Abstract
Objectives
To examine whether neighborhood income and neighborhood safety concerns influence multisystem physiological risk after adjusting for genetic and environmental selection effects that may have biased previous tests of this association.
Methods
We used structural equation modeling with a genetically informed sample of 686 male and female twin pairs in the Midlife in the United States Study II (2004).
Results
Controlling for additive genetic and shared environmental processes that may have biased neighborhood–health links in previous examinations, higher neighborhood safety concerns were associated with less physiological risk among women but not men.
Discussion
Our findings suggest a possible causal role of neighborhood features for a measure of physiological risk that is associated with the development of disease. Efforts to increase neighborhood safety, perhaps through increased street lighting or neighborhood watch programs, may improve community-level health.
Keywords: Between–within family models, Neighborhoods income, Neighborhood safety, Physiological risk, Twin data
Neighborhood features are associated with residents’ health. Neighborhood socioeconomic status (SES) indicates financial investment, which results in resource availability (housing) and amenities (green space). These resources relate to health through psychological, physiological, and behavioral pathways (Schulz & Northridge, 2004). Both objective (SES) and subjective neighborhood indicators, such as safety perceptions, relate to health (Robinette, Charles, Almeida, & Gruenewald, 2013; Robinette, Charles, & Gruenewald, 2016).
Despite evidence supporting these neighborhood-health links, selection processes may confound study conclusions. Individuals’ family background may relate both to health (Elder et al., 2009) and neighborhoods (Plomin, 2014). Some have argued that genetic propensities may result in neighborhood selection, and that common genetic variants may partially explain links between people’s environment and their traits (Plomin, 2014). The aforementioned gene–environment correlation may bias findings in previous neighborhood-health research.
Family studies can address these concerns, as twins from the same family share genes and early family environments, enabling adjustment for these biases in neighborhood-health studies (Plomin, 2014). We used a sample of twins from the Midlife in the United States Study II (MIDUS II) to examine relationships between neighborhood income and neighborhood safety concerns and multisystem physiological risk. Physiological risk assessments capture functioning across multiple regulatory systems (Gruenewald et al., 2012). Although there exist a variety of ways of assessing physiological risk (Wiley, Gruenewald, Karlamangla, & Seeman, 2016), a consensus emerges that increased physiological dysregulation increases individuals’ risk of mortality (Karlamangla, Singer, & Seeman, 2006) above other common risk factors, including age (Levine & Crimmins, 2018).
Neighborhoods and Health
People living in lower SES neighborhoods have worse health (Schulz & Northridge, 2004). Chronic health conditions are more prevalent in low-income neighborhoods, including those affecting infants (low birth weight), children (asthma), and adults (cardiovascular health; Pickett & Pearl, 2001). Older adults may be particularly vulnerable to neighborhood adversity due to reduced mobility, physiological well-being, and cognitive functioning with which to cope with chronic stressors (Glass & Balfour, 2003). Neighborhood features are related to cognition, physical functioning, mental and physical health, and mortality among older adults (Yen, Michael, & Perdue, 2009).
Fear of neighborhood crime is posited to constrain people’s behaviors (Raudenbush, 2003), many of which are health promoting (physical activity). This fear is further associated with health, including elderly mobility disability, self-rated health, and psychological distress (Clark et al., 2009). Women are more likely than men to report fear of neighborhood crime (Snedker, 2015), perhaps because they view themselves as more vulnerable to threats of harm, or because they provide care to others in their homes. Despite these gender differences, to the best of our knowledge, there has been no examination of gender differences in relationships between safety concerns and health. This is an important gap in the literature that is addressed in the present analyses.
Physiological risk, which includes indicators of inflammation and neuroendocrine and cardiovascular functioning, is elevated in low SES neighborhoods (Robinette, Charles, Almeida, et al., 2013) and neighborhoods perceived as unsafe (Robinette, Charles, & Gruenewald, 2016). These relations have been observed in both cross-sectional and longitudinal studies, suggesting both concurrent and lasting associations. Examination of physiological risk is informative for multiple reasons. First, mutual genetic variants explain the common co-occurrence of many single physiological indicators (e.g., blood glucose and lipids; Locke et al., 2015). Second, exposure to situations of stress is associated with changes across multiple physiological indicators, and it has been posited that mitochondrial dysfunction partially explains this integrated response to stress (Picard et al., 2015). For these reasons, it can be expected that dysregulation of multiple regulatory systems occurs together, and assessing multisystem physiological risk provides a more holistic impression of people’s health.
Common among many previous neighborhood-health studies is a concern that health is better explained by individual-level factors. Age, individual SES, and gender, for example, may relate to health, choice of neighborhood, or both. Chronic health conditions are disproportionately represented among older adults (Crimmins, 2015). Older adults are often less mobile, spending more time in their neighborhoods than younger adults, therefore, experiencing greater exposure to their neighborhoods than younger adults (Glass & Balfour, 2003). Low SES individuals have poor health (Gruenewald et al., 2012) and generally live in low SES neighborhoods (Pickett & Pearl, 2001). Finally, despite a lesser chance of being victimized, women report more fear of neighborhood crime than men (Snedker, 2015). Although researchers adjust for these characteristics in neighborhood-health research (Pickett & Pearl, 2001), it is implausible to adjust for a comprehensive list of potential selection confounds.
Selection Versus Causal Pathways
Genetic and environmental factors shared among family members may confound the putative causal health effects many researchers hypothesize. For instance, common genetic variants explain correlations among education and multiple health outcomes (Boardman, Domingue, & Daw, 2015). Furthermore, many SES correlates such as education (Lindahl, 2011) and cardiovascular disease are partially heritable (Elder et al., 2009). Growing evidence indicates that selection into certain environments is genetically mediated (Plomin, 2014). It is also feasible that people who grew up in neighborhoods with more resources for physical activity will select into similar types of neighborhoods as adults. Discerning the true effect of neighborhood features on residents’ health is challenging when potential gene–environment correlations are not taken into account.
Compounding this challenge is the general inability to randomly assign individuals to neighborhoods. One exception was the Moving to Opportunity (MTO) study (Sanbonmatsu et al., 2011). Moving randomly selected families from high- to low-poverty neighborhoods was related to decreased risk of obesity and diabetes at 10-year follow-up (Ludwig et al., 2011). Such true experiments, however, are rare, so alternative approaches are needed.
Genetically Informed Research Designs
Family studies represent a powerful alternative to random assignment, acting as a natural experiment, that can be used to address potential selection processes (Neale & Maes, 2004; Plomin, 2014). Many individual characteristics that may bias neighborhood-health links, including genes and early exposure to social, economic, and environmental factors, are correlated among twins from the same family. These shared experiences within twin pairs make family data an appealing alternative to random assignment. Twins from the same family serve as each other’s matched control, with differences in their adulthood neighborhoods serving as predictors of differences in their health. Significant neighborhood-health findings are adjusted for a wide range of unmeasured individual characteristics on which twins from the same family are similar.
Few researchers have used family data in neighborhood studies, and those that have generally focused on childhood (Caspi, Taylor, Moffitt, & Plomin, 2000). Academic achievement is more similar among children raised in the same family than those who grew up in the same neighborhood (Lindahl, 2011). In a study of adult twins, the twin living in a lower income neighborhood had a greater risk for ischemic heart disease than the twin living in a higher income neighborhood (Merlo et al., 2013). This study provides evidence for unique relations to neighborhood SES. Additional research using adult sibling data is needed to support causal arguments that neighborhoods influence health.
The Present Study
We used a sample of adult twins from MIDUS II to examine the relation between neighborhood income and neighborhood safety concerns and physiological risk (data collected between 2004 and 2009). Measures of physiological risk not only predict mortality (Karlamangla et al., 2006), but are often stronger predictors of mortality than age (Levine & Crimmins, 2018). We conducted between- and within-family models to test the hypothesis that neighborhood features influence physiological risk after adjusting for family-level factors that may bias hypothesized neighborhood-health links. If the twin living in a better neighborhood has lower physiological risk than his or her co-twin, then there is increased support for the causal influence of neighborhoods on health. If twins living in different neighborhoods have similar health, however, this would suggest that common familial processes alone explain previous neighborhood-health links.
This study builds on current understanding of neighborhood-health relationships in multiple ways. First, we addressed the issue of selection bias by using family data to examine whether family-level factors partly explain the correlation between neighborhoods and health. Second, we extended work examining youth (Caspi et al., 2000) by assessing a large sample of older adults. Physiological risk increases with age (Crimmins, Johnston, Hayward, & Seeman, 2003) and examining older adults may assist in determining how neighborhood features influence this aging process. Third, by examining physiological risk, we demonstrate the plausibility of early identification of those at greatest risk for the development of health problems and a potential physiological pathway to health. Fourth, we examine both objective and subjective neighborhood indicators for a broader assessment of neighborhood quality. Finally, given the gender differences in reports of neighborhood safety concerns, we tested for gender differences in our analyses.
Method
Sample and Procedures
MIDUS is a national survey of U.S. adults. Random digit dialing procedures were used to recruit the majority of the sample. Twin pairs were identified through the MIDUS Twin Screening Project involving telephone interviews assessing the presence of twins among a randomly selected set of 50,000 U.S. households. We report models using both the MIDUS full (n = 654) and twin samples (686 pairs), with 140 monozygotic female (MZF), 128 monozygotic male (MZM), 152 dizygotic female (DZF), 89 dizygotic male (DZM), and 177 dizygotic opposite sex (DZOS) twin pairs.
The MIDUS baseline survey assessed the behavioral, psychological, and social factors explaining health and aging. At the first 10-year follow-up, a subset of original MIDUS participants (N = 1,043) completed the Biomarker substudy consisting of an overnight stay in a General Clinical Research Center (University of California, Los Angeles; University of Wisconsin; and Georgetown University). Eligibility for the Biomarker Study was determined by ability and willingness to travel to one of those sites. Participants provided blood and urine and completed a physical exam, allowing for the construction of a physiological risk variable. The study was completed using ethical guidelines with the approval of each review board of the institutions involved.
Measures
Physiological risk
MIDUS researchers constructed a summary measure of physiological risk using a data-driven approach. First, using the physical exam and biological samples, values on each of 24 biomarkers were categorized into quartile ranges of the biomarker distributions (see Gruenewald et al., 2012). Given that elevated values on many physiological indicators are health compromising, the highest quartile was considered “at risk.” Only two exceptions, dehydroepiandrosterone sulfate and high-density lipoprotein cholesterol where lower values are health compromising, used the lowest quartile as “at risk.” Each of the 24 physiological indicators were given scores of 0 (no risk) or 1 (at risk) based on these risk categories.
Next, each of the 24 indicators was grouped by physiological system: the cardiovascular, sympathetic and parasympathetic nervous, hypothalamic–pituitary–adrenal axis, inflammatory, and lipid and glucose metabolism systems. Because these seven systems contained an uneven number of indicators, each subsystem for which data on at least half of the biomarkers of the subsystem were available was scored based on the proportion of within-system indicators with values in the “risk” quartiles. The system-specific scores thus ranged from 0, indicating none of the within-system indicators had values in the quartile of risk, through 1, indicating that all of the within-system indicators had values in the quartile of risk. Finally, the seven 0–1 system-specific scores were summed to create an overall risk score, which ranged from 0 to 7; females: M = 1.73 (SD = 1.08), range = 0–5.03; males: M = 1.64 (SD = 1.01), range = 0–4.50.
Neighborhood income
Census tract median household income was collected from the 2000 U.S. Census. The Biomarker Project was conducted between 2004 and 2009, making the 2000 U.S. Census decennial data the closest, albeit an imperfect, temporal match (females: M = $72,606 [SD = $32,413], range = $18,142–278,847; males: M = $69,437 [SD = $25,710], range = $25,176–178,171; based on 2016 dollars).
Perceived neighborhood safety
The MIDUS self-administered questionnaire included two questions assessing neighborhood safety: I feel safe being out alone in my neighborhood during the daytime (at night) (Keyes, 1998). Using a Likert-type scale, participants rated these questions with 1 = a lot to 4 = not at all. Items were reversed coded so that higher mean scores represented more neighborhood safety (females: M = 3.64 [SD = 0.44], range = 1.50–4; males: M = 3.85 [SD = 0.35], range = 2–4).
Additional covariates
We included a composite of self-reported income from personal wages, pensions, social security, and government assistance for both the participant and his or her spouse combined (females: M = $105,925 [SD = $85,440], range = $0–418,268; males: M = $119,315 [SD = $87,155], range = $0–418,268; based on 2016 dollars). We also adjusted for age (females: M = 54.32 [SD = 11.46], range = 34–81; males: M = 55.25 [SD = 11.88], range = 34–83).
Statistical Analyses
We begin by presenting the descriptive results of key variables. Gender comparisons were conducted using t tests when analyzing the MIDUS full sample, and linear mixed-effects regressions when analyzing the MIDUS twin sample given that correlation between twin pairs violates the independence of observation assumptions.
To replicate the method of Robinette, Charles, Almeida, et al. (2013), we estimated multilevel models (MLMs) in the MIDUS full sample to test whether neighborhood features significantly predicted physiological risk. This analysis established the basis for testing whether genetic and environmental processes attenuate the correlational effect between neighborhood indicators and physiological risk. To this aim, we conducted MLMs with proc mixed in SAS software, version 9.4. In these models, we included a random intercept, clustering individuals within families to provide estimates of the neighborhood indicator fixed effects.
Third, we present twin correlations to provide a first impression of genetic and environmental influences on physiological risk and the neighborhood variables. Cross-twin, cross-trait (CTCT) correlations are then presented to describe the genetic and environmental influences underlying the association between these variables. Monozygotic (MZ) twin correlations that are greater than dizygotic (DZ) twin correlations provide evidence for an additive genetic component. Evidence for a shared environmental process underlying the construct is obtained when the MZ correlations are equal to or less than DZ twin correlations.
Fourth, we fit structural equation models in Mplus version 8 (Muthén & Muthén, 1998–2017) using a five-group modeling approach to test for gender differences (MZM, MZF, DZM, DZF, and DZOS; Neale, Roysamb, & Jacobson, 2014). The variance in both physiological risk and the neighborhood indicators was decomposed into additive genetic (A), shared environment (C), and unique environment (E) latent variables for each twin. These models make three assumptions. First, A represents additive genetic effects that contribute to twin similarity; MZ twins are perfectly correlated and DZ twins are correlated .5 under the assumption that they share half of their genotype, on average. Second, C represents shared environmental effects that contribute to twin similarity and are perfectly correlated across MZ and DZ groups. Third, E represents any unique environmental effects that contribute to twin differences and includes measurement error and is uncorrelated between twins. In these models, latent variables were mean centered with variances constrained to 1, whereas factor loading were freely estimated. It is assumed that the A, C, and E latent variables are neither correlated nor interact with one another. The model functions under the assumption that maternal and paternal genetic backgrounds are uncorrelated (Neale et al., 2014).
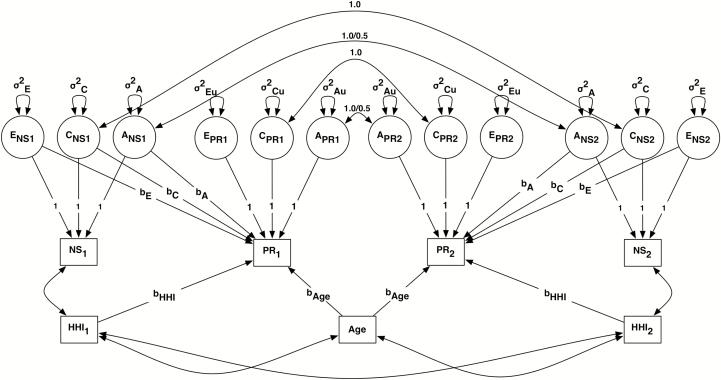
As a formal test of mediation, physiological risk is then regressed on the A, C, and E latent components of the neighborhood variables (see Figure 1) to test whether the genetic and environmental effects underlying the neighborhood variables significantly account for variability in physiological risk. If the E regression coefficient remains significant after adjusting for the effects of A and C, then there is support for the causal argument that neighborhood features influence health. We further adjusted for age and each twin’s adulthood household income; physiological risk was regressed on household income reported both by Twin 1 and Twin 2 from the same family as this factor is presumed to vary within twin pairs. In addition, we modeled correlations between all predictor variables (neighborhood indicator and covariates) within and across twins from the same family.
Figure 1.
Genetically informed regression model for neighborhood safety perceptions (and neighborhood income) and multisystem physiological risk. Shared environmental components are constrained to 1.0 for monozygotic (MZ) and all dizygotic (DZ) twin pairs. Additive genetic components are constrained to 1.0 for MZ and 0.5 for all DZ twin pairs. NS1 = Twin 1 neighborhood safety; PR1 = Twin 1 physiological risk; NS2 = Twin 2 neighborhood safety; PR2 = Twin 2 physiological risk; ENS1 = Twin 1 unique environmental component of neighborhood safety; CNS1 = Twin 1 shared environmental component of neighborhood safety; ANS1 = Twin 1 additive genetic component of neighborhood safety; ENS2 = Twin 2 unique environmental component of neighborhood safety; CNS2 = Twin 2 shared environmental component of neighborhood safety; ANS2 = Twin 2 additive genetic component of neighborhood safety. Correlations among all covariates were estimated, but not all are presented here for clarity of presentation.
With the twin sample, we first fit multiple regression models to examine neighborhood–health links, not taking into account potential genetic and environmental pathways. Next, individual A, C, and E regression effects were estimated. This procedure allowed for a test of whether the twin living in a lower income neighborhood or a neighborhood perceived as less safe would also have greater physiological risk after controlling for family-level factors. Finally, we fit a model that equated the regression effects between men and women to test whether neighborhood indicators influenced physiological risk equally between genders. All models were adjusted for individual income and age.
All multivariate models were fit using maximum likelihood with a robust standard error estimator (MLR). Exploratory data analysis revealed that univariate and multivariate normality assumptions were not met in the genetically informed structural models (Mardia’s test of multivariate skew = 9.83, p < .001 and Mardia’s test of multivariate kurtosis = 32.28, p < .001). MLR, thus, was used to minimize bias of the parameter estimates. Given violations of normality and the relatively small size per group, we used the Satorra–Bentler chi-square difference test (Satorra & Bentler, 2001) to compare nested models (e.g., comparing models where male and female parameters were equated or freely estimated). In addition, we used the root mean square error of approximation to assess absolute model fit (.05 is good and .08 is acceptable fit), and the Tucker–Lewis Index to assess incremental fit (greater than .95 is considered good fit; Hu & Bentler, 1999).
Results
A description of participants is provided in Table 1 by sex and sample (MIDUS full vs MIDUS twin). Both men and women had fairly low levels of physiological risk. Individual and neighborhood income span wide ranges. Men reported significantly higher perceptions of neighborhood safety than women in both the full and twin samples. Among the full sample, men reported significantly higher household income, and among the twin sample, women were significantly older than men.
Table 1.
Characteristics of Midlife in the United States Study (MIDUS) Full and Twin Samples
| MIDUS full sample | |||||
|---|---|---|---|---|---|
| Men (n = 307) | Women (n = 347) | ||||
| M (SD) | Range | M (SD) | Range | t | |
| Physiological risk | 1.67 (1.01) | 0.0–4.5 | 1.75 (1.07) | 0.0–5.03 | −0.84 |
| Neighborhood safety concerns | 3.85 (0.34) | 2.0–4.0 | 3.62 (0.47) | 1.5–4.0 | 7.63* |
| Neighborhood income ($) | 49,760 (18,997) | 10,457–147,585 | 50,698 (22,353) | 12,736–200,001 | −0.49 |
| Household income ($) | 81,499 (60,089) | 0–300,000 | 72,191 (59,638) | 0–300,000 | 2.53* |
| Age (year) | 55.83 (12.03) | 34–83 | 54.72 (11.60) | 34–84 | 1.37 |
| MIDUS twin sample | |||||
| Men (n = 218) | Women (n = 298) | ||||
| M (SD) | Range | M (SD) | Range | Estimate (SE) | |
| Physiological risk | 1.64 (1.01) | 0–4.5 | 1.73 (1.08) | 0.0–5.03 | 0.10 (0.09) |
| Neighborhood safety concerns | 3.85 (0.35) | 2.0–4.0 | 3.64 (0.44) | 1.5–4.0 | −0.21* (0.04) |
| Neighborhood income ($) | 49,804 (18,440) | 18,057–127,792 | 52,076 (23,248) | 13,012–200,001 | 2,857 (1,943) |
| Household income ($) | 85,578 (62,511) | 43,922–300,000 | 75,974 (61,281) | 0–300,000 | −8,856 (5,648) |
| Age (year) | 55.25 (11.88) | 34–83 | 54.32 (11.46) | 34–81 | 0.32 (0.12)* |
*p < 0.05.
Results of the MLMs predicting physiological risk among the MIDUS full sample are shown in Table 2. The first set of models indicated that people living in neighborhoods perceived as less safe had significantly higher physiological risk, adjusting for age, gender, and household income (Model 1a). The second set of models indicated that those living in lower income neighborhoods had significantly higher physiological risk (Model 1b). Neither of these neighborhood associations was moderated by gender, as indicated by the null interaction effects in Models 2a and 2b. Older adults and those with lower household income had significantly higher physiological risk.
Table 2.
Multilevel Models Predicting Physiological Risk in the Midlife in the United States Study Full Sample, Γ (SE)
| Neighborhood safety (n = 1,010) | Neighborhood income (n = 1,007) | |||
|---|---|---|---|---|
| Model 1a | Model 2a | Model 1b | Model 2b | |
| Intercept | 0.72 (0.36) | 0.84 (1.07) | 0.17 (0.21) | 0.09 (0.31) |
| Age (year) | 0.03*** (0.00) | 0.03*** (0.00) | 0.03*** (0.00) | 0.03*** (0.00) |
| Household income | −0.00*** (0.00) | −0.00*** (0.00) | −0.00*** (0.00) | −0.00*** (0.00) |
| Gendera | 0.05 (0.06) | −0.02 (0.60) | 0.10 (0.06) | 0.16 (0.16) |
| Safety concerns | −0.17** (0.07) | −0.21 (0.28) | ||
| Safety × gender | 0.02 (0.16) | |||
| Neighborhood income | −0.05** (0.01) | −0.03 (0.05) | ||
| Neighborhood income × gender | −0.01 (0.03) | |||
| Model fit −2 log likelihood | 2,809.7 | 2,811.5 | 2,818.2 | 2,841.7 |
aCompared to males, household income and neighborhood income coefficients in $10K increments.
*p < .05. **p< .01. ***p< .001.
Results of the linear regression in the twin sample suggested that women living in neighborhoods perceived as less safe had significantly greater physiological risk after adjusting for household income and age. This association was not observed among male twins. Both men and women living in lower income neighborhoods had significantly greater physiological risk. Older age, additionally, was significantly associated with greater physiological risk.
Next we present the twin correlations. There was stronger family resemblance for neighborhood income than neighborhood safety (see Table 3). Among men, the MZ correlations were twice the DZ correlations, suggesting an additive genetic process underlying neighborhood income. For neighborhood safety and physiological risk, however, DZ correlations were slightly larger than MZ correlations, suggesting shared environmental processes for both neighborhood measures. Among women, DZ correlations were similar to MZ correlations for neighborhood income, and were greater than MZ correlations for neighborhood safety, suggesting predominance of shared environmental influences on both neighborhood measures. Physiological risk had a greater additive genetic component as suggested by the larger MZ than DZ correlations.
Table 3.
Neighborhood Indicators and Multisystem Physiological Risk Within-Pair and Cross-Twin, Cross-Trait Correlations: Midlife in the United States Study twin sample
| Twin correlations | |||
|---|---|---|---|
| Neighborhood income | Neighborhood safety | Physiological risk | |
| MZM | .48 | −.02 | .54 |
| DZM | .24 | .08 | .60 |
| MZF | .51 | .20 | .54 |
| DZF | .53 | .44 | .08 |
| DZOS | .19 | .10 | .58 |
| Cross-twin, cross-trait correlations | |||
| Neighborhood income–physiological risk | Neighborhood safety–physiological risk | ||
| MZM | −.04 | −.29 | |
| DZM | .36 | .09 | |
| MZF | .03 | −.13 | |
| DZF | −.23 | −.02 | |
| DZOS | −.16 | .06 |
Note: DZF = dizygotic female; DZM = dizygotic male; DZOS = dizygotic opposite sex pair; MZF = monozygotic female; MZM = monozygotic male.
The CTCT correlations for both neighborhood safety and neighborhood income are unsystematic. In the male twins, the MZ CTCT correlations are negative for both neighborhood safety and neighborhood income. These correlations indicate that one twin’s higher neighborhood income and greater perceptions of neighborhood safety are correlated with lower physiological risk among his or her co-twin, as would be predicted. In contrast, the male DZ CTCT correlations are positive. Among the female twins, the MZ CTCT correlation between neighborhood income and physiological risk is nearly zero—no relationship—whereas female DZ CTCT correlations are negative, as would be predicted. For neighborhood safety, the CTCT correlations are negative for both MZ and DZ female twins. The opposite-sex twin-pair CTCT correlations were positive but small for neighborhood income whereas negative for neighborhood safety. Familial processes may mediate the bivariate relation between neighborhood features and physiological risk, but the pattern of univariate twin correlations combined with the unsystematic CTCT correlations do not make it possible to distinguish which familial processes underlie these correlations. For this reason, we adjusted our multivariate modeling approach and used a multigroup between- and within-family model that does not distinguish between genetic and shared environmental effects by equating A and C components.
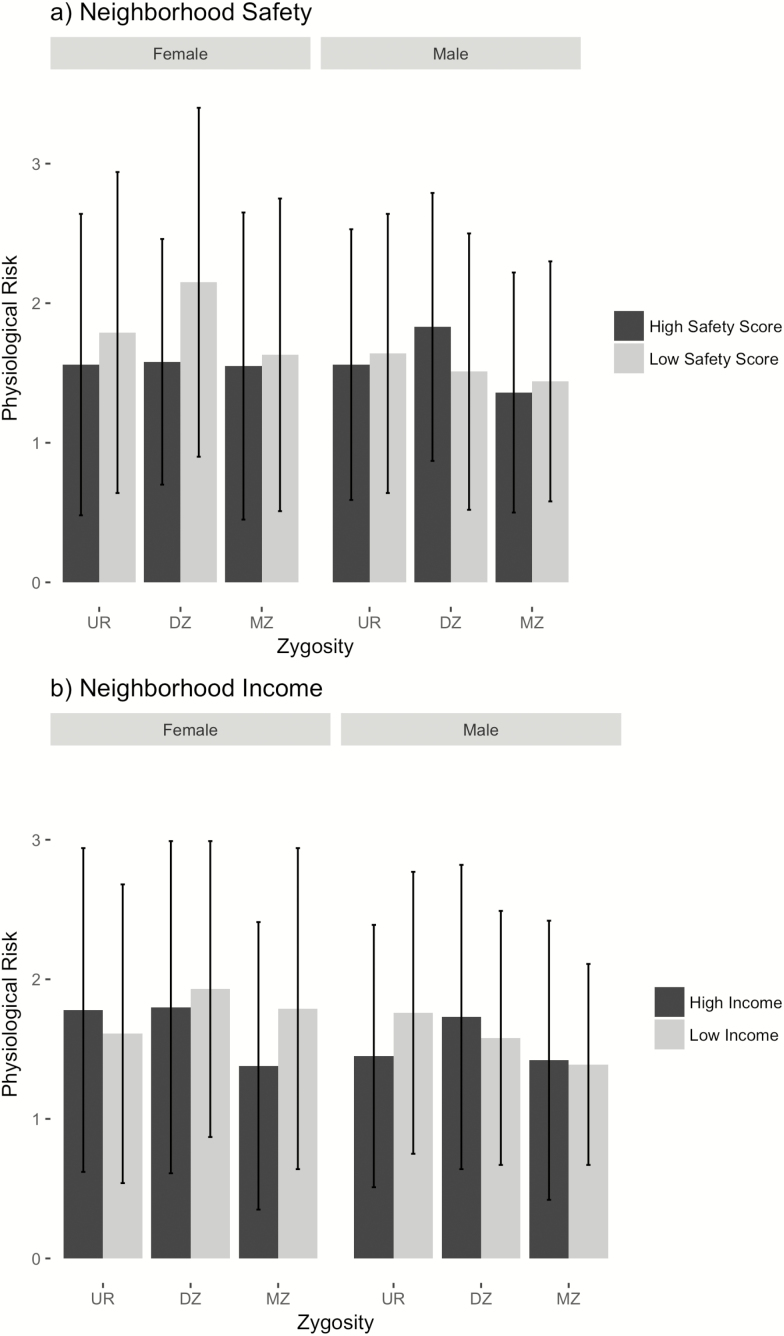
In the between- and within-family model that adjusts for any between-family confounds, the relation between neighborhood safety and physiological risk among women persisted; the female twin living in a neighborhood perceived as less safe had significantly greater physiological risk than her co-twin after controlling for family-level confounds (see Table 4). Neighborhood income, however, was no longer a significant predictor for either gender in the between- and within-model. As can be seen in Figure 2, twins within the same family were ordered from low to high on both neighborhood indicators. Panel A of Figure 2 demonstrates that, among female twins, the twin feeling less safe generally had greater physiological risk. This was less so for men. Panel B of Figure 2 revealed that female twins living in lower income neighborhoods consistently had higher physiological risk, but this was only sometimes the case among male twins. The statistically significant regression effect of safety on health in the female twins was largely driven by the difference in DZ twins. It is also worth pointing out that the standard errors are large, so the estimates lack precision.
Table 4.
Unstandardized Parameter Estimates for Phenotypic and Best-Fitting Genetically Informed Models Predicting Multisystem Physiological Risk: Midlife in the United States Study Twin Sample
| Neighborhood safety | Neighborhood income | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Multiple regression | ||||
| bphen | −0.04 (0.17) | −0.58* (0.14) | −0.40* (0.18) | −0.39* (0.18) |
| Structural equation model | ||||
| bBetween | −0.31 (0.45) | −0.31 (0.45) | −0.38 (0.28) | −0.38 (0.28) |
| b’phen | −0.05 (0.17) | −0.66** (0.23) | −0.37 (0.22) | −0.37 (0.22) |
| Covariates | ||||
| Household income1 | −0.05 (0.03) | 0.02 (0.03) | −0.05 (0.03) | 0.03 (0.03) |
| Household income2 | −0.02 (0.03) | −0.00 (0.03) | −0.02 (0.03) | −0.00 (0.04) |
| Age | 0.36*** (0.07) | 0.41*** (0.07) | 0.36*** (0.06) | 0.43*** (0.06) |
Note: bphen is the full phenotypic effect; b’phen is the within-family effect in the genetically informed structural equation models; bBetween represents the between-family effect of neighborhood indicators on physiological risk and represents the cumulative effect of genetic and environmental factors shared between both twins.
*p< .05. **p< .01. ***p< .001.
Figure 2.
Physiological risk by neighborhood safety (A) and neighborhood income (B) across zygosity groups: Midlife in the United States Study twin sample. MZ = monozygotic; DZ = dizygotic; UR = unrelated.
For neighborhood income, results of the Satorra–Bentler tests indicated that constraining the between-family and within-family regression effects across zygosity and gender was the best-fitting model (see Supplementary Table 1). This suggested that selection effects of neighborhood income on physiological risk were the same across genders and zygosity groups. For neighborhood safety, the best-fitting model constrained between-family, but freely estimated within-family regression effects across zygosity and gender.
Discussion
Neighborhood income and neighborhood safety concerns are associated with residents’ physiological health (Robinette, Charles, Almeida et al., 2013). With the exception of the MTO study (Sanbonmatsu et al., 2011), however, previous investigations of these relations have been correlational, limiting the ability to make causal inferences. We used a sample of twins to examine relations between physiological risk and two neighborhood features, neighborhood income and neighborhood safety concerns. Results of this genetically informed study indicated that female twins reporting more neighborhood safety had lower physiological risk than their co-twins. These results support causal arguments that this social neighborhood feature influences women’s health.
Neighborhood Safety and Health
We found evidence of a significant relation between lower neighborhood safety and greater physiological risk among women, a finding that has been observed among samples of unrelated individuals (Robinette, Charles, Almeida, et al., 2013; Robinette, Charles, & Gruenewald, 2016). We found this pattern of results after statistically adjusting for measured household income and age. Even after controlling for shared genetic and environmental factors in between- and within-family regressions, this finding persisted. Researchers are often concerned that individual characteristics that influence peoples’ selection of neighborhoods also influence their health. This is known as selection bias in the neighborhood–health literature, where characteristics of the individual, not features of the neighborhood, explain people’s health. Twins are often similar on many of the individual difference characteristics that influence their selection of neighborhoods. By comparing one twin to his or her co-twin, those individual difference characteristic are controlled, therefore isolating differences in the twins’ neighborhoods. Differences in the twins’ neighborhoods that are uniquely associated with differences in the twin’s health, such as was the case in the present study, garner support for the causal role neighborhood features on residents’ health.
Perhaps the most difficult methodological challenge in neighborhood–health research is that people are generally not randomly assigned to neighborhoods. When relationships between neighborhood safety concerns and residents’ physiological risk are observed, is it because exposure to neighborhood safety concerns is health-compromising? Or is it because the same characteristics that motivate people’s selection into certain neighborhoods also increase their risk for cardiometabolic health problems? Results from this study suggest, at least among women, that these concerns are causally linked with health. The present analysis represents the first genetically informed test of this question and may encourage policy-driven efforts to improve neighborhood conditions and therefore community-level health.
The association between neighborhood safety concerns and health was not observed among men. Other researchers have noted that women typically report more fear of crime than men (Snedker, 2015), and have posited that women more often than men appraise themselves as less able to cope with threats and experience fear for the safety of others. Although we did not assess fear of crime in the present study, we observed a similar pattern in the present study, with women reporting lower neighborhood safety than men. These perceptions were significantly related to women’s health. Extrapolating from the sociological literature noted earlier (Snedker, 2015), we believe the gender difference observed in the present study are explained by women’s relatively keener perception of safety hazards in their neighborhoods. Women are more likely than men to work in the home, which results in a greater amount of time spent in the home. Moreover, women are more likely the care providers of both children and older parents. Should women perceive their immediate surroundings as being unsafe, this would indicate a threat to their safety as well as the safety of their family members. Taken together, neighborhood safety concerns may serve as a more salient threat to women than men, one which may therefore have a stronger influence on women’s physiological regulation.
Neighborhood SES and Health
Although we found a significant relation between lower neighborhood income and greater physiological risk among both men and women in multiple regressions in the MIDUS full sample, this effect was not statistically significant after adjusting for genetic and environmental selection confounds in the between- and within-family models. The neighborhood income effect was likely attenuated because of gender differences. Among female twins, those living in higher income neighborhoods have lower physiological risk, supporting our hypothesis. Among male twins, however, the opposite pattern emerged, and this gender difference likely explained the null neighborhood income–physiological risk finding.
Limitations and Future Directions
We used a five-group approach in our modeling that resulted in small zygosity/sex groups. Replications are needed with larger samples of twins. As previously noted, physiological risk can be measured in various ways. Future tests of the present hypotheses should explore how the construction of a physiological risk measure may or may not change the pattern of associations with neighborhood indicators. Relatedly, future tests should incorporate a broader range of neighborhood indicators, including more comprehensive, objective measurement of safety, and indicators assessing neighborhood built, physical, and social environments. Finally, in the between- and within-family regression models, the parameter estimates may have been biased because the data were not multivariate normally distributed. Although a maximum likelihood estimator was chosen to minimize bias of the reported parameter estimates, further replication is needed. In addition, given that the present sample is primarily white, future tests of these questions should include more racially/ethnically heterogeneous samples and international family data.
Policy Implications
The present study is among the first to utilize family data to test an important epidemiological question regarding the relevance of the neighborhood environment for residents’ health. Results of the present study indicate that efforts to increase safety within neighborhoods may slow biological aging or reduce the burden of disease at community levels. Increasing street lighting, for example, may represent a cost-effective way to increase safety perceptions with minimal investment by civic institutions. Results of the present study suggest that feeling safer with well-lit streets may slow the development of physiological risk.
Funding
This research was based upon work supported by a National Institutes of Health (NIH)/National Institute on Aging training grant (T32-AG000037-37) and a National Institutes of Health/National Institute on Aging career development grant (1K99AG055699-01 to J. W. Robinette]. The research was further supported by the following grants M01-RR023942 (Georgetown), M01-RR00865 (University of California, Los Angeles) from the General Clinical Research Centers Program, and UL1TR000427 (University of Wisconsin) from the National Center for Advancing Translational Sciences, NIH.
Author Contributions
J. W. Robinette planned the study, conducted the data analysis, and wrote the article. C. R. Beam supervised all statistical analyses, contributed to revisions, and approved the final draft of the article.
Conflict of Interest
Authors have no conflicts of interest to report.
Supplementary Material
References
- Boardman J. D., Domingue B. W., & Daw J (2015). What can genes tell us about the relationship between education and health? Social Science & Medicine (1982), 127, 171–180. doi:10.1016/j.socscimed.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Taylor A., Moffitt T. E., & Plomin R (2000). Neighborhood deprivation affects children’s mental health: Environmental risks identified in a genetic design. Psychological Science, 11, 338–342. doi:10.1111/1467-9280.00267 [DOI] [PubMed] [Google Scholar]
- Clark C. R., Kawachi I., Ryan L., Ertel K., Fay M. E., & Berkman L. F (2009). Perceived neighborhood safety and incident mobility disability among elders: The hazards of poverty. BMC Public Health, 9, 162. doi:10.1186/1471-2458-9-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M. (2015). Lifespan and healthspan: Past, present, and promise. The Gerontologist, 55, 901–911. doi:10.1093/geront/gnv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Johnston M., Hayward M., & Seeman T (2003). Age differences in allostatic load: An index of physiological dysregulation. Experimental Gerontology, 38, 731–734. doi:10.1016/S0531-5565(03)00099-8 [DOI] [PubMed] [Google Scholar]
- Elder S. J., Lichtenstein A. H., Pittas A. G., Roberts S. B., Fuss P. J., Greenberg A. S., … Neale M. C (2009). Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. Journal of Lipid Research, 50, 1917–1926. doi:10.1194/jlr.P900033-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass T. A., & Balfour J. L (2003). Neighborhoods, aging, and functional limitations. In Kawachi I. & Berkman L. F. (Eds.), Neighborhoods and health (pp. 303–334). New York, NY: Oxford University Press. [Google Scholar]
- Gruenewald T. L., Karlamangla A. S., Hu P., Stein-Merkin S., Crandall C., Koretz B., & Seeman T. E (2012). History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine (1982), 74, 75–83. doi:10.1016/j.socscimed.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., & Bentler P. M (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternative. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. doi:10.1080/10705519909540118 [Google Scholar]
- Karlamangla A. S., Singer B. H., & Seeman T. E (2006). Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosomatic Medicine, 68, 500–507. doi:10.1097/01.psy.0000221270.93985.82 [DOI] [PubMed] [Google Scholar]
- Keyes C. L. M. (1998). Social well-being. Social Psychology Quarterly, 61, 121–137. doi:10.2307/2787065 [Google Scholar]
- Levine M. E., & Crimmins E. M (2018). Is 60 the new 50? Examining changes in biological age over the past two decades. Demography, 55, 387–402. doi:10.1007/s13524-017-0644-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L. (2011). A comparison of family and neighborhood effects on grades, test scores, educational attainment and income—Evidence from Sweden. The Journal of Economic Inequality, 9, 207–226. doi:10.1007/s10888-010-9144-1 [Google Scholar]
- Locke A. E., Kahali B., Berndt S. I., Justice A. E., Pers T. H., Day F. R., … Croteau-chonka D. C (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature, 518, 197–206. doi:10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J., Sanbonmatsu L., Gennetian L., Adam E., Duncan G. J., Katz L. F., … McDade T. W (2011). Neighborhoods, obesity, and diabetes–A randomized social experiment. The New England Journal of Medicine, 365, 1509–1519. doi:10.1056/NEJMsa1103216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo J., Ohlsson H., Chaix B., Lichtenstein P., Kawachi I., & Subramanian S (2013). Revisiting causal neighborhood effects on individual ischemic heart disease risk: A quasi-experimental multilevel analysis among Swedish siblings. Social Science and Medicine, 76, 39–46. doi:10.1016/j.socscimed.2012.08.034 [DOI] [PubMed] [Google Scholar]
- Muthén L. K., & Muthén B. O. (1998–2017). Mplus user’s guide(8th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Neale M. C., & Maes H. H. M (2004). Methodology for genetic studies of twins and families. Dordrecht, the Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Neale M. C., Roysamb E., & Jacobson K (2014). Multivariate genetic analysis of sex limitation and G x E interaction. Twin Research and Human Genetics, 9, 481–489. doi:10.1375/183242706778024937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M., McManus M. J., Gray J. D., Nasca C., Moffat C., Kopinski P. K. Wallace D. C. (2015). Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. PNAS, 112, E6614-E6623. doi:10.1073/pnas.1515733112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett K. E., & Pearl M (2001). Multilevel analyses of neighbourhood socioeconomic context and health outcomes: A critical review. Journal of Epidemiology and Community Health, 55, 111–122. doi:10.1136/jech.55.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R. (2014). Genotype-environment correlation in the era of DNA. Behavior Genetics, 44, 629–638. doi:10.1007/s10519-014-9673-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W. (2003). The quantitative assessment of neighborhood social environments. In Kawachi I. & Berkman L. F. (Eds.), Neighborhoods and health (pp. 112–131). New York, NY: Oxford University Press. [Google Scholar]
- Robinette J. W., Charles S. T., Almeida D. A., & Gruenewald T. L (2013). Neighborhood features and physiological risk: An examination of allostatic load. Health and Place, 41, 110–118. doi:10.1016/j.healthplace.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette J. W., Charles S. T., & Gruenewald T. L (2016). Vigilance at home: Longitudinal analyses of neighborhood safety perceptions and health. SSM-Population Health, 2, 525–530. doi:10.1016/j.ssmph.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbonmatsu L., Katz L. F., Ludwig J., Gennetian L. A., Duncan G. J., Kessler R. C., … Lindau S. T (2011). Moving to opportunity for fair housing demonstration program—Final impacts evaluation. U.S. Department of Housing and Urban Development; Retrieved 18 January 2017 from https://www.huduser.gov/publications/pdf/mtofhd_fullreport_v2.pdf. [Google Scholar]
- Satorra A., & Bentler P. M (2001). A scaled difference chi-square test statistic for moment structure analysis. Psychometrika, 66, 507–514. doi:10.1007/BF02296192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A., & Northridge M. E (2004). Social determinants of health: Implications for environmental health promotion. Health Education & Behavior: The Official Publication of the Society for Public Health Education, 31, 455–471. doi:10.1177/1090198104265598 [DOI] [PubMed] [Google Scholar]
- Snedker K. A. (2015). Neighborhood conditions and fear of crime: A reconsideration of sex differences. Crime & Delinquency, 61, 45–70. doi: 10.1177/0011128710392003 [Google Scholar]
- Wiley J. F., Gruenewald T. L., Karlamangla A. S., & Seeman T. E (2016). Modeling multisystem physiological dysregulation. Psychosomatic Medicine, 78, 290–301. doi:10.1097/PSY.0000000000000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen I. H., Michael Y. L., & Perdue L (2009). Neighborhood environment in studies of health of older adults: A systematic review. American Journal of Preventive Medicine, 37, 455–463. doi:10.1016/j.amepre.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.