Abstract
Background
Mycobacterium tuberculosis resides inside host macrophages during infection and adapts to resilient stresses generated by the host immune system. As a response, M. tuberculosis codes for short-chain dehydrogenases/reductases (SDRs). These SDRs are nicotinamide adenine dinucleotide-reliant oxidoreductases involved in cell homeostasis. The precise function of oxidoreductases in bacteria especially M. tuberculosis were not fully explored. This study aimed to know the detail functional role of one of the oxidoreductase Rv0148 in M. tuberculosis.
Results
In silico analysis revealed that Rv0148 interacts with Htdy (Rv3389) and the protein interactions were confirmed using far western blot. Gene knockout mutant of Rv0148 in M. tuberculosis was constructed by specialized transduction. Macrophage cell line infection with this knockout mutant showed increased expression of pro-inflammatory cytokines. This knockout mutant is sensitive to oxidative, nitrogen, redox and electron transport inhibitor stress agents. Drug susceptibility testing of the deletion mutant showed resistance to first-line drugs such as streptomycin and ethambutol and second-line aminoglycosides such as amikacin and kanamycin. Based on interactorme analysis for Rv0148 using STRING database, we identified 220 most probable interacting partners for Htdy protein. In the Rv0148 knockout mutants, high expression of htdy was observed and we hypothesize that this would have perturbed the interactome thus resulting in drug resistance. Finally, we propose that Rv0148 and Htdy are functionally interconnected and involved in drug resistance and cell homeostasis of M. tuberculosis.
Conclusions
Our study suggests that Rv0148 plays a significant role in various functional aspects such as intermediatory metabolism, stress, homeostasis and also in drug resistance.
Keywords: Mycobacterium tuberculosis, Tuberculosis, Oxidoreductases, Rv0148, Htdy, Drug resistance, Stress response
Background
Tuberculosis (TB) remains one of the world’s leading cause of death every year among infectious diseases [1]. In 2018, WHO reported that 10.4 million new cases and 1.8 million deaths occur worldwide each year [2]. With the increase in multidrug and extensive drug-resistance TB, there is a need to develop newer diagnostics and vaccines [3]. It is well known that Mycobacterium tuberculosis spreads through aerosol, survives in oxygen and nutrition depleted environments and persists for long periods in the host cells [4–6]. The change in the host metabolism is thereby continuously monitored by M. tuberculosis to perform its active replication and persistence [7, 8]. M. tuberculosis during infection is exposed to various anti-bacterial agents secreted by macrophages. In addition, macrophages produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) [9, 10]. The ROS interact directly and the resulting super oxides are converted into various oxidants like hypochlorite (HClO), peroxides (H2O2), peroxynitrite (ONOO−) and hydroxy radicals, which damage the bacterial cells [11]. The bacterial cells resist stress using in-built mechanism. M. tuberculosis uses various defence mechanisms to damage the cell. OxyR and soxR are two prokaryotic regulators against peroxides and superoxides. Due to the absence of oxyR in M. tuberculosis, peroxidase stress is managed by alkylhydroperoxidase reductase (AhpC) by detoxifying the peroxides [12]. In addition, other peroxidases such as KatG, superoxide dismutase, peroxiredoxin (AhpE) and thioredoxin reductase (Tpx) help in reducing peroxidase activity and also in controlling the oxidative and nitrosative stress conditions [13–15]. Though the components involved in the oxidative stress of M. tuberculosis have been identified, the functional importance remains unclear. Gene interaction and knockout studies thoroughly predict the functional interconnection between the genes and their role in the metabolism of bacteria.
In this study, for the first time, we attempted to predict the functional role of one of the hypothetical oxidoreductase Rv0148 of M. tuberculosis. Rv0148 is a hypothetical protein/putative short-chain dehydrogenase/reductase (SDR) and exhibits 100% similarity to M. bovis and M. africanum and 87% to M. avium [16]. Rv0148 possesses the conserved SDR domain. As aminoglycosides bind to SDR sites, Rv0148 might neutralize the overexpression of aminoglycosides [17]. Earlier studies reported that over expression of M. tuberculosis Rv0148 from multidrug-resistance isolates in Escherichia coli showed two- to three-folds of higher shift in MIC [18]. Previous studies from our lab identified that PknI, one of the 11 serine-threonine protein kinases (STPKs), interacts with two proteins Rv2159c and Rv0148 [19]. Rv2159c was characterized using gene knockdown studies, and its interaction with PknI increased its peroxidase activity several folds in the mutant strain [20].
As an extension to the previous study we have chosen Rv0148. It was characterized through bioinformatics tools using sequence and protein interaction analyses. In sequence analysis using Pfam database, we found Rv0148 carrying well-conserved nicotinamide adenine dinucleotide (NAD) domain, whereas other homologues possessed MaoC domain along with SDR. Furthermore, by in silico approach we identified that Rv0148 mainly interacts with hydroxyl acyl thioester dehydratase Htdy, a protein interaction which was confirmed by far western blotting (WB) and pull-down assay. We have constructed the gene knock out mutant of Rv0148 (Δ0148) by specialized transduction to understand the functional role of the gene. In vivo studies confirmed that this mutant induces pro-inflammatory cytokines and is susceptible to oxidative and nitrogen stress compounds. Δ0148 confers drug resistance to streptomycin, ethambutol, amikacin and kanamycin. This might be due to modified functional network in the absence of Rv0148. Overall, the study suggested that Rv0148, though a non-essential gene, is functionally involved in drug resistance and is interconnected with other drug-resistance genes. The absence of Rv0148 displays its prominent role in host immunity.
Results
Sequence homologues of Rv0148
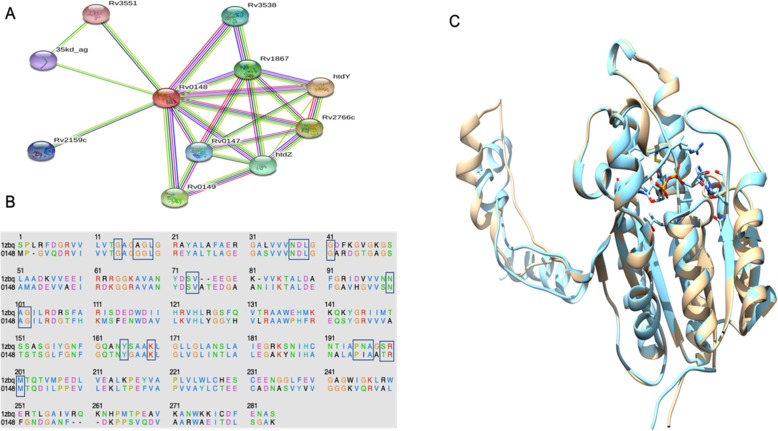
To identify the sequence homologues of Rv0148 in other organisms, blast search was performed against the non-reductant database in NCBI; 104 sequence homologues were selected and the sequences were reconfirmed based on short dehydrogenase domain in these sequences. Most of these sequences had the short dehydrogenase with a few sequences having both short dehydrogenase and MaoC dehydratase domain. A HMM profile based analysis (https://www.ebi.ac.uk/Tools/hmmer/search/hmmsearch) using the adh short domain profile from Pfam database identified 1270 significant sequences from Swissprot database (Table S1). Further, we searched the MaoC dehydratase sequence against mycobacterial genome and identified Htdy protein. Based on the analysis, we identified Rv0148 has only SDR. We searched for Rv0148 in STRING database and identified that Rv0148 interacts with Htdy protein (Fig. 1a). And this formed the basis for the subsequent analysis to predict the interaction of Rv0148 with Hdty using bioinformatics tools and then to validate the interaction using experimental methods.
Fig. 1.
a Protein-protein interaction from the STRING database for Rv0148 protein. b Sequence comparison of Rv0148 with a template sequence (1ZBQ). The black boxes are the mapping binding sites for Rv0148 based on the template sequence. c The structural superimposition between nicotinamide adenine dinucleotide (NAD)-docked RV0148 (brown) and template, 1ZBQ (cyan). The NAD is represented in stick form
Homology modelling of Rv0148
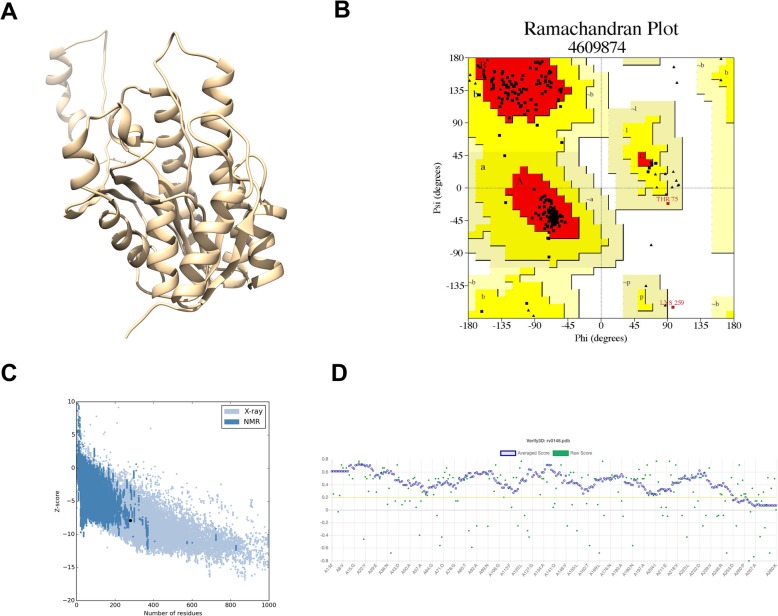
Because the structure of Rv0148 was not determined by experimental methods, we attempted to predict its 3D structure using the homology modelling approach. From the blast search results against PDB for a suitable template in complex with NAD, it was observed that Rv0148 showed the best alignment with human 17-beta-hydroxysteroid dehydrogenase type 4 in complex with NAD (PDB code 1ZBQ) and was chosen as the template. The sequence coverage between Rv0148 and 1ZBQ was 95% with a sequence identity of 52%, E-value of 2e-96) and gap of 2%. Both Rv0148 and 1ZBQ have short-chain dehydrogenase domain (PF00106). The 3D structure of Rv0148 was modelled (Fig. 2a) using the model-default python script in MODELLER software and the sequence alignment used for predicting the model structure is shown in Fig. 1b. The predicted model was validated using PROCHECK, profile-3D, and ProSA-web. Stereochemical properties of the predicted model were evaluated using PROCHECK to analyse the Ramachandran plot (Fig. 2b).
Fig. 2.
a Predicted 3D structure of Rv0148. b Ramachandran plot for the predicted model structure of Rv0148. c Plot showing residue energy with Z-score for the Rv0148 model. The blue and grey colours represent the experimentally determined structures using NMR and X-ray, respectively. The Z-score for Rv0148 model falls within the range of the experimentally determined structures. d Plot showing the average 3D-1D score for every residue of the model structure
The plot revealed that the occurrence of 92.3% amino acids was in the favoured region, 7.3% in the generously allowed region and 0.4% in the disallowed region. Z-score was − 7.89 for the model structure determined using ProSA-web, a program used for validating protein structure, and was located within the space determined by X-ray crystallography (Fig. 2c). Thus, the overall results from structural superimposition, Ramachandran plot, verify-3D, and ProSA-web reveal that the predicted model is satisfactory and thus considered for further analysis. Profile-3D of the Rv1048 model was 90.36, thus suggesting that 90.36% of the residues have an average 3D–1D score ≥ 2 (Fig. 2d).
Docking of NAD within the active site of Rv0148
The docking of NAD co-factor was performed using GOLD software. However, a primary docking of the bound NAD to the template structure 1ZBQ was re-docked in its binding site to evaluate the docking process. The RMSD of the resulting docking of NAD to 1ZBQ was 0.86 Å, suggesting that the docking program is able to reproduce a structure very close to the experimental structure. In order to discover the putative binding pose of NAD co-factor in Rv0148, the binding site for Rv0148 was mapped based on 1ZBQ (Fig. 1b). The best-predicted pose of NAD within the active site of Rv0148 had a score of 95.44 Å (Fig. 1c).
Rv0148 and Htdy interaction analysis
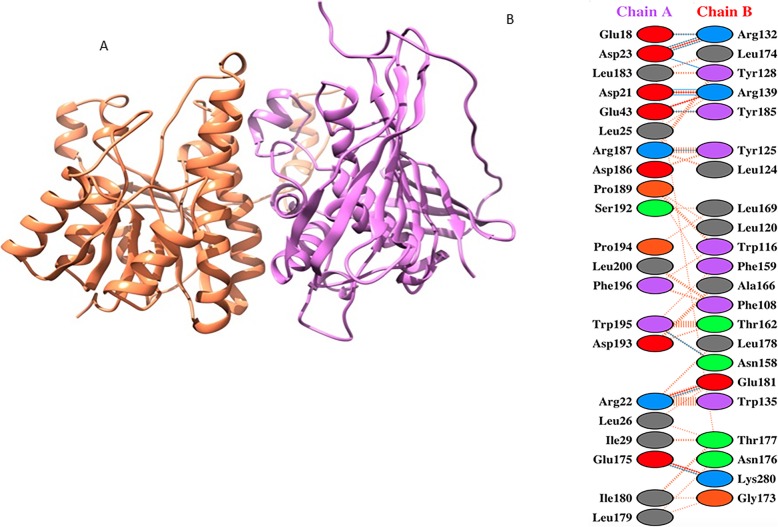
The crystallographically determined structure of Htdy (PDB code 3KHP) and the predicted model structure of Rv0148 were used for docking using the ClusPro server. The structure from the largest cluster and with the lowest energy was selected and examined for the interaction between the two complexes (Fig. 3a). The residues interacting between the two proteins Rv0148 and Htdy from the docked complex were then mapped to identify the active site (Fig. 3b).
Fig. 3.
a The predicted interaction of Rv0148 (orange) and Htdy (purple) protein structures using ClusPro protein-protein interaction server. b The figure showing interacting residues between the two proteins. Chain A & B, chain A represents Htdy and chain B represents Rv0148
Cloning, expression and purification of Rv0148 and Htdy
The coding region of the two genes was amplified by PCR and the product matches the size of Rv0148 with 858 bp and htdy with 969 bp. The E. coli strain (BL21) expressed pGBRJ3 Htdy as His-tagged protein and pGBRJ2 0148 as GST-tagged protein (Fig. S1). The protein was purified. The mass of Htdy is 31 kDa and Rv0148 GST is 55 kDa. These correspond to the expected mass of the above proteins through western blot.
Interaction of Rv0148 and Htdy
The pull-down assay with recombinant His-Htdy (PGBRJ3) lysate using Ni-NTA affinity chromatography confirmed that the Htdy was able to pull down GST-tagged Rv0148 (PGBRJ2). Simultaneously, the GST-tagged lysate was able to pull down His tag recombinant Htdy (Fig. 4). The pull-down assay and far western blot confirmed that Rv0148 interacts with Htdy protein.
Fig. 4.
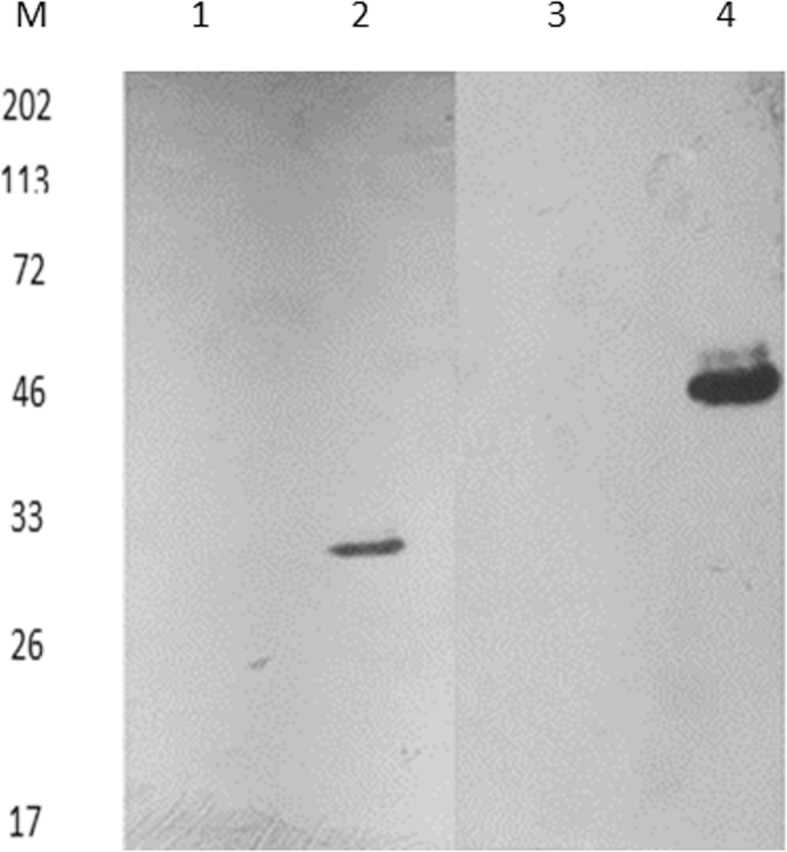
Rv0148 interacts with Htdy. Pull-down assay confirms the interaction between Rv0148 and Htdy. The crude lysates enclosing His-Htdy and GST-Rv0148 were incubated with Ni-NTA resin; unbound proteins (lane 1) were washed off and incubated with their corresponding substrates. The protein fractions were eluted (lanes 2 and 4) and immunoblotted with anti-His and anti-GST antibodies. As a control, the bait proteins were incubated with bovine serum albumin protein (lane 3). Lane M, molecular mass standard
Generation of ∆0148 knockout mutant in M. tuberculosis
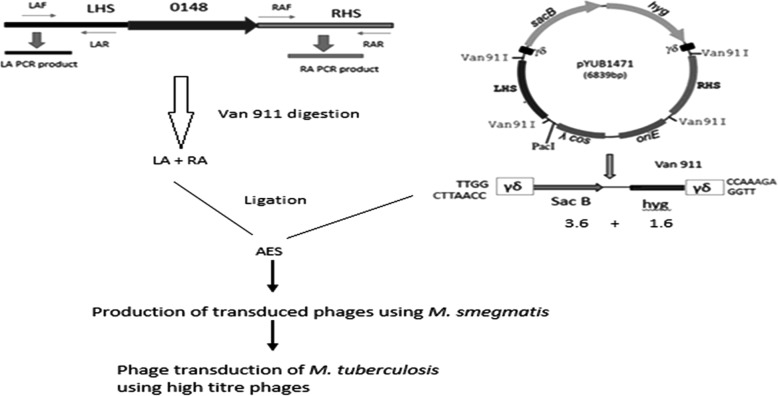
We constructed knockout mutant of M. tuberculosis for not expressing Rv0148 by phage-facilitated allelic exchange [21]. The coding region of the gene was replaced by the hygromycin cassette and four fragments were generated with 3.6, 1.6, 0.837 and 0.605 bp product sizes (Fig. S1). The 50 kb phAE159 shuttle phasmid with AES retains 6.642 kb (Fig. S2). The generated high-titre transducing phages were used for specialized transduction and the knockout mutant was generated successfully and named as Δ0148 (Fig. 5). We confirmed the knockout mutant using sequencing, PCR for hygromycin cassette and also through real-time PCR (RT-PCR) using gene-specific primers (Table S3).
Fig. 5.
Overview of gene knockout. Pictorial representation of steps followed for successful construction of gene knockout mutant
Colony morphology and growth kinetics
Gene disruption shows no distinct effect in the cell development of the knockout strain compared to H37Rv. To elucidate if the knockout of Rv0148 confers any change in vitro, we compared the growth kinetics of H37Rv, ∆0148 and CΔ0148 in 7H9-enriched media. All the strains showed similar growth and there was no distinct difference observed in in vitro survival of ∆0148.
Cytokines analysis using ELISA
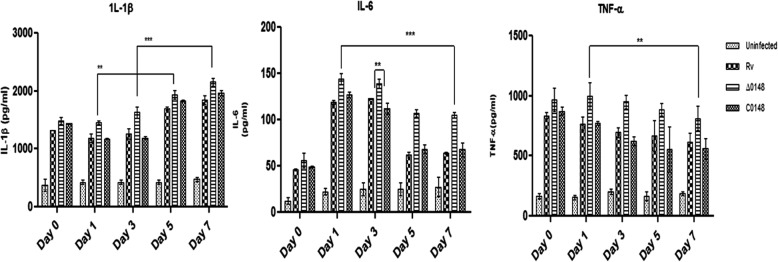
The cell-free supernatants after post-infection were analysed for the expression of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α and anti-inflammatory cytokines IL-10, IL-12p40, IL-12p70 and IL-4. Enhanced expression of IL-1β, IL-6 and TNF-α cytokines was observed in ∆0148 infected with THP-1 macrophages (Fig. 6a–c). Similarly, reduced level of expression of IL-10, IL-12p40, IL-12p70 and IL-4 was observed in ∆0148 compared to wild-type H37Rv (data not shown). The expression was analysed using appropriate statistical methods.
Fig. 6.
Cytokines secreted by macrophages infection. H37Rv, Δ0148 and CΔ0148 strains after post-infection cytokines were measured according to protocol. The capture antibody was coated onto the wells; to samples of 100 μL were added detection antibody followed by substrate solution to identify the specific cytokines. IL-1β, IL-6 and TNF-α. Two-way ANOVA was performed. The values were taken by considering the average of three triplicate independent experiments. Data represents mean, Standard error mean deviation (SEM) in each experiment and error bars indicate mean ± standard deviation SD. *** significant at P < 0.001 and ** significant at P < 0.01
Effect on inhibition of the electron transport chain in ∆0148
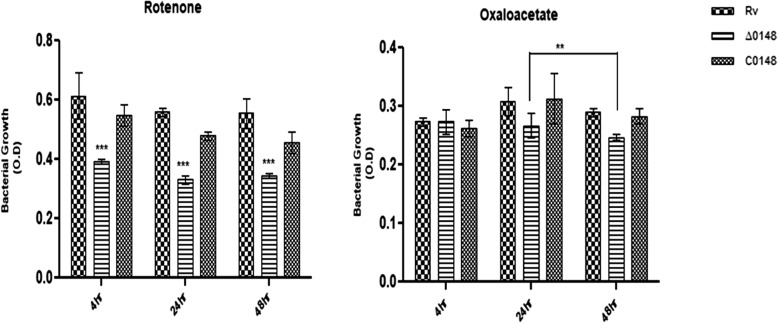
Rv0148 is predicted to be involved in the intermediatory metabolism of M. tuberculosis. The ETC plays an important role in the production of proton motive force (PMF) in the metabolism of bacteria. ETC consists of five complexes I, II, III, IV and V, and inhibition of these complexes could accommodate changes in the growth of bacteria. Rotenone is an inhibitor of complex I whereas oxaloacetate inhibits complex II. It was observed that the exposure of Δ0148 to 140 μM rotenone and 2 mM oxaloacetate significantly decreases the growth (Fig. 7a and b). The result suggests that ∆0148 strain has a distinct role in the ETC. The significance was reported using statistical tools.
Fig. 7.
Effect of electron chain inhibitors. H37Rv, Δ0148 and CΔ0148 strains were grown to mid-log phase and the culture diluted with 7H9 broth. The OD600 was measured after post-infection time points of 4, 24 and 48 h with 140 μM rotenone and 2 mM oxaloacetate. The represented data is the mean ± SD of three independent experiments. Two-Way ANOVA, standard error mean was performed. *** significant at P < 0.001 and ** significant at P < 0.01
Effect of oxidative and nitrosative stress in ∆0148
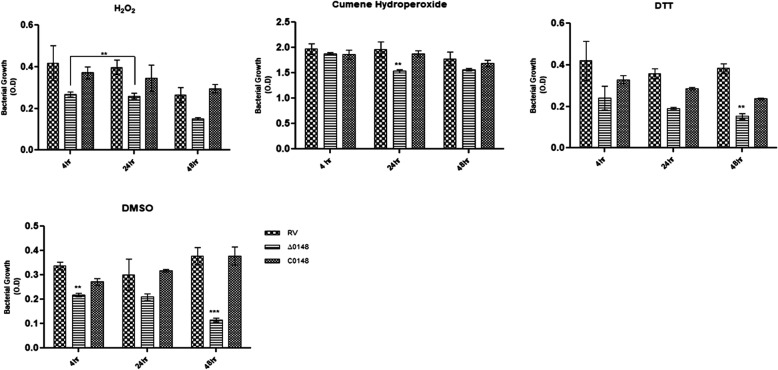
In diseased patients, mycobacterium resides in the alveolar macrophages in the initial stage of infection and secretes various ROS and RNS. We investigated whether these stresses affect the metabolism of ∆0148 strain when exposed to 5 mM hydrogen peroxide (H2O2), 5 mM cumene hydroperoxide, 2 mM thiol-reductant DTT and 1% sulphoxide (DMSO). ∆0148 is sensitive to oxidative stress components and to nitrogen derivatives and there is decrease in growth of Δ0148 compared to wild-type H37Rv (Fig. 8a–d). It was concluded that the growth of ∆0148 declined on exposure to ETC, oxidative and nitrosative stress agents. The result was considered using standard statistical methods.
Fig. 8.
Effect of stress in Δ0148. The mid-log phase cultures were subjected to oxidative and nitrogen stress compounds and OD values were measured after post-exposure with 5 mM H2O2, 5 mM cumene hydroperoxide, 2 mM dithiothreitol and 1% DMSO at 4, 24 and 48 h. The values were taken by performing three independent experiments. The bar graphs were plotted by considering the mean, SEM and using Two-Way ANOVA and error bars indicate mean ± SD. significant at P < 0.001 and ** significant at P < 0.01
Sensitivity and resistance to first-line and second-line drugs
BACTEC MGIT was performed with first-line and second-line drugs and shows that ∆0148 is resistant to streptomycin, ethambutol, amikacin, and kanamycin. H37Rv was maintained as a control for all these drug sensitivity assays (Table 1).
Table 1.
Resistance and sensitivity to drugs
| Name of sample | Strep (1 μg/mL) | INH (0.1 μg/mL) | Eth (5 μg/mL) | RIF (1 μg/mlL | KAN (2.5 μg/mL) | OFLO (2 μg/mL) | AMI (1 μg/mL) |
|---|---|---|---|---|---|---|---|
| H37Rv | S | S | S | S | S | S | S |
| Δ0148 | R | S | R | S | R | S | R |
Abbreviations: AMI amikacin, Eth ethambutol, INH isoniazid, KAN kanamycin, OFLO ofloxacin, R resistance, RIF rifampicin, S sensitive, Strep streptomycin
Interactomic analysis
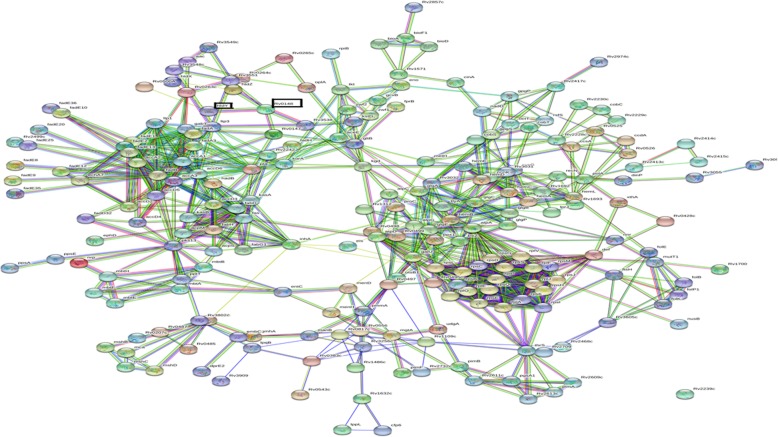
The STITCH database was used to identify M. tuberculosis proteins that interact with three anti-TB drugs streptomycin, kanamycin and amikacin. For each of the proteins interacting to the respective drugs identified from the STITCH database, we collected the interactomes with high confidence > 0.7 from STRING database to ensure a level of confidence for the interactions. Simultaneously, we collected the highest confidence interactomes for Rv0148, htdy and EIS genes from STRING (Table 2). Finally, we produced an interactome of 220 proteins that connect Rv0148, Htdy and EIS with the proteins that either interact directly or indirectly with the three drugs. The M. tuberculosis PPI (protein–protein interaction) network incorporating all the identified proteins is shown in Fig. 9. Comparing and analysing the intersection of proteins that connect the three drugs kanamycin, amikacin and streptomycin and the three proteins Rv0148, Htdy and EIS resulted in 57 proteins (Fig. S3). Analysing the 220 genes, except for streptomycin, we identified genes connected to kanamycin and amikacin as an intersection between interacting genes of Rv0148, htdy and EIS.
Table 2.
List of drug-resistance genes interacting with Rv0148
| Rv0148 vs kanamycin | Rv0148 vs EIS | EIS vs amikacin | Rv0148 vs Htdy |
|---|---|---|---|
| fadb | |||
| Rv3538 | accA2 | eis | accA2 |
| Fas | Rv2417c | Rv3538 | |
| Fas | kasA | Rv2414c | Rv0148 |
| Htdy | fabD | Rv3055 | fas |
| Htdx | kasB | Rv1312 | acps |
| Rv0130 | AccD3 | Rv1571 | kasA |
| mutT1 | eis | Rv3605c | Pks13 |
| Rv3839 | mbtB | ||
| Rv0428c | Rv0216 | ||
| Rv0495c | Rv2499c | ||
| Rv2242 | Htdx | ||
| hatB | |||
| fabD | |||
| Rv0130 | |||
| Rv3797 | |||
| FadE9 | |||
| fadE25 | |||
| fadE10 | |||
| fadE8 | |||
| fadE20 | |||
| FadE12 | |||
| fadA | |||
| FadA3 | |||
| Rv0147 | |||
| accD4 | |||
| Rv3548c | |||
| kasB | |||
| Rv2228c | |||
| mutT1 | |||
| accD2 | |||
| echA7 | |||
| accD3 | |||
| fadE13 |
Fig. 9.
Interactome showing 220 genes interacting with Rv0148
Differential expression of htdy
The interactome analysis predicted that htdy is also involved in drug resistance. Expression of htdy in the Δ0148 strain of M. tuberculosis was analysed using qPCR as mentioned earlier. The htdy expression in Δ0148 increased to 1.25-fold whereas the wild-type strain showed 0.7-fold (Fig. 10 and Table S4). The fold expression was calculated using SDS 2.4 software and relative quantity (RQ value) method.
Fig. 10.
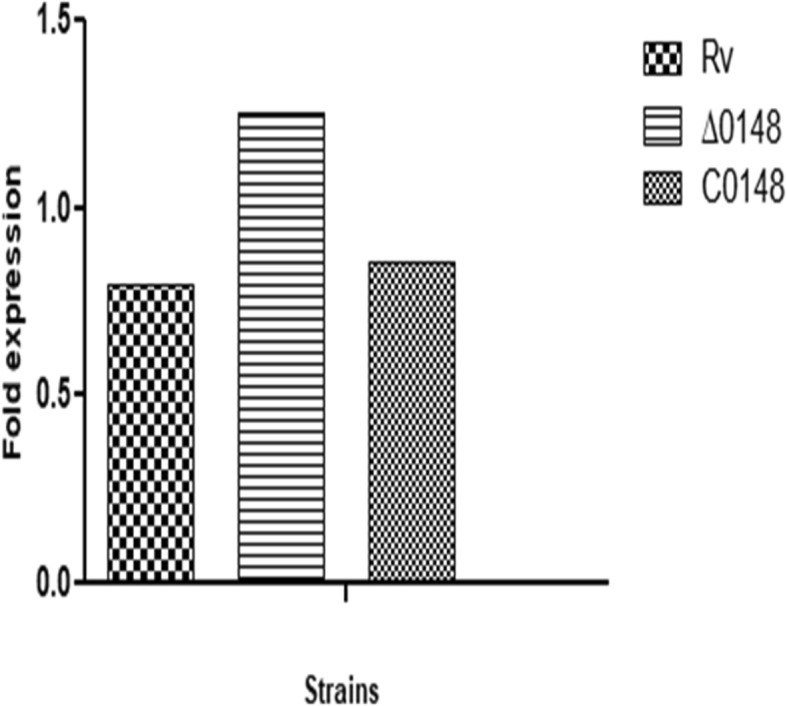
Expression of htdy in Δ0148 strain. The bar graphs represent the fold expression of htdy in H37Rv, Δ0148 and C0148. The expression were analysed using SDS software and relative quantity method
Discussion
In this study, we report the cloning, overexpression, purification, gene knockout and interacting partners of Rv0148, a hypothetical protein that belongs to the oxidoreductase family of M. tuberculosis. Through bio-informatics approaches, we concluded that Rv0148 is a NAD oxidoreductase protein with SDR domain. Rv0148 is predicted as interacting with a protein that has an MaoC dehydratase domain and the subsequent blast analysis led to the identification of Htdy protein. Further, the string analysis of Rv0148 displayed 10 interacting partners such as Rv3538, Rv0149, mbtB, fas, fadB, aacA2, Rv0147, Rv2228c, muT1 and htdy thus confirming our analysis. The predicted Htdy as an interacting partner of Rv0148 was further confirmed through far WB and pull-down assays.
We generated gene knockout mutant of Rv0148 in M. tuberculosis and show that Rv0148 is a non-essential gene as confirmed in the previous report [22]. ∆0148 grew similarly as the wild type under constant shaking in enriched 7H9 media. This indicates clearly that the loss of Rv0148 does not lead to any change in the growth of M. tuberculosis. There were no correlative morphological changes in cell structure owing to the deletion of Rv0148.
We infected the macrophage cell lines with mutants for cytokine profiling. Increased expression of pro-inflammatory cytokines predicts that Δ0148 might be involved in host immune responses [23, 24]. The lower level expression of anti-inflammatory cytokines IL-10, IL-4, IL-12p40 and IL-12p70 indicates that Δ0148 has no role in inflammation [25].
In aerobic organisms, respiratory chain possesses inner membrane complexes associated with energy production. Complex I known as NADH quinone oxidoreductase transfers electrons from NADH to quinone associated with proton transfer across the membrane. Complex II is involved in tricarboxylic acid cycle without transfer of protons. Complex III transfers electrons from quinone to cytochrome c. Complexes III and IV receive these electrons and transfer them to oxygen. Complex V using PMF synthesizes ATP molecules [26]. Rv0148 is a NAD oxidoreductase involved in the function of intermediary metabolism and respiration. The exposure of Δ0148 to components inhibiting ETC complexes I and II [27] revealed that ∆0148 responds to changes in the electron transport system, thus predicting its role in cell homeostasis. M. tuberculosis adapts to various stress conditions to survive as a successful pathogen inside the host [28]. Δ0148 interestingly showed decreased growth in hydrogen peroxide, CHP, DTT and DMSO, which indicates its sensitivity to oxidative and nitrogen stress. Thus, in response to stress, the differential growth of ∆0148 demonstrates that the gene plays an important role in the protective efficacy against oxidative and nitrogen stress [29].
Contradictory to previous findings, Rv0148 showed increased expression in MDR isolates [18]. In the present study, Δ0148 showed resistance to first-line streptomycin and ethambutol and second-line amikacin and kanamycin drugs and the result was correlated with its interaction towards drug-resistance genes. Interactome analysis in the study suggests that Rv0148 interacts with kanamycin resistance genes Rv3538, mutT1, fas, Rv0130, Rv3389 and htdx. The EIS gene is reported earlier to be involved in causing drug resistance of M. tuberculosis [30]. Our analysis reveals that Rv0148 interacts with eis genes accA2, fas, kasA, fabD, KasB and accD3. Rv0148 does not display any direct interaction with amikacin and ethambutol genes; however, the interactomes of EIS and amikacin share common interacting genes such as Rv2417c, Rv2414c, Rv3055, Rv1312, Rv1571, Rv3605c, Rv3839, Rv0428c, Rv0495c and Rv2242. As these proteins are connected to each other indirectly on a larger interactome space, we assume that drug resistance in Δ0148 strain could be due to some disruption of the interactome network.
The major findings from the current study prove that htdy is also involved in drug resistance and the increased expression of htdy in ∆0148 predicts that Rv0148 altered the expression of interacting partners in M. tuberculosis. Interactome analysis using STRING database predicts that Rv0148 has a strong interaction with kanamycin, eis and amikacin resistance genes. Further, interactome analysis reveals that Rv0148 and Htdy are connected to EIS and the interacting proteins of the drugs analysed in this study. Thus, we assumed that the absence of Rv0148 affects the interactome that might bring a major expression difference in these proteins resulting in drug resistance towards respective drugs. An additional transcriptome profiling of these strains will further help in identifying a clear mechanism towards drug resistance. Further, studies like double knockout mutant of Rv0148 and Rv3389 will provide the detailed functional role of these genes.
Conclusion
To summarize, Rv0148 is a non-essential gene belonging to the oxidoreductase family in M. tuberculosis. In silico approach and protein interaction studies reveal that Rv0148 interacts with Htdy, which is a protein of the hydroxyl acyl family. Htdy in the presence of Rv0148 is quite stable and fold expression of the gene is optimal. In the absence of Rv0148, increased fold expression of htdy proves that these two genes are functionally interconnected and furthermore it is experimentally proved. In addition to these findings, Rv0148 not only interacts with htdy, but also has a strong interactome network of 220 genes in M. tuberculosis with a high confidence of 0.7. The absence of Rv0148 showed resistance to first-line drugs streptomycin and ethambutol and second-line drugs amikacin and kanamycin. Increased expression of pro-inflammatory cytokines suggests that Δ0148 might play a role in targeting the immune response. Our study suggests that Rv0148 plays a significant role in various functional aspects such as intermediatory metabolism, stress and homeostasis.
Methods
Template selection and homology modelling
A suitable template for Rv0148 was identified by searching in the PDB database using the default settings of BLAST program in February, 2017. The sequence of Rv0148 was searched against Pfam database to further identify available PDB structures having the same domain. The final template (PDB Id: 1ZBQ) was chosen based on sequences having the highest sequence identity and domain coverage against Rv0148 sequence. The template 1ZBQ selected for modelling Rv0148, is the experimentally determined crystal structure of human 17-beta-hydroxysteroid dehydrogenase in complex with cofactor NAD. The template and the target sequence were aligned using the malign program in Modeller software [31]. Homology model of Rv0148 was constructed using Modeller and the quality of the model was further analysed using PROCHECK [32], ProSA-web [33] and verify 3D [34]. The model was also validated using root mean square deviation (RMSD).
Molecular docking
Docking was performed using NAD cofactor as the ligand with the predicted model of Rv0148 to understand its mode of interaction within the binding site. GOLD v5.2 (genetic optimization for ligand docking) is a widely used protein–ligand docking program [34]. GOLD uses a genetic algorithm methodology for protein–ligand docking that allows full ligand and partial protein flexibility. The homology model of Rv0148 protein was used as the receptor after the addition of hydrogen atoms before docking. The NAD ligand was corrected with Auto Edit Ligand option of GOLD program. Default genetic algorithm (GA) settings that ensure 100% search efficiency were used for docking. A total of 20 poses of the docked molecule were generated. Early termination of the number of GA runs was allowed when the RMSDs of the top three GA solutions were within 1.5 Å. The best pose of the docked ligand was selected based on Goldscore.
Protein–protein docking
The interactions between Rv0148 and Htdy structures were docked using ClusPro [35]. The ClusPro algorithm uses fast fourier transform correlation, making it feasible to generate billions of docked conformations by simple scoring functions. ClusPro filters the docked confirmations with near-native structures, ranks them based on their clustering properties and finally refines a limited number of structures by energy minimization. The server outputs the top predicted complexes based on energy and cluster size.
Bacterial strains and growth conditions
E. coli strains DH5α and BL21 (DE3) (Invitrogen, USA) were used for cloning and expression of recombinant proteins. E. coli cells were grown in Luria Bertani (LB) medium with constant shaking at 37 °C. M. smegmatis (mc2155) was grown in Middlebrook 7H9 broth (Difco, USA) with 0.2% glycerol and 0.05% Tween-80. The media was supplemented with 50 μg/mL ampicillin for E. coli whenever needed. M. tuberculosis was also grown in 7H9 broth with antibiotics carbenicillin 50 μg mL, cyclohexamide 10 μg mL, kanamycin 25 μg mL and hygromycin 50 μg mL, wherever required, along with OADC and Tween-80. Primers used in the study are listed in Table S2. The plasmids and strains constructed in the present study are mentioned in Table 3.
Table 3.
Plasmids and bacterial strains constructed in the study
| Plasmid | Source of description | Reference/origin |
|---|---|---|
| E. coli DH5α | F−endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG ϕ80dlacZΔM15 Δ(lacZYA-argF)U169, hsdR17(rK-mK+), λ- | Invitrogen |
| E. coli BL21 (DE3) | F−ompT gal dcm lon hsdSB(rB−mB−) λ(DE3 [lacI lacUV5-T7 gene 1 ind1 sam7 nin5] | Invitrogen |
| M. smegmatis mc2155 | Lab stock | |
| pGEX 4 T-1 | lacTq, 4.9 kb, Ampr, GST gene fusion vector | GE Healthcare |
| pRSET-B | lacTq, 2.8 kb, AmpR, 6xHis (N-term) gene fusion vector | Invitrogen |
| p0004- Sac B | Suicide recombination delivery vector carrying hygR-SacB for gene disruption, hygR | |
| PhAE159 | Conditionally replicating shuttle phasmid vector | William R Jacobs Jr (unpublished) |
| pMV261 | E. coli mycobacterial shuttle vector, KanR, hsp60 promoter | Strover et al. (1991) |
| Constructs in this study | ||
| pGBRJ1 | pRSET-B vector carrying the coding region of Rv0148 gene from M. tuberculosis, AmpR | This study |
| pGBRJ2 | pGEX 4 T-1 vector carrying the coding region of Rv0148 gene from M. tuberculosis, AmpR | This study |
| pGBRJ3 | pRSET-B vector carrying the coding region of Htdy gene from M. tuberculosis, AmpR | This study |
| Δ0148 | The deletion of Rv0148 gene from M. tuberculosis | This study |
| CΔ0148 | pMV261 vector carrying the coding region of Rv0148 gene from M. tuberculosis, KanR expressed in M. tuberculosis (complementation) | This study |
Cloning and expression of Rv0148 and htdy
M. tuberculosis genomic DNA was isolated using CTAB-NaCl standard method [36]. Coding region of Rv0148 from M. tuberculosis was amplified by polymerase chain reaction (PCR) using gene-specific oligonucleotides. The PCR product of htdy was further cloned into pRSET-B harbouring T7 promoter and Rv0148 in pGEX 4 T-1 vector harbouring tac promoter with Bam HI at 5′ and Xho I at 3′ end as restriction sites. The resultant clone was confirmed through restriction digestion and DNA sequencing. For recombinant protein expression, pGBRJ3 and pGBRJ2 clones were transformed into E. coli BL21 (DE3) cells and protein expression was induced with 0.5 mM isopropyl β-d-1 thiogalactopyranoside (Invitrogen, USA). Induced cells were grown for 4 h at 37 °C and harvested by centrifugation at 8000 rpm for 10 min. The cell pellet was re-suspended in ice-cold lysis buffer G (50 mM Tris pH 8, 300 mM NaCl, 20% glycerol,0.5 mM phenyl methyl sulphonyl fluoride and 10 mM imidazole) along with protease inhibitor cocktail Sigma fast (SIGMA, USA) and sonicated on ice using ultrasonic homogenizer (5 cycles: 30 s pulse and 1 min interval). Finally, the lysate was centrifuged at 10,000 rpm for 10 min at 4 °C to separate soluble and insoluble proteins.
Purification of Rv0148 and Htdy
The soluble lysate proteins were analysed on 12% SDS gel for confirmation of recombinant proteins and protein lysis. Lysate was purified using affinity column (Bio-RAD, CA, USA) and 6X histidine-tagged protein was bound to NI-NTA resin (Invitrogen, USA) and incubated at 12 °C for 2 h. The column was washed with 10 column volumes of wash buffer containing 40 mM imidazole and was eluted with a buffer containing 200 mM imidazole. Similarly, GST-tagged protein was bound to glutathione sepharose resin (GST, Takara, Japan) and eluted with 20 mM reduced glutathione. The eluted fractions were then dialysed and analysed by SDS-PAGE and WB.
Pull-down assay
Pull-down assay was performed using poly-prep chromatography column (Bio-Rad, USA) with NI-NTA resin. Recombinant pGBRJ3 His lysate was incubated with NI-NTA resin for 2 h at 12 °C with gentle agitation. The column was washed with buffer containing 40 mM imidazole and incubated overnight with pGBRJ2 GST lysate from E. coli BL21 (DE3) cells at 12 °C with gentle agitation at 10 rpm in an end-to-end shaker. Unbound proteins were removed by washing with 10 column volumes of buffer. The interacting proteins were eluted with buffer containing 200 mM imidazole.
Far western blot
To recognize the Rv0148 interacting proteins, the fraction eluted in pull-down assay was separated on a 12% SDS-PAGE and transferred to Immobilon-P (PVDF) membrane. The membrane was denatured by incubation with varying concentrations (6, 3, 1 and 0.1 M) of guanidine-HCl in AC buffer containing 5 M NaCl, 1 M Tris pH 7.5, 0.5 M EDTA, 10% Tween-20, 2% skimmed milk powder, 10% glycerol and 1 M dithiothreitol (DTT) for 30 min at room temperature followed by 5 min wash with phosphate buffer saline Tween 20 [37]. Then, the blot was completely renatured in AC buffer without guanidine-HCl and left overnight at 8 °C. The membrane was then blocked with 5% skimmed milk and incubated with bait protein for 7–8 h at 8 °C and further with 1:6000 anti-His monoclonal antibody and developed using enhanced chemiluminescent kit (Thermo Scientific, USA).
Construction of allelic exchange substrate
Gene disruption was carried out using Rv0148 left homology sequence (LHS) and right homology sequence (RHS) flanking regions carrying van911 as restriction site targeted for deletion and substitution. The gene was cloned onto either side of the selectable antibiotic cassette [γδ (sacB-hyg)γδ] to generate allelic exchange substrate for homologous recombination. Van911 restriction enzyme identifies a discontinuous palindrome interjected by five bases of sequence (CCAN_NNN^NTGG) to achieve a four-fragment ligation.
Construction of specialized transducing phages
Shuttle phasmid phAE159 proliferates at 30 °C and does not propagate in the infected mycobacterial cell at 37 °C. Shuttle phasmid phAE159 accommodates up to 10 kb of recombinant DNA into mycobacterial cells. The mycobacteriophage carrying AES disrupts a precise gene in mycobacteria through deletion–substitution. The AES with hygromycin resistance marker differs from the phasmid, and the use of in vitro lambda packaging extract (Epicentre, USA) helps in increasing the efficiency of transformation and also in the selection of phasmid DNA. Then the phasmid DNA was electroporated into M. smegmatis to produce specialized transducing phages (STPs) at permissive temperature of 30 °C. These phages were further propagated using MP buffer (1 M Tris pH 8, 5 M NaCl, 1 M MgCl2, 1 M CaCl2) to obtain high-titre allele-specific STPs.
Specialized transduction
Specialized transduction was performed at 37 °C for the augmentation of STPs. In this temperature transfer of DNA to the host (mycobacterium) occurs with homologous recombination. The transduced M. tuberculosis cells were selected on hygromycin plate (150 μg/mL) after transduction. A minimum of five colonies appeared on the plate and was confirmed by PCR and Sanger sequencing using hyg as a forward primer and RHS as a reverse primer to confirm homologous recombination. For complementation of Rv0148, the gene was cloned into pMV261 vector harbouring hsp60 promoter. The construct was then electroporated into the ∆0148 strain and colonies were screened on the 7H10 plate with antibiotic selection (kanamycin 20 mg mL). Complementation was confirmed by PCR using Rv0148 primers and the complemented strain labelled as CΔ0148.
In vitro growth kinetics of ∆0148
To check whether the disruption of Rv0148 fetched any changes in the in vitro growth of H37Rv, ∆0148 and CΔ0148 strains, log-phase cultures were grown in Middlebrook 7H9-OADC-Tween media and incubated at 37 °C with 180 rpm shaking. Aliquots of 100 μl culture were taken at different time points and OD values were measured at 600 nm using spectrophotometer (Eppendorf) on days 0, 3, 6, 9, 12 and 17. Survival of wild type, ∆0148 and CΔ0148 strains was analysed by plating the serial diluted broth cultures in 7H10 OADC agar without Tween-80. The plates were incubated for 3–4 weeks and the colony forming units were calculated.
Colony morphology
The colony morphology of the mutant was determined by comparing with complementary strain and wild type. The strains were grown in liquid 7H9 media to mid-log phase and 10 μl of culture was inoculated to 7H10 agar plate supplemented with hygromycin. The plates were incubated at 37 °C for 3–4 weeks for colony formation.
RNA extraction and qPCR
Bacterial culture 100 mL was grown in 7H9 media to mid-log phase and the growth was arrested in ice and centrifuged at 4 °C. The pellet was suspended in trizol (Invitrogen, USA). Cells were disrupted using 0.1 mm zirconium beads in a mini bead beater. The total RNA was extracted using the RNeasy purification kit (Qiagen, Germany) according to the manufacturer’s protocol and quantified using ND-1000 Nanodrop spectrophotometer (Nanodrop Technologies). It was stored at − 80 °C. To determine the relative mRNA through qPCR, the first strand cDNA was synthesized from 1 μg of RNA using Maxima first strand cDNA synthesis kit (Thermo Scientific, USA). Quantitative qPCR primers for the Rv0148, htdy and 16S rRNA genes were designed (Table S2). qPCR was carried out using SYBR Green Master Mix (Thermo Scientific, USA) according to manufacturer’s instructions using the Applied Biosystems 7300 real-time PCR system. Control reactions for each sample were carried out in the absence of reverse transcriptase to look over for DNA contamination. The amplification conditions for all reactions were 1 cycle of 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. qPCR data analysis was carried out by relative quantification of Rv0148, htdy and CΔ0148 gene expression using comparative calculated threshold (CT) method. For each qPCR run, the CT cycle was normalized to the internal control 16S rRNA gene amplified from the corresponding sample. Statistical analysis was carried out using SDS 2.4 software and relative quantity (RQ value) method. The data presented are averages of three independent experiments.
Infection of knockout strain in THP-1 cell lines
Intracellular viability of the knockout strain was determined using macrophage cell line infection studies. THP-1 cells were grown in RPMI media with 10% fetal bovine serum. Cells were further grown to reach 1 × 106 cells, seeded onto 24-well plates and differentiated into macrophages using 50 mM phorbol 12-myristate 13-acetate. Plates were incubated at 37 °C in the presence of 5% CO2 incubator for 2 days, washed with RPMI medium and left overnight. Macrophages were then infected in triplicate with H37Rv, ∆0148 and CΔ0148 with multiplicity of infection of 1:10. Phagocytosis was allowed to take place for 4 h, after which the infected macrophages were incubated with RPMI containing streptomycin to eliminate extracellular bacteria. Further, the cell-free culture supernatants were collected on days 0, 1, 3, 5 and 7 post-infection and stored at − 80 °C for cytokine analysis.
Elisa
The supernatants derived from THP-1 cell lines infected with H37Rv, ∆0148 and CΔ0148 at different intervals were evaluated for expression of cytokines IL-4, IL-6, IL-10, IL-1β, TNF, IL-2, INF-γ, IL-12 (p40) and IL-12 (p70) by sandwich ELISA using BD ELISA kit (BD pharmingen, USA) according to manufacturer’s protocol.
Role of knockout mutant during oxidative stress
To understand the role of oxidative and nitrogen stress in H37Rv, ∆0148 and CΔ0148, the strains were grown to mid-log phase in 7H9-OADC-T medium and exposed to various stress components such as electron transport chain (ETC) inhibitors, peroxides, sulphoxides, thiol stress with slight modification to the procedure by [27] and OD values were measured at 600 nm (OD600) for 4, 24 and 48 h time points. The graphs were plotted and significance was reported considering P < 0.01 and P < 0.001.
MGIT and drug susceptibility testing
The H37Rv and knockout strains were grown on LJ solid media, 0.5 McFarland culture suspension was used for MGIT inoculation and drug susceptibility testing (DST) was performed for all the culture-positive MGIT tubes after 4 days. The growth control (GC) was prepared by diluting the cultures in the ratio of 1:100. The test cultures were prepared using saline in 1:5 dilution; 800 μL of supplement was added to tubes according to manufacturer's protocol; 100µL streptomycin 1 μg mL, isoniazid 0.1 μg mL, rifampicin 1 μg mL and ethambutol 5 μg mL (SIRE kit, BD). Kanamycin 2.5 μg mL, ofloxacin 2 μg mL and amikacin 1 μg mL was added to the respective tubes; and finally 500 μL of culture was added to all the tubes and loaded in BACTEC MGIT 960. Tubes were recapped, mixed well and fixed into the BACTEC MGIT 960 using the antimicrobial susceptibility testing set entry protocol. BACTEC MGIT 960 instrument monitored susceptibility test until the growth unit of the GC reaches 400 [38].
Interactome analysis to predict drug-resistance partners
To predict the interacting genes involved in drug resistance, STITCH database was used and further analysed using a bioinformatics tool STRING.
Expression of htdy in the presence and absence of Rv0148
H37Rv, mutant and complimentary strains were grown in 7H9 media to log phase according to the growth conditions mentioned earlier and RNA was extracted. Individual and co-expression of htdy were analysed through qPCR in mutant and complementary strains and H37Rv. The Ct and R values were further considered for analysing the folds of gene expression.
Statistical analysis
The data represented all the three independent experiments carried out using replicates. Mean, standard error mean (SEM) and Two-way ANOVA was used to analyse the data obtained from in vitro kinetics, ELISA and oxidative stress assays (**P < 0.01 was said to be significant and ***P < 0.001 was said to be highly significant). Real time PCR data was analysed using SDS software and relative quantity method.
Supplementary information
Additional file 1: Table S1. HMM analysis files showing 1270 significant sequences from Swissprot database.
Additional file 2: Table S2. List of primers used in this study. Table S3. Data showing CT values for confirmation of gene knockout using real time PCR. Table S4. Data showing the CT values of htdy expression in gene knockout mutant.
Additional file 3: Figure S1. Restriction enzyme digestion of Rv0148 &Rv3389 and cloning. (A) Confirmation of recombinant clones in pGEX4T-1 M: 1 kb marker, Lane 1 to 4; pGEX4T-1 as control with 4.9 kb size, Lane 5to 8 recombinant clones, Lane 8 showing insert size 858 bp (B) Confirmation of recombinant clones using restriction digestion in pRSET-B M; marker, Lane 1; Control pRSET-B correlating 2.8 kb, Lane 2 to 6 showing recombinant clones with insert size 969 bp.
Additional file 4: Figure S2. Construction of knockout. (A) allelic exchange substrate construction M: 1 kb Marker, Lane 1: SacB digested with van911 showing four fragments 3.6 kb, 1.6 kb, 979 bp, 567 bp, Lane 2& 3: 0148 AES construct showing four fragments 3.6 kb, 1.6 kb, 837 RA, 605LA. (B) Packaging of AES with phAE159. M: 1 kb ladder, Lane 1: phAE159 digested with pac-I showing 50 kb and insert 3.8 kb, Lane 2& 3 clones without insert, Lane 4& 5 clone digested with pac-I showing phAE159 50 kb and 6.6 kb AES (C) Confirmation of knockout using PCR M: 1 kb ladder, Lane 1: Rv DNA amplified with right arm, Lane 2,4,5: knockout DNA amplified with hyg Forward primer and right arm reverse primer, Lane 6: Rv DNA not showed amplification with hyg & reverse primer.
Additional file 5: Figure S3. Intersection of genes and role in drug resistance. Intersection of Rv0148, Htdy and EIS with the three drugs kanamycin, amikacin and streptomycin resulting in 57 interacting proteins.
Acknowledgements
Not applicable.
Abbreviations
- SDRs
Short-chain dehydrogenases/reductases
- TB
Tuberculosis
- M. tuberculosis
Mycobacterium tuberculosis
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- AhpC
Alkylhydroperoxidase reductase
- AhpE
Peroxiredoxin
- Tpx
Thioredoxin reductase
- MIC
Minimum inhibitory concentration
- STPKs
Serine-threonine protein kinases
- NAD
Nicotinamide adenine dinucleotide
- PDB
Protein database
- RT-PCR
Real-time Polymerase chain reaction
- ELISA
Enzyme-linked immunosorbent assay
- MGIT
Mycobacteria growth indicator tube
Authors’ contributions
KP initiated and supervised the study. GB, SPT and KP designed the experiments. GB conducted the experiments. SH conducted the bioinformatics analysis. SB did the DST studies.GB, SH and KP has written the manuscript. All authors have read and approved the manuscript.
Funding
This work was supported by the ICMR-NIRT intramural research programme. GB awarded with PhD fellowship from the Department of Science and Technology INSPIRE programme. None of the funding bodies were involved in the design of the study, the collection, analysis, or interpretation of data, or in writing and communication of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
No clinical samples were used in this study as this was an entirely in-vitro study using bacterial strains ethical review is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12866-020-01763-1.
References
- 1.Kyu HH, Maddison ER, Henry NJ, Mumford JE, Mumford JE, Shields C. The global burden of tuberculosis: results from the global burden of disease study. Lancet Infect Dis. 2017;23:261-84. [DOI] [PMC free article] [PubMed]
- 2.WHO. Global Tuberculosis Report: World Health Organisation; 2018. https://apps.who.int/iris/handle/10665/274453.
- 3.Boshoff HI, Barry CE., 3rd Tuberculosis - metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3(1):70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 4.Vergne I, Chua J, Singh SB, Deretic V. Cell biology of mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 5.Uribe-Querol E, Rosales C. Control of phagocytosis by microbial pathogens. Front Immunol. 2017;8:1368. doi: 10.3389/fimmu.2017.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16(3):463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, Steyn AJ. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert Rev Mol Med. 2011;13:e39. doi: 10.1017/S1462399411002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi A, Singh N, Bhat SA, Gupta P, Kumar A. Redox biology of tuberculosis pathogenesis. Adv Microb Physiol. 2012;60:263–324. doi: 10.1016/B978-0-12-398264-3.00004-8. [DOI] [PubMed] [Google Scholar]
- 9.Voskuil MI, Bartek IL, Visconti K, Schoolnik GK. The response of mycobacterium tuberculosis to reactive oxygen and nitrogen species. Front Microbiol. 2011;2:105. doi: 10.3389/fmicb.2011.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94(10):5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nambi S, Long JE, Mishra BB, Baker R, Murphy KC, Olive AJ, Nguyen HP, Shaffer SA. The Oxidative Stress Network of Mycobacterium tuberculosis Reveals Coordination between Radical Detoxification Systems. Cell Host Microbe. 2015;17(6):829–837. doi: 10.1016/j.chom.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic V, Philipp W, Dhandayuthapani S, Mudd MH, Curcic R, Garbe T, Heym B, Via LE, Cole ST. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17(5):889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 13.Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, Tham KT, Lakey DL, Bochan MR, Kernodle DS. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164(12):2213–2219. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger T, Budde H, Flohe L, Menge U, Singh M, Trujillo M, Radi R. Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis. Arch Biochem Biophys. 2004;423(1):182–191. doi: 10.1016/j.abb.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Sherman DR, Mdluli K, Hickey MJ, Arain TM, Morris SL, Barry CE, 3rd, Stover CK. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science. 1996;272(5268):1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 16.Sharma D, Kumar B, Lata M, Joshi B, Venkatesan K, Shukla S, Bisht D. Comparative proteomic analysis of aminoglycosides resistant and susceptible Mycobacterium tuberculosis clinical isolates for exploring potential drug targets. PLoS One. 2015;10(10):e0139414. doi: 10.1371/journal.pone.0139414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma D, Singh R, Deo N, Bisht D. Interactome analysis of Rv0148 to predict potential targets and their pathways linked to aminoglycosides drug resistance: an insilico approach. Microb Pathog. 2018;121:179–183. doi: 10.1016/j.micpath.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Sharma D, Lata M, Faheem M, Khan AU, Joshi B, Venkatesan K, Shukla S, Bisht D: Cloning Expression and Correlation of Rv0148 to Amikacin & Kanamycin Resistance. Curr Proteomics 2015;12:96-100.
- 19.Venkatesan A, Hassan S, Palaniyandi K, Narayanan S. In silico and experimental validation of protein-protein interactions between PknI and Rv2159c from Mycobacterium tuberculosis. J Mol Graph Model. 2015;62:283–293. doi: 10.1016/j.jmgm.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Venkatesan A, Palaniyandi K, Sharma D, Bisht D, Narayanan S. Functional characterization of PknI-Rv2159c interaction in redox homeostasis of Mycobacterium tuberculosis. Front Microbiol. 2016;7:1654. doi: 10.3389/fmicb.2016.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain P, Hsu T, Arai M, Biermann K, Thaler DS, Nguyen A, Gonzalez PA, Tufariello JM, Kriakov J, Chen B, et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. mBio. 2014;5(3):e01245–e01214. doi: 10.1128/mBio.01245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48(1):77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 23.Russell-Goldman E, Xu J, Wang X, Chan J, Tufariello JM. A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes. Infect Immun. 2008;76(9):4269–4281. doi: 10.1128/IAI.01735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4(3):252–260. doi: 10.1038/mi.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 26.Ana MP, Melo TMB, Teixeira M. New insights into type II NAD(P)H:Quinone Oxidoreductases. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat SA, Iqbal IK, Kumar A. Imaging the NADH:NAD(+) homeostasis for understanding the metabolic response of Mycobacterium to physiologically relevant stresses. Front Cell Infect Microbiol. 2016;6:145. doi: 10.3389/fcimb.2016.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano T, Li LS, Weinstein E, Teh JS, Rubin H. Steady-state kinetics and inhibitory action of antitubercular phenothiazines on mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2) J Biol Chem. 2006;281(17):11456–11463. doi: 10.1074/jbc.M508844200. [DOI] [PubMed] [Google Scholar]
- 29.Bulatovic VM, Wengenack NL, Uhl JR, Hall L, Roberts GD, Cockerill FR, 3rd, Rusnak F. Oxidative stress increases susceptibility of Mycobacterium tuberculosis to isoniazid. Antimicrob Agents Chemother. 2002;46(9):2765–2771. doi: 10.1128/AAC.46.9.2765-2771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gikalo MB, Nosova EY, Krylova LY, Moroz AM. The role of eis mutations in the development of kanamycin resistance in Mycobacterium tuberculosis isolates from the Moscow region. J Antimicrob Chemother. 2012;67(9):2107–2109. doi: 10.1093/jac/dks178. [DOI] [PubMed] [Google Scholar]
- 31.Hatherley R, Brown DK, Glenister M, Tastan Bishop O. PRIMO: an interactive homology modeling pipeline. PLoS One. 2016;11(11):e0166698. doi: 10.1371/journal.pone.0166698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laskowski RA, Jablonska J, Pravda L, Varekova RS, Thornton JM. PDBsum: structural summaries of PDB entries. Protein Sci. 2018;27(1):129–134. doi: 10.1002/pro.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35(Web Server issue):W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cloete R, Kapp E, Joubert J, Christoffels A, Malan SF. Molecular modelling and simulation studies of the Mycobacterium tuberculosis multidrug efflux pump protein Rv1258c. PLoS One. 2018;13(11):e0207605. doi: 10.1371/journal.pone.0207605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozakov D, Hall DR, Xia B, Porter KA, Padhorny D, Yueh C, Beglov D, Vajda S. The ClusPro web server for protein-protein docking. Nat Protoc. 2017;12(2):255–278. doi: 10.1038/nprot.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baess I. Isolation and purification of deoxyribonucleic acid from mycobacteria. Acta Pathol Microbiol Scand B: Microbiol Immunol. 1974;82(6):780–784. doi: 10.1111/j.1699-0463.1974.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Li Q, Chen XZ. Detecting protein-protein interactions by far western blotting. Nat Protoc. 2007;2(12):3278–3284. doi: 10.1038/nprot.2007.459. [DOI] [PubMed] [Google Scholar]
- 38.NIRT . NIRT standard operating protocols. 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. HMM analysis files showing 1270 significant sequences from Swissprot database.
Additional file 2: Table S2. List of primers used in this study. Table S3. Data showing CT values for confirmation of gene knockout using real time PCR. Table S4. Data showing the CT values of htdy expression in gene knockout mutant.
Additional file 3: Figure S1. Restriction enzyme digestion of Rv0148 &Rv3389 and cloning. (A) Confirmation of recombinant clones in pGEX4T-1 M: 1 kb marker, Lane 1 to 4; pGEX4T-1 as control with 4.9 kb size, Lane 5to 8 recombinant clones, Lane 8 showing insert size 858 bp (B) Confirmation of recombinant clones using restriction digestion in pRSET-B M; marker, Lane 1; Control pRSET-B correlating 2.8 kb, Lane 2 to 6 showing recombinant clones with insert size 969 bp.
Additional file 4: Figure S2. Construction of knockout. (A) allelic exchange substrate construction M: 1 kb Marker, Lane 1: SacB digested with van911 showing four fragments 3.6 kb, 1.6 kb, 979 bp, 567 bp, Lane 2& 3: 0148 AES construct showing four fragments 3.6 kb, 1.6 kb, 837 RA, 605LA. (B) Packaging of AES with phAE159. M: 1 kb ladder, Lane 1: phAE159 digested with pac-I showing 50 kb and insert 3.8 kb, Lane 2& 3 clones without insert, Lane 4& 5 clone digested with pac-I showing phAE159 50 kb and 6.6 kb AES (C) Confirmation of knockout using PCR M: 1 kb ladder, Lane 1: Rv DNA amplified with right arm, Lane 2,4,5: knockout DNA amplified with hyg Forward primer and right arm reverse primer, Lane 6: Rv DNA not showed amplification with hyg & reverse primer.
Additional file 5: Figure S3. Intersection of genes and role in drug resistance. Intersection of Rv0148, Htdy and EIS with the three drugs kanamycin, amikacin and streptomycin resulting in 57 interacting proteins.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.