Abstract
Background
Isocitrate dehydrogenase 1/2 (IDH1/2), BAP1, ARID1A and PBRM1 have been reported as the most frequent mutant genes in intrahepatic cholangiocarcinoma (ICC), and their relationships with clinicopathological features and prognosis were researched in this study.
Methods
We collected clinical data of 130 ICC patients from January 2012 to December 2017. The IDH1/2 mutation and loss of BAP1, ARID1A and PBRM1 expressions were detected by DNA sequencing or immunohistochemical methods, and histological subtype of ICCs was determined by hematoxylin-eosin, Alcian blue and S100P staining.
Results
IDH1/2 mutation was related to decreased preoperative serum total bilirubin (P = 0.039), ferritin (P = 0.000) and higher histological differentiation (P = 0.024), and was associated with prolonged disease-free survival (P = 0.009) and a trend toward increased overall survival (P = 0.126) in small duct type of ICCs. Immunohistochemical staining results of MsMab-1 were generally consistent with DNA sequencing for IDH1/2 mutant in ICCs (κ = 0.691). Only BAP1 expression loss was correlated to prolonged disease-free survival (P = 0.031) and overall survival (P = 0.041) in large duct type of ICCs.
Conclusions
IDH1/2 mutation is a favorable predictor and may be related to iron metabolism in small duct type of ICCs. Furthermore, we suggest that the detection of IDH1/2 mutation is indispensable to determine targeted therapy in small duct type ICCs, while it is not necessary in large duct of ICCs. MsMab-1 is a relatively effective multi-specific antibody against IDH1/2 mutant in ICCs. BAP1 expression loss was correlated with improved prognosis only in large duct type ICCs.
Keywords: Intrahepatic cholangiocarcinoma (ICC), IDH1/2 mutation, BAP1, ARID1A, PBRM1, Large duct type, Small duct type
Background
Tumors originated from the bile ducts can be subdivided anatomically into three subgroups: distal, perihilar, and intrahepatic cholangiocarcinoma (ICC). The ICCs represent approximately 20% of bile duct malignancies and account for 15–20% of primary liver cancers [1]. ICC is related to a dismal prognosis with a postoperative 5-year overall survival (OS) rates of 17% [2]. Meanwhile, the incidence of ICC is increasing in recent years [3]. With the application of next-generation sequencing technology, many frequent genetic mutations have been discovered in ICCs, such as isocitrate dehydrogenase 1/2 (IDH1/2), KRAS, BAP1, ARID1A, PBRM1 and FGFR2-fusion [4, 5]. Almost all literatures reported that the incidences of their mutations are more than 10% in cases with ICCs [4–9].
In fact, the distinction between ICC and perihilar cholangiocarcinoma at second-order biliary branches is someway artificial and seems to take in consideration of surgical implications rather than molecular, histological and anatomical aspects [10]. As proposed by several investigators [10–15], ICCs are much heterogeneous and can be histologically classified into two subtypes according to the size of displayed bile duct: large duct type and small duct type (also known as bile duct type and cholangiolar type). Moreover, this category individuates ICC subgroups with different clinical and molecular features [12, 13]. For example, large duct type ICC representatively lacks IDH1/2 mutation and FGFR2-fusion, characteristics exclusively seen in small duct ICC [14]. Whereas, KRAS mutation was detected in 23% of large duct type and 1% of small duct type ICC respectively (P = 0.00003) [15].
IDH1 and IDH2 genes encode the NADP+-dependent isocitrate dehydrogenase in the cytoplasm and mitochondria respectively, catalyzing the conversion from isocitrate to α-ketoglutarate (α-KG). IDH1/2 mutations have been detected in many human cancers, including glioma, chondrosarcoma, acute myeloid leukemia, and cholangiocarcinoma [16–19]. These mutations acquire neomorphic activity that transforms α-KG into oncometabolite 2-hydroxyglutarate (2-HG). 2-HG accumulation may inhibit specific α-KG dependent dioxygenases and participate in tumorigenesis involving cell signaling, extracellular matrix maturation, and epigenetic regulation [20, 21]. Although previous studies have researched the association of IDH mutation with prognosis and pathology in ICC patients, the data are conflicting [4, 6, 7].
BAP1, ARID1A and PBRM1 represent the very frequently mutated histone modifying and chromatin remodeling genes in human cancer, such as renal cell carcinoma, ovarian carcinoma and ICC [4, 22, 23]. Due to the diversity of mutational hot-spots and the large scales of these genes, detection of these mutations is relatively difficult compared with IDH or KRAS. Alternatively, immunohistochemistry (IHC) staining is effective to detect the above mutations, because the presence of mutations correlates strongly with loss of the corresponding protein expressions [22, 24]. Some studies have estimated the association of these inactive mutations with clinical features and prognosis, but there are still many discrepancies [4, 8, 9, 25].
Here we investigated the mutational status of IDH1/2, BAP1, ARID1A and PBRM1, and for the first time, determined whether these mutations are correlated to clinicopathological characteristics and prognoses in each histological subtype of ICCs. Due to the heterogeneity of ICC, by analyzing their relationships in the subtypes, rather than the total cohort of ICCs, we could obtain more reasonable and accurate results to explain the inconsistencies reported previously [4, 6–9, 25].
Methods
Patients and tissue samples
For this study, we selected 130 consecutive patients with ICC who underwent surgical treatment at Tianjin Medical University Cancer Institute and Hospital from January 2012 to December 2017. Because of the challenge of determining exact anatomical origin of some tumors, the ICCs with similar features of perihilar carcinoma were excluded. Cases with combined HCC-CCA, composed of typical HCC and typical ICC, were excluded. All patients were reviewed to confirm the diagnosis of ICC and to restage according to the 8th edition 2017 AJCC staging system. After exclusion of 28 patients for prognostic analysis (including 11 cases lost to follow-up, 14 cases received non-R0 resection, 1 cases died of postoperative complications, and 2 cases died of some non-tumor-related causes), 130 and 102 patients were eventually used for comparisons of clinical characteristics and survival analyses respectively.
Formalin-fixed paraffin-embedded (FFPE) samples and hematoxylin-eosin (HE) staining slides of 130 surgical specimens were collected from Pathology Department of Tianjin Medical University Cancer Institute and Hospital. Tissue microarrays (TMAs) composed of 2-mm cores of FFPE tumor tissue were constructed for various staining by selecting a representative tumor areas and a typical peritumoral region from each case.
Clinical data
Clinical data, including the patients’ gender, age, surgical records, imaging examinations and laboratory tests were carefully reviewed and analyzed. All cases were regularly followed up every 3 months for tumor recurrence by screening of plasma tumor markers and imaging examinations. Disease-free survival (DFS) was measured from the date of surgery to the date of first recurrence or last follow-up, whereas OS was defined as the interval between the date of first diagnosis of ICC and the date of death or last follow-up.
Histopathology
HE staining was performed routinely on a set of TMAs. According to histological features, we subclassified ICCs into two types: large duct and small duct [11, 14, 15].
Type1 (large duct type) is made up of tall columnar tumor cells arranged in a large-sized glandular or tubular pattern and usually shows abundant desmoplastic stroma and low cellularity. Meanwhile, the tumor cells of type1 contain abundant eosinophilic, clear, or mucinous cytoplasm, and their nuclei are usually high grade. Extracellular mucin can be detected in glandular spaces (Fig. 1a).
Fig. 1.
The representative histology, mucin production and S100P expression of large duct type (a-c) and small duct type (d-f) of ICCs. These images were stained by HE (a, d), Alcian blue (b, e), and S100P (c, f), respectively. Scale bar represents 200 um
Type2 (small duct type) has histological features of small-sized tubular or acinar component composed of low columnar to cuboidal tumor cells and usually shows scant stroma and high cellularity. This type can also be arranged in cribrate or solid patterns. The tumor cells of Type2 have a higher nuclear-to-cytoplasmic ratio compared with type1 tumors and usually contain scanty amphophilic or eosinophilic cytoplasm (Fig. 1d).
If there was any doubt in the process of classification, we can refer to whole mount sections for further observation. Ultimately, some cases, not clearly classified, were termed undetermined type.
Mucin production was stained by Alcian Blue Stain Kit (pH 2.5) (G1560; Solarbio Science & Technology Co., Ltd., Beijing, China) on TMAs to distinguish subtypes of ICCs (Fig. 1b,e). Alcian blue staining was scored semiquantitatively by the proportion of glandular lumens with mucin production on a scale from 0 to 2: score 0, < 10%; score 1, 10 to 50%; and score 2, > 50% or frequent intracytoplasmic mucin [10, 26].
Immunohistochemistry
TMAs sections were dewaxed and rehydrated in xylene and gradient ethanol, respectively. After antigen retrieval and endogenous peroxidase activity blocking, the slides were incubated with primary antibodies (4 °C for 14 h and 37 °C for 1 h) and HRP-conjugated secondary antibody (37 °C for 1 h) in turn. Then the sections were visualized with 3,3′-diaminobenzidine (ZLI-9017; Zhongshan Goldbridge) for 5 min and counterstained with Hematoxylin. Appropriate internal or external positive controls and negative controls were designed and used for each round.
The primary antibodies used in the present study were as follows: S100P (clone EPR6142; dilution 1:300; Abcam, Cambridge, UK), IDH1/2 mutant (R132/172) (clone MsMab-1; dilution 1:200; EMD Millipore Corp., Billerica, MA, USA), BAP1 (ab199396; dilution 1:200; Abcam), ARID1A (clone EPR13501–73; dilution 1:500; Abcam), PBRM1 (HPA015629; dilution 1:200; Sigma-Aldrich).
The expression of S100P showed nuclear and cytoplasmic staining pattern and was evaluated semiquantitatively according to the percentage of positive tumor cells as follows: score 0, < 1%; score 1, 1 to 25%; score 2, 26 to 50%; score 3, 51 to 75%; and score 4, 76 to 100% [10]. (Fig. 1c,f).
IDH1/2 mutant exhibited cytoplasmic and mild nuclear expression, while only nuclear staining was interpreted as immunopositive in the evaluation of BAP1, ARID1A, and PBRM1 expression (Figs. 2 and 3). A semiquantitative scoring system was used for the above 4 factors in terms of the proportion of positive neoplastic cells: score 0 (negative), < 1%; score 1 (focal or regional positive), 1 to 90%; score 2 (diffuse positive), 91 to 100% [27, 28].
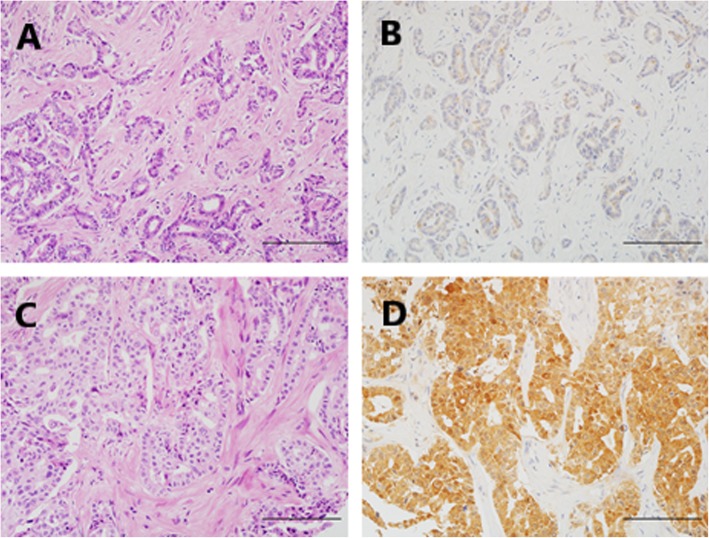
Fig. 2.
The representative HE and IHC images of IDH1/2 mutant negative (a, b) and positive (c, d) staining in cases with ICC. These corresponding images of HE (a, c) were presented as control of each IHC staining (b, d), respectively. Scale bar represents 200 um
Fig. 3.
The representative HE and IHC images of BAP1 (a-d), ARID1A (e-h) and PBRM1 (i-l) expression in cases with ICC. The nuclear staining was detected in cases with the normal expression of BAP1 (b), ARID1A (f) and PBRM1 (j). Similarly, the loss of BAP1 (d), ARID1A (h) and PBRM1 (l) expression were interpreted as inactive mutations of these genes. These corresponding images of HE (a, c, e, g, i, k) were presented as control of each IHC staining (b, d, f, h, j, l), respectively. Scale bar represents 200 um
Analysis of IDH1/2 mutation
Genomic DNA of ICCs was extracted from ten 10-um-thick sections micro-dissected from FFPE tissue blocks. Two pairs of primers were designed for hotspot mutations of IDH1/2 [10]. The first pair of primers for IDH1-R132: forward 5′- ACACGACGCTCTTCCGATCTACACATACAAGTTGGAAATTTCTGG-3′ and reverse 5′- GACGTGTGCTCTTCCGATCTAATCACCAAATGGCACCATAC-3′; the second pair of primers for IDH2-R140 and IDH2-R172: forward 5′-ACACGACGCTCTTCCGATCTCAGAGACAAGAGGATGGCTAGG-3′ and reverse 5′-GACGTGTGCTCTTCCGATCTTGTCCTCACAGAGTTCAAGCTG-3′ (Tsingke Biological Technology CO., Ltd., Beijing, China). The target DNA sequences were amplified by polymerase chain reaction using the primers above, and were further sequenced and analyzed for IDH1/2 mutation.
Statistical analysis
Categorical variables were presented as totals and frequencies, and evaluated by chi-square test or Fisher exact test, as appropriate; Continuous variables were described as means with standard deviations or medians with ranges, and compared with t test or Mann-Whitney U test, as appropriate. DFS and OS were calculated by Kaplan-Meier method, and assessed by log-rank test for univariate analysis. Cox proportional hazards model was conducted to adjust for the bias from tumor characteristics (e.g. TNM stage, bilateral involvement) in analyzing prognostic value of gene mutations and other clinical factors. The results were recorded as hazard ratios (HR) and 95% confidence intervals (CIs). A two-tailed P value less than 0.05 was considered significant. SPSS statistical program (IBM SPSS Statistics 24, Chicago, IL, USA) was used for data analysis.
Results
Large duct and small duct type of ICCs
Subclassification of ICCs
We classified 130 cases with ICC into 3 subtypes on the basis of histological features, S100P expression and Alcian blue staining in the first round. 12 cases (9.2%) were recognize as typical large duct type, which met three standards: HE, type1; S100P, score 3–4; and Alcian blue, score 1–2. Meanwhile, 73 cases (56.2%) were typical small duct type, which satisfied the following conditions: HE, type2; S100P, score 0–1; and Alcian blue, score 0–1. The remaining 45 cases (34.6%) were intermediate type that exhibited mixtures of two subtypes or indeterminate features (Supplementary Table S1).
In the second round, according to the above criteria, we set classification efficiency of three factors as follows: HE > S100P > Alcian blue. Then 45 cases of intermediate type were divided into 2 subcategories (Supplementary Table S2). Finally, we identified 27 (20.8%) and 103 (79.2%) cases as large duct and small duct type ICCs, respectively (Table 1) The concordant rate and κ value of interpretations between two observer were 96.9% and 0.904, respectively.
Table 1.
Integrated results of the ICC subclassification
| Subtype | HE | AB | S100P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Large | Small | underdetermined | 0 | 1 | 2 | 0 | 1 | 2 | 3 | 4 | |
| Large duct | 12 | 0 | 15 | 3 | 6 | 18 | 0 | 2 | 0 | 10 | 15 |
| Small duct | 0 | 86 | 17 | 92 | 8 | 3 | 68 | 20 | 6 | 9 | 0 |
Clinical characteristics and prognosis of ICC subtypes
Our study cohort included 71 males (54.6%) and 59 females (45.4%), with a mean age of 57.8 ± 9.7 years (range from 28 to 77). 71 cases (54.6%) underwent lymphadenectomy.
The large duct type was more likely to have higher level of CA19–9 (P = 0.002), higher frequencies of lymphadenectomy (P = 0.002), nerve invasion (P = 0.025), satellite lesions (P = 0.009), smaller size of tumor (P = 0.021) and more aggressive tumor stage of pT (P = 0.041), pM (P = 0.019), and TNM classification (P = 0.04) than small duct type (Supplementary Table S3).
The median follow-up period of 102 ICC cases was 25.2 months (range from 4.9 months to 100.0 months). Although patients with large duct type had worse prognosis than those with small duct type in univariate analysis (DFS, P = 0.063; OS, P = 0.031) (Figure S1), the histological subtype was not an independent prognostic factors of ICCs in multivariate analysis (P > 0.10).
IDH1/2 mutations in ICCs
IDH1/2 mutations by DNA sequencing and IHC
DNA sequencing showed 21 patients (16.1%) harbored IDH1/2 mutation, including IDH1-R132C, IDH1-R132G, IDH1-R132H, IDH1-R132L, IDH2-R172K, and IDH2-R172W. But no mutation of IDH2-R140 was detected. Meanwhile, IDH1/2 mutant was detected in 14 cases (10.8%) by MsMab-1, which is specific for IDH1-R132G, IDH1-R132H, and IDH2-R172W according to instructions (Table 2). IHC analysis verified the specificity of MsMab-1 according to results detected by DNA sequencing, and showed that sensitivity and specificity of MsMab-1 to detect specific types of IDH1/2 mutation were 81.8% (9 of 11) and 95.8% (114 of 119), respectively. Accordingly, MsMab-1 was a relatively effective multi-specific antibody against IDH1/2 mutant in ICCs (κ = 0.691).
Table 2.
Comparisons of IDH1/2 mutations in ICCs between DNA sequencing and IHC methods
| DNA sequencing | MsMab-1 staining | |||||
|---|---|---|---|---|---|---|
| Mutant gene | Nucleotide change | Amino acid change | Number | Positve | Negative | Reactivity by manual |
| IDH1 | CGT > TGT | R132C | 6 | 1 | 5 | No |
| IDH1 | CGT > GGT | R132G | 5 | 4 | 1 | Yes |
| IDH1 | CGT > CAT | R132H | 4 | 4 | 0 | Yes |
| IDH1 | CGT > CTT | R132L | 2 | 1 | 1 | No |
| IDH2 | AGG > TGG | R172W | 2 | 1 | 1 | Yes |
| IDH2 | AGG > AAG | R172K | 2 | 0 | 2 | No |
| Wild type | 109 | 3 | 106 | No | ||
Clinical implications of IDH1/2 mutation in small duct type of ICCs
IDH1/2 mutation was detected in 3.7% (1/27) of cases with large duct type and 19.4% (20/103) of patients with small duct type, respectively. Patients with IDH1/2 mutation had decreased TBIL (total bilirubin) (P = 0.039), Fe (ferritin) (P = 0.000) and higher histological differentiation (P = 0.024) in small duct type (Table 3).
Table 3.
Comparisons of clinicopathological characteristics between IDH1/2 mutant and wild type of ICCs
| Clinical variables | Total cohort | Small duct type | ||||
|---|---|---|---|---|---|---|
| IDH1/2 mutant type | IDH1/2 wild type | P-valve | IDH1/2 mutant type | IDH1/2 wild type | P-valve | |
| TBIL (umol/L) | 0.122 | 0.039 | ||||
| < 21 | 20 (95.2%) | 85 (78.7%) | 20 (100.0%) | 65 (79.3%) | ||
| ≥ 21 | 1 (4.8%) | 23 (21.3%) | 0 (0.0%) | 17 (20.7%) | ||
| INR | 0.025 | 0.054 | ||||
| <1.0 | 17 (81.0%) | 59 (54.6%) | 16 (80.0%) | 47 (56.6%) | ||
| ≥ 1.0 | 4 (19.0%) | 49 (45.4%) | 4 (20%) | 36 (43.4%) | ||
| CEA (ug/L) | 0.162 | 0.067 | ||||
| < 5 | 19 (90.5%) | 82 (75.9%) | 19 (100.0%) | 68 (81.9%) | ||
| ≥ 5 | 2 (9.5%) | 26 (24.1%) | 0 (0.0%) | 15 (18.1%) | ||
| Fe (ug/L) | 0.000 | 0.000 | ||||
| < 200 | 17 (85.0%) | 42 (41.2%) | 17 (89.5%) | 34 (44.2%) | ||
| ≥ 200 | 3 (15.0%) | 60 (58.8%) | 2 (10.5%) | 43 (55.8%) | ||
| Histological differentiation | 0.028 | 0.024 | ||||
| Low | 5 (23.8%) | 53 (50.0%) | 5 (25.0%) | 44 (53.0%) | ||
| Moderately to high | 16 (76.2%) | 53 (50.0%) | 15 (75.0%) | 39 (47.0%) | ||
| Histological subtype | 0.074 | |||||
| Large duct | 1 (4.8%) | 26 (23.9%) | ||||
| Small duct | 20 (95.2%) | 83 (76.1%) | ||||
Univariate and multivariate analyses exhibited that cases with IDH1/2 mutation had significantly improved prognosis than those without IDH1/2mutation in terms of DFS (P = 0.006) and OS (P = 0.031) in total cohort of ICCs (Fig. 4a-b, Supplementary Table S4). And more notably, Kaplan-Meier analysis showed IDH1/2 mutation was associated with prolonged DFS (P = 0.009) and a trend toward increased OS (P = 0.126) in small duct type of ICCs. Further multivariate analysis confirmed IDH1/2 mutation was a favorable independent predictor of DFS (P = 0.022, HR = 3.452, 95% CI = 1.191–10.004), and was not significantly related to OS (P = 0.114, HR = 2.686, 95%CI = 0.789–9.144) in patients with small duct type (Fig. 4c-d, Table 4).
Fig. 4.
Kaplan-Meier curves showing patients with IDH1/2 mutation had prolonged DFS (a) and OS (b) in total cohort of ICCs; and significant prolonged DFS (c) and a trend toward increased OS (d) were associated with IDH1/2 mutation in small duct type of ICCs
Table 4.
Univariate and multivariate analyses for prognostic value of frequent mutations in the subtypes of ICCs
| Clinical variables | DFS in large duct type | OS in large duct type | DFS in small duct type | OS in small duct type | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate P-value |
Multivariate P-valve/ HR (95% CI) |
Univariate P-value |
Multivariate P-valve/ HR (95% CI) |
Univariate P-value |
Multivariate P-valve/ HR (95% CI) |
Univariate P-value |
Multivariate P-valve/ HR (95% CI) |
|
| IDH1/2 | 0.009 | 0.022 | 0.126 | 0.114 | ||||
| Mutant vs. wild | 3..452 (1.191–10.004) | 2.686 (0.789–9.144) | ||||||
| BAP1 | 0.031 | 0.059 | 0.041 | 0.039 | 0.952 | 0.454 | ||
| Loss vs. retained | 1.711 (0.979–2.990) | 2.985 (1.038–4.186) | ||||||
| ARID1A | 0.432 | 0.580 | 0.357 | 0.626 | ||||
| Loss vs. retained | ||||||||
| PBRM1 | 0.672 | 0.159 | 0.284 | 0.614 | ||||
| Loss vs. retained | ||||||||
Expression status of BAP1, ARID1A and PBRM1 in ICCs
Loss of BAP1, ARID1A and PBRM1 expressions
BAP1 expression was retained in 72 cases (55.4%), while 47 cases (36.2%) and 11 cases (8.5%) showed complete and focal loss of BAP1 expression respectively. Complete or regional loss of ARID1A and PBRM1 staining was observed in 28 cases (21.5%) and 45 cases (34.6%), respectively. (Supplementary Table S5) There was obviously positive correlation among their expression loss (P < 0.05), especially between BAP1 and PBRM1 (P = 0.000) (Supplementary Table S6).
Clinical implications of BAP1, ARID1A and PBRM1 expression loss in subtypes of ICCs
The loss of BAP1 expression was associated with decreased ALT (alanine aminotransferase), AST (aspartate aminotransferase), TBIL and higher histological differentiation in large type of ICCs, while it was correlated to larger size of tumor in small duct type (Supplementary Table S7). All clinical factors significantly related to the loss of ARID1A expression, were within small duct type of ICCs, such as decreased ALT, AST, lower incidence of liver cirrhosis and earlier TNM stage (Supplementary Table S8). PBRM1 loss correlated decreased AST, TBIL, IBIL (indirect bilirubin), and CA19–9 in the large duct type of ICCs (Supplementary Table S9). In addition, the loss of BAP1, ARID1A and PBRM1 expressions had similar distribution in large duct and small duct type of ICC patients (P > 0.05) (Supplementary Table S7–9).
Kaplan-Meier curves showed loss of BAP1, ARID1A and PBRM1 expressions were not significantly associated DFS and OS in patients with ICCs (P > 0.05) (Supplementary Table S4) While univariate and multivariate analyses exhibited BAP1 expression loss was correlated to prolonged DFS (P = 0.031) and OS (P = 0.041) in large duct type of ICCs (Fig. 5). Besides this, none of inactive mutations was statistically associated with DFS or OS in either subtype of ICCs (P > 0.05) (Table 4).
Fig. 5.
Kaplan-Meier curves showing patients with loss of BAP1 had improved DFS (a) and OS (b) in large duct type of ICCs
A typical case of ICC patient with IDH1 mutation
A 61-year-old Chinese man was diagnosed with liver cancer and diffuse intrahepatic and abdominal lymph node metastasis by abdominal magnetic resonance (MR) in May 2017, and subsequently confirmed as ICC (stage IV) with IDH mutation (IDH1-R132C) by biopsy and gene sequencing. He received dasatinib (100 mg, once a day) combined with chemotherapy (gemcitabine, once every three weeks, twenty-five cycles) since June 2017. Abdominal MR in July 2017 showed stable disease (SD). Abdominal MR in January 2019 revealed progressive disease (PD). Therefore, in the same month, this patient was administered with lenvatinib (12 mg, once a day) instead of dasatinib and chemotherapy. Encouragingly, this case still survives with ECOG performance status 1 at the end of this study (September 2019). Figure 6 summarized the patient’s serial images of abdominal MR and diameter curves of representative tumors during follow-up period.
Fig. 6.
The serial images of abdominal MR (a) and diameter curves of 3 representative tumors (b) from a typical case during follow-up period. Three representative tumors were marked with corresponding colored arrows in the first MR image
Discussion
In the present study, we identified 27 (20.8%) and 103 (79.2%) cases as large duct and small duct type ICCs respectively, according to accepted classification criteria [10, 14], but the proportion of large duct type in ICCs was lower than previously reported [10, 15]. There are two possible explanations. Firstly, to ensure the accuracy of this research, we excluded many large duct type ICCs with characteristics similar to those of perihilar carcinoma. Secondly, ICC patients with cholelithiasis, often recognized as large duct type [15], were rarely admitted to our hospital.
Consistent with previous reports [11, 13, 15], we found that ICC patients with large duct type had worse prognosis than those with small duct type in univariate analysis. However, histopathological subtype was not an independent predictor of postoperative survival in multivariate analysis. Furthermore, the small duct type ICCs were closely associated with earlier tumor TNM stage. Accordingly, the bias of tumor stage from both subtypes may account for the inconsistency, and the histological subtype may not be an effective prognostic factor in patients with ICCs.
As reported in previous studies, IDH1/2 mutations were detected in 10.2–28.8% of ICCs [4, 6, 7, 29], and exclusively in small duct type of ICCs [10, 15]. In the present study, we found that 16.1% of patients with ICC harbored IDH1/2 mutation. Meanwhile, the incidence of IDH1/2 mutation in large duct and small duct type of ICCs was 3.7 and 19.4%, respectively. These results are consistent with previous reports. Consequently, the clinical features and prognostic value of IDH1/2 mutations should be investigated in small duct type, rather than in large duct type. However, previous studies have revealed inconsistent results regarding prognostic value of IDH mutation in ICCs. Yuchen Jiao, et al. [4] identified that ICC patients with IDH1/2 mutation had 3-year overall survival of 33% compared to 81% for patients with IDH1/2 wild-type (P = 0.0034), but Pu Wang, et al. [7] showed IDH1/2 mutation was significantly associated with improved prognosis in ICCs (4-years tumor recurrence rate was 45.3% vs. 71.5%; P = 0.046). At the same time, one study on advanced stage ICCs from Massachusetts General Hospital Cancer Center [29] found that there were no significant differences in DFS and OS between patients with IDH mutation and patients with IDH wild-type. In the present study, univariate and multivariate analyses showed patients with IDH1/2 mutation had significant prolonged DFS and a trend toward increased OS in small duct type of ICCs. Furthermore, IDH1/2 mutations were associated with higher histological differentiation, but not tumor stage. Therefore, these findings suggest that IDH1/2 mutation is a favorable predictor for small duct type ICCs. Due to worse prognosis of large duct type ICCs, cases with IDH1/2 mutation had significantly better DFS and OS than those with IDH1/2 wild-type in total cohort of ICCs. Our results may be account for the conflicting predictive values of IDH1/2 mutation in ICCs described above. In small duct type of ICCs, IDH1/2 mutations were correlated to a trend toward improved OS though the differences were not statistically significant. The inconsistence in DFS and OS maybe ascribed to the development of chemotherapy, immunotherapy, chemotherapy, local regional therapy and combination of different modalities, which obviously prolonged survival even after tumor recurrence. We further analyzed the treatments of ICC patients after recurrence and found that cases with small bile duct type were mostly to be treated with chemotherapy or palliative care. Coincidentally, a total of 9 patients underwent reoperation or radiofrequency ablation (RFA), all of which were classified as IDH wild-type. Therefore, this discrepancy between DFS and OS may be explained by the bias of different treatments after tumor recurrence in two groups. Moreover, the typical case described in methods who reached long-term (20 months) SD after treated with dasatinib also proved that ICC patient with IDH1/2 mutation had a better prognosis, even if the tumor is of advanced stage. In summary, IDH1/2 mutation may be a favorable predictor of small duct type ICCs, and have higher prognostic value for DFS than for OS.
Some investigators have found that IDH1/2 mutation was correlated to several clinical characteristics in ICCs, such as lower level of CA19–9, lower incidence of lymph node metastasis and smaller size of tumors [6, 29]. Interestingly, these clinical features were also observed in the small duct type of ICCs in our study. Since IDH1/2 mutations were typically detected in small duct type of ICCs, we considered these factors (CA19–9, lymph node metastasis and size of tumor) might be associated with small duct type, rather than IDH1/2 mutation. We firstly found that IDH1/2 mutation was strongly associated with decreased preoperative Fe and total bilirubin in small duct type of ICCs. Although the association of IDH1/2 mutation with iron and bilirubin metabolism has not been reported, previous studies have showed that in tricarboxylic acid cycle, iron homeostasis is regulated by haem and citric acid, both of which are closely related to the activity of IDH [30, 31]. Accordingly, the association between IDH1/2 mutation and iron metabolism may provide a new direction for the function study of IDH1/2 mutation in ICCs. Meanwhile, ICCs with IDH1/2 mutation, often categorized as small duct type, localized at the periphery of bile duct, rarely resulting in biliary obstruction in contralateral bile duct, which can explain the relationship between IDH1/2 mutation and lower level of serum total bilirubin.
It should be noted that IDH1/2 mutation is the most frequent mutant type of gliomas and can be detected by either DNA sequencing or IHC methods. Although DNA sequencing is regarded as gold standard, IHC seems to be more accurate, easier to perform and cheaper for detecting IDH1/2 mutation in glioma patients [32]. We firstly used MsMab-1 for detecting IDH1/2 mutation in ICCs by IHC method, and found that the results were basically consistent with those obtained by DNA sequencing. However, IDH1-R132C and IDH1-R132L, two common mutant types in ICCs, cannot be recognized by IHC so far, because their specific antibodies have not been commercially available yet. Therefore, multi-specific antibodies against hotspot mutations of IDH1/2 in ICCs need to be constructed and validated effectively in future.
Surgery remains the mainstream of potentially curative treatment for patients with resectable ICCs. Chemotherapy and radiotherapy are used as first-line therapy for advanced or relapsed ICC patients who are not candidate for surgery. Molecular targeted therapy and immunotherapy are developing very fast in the era of individualized and precision medicine [33]. Because of the low incidence of IDH1/2 mutations (3.7%), postoperative chemotherapy is recommended for ICC patients with large duct type without the need for gene sequencing. In contrast, due to the high incidence of IDH1/2 mutations (19.4%) in small bile duct type of ICCs, DNA sequencing should be recommend to detect IDH1/2 mutation and choose appropriate adjuvant therapies. If IDH1/2 mutation is detected, s small molecular medications that target IDH1/2 (dasatinib, ivosidenib or enasidenib) could be a promising treatment strategy [34–36]. With the development of specific antibodies against mutant IDH1/2, IHC will be an easy and cheap way to detect IDH1/2 mutation.
In addition, the clinical implications of other most frequent mutations (including BAP1, ARID1A and PBRM1) were investigated in each subtype of ICCs. Surprisingly, these inactive mutations were not significantly associated DFS and OS in small duct type of ICCs. Nonetheless, only BAP1 expression loss was correlated to prolonged DFS and OS in the large duct type of ICCs. Many studies [4, 25, 28] showed that these factors have no prognostic value in ICCs, but Sarcognato S et al. [8] found that the retained expression of BAP1 was correlated to poor prognosis, while another two studies [8, 9] got to conflicting conclusions about predictive value of ARID1A in ICCs. Accordingly, our study may explain the inconsistency in the prognostic value of BAP1: loss of BAP1 expression was correlated to improved survival in large duct type ICCs rather than in small duct type ICCs. When two subtypes were combined into one group, BAP1 can be considered as a favorable or irrelevant predictor in ICCs according to the ratio of large duct to small duct type ICCs.
There were limitations in this study. Firstly, the sample size is too small, especially for patients with large duct type ICC. This is why KRAS mutation, frequent in large duct type ICC, was not included in this study. Secondly, FGFR2-fusion, another frequent mutation in ICCs, was not investigated in this research.
Conclusion
IDH1/2 mutation is a favorable predictor and may be related to iron metabolism in small duct type of ICCs. Furthermore, we suggest that the detection of IDH1/2 mutation is indispensable to determine targeted therapy in small duct type ICCs, while it is not necessary in large duct of ICCs. MsMab-1 is a relatively effective multi-specific antibody against IDH1/2 mutant in ICCs. BAP1 expression loss was correlated with improved prognosis only in large duct type ICCs.
Supplementary information
Acknowledgements
We thank Fenglin Zang from Pathology Department of Tianjin Medical University Cancer Institute and Hospital for grading tissue sections.
Abbreviations
- ICC
Intrahepatic cholangiocarcinoma
- OS
Overall survival
- IDH
Isocitrate dehydrogenase
- α-KG
α-ketoglutarate
- 2HG
2-hydroxyglutarate
- IHC
Immunohistochemistry
- FFPE
Formalin-fixed paraffin-embedded
- HE
Hematoxylin-eosin staining
- TMA
Tissue microarray
- DFS
Disease-free survival
- HR
Hazard ratios
- CIs
Confidence intervals
- TBIL
Total bilirubin
- Fe
Ferritin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- IBIL
Indirect bilirubin
- MR
Magnetic resonance
- SD
Stable disease
- RFA
Radiofrequency ablation
- PD
Progressive disease
- DBIL
Direct bilirubin
- CEA
Carcinoembryonic antigen
Authors’ contributions
BM:study concept and design, data collection and analysis, experiment performing, manuscript writing, statistical analysis; HM: study concept and design, data analysis, manuscript writing; YT: data interpretation, technical and material support; YW, TS, TZ, QW, YC and HL: data collection; QL and WZ: study concept, data collection, data interpretation, and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by “National Natural Fund Project, 81572434” in constructing tissue microarrays and performing immunohistochemical analysis. The study was supported by “Ministry of Science and Technology, National Science and Technology Major Special Project: Prevention and Treatment of Major Infectious Diseases such as AIDS and Viral Hepatitis, 2018ZX10723204-007-001” in writing the manuscript. Generating DNA sequencing data was supported by a grant from “Young Medical Elites, Tianjin Health and Health Commission, 2018.12-2020.11”. The study was also supported by “Young Innovative Talents, Tianjin Medical University Cancer Institute and Hospital, 2017.1-2019.12” in sample collection and data interpretation. These funding sources have no role in study design.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The Medical Ethics Committee of Tianjin Medical University Cancer Institute and Hospital approved this study (Approval No.: bc2019065). Informed consents were obtained from all patients or their legal guardian.
Consent for publication
The typical ICC patient with IDH1 mutation provided informed written consent to the use of clinical data and MR images for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Zhang, Email: zhangweitjch@163.com.
Qiang Li, Email: liqiang6011@126.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-06804-6.
References
- 1.El Rassi ZE, Partensky C, Scoazec JY, et al. Peripheral cholangiocarcinoma: presentation, diagnosis, pathology and management. Eur J Surg Oncol. 1999;25:375–380. doi: 10.1053/ejso.1999.0660. [DOI] [PubMed] [Google Scholar]
- 2.Li YY, Li H, Lv P, et al. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15:608–613. doi: 10.1007/s11605-011-1419-8. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomark Prev. 2006;15:1198–1203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 4.Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5:2839–2852. doi: 10.18632/oncotarget.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu AX, Borger DR, Kim Y, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol. 2014;21:3827–3834. doi: 10.1245/s10434-014-3828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarcognato S, Gringeri E, Fassan M, et al. Prognostic role of BAP-1 and PBRM-1 expression in intrahepatic cholangiocarcinoma. Virchows Arch. 2019;474:29–37. doi: 10.1007/s00428-018-2478-y. [DOI] [PubMed] [Google Scholar]
- 9.Simbolo M, Vicentini C, Ruzzenente A, et al. Genetic alterations analysis in prognostic stratified groups identified TP53 and ARID1A as poor clinical performance markers in intrahepatic cholangiocarcinoma. Sci Rep. 2018;8:7119. doi: 10.1038/s41598-018-25669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi A, Misumi K, Shibahara J, et al. Distinct Clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic Cholangiocarcinoma. Am J Surg Pathol. 2016;40:1021–1030. doi: 10.1097/PAS.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 11.Aishima S, Kuroda Y, Nishihara Y, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31:1059–1067. doi: 10.1097/PAS.0b013e31802b34b6. [DOI] [PubMed] [Google Scholar]
- 12.Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 13.Akita M, Fujikura K, Ajiki T, et al. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol. 2017;30:986–997. doi: 10.1038/modpathol.2017.22. [DOI] [PubMed] [Google Scholar]
- 14.Kendall T, Verheij J, Gaudio E, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39:7–18. doi: 10.1111/liv.14093. [DOI] [PubMed] [Google Scholar]
- 15.Liau JY, Tsai JH, Yuan RH, et al. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27:1163–1173. doi: 10.1038/modpathol.2013.241. [DOI] [PubMed] [Google Scholar]
- 16.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai M, Nobusawa S, Ikota H, et al. Frequent IDH1/2 mutations in intracranial chondrosarcoma: a possible diagnostic clue for its differentiation from chordoma. Brain Tumor Pathol. 2012;29:201–206. doi: 10.1007/s10014-012-0085-1. [DOI] [PubMed] [Google Scholar]
- 18.McKenney AS, Levine RL. Isocitrate dehydrogenase mutations in leukemia. J Clin Invest. 2013;123:3672–3677. doi: 10.1172/JCI67266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao Q, Xia QY, Wang ZY, et al. Frequent co-inactivation of the SWI/SNF subunits SMARCB1, SMARCA2 and PBRM1 in malignant rhabdoid tumours. Histopathology. 2015;67:121–129. doi: 10.1111/his.12632. [DOI] [PubMed] [Google Scholar]
- 25.Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misumi K, Hayashi A, Shibahara J, et al. Intrahepatic cholangiocarcinoma frequently shows loss of BAP1 and PBRM1 expression, and demonstrates specific clinicopathological and genetic characteristics with BAP1 loss. Histopathology. 2017;70:766–774. doi: 10.1111/his.13127. [DOI] [PubMed] [Google Scholar]
- 27.Preusser M, Wöhrer A, Stary S, et al. Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1-R132H mutation in diffuse glioma biopsy specimens. J Neuropathol Exp Neurol. 2011;70:715–723. doi: 10.1097/NEN.0b013e31822713f0. [DOI] [PubMed] [Google Scholar]
- 28.Andrici J, Goeppert B, Sioson L, et al. Loss of BAP1 expression occurs frequently in intrahepatic Cholangiocarcinoma. Medicine (Baltimore) 2016;95:e2491. doi: 10.1097/MD.0000000000002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal L, Govindan A, Sheth RA, et al. Prognosis and Clinicopathologic features of patients with advanced stage Isocitrate dehydrogenase (IDH) mutant and IDH wild-type intrahepatic Cholangiocarcinoma. Oncologist. 2015;20:1019–1027. doi: 10.1634/theoncologist.2015-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerins MJ, Vashisht AA, Liang BX, et al. Fumarate mediates a chronic proliferative signal in Fumarate Hydratase-inactivated Cancer cells by increasing transcription and translation of ferritin genes. Mol Cell Biol. 2017;37:e00079–e00017. doi: 10.1128/MCB.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson S. Do mitochondria regulate cellular iron homeostasis through citric acid and haem production? Implications for cancer and other diseases. Med Hypotheses. 2003;60:106–111. doi: 10.1016/S0306-9877(02)00342-0. [DOI] [PubMed] [Google Scholar]
- 32.Babakoohi S, Lapidus RG, Faramand R, et al. Comparative analysis of methods for detecting Isocitrate dehydrogenase 1 and 2 mutations and their metabolic consequence, 2-Hydroxyglutarate, in different neoplasms. Appl Immunohistochem Mol Morphol. 2017;25:334–337. doi: 10.1097/PAI.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 33.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popovici-Muller J, Lemieux RM, Artin E, et al. Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9:300–305. doi: 10.1021/acsmedchemlett.7b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kats LM, Vervoort SJ, Cole R, et al. A pharmacogenomic approach validates AG-221 as an effective and on-target therapy in IDH2 mutant AML. Leukemia. 2017;31:1466–1470. doi: 10.1038/leu.2017.84. [DOI] [PubMed] [Google Scholar]
- 36.Saha SK, Gordan JD, Kleinstiver BP, et al. Isocitrate dehydrogenase mutations confer Dasatinib hypersensitivity and SRC dependence in intrahepatic Cholangiocarcinoma. Cancer Discov. 2016;6:727–739. doi: 10.1158/2159-8290.CD-15-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.