Abstract
-
•
Agriculture has huge impacts on human health, both positive and negative
-
•
Agriculture has not been oriented to achieving health outcomes
-
•
The livestock sector creates a disproportionate share of risks and benefits
-
•
Health problems related to farming and food require solutions that include these sectors
Keywords: Agriculture, Health, Emerging infectious disease, Zoonoses, Low and middle income countries
Abstract
Worldwide many people are in involved in agriculture and agri-food systems: change to most. Some people may produce food they don't consume people consume the food they produce. As well as providing nutrition and supporting livelihoods, a range of negative health impacts result from this. Among the most important health impacts of livestock are those resulting from infectious diseases: especially, zoonoses, emerging infectious diseases, foodborne disease and antimicrobial resistance. These categories overlap. There are complex linkages between agriculture and agriculture associated disease.
Zoonoses are diseases transmissible between animals and people and may be endemic (continually present) or epidemic (occur in outbreaks). Many zoonoses have mainly been controlled in high income countries but are serious health issues in low and middle income countries. Many zoonoses are considered neglected diseases.
Diseases emerging from agriculture typically get high levels of attention. Many originate in wildlife and then spillover to people, often using livestock as bridges. There is consensus that emerging zoonotic pathogens are best managed by One Health approaches in which human health, animal health and the environmental sectors work together. Recent epidemics and pandemics of emerging disease highlight the importance of good surveillance and rapid response.
The public health importance of foodborne disease is just starting to be recognised. The first global assessment of FBD, developed by the World Health Organisation, suggested the health burden of FBD was comparable to that of malaria, HIV-AIDS or tuberculosis. There are several strategies for managing foodborne disease including good practices, technologies and training.
Human infections that do not respond to treatment impose a large burden of illness and death as well as entailing enormous health care costs. An unknown but potentially substantial amount of this burden is due to the use of antimicrobials in agriculture. Multiple initiatives are underway to promote prudent use.
It is widely appreciated that agriculture development contributes significantly to public health outcomes. Collaborations that bridge the structural divisions between the agriculture and health sectors provides an opportunity for better managing these important diseases.
Introduction
In low and middle income countries (LMICs), around one-third of the population is engaged in agriculture, while in high income countries (HICs) only around 3% of the population are (World Bank, 2017). However, in HICs a far higher proportion of the population works in agri-food value chains, for example, around 12% of employment in Great Britain is in the food sector (outside of farming) (DFRA, 2017). Unsurprisingly, agriculture and the food sector have a large effect on human livelihoods and well-being.
Agricultural innovation has allowed massive expansion of people and their animals. Yet as the world population passed 7 billion in October 2011, two billion people lack micronutrients and nearly 2 billion are sickened each year from the food they ate (Havelaar et al., 2015). Millions more die from diseases that emerge from, or persist in, agricultural ecosystems: zoonoses (diseases transmissible between animals and man) and diseases recently emerged from animals make up 25% of the infectious disease burden in least developed countries and kill one in ten people who live there (Grace et al., 2012a). The burden in high income countries is much less (around 1% of the infectious disease burden), reflecting the reduced contact with sick livestock and better control of zoonoses.
Other urgent health problems related to agriculture include fungal toxins (mycotoxins) in crops and animal source foods; plant toxins; use of wastewater for agriculture; misuse of agricultural chemicals and antibiotics resulting in pathogens resistant to these chemicals; occupational hazards of food value chains; contribution of agriculture to climate change and impacts of this on disease; and, health impacts of agricultural alteration of ecosystems (such as irrigation practices that promote malaria). Table 1 summarises the deaths caused by some diseases associated with agriculture along with other causes for comparison.
Table 1.
Numbers of global deaths each year from selected causes in the early 21st century
| All deaths | 57,000,000 |
| Deaths from infectious disease | 18,000,000 |
| Child deaths in which malnutrition implicated | 5,000,000 |
| Diarrhoeal disease death (many cases are zoonotic) | 3,000,000 |
| Tuberculosis death (a small percentage is zoonotic) | 2,500,000 |
| HIV (a disease that emerged from animals) | 2,000,000 |
| Road traffic deaths | 1,200,000 |
| Foodborne disease | 410,000 |
| Fatal agricultural injuries | 170,000 |
| Rabies deaths | 55,000 |
| Cysticercosis (pig tapeworm) deaths | 50,000 |
| Trypanosomiasis (sleeping sickness) deaths | 40,000 |
| Liver cancer deaths attributable to aflatoxins | 35,000 |
| Extreme weather-related deaths | 20,000 |
Livestock and fish production and capture make essential contributions to the nutrition and livelihoods of billions of people. On the other hand, animal agriculture and capture is especially problematic as a generator of disease risks and a contributor to over-nutrition. Humans are animals and we share a majority of our diseases with them. Moreover, animal source food (ASF), while highly nutritious, provides a good medium for the survival and growth of pathogens. There are also many indirect linkages between animal agriculture and human health and nutrition. For example, because animal products are typically high value, they offer potential for income generation. At the same time, ready availability of markets may reduce the amount of nutritious animal source food available for household consumption. Animals require feeding, care and attention and this has implications for labour and time. In many cultures, women have an important role in animal husbandry and so this may directly compete with time available for child care. As well as contributing to diets and income, livestock produce many useful products and byproducts (such as manure, skins, fuel, power) and these products bring associated benefits and risks to health and well-being. For example, use of cow manure for fuel is widespread in India, which reduces costs of cooking but is associated with many diseases such as acute lower respiratory infections, chronic obstructive pulmonary disease, lung cancer, pulmonary tuberculosis, and asthma (Chakraborty et al., 2014).
Research has helped conceptualize the relations between agriculture and health: multiple benefits and multiple risks are linked through complex feedback loops, highly dependent on context and system (Fig. 1 ).
Figure 1.
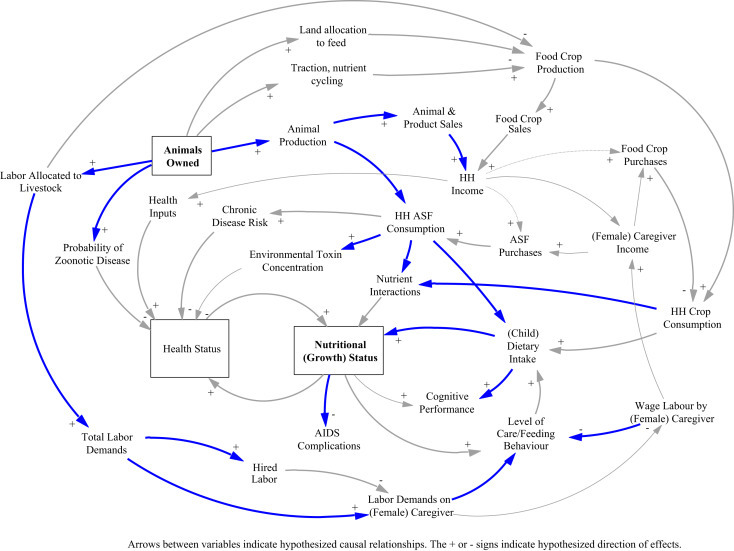
Complex interaction between health, nutrition, agriculture and livelihoods. HH = household, ASF = animal source food.
Adapted from Randolph et al. (2007).
The rest of this chapter will focus on four major sets of infectious diseases associated with agriculture:
-
1.
Endemic and epidemic zoonoses
-
2.
Emerging and re-emerging zoonoses
-
3.
Foodborne disease
-
4.
Antimicrobial resistance
Endemic and Epidemic Zoonoses
Zoonoses are diseases transmissible between animals (domestic and wildlife) and humans. Around 60% of all human diseases and around 75% of emerging infectious diseases are zoonotic (Taylor et al., 2001; Woolhouse and Gowtage-Sequeria, 2005). In aggregate, zoonoses have high impacts on human health, livelihoods, animals and ecosystems.
Endemic zoonoses are continually present to a greater or lesser degree in certain populations. Examples are cysticercosis (pig tapeworm), brucellosis, bovine tuberculosis, leptospirosis and food-borne zoonoses. They are common in poor populations and are responsible around a billion illnesses and millions of deaths every year (Grace et al., 2012b). Fig. 2 shows the positive association between poverty, livestock keeping and zoonoses. However, although endemic zoonoses have a huge impact on health and well-being, they have been neglected by the international donor, standard setting, and research communities.
Figure 2.
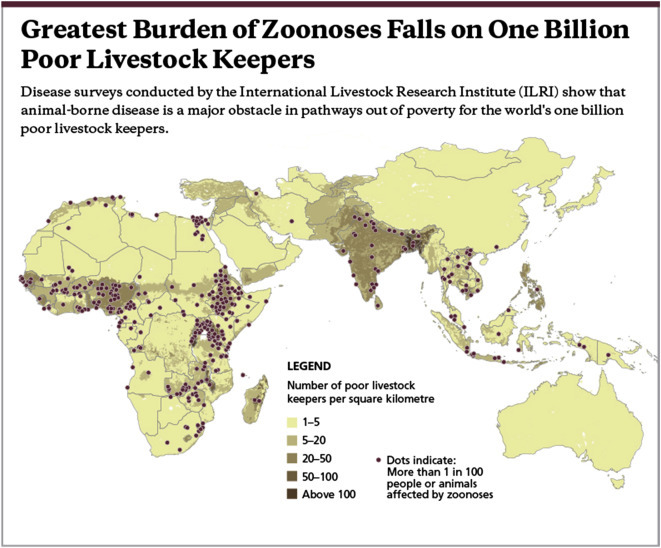
Greatest burden of zoonoses falls on one billion poor livestock keepers (Grace et al., 2012b).
In contrast to endemic diseases, outbreak or epidemic zoonoses typically occur intermittently. Examples are anthrax, rabies, Rift Valley fever, and leishmaniasis. Endemic zoonoses may occur as outbreaks in naïve populations or when triggered by events such as climate changes, flooding, waning immunity or concomitant hunger or disease. They typically have high temporal and spatial variability. Their overall impact in terms of morbidity, mortality and production loss is much less than endemic zoonoses but because they can ‘shock’ systems they are often of high priority to farmers and decision makers. They can also cause important economic losses, which are often related to reaction to the disease rather than the disease itself. Some diseases which now occur in endemic foci have in the past resulted in major outbreaks or epidemics.
Zoonotic diseases, whether endemic or epidemic, are caused by a range of viral, bacterial, mycotic, chlamydial, rickettsial and parasitic pathogens. Many are directly transmitted (by aerosols or contact) from animals to people and vice versa, but a great number are transmitted between animals and humans via food (e.g. milk and meat) and other animal products, water and waste. The term ‘zoonosis’ does not indicate the direction of transmission, i.e. from vertebrate animals to humans or vice versa. Some zoonoses cause (severe) disease and/or mortality in both livestock and in humans (e.g. bovine tuberculosis and anthrax); others are inapparent, mild or chronic in livestock but may cause prolonged disease in humans (e.g. sleeping sickness due to Trypanosoma brucei rhodesiense and many food-/water-borne diseases such as campylobacteriosis). Yet others are important production or devastating diseases in livestock but are mild in humans (e.g. Newcastle disease) or are the cause of important disease burden mainly in immuno-compromised people (e.g. cryptosporidiosis, toxoplasmosis and giardiasis).
Zoonoses have implications for food and nutrition security and sustainability beyond direct negative impacts caused by human sickness. Many zoonoses have negative impacts on animal health and productivity: these diseases directly reduce the availability and quality of animal source food. Zoonoses and their control also have complex upstream and downstream impacts on agri-food systems. For example, during the Rift Valley fever epidemic in east Africa in 2007, many actors beyond farmers (traders, processors, tea shop owners) suffered economic loss (Rich et al., 2011). When people become ill from zoonoses their nutrition suffers, as does the nutrition of family members depending on them.
There are efficacious tools for breaking the transmission cycle of zoonoses and thus preventing disease in people and infection of animals. Good vaccines against rabies in dogs, cysticercosis in pigs, and cystic echinococcosis in ruminants exist (although these vaccines will only be available in some years after in-depth field testing) as do sufficiently efficacious livestock vaccines against brucellosis and anthrax (Schelling et al., 2007). A test and slaughter strategy in livestock can eliminate a zoonosis from a region, especially once prevalence has been lowered, e.g. after vaccination against brucellosis. There are preventive and curative drugs for treating animals (e.g. canine echinococcosis, porcine cysticercosis) or human (e.g. taeniasis). Vector-control involving new technologies and community-participation is effective – for example with regard to trypanosomiasis where it is further combined with chemotherapy in the cattle reservoir.
However, while most important epidemic and endemic zoonoses have been well-controlled in high income countries, progress in LMICs lags and many zoonoses are still commonplace. Because control of zoonoses is well-understood, the problem is essentially one of mobilising interest and investments. Evidence on the economic benefits of control can stimulate this: one review on brucellosis, a common zoonosis, found that control invariably had more benefits than costs and that benefits from control in LMICs were relatively higher than in HICs (McDermott et al.). Zoonoses with only livestock hosts are promising candidates for eradication. For example, cysticercosis, caused by the pig tapeworm or echinococcosis caused by the dog tapeworm. On the other hand, pathogens which are widespread in the environment and in wildlife hosts are less feasible to eradicate but can be controlled.
Emerging Infectious Diseases
Emerging infectious diseases (EIDs) are infections that have newly appeared in a population or have existed previously but are rapidly increasing in incidence or geographical range (Morens et al., 2004). Some writers consider antimicrobial resistant infections to be EIDs (Courvalin, 2016). Most EIDs are zoonoses, that is they are transmissible between humans and animals. Some of the more important emerging zoonoses of recent decades include: bovine spongiform encephalopathy; sudden acute respiratory syndrome; highly pathogenic avian influenza; middle east respiratory syndrome; Ebola virus fever; and Henipa virus infection.
Currently one new human disease is emerging every 4 months; three-quarters of emerging infectious diseases are zoonotic. The costs of these emerging zoonotic diseases are estimated at $6.7 billion per year (World Bank, 2012). A worldwide pandemic could have impacts in the same order of magnitude as those caused by climate change.
Historically most diseases emerged from the western seaboards of north America and western Europe, possibly associated with high human density, high livestock density and good detection. More recently, China, Africa and Latin America are increasingly implicated as shown in Fig. 3 (Grace et al., 2012).
Figure 3.
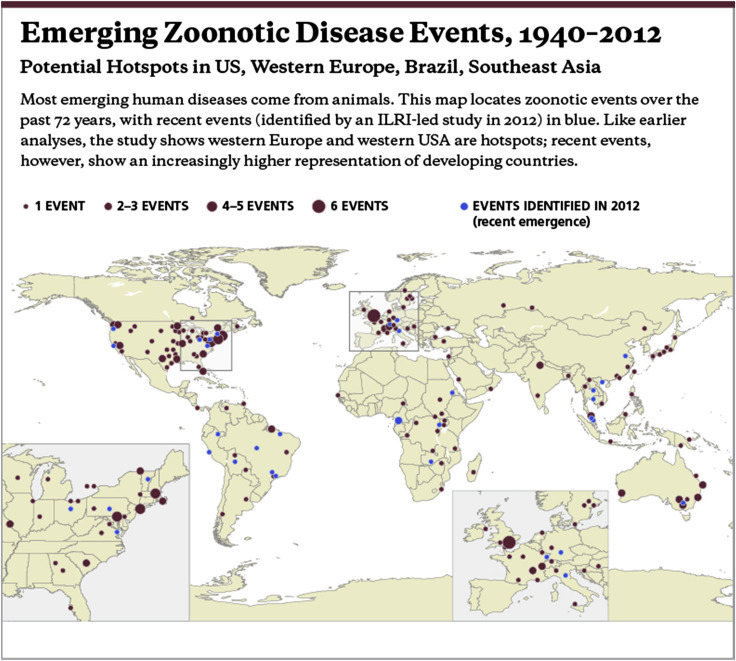
Emerging zoonotic disease events, 1940–2012 (Grace et al., 2012b).
Emerging diseases are caused by:
-
(i)
newly evolved pathogens – for example H5N1 highly pathogenic avian influenza virus in poultry (Vijaykrishna et al., 2008).
-
(ii)
pathogens that have spread to new areas or populations – including Rift Valley fever (RVF) virus that occurred for the first time outside Africa in southwest Saudi Arabia and northwest Yemen in the year 2000 to 2001 (Jupp et al., 2002); African swine fever (ASF), previously confined to sub-Saharan Africa, has been introduced into Caucasus including Georgia (Tomley and Shirley, 2009); spread to new areas or populations is often associated with genetic change.
-
(iii)
pathogens that have been responsible for disease throughout human history but have only recently been recognized as distinct diseases due to an infectious agent (Lyme disease and gastric ulcers are examples) (National Institutes of Health et al., 2007)
-
(iv)
re-emerging pathogens cause diseases that once were major health problems, and then declined dramatically, but are again becoming health problems for a significant proportion of the population (tuberculosis is an example).
Many factors have been identified that affect the risk of disease emergence from agriculture (Jones et al., 2013). Many of these factors are related to unsustainable agriculture or landuse practices. Some of the more important disease drivers include:
-
•
The majority of the most important emerging zoonoses have originated in wildlife but livestock have had an important role in spillover to humans. The growth in demand for milk and meat, mainly driven by urban consumers in developing countries, has been increasing in the last few decades and is projected to double the demand for livestock products by 2050. This “Livestock Revolution” is already leading to rapid increases in livestock populations in developing countries, which increases the likelihood of disease.
-
•
In those countries in which consumption has increased most rapidly, particularly in Asia and Latin America, there has been a trend towards larger-scale livestock production enterprises and greater intensification/industrialisation of production. This is especially the case for poultry and pigs. In those countries in which consumption has increased most rapidly, particularly in Asia and Latin America, there has been a trend towards larger-scale livestock production enterprises and greater intensification/industrialisation of production. This is especially the case for poultry and pigs.
-
•
Demand for livestock products and increased people movement and migration is leading to increased movement of livestock products. The 2001 UK outbreak of foot and mouth disease was widely believed to have been due to illegal imports of meat that was subsequently fed to pigs without adequate heat treatment.
-
•
Changing land use is a major driver by creating new conditions under which pathogens find susceptible hosts and are not checked by other ecosystem processes. New environments are been rapidly created through irrigation, incursion of agriculture into pristine ecosystems, reversion of farmland to leisure uses, urbanisation, and climate change.
-
•
Climate has a major influence on ecosystem conditions and is considered an important factor in the distribution of vector-borne diseases as well as pathogens which survive outside the host in soil or water.
Fig. 4 shows how some of these factors interact to facilitate spillover of pathogens from reservoir hosts (often wildlife) to humans. Livestock may act as a secondary host which facilitates transmission to humans. Vectors, such as mosquitoes or midges, may also be involved. Many spillover events do not result in sustained disease transmission: the infection is not well adapted to the new host and burns out. However, in some cases, the disease establishes in a new host.
Figure 4.
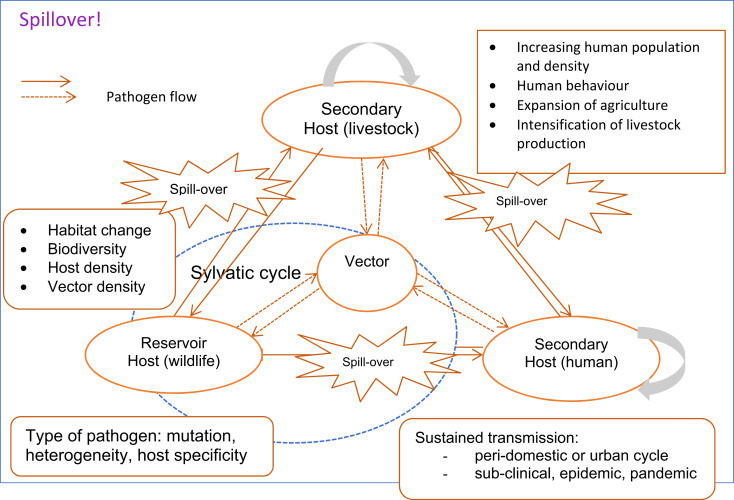
illustrating how diseases jump species from animals to humans.
Emerging zoonotic diseases have obvious implications for nutritional and food security. Widespread animal diseases reduce the availability of animal source food. But control of ZEIDs can also reduce animal source food available with likely nutritional consequences (Kavle et al., 2015). If people become ill their health and nutrition are obviously affected. Indirectly, the enormous cost of pandemic diseases slows economic growth and reduces ability to buy food.
There is consensus that emerging zoonotic pathogens are best managed by One Health approaches in which human health, animal health and the environmental sectors work together. Recent epidemics and pandemics of emerging disease highlight the importance of good surveillance and rapid response. A good understanding of the socio-cultural context underpins communication during outbreaks and technologies such as rapid tests, vaccines and information management are also key to bringing outbreaks under control. However, unless the underlying drivers of disease emergence are addressed, new diseases will continue to emerge and create enormous disruption and health burden.
Foodborne Disease
Foodborne diseases (FBD) are illnesses caused by contaminated, or naturally harmful, food or beverages. A food safety hazard is anything in food that can harm consumers' health. There are three major types of hazards:
-
•
Biological hazards are living organisms (including viruses, bacteria, protozoa, moulds and parasites), which have the ability to infect people or produce toxins injurious to health.
-
•Chemical hazards can be artificial chemicals produced by industry or natural chemicals (for example, those produced by heating food or toxic metals), which are injurious to health.
-
•Mycotoxins (chemical compounds produced by moulds) and phycotoxins (chemical compounds produced by algae and accumulated in sea foods) are considered biological hazards by some and chemical hazards by others.
-
•
-
•
Physical hazards include stones and fragments of metal or glass as well as sub-microscopic nanomaterials and radionuclides.
The health impacts of FBD can be measured in different ways, including annual cases of sickness and death. There is also a standard metric for measuring disease burden: the Disability Adjusted Life Year (DALY). One DALY is the equivalent of one lost year of healthy life. Measuring health impact in DALYs helps comparisons between dissimilar diseases and aids in prioritization. The first global assessment of FBD, developed by the World Health Organisation, suggested the health burden of FBD was comparable to that of malaria, HIV-AIDS or tuberculosis (Havelaar et al., 2015). The report found that most of the burden was caused by microbial disease, followed by helminths and toxins and that the great majority of the burden fell on developing countries (Fig. 5 ).
Figure 5.
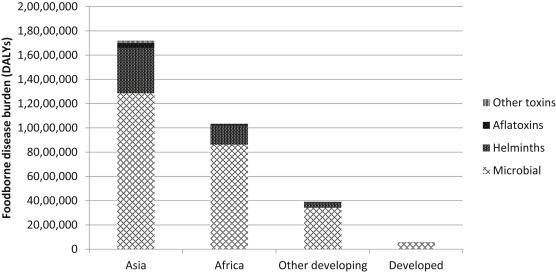
Foodborne disease burden by cause and region.
Derived from Havelaar et al. (2015).
Food borne disease has important implications for food and nutrition security. Stunting, or extreme shortness (very low height-for-age), is the result of a combination of long-term (chronic) poor dietary intake in terms of quality as well as quantity of food and repeated infectious disease episodes. Both wasting (extreme thinness, or low weight-for-age) and stunting are associated with increased mortality as well as poor health and longer-term development outcomes. FBD and hazards may contribute to both wasting and stunting through additional pathways, summarised in Grace (2017), for example:
-
•
Diarrhoea is associated with malnutrition, but a causal link is hard to demonstrate; a 9-country study found that 25% of stunting could be attributed to experiencing more than four episodes of diarrhoea before the age of 24 months. Studies find a strong peak in diarrhoea after the introduction of supplementary foods and find that weaning foods often have high levels of microbial contamination and adulteration.
-
•
Aflatoxins may directly contribute to stunting, and there are demonstrated associations between higher toxin levels and poorer growth in several contexts, although a causal relation, while plausible, is as yet unproven.
-
•
Ingestion of animal faecal material through food or from the environment may contribute to environmental enteric dysfunction1
Foodborne disease also has implications for sustainability because the most risky foods are also the most nutritious. In developed countries, most FBD results from consuming animal source foods (i.e. livestock products and food derived from aquatic animals) and contaminated produce (i.e. fresh fruits and vegetables) (Grace, 2015b). In developing countries, there is less good evidence on the cause of FBD but it seems that these foods are responsible for the majority of disease (Hoffmann et al., 2017). These foods, especially animal source foods, are often considered problematic from the perspective of sustainability because their production contributes to greenhouse gases and if not properly regulated may result in pollution, deforestation, and other adverse environmental impacts.
High income countries have been relatively successful in managing foodborne disease. There are four major lines of defence against FBD:
-
•
Improving the safety of inputs;
-
•
Improving the chemical and microbiological safety of raw foodstuffs;
-
•
Using food processing technologies that mitigate risk (pasteurization and irradiation) and prevent contamination;
-
•
Behaviour change aimed at food handlers, including home-based food handlers.
However, the limited literature on domestic food safety regulation in developing countries shows that we do not yet have good models for standards and approaches that can work at scale to assure food safety where risks are pervasive, costs of compliance are high and enforcement capacity is weak (Grace and Unnevehr, 2013). Given the very different farming systems and regulatory environments, the approaches used successfully in Europe cannot be directly applied to developing countries. A number of food safety interventions have been tried and evaluated with little evidence for benefit or sustainability. Nonetheless, other initiatives show promise, and a smaller number have been able to demonstrate sustained and scalable benefits. For example, introduction of new technologies such as disinfectants or training of food handlers have been shown to lead to reduced pathogen loads in food and in some cases to reduced human infections. Important approaches to improving food safety include risk analysis and Hazard Analysis Critical Control Points (HACCP). To be sustainable and scalable, food safety interventions should have an enabling policy environment and be accompanied by incentives that reward behaviour change.
Antimicrobial Resistance
Human infections that do not respond to treatment impose a large burden of illness and death as well as entailing enormous health care costs. Recent reports predict drug resistance will increase substantially; for example, that drug-resistant infections will cause 10 million extra deaths a year and cost the global economy up to $100 trillion by 2050 (Review on Antimicrobial Resistance, 2014). Technological breakthroughs are possible, for example, the announcement in 2015 of the discovery of a new class of antibiotics. However, over the last decades there has been a dramatic slow-down in the development of new drugs, which increases the need to safeguard existing drugs.
The use of antimicrobials in animal agriculture (both livestock and fish production) has been an issue of controversy for decades. In recent years, there had been increasing consensus on the links between veterinary drug use and drug resistance in human pathogens, and the need to reduce antimicrobial use in agriculture. However, nearly all the evidence relates to developed countries. It seems likely that, globally, more antimicrobials are used in agriculture (by weight) than in human health. In Europe, the USA and Australia, more than 75% of antibiotics are used in agriculture: however, in Sweden only around 16% is (Grace, 2015b). Moreover, antimicrobial use is predicted to increase rapidly in developing countries as animal systems intensive in response to the increasing demand for livestock products and fish: most of this growth will occur in the BRICs (Brazil, Russia, India, China) (Van Boeckel et al., 2015).
It has been clearly shown that resistance to drugs of human importance has been generated in animals and has spread to people. Moreover, meta-analyses suggest that reducing the use of antimicrobial in agriculture and reduce the level of antimicrobial resistance in bacteria in people (Tang et al., 2017). The importance of this is not known, because: there are few studies, many studies have a weak design; most studies were conducted in intensive farms in high income countries; no studies assessed human health outcomes. This is an area of intense interest and research is likely to shed light on these questions.
Most countries have policies and regulations in place to control the use of antimicrobials in livestock and fish production. Legislation, surveillance systems and bans on growth promotion are all trending upwards (Maron et al., 2013). There is increasing support for integrated “One Health” management of AMR which is articulated in the WHO and OIE strategies for better use of antimicrobials.
Experience from Europe shows dramatic declines in the use of antimicrobials are possible if there is adequate regulation and incentives in place for behaviour change. Moreover, these reductions were not prohibitively expensive and did not jeopardise food and nutrition security or animal welfare (Grace, 2015b). Many of these interventions were related to improving animal husbandry (e.g. decreasing stocking rates) but there are also promising technological solutions including new and better vaccines, improved diagnostics, and alternatives to antibiotics such as probiotics and pre-biotics. However, there is insufficient evidence to judge if interventions will have the same success in LMICs.
Reflecting the concern around antimicrobial resistance there are multiple initiatives at global, regional and country level to improve surveillance of antimicrobial resistance and to promote prudent drug use in both medical and veterinary use.
Conclusions
It is widely appreciated that agriculture development contributes significantly to public health outcomes. Collaborations that bridges the structural divisions between the agriculture and health sectors provides a largely untapped opportunity to improve the health and livelihoods of poor people, especially in rural, agricultural areas, where ill health is often the most important pathway for staying or becoming poor, and undermines the benefits of agricultural development.
Footnotes
An incompletely defined syndrome of inflammation, reduced absorption and barrier function of the small intestine.
References
- Courvalin P. Why is antibiotic resistance a deadly emerging disease? Clin. Microbiol. Infect. 2016;22(5):405–407. doi: 10.1016/j.cmi.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Chakraborty D., Mondal N.K., Datta J.K. Indoor pollution from solid biomass fuel and rural health damage: a micro-environmental study in rural area of Burdwan, West Bengal. Int. J. Sustain. Built Environ. 2014;3(2):262–271. [Google Scholar]
- DFRA . Department for Environment and Rural Affaires; UK: 2017. Food Statistics Pocket Book. [Google Scholar]
- Grace D., McDermott J. ILRI; Nairobi, Kenya: 2011. Agriculture-associated Diseases Research at ILRI: Food, Farming and Human Health. Livestock Exchange Issue Brief 10. [Google Scholar]
- Grace D., Gilbert J., Randolph T., Kang’ethe E. The multiple burdens of zoonotic disease and an ecohealth approach to their assessment. Trop. Animal Health Prod. 2012;44(S1):67–73. doi: 10.1007/s11250-012-0209-y. [DOI] [PubMed] [Google Scholar]
- Grace D., Mutua F., Ochungo P., Kruska R., Jones K., Brierley L., Lapar L., Said M., Herrero M., Phuc P.M., Thao N.B., Akuku I., Ogutu F. ILRI; Nairobi, Kenya: 2012. Mapping of Poverty and Likely Zoonoses Hotspots. Zoonoses Project 4. Report to the UK Department for International Development. [Google Scholar]
- Grace D., Unnevehr L. Tackling Aflatoxins: an overview of challenges and solutions. Focus Brief. 2013;20(14) Washington, DC: IFPRI. [Google Scholar]
- Grace D. Review of evidence on antimicrobial resistance and animal agriculture in developing countries. Rep. Produced by Int. Livest. Res. Inst. (ILRI) Evid. Demand Assistance UK. Dep. Int. Dev. (DFID) 2015 doi: 10.12774/eod_cr.june2015.graced. [DOI] [Google Scholar]
- Grace D. Food safety in developing countries: an overview. Rep. Produced by Int. Livest. Res. Inst. (ILRI) Evid. Demand Assistance UK. Dep. Int. Dev. (DFID) 2015 [Google Scholar]
- Grace D. 2017. White paper: Food Safety in Developing Countries: Research Gaps and Opportunities. Prepared for USAID, Washington. [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., Praet N., Bellinger D.C., de Silva N.R., Gargouri N., Speybroeck N., Cawthorne A., Mathers C., Stein C., Angulo F.J., Devleesschauwer B. World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12):e1001923. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S., Devleesschauwer B., Aspinall W., Cooke R., Corrigan T., Havelaar A.H., Angulo F., Gibb H., Kirk M., Lake R., Speybroeck N., Torgerson P., Hald T. Attribution of global foodborne disease to specific foods: findings from a World Health Organization structured expert elicitation. PLoS One. 2017;12(9):e0183641. doi: 10.1371/journal.pone.0183641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., Grace D., Kock R., Alonso S., Rushton J., Said M., McKeever D., Mutua F., Young J., McDermott J., Pfeiffer D. How do agricultural intensification and environmental change affect zoonoses with a wildlife-livestock interface? A systematic review. Proc. Natl. Acad. Sci. U. S. A. 2013;110(21):8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp P.G., Kemp A., Grobbelaar A., Leman P., Burt F.J., Alahmed A.M., Al Mujalli D., Al Khamees M., Swanepoel R. The 2000 epidemic of Rift Valley fever in Saudi Arabia: Mosquito vector studies. Med. Vet. Entomol. 2002;16:245–252. doi: 10.1046/j.1365-2915.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- Kavle J.A., El-Zanaty F., Landry M., Galloway R. The rise in stunting in relation to avian influenza and food consumption patterns in Lower Egypt in comparison to Upper Egypt: results from 2005 and 2008 Demographic and Health Surveys. BMC Public Health. 2015;15:285. doi: 10.1186/s12889-015-1627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D.F., Smith T.J., Nachman K.E. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Glob. Health. 2013;9:48. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D.M., Folkers G.K., Fauci A.S. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (US), Biological Sciences Curriculum Study, NIH Curriculum Supplement Series [Internet], Bethesda (MD): National Institutes of Health (US) 2007. Understanding Emerging and Re-emerging Infectious Diseases.https://www.ncbi.nlm.nih.gov/books/NBK20370/ Available from: [Google Scholar]
- O'Neill J. Review on Antimicrobial Resistance; London: 2014. Review on Antimicrobial Resistance Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations.https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf Available from: [Google Scholar]
- Randolph T.F., Schelling E., Grace D., Nicholson C., Cole D., Omore A., Dement M., Leroy J., Zinsstag J., Ruel M. The role of livestock in human health and nutrition for poverty reduction in developing countries. J. Animal Sci. 2007;85:2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- Rich K.M., Okike I., Randolph T., Akinwumi J., Ayele G., Mensah-Bonsu A., Okello J., Sudarman A. Poultry value chains and their linkages with HPAI risk factors: synthesis of case study findings. Draft Work. Pap. HPAI Pro-Poor DFID Funded Risk Reduct. Proj. 2011 [Google Scholar]
- Schelling E., Grace D., Willingham A.L., Randolph T.F. Which research approaches for pro-poor control of zoonoses? Food Nutr. Bull. 2007;(2 Suppl.):S345–S356. doi: 10.1177/15648265070282S214. [DOI] [PubMed] [Google Scholar]
- Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W., Polachek A.J., Ganshorn H., Sharma N., Kellner J.D., Ghali W.A. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1(8):e316–e327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomley F.M., Shirley M.W. Livestock infectious diseases and zoonoses. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:2637–2642. doi: 10.1098/rstb.2009.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel Global trends in antimicrobial use in food animals. PNAS. 2015;(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykrishna D., Bahl J., Riley S., Duan L., Jin X.Z., Chen H., Peiris J.S.M., Smith G.J.D., Guan Y. Evolutionary dynamics and emergence of panzootic H5N1 influenza viruses. PLoS Pathog. 2008;4:1–10. doi: 10.1371/journal.ppat.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M.E.J., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11(12):1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank . © World Bank; Washington, DC: 2012. People, Pathogens and Our Planet: The Economics of One Health.https://openknowledge.worldbank.org/handle/10986/11892 License: CC BY 3.0 Unported. [Google Scholar]
- World Bank . 2017. Data Bank.http://databank.worldbank.org/data/home.aspx [Google Scholar]


