 In depth knowledge of the physicochemical properties of different salt forms and respective solvates/hydrates has a significant impact on the pharmaceutical dosage form.
In depth knowledge of the physicochemical properties of different salt forms and respective solvates/hydrates has a significant impact on the pharmaceutical dosage form.
Abstract
Pritelivir (AIC316, BAY 57-1293) was discovered as a highly potent drug against herpes simplex viruses with a novel mode of action, i.e. inhibition of the viral helicase–primase. A side by side comparison of the oral form against Valtrex™ in patients with genital herpes, showed superiority in phase II testing for Pritelivir. A number of different solid forms have been generated for additional, e.g. systemic, or topical applications.
Herpes simplex viruses (HSV) types 1 and 2 are widely spread in the human population and cause benign and generally self-limiting blisters and ulcers mainly in the labial and genital area, respectively. However in immunocompromised patients or neonates, severe and life-threatening infections can occur and frequently recurrent labial or genital infections can severely impair the quality of life.1 Therefore, depending on the virus, which is infecting or reactivating, treatments for oral or topical application as well as for disease suppression are needed to serve the different patients' needs.
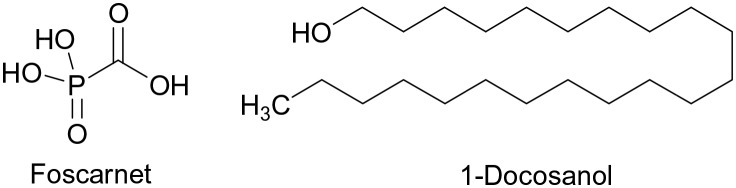
The marketed drugs against HSV1 and HSV2 are all nucleoside analogues, with the exception of foscarnet or docosanol (Fig. 1), a saturated fatty alcohol for topical treatment, with an unspecific mode of action. While the nucleoside analogs are prodrugs and require phosphorylation by a viral thymidine kinase and hence are inactive or poorly active in uninfected cells, foscarnet has significant toxicities.2
Fig. 1. Chemical structures of foscarnet and docosanol.
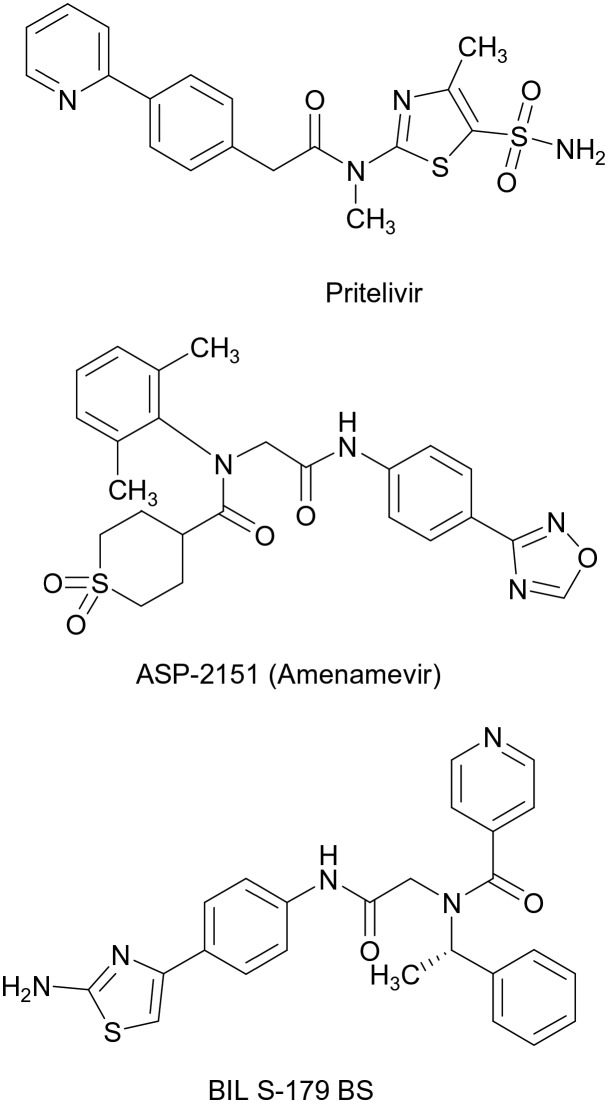
In an effort to offer novel treatment options, originally three different inhibitors of the viral helicase–primase of HSV were discovered independently (Fig. 2): Pritelivir (AIC316, BAY 57-1293,3), a tiazolylamide, amenamevir (ASP 2151,4), an oxadiazolphenyl and BILS-179 BS,5 an aminothiazolylphenyl derivative. The BILS compound was suspended from clinical development, amenamevir, initially discovered and developed by Astellas, showed in a phase II study in genital herpes a somewhat shortened time to lesion healing, but was suspended due to adverse events in Phase I. Interestingly – in contrast to Pritelivir and the BILS drug, ASP 2151 is also active against the varicella zoster virus VZV and clinical development against VZV was resumed by Maruho Co Ltd, in Japan for VZV patients. The drug was approved in Japan for shingles in 2017.
Fig. 2. Three non-nucleoside inhibitors against herpes-viruses addressing the viral helicase–primase as a novel target. Pritelivir (company code AIC-316) is end of phase II for HSV-1 and HSV-2; ASP-2151 (Amenamevir) is marketed in Japan for herpes zoster (VZV), the development of BIL S-179 BS was suspended.
In contrast to amenamevir and the BILS compound, Pritelivir was tested in 2 phase II studies in patients with recurrent genital herpes. In the first study6 upon oral administration of tablets a dose-dependent inhibition of viral shedding from the genital area together with a dose-dependent reduction in clinical lesion was seen. Due to the long half-life of the drug (80 h) a once weekly oral application of 400 mg was nearly equally effective as a once daily oral dose of 75 mg. In the 2nd study, a direct comparison of a 100 mg Pritelivir tablet administered orally once daily versus 500 mg Valtrex administered orally showed superiority in all relevant parameters analysed7 for Pritelivir.
In order to allow multiple patients to profit from Pritelivir, different solid phase forms such as pharmaceutical salts, solvates and hydrates as well as polymorphs thereof were investigated to develop oral and topical application forms with an optimized pharmaceutical profile.
Hence, pharmaceutical development is nowadays frequently facing the challenge of tailoring poorly soluble lipophilic drugs to allow sufficiently high oral in vivo absorption. This is even more demanding for early animal toxicological studies which typically require high doses being absorbed. Enabling formulation platforms are widely considered to mitigate this, and formulation scientists have a diverse tool-box available in this field, such as liquid formulation approaches, amorphous molecular dispersions, or particle size reduction technologies.8a Alternatively, going one step back from formulation development activities, modification of drug substance solid-state properties may be considered as a more direct tool to overcome poor solubility.
In case of Pritelivir the solubility of the free base at physiological pH of 7.4 is 0.0115 mg mL–1. Poor solubility is in many cases related to a low bioavailability.8b Pritelivir could be classified as a basic drug with the strongest acidic pKa of 8.58 (amino group) and the strongest basic pKa of 4.42 (pyridine group). The solubility of Pritelivir in different buffers and excipients is indicated in Tables 1 and 2.
Table 1. Comparative solubility of Pritelivir free and mesylate salt in the different excipients.
| Excipient | Saturated solubility (mg ml–1) |
|
| Pritelivir free base | Pritelivir mesylate salt | |
| Water | BLOQ a | 1.2 |
| pH 3.5 buffer | 0.1 mg | 1.3 |
| pH 4.5 buffer | 0.1 | 0.4 |
| pH 5.5 buffer | 0.1 | 0.2 |
| pH 7.0 buffer | 0.1 | BLOQ a |
aBLOQ: below limit of detection.
Table 2. Comparative solubility of Pritelivir free and mesylate salt in the different excipients.
| Excipient | Saturated solubility (mg ml–1) |
|
| Pritelivir free base | Pritelivir mesylate salt | |
| Ethanol | 0.3 | 0.7 |
| Isopropanol | 0.4 | BLOQ a |
| Benzyl alcohol | 2.2 | 2.0 |
| Phenoxyethanol | 2.2 | 10.7 |
| PEG 400 | 78.7 | 10.1 |
| Dimethyl isosorbide | 82.3 | BLOQ |
| Propylene glycol | 28.4 | 4.7 |
| Transcutol P | 44.8 | 1.3 |
| Oleyl alcohol | 0.1 | BLOQ |
| Glycerin | 0.5 | 13.1 |
| Isopropyl myristate | 0.1 | — b |
| Isopropyl palmitate | BLOQ | — |
| Crodamol GTC | 0.2 | BLOQ |
aBLOQ: below limit of detection.
bNo peak detected.
Hence, for overcoming solubility limitations from a drug substance solid-state perspective, amorphous phase and salt formation are the two most effective options. Whether the drug products are solutions, solids, or semi-solids, the use of a salt normally provides a higher concentration in solution than the free acid or free base (nonionized forms) due to a higher solubility. An estimated 50% of all drug molecules used in medicinal therapy are administered as salts.8c
Historically, the number of available salts was rather limited; however, today there is a wide range of chemical entities that are recognized as being safe, which can be used in the preparation of drug products. Pharmaceutical salt selection is extensively described in literature for overcoming poor solubility.9–11a,b In this context, sulfonate salts are advocated as especially useful counter ions for basic drugs.12,13a
Salts are formed when a compound that is ionized in solution forms a strong ionic interaction with an oppositely charged counterion, leading to crystallization of the salt form. Salt forms of drugs have a large effect on the drugs' quality, safety, and performance.13b Also, the properties of salt-forming species (i.e., counterions) significantly affect the pharmaceutical properties of a drug and the counter ion has to be pharmacologically safe.
In order to optimize the pharmaceutical development of Pritelivir, an extensive salt and polymorph screening was performed using the solvent/antisolvent approach as well as screening experiments. Based on physicochemical properties like crystallinity, solid phase stability, including thermal and photo stability, and thermodynamic solubility, several acceptable salt forms were obtained and investigated like the mesylate, maleate or sulfate salt. In addition, in some cases LSO stoichiometric changes were observed (1 : 1 and 1 : 2 ratio of Pritelivir and counter ion).16 In conclusion, salt and polymorph screening should be performed as early as possible during the development process.
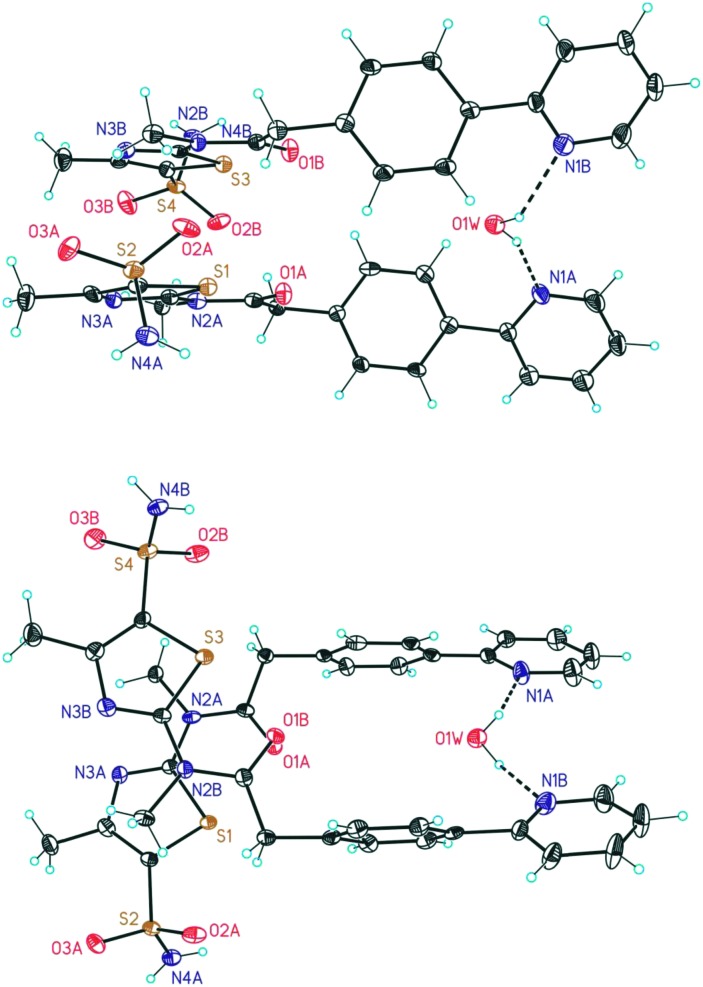
However, in the context of solid-state form control, salt formation often bears the liability of enhanced tendency for hydrate formation.14 The reason for high affinity of salt structures to incorporate water into their crystal lattice is a consequence of a strong interaction potential of water with ions in the salt structure.15 Also, in case of Pritelivir the hydrate formation was observed in most of the isolated salt form. During preliminary polymorphism studies of the mesylate salt, 9 different XRPD (X-ray powder diffraction) patterns (mainly solvates) were discovered. After assessing several properties of the obtained salt forms the mesylate monohydrate form (Fig. 3) was selected for further development of tablets17 and other applications18 due to the improved profile shown in the excipient's compatibility studies, accelerated and long term stability studies and an optimized dissolution and release profile, which was confirmed by later bioavailability and release studies in animals as well as in clinical studies.
Fig. 3. X-ray structure of Pritelivir mesylate monohydrate. The structure crystallizes in the triclinic space group P1[combining macron]. Hydrogen bonds form dimeric moieties which are linked by the CH3SO3 – and the H2O molecules.
Salt formation can also offer opportunities to overcome non-desirable particle properties of free form drugs, which may limit the active pharmaceutical ingredients' (API) manufacturability during drug product processing. In case of Pritelivir, after the upscaled manufacturing process in the GMP (good manufacturing practice) environment, a Pritelivir mesylate monohydrate is obtainable with optimized particle size distribution, optimal flowability properties and surface area (according Brunauer–Emmett–Teller, BET). The particle size distribution of Pritelivir mesylate monohydrate were determined as follows: non-micronized form: D(0.1) 45.5 μm, D(0.5) 114.7 μm, D(0.9) 226.4 μm; micronized form: D(0.1) 1.6 μm, D(0.5) 7.4 μm, D(0.9) 35.0 μm.
In addition, the selected salt form shows a very long stability. In addition to the development of Pritelivir tablets and vaginal ring devices a topical form was investigated for the treatment of labial herpes infections. During an extensive excipient compatibility program, the different solid forms of Pritelivir were investigated with selected excipients normally used in typical semi-solid forms like creams, gels and ointments. Surprisingly it was found that the free base showed much higher stability in combination with PEG 400 under forced degradation conditions (https://www.ich.org/products/guidelines.html) than the mesylate or other salt forms. Therefore, an additional polymorph screening program of the free base was conducted. Kinetically preferred polymorphs were examined using evaporation and cooling crystallizations, thermodynamically preferred polymorphs were examined using slurry type experiments. Several crystal forms have been observed for the free base Pritelivir.
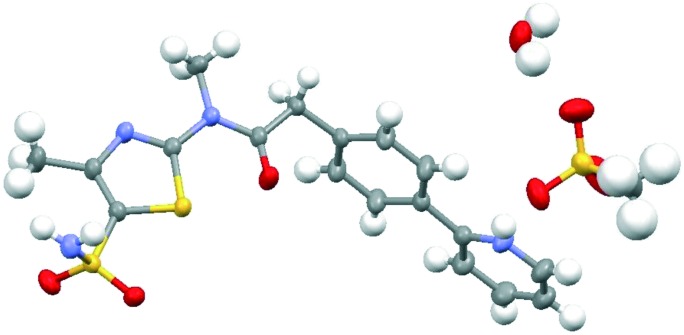
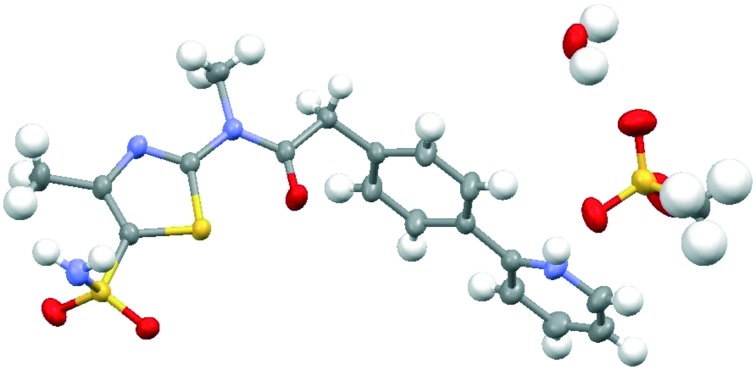
Besides the water free form, three hydrates were obtained and characterized: hemihydrate, monohydrate and dihydrate. The Pritelivir hemihydrate (form C, Fig. 4) was the hydrate with the highest stability under accelerated and long-term storage conditions and seems to be the thermodynamic most stable hydrate form of the free base. The hemihydrate was selected for further development and the respective ointment was tested in a clinical phase II study for the treatment of labial herpes.
Fig. 4. Ortep-plot (50%) showing the crystal structures of Pritelivir hemihydrate (form C) in two different views. The asymmetric unit contains two independent molecules of Pritelivir and one molecule of water.
Conclusions
The discovery and development of Pritelivir as a new in class anti-infective compound with a novel mode of action against herpes simplex 1 and 2 is an excellent example for the need to investigate different formulations and different solid forms early on, in order to bring the medicine to all patients, who need it and who do have different requirements. The involvement of early formulation work is becoming a more and more essential part for successful drug development to overcome physiochemical drawbacks like poor solubility at an early stage.
Conflicts of interest
H. Rübsamen-Schaeff was the founding CEO and headed AiCuris until 3/2015. She is now the Chair of AiCuris Scientific Expert Meeting. Helmut Buschmann is the Head of Chemistry, Pharmaceutical Formulation and Patent Affairs at AiCuris and Managing Director of Research, Development & Consultancy in Vienna.
Acknowledgments
This work was supported by AiCuris Anti-Infective Cures GmbH, Germany.
References
- Roizman B., Knipe D. M. and Whitley R. J., in Herpes Simplex Viruses, ed. D. M. Knipe and P. M. Howley, Fields Virology, Lippincott Williams & Wilkins, 5th edn, 2006, pp. 2502–2601. [Google Scholar]
- Rodriguez M. and Zachary K. C., UpToDate, MS Hirsch, Foscarnet: An overview, 2016, uptodate.com. [Google Scholar]
- Kleymann G., Fischer R., Betz U., Hendrix M., Bender W., Schneider U., Handke G., Eckenberg P., Hewlett G., Pevzner V., Baumeister J., Weber O., Henninger K., Keldenich J., Jensen A., Kolb J., Bach U., Popp A., Maeßen J., Frappa I., Häbich D., Lockhoff O., Ruebsamen-Waigmann H. Nat. Med. 2002;8:392. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- Katsumata K., Chono K., Kontani T., Sudo K., Shimizu Y. and Suzuki H., Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 2009, Poster V-1742. [Google Scholar]
- Crute J. J., Grygon C. A., Hargrave K. D., Simoneau B., Faucher A.-M., Bolger G., Kibler P., Liuzzi M., Cordingley M. G. Nat. Med. 2002;8:386. doi: 10.1038/nm0402-386. [DOI] [PubMed] [Google Scholar]
- Wald A., Corey L., Timmler B., Magaret A., Warren T., Tyring T., Johnston C., Kriesel J., Fife K., Galitz L., Stoelben S., Huang M.-L., Selke S., Stobernack H.-P., Ruebsamen-Schaeff H., Birkmann A. N. Engl. J. Med. 2014;370:201. doi: 10.1056/NEJMoa1301150. [DOI] [PubMed] [Google Scholar]
- Wald A., Timmler B., Magaret A., Warren T., Tyring S., Johnston C., Fife K., Selke S., Huang M. i.-L., Stobernack H.-P., Zimmermann H., Corey L., Birkmann A., Ruebsamen-Schaeff H. JAMA, J. Am. Med. Assoc. 2016;316:2495. doi: 10.1001/jama.2016.18189. [DOI] [PubMed] [Google Scholar]
- (a) Dai D., Pollock-Dove C., Dong L. C., Li S. Adv. Drug Delivery Rev. 2008;60:657–672. doi: 10.1016/j.addr.2007.10.017. [DOI] [PubMed] [Google Scholar]; (b) He Y., Orton E., Yang D. J. Pharm. Sci. 2018;107:419–425. doi: 10.1016/j.xphs.2017.10.032. [DOI] [PubMed] [Google Scholar]; (c) Makary P. UK J. Pharm. Biosci. 2014;2(4):01–04. [Google Scholar]
- Rodríguez-Spong B., Price C. P., Jayasankar A., Matzgar A. J., Rodríguez-Hornedo N. Adv. Drug Delivery Rev. 2004;56:241–274. doi: 10.1016/j.addr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Elder P., Holm R., Lopez de Diego H. Int. J. Pharm. 2013;50:6665–6672. [Google Scholar]
- (a) Handbook of pharmaceutical salts: properties, selection, and use, ed. P. H. Stahl and C. G. Wermuth, Verlag Helvetica Chimica Acta; Wiley-VCH, Zurich, Weinheim, 2nd rev, 2011. [Google Scholar]; (b) Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development, ed. R. Hilfiker and M. von Raumer, Wiley-VCH, Weinheim, 2019, ISBN: 978-3-527-34040-8. [Google Scholar]
- Serajuddin T. M. Adv. Drug Delivery Rev. 2007;59:603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- (a) Elder P., Delaney E., Teasdale A., Eyley S., Reif V. D., Jacq K., Facchine K. L., Schulte Oestrich R., Sandra P., David F. J. Pharm. Sci. 2010;99:2948–2961. doi: 10.1002/jps.22058. [DOI] [PubMed] [Google Scholar]; (b) Gupta D., Bhatia D., Dave V., Sutariya V., Varghese Gupta S. Molecules. 2018;23:1719. doi: 10.3390/molecules23071719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser J., The importance of solvates, in: Polymorphism in the Pharmaceutical Industry, ed. R. Hilfiker, Wiley-VCH, 2006. [Google Scholar]
- Morris R., Structural aspects of hydrates and solvates, in Polymorphism in Pharmaceutical Solids, ed. H. G. Brittain, Marcel Dekker Inc., 1999. [Google Scholar]
- Bachhav Y., Schwab W., Birkmann A., Bonsmann S. and Goldner T., Pharmaceutical formulations comprising maleate salt of N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetamide for the treatment of herpes viruses, WO2018096177, (Nov 28, 2017).
- Schwab W., Birkmann A., Voegtli K., Haag D., Lender A., Grunenberg A., Keil D. I., Birgit D. I. and Rehse J., Preparation of N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetamide mesylate monohydrate for the treatment of infectious diseases, EP2573086, (Sep 26, 2011).
- Bachhav Y., Schwab W., Birkmann A. and Voegtli K., A process for the preparation of aminosulfonyl-methylthiazolyl-methylpyridinyl-phenyl acetamide free base hemihydrate, WO2018096170, (Nov 28, 2017).