Abstract
Platinum-catalyzed selective C–H acylation of 2-aryloxypyridines with ethyl chlorooxoacetate provides an efficient way of introducing an α-keto ester functional group. The reaction is oxidant-free, additive-free, and, more significantly, free of any decarbonylative side reactions. The reaction tolerates a variety of substituents from strongly electron-donating to strongly electron-withdrawing groups. Double acylation is feasible for 2-phenoxypyridine and its derivatives with only one substituent at the para position. Although the reaction of 2-(2-methylphenoxy)pyridine with ethyl malonyl chloride did not produce the desired β-keto ester, the reaction with ethyl succinyl chloride proceeded smoothly to give the γ-keto ester. Ethyl chlorooxoacetate is much more reactive than ethyl succinyl chloride in this Pt-catalyzed C–H acylation reaction.
Introduction
α-Keto ester derivatives are important not only because they are present in many biologically and medicinally important organic molecules1−4 but also because α-keto esters are immediate precursors to other important organic compounds such as α-hydroxy acids and α-amino acids. α-Keto esters also have important applications in other organic synthesis including the synthesis of heterocycles.5 Introduction of an α-keto ester functional group is therefore of great importance, and various methods have been developed for this purpose.5,6
One of the most straightforward methods involves the use of readily accessible and inexpensive ethyl chlorooxoacetate that already possesses the α-keto ester functionality. There are two common ways to utilize this reagent. One involves the coupling of an organometallic reagent with ethyl chlorooxoacetate,7 but the drawback is that the commonly used organometallic reagents such as Grignard reagents are too reactive so the reaction has to be performed at very low temperatures or side products may be expected. The other is through the Friedel–Crafts acylation reaction of arenes with ethyl chlorooxoacetate.8−11 This method suffers from a limited substrate scope because of issues of reactivity and selectivity associated with Friedel–Crafts acylation and the use of excess amounts of strong Lewis acids. A potentially more attractive method is the transition metal-catalyzed C–H acylation12 reaction with ethyl chlorooxoacetate as the acylating reagent. However, so far, there has been no report of such a transition metal-catalyzed C–H acylation reaction to synthesize α-keto esters.
The challenge with using ethyl chlorooxoacetate in the transition metal-catalyzed C–H acylation reaction or cross coupling is probably the decarbonylation side reaction. In fact, decarbonylation is so common that it has been frequently exploited in metal-catalyzed decarbonylative coupling reactions.12−16 For example, in an attempt to synthesize α-keto esters through Pd-catalyzed acylation with ethyl glyoxylate as the acylating reagent and tert-butyl hydrogen peroxide as the oxidant, the desired product was not formed, but interestingly, the decarbonylative product was formed exclusively.16 Herein, we report a highly efficient method to introduce an α-keto ester functional group to 2-aryloxypyridines through platinum-catalyzed direct C–H functionalization with ethyl chlorooxoacetate. It should be noted that aryl heteroaryl ethers are frequently found in biologically active compounds.17−19 There has been considerable interest in modifying aryl heteroaryl ethers through transition metal-catalyzed C–H functionalization reactions,20−35 where the heteroaryl also serves as the directing group.
Results and Discussion
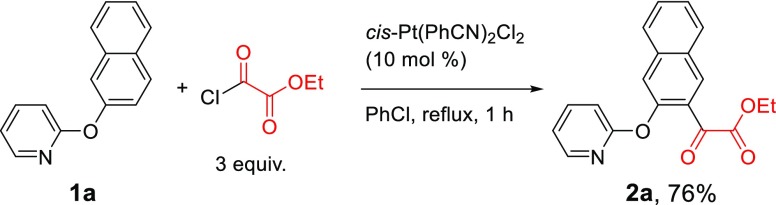
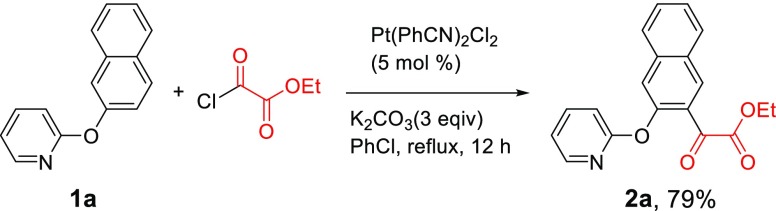
We have recently reported a unique Pt-catalyzed C–H acylation reaction with acyl chlorides,31 which, unlike other transition metal-catalyzed C–H acylation reactions, requires neither an oxidant nor additives. The reaction is accelerated with more electron-deficient acyl chlorides. We hypothesized that a chlorooxoacetate ester should possess higher reactivity because the electron-withdrawing ester group would make the carbonyl chloride more electron deficient. Although there was a concern of troublesome decarbonylation mentioned above, we performed the Pt-catalyzed C–H acylation reaction of 2-(naphthalen-2-yloxy)pyridine (1a) with ethyl chlorooxoacetate under the optimized conditions for the previous acylation reaction.31 To our delight, the reaction proceeded smoothly and was nearly complete within 1 h, producing the desired α-keto ester 2a (Scheme 1). The α-keto ester group was introduced to the 3-position of the naphthyl ring. The 1-position is obviously more sterically hindered. The reaction was very clean, and the gas chromatography (GC) analysis of the crude reaction mixture showed only the product peak along with a very small amount of starting material. However, initially, the product was isolated in only 57% yield. We attributed the low isolated yield to the possibility that some of the product 2a might have remained coordinated to the platinum after the reaction was complete. Indeed, when the reaction mixture was treated with pyridine before normal aqueous workup, where a ligand exchange with pyridine is expected to release the product 2a from the platinum, the isolated yield was improved to 76% (Scheme 1).
Scheme 1. Pt-Catalyzed C–H Acylation of 1a with Ethyl Chlorooxoacetate.
Another interesting finding is that cis-Pt(PhCN)2Cl236 was more reactive than trans-Pt(PhCN)2Cl237 in catalyzing this C–H acylation reaction. Although the reaction with cis-Pt(PhCN)2Cl2 as the catalyst resulted in 80% conversion in 1 h, the use of the trans-Pt(PhCN)2Cl2 achieved only about 30% conversion under the same conditions. The cause of the different reactivities of the two isomeric platinum complexes is not clear, and further mechanistic study is necessary to unveil this factor. In sharp contrast, palladium complexes including PdCl2, Pd(MeCN)2Cl2, and Pd(OAc)2 have been tested, but no reaction was observed under otherwise the same conditions. In a recent report on palladium-catalyzed C–H acylation of 1-naphthylamines with acyl chloride, it was also demonstrated that without a base additive such as NaOAc, the palladium catalyst alone was not effective at all.38 The possible reason for the failure of palladium catalysts could be that the 2-aryloxypyridines may not undergo cyclometalation with PdCl2 under the reaction conditions.39
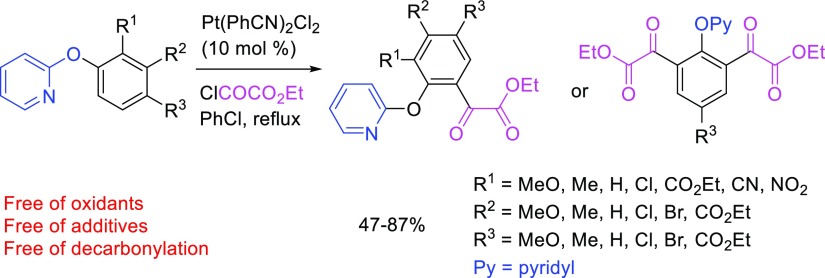
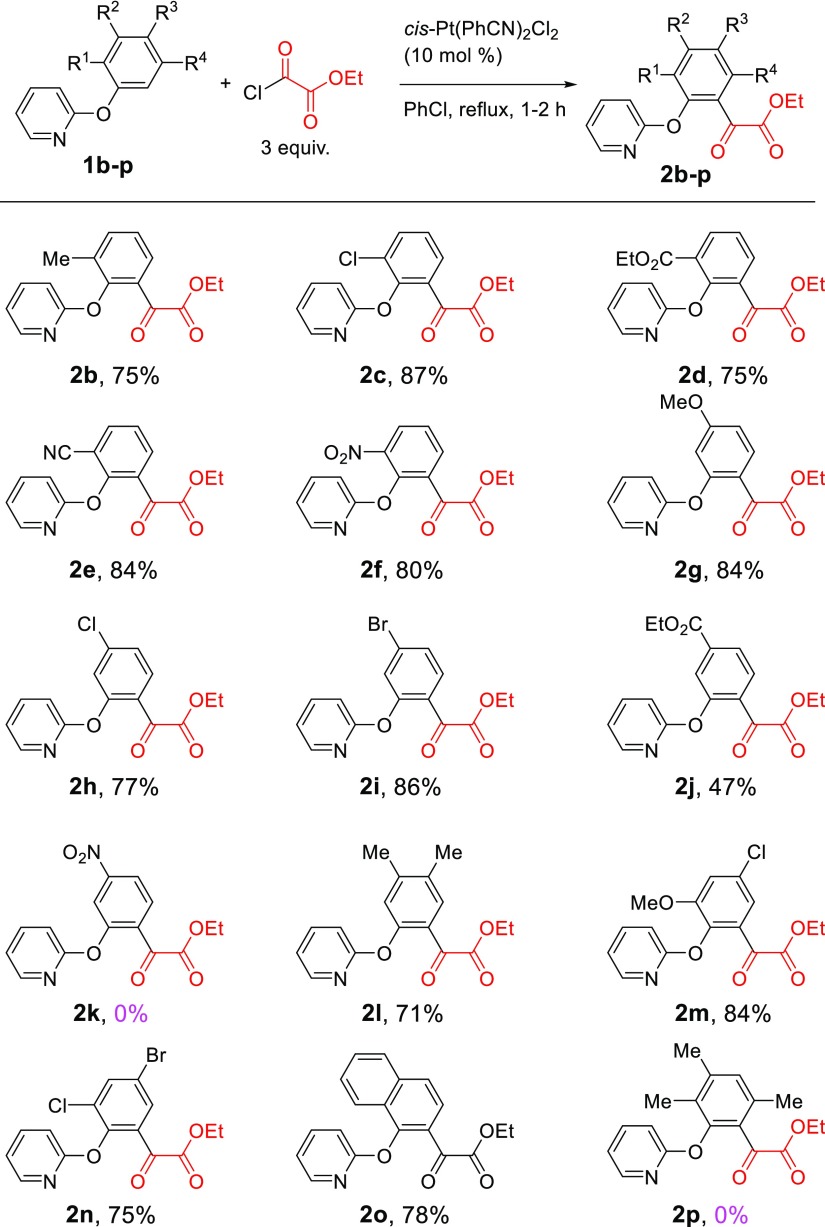
The reaction worked well with a variety of 2-aryloxypyridines. As shown in Scheme 2, for ortho-substituted 2-phenoxypyridines (1b–1f), the α-keto ester group was introduced to the other ortho position selectively in high yields (2b–2f, 75–87% yields). Substituents ranging from strongly electron-donating groups (MeO, 1b) to strongly electron-withdrawing groups (NO2, 1f) are well accommodated. All reactions were completed within 1–2 h. For the meta-substituted 2-aryloxypyridines (1g–1j) also, only one α-keto ester group was introduced to the ortho position that is less sterically hindered (2g–2j). Yields were generally high except for the substrates with a strongly electron-withdrawing group such as an ester group (2j). In particular, 2-(3-nitrophenoxy)pyridine (1k) did not undergo the Pt-catalyzed acylation reaction to give 2k (0%) under the same conditions. It appeared that the electronic effect of the meta-substituents is more pronounced than that of ortho-substituents of the aryloxypyridines. Substrates with multiple substituents (1l–1o) also participated in the reaction to give the desired products in high yields (2l–2o, 71–84% yields). The reaction of 2-(2,3,5-Trimethylphenoxy)pyridine (1p) did not produce the acylated product 2p (0% yield), clearly indicating that the meta-substituent prevents the acylation from occurring at its adjacent ortho position of the phenyl ring because of the steric effect. Although a more thorough screening of substrates is desirable, we noticed that 2-phenylpyridine and acetanilide did not undergo the Pt-catalyzed C–H acylation reaction.
Scheme 2. Pt-Catalyzed Monoacylation of 2-Aryloxypyridines with Ethyl Chloroacetate.
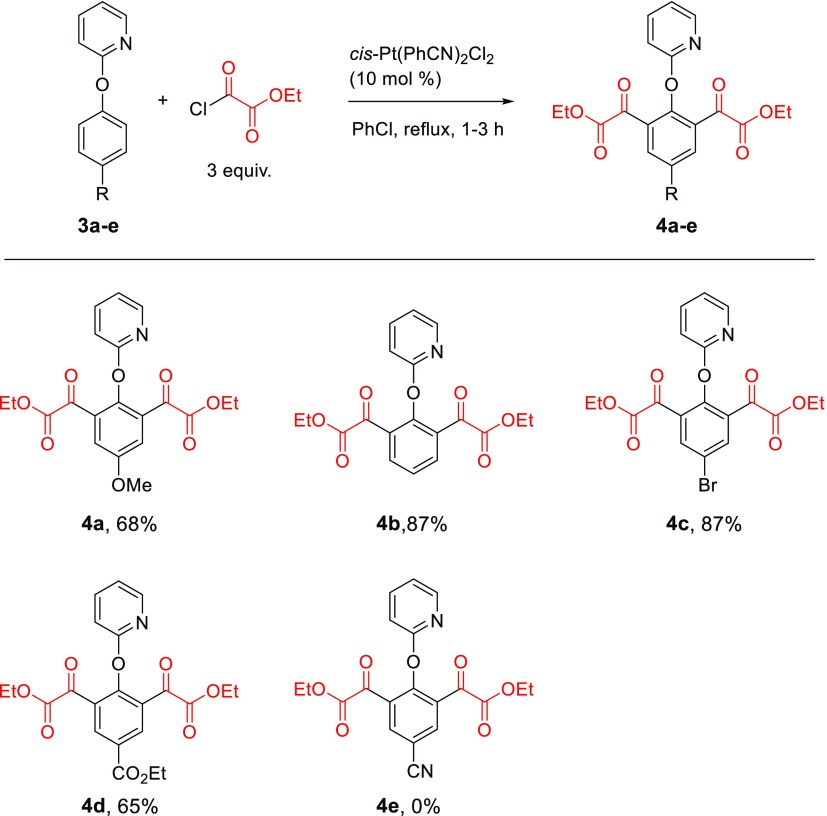
For 2-phenoxypyridine (3b) and para-substituted 2-aryloxypridines (3a, 3c, and 3d), two α-keto ester groups were introduced to both ortho positions to give di-α-keto esters 4a–4d in 65–87% yields (Scheme 3). Methoxy, bromo, and ester groups are tolerated in this double acylation reaction. Double acylation proceeded smoothly under the same reaction conditions used for monoacylation and were completed within 1–3 h. However, the more strongly electron-withdrawing cyano group (3e) prohibited the reaction. In this case, neither double acylation nor monoacylation reaction took place. This is in sharp contrast to the acylation of ortho-substituted 2-aryloxypyridines (1b–1f), in which both cyano- and nitro-substituted substrates 1e and 1f underwent acylation smoothly to give the desired α-keto ester 2e and 2f in high yields. One possible explanation is that the ortho-substituents do not exert efficient electronic effect, especially the resonance effect, because of the steric hindrance.
Scheme 3. Pt-Catalyzed Diacylation of 2-Aryloxypyridines with Ethyl Chlorooxoacetate.
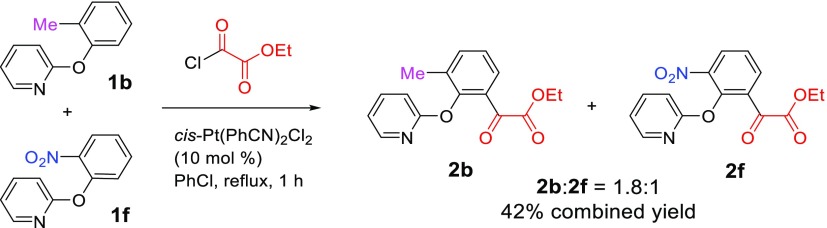
Tolerance of strongly electron-withdrawing groups CN and NO2 at the ortho position of 2-phenoxypyridine is highly interesting. Normally, electron-withdrawing groups retarded the acylation reaction, which is obvious with the meta- and para-substituted substrates because 2l (with the NO2 group) and 4e (with the CN group) were not formed. For the ortho-substituted 2-aryloxypyridines, it is difficult to tell the difference in reactivity between different substrates as their reactions were all complete within about an hour. Therefore, we carried out a competition experiment to assess the electronic effect of the substituents. As shown in Scheme 4, when equal molar amounts of 1b (with a Me group) and 1f (with a NO2 group) were combined in the same reaction vessel and reacted with ethyl chlorooxoacetate in the presence of the Pt catalyst, 2b and 2f were formed in the 1.8:1 ratio with a combined yield of 42%. This experiment clearly demonstrated that the electron-withdrawing group decelerated the reaction.
Scheme 4. Competing Acylation Reactions of 1b and 1f with Ethyl Chlorooxoacetate.
A catalyst loading of 10 mol % is necessary to achieve a high conversion. Lowering the catalyst loading to 5% led to less than 50% conversion even with a longer reaction time as the reaction became extremely slow after the first 1–2 h. There may be two contributing factors here: (1) the HCl generated from the reaction protonates the substrate and prevents the substrate from coordinating to the platinum and (2) the acylated product competes with the substrate coordinating to the platinum. It is hypothesized that the addition of a suitable HCl scavenger may be beneficial to the reaction. A few inorganic and organic bases were tested in the reaction of 1a, and it was found that the addition of 3 equiv of potassium carbonate allowed to achieve a reasonable conversion with reduced catalyst loading (5 mol %), albeit at the cost of a longer reaction time (Scheme 5). A few other substrates were also tested in this reaction. The results are summarized in Table 1 and compared with those obtained in the absence of a base. It can be seen from the table that reactions with the use of 3 equiv of K2CO3 and 5 mol % of the platinum catalyst gave either comparable or slightly lower yields, but longer reaction time (12 h) is required. It is noteworthy that in the presence of K2CO3, product degradation is minimized, allowing a longer reaction time. Without K2CO3, a longer reaction time resulted in product degradation. For example, without a base, the reaction of 1a with ethyl chlorooxoacetate in the presence of 10 mol % of the catalyst for 12 h produced 2a in 53% yield, while the 1 h reaction produced 2a in 76% yield. If the catalyst loading was lowered to 5%, the reaction produced 2a in only 39% yield. These results also suggested that the acid HCl generated from the reaction may be responsible for product degradation. Indeed, when acylated product 2a was mixed with 1 equiv of HCl (from concentrated hydrochloric acid) in chlorobenzene and refluxed for 1, 3, and 12 h, the product loss values of 5, 21, and 38% were observed, respectively. However, the degradation products were not identified because the NMR spectra of the mixtures showed very messy signals and thin-layer chromatography (TLC) did not show any significant spots that were isolatable. Sodium carbonate was much less effective, and cesium carbonate inhibited the reaction. Pyridine also inhibited the reaction, presumably because of the more favorable coordination of pyridine to the platinum than that of the substrate.
Scheme 5. Pt-Catalyzed C–H Acylation Reaction in the Presence of K2CO3.
Table 1. Comparison of the Yields of Acylated Products in the Presence or Absence of K2CO3a.
| with K2CO3 (3 equiv) |
without
base |
|||||
|---|---|---|---|---|---|---|
| product | catalyst (mol %) | time (h) | yield (%) | catalyst (mol %) | time (h) | yield (%) |
| 2a | 5 | 12 | 79 | 5 | 12 | 39 |
| 10 | 12 | 87 | 10 | 12 | 53 | |
| 10 | 1 | 76 | ||||
| 2b | 5 | 12 | 70 | 10 | 1 | 75 |
| 2c | 5 | 12 | 76 | 10 | 2 | 87 |
| 2e | 5 | 12 | 73 | 10 | 1 | 84 |
| 4a | 5 | 12 | 69 | 10 | 3 | 75 |
| 4b | 5 | 12 | 70 | 10 | 3 | 85 |
Yields are isolated yields.
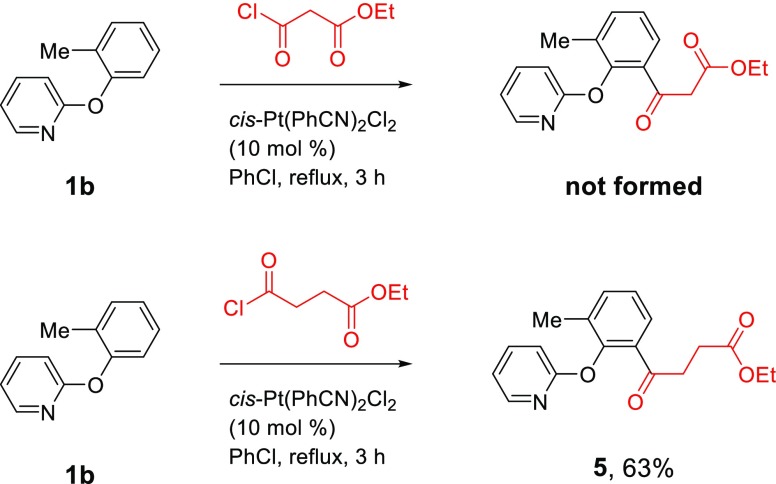
Following the successful introduction of the α-keto ester functional group through the Pt-catalyzed direct C–H acylation reaction, it became necessary to explore the introduction of β- and γ-keto ester functionalities with ethyl malonyl chloride and ethyl succinyl chloride as the acylating reagents, respectively. It was discovered that although the γ-keto esters can be synthesized successfully (Scheme 6), the use of ethyl malonyl chloride failed to produce the β-keto esters. The reaction of 2-(2-methylphenoxy)pyridine 1b with ethyl malonyl chloride in the presence of Pt(PhCN)2Cl2 under reflux for 3 h produced a dark orange/black mixture, and TLC and GC analysis of the reaction mixture showed that the starting material 1b remained, while no new product formation could be detected. A potential cause might be the instability of ethyl malonyl chloride because of the acidic protons on the α-carbon, which can be deprotonated by the basic 2-aryloxypyridine to form a ketene.40 The reaction of 1b with ethyl succinyl chloride proceeded to form the desired γ-keto ester 5 in 63% yield (Scheme 6).
Scheme 6. Platinum-Catalyzed C–H Acylation with Ethyl Malonyl Chloride and Succinyl Chloride.
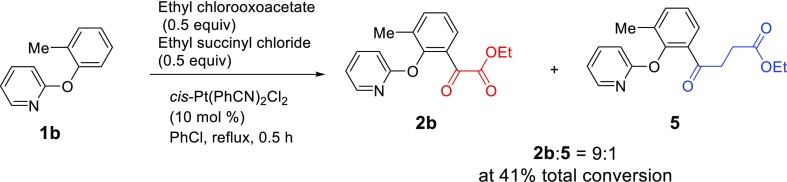
A competition experiment was designed (Scheme 7) to compare the relative reactivity of ethyl chlorooxoacetate and ethyl succinyl chloride. 2-(2-Methylphenoxy)pyridine (1b) was selected as the substrate for the competing reaction because the 1H NMR signals of the methyl group in the reactant 1b and the products 2b and 5 are very well separated for an accurate analysis. The reaction of 1b with ethyl chlorooxoacetate (0.5 equiv) and ethyl succinyl chloride (0.5 equiv) in the presence of cis-Pt(PhCN)2Cl2 (5 mol %) in chlorobenzene under reflux was run for 0.5 h and stopped. The crude mixture was analyzed using the 1H NMR spectrum of the reaction mixture, which showed that the ratio of 2b and 5 was 9 to 1, with a total of 41% conversion. This result is consistent with our previous observation that more electrophilic acyl chlorides are more reactive.31 In ethyl chlorooxoacetate, the ester group exhibits a stronger electron-withdrawing effect, making the acyl chloride more electrophilic. In ethyl succinyl chloride, the ester group is separated from the acyl chloride by two methylene groups, thus having a weaker electron-withdrawing ability only through an inductive effect.
Scheme 7. Competing Acylation Reactions of 1b with Ethyl Chlorooxoacetate and Ethyl Succinyl Chloride.
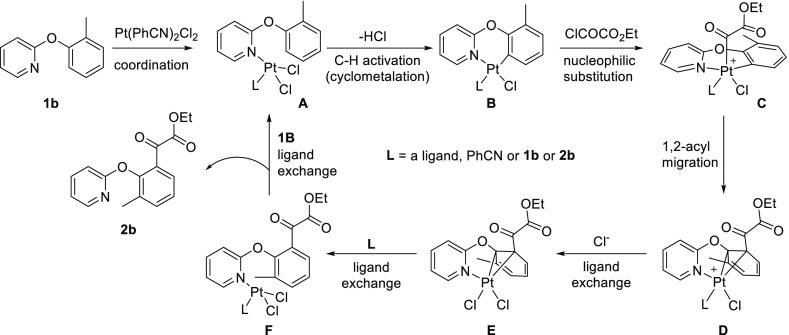
This reaction likely follows the similar mechanism to the Pt-catalyzed direct C–H acylation reaction reported previously.31 As demonstrated by our previous studies on the regiospecific acylation of cycloplatinated complexes,41,42 the reaction probably proceeds through two distinct steps: platinum-promoted C–H activation to form the cycloplatinated complex and the subsequent acylation reaction of the formed cycloplatinated complex. Based on our recent theoretical investigation43 of the acylation of a cycloplatinated complex, a plausible mechanism is proposed for the Pt-catalyzed acylation reaction of 1b (Scheme 8). The coordination of 1b to platinum forms the coordination complex A, which undergoes C–H activation to produce the cyclometalated complex B. The nucleophilic substitution of the ethyl chlorooxoacetate gives a five-coordinate acylplatinum complex C. The acyl group then undergoes subsequent 1,2-migration from the platinum to the metalated carbon, forming a cationic Pt-arene η2-complex D. This is followed by the ligand exchange with chloride, forming a more stable, neutral Pt-arene η2-complex E. Dissociation of the neutral Pt-arene complex occurs through ligand exchange to produce a coordination complex F. Finally, F undergoes the ligand exchange with 2b to release the acylated product 2b and regenerate A. Based on the structure of D, we suspect that the substituent effect may influence both carbons that bond to the platinum, which also explains the more significant substituent effect of electron-withdrawing groups at either the meta position or the para position of 2-phenoxypyridine (e.g., 1e and 3e). Detailed investigations on the reaction mechanism are currently underway, and the results will be reported in due course.
Scheme 8. Proposed Mechanism of Pt-Catalyzed Acylation of 1b.
Experimental Section
General Experimental Information
All reactions involving moisture- and/or oxygen-sensitive compounds were carried out under an argon atmosphere and anhydrous conditions. All anhydrous solvents used were purchased and used as received. cis-Pt(PhCN)2Cl236 and trans-Pt(PhCN)2Cl237 were prepared according to literature procedures. Aryloxypyridines 1a–1p and 3a–3e were prepared using a literature procedure.44 Compounds 1m, 1n, and 1p are new compounds and were fully characterized. All other reagents were purchased and were used as received. Thin layer chromatography was performed with silica gel 60 F254 plates. Gas chromatography was performed on a Shimadzu GC-2010 AFC equipped with an FID detector. 1H and 13C NMR spectra were recorded on a Bruker 400 MHz spectrometer at 298 K using CDCl3. Chemical shifts were reported relative to TMS (0.0 ppm for 1H) and chloroform-d (77.0 ppm for 13C). Elemental analyses were performed at Atlantic Microlab, Norcross, GA. Mass spectra were measured on a Waters UPLC/Micromass Quadrupole-ToF mass spectrometer. Melting points were measured on a Mel-temp apparatus.
Synthesis of 2-(4-Chloro-2-methoxyphenoxy)pyridine (1m)
General Procedure A44
A 250 mL, three-necked round-bottom flask with a condenser was dried and purged with argon and then charged with 4-chloro-2-methoxyphenol (5.71 g, 36 mmol), 2-bromopyridine (4.74 g, 30 mmol), CuI (0.57 g, 3 mmol), picolinic acid (0.74 g, 6 mmol), K3PO4 (12.70 g, 60 mmol), and anhydrous dimethyl sulfoxide (60 mL). The mixture was stirred and heated at 90 °C under argon for 24 h. The mixture was cooled to room temperature and quenched with H2O (100 mL). The aqueous layer was extracted with ethyl acetate (3 × 50 mL). The combined organic layer was washed with H2O (3 × 50 mL), 3 M NaOH (2 × 10 mL), and brine (3 × 25 mL) and dried over anhydrous Na2SO4. The organic solution was filtered, concentrated via a rotary evaporator, and purified by recrystallization from hexanes. Light brown solid, 4.98 g, 70.6% yield. mp 66–68 °C. 1H NMR (400 MHz, CDCl3): δ 8.15 (dd, J = 3.6, 1.3 Hz, 1H), 7.72–7.66 (m, 1H), 7.09 (d, J = 8.3 Hz, 1H), 7.02–6.94 (m, 4H), 3.77 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 163.5, 152.4, 147.5, 141.3, 139.3, 130.8, 123.9, 121.0, 118.3, 113.6, 110.9, 56.3. MS: calcd for C12H11ClNO2 (M + H+), 236.7; found, 236.6. Anal. Calcd for C12H10ClNO2: C, 61.16; H, 4.28; N, 5.94. Found: C, 60.96; H, 4.42; N, 5.85.
Synthesis of 2-(4-Bromo-2-chlorophenoxy)pyridine (1n)
This compound was synthesized according to General Procedure A and purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow solid, 69.1% yield. mp 65–67 °C. 1H NMR (400 MHz, CDCl3): δ 8.18–8.13 (m, 1H), 7.78–7.70 (m, 1H), 7.65 (d, J = 2.4 Hz, 1H), 7.45 (dd, J = 6.3, 2.3 Hz, 1H), 7.13 (d, J = 8.6 Hz, 1H), 7.06–7.00 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 162.7, 149.2, 147.5, 139.7, 133.2, 131.0, 128.6, 125.2, 118.9, 118.2, 111.3. MS: calcd for C11H8BrClNO (M + H+), 236.0, 238.0; found, 236.1, 238.1. Anal. Calcd for C11H7BrClNO: C, 46.43; H, 2.48; N, 4.92. Found: C, 46.37; H, 2.44; N, 4.99.
Synthesis of 2-(2,3,5-Trimethylphenoxy)pyridine (1p)
This compound was synthesized according to General Procedure A and purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): brown solid, 76.4% yield. mp 54–56 °C. 1H NMR (400 MHz, CDCl3): δ 8.23–8.19 (m, 1H), 7.70–7.64 (m, 1H), 6.93–6.87 (m, 1H), 6.89 (s, 1H), 6.85 (d, J = 8.3 Hz, 1H), 6.75 (s, 1H), 2.30 (s, 6H), 2.05 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 164.2, 152.0, 148.0, 139.3, 138.3, 136.1, 127.8, 126.1, 120.0, 117.7, 110.6, 20.95, 20.1, 12.2. MS: calcd for C14H16NO (M + H+), 214.1; found, 214.2. Anal. Calcd for C14H15NO: C, 78.84; H, 7.09; N, 6.57. Found: C, 79.00; H, 7.24; N, 6.56.
Synthesis of Ethyl 2-(2-(Pyridin-2-yloxy)naphth-3-yl)-2-oxoacetate (2a)
General Procedure B for the Pt-Catalyzed Acylation Reaction
A 50 mL, three-necked round-bottom flask equipped with a condenser was dried and purged with argon and then charged with 2-(naphthalen-2-yloxy)pyridine (222 mg, 1 mmol), cis-Pt(PhCN)2Cl2 (47 mg, 0.1 mmol), ethyl chlorooxoacetate (0.34 mL, 3 mmol), and anhydrous chlorobenzene (4 mL). A drying tube was placed on the top of the condenser, and the mixture was stirred and heated at reflux for 1 h. The temperature was lowered to 100 °C, and pyridine (1.0 mL) was added dropwise to the reaction mixture. After being stirred for 1 h, the mixture was cooled to room temperature and filtered through a pad of Celite. The filtrate was transferred to a 250 mL separatory funnel, H2O (20 mL) was added, and the aqueous layer was extracted with dichloromethane (3 × 15 mL). The organic layers were combined, dried over Na2SO4, filtered, and concentrated via a rotary evaporator. The product was purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 3:1) as the eluting solvent, yellow solid, 245.5 mg, 76.4%. mp 76–77.0 °C. 1H NMR (400 MHz, CDCl3): δ 8.57 (s, 1H), 8.22 (dd, J = 5.0, 1.7 Hz, 1H), 8.00 (d, J = 8.1, 1H), 7.82–7.75 (m, 2H), 7.64–7.59 (m, 2H), 7.56–7.50 (m, 1H), 7.12–7.07 (m, 1H), 7.01(d, J = 8.2, 1H), 4.19 (q, J = 7.1 Hz, 2H), 1.21 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 186.1, 164.2, 162.9, 149.9, 147.7, 139.8, 136.8, 133.4, 130.1, 129.7, 129.5, 127.4, 126.4, 126.3, 119.4, 118.9, 112.0, 62.2, 13.9. MS: calcd for C19H16NO4 (M + H+), 322.1; found, 322.3. Anal. Calcd for C19H15NO4: C, 71.02; H, 4.71; N, 4.36. Found: C, 71.08; H, 4.87; N, 4.32.
Synthesis of Ethyl 2-(3-Methyl-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2b)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow oil, 74.5% yield. 1H NMR (400 MHz, CDCl3): δ 8.13 (dd, J = 6.3, 2.5 Hz, 1H), 7.86 (dd, J = 6.8, 4.0 Hz, 1H), 7.77–7.71 (m, 1H), 7.56 (dd, J = 6.6, 1.0 Hz, 1H), 7.36–7.29 (m, 1H), 7.04–6.99 (m, 1H), 6.93 (d, J = 9.0 Hz, 1H), 4.16 (q, J = 7.8 Hz, 2H), 2.1 (s, 3H), 1.21 (t, J = 8.1 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 164.5, 162.7, 152.1, 147.6, 139.7, 137.6, 132.6, 128.7, 127.92, 125.8, 118.7, 110.6, 62.0, 16.6, 13.9 (the signal for the keto carbon is too weak to be assessed). MS: calcd for C16H16NO4 (M + H+), 286.1; found, 286.1. Anal. Calcd for C16H15NO4: C, 67.36; H, 5.30; N, 4.91. Found: C, 67.08; H, 5.31; N, 4.83.
Synthesis of Ethyl 2-(3-Chloro-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2c)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 3:1): yellow oil, 87.0% yield. 1H NMR (400 MHz, CDCl3): δ 8.01 (dd, J = 5.0, 2.0 Hz, 3H), 7.83 (dd, J = 7.9, 1.5 Hz, 2H), 7.70–7.61 (m, 2H), 7.28 (t, J = 8.0 Hz, 1H), 6.98–6.89 (m, 2H), 4.06 (q, J = 7.2 Hz, 1H), 1.11 (t, J = 7.2 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 185.1, 162.8, 161.2, 149.0, 146.3, 138.8, 135.8, 128.8, 128.2, 128.1, 125.5, 118.3, 109.9, 61.2, 12.8. MS: calcd for C15H12ClNO4 (M + H+), 306.1; found, 306.0. Anal. Calcd for C15H12ClNO4: C, 58.93; H, 3.96; N, 4.58. Found: C, 58.94; H, 4.11; N, 4.51.
Synthesis of Ethyl 2-(3-Ethoxycarbonyl-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2d)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 2:1): clear oil, 74.8% yield. 1H NMR (400 MHz, CDCl3): δ 8.26 (dd, J = 8.0, 4.0 Hz, 1H), 8.15 (dd, J = 6.4, 2.56 Hz, 1H), 8.08–8.08 (m, 1H), 7.79–7.73 (m, 1H), 7.48 (t, J = 7.7 Hz, 1H), 7.05–6.98 (m, 2H), 4.14–4.02 (m, 4H), 1.19 (t, J = 7.4 Hz, 3H), 1.06 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 186.0, 164.5, 163.8, 163.0, 152.5, 147.0, 139.6, 137.3, 134.5, 129.3, 126.2, 125.5, 118.9, 111.1, 62.1, 61.3, 13.8. MS: calcd for C18H18NO6 (M + H+), 344.1; found, 344.1. Anal. Calcd for C18H17NO6: C, 62.97; H, 4.99; N, 4.08. Found: C, 62.73; H, 5.17; N, 4.22.
Synthesis of Ethyl 2-(3-Cyano-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2e)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 2:1): yellow solid, 84.2% yield. mp 71–73 °C. 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 6.5, 3.5 Hz, 1H), 8.12 (dd, J = 7.2, 3.2 Hz, 1H), 7.95 (dd, J = 7.9, 4.0 Hz, 1H), 7.81 (td, J = 7.0, 3.2 Hz, 1H), 7.51 (t, J = 8.5 Hz, 1H), 7.16–7.09 (m, 2H), 4.17 (q, J = 7.9 Hz, 2H), 1.22 (t, J = 7.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 183.6, 163.1, 161.9, 155.2, 147.1, 140.4, 138.7, 135.0, 129.4, 126.0, 120.3, 114.6, 111.5, 109.5, 62.5, 13.9. MS: calcd for C16H13N2O4 (M + H+), 297.1; found, 297.1. Anal. Calcd for C16H12N2O4: C, 64.86; H, 4.08; N, 9.46. Found: C, 64.56; H, 4.29; N, 9.36.
Synthesis of Ethyl 2-(3-Nitro-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2f)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): white solid, 79.5% yield. mp 75–77 °C. 1H NMR (400 MHz, CDCl3): δ 8.31 (dd, J = 9.2, 1.0 Hz, 1H), 8.22 (dd, J = 7.5 Hz, 0.4 Hz, 1H), 8.03 (dd, J = 6.7, 1.5 Hz, 1H), 7.83–7.76 (m, 1H), 7.55 (t, J = 8.6 Hz, 1H), 7.11–7.03 (m, 2H), 4.13 (q, J = 7.8 Hz, 2H), 1.21 (t, J = 7.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 184.7, 163.0, 161.1, 147.0, 146.4, 143.2, 140.3, 135.5, 130.8, 130.6, 125.7, 120.0, 111.1, 62.5, 13.9. MS: calcd for C15H12N2O6 (M + H+), 317.1; found, 317.2. Anal. Calcd for C15H12N2O6: C, 56.97; H, 3.82; N, 8.86. Found: C, 56.78; H, 3.82; N, 8.86.
Synthesis of Ethyl 2-(4-Methoxy-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2g)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 2:1): brown oil, 83.8% yield. 1H NMR (400 MHz, CDCl3): δ 8.24 (dd, J = 3.2, 1.8 Hz, 1H), 8.02 (d, J = 8.8 Hz, 1H), 7.80–7.73 (m, 1H), 7.12–7.07 (m, 1H), 6.99 (d, J = 8.3 Hz, 1H), 6.87 (dd, J = 6.5, 2.0 Hz, 1H), 6.65 (d, J = 2.4 Hz, 1H), 4.41 (q, J = 7.2 Hz, 2H), 3.85 (s, 3H), 1.17 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 184.7, 165.8, 165.3, 162.4, 156.8, 147.9, 139.9, 132.6, 119.7, 119.2, 112.2, 111.7, 107.0, 61.8, 55.8, 13.9. MS: calcd for C16H16NO5 (M + H+), 302.1; found, 302.1. Anal. Calcd for C16H15NO5: C, 63.78; H, 5.02; N, 4.65. Found: C, 63.66; H, 5.15; N, 4.68.
Synthesis of Ethyl 2-(4-Chloro-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2h)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): white solid, 76.7% yield. mp 51–53 °C. 1H NMR (400 MHz, CDCl3): δ 8.25 (dd, J = 5.0, 2.0 Hz, 1H), 7.96 (d, J = 8.5 Hz, 1H), 7.83–7.77 (m, 1H), 7.33 (dd, J = 8.6, 1.9 Hz, 1H), 7.24 (d, J = 1.9 Hz, 1H), 7.17–7.12 (m, 1H), 7.00 (d, J = 8.4 Hz, 1H), 4.16 (q, J = 7.2 Hz, 2H), 1.19 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 185.0, 164.2, 161.8, 154.9, 147.9, 141.4, 140.2, 131.7, 125.6, 124.8, 122.5, 120.3, 112.0, 62.1, 13.90. MS: calcd for C15H13ClNO4 (M + H+), 306.1; found, 306.0. Anal. Calcd for C15H12ClNO4: C, 58.93; H, 3.96; N, 4.58. Found: C, 58.82; H, 4.10; N, 4.52.
Synthesis of Ethyl 2-(4-Bromo-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2i)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): white solid, 86.1% yield. 1H NMR (400 MHz, CDCl3): δ 8.24 (s, 1H), 7.88 (d, J = 8.1 Hz, 1H), 7.79 (t, J = 7.4 Hz, 1H), 7.49 (dd, J = 6.8, 1.8 Hz, 1H), 7.39 (d, J = 1.2 Hz, 1H), 7.13 (t, J = 6.1 Hz, 1H), 6.99 (d, J = 8.2 Hz, 1H), 4.13 (q, J = 7.2 Hz, 2H), 1.17 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 185.1, 164.0, 161.5, 154.7, 147.7, 140.1, 131.6, 129.6, 128.4, 125.1, 125.3, 120.2, 112.1, 62.1, 13.7. MS: calcd for C15H13BrNO4 (M + H+), 350.0, 352.0; found, 350.1; 352.1. Anal. Calcd for C15H12BrNO4: C, 51.45; H, 3.45; N, 4.00. Found: C, 51.71; H, 3.42; N, 4.11.
Synthesis of Ethyl 2-(4-Ethoxycarbonyl-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2j)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): light orange oil, 47.4% yield. 1H NMR (400 MHz, CDCl3): δ 8.25 (dd, J = 5.2, 3.6 Hz, 1H), 8.05–7.96 (m, 2H), 7.89–7.87 (m, 1H), 7.79 (dd, J = 8.7, 3.1 Hz, 1H), 7.12 (dd, J = 7.3, 1.8 Hz, 1H), 7.01 (d, J = 8.0 Hz, 1H), 4.41 (q, J = 7.3, 2H), 4.16 (q, J = 7.5 Hz, 2H), 1.40 (t, J = 7.3 Hz, 3H), 1.14 (t, J = 7.5 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 185.6, 164.5, 163.7, 162.1, 154.2, 147.7, 140.0, 136.6, 130.7, 129.9, 125.6, 123.5, 119.9, 112.0, 62.2, 61.8, 14.3, 13.8. MS: calcd for C18H18NO6 (M + H+), 344.1; found, 344.1. Anal. Calcd for C18H17NO6: C, 62.97; H, 4.99; N, 4.08. Found: C, 63.16; H, 5.16; N, 4.02.
Synthesis of Ethyl 2-(4,5-Dimethyl-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2l)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 3:1): clear oil, 70.8% yield. 1H NMR (400 MHz, CDCl3): δ 8.23–8.20 (m, 1H), 7.79–7.72 (m, 2H), 7.08–7.04 (m, 1H), 6.97–6.93 (m, 2H), 4.13 (q, J = 7.2 Hz, 2H), 2.32 (s, 6H), 1.16 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 185.9, 165.0, 162.7, 152.6, 147.8, 146.3, 139.7, 134.0, 131.2, 123.8, 123.3, 119.4, 111.8, 61.8, 20.6, 19.1, 13.9. MS: calcd for C17H18NO4 (M + H+), 300.1; found, 300.3. Anal. Calcd for C17H17NO4: C, 68.22; H, 5.72; N, 4.68. Found: C, 68.29; H, 5.68; N, 4.68.
Synthesis of Ethyl 2-(5-Chloro-3-methoxy-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2m)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 2:1): brown oil, 83.8% yield. 1H NMR (400 MHz, CDCl3): δ 8.14–8.10 (m, 1H), 7.76–7.70 (m, 1H), 7.56 (d, J = 5.3 Hz, 1H), 7.20 (d, J = 5.1 Hz, 1H), 7.06–7.02 (m, 1H), 6.96 (d, J = 7.9 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 3.74 (s, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 185.0, 163.8, 162.4, 152.8, 147.3, 142.3, 139.5, 131.7, 129.0, 121.0, 119.1, 118.6, 110.5, 62.2, 56.5, 13.8. MS: calcd for C16H15ClNO5 (M + H+), 336.1; found, 336.0. Anal. Calcd for C16H14ClNO5: C, 57.24; H, 4.20; N, 4.17. Found: C, 57.26; H, 4.38; N, 4.15.
Synthesis of Ethyl 2-(5-Bromo-3-chloro-2-(pyridin-2-yloxy))phenyl-2-oxoacetate (2n)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow solid, 75.1% yield. mp 74–76 °C. 1H NMR (400 MHz, CDCl3w): δ 8.13–8.07 (m, 1H), 8.02 (d, J = 2.4 Hz, 1H) 7.86 (d, J = 1.0 Hz, 1H), 7.81–7.75 (m, 1H), 7.11–7.00 (m, 2H), 4.17 (q, J = 7.2 Hz, 2H), 1.21 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 183.8, 163.1, 161.8, 149.1, 147.3, 140.0, 138.3, 131.8, 131.0, 130.4, 119.6, 119.0, 110.9, 62.4, 14.0. MS: calcd for C15H12BrClNO4 (M + H+), 384.0, 386.0; found, 384.2, 386.2. Anal. Calcd for C15H11BrClNO4: C, 46.84; H, 2.88; N, 3.64. Found: C, 47.10; H, 2.84; N, 3.68.
Synthesis of Ethyl 2-(1-(Pyridin-2-yloxy)naphth-2-yl)-2-oxoacetate 2o
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow solid, 78.0% yield. mp 120–122 °C. 1H NMR (400 MHz, CDCl3): δ 8.09–8.02 (m, 2H), 7.93 (d, J = 4.2 Hz, 1H), 7.89–7.80 (m, 2H), 7.79–7.74 (m, 1H), 7.66–7.60 (m, 1H), 7.46 (td, J = 7.2, 1.1 Hz, 1H), 7.07–7.01 (m, 2H), 4.22 (q, J = 7.1 Hz, 2H), 1.23 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 186.3, 164.7, 169.0, 152.4, 147.8, 139.8, 137.0, 129.4, 128.4, 127.3, 127.1, 126.3, 124.6, 124.0, 123.7, 119.1, 110.5, 62.1, 13.9. MS: calcd for C19H16NO4 (M + H+), 322.2; found, 322.1. Anal. Calcd for C19H15NO4: C, 71.02; H, 4.71; N, 4.36. Found: C, 70.58; H, 4.69; N, 4.33.
Synthesis of Diethyl (5-Methoxy-2-(pyridin-2-yloxy))benzene-1,3-diglyoxylate (4a)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow oil, 69.9% yield. 1H NMR (400 MHz, CDCl3): δ 8.04 (dd, J = 5.2, 1.8 Hz, 1H), 7.77–7.71 (m, 1H), 7.67 (s, 2H), 7.06–7.02 (m, 1H), 6.92 (d, J = 8.3 Hz, 1H), 4.18 (q, J = 7.2 Hz, 4H), 3.94 (s, 3H), 1.23 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 184.6, 162.8, 162.4, 156.7, 146.8, 145.6, 140.0, 130.0, 120.8, 119.7, 111.1, 62.5, 56.2, 13.9. MS: calcd for C20H20NO8 (M + H+), 402.1; found, 402.2. Anal. Calcd for C20H19NO8: C, 59.85; H, 4.77; N, 3.49. Found: C, 60.11; H, 5.03; N, 3.43.
Synthesis of Diethyl (2-(Pyridin-2-yloxy))benzene-1,3-diglyoxylate (4b)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow oil, 86.6% yield. 1H NMR (400 MHz, CDCl3): δ 8.16 (d, J = 7.7 Hz, 2H), 8.04 (dd, J = 5.1, 2.2 Hz, 1H), 7.80–7.73 (m, 1H), 7.53 (t, J = 7.7 Hz, 1H), 7.09–7.04 (m, 1H), 6.95 (d, J = 8.3 Hz, 1H), 4.17 (q, J = 7.2, 4H), 1.23 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 184.6, 162.7, 161.9, 152.0, 149.8, 146.9, 140.2, 136.0, 129.1, 125.7, 123.9, 114.9, 111.2, 62.5, 13.8. MS: calcd for C19H18NO7 (M + H+), 372.1; found, 372.1. Anal. Calcd for C19H17NO7: C, 61.45; H, 4.61; N, 3.77. Found: C, 61.31; H, 4.67; N, 3.68.
Synthesis of Diethyl (5-Bromo-2-(pyridin-2-yloxy))benzene-1,3-diglyoxylate 4c
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1): yellow oil, 86.6% yield. 1H NMR (400 MHz, CDCl3): δ 8.20 (s, 2H), 8.00 (dd, J = 5.0, 1.6 Hz, 1H), 7.76 (t, J = 7.7 Hz, 1H), 7.07 (t, J = 6.1 Hz, 1H), 6.92 (d, 8.3 Hz, 1H), 4.15 (q, J = 7.1 Hz, 4H), 1.19 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 183.1, 162.1, 161.3, 150.4, 146.7, 140.4, 138.1, 130.8, 120.1, 118.9, 111.1, 62.6, 13.9. MS: calcd for C19H17BrNO7 (M + H+), 450.0, 452.0; found, 450.0, 452.0. Anal. Calcd for C19H16BrNO7: C, 50.69; H, 3.58; N, 3.11. Found: C, 50.88; H, 3.65; N, 3.15.
Synthesis of Diethyl (5-Ethoxycarbonyl-2-(pyridin-2-yloxy))benzene-1,3-diglyoxylate (4d)
Purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 2:1): yellow oil, 65.0% yield. 1H NMR (400 MHz, CDCl3): δ 8.81–8.74 (m, 2H), 8.02 (dd, J = 5.0, 1.9 Hz, 1H), 7.82–7.75 (m, 1H), 7.12–7.07 (m, 1H), 6.98 (d, J = 8.3 Hz, 1H), 4.47 (q, J = 7.3 Hz, 2H), 4.18 (q, J = 7.2 Hz, 4H), 1.45 (t, J = 7.3 Hz, 4H), 1.24 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 183.8, 164.0, 162.2, 161.3, 154.5, 146.7, 140.5, 136.5, 129.5, 128.2, 120.3, 111.2, 62.6, 61.9, 14.3, 13.8. MS: calcd for C22H22NO9 (M + H+), 444.1; found, 444.1. Anal. Calcd for C22H21NO9: C, 59.59; H, 4.77; N, 3.16. Found: C, 58.74; H, 4.83; N, 3.18.
Competing Reaction of 1b and 1f with Ethyl Chlorooxoacetate
A 50 mL, three-necked round-bottom flask equipped with a condenser was dried and then charged with 1b (93 mg, 0.5 mmol), 1f (108 mg, 0.5 mmol), cis-Pt(PhCN)2Cl2 (48 mg, 0.1 mmol), ethyl chlorooxoacetate (0.34 mL, 3 mmol), and anhydrous chlorobenzene (6.0 mL). A drying tube was placed on the top of the condenser, and the mixture was then stirred and heated at reflux for 1 h. The temperature was lowered to 100 °C, and pyridine (1.0 mL) was added dropwise. After stirring it for 30 min, the mixture was quenched with H2O (30 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic solution was washed with water (3 × 20 mL) and brine (1 × 20 mL), dried over Na2SO4, and concentrated via a rotary evaporator. The crude residue was analyzed by 1H NMR, which indicated that the ratio of 2b/2f is 1.8:1 (Figure S22, Supporting Information). The crude product was purified via column chromatography on silica gel with hexanes–ethyl acetate (v/v = 4:1). The fractions containing both products were combined, and the solvent was evaporated using a rotary evaporator, yellow oil, 121.2 mg, with a combined isolated yield of 42%.
Synthesis of 4-(3-Methyl-2-(pyridine-2-yloxy))phenyl-4-oxobutanoate (5)
A 50 mL, three-necked round-bottom flask with a condenser was dried and then charged with 2-(2-methylphenoxy)pyridine (93 mg, 0.5 mmol), cis-(PhCN)2PtCl2 (24 mg, 0.05 mmol), ethyl succinyl chloride (0.29 mL, 1.5 mmol), and anhydrous chlorobenzene (3 mL). The mixture was stirred and heated at reflux for 3 h. The temperature was lowered to 100 °C, and the reaction mixture was treated with pyridine (1.0 mL), which was added dropwise. After being stirred for 1 h, the mixture was filtered through a Celite. The filtrate was transferred to a 250 mL separatory funnel, water (20 mL) was added, and the aqueous layer was extracted with ethyl acetate (3 × 20 mL). The organic layers were combined and washed with water and brine, dried over Mg2SO4, filtered, and concentrated via a rotary evaporator. The product 5 was purified via column chromatography on silica gel with hexanes–ethyl acetate (3:1) as the eluting solvent, yellow oil, 98 mg, 63%. 1H NMR (400 MHz, CDCl3): δ 8.13 (dd, J = 5.0, 1.2 Hz, 1H), 7.74–7.68 (m, 2H), 7.45 (d, J = 7.5 Hz, 1H), 7.25 (d, J = 7.7 Hz, 1H), 7.00–6.94 (m, 2H), 4.11 (q, J = 7.1 Hz, 2H), 3.17 (t, J = 6.7 Hz, 2H), 2.60 (t, J = 6.7 Hz, 2H), 2.15 (s, 3H), 1.23 (t, J = 7.1 Hz, 3H). 13C NMR (400 MHz, CDCl3): δ 199.5, 172.7, 163.0, 150.0, 147.7, 139.7, 135.0, 132.7, 132.6, 127.6, 125.5, 118.3, 110.6, 60.5, 37.3, 28.6, 16.7, 14.2. MS: calcd for C18H20NO4 (M + H+), 315.13; found, 315.14. Anal. Calcd for C18H19NO4: C, 69.00; H, 6.11; N, 4.47. Found: C, 69.04; H, 6.23; N, 4.49.
Competing Reaction of 1b with Ethyl Chlorooxoacetate and Ethyl Succinyl Chloride
A 25 mL, three-necked round-bottom flask equipped with a condenser was dried and then charged with 1b (93 mg, 0.5 mmol), cis-Pt(PhCN)2Cl2 (12 mg, 0.025 mmol), ethyl chlorooxoacetate (0.028 mL, 0.25 mmol), ethyl succinyl chloride (0.036 mL, 0.25 mmol), and anhydrous chlorobenzene (3.0 mL). A drying tube was placed on the top of the condenser, and the mixture was then stirred and heated at reflux for 30 h. The temperature was lowered to 100 °C, and pyridine (1.0 mL) was added dropwise. After stirring it for 30 min, the mixture was quenched with H2O (30 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic solution was washed with water (3 × 20 mL) and brine (1 × 20 mL), dried over Na2SO4, and concentrated via a rotary evaporator. The crude residue was analyzed by 1H NMR, which indicated that the ratio of 2b/5 is 9:1, and the total conversion was calcd to be 41% based on the relative amounts of the products and the remaining reactant (Figure S24, Supporting Information).
Acknowledgments
The authors would like to acknowledge the financial support by the National Science Foundation (NSF-CHE-1900102). J.N. is grateful to the financial support provided by the National Science Foundation REU program (NSF-CHE-1851844).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00982.
1H and 13C NMR spectra (PDF)
Author Present Address
† Department of Chemistry, Purdue University, West Lafayette, IN 47907.
The authors declare no competing financial interest.
Supplementary Material
References
- Ocain T. D.; Rich D. H. α-Keto Amide Inhibitors of Aminopeptidases. J. Med. Chem. 1992, 35, 451–456. 10.1021/jm00081a005. [DOI] [PubMed] [Google Scholar]
- Elsebai M. F.; Kehraus S.; Lindequist U.; Sasse F.; Shaaban S.; Gütschow M.; Josten M.; Sahl H.-G.; König G. M. Antimicrobial Phenalenone Derivatives from the Marine-derived Fungus Coniothyrium Cereal. Org. Biomol. Chem. 2011, 9, 802–808. 10.1039/c0ob00625d. [DOI] [PubMed] [Google Scholar]
- Nie Y.; Xiao R.; Xu Y.; Montelione G. T. Novel anti-Prelog Stereospecific Carbonyl Reductases from Canadian Parapsilosis for Asymmetric Reduction of Prochiral Ketones. Org. Biomol. Chem. 2011, 9, 4070–4078. 10.1039/c0ob00938e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir M.; El Maddah F.; Kehraus S.; Egereva E.; Piel J.; Brachmann A. O.; König G. M. Phenalenones: Insight into the Biosynthesis of Polyketides from the Marine Alga-derived Fungus Coniothyrium Cereal. Org. Biomol. Chem. 2015, 13, 8071–8079. 10.1039/c5ob00844a. [DOI] [PubMed] [Google Scholar]
- Eftekhari-Sis B.; Zirak M. Chemistry of α-Oxoesters: A Powerful Tool for the Synthesis of Heterocycles. Chem. Rev. 2015, 115, 151–264. 10.1021/cr5004216. [DOI] [PubMed] [Google Scholar]
- Kovacs L. Methods for the Synthesis of a-Keto Esters. Recl. Trav. Chim. Pays-Bas 1993, 112, 471–496. 10.1002/recl.19931120902. [DOI] [Google Scholar]
- Babudri F.; Fiandanese V.; Marchese G.; Punzi A. A General and Straightforward Approach to α,ω-ketoesters. Tetrahedron 1996, 52, 13513–13520. 10.1016/0040-4020(96)00805-8. [DOI] [Google Scholar]
- Sprenger R. D.; Ruoff P. M.; Frazer A. H. Citrinin Studies. Hydroxy- and Methoxy-phenylglyoxylic Acids. J. Am. Chem. Soc. 1950, 72, 2874–2876. 10.1021/ja01163a017. [DOI] [Google Scholar]
- Yoshikawa N.; Doyle A.; Tan L.; Murry J. A.; Akao A.; Kawasaki M.; Sato K. An Efficient and Scalable Synthesis of Substituted Phenanthrenequinones by Intramolecular Friedel-Crafts Reaction of Imidazolides. Org. Lett. 2007, 9, 4103–4106. 10.1021/ol071261h. [DOI] [PubMed] [Google Scholar]
- Wadhwa K.; Yang C.; West P. R.; Deming K. C.; Chemburkar S. R.; Reddy R. E. Synthesis of Arylglyoxylic Acids and Their Collision-Induced Dissociation. Synth. Commun. 2008, 38, 4434–4444. 10.1080/00397910802369554. [DOI] [Google Scholar]
- Xiang J. M.; Li B. L. Solvent-free synthesis of some ethyl arylglyoxylates. Chin. Chem. Lett. 2009, 20, 55–57. 10.1016/j.cclet.2008.10.003. [DOI] [Google Scholar]
- Wu X.-F. Acylation of (Hetero)Arenes through C¢H Activation with Aroyl Surrogates. Chem.—Eur. J. 2015, 21, 12252–12265. 10.1002/chem.201501548. [DOI] [PubMed] [Google Scholar]
- Correa A.; Cornella J.; Martin R. Nickel-Catalyzed Decarbonylative C-H Coupling Reactions: A Strategy for Preparing Bis(heteroaryl)backbones. Angew. Chem., Int. Ed. 2013, 52, 1878–1880. 10.1002/anie.201208843. [DOI] [PubMed] [Google Scholar]
- Allen C. L.; Williams J. M. J. Ruthenium-Catalyzed Alkene Synthesis by the Decarbonylative Coupling of Aldehydes with Alkynes. Angew. Chem., Int. Ed. 2010, 49, 1724–1725. 10.1002/anie.200906896. [DOI] [PubMed] [Google Scholar]
- Malapit C. A.; Ichiishi N.; Sanford M. S. Pd-Catalyzed Decarbonylative Cross-Couplings of Aroyl Chlorides. Org. Lett. 2017, 19, 4142–4145. 10.1021/acs.orglett.7b02024. [DOI] [PMC free article] [PubMed] [Google Scholar]; and references cited therein
- Wang S.; Yang Z.; Liu J.; Xie K.; Wang A.; Chen X.; Tan Z. Efficient Synthesis of Anthranilic Esters via Pd-Catalyzed Dehydrogenative/Decarbonylative Coupling of Anilides and Glyoxylates. Chem. Commun. 2012, 48, 9924–9926. 10.1039/c2cc34473d. [DOI] [PubMed] [Google Scholar]
- am Ende C. W.; Knudson S. E.; Liu N.; Childs J.; Sullivan T. J.; Boyne M.; Xu H.; Gegina Y.; Knudson D. L.; Johnson F.; Peloquin C. A.; Slayden R. A.; Tonge P. J. Synthesis and in vitro Antimycobacterial Activity of B-ring Modified Diaryl Ether InhA Inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 3029–3033. 10.1016/j.bmcl.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dounay A. B.; Barta N. S.; Campbell B. M.; Coleman C.; Collantes E. M.; Denny L.; Dutta S.; Gray D. L.; Hou D.; Iyer R.; Maiti S. N.; Ortwine D. F.; Probert A.; Stratman N. C.; Subedi R.; Whisman T.; Xu W.; Zoski K. Design, Synthesis, and Pharmacological Evaluation of Phenoxy Pyridyl Derivatives as Dual Norepinephrine Reuptake Inhibitors and 5-HT1A Partial Agonists. Bioorg. Med. Chem. Lett. 2010, 20, 1114–1117. 10.1016/j.bmcl.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Chao H.; Turdi H.; Herpin T. F.; Roberge J. Y.; Liu Y.; Schnur D. M.; Poss M. A.; Rehfuss R.; Hua J.; Wu Q.; Price L. A.; Abell L. M.; Schumacher W. A.; Bostwick J. S.; Steinbacher T. E.; Stewart A. B.; Ogletree M. L.; Huang C. S.; Chang M.; Cacace A. M.; Arcuri M. J.; Celani D.; Wexler R. R.; Lawrence R. M. Discovery of 2-(Phenoxypyridine)-3-phenylureas as Small Molecule P2Y1 Antagonists. J. Med. Chem. 2013, 56, 1704–1714. 10.1021/jm301708u. [DOI] [PubMed] [Google Scholar]
- Jia X.; Zhang S.; Wang W.; Luo F.; Cheng J. Palladium-Catalyzed Acylation of sp2 C-H bond: Direct Access to Ketones from Aldehydes. Org. Lett. 2009, 11, 3120–3123. 10.1021/ol900934g. [DOI] [PubMed] [Google Scholar]
- Gu S.; Chen C.; Chen W. Ortho-Functionalization of 2-Phenoxypyrimidines via Palladium-Catalyzed C-H Bond Activation. J. Org. Chem. 2009, 74, 7203–7206. 10.1021/jo901316b. [DOI] [PubMed] [Google Scholar]
- Chu J.-H.; Lin P.-S.; Wu M.-J. Palladium(II)-Catalyzed Ortho Arylation of 2-Phenoxypyridines with Potassium Aryltrifluoroborates via C-H Functionalization. Organometallics 2010, 29, 4058–4065. 10.1021/om100494p. [DOI] [Google Scholar]
- Yao J.; Feng R.; Wu Z.; Liu Z.; Zhang Y. Palladium-Catalyzed Decarboxylative Coupling of α-Oxocarboxylic Acids with C(sp2)-H of 2-Aryloxypyridines. Adv. Synth. Catal. 2013, 355, 1517–1522. 10.1002/adsc.201300078. [DOI] [Google Scholar]
- Liu B.; Jiang H.-Z.; Shi B.-F. Palladium-Catalyzed Oxidative Olefination of Phenols Bearing Removable Directing Groups under Molecular Oxygen. J. Org. Chem. 2014, 79, 1521–1526. 10.1021/jo4027403. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Zhang J.; Ren S.; Liu Y. Palladium-Catalyzed Aromatic C–H Bond Nitration Using Removable Directing Groups: Regiospecific Synthesis of Substituted o-Nitrophenols from Related Phenols. J. Org. Chem. 2014, 79, 11508–11516. 10.1021/jo502145v. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Sun P. Palladium-Catalyzed ortho-Sulfonylation of 2-Aryloxypyridines and Subsequent Formation of ortho-Sulfonylated Phenols. J. Org. Chem. 2014, 79, 8457–8461. 10.1021/jo5014146. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Liu P.; Li S.-L.; Sun P. Palladium-Catalyzed Direct C(sp2)–H Alkoxylation of 2-Aryloxypyridines Using 2-Pyridyloxyl as the Directing Group. J. Org. Chem. 2015, 80, 1269–1274. 10.1021/jo5026095. [DOI] [PubMed] [Google Scholar]
- Chu J.-H.; Chen S.-T.; Chiang M.-F.; Wu M.-J. Palladium-Catalyzed Direct Ortho Aroylation of 2-Phenoxypyridines with Aldehydes and Catalytic Mechanism Investigation. Organometallics 2015, 34, 953–966. 10.1021/om501330h. [DOI] [Google Scholar]
- Lou S.-J.; Chen Q.; Wang Y.-F.; Xu D.-Q.; Du X.-H.; He J.-Q.; Mao Y.-J.; Xu Z.-Y. Selective C–H Bond Fluorination of Phenols with a Removable Directing Group: Late-Stage Fluorination of 2-Phenoxyl Nicotinate Derivatives. ACS Catal. 2015, 5, 2846–2849. 10.1021/acscatal.5b00306. [DOI] [Google Scholar]
- Wang L.; Pan L.; Huang Y.; Chen Q.; He M. Palladium-Catalyzed Regioselective C–H Acetoxylation of 2- Aryloxypyridines with 2-Pyridyloxy as a Removable Directing Group. Eur. J. Org. Chem. 2016, 2016, 3113–3118. 10.1002/ejoc.201600508. [DOI] [Google Scholar]
- McAteer D. C.; Javed E.; Huo L.; Huo S. Platinum-Catalyzed Double Acylation of 2-(Aryloxy)pyridines via Direct C–H Activation. Org. Lett. 2017, 19, 1606–1609. 10.1021/acs.orglett.7b00423. [DOI] [PubMed] [Google Scholar]
- Wang L.; Yang Z.; Yang M.; Tian M.; Kuai C.; Cui X. A Facile Route to Ortho-Hydroxyanilines through an IrIII-Catalyzed Direct C-H Amidation of 2-Phenoxypyridines. Chem.–Asian J. 2017, 12, 2634–2643. 10.1002/asia.201701028. [DOI] [PubMed] [Google Scholar]
- Wang L.; Yu Y.; Yang M.; Kuai C.; Cai D.; Yu J.; Cui X. Rhodium-Catalyzed Synthesis of Multiaryl-substituted Naphthols via a Removable Directing Group. Adv. Synth. Catal. 2017, 359, 3818–3825. 10.1002/adsc.201700726. [DOI] [Google Scholar]
- Wang L.; Yang Z.; Yang M.; Zhang R.; Kuai C.; Cui X. Iridium-Catalyzed Direct C–H Amidation of Anilines with Sulfonyl Azides: Easy Access to 1,2- Diaminobenzenes. Org. Biomol. Chem. 2017, 15, 8302–8307. 10.1039/c7ob01899a. [DOI] [PubMed] [Google Scholar]
- Gao C.; Li H.; Liu M.; Ding J.; Huang X.; Wu H.; Gao W.; Wu G. Regioselective C–H Chlorination: Towards the Sequential Difunctionalization of Phenol Derivatives and Late-stage Chlorination of Bioactive Compounds. RSC Adv. 2017, 7, 46636–46643. 10.1039/c7ra09939h. [DOI] [Google Scholar]
- Kiyooka S.-i.; Matsumoto S.; Shibata T.; Shinozaki K.-i. Platinum(II) complex-catalyzed enantioselective aldol reaction with ketene silyl acetals in DMF at room temperature. Tetrahedron 2010, 66, 1806–1816. 10.1016/j.tet.2010.01.036. [DOI] [Google Scholar]
- Anderson G. K.; Lin M.. Inorganic Synthesis; Angelici R. J., Ed.; Wiley and Sons, 1990; Vol. 28, pp 60–63. [Google Scholar]
- Yu X.; Yang F.; Wu Y.; Wu Y. Palladium-Catalyzed C8-H Acylation of 1-Naphthylamines with Acyl Chlorides. Org. Lett. 2019, 21, 1726–1729. 10.1021/acs.orglett.9b00283. [DOI] [PubMed] [Google Scholar]
- de Geest D. J.; O’Keefe B. J.; Steel P. J. Cyclometallated Compounds. XIII. Cyclopalladation of 2-Phenoxypyridine and Structurally-related Compounds. J. Organomet. Chem. 1999, 579, 97–105. 10.1016/s0022-328x(98)01203-0. [DOI] [Google Scholar]
- Ziegler E.; Sterk H. Über die thermische Bildung von Ketencarbonsäurederivaten. Monatsh. Chem. 1967, 98, 1104–1107. 10.1007/bf00901414. [DOI] [Google Scholar]
- Carroll J.; Woolard H. G.; Mroz R.; Nason C. A.; Huo S. Regiospecific Acylation of Cycloplatinated Complexes: Scope, Limitations, and Mechanistic Implications. Organometallics 2016, 35, 1313–1322. 10.1021/acs.organomet.6b00174. [DOI] [Google Scholar]
- Carroll J.; Gagnier J. P.; Garner A. W.; Moots J. G.; Pike R. D.; Li Y.; Huo S. Reaction of N-Isopropyl-N-phenyl-2,2′-bipyridin-6-amine with K2PtCl4: Selective C–H Bond Activation, C–N Bond Cleavage, and Selective Acylation. Organometallics 2013, 32, 4828–4836. 10.1021/om400540y. [DOI] [Google Scholar]
- Warden E.; Bartolotti L.; Huo S.; Li Y. Theoretical Probe to the Mechanism of Pt-Catalyzed C–H Acylation Reaction: Possible Pathways for the Acylation Reaction of a Platinacycle. Inorg. Chem. 2020, 59, 555–562. 10.1021/acs.inorgchem.9b02835. [DOI] [PubMed] [Google Scholar]
- Maiti D.; Buchwald S. L. Cu-Catalyzed Arylation of Phenols: Synthesis of Sterically Hindered and Heteroaryl Diaryl Ethers. J. Org. Chem. 2010, 75, 1791–1794. 10.1021/jo9026935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.