Abstract
Ammonia is one of the principal kairomones originating from human and other animal emanations and in that context, plays an essential role in the host-seeking behaviors of the malaria vector mosquito Anopheles gambiae. Nevertheless, despite its importance in directing host-seeking, the mechanisms underlying ammonia detection in the mosquito olfactory system remains largely unknown. In addition to ongoing efforts to identify and characterize the molecular receptors that underlie ammonia sensitivity, previous studies have revealed a prominent role for ammonium transporters (Amt) in modulating antennal and behavioral responses in Drosophila melanogaster and An. gambiae. In the former, localization of DmAmt in antennal sensilla to auxiliary cells surrounding the ammonia sensory neurons led to the hypothesis that its role was to clear excess ammonium ions in the sensillar lymph. In the latter, RT-PCR and heterologous expression have been used to examine the expression and functional characteristics of the An. gambiae ammonium transporter, AgAmt. We now employ advanced transgenic tools to comprehensively examine AgAmt spatial localization across the peripheral chemosensory appendages in larvae and adult female An. gambiae. In the larval antennae, AgAmt appears localized in both neuronal and auxiliary cells. In contrast to D. melanogaster, in the adult antennae, AgAmt-derived signals are observed in both non-neuronal auxiliary cells and in sensory neurons in ammonia-responsive basiconic and coeloconic sensilla. In the maxillary palps, labella, and tarsi, AgAmt appears restricted to sensory neurons. We have also characterized the responses to ammonia of adult antennal coeloconic sensilla and maxillary palp capitate pegs revealing a correlation between sensillar AgAmt expression and ammonia sensitivity. Taken together, these data suggest that AgAmt may play heterogeneous roles in the adult and larval chemosensory apparatus and potentially broad utility as a supra-receptor target in mosquito control.
Keywords: Anopheles gambiae ammonium transporter, chemosensory system, cellular organization, Q system, phiC31 integration
1. Introduction
Mosquito vectors are responsible for the transmission of a variety of deadly human diseases, including dengue fever, yellow fever, Zika virus, West Nile virus, lymphatic filariasis, and malaria (Van Der Goes Van Naters and Carlson, 2006). In 2016, more than 200 million cases of human malaria occurred worldwide (Alonso and Noor, 2017). The malaria mosquito Anopheles gambiae is one of the primary vectors of the most prevalent malaria parasite Plasmodium falciparum (Molina-Cruz et al., 2016; Report, 1980). Female mosquitoes ingest blood meals that are required for egg development and through which the parasite is vectored by Anopheles mosquitoes and is transmitted from human to human (Cox, 2010). Mosquitoes rely on their acute olfactory system to detect volatiles, including CO2, ammonia, and other specific odors to locate humans and other mammals which represent potential blood meal hosts (VanDer Goes Van Naters and Carlson, 2006; Zwiebel and Takken, 2004).
In mosquitoes, the primary head appendages involved in odorant detection are the antennae, maxillary palps, and the labella of the proboscis. Dispersed along the surface of those appendages are hollow sensory hairs known as sensilla. A large variety of olfactory sensory neurons (OSNs) are housed in the sensilla and respond to distinct spectrums of odorants (Guidobaldi et al., 2014; Montell and Zwiebel, 2016). Based on the morphological features, olfactory sensilla are categorized as trichoid, basiconic (also known as grooved pegs), and coeloconic (Pitts and Zwiebel, 2006). Although different functional subgroups are present within these classes of sensilla, grooved pegs are generally tuned to acids and amines, trichoid sensilla respond to a broader spectrum of odors (Qiu et al., 2006), while coeloconic sensilla have been associated with both chemo- and thermosensory pathways (McIver, 1973). Within chemosensory sensilla, OSNs are positioned in an aqueous lymph that fills the luminal space and contains odorant binding proteins (OBPs) and odorant-degrading enzymes (ODEs) and other components that are required for odorant recognition and clearage (Leal, 2013). On OSN dendritic membranes, several families of ligand-gated ion channels including odorant receptors (ORs), gustatory receptors (GRs) and ionotropic receptors (IRs), play central roles in olfactory signal transduction as they are activated by specific odorants to generate the action potentials representing peripheral odor coding (Suh et al., 2014).
Ammonia is a volatile component of mammalian sweat and electrophysiological studies have revealed a broad set of neuronal responses to ammonia in antennal trichoid sensilla and grooved pegs (Meijerink et al., 2001; Qiu et al., 2006). As a component of a hyper-effective five-compound mosquito blend, ammonia has been shown to attract female An. gambiae (Boverhof et al., 2008; Mukabana et al., 2012; Smallegange et al., 2005, 2002). While studies in D. melanogaster have suggested DmIr92a is a functional ammonia receptor, little is known about the molecular and indeed the neuronal receptors responsible for ammonia detection in mosquitoes (Benton et al., 2009; Min et al., 2013), especially as no clear homologs of DmIr92a are encoded in mosquito genomes.
Nitrogen is essential for the survival of mosquitoes and indeed all living organisms and ammonium is the principal ionic target for its fixation and subsequent incorporation into biological molecules. At the same time, ammonia is toxic when it accumulates, which makes it critical for cells to have efficient mechanisms for the uptake and transport of ammonium (Bittsánszky et al., 2015; Crawford and Forde, 2002). Because ammonium is not able to penetrate the cell membrane passively, ammonium transport proteins, including ammonium transporters (Amts), methylammonium/ammonium permeases (Meps), and Rhesus (Rh) proteins, facilitate the cross-membrane transportation of ammonium (Andrade and Einsle, 2007). Recently, several groups have reported the characterization of antennal-expressed Amt proteinsin both Drosophila and Anopheles (Menuz et al., 2014; Pitts et al., 2014).
In D. melanogaster, DmAmt has been shown to be directly involved in ammonia detection as null mutants show significantly reduced olfactory responses to ammonia than the wild type (Menuz et al., 2014). Localization of DmAmt in the fruit fly antennae revealed that it is expressed in the auxiliary cells surrounding ammonia sensitive neurons, suggesting that DmAmt is not the receptor/sensor but rather is involved in ammonium clearance from the sensillar lymph (Menuz et al., 2014). This finding suggests that DmAmt’s role is to prevent DmIr92a from being prematurely desensitized by the accumulation of environmental (sensory) ammonia or ammonia otherwise produced by cellular metabolism (Menuz et al., 2014; Trussell and Fischbach, 1989).
In Aedes aegypti, the reduced expression of AeAmt1 caused accumulation of ammonium in the mosquito larval hemolymph which suggested its role in ammonium excretion (Chasiotis et al., 2016). In An. gambiae, while the functional role of AgAmt in mediating antennal ammonia sensitivity remains unclear, its transcripts are highly abundant in female antennae and have been functionally shown to modulate ammonium cross-membrane transportation using Xenopus heterogeneous expression system (Pitts et al., 2014). In addition, AgAmt was shown to phenotypically rescue the defect of ammonia responses in DmAmt mutants (Menuz et al., 2014).
In order to better understand the role of AgAmt in ammonia detection in An. gambiae, we now have characterized its tissue and cellular localization utilizing several advanced transgenic tools, including the newly developed “Q system” (Potter et al., 2010). The comprehensive cellular localization of AgAmt in the chemosensory system of larval and adult-stage An. gambiae informs our understanding of the role of this transporter in host-seeking and other important behaviors.
2. Material and Methods
2.1. Mosquito maintenance
An. gambiae phiC31 docking line E was acquired from The Malaria Research and Reference Reagent Resource Center (Meredith et al., 2011). An. gambiae effector line (QUAS-mCD8:GFP) was a generous gift from the lab of Dr. C. Potter at The Johns Hopkins University School of Medicine (Riabinina et al., 2016). All the mosquito lines were reared at 27°C, 75% relative humidity under a 12:12 light-dark cycle and supplied with 10% sugar water in the Vanderbilt University Insectary.
2.2. AgAmt promoter-QF2 construct
The 1kb/3kb AgAmt upstream sequence was amplified from the wild type An. gambiae genomic DNA using the forward primers (AgAmt_1kb_F: 5’- AAT CCG GAA CAA GCA TCA TCA GAG CGA T −3’; AgAmt_3kb_F: 5’- AAT CCG GAC CCA AGT AAT TAA GTA GTG CT −3’) and the reverse primer AgAmt _R (5’- AAG GCG CGC CTG CAG TGC TAA TCA AAC CAA C −3’). The 1kb/3kb AgAmt promoter (AgAmtP) amplicons were restriction enzyme digested and inserted into the BspEI/AscI restriction site on a pBattB-DsRed construct (Supplementary data). Likewise, the QF2 sequence was sequentially amplified from the pXL-BACII-DsRed-QF2-hsp70 construct (Riabinina et al., 2016) using the primer pair QF2for (5’- AAG GCC GGC CAT GCC ACC CAA GCG CAA AAC- 3’) and QF2rev (5’- AAG CGA TCG CTC ACT GTT CGT ATG TAT TAA TG- 3’) and inserted into the FseI/AsiSi restriction site which is downstream of the AgAmtP insertion site.
2.3. Mosquito transgenics
The detailed microinjection protocol was described previously (Pondeville et al., 2014). Briefly, newly laid (approximately 1hr-old) embryos of the phiC31 docking line (Meredith et al., 2011) were immediately collected and aligned on a filter paper moistened with 25mM sodium chloride solution. All the embryos were fixed on a coverslip with double-sided tape and a drop of halocarbon oil 27 was applied to cover the embryos. The coverslip was further fixed on a slide under a Zeiss Axiovert 35 microscope with a 40X objective. The microinjection was performed using Eppendorf FemtoJet 5247 and quartz needles (Sutter Instrument, Novato, CA). The phiC31 integrase was provided by the pENTR R4-vas2-integrase-R3 helper plasmid (a kind gift from Eric Marois via Addgene plasmid #62299) which was diluted to 200ng/μl in 1x microinjection buffer (5mM KCl, 0.1mM sodium phosphate buffer, pH7.2) before being co-injected with the pBattB-DsRed-AgAmtP-QF2 plasmid at 400ng/μl. The injected embryos were placed into deionized water with artificial sea salt (0.3g/L) and reared in the lab condition.
First generation (G0) of injected adults were separated based on gender and crossed to 5X phiC31 docking line gender counterparts. Their offspring (F1) was screened for DsRed-derived red eye fluorescence. Red-eyed F1 males were individually crossed to 5X docking line females to establish a stable transgenic line. PCR analysesof all individuals were performed (after mating) to validate phiC31 integration using the primer pairs attR_F (5’- TCA AAC TAA GGC GGA GTG G −3’) and attR_R (5’- GAT GGG TGA GGT GGA GTA CG −3’); attL_F (5’- GAG GTC GAC GAT GTA GGT CAC −3’) and attL_R (5’- ACC TTT TCT CCC TTG CTA CTG AC −3’) that covers the junctions between the integrated and endogenous sequences (Meredith et al., 2011). The presence of 1kb/3kb AgAmtP-QF2 sequences was PCR validated using primers 1kb_F (5’- GCC ATC CAA CTC ACC ACA CA −3’), 3kb_F (5’- CGG CAA AAG AAG GGT TTC GG −3’), and QF_R (5’- CAG GGT CGT AGT TGT GGG TC −3’).
2.4. Whole-mount appendage imaging
Because the driver line was not homozygous, all the offspring from the cross between the AgAmtP-QF2 driver line and the QUAS-mCD8:GFP effector line (Riabinina et al., 2016) were collected and screened for the presence of DsRed in the eye. Whole antennae from adult females and larvae were thereafter dissected into 4% formaldehyde in PBST (0.1% Triton X-100 in PBS) and fixed on ice for 30mins. Samples were washed 3X in PBST for 10mins each and transferred onto slides and mounted in Vectashield fluorescent medium (Vector Laboratories, Burlingame, CA).
2.5. Immunocytochemistry
Antibody staining was performed as previously (Pitts et al., 2004) with minor modifications. Antennae, labella, maxillary palps, and tarsi were dissected into 4% formaldehyde in PBST and fixed on ice for 30mins. Samples were wash 3X in PBST for 10mins each and then embedded in TFM Tissue Freezing Medium (General Data Company Inc., Cincinnati, OH). Cryosections were obtained at −20°C with a CM1900 cryostat (Leica Microsystems, Bannockburn, IL). Samples were sectioned at ~10μm and transferred onto Superfrost plus slides (VWR Scientific, Radnor, PA). Slides were air-dried at room temperature (RT) for 30mins and fixed in 4% formaldehyde in PBST for 10mins, followed by 3X rinsing in PBST for 10mins each. Thereafter, 5% normal goat serum (Sigma-Aldrich, St. Louis, MO) in PBST was applied and the slides were blocked in dark at RT for 1hr with HybriWell sealing chambers (Grace Bio-Labs, Bend, OR). Primary antibody (Rabbit α-Orco/Goat α-HRP-Cy3) was diluted 1:500 in 5% normal goat serum in PBST and applied on the slides and incubated overnight at 4°C. After primary antibody staining, slides were washed 3X in PBST for 10mins each and stained with secondary antibody Goat α-Rabbit-Cy3 (Jackson ImmunoResearch, West Grove, PA) 1:500 in 5% normal goat serum PBST for 2hrs at RT and then rinsed 3X. Nuclei were stained with 300nM DAPI (Invitrogen, Carlsbad, CA) at RT for 10mins. Slides were briefly washed and mounted in Vectashield fluorescent medium (Vector Laboratories, Burlingame, CA). α-HRP-Cy3 staining was directly visualized without the use of secondary antibody.
The whole-mount larval antennae were dissected into 4% formaldehyde in PBST and fixed on ice for 30mins. Samples were washed 3X in PBST for 10mins each and blocked with 5% normal goat serum (Sigma-Aldrich, St. Louis, MO) in PBST overnight at 4°C. Then 1:500 primary antibody (Rabbit α-Orco/Goat α-HRP-Cy3) was added and the samples were incubated overnight at 4°C. After primary antibody staining, samples were washed 3X in PBST for 10mins each and stained with secondary antibody Goat α-Rabbit-Cy3 (Jackson ImmunoResearch, West Grove, PA) 1:500 in 5% normal goat serum PBST overnight at 4°C and then rinsed 3X. Nuclei were stained with 300nM DAPI (Invitrogen, Carlsbad, CA) at 4°C for 1hr. Samples were transferred onto the slides and mounted in Vectashield fluorescent medium (Vector Laboratories, Burlingame, CA). α-HRP-Cy3 staining was directly visualized without the use of secondary antibody.
2.6. Fluorescence In-situ hybridization (FISH)
The TOPO-TA dual promoter vector (Invitrogen, Carlsbad, CA) was used to subclone full length AgIr76b as described in the previous study (Pitts et al., 2017). Similarly, the full length AgGr15 coding sequence was cloned into the TOPO-TA dual promoter vector and verified by DNA sequence analysis. Antisense/sense probes were thereafter synthesized using SP6/T7 RNA polymerase (NEB, Ipswich, MA) with Digoxigenin (DIG) RNA Labeling Kit (Roche, Switzerland).
Antennal samples were sectioned and fixed as described above. Sequentially, the slides were incubated in acetylation solution (0.1M triethanolamine, 0.65% HCl, 0.375% acetic anhydride) for 10mins at RT. After being washed 3X in PBST, slides were incubated in pre-heated (65°C) hybridization buffer (50% deionized formamide, 5X saline sodium citrate (SSC), 50μg/mL heparin sodium salt, 0.1% tween-20) for 40mins at RT. RNA probes were diluted in the hybridization buffer at 500ng/mL and applied onto the slides which were further incubated with sealing chambers at 65°C for 18hrs.
Following the hybridization, slides were washed 3X in 0.2X SSC at 65°C for 20mins each and then placed in TNT buffer (0.1M Tris-HCl, 150mM NaCl, 0.05%Tween-20) at RT for 10mins. TNB buffer (0.1M Tris-HCl, 150mM NaCl, 0.05%Tween-20, 1% blocking reagent from PerkinElmer) was subsequently applied to the slides for blocking for 1hr at RT inside sealed hybridization chambers. Due to the extreme overnight incubation temperature of FISH (65°C) that bleached the fluorescence of GFP, the GFP signal was recovered using GFP antibody (α-GFP) after the FISH experiment. Primary antibodies including 1:200 Chicken α-GFP (Vanderbilt Antibody and Protein Resource) and 1:500 Sheep α-DIG-POD (Roche, Switzerland) in TNB were applied to the slides which were thereafter incubated at 4°C overnight. After washing (3X) in TNT at RT for 5mins each, secondary antibody (1:1000 Donkey α-Chicken-Alexa488; Jackson ImmunoResearch, West Grove, PA) in 5% normal donkey serum TNT was applied to the slides which were incubated for 2hrs at RT. The slides were subsequently washed 3X in TNT at RT for 5mins each. TSA-Cy3 amplification (PerkinElmer, Waltham, MA) was performed according to the manufacturer’s instructions at 1:50 in amplification working buffer at dark, following by 3X washing in TNT for 5mins each and mounting in Vectashield fluorescent medium (Vector Laboratories, Burlingame, CA).
2.7. Confocal microscopy
Confocal microscopy images at 1024*1024 pixel resolution were collected on an Olympus FV-1000 equipped with a 100X oil objective at the Vanderbilt University Cell Imaging Shared Resource Core. Lasers wavelengths of 405nm, 488nm, and 543nm were used to detect DAPI, GFP, and Cy3, respectively.
2.8. Electrophysiology
Single sensillum recording (SSR) were carried out as previously described (Liu et al., 2013) with minor modifications. Mated female mosquitoes (4–10 days after eclosion) were mounted on a microscope slide (76 × 26 mm) (Ghaninia et al., 2007). The antennae were fixed using double-sided tape to a cover slip resting on a small bead of dental wax to facilitate manipulation and the cover slip was placed at approximately 30 degrees to the mosquito head. Once mounted, the specimen was placed under an Olympus BX51WI microscope and the antennae viewed at high magnification (1000×). Two tungsten microelectrodes were sharpened in 10% KNO2 at 10 V. The grounded reference electrode was inserted into the compound eye of the mosquito using a WPI micromanipulator and the recording electrode was connected to the preamplifier (10×, Syntech) and inserted into the shaft of the olfactory sensillum to complete the electrical circuit to extracellularly record OSN potentials (Den Otter et al., 1980). Controlled manipulation of the recording electrode was performed using a Burleigh micromanipulator (Model PCS6000). The preamplifier was connected to an analog to digital signal converter (IDAC-4, Syntech), which in turn was connected to a computer for signal recording and visualization.
Ammonium hydroxide (Sigma-Aldrich, St. Louis, MO) was serially diluted (in water) to 0.01%, 0.05%, 0.1%, 0.5%, 1%, and 5% ammonia solutions. For each concentration, a 10μl aliquot was applied onto a filter paper (3 × 10mm) which was then inserted into a Pasteur pipette to create the stimulus cartridge. A sample containing the solvent (water) alone served as the control. The airflow across the antennae was maintained at a constant 20 ml/s throughout the experiment. Purified and humidified air was delivered to the preparation through a glass tube (10-mm inner diameter) perforated by a small hole 10cm away from the end of the tube into which the tip of the Pasteur pipette could be inserted. The stimulus was delivered to the sensilla by inserting the tip of the stimulus cartridge into this hole and diverting a portion of the air stream (0.5L/min) to flow through the stimulus cartridge for 500ms using a stimulus controller (Syntech). The distance between the end of the glass tube and the antennae was ⩽1cm. Signals were recorded for 10s starting 1 second before stimulation, and the action potentials were counted off-line over a 500ms period before and after stimulation. Spike rates observed during the 500ms stimulation were subtracted from the spontaneous activities observed in the preceding 500ms and counts recorded in units of spikes/s.
3. Results
3.1. Generation of AgAmtP-QF2 driver line
Significant efforts to directly localize AgAmt protein or AgAmt-derived transcripts proved unsuccessful due to the paucity of specific immunological reagents and riboprobes. To address this deficit, the “Q” binary expression system recently brought to An. gambiae (Riabinina et al., 2016) was utilized to indirectly visualize AgAmt expression. Here an AgAmtP-QF2 driver line was generated and subsequently crossed to a QUAS-mCD8:GFP effector line to generate AgAmtP-QF2, QUAS-mCD8:GFP progeny lines. In this way, AgAmtP elements regulate the expression of QF2 which in turn binds to QUAS elements to robustly drive the expression of visible mCD8:GFP markers to indirectly reveal the likely sites of AgAmt expression. Inasmuch as subsequent studies rely on these indirect binary markers, we acknowledge the inherent caveats of this system in our characterization of AgAmt expression.
To begin with we took advantage of the site-specific integration phiC31 system to generate a driver line by integrating the AgAmtP-QF2 construct into a pre-defined genomic site (Meredith et al., 2011). Because of our imprecise understanding of the all the features of the AgAmtP, we initially chose to incorporate 1kb and 3kb upstream sequences from the AgAmt start codon as potential AgAmt regulatory sequences to generate two independent transgenic driver lines (1kb/3kb AgAmtP-QF2 drivers). Microinjection of preblastoderm embryos was used to deliver the AgAmtP-QF2 construct containing an attB attachment site and a 3xP3-DsRed marker (pBattB-DsRed-1kb/3kb AgAmtP-QF2) along with a phiC31 integrase encoding helper plasmid regulated by the vasa2 promoter to induce the integration in germ cells (Table 1). Individual males with red fluorescence in the eye and ventral nerve cord fluorescence were crossed to docking line females to establish transgenic lines. The successful integration was confirmed using genomic DNA PCR to show amplicons that cover the junctions between the endogenous and integrated sequences (Figure 1A–1G).
Table 1.
Details of embryo microinjection and the efficiency of phiC31 integration. The survived adults were pooled and crossed to the docking line opposite gender mosquitoes, and therefore the number of founders is the minimum.
| Injected construct | Injected embryos | Survived adults | Founders |
|---|---|---|---|
| pBattB-DsRed-1 kb | 1176 | 14 males | ≥ 7.4% (2/27) |
| pBattB-DsRed-3kb | 572 | 5 females | ≥ 20% (1/5) |
Figure 1.

Schematics of PCR strategies to validate: (A) the phiC31 site specific integration using two primer pairs that cover the integration junctions; (B) the unintegrated attP attachment site; (C) the 1kb AgAmtP using a primer pair that spans the 1kb promoter-QF sequences; (D) the 3kb AgAmtP using a primer pair that spans the 3kb promoter-QF sequences. (E-G) The gel image of the PCR validation on 1kb drivers (E), 3kb drivers (F), and docking lines (G). For each mosquito line, the lanes from left to right are using primer pairs: attR_F/attR_R (AR, 224bp), attL_F/attL_R (AL, 301 bp), attP_F/attP_R (B, 391bp), 1kb_F/QF_R (C, 792bp), and 3kb_F/QF_R (D, 1.7kb), respectively.
3.2. Specific AgAmt localization driven by the “Q system”
We first examined whole-mount female antennal samples of both the 1kb/3kb
AgAmtP-GFP progeny where AgAmtP-driven expression was specific to the grooved pegs and coeloconic sensilla (1kb AgAmtP-GFP: Figure 2A, 2B; 3kb AgAmtP-GFP: Figure 2C, 2D). Importantly, except for the nonspecific expression inherent to the effector line (Riabinina et al., 2016), no GFP expression was observed in chemosensory appendages in either parental strain (Supplementary data; Figure S1).
Figure 2.
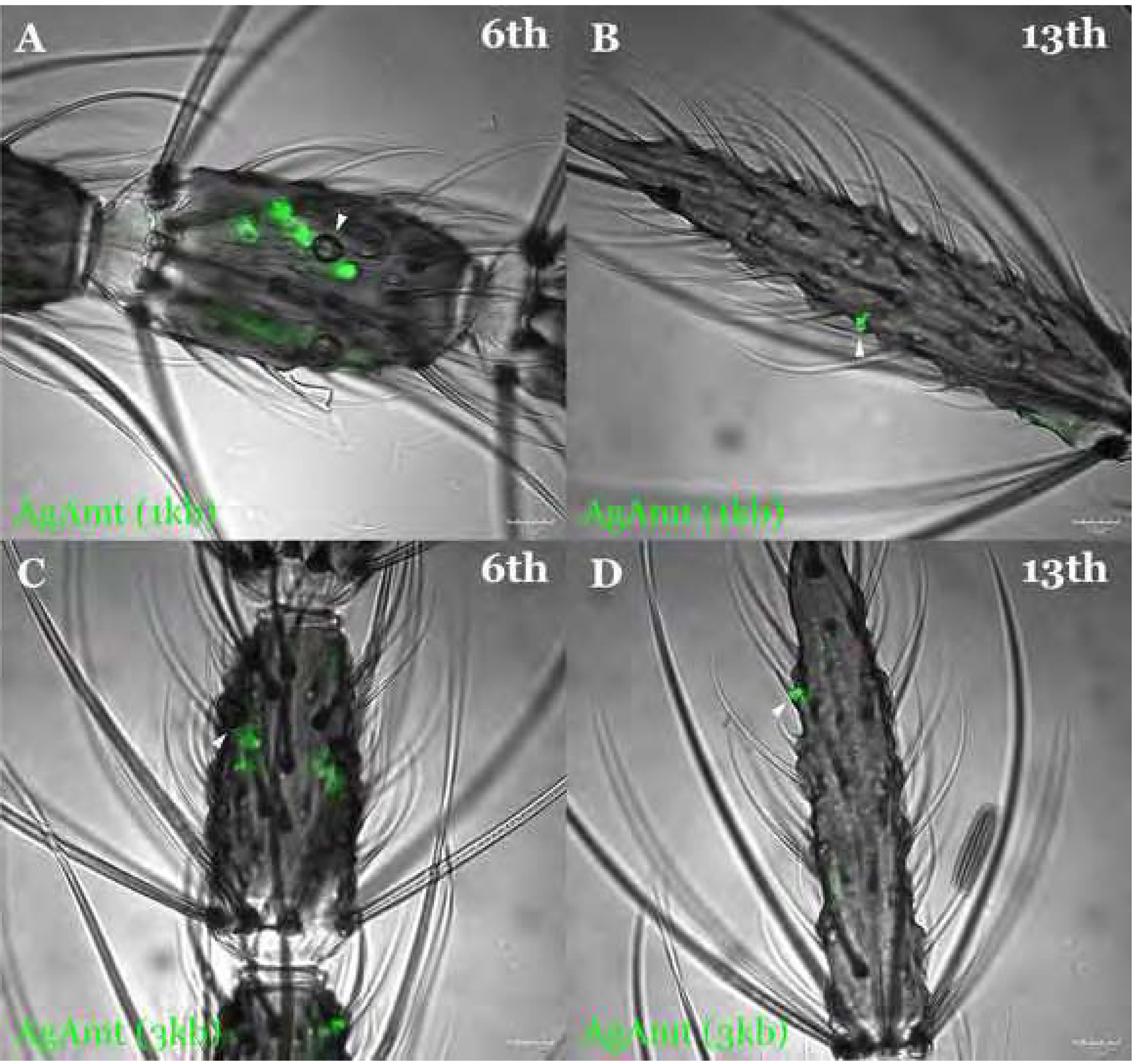
The confocal optical section of the whole-mount female An. gambiae antennae carrying QUAS-GFP and either 1kb AgAmtP-QF2 (A, B; green) or 3kb AgAmtP-QF2 (C, D; green) constructs showing specific expression of GFP in coeloconic sensilla on the 6th flagellomere (A, C) and grooved pegs on the 13th flagellomere (B, D). Sensilla are highlighted with white arrows. Scale bars = 10μm.
While AgAmtP-driven GFP is expressed in all coeloconic sensilla, it was only observed in a subset of grooved pegs. In order to assess the relative GFP expression patterns of both these lines, the total number of GFP-labelled grooved pegs (Supplementary data; Table S1 & Figure S2A) and coeloconic sensilla (Supplementary data; Table S2 & Figure S2B) were counted and compared between the 1kb AgAmtP-GFP progeny and the 3kb AgAmtP-GFP progeny. These quantitative data suggest there is no significant difference between the percentage of GFP-labelled grooved pegs (Supplementary data; Table S3; p-value = 0.1061) in the 1kb (99.30% as the observed percentage in the statistical test) and 3kb (96.97% as the expected percentage in the statistical test) AgAmtP-GFP progeny. This suggests that the major regulatory elements that comprise the AgAmtP are likely to be contained within the region that is 1kb upstream from the start codon. However, inasmuch as this comparison was not based on cellular expression, we appreciate that there may still be subtle GFP signal expression/intensity differences between the two lines. Nevertheless, in the absence of significant expression differences, all studies were henceforth carried out using the 1kb AgAmtP-GFP progeny.
3.3. Heterogeneous AgAmt expression in olfactory appendages
We first investigated whether AgAmt is expressed in non-neuronal auxiliary cells where, as was observed for DmAmt (Menuz et al., 2014) it might function in clearing ammonium from the sensillar lymph. Neurons were labelled using a horseradish peroxidase antibody (α-HRP-Cy3), which has been previously used as a general neuronal marker in Drosophila and mosquitoes (Jan and Jan, 1982; Loesel et al., 2006; Pitts et al., 2004). An examination of female antennal cryosections revealed that AgAmtP-driven GFP is expressed in both neuronal and non-neuronal cells across multiple flagellomeres on antennae (Figure 3A–3D). Z-axis projections of whole-mount antennal images revealed both coeloconic sensilla and grooved pegs are innervated with likely AgAmt-expressing neurons (Figure 3E–3H). Here, we observed characteristically distinct cellular morphology between AgAmtP-driven GFP labelled neurons and auxiliary cells. While the likely AgAmt-expressing neuron cell bodies are typically circular, the shapes of the non-neuronal cells are more irregular, consistent with the expected morphology of thecogen, tormogen, and trichogen sensillar auxiliary cells (Mclver, 1982; Shanbhag et al., 2000). In some instances, a partial overlap between AgAmt and α-HRP labelling was observed, which might be due to cell stacking on the z-axis. This also supports a close association between potentially AgAmt-expressing auxiliary cells and sensory neurons (Figure 3I–3L).
Figure 3.
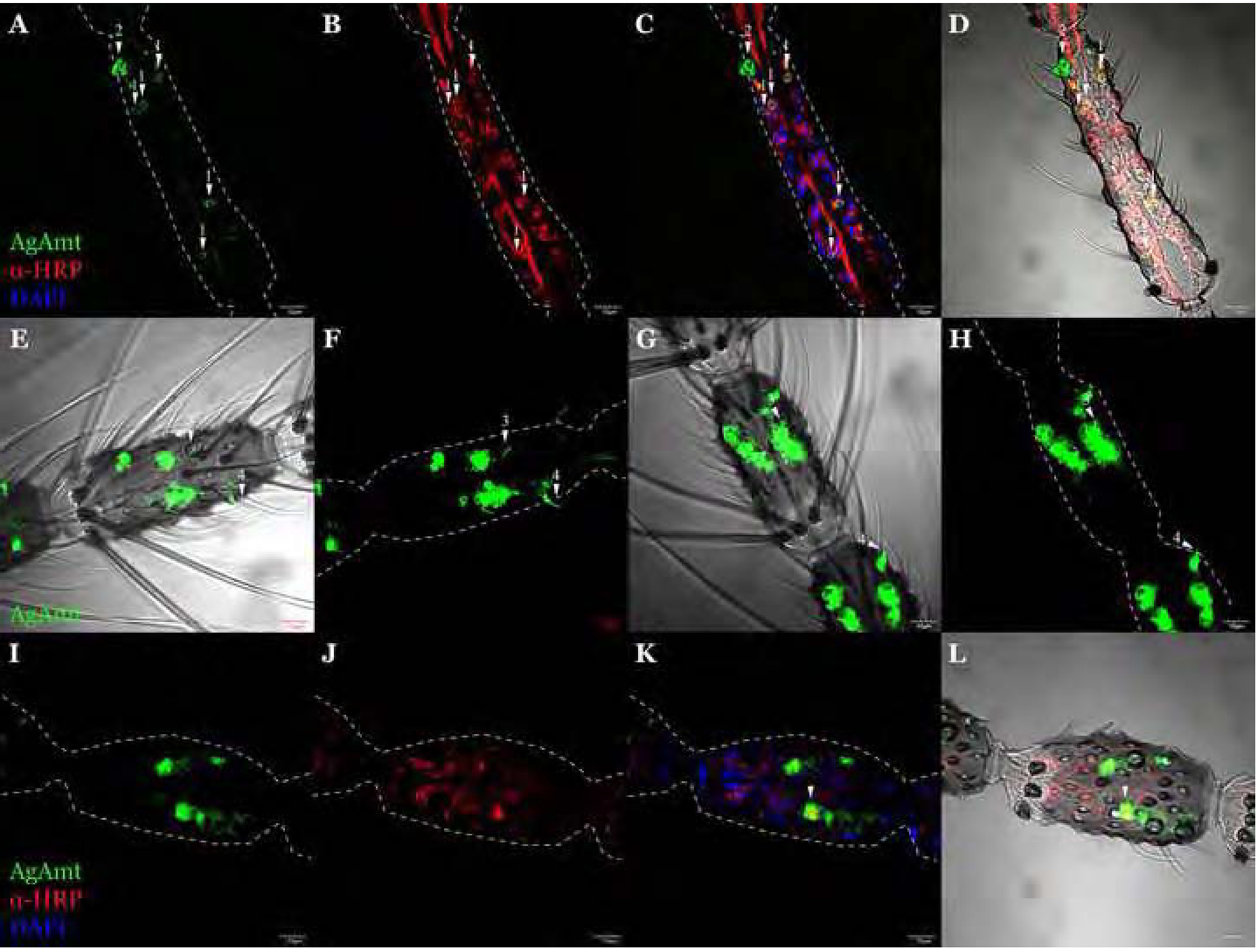
The confocal optical section of immunohistochemistry staining on the female antennae using α-HRP (red) showing AgAmtP-driven GFP (green) is expressed in both neuronal (arrow 1) and non-neuronal cells (arrow 2) (A-D). Z-axis projection of the whole-mount antennal female An. gambiae 6th flagellomere showing AgAmt dendritic labelling in coeloconic sensilla (arrow 3) and grooved pegs (arrow 4) of 1kb AgAmtPGFP progeny (E, F) and 3kb AgAmtP-GFP progeny (G, H). The confocal optical section showing a partial overlapping between GFP and α-HRP labeled cells (I-L). Scale bars = 10μm.
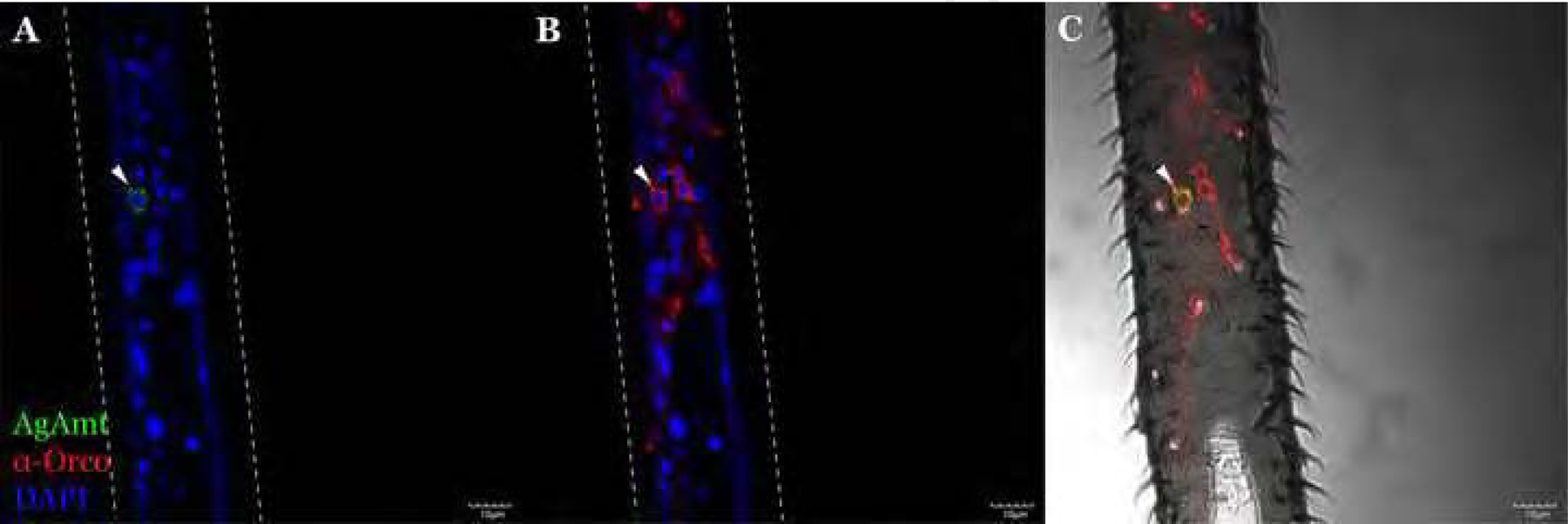
Immunohistochemistry and fluorescence in situ hybridization (FISH) were used to further investigate the relationship between AgAmt and discrete sets of chemosensory receptors to discriminate subclasses of chemosensory neurons. In these studies, polyclonal antibodies to the Anopheline orthologs of the OR co-receptor (AgOrco) and riboprobes to the IR co-receptor (AgIr76b) allowed us to label antennal odorant receptor neurons (ORNs) (Pitts et al., 2004) and ionotropic receptor neurons (IRNs) independently (Pitts et al., 2017). We elected to focus on AgIr76b as its homolog in Drosophila is expressed in the same neurons as the ammonia receptor, DmIr92a (Benton et al., 2009). These studies revealed neither co-localization nor a close association between AgAmt- and Orco-expressing cells (Figure 4A–4D), while AgIr76b antisense riboprobes shows a close association between Ir76b-expressing IRNs and potentially AgAmt-expressing auxiliary cells (Figure 5A, 5B). Taken together, these data suggest that AgAmt is likely to be expressed in auxiliary cells surrounding IR-expressing IRNs. As a negative control, AgIr76b sense riboprobes failed to label any cells or structures other than the antennal cuticle (Figure 5C, 5D). Surprisingly, in contrast to the labelling studies of the antennae, AgAmtP-driven GFP signals were co-localized with a discrete subset of Orco-expressing ORNs in the female maxillary palps where only a very small portion of ORNs (approximately less than 5%) were likely to be AgAmt-positive (Figure 6A–6C).
Figure 4.

A representative confocal optical section from adult An. gambiae female antennae immunohistochemically stained using α-Orco antibodies (red) showing that AgAmtP-driven GFP (green) and Orco are expressed in distinct cells (A-D). Scale bars = 10μm.
Figure 5.
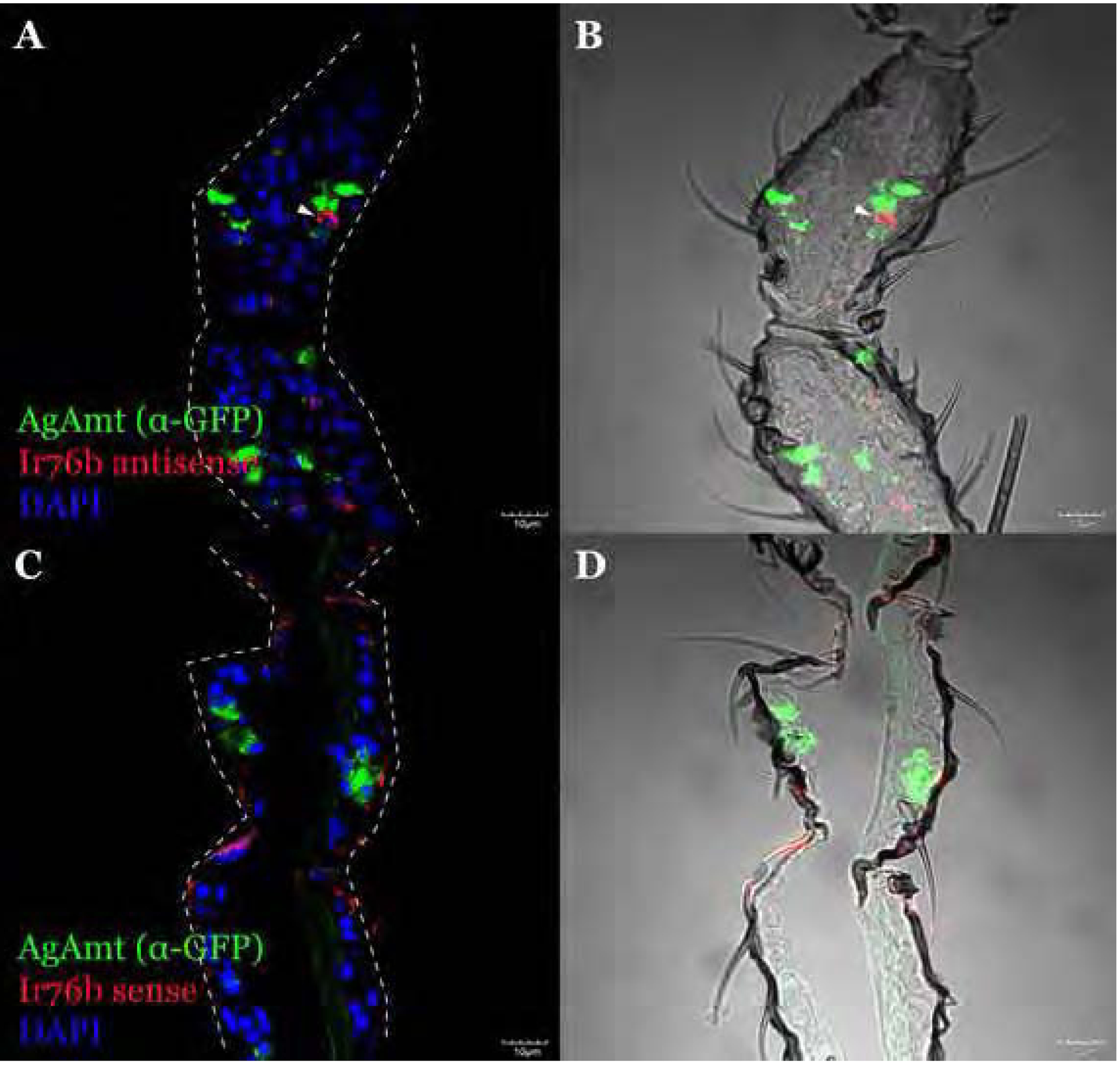
A representative confocal optical section of FISH on the female antennae using Ir76b antisense riboprobe (red) and α-GFP showing AgAmtP-driven GFP (green) is expressed in auxiliary cells closely associated with Ir76b-expressing neurons (A, B). Ir76b sense riboprobe was used as the negative control for riboprobe specificity (C, D). An Ir76b-expressing cell is highlighted by arrows. Scale bars = 10μm.
Figure 6.
A representative confocal optical section of adult female An. gambiae maxillary palps immunohistochemically stained with α-Orco antibodies (red) showing co-localization of AgAmtP-driven GFP (green) and Orco. An AgAmtP-derived GFP and Orco co-expressing cell is highlighted by arrows. Scale bars = 10μm.
3.4. Olfactory responses of capitate pegs and coeloconic sensilla to ammonia
The localization of AgAmtP-driven GFP in subpopulations of coeloconic sensilla on the antennae and maxillary palp capitate pegs begs the question as to ammonia response profiles of those structures. Single sensillum recordings (SSRs) were carried out to investigate dose-dependent responses of these sensilla to ammonia at 6 different concentrations (0.01%−5%), with a specific focus on the 5th-8th antennal flagellomeres where consistent AgAmtP-derived GFP expression in coeloconic sensilla was observed. SSR studies identified significant and dose-dependent ammonia responses in all tested antennal coeloconic sensilla (n=14) (Figure 7A, 7B). In maxillary palp capitate pegs, the cpB and cpC neurons typically display lower spike amplitudes than cpA neurons (Lu et al., 2007), and were therefore counted together due to the technical difficulty of separation. Dose-dependent excitatory responses to ammonia were identified in only cpB/C neurons; in contrast to either no response or slightly inhibitory responses displayed in cpA neurons (n=6) (Lu et al., 2007) (Figure 7C, 7D).
Figure 7. (A).

Single sensillum recording signals from a coeloconic sensillum to different concentrations of ammonia and the solvent. The vertical bar denotes the amplitude and the horizontal bar denotes the 0.5s duration of chemical stimulation; (B) Mean dose-dependent single sensillum responding patterns of coeloconic sensilla to six concentrations of ammonia; (C) Single sensillum recording signals from a capitate peg to different concentrations of ammonia and the solvent. The vertical bar denotes the amplitude and the horizontal bar denotes the 0.5s duration of chemical stimulation; (D) Mean dose-dependent single sensillum responding patterns of cpA neurons and cpB/C neurons in capitate pegs to six concentrations of ammonia. Error bars = Standard error of the mean.
3.5. Neuronal AgAmt expression in gustatory appendages
While the labella and tarsi are considered to be the primary gustatory appendages of the mosquito, previous studies have revealed robust olfactory responses in the labial sensilla of An. gambiae (Kwon et al., 2006; Saveer et al., 2018). To investigate the potential role of AgAmt in these appendages, cryosections were examined using immunohistochemistry and FISH. To begin with, GFP-labelled cells were counted and compared in labella between the 1kb AgAmtP-GFP and the 3kb AgAmtP-GFP lines (Supplementary data; Table S4). Analysis using unpaired t-tests suggests there is no significant difference in the number of GFP-labelled cells between these lines (Supplementary data; Figure S3; p-value = 0.874). Z-axis projections of labellum whole-mounts showed intensive AgAmtP-driven GFP expression that was relatively uniform throughout the entire appendage where AgAmtP-derived GFP expressing neurons were found across the gustatory T1 sensilla (Figure 8A–8D) (Saveer et al., 2018), a pattern that is markedly different from the antennae. Furthermore, α-HRP-Cy3 (Figure 9A–9D), α-Orco immunostaining (Figure 9E–9H) and AgIr76b FISH (Figure 10), revealed that AgAmtP-derived GFP expression in the labellum is strictly neuronal, distributed across distinct populations of AgOrco-expressing ORNs and AgIr76b-expressing IRNs.
Figure 8.

Z-axis projection of whole-mount adult female An. gambiae labellum carrying QUAS-GFP and either 1kb AgAmtP-QF2 (A-B) or 3kb AgAmtP-QF2 (C-D) constructs showing specific expression of GFP. Dendritic labelling of AgAmt in T1 sensilla is highlighted by arrows. Scale bars = 10μm.
Figure 9.

A representative confocal optical section of adult female labellum immunohistochemically stained with α-HRP (A-D; red) and α-Orco (E-H; red) showing AgAmtP-driven GFP (green) is expressed in neurons and partially in ORNs. AgAmtP-derived GFP and Orco co-expressing cells are highlighted by arrows. Scale bars = 10μm.
Figure 10.

A representative confocal optical section of the adult female labellum stained for FISH using Ir76b antisense riboprobes (red) and α-GFP showing partial AgAmtP-derived GFP (green) expression in Ir76b-expressing neurons (A-D). Ir76b sense riboprobes were used as the negative control for riboprobe specificity (E-H). AgAmtP- derived GFP and Ir76b co-expressing cells are highlighted by arrows. Scale bars = 10μm.
In the D. melanogaster labellum, DmAmt is not involved in volatile or contact-based ammonia sensing but instead is expressed in sugar sensing Gr5a neurons (Delventhal et al., 2017). To investigate whether AgAmt has a similar expression pattern, we used FISH to localize AgGr15 which is the homolog to DmGr5a. In these studies, we found extensive co-localization between AgAmtP-driven GFP and AgGr15 (Figure 11A–11D; negative control with AgGr15 sense riboprobe: Figure 11E–11H). In the An. gambiae tarsi, AgAmtP-derived GFP expression was uniformly observed across all 5 tarsal segments of prothoracic, mesothoracic, and metathoracic tarsi where it co-localizes with α-HRP-Cy3, indicating once more that AgAmtP-derived GFP is expressed in neurons (Figure 12A–12C). These likely AgAmt-expressing cells are localized to the distal half of each tarsal segment in close proximity to the joints between segments where a cluster of neurons are localized.
Figure 11.
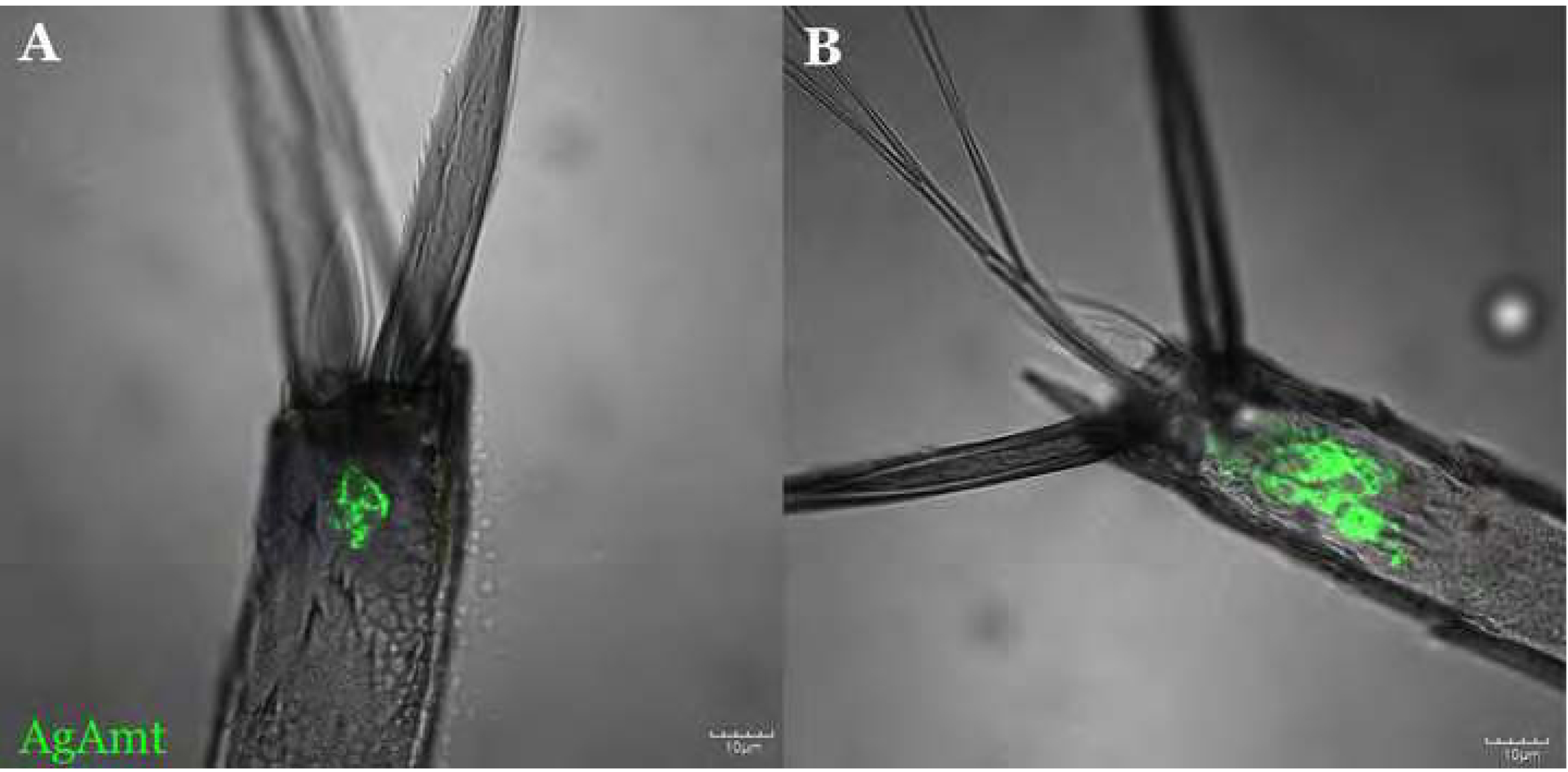
A representative confocal optical section of the adult female labellum stained for FISH using Gr15 antisense riboprobes (red) and α-GFP (green) showing partial AgAmtP-derived GFP expression in the Gr15-expressing neurons (A-D). Gr15 sense riboprobes were used as the negative control for riboprobe specificity (E-H). AgAmtP-driven GFP and Gr15 co-expressing cells are highlighted by arrows. Scale bars = 10μm.
Figure 12.

A representative confocal optical section of adult female tarsi immunohistochemically stained with α-HRP (red) showing AgAmtP-GFP (green) is expressed in neurons in all five segments (1st-5th labelled on images) of protarsi (A), mesotarsi (B), and metatarsi (C). Scale bars = 10μm.
3.6. Larval AgAmt expression
Larval progeny from both the 1kb and 3kb AgAmtP-QF2 driver lines house a cluster of GFP+ potentially AgAmt-expressing cells proximal to the sensory cone (Figure 13), which is the major olfactory structure on the multi-articulated larval antennae (Xia et al., 2008). Interestingly, significantly fewer cells were labelled in the 1kb AgAmtP-GFP progeny (Figure 13A) as compared to the 3kb progeny (Figure 13B), suggesting the presence of larval specific transcriptional control elements between 1kb and 3kb upstream of the AgAmt translational start site. As such, sequential experiments were conducted using the 3kb AgAmtP-GFP progeny. Consistent with the adult female antennae, whole-mount immunostaining with α-HRP-Cy3 revealed that AgAmtP-driven GFP is expressed in both neuronal and auxiliary cells in the larval antennae (Figure 14A–14D). The AgAmtP-driven GFP expressing auxiliary cells surround, or are proximate to, most if not all of the α-HRP labelled neurons, which is consistent with a previous description of larval auxiliary cells (Figure 14A–14D) (Zacharuk et al., 1971). As expected, α-Orco polyclonal antibodies marked ORNs comprise a subset of these larval neurons (Figure 14B, 14F) and a close association between ORNs and what are likely to be AgAmt-expressing larval auxiliary cells was observed (Figure 14E–14H).
Figure 13.

A representative confocal optical section of whole-mount An. gambiae larval antennae carrying either 1kb (A) or 3kb (B) AgAmtP-QF2 and QUAS-GFP showing AgAmtP-GFP (green) expression closely associated with the antennal sensory cone. Scale bars = 10μm.
Figure 14.
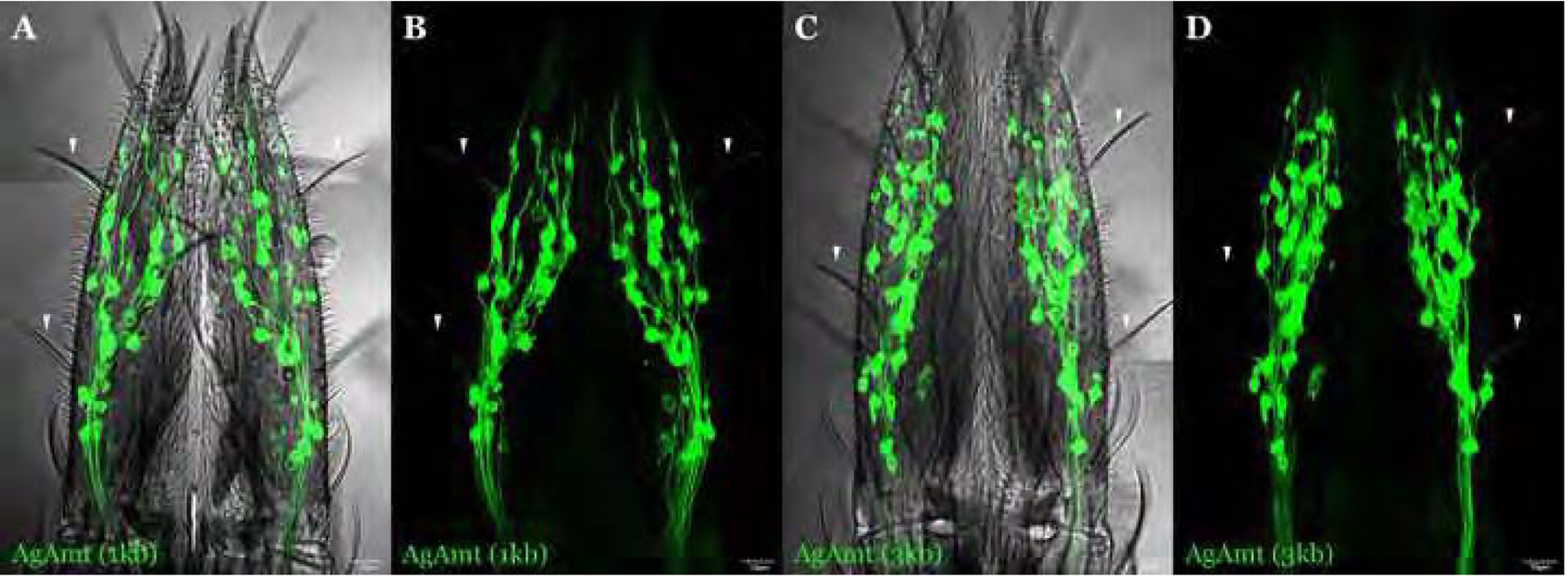
A representative confocal optical section of whole-mount An. gambiae larval antennae from 3kb AgAmtP-GFP (green) progeny immunohistochemically stained with α-HRP (A-D; red) and α-Orco (E-H; red(showing AgAmtP-driven GFP expression in both neuronal cells and auxiliary cells which envelope the neurons. An AgAmtP-GFP-expressing neuron is highlighted by arrows. Scale bars = 10μm.
4. Discussion
4.1. Heterogeneous localization of AgAmt in Olfactory and Gustatory Appendages
From a technical perspective, the generation of a set of AgAmtP-QF2 driver lines involved the development of a high-efficiency adaptation that brings together the phiC31 integration (Meredith et al., 2011) and Q system (Riabinina et al., 2016) in the malaria vector An. gambiae which until recently has been largely refractory to such genetic manipulations. We have utilized the phiC31 site-specific integration system to generate a Q system driver line thereby avoiding positional effects and potential fitness costs introduced by random-insertion transposon systems (Labbé et al., 2010). Additionally, the large integration capacity of the phiC31 system ensures the practicability of generating driver lines with larger transcriptional regulatory elements (Nimmo et al., 2006). Indeed, the relatively high integration efficiency of driver constructs we observed in An. gambiae is likely due to the use of the phiC31 helper plasmid as the source of integrase, as the plasmid is likely to be more stable and therefore better able to express integrase activity than co-injected mRNA (Gratz et al., 2014; Kistler et al., 2015). In this manner, we have developed a technical workflow where the driver line, and potentially, a modified effector line can be rapidly generated in An. gambiae. These substantial improvements in utility result in a highly efficient Q system that represents a powerful tool for the genetic characterization and manipulation of An. gambiae target genes in future studies.
In order to further characterize the role of AgAmt insofar as ammonia sensitivity and metabolic processes that are salient for host-seeking and other mosquito behaviors, we examined the spatial localization in the chemosensory appendages of the adult and larval peripheral nervous system of An. gambiae. Previous studies examining the localization of DmAmt, the Amt homolog in the chemosensory appendages of D. melanogaster, revealed an unexpected heterogeneity in which DmAmt is found in nonneuronal antennal auxiliary cells whereas in the labella and tarsi, DmAmt is neuronal (Delventhal et al., 2017; Menuz et al., 2014). Importantly, these Drosophila studies also revealed DmAmt’s functional heterogeneity; in which antennal expression is required for olfactory responses to ammonia, while null mutants which showed no labellum-expression also displayed wild-type gustatory responses to ammonium solutions (Delventhal et al., 2017; Menuz et al., 2014).
While acknowledging the reliance on AgAmtP-driven GFP signals, our localization studies in female An. gambiae antennae build upon the theme of heterogeneity by demonstrating that AgAmtP-derived GFP is widely expressed in both neuronal and non-neuronal cells (Figures 2–5). More specifically, and in contrast to DmAmt which is expressed only in a narrow subtype of antennal coeloconic sensilla (ac1) (Menuz et al., 2014), AgAmtP-driven GFP is expressed in the auxiliary cells in all coeloconic sensilla as well as what appears to be the majority of grooved pegs. Even more complexity is evident as neuronal AgAmtP-derived GFP expression seems to be localized to non-OSNs as these cells are not labelled by either α-Orco antibodies or Ir76b riboprobes. As is the case in Drosophila antennae (Menuz et al., 2014), we did not observe AgAmtP-derived GFP expression in any trichoid sensilla despite studies demonstrating ammonia sensitivity across these sensilla (Qiu et al., 2006). Therefore the significant diversity we observed in AgAmtP-driven GFP expression presumably correlates with heterogenous function along the antennae of An. gambiae.
The chemosensory ultrastructure of the An. gambiae maxillary palps has long been thought to contain a single, homogeneous class of olfactory sensilla, the capitate pegs, in which 3 distinct neurons are present: two ORNs and one CO2 sensing gustatory receptor neuron (GRN) (Lu et al., 2007). However, our expression data challenges this model as in contrast to the antennae, where AgAmt appears to be expressed in auxiliary cells and non-ORN neurons, AgAmt-driven GFP is expressed only in a subset of the maxillary palp ORNs. This suggests there is a previously undescribed heterogeneity among the maxillary palp capitate peg ORNs of An. gambiae that may impact functionality and sensitivity to ammonia and perhaps other stimuli on this important chemosensory appendage.
In the proboscis and tarsi that encompass the adult gustatory appendages, the complete spectrum of labial chemosensory neurons (ORNs, IRNs, and GRNs) appear to express AgAmt. Moreover, all segments in the tarsi display AgAmtP-driven GFP expression compared to only up to 4 DmAmt+ segments in Drosophila (Delventhal et al., 2017). Consistent with studies showing that DmAmt is expressed in the sugar sensing DmGr5a neurons instead of the ammonium sensing DmGr66a neurons (Delventhal et al., 2017), AgAmtP-driven GFP is expressed in the putative mosquito sugar sensing AgGr15 neurons in the labellum. Inasmuch as mosquito tarsi have not been shown to display ammonia sensitivity, these data raise the possibility that the function of neuronal AgAmt is fundamentally different from the auxiliary cell AgAmt and indeed may not be directly involved in environmental ammonium sensing.
The An. gambiae larval antennae is a single tubular appendage in which the primary olfactory structure, the sensory cone, is located at the distal tip and is innervated by a group of subtended chemosensory neurons that are enveloped by auxiliary cells (Xia et al., 2008; Zacharuk et al., 1971). Although studies have revealed the potential olfactory function of the sensory cone (Xia et al., 2008), no electrophysiological studies have thus far been conducted to characterize the chemosensory responses of neurons in the larval antennae (Liu et al., 2010; Xia et al., 2008). Given that AgAmtP-driven GFP is localized in auxiliary cells surrounding a group of α-HRP labelled neurons among which ORNs only compose a subset, our data suggests that in larvae, AgAmt functionality is associated with other chemosensory neurons besides ORNs. Therefore, given the strong correlation between Ag/DmAmt expression and ammonia sensitivity that we and others have demonstrated (Delventhal et al., 2017; Menuz et al., 2014), it is reasonable to speculate that other structures/chemosensory neurons on the larval antennae, along with the ORN-innervated sensory cone (Xia et al., 2008), are involved in chemosensory processing of ammonia-based stimuli (Zacharuk and Blue, 1971).
In addition to the expression of AgOrco and AgORs that define ORNs, AgIR expression has been reported in larval IRNs and shown to mediate larvae behaviors (Liu et al., 2010). In light of previous studies in the larval antennae where ORNs seem to be exclusively present in the sensory cone (Xia et al., 2008) and in the adult chemosensory system that suggest IRNs and ORNs are distinct cell populations (Pitts et al., 2017) it is reasonable to speculate that larval AgIRs are expressed in chemosensory appendages other than the ORN-rich sensory cone that may correlate to larval ammonia sensitivity.
4.2. A Model for Neuronal Amt function
While it has been suggested that DmAmt expression in Drosophila antennal auxiliary cells is involved in ammonium clearance from the sensillar lymph to ameliorate receptor desensitization (Menuz et al., 2014), the function of neuronal DmAmt on the labellum remains cryptic (Delventhal et al., 2017). In contrast, apart from our observation of non-neuronal AgAmtP-driven GFP expression in antennal coeloconic and basiconic auxillary cells, AgAmt is largely neuronal across all the chemosensory appendages of An. gambiae. This begs the general question as to the function of neuronal ammonium transporters in the malaria mosquito.
It is reasonable to suggest that insect chemosensory neurons must transport ammonium for a variety of metabolic and/or neurobiological purposes that could be addressed by bidirectional Amt transporters (Durant and Donini, 2018; Soupene et al., 2002). Indeed, in honeybees it has been observed that ammonium is produced in the conversion of glutamine to glutamate in glutamatergic neurons and is released from photoreceptor neurons and transported to glial cells (Marcaggi and Coles, 2001; Tsacopoulos et al., 1997), where neuronal expressing Amt may be involved. While the ammonium excretion from neurons remains largely uncharacterized, it was suggested that the Na+-K+−2Cl− co-transporter plays a role in transporting ammonia into the honeybee glial cells by substituting the K+ with ammonium (Bak et al., 2006; Marcaggi and Coles, 2001). However, this co-transporter has a moderate affinity for ammonium and is therefore less efficient in its transport (Bakouh et al., 2006). It is therefore possible that Amt is acting as a potential supporting element in this process. While glutamatergic interneurons have been characterized in the antennal lobes of Drosophila, no glutamatergic OSNs have been described in other insects. In addition to glutamate, gamma-aminobutyric acid (GABA) has also been shown to serve as an inhibitory neurotransmitter in insects (Wilson and Laurent, 2005). The GABAergic neurons requires a similar ammonia clearance system as the glutamatergic neurons where Amt can also be functional (Bak et al., 2006). While many studies suggest a large portion of glutamatergic/GABAergic cells act as local interneurons (LNs) concentrated in the insect antennal lobes, antennal GABAergic neurons have also been identified in the sphinx moth (Hoskins et al., 1986). Additionally, it is possible that neuronal Amt plays a role in the metabolism of biogenic amines which modulate neuronal activity (Zhukovskaya and Polyanovsky, 2017). Moreover, serotoninergic neurons were found in olfactory and gustatory appendages in mosquitoes (Siju et al., 2008; Zhukovskaya and Polyanovsky, 2017), where the uptake/excretion of ammonia could be essential for the regulation of serotonin synthesis (Coleman and Neckameyer, 2005; Grippon et al., 1986). While the function of DmAmt in gustatory appendages remains unknown, the high labial expression level of Amt in Drosophila, Anopheles, and Aedes suggests that a significant role of ammonium transporters in gustation or in labial-based olfaction that has been shown to occur in An. gambiae (Kwon et al., 2006; Matthews et al., 2016; Menuz et al., 2014; Pitts et al., 2014; Saveer et al., 2018).
4.3. IRs are implicated in ammonia detection
While Anopheline ORs are widely expressed on antennae and have been shown to recognize diverse combinations of chemical stimuli, they are not expressed in the ammonia-sensitive grooved pegs (Carey et al., 2010; Pitts et al., 2004; Wang et al., 2010). The characterization of Drosophila IRs as amine and acid receptors revealed DmIr92a is co-expressed with DmIr76b and DmIr25a co-receptors that together are responsible for olfactory responses to ammonia in ac1 coeloconic sensilla (Ai et al., 2010; Benton et al., 2009; Min et al., 2013). Recently, several similar amine and acid-sensitive An. gambiae IRs have been characterized (Pitts et al., 2017) although in the absence of a clear DmIr92a homolog, the Anopheline ammonia receptor has not yet been identified.
We have found a strong association between AgAmt and ammonia sensing neurons. In addition to the grooved pegs as previously demonstrated (Qiu et al., 2006), all of the putative AgAmt-expressing sensilla on the antennae and maxillary palps showed neuronal responses to ammonia. Moreover, the localization of antennal AgAmtP-driven GFP is highly correlated with AgIr76b, which suggests that, in keeping with the data from Drosophila (Menuz et al., 2014), mosquito ammonia receptors are likely to be IRs. In light of the high correlation between the response spectrum of DmIRs and AgIRs and the odor response profiles of An. gambiae grooved pegs (Benton et al., 2009; Pitts et al., 2017; Qiu et al., 2006), which are orthologous to Drosophila coeloconic sensilla (Ray, 2015), it is reasonable to suggest these sensilla are innervated by IRNs instead of ORNs, and that AgIRs are ammonia sensing receptors in Anopheline grooved pegs.
It is noteworthy that we did not observe AgAmtP-driven GFP expression in the trichoid sensilla, a subpopulation of which has been shown to house ammonia sensitive neurons (Qiu et al., 2006). This suggests there are AgAmt-independent ammonia sensing pathways in An. gambiae. Furthermore, inasmuch as An. gambiae trichoid sensilla express Orco and presumably a range of tuning ORs, it is likely this alternative pathway is OR-mediated and perhaps involves the antennal-expressed Rh50 transporter (Pitts et al., 2014).
Supplementary Material
Highlights.
Comprehensive cellular localization of AgAmt in primary and accessory chemosensory appendages of mosquitoes using transgenic Q system;
Heterogeneous expression pattern of AgAmt in both neuronal and non-neuronal cells;
Significant ammonia sensitivity in AgAmt-expressing sensilla.
Acknowledgements
We thank Zhen Li for mosquito rearing, Dr. Ahmed Saveer (North Carolina State University) and Dr. Zhiwei Zhang (Shanxi Agricultural University) for technical training, and all members of the Zwiebel lab for valuable advice and comments throughout the course of this work. We also thank Dr. Julian Hillyer, Dr. Maulik Patel, Dr. Wenbiao Chen, and Dr. Patrick Abbot (Vanderbilt University) for critical suggestions. We are especially grateful for the generous gift of expertise and Q system mosquito lines from Dr. Christopher Potter (the Johns Hopkins University School of Medicine). Lastly, we acknowledge The Vanderbilt University Cell Imaging Shared Resource Core for training and use of the Olympus FV-1000 confocal microscope and Dr. James Patton (Vanderbilt University) for cryostat access. This work was conducted with the support of Vanderbilt University and funded by the National Institutes of Health (NIAID, R21-113960) to LJZ.
Abbreviations
- AgAmt
Anopheles gambiae ammonium transporter
- DmAmt
Drosophila melanogaster ammonium transporter
- AgAmtP
AgAmt promoter
- IR
ionotropic receptor
- OR
odorant receptor
- GR
gustatory receptor
- ORN
odorant receptor neuron
- IRN
ionotropic receptor neuron
- OSN
olfactory sensory neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GSB, 2010. Acid sensing by the Drosophila olfactory system. Nature 468, 691–695. 10.1038/nature09537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso P, Noor AM, 2017. The global fight against malaria is at crossroads. Lancet 390, 2532–2534. 10.1016/S0140-6736(17)33080-5 [DOI] [PubMed] [Google Scholar]
- Andrade SLA, Einsle O, 2007. The Amt/Mep/Rh family of ammonium transport proteins (Review). Mol. Membr. Biol 24, 357–365. 10.1080/09687680701388423 [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS, 2006. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem 98, 641–653. 10.1111/j.1471-4159.2006.03913.x [DOI] [PubMed] [Google Scholar]
- Bakouh N, Benjelloun F, Cherif-Zahar B, Planelles G, 2006. The challengeof understanding ammonium homeostasis and the role of the Rh glycoproteins. Transfus. Clin. Biol 13, 139–146. 10.1016/j.tracli.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB, 2009. Variant Ionotropic Glutamate Receptors as Chemosensory Receptors in Drosophila. Cell 136, 149–162. 10.1016/j.cell.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittsánszky A, Pilinszky K, Gyulai G, Komives T, 2015. Overcoming ammonium toxicity. Plant Sci. 231, 184–190. 10.1016/j.plantsci.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Wiescinski CM, Botham P, Lees D, Debruyne E, Repetto-Larsay M, Ladics G, Hoban D, Gamer A, Remmele M, Wang-Fan W, Ullmann LG, Mehta J, Billington R, Woolhiser MR, 2008. Interlaboratory validation of 1% pluronic L92 surfactant as a suitable, aqueous vehicle for testing pesticide formulations using the murine local lymph node assay. Toxicol. Sci 105, 79–85. 10.1093/toxsci/kfn117 [DOI] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR, 2010. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71. 10.1038/nature08834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis H, Ionescu A, Misyura L, Bui P, Fazio K, Wang J, Patrick M, Weihrauch D, Donini A, 2016. An animal homolog of plant Mep/Amt transporters promotes ammonia excretion by the anal papillae of the disease vector mosquito Aedes aegypti. J. Exp. Biol 219, 1346–1355. 10.1242/jeb.134494 [DOI] [PubMed] [Google Scholar]
- Coleman CM, Neckameyer WS, 2005. Serotonin synthesis by two distinct enzymes in Drosophila melanogaster. Arch. Insect Biochem. Physiol 59, 12–31. 10.1002/arch.20050 [DOI] [PubMed] [Google Scholar]
- Cox FE, 2010. History of the discovery of the malaria parasites and their vectors. Parasites and Vectors 3. 10.1186/1756-3305-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Forde BG, 2002. Molecular and Developmental Biology of Inorganic Nitrogen Nutrition. Arab. B 1, e0011 10.1199/tab.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delventhal R, Menuz K, Joseph R, Park J, Sun JS, Carlson JR, 2017. The taste response to ammonia in Drosophila. Sci. Rep. 7 10.1038/srep43754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Otter CJ, Behan M, Maes FW, 1980. Single cell responses in female Pieris brassicae (Lepidoptera: Pieridae) to plant volatiles and conspecific egg odours. J. Insect Physiol 26, 465–472. 10.1016/0022-1910(80)90117-1 [DOI] [Google Scholar]
- Durant AC, Donini A, 2018. Ammonia excretion in an osmoregulatory syncytium is facilitated by AeAmt2, a novel ammonia transporter in Aedes aegypti larvae. Front. Physiol 9 10.3389/fphys.2018.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaninia M, Ignell R, Hansson BS, 2007. Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti. Eur. J. Neurosci 26, 1611–1623. 10.1111/j.1460-9568.2007.05786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, Oconnor-Giles KM, 2014. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961–971. 10.1534/genetics.113.160713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippon P, le Poncin Lafitte M, Boschat M, Wang S, Faure G, Dutertre D, Opolon P, 1986. Evidence for the role of ammonia in the intracerebral transfer and metabolism of tryptophan. Hepatology 6, 682–686. 10.1002/hep.1840060424 [DOI] [PubMed] [Google Scholar]
- Guidobaldi F, May-Concha IJ, Guerenstein PG, 2014. Morphology and physiology of the olfactory system of blood-feeding insects. J. Physiol. Paris 108, 96–111. 10.1016/j.jphysparis.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Hoskins SG, Homberg U, Kingan TG, Christensen TA, Hildebrand JG, 1986. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res. 244, 243–252. 10.1007/BF00219199 [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN, 1982. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl. Acad. Sci. U. S. A 79, 2700–2704. 10.1073/pnas.79.8.2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler KE, Vosshall LB, Matthews BJ, 2015. Genome engineering with CRISPR Cas9 in the mosquito Aedes aegypti. Cell Rep. 11, 51–60. 10.1016/j.celrep.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HW, Lu T, Rützler M, Zwiebel LJ, 2006. Olfactory response in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A 103, 13526–13531. 10.1073/pnas.0601107103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé GMC, Nimmo DD, Alphey L, 2010. Piggybac- and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse). PLoS Negl. Trop. Dis 4 10.1371/journal.pntd.0000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal WS, 2013. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol 58, 373–391. 10.1146/annurev-ento-120811-153635 [DOI] [PubMed] [Google Scholar]
- Liu C, Pitts RJ, Bohbot JD, Jones PL, Wang G, Zwiebel LJ, 2010. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 8, 27–28. 10.1371/journal.pbio.1000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chen L, Appel AG, Liu N, 2013. Olfactory responses of the antennal trichoid sensilla to chemical repellents in the mosquito, Culex quinquefasciatus. J. Insect Physiol 59, 1169–1177. 10.1016/j.jinsphys.2013.08.016 [DOI] [PubMed] [Google Scholar]
- Loesel R, Weigel S, Bräunig P, 2006. A simple fluorescent double staining method for distinguishing neuronal from non-neuronal cells in the insect central nervous system. J. Neurosci. Methods 155, 202–206. 10.1016/j.jneumeth.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJA, Takken W, Carlson JR, Zwiebel LJ, 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol 17, 1533–1544. 10.1016/j.cub.2007.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Coles JA, 2001. Ammonium in nervous tissue: Transport across cell membranes, fluxes from neurons to glial cells, and role in signalling. Prog. Neurobiol 64, 157–183. 10.1016/S0301-0082(00)00043-5 [DOI] [PubMed] [Google Scholar]
- Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB, 2016. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics 17 10.1186/s12864-015-2239-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SB, 1973. Fine structure of antennal sensilla coeloconica of culicine mosquitoes. Tissue Cell 5, 105–112. 10.1016/S0040-8166(73)80009-6 [DOI] [PubMed] [Google Scholar]
- Mclver SB, 1982. Sensilla of mosquitoes (Diptera: Culicidae)1, 2. J. Med. Entomol 19, 489–535. 10.1093/jmedent/19.5.489 [DOI] [PubMed] [Google Scholar]
- Meijerink J, Braks MAH, Van Loon JJA, 2001. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J. Insect Physiol 47, 455–464. 10.1016/S0022-1910(00)00136-0 [DOI] [PubMed] [Google Scholar]
- Menuz K, Larter NK, Park J, Carlson JR, 2014. An RNA-Seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 10 10.1371/journal.pgen.1004810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JM, Basu S, Nimmo DD, Larget-Thiery I, Warr EL, Underhill A, McArthur CC, Carter V, Hurd H, Bourgouin C, Eggleston P, 2011. Site-specific integration and expression of an anti-malarial gene in transgenic Anopheles gambiae significantly reduces Plasmodium infections. PLoS One 6 10.1371/journal.pone.0014587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Ai M, Shin SA, Suh GSB, 2013. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc. Natl. Acad. Sci. U. S. A 110, 1321–1329. 10.1073/pnas.1215680110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Zilversmit MM, Neafsey DE, Hartl DL, Barillas-Mury C, 2016. Mosquito vectors and the globalization of Plasmodium falciparum malaria. Annu. Rev. Genet 50, 447–465. 10.1146/annurev-genet-120215-035211 [DOI] [PubMed] [Google Scholar]
- Montell C, Zwiebel LJ, 2016. Mosquito sensory systems. Adv. In Insect Phys 51, 293–328. 10.1016/bs.aiip.2016.04.007 [DOI] [Google Scholar]
- Mukabana WR, Mweresa CK, Otieno B, Omusula P, Smallegange RC, van Loon JJA, Takken W, 2012. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J. Chem. Ecol 38, 235–244. 10.1007/s10886-012-0088-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo DD, Alphey L, Meredith JM, Eggleston P, 2006. High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol. Biol 15, 129–136. 10.1111/j.1365-2583.2006.00615.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Derryberry SL, Pulous FE, Zwiebel LJ, 2014. Antennal-expressed ammonium transporters in the malaria vector mosquito Anopheles gambiae. PLoS One 9 10.1371/journal.pone.0111858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Derryberry SL, Zhang Z, Zwiebel LJ, 2017. Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxylic acids. Sci. Rep 7 10.1038/srep40297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, Zwiebeil LJ, 2004. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A 101, 5058–5063. 10.1073/pnas.0308146101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Zwiebel LJ, 2006. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar. J 5 10.1186/1475-2875-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondeville E, Puchot N, Meredith JM, Lynd A, Vernick KD, Lycett GJ, Eggleston P, Bourgouin C, 2014. Efficient φc31 integrase-mediated site-specific germline transformation of Anopheles gambiae. Nat. Protoc 9, 1698–1712. 10.1038/nprot.2014.117 [DOI] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L, 2010. The Q system: A repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141, 536–548. 10.1016/j.cell.2010.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT, van Loon JJA, Takken W, Meijerink J, Smid HM, 2006. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem. Senses 31, 845–863. 10.1093/chemse/bjl027 [DOI] [PubMed] [Google Scholar]
- Ray A, 2015. Reception of odors and repellents in mosquitoes. Curr. Opin. Neurobiol 34, 158–164. 10.1016/j.conb.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Report WM, 1980. World. Chem. Eng. News 58, 28 10.1016/S0264-410X(07)01183-8 [DOI] [PubMed] [Google Scholar]
- Riabinina O, Task D, Marr E, Lin CC, Alford R, O’Brochta DA, Potter CJ, 2016. Organization of olfactory centres in the malaria mosquito Anopheles gambiae. Nat. Commun 7 10.1038/ncomms13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saveer AM, Pitts RJ, Ferguson ST, Zwiebel LJ, 2018. Characterization of chemosensory responses on the labellum of the malaria vector mosquito, Anopheles coluzzii. Sci. Rep 8 10.1038/s41598-018-23987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag SR, Müller B, Steinbrecht RA, 2000. Atlas of olfactory organs of Drosophila melanogaster 2. Internal organization and cellular architecture of olfactory sensilla. Arthropod Struct. Dev 29, 211–229. 10.1016/S1467-8039(00)00028-1 [DOI] [PubMed] [Google Scholar]
- Siju KP, Hansson BS, Ignell R, 2008. Immunocytochemical localization of serotonin in the central and peripheral chemosensory system of mosquitoes. Arthropod Struct. Dev 37, 248–259. 10.1016/j.asd.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Smallegange RC, Geier M, Takken W, 2002. Behavioural responses of Anopheles gambiae to ammonia, lactic acid and a fatty acid in a y-tube olfactometer. Proc. Exp. Appl. Entomol 13, 147–152. [Google Scholar]
- Smallegange RC, Qiu YT, van Loon JA, Takken W, 2005. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae). Chem. Senses 30, 145–152. 10.1093/chemse/bji010 [DOI] [PubMed] [Google Scholar]
- Soupene E, Lee H, Kustu S, 2002. Ammonium/methylammonium transport (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc. Natl. Acad. Sci. U. S. A 99, 3926–3931. 10.1073/pnas.062043799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh E, Bohbot JD, Zwiebel LJ, 2014. Peripheral olfactory signaling in insects. Curr. Opin. Insect Sci 6, 86–92. 10.1016/j.cois.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Fischbach GD, 1989. Glutamate receptor desensitization and its role in synaptic transmission. Neuron 3, 209–218. 10.1016/0896-6273(89)90034-2 [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry-Yamate CL, Poitry S, 1997. Ammonium and glutamate released by neurons are signals regulating the nutritive function of a glial cell. J. Neurosci 17, 2383–2390. 10.1523/jneurosci.17-07-02383.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Goes Van Naters W, Carlson JR, 2006. Insects as chemosensors of humans and crops. Nature 444, 302–307. 10.1038/nature05403 [DOI] [PubMed] [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ, 2010. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A 107, 4418–4423. 10.1073/pnas.0913392107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Laurent G, 2005. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci 25, 9069–9079. 10.1523/JNEUROSCI.2070-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, Zwiebel LJ, 2008. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl. Acad. Sci. U. S. A 105, 6433–6438. 10.1073/pnas.0801007105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharuk RY, Blue SG, 1971. Ultrastructure of a chordotonal and a sinusoidal peg organ in the antenna of larval Aedes aegypti (L.). Can. J. Zool 49, 1223–1230. 10.1139/z71-185 [DOI] [PubMed] [Google Scholar]
- Zacharuk RY, Yin LR, Blue SG, 1971. Fine structure of the antenna and its sensory cone in larvae of Aedes aegypti (L.). J. Morphol 135, 273–297. 10.1002/jmor.1051350303 [DOI] [PubMed] [Google Scholar]
- Zhukovskaya MI, Polyanovsky AD, 2017. Biogenic amines in insect antennae. Front. Syst. Neurosci 11 10.3389/fnsys.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiebel LJ, Takken W, 2004. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol 34, 645–652. 10.1016/j.ibmb.2004.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


