Abstract

A method for simple and fast (30–60 s) synthesis of spherical “Fe3O4 core–carbon shell” structures by atmospheric pressure aerosol pyrolysis of benzoic acid in dimethylformamide solutions containing dispersed Fe3O4 nanoparticles is described. It has been experimentally shown that it is possible to control both the size of the core–shell particles and the size of Fe3O4 grains and their amount in the particle core by the variation of benzoic acid concentration in solution and using pre-stabilized by mannitol iron oxide nanoparticles. It has been found that particles with an average size of 250–350 nm are formed at the concentration of benzoic acid in the range 0.5–1 mol/L. At a concentration of about 1 mol/L, preliminary stabilization of iron oxide nanoparticles by mannitol with a size of about 180 nm is performed.
Introduction
The technological importance of iron oxide nanosystems in view of different technological end-uses leads to the development of new synthesis methods.1−4 In this letter, we report a new method for the synthesis of core–shell structures based on Fe3O4 nanoparticles and carbon, which can be considered as promising materials for various applications in industry and medicine.5−8 Among other iron oxides, magnetite (Fe3O4) is more stable and has higher magnetic characteristics. It can be used, for example, in medicine in diagnostic systems (MRI), targeted delivery, protein immobilization, biolabels, and so forth. Existing methods for the preparation of nanomaterials with a “Fe3O4 core–carbon shell” structure are usually time consuming and difficult to implement. They can provide high productivity of the material with required characteristics and composition only in rare cases.
The aerosol-assisted chemical vapor deposition (AACVD) can be considered as an attractive method for the fast formation of different nanomaterials9 including core–shell nanostructures in the “iron oxide–carbon” system. In this case, one can use solutions of carbon-containing reagents required for the “carbon shell” formation with dispersed Fe3O4 particles as initial substances. The main advantages of this approach compared to other methods are simplicity of the synthesis procedure and required equipment, as well as significantly lower reagent consumption. Variations of process parameters allow to smoothly and predictably change the structural characteristics of the obtained particles. However, there are only a few papers dedicated to the study of principal possibility to synthesize carbon nanoparticles using this method10 and to fill porous carbon nanospheres with metal oxide particles.11 The AACVD method has not been used for the synthesis of the “Fe3O4 core–C shell” nanostructures. The aim of this work was to study the influence of the main technological parameters of the aerosol chemical deposition process on the composition and structure of the formed nanoparticles with a “Fe3O4 core–carbon shell” structure.
Results and Discussion
The AACVD process was carried out in a vertical flow reactor with “hot walls” (see Experimental section). To obtain powdered nanomaterials with a “Fe3O4 core–carbon shell” particle structure, a solution of benzoic acid in dimethylformamide (DMF) containing Fe3O4 nanoparticles dispersed in it was used. Magnetite nanoparticles were preliminarily synthesized in accordance with the method described in ref (11), with and without the use of mannitol as a stabilizer. Then, the aqueous solution base was replaced by a solution of benzoic acid in DMF. Unstabilized iron oxide nanoparticles form agglomerates of about 100 nm in size before the base is replaced. The use of mannitol allows to obtain particles in aqueous medium with an average size of about 10 nm.12 When the medium is replaced from aqueous to solution of benzoic acid in DMF, mannitol is dissolved and Fe3O4 nanoparticles are also subjected to agglomeration, however, with a lower intensity comparing to water because DMF acts as a stabilizer.13 The concentration of the benzoic acid solution in DMF was 1 mol/L (in several experiments it was different and this is highlighted in the text). The content of iron oxide nanoparticles in solutions was about 1 mg/mL.
The temperature along the reactor was set at 750 °C. The residence time of the reagents in the reaction (heated) zone was controlled in the range of 20–60 s by changing the carrier gas flow rate in the range of 0.3–1 L/min.
To establish the time required for the formation of carbon in the “iron oxide–benzoic acid solution in the DMF” system, we studied the IR spectra (Figure 1, spectra were vertically shifted for better visualization) of synthesized composite powders and carbon-containing powders obtained by the AACVD method from a solution of benzoic acid in DMF. It was found that the intensities of the absorption bands corresponding to vibrations of C–Hx fragments (Table 1) decrease in the IR spectra when the residence time of the reagents in the reaction zone is increased and therefore a more complete decomposition of the carbon-containing reagent occurs.
Figure 1.

IR spectra of powders obtained at different times: (a) composite and (b) carbon-containing.
Table 1. Wavenumbers Corresponding to the Absorption Bands in the IR Spectra of Synthesized Powders14.
| ν, cm–1 | vibration type |
|---|---|
| 680–890 | C–Cbend ring; C=Obend |
| 1120 | C–Hbend |
| 1590 | C–Cstretch ring |
| 1750 | C=Ostretch |
| 2780–3200 | C–Hstretch ring |
The results of EDX-analysis of the particles obtained in the reaction system “iron oxide–benzoic acid solution in DMF” with a residence time of about 40 s showed that particles contained iron, oxygen, and carbon. Figure 2 shows the scanning electron microscopy (SEM) image and the data of EDX analysis of the single particle in accordance with the marked profile line.
Figure 2.

Elemental EDX line scans for various elements along the yellow line marked in the upper SEM image.
According to X-ray photoelectron spectroscopy (XPS), the composition of iron oxide in the obtained particles corresponds to Fe3O4 (see Experimental section). Figure 3 shows the images of the synthesized particles obtained with the use of transmission electron microscopy (TEM). The size of the particles was in the range 200–600 nm, and more than 80% of them had a size in the range 300–400 nm (Figure 3a,c). As one can see from the high-resolution TEM (HR-TEM) image (Figure 3d) of the synthesized particles, there is a lighter shell and a dark core inside usually consisting of several 80–100 nm grains (Figure 3b). This fact allowed us to suggest that the compositions of the shell and the inner core are different. The image of the substance forming the shell with a thickness of about 2–2.5 nm (Figure 3d) did not contain clearly visible atomic planes. This is evidence of its amorphous structure, and a lighter color probably indicates that it consists of lighter atoms compared to the core. The results of electron diffraction (Figure 3d) showed that the sample contains iron oxide with the structure of magnetite.15 HR-TEM allowed to estimate the value of an interplanar spacing of 4.86 Å (Figure 3d), which corresponds to the (111) planes in the Fe3O4 phase.16,17 Comparing the data of EDX-analysis, XPS, and TEM, one can conclude that the synthesized particles have a “Fe3O4 core–C shell” structure.
Figure 3.
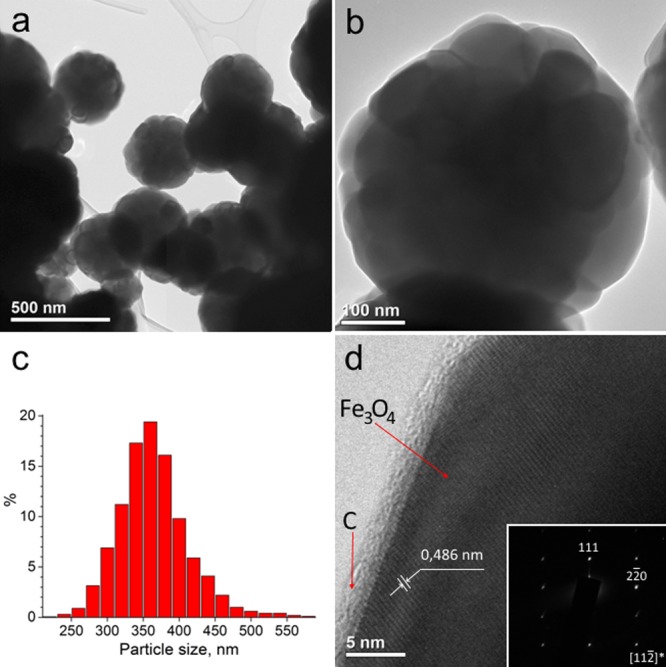
TEM images of particles with the “Fe3O4 core–C shell” structure (a,b), particle size distribution (c), HR-TEM image and insertion of the diffraction pattern of iron oxide, which is part of the particles (d), obtained in the system “iron oxide–benzoic acid solution in DMF”.
The scheme shown in Figure 4 illustrates the mechanism of Fe3O4–C nanoparticle formation in the system “iron oxide–benzoic acid solution in DMF”. An aerosol particle generated with the use of an ultrasonic nebulizer contains several Fe3O4 nanoparticles surrounded by benzoic acid molecules. When aerosol particles are introduced into the reactor, the solvent is evaporated from the surface of the aerosol particle and a solid layer of carbon-containing substances are gradually formed. Then, it is subjected to pyrolysis with the formation of a carbon shell. It is the most probably that carbon in the system participates not only in the formation of the particle shell, but also in the binding of Fe3O4 grains to each other.
Figure 4.

Schematic presentation of the synthesis of “Fe3O4 core–C shell” nanoparticles in the system “iron oxide–benzoic acid solution in DMF”.
Particles with an average size of about 250 nm are formed when the benzoic acid concentration is lowered to 0.5 mol/L (Figure 5a). The use of pre-stabilized mannitol Fe3O4 nanoparticles allows to reduce the average size of the formed core–shell particles to about 180 nm with a more narrow size distribution (Figure 5b). Because of the lower tendency of the iron oxide particles to agglomerate in DMF, smaller grains are formed (Figure 5c). It is possible also, that mannitol in the solution can play the role as an additional carbon-containing reagent in the system. It can be converted into sugars (mannose and galactose) which then are transformed to carbon because of pyrolysis at a higher temperature.18
Figure 5.
SEM and TEM images of core–shell nanoparticles formed during 40 s at the benzoic acid concentration in DMF of 0.5 mol/L (a) and 1 mol/L using stabilized Fe3O4 nanoparticles (b,c) in the system “iron oxide–benzoic acid solution in DMF”.
Conclusions
A method for “Fe3O4–shell C core” spherical structure synthesis by the pyrolysis of aerosols of a solution of benzoic acid in DMF with dispersed Fe3O4 nanoparticles has been developed. It was experimentally shown that by changing the concentration, it is possible to control the size of the formed particles. A decrease in the particle size and the number of Fe3O4 grains in the core, as well as the formation time, is possible in the case of preliminary stabilization of iron oxide nanoparticles by mannitol, which is also an additional carbon-containing component in the system. Detailed step-by-step explanation of this process will be featured in an upcoming publication. Further research activities on the proposed method will be aimed at studying the sorption ability of particles, magnetic characteristics, and at increasing the synthesis rate and yield of the final product.
Experimental Section
Nanoparticle Formation
Powdered nanomaterials with the “Fe3O4 core–C shell” structure were obtained by the AACVD in the experimental setup shown in Figure 6. The liquid reagents from reservoir 1, where they were transferred to aerosol form with the use of piezoelectric nebulizer, were transported into the reaction zone by argon used as a carrier gas. “Hot wall” vertical reactor 3 is equipped with heating elements that allow setting different temperatures in the reactor sections. Single-crystal silicon substrates were placed on the inner surface of nickel cylinder 4, which together with electrode 5 works as an electrostatic filter for collecting of the synthesized products.
Figure 6.

Schematic presentation of the experimental setup.
Materials Characterization
Size and structure analysis of the nanoparticles was carried out according to the SEM images (SUPRA 55VP). Statistical analysis of particle size characteristics was carried out by processing SEM images using the Digimizer software package in at least 200 measurements. The study of the particle structure was carried out using a transmission electron microscope (Jeol JEM-2100F). TEM samples were prepared by dry transfer of the deposited particles onto a carbon grid suspended on a copper TEM grid. In order to analyze the structure of the material, bright-field images and high-resolution images, as well as electron diffraction patterns, were obtained. To determine the nature of chemical bonds in the deposited product and to qualitatively evaluate the composition of powder materials, IR Fourier spectrometry (FSM-1201 FTIR spectrometer) was used. The composition of the particles was studied by means of energy-dispersive spectrometry (Oxford Instruments X-Max) and X-ray photoelectron spectroscopy (SPECS). The energy scale of a XPS spectrometer was calibrated using the 4f7/2 gold line, the binding energy of which was set to 84.00 eV and Mg Kα radiation was used. The charging effect was taken into account along the carbon line corresponding to C–H bonds, and a good agreement was observed between the positions of the lines of the remaining elements in the sample and their expected chemical state.
XPS Spectra Interpretation
According to the overall spectrum obtained by the XPS method, the content of Fe in the test sample is 3.6 at. %, O—32.0 at. % and C—64.4 at. %. The 2p3/2 Fe line (Figure 7, left) is fitted with asymmetric Gaussian–Lorentzian peak with the maximum at 710.58 eV. The 1s oxygen line (Figure 7, right, red curve) can be interpreted as a superposition (brown dash curve) of four components with different binding energies while 530.31 eV peak correspond to the “oxygen–iron” bond.19 The area under this curve is 15.0% or 4.8 at. %, which allows to conclude that the ratio of iron and oxygen associated with iron in the test sample is 3:4 (3.6 at. % Fe and 4.8 at. % O) corresponding to iron oxide (II, III). Binding energies of 530.31 and 710.58 eV correspond to the iron and oxygen in magnetite.19 The Fe 2p3/2 710.58 eV peak has no satellites, which is typical, unlike other iron oxides, for Fe3O4.19,20
Figure 7.

Fe 2p3/2 and O 1s peaks and XPS of synthesized nanoparticles.
The authors declare no competing financial interest.
References
- Mantovan R.; Lamperti A.; Georgieva M.; Tallarida G.; Fanciulli M. CVD Synthesis of polycrystalline magnetite thin films: structural, magnetic and magnetotransport properties. J. Phys. D: Appl. Phys. 2010, 43, 065002. 10.1088/0022-3727/43/6/065002. [DOI] [Google Scholar]
- Warwick M. E. A.; Kaunisto K.; Barreca D.; Carraro G.; Gasparotto A.; Maccato C.; Bontempi E.; Sada C.; Ruoko T.-P.; Turner S.; Van Tendeloo G. Vapor phase processing of α-Fe2O3 Photoelectrodes for water splitting: an insight into the structure/property interplay. ACS Appl. Mater. Interfaces 2015, 7, 8667–8676. 10.1021/acsami.5b00919. [DOI] [PubMed] [Google Scholar]
- Barreca D.; Carraro G.; Gasparotto A.; MacCato C.; Rossi F.; Salviati G.; Tallarida M.; Das C.; Fresno F.; Korte D.; Štangar U. L.; Franko M.; Schmeisser D. Surface functionalization of nanostructured Fe2O3 polymorphs: from design to light-activated applications. ACS Appl. Mater. Interfaces 2013, 5, 7130–7138. 10.1021/am401475g. [DOI] [PubMed] [Google Scholar]
- Carraro G.; Sugrañez R.; Maccato C.; Gasparotto A.; Barreca D.; Sada C.; Cruz-Yusta M.; Sánchez L. Nanostructured iron(III) oxides: from design to gas- and liquid-phase photo-catalytic applications. Thin Solid Films 2014, 564, 121–127. 10.1016/j.tsf.2014.05.048. [DOI] [Google Scholar]
- Wang C.; Zhong H.; Wu W.; Pan C.; Wei X.; Zhou G.; Yang F. Fe3O4@C core-shell carbon hybrid materials as magnetically separable adsorbents for the removal of dibenzothiophene in fuels. ACS Omega 2019, 4, 1652–1661. 10.1021/acsomega.8b03157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Jin H.; Uchaker E.; Xie Z.; Wang Y.; Cao G.; Hou S.; Li J. One-pot synthesis of in-situ carbon-coated Fe3O4 as a long-life lithium-ion battery anode. Nanotechnology 2017, 28, 155603. 10.1088/1361-6528/aa6143. [DOI] [PubMed] [Google Scholar]
- Wang S.; Huang F.; Zhang M.; Kong X.; Zi Z.; Liu Q. Fe3O4/carbon chain-like core/shell composites: synthesis and microwave absorption properties. Integr. Ferroelectr. 2018, 190, 76–84. 10.1080/10584587.2018.1456176. [DOI] [Google Scholar]
- Huang Y.-K.; Su C.-H.; Chen J.-J.; Chang C.-T.; Tsai Y.-H.; Syu S.-F.; Tseng T.-T.; Yeh C.-S. Fabrication of silica-coated hollow carbon nanospheres encapsulating Fe3O4 cluster for magnetical and MR imaging guided NIR light triggering hyperthermia and ultrasound imaging. ACS Appl. Mater. Interfaces 2016, 8, 14470–14480. 10.1021/acsami.6b04759. [DOI] [PubMed] [Google Scholar]
- Kaskela A.; Nasibulin A. G.; Timmermans M. Y.; Aitchison B.; Papadimitratos A.; Tian Y.; Zhu Z.; Jiang H.; Brown D. P.; Zakhidov A.; Kauppinen E. I. Aerosol-synthesized SWCNT networks with tunable conductivity and transparency by a dry transfer technique. Nano Lett. 2010, 10, 4349–4355. 10.1021/nl101680s. [DOI] [PubMed] [Google Scholar]
- Ardekani S. R.; Aghdam A. S. R.; Nazari M.; Bayat A.; Yazdani E.; Saievar-Iranizad E. A Comprehensive review on ultrasonic spray pyrolysis technique: mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. 10.1016/j.jaap.2019.104631. [DOI] [Google Scholar]
- Atkinson J. D.; Fortunato M. E.; Dastgheib S. A.; Rostam-Abadi M.; Rood M. J.; Suslick K. S. Synthesis and characterization of iron-impregnated porous carbon spheres prepared by ultrasonic spray pyrolysis. Carbon 2011, 49, 587–598. 10.1016/j.carbon.2010.10.001. [DOI] [Google Scholar]
- Tyurikova I. A.; Demidov A. I. Synthesis of water-based Fe3O4 magnetic nanoparticles, stabilized by oleic acid and mannitol. Inorg. Mater. 2017, 53, 413–418. 10.1134/s0020168517040185. [DOI] [Google Scholar]
- Azuma R.; Nakamichi S.; Kimura J.; Yano H.; Kawasaki H.; Suzuki T.; Kondo R.; Kanda Y.; Shimizu K.-i.; Kato K.; Obora Y. Solution synthesis of N,N-dimethylformamide-stabilized iron-oxide nanoparticles as an efficient and recyclable catalyst for alkene hydrosilylation. ChemCatChem 2018, 10, 2378–2382. 10.1002/cctc.201800161. [DOI] [Google Scholar]
- Stepanian S. G.; Reva I. D.; Radchenko E. D.; Sheina G. G. Infrared spectra of benzoic acid monomers and dimers in argon matrix. Vib. Spectrosc. 1996, 11, 123–133. 10.1016/0924-2031(95)00068-2. [DOI] [Google Scholar]
- Lafuente B.; Downs R. T.; Yang H.; Stone N.. The power of databases: the RRUFF project. In Highlights in mineralogical crystallography; Armbruster T.; Danisi R. M., Eds.; Walter de Gruyter GmbH, 2016; pp 1–29. [Google Scholar]
- Rudman R. Handbook of X-rays, for diffraction, emission, absorption, and microscopy. J. Chem. Educ. 1968, 45, 443–444. 10.1021/ed045p443.2. [DOI] [Google Scholar]
- Tsurin V. A.; Yermakov A. Y.; Uimin M. A.; Mysik A. A.; Shchegoleva N. N.; Gaviko V. S.; Maikov V. V. Synthesis, structure, and magnetic properties of iron and nickel nanoparticles encapsulated into carbon. Phys. Solid State 2014, 56, 287–301. 10.1134/s1063783414020309. [DOI] [Google Scholar]
- Anastasakis K.; Ross A. B.; Jones J. M. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 2011, 90, 598–607. 10.1016/j.fuel.2010.09.023. [DOI] [Google Scholar]
- Yamashita T.; Hayes P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. 10.1016/j.apsusc.2007.09.063. [DOI] [Google Scholar]
- Muhler M.; Schütze J.; Wesemann M.; Rayment T.; Dent A.; Schlögl R.; Ertl G. The nature of the iron oxide-based catalyst for dehydrogenation of ethylbenzene to styrene. I. Solid-state chemistry and bulk characterization. J. Catal. 1990, 126, 339–360. 10.1016/0021-9517(90)90003-3. [DOI] [Google Scholar]



