differences between Z-DNA and B-DNA (ΔGZB) of each oligo in various salt concentrations ranging from no salt to 4 M salt are reported in Table 1. Free energy calculation was generally consistent with the results from CD and NMR experiments such that B-DNA is more energetically favorable than Z-DNA, except for 8oxG–CG5. In the case of 8oxG–CG5, the calculation outcome deviates from the experimental result and overestimates that Z-DNA is more stable than canonical B-DNA even without NaCl, represented by a negative value of ΔGZB. As a result of increasing NaCl concentration, the differences in energy between Z-DNA and B-DNA of 5mC–CG5, 5hmC–CG5, 5fC–CG5, and 5caC–CG5 are reduced. This result is in agreement to the fact that Na+ help screen negative charges of phosphate backbone in Z-DNA, therefore stabilizing the left-handed conformation. Nevertheless, B-DNA of 5hmC–CG5, 5fC–CG5, and 5caC–CG5 remain more energy favorable even with the salt up to 4 M as indicated with calculated ΔGZB equal to 13.68, 17.52, and 13.45, respectively. This calculation supports the experimental result that 5hmC, 5fC, and 5caC do not have a Z-DNA-promoting effect and cannot facilitate B-DNA-to-Z-DNA conversion. In the case of 5mC, although MM-PBSA calculation underestimates the effect of methylation on B-DNA-to-Z-DNA transition, e.g., in CD experiment, 2 M NaCl was sufficient to promote Z-DNA formation of 5mC–CG5 but the calculated ΔGZB is positive and describes 5mC–CG5 as less favorable for Z-DNA conformation when compared to the unmodified CG5, the free energy calculation indicates some degree of Z-DNA-promoting effect from the methyl substitution such that in the case of 4 M salt, ΔGZB was 0.96, suggesting comparable energy level of B-DNA and Z-DNA. While the free energy obtained from MM-PBSA has shown a slight discrepancy to experimental studies, the calculation described a similar trend to what we have found with CD and NMR. Ranking of ΔGZB suggests that 8oxG is the most potent modification to promote Z-DNA formation, followed by 5mC, while relatively large, positive ΔGZB of the rest of modified oligos indicate that 5hmC, 5fC, and 5caC do not have Z-DNA-promoting effect, as their precursor has.
Table 1. Free Energy (kcal mol–1) Values of Unmodified and Epigenetic Base Containing CG Decamers Calculated Using MM-PBSA.
| 0.0 M NaCl |
0.2 M NaCl |
2.0 M NaCl |
4.0 M NaCl |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| oligos | GZ | GB | ΔGZB | GZ | GB | ΔGZB | GZ | GB | ΔGZB | GZ | GB | ΔGZB |
| 5mC–CG5 | –4848.57 | –4843.00 | 5.57 | –4846.43 | –4849.05 | 2.62 | –4845.92 | –4847.40 | 1.48 | –4849.06 | –4848.10 | 0.96 |
| 5hmC–CG5 | –4950.50 | –4930.36 | 20.14 | –4933.86 | –4950.56 | 16.70 | –4934.38 | –4948.55 | 14.17 | –4950.09 | –4936.41 | 13.68 |
| 5fC–CG5 | –5040.77 | –5019.00 | 21.77 | –5022.51 | –5041.58 | 19.07 | –5021.50 | –5039.06 | 17.56 | –5041.20 | –5023.68 | 17.52 |
| 5caC–CG5 | –5157.58 | –5139.76 | 17.82 | –5144.46 | –5158.94 | 14.48 | –5142.62 | –5156.54 | 13.92 | –5158.17 | –5144.72 | 13.45 |
| 8oxG–CG5 | –5021.14 | –5021.41 | –0.27 | –5024.25 | –5021.53 | –2.72 | –5024.08 | –5020.33 | –3.75 | –5021.55 | –5025.11 | –3.56 |
| CG5 | –4647.07 | –4649.11 | 2.04 | –4649.62 | –4649.81 | 0.19 | –4649.63 | –4648.75 | –0.88 | –4650.83 | –4649.17 | –1.66 |
Conclusions
The epigenetic 5mC was discovered to be a modification that plays an important part in complex genetic regulation process. By historical context, the removal of 5mC was believed by the research community that passive dilution via replication and base excision was the only mechanistic means. With advances in detection technique along with further insight into how TET works, oxidative products of 5mC including 5hmC, 5fC, and 5caC were identified and their production has been known to regulate the presence of 5mC in genome. In addition to 5mC reversal, expanding evidence suggests the epigenetic role of TET-catalyzed 5mC products such that they may have their own role in gene regulation. Replacing 5mC on DNA strand with its oxidative products causes changes in term of chemical feature, local structure, and perhaps global structure, which, in turn, alter the function of macromolecules. For instance, stabilization46 and destabilization47−49 of DNA duplex by TET-catalyzed 5mC products have been reported in separate studies, hence may provide diverse effects on DNA integrity and function. It has been reported that lost recognition of 5hmC to DNA binding protein such as MeCP2, transcription repressor MBP, hints release of gene repression by 5mC.50 Different excision rate of 5mC products by thymine DNA glycosylase51 (TDG), which demethylates 5mC, was also an outcome of TET catalysis. A study on DNA polymerase β fidelity and activity in the presence of 5hmC, 5fC, and 5caC has been reported recently, with unaffected fidelity for all base modifications but dramatic drop in efficiency for 5caC. Global effect of epigenetic bases on DNA conformation is also another interesting outcome that may implicate epigenetic regulation as demonstrated in 5mC which stabilizes G-quadruplex DNA in bcl-2 promoter.52 Z-DNA conformation is our focus since epigenetic bases, i.e., 5mC and 8oxG, and CpG, repeated as CG islands found in transcription starting site involved in an epigenetic event,53,54 are both Z-DNA-promoting factors. Additionally, there are several studies that demonstrate the necessity of Z-DNA-forming sequences in gene expression16,55 and repression.18,19 In summary, according to the result from this study, no TET-catalyzed oxidative products of 5mC facilitates B-DNA-to-Z-DNA transition. Intriguingly, 5hmC, 5fC, and 5caC seem to inhibit Z-DNA formation in our setting when compared to unmodified CG decamer. The insight obtained here recaptured 5mC as Z-DNA promoter while described its oxidative product as suppressor. This finding has lit interesting prospect on potential of Z-DNA involvement in mechanistic control over epigenetic regulation as a switch-on/switch-off process through B-DNA-to-Z-DNA transition as proposed in Figure 6. In addition to chemistry alteration and local structure change that modify DNA function, conformational control through Z-DNA could be another mechanism in epigenetics. Nevertheless, an extensive study is mandatory to answer this initial hypothesis. A near-physiological in vitro setting such as a B–Z transition study with Z-DNA binding protein at low salt concentration would be the next step to verify what has been found in this study before extrapolating the effect of 5hmC, 5fC, and 5caC on Z-DNA to in vivo setting. Subsequently, cellular imaging of DNA conformation and gene expression studies with the presence of epigenetic bases is our next step. This work serves as a preliminary stage toward a more insightful investigation of the role of Z-DNA in epigenetic regulation.
Figure 6.
Z-DNA switch-on/switch-off model regulated by methylated cytosine and its TET oxidative products.
Methods
Epigenetic Bases Containing Oligomers
Oligomers including the unmodified CG5, 5mC-, 5hmC-, 5fC-, 5caC-, and 8oxG-containing CG5 were purchased from Hokkaido System Science. Each oligomer contains single modified base at C5 for all modified cytosines, and G6 for 8oxG (Figure 1) CG5 sequence has been used as a model in this study for comparison purposes with previous works on the modified base capable of promoting Z-DNA formation. The synthesized oligomers were purified by HPLC and characterized by mass spectrometry in negative ion mode. Mass spectra of 5mC–CG5, 5hmC–CG5, 5fC–CG5, 5caC–CG5, and 8oxG–CG5 showing m/z 3043.29, 3059.38, 3057.40, 3073.38, and 3045.28, respectively, indicate the correct identity of the modified oligonucleotides.
Circular Dichroism
CD spectra of CG5, 5mC–CG5, 5hmC–CG5, 5fC–CG5, 5caC–CG5, and 8oxG–CG5 were obtained by Jasco J-815 CD spectropolarimeter with CDF-426L/15 temperature controller. Samples with oligomer concentration of 20 μM were prepared in 50 mM phosphate buffer pH 7.4 with NaCl concentration ranging between 50 mM and 5 M and were contained in sample cells with 1 mm path length. CD spectrum of each sample was recorded from 230 to 310 nm at 283 K with five accumulations and a scanning rate of 50 nm min–1. Transition from B-DNA to Z-DNA can be monitored by change in the CD signal of B-DNA to Z-DNA. Observable from CD, B-DNA shows the characteristic positive ellipticity at approximately 280 and a negative ellipticity at approximately 250 nm. CD spectrum of Z-DNA shows a hallmark negative ellipticity at approximately 290 nm and a positive ellipticity at approximately 250 nm.
NMR
NMR experiments were performed with a 600 MHz NMR spectrometer (AVANCE-600, Bruker Biospin). At 283 K, conformation of the oligomers (approximately 50 μM oligo samples of CG5, 5mC–CG5, 5fC–CG5, 5caC–CG5, 8oxG–CG5, and 30 μM sample of 5hmC–CG5 in 20 mM phosphate buffer pH 6.5 and 5% v/v D2O) with different salt concentrations (50 mM, 0.5 M, 1.0 M, and 1.5 M for modified CG5) was characterized by 1H NMR, nuclear Overhauser effect spectroscopy (NOESY), and homonuclear Hartmann–Hahn spectroscopy (HOHAHA). For NOESY and HOHAHA measurements, mixing times of 300 and 50 ms were used, respectively. The water signal was suppressed by the 3–9–19 pulse sequence.56 The NMR data on the sample with a salt concentration above 1.5 M could not be achieved due to the limitation of the NMR spectrometer.
MD Simulation and Free Energy Calculation
Duplexes of 5mC–CG5, 5hmC–CG5, 5fC–CG5, and 5caC–CG5 in B-DNA and Z-DNA conformations were built in Chimera by replacing C5 and C15 of CG5 duplex with the corresponding modified cytosines. G6 and G16 of CG5 duplex were replaced with 8oxG to yield the 8oxG–CG5 structure. PDB coordinates of the modified CG5 were used in AMBER 12 for further preparation of MD simulation. Antechamber and Parmchk equipped in AMBER 12 were then applied to parameterize force field of 5mC, 5hmC, 5fC, 5caC, and 8oxG. Atom types were consistent with the parm99.dat force field. Coordinates and force-field parameters of the modified bases were loaded into tleap to generate topology and coordinate files with TIP3PBOX for MD simulation. Note that in the case of 5caC-containing CG5, the carboxylic protons were removed and thus prepared as carboxylates since this is the form they are present under physiological conditions. The simulation protocol started with minimizing the energy of the whole system with 50 steps of steepest descent and then 950 steps of conjugated gradient minimization. In addition, periodicity with 9 Å cutoff was used in the minimization. Next step, 20 ps MD with the DNA fixed and increase temperature to 300 K was performed to equilibrate water and counterions followed by 100 ps MD in the isothermal–isobaric ensemble (300 K, 1 atm) with a 9 Å cutoff. A further 100 ps MD with decrease harmonic restraint of solute was performed to relax the system. Production runs of 20 ns duration, at constant temperature and constant pressure (300 K, 1 atm), were performed after the final equilibration step. MM-PBSA was used to calculate the solvation free energy of the oligomers in the solution from MD trajectories of both B and Z forms. Snapshots for the MM-PBSA calculation were created from 20 ns MD simulation. The first ns was excluded from the analysis. The rest of 19 ns MD run was used to extract the snapshots in 50 ps intervals, yielding 190 snapshots per MD trajectory. Salt concentrations 0.0, 0.2, 2.0, and 4.0 M were assigned in the MM-PBSA input script for the calculation of the free energy of oligos with different salt concentrations.
Acknowledgments
This work was supported by grant for join funding, Ratchadaphiseksomphot endowment fund. In addition, the study received support from the Chiba Institute of Technology International Academic Fellowship Program for Cooperative Universities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00120.
Proton NMR spectra of the oligonucleotides and MM-PBSA energy term of the oligonucleotides; 1D 1H NMR of CG5, 5mC–CG5, 5fC–CG5, 5caC–CG5, and 8oxG–CG5 in 20 mM phosphate buffer pH 6.5 and 5% D2O with 50 mM, 500 mM, 1 M, and 1.5 M (with exception of CG5) NaCl; MM-PBSA energy term of CG5, 5mC–CG5, 5fC–CG5, 5caC–CG5, and 8oxG–CG5 in B-DNA and Z-DNA with the ionic strength of 0 mM, 0.2 mM, 2.0 M, and 4.0 M NaCl (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hermann A.; Gowher H.; Jeltsch A. Biochemistry and Biology of Mammalian DNA Methyltransferases. Cell. Mol. Life Sci. 2004, 61, 2571–2587. 10.1007/s00018-004-4201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. DNA Methylation Patterns and Epigenetic Memory. Genes Dev. 2002, 16, 6–21. 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Jones P. A.; Takai D. The Role of DNA Methylation in Mammalian Epigenetics. Science 2001, 293, 1068–1070. 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Tahiliani M.; Koh K. P.; Shen Y.; Pastor W. A.; Bandukwala H.; Brudno Y.; Agarwal S.; Iyer L. M.; Liu D. R.; Aravind L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935. 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S.; D’Alessio A. C.; Taranova O. V.; Hong K.; Sowers L. C.; Zhang Y. Role of Tet Proteins in 5mC to 5hmC Conversion, ES-Cell Self-Renewal and Inner Cell Mass Specification. Nature 2010, 466, 1129–1133. 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S.; Shen L.; Dai Q.; Wu S. C.; Collins L. B.; Swenberg J. A.; He C.; Zhang Y. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science 2011, 333, 1300–1303. 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M.; Jiang L.; Akatsuka S.; Suyama M.; Toyokuni S. Genome-Wide Profiling of 8-Oxoguanine Reveals Its Association with Spatial Positioning in Nucleus. DNA Res. 2014, 21, 603–612. 10.1093/dnares/dsu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Fleming A. M.; Burrows C. J. Sequencing the Mouse Genome for the Oxidatively Modified Base 8-Oxo-7,8-Dihydroguanine by OG-Seq. J. Am. Chem. Soc. 2017, 139, 2569–2572. 10.1021/jacs.6b12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. P. G.; Toomire K. J.; Strauss P. R. DNA Modifications Repaired by Base Excision Repair Are Epigenetic. DNA Repair 2013, 12, 1152–1158. 10.1016/j.dnarep.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Fleming A. M.; Ding Y.; Burrows C. J. Oxidative DNA Damage Is Epigenetic by Regulating Gene Transcription via Base Excision Repair. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 2604–2609. 10.1073/pnas.1619809114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifermann M.; Epe B. Oxidatively Generated Base Modifications in DNA: Not Only Carcinogenic Risk Factor but Also Regulatory Mark?. Free Radical Biol. Med. 2017, 107, 258–265. 10.1016/j.freeradbiomed.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Fleming A. M.; Burrows C. J. 8-Oxo-7,8-Dihydroguanine, Friend and Foe: Epigenetic-like Regulator versus Initiator of Mutagenesis. DNA Repair 2017, 56, 75–83. 10.1016/j.dnarep.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiber E.-A.; Beraldi D.; Ficz G.; Burgess H. E.; Branco M. R.; Murat P.; Oxley D.; Booth M. J.; Reik W.; Balasubramanian S. Genome-Wide Distribution of 5-Formylcytosine in Embryonic Stem Cells Is Associated with Transcription and Depends on Thymine DNA Glycosylase. Genome Biol. 2012, 13, R69 10.1186/gb-2012-13-8-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M. J.; Branco M. R.; Ficz G.; Oxley D.; Krueger F.; Reik W.; Balasubramanian S. Quantitative Sequencing of 5-Methylcytosine and 5-Hydroxymethylcytosine at Single-Base Resolution. Science 2012, 336, 934–937. 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- Booth M. J.; Marsico G.; Bachman M.; Beraldi D.; Balasubramanian S. Quantitative Sequencing of 5-Formylcytosine in DNA at Single-Base Resolution. Nat. Chem. 2014, 6, 435–440. 10.1038/nchem.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.; Liu H.; Chen X.; Kirby M.; Brown P. O.; Zhao K. Regulation of CSF1 Promoter by the SWI/SNF-like BAF Complex. Cell 2001, 106, 309–318. 10.1016/S0092-8674(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Wittig B.; Wolfl S.; Dorbic T.; Vahrson W.; Rich A. Transcription of Human C-myc in Permeabilized Nuclei Is Associated With Formation of Z-DNA in Three Discrete Regions of the Gene. EMBO J. 1992, 11, 4653–4663. 10.1002/j.1460-2075.1992.tb05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K.; Dhar S.; Shakya A.; Ray A. Z-DNA-Forming Silencer in the First Exon Regulates Human ADAM-12 Gene Expression. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 103–108. 10.1073/pnas.1008831108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K.; Dhar S.; Henry C.; Rich A.; Ray A. Epigenetic Regulation by Z-DNA Silencer Function Controls Cancer-Associated ADAM-12 Expression in Breast Cancer: Cross-Talk between MeCp2 and NF1 Transcription Factor Family. Cancer Res. 2013, 73, 736–744. 10.1158/0008-5472.CAN-12-2601. [DOI] [PubMed] [Google Scholar]
- Pohl F. M.; Jovin T. M. Salt-Induced Coperative Conformational Change of a Synthetic DNA: Equilibrium and Kinetic Studies with Poly (dG-dC). J. Mol. Biol. 1972, 67, 375–396. 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Behe M.; Felsenfeld G. Effects of Methylation on a Synthetic Polynucleotide: The B-Z Transition in Poly(dG-m5dC).Poly(dG-m5dC). Proc. Natl. Acad. Sci. U.S.A. 1981, 78, 1619–1623. 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner R. V.; Quigley G. J.; Wang A. H.; van der Marel G. A.; van Boom J. H.; Rich A. Structural Basis for Stabilization of Z-DNA by Cobalt Hexaammine and Magnesium Cations. Biochemistry 1985, 24, 237–240. 10.1021/bi00323a001. [DOI] [PubMed] [Google Scholar]
- Chatake T.; Sunami T. Direct Interactions between Z-DNA and Alkaline Earth Cations, Discovered in the Presence of High Concentrations of MgCl2 and CaCl2. J. Inorg. Biochem. 2013, 124, 15–25. 10.1016/j.jinorgbio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Russell W. C.; Precious B.; Martin S. R.; Bayley P. M. Differential Promotion and Suppression of Z Leads to B Transitions in Poly[d(G-C)] by Histone Subclasses, Polyamino Acids and Polyamines. EMBO J. 1983, 2, 1647–1653. 10.1002/j.1460-2075.1983.tb01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. J.; Messner R. P. A Left-Handed (Z) Conformation of Poly(dA-dC).Poly(dG-dT) Induced by Polyamines. Nucleic Acids Res. 1986, 14, 6721–6733. 10.1093/nar/14.16.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. J.; Gunnia U. B.; Thomas T. Polyamine-Induced B-DNA to Z-DNA Conformational Transition of a Plasmid DNA with (dG-dC)n Insert. J. Biol. Chem. 1991, 266, 6137–6141. [PubMed] [Google Scholar]
- Ohishi H.; Kunisawa S.; van der Marel G.; van Boom J. H.; Rich A.; Wang A. H.; Tomita K.; Hakoshima T. Interaction between the Left-Handed Z-DNA and Polyamine. The Crystal Structure of the d(CG)3 and N-(2-Aminoethyl)-1,4-Diamino-Butane Complex. FEBS Lett. 1991, 284, 238–244. 10.1016/0014-5793(91)80694-X. [DOI] [PubMed] [Google Scholar]
- Hasan R.; Alam M. K.; Ali R. Polyamine Induced Z-Conformation of Native Calf Thymus DNA. FEBS Lett. 1995, 368, 27–30. 10.1016/0014-5793(95)00591-V. [DOI] [PubMed] [Google Scholar]
- Kang Y.-M.; Bang J.; Lee E. H.; Ahn H.-C.; Seo Y.-J.; Kim K. K.; Kim Y.-G.; Choi B.-S.; Lee J.-H. NMR Spectroscopic Elucidation of the B-Z Transition of a DNA Double Helix Induced by the Z alpha Domain of Human ADAR1. J. Am. Chem. Soc. 2009, 131, 11485–11491. 10.1021/ja902654u. [DOI] [PubMed] [Google Scholar]
- Kim H.-E.; Ahn H.-C.; Lee Y.-M.; Lee E. H.; Seo Y.-J.; Kim Y.-G.; Kim K. K.; Choi B.-S.; Lee J.-H. The Zbeta Domain of Human DAI Binds to Z-DNA via a Novel B-Z Transition Pathway. FEBS Lett. 2011, 585, 772–778. 10.1016/j.febslet.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Ikeda R.; Sugiyama H. 8-Methylguanosine: A Powerful Z-DNA Stabilizer. J. Am. Chem. Soc. 2003, 125, 13519–13524. 10.1021/ja036233i. [DOI] [PubMed] [Google Scholar]
- Vongsutilers V.; Phillips D. J.; Train B. C.; McKelvey G. R.; Thomsen N. M.; Shaughnessy K. H.; Lewis J. P.; Gannett P. M. The Conformational Effect of Para-Substituted C8-Arylguanine Adducts on the B/Z-DNA Equilibrium. Biophys. Chem. 2011, 154, 41–48. 10.1016/j.bpc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Vongsutilers V.; Gannett P. M. C8-Guanine Modifications: Effect on Z-DNA Formation and Its Role in Cancer. Org. Biomol. Chem. 2018, 16, 2198–2209. 10.1039/C8OB00030A. [DOI] [PubMed] [Google Scholar]
- Fujii S.; Wang A. H.; van der Marel G.; van Boom J. H.; Rich A. Molecular Structure of (m5 dC-dG)3: The Role of the Methyl Group on 5-Methyl Cytosine in Stabilizing Z-DNA. Nucleic Acids Res. 1982, 10, 7879–7892. 10.1093/nar/10.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest D.; Hartmann B.; Thuong N. T.; Ptak M.; Leng M. Thermal Stability of the Z-Conformation of the Tetranucleoside Triphosphate (m5dC-dG). Biochem. Biophys. Res. Commun. 1984, 125, 803–811. 10.1016/0006-291X(84)90610-7. [DOI] [PubMed] [Google Scholar]
- Zarling D. A.; Arndt-Jovin D. J.; Robert-Nicoud M.; McIntosh L. P.; Thomae R.; Jovin T. M. Immunoglobulin Recognition of Synthetic and Natural Left-Handed Z DNA Conformations and Sequences. J. Mol. Biol. 1984, 176, 369–415. 10.1016/0022-2836(84)90495-9. [DOI] [PubMed] [Google Scholar]
- Zacharias W.; Caserta M.; O’Connor T. R.; Larson J. E.; Wells R. D. Cytosine Methylation as an Effector of Right-Handed to Left-Handed DNA Structural Transitions. Gene 1988, 74, 221–224. 10.1016/0378-1119(88)90291-0. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wang S.; Zhong C.; Tian T.; Zhou X. Novel Insights into a Major DNA Oxidative Lesion: Its Effects on Z-DNA Stabilization. Org. Biomol. Chem. 2015, 13, 8996–8999. 10.1039/C5OB01340B. [DOI] [PubMed] [Google Scholar]
- Hardwick J. S.; Ptchelkine D.; El-Sagheer A. H.; Tear I.; Singleton D.; Phillips S. E. V.; Lane A. N.; Brown T. 5-Formylcytosine Does Not Change the Global Structure of DNA. Nat. Struct. Mol. Biol. 2017, 24, 544–552. 10.1038/nsmb.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiber E.-A.; Murat P.; Chirgadze D. Y.; Beraldi D.; Luisi B. F.; Balasubramanian S. 5-Formylcytosine Alters the Structure of the DNA Double Helix. Nat. Struct. Mol. Biol. 2015, 22, 44–49. 10.1038/nsmb.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepierre M.; Langlois D’Estaintot B.; Igolen J.; Roques B. P. Conformational Studies of d(m5CpGpm5CpG) and d(CpGpCpG) by 1H and 31P NMR. Eur. J. Biochem. 1986, 161, 571–577. 10.1111/j.1432-1033.1986.tb10480.x. [DOI] [PubMed] [Google Scholar]
- Vongsutilers V.; Sawaspaiboontawee K.; Tuesuwan B.; Shinohara Y.; Kawai G. 5-Methylcytosine Containing CG Decamer as Z-DNA Embedded Sequence for a Potential Z-DNA Binding Protein Probe. Nucleosides, Nucleotides Nucleic Acids 2018, 37, 485–497. 10.1080/15257770.2018.1498512. [DOI] [PubMed] [Google Scholar]
- Liebl K.; Zacharias M. How Methyl-Sugar Interactions Determine DNA Structure and Flexibility. Nucleic Acids Res. 2018, 56142668 10.1093/nar/gky1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbons L. P.; van der Marel G. A.; van Boom J. H.; Altona C. The B and Z Forms of the d(m5C-G)3 and d(Br5C-G)3 Hexamers in Solution. A 300-MHz and 500-MHz Two-Dimensional NMR Study. Eur. J. Biochem. 1986, 160, 131–139. 10.1111/j.1432-1033.1986.tb09949.x. [DOI] [PubMed] [Google Scholar]
- Couch G. S.; Hendrix D. K.; Ferrin T. E. Nucleic Acid Visualization with UCSF Chimera. Nucleic Acids Res. 2006, 34, e29 10.1093/nar/gnj031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumino M.; Ohkubo A.; Taguchi H.; Seio K.; Sekine M. Synthesis and Properties of Oligodeoxynucleotides Containing 5-Carboxy-2′-Deoxycytidines. Bioorg. Med. Chem. Lett. 2008, 18, 274–277. 10.1016/j.bmcl.2007.10.081. [DOI] [PubMed] [Google Scholar]
- Thalhammer A.; Hansen A. S.; El-Sagheer A. H.; Brown T.; Schofield C. J. Hydroxylation of Methylated CpG Dinucleotides Reverses Stabilisation of DNA Duplexes by Cytosine 5-Methylation. Chem. Commun. 2011, 47, 5325–5327. 10.1039/c0cc05671e. [DOI] [PubMed] [Google Scholar]
- Xuan S.; Wu Q.; Cui L.; Zhang D.; Shao F. 5-Hydroxymethylcytosine and 5-Formylcytosine Containing Deoxyoligonucleotides: Facile Syntheses and Melting Temperature Studies. Bioorg. Med. Chem. Lett. 2015, 25, 1186–1191. 10.1016/j.bmcl.2015.01.070. [DOI] [PubMed] [Google Scholar]
- Dai Q.; Sanstead P. J.; Peng C. S.; Han D.; He C.; Tokmakoff A. Weakened N3 Hydrogen Bonding by 5-Formylcytosine and 5-Carboxylcytosine Reduces Their Base-Pairing Stability. ACS Chem. Biol. 2016, 11, 470–477. 10.1021/acschembio.5b00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriukienė E.; Liutkeviciute Z.; Klimasauskas S. 5-Hydroxymethylcytosine--the Elusive Epigenetic Mark in Mammalian DNA. Chem. Soc. Rev. 2012, 41, 6916–6930. 10.1039/c2cs35104h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulik M. W.; Pallan P. S.; Nocek B.; Voehler M.; Banerjee S.; Brooks S.; Joachimiak A.; Egli M.; Eichman B. F.; Stone M. P. Differential Stabilities and Sequence-Dependent Base Pair Opening Dynamics of Watson-Crick Base Pairs with 5-Hydroxymethylcytosine, 5-Formylcytosine, or 5-Carboxylcytosine. Biochemistry 2015, 54, 1294–1305. 10.1021/bi501534x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.; Hou J.; Xiang H.; Yan Y.; Gu Y.; Tan J.; Li D.; Gu L.; Ou T.; Huang Z. Stabilization of G-Quadruplex DNA by C-5-Methyl-Cytosine in Bcl-2 Promoter: Implications for Epigenetic Regulation. Biochem. Biophys. Res. Commun. 2013, 433, 368–373. 10.1016/j.bbrc.2012.12.040. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Functions of DNA Methylation: Islands, Start Sites, Gene Bodies and Beyond. Nat. Rev. Genet. 2012, 13, 484–492. 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Brenet F.; Moh M.; Funk P.; Feierstein E.; Viale A. J.; Socci N. D.; Scandura J. M. DNA Methylation of the First Exon Is Tightly Linked to Transcriptional Silencing. PLoS One 2011, 6, e14524 10.1371/journal.pone.0014524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfl S.; Wittig B.; Rich A. Identification of Transcriptionally Induced Z-DNA Segments in the Human c-myc Gene. Biochim. Biophys. Acta 1995, 1264, 294–302. 10.1016/0167-4781(95)00155-7. [DOI] [PubMed] [Google Scholar]
- Piotto M.; Saudek V.; Sklenar V. Gradient-Tailored Excitation for Single-Quantum NMR Spectroscopy of Aqueous Solutions. J. Biomol. NMR 1992, 2, 661–665. 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.