Abstract
Traumatic brain injury (TBI) affects millions of individuals every year. Many of these injuries lead to lasting effects, particularly impairments in domains broadly classified as executive functions, such as impulse control and decision-making. While these impairments have been historically associated with frontal brain damage, other injuries such as concussion or parietal injury also contribute to similar dysfunction. However, it is unknown whether animal models of TBI would replicate these broad effects that are observed in human patients. In the current study, we delivered a unilateral parietal controlled cortical impact injury and assessed the performance of rats on a motoric task (rotarod) and a test of decision-making and impulsivity (rodent gambling task). TBI rats demonstrated significant motor impairments on the rotarod task; however, this did not extend to difficulties inhibiting motor actions (impulsivity). In addition, TBI caused chronic alterations to risk-based decision-making, extending out to 12 weeks post-injury. Specifically, rats with TBI preferred the riskiest, and most suboptimal option over all others. The current data suggest that models of unilateral TBI are sufficient for replicating some aspects of executive dysfunction (risky decision-making), while others are limited to frontal damage (impulsivity). These models may be used to develop therapeutics targeted at the chronic post-injury period when these symptoms often manifest in patients, a critically understudied area in preclinical TBI research.
Keywords: Decision-making, impulsivity, executive function, controlled cortical impact
Introduction
Traumatic brain injury (TBI), defined as an external force applied to the brain, affects more than 2.8 million people each year in the United States alone [1, 2]. Although most patients with TBI recover with little to no complications, approximately 20% suffer chronic behavioral deficits [3]. In addition, TBI is a major risk factor for the development of mood, attention-deficit, substance use, and neurodegenerative disorders [4–11]. Importantly, TBI also contributes to numerous deficits that may not rise to the level of diagnosis for a given disorder, including a wide range of executive function impairments [see 12 for review]. Of these subclinical consequences, one that has wide-ranging detrimental effects across multiple aspects of life is impairment to decision-making.
Decision-making is a multifaceted construct that includes weighing various costs and benefits associated with choice alternatives. After brain injury, impulsive choice is frequently increased. Impulsive choice refers to decisions that result in smaller, immediate gains at the cost of larger, delayed benefits. In general, TBI is associated with an increase in choice for smaller, more immediate reinforcers over larger, delayed reinforcers—an effect that has been demonstrated in both humans and rodents post-TBI [13–16]. Such deficits in impulsive decision-making may result in poorer quality of life for patients with TBI, including financial and physical-health problems [17, 18]. Moreover, these impairments are likely the result of multiple causes, such as deficits in timing (e.g., over-estimation of time), inability to connect distal consequences with current action, and/or inattention, all of which have implications for other types of decision-making.
Risk-based decision-making, which includes larger reinforcers that are associated with risk rather than delays, is also impaired after brain injury [19, 20]. Given that risk-based decision-making does not include a temporal component but rather immediate feedback, it seems that TBI affects decision-making ability broadly. The most common task for assessing risk-based decision-making in humans is the Iowa Gambling Task [IGT; 21]. During the IGT, choices are presented between four decks of cards across numerous trials. Participants choose from one of the decks during each trial and either gain or lose hypothetical money. Two of the decks include larger values of money that can be “won”, but these decks are also associated with larger losses, making them risky alternatives. The other two decks include smaller values of money that can be won and are associated with smaller losses, making them safer alternatives. In general, patients with TBI show increased choice for the two risky alternatives compared to non-TBI patients [19, 22, 23]. However, it is difficult in patient populations to determine whether impairments in risk-based decision-making are a direct result of TBI or if such decision-making impairments are a precursor to suffering a TBI. In addition, clinical reports seldom specify the locations) of injury, and thus, work is needed to determine the degree to which preclinical research translates to the human condition. Thus, a direct analog of the IGT has been used to investigate the phenomenon in rodents, allowing for greater experimental control and assessment of directionality of effects, called the Rodent Gambling Task [RGT; 24].
The primary difference between the human IGT and the RGT are that during the RGT, there is one optimal choice out of the four alternatives (i.e., the choice that produces the highest rate of reinforcement; Fig 1). In addition to assessing risk-based decision-making, the RGT includes a component for simultaneously assessing motor impulsivity (the ability to inhibit action) making it a robust procedure for the assessment of rodent behavior. Recently, our laboratory assessed the utility of using the RGT to evaluate the effects of TBI on risk-based decision-making. Results from this study demonstrated that severe bilateral frontal TBI decreased optimal decision-making, and shifted choice to both, riskier and safer (but suboptimal), choice alternatives [20]. In addition to effects on risk-based decision-making, frontal TBI also increased motor impulsivity on this task. However, given the sparse evidence for effects of TBI on risk-based decision-making in animal models, the current study was designed to evaluate whether effects of TBI on risky decision-making are a generalized phenomenon of brain injuries, or if they are specific to frontal damage. Therefore, unilateral parietal controlled cortical impact TBIs were induced in rats prior to behavioral training and testing, and RGT performance was evaluated through the chronic post-injury period.
Figure 1.
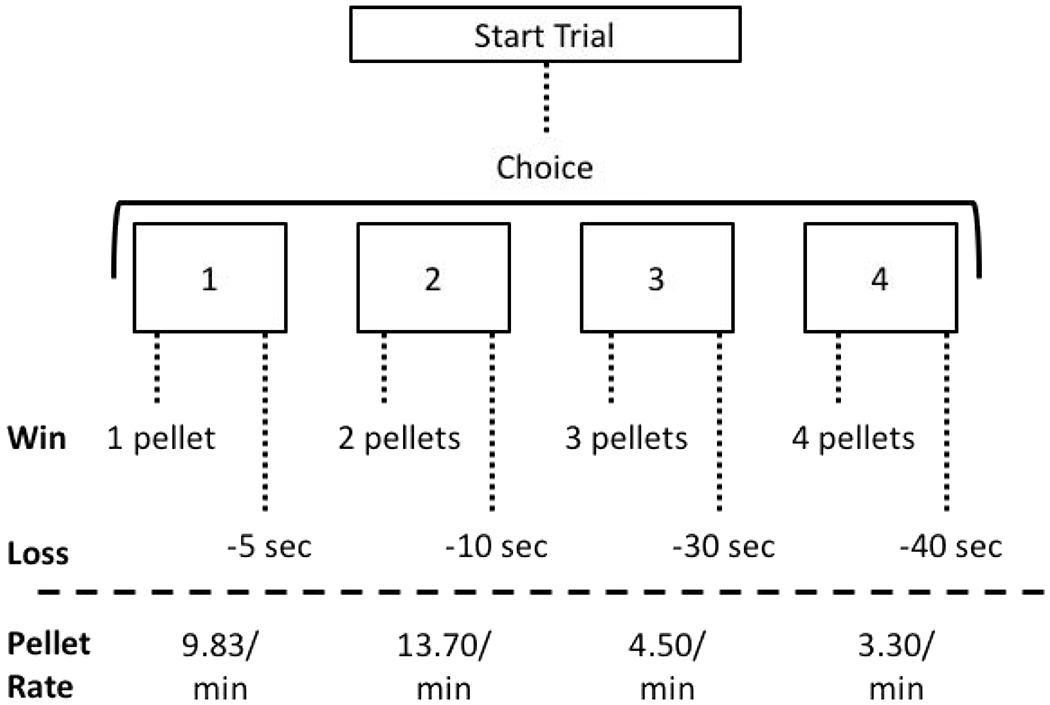
Task diagram. On the rodent gambling task (RGT), rats choose between four reinforcer/punisher options. The 1-pellet option is “safe”; the 2-pellet option is “safe” and the most optimal choice; the 3-pellet option is “risky”; the 4-pellet option is “risky” and the least optimal choice.
Methods
Subjects
Subjects were 23 male Long-Evans rats (Charles River, Wilmington, MA). Rats were approximately 4 months old at the time of injury, and 7 months at euthanasia. Rats were pair-housed pre-injury and single-housed post-injury in controlled environmental conditions and standard cages (temperature, 24°C; 12-h reverse light/dark cycle). Rats had continuous access to water in home cages but were food restricted at approximately 14g/day post-session. All procedures were approved by the institutional Animal Care and Use Committee, under the guidance of the NIH Guide for Care and Use of Laboratory Animals.
Rotarod
The rotarod task is a sensitive measure of motor impairment following TBI [e.g., 25], which consists of a cylinder located above a foam pad (Rota Rod Rotamex 5, Columbus Instruments, Columbus, OH). The cylinder is controlled mechanically to rotate and accelerate. To evaluate motor function, the rotarod task was administered for two sessions immediately preceding injury (training) and again for three sessions on post-injury days 7-9 (testing). At the start of the first session, rats were required to stand on the stationary cylinder for 60 s without falling to habituate them to the task. After meeting this criterion, training began immediately. During each trial, rats were placed on the cylinder as it began rotating at a rate of 5 rpm for 20 s. After 20 s, the cylinder began accelerating from 5 rpm to 50 rpm over a period of 300 s (acceleration rate of 0.055 cm/s2 or 0.15 rpm/s). Four trials were conducted per rat per day with an approximate intertrial interval of 10 min. Latency to fall was the outcome measure of interest and was calculated by averaging the latencies for all four trials during a single session.
TBI Surgery
Rats were matched for performance on the rotarod and then randomly assigned to TBI (n = 9) or Sham (n = 14) groups. Controlled cortical impact (CCI) procedures were carried out aseptically, as previously described [26]. Briefly, rats were anesthetized with isoflurane (5% induction, 2-4% maintenance) in 0.5 L/min oxygen. Local (bupivacaine, 0.25%) and general (ketoprofen, 5 mg/kg) analgesic were given subcutaneously. Rats were placed in a stereotaxic frame, the surgical site sterilized, and a midline incision performed. After retracting the periosteum, a 5 mm circular craniectomy was measured out in the left hemisphere, centered at +2.4 mm, −2.4 mm from bregma and performed using a surgical drill. A severe, unilateral parietal CCI (4 mm in diameter, 2.5 mm depth, 3 m/s velocity, 500 ms dwell time) was induced using a Leica Impact One CCI device (Leica Biosystems, Buffalo Grove, IL). Bleeding was stopped, incision site sutured closed, and triple-antibiotic ointment applied to the site. Sham surgeries were identical to TBI surgery with exception of the impact. Rats were placed on free-feeding for four days following surgery, after which they were leaned back down to 14g/day to motivate behavior on the RGT.
Rodent Gambling Task (RGT)
Apparatus.
Behavioral testing took place in 16 standard five-hole operant conditioning chambers for rats, each enclosed in a sound-attenuating cubicle (Med Associates, St. Albans, VT). Each chamber included a stimulus light at the back of each 5-choice hole and infrared beams to record nose pokes. A food magazine with a light was on the wall opposite to the working wall with a pellet dispenser and houselight above it. Chambers were controlled by custom software written in Med-PC IV. Sucrose pellets (45 mg, BioServ, Fleming, NJ) were used as reinforcers.
Training.
After seven days of recovery from surgery, RGT training was conducted as described previously [20, 24]. First, 20-min habituation sessions were conducted in which sucrose pellets were placed manually into all five nose-poke holes and the food magazine. Once rats consumed all of the pellets during one session, training began to shape responses to the presentation of cue lights in the nose-poke holes. To initiate a trial, rats nose-poked into the food magazine. After a 5-s delay, a cue light turned on in one of the five nose-poke holes for 30 s. A response to the corresponding nose-poke hole resulted in the delivery of a single sucrose pellet. Premature responses before the cue light turned on, incorrect responses, or omitted responses resulted in a 5-s timeout. Sessions were terminated following 30 min or 100 reinforcers, whichever came first. Once rats completed at least 30 trials, the cue light duration was reduced from 30 s to 20 s and eventually to 10 s [27]. RGT training began when rats completed at least 50 trials with 80% accuracy at the 10-s cue light duration.
Prior to beginning the full RGT, training was conducted using a “forced-choice” trials procedure for seven sessions (weeks 3 and 4 post-injury). One at a time, four choice options were presented, each associated with a different probability and magnitude of reinforcement and punishment (see Fig 1). Choice PI had a 90:10% chance of one sucrose pellet or a 5-s timeout. Choice P2 had an 80:20% chance of two sucrose pellets or a 10-s timeout. Choice P3 had a 60:40% chance of three sucrose pellets or a 30-s timeout. Choice P4 had a 40:60% chance of four sucrose pellets or a 40-s timeout. “Forced-choice” sessions lasted 30 min and each trial included only one available choice option to familiarize rats with the different contingencies. Rats were required to nose poke into the food magazine to initiate atrial. After a 5-s delay, the cue light for one nose-poke hole was turned on. Premature responses made before the cue light came on were punished with a 5-s timeout. Following a correct response to the corresponding hole, either the associated number of pellets would be delivered (“win”) or no pellets would be delivered (“loss”) and the choice hole would slowly (1 Hz) flash for the duration of the timeout. Contingency locations were kept consistent for individual rats throughout the study and were counterbalanced across rats using two versions of the program.
Testing.
Testing on the full RGT began during week 5 post-injury. Sessions during the full RGT were identical to those during “forced-choice” training with except that all four nose-poke holes were illuminated on each trial and rats could choose freely between the four options (Pf-P4). Full RGT responding was assessed until 12 weeks post-injury. To maximize reinforcement, the P2 option should be chosen 100% of the time as it is the most optimal alternative.
Lesion Analysis
Following behavioral assessment (12 weeks post-injury), rats were transcardially perfused with 0.9% phosphate buffered saline, followed by 3.7% phosphate buffered formaldehyde. After perfusions, brains were post-fixed in 3.7% formaldehyde for 24 h and placed in 30% sucrose solution for 48 h. Brains were them embedded in a gel matrix (15% gelatin) with between three and four brains per gel block, and then sliced, frozen, on a sliding microtome at 30 pm.
For lesion analysis, four sections were selected (0.0, −1.0, −2.0, and −3.0 from bregma) and mounted to slides before cresyl violet or thionin staining. In brief, slides were rehydrated through alcohols with increasing concentrations of water, stained with thionin or cresyl violet, and then dehydrated through alcohols with decreasing concentrations of water. Images were captured on a Konica Minolta copier at 600 DPI and the area of each hemisphere was measured using ImageJ (NIH, Bethesda, MD). Volumes for each hemisphere were calculated by multiplying the mean area from the four coordinates by the thickness of sections (30 pm) and total number of sections (4). Then, extent of injury was determined by calculating the percent reduction from the contralateral hemisphere using the formula: 100−(ipsilateral hemisphere volume/contralateral hemisphere volume)* 100.
Data Analysis
Before analyzing statistically, dependent variables were tested for normality with a Box-Cox test and transformations were applied as appropriate to ensure normality.
For nose-poke training, sessions required to meet criterion to progress to the forced-choice RGT procedure were analyzed as a function of group (FBI vs. Sham) using an independent-samples t-test. Omissions (motivation) during nose-poke training were analyzed using linear mixed-effects regression, examining effects of Group, Session, and Group x Session interactions. For forced-choice RGT training, omitted and premature responses (motor impulsivity) across the seven sessions were analyzed as a function of group using independent samples t-tests.
The primary dependent measure from the full RGT procedure was percent choice among the four nose-poke options. However, premature responses (motor impulsivity), omitted responses (motivation), pellets earned (overall efficiency), trials completed, win-stay percentages, and lose-shift percentages were also recorded and analyzed. Win-stay percentages were calculated as the percent of trials in which a “win” was followed by a subsequent response on the same nose poke. Lose-shift percentages were calculated as the percent of trials in which a “loss” was followed by a subsequent response on an alternative nose poke. The arcsine-square root transformation was applied to choice data, log transformation for premature responses, pellets earned, trials completed, and lose-shift percentages, inverse square-root transformation for omitted trials, and square transformation for win-stay percentages. Then, RGT outcome measures and latency to fall (s) from the rotarod were analyzed using linear mixed-effects regression, examining effects of Group, Time, and Group x Time interactions. Linear mixed-effects regressions were used because they account for the nested nature of individual subjects within groups; given that parameters are allowed to vary at each nested level, precise estimates and strong models are obtained. For lesion analysis, the percent reduction score for the ipsilateral hemisphere was analyzed using an independent-samples t-test. All analyses were performed using R statistical software (http://www.r-project.org/) in the MASS, lme4, lmerTest, and stats libraries. All outcomes were considered statistically significant at the level of p < 0,05.
Results
Rotarod
Although latency to fall increased significantly over the course of the testing period for both groups, suggesting that rats improved on the task (β = 0.05, SE = 0.02, t = 2,48, p = 0.015; Fig 2), FBI rats were significantly impaired post-injury and did not leam at the rate of their Sham counterparts (β = 0.08, SE = 0.03, t = 3.26, p = 0.002; Fig 2).
Figure 2.
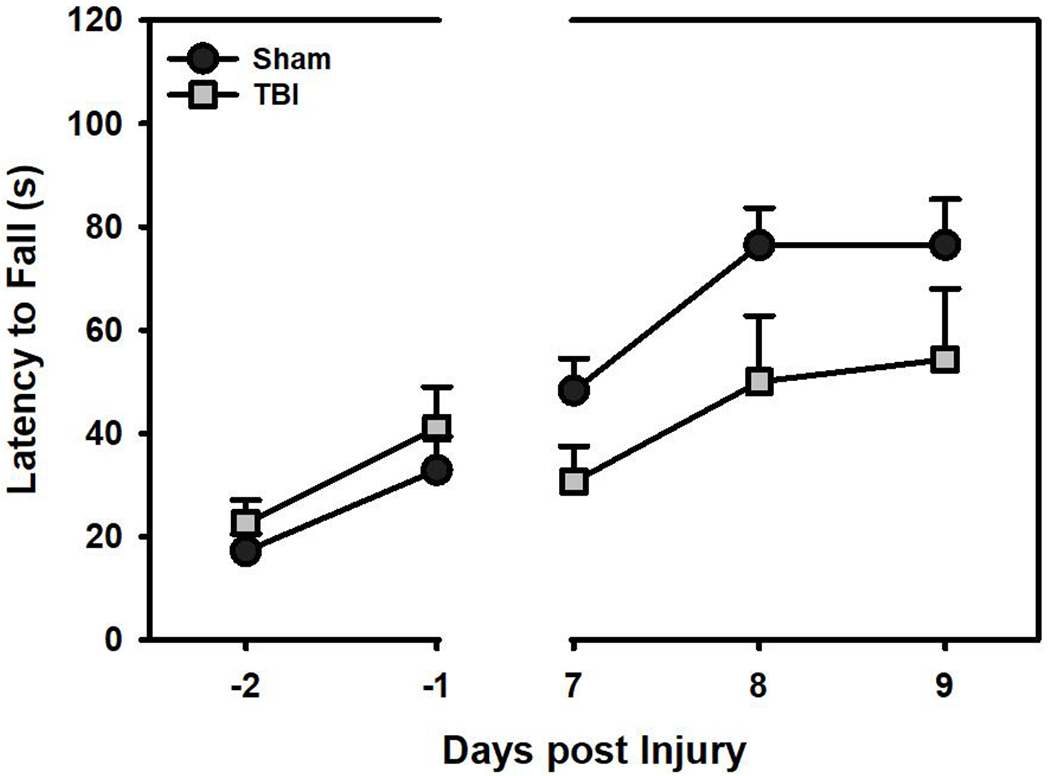
Latency to fall on the rotarod task (mean+SEM). TBI rats were significantly impaired compared to Sham rats after injury (p < 0.05).
RGT Training
TBI rats required significantly more sessions to meet criterion for successful nose-poke training than Sham rats (t(22) = −2.75, p = 0.014; Fig 3A). A linear mixed-effects regression (Group*Session) revealed no significant group differences in premature responses (β = −0.27, SEM = 0.38, t = −0.72, p = 0.475; Fig 3B) or omitted responses (β = 0.07, SEM = 0.42, t = −0.19, p = 0.852; Fig 3C). For forced-choice RGT training, there were no group significant differences with regard to premature responses (β = 1.11, SEM = 0.80, t = 1.39, p = 0.167; Fig 3B) or omitted trials (β = 0.20, SEM = 0.83, t = 0.24, p = 0.809; Fig 3C).
Figure 3.
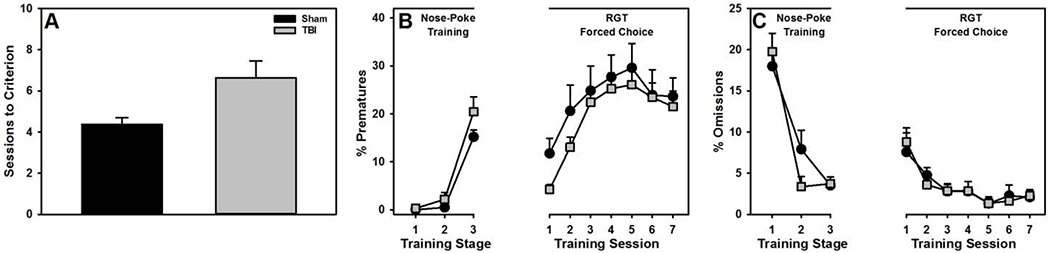
Performance during training (mean+SEM). A) TBI rats took significantly more sessions to meet criterion for nose-poke training (p = 0.014). However, there were no significant group differences in B) premature responses (p = 0.100) or C) omitted responses (p = 0.959).
RGT
Full statistical parameters for all outcome measures are shown in Table 1.
Table 1.
Statistical results for effects of TBI on RGT behavioral variables.
| RGT Behavior | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Choice |
Other Variables |
||||||||||
| β | SE | t | p | β | SE | t | p | ||||
| PI | Group | 0.19 | 0.13 | 1.50 | 0.133 | Prematures | Group | 0.06 | 0.25 | 0.23 | 0.821 |
| Week | −0.04 | 0.02 | −2.19 | 0.029 | Week | −0.09 | 0.02 | −5.75 | <0.001 | ||
| Group*Week | 0.15 | 0.03 | 5.57 | <0.001 | Group* Week | −0.02 | 0.03 | −0.65 | 0.515 | ||
| P2 | Group | −0.37 | 0.13 | −2.87 | 0.004 | Omissions | Group | −0.16 | 0.26 | −0.61 | 0.547 |
| Week | 0.06 | 0.02 | 3.59 | <0.001 | Week | 0.03 | 0.02 | 2.02 | 0.044 | ||
| Group* Week | −0.11 | 0.03 | −4.17 | <0.001 | Group* Week | 0.03 | 0.03 | 1.03 | 0.303 | ||
| P3 | Group | −0.19 | 0.13 | −1.49 | 0.136 | Pellets | Group | −0.16 | 0.26 | −0.61 | 0.547 |
| Week | 0.04 | 0.02 | 2.18 | 0.030 | Week | 0.03 | 0.02 | 2.05 | 0.044 | ||
| Group* Week | −0.03 | 0.03 | −1.08 | 0.282 | Group* Week | 0.03 | 0.03 | 1.03 | 0.303 | ||
| P4 | Group | 0.19 | 0.13 | 1.50 | 0.133 | Trials | Group | 0.09 | 0.30 | 0.31 | 0.761 |
| Week | −0.04 | 0.02 | −2.19 | 0.029 | Week | 0.07 | 0.01 | 5.62 | <0.0001 | ||
| Group* Week | 0.15 | 0.03 | 5.57 | <0.001 | Group* Week | −0.15 | 0.02 | −0.36 | <0.0001 | ||
| Win-Stay | Group | −0.43 | 0.33 | −1.30 | 0.205 | ||||||
| Week | −0.01 | 0.02 | −0.36 | 0.720 | |||||||
| Group* Week | 0.06 | 0.03 | 1.77 | 0.078 | |||||||
| Lose-Shift | Group | 0.15 | 0.30 | 0.51 | 0.617 | ||||||
| Week | 0.03 | 0.02 | 2.02 | 0.044 | |||||||
| Group* Week | 0.05 | 0.03 | 1.67 | 0.096 | |||||||
Choice.
A linear mixed-effects regression (Percent Choice ~ Choice Option*Group*Week) revealed an omnibus Group*Week*Choice Option interaction (F(3,3476) = 16.59, p < 0.001), so each choice option was compared separately to identify significant Group and Group*Week effects. Preference for the 1-pellet option decreased for both groups over time, but TBI rats continued to prefer it into the chronic post-injury period (p < 0.001; Fig 4A). Preference for the 2-pellet option (i.e., the most optimal alternative) increased over time for Sham rats but decreased for FBI rats (p < 0.001; Fig 4B). Preference for the 3-pellet option increased slightly overtime, with no significant differences between the groups (p’s > 0,136; Fig 4C). Finally, preference for the 4-pellet option (i.e., the most risky alternative) remained low and stable for Sham rats but increased for FBI rats over time (p < 0.001; Fig 4D).
Figure 4.
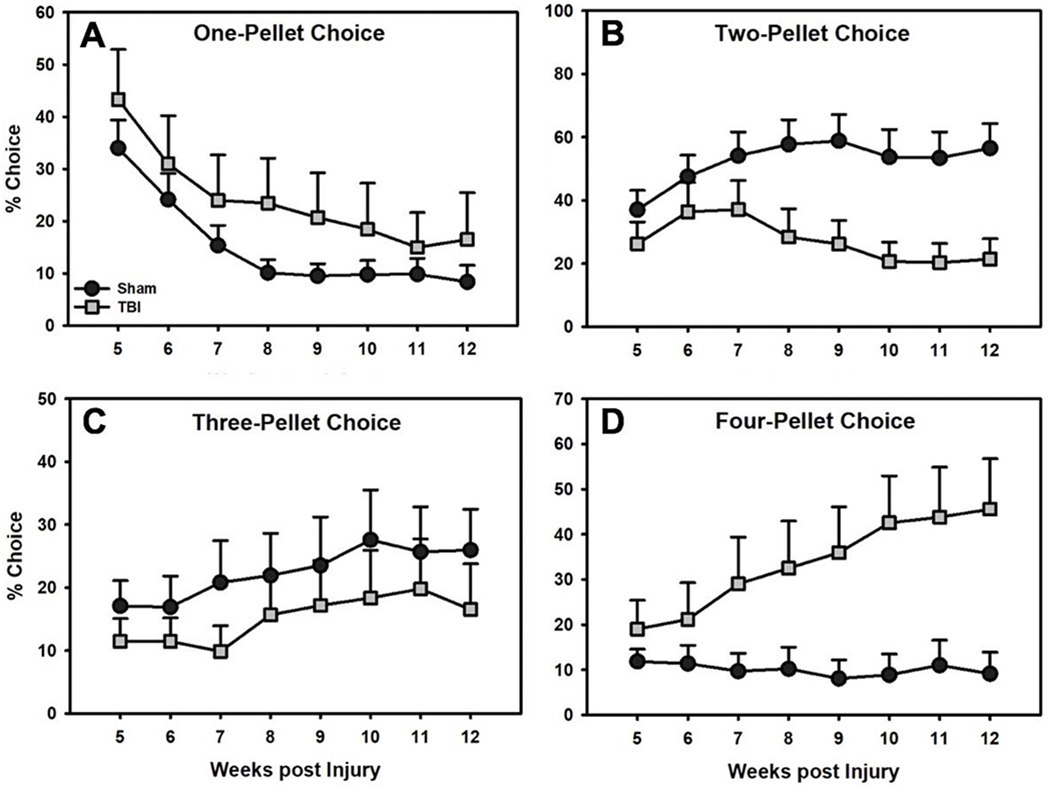
Percent choice on the RGT for A) 1-pellet (“safe”), B) 2-pellet (“safe” and most optimal), C) 3-pellet (“risky”), and D) 4-pellet (“risky” and least optimal) options over time (mean+SEM). TBI rats increased preference for the 1-pellet (A) and 4-pellet options (D) and decreased preference for the 2-pellet (B) option (p’s < 0.001). There were no group differences on the 3-pellet option (C).
Other Variables.
Linear mixed-effects regression models (Outcome ~ Group*Week) were examined for premature responses, omitted responses, pellets earned, trials completed, win-stay percentages, and lose-shift percentages. TBI rats completed fewer trials and earned fewer pellets than Sham rats across the course of testing (p’s < 0.001), likely due to their choice profile, but did not differ with regard to premature responses, omitted responses, win-stay percentages, or lose-shift percentages (Fig 5).
Figure 5.
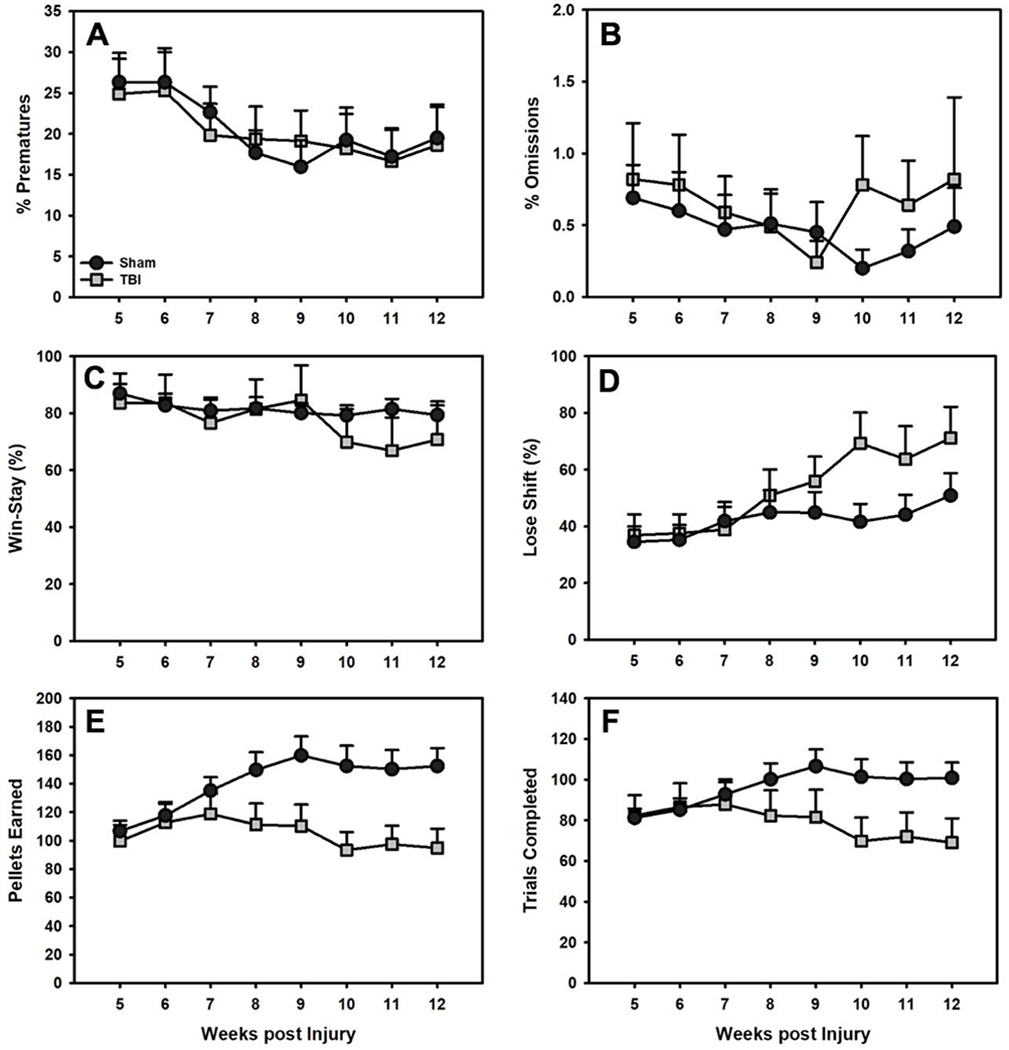
Performance on other RGT outcome measures (mean+SEM). There were no differences in A) premature responses, B) omitted responses, C) win-stay, or D) lose-shift behavior between TBI and Sham rats (p’s > 0.05). However, TBI rats E) earned significantly fewer pellets and F) completed significantly fewer trials than Sham rats over time (p’s < 0.001).
Lesion Analysis
TBI caused significant cavity formation and tissue loss compared to Sham rats (t(22) = −.61, p < 0.001; Fig 6).
Figure 6.
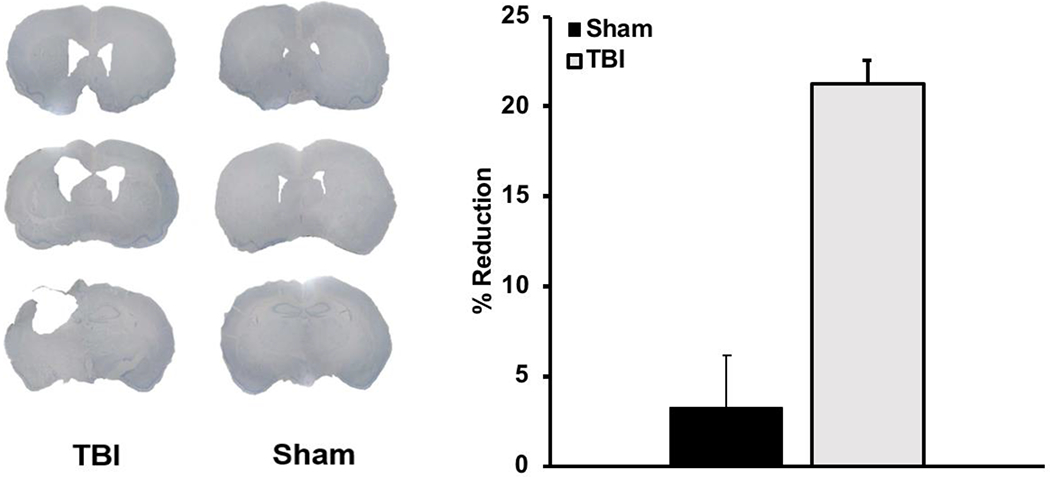
Left: Representative images of unilateral parietal lesions in Sham and TBI rats (0.0, −1.0, −2.0 from bregma). Right: Average lesion size (percent reduction +SEM) was significantly larger for TBI rats relative to Shams (p < 0.001).
Discussion
The core question of the current study was whether unilateral parietal TBI would produce psychiatric-like symptoms, namely deficits in risk-based decision-making and behavioral inhibition (i.e., premature responding), or whether these were limited to the frontal injuries we have previously reported on [20]. Our core findings demonstrate that unilateral FBI-induced deficits in risk-based decision-making and gross locomotor function, but not behavioral inhibition. Such deficits in decision-making for TBI rats became more pronounced across time and were stable at 12 weeks post-injury, suggesting a long-lasting phenotype, similar to clinical reports. The stability of these effects may be attributed, in part, to the use of the RGT, which has strong translational validity because it is analogous to the IGT used in patient populations. Moreover, it also has the advantage of dissociating risk-based decision-making from motor impulsivity in the same task.
Patients with TBI demonstrate preference for, or higher tolerance to, risk when tested on the IGT [19, 28, 29]. However, in the case of the IGT, the four options are normalized to two identical rates of return (providing two “good” and two “bad” choices). Thus, this preference for risk may merely be a manifestation of an inability to distinguish between outcomes on the different alternatives. Prior reports provide some support for this hypothesis and have indicated that patients with TBI may be less sensitive to environmental contingencies than those without TBI [30, 31], and injured rats are also impaired in their ability to discriminate [32, 33]. In contrast to the IGT, the RGT has four distinct outcomes – an optimal/safe (2-pellet), suboptimal/safe (1-pellet), and two suboptimal/risky (3-and 4-pellet) choices. This provides the ability to distinguish between risk-preference and general insensitivity to outcomes. In our prior research in frontal TBI [20], and in our current data on unilateral TBI, injured rats shifted preference from the optimal choice (2-pellet) to suboptimal alternatives, but not explicitly “safer” or “riskier”. In the current study, preference of TBI rats moved toward the safest (1-pellet) and riskiest (4-pellet) alternatives, with the highest degree of preference for the 4-pellet option. These data provide some support for the idea that brain injury generally impairs discrimination of outcomes, but also that risk-seeking is differentially affected. Given that the 4-pellet option was associated with the largest reinforcer magnitude and timeout duration, such a finding may indicate that unilateral parietal TBI increases salience of reinforcing events and/or reduces sensitivity to punishing events. Further studies will be needed to determine which of these alternatives is at work in TBI.
Although considerable similarities to frontal brain injuries were found in decision-making, a strong dissociation with prior work was present in behavioral inhibition. Unilateral TBI induced clear motoric deficits (i.e., reduced latency to fall on the rotarod task), however, injured rats did not differ from shams in premature responses. This finding is in contrast to prior reports in frontal TBI, which demonstrated substantial increases in premature responding after severe [20, 34, 35], and even milder, concussive injuries [36]; although, it should be noted that other concussive studies have also provided equivocal findings [37]. Together, these findings suggest that, although both impulsivity and decision-making are often considered “frontal” functions, distinct neural mechanisms likely govern these behaviors. Literature from the field of behavioral neuroscience has identified that, despite relations between these two functions [38], there are clear regional and neurotransmitter differences [24, 39]. However, some caution should be given to not over-interpret motor impulsivity findings on the RGT, given that rats are provided with ample time (10 s) to respond after response holes become illuminated. Thus, the RGT does not generate a strong prepotent response and it is possible that more sensitive measures, such as those designed specifically to measure motor impulsivity, would reveal deficits even after unilateral TBI.
One core question that arises out of the current data is: why do frontally-mediated deficits occur after a unilateral, parietal brain injury? Notably, deficits in attention and behavioral-flexibility also occur among rats after unilateral parietal TBI [40]. However, given that win-stay and lose-shift behavior did not differ between TBI and Sham rats in the current study, changes to risk-based decision-making cannot be solely explained by response perseveration or deficits in flexibility. Thus, our results expand upon the existing experimental TBI literature on executive function, suggesting that decision-making deficits also occur following unilateral brain injury. Initial reports in clinical and preclinical populations specified that risk-based decision-making is mediated primarily by the prefrontal cortex [21, 41–44]; however, research with TBI patients has shown similar levels of impairment regardless of injury severity or lesion location [19,23]. Together, these data challenge the simplified notion that decision-making specifically, and executive function broadly, are solely the domain of the frontal cortex. Instead, complex behaviors such as these represent the interplay of several interconnected regions, including the ventral striatum/nucleus accumbens (motivation), frontal cortex (action selection, comparison with past outcomes), and dorsal striatum (habitual action, motor control) [45]. When considering the circuit that is involved, it becomes less surprising that TBI might cause these deficits, even when the impact occurs distal from the frontal cortex. The experimental evidence supporting this notion is robust as well. In particular, unilateral injuries cause deficits in dopamine neurotransmission along the mesocortical pathway, such as reduced dopamine release in the striatum and frontal cortex [46, 47], leading to inefficient clearance from the synapse, and ultimately, dopamine transporter down-regulation [46–49]. Moreover, similar changes, on a smaller scale, may influence the (often subtler) deficits in executive function observed after concussive TBI [15]. Finally, evidence in human patients suggests that disruptions to core networks identifiable by functional magnetic resonance imaging may account for many of these observed deficits [50].
Due to its role in these complex behaviors and long-term changes after TBI, the dopamine system has been a target of interest for treatments for a number of years [12, 51]. Recently, dopaminergic drugs, such as amantadine, have been assessed in clinical trials acutely after TBI, but failed to provide any benefit [52, 53]. Despite these failures at early treatment time points, there is still considerable interest in the use of dopaminergic drugs for chronic deficits [54]. Reasonable effects have been observed in small-scale studies with methylphenidate [55, 56], but these chronic studies are surprisingly few in number given the impact of risk-taking and impulsive behavior on daily function in patients [57, 58]. Because more knowledge is needed to understand the specific conditions under which executive-function deficits develop, behavioral tests such as the RGT provide an ideal platform for assessing preclinical therapeutics for executive deficits in TBI. Moreover, the deficits observed here develop over time, and are long-lasting, providing a mirror for the human condition and multiple points at which pharmacological intervention could be introduced (i.e., acute versus chronic post-injury phase). Moreover, while the current data provide an example of learning, many operant behaviors (when trained to stability), including the RGT, provide a robust assessment of trait-like conditions (i.e., those that are chronic in nature), which may be more relevant for patient populations [59–61]. Given that a substantial number of patients with TBI suffer from long-term, debilitating psychiatric conditions [3], developing treatments for chronic symptoms is of crucial importance for improving the lives of millions living with cognitive deficits as the result of brain injury.
Highlights.
Unilateral parietal traumatic brain injury (TBI) caused chronic deficits in risk-based decision-making, with TBI rats preferring the riskiest choice alternatives.
Unilateral parietal TBI caused motor impairments on the rotarod task but had no effect on motor impulsivity.
Though primarily mediated by the prefrontal cortex, unilateral parietal TBI is sufficient to impair risk-based decision-making.
Acknowledgements
We would like to thank the Injury and Recovery Laboratory members who contributed to the behavioral testing of animals in this project. This work was funded by the National Institutes of Health (NIGMS P20-GM109098; NINDS R01-NS110905).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no commercial interests.
References
- 1.Diaz-Arrastia R and Kenney K, Epidemiology of traumatic brain injury, in Traumatic Brain Injury, Vos P and Diaz-Arrastia R, Editors. 2014, Wiley-Blackwell: Oxford, UK. p. 183–191. [Google Scholar]
- 2.Rosenbaum SB and Lipton ML, Embracing chaos: The scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging and Behavior, 2012. 6(2): p. 255–282. [DOI] [PubMed] [Google Scholar]
- 3.Stocchetti N and Zanier ER, Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Critical Care Medicine, 2016. 20(1): p. 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semchuk KM, Love EJ, and Lee RG, Parkinson’s disease: A test of the multifactorial etiologic hypothesis. Neurology, 1993. 43: p. 1173–1180. [DOI] [PubMed] [Google Scholar]
- 5.Plassman BL, et al. , Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology, 2000. 55(8): p. 1158–1166. [DOI] [PubMed] [Google Scholar]
- 6.Moor E, et al. , Impairment of the ability of the injured aged brain in elevating urate and ascorbate. Experimental Gerontology, 2006. 41(3): p. 303–311. [DOI] [PubMed] [Google Scholar]
- 7.Vaishnavi S, Rao V, and Fann JR, Neuropsychiatric problems after traumatic brain injury: Unraveling the silent epidemic. Psychosomatics, 2009. 50(3): p. 198–205. [DOI] [PubMed] [Google Scholar]
- 8.Rao V, et al. , Neuropsychiatric symptoms in dementia patients with and without a history of traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 2010. 22(2): p. 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konrad C, et al. , Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychological medicine, 2011. 41(6): p. 1197. [DOI] [PubMed] [Google Scholar]
- 10.Reeves RR and Panguluri RL, Neuropsychiatric complications of traumatic brain injury. Journal of Psychosocial Nursing and Mental Health Services, 2011. 49(3): p. 42–50. [DOI] [PubMed] [Google Scholar]
- 11.Zgaljardic DJ, et al. , Psychiatric Disease and Post-Acute Traumatic Brain Injury. Journal of Neurotrauma, 2015. 32(23): p. 1911–25. [DOI] [PubMed] [Google Scholar]
- 12.Ozga JE, et al. , Executive (dys)function after traumatic brain injury: Special considerations for behavioral pharmacology. Behavioural Pharmacology, 2018. 29(7): p. 617–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon MR, et al. , Impulsivity, self-control, and delay discounting in persons with acquired brain injury. Behavioral Interventions, 2005. 20(1): p. 101–120. [Google Scholar]
- 14.McHugh L and Wood RL, Using a temporal discounting paradigm to measure decision-making and impulsivity following traumatic brain injury: A pilot study. Brain Injury, 2008. 22(9): p. 715–721. [DOI] [PubMed] [Google Scholar]
- 15.Vonder Haar C, et al. , Repetitive closed-head impact model of engineered rotational acceleration (CHIMERA) injury in rats increases impulsivity, decreases dopaminergic innervation in the olfactory tubercle and generates white matter inflammation, tau phosphorylation and degeneration. Experimental Neurology, 2019. 317: p. 87–99. [DOI] [PubMed] [Google Scholar]
- 16.Vonder Haar C, et al. , Frontal traumatic brain injury increases impulsive decision making in rats: A potential role for the inflammatory cytokine interleukin-12. Journal of Neurotrauma, 2017. 34(19): p. 2790–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton KR and Potenza MN, Relations among delay discounting, addictions, and money mismanagement: implications and future directions. American Journal on Drug and Alcohol Abuse, 2012. 38(1): p. 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle PA, et al. , Temporal discounting is associated with an increased risk of mortality among community-based older persons without dementia. PLoS One, 2013. 8(6): p. e67376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotrena C, et al. , Impaired decision-making after traumatic brain injury: The Iowa Gambling Task. Brain Injury, 2014. 28(8): p. 1070–1075. [DOI] [PubMed] [Google Scholar]
- 20.Shaver TK, et al. , Long-term deficits in risky decision-making after traumatic brain injury on a rat analog of the Iowa gambling task. Brain Research, 2019. 1704: p. 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechara A, et al. , Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 1994. 50(1–3): p. 7–15. [DOI] [PubMed] [Google Scholar]
- 22.Levine B, et al. , Gambling task performance in traumatic brain injury: Relationships to injury severity, atrophy, lesion location, and cognitive and psychosocial outcome. Cognitive and Behavioral Neurology, 2005. 18(1): p. 45–54. [PubMed] [Google Scholar]
- 23.Sigurdardottir S, et al. , Olfactory dysfunction, gambling task performance and intracranial lesions after traumatic brain injury. Neuropsychology, 2010. 24(4): p. 504. [DOI] [PubMed] [Google Scholar]
- 24.Zeeb FD, Robbins TW, and Winstanley CA, Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology, 2009. 34(10): p. 2329–2343. [DOI] [PubMed] [Google Scholar]
- 25.Hamm RJ, et al. , The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. Journal of Neurotrauma, 1994. 11(2): p. 187–196. [DOI] [PubMed] [Google Scholar]
- 26.Vonder Haar C, Emery MA, and Hoane MR, Chronic folic acid administration confers no treatment effects in either a high or low dose following unilateral controlled cortical impact injury in the rat. Restorative Neurology and Neuroscience, 2012. 30(4): p. 291–302. [DOI] [PubMed] [Google Scholar]
- 27.Carli M, et al. , Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behavioural Brain Research, 1983. 9(3): p. 361–380. [DOI] [PubMed] [Google Scholar]
- 28.MacPherson SE, et al. , Iowa Gambling task impairment is not specific to entromedial prefrontal lesions. The Clinical Neuropsychologist, 2009. 23(3): p. 510–522. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L, et al. , Is there a recovery of decision-making function after frontal lobe damage? A study using alternative versions of the Iowa Gambling Task. Journal of Clinical and Experimental Neuropsychology, 2013. 35(5): p. 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlund MW and Pace G, The effects of traumatic brain injury on reporting and responding to causal relations: An investigation of sensitivity to reinforcement contingencies. Brain Injury, 2000. 14(6): p. 573–583. [DOI] [PubMed] [Google Scholar]
- 31.Larson MJ, et al. , Reward context sensitivity impairment following severe TBI: An event-related potential investigation. Journal of the International Neuropsychological Society, 2007. 13(4): p. 615–625. [DOI] [PubMed] [Google Scholar]
- 32.Vonder Haar C, et al. , Deficits in discrimination following experimental frontal brain injury are mediated by motivation and can be improved by nicotinamide administration. Journal of Neurotrauma, 2014. 31(20): p. 1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens KM, et al. , A discrimination task used as a novel method of testing decision-making behavior following traumatic brain injury Journal of Neurotrauma, 2012. 29: p. 2505–2512. [DOI] [PubMed] [Google Scholar]
- 34.Martens KM, et al. , Cathodal transcranial direct-current stimulation selectively reduces impulsivity after TBI. Journal of Neurotrauma, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vonder Haar C, et al. , Frontal traumatic brain injury in rats causes long-lasting impairments in impulse control that are differentially sensitive to pharmacotherapeutics and associated with chronic neuroinflammation. ACS Chemical Neuroscience, 2016. 7(11): p. 1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mychasiuk R, Hehar H, and Esser MJ, A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats. Behavioural Brain Research, 2015. 286: p. 285–292. [DOI] [PubMed] [Google Scholar]
- 37.Arulsamy A, Corrigan F, and Collins-Praino LE, Age, but not severity of injury, mediates decline in executive function: Validation of the rodent touchscreen paradigm for preclinical models of traumatic brain injury. Behavioural Brain Research, 2019. 368: p. 111912. [DOI] [PubMed] [Google Scholar]
- 38.Barrus MM, et al. , Disadvantageous decision-making on a rodent gambling task is associated with increased motor impulsivity in a population of male rats. Journal of Psychiatry & Neuroscience, 2015. 40(2): p. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baarendse PJJ, Winstanley CA, and Vanderschuren LJMJ, Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology, 2013. 225(3): p. 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bondi CO, et al. , Old dog, new tricks: The attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. Journal of Neurotrauma, 2014. 31(10): p. 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bechara A, Tranel D, and Damasio H, Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain, 2000. 123(11): p. 2189–2202. [DOI] [PubMed] [Google Scholar]
- 42.Fellows LK, The role of orbitofrontal cortex in decision making: a component rocess account. Annals of the New York Academy of Science, 2007. 1121(1): p. 421–330. [DOI] [PubMed] [Google Scholar]
- 43.Chudasama Y and Robbins TW, Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological Psychology, 2006. 73(1): p. 19–38. [DOI] [PubMed] [Google Scholar]
- 44.Robbins TW and Arnsten AFT, The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annual Review of Neuroscience, 2009. 32(1): p. 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Euston DR, Gruber AJ, and McNaughton BL, The role of medial prefrontal cortex in memory and decision making. Neuron, 2012. 76(6): p. 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner AK, et al. , Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. Journal of Neurochemistry, 2005. 95(2): p. 457–465. [DOI] [PubMed] [Google Scholar]
- 47.Yan HQ, et al. , Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. Neuroreport, 2002. 13(15): p. 1899–901. [DOI] [PubMed] [Google Scholar]
- 48.Shimada R, et al. , Changes in dopamine transporter expression in the midbrain following traumatic brain injury: An immunohistochemical and in situ hybridization study in a mouse model. Neurological research, 2014. 36(3): p. 239–246. [DOI] [PubMed] [Google Scholar]
- 49.Huang EY, et al. , Remote effects on the striatal dopamine system after fluid percussion injury. Behavioural Brain Research, 2014. 267: p. 156–172. [DOI] [PubMed] [Google Scholar]
- 50.Sharp DJ, Scott G, and Leech R, Network dysfunction after traumatic brain injury. Nature Reviews Neurology, 2014. 10(3): p. 156–166. [DOI] [PubMed] [Google Scholar]
- 51.Lan Y-L, et al. , The potential roles of dopamine in traumatic brain injury: A preclinical and clinical update. American Journal of Translational Research, 2019. 11(5): p. 2616–2631. [PMC free article] [PubMed] [Google Scholar]
- 52.Ghalaenovi H, et al. , The effects of amantadine on traumatic brain injury outcome: a double-blind, randomized, controlled, clinical trial. Brain Injury, 2018. 32(8): p. 1050–1055. [DOI] [PubMed] [Google Scholar]
- 53.Hammond FM, et al. , Amantadine did not positively impact cognition in chronic traumatic brain injury: A multi-site, randomized, controlled trial. Journal of Neurotrauma, 2018. 35(19): p. 2298–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bales JW, et al. , Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neuroscience & Biobehavioral Reviews, 2009. 33(7): p. 981–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAllister TW, et al. , Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology, 2016. 41(5): p. 1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreno-López L, et al. , Anything goes? Regulation of the neural processes underlying response inhibition in TBI patients. European Neuropsychopharmacology, 2016. 27(2): p. 159–169. [DOI] [PubMed] [Google Scholar]
- 57.James LM, Strom TQ, and Leskela J, Risk-taking behaviors and impulsivity among veterans with and without PTSD and mild TBI. Military Medicine, 2014. 179(4): p. 357–363. [DOI] [PubMed] [Google Scholar]
- 58.Rochat L, et al. , Assessment of impulsivity after moderate to severe traumatic brain injury. Neuropsychological Rehabilitation, 2010. 20(5): p. 778–797. [DOI] [PubMed] [Google Scholar]
- 59.Brevers D, et al. , Impaired self-awareness in pathological gamblers. Journal of Gambling Studies, 2013. 29(1): p. 119–129. [DOI] [PubMed] [Google Scholar]
- 60.Gansler DA, et al. , Does the iowa gambling task measure executive function? Archives of Clinical Neuropsychology, 2011. 26(8): p. 706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sevy S, et al. , Iowa gambling task in schizophrenia: A review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophrenia Research, 2007. 92(1–3): p. 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


