Section A: Canine Lymphoma and Lymphocytic Leukemias
David M. Vail, Marie Pinkerton, and Karen M. Young
Lymphoma
Lymphoma (malignant lymphoma or lymphosarcoma) comprises a diverse group of neoplasms that have in common their origin from lymphocytes. The neoplasms usually arise in lymphoid tissues such as lymph nodes (LNs), spleen, and bone marrow; however, they may arise in almost any tissue in the body. Although the annual incidence of lymphoma is difficult to predict in the absence of a national canine tumor registry, it is clear that it represents one of the most common neoplasms seen in the dog. The annual incidence has been estimated to range between 13 and 114 per 100,000 dogs at risk. The rates at specific ages are estimated to be 1.5 per 100,000 for dogs less than 1 year of age and 84 per 100,000 for dogs 10 to 11 years old.1, 2, 3, 4 Lymphoma comprises approximately 7% to 24% of all canine neoplasias and 83% of all canine hematopoietic malignancies.5, 6 In a review of the Veterinary Medical Database Program (VMDP) at Purdue University from 1987 to 1997, the frequency of dogs presented with lymphoma to 20 veterinary institutions increased from 0.75% to 2.0% of total case load, and it appears the frequency is continuing to increase. A similar trend is present in physician-based oncology; non-Hodgkin’s lymphoma (NHL) represents 5% of all new cancer cases, the fifth leading cause of cancer death, and the second fastest growing cancer in terms of mortality in humans.7 Middle-aged to older (median age of 6–9 years) dogs are primarily affected, although dogs with T-cell lymphoma tend to be younger.8 A decreased risk for lymphoma is reported for intact females.9 Breeds reported to have a higher incidence include boxers, bullmastiffs, basset hounds, St. Bernards, Scottish terriers, Airedales, pitbulls, Briards, Irish setters, Rottweilers, and bulldogs; breeds at lower risk include dachshunds and Pomeranians.8, 10, 11 See Box 33.1 .
BOX 33.1. Key Clinical Summary Points: Canine Lymphoma.
-
•
Lymphoma is a catch-all term for approximately two dozen lymphocyte cancer subtypes (Table 33.1).
-
•
Most are intermediate or high grade, but indolent forms exist.
-
•
Dogs with lymphoma most commonly have peripheral lymphadenopathy, although varied anatomic locations can be affected (Box 33.2).
-
•
For nodal disease, needle aspirate cytology is a good first screening step; ancillary diagnostics are required to subtype for prognosis or to confirm diagnosis in equivocal cases (Figs. 33.3, 33.8).
-
•
Many treatment protocols exist, but most involve CHOP-based combination chemotherapy (Table 33.4).
-
•
Initially gratifying to treat, as response rates are high and often durable (>6 months); however, cures are rare (<10%) and it is ultimately a uniformly fatal disease.
-
•
Dogs with indolent subtypes may live years, often without therapeutic intervention.
-
•
A veterinary oncology specialist should be consulted on individual cases, as the clinical, diagnostic, and therapeutic landscape changes rapidly.
Etiology
The etiology of canine lymphoma is likely multifactorial and largely unknown; however, investigations are currently shedding significant light on the subject.
Genetic and Molecular Factors
Advances in molecular cytogenetics (see Chapter 1, Section A), including array-comparative genomic hybridization and chromosome painting, have been and are currently being applied to investigations of chromosomal aberrations in dogs with lymphoma.12, 13, 14, 15, 16, 17, 18 Publication of the canine genome and commercial availability of canine gene microarrays (GeneChip Canine Genome 2.0 Array, Affymetrix, Inc.) have led to advances in our understanding of deregulations of gene expression occurring in lymphoma.19 Gains of canine chromosomes 13 and 31 and loss of chromosome 14 have been documented as the most common aberrations in a group of 25 cases analyzed.17 Chromosomal aberrations have also been associated with prognosis in dogs with lymphoma. A study of 61 dogs with lymphoma demonstrated a prognostic advantage in dogs with trisomy of chromosome 13 (25% of the dogs studied), as evidenced by increase in duration of first remission and overall survival time (ST).20 Germline and somatic genetic mutations and altered oncogene/tumor suppressor gene expression, epigenetic changes (e.g., DNA hypomethylation), signal transduction, and death-pathway alterations (e.g., Bcl-2 family) are common in human lymphomas and have been reported in the dog as well (see Chapter 1, Section A, and Chapter 15, Section B).21, 22, 23, 24, 25 These include N-ras, p53, Rb, p16 cyclin-dependent kinase, telomerase, and NF-κB among others.22, 26, 27, 28, 29, 30, 31 Somatic mutations, as determined by exome sequencing, have shown much overlap in canine breeds with respect to B-cell lymphoma, specifically mutations in TRAF3-MAP3K14, FBXW7, and POT1, but little overlap in somatic mutations among breeds with T-cell lymphoma.21 In addition, differences in the prevalence of immunophenotypic subtypes of lymphoma among different breeds indicate heritable risks.32 Telomerase activity (see Chapter 2) has also been documented in canine lymphoma tissues.33, 34, 35 As somatic mutations are often implicated, it is not surprising that alterations or deficiencies in DNA repair mechanisms would also be implicated, as has been demonstrated in golden retrievers with lymphoma.36
Infectious Factors
The hypothesis that a retrovirus may be involved in the pathogenesis of canine lymphoma has not been confirmed. Epstein–Barr virus, a gammaherpesvirus linked to some forms of lymphoma in humans, has also been investigated in canine lymphoma; however, there was no association between serologic or molecular detection of gammaherpesvirus and development of lymphoma.37, 38
In humans, a direct association between Helicobacter sp. infections and development of gastric lymphoma has been made.39 Although this has not been definitively shown in dogs, there is evidence of Helicobacter sp. infection in laboratory beagle dogs resulting in gastric lymphoid follicle formation that is considered a precursor of mucosa-associated lymphoid tissue (MALT) lymphoma in humans.40
Alterations in the gut microbiome have been implicated as playing a role in susceptibility to certain tumors. Fecal microbiota of dogs with lymphoma have been shown to be significantly different than control dogs, although a cause–effect relationship is unclear.41
Environmental Factors
In humans, evidence has accumulated implicating phenoxyacetic acid herbicides, in particular 2,4-dichlorophenoxyacetic acid (2, 4-D), in the development of NHL. Some epidemiologic evidence also implicates lawn herbicide use and occurrence of lymphoma incidence in dogs.42, 43, 44, 45 In one case-control study, the risk of canine lymphoma was reported to rise two-fold (odds ratio [OR] = 1.3) with four or more yearly owner applications of 2,4-D. The results of this study have come under criticism, and three additional follow-up investigations have not validated this increased risk.46, 47, 48 In another study, dogs exposed to lawn treatment within 7 days of application were greater than 50 times more likely to have 2,4-D urinary levels of 50 μg/L or higher.45 In an environmental case-control study performed in Europe, two variables, residency in industrial areas and use of chemicals (defined as paints or solvents) by owners, modestly increased the risk of developing lymphoma; however, no link was found with pesticide use.49 A more recent epidemiologic study investigating multiple environmental factors showed increased risk of canine lymphoma with use of lawn care products, in particular professionally applied pesticides.43 This study did not find an association with flea and tick control products.
A weak association between lymphoma in dogs and exposure to strong magnetic fields was observed in a preliminary epidemiologic study.50 In this hospital-based case-control study, the risk of developing lymphoma with high or very high exposure was increased (OR = 1.8). More thorough studies are necessary to evaluate this association further. Proximity to environmental waste was implicated in two European studies; however, it was felt to be a risk indicator rather than a risk factor and would require further case-control investigations.51, 52 Exposure to tobacco smoke was also implicated in one study.53
Immunologic Factors
Impaired immune function has also been implicated in dogs with lymphoma. Immune system alterations, such as immune-mediated thrombocytopenia, independent of age and sex, have been associated with a higher risk of subsequently developing lymphoma compared with the normal population.54, 55 Additional evidence comes from observations in human and feline transplantation patients.56, 57, 58 In a case-control study of cats undergoing renal transplant, 24% of cases developed cancer (36% of those were lymphoma) while on cyclosporine immunosuppressive therapy compared with 5.1% of control cats, none of which developed lymphoma (OR, 6.1; p = 0.001).58 A case of lymphoma developing in a dog after treatment with cyclosporine also exists.57 One report suggests an association between the immunodysregulation observed in dogs with atopic dermatitis and the risk of developing epitheliotropic T-cell lymphoma; whether lymphoma is associated with the primary disease or the immunomodulatory treatments commonly applied is unknown.59
Classification and Pathology
Classification of malignant lymphoma in dogs is based on anatomic location, histologic criteria, and immunophenotypic characteristics. The most common anatomic forms of lymphoma, in order of decreasing prevalence, are multicentric, gastrointestinal (GI), mediastinal, and cutaneous forms.60 Primary extranodal forms, which can occur in any location outside the lymphatic system, include the eyes, skin, central nervous system (CNS), bone marrow, bladder, heart, and nasal cavity. The pathologic characteristics of the various anatomic classifications will be discussed in this section and clinical characteristics will be described in subsequent sections.
More than 80% of dogs with lymphoma are presented with the multicentric form, which is usually characterized by the presence of peripheral lymphadenopathy (Fig. 33.1 ).60 The alimentary form of lymphoma is much less common, accounting for 5% to 7% of all canine lymphomas. Primary GI lymphoma in dogs may occur focally, but more often affects multiple segments with thickening of the wall, narrowing of the lumen, and frequently mucosal ulceration.61, 62 Histologically, there is infiltration of neoplastic lymphocytes throughout the mucosa and submucosa with occasional transmural infiltration. Liver and local LNs are often secondarily involved. Lymphocytic-plasmacytic inflammatory bowel disease (LP-IBD) can be seen adjacent to or distant from the primary tumor. Pathologically, some of these neoplasms may resemble plasma cell tumors and aberrant production of immunoglobulins may occur. Histologically, distinguishing between GI lymphoma and LP-IBD can be difficult. Some have suggested that LP-IBD may be a prelymphomatous change in the GI tract. A syndrome of immunoproliferative intestinal disease characterized by LP-IBD has been described in Basenjis, which subsequently develop GI lymphoma.63 In addition, plasma cell–rich areas with heterogeneous lymphomatous infiltration may resemble lesions of LP-IBD. Only a few reports specifically identify the immunophenotype of the lymphocyte subpopulations in GI lymphoma in dogs. Historically, it was presumed that they most likely originate from B cells; however, recent evidence suggests that most GI lymphomas in dogs arise from T cells and often exhibit epitheliotropism.62, 64, 65 The boxer and Shar-pei breeds may be overrepresented in cases of alimentary lymphoma.65, 66
Fig. 33.1.

A dog with obvious mandibular lymphadenopathy resulting from multicentric lymphoma.
The mediastinal form of the disease occurs in approximately 5% of cases.60 This form is characterized by enlargement of the cranial mediastinal LNs, thymus, or both (Fig. 33.2 ). Hypercalcemia is reported to occur in 10% to 40% of dogs with lymphoma and is most common with the mediastinal form. In a study of 37 dogs with lymphoma and hypercalcemia, 16 (43%) had mediastinal lymphoma.67 The mediastinal form in dogs is most commonly associated with a T-cell phenotype.68, 69 A single case of mediastinal γδT-cell lymphoma with large granular lymphocyte morphology has been reported.70
Cutaneous lymphoma can be solitary or more generalized and is usually classified as epitheliotropic (mycosis fungoides) or nonepitheliotropic.71, 72 Canine epitheliotropic cutaneous lymphoma originates from T cells,73, 74, 75, 76, 77 similar to the case in humans. In dogs, these more commonly represent CD8+ cells, whereas in humans they are typically CD4+ cells.76 A rare form of cutaneous T-cell lymphoma, characterized by skin involvement with evidence of peripherally circulating large (15–20 μm in diameter) malignant T cells with folded, grooved nuclei, has been described. In humans, this is referred to as Sézary syndrome and has been reported in both dogs and cats.78, 79, 80 Nonepitheliotropic cutaneous lymphomas form single or multiple dermal or subcutaneous nodules or plaques; histologically, they spare the epidermis and papillary dermis and affect the middle and deep portions of the dermis and subcutis.72 An inflamed form of nonepitheliotropic cutaneous T-cell lymphoma (NE-CTCL) is more pleocellular and can be difficult to differentiate from reactive histiocytosis.71
Atypical Anatomic Forms of Lymphoma
Hepatosplenic lymphoma is a relatively uncommon, distinct presentation in the dog marked by a lack of significant peripheral lymphadenopathy in the face of hepatic, splenic, and bone marrow infiltration with malignant lymphocytes, usually of T-cell origin.81, 82, 83 Biologically, this form of lymphoma is extremely aggressive and poorly responsive to therapy. In humans and dogs, the tumor usually is composed of γδT cells (i.e., T cells that express the γδT-cell receptor).81, 82
Intravascular (angiotropic, angioendotheliomatosis) lymphoma is a distinct form of lymphoma defined as proliferations of neoplastic lymphocytes within the lumen and wall of blood vessels in the absence of a primary extravascular mass or leukemia. It has been reported several times in the veterinary literature and often involves the CNS and peripheral nervous system (PNS), including the eye.84, 85, 86, 87, 88, 89 The B-cell immunophenotype is most common in humans; however, in most reported cases in dogs, the origin is either T cell or null cell (neither B nor T cell), although one case of a B-cell phenotype has been reported.
Pulmonary lymphomatoid granulomatosis (PLG), also termed angiocentric B-cell lymphoma, is a rare neoplasm of the lung and occasionally other tissues, characterized by a heterogeneous accumulation of lymphocytes (both B and T cell), neutrophils, plasma cells, and macrophages, often arranged angiocentrically.90, 91, 92, 93, 94 Clinical signs are related to respiratory compromise, and various chemotherapeutic protocols have been used with reported results varying from rapid progression to long-term clinical remissions.
Histologic Classification Systems
Lymphomas arise from clonal expansion of lymphocytes with distinctive morphologic and immunophenotypic features. Many histologic systems have been used to classify NHL in humans, and some of these have been applied to lymphoma in the dog and other species. Histologic classification of lymphoma currently follows the Revised European American Lymphoma/World Health Organization (REAL/WHO) system, which incorporates anatomic, morphologic (cytology and histology), and immunophenotypic criteria (B- and T-cell immunophenotype), with the goal of enabling accurate and reproducible diagnosis of specific neoplastic entities.90, 95, 96 Fig. 33.3 represents an overall histologic approach to the classification of various subtypes in the dog as reviewed by Seelig et al.96, 97 This theoretically should assist in better tailoring of treatment protocols, better correlation of prognosis with subtype, and better comparative capabilities once larger data sets with correlate outcomes are generated. Table 33.1 shows some of the WHO categories in three different surveys, including a 2-year survey (2008–2009) of canine necropsy and biopsy cases at the University of Wisconsin–Madison Veterinary Care Hospital29, 95, 96, 98; some of the less common categories in the REAL/WHO system were not represented and are not listed. Most canine lymphomas fall into the following categories, in decreasing frequency: diffuse large B-cell lymphoma (DLBCL), peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), T-zone lymphoma (TZL), T-lymphoblastic lymphoma (also called “precursor T-cell neoplasia”), and marginal zone lymphoma (MZL).90 The REAL/WHO system provides accurate and consistent reproducible diagnostic results similar to the system used in human pathology; agreement among a group of pathologists examining 300 cases was 83%, and accuracy in evaluating the six most common diagnoses (80% of the cases) was 87%.99 It is clear that lymphoma is not a single disease, and classification by subtype will become increasingly important as clinical studies are performed to correlate the various categories of disease with biologic behavior, response to treatment, and prognosis. Preliminary results indicate that dogs with indolent lymphoma (e.g., MZL, follicular lymphoma, B- or T-cell small cell lymphoma, T-cell–rich B-cell lymphoma, and TZL) maintain normal activity and appetite levels even during advanced stages of disease and experience long-term survival even with limited or no therapy.90, 99, 100, 101, 102
Fig. 33.3.
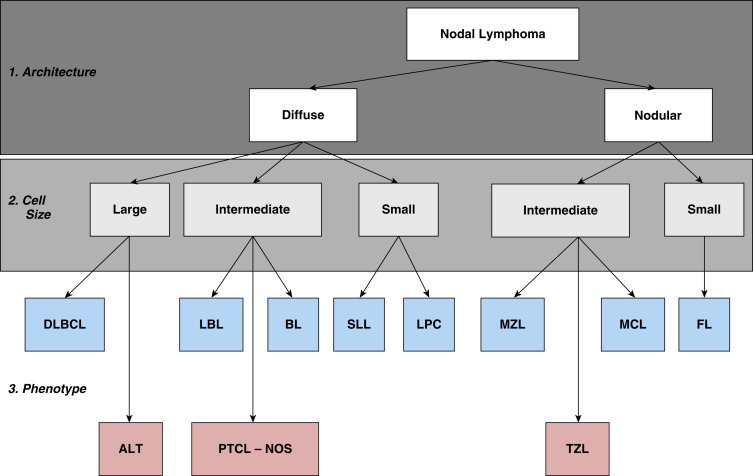
The histologic approach toward the classification of canine nodal lymphoma. Using excisional lymph node sections, lymphoma is initially divided into diffuse (effacing) or nodular (noneffacing) forms of the disease. Next, using a red blood cell or a small lymphocyte as a guideline, the neoplastic population is divided into large, small, and intermediate forms of the disease. Finally, using knowledge of additional cellular and nuclear features, including mitotic rate and immunophenotype (B cell, blue boxes; T cell, red boxes), a final diagnosis is established. ALT, Anaplastic large cell T-cell lymphoma; BL, Burkitt lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; LBL, lymphoblastic lymphoma; LPC, lymphoplasmacytoid lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; PTCL, NOS, peripheral T-cell lymphoma, not otherwise specified; SLL, small lymphocytic lymphoma; TZL, T-zone lymphoma.
Reproduced and modified with permission from Seelig DM, Avery AC, Ehrhart EJ, Linden MA. The comparative diagnostic features of canine and human lymphoma. Vet Sci. 2016;3(2). Epub 2017/04/25. https://doi.org/10.3390/vetsci3020011. PubMed PMID: 28435836; PubMed Central PMCID: PMCPMC5397114.
TABLE 33.1.
World Health Organization Classification System for Canine Lymphoma
| Category | Percentage |
||
|---|---|---|---|
| Seelig et al96 (n = 3 data sets) |
Vezzali et al98 (n = 123) |
University of Wisconsin (n = 122) |
|
| B-Cell Neoplasms | 69 | 78.9 | 59.0 |
| Precursor B lymphoblastic leukemia/lymphomaa | — | 2.4 | 8.2 |
| B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma | — | 2.4 | 0.8 |
| Lymphocytic lymphoma—intermediate type | — | 0.8 | — |
| Lymphoplasmacytic lymphoma | — | 3.3 | 0.8 |
| Mantle cell lymphoma | 2 | 1.6 | — |
| Follicular center cell lymphomas | — | 2.4 | — |
| Marginal zone lymphoma (splenic, nodal, mucosa-associated lymphoid tissue) | 8 | 3.3 | 2.5 |
| Plasma cell myeloma/plasmacytoma | — | 16.3 | 9.8 |
| Diffuse large cell lymphoma | 52 | 33.3 | 24.6 |
| T-cell–rich, B-cell lymphoma | — | 0.8 | — |
| Large cell immunoblastic lymphoma | — | 10.6 | 10.7 |
| Mediastinal (thymic) large B-cell lymphoma | — | 0.8 | — |
| Burkitt’s lymphoma/leukemia | — | 0.8 | 1.6 |
| Other B cell | 8d | — | — |
| T-Cell and Natural Killer (NKb) Cell Lymphomas | 31 | 21.1 | 41.0 |
| Precursor T lymphoblastic lymphoma/leukemiaa | 3 | 6.5 | 9.8 |
| T-cell chronic lymphocytic leukemia (CLL) | — | 3.3 | 0.8 |
| Intestinal T-cell lymphoma | — | 4.1 | 4.1 |
| Mycosis fungoides/Sézary syndrome | — | 1.6 | 11.5 |
| Cutaneous nonepitheliotropic lymphoma | — | 3.3 | — |
| Anaplastic large cell lymphoma | — | — | 0.8 |
| Peripheral T-cell lymphoma-not otherwise specified | 15 | 2.4 | 13.1c |
| T-zone lymphoma | 4 | ||
| Other T cell | 7d | — | — |
| Other | 3d | ||
Acute leukemias and lymphoblastic lymphomas of both B and T derivation are also classified as lymphoid “precursor neoplasms.”
Non-B, non-T lymphomas.
Includes T-zone lymphoma in this data set.
Other in Seelig include those not otherwise subclassified.
Other classification systems that have been used include the National Cancer Institute Working Formulation (WF)103 and the updated Kiel system.104 The WF was developed to allow investigators to “translate” among the numerous classification systems so that clinical trials could be compared in humans. Most of the larger compilations agree that most canine lymphomas are intermediate or high grade. The WF categorizes tumors according to pattern (diffuse or follicular) and cell type (e.g., small cleaved cell, large cell, immunoblastic), but it does not include information about the immunophenotype of the tumor.103 The WF subtypes are related to the biology of the tumor and patient survival. The updated Kiel classification includes the architectural pattern, morphology (centroblastic, centrocytic, or immunoblastic), and immunophenotype (B or T cell) of the tumor cells.104 In both systems, the tumors can then be categorized as low-grade, intermediate-grade, or high-grade malignancies. Low-grade lymphomas composed of small cells with a low mitotic rate typically progress slowly and are associated with long STs, but are ultimately incurable. High-grade lymphomas with a high mitotic rate progress rapidly, but are more likely to respond initially to chemotherapy and, in humans, are potentially curable. In the REAL/WHO system, each subtype of lymphoma is classified as a distinct disease based on characteristics that include biologic behavior (indolent versus aggressive, response to treatment).90
Several features of canine lymphomas become apparent when these classification systems are applied. The most striking difference between canine and human lymphomas is the scarcity of follicular lymphomas in the dog. The most common form of canine lymphoma is DLBCL, a high-grade tumor.90, 98, 99, 105 A small percentage of canine lymphomas (5.3%–29%) are considered low-grade.
A documented difference exists in the prevalence of the various immunophenotypes based on breed.32, 106, 107 For example, cocker spaniels and Doberman pinschers are more likely to develop B-cell lymphoma, boxers are more likely to have T-cell lymphoma, and golden retrievers appear to have an equal likelihood of B- and T-cell tumors.
To be clinically useful, these classification systems in the end must yield information about response to therapy, maintenance of remission, and survival. In most studies, high-grade lymphomas achieve a complete response (CR) to chemotherapy significantly more often than low-grade tumors. However, dogs with low-grade tumors may live years without aggressive chemotherapy.100, 101, 102, 108, 109, 110, 111 Dogs with T-cell lymphomas have shown a lower rate of CR to chemotherapy and shorter remission and STs than dogs with B-cell tumors (with the exception of low-grade T-cell subtypes).68, 69, 112, 113 Furthermore, T-cell lymphomas are more commonly associated with hypercalcemia.8, 114, 115
In the veterinary literature, 60% to 80% of canine lymphomas are of B-cell origin; T-cell lymphomas account for 10% to 38%; mixed B- and T-cell lymphomas account for as many as 22%; and null-cell tumors represent fewer than 5%.8, 68, 69, 116, 117, 118 The development of monoclonal antibodies to detect specific markers on canine lymphocytes has made immunophenotyping of tumors in dogs routinely available in many commercial laboratories. Such techniques can be performed on paraffin-embedded samples, from tissue microarrays, on cytologic specimens obtained by fine-needle aspiration (FNA) of lesions, or by flow cytometric analysis of cellular fluid samples (e.g., peripheral blood, effusions) and lesion aspirates.
One criticism of the Kiel and WF classification systems is that they fail to include extranodal lymphomas as a separate category. The REAL/WHO system does include anatomic location as a factor in determining certain categories. Although differences between nodal and extranodal tumors in biologic behavior and prognosis are well recognized, comparative information about the histogenesis of these tumors is lacking. For example, in humans, small-cell lymphomas arising from MALT are composed of cells with a different immunophenotype than that of other small-cell lymphomas (i.e., MALT lymphomas typically are negative for both CD5 and CD10). With the exception of cutaneous lymphoid neoplasms, detailed characterization of extranodal lymphomas in dogs has not been done. Although cutaneous lymphoma is a heterogeneous group of neoplasms that includes an epitheliotropic form resembling mycosis fungoides and a nonepitheliotropic form, most cutaneous lymphomas have a T-cell phenotype.119
To summarize, it is important to determine the histologic grade of canine lymphomas as low (small lymphocytic or centrocytic lymphomas), intermediate, or high (diffuse large cell, centroblastic, and immunoblastic lymphomas), and the architecture as diffuse or nodular/follicular. Furthermore, determining the immunophenotype of the tumor provides useful information and is essential to accurately subtype lymphoma. Response rates to chemotherapy are, in general, better in animals with B-cell tumors and intermediate- to high-grade lymphomas. Dogs with low-grade indolent lymphomas can have long STs without aggressive therapy.
History and Clinical Signs
The clinical signs associated with canine lymphoma are variable and depend on the extent and location of the tumor. Multicentric lymphoma, the most common form, is usually distinguished by the presence of generalized peripheral lymphadenopathy (see Fig. 33.1). Enlarged LNs are usually painless, rubbery, and discrete. In addition, hepatosplenomegaly and bone marrow involvement can be associated with generalized lymphadenopathy. Most dogs with multicentric lymphoma are presented without dramatic signs of systemic illness (WHO substage a) (Box 33.2 ); however, a diversity of nonspecific signs such as anorexia, weight loss, vomiting, diarrhea, emaciation, ascites, dyspnea, polydipsia, polyuria, and fever can occur (WHO substage b). Dogs with T-cell lymphoma are more likely to have constitutional (i.e., substage b) signs. Most veterinary oncologists consider mild-moderate severity of clinical signs sufficient for a substage b designation.120 Polydipsia and polyuria are often evident in dogs with hypercalcemia of malignancy. Dogs may also be presented with clinical signs related to blood dyscrasias secondary to marked tumor infiltration of bone marrow (myelophthisis) or paraneoplastic anemia, thrombocytopenia, or neutropenia. These could include fever, sepsis, anemia, and hemorrhage. Diffuse pulmonary infiltration, as detected by radiographic changes, is seen in 27% to 34% of dogs with the multicentric form (Fig. 33.4 ).121, 122 Based on bronchoalveolar lavage, the actual incidence of lung involvement may be higher.123, 124
BOX 33.2. World Health Organization’s Clinical Staging System for Lymphoma in Domestic Animals.
-
1.Anatomic site
-
A.Generalized
-
B.Alimentary
-
C.Thymic
-
D.Skin
-
E.Leukemia (true)a
-
F.Others (including solitary renal)
-
A.
-
2.Stage (to include anatomic site)
-
I.Involvement limited to a single node or lymphoid tissue in a single organb
-
II.Involvement of many lymph nodes in a regional area (± tonsils)
-
III.Generalized lymph node involvement
-
IV.Liver and/or spleen involvement (± Stage III)
-
V.Manifestation in the blood and involvement of bone marrow and/or other organ systems (± Stage I–IV)
-
I.
Each stage is subclassified into:
-
a.
Without systemic signs
-
b.
With systemic signs
Fig. 33.4.
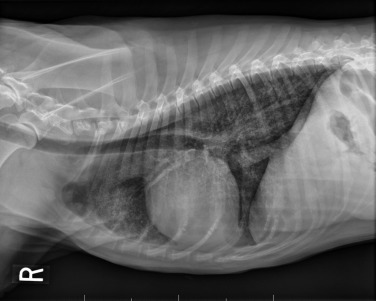
Lateral projection of a thoracic radiograph of a dog with diffuse interstitial infiltration with lymphoma secondary to multicentric lymphoma.
Dogs with GI lymphoma usually present with nonspecific GI signs, such as vomiting, diarrhea, weight loss, and malabsorption.61, 64, 125, 126, 127, 128 Mesenteric LNs, spleen, and liver may be involved.
The mediastinal form of lymphoma is characterized by enlargement of the cranial mediastinal structures and/or thymus (see Fig. 33.2), and clinical signs are associated with the extent of disease with resulting respiratory compromise or polydipsia/polyuria from hypercalcemia. In advanced cases, dogs present with respiratory distress caused by a space-occupying mass and pleural effusion, exercise intolerance, and possibly regurgitation. In addition, dogs with mediastinal lymphoma may have precaval syndrome, characterized by pitting edema of the head, neck, and forelimbs secondary to tumor compression or invasion of the cranial vena cava (Fig. 33.5 ).
Fig. 33.2.
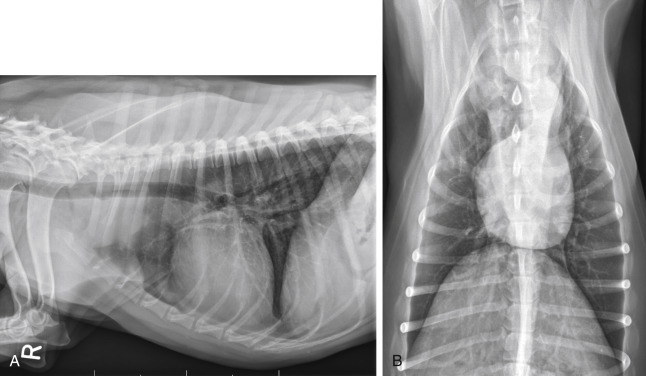
(A) Lateral radiographic projection of a dog with mediastinal lymphoma. (B) Ventrodorsal projection of the same dog.
Fig. 33.5.
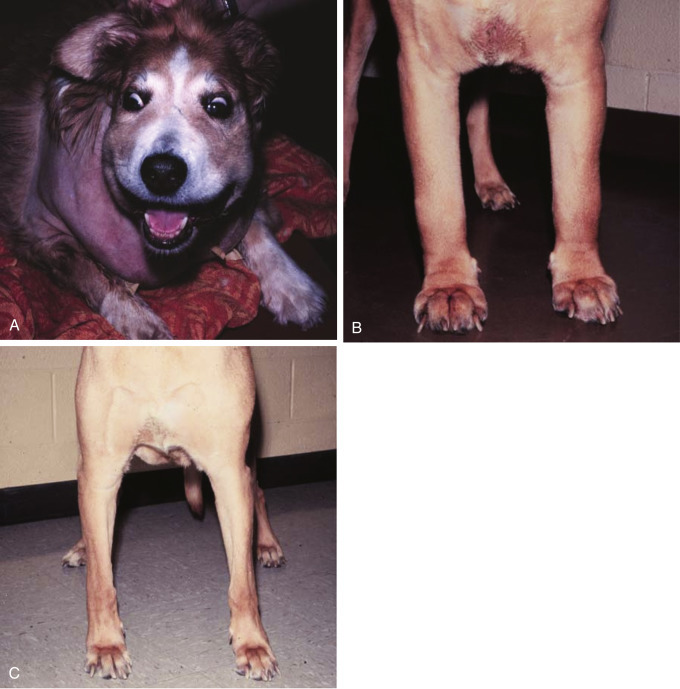
(A) Facial edema in a dog with precaval syndrome secondary to mediastinal lymphoma. (B) Forelimb edema in a dog with precaval syndrome secondary to mediastinal lymphoma. (C) The dog in (B) 24 hours after radiation therapy to the cranial mediastinal mass, showing resolution of pitting edema.
Clinical signs in dogs with extranodal lymphoma depend on the specific organ involved. Cutaneous lymphoma can be mucocutaneous, cutaneous, or both. Lesions can be solitary, generalized, or multifocal.71, 74, 75, 76, 129, 130, 131, 132 Tumors occur as nodules, plaques, ulcers, and erythemic or exfoliative dermatitis with focal hypopigmentation and alopecia. Epitheliotropic T-cell lymphoma (e.g., mycosis fungoides) typically has a clinical course with three apparent clinical stages. Initially, there will be scaling, alopecia, and pruritus (Fig. 33.6A ), which can mimic a variety of other skin conditions. As the disease progresses, the skin becomes more erythematous, thickened, ulcerated, and exudative. The final stage is characterized by proliferative plaques and nodules with progressive ulceration (Fig. 33.6B). Oral mucocutaneous involvement may also occur and this can appear as multicentric erythematous plaque-like hypopigmented lesions or nodules associated with the gum and lips (Fig. 33.6C). Extracutaneous involvement can also occur, most often in the LNs, spleen, liver, and bone marrow/peripheral blood. Nonepitheliotropic cutaneous lymphomas are also quite variable in appearance and can form single or multiple dermal or subcutaneous nodules or plaques that may be nonpuritic, ulcerated, or alopecic with crusts.71 The face (lips, nasal planum, eyelids), lower extremities (paws, interdigital folds), neck, and trunk are often affected.
Fig. 33.6.
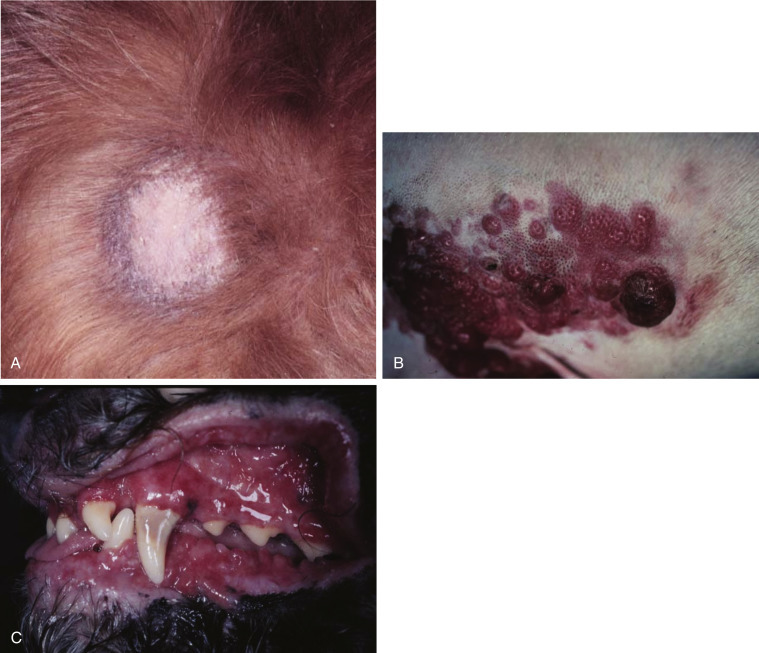
(A) Early epitheliotropic cutaneous lymphoma in the scaly, plaque stage in a dog. (B) Advanced epitheliotropic cutaneous lymphoma in the nodular stage in a dog. (C) Oral mucosal epitheliotropic cutaneous lymphoma in a dog.
Dogs with CNS lymphoma may be presented with either multifocal or solitary involvement.133, 134, 135 The majority of cases involve secondary extension into the CNS. Most have a B-cell immunophenotype and have meningeal, perivascular, and periventricular locations, whereas T-cell varieties are more likely to involve the peripheral nerves.136 Seizures, paralysis, and paresis may be noted.
Ocular lymphoma is characterized by infiltration and thickening of the iris, uveitis, hypopyon, hyphema, posterior synechia, and glaucoma, and is discussed in more detail in Chapter 32.137, 138, 139 Although it is often secondary to multicentric systemic lymphoma, in a compilation of 100 cases, 61% were presumed solitary ocular lymphoma (PSOL) without systemic involvement at diagnosis and no progression postenucleation.137 Peripheral T-cell lymphoma and DLBCL are the most common subtypes. Importantly, dogs with PSOL had median survival times (MSTs) of 769 days versus 103 days for dogs having systemic involvement at diagnosis. In one study of 94 cases of canine multicentric lymphoma, 37% had ocular changes consistent with lymphoma; and, in a series of 102 cases of uveitis in dogs, 17% were secondary to lymphoma.139 Anterior uveitis was most commonly seen in the advanced stage of disease (stage V).
Dogs with intravascular lymphoma usually present with signs related to CNS, PNS, or ocular involvement,84, 85, 86, 87, 88 including paraparesis, ataxia, hyperesthesia, seizures, blindness, lethargy, anorexia, weight loss, diarrhea, polyuria, polydipsia, and intermittent fever. Finally, dogs with pure hepatosplenic lymphoma usually present with nonspecific signs of lethargy, inappetence and weakness, and often are icteric.81, 82, 83
Canine lymphoma also may be associated with paraneoplastic syndromes (see Chapter 5). Anemia is the most common lymphoma-related paraneoplastic syndrome.140 Paraneoplastic hypercalcemia is also common and is characterized clinically by anorexia, weight loss, muscle weakness, lethargy, polyuria, polydipsia, and, rarely, CNS depression and coma. Lymphoma-induced hypercalcemia in most cases results from parathyroid hormone–related peptide (PTHrP), elaborated by neoplastic cells; however, it can also be related to the production of several other humoral factors, including interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), and vitamin D analogs (e.g., 1,25-dihydroxyvitamin D).114, 115, 141, 142, 143 As previously discussed, hypercalcemia is most commonly associated with the T-cell immunophenotype. Other paraneoplastic syndromes that may be encountered include monoclonal gammopathies, neuropathies, and cancer cachexia.
Diagnostics
For dogs with suspected lymphoma, the diagnostic evaluation should include a thorough physical examination; complete blood count (CBC), including differential leukocyte and platelet counts; a serum biochemical profile; and urinalysis. Optimally, plasma ionized calcium concentration should be measured. Ultimately, obtaining tissue or cytologic specimens (or both) for a definitive diagnosis is essential. The differential diagnosis of lymphadenopathy depends on the travel history of the dog (i.e., relative to infectious disease) and the size, consistency, and location of affected LNs. Other causes of lymphadenopathy include infections caused by bacteria, viruses, protozoa (Toxoplasma sp., Leishmania sp.), rickettsial organisms (salmon poisoning, Ehrlichia sp.), and fungal agents (Blastomyces and Histoplasma sp.). The potential for hypercalcemia to accompany systemic fungal diseases may further complicate differentiation from lymphoma. Discrete, hard, asymmetric LNs, particularly if they are fixed to underlying tissues, may indicate metastatic tumors such as mast cell tumor or carcinoma. Immune-mediated diseases (e.g., pemphigus, systemic lupus erythematosus, and immune-mediated polyarthropathy) also may result in mildly to moderately enlarged LNs. The various differential diseases or conditions that can resemble canine lymphoma are listed in Table 33.2 .
TABLE 33.2.
Differential Diseases or Conditions That Can Resemble Canine Lymphoma
| Form of Lymphoma | Other Disorders |
|---|---|
| Multicentric | Disseminated infections: bacterial, viral, rickettsial, parasitic, fungal Immune-mediated disorders: dermatopathies, vasculitis, polyarthritis, lupus erythrematosus Tumors metastatic to nodes Other hematopoietic tumors: leukemia, multiple myeloma, malignant or systemic histiocytosis |
| Mediastinal | Other tumors: thymoma, chemodectoma, ultimobranchial cyst, ectopic thyroid carcinoma, pleural carcinomatosis, pulmonary lymphomatoid granulomatosisa Infectious disease: granulomatous disease, pyothorax Miscellaneous: congestive heart failure, chylothorax, hemothorax |
| Alimentary | Other gastrointestinal tumors, foreign body, lymphangiectasia, lymphocytic-plasmacytic enteritis, systemic mycosis, gastroduodenal ulceration |
| Cutaneous | Infectious dermatitis: advanced pyoderma Immune-mediated dermatitis: pemphigus Other cutaneous neoplasms (in particular histiocytic disorders) |
| Extranodal | Variable, depending on organ/system involved |
The existence of this disease is controversial; in most cases the disease has been reclassified as a lymphoid neoplasm.
Physical Examination
A thorough physical examination should include palpation of all assessable LNs and rectal examination, as in the authors’ experience, a significant proportion of dogs will have rectal polyps consisting of aggregates of neoplastic lymphocytes. Inspection of mucous membranes for pallor, icterus, petechiae, and ulceration should be undertaken as these signs may indicate anemia or thrombocytopenia secondary to myelophthisis or immune-mediated disease or may be evidence of major organ failure or uremia. Abdominal palpation may reveal organomegaly, intestinal wall thickening (if marked), or mesenteric lymphadenopathy. The presence of a mediastinal mass and/or pleural effusion can be suspected after thoracic auscultation. Ocular examination, including funduscopic assessment, may reveal abnormalities (e.g., uveitis, retinal hemorrhage, ocular infiltration, glaucoma) in approximately one-third to one-half of dogs with lymphoma.139, 144
Complete Blood Count, Biochemical Profile, and Urinalysis
Anemia, the most common lymphoma-related hematologic abnormality, is usually normochromic and normocytic (nonregenerative), consistent with anemia of chronic disease140; however, hemorrhagic and hemolytic anemias may also occur, and regenerative anemias may reflect concomitant blood loss or hemolysis. In addition, if significant myelophthisis is present, anemia may be accompanied by thrombocytopenia and leukopenia.145, 146 In animals with anemia or evidence of bleeding, in addition to a platelet count, a reticulocyte count and coagulation testing may be indicated. Thrombocytopenia occurs in 30% to 50% of cases, but bleeding is seldom a clinical problem. Neutrophilia occurs in 25% to 40% of dogs and lymphocytosis occurs in approximately 20% of affected dogs. Circulating atypical lymphocytes may be indicative of bone marrow involvement and leukemia. It is important to differentiate multicentric lymphoma with bone marrow involvement (i.e., stage V disease) from primary lymphocytic leukemia because the prognosis for each may be different. Hypoproteinemia is observed more frequently in animals with alimentary lymphoma. In dogs with a high total protein concentration or evidence of an increased globulin fraction on a biochemical profile, serum proteins may be evaluated by serum protein electrophoresis. Monoclonal gammopathies have been reported to occur in approximately 6% of dogs with lymphoma.147
Serum biochemical abnormalities often reflect the anatomic site involved as well as paraneoplastic syndromes, such as hypercalcemia. In dogs with lymphoma, ionized calcium concentrations should be obtained, as they may be increased even if the total calcium concentration is within the reference interval. In cases of hypercalcemia of unknown origin, lymphoma should always be considered high on the differential disease list and diagnostic testing directed at this possibility should be undertaken (see Chapter 5). In addition, the presence of hypercalcemia can serve as a biomarker for response to therapy and relapse. Increased urea nitrogen and creatinine concentrations can occur secondary to renal infiltration with tumor, hypercalcemic nephrosis, or prerenal azotemia from dehydration. Increases in liver-specific enzyme activities or bilirubin concentrations may result from hepatic parenchymal infiltration. Increased serum globulin concentrations, usually monoclonal, occur infrequently with B-cell lymphoma.
Urinalysis is part of the minimum database used to assess renal function and the urinary tract. For example, isosthenuria and proteinuria in the absence of an active sediment may indicate renal disease, and hematuria may result from a hemostatic abnormality. It is important to note that isosthenuria in hypercalcemic dogs is not necessarily indicative of renal disease, as high calcium concentrations interfere with tubular concentrating capabilities through impairment of response to antidiuretic hormone; however, clinicians should be aware that there is a risk of renal calcification and subsequent failure with sustained high calcium concentrations.
Histologic and Cytologic Evaluation of Lymph Nodes
Morphologic and phenotypic examination of the tissue and cells that constitute the tumor is essential to the diagnosis and subtyping of lymphoma. In humans, a combination of histologic, immunophenotypic, clinical, and genetic features are used in the diagnosis and subtyping of NHL. An excellent review of the morphologic and immunophenotypic diagnostic features of canine lymphoma has recently been published.96 In veterinary medicine, care should be taken to avoid sampling LNs from reactive areas (e.g., mandibular LNs), unless those nodes are the only ones enlarged; the prescapular or popliteal LNs are preferable if also involved. Also, lymphocytes are fragile, and in preparing smears of aspirated material only gentle pressure should be applied in spreading material on the slides. As the majority of dogs with nodal lymphomas are presented with multicentric effacement of peripheral LNs by intermediate or large lymphocytes, cytologic examination of FNAs of affected LNs or other tissues is a highly sensitive and specific first-line or screening diagnostic step.96 Typically, most of the cells are large lymphocytes (>2 times the diameter of a red blood cell or larger than a neutrophil), and they may have visible nucleoli and basophilic cytoplasm with or without paranuclear clear zones (Fig. 33.7A ) or fine chromatin with indistinct nucleoli. Because tissue architecture is not maintained in cytologic specimens, effacement of the node or capsular disruption cannot be detected. Therefore marked reactive hyperplasia characterized by increased numbers of large lymphocytes may be difficult to distinguish from lymphoma. In some forms of lymphoma, intermediate lymphocytes that are similar in diameter to neutrophils predominate; these specimens can be more challenging for novice cytopathologists. Small-cell lymphomas may have few cytologic clues that point to malignancy. Therefore classification of lymphoma into subtypes that make up the low-, intermediate-, and high-grade forms can be attempted using cytologic appearance and immunophenotypic analysis of cytologic specimens (Fig. 33.7B),96, 148, 149, 150 but is performed most accurately on histologic sections. Although cytologic findings identified by an experienced cytopathologist may suggest a particular subtype of lymphoma, subsequent analysis that may include flow cytometry, immunocytochemistry, biopsy for histologic examination, clonality assays, and cytogenetic analysis are required to further subtype the lymphoma or to confirm or establish a diagnosis in equivocal cases. Fig. 33.8 presents a diagnostic algorithm for assessing peripheral lymphadenopathy in dogs applying these techniques, beginning with initial screening by cytologic examination.
Fig. 33.7.
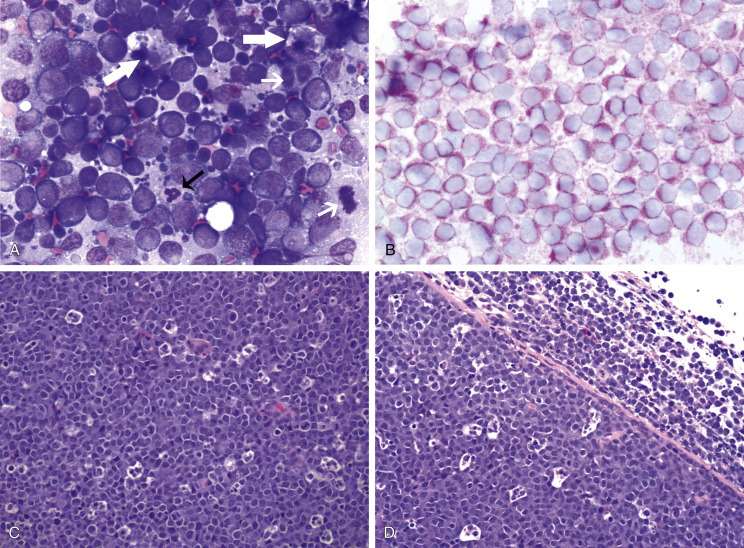
Lymph nodes from dogs with lymphoma. (A) Fine-needle aspirate. Note the homogeneous population of large lymphoid cells with prominent nucleoli and basophilic cytoplasm. These cells are larger than the neutrophil (black arrow) in the field. Mitotic figures (thin white arrows) and tingible-body macrophages (thick white arrows) also are present. (Wright’s stain, ×60 objective.) (B) Fine-needle aspirate stained for immunoreactivity for CD79a. Note that nearly all of the lymphocytes express CD79a. The diagnosis was B-cell lymphoma. (Alkaline phosphatase/Fast Red, ×60 objective.) (C) Histologic section. Note effacement of normal architecture. The white spaces are macrophages, giving a “starry sky” appearance to the lymph node. (H&E, ×20 objective.) (D) Histologic section. Note the presence of tumor cells outside the capsule of the lymph node. (H&E, ×20 objective.)
Fig. 33.8.
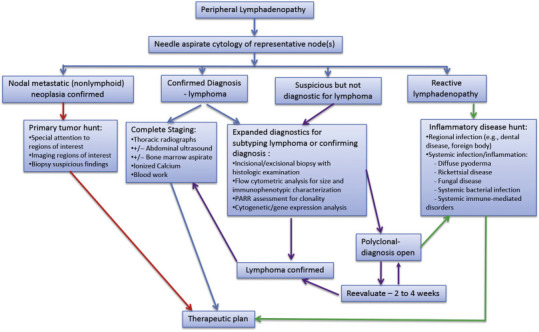
Diagnostic algorithm for peripheral lymphadenopathy in dogs. PARR, polymerase chain reaction for receptor gene rearrangement.
For accurate histopathologic evaluation, an entire LN, including the capsule, should be removed, placed in buffered formalin, and submitted to a pathologist. Needle-core biopsies are generally inadequate to evaluate nodal morphology. Effacement of normal nodal architecture by neoplastic lymphocytes and capsular disruption are characteristic findings (Fig. 33.7C, D).
Histologic and Cytologic Evaluation of Extranodal Sites
Diagnostic ultrasonography and ultrasound-guided FNA or needle biopsy have been useful for evaluation of involvement of the liver, spleen, or abdominal LNs.151, 152, 153 Aspiration of ultrasonographically normal splenic tissue is rarely contributory to a diagnosis. If possible, the diagnosis should be made by sampling peripheral nodes, avoiding percutaneous biopsies of the liver and spleen. However, if there is no peripheral node involvement, it is appropriate to biopsy affected tissues in the abdominal cavity.
When GI lymphoma is suspected, an open surgical wedge biopsy of the intestine is preferred in most cases to differentiate lymphoma from lymphocytic enteritis. If associated abdominal LNs also appear involved, image-guided biopsies may be obtained with less morbidity than intestinal biopsies. Multiple samples may be necessary to accurately diagnose segmental disease. Endoscopic biopsies may be inadequate as only a superficial specimen is obtained; however, more aggressive endoscopic biopsy techniques combined with more accurate histopathologic, immunophenotypic, and molecular assessments are improving the diagnostic yield of these less invasive techniques.125, 126, 154, 155, 156, 157, 158, 159, 160, 161, 162 In many dogs with primary GI lymphoma, an inflammatory nonneoplastic infiltrate (i.e., LP-IBD) may be misdiagnosed on biopsy specimens that are too superficial. The application of assays for clonal expansion (e.g., receptor gene rearrangement [PARR]) does not appear as yet to be as accurate for endoscopically derived intestinal biopsies as it is for other solid lymphoid tumors in dogs.
Cytologic examination of cerebrospinal fluid (CSF), thoracic fluid, or mass aspirates is indicated in animals with CNS disease, pleural effusion, or an intrathoracic mass, respectively. In two studies, CSF analysis was diagnostic of lymphoma in 74% of 27 samples.133, 136 Characteristics of the CSF in one study included an increased nucleated cell count in seven dogs with 95% to 100% of the cells comprising atypical lymphocytes.133 CSF protein concentration was increased in five of the dogs, ranging from 34 to 310 mg/dL (reference interval <25 mg/dL). Flow cytometric and molecular diagnostic procedures may also be applied to CSF samples,136 although cell counts may be a limiting factor as some of these assays require at least 10,000 cells.
For cutaneous lymphoma, dermal punch biopsies (4–8 mm) should be taken from the most representative and infiltrative, but not secondarily infected, skin lesions. Application of immunophenotypic and clonality assessments of cutaneous biopsies can aid in differentiating lymphoma from benign lymphocytic lesions.71, 75, 77, 163
Molecular Diagnostic Techniques
Molecular techniques can be used to establish a diagnosis of lymphoma, but are best used to further characterize the tumor after the initial diagnosis is made. Indeed, in people, genetic characterization of NHL are often used in diagnosis and subtyping.96 Tissues and cells from peripheral blood, LNs, nonlymphoid sites, and effusions can be analyzed by various molecular and cytogenetic means to aid in categorization of subtypes and in cases that represent a more difficult diagnostic challenge, particularly in cases where reactive lymphocytosis and lymphoma are both possible based on standard histologic or cytologic assessment. These include histochemical and cytochemical, immunohistochemical (IHC) and immunocytochemical, flow cytometric, polymerase chain reaction (PCR), and cytogenetic techniques. For example, the immunophenotype (B vs T cell),118, 164, 165, 166, 167, 168, 169, 170, 171 proliferation rate (e.g., expression of Ki67, proliferating cell nuclear antigen expression, argyrophilic nucleolar organizer regions [AgNOR]),68, 74, 101, 154, 171, 172, 173, 174, 175, 176, 177 and clonality (PCR for antigen PARR)81, 160, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187 of the tumor can be determined. Genetic characterizations of canine lymphoma samples have been investigated and are showing potential for both diagnostic and prognostic utility, but are not widely applied and clinical correlates are currently preliminary.21, 22, 24, 178, 179, 188, 189 The availability of molecular and genetic analyses is increasing in veterinary oncology; however, at present, only immunophenotype and PARR clonality assays are routinely used in dogs to inform clinical decision making.
Immunophenotyping
Immunophenotyping is used to determine the type of cells that comprise the tumor, but this technique also can be helpful for making the initial diagnosis and predicting outcome.96, 164, 166, 167, 168, 169, 170, 171, 190, 191 When a heterogeneous population of lymphocytes is expected in a tissue, documentation of a homogeneous population of the same immunophenotype is supportive of a neoplastic process. The immunophenotype of a lymphocyte is identified by determining the expression of molecules specific for B cells (e.g., CD79a, CD20) and T cells (e.g., CD3), and have been recently reviewed.96 Although tumor cells sometimes have morphologic characteristics that typify a particular immunophenotype, exceptions occur and morphologic appearance cannot be used as the sole determinant of immunophenotype. For example, in a series of nine high-grade T-cell lymphomas and leukemias in dogs, the cells had a plasmacytoid appearance typically associated with B-cell lymphoma.123, 192 Similarly, anatomic location does not always predict the immunophenotype.
For accurate determination of immunophenotype, antibodies against lymphocyte markers are applied to tissue sections (IHC), cytologic specimens (immunocytochemistry), or individual cells in a fluid medium (flow cytometry). Flow cytometric evaluation of cells obtained by needle aspiration is also feasible. For T cells, markers include CD3 (pan T), CD4 (helper T), and CD8 (cytotoxic T); for B cells, the markers are CD79a (see Fig. 33.7B), CD20, and CD21, although dogs with indolent TZL can express CD21.178 Increasingly, aberrant expression of CD molecules has been reported in canine lymphoma. In a study of 59 dogs with lymphoma, tumor cells from six dogs were positive for both T- and B-cell markers; however, a clonality assay revealed clonality either of the T-cell or the immunoglobulin receptor, but not both. This indicates that, in some cases, the malignant cells may coexpress B- and T-cell markers.118 Antibodies against these molecules are used to determine the immunophenotype; however, they also have potential utility as a therapeutic modality if tumor cells could be targeted using these antibodies. Table 33.3 presents the histologic and immunophenotypic characteristics of the more common lymphoma subtypes in dogs.
TABLE 33.3.
Histologic and Immunophenotypic Characteristics of Common Canine Non-Hodgkin’s Lymphomas in Relative Order of Frequency
| Subtype | Typical Location | Histologic Architecture | Cellular Features | Immunophenotype |
|---|---|---|---|---|
| Diffuse large B-cell (DLBCL) | Usually multicentric lymphadenopathy | Diffuse | Large cells; round nuclei; one (central) or multiple nucleoli; high mitotic rate; “starry sky” appearance | CD1+, CD20+, CD21+, CD45+, CD79a+, Pax5+, MHCII+, CD18low |
| Peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) | Usually multicentric lymphadenopathy | Diffuse | Variable size (small to large); irregular nuclei, variable chromatin, prominent nucleoli; varied mitotic activity | CD3+, CD79a–, CD21–, CD45+, CD5+, CD4+/–, CD8+/–, CD18high, TCRαβ |
| Marginal zone lymphoma (MZL) | Nodal (nMZL) or splenic (sMZL) or extranodal mucosal | Nodular/ follicular | Mostly intermediate- sized cells- abundant pale cytoplasm; irregular nuclei with peripheralized chromatin and a single central nucleolus; rare mitotic figures (except nMZL) | CD1+, CD20+, CD21+, CD45+, CD79a+, MHCII+, CD18intermediate |
| T-zone lymphoma (TZL) | Usually multicentric Lymphadenopathy | Nodular, paracortical, progressing to diffuse | Small to intermediate- sized cells; moderate amount of pale cytoplasm; oval to elliptical nuclei with sharp, shallow indentations; nucleoli and mitotic figures are sparse | CD45–, CD3+, CD5+, CD21+, CD4+/–, CD8+/– |
| Precursor lymphomaa | Multicentric and/or leukemia | Diffuse and/or leukemia | Intermediate-sized cells; round nuclei; scant Cytoplasm; high mitotic rate |
If T-cell: CD45+, CD34+/–, CD5+/–, CD3+/–, CD4+/–, CD8– If B-cell: CD45+, CD18+, CD34+/–, CD79a+, CD21+/–, CD20+/– |
| Mantle cell lymphoma (MCL) | Splenic white pulp | Nodular/ follicular | Small to intermediate- sized cells; scant cytoplasm; round nuclei with dense chromatin, inconspicuous nucleoli; varied mitotic rate | CD20+, CD21+, CD45+, CD79a+, MHCII+ |
| Follicular lymphoma | Lymphadenopathy, solitary or multiple | Nodular/ follicular | Mixed—mostly small cells with clear cytoplasm, pale chromatin, and inconspicuous nucleoli (centrocytes) with fewer large cells with dark blue cytoplasm, vesicular nuclei, and 1–3 nucleoli (centroblasts) | CD20+, CD21+, CD45+, CD79a+, MHCII+ |
Precursor lymphoma includes lymphoblastic B- or T-cell lymphomas and B- or T-cell acute leukemias.
Adapted with permission from Seelig DM, Avery AC, Ehrhart EJ, Linden MA. The comparative diagnostic features of canine and human lymphoma. Vet Sci. 2016;3: Epub ahead of print. https://doi.org/10.3390/vetsci3020011; and Burkhard MJ, Bienzle D. Making sense of lymphoma diagnostics in small animal patients. Vet Clin North Am Small Anim Pract. 2013;43:1331-1347.
Clonality Assays
Information about the clinical presentation and morphologic and immunophenotypic characteristics of the lymphocytes, obtained by IHC or flow cytometric analysis, must be integrated to select appropriate targets for clonality testing and to interpret results accurately.193 Occasionally, diagnosis of lymphoma and differentiation of malignant versus benign proliferation of lymphocytes are not possible based on standard histologic and cytologic criteria. In these cases, advanced molecular analyses may be helpful to confirm a diagnosis. Clonality is the hallmark of malignancy; that is, the malignant cell population theoretically should be derived from expansion of a single malignant clone characterized by a particular DNA region unique to that tumor. For example, in a dog with T-cell lymphoma, all the malignant cells theoretically should have the same DNA sequence for the variable region of the T-cell receptor gene. Likewise, in a dog with B-cell lymphoma, the tumor cells should have identical DNA sequences in the variable region of the immunoglobulin (Ig) receptor gene. Conversely, in reactive lymphocytosis, the cells are polyclonal for their antigen receptors. Using this knowledge, investigators have used PCR technology to amplify the variable regions of the T-cell and immunoglobulin receptor genes to detect the presence of clonal lymphocyte populations in dogs (see Fig. 8.3 of Chapter 8). These techniques are reviewed in Chapter 8 and elsewhere.158, 180, 193, 194, 195 In physician-based medicine, such assays of clonality are approximately 70% to 90% sensitive and have a false-positive rate of approximately 5%, and recent studies report similar rates in dogs. False-negative and false-positive results can occur with clonality assays. For example, cells from a dog with lymphoma may be negative for clonality if the clonal segment of DNA is not detected with the PCR primers used, mutation of the primer site has occurred, there are background nonneoplastic lymphocytes (noise) within the tumor, the malignant cells are natural killer (NK) cells (rare), or the malignant cells are present in too low a frequency to be detected. False positives occur rarely in some infectious diseases (e.g., ehrlichiosis and leishmaniasis). In these cases, a diagnosis should be made only after considering the results of all the diagnostic tests, including histologic/cytologic evaluation, immunophenotyping, and clonality studies in conjunction with signalment and physical examination findings. These molecular techniques, although helpful for diagnosis, could also have utility in detecting relapse and in determining more accurate clinical stage and so-called “molecular remission rates” because they are more sensitive than standard cytologic assessment of peripheral blood, bone marrow, or LNs.
Other Immunohistochemical and Immunocytochemical Assessments
Assessments of several markers of multidrug resistance and apoptotic pathways (e.g., P-glycoprotein, p53, Bcl-2 proteins) have been evaluated in dogs with lymphoma29, 30, 174, 196, 197; however, their clinical significance and utility have not been established.
Proteomics and Serum Biomarkers
Proteomics comprises, simplistically, methodologies that analyze the entire protein component or protein signature of cells (the proteome). Protein components of a cell (normal or malignant) change over time with upregulation and downregulation of gene expression in response to varied stimuli (e.g., growth factors, environmental cues). It may therefore be possible to use the field of proteomics to identify serum biomarkers of malignancy (i.e., cancer-specific protein markers) and to further analyze response to therapy or even to predict which therapies are appropriate for an individual patient’s tumor. Although in its infancy in veterinary oncology, preliminary investigations of the proteome of dogs with lymphoma have been reported198, 199, 200, 201; however, they have yet to reach the level of sophistication in which useful output would have a significant effect on clinical decision making.
Several analytes in serum have been explored as biomarkers of lymphoma in the dog and have been reviewed.202 These include tumor and metabolic products, cytokines, cellular leakage enzymes, and serum proteins. Examples include thymidine kinase 1, C-reactive protein, alpha-fetoprotein, alpha-1 glycoprotein levels, zinc, chromium, iron, endostatin, vascular endothelial growth factor (VEGF), lactate dehydrogenase, haptoglobin, and antioxidants/oxidative stress markers.203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214 Although some have been grouped and commercialized (e.g., TK Canine Cancer Panel [VDI Labs, Simi Valley, CA, USA], Canine Lymphoma Blood Test [cLBT; Avacta Animal Health, Whetherby, UK]), the clinical, biologic, and prognostic significance of these assays has yet to be definitively characterized. Intuitively, use of biomarkers to detect early relapse would have clinical utility if meaningful therapeutic decisions and options were identified that would result in enhancement of quantity and quality of life. Currently, the lead-time provided over standard clinical diagnosis of relapse is relatively short, limiting their routine utility; definitive studies to support their application in larger and more varied general populations of dogs with lymphoma are currently lacking.
Clinical Staging
After a diagnosis has been established, the extent of disease should be determined and categorized by clinical staging. The WHO staging system routinely used to stage dogs with lymphoma is presented in Box 33.2. Most dogs (>80%) are presented in advanced stages (III–IV). Diagnostic imaging and assessment of bone marrow involvement may be indicated for staging. The degree to which thorough staging is implemented depends on whether the result will alter the treatment plan, whether relevant prognostic information is gleaned, and whether the clients need to know the stage before initiating (or declining) a treatment plan. In addition, when comparing different treatment protocols with respect to efficacy, consistent and similar staging diagnostics should be used to avoid so-called “stage migration,” which results when one staging methodology is more accurate than another.215 The effect of stage migration on prognosis should be considered when comparing different published outcomes.
Bone Marrow Evaluation
A bone marrow aspirate or core biopsy (from the proximal humerus or iliac crest) is recommended for complete staging and prognostication and may be indicated in dogs with anemia, lymphocytosis, circulating atypical lymphocytes, or other peripheral cytopenias. In one study of 53 dogs with lymphoma, 28% had circulating malignant cells and were considered leukemic, whereas bone marrow examination indicated involvement in 57% of the dogs.216 The presence of a few prolymphocytes and large lymphocytes with nucleoli in the circulation of dogs with lymphoma may indicate bone marrow involvement. It is important to remember these cells also can be seen with immune-mediated and inflammatory/infectious diseases. As discussed previously, tumor cells within the peripheral and bone marrow compartments can also be identified using clonality assays (PARR) that are more sensitive than routine microscopic examination in detecting malignant cells; however, the prognostic significance of the knowledge gained with more sensitive staging methodologies has yet to be determined.216, 217, 218 Although bone marrow evaluation may offer prognostically valuable information, it is not necessary to perform the procedure if the client is committed to treat regardless of stage.
Imaging
Evaluation of thoracic and abdominal radiographs may be important in determining the extent of internal involvement (Fig. 33.9 ).121, 219 Approximately 60% to 75% of dogs with multicentric lymphoma have abnormalities on thoracic radiographs, with one-third having evidence of pulmonary infiltrates (see Fig. 33.4) and two-thirds having thoracic lymphadenopathy (sternal and tracheobronchial LNs [see Fig. 33.9]) and widening of the cranial mediastinum (see Fig. 33.2).121, 122 Pulmonary infiltrates usually are represented by an interstitial and/or alveolar pattern; however, nodules (rarely) and bronchial infiltrates can also occur.220 Pleural effusion may also be present. Cranial mediastinal lymphadenopathy is detected in 20% of dogs with lymphoma.122, 220 Abdominal radiographs reveal evidence of involvement of medial iliac (sublumbar) and/or mesenteric LNs, spleen, or liver in approximately 50% of cases. In the authors’ practice, for the typical cases of canine multicentric lymphoma, imaging is limited to thoracic radiographs as there is no prognostic difference between dogs with stage III and IV disease (i.e., liver/spleen involvement); however, cranial mediastinal lymphadenopathy is of prognostic significance. If there are clinical signs attributable to abdominal disease, if complete staging is necessary (e.g., for clinical trial inclusion), or if peripheral lymphadenopathy is not part of the presentation, further imaging of the abdomen is warranted. Abdominal ultrasonography can be important for obtaining ultrasound-guided intraabdominal samples for diagnosis if more peripheral lesions are not evident (e.g., GI, abdominal nodal, and hepatosplenic lymphoma) or if complete clinical staging is required.152 Ultrasonographic (including Doppler ultrasound) assessment of peripheral LNs has also been explored153; however, its clinical applicability is questionable because cytologic assessment of peripheral nodes is easy, inexpensive, and of higher diagnostic utility.
Fig. 33.9.
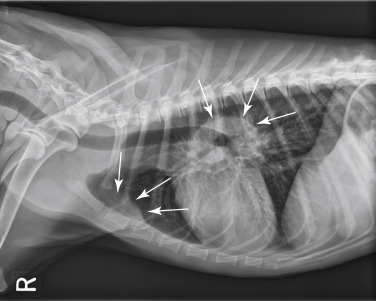
Lateral radiographic projection of a dog with sternal and hylar lymphadenopathy due to lymphoma.
Advanced imaging modalities, including computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), or PET/CT and PET/MR imaging, are becoming more commonplace in veterinary practice and their utility is only now being determined.221, 222, 223, 224, 225, 226 PET/CT imaging is the current standard of care for following and predicting durability of treatment response in human patients with lymphoma, and both [18F]fluorothymidine (18FLT) PET/CT and [18F]fluoro-d-glucose (18FDG) PET imaging have been reported in dogs with lymphoma.224–226 18FLT-PET/CT functional and anatomic imaging shows promise for the evaluation of response to cytotoxic chemotherapy in dogs with lymphoma and for predicting relapse before standard clinical and clinicopathologic confirmation (Fig. 33.10 ).
Fig. 33.10.
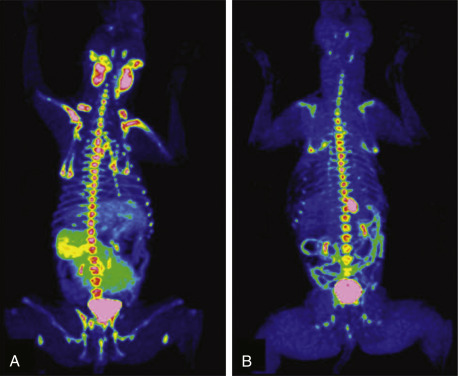
(A) FLT-PET/CT image of a 3-year-old MN Hound cross illustrating FLT uptake in the peripheral nodes, bone marrow, kidneys bladder and spleen. (B) FLT-PET/CT image of the same dog 3 weeks after his final dose of chemotherapy. The lymph nodes were small on CT with minimal FLT uptake on PET images. Note the persistent uptake in the bone marrow, kidneys, and bladder.
Reprinted with permission from Lawrence J, Vanderhoek M, Barbee D, et al Use of 3′-deoxy-3′-[18F]fluorothymidine PET/CT for evaluating response to cytotoxic chemotherapy in dogs with non-Hodgkin’s lymphoma. Vet Radiol Ultrasound. 2009;50:660–668.
Treatment of Multicentric Lymphoma
The therapeutic approach to a particular patient with lymphoma is determined by the subtype, stage, and substage of disease, the presence or absence of paraneoplastic disease, the overall physiologic status of the patient, financial and time commitment of the clients, and their level of comfort with respect to likelihood of treatment-related success and/or side effects. Without treatment, most dogs with intermediate- or high-grade lymphoma will die of their disease within 4 to 6 weeks of diagnosis, although significant variability exists.147 With few exceptions, canine lymphoma is considered a systemic disease and therefore requires systemic therapy to achieve remission and prolong survival. The majority of canine multicentric lymphomas are intermediate- to high-grade, and, currently, histologic and immunophenotypic characterization does not play a significant role in determining the initial treatment protocol unless a diagnosis of indolent or low-grade lymphoma is confirmed. It is hoped that in the near future, as more clinically correlative information on the significance of the various subclassifications of lymphoma in dogs is acquired, more tailored therapeutic approaches may become available.
Systemic multiagent chemotherapy continues to be the therapy of choice for canine intermediate- and high-grade lymphoma. In general, combination chemotherapy protocols are superior in efficacy to single-agent protocols. In rare cases in which lymphoma is limited to one site (especially an extranodal site), the animal can be treated with a local modality such as surgery or radiation therapy (RT) as long as the client and clinician are committed to diligent reevaluation (active surveillance) to document subsequent progression to systemic involvement, should it occur.
Multidrug Combination Protocols
Many chemotherapeutic protocols for dogs with lymphoma have been developed over the past 30 years (Table 33.4 ).116, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248, 249, 250, 251 Significant limitations arise when comparing efficacy studies in the veterinary literature for the various published protocols. Few of these studies include sufficient numbers of dogs for adequate statistical power and even fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably among reports. Therefore evaluations of efficacy among various protocols are subject to substantial bias, making direct comparisons difficult and indeed precarious. A recurring theme in the concluding statement in most of these published protocols is some variation of “prospective randomized trials will be required to confirm these suggestive findings.” Despite the plethora of available combination protocols, most are modifications of CHOP protocols initially designed for human oncologic use, and currently randomized prospective evidence does not exist in dogs to clearly recommend one over the other as long as the basic CHOP components are present. CHOP represents combinations of cyclophosphamide (C), doxorubicin (H, hydroxydaunorubicin), vincristine (O, Oncovin), and prednisone (P). In the 1980s and early 1990s, physicians treating human patients with advanced, intermediate- or high-grade lymphoma faced a similar dilemma in that many different variations of CHOP existed and no randomized data were available to determine which protocols were superior. Eventually, a national randomized trial involving more than 1000 people with intermediate/high grade NHL was conducted comparing the plethora of protocols available, and the results indicated that CHOP was as effective as any of the more complicated protocols and had the safest adverse event profile.252 CHOP subsequently became (and remains, with the addition of monoclonal antibody therapy) the standard of care for people with intermediate-/high-grade NHL.
TABLE 33.4.
Summary of First Remission Outcomes of Combination or Single-Agent Doxorubicin Lymphoma Chemotherapy Protocolsa
| PRIVATE Protocol | No. of Dogs | Remission Rate (%) | Median Remission Duration (Months) | % 1-Year Survival | References |
|---|---|---|---|---|---|
| COP | 77 | 75 | 6.0 | 19 | 248 |
| A | 37 | 59 | 4.4 | NR | 250 |
| Ab | 121 | 85 | 4.3 | NR | 246 |
| A | 42 | 74 | 4.9 | NR | 239 |
| A + piroxicam | 33 | 79 | 4.3 | NR | 239 |
| VMC-L | 59 | 90 | 4.4 | 25 | 236 |
| VMC-L | 147 | 77 | 4.7 | 25 | 237 |
| VCA-L | 112 | 73 | 7.9 | 50 | 116 |
| L-COPA | 41 | 76 | 11.0c | 48 | 251 |
| L-COPA(II) | 68 | 75 | 9.0c | 27 (13 at 2 yr) | 240 |
| COPLA/LVP | 75 | 92 (80c) | 5.8 | 17 | 415 |
| VELCAP-SC | 94 | 70 | 5.6 | 44 | 238 |
| VLCAP-Long | 98 | 69 | 12.5c | NR | 247 |
| L-VCAMP (UW-Madison CHOP) | 55 | 84 | 8.4 | 50 (24 at 2 yr) | 235 |
| L-VCAMP (continuous maintenance CHOP) | 96 86 |
79 (CR) 90 |
9 6.8c |
NR 35c |
234 243 |
| L-VCAMP (+/– intensification CHOP) | 130 | 94.6 | 7.3d | NR | 245 |
| L-VCAP (25-week CHOP) | 51 | 94 | 9.1 | NR | 232 |
| L-VCAP-Mx | 65 | 94 | 10 | NR | 231 |
| L-VCAP | 71 | 88 | 9.7c | 32 (13 at 2 yr) | 241 |
| L-VCAP (12-week CHOP) | 77 | 89 | 8.1c | 28c | 244 |
| VCAP (15-week CHOP) | 31 134 |
100 (84 CR) 98 (78 CR) |
4.7d 5.9d |
NR NR |
227 228 |
| L-VCAP/CCNU/MOPrP | 66 | 94 | 10.6c | 46 (35 at 2 yr) | 241 |
| COArP | 71 | 92 | 3 | NR | 233 |
| L-VCADP | 39 | 100 | 11d | NR | 242 |
| L-VCEP | 97 | 100 (96 CR) | 7.2 | NR | 229 |
| RA | 54 | 98 (68 CR) | 6.5d | NR | 230 |
eQuestionable (only one-third reportedly finished).
A, Adriamycin (doxorubicin); Ar, cytosine arabinoside; C, cyclophosphamide; CR, complete response; D, dactinomycin; E, epirubicin; L, l-asparaginase; M, methotrexate; Mx, mitoxantrone; NR, not reported; O, Oncovin (vincristine); P, prednisone; Pr, procarbazine; R, rabacfosadine; V, vincristine.
Minimum of 30 cases required for inclusion. Few of these protocols include sufficient numbers for adequate statistical power and fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably between protocols presented. Therefore evaluations of efficacy between the various protocols are subject to bias, making direct comparisons difficult and indeed precarious.
With COP rescue.
Only durations of cases achieving CR reported.
Time to progression.
Conventional CHOP-based chemotherapy induces remission in approximately 80% to 95% of dogs, with overall MSTs of 10 to 12 months. Approximately 20% to 25% of treated dogs will be alive 2 years after initiation of these protocols (Fig. 33.11 ). Response rates and durations of response vary according to the presence or absence of prognostic factors discussed in the text that follows. The relative cost of the various protocols to the client depends on the drug(s) selected, the size of the animal, the frequency of administration, and the laboratory tests required to monitor adverse events and response.
Fig. 33.11.
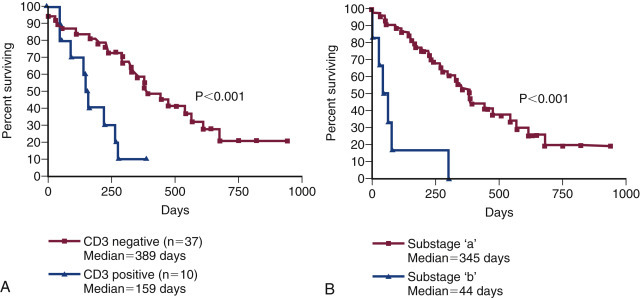
(A) Kaplan–Meier survival duration estimates for a group of 55 dogs with lymphoma treated with an identical CHOP-based combination chemotherapy protocol. Dogs with CD3 immunoreactive (T-cell) lymphoma had significantly shorter survival durations. (B) Kaplan–Meier survival duration estimates for a group of 55 dogs with lymphoma treated with an identical CHOP based combination chemotherapy protocol at the University of Wisconsin. Dogs with substage b disease (i.e., clinically ill) had significantly shorter survival durations.
From Vail DM. Hematopoietic tumors. In Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis: Elsevier; 2005.
In an attempt to better standardize response criteria and outcome reporting of future trials, the Veterinary Cooperative Oncology Group (VCOG) published response evaluation criteria (v1.0)253 that can be applied in the routine practice setting. The greatest obstacle to performance of prospective randomized comparative lymphoma trials in veterinary oncology is financial; that is, clinical trials are inherently costly, and because most of the known effective drugs are unregistered off-label human generic (i.e., off-patent) drugs, the incentive for pharmaceutical-funded, sufficiently powered, randomized field trials is low, resulting in a general lack of comparative data.
Dogs responding to chemotherapy and undergoing complete “clinical” remission are usually free of clinical signs associated with lymphoma and subsequently return to a very good quality of life, making the treatment of dogs with lymphoma initially gratifying. Most dogs tolerate chemotherapy well, and although dose reductions and treatment breaks (“treatment holidays”) are sometimes required in individual cases, only a minority of dogs develop significant adverse events requiring hospitalization.254, 255 Studies assessing client perceptions of medical treatment for cancer in general and lymphoma in particular report a positive experience; most owners feel treatment was worthwhile, that it resulted in improvement in the well-being of their pet, and that quality of life during treatment was good.256, 257 Very few clients express regret about treating lymphoma using a multidrug protocol.
Importantly, it must be realized that cures are rare, and though complete clinical remissions are the norm, complete molecular remissions (iCR), which can be documented only with molecular techniques, are rarely achieved in dogs; thus the utility of documenting iCR, in the absence of meaningful therapeutic options, is limited to investigative trials striving to achieve them.
With lymphoma, the fundamental goals of chemotherapy are to induce a complete durable (>6 months) first remission (termed induction), to reinduce a remission when the tumor recrudesces (the patient relapses) after achievement of a remission (termed reinduction), and, finally, to induce remissions when the cancer fails to respond to induction or reinduction using drugs not present in the initial protocols (termed rescue).
An unanswered question in the treatment of lymphoma has been whether long-term maintenance chemotherapy is useful after an initial course of aggressive induction chemotherapy lasting 6 months or less. Long-term maintenance chemotherapy has not been shown to be of significant value in humans with most aggressive forms of NHL; however, in humans, the initial induction course of chemotherapy is much more aggressive than that used in veterinary patients. Although no randomized prospective studies have been performed to address the therapeutic benefit of long-term maintenance chemotherapy in dogs, most comparisons of dogs treated with CHOP-based protocols do not show any clear advantage for a maintenance or consolidation phase after induction therapy.229, 231, 232, 234, 240, 242, 243, 244, 245, 247, 258, 259, 260, 261, 262 Indeed, in most reports, dogs receiving shorter, less costly protocols that do not include a prolonged maintenance phase have comparable remission and progression-free survival (PFS) durations and appear to more readily achieve second remissions when they relapse after completion of chemotherapy than their counterparts receiving long-term maintenance. These data, taken together, suggest that maintenance therapy is not beneficial for most dogs with lymphoma. Until well-designed randomized prospective trials indicate otherwise, the author (DMV) prefers protocols that utilize an aggressive induction without maintenance.
Single-Agent Chemotherapy with Known Activity for Dogs with Lymphoma
The most effective currently available chemotherapeutic agents for canine lymphoma include doxorubicin (DOX), l-asparaginase, vincristine, cyclophosphamide, and prednisone, most of which are represented to one degree or another in most first-line multiagent chemotherapy protocols. Other drugs that have documented activity are often considered second-line agents and include rabacfosadine (Tanovea-CA1), lomustine, vinblastine, actinomycin-D, mitoxantrone, mustargen, chlorambucil, methotrexate, dacarbazine (DTIC), 9-aminocamptothecin, ifosfamide, cytosine arabinoside, procarbazine, bleomycin, and gemcitabine. Of these, cytosine arabinoside,263 ifosfamide,264 bleomycin,265 and gemcitabine266 appear to have minimal activity. With the exception of DOX, single-agent induction therapy does not typically result in durable remission durations compared with standard combination protocols. Incorporation of other standard cytotoxic drugs with single-agent activity into standard CHOP-based protocols has not resulted in significant gains or has not been adequately evaluated, and most are reserved for subsequent rescue settings.
The use of rabacfosadine (Tanovea-CA1) warrants a brief discussion as it is the only chemotherapy agent currently approved, albeit conditionally, by the US Food and Drug Administration (FDA) for the treatment of dogs with lymphoma (see Chapter 12 for a specific discussion of rabacfosadine). Rabacfosadine has been evaluated in hundreds of dogs with lymphoma and activity has been documented as a single-agent for treatment of cutaneous lymphoma, multiple myeloma, and naïve and relapsed multicentric lymphoma, as well as in combination with DOX for lymphoma.226, 230, 267, 268, 269, 270 Currently, rabacfosadine is most commonly used as a rescue agent at relapse or as a first-line treatment in combination with DOX owing to a less intense treatment protocol while maintaining similar remission durations compared with CHOP in a nonrandomized fashion.230
Overall Chemotherapy Recommendations for Multicentric Lymphoma (Author [DMV] Preference)
Several factors should be considered and discussed with caregivers on a case-by-case basis when choosing the treatment protocol. These factors include cost, time commitment involved, efficacy, adverse event profiles, and experience of the clinician with the protocols under consideration.
Induction in Treatment-Naïve Patients
It is now clearly established that “standard-of-care” combination protocols used in dogs with intermediate- and high-grade lymphoma are essentially variations of CHOP protocols (see Table 33.4). Specific details regarding dose and timing of the CHOP protocol currently preferred by the author (DMV) are outlined in Box 33.3 . This protocol does not have a maintenance component and all treatments cease at 19 weeks, provided the animal is in complete clinical remission. Although several other CHOP-based protocols include l-asparaginase either at initiation or at varying times throughout the protocol, several studies suggest this does not result in clinically relevant increases in remission rate, speed of attaining remission, or first-remission duration, and therefore the author reserves its use for rescue situations.246, 260, 271, 272
BOX 33.3. Current Canine Lymphoma Protocol (UW-Madison-19).
Week 1: Vincristine, 0.7 mg/m2 IV
Prednisone, 2 mg/kg, PO, daily
Week 2: Cyclophosphamide,a 250 mg/m2 IV or PO
Prednisone, 1.5 mg/kg, PO, daily
Week 3: Vincristine, 0.7 mg/m2 IV
Prednisone, 1.0 mg/kg, PO, daily
Week 4: Doxorubicin,b 30 mg/m2 IV
Prednisone, 0.5 mg/kg, PO, daily
Week 6: Vincristine, 0.7 mg/m2 IV
Week 7: Cyclophosphamide,a 250 mg/m2 IV or PO
Week 8: Vincristine, 0.7 mg/m2 IV
Week 9: Doxorubicin,b 30 mg/m2 IV
Week 11: Vincristine, 0.7 mg/m2 IV
Week 12: Cyclophosphamide,a 250 mg/m2 IV or PO
Week 13: Vincristine, 0.7 mg/m2 IV
Week 14: Doxorubicin,b 30 mg/m2 IV
Week 16: Vincristine, 0.7 mg/m2 IV
Week 17: Cyclophosphamide,a 250 mg/m2 IV or PO
Week 18: Vincristine, 0.7 mg/m2 IV
Week 19: Doxorubicin,b 30 mg/m2 IV
-
1.
All treatments are discontinued after week 19 if in complete remission.
-
2.
A complete blood count (CBC) should be performed before each chemotherapy. If neutrophil count is <1500 wait 5 to 7 days and repeat CBC.
-
3.
If sterile hemorrhagic cystitis occurs on cyclophosphamide, discontinue and substitute chlorambucil (1.4 mg/kg PO) for subsequently scheduled cyclophosphamide treatments.
-
4.
For acute lymphocytic leukemia (ALL)–administer l-asparaginase 400 IU/kg SQ with each vincristine injection, until a complete response is achieved.
If client or other considerations preclude a CHOP-based protocol, single-agent DOX (30 mg/m2, intravenous [IV], every 3 weeks for five total treatments) is offered along with a 4-week tapering oral prednisone regimen (same prednisone regimen in Box 33.3) as a less aggressive, less time-consuming, and less costly approach. The expected CR rate for the single-agent DOX protocol will range from 50% to 75%, with an anticipated MST of 6 to 8 months.246, 250, 273, 274 The addition of oral cyclophosphamide (50 mg/m2 daily for 3 days starting on the same day as DOX) to single-agent DOX resulted in a numerically, but not statistically, improved outcome in a randomized trial comparing DOX/prednisone with DOX/cyclophosphamide/prednisone (PFS of 5.6 months vs 8.2 months, respectively).274 This trial was powered only to detect a three-fold difference in PFS; therefore larger trials should be undertaken to confirm any benefit. Alternatively, a less time-intense protocol with treatments every 3 weeks involves the previously mentioned rabacfosadine/DOX protocol.230
If clients are reticent to include IV medications, the author often recommends a protocol of either oral lomustine (CCNU; 70 mg/m2 by mouth [PO] every 3 weeks for five treatments) and prednisone or oral cyclophosphamide (250 mg/m2 [PO] every 2–3 weeks) with prednisone. The CCNU protocol has been associated with short median remissions (40 days) in one small case series275; however, in the author’s experience, a subset of dogs have remained in remission for several months on this protocol when clients decline IV medication.
If financial or other client concerns preclude the use of systemic chemotherapy, prednisone alone (2 mg/kg PO, daily) will often result in short-lived remissions of approximately 1 to 2 months; however, an occasional durable remission will result. In these cases, it is important to educate clients that, should they decide to pursue more aggressive therapy at a later date, dogs receiving single-agent prednisone therapy are more likely to develop multidrug resistance (MDR) and experience shorter remission and survival durations with subsequent combination protocols.258, 276, 277, 278 This is especially true after long-term prednisone use or in dogs that have experienced a relapse while receiving prednisone. Therefore the earlier that clients opt for more aggressive therapy, the more likely a durable response will result.
A CBC should be performed before each chemotherapy treatment. Dogs should have a minimum of 1500 neutrophils/μL (some oncologists use a cut-off of 2000 neutrophils/μL) and 50,000 platelets/μL before the administration of myelosuppressive chemotherapy.279 If the neutrophil count is lower than 1500/μL, it is recommended to wait 5 to 7 days and repeat the CBC; if the neutrophil count has increased to more than 1500 cells/μL, the drug can be safely administered. A caveat to these restrictions is that for dogs presenting before initiation of chemotherapy with low neutrophil and platelet counts due to bone marrow effacement (myelophthisis), myelosuppressive chemotherapy is instituted in the face of cytopenias to clear the bone marrow of neoplastic cells and allow hematopoiesis to normalize.
In those breeds likely to have homozygous MDR1 gene mutations (e.g., collies; see Chapter 12), and therefore to be at risk for serious chemotherapy toxicity,280 the author [DMV] will initiate a CHOP protocol out of sequence, beginning with non-MDR1–substrate drugs, such as cyclophosphamide. This ensures treatment of the lymphoma while allowing sufficient time for analysis of MDR1 gene mutations before initiating MDR1 substrate drugs. No specific protocols have been scrutinized for treating dogs that are double-mutant for MDR1; however, if using MDR1-substrate drugs, the author initiates treatment using a 40% to 50% dose reduction. Subsequent dose modifications (increased or decreased dosage) can be implemented, depending on the level of adverse events observed, particularly low neutrophil counts at nadir. The author does not dose-modify for heterozygous MDR1 gene mutations as little documented clinically significant chemosensitivity exists in these animals.
The Case for Treating T-Cell Lymphoma Differently
With some exceptions (e.g., TZL), multicentric T-cell lymphoma, compared with multicentric B-cell lymphoma, is associated with similar initial response rates, but significantly lower response durations (e.g., PFS) after chemotherapy (including CHOP-based protocols).68, 112, 113, 116, 123, 226, 242, 244, 277, 281, 282, 283 In addition, the effectiveness of a single treatment of DOX in the treatment of naïve lymphoma in one retrospective case series suggested a lower initial response rate for T-cell, compared with B-cell, immunophenotypes; however, this study performed only a single day 7 evaluation.281 Many question whether dogs diagnosed with T-cell lymphoma should be treated with standard CHOP-based protocols or with alternative protocols. This is a valid question; however, the answer remains elusive because adequately powered randomized controlled trials do not currently exist to demonstrate if an alternate protocol is better for this immunophenotype. Several alternative protocols (MOPP, LOPP, VELCAP-TSC) have been reported and reviewed for induction therapy in dogs with multicentric T-cell lymphoma.110, 284, 285, 286 These alternative protocols tend to add or substitute alkylating agents (e.g., nitrogen mustard, lomustine, procarbazine) for DOX. Although reports have suggested improvements in remission durations in dogs with either confirmed T-cell lymphoma or lymphoma with hypercalcemia and no immunophenotypic classification, the confidence intervals of the medians all overlap with data from CHOP-based protocols, and differences in determining PFS, response evaluation, and study population (in particular, some do not adequately distinguish indolent multicentric lymphoma) in these reports preclude confident comparisons. As yet, no controlled, randomized trials have documented improvement with this approach. Ultimately, the development of better protocols for treating T-cell lymphoma awaits careful, randomized, prospective trial assessment. Until such time, the author prefers to initiate CHOP-based induction and switch to lomustine-based rescue at the first sign of progression or to enter dogs into clinical trials with novel agents. Although species differences may exist, in people with aggressive T-cell NHL, alternative protocols rich in alkylating agents have not shown superiority over CHOP-based protocols, and the National Comprehensive Cancer Network recommend human patient participation in clinical trials as the “gold standard” for aggressive nonindolent T-cell lymphomas.
Evaluation of Treatment Response
VCOG has published criteria for evaluation of treatment response (v1.0) to standardize reporting of outcome results and comparisons among protocols for peripheral nodal disease using criteria readily available in the practice setting.253 The most important of these outcome measures and the preferred temporal outcome criterion for assessing protocol activity is now considered to be PFS, which is defined as being the time from treatment initiation to tumor progression or death from any cause. This brings veterinary outcome reporting more in line with human standards. Because the majority of dogs with lymphoma eventually experience relapse after chemotherapy-induced remissions and because methodology for differentiating complete and partial responses is analysis dependent on how responses are determined, PFS removes many sources of bias. Further, overall survival in published reports invariably includes patients who go on to receive varied rescue protocols that bias the overall result, making it a less comparable outcome. Widespread application of these standardized criteria should permit more suitable comparisons in the future. True iCR rarely occur in dogs with lymphoma; documenting iCRs is limited to investigative trials striving to achieve them because we lack meaningful therapeutic options.
Improved methods of detecting minimal residual disease (MRD) or early recurrence have been investigated in dogs with lymphoma and include advanced imaging and detection of molecular and biologic markers of MRD. Advanced functional and anatomic imaging techniques (i.e., PET/CT) are the current standard for assessing treatment response and early relapse of lymphoma in humans and have also been investigated in dogs (see Fig. 33.10).221, 223, 224, 225, 226 As these techniques become available to a broader veterinary population, their clinical application will surely increase. Molecular detection of MRD applies clonality and PCR techniques. Beyond diagnostic applications, these techniques have been applied to determine cytoreductive efficacy of various chemotherapeutic drugs and to document and predict early relapse in patients before more conventional methods.123, 217, 246, 287, 288, 289, 290, 291, 292, 293 Regarding biomarkers of MRD, preliminary investigations have suggested serum lactate dehydrogenase activity, thymidine kinase 1 activity, haptoglobin, and serum C-reactive protein may be candidates in the dog.202, 203, 204, 205, 206, 294
As we become more proficient at defining MRD, the pressing clinical question becomes how we use this information. Theoretically, such information could suggest when more aggressive therapy or alternative therapy should be instituted in patients who have not achieved a “molecular remission” or who are undergoing early relapse; however, until we determine what these interventions should be based on prospective trial assessment, the clinical utility of MRD analytics remain theoretical.
Reinduction and Rescue Chemotherapy
Eventually, the vast majority of dogs that achieve a remission will relapse or experience recrudescence of lymphoma. This usually represents the emergence of tumor clones or tumor stem cells (see Chapter 2) that are inherently more resistant to chemotherapy than the original tumor, the so-called MDR clones that either were initially drug resistant or became so after exposure to selected chemotherapy agents.295 Evidence suggests that in dogs with relapsed lymphoma, tumor cells are more likely to express genes (e.g., MDR1) that encode ABC-transporter protein transmembrane drug pumps often associated with MDR.196, 197, 296, 297, 298, 299 MDR1 represents only one of the plethora of mechanisms that lead to drug-resistant disease (see Chapter 12). Other causes of relapse after chemotherapy include inadequate dosing and/or frequency of administration of chemotherapy, failure to achieve high concentrations of chemotherapeutic drugs in certain sites such as the CNS, and initial treatment with prednisone alone.
At the first recurrence of lymphoma, it is recommended that reinduction be attempted first by reintroducing the induction protocol that was initially successful, provided the recurrence occurred temporally far enough from the conclusion of the initial protocol (e.g., ≥2 months) to make reinduction likely. The cumulative dose of DOX that will result from reinduction, baseline cardiac assessment, the use of cardioprotectants, alternative drug choices, and client education should all be considered. In general, the duration of reinduction remission will be half that encountered in the initial therapy; however, a subset of animals will enjoy long-term reinductions, especially if the dog completed the initial induction treatment protocol and was currently not receiving chemotherapy for several months when relapse occurred. Reinduction rates of nearly 80% to 90% can be expected in dogs that have completed CHOP-based protocols and then relapse while not receiving therapy.232, 300 The duration of a second CHOP-based remission in one report was predicted by the duration of the interval between protocols and the duration of the first remission.123, 300
If reinduction fails or the dog does not respond to the initial induction, the use of so-called “rescue” agents or “rescue” protocols may be attempted. These are single drugs or drug combinations that are typically not found in standard CHOP protocols and are withheld for use in the drug-resistant setting. The most common rescue protocols used in dogs include single-agent use or a combination of rabacfosadine, actinomycin D, mitoxantrone, DOX (if DOX was not part of the original induction protocol), dacarbazine (DTIC), temozolomide, lomustine (CCNU), l-asparaginase, mechlorethamine, vincristine, vinblastine, procarbazine, prednisone, and etoposide. Some rescue protocols are relatively simple and convenient single-agent treatments, whereas others are more complicated (and expensive) multiagent protocols, such as MOPP. Overall rescue response rates of 40% to 90% are reported; however, responses are usually not durable with median response durations of 1.5 to 2.5 months being typical regardless of the complexity of the protocol. A small (<20%) subset of animals will enjoy longer rescue durations. Table 33.5 provides a summary of canine rescue protocols and published results.270, 301, 302, 303, 304, 305, 306, 307, 308, 309, 310, 311, 312, 313, 314, 315, 316, 317, 318, 319, 320 Current published data from rescue protocols do not include sufficient numbers for adequate statistical power, nor do they compare protocols in a controlled, randomized prospective fashion. Therefore comparative evaluations of efficacy among various protocols are subject to substantial bias, making direct comparisons difficult. Choice of a particular rescue protocol should depend on several factors, including cost, time commitment required, efficacy, adverse event profile, and experience of the clinician with the protocols in question. As the complexity of rescue protocols does not yet appear to be associated with significant gains in rescue durability, the author (DMV) tends to choose simpler and less costly protocols (e.g., CCNU/l-asparaginase/prednisone) (Table 33.6 ); however, the use of multiple varied rescue protocols, switching as needed based on response, continues as long as clients are comfortable with their dog’s quality of life. This sequential application of several different rescue protocols can result in several months of extended survival with acceptable quality of life.
TABLE 33.5.
Summary of Response for Rescue Protocolsa
| PRIVATE Protocol | Number of Animals | Overall Response (%) | Complete Response (%) | Median Response Durationc | Median Duration of Complete Response | References |
|---|---|---|---|---|---|---|
| Actinomycin-D | 25 | 0 | 0 | 0 days | 0 days | 316 |
| Actinomycin-D | 49b | 41 | 41 | 129 days | 129 days | 310 |
| Dacarbazine | 40 | 35 | 3 | 43 days | 144 days | 313 |
| Dacarbazine or temozolomide-anthracycline | 63 | 71 | 55 | 45 days | NR | 311 |
| DMAC (dexamethasone, melphalan, actinomycin-D, cytosine arabinoside) | 54 86 |
72 43 |
44 16 |
61 days 24 days |
112 days NR |
309 306 |
| Lomustine (CCNU) | 43 | 27 | 7 | 86 days | 110 days | 314 |
| Lomustine, l-asparaginase, prednisone | 48 | 77 | 65 | 70 days | 90 days | 319 |
| Lomustine, l-asparaginase, prednisone | 31 | 87 | 52 | 63 days | 111 days | 320 |
| Lomustine, DTIC | 57 | 35 | 23 | 62 days | 83 days | 312 |
| Mitoxantrone | 44 | 41 | 30 | NR | 127 days | 315 |
| MOPP (mechlorethamine, vincristine, procarbazine, prednisone) | 117 | 65 | 31 | 61 days | 63 days | 318 |
| MPP (mechlorethamine, procarbazine, prednisone) | 41 | 34 | 17 | 56 days | 238 days | 317 |
| MOMP (mechlorethamine, vincristine, melphalan, prednisone) | 88 | 51 | 12 | 56 days | 81 days | 301 |
| LOPP (lomustine, vincristine, procarbazine, prednisone) | 33 | 61 | 36 | 84 days | NR | 302 |
| Vinblastine (second rescue) | 39 | 25.6 | 7.7 | 30 days | NR | 304 |
| Rabacfosadine (B cell only) | 50 | 74 | 45 | 108 days | 203 days | 270 |
| LPP (lomustine, procarbazine, prednisone) | 41 | 61 | 29 | 34 days | 84 days | 307 |
| Temozolomide | 26 | 32 | 13 | 15 days | NR | 308 |
Minimum of 25 cases. Few of these protocols include sufficient numbers for adequate statistical power and fewer compare treatment protocols in a randomized prospective fashion. In addition, staging, inclusion, and response criteria vary considerably between protocols presented. Therefore evaluations of efficacy between the various protocols are subject to bias making direct comparisons difficult and indeed precarious.
Prednisone often used concurrently.
Various temporal response end-points were used including disease-free interval, time to progression, progression-free survival, time to discontinuation, and remission duration.
TABLE 33.6.
First-Line Rescue Protocola
| Cycle 1 | |
| Week 1 |
Baseline ALT ________ Units/Ll-Asparaginase, 400 Units/kg SC CCNUb 70 mg/m2 PO Prednisone, 2 mg/kg PO, once daily |
| Week 2 | Prednisone, 1.5 mg/kg PO, once daily |
| Week 3 | Prednisone, 1.0 mg/kg PO, once daily |
| Cycle 2 | |
| Week 1 |
Optional ALT ________ Units/Ll-Asparaginase, 400 U/kg SC CCNU 70 mg/m2 PO Prednisone, 1.0 mg/kg PO, EOD |
| Week 2 | Prednisone, 1.0 mg/kg PO, EOD |
| Week 3 | Prednisone, 1.0 mg/kg PO, EOD |
| Cycle 3–5 | |
| Week 1 |
Mandatory ALT ________ Units/L CCNU 70 mg/m2 PO Prednisone, 1.0 mg/kg PO, EOD |
| Week 2 | Prednisone, 1.0 mg/kg PO, EOD |
| Week 3 | Prednisone, 1.0 mg/kg PO, EOD |
ALT, Alanine aminotransferase.
Treatment discontinuation criteria: (1) After completion of protocol, two treatments beyond complete response (CR); (2) progressive disease; (3) increase in ALT activity >2× upper limit of normal (or 2× baseline if higher than baseline at initiation)—institute drug discontinuation and reinstitution/dose reduction dependent on normalization of ALT.
Prophylactic liver protectants recommended (e.g., Denamarin).
Strategies to Enhance Effectiveness of Therapy in Lymphoma
Despite the plethora of published chemotherapeutic protocols for dogs with lymphoma, it appears we have achieved as much as we can from currently available cytotoxic chemotherapeutics in standard settings. The 12-month median survival “wall” and the 20% to 25% 2-year survival rates have not improved dramatically. Further advances in remission and survival durations await the development of new methods of delivering or targeting traditional chemotherapeutic drugs, new generations of chemotherapeutic drugs, or novel nonchemotherapeutic treatment modalities; in particular, the development of targeted immunotherapies, which is the standard of care in physician-based oncology. Mechanisms of avoiding or abrogating MDR, enhancing tumor apoptosis (programmed cell death), tumor ablation, and immune-system reconstitution, as well as novel immunomodulatory therapies for lymphoma, are all active areas of investigation in both human and veterinary medicine.
Treatment Approaches Using Immunologic or Biologic Agents
Monoclonal Antibody Approaches
Enhanced durability of first remissions in humans with B-cell NHL has been achieved primarily through the institution of monoclonal antibody (mAb)-based therapies (so-called R-CHOP protocols that are the current “standard-of-care” in people). The “R” refers to rituximab (Rituxan), a recombinant chimeric murine/human antibody directed against the CD20 antigen, a hydrophobic transmembrane protein located on normal pre-B and mature B lymphocytes. After binding, rituximab triggers a host cytotoxic immune response against CD20-positive cells. Unfortunately, rituximab does not have therapeutic activity in dogs because of a lack of external recognition of a similar antigen on canine lymphoma cells and the inherent antigenicity of human-derived antibodies in dogs.321, 322 Recently, caninized mAb designed to target either B-cell (blontuvetmab; Blontress) or T-cell (tamtuvetmab; Tactress) lymphomas were conditionally approved by the USDA for use in dogs with lymphoma; however, after being assessed in prospective clinical trials involving a large number of dogs, their target specificity was found to be inadequate to effect clinical efficacy, and they are no longer available and are not currently recommended for use. Several laboratories throughout the world are working to characterize and develop more specific and effective mAb therapies for use in canine lymphoma,323, 324, 325, 326 and practitioners await their development.
Antitumor Vaccine Approaches
Several antitumor vaccine approaches have been investigated in dogs with lymphoma, including tumor vaccine extract using killed lymphoma cells combined with Freund’s adjuvant327, 328 and autologous killed and/or gene engineered lymphoma tumor cell vaccines329, 330; however, no significant gains in remission times or overall survival have been documented. Exploratory vaccines targeting telomerase331, 332 (see Chapter 15, Section D), heat shock proteins,333, 334 and RNA-loaded CD40-activated B cells335, 336 in dogs with lymphoma have also been conducted. These studies involved small numbers of nonrandomized patients and lacked controlled populations for comparison. A xenogeneic DNA vaccine designed to target canine CD20 is currently undergoing clinical trials in the United States. Although preliminary activity is suggested in many of these reports and they are serving to enhance our basic understanding of immunotherapeutic methodologies, their development is still early; complete safety and efficacy trials have not been completed to date.
Adaptive Immunotherapy Approaches
Much excitement has been generated in physician-based medicine about adaptive immunotherapy approaches, in particular the application of chimeric antigen receptor T cells (CAR-T; see Chapter 14). These approaches are currently the subject of several proof-of-concept trials in dogs with NHL.337, 338
Surgery
Most dogs with lymphoma have multicentric disease and therefore require systemic chemotherapy to effectively treat their disease. However, surgery has been used to treat solitary lymphoma (stage I) or solitary extranodal disease. Careful staging is necessary in such cases to rule out multicentric involvement before treating local disease. Surgery has also occasionally been applied for palliative removal of nodes that are mechanical obstructions in drug resistant settings.
The benefit of surgical removal of the spleen in dogs with massive splenomegaly remains unclear; however, for indolent lymphomas confined to the spleen, long-term survival after splenectomy is the norm.100, 102, 339, 340 In an older report, 16 dogs with lymphoma underwent splenectomy to remove a massively enlarged spleen and were subsequently treated with chemotherapy.340 Within 6 weeks of splenectomy, five of the 16 dogs died of disseminated intravascular coagulation (DIC) and sepsis. The remaining 11 dogs (66%) had a CR, and seven dogs had a MST of 14 months. No staging or histologic information was provided, so the information appears of limited usefulness, although those with follow-up lived approximately 1 year. In two reports of indolent nodular lymphoma of the spleen (MZL and mantle cell lymphoma [MCL]), outcome was available on seven MZL cases, including three cases that did not receive adjuvant chemotherapy after surgery,100, 102 and only one died of lymphoma after splenectomy. In a recent report of indolent lymphomas, four splenic lymphomas (three MZL and one MCL) underwent splenectomy alone and all survived more than 1 year, with none dying of their primary disease.101 In another report of 41 dogs undergoing splenectomy for lymphoma, those dogs with indolent forms enjoyed long-term survival.339 Splenectomy should be considered if the lymphoma is not documented in other sites after thorough staging, if lymphoma is an indolent form, or if splenic rupture has occurred. Of note, no control population consisting of dogs that did not undergo splenectomy exists, so the natural history of indolent splenic lymphoma remains uncertain.
Radiation Therapy
Radiation therapy, although of limited routine use in the treatment of lymphoma, may be indicated in selected cases.341, 342, 343, 344, 345, 346, 347, 348, 349, 350, 351 Potential indications are as follows:
-
1.
Curative intent therapy for stage I LN and solitary extranodal disease (i.e., nasal, cutaneous, spinal lymphoma)
-
2.
Palliation for local disease (e.g., mandibular lymphadenopathy, rectal lymphoma, mediastinal lymphoma where precaval syndrome is present, localized bone involvement)
-
3.
Total body radiation combined with bone marrow or stem cell reconstitution
-
4.
Whole or staged half-body RT after chemotherapy-induced remissions
In the latter case, staged half-body irradiation sandwiched between chemotherapy cycles or after attainment of remission by induction chemotherapy has been preliminarily investigated as a form of consolidation or maintenance.261, 344, 345, 349, 352, 353, 354 RT is delivered to either the cranial or caudal half of the body in 4 to 8 Gy fractions and, after a 2- or 4-week rest, the other half of the body is irradiated in a similar fashion. Although these preliminary investigations were not randomized, they suggest that RT applied when dogs are in either complete or partial remission is safe and warrants further investigation to determine whether significant therapeutic gain can be realized. A pilot study of low-dose (1 Gy) single-fraction total body irradiation in seven dogs with relapsed drug-resistant lymphoma, although safely applied, resulted in only partial nondurable (1–4 week) remissions.341
Total body irradiation (and/or ablative chemotherapy) for complete or partial bone marrow ablation followed by reconstitution with bone marrow or stem-cell transplant in dogs, although a recognized model in comparative research settings,355 is still in its early phases of development and application in clinical veterinary practice.356, 357, 358, 359 Because of the high cost, limited accessibility to relatively sophisticated equipment, and management requirements, these types of procedures are limited to preliminary investigations at a few centers. Currently, long-term results documenting significantly enhanced efficacy in sufficient numbers of treated cases have yet to be presented.
Treatment of Extranodal Lymphoma
In general, the veterinary literature contains little information on treating various extranodal forms of lymphoma in dogs, and our ability to predict outcome is thus limited. In general, it is recommended that, after extensive staging, in those cases where disease is shown to be localized to a solitary site, local therapies (e.g., surgery, local RT) can be used while withholding systemic therapies (i.e., chemotherapy) until systemic progression or recurrence is documented. In contrast, if multiple extranodal sites are involved or they are part of a more generalized process, systemic chemotherapy should be chosen.
Alimentary Lymphoma
Most dogs with alimentary lymphoma are presented with diffuse involvement of the intestinal tract, and involvement of local LNs and liver is common. Chemotherapy in dogs with diffuse intermediate- or high-grade disease has been reported to be unrewarding for the most part61, 126, 360, 361 with MSTs of only a few months after CHOP-based chemotherapy; however, durable remissions in a small subset of cases have been reported. A small-cell T-cell intestinal lymphoma has been reported in dogs that appears to have a more indolent course similar to small-cell T-cell intestinal lymphoma in cats.64, 125 In reports of 17 and 20 dogs, MSTs after receiving conservative treatment (prednisone and chlorambucil most commonly) were 1.5 to 2.0 years.64, 125 Solitary alimentary lymphoma is rare in the dog; however, if the tumor is localized and can be surgically removed, results (with or without follow-up chemotherapy) can be encouraging. Colorectal lymphoma, generally a high-grade B-cell phenotype, is also associated with an indolent outcome with median progression-free and overall survival times greater than 3 years after initiation of chemotherapy.362, 363
Primary Central Nervous System Lymphoma
CNS lymphoma in dogs usually results from extension of multicentric lymphoma; however, primary CNS lymphoma has been reported.87, 133, 135, 136, 364 If tumors are localized (rare), local RT should be considered. Few studies have reported the use of chemotherapy. In one study, cytosine arabinoside (Ara-C) at a dosage of 20 mg/m2 was given intrathecally; this treatment was combined with systemic chemotherapy and CNS RT.133 Overall, the response rates are low and of short duration (several weeks to months), although occasional durable responses are encountered.
Cutaneous Lymphoma
The cutaneous lymphomas represent an assorted group of clinical entities that vary considerably in presentation and outcome.71, 74, 77, 119, 129, 130, 365, 366 Epitheliotropic cutaneous lymphoma is most common and has been categorized into two clinically separate entities (mucocutaneous and cutaneous) based on outcome differences, with the mucocutaneous form appearing to have better overall outcomes.129 Treatment of cutaneous and mucocutaneous lymphoma depends on the extent of disease. Solitary lesions have a better prognosis and may be treated with surgical excision or RT, although thorough staging for systemic disease should be undertaken before local therapy and active surveillance for subsequent development of recurrent or systemic involvement should be implemented. Fractionated RT has been associated with long-term control.366, 367 Diffuse cutaneous lymphoma is best managed with systemic therapy, although the rate and durability of response is generally less than in multicentric lymphoma. The most widely used protocols for epitheliotropic and nonepitheliotropic cutaneous T-cell lymphoma (CTCL) include CCNU (with or without l-asparaginase [see Table 33.6]) along with prednisone, pegylated l-asparaginase (very costly), and oral retinoic acid analogs (limited availability; acitretin, etretinate, isotretinoin).269, 366, 368, 369, 370, 371, 372 Multiagent protocols (generally CHOP-based) may also be used, but have generally been instituted after single-agent therapies have failed. Although reported response rates can range from 40% to 80%, median remission durations are generally short (approximately 3–6 months); occasionally, durable remissions are encountered. Sporadic reports of other therapies for cutaneous lymphoma in small numbers of cases include the use of COAP (cyclophosphamide, vincristine [Oncovin], Ara-C, and prednisone), topical mechlorethamine (Mustargen), rabacfosadine, recombinant human interferon-alpha, and masitinib.132, 269, 368, 373 All these reports involved small numbers of cases and resulted in relatively short response durations. Whole-body surface RT has also been explored for the treatment of diffuse cutaneous lymphoma in preliminary trials.348, 374
A form of cutaneous lymphocytic infiltration has been characterized as an indolent T-cell lymphoma based on clonality.77 It is associated with slow progression and long-term survival after corticosteroid management; however, it does have the potential to progress to high-grade lymphoma.
Prognosis
The prognosis for dogs with lymphoma is highly variable and depends on a wide variety of factors that are documented or presumed to affect response to therapy. Although rarely curable (<10% of cases), CRs and a good quality of life during extended remissions and survival are typical. Factors that have been shown to influence treatment response and survival for peripheral nodal lymphoma are summarized in Tables 33.7 and 33.8 .a The prognostic factors most consistently identified for peripheral nodal lymphoma are immunophenotype, WHO substage (see Fig. 33.11B), and an indolent subclassification (Fig. 33.12 ). Many reports have confirmed that dogs with nonindolent intermediate- and high-grade T-cell lymphomas have significantly shorter remissions and survival durations than dogs with intermediate or high-grade B-cell disease.68, 69, 112, 113, 116, 277, 391 This holds true primarily for dogs with multicentric lymphoma because the immunophenotype of solitary or extranodal forms of lymphoma has not been thoroughly investigated with respect to prognosis. In addition, it has been shown that dogs with B-cell lymphomas that express lower than normal levels of B5 antigen (expressed in 95% of nonneoplastic lymphocytes) or low levels of class II MHC expression experience shorter remissions and survival durations.69, 391 Dogs presented with WHO substage b disease (i.e., clinically ill) also do poorly compared with dogs with substage a disease.68, 112, 116, 235, 387 Dogs with stage I and II disease have a better prognosis than those dogs in more advanced stages (stage III, IV, and V).
TABLE 33.7.
Prognostic Factors for Peripheral Nodal Lymphoma in Dogs
| Factor | Strong Association | Modest Association Requiring Further Investigation | Comments | References |
|---|---|---|---|---|
| Histopathology/Subclassification | X | High/intermediate-grade: unfavorable Indolent/low-grade: favorable |
29,90,96,98–102,107–113,116–119 | |
| Immunophenotype | X | T cell phenotype: unfavorable (except TZL) Low MHC II expression: unfavorable |
68,69,112,113,116,277,365,379,391 | |
| WHO clinical stage | X | Stage I/II: favorable Stage V with significant bone marrow involvement: unfavorable |
248,273,379,387 | |
| WHO clinical substage | X | Substage b (clinically ill): unfavorable | 68,112,116,235,379,387 | |
| Sex | X | Females/neutered females: unfavorable | 235,237 | |
| Genetic/gene expression analysis | X | Some signatures highly predictive of outcome | 14,20,29,378,384,385 | |
| Anatomic location | X | Leukemia, diffuse cutaneous and alimentary, hepatosplenic forms: unfavorable | See text for extra nodal sites | |
Peripheral blood counts at presentation
|
X |
X X X X |
Unfavorable Unfavorable Low: unfavorable Unfavorable Low: unfavorable |
277,375,376,379,386,390 376 379 375 382 |
| Molecular assessment of minimal residual disease (e.g., PCR, flow) | X | Likely to become more important when “curative” therapeutic approaches are developed and instituted | 123,217,287–290,293,393,416 | |
| Measures of proliferation | X | Contradictory reports exist | 123,177,380,388,389 | |
| Steroid pretreatment | X | Prolonged steroid pretreatment: unfavorable | 276,278 | |
| Cranial mediastinal lymphadenopathy | X | Present: unfavorable | 122 | |
| Chemotherapy-induced hematologic toxicity | X | Grade III or IV chemotherapy-induced neutropenias: favorable | 381,392 | |
| Geographic location | X | Mixed | 383 |
TABLE 33.8.
Circulating (Serum/Plasma) Biomarkers as Prognostic Indices in Dogs with Lymphoma
| Biomarker | Comments | References |
|---|---|---|
| Lactate dehydrogenase activity | Increased: unfavorable | 202,210 |
| Thymidine kinase activity | Increased: unfavorable | 202,205,206,396,397 |
| Haptoglobin | Increased: unfavorable | 202–204 |
| Serum VEGF | Increased: unfavorable | 213,417 |
| Glutathione-S-transferase | Increased: unfavorable | 418 |
| Hypercalcemia | Unfavorable | 116,395,419,420 |
| Serum cobalamin | Decreased: unfavorable | 395 |
| Serum albumin | Decreased: unfavorable | 375,377 |
| Serum C-reactive protein | Although it may be used to characterize remission status, variable levels preclude utility. | 202–205,294 |
VEGF, Vascular endothelial growth factor.
Fig. 33.12.
Kaplan–Meier curves illustrating time to relapse adjusted for clinical stage and immunophenotype among dogs treated for low-grade (n = 17) (blue line) or high-grade (n = 51) (red line) Kiel classification lymphoma.
From Teske E, van Heerde P, Rutteman GR, et al. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc. 1994;205:1722-1728.
Proliferative assays such as analysis of bromodeoxyuridine (BrdU) uptake, Ki67 antibody reactivity, and AgNOR indices to measure proliferative activity of tumor cells have been shown to provide prognostic information in dogs treated with combination chemotherapy; however, different studies are contradictory and the information is rarely helpful clinically. In addition, in one report, the proportion of tumor cells undergoing apoptosis was modestly predictive of remission duration.123, 174
The anatomic site of disease is also of considerable prognostic importance. Primary diffuse cutaneous, diffuse GI, hepatosplenic, and primary CNS lymphomas tend to be associated with a poor prognosis. Dogs with indolent cutaneous T-cell lymphocytic infiltration experience long-term survivals.77 Sex has been shown to influence prognosis in some studies.235, 237 Neutered females tend to have a better prognosis; male dogs may have a higher incidence of the T-cell phenotype, which may account for the poorer prognosis.
Reported biomarkers of prognosis, summarized in Table 33.8, include increased circulating levels of glutathione-S-transferase, thymidine kinase, lactate dehydrogenase, C-reactive proteins, and VEGF. Finally, one report suggests that a history of chronic inflammatory disease of several types predicts likelihood of early relapse.202, 398 These putative prognostic indicators require further confirmation in larger trials.
Of particular interest is the capacity of gene expression analysis to predict and indeed subcategorize the lymphomas into prognostic categories.14, 20, 178, 179, 384 Although these types of analysis are not currently widely available in the veterinary clinical setting, they are routinely used for prognostication and therapeutic decision making in people and are likely to become more readily available to veterinary clinicians in the next decade. The potential of genetic molecular profiling in veterinary oncology is illustrated by Frant et al178: they were able to subcategorize dogs with peripheral nodal lymphoma into three prognostic categories based on a benchtop quantitative real-time (qRT)-PCR diagnostic analysis of expression of only four genes.178
The Indolent Lymphomas
Histologic grade (subtype) greatly influences prognosis. Dogs with lymphoma classified as intermediate or high grade tend to respond to chemotherapy, but can relapse early. Dogs with low-grade indolent lymphomas have a poorer response to chemotherapy, yet have a survival advantage over dogs with intermediate- and high-grade lymphomas (see Fig. 33.12). Several case compilations have documented that dogs with indolent lymphoma (e.g., MZL, MCL, TZL) experience prolonged STs, often in the absence of any or aggressive chemotherapy; that is, they often enjoy prolonged survival despite intervention rather than because of intervention.100, 101, 102 One caveat is that canine nodal marginal zone lymphoma, which is designated as an indolent disorder, is generally more aggressive with median progression-free intervals of 5 months and overall STs of only 8.5 months; this is substantially less than splenic MZL, which is associated with long-term survival or cure after splenectomy.108 Many dogs with indolent lymphoma will live near normal life-spans and ultimately die of non–lymphoma-related disorders. Generally, with the indolent lymphomas, unless the presence of disease is having an effect on the quality of life or results in clinically significant cytopenias (myelophthisis), active surveillance in the absence of treatment is recommended. If treatment is deemed necessary, more conservative protocols (e.g., chlorambucil/prednisone or cyclophosphamide/prednisone) are initiated. The goal of therapy is to control the disease (stable disease or partial response) as CRs are unusual with the indolent lymphomas.
Lymphocytic Leukemia
Lymphocytic leukemia is typically defined as proliferation of neoplastic lymphocytes in bone marrow. Neoplastic cells usually originate in the bone marrow, but occasionally in the spleen, and may or may not be circulating in the peripheral blood. Our ability to diagnose lymphocytic leukemias using morphologic, flow cytometric, immunophenotypic, and cytochemical techniques has increased significantly in the past decade. Although little information on treatment and prognosis is available for acute lymphocytic leukemias (ALL), clinically relevant information on chronic lymphocytic leukemias (CLL), their prognosis, and treatment have recently come to light. Differentiating between true leukemia and stage V lymphoma can be difficult and arbitrary, and is often based on lack of significant lymphadenopathy, degree of blood and bone marrow involvement, and immunophenotypic characteristics.
Incidence, Risk Factors, and Etiology
Lymphocytic leukemia is more common than acute myeloid leukemia and myeloproliferative disorders, but the true incidence is unknown. Overall, CLL is much more common than ALL. Smaller reports state that German shepherd dogs and golden retrievers may be overrepresented.170, 399 Based on a recent compilations of more than 400 dogs with B-cell CLL (B-CLL), small-breed dogs are much more likely to be affected.106 Lymphocytic leukemia can occur in dogs of any age, but typically occurs in middle-aged to older dogs (mean of 7–10 years of age); CLL usually occurs in older dogs (mean of 10–11 years of age),106, 170, 394, 399, 400 although a distinct form of B-CLL in English bulldogs occurs in younger dogs (mean, 6 years of age).106 A significant sex predilection is not reported. As with lymphoma, the etiology of lymphocytic leukemia is for the most part unknown. Genetic factors likely play a role and have been compared between dogs and humans.12
Pathology and Classification
Lymphocytic leukemias can be subdivided based on cell size, maturity, genetic aberrations, microRNA expression, and immunophenotype.12, 170, 180, 394, 399, 400, 401, 402 The simplest classification divides leukemia into two groups: chronic (small cells with a mature cytologic phenotype) and acute (large cells with an immature cytologic phenotype). Immunophenotypic assessment using flow cytometric and molecular assays can further characterize these two major subtypes.
Of the CLLs, approximately two-thirds are T cell (T-CLL) and one-third are B-CLL. Three primary subtypes of CLL, based primarily on immunophenotyping, have been reported106, 164, 170, 394, 400: (1) T-CLL, which is the most common form, with cells in the majority of cases being CD3+/CD8+ granular lymphocytes; (2) B-CLL (CD21+), which is the next most common subtype; and (3) atypical CLL, which represents a combination of immunophenotypes (CD3−, CD8+; CD3+, CD4−, CD8−; CD3+, CD4+, CD8+, and CD3+ + CD21+). This is in contrast to CLL in humans, which is primarily a disease of B cells. In CLL, lymphocytes often are indistinguishable morphologically from normal small lymphocytes (Fig. 33.13 ) and have a low rate of proliferation; accumulation of lymphocytes likely results from their prolonged lifespan.
Fig. 33.13.
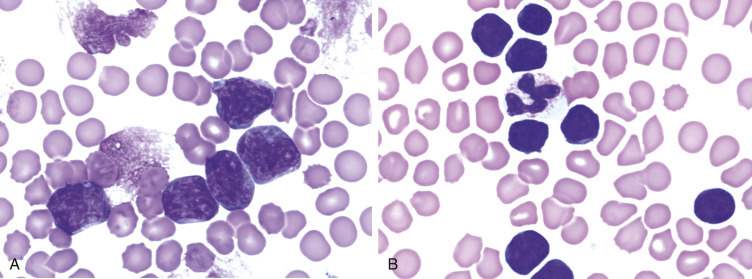
(A) Peripheral blood from a dog with acute lymphocytic leukemia (ALL). Note the large lymphoid cells with visible nucleoli. Chromatin from disintegrated cells also is visible. (Wright’s stain, ×60 objective.) (B) Peripheral blood from a dog with chronic lymphocytic leukemia (CLL). Note the small lymphocytes of normal mature morphology (smaller than the neutrophil). (Wright’s stain, ×60 objective.)
ALL has also been classified as a lymphoid precursor neoplasm and can be derived from either B cells (B-ALL) or T cells (T-ALL). The majority are B-ALL (CD21+, CD3−, CD4−, CD8−), although a smaller percentage (<10%) are T-ALL (CD3+, CD4−, CD8−, CD21−).170 In general, these cells tend to be intermediate or large cells with moderate amounts of basophilic cytoplasm. Perhaps the most distinguishing feature of the large lymphocytes is the nuclear chromatin pattern, which typically is less condensed than the chromatin in small lymphocytes but more condensed than the chromatin in myeloblasts. Large lymphocytes are larger than neutrophils, have a high nuclear-to-cytoplasmic ratio, and contain basophilic cytoplasm (see Fig. 33.13). Nucleoli, although present, are less prominent in large lymphocytes than in myeloblasts. Nevertheless, these cells cannot be distinguished easily from immature cells of other hematopoietic lineages, and identification of lineage-specific markers by immunocytochemical, flow cytometric, or molecular/genetic analysis is required to ascertain their lineage.401, 403, 404 If the cells express CD34, a stem-cell marker, an acute phenotype is implied170, 394, 399; however, both myeloid and lymphoid lineages express CD34 and our ability to differentiate ALL from acute myeloid leukemia relies on detection of other markers, including T- and B-cell markers and myeloperoxidase, a myeloid marker. Furthermore, some T-ALLs do not express CD34.404
History and Clinical Signs
Dogs with CLL are often asymptomatic and a diagnosis is pursued based on an incidental finding of increased circulating mature lymphocytes on routine CBCs. In more advanced disease, some owners report lethargy and decreased appetite. Mild lymphadenopathy and splenomegaly may be present, although late in the disease splenomegaly may be marked.405 In a compilation of nearly 500 dogs with B-CLL, 50% had some degree of peripheral lymphadenopathy, 50% had splenomegaly, 30% had hepatomegaly, 23% had visceral lymphadenopathy, and 3% had a mediastinal mass.106 The white blood cell (WBC) count is usually >30,000 cells/μL but can vary from normal to more than 100,000 cells/μL because of an increase in circulating mature lymphocytes. Lymphocytosis is persistent, and granulocytes are usually present in normal numbers. Other than lymphocytosis, hemograms of dogs with CLL tend to have few abnormalities when lymphocytes are less than 30,000/μL.170, 394, 400 Mild anemia, neutropenia, and thrombocytopenia are common, but may become marked as the disease progresses and lymphocyte counts increase above 30,000/μL. In B-CLL, the median lymphocyte count was 24,600/μL and neutropenia and thrombocytopenia were uncommon (1% and 7%, respectively).106 Despite the well-differentiated appearance of the lymphocytes in CLL, these cells may function abnormally. Paraneoplastic syndromes include monoclonal gammopathies, immune-mediated hemolytic anemia, pure red cell aplasia, and, rarely, hypercalcemia; 80% of dogs with B-CLL were reported to be hyperglobulinemic and 13% were hypercalcemic.106, 406, 407 In 22 dogs with CLL, 68% had monoclonal gammopathies (usually IgM or IgA).407 The immunophenotypes were not reported in this latter report, but a monoclonal gammopathy would be more likely to occur in B-CLL.
Dogs with ALL usually present with clinical signs of anorexia, weight loss, and lethargy. Splenomegaly is typical and other physical abnormalities may include hemorrhage, lymphadenopathy, and hepatomegaly.408 Infiltration of bone marrow by neoplastic lymphocytes may be extensive, resulting in significant depression of normal hematopoietic elements or myelophthisis.170, 394, 399, 404, 408, 409 Anemia, neutropenia, and thrombocytopenia are typically much more severe than with CLL and may become life threatening. Infiltration of extramedullary sites, such as the CNS, bone, and GI tract, may also occur and can result in neuropathies, bone pain, and GI signs, respectively.
Diagnostics and Clinical Staging
Consideration of signalment, history, physical findings, and morphologic appearance and immunophenotype of cells is essential in making an accurate diagnosis. It is helpful to know the profile of lymphocyte subsets in the peripheral blood of normal dogs to determine whether a particular subset has expanded. Approximately 80% of circulating lymphocytes in normal dogs are T cells and about 15% are B cells. NK cells and double-negative (CD4−, CD8−) T cells constitute the remaining fraction. In the T-cell fraction, helper T cells (CD4+) outnumber cytotoxic T cells (CD8+).407 Lymphocytic leukemia should be a consideration if atypical lymphocytes are in circulation, the immunophenotype of the lymphocytes in circulation is homogeneous as determined by flow cytometric analysis, a phenotype typically present in low frequency has increased, or if clonality is documented (e.g., by PARR analysis). Other differential diagnoses for lymphocytosis include infectious diseases, such as chronic ehrlichiosis, postvaccinal responses in young dogs, IL-2 administration, and transient physiologic or epinephrine-induced lymphocytosis. In some cases, reactive and neoplastic lymphocytoses are difficult to distinguish.
Expansion of neoplastic lymphocytes in bone marrow is the hallmark of ALL and, in most cases, CLL. Careful examination of peripheral blood and bone marrow by an experienced cytopathologist is important in establishing a diagnosis of lymphocytic leukemia; in cases of marked lymphocytosis with atypia, peripheral blood can be used for analysis of immunophenotype and clonality, and examination of bone marrow is not essential. If diagnostic bone marrow cannot be adequately obtained by aspiration, bone marrow core biopsy should be performed. In ALL, large lymphocytes predominate in the bone marrow and are also present in peripheral blood, and other lineages are decreased. In B- and T-cell CLL, lymphocytes are small mature cells that occur in excessive numbers in bone marrow (≥30% of all nucleated cells) early in the disease.405 In T-CLL, lymphocytes may contain pink granules. Infiltration becomes more extensive as the disease slowly progresses, and eventually the neoplastic cells replace normal marrow.
A separate clinical staging system has not been developed for lymphocytic leukemias. Currently, all dogs with leukemia are classified as stage V based on the WHO Staging System for lymphoma as presented in Box 33.2.
Treatment of Chronic Lymphocytic Leukemia
Because of the indolent and often asymptomatic nature of CLL, the decision to treat is often based on the clinical and laboratory findings in the individual dog. Most oncologists recommend active surveillance (monthly or bimonthly physical examination and CBC) over active therapy when CLL is identified incidentally, there are no accompanying clinical signs, and other significant hematologic abnormalities are not identified. If the dog is significantly anemic or thrombocytopenic or is showing evidence of significant lymphadenopathy or hepatosplenomegaly, therapy should be considered. There is no consensus on what degree of lymphocytosis is used to initiate therapy; the definition of “excessively high” varies among oncologists, and a standard has not been established in veterinary medicine. The author (DMV) prefers to base treatment decisions on the presence of significant constitutional signs and peripheral cytopenias rather than an absolute lymphocyte count. Currently, the most effective drug available for treatment of CLL, once therapy is deemed necessary, is chlorambucil.400, 405 Chlorambucil is given orally at a dose of 0.2 mg/kg or 6 mg/m2 PO once daily for 7 to 14 days; the dose can then be reduced to 0.1 mg/kg or 3 mg/m2 PO daily. For long-term maintenance, a dose of 2.0 mg/m2 every other day can be used. The dose is adjusted based on clinical response and bone marrow tolerance. Pulse-dose chlorambucil (20–30 mg/m2 q 2 weeks) has been anecdotally used for CLL in some cases; however, no compilations of cases have been reported to assess effectiveness of this protocol. Oral prednisone is used concurrently with chlorambucil at doses of 1 mg/kg daily for 1 to 2 weeks, then 0.5 mg/kg every other day thereafter. The addition of vincristine or the substitution of cyclophosphamide for chlorambucil has been advocated in animals that do not respond to chlorambucil.
Treatment of CLL, once initiated, is primarily palliative with rare complete remissions and a uniformly fatal course. Owing to the indolent nature of this disease, however, STs have been in the range of 1 to 3 years with a good quality of life.400, 405, 410 The phenotype of CLL is usually stable over months to years; however, the disease may evolve into an acute phase, and some dogs will develop a form of lymphoma that is rapidly progressive and characterized by the presence of pleomorphic large lymphocytes411, 412; in humans, this is termed Richter’s syndrome. 413 Based on a data set of 153 cases, 2% of T-CLLs and 10% of B-CLLs progressed to a Richter-like acute disease often characterized by lymphadenopathy, coughing, vomiting, weight loss, and neurologic signs.412 In these eight dogs, the progression to acute disease occurred 2 to 16 months after initial diagnosis of their CLL and MST after this progression was only 41 days despite aggressive (CHOP-based) chemotherapy in half of the cases.412
Treatment of Acute Lymphocytic Leukemia
Much of the morbidity in dogs with ALL results from effacement of bone marrow (myelophthisis) and subsequent life-threatening peripheral cytopenias. Neutropenia, thrombocytopenia, and anemia may be severe. Dogs often require supportive therapy, such as fresh whole-blood transfusions, broad-spectrum antibiotics, fluid therapy, and nutritional support. Careful monitoring for sepsis, hemorrhage, and DIC is important. Specific treatment of ALL would require aggressive chemotherapy; however, consistently efficacious protocols have not been developed in veterinary medicine, and there are few published reports. CHOP-based protocols, similar to those used for lymphoma (see Table 33.4), have been used by the author (DMV) and others for dogs with ALL; however, responses and durability of response are generally disappointing. The standard of care in humans with acute leukemia generally involves bone marrow ablative treatments with stem cell or marrow replacement, technology that is not widely available or often pursued in veterinary oncology.
Prognosis
In general, CLL is an indolent disease (with the aforementioned exception of atypical CLL), and many dogs will not require therapy for some time after diagnosis; several dogs have been reported to survive a year or more without treatment.400, 414 For those dogs that are treated, normalization of lymphocyte counts can be expected in 70% of cases. In one report of 17 dogs treated with vincristine, chlorambucil, and prednisone, MST was approximately 12 months with an expected 30% survival at 2 years.405 In larger compilations of cases that include immunophenotypic analysis, treatment protocols were poorly documented, although most received chlorambucil and prednisone. The immunophenotype of CLL has been shown to be prognostic; in a report of 43 cases, MSTs of 930 days, 480 days, and 22 days were reported for T-CLL, B-CLL, and atypical CLL, respectively.400 In this group of dogs, young age and anemia were also associated with a poor prognosis. In another series with limited treatment information, dogs with CLL of a CD8+ immunophenotype that presented with less than 30,000 lymphocytes/μL or greater than 30,000 lymphocytes/μL had MSTs of 1098 and 131 days, respectively.400
Prognosis for dogs with ALL is generally very poor. In a study of 21 dogs treated with vincristine and prednisone, dogs achieving complete or partial remission (29%) had an MST of 120 days, and few dogs survived longer than 8 months.408 In one report of 46 cases of ALL with a CD34+ phenotype, dogs had a MST of 16 days (range, 3–128 days), even though the majority received a CHOP-based treatment protocol.394 In addition, dogs with B-cell ALL (CD21+) in which the lymphocytes were large cells (forward scatter lymphocyte/forward scatter neutrophil ratio of greater than 0.58 by flow cytometric analysis) had a MST of only 129 days, independent of treatment protocol.394
Section B: Feline Lymphoma and Leukemia
David M. Vail and Marie Pinkerton
Lymphoma
Lymphoma (malignant lymphoma or lymphosarcoma) comprises a diverse group of neoplasms that have in common their origin from lymphocytes. The neoplasms usually arise in lymphoid tissues such as lymph nodes, spleen, and bone marrow; however, they may arise in almost any tissue in the body. Lymphoma is one of the most common neoplasms seen in the cat. See Box 33.4 .
BOX 33.4. Key Clinical Summary Points: Feline Lymphoma.
-
•
A varied group of lymphoid cancers discussed in three major groups based on varied presentation, diagnosis, management, and outcome (Table 33.9): Alimentary/Gastrointestinal, Peripheral Nodal, and Extranodal.
-
•
Feline leukemia virus (FeLV) infection, a major etiologic agent of the disease, is no longer relevant in the majority of cases because of viral elimination and vaccination programs instituted in the 1980s.
-
•
The alimentary/gastrointestinal forms predominate, and distinct subtypes exist representing both indolent (majority) and aggressive (intermediate-/high-grade) lymphomas (Table 33.10).
-
•
Indolent (low-grade) subtypes in any anatomic site are managed with conservative chemotherapy (chlorambucil and prednisolone), with high response rates and durable survival durations (≥1.5–3 years) expected.
-
•
Intermediate- or high-grade subtypes of any anatomic site are managed with more aggressive CHOP- or COP-based chemotherapy protocols (TABLE 33.11, TABLE 33.12), with moderate response rates (50%–65%) and less durable (<1 year) survival durations expected.
-
•
Cats with intermediate- or high-grade lymphoma that achieve a complete response with chemotherapy (approximately 35% of cases) often enjoy more durable (>1 year) survival durations.
-
•
Solitary forms of lymphoma may be managed with local therapies (i.e., surgery, radiation therapy) provided thorough staging has ruled out systemic spread and active surveillance for identifying recurrence is instituted.
Incidence
Epidemiologic reports before 1990 suggested that lymphoma accounted for 50% to 90% of all hematopoietic tumors in the cat,421, 422 and because hematopoietic tumors (lymphoid and myeloid) represent approximately one-third of all feline tumors, it was estimated that lymphoid neoplasia accounted for an incidence of 200 per 100,000 cats at risk.423 In one series of 400 cats with hematopoietic tumors, 61% had lymphoma and 39% had leukemias and myeloproliferative diseases (MPDs), of which 21% were categorized as undifferentiated leukemias, most likely myeloid in origin.424 However, a significant change in the epidemiology and characteristics of lymphoma in cats coincided with the widespread integration of feline leukemia virus (FeLV) diagnostic assays and affected animal elimination regimens of the late 1970s and 1980s, and was further enhanced by the commercially available FeLV vaccines appearing in the late 1980s. The decline in FeLV-associated lymphoma is mirrored by a global decline in the overall prevalence per year of FeLV positivity in cats tested.425, 426, 427, 428, 429 Importantly, many of these studies reveal that despite a sharp drop in FeLV-associated lymphoma, the overall prevalence of lymphoma in cats is increasing. The increased prevalence appears because of an increase in the number and relative frequency of the alimentary (and, in particular, the intestinal) and extranodal anatomic forms of lymphoma.426, 430, 431, 432, 433 This is supported by an epidemiologic survey of several hundred cases of feline intestinal lymphoma; 534 (86%) were from the 20 years after 1985 and only 14% were from cases diagnosed in the 20 years before 1985.432 This change in incidence has also been observed in Europe.426, 431
The true annual incidence rate for lymphoma in cats is currently unknown. With respect to feline pediatric (<1 year of age) tumors, a study in the United Kingdom (n = 233 pathology specimens) found that 73 (31%) represented hematopoietic tumors, of which 51 (70%) were lymphoma; note that FeLV status was unavailable for this compilation of cases.433
The typical signalment for cats with lymphoma cannot be uniformly stated as it varies widely based on anatomic site and FeLV status, and therefore will be discussed individually under site-specific discussions. In general, based on two large compilations (n = 850) of cases in North America and Europe,427, 428 domestic shorthair (DSH) cats are most commonly affected and Siamese cats appear overrepresented in some reports. A 1.5:1 male-to-female ratio was observed in two studies, with no association with gender or neutering status observed in one.427, 428 In a large compilation of Australian cases, male cats and the Siamese/oriental breeds were overrepresented,434 and similar breed findings have been observed in North America, although similar gender predilections have not been found. Within the Siamese/oriental breeds, there appears to be a predisposition for a mediastinal form that is not FeLV-associated and represents a younger population (median of 2 years).
Etiology
Viral Factors
FeLV was the most common cause of hematopoietic tumors in the cat in the so-called “FeLV era” of the 1960s through the 1980s, when approximately two-thirds of lymphoma cases were associated with FeLV antigenemia. Several studies have documented the potential molecular means by which FeLV can result in lymphoid neoplasia (see Chapter 1, Section C). As one would predict, along with a shift away from FeLV-associated tumors came a shift away from traditional signalment and relative frequency of anatomic sites. This is also supported outside of North America by similar signalment and anatomic frequency data observed in Australia, where FeLV infection is less common, and in Europe, where FeLV incidence has declined and signalment and anatomic site prevalence has shifted accordingly.426, 431, 434, 435 The median age of approximately 11 to 12 years now reported in North America and Europe is considerably higher than the median ages of 3 to 5 years reported in the FeLV era.421, 422, 426, 427, 428, 429, 431 The median age of cats within various anatomic tumor groupings has not changed, and anatomic forms traditionally associated with FeLV, such as the mediastinal form, may still occur in younger, FeLV antigenemic cats. Similarly, the alimentary form and extranodal forms occur most often in older, FeLV-negative cats.436, 437, 438, 439, 440, 441 Table 33.9 presents an overview of the characteristics of the various anatomic sites of lymphoma in cats. As our ability to interrogate FeLV associations on a molecular basis has improved (e.g., PCR amplification and fluorescent in situ hybridization), several reports exist defining the role or potential role of FeLV in cats with and without FeLV antigenemia.442, 443, 444, 445, 446, 447 Collectively, these studies indicate FeLV proviral insertion exists in a proportion of feline lymphoma tissues and is more common in those of T-cell origin, particularly the thymic and peripheral lymph node anatomic forms. They also suggest that several common FeLV integration sites exist.
TABLE 33.9.
General Characteristics of the Most Commonly Encountered Anatomic Forms of Lymphoma in Cats
| Anatomic Form | Relative Frequency | Median Age (yr) | Immuno-Phenotype (Generally) | FeLV Antigenicity | Local versus Diffuse/Multicentric | Biologic Behavior | General Prognosis |
|---|---|---|---|---|---|---|---|
| Alimentary/ Gastrointestinal LGAL I/HGAL LGL |
Common Uncommon Uncommon |
10–13 12 9 |
T-cell, small B-cell, large T-cell, large |
Rare Rare Rare |
Diffuse Generally Diffuse Generally Diffuse |
Indolent Aggressive Aggressive |
Good Poor–Fair Poor |
| Nasal | Uncommon | 9–10 | B cell (75%) | Rare | Local common | More indolent | Good–Fair |
| Mediastinal | Uncommon | 2–4 | T-cell, large | More common | Local common | Indolent or aggressive forms | Fair–Poor |
| Peripheral nodal Non-Hodgkin’s Hodgkin’s-like |
Uncommon Rare |
3–4 11 |
B-cell (75%), large T-cell rich B-cell, large |
More common Rare |
Multicentric Local initially |
Aggressive Indolent |
Fair–Poor Good– Fair |
| Laryngeal/Tracheal | Rare | 9 | ID | Rare | Local common | ID | Fair–Good |
| Renal | Uncommon | 9 | B-cell | Rare | Multicentric | Aggressive | Poor–Fair |
| CNS | Rare | 4–10 | ID | Rare | Multicentric | Aggressive | Poor |
| Cutaneous | Rare | 10–13 | T-cell | Rare | Local initially | Indolent to aggressive | Fair |
| Subcutaneous | Rare | 10–13 | B-cell, large | Rare | Local initially | Aggressive | Fair |
| Ocular (PSOL) | Rare | 10–11 | B-cell | Rare | Local | Often indolent | Good–Fair |
Common = >50% of clinical presentations; Moderate = 20%–50% of clinical presentations;
Uncommon = 5%–20% of clinical presentation; Rare = <5% of clinical presentations.
ID, Insufficient data; I/HGAL, intermediate-/high-grade alimentary lymphoma; LGAL, low-grade alimentary lymphoma; LGL, = large granular lymphoma; PSOL, presumed solitary ocular lymphoma.
There is also evidence that feline immunodeficiency virus (FIV) infection can increase the incidence of lymphoma in cats.425, 448, 449, 450, 451, 452, 453, 454, 455, 456 In contrast to the direct role of FeLV in tumorigenesis, most evidence suggests an indirect role for FIV secondary to the immunosuppressive effects of the virus. Shelton et al determined that FIV infection alone in cats was associated with a five-fold increased risk for development of lymphoma.454 Coinfection with FeLV further potentiates the development of lymphoproliferative disorders. Experimentally, cats infected with FIV have developed lymphoma in the kidney, alimentary tract, liver, and multicentric sites. FIV-associated lymphoma is more likely to be of B-cell immunophenotype, as opposed to the T-cell predominance associated with FeLV. It has been suggested that FIV infection may be associated more commonly with alimentary lymphoma of B-cell origin,449, 457 and this may be related to chronic dysregulation of the immune system or the activation of oncogenic pathways; however, FIV antigenemia was only rarely associated with alimentary lymphoma in other large compilations of cases.428, 458, 459, 460, 461
Interrogations of gammaherpesvirus 1(FcGHV1) in cats with lymphoma did not show an association; however, FcGHV1 antigenemia was associated with an overall poorer prognosis for cats with lymphoma, the causality of which is speculative.462
Genetic and Molecular Factors
As discussed in Section A of this chapter (canine lymphoma), recent advances in molecular cytogenetics (see also Chapter 1, Section A, and Chapter 8), including gene microarray techniques, have and are currently being applied to investigations of chromosomal aberrations and gene expression changes in veterinary species with lymphoma. Indeed, a predisposition of the oriental cat breeds to develop lymphoma suggests a genetic predisposition and indicates heritable risk.427, 435, 436 Altered oncogene/tumor suppressor gene expression, epigenetic changes, signal transduction, and cell death-pathway alterations are common in lymphomas of humans and are likely also involved in the cat. Several genetic factors have already been discussed as they relate to FeLV associations. In addition, N-ras aberrations have been implicated, although they are rare in cats.463 Furthermore, telomerase activity (see Chapter 2) has been documented in feline lymphoma tissues.464, 465 Alterations in cellular proliferation and in cell-cycle and death (apoptosis) pathways, in particular the cyclin-dependent kinase cell-cycle regulators and the Bcl-2 family of proapoptotic and antiapoptotic governing molecules, have also been implicated in feline lymphoma.466, 467, 468
Environmental Factors
Evidence for exposure to environmental tobacco smoke (ETS) as a risk factor for lymphoma in humans has prompted investigations in cats. In one report, the relative risk of developing lymphoma in cats with any exposure to ETS and with 5 or more years of exposure to ETS was 2.4 and 3.2, respectively.469 A large European study documenting an association between proximity of waste management and cancer in dogs failed to show increased risk in cats.470
Immunosuppression
Immune system alterations in the cat, such as those accompanying FIV infection, has been implicated in the development of lymphoma.450, 452, 454, 471 As is the case in immunosuppressed human organ transplantation patients, reports of immunosuppressed feline renal transplant recipients document increased risk of lymphoma after transplant and associated immunosuppressive therapy.472, 473, 474 In these studies, approximately 10% of transplanted cats developed de novo malignant lymphoma and, in one report, all cats had intermediate- or high-grade multicentric B cell lymphoma.
Chronic Inflammation
Although definitive proof is lacking, there is a growing body of indirect evidence to suggest that lymphoma can be associated with the presence of chronic inflammation, which theoretically could be the case with intestinal and nasal lymphoma. In particular, an association has been suggested between intestinal lymphoma and inflammatory bowel disease427, 475; however, others have not found support for this concept.476 In addition, an association between gastric Helicobacter infection and gastric mucosa-associated lymphoid tissue (MALT) lymphoma in cats is suggested in one study, and it warrants further investigation because this is a recognized syndrome in humans.477, 478 In a case-control study investigating mucosa-invading and intravascular bacteria in feline intestinal lymphomas, statistically significant differences were found in prevalence of mucosa-invading bacteria, which were identified in 82%, 18% and 3% of large cell lymphoma, small cell lymphoma and lymphocytic-plasmacytic enteritis biopsy samples, respectively.477 Furthermore, intravascular bacteria were only present in cases of large cell intestinal lymphoma (29% of cases). The etiologic significance of this has not been explored, but is also warranted. Finally, a suggestion that chronic inflammation from injection sites may be involved in the risk of developing subcutaneous lymphoma in cats,431 similar to its documented association with subcutaneous soft tissue sarcomas.
Diet and Intestinal Lymphoma
Although no direct evidence exists, a link between diet and the development of intestinal lymphoma in cats has been suggested.427 Support is offered by the relative and absolute increase in the alimentary form of lymphoma in the past 20 years and the fact that several dietary modifications in cat food have occurred in a similar timeframe in response to diseases, such as urinary tract disease. Further investigation is warranted to prove or disprove such assertions.
Pathology and Natural Behavior
Lymphoma can be classified based on anatomic location and histologic and immunophenotypic (flow cytometric or immunohistochemical) criteria; often, the two are intimately associated because certain histologic and immunophenotypic types are commonly associated with specific anatomic locations (see Table 33.9). The interrogation of lymphoma subtypes by flow cytometric analysis, PARR clonality analysis and genetic characterization, as is the standard of practice for human lymphoma and becoming so in canine lymphoma, is less well described and applied in the feline lymphomas, partly due to the prevalence of intrabdominal anatomic forms making sampling more difficult and partly due to variable PCR primer availability and sensitivity.479, 480 The largest compilation of feline cases subjected to rigorous histologic classification was reported by Valli and others using the NCI Working Formulation.481 The WHO has also published a histologic classification system that uses the REAL system as a basis for defining histologic categories of hematopoietic tumors in domestic animals.482, 483 This system incorporates both histologic criteria and immunohistologic criteria (e.g., B- and T-cell immunophenotype) and was discussed in length in Section A of this chapter. The updated Kiel classification system has also been used to classify feline lymphoma.484, 485 Regarding anatomic location, a profound change in presentation, signalment, FeLV antigenemia, immunophenotype, and frequency of anatomic sites has occurred in cats with lymphoma in the “post-FeLV” era (see Table 33.9). Because of this shift, characteristics of feline lymphoma discussed in this chapter will be primarily limited to reports collected from cases presenting after 1995.
Several anatomic classifications exist for lymphoma in the cat, and some categorize the disease as mediastinal, alimentary, multicentric, nodal, leukemic, and individual extranodal forms. Others have combined various nodal and extranodal forms into categories of atypical, unclassified, and mixed, and others have combined intestinal, splenic, hepatic, and mesenteric nodal forms into one category termed intraabdominal. Some discrepancies in the discussion of frequency will inevitably result from the variations in classification used in the literature. The relative frequency of anatomic forms and their associated immunophenotype may also vary with geographic distribution and may be related to genetic and FeLV strain differences, as well as prevalence of FeLV vaccine use. For the purposes of this chapter, the feline lymphomas will be discussed in three separate sections: alimentary/gastrointestinal, peripheral nodal, and extranodal. Signalment, clinical presentation, diagnosis, treatment, and prognosis will be discussed individually under each of these three sections.
Alimentary/Gastrointestinal Lymphoma
Lymphoma is the most common tumor type found in the GI tract of cats, representing 55% of cases in an epidemiologic survey of 1129 intestinal tumors in the species.432 The Siamese breed is reported at increased risk; however, the majority of cases occur in DSH cats.427, 432, 436, 441 Although lymphoma may occur in cats of any age, it is primarily a disease of aged cats with a mean of 10 to 13 years for T-cell alimentary lymphoma and 12 years for B-cell lymphoma.427, 432, 441, 486, 487 Alimentary/GI lymphoma can be confined to intestinal/gastric infiltration or a combination of intestinal, mesenteric lymph node, and hepatosplenic involvement. Uncommonly, cats may be presented with extraabdominal coinvolvement. The tumors can be solitary, but more commonly are diffuse throughout the intestines. No consistent sex bias is noted. Anatomically, alimentary lymphoma is nearly four times more likely to occur in the small intestine than the large intestine.486 In a series of colonic neoplasia in cats, lymphoma was the second most common malignancy (41%), second only to adenocarcinoma.459 Most feline GI lymphomas can be categorized into one of three types based on histopathology and immunohistopathology: (1) low-grade alimentary lymphoma (LGAL), (2) intermediate- or high-grade alimentary lymphoma (I/HGAL), and (3) large granular lymphoma (LGL). Salient, generalizable characteristics of each are presented in Table 33.10 .
TABLE 33.10.
Characteristics of the Three Most Common Forms of Alimentary/Gastrointestinal Lymphoma in Cats
| Characteristic | Low-Grade Alimentary Lymphoma (LGAL) | Intermediate-/High-Grade Alimentary Lymphoma (I/HGAL) | Large Granular Lymphoma (LGL) |
|---|---|---|---|
| Incidence | 50%–80% of cases | ≈20% of cases | ≈10% of cases |
| Clinical presentation | Nonspecific gastrointestinal signs (anorexia, weight loss, diarrhea, inappetence) | Nonspecific gastrointestinal signs; vomiting common if gastric; hematochezia more common if large bowel | Nonspecific gastrointestinal signs; vomiting more common |
| Clinical course | Indolent clinical progression | Acute clinical progression | Acute clinical progression |
| Abdominal palpation | Generally normal, modest intestinal thickening and abdominal lymphadenopathy possible | More common to palpate gastric/intestinal mass, mesenteric lymphadenopathy, organomegaly | More common to palpate gastric/intestinal mass, mesenteric lymphadenopathy |
| Abdominal ultrasound findings | Often unremarkable; diffuse intestinal wall thickening if present is limited to muscularis propria /submucosa; normal intestinal wall layering; mild lymphadenopathy/organomegaly possible | More commonly thickened transmural intestinal wall; loss of normal intestinal wall layering; mass effect more likely; mesenteric lymphadenopathy more likely | More commonly thickened transmural intestinal wall; loss of normal intestinal wall layering; mass effect more likely; mesenteric lymphadenopathy more likely; effusion uncommon but more likely |
| Topographya |  |
 |
 |
| General diagnostics | Cytology generally not helpful; biopsy (full thickness preferred, but endoscopic helpful) with histopathology, immunophenotype, and clonality analysis often helpful to differentiate from LPE | Cytology (mass/lymph node) often diagnostic; biopsy with histopathology, immunophenotype, and clonality analysis less commonly required. | Cytology (mass/lymph node) often diagnostic; biopsy with histopathology, immunophenotype, and clonality analysis less commonly required. |
| Cell size | >80% small, <20% large | >90% intermediate/large | Intermediate/large |
| Immunophenotype | >80% T-cell (CD3+) | ≈100% B-cell (CD79a+) | Cytotoxic T-cell (CD3+/CD8+/CD79a–), or NK cell (CD3–/CD79a–); often CD103+ and granzyme B+ |
| Clonality | >90% clonal or oligoclonal | >70% clonal or oligoclonal | >90% clonal or oligoclonal |
| WHO EATCL classification | 90% type II (mucosal) 10% type I (transmural) |
90% type I (transmural) 10% type II (mucosal) |
≥90% type I (transmural) |
| Epitheliotropism | Common | Rare | Common |
| Recommended treatment | Chlorambucil/prednisolone | CHOP- or COP-based chemotherapy; surgery considered if large discreet lesion prechemo; surgery performed if obstruction/perforation | CHOP- or COP-based chemotherapy; surgery considered if large discreet lesion prechemo; surgery performed if obstruction/perforation |
| Chemotherapy response and outcome | >80% response; median survival 1.5–3 years | ≈50%–60% response (30% CR); median survival 3–10 months; more durable if CR | ≈30% response; median survival 45–90 days; occasionally more durable |
CR, Complete response; LPE, lymphocytic-plasmacytic enteritis; WHO EATCL, World Health Organization enteropathy-associated T-cell lymphoma.
Topography diagrams used with permission from: Moore PF, Rodriguez-Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol. 2012;49:658-668.
Numbers indicate number of cases having lymphoma at that location. Areas in red indicate most commonly affected regions of the intestine.
Pathology and Natural Behavior
Low-Grade Alimentary Lymphoma
It is now clear that the vast majority of LGALs represent mucosal, epitheliotropic, small T-cell immunophenotypes that arise primarily from MALT.436, 441, 487, 488, 489, 490, 491, 492 Thus the major differential for LGAL is benign lymphocytic-plasmacytic enteritis (LPE; commonly referred to as inflammatory bowel disease [IBD]), which is also most commonly characterized by a small, T-cell, epitheliotropic infiltration. The largest compilation of LGAL (n = 120) classified GI lymphoma based on immunophenotype, then as either mucosal (infiltrate confined to mucosa and lamina propria with minimal submucosal extension) or transmural (significant extension into submucosa and muscularis propria).487 They then compared infiltration patterns with the WHO classification scheme493 as well as documenting anatomic location, cell size, presence of epitheliotropism, clonality, and outcome data. This information is summarized in Table 33.10. Of the 120 cases, none tested serologically positive for FeLV and only three cats tested positive for FIV. Four cats had large B-cell lymphoma (gastric, cecal, or colonic) concurrent with small T-cell lymphoma of the small intestine. Topographically, T-cell variants are much more likely to occur in the small intestine (94%) and rarely in the stomach or large intestine. The majority of T-cell variants are mucosal (equivalent to WHO enteropathy-associated T-cell lymphoma [WHO EATCL] type II), and the majority of B-cell tumors are transmural (equivalent to WHO EATCL type I classification). Regarding cell size, nearly all mucosal T-cell tumors were composed of small lymphocytes, and slightly more than half of transmural T-cell and all B-cell variants were composed of larger cells. Epitheliotropism is common with LGAL T-cell tumors, but is rare in B-cell tumors. Other abdominal organ involvement is common, and in one report of 29 cases of low-grade T-cell intestinal lymphoma, liver and mesenteric node involvement was documented in 53% and 33% of cases, respectively.494 Hepatic lymphoma can occur concurrently with GI lymphoma or be confined solely to the liver.486, 495 Most are T-cell and clonal or oligoclonal based on PCR analysis.
Intermediate- or High-Grade Alimentary Lymphoma
Unlike LGAL, the majority of I/HGALs are large or intermediate sized B-cell lymphomas. They arise from organized lymphoid tissues; MALT in the stomach and Peyer’s patches and mucosal lymphoid nodules concentrated in the distal small intestine, cecum, and colon.436, 487, 488, 496, 497 Therefore I/HGAL is more common in the stomach, distal small intestine, cecum, and colon (see Table 33.10). These B-cell variants can be solitary or at multiple sites that occur simultaneously within the stomach, small intestine, and ileocecocolic junction. The majority are transmural (equivalent to WHO EATCL type I classification) and epitheliotropism is rarely observed.
Large Granular Lymphoma
LGL represents a less common, distinct form of alimentary lymphoma occurring in older (median age 9–10 years) cats.436, 438, 487, 497, 498, 499, 500, 501 These granulated round cell tumors have also been termed globule leukocyte tumors, although they are likely variations of the same disease. LGL is characterized by lymphocytes described as 12 to 20 μm in diameter with a round, clefted, or cerebriform nucleus, variably distinct nucleoli, finely granular to lacey chromatin, and a moderate amount of basophilic granular cytoplasm that is occasionally vacuolated.500 Prominent magenta or azurophilic granules are characteristic (Fig. 7.34, Chapter 7). They are usually granzyme B positive by immunohistochemistry.487 This population of cells includes cytotoxic T cells and occasionally NK cells: most are CD3+, CD8+, and CD20− and have T-cell receptor gene rearrangements.487, 501 In one report, nearly 60% expressed CD103 (integrin alpha E).501, 502 LGL was confined to the intestines in 93% of cases in a large compilation,438 but can occur extraabdominally (e.g., nasal).438, 498 Approximately 10% express neither B- or T-cell markers and are thus classified as NK cells. These NK tumors commonly originate in the small intestine, especially the jejunum, are transmural, often exhibit epitheliotropism, and at least two-thirds present with other organs involved; most with mesenteric lymph node involvement and many with liver, spleen, kidney, peritoneal malignant effusions, and bone marrow infiltration. Also, thoracic involvement may occur with malignant pleural effusion and a mediastinal mass present. Peripheral blood involvement was present in 10% to 15% in some reports438, 500 and as often as 86% in another report.501 Affected cats are generally FeLV/FIV negative.
History, Clinical Signs, and Physical Examination Findings
Low-Grade Alimentary Lymphoma
LGAL is most commonly associated with nonspecific signs associated with the GI tract; most cats present with weight loss (>80%), vomiting and/or diarrhea (70%–90%), and hyporexia (70%–90%), whereas icterus is uncommon (7%).436, 486, 503, 504 Abdominal palpation is often unremarkable, but intestinal thickening, mesenteric lymphadenopathy, and organomegaly can occasionally be appreciated. Clinical signs are usually present for several months before diagnosis (median, 6 months).504
Intermediate- or High-Grade Alimentary Lymphoma
I/HGAL tends to cause similar clinical signs as LGAL; however, they tend to progress more acutely and are more likely to present with a palpable abdominal mass originating from the GI tract, enlarged mesenteric lymph nodes, or liver.436, 439, 461, 496, 503, 505 Icterus is also more common in large cell forms. Hematochezia and tenesmus may also be present if the colon is involved.459 Rarely, cats may present with signs consistent with an acute abdomen due to intestinal obstruction or perforation and concurrent peritonitis.
Large Granular Lymphoma
Cats with intestinal LGL have typical GI clinical signs, but are also more likely to be acutely presented.438, 498, 500, 501 A palpable abdominal mass is present in approximately half of cases, and hepatomegaly, splenomegaly, and renomegaly are common. Abdominal and pleural effusions, and icterus are observed in nearly 10% of cases.
Diagnosis and Clinical Staging
For most cats with suspect alimentary/GI lymphoma, the diagnostic evaluation should include a baseline assessment consisting of a CBC with differential cell and platelet count, serum biochemistry profile, urinalysis, and retroviral (FeLV/FIV) screen. Anemia and neutrophilia are common findings in all forms of alimentary lymphoma; however, they tend to be more profound in I/HGAL and LGL.436, 438, 488, 498 Circulating neoplastic lymphocytes are rare with LGAL, but may be observed in up to 15% of I/HGAL and LGL. Serum biochemistry profiles can help establish the overall health of the animal and suggest extra-GI involvement (e.g., liver enzymes elevations/icterus may indicate hepatic infiltration; azotemia may indicate renal involvement). For cats with alimentary lymphoma, hypoproteinemia and anemia are reported to occur in up to 23% and 76% of cases, respectively.461, 486, 506 Hypercalcemia is rarely seen in cats, but has been reported in cats with lymphoma at various anatomic sites. Hypoglycemia, hypoalbuminemia, hyperglobulinemia, abnormal serum folate (high or low), elevated lactate dehydrogenase (LDH), and hypocobalaminemia are often reported.436, 438, 506, 507
Low-Grade Alimentary Lymphoma
LGAL must be differentiated from LPE, which have similar clinical presentations and histologic cell populations.441, 487, 488, 489, 490, 491, 508 LGAL is more commonly associated with modest (or palpably absent) intestinal thickening without mass effect similar if not identical in presentation to LPE. The key elements necessary for the diagnosis of LGAL (and differentiation from IBD) include procurement of tissue for histopathology, and if necessary, assessment of immunophenotype and clonality.
Abdominal ultrasound is by no means pathognomonic as both LGAL and LPE can have normal ultrasound appearance or reveal modest intestinal wall thickening with preservation of wall layering.436, 461, 486, 488, 508, 509, 510 Changes, if present, predominantly involves the muscularis propria and submucosal layers, although mucosal thickening can also occur. Focal mural masses are uncommon. Mesenteric lymphadenopathy is also common and reported in 45% to 80% of affected cats. LGAL generally involves the small intestine and, less commonly, the stomach and large intestine. Cats with LGAL will uncommonly have ultrasonographic abnormalities in other abdominal organs such as the stomach, liver, spleen, colon, and pancreas, and occasionally, mild effusions are observed.
Cytologic evaluation of thickened bowel or associated mesenteric lymphadenopathy alone is generally not sufficient for differentiating LGAL from LPE.486, 488, 502 Therefore tissue procurement is required for diagnosis (and differentiation from LPE). The debate still exists as to whether endoscopically obtained tissue is sufficient for diagnosis or if full-thickness tissue procured during laparotomy or laparoscopy is necessary in light of similarities with LPE.436, 487, 491, 509, 511, 512 Although histologic morphology and intestinal infiltrative patterns (e.g., villous nests or plaques) can be highly suggestive of lymphoma, they may not provide a definitive diagnosis. Most agree that although full thickness biopsies are preferred, less invasive endoscopic biopsies, with ancillary immunophenotypic and molecular (i.e., PARR; see Chapter 8) assessments, are sufficient in the majority of cases (see Table 33.10). If the differentiation of lymphoma and LPE is equivocal after standard histopathologic assessment, the addition of immunophenotypic and clonality analysis in a stepwise fashion, as proposed by several reports, enhances specificity and sensitivity and usually provides a definitive diagnosis.436, 441, 479, 480, 487, 489, 491, 513, 514 As LGAL most commonly involves the jejunum and ilium, endoscopic biopsy by both gastroduodenoscopy and ileocolonoscopy may be necessary to procure representative samples.
Intermediate- or High-Grade Alimentary Lymphoma and Large Granular Lymphoma
The diagnosis of I/HGAL and LGL is generally less complicated than for LGAL.436, 438, 488, 498 The former are often diagnosed with physical examination, abdominal imaging (e.g., ultrasound), and cytologic or histologic assessment of needle aspirate or needle biopsy samples from intestinal masses, enlarged mesenteric lymph nodes, or liver because mass lesions and gross lymphadenopathy are more commonly present. Ultrasonographically, I/HGAL is more likely to involve the stomach and colon than LGAL. In a series of 16 cats with I/HGAL of the stomach, all had either ultrasonic evidence of wall thickening or the presence of a mass, and 20% had abdominal lymphadenopathy.439 Less commonly, abdominal exploration is necessary if lesions are subtle or not amenable to transabdominal sampling. Further staging via thoracic imaging, peripheral lymph node aspiration, and bone marrow assessment may be performed, but rarely contributes prognostic information or alters treatment decisions because the disease is already widespread and systemic therapy is required.
Treatment and Prognosis
In general, cats tolerate chemotherapy for lymphoma quite well, most clients are happy with their choice to initiate treatment, and quality of life generally improves after commencement of therapy.515, 516
Low-Grade Alimentary Lymphoma
LGAL is a gratifying disease to treat as durable remissions are generally achieved with well-tolerated, conservative treatment protocols (e.g., oral chlorambucil and prednisolone).486, 487, 488, 504, 517, 518 Chlorambucil (20 mg/m2 PO every 2 weeks [preferred by the author] or 2 mg PO every other day) and prednisolone (initially 1–2 mg/kg PO daily, reduced to 0.5–1.0 mg/kg every other day over several weeks) results in response rates (i.e., resolution of clinical signs) of greater than 80% and MSTs of approximately 1.5 to 3.0 years.486, 488, 491, 504, 517, 518 Most clinical oncologists continue these conservative protocols for 2 years or longer; however, one report discontinued chlorambucil/prednisolone therapy at 1 year.517 Cats that relapse while receiving this protocol often will subsequently respond to alternative alkylating agents, such as cyclophosphamide (200–250 mg/m2, PO, q2–3 weeks) or lomustine, or to reintroduction of chlorambucil if this had been discontinued.505, 517, 518, 519 Rescue protocols have reported MSTs ranging from 9 to 29 months. Anecdotally, many will also respond to vinblastine chemotherapy if they no longer are responsive to alkylating agents. Ultimately, more aggressive CHOP- or MOPP-based protocols may be utilized when more conservative protocols are no longer effective.
Prognostic factors for cats with LGAL are not well-defined owing to the indolent nature of the disease and typical long-term survival. Only lack of response to initial induction chemotherapy is consistently observed as a negative factor, although transmural extension may also be associated with shorter STs.487
Intermediate- or High-Grade Alimentary Lymphoma
More aggressive multiagent combination chemotherapy is recommended for I/HGAL and LGL subtypes of lymphoma. The agents used most commonly to treat intermediate- or high-grade lymphoma in cats are similar to those used for dogs with lymphoma (see Section A in this chapter), and induction protocols currently employed in cats are modifications of CHOP protocols initially designed for humans.428, 438, 439, 440, 488, 496, 498, 515, 520, 521, 522, 523, 524, 525, 526, 527, 528 CHOP represents combinations of cyclophosphamide (C), doxorubicin (H, hydroxydaunorubicin), vincristine (O, Oncovin) and prednisone (P). In general, CHOP-based protocols are appropriate for cats with intermediate- and high-grade lymphoma involving any anatomic site (e.g., peripheral nodal, mediastinal, and extranodal forms), but should not be first-line therapy for low-grade variants such as LGAL. As in the dog, a plethora of CHOP-based protocols have been reported for use in cats, although virtually no high-quality comparative data exist to compare outcomes. As such, the protocol used should be based on cost, ease, client/veterinarian preference, and level of comfort. One report found that cats may better tolerate CHOP protocols that substitute vinblastine for vincristine; GI adverse events were less frequent and of lesser grade in cats receiving vinblastine.529 The current CHOP-based protocol in use by the author for cats is presented in Table 33.11 . This protocol has been used in many cats with various forms of intermediate- and high-grade lymphoma and is generally well tolerated. At present, most canine lymphoma protocols involve a 12- to 25-week induction phase whereupon chemotherapy is discontinued and no maintenance chemotherapy is used. Although data exist in dogs for a maintenance-free approach, similar comparative data do not currently exist in the cat. DOX alone (25 mg/m2 every 3 weeks for five total treatments), CCNU (lomustine; 40–50 mg/m2 PO q 3 weeks),440 or palliative prednisone therapy is offered if clients decline more aggressive CHOP-based therapy. 440, 530, 531 Cats are generally less tolerant of DOX than are dogs; therefore a lower dosage (25 mg/m2 or 1 mg/kg IV) is used (see Chapter 12). Cardiac toxicity does not appear to be a clinically significant problem in cats, although renal toxicity is more commonly encountered,532 and renal function should be monitored (i.e., serial blood urea nitrogen [BUN], creatinine, and urine specific gravity) closely before and during therapy. The use of COP (i.e., CHOP without the addition of DOX) is often used in cats in Europe, and one compilation reported similar results to CHOP.533 A COP (cyclophosphamide, vincristine, and prednisolone) protocol commonly employed in cats is presented in Table 33.12 ; however, several studies have reported that the inclusion of DOX is important for predicting more durable responses. 488, 527, 534 The use of intraperitoneal-delivered COP in a small number of cats (n = 26) was reported; three-quarters achieved a CR with a MST of 1 year.535 This study included only three GI cases and did not histologically or immunophenotypically subtype cases beyond saying all were “large cell”; therefore larger, more controlled studies would be necessary to establish/confirm efficacy of this protocol.
TABLE 33.11.
The CHOP-Based Chemotherapy Protocol for Cats with Intermediate/High-grade Lymphoma Employed by the Author
| Treatment Week | Drug, Dosage, and Route |
|---|---|
| 1 | Vincristine, 0.5–0.7 mg/m2, IV l-Asparaginase, 400 Units/kg, SC Prednisolone, 2.0 mg/kg, PO |
| 2 | Cyclophosphamide 200 mg/m2, PO Prednisolone, 2.0 mg/kg, PO |
| 3 | Vincristine, 0.5–0.7 mg/m2, IV Prednisolone, 1.0 mg/kg, PO |
| 4 | Doxorubicin, 25 mg/m2, IV Prednisolone, 1.0 mg/kg, POa |
| 6 | Vincristine, 0.5–0.7 mg/m2, IV |
| 7 | Cyclophosphamide 200 mg/m2, PO |
| 8 | Vincristine, 0.5–0.7 mg/m2, IV |
| 9b | Doxorubicin, 25 mg/m2, IV |
| 11 | Vincristine, 0.5–0.7 mg/m2, IV |
| 13 | Cyclophosphamide 200 mg/m2, PO |
| 15 | Vincristine, 0.5–0.7 mg/m2, IV |
| 17 | Doxorubicin, 25 mg/m2, IV |
| 19 | Vincristine, 0.5–0.7 mg/m2, IV |
| 21 | Cyclophosphamide 200 mg/m2, PO |
| 23 | Vincristine, 0.5–0.7 mg/m2, IV |
| 25c | Doxorubicin, 25 mg/m2, IV |
CHOP, Combinations of cyclophosphamide (C), doxorubicin (H, hydroxydaunorubicin), vincristine (O, Oncovin), and prednisolone (P); IV, intravenous; PO, by mouth; SQ, subcutaneous.
Note: A complete blood count (CBC) should be performed before each chemotherapy. If neutrophils are <1500 cells/μL, wait 5–7 days, repeat CBC, then administer the drug if neutrophils have risen above the 1500 cell/μL cutoff.
Prednisolone is continued (1 mg/kg, PO) every other day from this point on.
If in complete remission at week 9, continue to week 11.
If in complete remission at week 25, therapy is discontinued after this doxorubicin, and cat is rechecked monthly for recurrence.
TABLE 33.12.
COP Protocol for Lymphoma in Cats
| Drug | Frequency of Drug Delivery |
|---|---|
| Cyclophosphamide: 250–300 mg/m2, POa | Given every 3 weeks on the day after vincristine |
| Vincristine: 0.7 mg/m2, IV | Given weekly on weeks 1, 2, 3, and 4, then given every 3 weeks thereafter on the days before cyclophosphamide. Discontinue if in remission at 1 year. |
| Prednisolone: 1–2 mg/kg, PO | Given daily for 1 year. |
Note: A complete blood count (CBC) should be performed before each chemotherapy. If neutrophils are <1500 cells/μL, wait 5–7 days, repeat CBC, then administer the drug if neutrophils have risen above the 1500 cell/μL cutoff.
Some divide the cyclophosphamide over 3 consecutive days.
Response rates and durability of response for cats with I/HGAL treated with combination protocols are generally not as good as in dogs with intermediate- or high-grade peripheral nodal lymphoma. Remission rates of 50% to 65% can be expected with approximately one-third achieving CR. Remission and survival are only durable in cases achieving a CR; MSTs for cats in CR are approximately 7 to 10 months with a subset living to 1 year or longer.439, 440, 488, 496, 520, 521, 522, 529,428, 515, 523, 524, 525, 526, 527, 528 Rescue protocols involving alternate drugs (e.g., melphalan, lomustine, mechlorethamine, actinomycin-D, cytarabine) or reinduction with CHOP generally do not result in durable subsequent remissions.519, 536, 537
Several prognostic factors have been reported for cats with I/HGAL; however, by far the most predictive is whether a CR is achieved.439, 440, 487, 488, 491, 496, 520, 521, 522, 529 Negative prognostic factors identified for I/HGAL include transmural extension, FeLV antigenemia, weight loss, elevated LDH, hypoalbuminemia, hypocobaliminemia, and bicavitary involvement, while stage I disease (rare) is associated with a more favorable prognosis. Factors not found to be prognostic were immunophenotype and various proliferation indices (e.g., PCNA, AgNOR, Ki67).
Large Granular Lymphoma
Cats with LGL typically have the lowest reported response rates and shortest response durations of any of the GI lymphomas. Approximately one-third of cases will experience a response and MSTs in larger reports of cases were only 21 days; cats receiving CHOP-based or CCNU-based protocols experienced MSTs of 45 to 90 days.438, 488, 500 That being said, a small subset (7% in this report) enjoyed more durable (>6 month survivals) responses and, in one small study (n = 6), a MST of 9 months was reported after a variety of interventions.498
The Role of Surgery in Cats with Gastrointestinal Lymphoma
Surgery is primarily reserved for I/HGAL that have discrete lesions and in cats that are presented with intestinal perforation and obstructive lesions.439, 488, 496 Of current debate is whether surgery should be performed on discrete lesions that are not perforated or obstructed before initiating systemic chemotherapy. This question is currently unanswered as comparative trials have not clearly established a benefit or detriment to this approach. The motivation behind performing surgery before chemotherapy often lies with concern for GI perforation resulting from robust chemotherapy response in cases with full-thickness involvement with lymphoma. Crouse reported on 23 cats with discrete I/HGAL undergoing chemotherapy without surgery. Although four cats (17%) experienced perforation, these events occurred 2 to 87 days after initiation of chemotherapy rather than in the acute postchemotherapy period, they were not associated with size or degree of hypoalbuminemia, and progressive disease was documented in three cats and only a partial response in the other cat at the time of perforation.437 Taken together, this implies that perforations were likely due to progressive disease rather than robust response to chemotherapy. An additional motivation for surgical intervention is the theoretical advantage of immediate creation of a “minimal residual disease” state if staging does not reveal disease distant from a large GI primary tumor; chemotherapy is generally thought to result in more favorable outcomes in the minimal disease state, rather than in the macroscopic disease state. The author generally recommends surgery, albeit in the absence of convincing support in the literature, if lesions are large, discrete, and no (or minimal) involvement is documented outside the primary mass after complete staging. Multiagent chemotherapy is then initiated at suture removal owing to the high-grade nature and overall short STs associated with I/HGAL.
The Role of Radiation Therapy in Cats with Gastrointestinal Lymphoma
Radiation therapy (RT) for GI lymphoma in cats has not been thoroughly explored and is generally reserved for consolidation therapy (during or after chemotherapy) or as a rescue modality.538, 539 In one report, 11 cats (six small cell, four large cell, and one LGL) that progressed after chemotherapy received abdominal radiation (8 Gy in two fractions over 2 days) and experienced a MST of 7 months, although numbers were small and 40% were lost to follow-up.538 In a second report, eight cats (seven with I/HGAL) underwent 6 weeks of CHOP-based combination chemotherapy followed 2 weeks later by whole abdomen radiation consisting of 10 daily 1.5 Gy fractions.539, 540 Although three cats died within 3 weeks of RT, five experienced durable remissions. These preliminary results warrant further investigation before RT can be recommended as standard care.
Supportive Care for Cats with Gastrointestinal Lymphoma
Intuitively, GI disease may compromise the nutritional status of affected cats. As such, careful and repeated assessments of nutritional state, caloric intake, and body weight should be undertaken. Nutritional support (see Chapter 16, Section B) should be instituted sooner rather than later in affected cats. A good plan of nutrition should help maintain or improve quality of life, immunologic status, and tolerance of chemotherapy. Cobalamin supplementation should be instituted in those cats with documented hypocobalaminemia.
Peripheral Nodal Lymphoma in Cats
Involvement limited to peripheral lymph nodes is unusual in cats with lymphoma, representing approximately 4% to 10% of cases.427, 428 In contrast, approximately one-quarter of all other anatomic forms of lymphoma have some component of lymph node involvement. One-third of cats with nodal lymphoma are T-cell immunophenotype and FeLV antigenemic; however, complete categorizations have not occurred in the post-FeLV era and this may no longer be true.427, 428, 435, 492 Peripheral nodal lymphoma was the most common anatomic form of lymphoma reported in a compilation of cases in cats under the age of 1 year, representing one-third of cases in this age group.433 As lymphoma progresses, bone marrow and hepatic infiltration may develop. Clinical staging and diagnostic approach for peripheral lymphadenopathy and peripheral nodal lymphoma is similar to the dog (see Section A of this chapter).
Cats with the nodal form of lymphoma present with variable clinical signs depending on the extent of disease; however, they are often depressed and lethargic. Peripheral lymphadenopathy, as the only physical finding, is an uncommon presentation.
An uncommon and distinct form of nodal lymphoma in cats referred to as “Hodgkin’s-like” lymphoma has been reported.541, 542 This form typically involves solitary or regional lymph nodes of the head and neck (Fig. 33.14 ) and histologically resembles Hodgkin’s lymphoma in humans. Affected cats generally present with enlargement of one or two mandibular or cervical nodes initially, and tumors are immunophenotypically classified as T-cell–rich, B-cell lymphoma. Histologically, lymph nodes can be effaced by either nodular or diffuse small to large lymphocytes with characteristic bizarre or multinucleated cells (Reed–Sternberg-like cells) (Fig. 33.15 ). No association with FeLV or FIV has been documented. Cats with Hodgkin’s-like nodal lymphoma usually present without overt clinical signs.541, 542 Inguinal node, multicentric nodal, subcutaneous, and conjunctival involvement have been reported.542, 543, 544, 545 Interestingly, in both reports of subcutaneous Hodgkin’s lymphoma in cats, spontaneous remissions were observed suggesting these may not have been be true lymphoid neoplasia.543, 544 The clinical course of Hodgkin’s like lymphoma is generally more indolent than for peripheral nodal non-Hodgkin’s lymphoma in the cat.
Fig. 33.14.
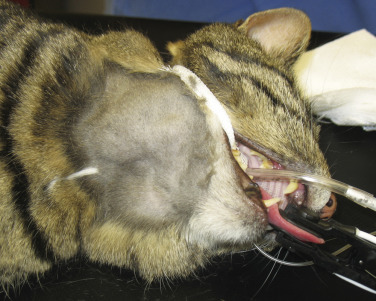
A cat presented with mandibular lymphadenopathy that was confirmed to be Hodgkin’s-like lymphoma after histologic assessment.
Fig. 33.15.
Fine-needle aspirate cytology of a lymph node in a cat with Hodgkin’s-like lymphoma. The large lymphocytes have prominent nucleoli and smooth basophilic cytoplasm. Several binucleate lymphocytes are present.
The treatment choice for peripheral nodal lymphoma in cats depends on whether the individual case represents a low-grade (e.g., indolent [rare]) versus an intermediate- or high-grade (e.g., intermediate/large cell) lymphoma; the latter are best treated with CHOP- or COP-based protocols (discussed under I/HGAL earlier) and carry a less favorable prognosis, whereas the former generally respond to less aggressive chlorambucil/corticosteroid protocols and enjoy durable responses. Less is known regarding the treatment of Hodgkin’s-like lymphoma involving solitary or regional nodes of the head and neck.541, 542 Clinical outcome after surgical extirpation of or RT to the affected node (or nodes if a reasonable number) is often associated with long-term disease control and MSTs of approximately 1 year, suggesting that it is a more indolent form of lymphoma. Eventual recurrence in distal nodes after surgical excision or RT is common, and the author currently offers clients the option of adjuvant chlorambucil/corticosteroids after surgery. This theoretically may have benefit; however, insufficient data currently exist to document a survival advantage with this approach.
Mediastinal Lymphoma
The mediastinal form can involve the thymus and mediastinal and sternal lymph nodes. Pleural effusion is common. In two large compilations, 63% of cats with thymic disease and 17% of cats with pleural effusion were documented as having lymphoma.546, 547 Hypercalcemia occurs frequently with mediastinal lymphoma in dogs, but is rare in cats. The majority of cats with mediastinal lymphoma in older reports were young (median age, 2–4 years), FeLV positive, and had T-cell immunophenotype tumor; however, many reports did not report immunophenotypic data.427, 428, 433, 434, 435, 548 The disease is confined to the mediastinum in most cases. A form of mediastinal lymphoma also occurs primarily in young, FeLV-negative Siamese cats that appears to be less biologically aggressive and more responsive to chemotherapy than FeLV-associated forms.533 In a more recent report of 55 cats with mediastinal lymphoma from the United Kingdom, the majority were antigenically FeLV/FIV negative, young (median age, 3 years), male (3.2:1 male-to-female ratio), and nearly one-third were Siamese.548 In this large cohort, immunophenotype was not reported.
The clinical signs associated with mediastinal lymphoma include dyspnea (80%), tachypnea, and a noncompressible cranial thorax with dull heart and lung sounds.549 Rarely, a Horner’s syndrome and precaval syndrome may be observed. Pleural effusion is observed in 50% of cases and characterized by serohemorrhagic to chylous effusion and, in most cases, neoplastic cells (lymphoblasts) are identified.546, 548, 550 Diagnostic suspicion may begin with a noncompressible cranial thorax on physical examination and confirmation of a mediastinal mass/pleural effusion on thoracic radiographs. Fine-needle aspirate (FNA) cytology of the mass or cytologic evaluation of pleural fluid may be sufficient to establish a diagnosis. In most cats, the finding of a monotonous population of intermediate or large lymphoid cells will establish a diagnosis; however, definitive diagnosis of lymphoma in cats with a mediastinal mass and concurrent chylothorax can be challenging. CT appearance may be helpful, but generally does not contribute to a definitive diagnosis. If large lymphocytes are not identified in the pleural chylous effusion, then cholesterol and triglyceride concentrations can be measured.551 In chylous effusions, the pleural fluid triglyceride concentration will be greater than in the serum; however, anorectic cats may have lower triglyceride levels in the pleural fluid. A major differential for mediastinal lymphoma is thymoma. The cytologic features of thymoma can be distinct from lymphoma in many cases, but the diagnosis can be challenging because of a preponderance of small lymphocytes in thymoma. Mast cells can also be seen in up to 50% of aspirations from thymomas. The addition of immunophenotypic and clonality assessment may be helpful in equivocal cases.
In the largest report, cats with mediastinal lymphoma treated with either COP- or CHOP-based protocols experienced an overall response rate of 95% with a MST of 373 days (980 days if CR was achieved).548 In contrast, mediastinal lymphoma in young FeLV-positive cats is generally associated with a poor prognosis and MSTs of approximately 2 to 3 months are expected after treatment with CHOP- or COP-based protocols.428, 524
Extranodal Lymphoma
Collectively, extranodal lymphoma represents the second most common site of lymphoma after GI lymphoma in cats.431 The most common extranodal sites for lymphoma in cats include nasal (including nasopharyngeal and sinonasal), kidney, CNS, laryngeal and tracheal, cutaneous, subcutaneous, and ocular.
The clinical signs associated with feline extranodal lymphoma are variable and depend on anatomic location and extent of disease. Many, if confined to the primary site (stage I), will appear clinically healthy. However, cats with lymphoma, regardless of site, may present with nonspecific constitutional signs including anorexia, weight loss, lethargy, or depression. Secondary bone marrow infiltration, although uncommon, may lead to anemia. Signs related to paraneoplastic hypercalcemia (polyuria/polydipsia [PU/PD]) can occur in cats, however, much less commonly than in the dog. In one survey of hypercalcemia in cats, approximately 10% were diagnosed with lymphoma of various anatomic types.552
For most cats with suspected extranodal lymphoma, the diagnostic evaluation should include a baseline assessment consisting of a CBC with differential cell count, platelet count, serum chemistry profile, urinalysis, and retroviral (FeLV/FIV) screen. Serum biochemistry profiles can help establish the overall health of the animal, as well as, in some cases, suggest site-specific tumor involvement. For example, increased liver enzymes levels may indicate hepatic infiltration and increased BUN and creatinine may indicate renal lymphoma. Hypoglycemia was reported in approximately one-third of cats with lymphoma in one Australian study.506 In a series of cats with various anatomic forms of lymphoma, serum albumin concentrations were significantly lower and β-globulin concentrations (as measured by protein electrophoresis) were significantly higher than a healthy control population.507
The use of various imaging modalities in cats with lymphoma depends on the anatomic site and will be discussed in site-specific discussions to follow.
Cytopathologic or histopathologic evaluation of involved lymph node(s) or involved organ tissue, procured via FNA cytology (see Chapter 7) or surgical, endoscopic, or needle-core biopsy (see Chapter 9), is required for a definitive diagnosis. Cytology alone may not be sufficient in some cases, owing to difficulties encountered in distinguishing lymphoma from benign hyperplastic or reactive lymphoid conditions. In such cases, incisional or excisional biopsy is preferred as orientation and information regarding invasiveness and architectural abnormalities may be necessary for diagnosis. In addition, involved tissue, needle aspirate, and fluid samples can be further interrogated by various histochemical, immunohistochemical, flow cytometric analysis (e.g., size and immunophenotypic assessment), and molecular techniques (e.g., PARR to assess clonality) to further characterize the disease process and refine the diagnosis in equivocal cases. The reader is referred to Chapter 8 for a general discussion of flow cytometric analysis and molecular diagnostic techniques, as well as Section A of this chapter for specific applications to lymphoma. PARR in cats is approximately 80% sensitive for the diagnosis of lymphoma553; however, assessment of specificity has not been clearly established. Clonality assessment tools (e.g., primers) for both Ig and T-cell receptor variable region genes have been developed in cats.479, 480, 554, 555, 556, 557
Assessment of tumor proliferation rates (e.g., Ki67, PCNA, AgNOR), telomerase activity, and serum protein electrophoresis can also be performed on involved tissues in cats; however, consistent prognostic value across the anatomic, histopathologic, and immunophenotypic variants of lymphoma in cats is not well characterized.
Thorough staging, including a bone marrow aspiration or biopsy, peripheral lymph node assessment (clinically normal or abnormal nodes), and thoracic and/or abdominal imaging, is indicated when (1) solitary site disease is suspected (stage I) and a decision between locoregional therapy (i.e., surgery and/or RT) versus systemic therapy (i.e., chemotherapy) is being considered; (2) it provides prognostic information that will help a caregiver make treatment decisions; and (3) complete staging of the extent of disease is required as part of a clinical trial. Bone marrow evaluation may be of particular interest if anemia, cellular atypia, and/or leukopenia are present. A WHO staging system exists for the cat that is similar to that used in the dog (see Box 33.2); however, because of the high incidence of visceral/extranodal involvement in cats, a separate staging system has been evaluated and is often used (Box 33.5 ).558 Because lymphoma in cats is more varied with respect to anatomic locations, staging systems are generally less helpful for predicting response.
BOX 33.5. Clinical Staging System for Feline Lymphoma558.
Stage 1
-
•
A single tumor (extranodal) or single anatomic area (nodal)
-
•
Includes primary intrathoracic tumors
Stage 2
-
•
A single tumor (extranodal) with regional lymph node involvement
-
•
Two or more nodal areas on the same side of the diaphragm
-
•
Two single (extranodal) tumors with or without regional lymph node involvement on the same side of the diaphragm
-
•
A resectable primary gastrointestinal tract tumor, usually in the ileocecal area, with or without involvement of associated mesenteric nodes only
Stage 3
-
•
Two single tumors (extranodal) on opposite sides of the diaphragm
-
•
Two or more nodal areas above and below the diaphragm
-
•
All extensive primary unresectable intraabdominal disease
-
•
All paraspinal or epidural tumors, regardless of other tumor site or sites
Stage 4
-
•
Stages 1–3 with liver and/or spleen involvement
Stage 5
-
•
Stages 1–4 with initial involvement of CNS or bone marrow or both
Our knowledge base for treating cats with extranodal lymphoma is not well established, and outcomes are less predictable than that in dogs, primarily due to the greater variation in histologic type and anatomic location observed in cats. This is further complicated by the plethora of papers that include very small numbers of cases representing multiple anatomic/immunophenotypic and histologic subtypes (e.g., small cell vs large cell variants) together when reporting survival analysis after chemotherapy. This provides only general observations rather than specific outcome information (i.e., response rate and durability of response) that can vary significantly with respect to anatomic and histologic subtype. Most treatment decisions should be based on assessment of whether the individual case represents a low-grade (e.g., indolent) versus an intermediate- or high-grade (e.g., large cell) lymphoma and whether the disease is limited to the local extranodal site. Chemotherapy protocols for the cat have been previously discussed under sections in GI lymphoma; low-grade tumors are generally treated with chlorambucil/prednisolone protocols and intermediate- or high-grade tumors with CHOP- or COP-based protocols. Finally, much of the early work on chemotherapy protocol development for cats with lymphoma occurred during the FeLV era and care should be exercised when applying this information in the post-FeLV era.
Nasal Lymphoma
Nasal lymphoma is the most common extranodal lymphoma in cats.559 It is usually a localized disease; however, 20% have local extension or distant metastasis at necropsy.560 The majority of nonviral nasal/paranasal diseases in cats are neoplasia, and lymphoma represents one-third to one-half of these cases.561, 562, 563, 564 It occurs primarily in older (median age, 9–10 years; range, 3–17 years), FeLV/FIV antigenemic negative cats, and at least three-quarters are B-cell in origin, although T-cell and mixed B-cell/T-cell immunophenotypes are reported in 10% to 15% of cases.428, 559, 560, 561, 565 An Italian report documented FeLV antigen (p27 or gp70) expression in nasal lymphoma tissues by immunohistochemistry (IHC); however, FeLV antigenemia was not reported in this cohort.561 Siamese cats appear overrepresented and a 2:1 male-to-female ratio has also been observed.561, 565 Most are of intermediate- or high-grade histology; however, small-cell low-grade variants have been reported in up to 25% of some cohorts.560, 561, 565 Epitheliotropism is common if the epithelium is present in the biopsy.
Cats with nasal lymphoma present with nasal discharge (60%–85%), sneezing (20%–70%), upper respiratory noise (stridor, stertor, wheezing; 20%–60%), facial deformity (0%–20%), hyporexia (10%–60%), epiphora (10%–30%), and occasionally increased respiratory effort and coughing.559, 560, 561, 565 The nasal discharge is usually mucopurulent, although epistaxis is present in up to one-third of cases. Regional lymphadenopathy can also occur. The median duration of clinical signs before diagnosis is 2 months (range, 1–1800 days).
If nasal lymphoma is suspected, advanced imaging (CT, MRI), rhinoscopy, and biopsy are usually necessary for diagnosis (see Chapter 24, Section B). CT or MRI is useful to determine the extent of involvement and to help plan biopsy procurement and RT if that treatment option is pursued. CT characteristics include the presence of a unilateral or bilateral nasal/sinus mass or fluid, bulla effusion, and lysis of associated bony structures.561, 566, 567, 568 A biopsy can be procured either by intranasal procurement (with or without rhinoscopy) or by flushing one hemicavity with a bulb syringe and saline while occluding the contralateral cavity and collecting samples flushed out of the nasopharynx (Fig. 33.16 ). Thorough staging (i.e., regional node assessment, thoracic and abdominal staging, and bone marrow assessment) to ensure the disease is confined to the nasal passages is recommended if local RT without systemic chemotherapy is being considered. IHC may be necessary to differentiate nasal carcinoma from nasal lymphoma in a subset of cases; approximately 7% of samples required IHC to differentiate carcinoma from lymphoma in one large cohort of cases.569
Fig. 33.16.
Flush biopsy of nasal lymphoma. Note the large sample (arrow) procured by retrograde flushing of saline through on nares while occluding the contralateral nares. The sample is flushed through the pharynx and out the mouth.
In one report, 38 cats with nasal lymphoma that were not treated had MSTs of only 53 days.561 However, in cats that are treated, durable remissions and lengthy MSTs can be expected in the majority of cases.428, 502, 559, 565, 570, 571, 572, 573, 574 If disease is documented to be confined to the nasal cavity (i.e., stage I) after thorough staging, then RT is the treatment of choice. CR rates of 75% to 95% are reported with MSTs after RT of 1.5 to 3.0 years.565, 573 Cats that do not achieve a CR with RT have an MST of approximately 4.5 months. Total radiation dosage does affect STs, and a total dose greater than 32 Gy is recommended. The addition of chemotherapy to RT has not been definitively shown to enhance STs for cats with locally confined disease; combinations of RT and chemotherapy result in similar response rates and STs.565, 570, 571, 573, 574 Chemotherapy (COP- or CHOP-based protocols) is a reasonable alternative to RT, with CR rates of approximately 75% and MSTs of approximately 2 years for cats achieving CR.559 The author’s preference is to initiate systemic chemotherapy only for cases that have confirmed disease beyond the nasal passage, cases that relapse after RT, or cases in which RT is unavailable or declined.
Renal Lymphoma
Renal lymphoma is the second most common form of extranodal lymphoma, occurring in approximately one-third of cases.559, 572 Although it can present as confined to the kidneys (<25%), it more commonly presents as concurrent with alimentary or multicentric lymphoma. The median age at presentation is 9 years, although 6% occur in cats under 1 year of age.559, 575 The majority of cases are not associated with FeLV and, although most are not associated with FIV, approximately one-half of cats reported in an Australian study were FIV positive. Little contemporary information exists on the immunohistologic classification of renal lymphoma; however, the majority are of high-grade B-cell immunophenotype.428, 435 Extension to the CNS was frequent in one report, but not similarly reported elsewhere.575
Cats with renal lymphoma present with signs consistent with renal insufficiency: hyporexia, weight loss, and polyuria/polydipsia.559, 575 On physical examination, marked renomegaly (bilateral, lumpy, and irregular; although a smooth variant has been observed) is palpated in the majority of cases (Fig. 33.17 ). Radiographic appearance is smooth-to-irregular renomegaly (Fig. 33.17A). Ultrasonographic imaging usually reveals bilateral (>80%), irregular renomegaly with hypoechoic subcapsular thickening.156 Approximately one-third of cases will have ultrasonographic evidence of other abdominal organ involvement. The disease is usually diffuse throughout the renal cortex (Fig. 33.17B) and transabdominal FNA cytology or core biopsy is diagnostic in most cases.576
Fig. 33.17.
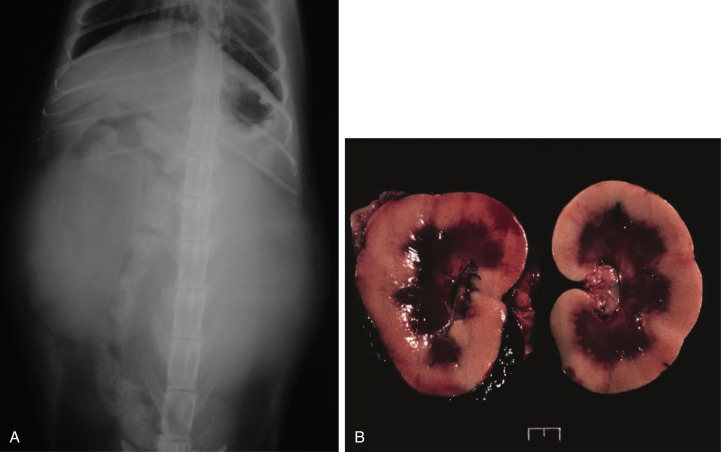
(A) Ventrodorsal projection of a cat with real lymphoma. Massive bilateral renomegaly is observed. (B) Necropsy specimen of a cat with bilateral renal lymphoma illustrating the diffuse cortical nature of the disease that is most common.
Treatment and outcome appears similar to other high-grade lymphomas in the cat; approximately two-thirds will experience clinical benefit with COP- or CHOP-based protocols with MSTs reported from 4 to 7 months. Owing to an inability to differentiate how much of the renal insufficiency at presentation is lymphoma-related versus due to underlying renal disease of older cats, most oncologists will start COP-based protocols and only add in DOX if renal values normalize during remission because of the potential for renal toxicity with DOX in cats.
CNS Lymphoma
CNS lymphoma can be intracranial, extracranial (i.e., spinal), or both.157 CNS lymphoma accounted for 14% of 110 reported cases of extranodal lymphoma,559 15% to 31% of intracranial tumors,158, 577 and 39% of spinal cord tumors,578 making it one of the most common malignancies encoutered in the CNS in cats.579 Although some discordance exists in the literature, cats with CNS lymphoma are younger (median age, 4.0–10.5 years) and 17%580 to 50% of cases are FeLV antigenemic.577, 578, 581 As most reports are older, these data may no longer hold true in the current era. Approximately two-thirds of intracranial cases also have multicentric and extracranial disease; and approximately 40% of spinal lymphoma cases occur in multiple spinal cord sites with one-third also involving intracranial locations.559, 577, 578, 579, 581 In a compilation of 160 cases of intracranial tumors in cats, diffuse cerebral and brainstem involvement was most common for lymphoid malignancies.577 Spinal lesions are usually both extradural and intradural, although they can be limited to one or the other compartment.578 Feline CNS lymphoma may be primary, but more commonly (approximately 80%) represents a multicentric process (especially with renal or bone marrow involvement).577, 581, 582, 583 A paucity of information exists on the immunophenotype of CNS lymphoma.
Cats with CNS lymphoma can present with constitutional signs (hyporexia, lethargy) and signs referring to intracranial lesions, spinal lesions, or both.577, 578, 579, 580, 582, 583 Intracranial signs may include ataxia, altered consciousness, aggression, central blindness, and vestibular abnormalities. In a study of cats with seizures, of those diagnosed with intracranial lesions, 8% were due to lymphoma.580 Clinical signs referring to spinal cord involvement may include paresis or paraplegia (>80% with tetraparesis in 20%), ataxia, pain, constipation, and nonspecific constitutional signs (e.g., hyporexia, lethargy, weight loss).578, 582 Neurologic examination may further reveal lower or upper motor neuron bladder, tail flaccidity, and absent deep pain; approximately one-third of cats will be asymmetric and most refer to thoracolumbar involvement. The neurologic dysfunction may be insidious or progress rapidly.
In cats with suspected spinal lymphoma, survey radiographs of the spine will rarely reveal osseous lesions. CT or MRI are preferred and, in approximately 75% of the cases, an extradural or intradural mass will be detected.577, 579, 580, 582, 583, 584 Most lesions occur at a thoracolumbar or lumbosacral location and are often multifocal. Image-guided FNA of epidural lesions may yield a cytologic diagnosis. CT or MRI also reveals multifocal disease in the majority of cats with intracranial lymphoma.577, 580 CSF analysis may be helpful and could provide a definitive diagnosis in some cases. One of 11 cats with confirmed spinal lymphoma in one study578 and six of 17 with confirmed intracranial lymphoma in another study577 had evidence of neoplastic lymphocytes in the CNS, and an increased protein content was commonly found. Bone marrow and renal involvement are often present, and cytologic assessment of these or other more accessible involved organs is generally more easily attainable than from spinal sites.
Few cases report chemotherapy treatment and outcome for CNS lymphoma, and although an occasional case has experienced durable response to systemic chemotherapy, generally fewer than 50% will respond and MSTs of 1 to 4 months can be expected.528, 559, 572, 577, 578 RT may be used and generally brisk responses would be expected owing to the inherent radiosensitivity of lymphocytes. Adjuvant chemotherapy should be considered, as many cases with CNS lymphoma have documented bone marrow or renal involvement.582, 583
Cutaneous Lymphoma
Cutaneous lymphoma is a rarely encountered anatomic form in the cat. It is usually seen in older cats (median age, 10.0–13.5 years) with no sex or breed predominance, and is not associated with FeLV/FIV.585, 586 Cutaneous lymphoma may be solitary or diffuse with a varied presentation.585, 587 In decreasing order of likelihood, lesions may include erythematous patches, alopecia, scaling, dermal nodules, or ulcerative plaques. Nasal hypopigmentation, miliary dermatitis, and mucosal lesions are rarely observed. Peripheral lymphadenopathy may also be present. In most cats, the duration of signs will be prolonged, lasting several months.
Cutaneous lymphoma often affects the head and face and is generally an indolent disease. Two forms have been distinguished histologically and immunohistochemically. Most reports in the cat are epitheliotropic and consist of T cells, although, unlike in dogs, adnexal structures are often spared. A report of nonepitheliotropic cutaneous lymphoma in cats also found 5 of 6 cases to be of T-cell derivation.588 “Cutaneous lymphocytosis,” an uncommon disease histologically resembling well-differentiated lymphoma, was characterized in 23 cats.502 Solitary lesions were most common. All were composed primarily of T cells, with two-thirds having some B-cell aggregates. Cutaneous lymphocytosis was characterized as a slowly progressive disorder; however, a few cases went on to develop internal organ infiltration. Two case reports exist of cats with cutaneous T-cell lymphoma and circulating atypical lymphocytes.589, 590 The circulating cells were lymphocytes with large, hyperchromatic, grooved nuclei, and one case was immunophenotyped as a CD3/CD8 population. In humans, cutaneous T-cell lymphoma with circulating malignant cells is termed Sézary syndrome.
For cats suspected of cutaneous lymphoma, dermal punch biopsies (4–8 mm) should be taken from the most representative and infiltrative sites, while avoiding overtly infected skin lesions. Immunophenotypic and PARR analysis are often helpful in definitive diagnosis. Complete staging to rule out systemic disease is also recommended for cats with cutaneous lymphoma, as local therapy may be applied in cases of solitary disease.
Very little has been published regarding the treatment of cutaneous lymphoma in cats585; however, a report of a CR to lomustine exists.591 Cats with a solitary disease could theoretically be treated with surgical excision or RT, although clinical staging is necessary to rule out possible further systemic involvement. For multiple sites, combination chemotherapy should be considered.
Subcutaneous Lymphoma
Recently several retrospective compilations of a subcutaneous form of lymphoma (SC-L) have been reported.431, 592, 593 Although these have been referred to as “cutaneous” lymphoma in some reports, their clinical and histologic characteristics imply a SC localization. Most affected cats are older DSH cats, and males may be overrepresented. Overall, this appears to be an uncommon presentation, representing 0.4% of all cutaneous/SC masses submitted in a report of 97 cases.431 Retroviral (FeLV/FIV) antigenemia is rare, although in one report of 17 cats, FeLV gp70 and/or p27 protein was expressed in the majority of tumor tissues.593
Cats with SC-L are presented with firm, painless SC nodules with a predilection for lateral thoracic, lateral abdominal wall, intrascapular, and tarsal locations (Fig. 33.18 ). Histologically, they are characterized by deep SC invasion with a monomorphic round cell population, extension into underlying tissues and overlying superficial tissue (but not epitheliotropic), and with extensive central necrosis and peripheral inflammation (“collaring”). Angiocentricity and angioinvasion are often observed. Mitotic index ranges from 3 to more than 25/10 HPF. The round cell population has been characterized as a large high-grade B cell in approximately two-thirds of cases and high-grade T cell in one-third, with an occasional NK immunophenotype reported.431, 592, 593
Fig. 33.18.
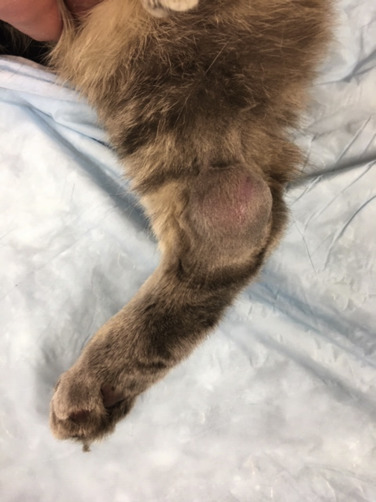
Subcutaneous lymphoma of the tarsus in a cat.
Image courtesy Dr. Samuel Hocker.
Most animals are otherwise healthy (substage a) and the disease is generally confined to the local site at presentation (stage I), although in one report the tarsal location was associated with regional popliteal lymph node involvement in nearly 20% of cases.592 In two cases, concurrent feline injection-site sarcoma (FISS) was found at other sites. SC-L has several similarities to FISS, including clinical presentation, site of occurrence, poor demarcation, central necrosis and peripheral inflammation, although macrophages with phagocytized vaccine-like product has not been observed within the inflammatory component.431 These similarities suggest the possibility that injection-site inflammation may play a role in the disease etiology; however, this has not been confirmed. Regardless, SC-L should remain an important differential in consideration of FISS.
The treatment of SC-L in the literature is varied and, as such, a standard of care is not currently established.431, 592, 593 Although the disease is initially confined to the local primary site in most cases, recurrence after local therapy, whether surgical excision, RT, or both, occurs in nearly half of cases and eventual distant metastasis occurs in one-third of cases. Approximately 75% of affected cats go on to die of their lymphoma; therefore SC-L should be considered to have an aggressive biologic behavior. In the largest report, median progression-free and overall STs after primary site surgical removal were 101 days and 148 days respectively.431 In the case of tarsal SC-L, even with hindlimb amputation in three cats, regional nodal or distant involvement was documented in all cases (at 56, 350, and 525 days), albeit durable disease-free intervals were observed in two cats. In a limited number of cats treated with RT, responses were brisk, but some progressed beyond the radiation field. The efficacy of chemotherapy is currently not well known; in a small number of cases receiving a variety of chemotherapy protocols, although many responded or stabilized, durability was poor and MSTs were approximately 6 months.592 Seven cases of tarsal SC-L received chemotherapy and RT with an MST of 216 days. Because the disease has a high recurrence and metastatic behavior, it is likely that a multimodality approach involving local therapy (surgery and/or RT) and systemic chemotherapy would intuitively provide the best outcomes.
Laryngeal/Pharyngeal/Tracheal Lymphoma
Laryngeal lymphoma comprised 10% of 110 cases of extranodal forms in one report and represented 11% of all laryngeal disease in the species.559, 572, 594 It occurs in older cats (median age, 9 years), is not associated with FeLV, and may be a solitary lesion or occur in the presence of other multicentric sites. No information on immunophenotype is currently available. Signs associated with this location in affected cats include dyspnea, dysphonia, stridor, gagging or retching, and rarely, coughing.559, 594 Although it is generally localized to the primary site (stage I), approximately 25% had regional nodal involvement in one report. The vast majority of cats with laryngeal or tracheal lymphoma respond to either RT (if localized) or systemic chemotherapy (90% CR to COP- or CHOP-based protocols) (Fig. 33.19 ).528, 559 Whereas the author’s experience is that most have durable responses and STs typically approach or exceed 1 year, published reported MSTs range from 5.5 to 9.0 months after achievement of a CR.561
Fig. 33.19.
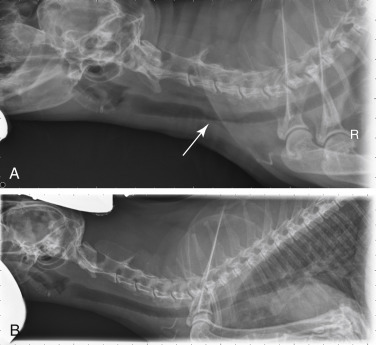
(A) Lateral radiographic projection of a cat with tracheal lymphoma before treatment. (B) The same cat 6 weeks after initiation of CHOP-based chemotherapy protocol.
Ocular Lymphoma
In a compilation of 75 cases of intraocular tumors of cats, 15 (20%) were lymphoma (seven B-cell, four T-cell and four not evaluated).506 It was presumed, but not proved, that the majority of these were part of a systemic multicentric process. Indeed, lymphoma was the most common metastatic cancer in the feline eye. In 26 cats surveyed with systemic lymphoma, nearly half had some ocular changes with uveitis being most common, followed by exophthalmos, corneal ulceration, and chemosis.595 In a histologic characterization of uveitis in cats, lymphoma was diagnosed in approximately one-third of cases; however, whether these were part of a systemic process or limited to the eye was unclear.596 Nearly half of these cases were documented before 1988 and because nearly half were FeLV antigenemic cats, these data likely have little bearing on modern incidence of the disease.
Presumed solitary ocular lymphoma (PSOL) is rare in cats and was identified in 5 of 110 cases of extranodal lymphoma in one report.559 Cats with ocular lymphoma are presented with uveitis or iridial masses, as well as signs related to systemic involvement of disease.
Only sporadic reports appear in the literature with the majority (approximately 75%) being B-cell immunophenotyped. One case of LGL PSOL is documented.559 Intraocular, retinal, and conjunctival locations are reported.597, 598, 599, 600 Outcomes are poorly defined in reports; those cases that underwent enucleation often experienced long-term outcomes with STs of 6 months to 4 years reported.
General Summary of Prognosis for Cats with Lymphoma
As previously discussed, the prediction of outcome in cats with lymphoma is not generalizable because of the wide spectrum of histologic and anatomic subtypes encountered. Much has been mentioned in the previous treatment sections, and Tables 33.9 and 33.10 summarize prognostic parameters for lymphoma in cats.
Feline Leukemias, Myeloproliferative Disorders, and Myelodysplasia
For a complete discussion of leukemias and MPDs, including a general discussion of hematopoiesis, etiologies, lineage classification and descriptions, see Section C of this chapter. The classification of leukemias in cats is difficult because of the similarity of clinical and pathologic features and the transition, overlap, or mixture of cell types involved.601, 602, 603, 604, 605 Most cases are from the FeLV era and generally only single case reports exist from the more contemporary post-FeLV era, which further confuses our understanding of their biology and outcome. For this reason, only a simplistic discussion, primarily relating to the lymphoid leukemias will be presented here and the interested reader is again referred to Section C of this chapter for a general discussion of nonlymphoid leukemia.
For cats with suspected leukemia, peripheral blood assessment (e.g., CBC with differential, flow cytometric analysis for size and immunophenotype, and PARR [for lymphoid leukemias]), and bone marrow aspiration or biopsy may contribute to a diagnosis. Cats with acute leukemia are likely to have malignant cellular infiltrates in organs other than bone marrow.603 A bone marrow aspirate with greater than 30% abnormal blast cells is sufficient to make a diagnosis of an acute leukemia. In cats with suspected CLL, infiltration of the bone marrow with more than 15% mature lymphocytes helps support the diagnosis.606 All cats with leukemia should be tested for FeLV/FIV. Determining the lineage of some leukemias can be challenging; most can be distinguished by histologic appearance, histochemical stains, or immunohistochemical or flow cytometric analysis of the leukemic cells for cellular antigens that identify their lineage (see Chapter 8 and Section C of this chapter).601, 605, 607 In addition, examination of blast cells by electron microscopy may reveal characteristic ultrastructural features. The French–American–British (FAB) classification system is considered useful in cats with myelodysplastic syndromes and almost all reported cases have been FeLV antigenemic.607, 608
Lymphoid Leukemia
ALL was the most commonly encountered type of leukemia in cats in the FeLV era; however, it is much less common today. ALL is characterized by poorly differentiated lymphoblasts and prolymphocytes in blood and bone marrow. Approximately 60% to 80% of cats with ALL are FeLV positive, and most malignant cells have T-cell immunophenotypes609; however, little information is available in the contemporary literature.
CLL is rarely reported in cats and is characterized by well-differentiated, small, mature lymphocytes in peripheral blood and bone marrow.606, 607, 610, 611 In the largest compilation, most cats were older, half were presented incidentally, and half with nonspecific constitutional signs (weight loss, hyporexia).606 No specific serum biochemical abnormalities were noted; however, 50% were anemic, 10% thrombocytopenic, and all had peripheral lymphocyte counts of greater than 9000/μL (although this was the cutoff required for diagnosis). All cats tested (n = 13) were negative for FeLV/FIV. Median peripheral lymphocyte counts were 34,200/μL (range, 9 to >300,000/μL). Whereas most are of the T-cell lineage, B-cell CLL has also been reported in cats.606, 607, 610, 611 In one report of 18 cases of CLL in cats, most were found to be T-helper cells (CD3+/CD4+/CD8–).611
Treatment of Leukemias
The use of chemotherapy to treat ALL has been disappointing. Using COP-based protocols, a 27% CR rate has been reported.612
CLL can be treated with chlorambucil (0.2 mg/kg PO or 2 mg/cat qod; alternatively, 20 mg/m2 q2 weeks) and prednisolone (1 mg/kg PO daily). In 16 cats treated with chlorambucil and prednisolone, approximately 90% responded with a median remission duration of 6 months; however, half achieved CR with a median remission duration of 14 months.606 Several of these cats were rescued with various protocols after recurrence, many with prolonged second remissions. As in humans and dogs, if significant clinical signs or profound cytopenias are not present, treatment can be withheld; one cat with CLL remained stable without chemotherapy for over a year.610
The prognoses for feline ALLs are generally very poor, although some exceptions exist in case report form in the historic literature.
Section C: Canine Acute Myeloid Leukemia, Myeloproliferative Neoplasms, and Myelodysplasia
David M. Vail and Karen M. Young
Myeloproliferative disorders (MPDs) are a group of neoplastic diseases of bone marrow in which there are clonal disorders of hematopoietic stem cells.613 Aberrant proliferation of cells with defective maturation and function leads to reduction of normal hematopoiesis and invasion of other tissues. These disorders have been classified based on biologic behavior, degree of cellular differentiation, and lineage of the neoplastic cells (granulocytic, monocytic, erythroid, megakaryocytic, or mixed). Newer classification systems in humans have incorporated genetics and molecular genetic analysis; these are currently areas of active investigation in the study of animal MPDs.614 In 1991, the Animal Leukemia Study Group made recommendations for classifying nonlymphoid leukemias in dogs and cats.615 More recently, the Oncology Committee of the American College of Veterinary Pathologists (ACVP) has been reexamining criteria for a classification system and spearheading large multiinstitutional studies to validate the criteria. Long-term objectives of these studies are to define molecular lesions, establish prognostic markers, and identify effective therapeutic approaches.616 See Box 33.6 .
BOX 33.6. Key Clinical Summary Points: Myeloid Leukemia, Myeloproliferative Neoplasms, and Myelodysplasia.
-
•
Myeloid leukemias and myeloproliferative disorders are rare neoplastic diseases of bone marrow in which there are clonal disorders of hematopoietic stem cells (Table 33.13).
-
•
Clonal disorders of bone marrow include myeloaplasia (usually referred to as aplastic anemia), myelodysplasia, and myeloproliferation.
-
•
The terms acute and chronic refer to the degree of cellular differentiation of the leukemic cells, but these terms also correlate with the biologic behavior of the neoplasm.
-
•
The acute myeloid leukemias (AMLs) are aggressive leukemias that progress rapidly, are poorly responsive to treatment, and are associated with short survival times (typically <2 months).
-
•
In AML, determination of the leukemic lineage (i.e., neutrophilic, monocytic, erythroid, or megakaryocytic origin) requires advanced diagnostic testing (cytochemical staining, flow cytometric analysis, clonality testing, or genetic analysis).
-
•
Myeloproliferative neoplasms (MPNs), previously termed chronic myeloproliferative disorders, are characterized by excessive production of differentiated bone marrow cells, resulting in the accumulation of erythrocytes (polycythemia vera), granulocytes and/or monocytes (chronic myelogenous leukemia and its variants), or platelets (essential thrombocythemia).
-
•
Because of the degree of differentiation of cells in MPNs, these disorders must be distinguished from nonneoplastic causes of increases in these cell types.
-
•
Compared with AMLs, MPNs and myelodysplasias have a more chronic course and may have better responses to therapy, although most will progress and ultimately result in mortality.
Incidence and Risk Factors
Myeloid neoplasms are uncommon or rare in the dog and occur 10 times less frequently than lymphoproliferative disorders.617 Accurate information about incidence and other epidemiologic information has been generally lacking owing to a lack of a consistent use of a uniform classification system; however, several larger compilations have been published recently.618, 619, 620, 621, 622 There is no consistent breed predilection; most are large-breed dogs with a median age of 7 to 8 years, although acute myeloid leukemia (AML) can occur in dogs as young as 7 months of age. AML occurred more frequently in males than females (2:1 ratio) in two large compilations.618, 621 In dogs, the etiology of spontaneously occurring leukemia is unknown. It is likely that genetic and environmental factors (including exposure to radiation, drugs, or toxic chemicals) play a role. In humans, acquired chromosomal derangements lead to clonal overgrowth with arrested development.623 Chromosomal abnormalities have been reported in dogs with AML, chronic myelogenous leukemia (CML), and lymphoid leukemia.624–627However, because karyotyping is difficult to perform in dogs because of the large number and morphologic similarity of their chromosomes and their resistance to banding, defining genetic factors in canine myeloid neoplasms has awaited application of molecular technologies and use of the canine genome mapping.614, 626, 628, 629, 630 Certain forms of leukemia in dogs have been produced experimentally after irradiation.631, 632, 633 In contrast to MPDs in cats, no causative viral agent has been demonstrated in dogs, although retrovirus-like budding particles were observed in the neoplastic cells of a dog with granulocytic leukemia.634
Pathology and Natural Behavior
A review of normal hematopoiesis will aid in understanding the various manifestations of MPDs. Hematopoiesis is the process of proliferation, differentiation, and maturation of stem cells into terminally differentiated blood cells. A simplified scheme is presented in Fig. 33.20 . Pluripotent stem cells differentiate into either lymphopoietic or hematopoietic multipotent stem cells.635 Under the influence of specific regulatory and microenvironmental factors, multipotent stem cells in bone marrow differentiate into progenitor cells committed to a specific hematopoietic cell line, for example, erythroid, granulocytic-monocytic, or megakaryocytic. Maturation results in the production of terminally differentiated blood cells (erythrocytes, granulocytes, monocytes, and platelets) that are delivered to the circulation. In some cases, as in the maturation of reticulocytes to erythrocytes, final development may occur in the spleen.
Fig. 33.20.
A simplified scheme of hematopoiesis. BFU, Blast-forming units; CFU, colony-forming units; E, erythroid; EO, eosinophil; GM, granulocytic-monocytic; MEG, megakaryocyte.
The proliferation and differentiation of hematopoietic cells are controlled by a group of regulatory growth factors.635, 636 Of these, erythropoietin is the best characterized; it regulates erythroid proliferation and differentiation and is produced in the kidney, where changes in oxygen tension are detected. The myeloid compartment depends on a group of factors, collectively referred to as colony-stimulating factors (CSFs). These factors act at the level of the committed progenitor cells, but also influence the functional capabilities of mature cells. Some of these factors have a broad spectrum of activity; others are more restricted in their target cells and actions. CSFs are produced in vitro by a multitude of cell types, including monocytes, macrophages, lymphocytes, and endothelial cells, and these cells likely play a role in the production and regulation of these factors in vivo. The gene for thrombopoietin also has been cloned, and it appears that this hormone alone can induce differentiation of megakaryocytes and platelet production.637 Recombinant forms of many of these growth factors are increasingly available.
Clonal disorders of bone marrow include myeloaplasia (usually referred to as aplastic anemia), myelodysplasia, and myeloproliferation. A preleukemic syndrome, characterized by peripheral pancytopenia and bone marrow hyperplasia with maturation arrest, is more correctly termed myelodysplasia because the syndrome does not always progress to overt leukemia. This syndrome has been described in cats, usually in association with FeLV infection, but is rarely recognized in dogs.638, 639, 640, 641 These clonal disorders may be manifested by abnormalities in any or all lineages because hematopoietic cells share a common stem cell. In addition, transformation from one form to another may occur.642
Myeloid neoplasms are classified in several ways. The terms acute and chronic refer to the degree of cellular differentiation of the leukemic cells, but these terms also correlate with the biologic behavior of the neoplasm.643 Disorders resulting from uncontrolled proliferation or decreased apoptosis of cells incapable of maturation lead to the accumulation of poorly differentiated or “blast” cells. These disorders are included under the umbrella term of AML. Disorders resulting from unregulated proliferation of cells that exhibit progressive, albeit incomplete and defective, maturation lead to the accumulation of differentiated cells and thus are called chronic disorders. These disorders are termed myeloproliferative neoplasms (MPN) and include polycythemia vera, CML and its variants, essential thrombocythemia, and possibly primary myelofibrosis. Myeloid neoplasms are further classified by the lineage of the dominant cell type(s), defined by Romanowsky stains, special cytochemical stains, ultrastructural features, flow cytometric analysis, molecular genetic analysis, and immunologic cell markers, and they have been classified into subtypes.
AML has a more sudden onset and is more clinically aggressive. In both acute and chronic disorders, however, abnormalities in proliferation, maturation, and functional characteristics can occur in any hematopoietic cell line.613 In addition, normal hematopoiesis is adversely affected. Animals with acute leukemias usually have decreased numbers of circulating normal cells. The pathogenesis of cytopenias is complex and may result in part from production of inhibitory factors. Eventually, neoplastic cells displace normal hematopoietic cells, termed myelophthisis. Anemia and thrombocytopenia are particularly common. Neutropenia and thrombocytopenia result in infection and hemorrhage, respectively, which may be more deleterious to the animal than the primary disease process.
Acute Myeloid Leukemia
AML is rare and is characterized by aberrant proliferation and/or decreased apoptosis of a clone of cells without maturation. This results in accumulation of immature blast cells in bone marrow and peripheral blood (Fig. 33.21 ). The white blood cell (WBC) count is variable and ranges from leukopenia to counts greater than 250,000/μL. Spleen, liver, and lymph nodes are frequently involved, and other tissues, including tonsils, kidney, heart, and the CNS, may be infiltrated as well.621 The median age is approximately 7 to 8 years; however, young dogs may be affected.618, 619, 620, 621, 644 The clinical course of these disorders tends to be rapid. Production of normal peripheral blood cells is usually diminished or absent, and anemia, neutropenia, and thrombocytopenia are common with infection and hemorrhage occurring as frequent sequelae. Occasionally, neoplastic blasts are present in bone marrow, but not in peripheral blood. This is termed aleukemic leukemia, whereas subleukemic suggests a normal or decreased WBC count with some neoplastic cells in circulation.
Fig. 33.21.
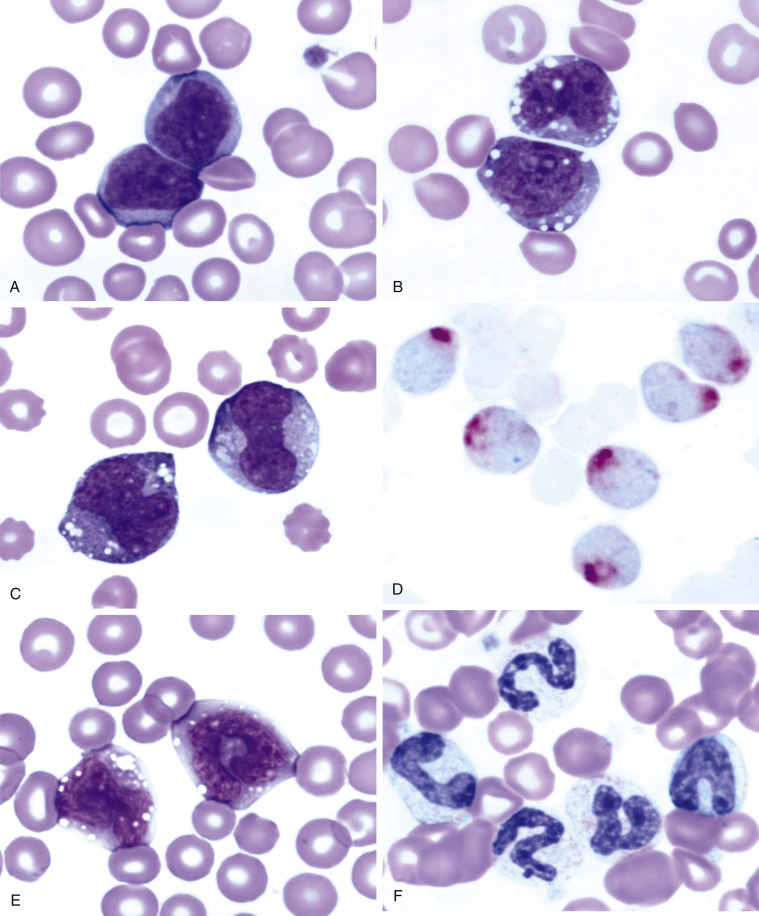
Peripheral blood from dogs with myeloid neoplasms. All diagnoses were confirmed by cytochemical staining. Note how similar the blast cells appear in A–C. (A) Acute myeloblastic leukemia (M1). Wright’s stain, ×100 objective. (B) Acute myelomonocytic leukemia (M4). Wright’s stain, ×100 objective. (C) Acute monocytic leukemia (M5a). Wright’s stain. (D) Acute monocytic leukemia (M5a). Cytochemical stain: α-naphthyl butyrate esterase (nonspecific esterase) with red reaction product. (E) Acute monocytic leukemia with some differentiation (M5b). Wright’s stain, ×100 objective. (F) Chronic myelogenous leukemia (CML). Wright’s stain, ×100 objective.
In 1985, the Animal Leukemia Study Group was formed under the auspices of the American Society for Veterinary Clinical Pathology to develop specific morphologic and cytochemical criteria for classifying acute nonlymphocytic leukemias. Recognition of specific subtypes of leukemia is required to compile accurate and useful information about prognosis and response to treatment, as well as to compare studies from different sites. In 1991 this group proposed a classification system following adaptation of the FAB system and criteria established by the NCI Workshop.615 Group members examined blood and bone marrow from 49 dogs and cats with myeloid neoplasms. Romanowsky-stained specimens were examined first to identify blast cells and their percentages. Lineage specificity was then determined using cytochemical markers. The percentage of blasts and the information about lineage specificity were used in combination to classify disorders as acute undifferentiated leukemia (AUL), AML (subtypes M1–M5 and M7), and erythroleukemia with or without erythroid predominance (M6 and M6Er). A description of these subtypes is presented in Table 33.13 .
TABLE 33.13.
Subtypes of Leukemias and Dysplasias Adapting the FAB System
| Subtype |
Description |
|---|---|
| Acute Leukemias | |
| AUL | Acute undifferentiated leukemia (formerly called reticuloendotheliosis) |
| M1 | Myeloblastic leukemia, without differentiation |
| M2 | Myeloblastic leukemia, with some neutrophilic differentiation |
| M3 | Promyelocytic leukemia (not recognized in animals) |
| M4 | Myelomonocytic leukemia |
| M5a | Monocytic leukemia, without differentiation |
| M5b | Monocytic leukemia, with some monocytic differentiation |
| M6 | Erythroleukemia |
| M6Er | Variant of M6 with erythroblasts comprising erythroid component |
| M7 | Megakaryoblastic leukemia |
| Chronic Myeloid Leukemias | |
| CML | Chronic myelogenous leukemia |
| CMML | Chronic myelomonocytic leukemia |
| CMoL | Chronic monocytic leukemia |
| Hematopoietic Dysplasia | |
| MDS | Myelodysplastic syndrome |
| MDS-Er | Myelodysplastic syndrome with erythroid predominance |
Because the modified FAB system has been adopted only recently, the names given to these disorders in the literature vary considerably. In addition, in the absence of cytochemical staining, immunophenotyping, or electron microscopic evaluation, the specific subtype of leukemia has often been uncertain, making retrospective analysis of epidemiologic information, prognosis, and response to therapy confusing at best. Although defining specific subtypes may seem to be an academic exercise owing to the uniformly poor prognosis of acute leukemias, this information is critical to improving their management. Because of the low incidence of AML, national and international cooperative efforts will be required to accumulate information on the pathogenesis and response to different treatment modalities of specific subtypes. Utilization of a uniform classification system is an essential first step. Different forms of AML are demonstrated in Fig. 33.21. With the exception of acute promyelocytic leukemia or M3, all AML subtypes have been described in dogs. Combining three recent compilations of 85 dogs with AML, the relative frequency of subtypes in decreasing order were: 42% monocytic leukemia (M5a, M5b), 33% myelomonocytic leukemia (M4), 13% myeloblastic leukemia without differentiation (M1), 5% megakaryoblastic leukemia (M7), and one each of myeloblastic leukemia with some differentiation (M2) and erythroleukemia (M6).619, 620, 621 AML of mixed lineages comprised 5% of cases. Many single case or small case series reports also exist describing various subtypes in dogs.617, 632, 633, 634, 635, 636, 637, 639, 640, 641, 642, 643, 644, 645, 646, 647, 648, 649, 650, 651, 652, 653, 654, 655, 656, 657, 658, 659, 660, 661, 662, 663, 664, 665, 666, 667, 668, 669, 670, 671 Monocytic leukemias have likely included those with and without monocytic differentiation (M5a and M5b),672, 673 but in some cases the diagnosis may have been chronic myelomonocytic or chronic monocytic leukemia. There are few reports in dogs of spontaneously occurring erythroleukemia (M6) in which the leukemic cells include myeloblasts, monoblasts, and erythroid elements.674, 675, 676 AULs have uncertain lineages because they are negative for all cytochemical markers. These leukemias should be distinguished from lymphoid leukemias by flow cytometric analysis of the leukemic cells for cellular antigens that identify their lineage.620, 677 In addition, examination of blast cells by electron microscopy may reveal characteristic ultrastructural features.
Canine karyotyping is difficult, but with advancements in molecular cytogenetic analysis, chromosome painting, and genomic hybridization, AML in dogs can now be analyzed at the base-pair level,626, 628, 629 and missense mutations in flt3, c-kit, and ras sequences have been identified in dogs with AML, similar to what has been found for human AML.678 BCR–ABL translocation is also reported in dogs with acute myeloblastic leukemia.679 Recurrent DNA copy number abnormalities (CNA) have been interrogated in 24 dogs with AML and there is potential for CNA clustering to be used in diagnostic models.626 In addition to serving as diagnostic and prognostic markers, cytogenetic lesions may be therapeutic targets. As cytogenetic abnormalities continue to be identified, this information will need to be incorporated into classification schemes.
Myeloproliferative Neoplasms
Myeloproliferative neoplasms (MPNs), previously termed chronic myeloproliferative disorders, are characterized by excessive production of differentiated bone marrow cells, resulting in the accumulation of erythrocytes (polycythemia vera), granulocytes and/or monocytes (CML and its variants), or platelets (essential thrombocythemia). Primary myelofibrosis, a clonal disorder of bone marrow stromal cells characterized by proliferation of megakaryocytes and granulocytic precursors with accumulation of collagen in bone marrow, has been recognized only rarely in animals. Phenotypic-driver mutations in the JAK2, CALR, and MPL genes have been identified in people with polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis.680 Only JAK2 mutations have been investigated in dogs with PV, and an identical mutation to that in people was found in 1 of 5 cases in dogs.681 Myelofibrosis is considered a response to injury and may occur secondary to other neoplasms, systemic inflammation, drug exposure, or FeLV infection in cats.
Polycythemia Vera
Polycythemia vera (PV) is a clonal disorder of stem cells, although whether the defect is in the pluripotent stem cell or the hematopoietic multipotent stem cell is still not clear. The disease is rare and must be distinguished from more common causes of polycythemia. In PV, there is neoplastic proliferation of the erythroid series with terminal differentiation to RBCs. The disease has been reported in dogs that tend to be middle-aged with no breed or sex predilection682, 683, 684, 685, 686, 687, 688, 689, 690, 691, 692 and is characterized by an increased RBC mass evidenced by an increased packed cell volume (PCV), RBC count, and hemoglobin concentration. The PCV is typically in the range of 65% to 85%. The bone marrow is hyperplastic, although the myeloid-to-erythroid (M:E) ratio tends to be normal. In contrast to the disease in humans, other cell lines do not appear to be involved and transformation to other MPNs has not been reported. The disease in dogs may be more appropriately termed primary erythrocytosis. In humans, progenitor cells have an increased sensitivity to insulin-like growth factor 1, which stimulates hematopoiesis.691 It is not known whether this hypersensitivity is the primary defect or is secondary to another gene mutation. In any case, the result is overproduction of red blood cells (RBCs). Acquired JAK2 gene mutations are identified in 90% of humans with PV, and an identical mutation in the JAK2 gene of 1 of 5 dogs with PV was reported.681
Chronic Myelogenous Leukemia
In dogs, CML is more similar to chronic neutrophilic leukemia, a rare form of MPN in humans, than to CML in humans because it is a neoplastic proliferation of the neutrophil series, although concurrent eosinophilic and basophilic differentiation can occur. CML can occur in dogs of any age.693, 694, 695, 696, 697, 698 Neutrophils and neutrophilic precursors accumulate in bone marrow and peripheral blood as well as in other organs. The peripheral WBC count is usually, but not always, greater than 100,000/μL. Both immature and mature neutrophils are present (see Fig. 33.21F). Mature forms are usually more numerous, but sometimes an “uneven” left shift is present. Signs of dysplasia may be evident, including hypersegmentation, ringed nuclei, and giant forms. Eosinophils and basophils may also be increased. The bone marrow is characterized by granulocytic hyperplasia and morphologic abnormalities may not be present. Erythroid and megakaryocytic lines may be affected, resulting in anemia, thrombocytopenia, or less commonly, thrombocytosis. This disorder must be distinguished from severe or extreme neutrophilic leukocytosis (“leukemoid reactions”) caused by inflammation or immune-mediated diseases. Extreme neutrophilia can also occur as a paraneoplastic syndrome. In humans with CML, characteristic cytogenetic abnormalities are present in all bone marrow cells, signifying a lesion at the level of an early multipotent stem cell. Typically, these individuals have a chromosomal translocation, resulting in the Philadelphia chromosome or BCR–ABL translocation between chromosomes 9 and 22.699 The analogous chromosomes in dogs are chromosomes 9 and 26, and BCR–ABL translocation, termed the “Raleigh chromosome,” has been reported in several cases of CML in dogs.614, 627, 700, 701 Variants of CML are chronic myelomonocytic leukemia and chronic monocytic leukemia (CMoL).702, 703, 704 CMoL has also been associated with BCR–ABL translocation in the dog.702 These diagnoses are made based on the percentage of monocytes in the leukemic cell population. An infrequent myeloproliferative neoplasm, atypical chronic myeloid leukemia, has been reported in a dog and had features of both myelodysplastic syndrome and chronic leukemia.700 In this dog, BCR–ABL translocation was present in fewer than 10% of cells, considered a negative finding.
In addition to accumulating in bone marrow and peripheral blood, leukemic cells also are found in the red pulp of the spleen, the periportal and sinusoidal areas of the liver, and sometimes lymph nodes. Other organs, such as the kidney, heart, and lung, are less commonly affected. In addition, extramedullary hematopoiesis may be present in the liver and spleen. Death is usually due to complications of infection or hemorrhage secondary to neutrophil dysfunction and thrombocytopenia, respectively. In some cases, CML may terminate in “blast crisis,” in which there is a transformation from a predominance of well-differentiated granulocytes to excessive numbers of poorly differentiated blast cells in peripheral blood and bone marrow. This phenomenon is well documented in the dog.693, 696, 698
Basophilic and Eosinophilic Leukemia
Basophilic leukemia, although rare, has been reported in dogs and is characterized by an increased WBC count with a high proportion of basophils in peripheral blood and bone marrow.705, 706, 707 Hepatosplenomegaly, lymphadenopathy, and thrombocytosis may be present. All the dogs have been anemic. Basophilic leukemia should be distinguished from mast cell leukemia (mastocytosis). Whether dogs develop eosinophilic leukemia remains in question. Reported cases have had high blood eosinophil counts and eosinophilic infiltrates in organs.708, 709 One dog responded well to treatment with corticosteroids. The distinction between neoplastic proliferation of eosinophils and idiopathic hypereosinophilic syndrome remains elusive. Nonmyeloproliferative disorders associated with eosinophilia such as parasitism, skin diseases, or diseases of the respiratory and GI tracts should be considered first in an animal with eosinophilia. One distinguishing feature should be clonality, with reactive eosinophilia comprising polyclonal cells and the neoplastic condition arising from a single clone. As clonality assays become more available, this discrepancy may be resolved.
Essential Thrombocythemia
In humans, ET, or primary thrombocytosis, is characterized by platelet counts that are persistently greater than 600,000/μL. There are no blast cells in circulation and marked megakaryocytic hyperplasia of the bone marrow without myelofibrosis is present. Thrombosis and bleeding are the most common sequelae, and most patients have splenomegaly. Other MPDs, especially PV, should be ruled out, and importantly, there should be no primary disorders associated with reactive thrombocytosis,710 including inflammation, hemolytic anemia, iron deficiency anemia, malignancies, recovery from severe hemorrhage, rebound from immune-mediated thrombocytopenia, and splenectomy. In addition, certain drugs such as vincristine can induce thrombocytosis. ET has been recognized in dogs.642, 711, 712, 713, 714 In one dog, the platelet count exceeded 4 million/μL and bizarre giant forms with abnormal granulation were present. The bone marrow contained increased numbers of megakaryocytes and megakaryoblasts, but circulating blast cells were not seen. Other findings included splenomegaly, GI bleeding, and increased numbers of circulating basophils. Causes of secondary or reactive thrombocytosis were ruled out.713 Basophilia was also reported in a more recent case.711 In another dog, ET was diagnosed and then progressed to CML.642 In some cases reported in the literature as ET, the dogs had microcytic hypochromic anemias. Because iron deficiency anemia is associated with reactive or secondary thrombocytosis, care must be taken to rule out this disorder. However, spurious microcytosis may be reported if a dog has many giant platelets that are counted by some analyzers as small RBCs.712 Microscopic review of the blood film may be helpful in these cases.
Other Bone Marrow Disorders
Myelofibrosis
Primary myelofibrosis has been reported only rarely in dogs, and myelofibrosis is more typically a secondary, or reactive, process.715, 716 In humans, myelofibrosis is characterized by collagen deposition in bone marrow and increased numbers of megakaryocytes and granulocytic precursors, many of which exhibit morphologic abnormalities. In fact, breakdown of intramedullary megakaryocytes and subsequent release of factors that promote fibroblast proliferation or inhibit collagen breakdown may be the underlying pathogenesis of the fibrosis.717 Focal osteosclerosis is sometimes present. Anemia, thrombocytopenia, splenomegaly, and myeloid metaplasia (production of hematopoietic cells outside the bone marrow) are consistent features.
In dogs, myelofibrosis occurs secondary to MPDs, radiation damage, and congenital hemolytic anemias.718, 719, 720, 721 In some cases, the inciting cause is unknown (idiopathic myelofibrosis). There may be concurrent marrow necrosis in cases of ehrlichiosis, septicemia, or drug toxicity (estrogens, cephalosporins), and there is speculation that fibroblasts proliferate in response to release of inflammatory mediators associated with the necrosis.715 Myeloid metaplasia has been reported to occur in the liver, spleen, and lung.721 Extramedullary hematopoiesis is ineffective in preventing or correcting the pancytopenia that eventually develops.
Myelodysplastic Syndrome
Dysfunction of the hematopoietic system can be manifested by a variety of abnormalities that constitute myelodysplastic syndrome (MDS). In dogs, in which the syndrome is rare, there usually are cytopenias in two or three lines in the peripheral blood (anemia, neutropenia, and/or thrombocytopenia). Other blood abnormalities can include macrocytic erythrocytes and metarubricytosis. The bone marrow is typically normocellular or hypercellular, and dysplastic changes are evident in several cell lines. If blast cells are present, they make up fewer than 30% of all nucleated cells,614 although this threshold is being changed to less than 20%.616, 722 Myelodysplasia is sometimes referred to as preleukemia because, in some cases, it may progress to acute leukemia.639, 640, 641 Based on reported cases, poor prognostic factors include increased percentage of blast cells, cytopenias involving more than one lineage, and cellular atypia.616 Primary MDSs are clonal disorders and are considered neoplastic. Complex classification schemes for human MDS, based on percentages of blasts in bone marrow, cytogenetic analysis, cytopenias, need for transfusions, and other variables, comprise at least nine subtypes; their applicability to veterinary medicine is unknown.617 Three subtypes are proposed for dogs and cats and include MDS with excessive blasts (MDS-EB), in which blast percentages are greater than 5% and less than 20%, and progression to AML may occur; MDS with refractory cytopenia (MDS-RC) with blast percentages less than 5% and cytopenias in one or more lineages; and MDS with erythroid predominance (MDS-ER) in which the M:E ratio is less than one and prognosis is poor.616 Larger studies are needed to determine the utility of this classification scheme and other potential prognostic factors, such as sex and age and, in cats, FeLV positivity. In addition to accumulating enough cases, another confounding factor to studying and classifying MDS is the presence of reversible MDSs that occur secondary to immune-mediated, infectious, and other diseases in both dogs and cats.
History and Clinical Signs
Dogs with myeloid neoplasms have similar presentations regardless of the specific disease entity, although animals with AML have a more acute onset of illness and a more rapid clinical course. A history of constitutional signs (e.g., lethargy, hyporexia, and weight loss) is common.618, 619, 620, 621 Clinical signs include emaciation, persistent fever, pallor, and petechiation. Peripheral lymphadenopathy is reported in 40% to 75% of cases and hepatosplenomegaly in approximately 40% of cases. Shifting leg lameness, ocular lesions, and recurrent infections are also seen. Vomiting, diarrhea, dyspnea, and neurologic signs are variable features. Serum biochemical analytes may be within reference intervals, but can change if significant organ infiltration occurs. Animals with MDS may be lethargic and anorectic and have pallor, fever, and hepatosplenomegaly. In PV, dogs often have erythema of mucous membranes owing to the increase in RBC mass. Some dogs are polydipsic. In addition, neurologic signs such as disorientation, ataxia, or seizures may be present and are thought to be the result of hyperviscosity or hypervolemia.690 Hepatosplenomegaly is usually absent.
Peripheral blood abnormalities are consistently found in more than 90% of cases.615, 618, 619, 620, 621 In addition to the presence of neoplastic cells, other abnormalities, including bi- and pancytopenia, may be present. Low numbers of nucleated RBCs are present in the blood of about half the dogs with acute nonlymphocytic leukemia. Nonregenerative anemia and thrombocytopenia are present in most cases. Anemia is usually normocytic and normochromic, although macrocytic anemia is sometimes present. Pathogenic mechanisms include effects of inhibitory factors leading to ineffective hematopoiesis, myelophthisis, immune-mediated anemia secondary to neoplasia, and hemorrhage secondary to thrombocytopenia, platelet dysfunction, or disseminated intravascular coagulation. Anemia is most severe in AML, although both anemia and thrombocytopenia may be milder in animals with the M5 subtype (acute monocytic leukemia). In myelofibrosis, anemia is characterized by anisocytosis and poikilocytosis. In addition, pancytopenia and leukoerythroblastosis, in which immature erythroid and myeloid cells are in circulation, may be present. These phenomena probably result from replacement of marrow by fibrous tissue with resultant shearing of red cells and escape of immature cells normally confined to bone marrow. In PV, the PCV is increased, usually in the range of 65% to 85%. The bone marrow is hyperplastic and the M:E ratio is usually in the normal range.
Neoplastic cells are often defective functionally. Platelet dysfunction has been reported in a dog with acute megakaryoblastic leukemia (M7)658; and, in CML, neutrophils have decreased phagocytic capacity and other abnormalities. One exception to this was a report of CML in a dog in which the neutrophils had enhanced phagocytic capacity and superoxide production.723 The authors hypothesized that increased synthesis of granulocyte-macrophage (GM)-CSF resulted from a lactoferrin deficiency in the neoplastic neutrophils and mediated the enhanced function of these cells.
Diagnostic Techniques and Workup
In all cases of myeloid neoplasms, diagnosis depends on examination of peripheral blood and bone marrow. AML is diagnosed on the basis of finding blast cells with clearly visible nucleoli in blood and bone marrow. Most dogs with acute leukemia have circulating blasts. These cells may be present in low numbers in peripheral blood, and careful examination of the smear, especially at the feathered edge, should be made. Even if blasts are not detected in circulation, indications of bone marrow disease such as nonregenerative anemia or thrombocytopenia are usually present. Occasionally, neoplastic cells can be found in CSF in animals with invasion of the CNS. Smears of aspirates from tissues such as the lymph nodes, spleen, or liver may contain blasts but usually contribute little to the diagnostic workup.
Examination of blasts stained with standard Romanowsky stains may give clues as to the lineage of the cells (see Fig. 33.21A–C, and E). In myelomonocytic leukemia, the nuclei of the blasts are usually pleomorphic, with round to lobulated forms. In some cells, the cytoplasm may contain large azurophilic granules or vacuoles. Blasts in megakaryocytic leukemia may contain vacuoles and have cytoplasmic blebs. In addition, bizarre macroplatelets may be present. Although these distinguishing morphologic features may suggest a definitive diagnosis, cytochemical staining, immunophenotyping, flow cytometric analysis, clonality testing, and genetic analysis are usually required to definitively define the lineage of the blasts; the reader is referred to several large compilations for which these methodologies have been discussed and applied in dogs.618, 619, 620, 621, 626, 671, 724, 725, 726 Several investigators have reported modification of diagnoses after cytochemical staining. It is especially important to distinguish AML from lymphocytic leukemia to provide accurate prognostic information to the owner and institute appropriate therapy.
The Animal Leukemia Group has recommended the following diagnostic criteria, summarized in Fig. 33.22 .615 Using well-prepared Romanowsky-stained blood and bone marrow films, a minimum of 200 cells are counted to determine the leukocyte differential in blood and the percentage of blast cells in bone marrow and/or blood. In bone marrow, blast cells are calculated both as a percentage of all nucleated cells (ANC) and nonerythroid cells, and are further characterized using cytochemical markers.724, 725, 727 Neutrophil differentiation is identified by positive staining of blasts for peroxidase, Sudan Black B, and chloracetate esterase. Nonspecific esterases (α-naphthyl acetate esterase or α-naphthyl butyrate esterase), especially if they are inhibited by sodium fluoride, mark monocytes. Canine monocytes may also contain a few peroxidase-positive granules. Acetylcholinesterase is a marker for megakaryocytes in dogs and cats. In addition, positive immunostaining for von Willebrand’s factor (factor VIII–related antigen) and platelet glycoproteins on the surface of blasts identifies them as megakaryocyte precursors.648, 658, 659, 661, 665, 667 Alkaline phosphatase (ALP) only rarely marks normal cells in dogs and cats, but is present in blasts cells in acute myeloblastic and myelomonocytic leukemias. However, owing to reports of ALP activity in lymphoid leukemias in dogs, its specificity as a marker for myeloid cells is not certain. A recent study indicated that ALP was a useful marker for the diagnosis of AML if neoplastic cells express only CD34.728 Omega exonuclease is a specific marker for basophils, which are also positive for chloracetate esterase activity.707
Fig. 33.22.
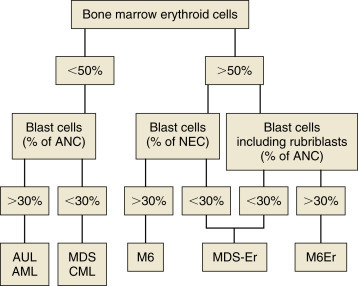
A scheme to classify myeloid neoplasms and myelodysplastic syndromes in dogs and cats. AML, Acute myeloid leukemias M1–M5 and M7; ANC, all nucleated cells in bone marrow, including lymphocytes, plasma cells, macrophages, and mast cells; AUL, acute undifferentiated leukemia; Blast cells, myeloblasts, monoblasts, and megakaryoblasts; CML, chronic myeloid leukemias, including chronic myelogenous, chronic myelomonocytic, and chronic monocytic leukemias; M6, erythroleukemia; M6Er, erythroleukemia with erythroid predominance; MDS, myelodysplastic syndrome; MDS-Er, myelodysplastic syndrome with erythroid predominance; NEC, nonerythroid cells in bone marrow.
Reprinted with permission from Jain NC, Blue JT, Grindem CB, et al. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet Clin Pathol. 1991;20(3):63-82.
Blood and bone marrow differential counts and cytochemical staining should be performed and interpreted by experienced veterinary cytopathologists. If erythroid cells are less than 50% of ANC and the blast cells are greater than 20%, a diagnosis of AML or AUL is made. If erythroid cells are greater than 50% of ANC and the blast cells are greater than 20%, a diagnosis of erythroleukemia (M6) is made. If rubriblasts are a significant proportion of the blast cells, a diagnosis of M6Er, or erythroleukemia with erythroid predominance, can be made. It should be noted that in the human AML classification system, the blast threshold has been lowered from 30% to 20%, and similar recommendations are now made for AML in dogs and cats.
In some cases, electron microscopy is required to identify the lineage of the blast cells. For example, megakaryocyte precursors are positive for platelet peroxidase activity and contain demarcation membranes and alpha granules.648, 665 Both of these features are detected at the ultrastructural level. Increasingly, cytogenetic abnormalities are being identified in animal leukemias; cytogenetic analysis may yield important diagnostic and prognostic information and become a valuable tool for identifying targeted therapeutic approaches.
Although morphologic and cytochemical analyses have formed the mainstay of cell identification, newer technologies now are routinely used to classify leukemias by using monoclonal antibodies to detect antigens associated with certain cell types. Cells can be immunophenotyped using flow cytometric analysis or immunocytochemistry.618, 619, 620, 621, 671, 677, 722, 729, 730, 731, 732 Cells from both acute lymphoid leukemia and AML are positive for CD34. Many lymphocyte markers, including CD3, CD4, CD8, CD21, CD79, and IgG, are available for dogs and can be used to rule out lymphoblastic leukemia in dogs with acute leukemias. Other markers include myeloperoxidase (MPO) and CD11b for myeloid cells and CD41 for megakaryoblasts. There is some overlap in expression of these cellular antigens. For example, canine (but not human) granulocytes express CD4. It is highly recommended to use a panel of antibodies (similar to using a battery of cytochemical stains) because antigens are often expressed on multiple lineages, and lineage infidelity can occur. These tests have become more valuable with the availability of canine reagents. Currently, the ACVP Oncology Committee recommends that the following immunophenotyping panel be done on bone marrow and/or blood smears to characterize animal leukemias: for B lymphocytes, CD79a; for T lymphocytes, CD3; for myeloid cells, MPO and CD11b; for megakaryoblasts, CD41; for dendritic cells, CD1c; and for acute leukemias, CD34.632 In 2 large reports of 60 cases of AML in dogs, most were CD45/CD18/CD34 positive and, in one report, 64% had clonal or biclonal rearrangements of either the T- or B-cell receptor.619, 621
Because of the degree of differentiation of cells in MPN, these disorders must be distinguished from nonneoplastic causes of increases in these cell types. To make a diagnosis of PV, it must first be established that the polycythemia is absolute rather than relative. In relative polycythemias, plasma volume is decreased from hemoconcentration, dehydration, or hypovolemia, and the absolute RBC mass is not increased. Splenic contraction can also result in relative polycythemia. Absolute polycythemia, in which RBC mass is increased, is usually secondary to tissue hypoxia, causing appropriately increased production of erythropoietin. Rarely, erythropoietin may be produced inappropriately by a tumor (e.g., renal cell carcinoma) or in renal disease (pyelonephritis) or localized renal hypoxia.733, 734, 735 These causes of polycythemia should be eliminated by appropriate laboratory work, thoracic radiographs, arterial blood gas analysis, and renal ultrasonography. In humans with PV, plasma erythropoietin (EPO) concentrations are low. EPO concentrations in dogs with PV tend to be low or low-normal, whereas in animals with secondary absolute polycythemia, the levels are high.736, 737 Samples for determination of EPO concentrations should be taken before therapeutic phlebotomy used to treat hyperviscosity and, owing to fluctuations in EPO concentrations, should be repeated if results are incongruous with other information.
There are no pathognomonic features of CML in dogs, and other common causes for marked leukocytosis with a left shift (extreme neutrophilia) and granulocytic hyperplasia of bone marrow must be eliminated. These include infections, especially pyogenic infections; immune-mediated diseases; and some neoplasms that cause neutrophilia by elaborating CSFs. In CML, maturation sometimes appears disorderly, and there may be variation in the size and shape of neutrophils at the same level of maturation. In addition, neoplastic leukocytes may disintegrate more rapidly and appear vacuolated.697 Because of the invasive nature of CML, biopsy of liver or spleen may also help distinguish true leukemia from a leukemoid reaction, assuming the animal can tolerate the procedure. Fluorescent in situ hybridization analysis is available to identify chromosomal rearrangements, including translocations (e.g., Raleigh chromosome), inversions, and deletions, in dogs; some of these aberrations are associated with certain forms of leukemia, and continued investigations will likely yield a larger database of cytogenetic abnormalities and their links to hematologic malignancies.614, 624, 625, 626, 627, 628, 629, 630, 678, 679, 681, 699, 700, 701, 702
Basophilic leukemia is diagnosed by finding excessive numbers of basophils in circulation and in bone marrow. Basophilic leukemia must be differentiated from mastocytosis based on the morphology of the cell type present. Basophils have a segmented “ribbon-like” nucleus and variably sized granules, whereas mast cells have a round-to-oval nucleus that may be partially or totally obscured by small, round, metachromatic-staining granules. This distinction is usually easy to make; however, in basophilic leukemia, changes in the morphology of the nucleus and granules make the distinction less clear.706
ET has been diagnosed based on finding persistent and excessive thrombocytosis (>600,000/μL) without circulating blast cells and in the absence of another MPD (e.g., PV), myelofibrosis, or disorders known to cause secondary thrombocytosis,710 including iron deficiency anemia, chronic inflammatory diseases, recovery from severe hemorrhage, rebound from immune-mediated thrombocytopenia, and absence of a spleen. Thrombocytosis is transient in these disorders or abates with resolution of the primary disease. In ET, platelet morphology may be abnormal with bizarre giant forms and abnormal granulation.713 In the bone marrow, megakaryocytic hyperplasia is a consistent feature and dysplastic changes may be evident in megakaryocytes.712 Spurious hyperkalemia may be present in serum samples from dogs with thrombocytosis from any cause due to the release of potassium from platelets during clot formation.738 Measuring potassium in plasma is recommended in these cases and usually demonstrates a potassium concentration within reference interval. Platelet aggregability has been variably reported as impaired713 or enhanced.712 In the one dog in which it was measured, plasma thrombopoietin (TPO) concentration was normal.711 It is unclear whether TPO plays a role in ET or is suppressed by the high platelet mass.
In MDS, abnormalities in two or three cell lines are usually manifested in peripheral blood as neutropenia with or without a left shift, nonregenerative anemia, or thrombocytopenia. Other changes include macrocytosis and metarubricytosis. The bone marrow is typically normocellular or hypercellular with an increased M:E ratio, and blasts cells, although increased, constitute less than 20% of nucleated cells; in a report of 13 dogs with primary or secondary MDS, in all but one dog the blast cell percentage was less than 20%.739 Dysplastic changes can be detected in any cell line. Dyserythropoiesis is characterized by asynchronous maturation of erythroid cells typified by large hemoglobinized cells with immature nuclei (megaloblastic change). If the erythroid component is dominant, the MDS is called MDS-Er (see Table 33.13).615, 638 In dysgranulopoiesis, giant neutrophil precursors and abnormalities in nuclear segmentation and cytoplasmic granulation can be seen. Finally, dysthrombopoiesis is characterized by giant platelets and micromegakaryocytes.
Myelofibrosis should be suspected in animals with nonregenerative anemia or pancytopenia, abnormalities in erythrocyte morphology (especially shape), and leukoerythroblastosis. Bone marrow aspiration is usually unsuccessful, resulting in a “dry tap.” This necessitates a bone marrow biopsy obtained with a Jamshidi needle.740 The specimen is processed for routine histopathologic examination and, if necessary, special stains for fibrous tissue can be used. Because myelofibrosis occurs secondary to other diseases of bone marrow, such as chronic hemolytic anemia or bone marrow necrosis, the clinician should look for a primary disease process.
The concept of clinical staging of patients with AML, MPD, and MDS is obviously much different than that of patients with solid tumors. As these hematologic tumors are “liquid,” that is they involve primarily the peripheral blood and bone marrow compartments, clinical staging is generally not performed beyond these two compartments. Certainly, infiltration of peripheral nodes and other organs occurs; however, documentation of their involvement with advanced imaging or tissue aspirates does not alter treatment or prognosis in any significant way. Two studies have documented the proof-of-concept use of 3-T body MRI to distinguish diffuse versus focal bone marrow and/or parenchymal involvement of hematopoetic neoplasia; however, the clinical utility of this methodology is currently unknown.741, 742
Treatment
Acute Myeloid Leukemia
Treatment of acute nonlymphocytic leukemias has been unrewarding to date. There is limited information on the response of specific subtypes of leukemia to uniform chemotherapeutic protocols, in part owing to the rarity of these diseases and the paucity of cases in the literature. Veterinarians are advised to contact a veterinary oncologist for discussion of new protocols and appropriate management of these cases, as novel agents are currently in development and may become available in the future.
The overriding therapeutic goal is to eradicate leukemic cells and reestablish normal hematopoiesis. Currently, this is best accomplished by cytoreductive chemotherapy and the agents most commonly utilized include combinations of anthracyclines, such as doxorubicin, cyclophosphamide, vincristine, 6-thioguanine, and prednisone.618, 619, 620, 640, 644, 646, 707, 743, 744, 745, 746 In dogs, cytosine arabinoside (Ara-C), 100 to 200 mg/m2, given by slow infusion (12–24 hours) daily for 3 days and repeated weekly, has been used, as well as several other variations using subcutaneous injections of Ara-C (see Chapter 12). Several variations of CHOP- or COP-based protocols (see Section A of this chapter), with or without Ara-C, have been used as well. The overall prognosis with currently available treatment is poor. Although response rates to multiagent protocols are relatively high (50%–70%), responses are not durable and MSTs, despite aggressive protocols, are generally 0.5 to 2.0 months.618, 619, 620 Obviously, effective therapies for AML in dogs await further investigation.
Regardless of the chemotherapy protocol used, significant cytopenias either persist or are sequela to chemotherapy, and intensive supportive care will be necessary. Transfusions of whole blood or platelet-rich plasma may be required to treat anemia and thrombocytopenia, and infection should be managed with aggressive antibiotic therapy. Because of the generally poor response, many clients will choose palliative supportive care; however, the acute progression of disease does not allow for prolonged palliation in most cases and MSTs with supportive care are generally only 1 to 2 weeks.
Polycythemia Vera
In treating PV, therapy is directed at reducing RBC mass. The PCV should be reduced to 50% to 60% or by one-sixth of its starting value. Phlebotomies should be performed as needed, administering appropriate colloid and crystalloid solutions to replace lost electrolytes; 20 mL of whole blood/kg of body weight can be removed at regular intervals.687 In humans, phlebotomy continues to be the therapeutic approach used most frequently.
The chemotherapeutic drug of choice is hydroxyurea, an inhibitor of DNA synthesis. This drug should be administered at an initial dose of 30 mg/kg for 10 days and then reduced to 15 mg/kg PO daily.690 The major goal of treatment is to maintain the PCV as close to normal as possible. Radiophosphorus (32P) has been shown to provide long-term control in people with PV and ET but has seen only limited use in veterinary medicine.747, 748 A JAK2 inhibitor, ruxolitinib (Jakafi), has been approved for second-line use in people with PV.680 A mutation in the JAK2 gene that is identical to that observed in people was documented in one of five dogs with PV681; therefore one could speculate that oclacitinib (Apoquel), which has some JAK2 inhibitory activity and is FDA-approved for use in dogs with atopy, could have therapeutic potential for PV in some dogs. This potential application for oclacitinib has not, as yet, been investigated.
Chronic Myelogenous Leukemia
It has now been documented that a subset of CML in dogs is associated with a BCR–ABL chromosomal abnormality (“Raleigh chromosome”) similar to the “Philadelphia chromosome” translocation responsible for a large majority of CML in humans. Imatinib mesylate (Gleevec), a tyrosine kinase inhibitor, is known to be an effective therapy for CML in humans. For dogs with CML that have the Raleigh chromosomal abnormality, it is intuitive that these types of drugs may have activity, and indeed tyrosine kinase inhibitors have been investigated in dogs with BCR–ABL translocation CML.701 One dog with chronic monocytic leukemia treated with toceranib (Palladia) and prednisone therapy achieved a clinical remission (before developing progressive disease) and a partial cytogenetic response. In addition, molecular techniques may be used to monitor cytogenetic aberrations, such as DNA copy number aberrations and BCR–ABL translocations, after treatment to gauge the cytogenetic response to therapy.627, 701 The author (DMV) and others have anecdotally used toceranib and/or imatinib in a handful of CML cases with responses that have lasted several months; the true activity and durability of response with these agents in dogs awaits further investigation.
CML has also been managed with chemotherapy to control the proliferation of the abnormal cell line and improve the quality of life. Hydroxyurea is the most effective agent for treating CML during the chronic phase.627, 696, 749 The initial dosage is 20 to 25 mg/kg twice daily. Treatment with hydroxyurea should continue until the leukocyte count falls to 15,000 to 20,000 cells/μL.694, 696, 705 Then the dosage of hydroxyurea can be reduced by 50% on a daily basis or to 50 mg/kg given biweekly or triweekly. In humans, the alkylating agent busulfan can be used as an alternative.750 An effective dosage has not been established in the dog, but following human protocols, 0.1 mg/kg/day PO is given until the leukocyte count is reduced to 15,000 to 20,000 cells/μL. Vincristine and prednisone therapy resulted in a short remission in one dog with CML.627
Despite response to chemotherapy and control for many months, most dogs with CML will eventually enter a terminal phase of their disease. In one study of seven dogs with CML, 4 dogs underwent terminal phase blast crisis.696 In humans, blast crisis may be lymphoid or myeloid.751 Dogs with blast crisis have a poor prognosis, despite rescue with more aggressive multiagent chemotherapy.
Essential Thrombocythemia
Few cases have been reported, but one dog was treated successfully with a combination chemotherapy protocol that included vincristine, Ara-C, cyclophosphamide, and prednisone.714 Treatment is controversial in humans because of the lack of evidence that asymptomatic patients benefit from chemotherapy. Patients with thrombosis or bleeding are given cytoreductive therapy. Hydroxyurea is the drug of choice for initially controlling the thrombocytosis.710 JAK2 small molecule inhibitors have been used in people with ET,680 and although no studies have investigated JAK2 mutations in dogs with ET, one could speculate on the use of oclacitinib (Apoquel) in dogs. Radiophosphorus treatment is also occasionally used in people with ET.747
Myelodysplastic Syndrome
There is no standard therapeutic regime for MDS. Often, humans receive no treatment if the cytopenias do not cause clinical signs. Transfusions are given when necessary, and patients with fever are evaluated aggressively to detect infections. Growth factors, such as EPO, GM-CSF, G-CSF, and IL-3, are sometimes used in patients who require frequent transfusions to increase their blood cell counts and enhance neutrophil function.752, 753 In one case report, human EPO was administered (100 U/kg SQ q48 hours) to a dog with MDS because of profound anemia. The rationale for use of EPO was to promote terminal differentiation of dysplastic erythrocytes. Human recombinant EPO should be used with caution in animals, as anti-EPO antibodies may be induced and target endogenous EPO. The PCV increased from 12% to 34% by day 19 of EPO treatment. This dog remained in remission for more than 30 months.638 Other factors that induce differentiation of hematopoietic cells include retinoic acid analogs, 1,25 dihydroxyvitamin D3, interferon-α, and conventional chemotherapeutic agents, such as 6-thioguanine and Ara-C.754, 755, 756 The propensity of these factors to enhance progression to leukemia is not known in many cases, but the potential risk exists.
Prognosis
In general, the prognosis for animals with MPN is better than for dogs with AML, in which it is grave. The prognosis for PV and CML is guarded, but significant remissions have been achieved with certain therapeutic regimes and careful monitoring. Animals commonly survive a year or more.696, 714
Section D: Myeloma-Related Disorders
David M. Vail
Myeloma-related disorders (MRDs) arise when a cell of the plasma cell or immunoglobulin-producing B-lymphocyte precursor lineage transforms and proliferates to form a clonal neoplastic population of similar cells. This population is believed in most instances to be monoclonal (i.e., derived from a single cell) because they typically produce homogeneous immunoglobulin, although some examples of biclonal and polyclonal MRD neoplasms exist. A wide variety of clinical syndromes are represented by MRDs, including multiple myeloma (MM), extramedullary plasmacytoma (EMP [both cutaneous and noncutaneous]), IgM (Waldenström’s) macroglobulinemia, solitary osseous plasmacytoma (SOP), and Ig-secreting lymphomas and leukemias (including plasma cell leukemia). MM is the most important MRD based on clinical incidence and severity. There appears to be some discordance and blurring of the distinction between MM and multicentric noncutaneous EMP in cats and these two MRDs will be discussed together in this species. See Box 33.7 .
BOX 33.7. Key Clinical Summary Points: Myeloma-Related Disorders.
-
•
Myeloma-related disorders (MRDs) represent clonal neoplastic populations of plasma cells.
-
•
They include multiple myeloma (MM), extramedullary plasmacytoma (EMP), (Waldenström’s) macroglobulinemia, solitary osseous plasmacytoma (SOP), and plasma cell leukemia.
-
•
Dogs and cats with MM are presented with a wide variety of clinical signs (TABLE 33.14, TABLE 33.15) and clinicopathologic abnormalities (Table 33.16) resulting from high levels of circulating M component, organ or bone infiltration with neoplastic cells, or both.
-
•
Diagnosis of MM usually follows the demonstration of bone marrow or visceral organ plasmacytosis (Fig. 33.23), the presence of osteolytic bone lesions (Fig. 33.25, Fig. 33.26), and the demonstration of serum or urine myeloma proteins (M component; Fig. 33.24).
-
•
Most (>80%) dogs with MM respond to chemotherapy (melphalan/prednisone) and enjoy durable remissions, with median survival times of 1.5 to 2.5 years. Most (50%–80%) cats also respond to chemotherapy (cyclophosphamide/prednisolone) although the durability of response tends to be shorter, with median survival times reported from 4 to 13 months
-
•
Cutaneous and oral solitary EMPs are usually cured after surgical excision.
-
•
Cutaneous plasmacytosis, however, is associated with multiple lesions (10s to 100s), is a biologically aggressive disease, with treatment and outcomes more like those for MM.
-
•
Noncutaneous/nonoral EMPs and SOPs can be initially confined to local sites and respond to local therapy (i.e., surgery, radiation therapy); however, frequent rechecks are necessary, because many will eventually progress systemically.
Multiple Myeloma
Incidence and Etiology
Although MM represents fewer than 1% of all malignant tumors in animals, it is responsible for approximately 8% of all hematopoietic tumors and 3.6% of all primary and secondary tumors affecting bone in dogs.757, 758 In a compilation of bone marrow disorders in dogs (n = 717), MM represented 4.4% and 19.8% of all abnormal samples and neoplastic processes, respectively.759 Furthermore, in a compilation of serum protein electrophoretic samples (n = 147 dogs), MM accounted for 4.3% of abnormal and 28.5% of neoplastic processes encountered, respectively.760 Several compilations have suggested a male predisposition,761, 762, 763 whereas others have not observed this.758, 764 Older dogs are affected with an average age of between 9 and 10 years (range, 3–14 years).758, 761, 762, 763, 764 In one large case series, German shepherd dogs were overrepresented based on the hospital population.758 The true incidence of MM in the cat is unknown; however, it is a more rare diagnosis than in the dog, representing only 1 of 395 and 4 of 3248 tumors in two large compilations of feline malignancies, and 0.9% of all malignancies and 1.9% of hematologic malignancies in another report.765, 766, 767 MM represented 1.4% and 14% of abnormal and malignant serum protein electrophoretic samples, respectively, in a compilation of 155 feline samples.768 MM occurs in aged cats (median age 12–14 years), most commonly in domestic short hair cats, and no sex predilection has been consistently reported, although a male preponderance may exist.764, 767, 769, 770, 771 MM has not been associated with coronavirus, FeLV, or FIV infections.
The etiology of MM is for the most part unknown. Genetic predispositions, molecular aberrations (e.g., c-kit), viral infections, chronic immune stimulation, and carcinogen exposure have all been suggested as contributing factors.764, 772, 773, 774, 775, 776, 777, 778, 779 Suggestion of a familial association in cats follows cases reported among siblings.770 Evidence exists that molecular mechanisms of cellular control, including overexpression of cell cycle control components like cyclin D1 (see Chapter 2), and receptor tyrosine kinase dysregulation may be involved in canine MM and plasma cell tumors.774, 776 In rodent models, chronic immune stimulation and exposure to implanted silicone gel have been associated with development of MM,778, 779 as have chronic infections and prolonged hyposensitization therapy in humans.775 Viral Aleutian disease of mink results in monoclonal gammopathies in a small percentage of cases.777 Exposure to the agricultural industry, petroleum products, and irradiation are known risk factors for development in humans.780, 781, 782 In addition, progression of solitary plasma cell tumors to MM has been reported in both dogs and cats, and a single case of a B-cell lymphoma progressing to MM exists in the dog.783, 784, 785
Pathology and Natural Behavior
MM is a systemic proliferation of malignant plasma cells or their precursors arising as a clone of a single cell that usually involves multiple bone marrow sites in dogs. In cats, as previously stated, a blurring of the distinction of MM and multicentric noncutaneous EMP within the MRD occurs because widespread abdominal organ involvement without significant bone marrow infiltration has been described in a proportion of cases in European compilations.771, 786 Because both MM and multicentric noncutaneous EMP have a similar clinical course and widespread systemic involvement with hyperglobulinemia in cats, they will be discussed as MM in this chapter. Malignant plasma cells can have a varied appearance on histologic sections and cytologic preparations. The degree of differentiation ranges from those resembling normal plasma cells in late stages of differentiation (Fig. 33.23 ) to very large anaplastic round cells (often referred to as plasmablasts) with a high mitotic index representing early stages of differentiation.763, 764, 767, 786 Binucleate and multinucleate cells are often present (see Fig. 7.32, Chapter 7). In 16 cats with MM in one case series,787 the majority (83%) of plasma cells were immature and had marked atypia, including increased size, multiple nuclei, clefted nuclei, anisocytosis, anisokaryosis, variable nuclear: cytoplasmic ratios, decreased chromatin density, and variable nucleoli; nearly one quarter had “flame cell” morphology characterized by peripheral eosinophilic cytoplasmic processes.767 However, in a European compilation of feline multicentric noncutaneous MRD cases (n = 17), 78% had well-differentiated morphologies.786 The authors of this latter case series developed a grading system dependent on the percentage of plasmablasts within the neoplastic cells in which well-differentiated, intermediate-grade, and poorly differentiated MMs have less than 15%, 15% to 49%, and 50% or more plasmablasts, respectively.786 Malignant plasma cells typically produce an overabundance of a single type of or component of immunoglobulin, which is referred to as the M component (Fig. 33.24 ). The M component can be represented by any class of the entire immunoglobulin or only a portion of the molecule, such as the light chain (Bence Jones protein) or heavy chain (heavy chain disease) of the molecule. In the dog, the M component is usually represented by either IgA or IgG immunoglobulin types in nearly equal incidence, whereas the ratio of IgGtoIgA in cats is approximately 5:1 in some reports and approximately 1:1 in others.757, 763, 764, 765, 766, 767, 786, 788 However, in two recent compilations of dogs with MM, including 27 dogs in which the immunoglobulin was typed, the vast majority (78%) were of the IgA type.761, 762 If the M component is the IgM type, the term macroglobulinemia (Waldenström’s) is often applied. Several cases of biclonal gammopathy in dogs and cats have been reported,761, 767, 770, 771, 789, 790, 791, 792, 793, 794, 795 and several cases of nonsecretory MM have been reported in dogs.762, 796, 797, 798 Rarely, cryoglobulinemia occurs in dogs with MM and IgM macroglobulinemia, and this has also been reported in a cat with IgG myeloma.764, 799, 800, 801 Cryoglobulins are paraproteins that are insoluble at temperatures below 37°C and require blood collection and clotting to be performed at 37°C before serum separation. If whole blood is allowed to clot at temperatures below this, the protein precipitates in the clot and is lost. Pure light-chain M component is rare, but has been reported in both dogs and cats.762, 802, 803
Fig. 33.23.
Bone marrow aspirate from a dog with multiple myeloma showing an overabundance of large neoplastic plasma cells with characteristic paranuclear clear zone representing the Golgi apparatus (arrow). (Dif-quick stain, ×100 objective.)
Fig. 33.24.
Serum protein electrophoresis from a cat with multiple myeloma. Stained cellulose acetate electrophoretic strip (upper right corner) with accompanying densitogram. Note large M-component spike (representing an IgG monoclonal gammopathy) present in the gamma region.
Courtesy Dr. Frances Moore, Marshfield Laboratories, Marshfield, WI.
The pathology associated with MM is a result of either high levels of circulating M component, organ or bone infiltration with neoplastic cells, or both. Associated pathologic conditions include bone disease, bleeding diathesis, hyperviscosity syndrome (HVS), renal disease, hypercalcemia, immunodeficiency (and subsequent susceptibility to infections), cytopenias secondary to myelophthisis, and cardiac failure.
Bone lesions can be isolated, discrete osteolytic lesions (including pathologic fractures) (Fig. 33.25A ), diffuse osteopenias, or both (Fig. 33.26 ). Approximately one-quarter to two-thirds of dogs with MM have radiographic evidence of bony lysis or diffuse osteoporosis.757, 761, 763, 764 The incidence of radiographic skeletal lesions in cats varies tremendously within reports from as few as 8% in some case series to as high as 65% in others.765, 767, 770, 771, 788 Those bones engaged in active hematopoiesis are more commonly affected and include the vertebrae, ribs, pelvis, skull, and the metaphyses of long bones. Skeletal lesions are rare with IgM (Waldenström’s) macroglobulinemia, in which malignant cells often infiltrate the spleen, liver, and lymphoid tissue rather than bone.764, 804, 805
Fig. 33.25.
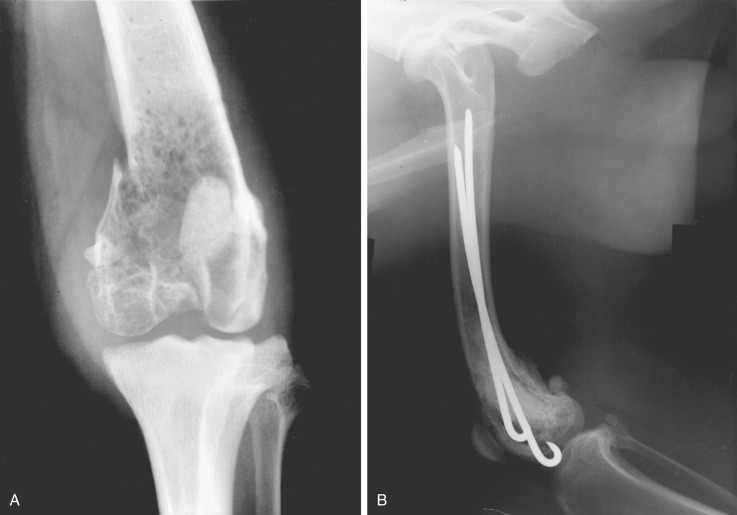
(A) Radiograph of a distal femur in a dog demonstrating severe osteolysis and a pathologic fracture secondary to a plasma cell tumor. (B) Radiograph of the same pathologic fracture after surgical repair with Rush rods and bone cement. Local site was treated with adjuvant radiation. The dog was continued on chemotherapy for 2 more years and did well.
Fig. 33.26.
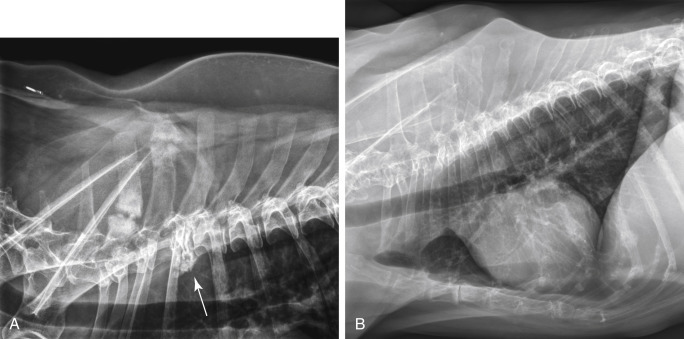
(A) Lateral thoracic radiographs of a dog showing multiple expansile lytic lesions and pathologic fractures of the dorsal spinous processes and collapse fracture (arrow) of the third thoracic vertebral body. (B) Lateral thoracic radiographs of a dog with diffuse osteopenia secondary to multiple myeloma. Note the overall decreased opacity of the lumbar vertebrae and dorsal spinous processes secondary to diffuse marrow involvement causing loss of bone trabeculae and thinning of the cortices.
Bleeding diathesis can result from one or a combination of events. M components may interfere with coagulation by (1) inhibiting platelet aggregation and the release of platelet factor-3; (2) causing adsorption of minor clotting proteins; (3) generating abnormal fibrin polymerization; and (4) producing a functional decrease in calcium.764, 806, 807 Approximately 10% to 30% of dogs and up to one-quarter of cats have clinical evidence of hemorrhage.757, 761, 767, 770, 771 In dogs, nearly half have abnormal prothrombin (PT) and partial thromboplastin (PTT) times. Thrombocytopenia may also play a role if bone marrow infiltration is significant (i.e., myelophthisis).
HVS represents one of a constellation of clinicopathologic abnormalities resulting from greatly increased serum viscosity. The magnitude of viscosity changes is related to the type, size, shape, and concentration of the M component in the blood. HVS is more common with IgM macroglobulinemia because of the high molecular weight of this class of immunoglobulin. IgA-secreting myelomas (IgA is usually present as a dimer in the dog) may undergo polymerization resulting in increased serum viscosity.757, 764, 808 IgG-associated HVS can also occur, albeit less frequently. High serum viscosity occurs in approximately 20% to 40% of dogs with MM and can result in bleeding diathesis, neurologic signs (e.g., dementia, depression, seizure activity, coma), ophthalmic abnormalities (e.g., dilated and tortuous retinal vessels, retinal hemorrhage [Fig. 33.27 ], retinal detachment), and increased cardiac workload with the potential for subsequent development of cardiomyopathy.757, 761, 764, 804, 805, 808, 809, 810, 811 In a retrospective compilation of 83 dogs with retinal hemorrhage, 5% were due to MM.812 These consequences of HVS are thought to be a result of sludging of blood in small vessels, ineffective delivery of oxygen and nutrients, and coagulation abnormalities. HVS has been reported in cats with IgG-, IgA-, and IgM-secreting tumors.764, 765, 813, 814, 815, 816, 817, 818 In several of these cases, relative serum viscosity was increased above control ranges.
Fig. 33.27.
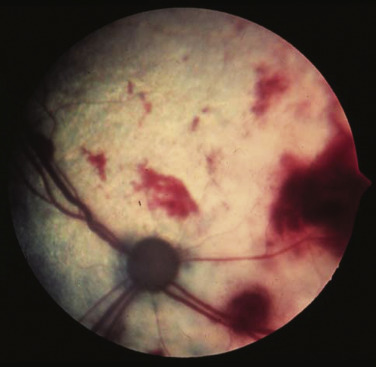
Multiple retinal hemorrhages on the fundus in a cat with hyperviscosity syndrome secondary to multiple myeloma.
Renal disease is present in approximately one-quarter to one-half of dogs with MM, and azotemia is observed in 30% to 40% of cats.757, 761, 763, 767, 769, 771 The pathogenesis of renal failure is often multifactorial and can ensue as a result of Bence Jones (light-chain) proteinuria, tumor infiltration into renal tissue, hypercalcemia, amyloidosis, diminished perfusion secondary to HVS, dehydration, or ascending urinary tract infections.757, 764, 806, 807 Normally, heavy- and light-chain synthesis is well balanced in nonneoplastic immunoglobulin production. In the case of MM, an unbalanced excess of light-chain products may be produced. Light chains are of low molecular weight and are normally filtered by the renal glomerulus, and their presence in urine can result in protein precipitates and subsequent renal tubular injury. The presence of light chains in urine without a concomitant monoclonal spike in serum, although rare, is indicative of pure light-chain disease.802 Tubules become obstructed by large laminated casts containing albumin, immunoglobulin, and light chains. Bence Jones proteinuria occurs in approximately 25% to 40% of dogs with MM.757, 763, 764 Bence Jones proteinuria is reported to occur in approximately 40% of cats with MM/MRD.767, 768 Hypercalcemia is reported in 15% to 50% of dogs with MM and is thought to result primarily from the production of osteoclast-activating factor by neoplastic cells.757, 761, 762, 764, 819 Other factors, including increased levels of various cytokines, TNF-α, IL-1, and IL-6, have been implicated in human MM. In two dogs with MM and hypercalcemia, serum elevations in circulating N-terminal parathyroid hormone-related peptide were noted.820 Hypercalcemia may also be exacerbated by associated renal disease. Hypercalcemia, initially thought to be a rare event in cats with MM, occurred in 10% to 25% of recently reported cases.767, 769, 770, 771, 821
Susceptibility to infection and immunodeficiency have long been associated with MM and are often the ultimate cause of death in affected animals.757, 764, 788 Infection rates in humans with MM are 15 times higher than normal and usually present as pneumonia or urinary tract infections.822 Response to vaccination has also been shown to be suppressed in humans with MM.822 “Normal” immunoglobulin concentrations are often severely depressed in affected animals.764 In addition, leukopenia may be present secondary to myelophthisis. Reports of multiple concurrent infections in both dogs and cats affected with MM exist and, in one dog with several concurrent infections, a polyclonal and a monoclonal gammopathy existed pretreatment, with the former persisting after successful treatment of the myeloma.772, 773
Variable cytopenias may be observed in association with MM. A normocytic, normochromic, nonregenerative anemia is encountered in approximately one-half to two-thirds of dogs with MM.757, 761, 762, 763, 764 This can result from marrow infiltration (myelophthisis), blood loss from coagulation disorders, anemia of chronic disease, or increased erythrocyte destruction secondary to high serum viscosity. Rare erythrophagocytic forms of MM have also been reported in both dogs and cats and may contribute to anemia.823, 824, 825 Similar factors lead to thrombocytopenia and leukopenia in 30% and 80% of dogs with MM, respectively; and in cats, approximately two-thirds, one-half, and one-third will be anemic, thrombocytopenic, and neutropenic, respectively.761, 762, 767, 769, 770, 771
Cardiac disease, if present, is usually a result of excessive cardiac workload and myocardial hypoxia secondary to hyperviscosity. Myocardial infiltration with amyloid and anemia may be complicating factors. Nearly one-half of cats with MM in two reports presented with a cardiac murmur, the etiology of which was not established.767, 769 Three cats with HVS presented with congestive heart failure, murmurs, and echocardiographic signs consistent with hypertrophic cardiomyopathy.813
History and Clinical Signs
Clinical signs of MM may be present up to a year before diagnosis with a median duration of 1 month reported in dogs.757, 764 In one cat, M-component elevations were detected 9 years before clinical presentation.767 In this latter case, the M-component elevation was consistent with monoclonal gammopathy of unknown significance (MGUS). MGUS (i.e., benign, essential, or idiopathic monoclonal gammopathy) is a benign monoclonal gammopathy that is not associated with osteolysis, bone marrow infiltration, or Bence Jones proteinuria. MGUS has also been reported in dogs.826, 827 Signs of MM can be variable based on the wide range of pathologic effects possible. TABLE 33.14, TABLE 33.15 list the relative frequencies of clinical signs observed in the dog and cat, respectively, based on a compilation of several reports.757, 761, 762, 764, 767, 769, 770, 771, 788 Bleeding diathesis is usually represented by epistaxis and gingival bleeding. Funduscopic abnormalities may include retinal hemorrhage (see Fig. 33.27), venous dilatation with sacculation and tortuosity, retinal detachment, and blindness.757, 761, 6, 764, 769, 771, 808, 809, 810, 811, 812 CNS signs may include dementia, seizure activity, tremors, and deficiencies in midbrain or brainstem localizing reflexes secondary to HVS or extreme hypercalcemia. Signs reflective of transverse myelopathies secondary to vertebral column infiltration, pathologic fracture, or extradural mass compression can also occur.757, 764, 799, 828, 829 One case of ataxia and seizure activity in a dog with EMP secondary to tumor-associated hypoglycemia has been reported.830 In addition, paraneoplastic polyneuropathy has been reported in a dog with MM.831 A history of chronic respiratory infections and persistent fever may also be present in cats. Hepatosplenomegaly and renomegaly can occur due to organ infiltration. Bleeding diathesis due to HVS is less common in the cat; however, epistaxis, pleural and peritoneal hemorrhagic effusions, retinal hemorrhage, and central neurologic signs have been reported in both dogs and cats.761, 764, 765, 769, 813, 814, 815, 816, 817, 818 Polydipsia and polyuria can occur secondary to renal disease or hypercalcemia, and dehydration may develop. Hindlimb paresis secondary to osteolysis and instability of lumbar vertebral bodies or extradural compression has been reported in cats.770, 832
TABLE 33.14.
| Clinical Sign | Frequency Reported (%) |
|---|---|
| Lethargy and weakness | 58 |
| Inappetence and weight loss | 36 |
| Lameness | 35 |
| Bleeding diathesis | 28 |
| Funduscopic/ocular abnormalities | 32 |
| Polyuria/polydipsia | 30 |
| CNS deficits | 8 |
CNS, Central nervous system.
TABLE 33.15.
Approximate Frequency of Clinical Signs Reported for Cats with Myeloma-Related Disorders (n = 68)764, 767, 769, 771, 788
| Clinical Sign | Frequency Range Reported (%) |
|---|---|
| Lethargy and weakness | 40–100 |
| Anorexia | 33–100 |
| Pallor | 30–100 |
| Polyuria/polydipsia | 13–40 |
| Vomiting/diarrhea | 10–30 |
| Dehydration | 20–33 |
| Palpable organomegaly | 20–25 |
| Lameness | 7–25 |
| Heart murmur | 0–45 |
| Hind limb paresis/paralysis | 0–45 |
| Bleeding diathesis | 0–40 |
| CNS signs | 13–30 |
| Concurrent cutaneous plasma cell tumor | 0–30 |
| Fundic/ocular changes | 13–33 |
| Lymphadenopathy | 0–10 |
CNS, Central nervous system.
Diagnosis and Staging
The diagnosis of MM in dogs usually follows the demonstration of bone marrow plasmacytosis (see Fig. 33.23), the presence of osteolytic bone lesions (see Fig. 33.25, Fig. 33.26), and the demonstration of serum or urine myeloma proteins (M component) (see Fig. 33.24). In the absence of osteolytic bone lesions, a diagnosis can also be made if marrow plasmacytosis is associated with a progressive increase in the M-component or if plasma cell clonality (e.g., PARR) is documented. In the cat, because the degree of bone marrow infiltration may not be as marked, it has been suggested that consideration of plasma cell morphology and visceral organ infiltration (Fig. 33.28 ) be given in cases with demonstrable M-component disease in the absence of marked (<20%) marrow plasmacytosis.767, 771, 786
Fig. 33.28.
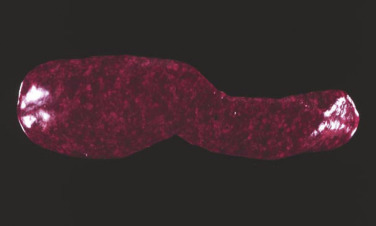
Necropsy specimen of a spleen from a cat with multiple myeloma showing diffuse plasma cell infiltration.
All animals suspected of plasma cell tumors should receive a minimal diagnostic evaluation including a CBC, platelet count, ionized calcium, serum biochemistry profile, and urinalysis. Particular attention should be paid to renal function and serum calcium levels. If clinical hemorrhage is present, a coagulation assessment (e.g., platelet count, PT, PTT) and serum viscosity measurements are indicated. All animals should undergo a careful funduscopic examination. Serum electrophoresis and immunoelectrophoresis are performed to determine the presence of a monoclonal M-component (see Fig. 33.24) and to categorize the immunoglobulin class involved. Heat precipitation and electrophoresis of urine may be performed to determine presence of Bence Jones proteinuria because commercial urine dipstick methods are not capable of this determination. Definitive diagnosis usually follows the performance of a bone marrow aspiration in the dog. A bone marrow core biopsy or multiple aspirations may be necessary because of the possibility of uneven clustering or infiltration of plasma cells in the bone marrow. Normal marrow contains less than 5% plasma cells, whereas myelomatous marrow often greatly exceeds this level. Current recommendations require more than 20% marrow plasmacytosis to be present, although a 10% cutoff in cats has been recently recommended with special attention to cellular atypia.767 Even the 10% threshold may be problematic in cats, and cellular atypia and visceral organ involvement (assessed through needle aspiration cytology or tissue biopsy) should be considered equally important in this species.767, 771, 786 Rarely, biopsy of osteolytic lesions (i.e., Jamshidi core biopsy; see Chapter 25) is necessary for diagnosis in the dog. In one case of MM in a dog, splenic aspirates were diagnostically helpful.833 Overall frequencies of clinical diagnostic abnormalities for dogs and cats with MM are compiled from published series having at least five cases each and are listed in Table 33.16 .
TABLE 33.16.
Approximate Frequency of Clinical Diagnostic Abnormalities for Dogs and Cats with Multiple Myeloma (n = 68 cats, 134 dogs)757, 761, 762, 764, 767, 769, 771, 788
| Abnormality | Frequency Range Reported (%) |
|
|---|---|---|
| Dogs | Cats | |
| Increased M component | 99 | 94–98 |
|
95 | 77–100 |
|
5%a | 16–23 |
|
40 | 84 |
|
60 | 16 |
| Noncutaneous extramedullary extension | NR | 65–100b |
| Marrow plasmacytosis (>10%) | 100 | 50–100 |
| Complete blood count (CBC) abnormalities | ||
| Anemia (nonregenerative) | 61 | 50–80 |
| Thrombocytopenia | 39 | 50 |
| Neutropenia | 26 | 37 |
| Circulating plasma cells (leukemia) | 7 | 5–25 |
| Hypoalbuminemia | 62 | 36–60 |
| Hypocholesterolemia | NR | 68 |
| Proteinuria | 45 | 71–91 |
| Bence Jones proteinuria | 38 | 40–59 |
| Bone lysis | 64 | 5–45 |
| Serum hyperviscosity | 35 | 35–44 |
| Azotemia | 29 | 22–40 |
| Hypercalcemia | 27 | 10–25 |
| Increased activities of liver enzymes | NR | 43–50 |
NR, Not reported.
Several single case reports exist for biclonal gammopathy in dogs with MM.
11 of 11 in one report had evidence of infiltration in either spleen, lymph node, or liver.
Immunohistochemical and Molecular Diagnostics
Histochemical and IHC analyses of cells or tissues suspected of MRD are more often applied in the case of solitary plasmacytomas or where EMP is suspected in the absence of marrow involvement and will be discussed in subsequent sections; however, they have been occasionally useful in the diagnosis of MM. Molecular diagnostic techniques for MM have received limited use thus far in veterinary oncology; however, determining clonality of the immunoglobulin heavy chain variable region gene has been performed in feline and canine plasmacytoma and myeloma using PARR techniques (see Chapter 8),762, 834 and use of this technology in cases where diagnosis is not straightforward is expanding. The author has used PARR analysis both before treatment and after clinical remission in a small number of dogs with MM involved in clinical trials and documented its utility for (1) initial diagnosis and (2) to characterize molecular remission.762
Imaging
Routine thoracic and abdominal radiographs are recommended in suspected cases. Occasionally, bony lesions can be observed in skeletal areas on these standard films, and organomegaly (liver, spleen, kidney) is observed in the majority of cats.767, 769, 771 Abdominal ultrasound is recommended in all cats suspected of MM because this modality reveals involvement of one or more abdominal organs in the majority of cases. These include splenomegaly with or without nodules, diffuse hyperechoic hepatomegaly with or without nodules, renomegaly, and iliac lymph node enlargement. In one case series in cats with MM, 85% of organs with ultrasonographic abnormalities were subsequently confirmed to have plasma cell infiltration.771 Skeletal survey radiographs are recommended to determine presence and extent of osteolytic lesions, which may have diagnostic, prognostic, and therapeutic implications. Although nuclear scintigraphy (bone scan) for clinical staging of dogs with MM has been performed, because of the predominant osteolytic activity with osteoblastic inactivity present, scans seldom give positive results and are therefore not useful for routine diagnosis.835 In physician-based oncology, bone mineral density analysis (dual-energy x-ray absorptiometry [DEXA] scan) to document osteoporosis, MRI of bone marrow, and PET/CT are commonly used for staging; however, these modalities have not been applied consistently in the veterinary literature. A clinical staging system for canine MM has been suggested757; however, at present, no prognostic significance has been attributed to it.
Differential Diagnosis of MM
Disease syndromes other than plasma cell tumors can be associated with monoclonal gammopathies and should be considered in any list of differentials. These include other lymphoreticular tumors (B-cell lymphoma, extramedullary plasmacytoma, and chronic and acute B-lymphocytic leukemia), chronic infections (e.g., ehrlichiosis, leishmaniasis, feline infectious peritonitis), and MGUS.763, 767, 826, 71, 836, 837, 838, 839
Treatment
Initial Therapy of Multiple Myeloma
Therapy for MM is directed at both the tumor cell mass and the secondary systemic effects they elicit. With the exception of treating life-threatening sequelae (e.g., marked hypercalcemia, infection, renal failure), all diagnostic procedures to confirm MM should be completed before initiating primary therapy to ensure a diagnosis is confirmed and baseline values are procured for monitoring response. Chemotherapy is effective at reducing malignant cell burden, relieving bone pain, allowing for skeletal healing, and reducing levels of serum immunoglobulins in the majority of dogs with MM, and will greatly extend both the quality and quantity of most patients’ lives. MM in dogs is initially a gratifying disease to treat for both the clinician and the companion animal caregiver as durable remissions are the norm, although complete elimination of neoplastic myeloma cells is rarely achieved and eventual relapse is to be expected. Although most cats with MM will also initially respond to chemotherapy, overall response rates and durability of response and overall survival times are generally not as high nor as durable as in the dog; although a more recent compilation of 15 cats had a more favorable response to chemotherapy (see Prognosis section to follow).769
Melphalan, an alkylating agent, is the chemotherapeutic of choice for the treatment of multiple myeloma in the dog.757, 761, 764 Two different melphalan protocols can be used. A continuous daily dosing regimen has been historically used in the dog with an initial starting dose of 0.1 mg/kg PO, once daily for 10 days, which is then reduced to 0.05 mg/kg PO, once daily continuously. The author prefers a pulse-dose protocol that was recently shown to result in statistically similar (albeit numerically superior) efficacy and with a similar adverse event profile.761 The pulse-dosing regimen uses melphalan at 7 mg/m2 PO, daily for 5 consecutive days every 3 weeks. This protocol also has the advantage of requiring the caregiver to administer fewer treatments with less overall exposure to chemotherapy during delivery and in body fluids. This protocol has been used successfully by the author in a small number of cases in which myelosuppression was limiting more traditional continuous low-dose therapy.
The addition of prednisone or prednisolone is thought to increase the efficacy of melphalan therapy. Prednisone is initiated at a dosage of 0.5 mg/kg PO, once daily for 10 days, then reduced to 0.5 mg/kg every other day before discontinuation after 60 days of therapy, although some continue every other day prednisone continuously. Melphalan is continued indefinitely until clinical relapse occurs or myelosuppression necessitates a dose reduction or discontinuation. The vast majority of dogs on melphalan and prednisone combination therapy tolerate the regimen well. The most clinically significant toxicity of melphalan is myelosuppression, in particular a delayed thrombocytopenia which can be slow to recover and in some cases irreversible. CBCs, including platelet counts, should be performed biweekly for 2 months of therapy and monthly thereafter in dogs on continuous protocols and just before pulse-dose when using the alternate protocol. If significant myelosuppression occurs (usually thrombocytopenia or neutropenia), a drug holiday is instituted with reintroduction at a lesser dose after marrow recovery. Alternatively, a different alkylating agent (e.g., cyclophosphamide, lomustine) may be substituted.
Although melphalan and prednisolone therapy can also be used in cats with multiple myeloma, it appears this protocol is more myelosuppressive than in the dog and many clinicians prefer to use a cyclophosphamide (250 mg/m2 PO or IV every 2–3 weeks) and prednisolone (1 mg/kg PO daily for 2 weeks and then every other day) protocol or a COP protocol (see Section B of this chapter) in this species. Alternatively, some have used cyclophosphamide at a dose of 25 mg/cat twice weekly.769, 771 If using melphalan in the cat, a dosing schedule similar to the dog has been reported; 0.1 mg/kg once daily for 10 to 14 days, then every other day until clinical improvement or leukopenia develop. Long-term continuous maintenance (0.1 mg/kg, once every 7 days) has been advocated.770 An alternative protocol advocated in the cat uses melphalan at 2 mg/m2, once every 4 days continuously, and appears to be well tolerated.771
Cyclophosphamide has been used as an alternative alkylating agent or in combination with melphalan in dogs and cats with MM.757, 764, 769, 771 There is no evidence to suggest that it is superior to melphalan in the dog. In the author’s practice, cyclophosphamide in dogs with MM is limited to those cases presenting with severe hypercalcemia or with widespread systemic involvement in which a faster acting alkylating agent may more quickly alleviate systemic effects of the disease. Cyclophosphamide is initiated at a dosage of 200 to 250 mg/m2 IV or PO, once, at the same time oral melphalan is started. Because cyclophosphamide is less likely to affect platelets, it may be substituted in those patients in which thrombocytopenia has developed secondary to long-term melphalan use.
Chlorambucil, another alkylating agent, has been used successfully for the treatment of IgM macroglobulinemia in dogs at a dosage of 0.2 mg/kg PO, once daily.764, 804 Little or no clinical signs of toxicity result from this dosing schedule. Chlorambucil has also been used in cats with MRD.771
Lomustine (CCNU), yet another alkylating agent, has been used in a limited number of cats with MM and a partial response has been reported after dosing at 50 mg/m2 PO, every 21 days.840
Evaluation of Response to Therapy
Evaluation of response to systemic therapy for MM is based on improvement in clinical signs, clinicopathologic parameters, radiographic improvement of skeletal lesions, ultrasonographic improvement of organ involvement, and, in some cases, bone marrow reassessment with molecular analysis of clonality.757, 761, 762, 764, 771 Subjective improvement in clinical signs of bone pain, lameness, lethargy, and hyporexia should be evident within 3 to 4 weeks after initiation of therapy. Objective laboratory improvement, including reduction in serum total globulin, M component and calcium, along with normalization of the hemogram, is usually noted within 3 to 6 weeks (Fig. 33.29 ). Radiographic improvement in osteolytic bone lesions may take months and resolution may only be partial. Ophthalmic complications (including long-standing retinal detachments) and paraneoplastic neuropathies often resolve along with tumor mass.810, 831 In cats responding to chemotherapy, clinical improvement is noted in 2 to 4 weeks and serum protein and radiographic bone abnormalities were greatly improved by 8 weeks.770, 771
Fig. 33.29.
Clinicopathologic data changes over time (weeks) after initiation of cytotoxic chemotherapy in three dogs with IgA multiple myeloma. Light blue area, Normal reference range. (A) Serum IgA (mg/dL); (B) Total globulins (g/dL). (C) Hematocrit (%). (D) Platelets/dL. (E) Serum calcium (mg/dL). (F) Total white blood cells/dL.
As previously discussed, complete resolution of MM does not generally occur and a good response is defined as a reduction in measured M-component (i.e., immunoglobulin or Bence Jones proteins) of at least 50% of pretreatment values.764 Reduction in serum immunoglobulin levels may lag behind reductions in Bence Jones proteinuria because the half-lives are 15 to 20 days and 8 to 12 hours, respectively.841 For routine follow-up, quantification of the increased serum globulin, immunoglobulin, or urine Bence Jones protein is performed monthly until a good response is noted and then every 2 to 3 months thereafter. Repeat bone marrow aspiration or imaging (in the case of visceral disease) for evaluation of plasma cell infiltration may be occasionally necessary. Bone marrow reevaluation is particularly prudent when cytopenias develop during chemotherapy, and drug-induced myelosuppression must be differentiated from myelophthisis due to neoplastic marrow recurrence.
Therapy Directed at Complications of Multiple Myeloma
The long-term control of complications, including hypercalcemia, HVS, bleeding diathesis, renal disease, immunosuppression with infection, ophthalmic complications, and pathologic skeletal fractures, depend on controlling the primary tumor mass. However, therapy directed more specifically at these complications may be indicated in the short term.
If hypercalcemia is marked and significant clinical signs exist, standard therapies, including fluid diuresis, with or without pharmacologic agents (e.g., zoledronate, calcitonin), may be indicated (see Chapter 5). Moderate hypercalcemia will typically begin to improve within 2 to 3 days after initiation of melphalan/prednisone chemotherapy without the addition of therapies directed at hypercalcemia specifically.
HVS is best treated in the short term by plasmapheresis.764, 808, 813, 838, 844, 845, 846 Whole blood is collected from the patient and centrifuged to separate plasma from packed cells. Packed red cells are resuspended in normal saline or another crystalloid and reinfused into the patient. More advanced plasmapheresis methods have also been used in dogs with HVS.842 Bleeding diathesis will usually resolve along with HVS; however, platelet-rich plasma transfusions may be necessary in the face of thrombocytopenia.
Renal impairment may necessitate aggressive fluid therapy in the short term and maintenance of adequate hydration in the long term. Careful attention to secondary urinary tract infections and appropriate antimicrobial therapy is indicated. Ensuring adequate water intake at home is important, and occasionally, educating owners in subcutaneous fluid administration is indicated. Continued monitoring of renal function is recommended along with follow-up directed at tumor response.
Patients with MM can be thought of as immunologically impaired. Some have recommended prophylactic antibiotic therapy in dogs with MM764; however, in humans, no benefit for this approach over diligent monitoring and aggressive anti-microbial management when indicated has been observed.806 Cidal antimicrobials are preferred over static drugs, and avoidance of nephrotoxic antimicrobials is recommended.
Pathologic fractures of weight-bearing long bones and vertebrae resulting in spinal cord compression may require immediate surgical intervention in conjunction with systemic chemotherapy. Orthopedic stabilization of fractures should be undertaken and may be followed with RT (see Fig. 33.25). Inhibition of osteoclast activity by bisphosphonate drugs (in particular zoledronate), has been shown in several meta-analyses to reduce the incidence and severity of skeletal complications (e.g., bone pain, pathologic fracture) of MM in humans and to result in some prolongation of overall survival.835, 845, 846 This class of drugs may hold promise for use in dogs and cats with various skeletal tumors; however, they have not been adequately evaluated for time-to-event efficacy in MRD in companion species.
Rescue Therapy
When MM eventually relapses in dogs and cats undergoing initial melphalan or alternative alkylator therapy or in the uncommon case that is initially resistant to alkylating agents, rescue therapy may be attempted. Switching to an alternate alkylating agent (e.g., cyclophosphamide, lomustine, chlorambucil) may be effective.761, 769 The author has also had limited success with VAD, which is a combination of doxorubicin (30 mg/m2 IV, every 21 days), vincristine (0.7 mg/m2 IV, days 8 and 15), and dexamethasone sodium phosphate (1.0 mg/kg IV, once a week on days 1, 8, and 15), given in 21-day cycles. Whereas most dogs initially respond to rescue protocols, the duration of response tends to be short, lasting only a few months. Liposomal doxorubicin has produced a long-term remission in a dog with MM previously resistant to native doxorubicin.847
Investigational Therapies
MM is ultimately a uniformly fatal disease in most species and thus significant effort is being placed on investigational therapies for this disease. Currently, bone marrow ablative therapy and marrow or stem cell rescue, thalidomide (and other antiangiogenic therapies), bortezomib (a proteasome inhibitor), arsenic trioxide, bisphosphonates, and several molecular targeting therapies are under investigation; however, their use in veterinary species is limited or completely absent at present. Bortezomib has been shown to have activity against canine melanoma in cell culture and mouse xenograft models.848 A bortezomib protocol that is well-tolerated in dogs has been used successfully in the treatment of golden retriever muscular dystrophy but has not been, as yet, reported in dogs with MM.849 One case report exists of a dog with MM that was resistant to melphalan, prednisone, and doxorubicin that subsequently achieved a partial response to tyrosine kinase inhibitor therapy (toceranib) that was maintained for 6 months.776 Rabacfosadine (Tanovea-CA1) has been used investigationally in dogs with MM, either as induction therapy or for rescue in melphalan-resistant disease and significant efficacy, including durable molecular complete responses, was noted.762 However, rabacfosadine is currently conditionally approved for use only in dogs with lymphoma.
Prognosis
The prognosis for dogs with MM is good for initial control of tumor and a return to good quality of life.757, 761, 762 In a group of 60 dogs with MM, approximately 43% achieved complete remission (i.e., serum immunoglobulins normalized), 49% achieved a partial remission (i.e., immunoglobulins <50% pretreatment values), and only 8% did not respond to melphalan and prednisone chemotherapy.757 Long-term survival is the norm, with a median survival time of 540 days reported (Fig. 33.30 ). More recently, in 38 dogs treated with melphalan/prednisone, 86% had objective responses (94% for pulse-dose protocol and 79% for continuous daily protocol) with a median progression-free and overall survival time (MST) of 601 and 930 days, respectively.761 The 1-, 2-, and 3-year survival rates were 81%, 55%, and 30%, respectively. The presence of hypercalcemia, Bence Jones proteinuria, and extensive bony lysis were found to be negative prognostic factors in the dog in one large cohort,757 but not in another.761 Only the presence of renal disease and a high peripheral neutrophil:lymphocyte ratio at diagnosis were negative indices in the latter report.761 Despite long-term durable responses in treated dogs, MM is generally a uniformly fatal disorder as drug-resistant recurrence of tumor mass and associated clinical signs is expected. Eventually, the tumor is no longer responsive to available chemotherapeutics and death follows from infection, renal failure, or euthanasia for intractable bone or spinal pain.757, 764
Fig. 33.30.
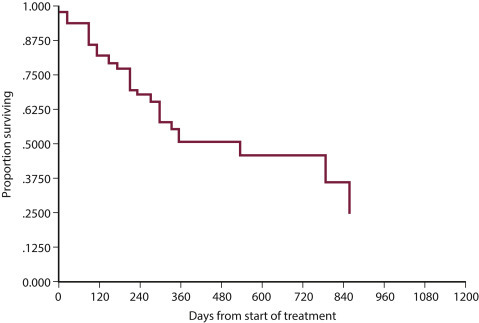
Survival curve of 37 dogs with multiple myeloma treated with chemotherapy. The median survival time (MST) is 540 days.
From Matus RE, Leifer CE, MacEwen EG, et al. Prognostic factors for multiple myeloma in the dog. J Am Vet Med Assoc. 1986;188:1288-1292.
The prognosis for MM in the cat is not as favorable as it is in the dog.764, 767, 769, 770, 771, 788 That being said, approximately 50% to 83% of cats with MM will respond to chemotherapy and although older compilations report MSTs of approximately 4 months, several long-term responses (i.e., >1 year) have been reported and a recent compilation documented MSTs of 8 to 13 months with treatment.764, 767, 769, 770, 771, 788, 790, 821 One investigator grouped MM in cats into two prognostic categories (Table 33.17 ) based on criteria known to predict behavior in dogs.770 Although no rigorous statistical analysis was performed on this small group of nine cats, the MST for cats in “aggressive” and “nonaggressive” categories was 5 days and 387 days, respectively.
TABLE 33.17.
Classification of Multiple Myeloma in Cats Based on Clinical and Diagnostic Criteria Suspected of Predicting Prognosis770
| Behavior Category | Criteria |
|---|---|
| Aggressive | Hypercalcemia, presence of bony lesions with pathologic fracture, low packed cell volume (PCV), presence of light-chain Bence Jones protein in urine, azotemia, hypercreatinemia, persistence of high serum protein level after 8 weeks of treatment, little or no clinical improvement |
| Less aggressive | Normal serum calcium, normal creatinine, blood urea nitrogen, PCV levels, presence of bony lesions without pathologic fractures, absence of light-chain Bence Jones protein, normalization of serum protein level after 8 weeks of treatment. |
Experience in dogs with IgM macroglobulinemia is limited.764, 804, 805 Response to chlorambucil is to be expected, and in nine treated dogs, 77% achieved remission with an MST of 11 months.764
Solitary and Extramedullary Plasmacytic Tumors
Solitary collections of monoclonal plasmacytic tumors can originate in soft tissues or bone and are referred to as extramedullary plasmacytoma (EMP) and solitary osseous plasmacytoma (SOP), respectively. The systemic, multicentric, biologically aggressive EMP syndrome encountered in cats has been discussed in the MM section and will only receive limited discussion in this section.771, 786 A number of large case compilations of cutaneous plasmacytoma have been reported in the dog.774, 783, 850, 851, 852, 853, 854, 855, 856, 857, 858, 859, 860, 861 The most common locations for EMP in the dog are cutaneous (86%; Fig. 33.31 ), mucous membranes of the oral cavity and lips (9%; Fig. 33.32 ), and the gastrointestinal tract (4%). The skin of the limbs and head (including the ears) are the most frequently reported cutaneous sites.783 Oral plasmacytoma represents 5% of oral tumors, 2% of lingual tumors, and approximately 20% of all EMPs.853 Other EMP sites uncommonly encountered include spleen, genitalia, eye, uterus, liver, larynx, trachea, third eyelid, sinonasal cavity (one case reported in the cat862), and intracranial sites.863, 864, 865, 866, 867, 868, 869, 870, 871 The American cocker spaniel, English cocker spaniel, and West Highland white terrier (and perhaps Yorkshire terriers, boxers, German shepherds, and Airedale terriers) have been reported inconsistently to be at increased risk for developing plasmacytomas and the median age of affected dogs is 9 to 10 years of age. 783, 853
Fig. 33.31.
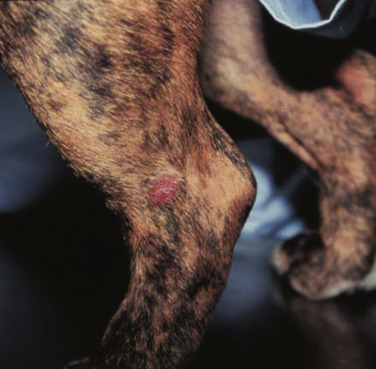
A cutaneous plasmacytoma on the limb of a dog.
Fig. 33.32.
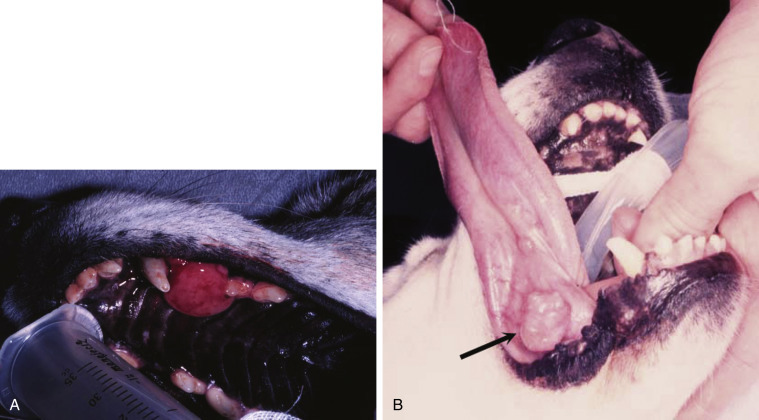
Examples of oral solitary plasmacytoma in dogs; one involving the maxilla (A), the other involving the underside of the tongue (B). Both dogs were cured after surgical excision.
Pathology and Natural Behavior of Solitary and Extramedullary Plasmacytic Tumors
Cutaneous and oral EMP in dogs are typically benign tumors that are highly amenable to local therapy.783, 851, 853, 862, 872 There exists, however, an uncommon form of multiple cutaneous plasmacytomas in the absence of MM referred to as cutaneous plasmacytosis in dogs that is a biologically aggressive disease with treatment and outcomes more like MM.850, 873, 874 Three dogs with multiple oral plasmacytomas have been reported.871 These were locally aggressive but did not metastasize and these dogs enjoyed long-term survival after surgical excision.
The natural behavior of noncutaneous/nonoral EMP appears to be somewhat more aggressive in the dog. Gastrointestinal EMP have been reported on a number of occasions in the veterinary literature, including the esophagus,870 stomach,874, 875, 876, 877 and small877 and large intestine.876, 877, 878, 879, 880 Metastasis to associated lymph nodes is more common in these cases; however, bone marrow involvement and monoclonal gammopathies are less commonly encountered. Colorectal EMPs tend to be of low biologic aggressiveness, and most do not recur after surgical excision.879 Conversely, the majority of SOPs eventually progress to systemic MM; however, the time course from local tumor development to systemic MM may be many months to years.797, 881 SOPs have been reported in the dog involving the appendicular skeleton, as well as the zygomatic arch, and ribs.797
SOPs are less common in cats, and fewer reports exist in the literature.771, 785, 882, 883, 884, 885, 886 They occur in older cats (mean ages 9–14 years), with no significant sex predilection. The skin is the most common site; however, other sites include the oral cavity, eye, GI tract, liver, subcutaneous tissues, and brain. Reports exist of cutaneous EMP in cats that progressed to systemic MRD.771, 785, 886
History and Clinical Signs of Solitary and Extramedullary Plasmacytic Tumors
Clinical signs associated with EMPs and SOPs relate to the location of involvement, or in those rare cases with high levels of M component, HVS may occur. Most cutaneous plasmacytomas are solitary, smooth, raised pink, variably alopecic nodules from 1 to 2 cm in diameter (see Fig. 33.31), although tumors as large as 10 cm have been reported. Combining large series, greater than 95% occur as solitary masses and less than 1% occur as part of a systemic MM process.774,98, 861,873,874 Cutaneous and oral EMPs usually have a benign course with no related clinical signs.
Cutaneous plasmacytosis, however, is associated with multiple lesions, often with more than 10 and up to hundreds of lesions.850 Some are ulcerated on presentation, but 81% were asymptomatic at presentation. Gastrointestinal EMPs typically have nonspecific signs which may suggest alimentary involvement. One dog with GI EMP was presented with intussusception.878 Colorectal plasmacytomas often cause rectal bleeding, hematochezia, tenesmus, and rectal prolapse.879 One case of ataxia and seizure activity in a dog with EMP secondary to tumor-associated hypoglycemia has been reported.830 SOP is usually associated with pain and lameness if the appendicular skeleton is affected or neurologic signs if vertebral bodies are involved.
Diagnosis for Solitary and Extramedullary Plasmacytic Tumors
The diagnosis of SOP and EMP usually requires tissue biopsy or needle aspiration cytology for diagnosis. Cells making up solitary plasmacytic tumors in both cats and dogs have been histologically classified into mature, hyaline, cleaved, asynchronous, monomorphous blastic and polymorphous blastic cell types; however, no prognostic significance has been observed after classification, although it has been suggested that the polymorphous-blastic type may act more aggressively in the dog.774, 852, 859, 880 A divergent pseudoglandular histologic subtype has been reported in a small number of dogs that may be confused with epithelial neoplasia.851 Intravascular tumor cells were observed in 16% of cutaneous plasmacytoma samples in one report, but this was not found to correlate with outcome and most were behaviorally benign.783 A different classification was proposed for EMP in cats based on percentage of plasmablasts, and some prognostic importance has been documented.786 In the case of poorly differentiated plasmacytic tumors, IHC studies, directed at detecting immunoglobulin, light and heavy chains, MM-1/interferon regulatory factor-4 (MUM1/IRF4), and thioflavin T, may be helpful in differentiation from other round cell tumors.797, 857, 859, 882, 883, 887, 888, 889 Of note, canine cutaneous histiocytoma (not histiocytic sarcoma) can be immunoreactive for MUM1 and therefore should be considered in any MUM1+ differential.853 Immunoreactivity has been demonstrated for canine IgG F(ab)2 and vimentin.854 A variant characterized by an IgG-reactive amyloid interspersed with the neoplastic cells has also been described.861 A panel of monoclonal antibodies (recognizing tryptase, chymase, serotonin, CD1a, CD3, CD79a, CD18, and MHC class II) in association with a histochemical stain (naphthol AS-D chloroacetate) has been advocated for use on formalin-fixed, paraffin-embedded sections of cutaneous round cell tumors to help classify poorly differentiated round cell tumors (mast cell tumors, histiocytomas, lymphomas, and plasmacytomas).888 In addition, clonality of the immunoglobulin heavy chain variable region gene can be performed in plasmacytomas and myelomas using PCR technology, and this may have some diagnostic utility in difficult cases.762
It is important to thoroughly stage dogs and cats with plasmacytomas that are at higher risk for systemic spread if contemplating local or locoregional therapy without systemic therapy. This should include bone marrow aspiration cytology, serum electrophoresis, abdominal ultrasound, and skeletal survey radiographs to ensure the disease is confined to a local site before initiation of therapy. Several, albeit rare, instances of monoclonal gammopathy or plasma cell leukemia have been reported in dogs with cutaneous plasmacytoma, cutaneous plasmacytosis and gastrointestinal EMP.850, 874, 890, 891 Staging is likely most important in cases of cutaneous plasmacytosis, SOP, and GI EMP due to their relatively high metastatic rate, but is less important for cutaneous, oral, and colorectal plasmacytomas because of their more typical benign behavior. Cutaneous plasmacytosis was associated with lymph node or abdominal viscera involvement in approximately 30% of cases; however, no cases in a large compilation had positive bone marrow aspirates and only one dog had a monoclonal gammopathy.850 For GI EMP (including colorectal EMP), endoscopic evaluation of the entire GI tract is recommended. A single case report of the use of PET/CT imaging for extramedullary splenic plasmacytoma in a dog exists; however, its utility remains unknown.892
Therapy for Solitary and Extramedullary Plasmacytic Tumors
Cutaneous and oral plasma cell tumors in the dog are almost always benign and carry an excellent prognosis after conservative surgical excision.774, 783, 853, 859, 860, 879, 893 The exception is cutaneous plasmacytosis where excision is not possible due to the number of lesions and the fact that 30% of cases have documented systemic disease.850 Systemic chemotherapy is therefore indicated for dogs with cutaneous plasmacytosis; approximately three-quarters of 14 cases experienced objective responses to either melphalan or lomustine and median progression-free and overall STs were 153 and 542 days, respectively.850 EMPs of the trachea, liver, and uterus have also been reported in dogs, and all had a benign course after local resection.864, 865, 866 Successful therapy with melphalan and prednisone has been rarely applied for a local recurrence or incomplete margins in dogs and cats. RT has been used infrequently for cases that are not surgical candidates, including the application of strontium-90 plesiotherapy for lingual plasmacytoma in a dog.894 Surgery is recommended, in combination with RT, for those cases of SOP in which the lesion results in an unstable, long bone fracture (see Fig. 33.25), or the patient is nonambulatory from neurologic compromise resulting from a vertebral body SOP. In the latter case, spinal cord decompression, mass excision, and possibly spinal stabilization may be necessary.829 RT can be used alone (i.e., without surgery) in those cases where fractures are stable, as a palliative measure for bone pain, or in the case of vertebral SOP if the patient is ambulatory and stable. Good local control is usually achieved; however, most progress to systemic MM.797, 828, 881 SOP of the axial skeleton can be managed by excision or RT alone. There is controversy as to whether systemic chemotherapy should be initiated at the time of local therapy for SOP when systemic involvement is not documented. Systemic spread may not occur for many months to even years beyond primary SOP diagnosis in humans and dogs, and studies in humans reveal no benefit to initiating systemic chemotherapy before progression to systemic disease.807, 828 Two cases of SOP in cats were recently reported; one was treated with external-beam RT and one managed with melphalan chemotherapy and both enjoyed durable remissions of greater than 4 years.895 Similarly, EMP of the GI tract in humans are treated most commonly by surgical excision and thorough staging of disease. Systemic therapy is not initiated unless systemic involvement is documented. Systemic chemotherapy has been used after gastric EMP in a cat; however, the utility of adjuvant therapy in the species is unknown.896
Long-term follow-up of patients with SOP is indicated to recognize both recurrence of disease and systemic spread. Careful attention is given to serum globulin levels, bone pain, and radiographic appearance of bone healing in cases of SOP. Restaging of disease, including bone marrow evaluation, is indicated if systemic spread is suspected.
Prognosis for Solitary and Extramedullary Plasmacytic Tumors
Prognosis for solitary plasma cell tumors is generally good. Cutaneous and mucocutaneous plasmacytomas are usually cured after surgical excision.774, 859, 893 In large compilations of cases in dogs, the local recurrence rate was approximately 5%, and nodal or distant metastasis occurred in only 7 of 349 cases (2%).774, 783, 98, 855, 859, 860 New cutaneous plasmacytomas at sites distant from the primary developed in fewer than 2% of cases. Neither tumor cell proliferation rate (as measured by Ki67 IHC), intravascular tumor infiltration/emboli in the dog, nor histopathologic grading in dogs and cats were prognostic in large compilations of cases, although it has been suggested that the polymorphous-blastic and plasmablastic type may act more aggressively in the dog and cat.774, 783, 786, 859 The presence of amyloid and overexpression of cyclin D1 (prognostic in human plasmacytomas) were not shown to be of prognostic value in dogs.774 Dogs with EMP of the alimentary tract and other abdominal organs (e.g., liver, uterus) treated by surgical excision alone or in combination with systemic chemotherapy (if metastasis is present) can enjoy long-term survival in the majority of cases.797, 864, 865, 866, 870, 875, 876, 877, 880 In a compilation of nine dogs with colorectal plasmacytoma, two dogs had local recurrence at 5 and 8 months after surgery, and the overall MST was 15 months after surgery alone.879 DNA ploidy and Myc oncoprotein expression in biopsy samples were determined to be prognostic for EMPs in dogs; however, those that were malignant were all from noncutaneous sites (i.e., lymph node, colon, spleen). Therefore location appears to be as predictive.897 As previously discussed, the majority of cases of SOP will eventually develop systemic disease; however, long disease-free periods usually precede the event.
The prognosis in cats is less well-defined because of the paucity of reported cases. If disease is confined to a local site and/or regional nodes, surgical excision and chemotherapy can result in long-term control; however, early, widespread metastasis and progression to MM is also reported in cats.771, 785, 857, 882, 884, 885, 896
Only blood and bone marrow involved.
Excluding bone marrow.
Furosemide (1–2 mg/kg) is given IV or PO, concurrent with cyclophosphamide to lessen the incidence of sterile hemorrhagic cystitis.
In dogs less than 15 kg in body weight, a doxorubicin dose of 1 mg/kg is substituted for 30 mg/m2
References
- 1.Boerkamp K.M., Teske E., Boon L.R. Estimated incidence rate and distribution of tumours in 4,653 cases of archival submissions derived from the Dutch golden retriever population. BMC Vet Res. 2014;10(34) doi: 10.1186/1746-6148-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorn C.R., Taylor D.O., Frye F.L. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst. 1968;40:295–305. [PubMed] [Google Scholar]
- 3.Dorn C.R., Taylor D.O., Schneider R. The epidemiology of canine leukemia and lymphoma. Bibl Haematol. 1970:403–415. doi: 10.1159/000391733. [DOI] [PubMed] [Google Scholar]
- 4.Merlo D.F., Rossi L., Pellegrino C. Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. J Vet Intern Med. 2008;22:976–984. doi: 10.1111/j.1939-1676.2008.0133.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser H. Animal neoplasia: a systemic review. In: Kaiser H., editor. neoplasms-comparative pathology in animals, plants and man. Wiliams & Wilkins; Baltimore: 1981. [Google Scholar]
- 6.Moulton J.E., Harvey H.J. Tumors of lymphoid and hematopoietic tissue. In: Moulton J.E., editor. tumors of domestic animals. ed 3. Univeristy of California Press; Berkley, CA: 1990. [Google Scholar]
- 7.Jemal A., Tiwari R.C., Murray T. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Ernst T., Kessler M., Lautscham E. Multicentric lymphoma in 411 dogs - an epidemiological study. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2016;44:245–251. doi: 10.15654/TPK-150338. [DOI] [PubMed] [Google Scholar]
- 9.Villamil J.A., Henry C.J., Hahn A.W. Hormonal and sex impact on the epidemiology of canine lymphoma. J Cancer Epidemiol. 2009;2009:591753. doi: 10.1155/2009/591753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards D.S., Henley W.E., Harding E.F. Breed incidence of lymphoma in a UK population of insured dogs. Vet Comp Oncol. 2003;1:200–206. doi: 10.1111/j.1476-5810.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 11.Priester W.A., McKay F.W. The occurrence of tumors in domestic animals. Natl Cancer Inst Monogr. 1980:1–210. [PubMed] [Google Scholar]
- 12.Breen M., Modiano J.F. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans—man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 13.Devitt J.J., Maranon D.G., Ehrhart E.J. Correlations between numerical chromosomal aberrations in the tumor and peripheral blood in canine lymphoma. Cytogenet Genome Res. 2009;124:12–18. doi: 10.1159/000200083. [DOI] [PubMed] [Google Scholar]
- 14.Thomas R., Fiegler H., Ostrander E.A. A canine cancer-gene microarray for CGH analysis of tumors. Cytogenet Genome Res. 2003;102:254–260. doi: 10.1159/000075758. [DOI] [PubMed] [Google Scholar]
- 15.Thomas R., Seiser E.L., Motsinger-Reif A. Refining tumor-associated aneuploidy through ‘genomic recoding’ of recurrent DNA copy number aberrations in 150 canine non-Hodgkin lymphomas. Leuk Lymphoma. 2011;52:1321–1335. doi: 10.3109/10428194.2011.559802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas R., Smith K.C., Gould R. Molecular cytogenetic analysis of a novel high-grade canine T-lymphoblastic lymphoma demonstrating co-expression of CD3 and CD79a cell markers. Chromosome Res. 2001;9:649–657. doi: 10.1023/a:1012904307579. [DOI] [PubMed] [Google Scholar]
- 17.Thomas R., Smith K.C., Ostrander E.A. Chromosome aberrations in canine multicentric lymphomas detected with comparative genomic hybridisation and a panel of single locus probes. Br J Cancer. 2003;89:1530–1537. doi: 10.1038/sj.bjc.6601275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starkey M.P., Murphy S. Using lymph node fine needle aspirates for gene expression profiling of canine lymphoma. Vet Comp Oncol. 2010;2010(8):56–71. doi: 10.1111/j.1476-5829.2009.00205.x. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad-Toh K., Wade C.M., Mikkelsen T.S. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 20.Hahn K.A., Richardson R.C., Hahn E.A. Diagnostic and prognostic importance of chromosomal aberrations identified in 61 dogs with lymphosarcoma. Vet Pathol. 1994;31:528–540. doi: 10.1177/030098589403100504. [DOI] [PubMed] [Google Scholar]
- 21.Elvers I., Turner-Maier J., Swofford R. Exome sequencing of lymphomas from three dog breeds reveals somatic mutation patterns reflecting genetic background. Genome Res. 2015;25:1634–1645. doi: 10.1101/gr.194449.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudaliar M.A., Haggart R.D., Miele G. Comparative gene expression profiling identifies common molecular signatures of NF-kappaB activation in canine and human diffuse large B cell lymphoma (DLBCL) PLoS One. 2013;8:e72591. doi: 10.1371/journal.pone.0072591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas R., Demeter Z., Kennedy K.A. Integrated immunohistochemical and DNA copy number profiling analysis provides insight into the molecular pathogenesis of canine follicular lymphoma. Vet Comp Oncol. 2017;15:852–867. doi: 10.1111/vco.12227. [DOI] [PubMed] [Google Scholar]
- 24.Tonomura N., Elvers I., Thomas R. Genome-wide association study identifies shared risk loci common to two malignancies in golden retrievers. PLoS Genet. 2015;11:e1004922. doi: 10.1371/journal.pgen.1004922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulve R., Rault M., Bahin M. Discovery of human-similar gene fusions in canine cancers. Cancer Res. 2017;2017(77):5721–5727. doi: 10.1158/0008-5472.CAN-16-2691. [DOI] [PubMed] [Google Scholar]
- 26.Nasir L., Argyle D.J. Mutational analysis of the tumour suppressor gene p53 in lymphosarcoma in two bull mastiffs. Vet Rec. 1999;145:23–24. doi: 10.1136/vr.145.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Pelham J.T., Irwin P.J., Kay P.H. Genomic hypomethylation in neoplastic cells from dogs with malignant lymphoproliferative disorders. Res Vet Sci. 2003;74:101–104. doi: 10.1016/s0034-5288(02)00179-0. [DOI] [PubMed] [Google Scholar]
- 28.Setoguchi A., Sakai T., Okuda M. Aberrations of the p53 tumor suppressor gene in various tumors in dogs. Am J Vet Res. 2001;62:433–439. doi: 10.2460/ajvr.2001.62.433. [DOI] [PubMed] [Google Scholar]
- 29.Sueiro F.A., Alessi A.C., Vassallo J. Canine lymphomas: a morphological and immunohistochemical study of 55 cases, with observations on p53 immunoexpression. J Comp Pathol. 2004;131:207–213. doi: 10.1016/j.jcpa.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Veldhoen N., Stewart J., Brown R. Mutations of the p53 gene in canine lymphoma and evidence for germ line p53 mutations in the dog. Oncogene. 1998;16:249–255. doi: 10.1038/sj.onc.1201489. [DOI] [PubMed] [Google Scholar]
- 31.Sokolowska J., Cywinska A., Malicka E. p53 expression in canine lymphoma. J Vet Med A Physiol Pathol Clin Med. 2005;52:172–175. doi: 10.1111/j.1439-0442.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- 32.Modiano J.F., Breen M., Burnett R.C. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 2005;65:5654–5661. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- 33.Carioto L.M., Kruth S.A., Betts D.H. Telomerase activity in clinically normal dogs and dogs with malignant lymphoma. Am J Vet Res. 2001;62:1442–1446. doi: 10.2460/ajvr.2001.62.1442. [DOI] [PubMed] [Google Scholar]
- 34.Nasir L., Devlin P., McKevitt T. Telomere lengths and telomerase activity in dog tissues: a potential model system to study human telomere and telomerase biology. Neoplasia. 2001;3:351–359. doi: 10.1038/sj.neo.7900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yazawa M., Okuda M., Kanaya N. Molecular cloning of the canine telomerase reverse transcriptase gene and its expression in neoplastic and non-neoplastic cells. Am J Vet Res. 2003;64:1395–1400. doi: 10.2460/ajvr.2003.64.1395. [DOI] [PubMed] [Google Scholar]
- 36.Thamm D.H., Grunerud K.K., Rose B.J. DNA repair deficiency as a susceptibility marker for spontaneous lymphoma in golden retriever dogs: a case-control study. PLoS One. 2013;8(e69192) doi: 10.1371/journal.pone.0069192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waugh E.M., Gallagher A., McAulay K.A. Gammaherpesviruses and canine lymphoma: no evidence for direct involvement in commonly occurring lymphomas. J Gen Virol. 2015;96:1863–1872. doi: 10.1099/vir.0.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milman G., Smith K.C., Erles K. Serological detection of Epstein-Barr virus infection in dogs and cats. Vet Microbiol. 2011;150:15–20. doi: 10.1016/j.vetmic.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Farinha P., Gascoyne R.D. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 40.Rossi G., Rossi M., Vitali C.G. A conventional beagle dog model for acute and chronic infection with Helicobacter pylori. Infect Immun. 1999;67:3112–3120. doi: 10.1128/iai.67.6.3112-3120.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavazza A., Rossi G., Lubas G. Faecal microbiota in dogs with multicentric lymphoma. Vet Comp Oncol. 2017 doi: 10.1111/vco.12367. [DOI] [PubMed] [Google Scholar]
- 42.Ruple A., Avery A.C., Morley P.S. Differences in the geographic distribution of lymphoma subtypes in Golden retrievers in the USA. Vet Comp Oncol. 2017;15:1590–1597. doi: 10.1111/vco.12258. [DOI] [PubMed] [Google Scholar]
- 43.Takashima-Uebelhoer B.B., Barber L.G., Zagarins S.E. Household chemical exposures and the risk of canine malignant lymphoma, a model for human non-Hodgkin’s lymphoma. Environ Res. 2012;112:171–176. doi: 10.1016/j.envres.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes H.M., Tarone R.E., Cantor K.P. Case-control study of canine malignant lymphoma: positive association with dog owner’s use of 2,4-dichlorophenoxyacetic acid herbicides. J Natl Cancer Inst. 1991;83:1226–1231. doi: 10.1093/jnci/83.17.1226. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds P.M., Reif J.S., Ramsdell H.S. Canine exposure to herbicide-treated lawns and urinary excretion of 2,4-dichlorophenoxyacetic acid. Cancer Epidemiol Biomarkers Prev. 1994;3:233–237. [PubMed] [Google Scholar]
- 46.Carlo G.L., Cole P., Miller A.B. Review of a study reporting an association between 2,4-dichlorophenoxyacetic acid and canine malignant lymphoma: report of an expert panel. Regul Toxicol Pharmacol. 1992;16:245–252. doi: 10.1016/0273-2300(92)90004-s. [DOI] [PubMed] [Google Scholar]
- 47.Garabrant D.H., Philbert M.A. Review of 2,4-dichlorophenoxyacetic acid (2,4-D) epidemiology and toxicology. Crit Rev Toxicol. 2002;32:233–257. doi: 10.1080/20024091064237. [DOI] [PubMed] [Google Scholar]
- 48.Kaneene J.B., Miller R. Re-analysis of 2,4-D use and the occurrence of canine malignant lymphoma. Vet Hum Toxicol. 1999;41:164–170. [PubMed] [Google Scholar]
- 49.Gavazza A., Presciuttini S., Barale R. Association between canine malignant lymphoma, living in industrial areas, and use of chemicals by dog owners. J Vet Intern Med. 2001;15:190–195. [PubMed] [Google Scholar]
- 50.Reif J.S., Lower K.S., Ogilvie G.K. Residential exposure to magnetic fields and risk of canine lymphoma. Am J Epidemiol. 1995;141:352–359. doi: 10.1093/aje/141.4.352. [DOI] [PubMed] [Google Scholar]
- 51.Marconato L., Leo C., Girelli R. Association between waste management and cancer in companion animals. J Vet Intern Med. 2009;23:564–569. doi: 10.1111/j.1939-1676.2009.0278.x. [DOI] [PubMed] [Google Scholar]
- 52.Pastor M., Chalvet-Monfray K., Marchal T. Genetic and environmental risk indicators in canine non-Hodgkin’s lymphomas: breed associations and geographic distribution of 608 cases diagnosed throughout France over 1 year. J Vet Intern Med. 2009;23:301–310. doi: 10.1111/j.1939-1676.2008.0255.x. [DOI] [PubMed] [Google Scholar]
- 53.Pinello K.C., Santos M., Leite-Martins L. Immunocytochemical study of canine lymphomas and its correlation with exposure to tobacco smoke. Vet World. 2017;10:1307–1313. doi: 10.14202/vetworld.2017.1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller E.T. Immune-mediated disease as a risk factor for canine lymphoma. Cancer. 1992;70:2334–2337. doi: 10.1002/1097-0142(19921101)70:9<2334::aid-cncr2820700920>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 55.Foster A.P., Sturgess C.P., Gould D.J. Pemphigus foliaceus in association with systemic lupus erythematosus, and subsequent lymphoma in a cocker spaniel. J Small Anim Pract. 2000;41:266–270. doi: 10.1111/j.1748-5827.2000.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 56.Aronson L.R. Update on the current status of kidney transplantation for chronic kidney disease in animals. Vet Clin North Am Small Anim Pract. 2016;46:1193–1218. doi: 10.1016/j.cvsm.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Blackwood L., German A.J., Stell A.J. Multicentric lymphoma in a dog after cyclosporine therapy. J Small Anim Pract. 2004;45:259–262. doi: 10.1111/j.1748-5827.2004.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 58.Schmiedt C.W., Grimes J.A., Holzman G. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immunosuppression. Vet Comp Oncol. 2009;7:45–53. doi: 10.1111/j.1476-5829.2008.00172.x. [DOI] [PubMed] [Google Scholar]
- 59.Santoro D., Marsella R., Hernandez J. Investigation on the association between atopic dermatitis and the development of mycosis fungoides in dogs: a retrospective case-control study. Vet Dermatol. 2007;18:101–106. doi: 10.1111/j.1365-3164.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 60.Madewell B. Hematopoietic neoplasms, sarcomas and related conditions. In: Theilen G.H., Madewell B., editors. Veterinary Cancer Medicine. ed 2. Lea and Febiger; Philadelphia: 1987. [Google Scholar]
- 61.Couto C.G., Rutgers H.C., Sherding R.G. Gastrointestinal lymphoma in 20 dogs. A retrospective study. J Vet Intern Med. 1989;1989(3):73–78. doi: 10.1111/j.1939-1676.1989.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 62.Ozaki K., Yamagami T., Nomura K. T-cell lymphoma with eosinophilic infiltration involving the intestinal tract in 11 dogs. Vet Pathol. 2006;43:339–344. doi: 10.1354/vp.43-3-339. [DOI] [PubMed] [Google Scholar]
- 63.Breitschwerdt E.B., Waltman C., Hagstad H.V. Clinical and epidemiologic characterization of a diarrheal syndrome in Basenji dogs. J Am Vet Med Assoc. 1982;180:914–920. [PubMed] [Google Scholar]
- 64.Couto K.M., Moore P.F., Zwingenberger A.L. Clinical characteristics and outcome in dogs with small cell T-cell intestinal lymphoma. Vet Comp Oncol. 2018 doi: 10.1111/vco.12384. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coyle K.A., Steinberg H. Characterization of lymphocytes in canine gastrointestinal lymphoma. Vet Pathol. 2004;41:141–146. doi: 10.1354/vp.41-2-141. [DOI] [PubMed] [Google Scholar]
- 66.Steinberg H., Dubielzig R.R., Thomson J. Primary gastrointestinal lymphosarcoma with epitheliotropism in three Shar-pei and one boxer dog. Vet Pathol. 1995;32:423–426. doi: 10.1177/030098589503200413. [DOI] [PubMed] [Google Scholar]
- 67.Rosenberg M.P., Matus R.E., Patnaik A.K. Prognostic factors in dogs with lymphoma and associated hypercalcemia. J Vet Intern Med. 1991;5:268–271. doi: 10.1111/j.1939-1676.1991.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 68.Vail D.M., Kisseberth W.C., Obradovich J.E. Assessment of potential doubling time (Tpot), argyrophilic nucleolar organizer regions (AgNOR), and proliferating cell nuclear antigen (PCNA) as predictors of therapy response in canine non-Hodgkin’s lymphoma. Exp Hematol. 1996;24:807–815. [PubMed] [Google Scholar]
- 69.Ruslander D.A., Gebhard D.H., Tompkins M.B. Immunophenotypic characterization of canine lymphoproliferative disorders. Vivo. 1997;11:169–172. [PubMed] [Google Scholar]
- 70.Ortiz A.L., Carvalho S., Leo C. Gamma delta T-cell large granular lymphocyte lymphoma in a dog. Vet Clin Pathol. 2015;44:442–447. doi: 10.1111/vcp.12265. [DOI] [PubMed] [Google Scholar]
- 71.Moore P.F., Affolter V.K., Keller S.M. Canine inflamed nonepitheliotropic cutaneous T-cell lymphoma: a diagnostic conundrum. Vet Dermatol. 2013;24:204–211. doi: 10.1111/j.1365-3164.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 72.Gross T., Ihrke P.J., Walder E.J. Lymphocytic neoplasms. In: Gross T.L., Ihrke P.J., Walder E.J., editors. Skin diseases of the dog and cat. ed 2. Blackwell; Oxford: 2005. pp. 866–893. [Google Scholar]
- 73.Broder S., Muul L., Marshall S. Neoplasms of immunoregulatory T cells in clinical investigation. J Invest Dermatol. 1980;74:267–271. doi: 10.1111/1523-1747.ep12543356. [DOI] [PubMed] [Google Scholar]
- 74.Fontaine J., Heimann M., Day M.J. Canine cutaneous epitheliotropic T-cell lymphoma: a review of 30 cases. Vet Dermatol. 2010;21:267–275. doi: 10.1111/j.1365-3164.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 75.Moore P.F., Affolter V.K., Graham P.S. Canine epitheliotropic cutaneous T-cell lymphoma: an investigation of T-cell receptor immunophenotype, lesion topography and molecular clonality. Vet Dermatol. 2009;20:569–576. doi: 10.1111/j.1365-3164.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 76.Moore P.F., Olivry T., Naydan D. Canine cutaneous epitheliotropic lymphoma (mycosis fungoides) is a proliferative disorder of CD8+ T cells. Am J Pathol. 1994;144:421–429. [PMC free article] [PubMed] [Google Scholar]
- 77.Affolter V.K., Gross T.L., Moore P.F. Indolent cutaneous T-cell lymphoma presenting as cutaneous lymphocytosis in dogs. Vet Dermatol. 2009;20:577–585. doi: 10.1111/j.1365-3164.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 78.Foster A.P., Evans E., Kerlin R.L. Cutaneous T-cell lymphoma with Sezary syndrome in a dog. Vet Clin Pathol. 1997;26:188–192. doi: 10.1111/j.1939-165x.1997.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 79.Schick R.O., Murphy G.F., Goldschmidt M.H. Cutaneous lymphosarcoma and leukemia in a cat. J Am Vet Med Assoc. 1993;203:1155–1158. [PubMed] [Google Scholar]
- 80.Thrall M.A., Macy D.W., Snyder S.P. Cutaneous lymphosarcoma and leukemia in a dog resembling Sezary syndrome in man. Vet Pathol. 1984;21:182–186. doi: 10.1177/030098588402100209. [DOI] [PubMed] [Google Scholar]
- 81.Keller S.M., Vernau W., Hodges J. Hepatosplenic and hepatocytotropic T-cell lymphoma: two distinct types of T-cell lymphoma in dogs. Vet Pathol. 2013;50:281–290. doi: 10.1177/0300985812451625. [DOI] [PubMed] [Google Scholar]
- 82.Cienava E.A., Barnhart K.F., Brown R. Morphologic, immunohistochemical, and molecular characterization of hepatosplenic T-cell lymphoma in a dog. Vet Clin Pathol. 2004;33:105–110. doi: 10.1111/j.1939-165x.2004.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 83.Fry M.M., Vernau W., Pesavento P.A. Hepatosplenic lymphoma in a dog. Vet Pathol. 2003;40:556–562. doi: 10.1354/vp.40-5-556. [DOI] [PubMed] [Google Scholar]
- 84.Dargent F.J., Fox L.E., Anderson W.I. Neoplastic angioendotheliomatosis in a dog: an angiotropic lymphoma. Cornell Vet. 1988;78:253–262. [PubMed] [Google Scholar]
- 85.McDonough S.P., Van Winkle T.J., Valentine B.A. Clinicopathological and immunophenotypical features of canine intravascular lymphoma (malignant angioendotheliomatosis) J Comp Pathol. 2002;126:277–288. doi: 10.1053/jcpa.2002.0553. [DOI] [PubMed] [Google Scholar]
- 86.Ridge L., Swinney G. Angiotrophic intravascular lymphosarcoma presenting as bi-cavity effusion in a dog. Aust Vet J. 2004;82:616–618. doi: 10.1111/j.1751-0813.2004.tb12604.x. [DOI] [PubMed] [Google Scholar]
- 87.Bush W.W., Throop J.L., McManus P.M. Intravascular lymphoma involving the central and peripheral nervous systems in a dog. J Am Anim Hosp Assoc. 2003;39:90–96. doi: 10.5326/0390090. [DOI] [PubMed] [Google Scholar]
- 88.Cullen C.L., Caswell J.L., Grahn B.H. Intravascular lymphoma presenting as bilateral panophthalmitis and retinal detachment in a dog. J Am Anim Hosp Assoc. 2000;36:337–342. doi: 10.5326/15473317-36-4-337. [DOI] [PubMed] [Google Scholar]
- 89.Summers B.A., deLahunta A. Cerebral angioendotheliomatosis in a dog. Acta Neuropathol. 1985;68:10–14. doi: 10.1007/BF00688949. [DOI] [PubMed] [Google Scholar]
- 90.Valli V.E., Kass P.H., San Myint M. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol. 2013;50:738–748. doi: 10.1177/0300985813478210. [DOI] [PubMed] [Google Scholar]
- 91.Berry C.R., Moore P.F., Thomas W.P. Pulmonary lymphomatoid granulomatosis in seven dogs (1976-1987) J Vet Intern Med. 1990;4:157–166. doi: 10.1111/j.1939-1676.1990.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 92.Hatoya S., Kumagai D., Takeda S. Successful management with CHOP for pulmonary lymphomatoid granulomatosis in a dog. J Vet Med Sci. 2011;73:527–530. doi: 10.1292/jvms.10-0301. [DOI] [PubMed] [Google Scholar]
- 93.Park H.M., Hwang D.N., Kang B.T. Pulmonary lymphomatoid granulomatosis in a dog: evidence of immunophenotypic diversity and relationship to human pulmonary lymphomatoid granulomatosis and pulmonary Hodgkin’s disease. Vet Pathol. 2007;44:921–923. doi: 10.1354/vp.44-6-921. [DOI] [PubMed] [Google Scholar]
- 94.Fitzgerald S.D., Wolf D.C., Carlton W.W. Eight cases of canine lymphomatoid granulomatosis. Vet Pathol. 1991;28:241–245. doi: 10.1177/030098589102800308. [DOI] [PubMed] [Google Scholar]
- 95.Ponce F., Marchal T., Magnol J.P. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet Pathol. 2010;47:414–433. doi: 10.1177/0300985810363902. [DOI] [PubMed] [Google Scholar]
- 96.Seelig D.M., Avery A.C., Ehrhart E.J. The comparative diagnostic features of canine and human lymphoma. Vet Sci. 2016;3:11. doi: 10.3390/vetsci3020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burkhard M.J., Bienzle D. Making sense of lymphoma diagnostics in small animal patients. Vet Clin North Am Small Anim Pract. 2013;43:1331–1347. doi: 10.1016/j.cvsm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Vezzali E., Parodi A.L., Marcato P.S. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet Comp Oncol. 2010;8:38–49. doi: 10.1111/j.1476-5829.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 99.Valli V.E., San Myint M., Barthel A. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol. 2011;48:198–211. doi: 10.1177/0300985810379428. [DOI] [PubMed] [Google Scholar]
- 100.Stefanello D., Valenti P., Zini E. Splenic marginal zone lymphoma in 5 dogs (2001-2008) J Vet Intern Med. 2011;25:90–93. doi: 10.1111/j.1939-1676.2010.0639.x. [DOI] [PubMed] [Google Scholar]
- 101.Flood-Knapik K.E., Durham A.C., Gregor T.P. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet Comp Oncol. 2013;11:272–286. doi: 10.1111/j.1476-5829.2011.00317.x. [DOI] [PubMed] [Google Scholar]
- 102.Valli V.E., Vernau W., de Lorimier L.P. Canine indolent nodular lymphoma. Vet Pathol. 2006;43:241–256. doi: 10.1354/vp.43-3-241. [DOI] [PubMed] [Google Scholar]
- 103.National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer. 1982;49:2112–2135. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 104.Lennert K.F.A. ed 2. Springer-Verlag; Berlin: 1990. Histopathology of non-hodgkin’s lymphomas (based on the updated kiel classification) [Google Scholar]
- 105.Carter R.F., Valli V.E., Lumsden J.H. The cytology, histology and prevalence of cell types in canine lymphoma classified according to the National Cancer Institute Working Formulation. Can J Vet Res. 1986;50:154–164. [PMC free article] [PubMed] [Google Scholar]
- 106.Bromberek J.L., Rout E.D., Agnew M.R. Breed distribution and clinical characteristics of b cell chronic lymphocytic leukemia in dogs. J Vet Intern Med. 2016;30:215–222. doi: 10.1111/jvim.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hughes K.L., Labadie J.D., Yoshimoto J.A. Increased frequency of CD45 negative T cells (T zone cells) in older Golden retriever dogs. Vet Comp Oncol. 2018;16:E109–E116. doi: 10.1111/vco.12343. [DOI] [PubMed] [Google Scholar]
- 108.Cozzi M., Marconato L., Martini V. Canine nodal marginal zone lymphoma: descriptive insight into the biological behaviour. Vet Comp Oncol. 2018;16:246–252. doi: 10.1111/vco.12374. [DOI] [PubMed] [Google Scholar]
- 109.Mizutani N., Goto-Koshino Y., Takahashi M. Clinical and histopathological evaluation of 16 dogs with T-zone lymphoma. J Vet Med Sci. 2016;78:1237–1244. doi: 10.1292/jvms.15-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moore A.S. Treatment of T cell lymphoma in dogs. Vet Rec. 2016;179:277. doi: 10.1136/vr.103456. [DOI] [PubMed] [Google Scholar]
- 111.Sayag D., Fournel-Fleury C., Ponce F. Prognostic significance of morphotypes in canine lymphomas: a systematic review of literature. Vet Comp Oncol. 2018;16:12–19. doi: 10.1111/vco.12320. [DOI] [PubMed] [Google Scholar]
- 112.Teske E., van Heerde P., Rutteman G.R. Prognostic factors for treatment of malignant lymphoma in dogs. J Am Vet Med Assoc. 1994;205:1722–1728. [PubMed] [Google Scholar]
- 113.Appelbaum F.R., Sale G.E., Storb R. Phenotyping of canine lymphoma with monoclonal antibodies directed at cell surface antigens: classification, morphology, clinical presentation and response to chemotherapy. Hematol Oncol. 1984;2:151–168. doi: 10.1002/hon.2900020205. [DOI] [PubMed] [Google Scholar]
- 114.Rosol T.J., Capen C.C. Mechanisms of cancer-induced hypercalcemia. Lab Invest. 1992;67:680–702. [PubMed] [Google Scholar]
- 115.Weir E.C.G.P., Matus R. Hypercalcemia in canine lymphosarcoma is associated with the T cell subtype and with secretion of a PTH-like factor. J Bone Miner Res. 1988;3 [Google Scholar]
- 116.Greenlee P.G., Filippa D.A., Quimby F.W. Lymphomas in dogs. A morphologic, immunologic, and clinical study. Cancer. 1990;66:480–490. doi: 10.1002/1097-0142(19900801)66:3<480::aid-cncr2820660314>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 117.Fournel-Fleury C., Ponce F., Felman P. Canine T-cell lymphomas: a morphological, immunological, and clinical study of 46 new cases. Vet Pathol. 2002;39:92–109. doi: 10.1354/vp.39-1-92. [DOI] [PubMed] [Google Scholar]
- 118.Wilkerson M.J., Dolce K., Koopman T. Lineage differentiation of canine lymphoma/leukemias and aberrant expression of CD molecules. Vet Immunol Immunopathol. 2005;106:179–196. doi: 10.1016/j.vetimm.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 119.Day M.J. Immunophenotypic characterization of cutaneous lymphoid neoplasia in the dog and cat. J Comp Pathol. 1995;112:79–96. doi: 10.1016/s0021-9975(05)80091-x. [DOI] [PubMed] [Google Scholar]
- 120.Barber L.G., Weishaar K.M. Criteria for designation of clinical substage in canine lymphoma: a survey of veterinary oncologists. Vet Comp Oncol. 2016;14(Suppl 1):32–39. doi: 10.1111/vco.12086. [DOI] [PubMed] [Google Scholar]
- 121.Blackwood L., Sullivan M., Lawson H. Radiographic abnormalities in canine multicentric lymphoma: a review of 84 cases. J Small Anim Pract. 1997;38:62–69. doi: 10.1111/j.1748-5827.1997.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 122.Starrak G.S., Berry C.R., Page R.L. Correlation between thoracic radiographic changes and remission/survival duration in 270 dogs with lymphosarcoma. Vet Radiol Ultrasound. 1997;38:411–418. doi: 10.1111/j.1740-8261.1997.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 123.Congress ‘99: what’s new in the treatment of lymphoma? J Small Anim Pract. 1999;40:51. doi: 10.1111/j.1748-5827.1999.tb03259.x. [DOI] [PubMed] [Google Scholar]
- 124.Yohn S.E., Hawkins E.C., Morrison W.B. Confirmation of a pulmonary component of multicentric lymphosarcoma with bronchoalveolar lavage in two dogs. J Am Vet Med Assoc. 1994;204:97–101. [PubMed] [Google Scholar]
- 125.Lane J., Price J., Moore A. Low-grade gastrointestinal lymphoma in dogs: 20 cases (2010 to 2016) J Small Anim Pract. 2018;59:147–153. doi: 10.1111/jsap.12769. [DOI] [PubMed] [Google Scholar]
- 126.Sogame N., Risbon R., Burgess K.E. Intestinal lymphoma in dogs: 84 cases (1997-2012) J Am Vet Med Assoc. 2018;252:440–447. doi: 10.2460/javma.252.4.440. [DOI] [PubMed] [Google Scholar]
- 127.Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract. 2011;41:419–432. doi: 10.1016/j.cvsm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Leib M.S. Alimentary lymphosarcoma in a dog. Compend Pract Vet Contin Educ. 1987;9:809–815. [Google Scholar]
- 129.Chan C.M., Frimberger A.E., Moore A.S. Clinical outcome and prognosis of dogs with histopathological features consistent with epitheliotropic lymphoma: a retrospective study of 148 cases (2003-2015) Vet Dermatol. 2018;29:154–159. doi: 10.1111/vde.12504. [DOI] [PubMed] [Google Scholar]
- 130.Fontaine J., Bovens C., Bettenay S. Canine cutaneous epitheliotropic T-cell lymphoma: a review. Vet Comp Oncol. 2009;7:1–14. doi: 10.1111/j.1476-5829.2008.00176.x. [DOI] [PubMed] [Google Scholar]
- 131.Fontaine J., Heimann M., Day M.J. Cutaneous epitheliotropic T-cell lymphoma in the cat: a review of the literature and five new cases. Vet Dermatol. 2011;22:454–461. doi: 10.1111/j.1365-3164.2011.00972.x. [DOI] [PubMed] [Google Scholar]
- 132.McKeever P.J., Grindem C.B., Stevens J.B. Canine cutaneous lymphoma. J Am Vet Med Assoc. 1982;180:531–536. [PubMed] [Google Scholar]
- 133.Couto C.G., Cullen J., Pedroia V. Central nervous system lymphosarcoma in the dog. J Am Vet Med Assoc. 1984;184:809–813. [PubMed] [Google Scholar]
- 134.Dallman M.J., Saunders G.K. Primary spinal cord lymphosarcoma in a dog. J Am Vet Med Assoc. 1986;189:1348–1349. [PubMed] [Google Scholar]
- 135.Rosin A. Neurologic diseases associated with lymphosarcoma in ten dogs. J Am Vet Med Assoc. 1982;181:50–53. [PubMed] [Google Scholar]
- 136.Siso S., Marco-Salazar P., Moore P.F. Canine nervous system lymphoma subtypes display characteristic neuroanatomical patterns. Vet Pathol. 2017;54:53–60. doi: 10.1177/0300985816658101. [DOI] [PubMed] [Google Scholar]
- 137.Lanza M.R., Musciano A.R., Dubielzig R.D. Clinical and pathological classification of canine intraocular lymphoma. Vet Ophthalmol. 2018;21:167–173. doi: 10.1111/vop.12492. [DOI] [PubMed] [Google Scholar]
- 138.Swanson J.F. Ocular manifestations of systemic disease in the dog and cat. Recent developments. Vet Clin North Am Small Anim Pract. 1990;20:849–867. doi: 10.1016/s0195-5616(90)50065-0. [DOI] [PubMed] [Google Scholar]
- 139.Krohne S.D.G.V.W., Richardson R.C. Ocular involvement in canine lymphosarcoma: a retrospecive study of 94 cases. Am College Vet Ophth Proc. 1987:68–84. [Google Scholar]
- 140.Madewell B.R., Feldman B.F. Characterization of anemias associated with neoplasia in small animals. J Am Vet Med Assoc. 1980;176:419–425. [PubMed] [Google Scholar]
- 141.Kubota A., Kano R., Mizuno T. Parathyroid hormone-related protein (PTHrP) produced by dog lymphoma cells. J Vet Med Sci. 2002;64:835–837. doi: 10.1292/jvms.64.835. [DOI] [PubMed] [Google Scholar]
- 142.Rosol T.J., Nagode L.A., Couto C.G. Parathyroid hormone (PTH)-related protein, PTH, and 1,25-dihydroxyvitamin D in dogs with cancer-associated hypercalcemia. Endocrinology. 1992;131:1157–1164. doi: 10.1210/endo.131.3.1505457. [DOI] [PubMed] [Google Scholar]
- 143.Gerber B., Hauser B., Reusch C.E. Serum levels of 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol in dogs with hypercalcaemia. Vet Res Commun. 2004;28:669–680. doi: 10.1023/b:verc.0000045954.71403.74. [DOI] [PubMed] [Google Scholar]
- 144.Massa K.L., Gilger B.C., Miller T.L. Causes of uveitis in dogs: 102 cases (1989-2000) Vet Ophthalmol. 2002;5:93–98. doi: 10.1046/j.1463-5224.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 145.Grindem C.B., Breitschwerdt E.B., Corbett W.T. Thrombocytopenia associated with neoplasia in dogs. J Vet Intern Med. 1994;8:400–405. doi: 10.1111/j.1939-1676.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 146.BR m. Hematological and bone marrow cytological abnormalities in 75 dogs with malignant lymphoma. J Am Anim Hosp Assoc. 1986;22:235–240. [Google Scholar]
- 147.MacEwen E.G., Hurvitz A.I. Diagnosis and management of monoclonal gammopathies. Vet Clin North Am. 1977;7:119–132. doi: 10.1016/s0091-0279(77)50010-x. [DOI] [PubMed] [Google Scholar]
- 148.Ku C.K., Kass P.H., Christopher M.M. Cytologic-histologic concordance in the diagnosis of neoplasia in canine and feline lymph nodes: a retrospective study of 367 cases. Vet Comp Oncol. 2017;2017(15):1206–1217. doi: 10.1111/vco.12256. [DOI] [PubMed] [Google Scholar]
- 149.Sapierzynski R., Kliczkowska-Klarowicz K., Jankowska U. Cytodiagnostics of canine lymphomas - possibilities and limitations. Pol J Vet Sci. 2016;19:433–439. doi: 10.1515/pjvs-2016-0055. [DOI] [PubMed] [Google Scholar]
- 150.Sozmen M., Tasca S., Carli E. Use of fine needle aspirates and flow cytometry for the diagnosis, classification, and immunophenotyping of canine lymphomas. J Vet Diagn Invest. 2005;17:323–330. doi: 10.1177/104063870501700404. [DOI] [PubMed] [Google Scholar]
- 151.Crabtree A.C., Spangler E., Beard D. Diagnostic accuracy of gray-scale ultrasonography for the detection of hepatic and splenic lymphoma in dogs. Vet Radiol Ultrasound. 2010;51:661–664. doi: 10.1111/j.1740-8261.2010.01725.x. [DOI] [PubMed] [Google Scholar]
- 152.Kinns J., Mai W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. Vet Radiol Ultrasound. 2007;48:565–569. doi: 10.1111/j.1740-8261.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 153.Nyman H.T., Lee M.H., McEvoy F.J. Comparison of B-mode and Doppler ultrasonographic findings with histologic features of benign and malignant superficial lymph nodes in dogs. Am J Vet Res. 2006;67:978–984. doi: 10.2460/ajvr.67.6.978. [DOI] [PubMed] [Google Scholar]
- 154.Carrasco V., Rodriguez-Bertos A., Rodriguez-Franco F. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet Pathol. 2015;52:668–675. doi: 10.1177/0300985814559398. [DOI] [PubMed] [Google Scholar]
- 155.Maeda S., Tsuboi M., Sakai K. Endoscopic cytology for the diagnosis of chronic enteritis and intestinal lymphoma in dogs. Vet Pathol. 2017;54:595–604. doi: 10.1177/0300985817705175. [DOI] [PubMed] [Google Scholar]
- 156.Ohmi A., Ohno K., Uchida K. Significance of clonal rearrangements of lymphocyte antigen receptor genes on the prognosis of chronic enteropathy in 22 Shiba dogs. J Vet Med Sci. 2017;79:1578–1584. doi: 10.1292/jvms.16-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ohmura S., Leipig M., Schopper I. Detection of monoclonality in intestinal lymphoma with polymerase chain reaction for antigen receptor gene rearrangement analysis to differentiate from enteritis in dogs. Vet Comp Oncol. 2017;15:194–207. doi: 10.1111/vco.12151. [DOI] [PubMed] [Google Scholar]
- 158.Takanosu M., Kagawa Y. Comparison of primer sets for T-cell clonality testing in canine intestinal lymphoma. J Vet Diagn Invest. 2015;27:645–650. doi: 10.1177/1040638715600197. [DOI] [PubMed] [Google Scholar]
- 159.Fukushima K., Ohno K., Koshino-Goto Y. Sensitivity for the detection of a clonally rearranged antigen receptor gene in endoscopically obtained biopsy specimens from canine alimentary lymphoma. J Vet Med Sci. 2009;71:1673–1676. doi: 10.1292/jvms.001673. [DOI] [PubMed] [Google Scholar]
- 160.Kaneko N., Yamamoto Y., Wada Y. Application of polymerase chain reaction to analysis of antigen receptor rearrangements to support endoscopic diagnosis of canine alimentary lymphoma. J Vet Med Sci. 2009;71:555–559. doi: 10.1292/jvms.71.555. [DOI] [PubMed] [Google Scholar]
- 161.Kleinschmidt S., Meneses F., Nolte I. Retrospective study on the diagnostic value of full-thickness biopsies from the stomach and intestines of dogs with chronic gastrointestinal disease symptoms. Vet Pathol. 2006;43:1000–1003. doi: 10.1354/vp.43-6-1000. [DOI] [PubMed] [Google Scholar]
- 162.Miura T., Maruyama H., Sakai M. Endoscopic findings on alimentary lymphoma in 7 dogs. J Vet Med Sci. 2004;66:577–580. doi: 10.1292/jvms.66.577. [DOI] [PubMed] [Google Scholar]
- 163.Chaubert P., Baur Chaubert A.S., Sattler U. Improved polymerase chain reaction-based method to detect early-stage epitheliotropic T-cell lymphoma (mycosis fungoides) in formalin-fixed, paraffin-embedded skin biopsy specimens of the dog. J Vet Diagn Invest. 2010;22:20–29. doi: 10.1177/104063871002200104. [DOI] [PubMed] [Google Scholar]
- 164.Comazzi S., Gelain M.E. Use of flow cytometric immunophenotyping to refine the cytological diagnosis of canine lymphoma. Vet J. 2011;188:149–155. doi: 10.1016/j.tvjl.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 165.Culmsee K., Simon D., Mischke R. Possibilities of flow cytometric analysis for immunophenotypic characterization of canine lymphoma. J Vet Med A Physiol Pathol Clin Med. 2001;48:199–206. doi: 10.1046/j.1439-0442.2001.00351.x. [DOI] [PubMed] [Google Scholar]
- 166.Fisher D.J., Naydan D., Werner L.L. Immunophenotyping lymphomas in dogs: a comparison of results from fine needle aspirate and needle biopsy samples. Vet Clin Pathol. 1995;24:118–123. doi: 10.1111/j.1939-165x.1995.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 167.Gelain M.E., Mazzilli M., Riondato F. Aberrant phenotypes and quantitative antigen expression in different subtypes of canine lymphoma by flow cytometry. Vet Immunol Immunopathol. 2008;121:179–188. doi: 10.1016/j.vetimm.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 168.Gibson D., Aubert I., Woods J.P. Flow cytometric immunophenotype of canine lymph node aspirates. J Vet Intern Med. 2004;18:710–717. doi: 10.1892/0891-6640(2004)18<710:fciocl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 169.Lana S., Plaza S., Hampe K. Diagnosis of mediastinal masses in dogs by flow cytometry. J Vet Intern Med. 2006;20:1161–1165. doi: 10.1892/0891-6640(2006)20[1161:dommid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 170.Tasca S., Carli E., Caldin M. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002-2006) Vet Clin Pathol. 2009;38:2–12. doi: 10.1111/j.1939-165X.2008.00099.x. [DOI] [PubMed] [Google Scholar]
- 171.Vail D.M., Kravis L.D., Kisseberth W.C. Application of rapid CD3 immunophenotype analysis and argyrophilic nucleolar organizer region (AgNOR) frequency to fine needle aspirate specimens from dogs with lymphoma. Vet Clin Pathol. 1997;26:66–69. doi: 10.1111/j.1939-165x.1997.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 172.Bauer N.B., Zervos D., Moritz A. Argyrophilic nucleolar organizing regions and Ki67 equally reflect proliferation in fine needle aspirates of normal, hyperplastic, inflamed, and neoplastic canine lymph nodes (n = 101) J Vet Intern Med. 2007;21:928–935. doi: 10.1892/0891-6640(2007)21[928:anorak]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 173.Fournel-Fleury C., Magnol J.P., Chabanne L. Growth fractions in canine non-Hodgkin’s lymphomas as determined in situ by the expression of the Ki-67 antigen. J Comp Pathol. 1997;117:61–72. doi: 10.1016/s0021-9975(97)80066-7. [DOI] [PubMed] [Google Scholar]
- 174.Phillips B.S., Kass P.H., Naydan D.K. Apoptotic and proliferation indexes in canine lymphoma. J Vet Diagn Invest. 2000;12:111–117. doi: 10.1177/104063870001200202. [DOI] [PubMed] [Google Scholar]
- 175.Vajdovich P., Psader R., Toth Z.A. Use of the argyrophilic nucleolar region method for cytologic and histologic examination of the lymph nodes in dogs. Vet Pathol. 2004;41:338–345. doi: 10.1354/vp.41-4-338. [DOI] [PubMed] [Google Scholar]
- 176.Hung L.C., Pong V.F., Cheng C.R. An improved system for quantifying AgNOR and PCNA in canine tumors. Anticancer Res. 2000;20:3273–3280. [PubMed] [Google Scholar]
- 177.Poggi A., Miniscalco B., Morello E. Prognostic significance of Ki67 evaluated by flow cytometry in dogs with high-grade B-cell lymphoma. Vet Comp Oncol. 2017;15:431–440. doi: 10.1111/vco.12184. [DOI] [PubMed] [Google Scholar]
- 178.Frantz A.M., Sarver A.L., Ito D. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Vet Pathol. 2013;50:693–703. doi: 10.1177/0300985812465325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Su Y., Nielsen D., Zhu L. Gene selection and cancer type classification of diffuse large-B-cell lymphoma using a bivariate mixture model for two-species data. Hum Genomics. 2013;7(2) doi: 10.1186/1479-7364-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Avery A. Molecular diagnostics of hematologic malignancies. Top Companion Anim Med. 2009;24:144–150. doi: 10.1053/j.tcam.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 181.Avery P.R., Avery A.C. Molecular methods to distinguish reactive and neoplastic lymphocyte expansions and their importance in transitional neoplastic states. Vet Clin Pathol. 2004;33:196–207. doi: 10.1111/j.1939-165x.2004.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 182.Burnett R.C., Vernau W., Modiano J.F. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol. 2003;40:32–41. doi: 10.1354/vp.40-1-32. [DOI] [PubMed] [Google Scholar]
- 183.Dreitz M.J., Ogilvie G., Sim G.K. Rearranged T lymphocyte antigen receptor genes as markers of malignant T cells. Vet Immunol Immunopathol. 1999;69:113–119. doi: 10.1016/s0165-2427(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 184.Gentilini F., Calzolari C., Turba M.E. GeneScanning analysis of Ig/TCR gene rearrangements to detect clonality in canine lymphomas. Vet Immunol Immunopathol. 2009;127:47–56. doi: 10.1016/j.vetimm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 185.Keller R.L., Avery A.C., Burnett R.C. Detection of neoplastic lymphocytes in peripheral blood of dogs with lymphoma by polymerase chain reaction for antigen receptor gene rearrangement. Vet Clin Pathol. 2004;33:145–149. doi: 10.1111/j.1939-165x.2004.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 186.Tamura K., Yagihara H., Isotani M. Development of the polymerase chain reaction assay based on the canine genome database for detection of monoclonality in B cell lymphoma. Vet Immunol Immunopathol. 2006;110:163–167. doi: 10.1016/j.vetimm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 187.Yagihara H., Tamura K., Isotani M. Genomic organization of the T-cell receptor gamma gene and PCR detection of its clonal rearrangement in canine T-cell lymphoma/leukemia. Vet Immunol Immunopathol. 2007;115:375–382. doi: 10.1016/j.vetimm.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 188.Davis B., Schwartz M., Duchemin D. Validation of a multiplexed gene signature assay for diagnosis of canine cancers from formalin-fixed paraffin-embedded tissues. J Vet Intern Med. 2017;31:854–863. doi: 10.1111/jvim.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Drazovska M., Sivikova K., Dianovsky J. Comparative genomic hybridization in detection of DNA changes in canine lymphomas. An Sci J. 2017;88:27–32. doi: 10.1111/asj.12582. [DOI] [PubMed] [Google Scholar]
- 190.Comazzi S., Avery P.R., Garden O.A. European canine lymphoma network consensus recommendations for reporting flow cytometry in canine hematopoietic neoplasms. Cytometry Part B. 2017;92:411–419. doi: 10.1002/cyto.b.21382. [DOI] [PubMed] [Google Scholar]
- 191.Fournel-Fleury C., Magnol J.P., Bricaire P. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin’s lymphomas. J Comp Pathol. 1997;117:35–59. doi: 10.1016/s0021-9975(97)80065-5. [DOI] [PubMed] [Google Scholar]
- 192.Ponce F., Magnol J.P., Marchal T. High-grade canine T-cell lymphoma/leukemia with plasmacytoid morphology: a clinical pathological study of nine cases. J Vet Diagn Invest. 2003;15:330–337. doi: 10.1177/104063870301500405. [DOI] [PubMed] [Google Scholar]
- 193.Keller S.M., Vernau W., Moore P.F. Clonality testing in veterinary medicine: a review with diagnostic guidelines. Vet Pathol. 2016;53:711–725. doi: 10.1177/0300985815626576. [DOI] [PubMed] [Google Scholar]
- 194.Langner K.F., Joetzke A.E., Nerschbach V. Detection of clonal antigen receptor gene rearrangement in dogs with lymphoma by real-time polymerase chain reaction and melting curve analysis. BMC Vet Res. 2014;10(1) doi: 10.1186/1746-6148-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Waugh E.M., Gallagher A., Haining H. Optimisation and validation of a PCR for antigen receptor rearrangement (PARR) assay to detect clonality in canine lymphoid malignancies. Vet Immunol Immunopathol. 2016;182:115–124. doi: 10.1016/j.vetimm.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bergman P.J., Ogilvie G.K., Powers B.E. Monoclonal antibody C219 immunohistochemistry against P-glycoprotein: sequential analysis and predictive ability in dogs with lymphoma. J Vet Intern Med. 1996;10:354–359. doi: 10.1111/j.1939-1676.1996.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 197.Moore A.S., Leveille C.R., Reimann K.A. The expression of P-glycoprotein in canine lymphoma and its association with multidrug resistance. Cancer Invest. 1995;13:475–479. doi: 10.3109/07357909509024910. [DOI] [PubMed] [Google Scholar]
- 198.Gaines P.J., Powell T.D., Walmsley S.J. Identification of serum biomarkers for canine B-cell lymphoma by use of surface-enhanced laser desorption-ionization time-of-flight mass spectrometry. Am J Vet Res. 2007;68:405–410. doi: 10.2460/ajvr.68.4.405. [DOI] [PubMed] [Google Scholar]
- 199.McCaw D.L., Chan A.S., Stegner A.L. Proteomics of canine lymphoma identifies potential cancer-specific protein markers. Clin Cancer Res. 2007;13:2496–2503. doi: 10.1158/1078-0432.CCR-06-2699. [DOI] [PubMed] [Google Scholar]
- 200.Ratcliffe L., Mian S., Slater K. Proteomic identification and profiling of canine lymphoma patients. Vet Comp Oncol. 2009;7:92–105. doi: 10.1111/j.1476-5829.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- 201.Wilson C.R., Regnier F.E., Knapp D.W. Glycoproteomic profiling of serum peptides in canine lymphoma and transitional cell carcinoma. Vet Comp Oncol. 2008;6:171–181. doi: 10.1111/j.1476-5829.2008.00158.x. [DOI] [PubMed] [Google Scholar]
- 202.Bryan J.N. The current state of clinical application of serum biomarkers for canine lymphoma. Frontie Vet Sci. 2016;3:87. doi: 10.3389/fvets.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Alexandrakis I., Tuli R., Ractliffe S.C. Utility of a multiple serum biomarker test to monitor remission status and relapse in dogs with lymphoma undergoing treatment with chemotherapy. Vet Comp Oncol. 2017;15:6–17. doi: 10.1111/vco.12123. [DOI] [PubMed] [Google Scholar]
- 204.Mirkes E.M., Alexandrakis I., Slater K. Computational diagnosis and risk evaluation for canine lymphoma. Comput Biol Med. 2014;53:279–290. doi: 10.1016/j.compbiomed.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 205.Selting K.A., Ringold R., Husbands B. Thymidine kinase type 1 and C-reactive protein concentrations in dogs with spontaneously occurring cancer. J Vet Intern Med. 2016;30:1159–1166. doi: 10.1111/jvim.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sharif H., von Euler H., Westberg S. A sensitive and kinetically defined radiochemical assay for canine and human serum thymidine kinase 1 (TK1) to monitor canine malignant lymphoma. Vet J. 2012;194:40–47. doi: 10.1016/j.tvjl.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 207.Hahn K.A., Freeman K.P., Barnhill M.A. Serum alpha 1-acid glycoprotein concentrations before and after relapse in dogs with lymphoma treated with doxorubicin. J Am Vet Med Assoc. 1999;214:1023–1025. [PubMed] [Google Scholar]
- 208.Kazmierski K.J., Ogilvie G.K., Fettman M.J. Serum zinc, chromium, and iron concentrations in dogs with lymphoma and osteosarcoma. J Vet Intern Med. 2001;15:585–588. doi: 10.1892/0891-6640(2001)015<0585:szcaic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 209.Lechowski R., Jagielski D., Hoffmann-Jagielska M. Alpha-fetoprotein in canine multicentric lymphoma. Vet Res Commun. 2002;26:285–296. doi: 10.1023/a:1016086508286. [DOI] [PubMed] [Google Scholar]
- 210.Marconato L., Crispino G., Finotello R. Clinical relevance of serial determinations of lactate dehydrogenase activity used to predict recurrence in dogs with lymphoma. J Am Vet Med Assoc. 2010;236:969–974. doi: 10.2460/javma.236.9.969. [DOI] [PubMed] [Google Scholar]
- 211.Mischke R., Waterston M., Eckersall P.D. Changes in C-reactive protein and haptoglobin in dogs with lymphatic neoplasia. Vet J. 2007;174:188–192. doi: 10.1016/j.tvjl.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 212.Winter J.L., Barber L.G., Freeman L. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Intern Med. 2009;23:311–316. doi: 10.1111/j.1939-1676.2009.0273.x. [DOI] [PubMed] [Google Scholar]
- 213.Zizzo N., Patruno R., Zito F.A. Vascular endothelial growth factor concentrations from platelets correlate with tumor angiogenesis and grading in a spontaneous canine non-Hodgkin lymphoma model. Leuk Lymphoma. 2010;51:291–296. doi: 10.3109/10428190903452818. [DOI] [PubMed] [Google Scholar]
- 214.Rossmeisl J.H., Jr., Bright P., Tamarkin L. Endostatin concentrations in healthy dogs and dogs with selected neoplasms. J Vet Intern Med. 2002;16:565–569. doi: 10.1892/0891-6640(2002)016<0565:ecihda>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 215.Flory A.B., Rassnick K.M., Stokol T. Stage migration in dogs with lymphoma. J Vet Intern Med. 2007;21:1041–1047. doi: 10.1892/0891-6640(2007)21[1041:smidwl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 216.Raskin R.E., Krehbiel J.D. Prevalence of leukemic blood and bone marrow in dogs with multicentric lymphoma. J Am Vet Med Assoc. 1989;194:1427–1429. [PubMed] [Google Scholar]
- 217.Aresu L., Arico A., Ferraresso S. Minimal residual disease detection by flow cytometry and PARR in lymph node, peripheral blood and bone marrow, following treatment of dogs with diffuse large B-cell lymphoma. Vet J. 2014;200:318–324. doi: 10.1016/j.tvjl.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 218.Weiss D.J. Evaluation of proliferative disorders in canine bone marrow by use of flow cytometric scatter plots and monoclonal antibodies. Vet Pathol. 2001;38:512–518. doi: 10.1354/vp.38-5-512. [DOI] [PubMed] [Google Scholar]
- 219.Ackerman N., Madewell B.R. Thoracic and abdominal radiographic abnormalities in the multicentric form of lymphosarcoma in dogs. J Am Vet Med Assoc. 1980;176:36–40. [PubMed] [Google Scholar]
- 220.Geyer N.E., Reichle J.K., Valdes-Martinez A. Radiographic appearance of confirmed pulmonary lymphoma in cats and dogs. Vet Radiol Ultrasound. 2010;51:386–390. doi: 10.1111/j.1740-8261.2010.01683.x. [DOI] [PubMed] [Google Scholar]
- 221.Jones I.D., Daniels A.D., Lara-Garcia A. Computed tomographic findings in 12 cases of canine multi-centric lymphoma with splenic and hepatic involvement. J Small Anim Pract. 2017;58:622–628. doi: 10.1111/jsap.12714. [DOI] [PubMed] [Google Scholar]
- 222.Yoon J., Feeney D.A., Cronk D.E. Computed tomographic evaluation of canine and feline mediastinal masses in 14 patients. Vet Radiol Ultrasound. 2004;45:542–546. doi: 10.1111/j.1740-8261.2004.04093.x. [DOI] [PubMed] [Google Scholar]
- 223.Bassett C.L., Daniel G.B., Bochsler P.N. Characterization of uptake of 2-deoxy-2-[18F]fluoro-D-glucose by fungal-associated inflammation: the differential uptake ratio for blastomyces-associated lesions is as high as for lymphoma and higher than for turpentine abscesses in experimentally induced lesions in rats. Mol Imaging Biol. 2002;4:193–200. doi: 10.1016/s1536-1632(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 224.Lawrence J., Vanderhoek M., Barbee D. Use of 3’-deoxy-3’-[18F]fluorothymidine PET/CT for evaluating response to cytotoxic chemotherapy in dogs with non-Hodgkin’s lymphoma. Vet Radiol Ultrasound. 2009;50:660–668. doi: 10.1111/j.1740-8261.2009.01612.x. [DOI] [PubMed] [Google Scholar]
- 225.LeBlanc A.K., Jakoby B.W., Townsend D.W. 18FDG-PET imaging in canine lymphoma and cutaneous mast cell tumor. Vet Radiol Ultrasound. 2009;50:215–223. doi: 10.1111/j.1740-8261.2009.01520.x. [DOI] [PubMed] [Google Scholar]
- 226.Vail D.M., Thamm D.H., Reiser H. Assessment of GS-9219 in a pet dog model of non-Hodgkin’s lymphoma. Clin Cancer Res. 2009;15:3503–3510. doi: 10.1158/1078-0432.CCR-08-3113. [DOI] [PubMed] [Google Scholar]
- 227.Burton J.H., Garrett-Mayer E., Thamm D.H. Evaluation of a 15-week CHOP protocol for the treatment of canine multicentric lymphoma. Vet Comp Oncol. 2013;11:306–315. doi: 10.1111/j.1476-5829.2012.00324.x. [DOI] [PubMed] [Google Scholar]
- 228.Curran K., Thamm D.H. Retrospective analysis for treatment of naive canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet Comp Oncol. 2016;14(Suppl 1):147–155. doi: 10.1111/vco.12163. [DOI] [PubMed] [Google Scholar]
- 229.Elliott J.W., Cripps P., Marrington A.M. Epirubicin as part of a multi-agent chemotherapy protocol for canine lymphoma. Vet Comp Oncol. 2013;11:185–198. doi: 10.1111/j.1476-5829.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 230.Thamm D.H., Vail D.M., Post G.S. Alternating rabacfosadine/doxorubicin: efficacy and tolerability in naive canine multicentric lymphoma. J Vet Intern Med. 2017;31:872–878. doi: 10.1111/jvim.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Daters A.T., Mauldin G.E., Mauldin G.N. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP-MA) for the treatment of canine lymphoma. Vet Comp Oncol. 2010;8:11–22. doi: 10.1111/j.1476-5829.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 232.Garrett L.D., Thamm D.H., Chun R. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2002;16:704–709. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 233.Hosoya K., Kisseberth W.C., Lord L.K. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1355–1363. doi: 10.1892/06-284.1. [DOI] [PubMed] [Google Scholar]
- 234.Kaiser C.I., Fidel J.L., Roos M. Reevaluation of the University of Wisconsin 2-year protocol for treating canine lymphosarcoma. J Am Anim Hosp Assoc. 2007;43:85–92. doi: 10.5326/0430085. [DOI] [PubMed] [Google Scholar]
- 235.Keller E.T., MacEwen E.G., Rosenthal R.C. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J Vet Intern Med. 1993;7:289–295. doi: 10.1111/j.1939-1676.1993.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 236.MacEwen E.G., Brown N.O., Patnaik A.K. Cyclic combination chemotherapy of canine lymphosarcoma. J Am Vet Med Assoc. 1981;178:1178–1181. [PubMed] [Google Scholar]
- 237.MacEwen E.G., Hayes A.A., Matus R.E. Evaluation of some prognostic factors for advanced multicentric lymphosarcoma in the dog: 147 cases (1978-1981) J Am Vet Med Assoc. 1987;190:564–568. [PubMed] [Google Scholar]
- 238.Morrison-Collister K.E., Rassnick K.M., Northrup N.C. A combination chemotherapy protocol with MOPP and CCNU consolidation (Tufts VELCAP-SC) for the treatment of canine lymphoma. Vet Comp Oncol. 2003;1:180–190. doi: 10.1111/j.1476-5810.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 239.Mutsaers A.J., Glickman N.W., DeNicola D.B. Evaluation of treatment with doxorubicin and piroxicam or doxorubicin alone for multicentric lymphoma in dogs. J Am Vet Med Assoc. 2002;220:1813–1817. doi: 10.2460/javma.2002.220.1813. [DOI] [PubMed] [Google Scholar]
- 240.Myers N.C., 3rd, Moore A.S., Rand W.M. Evaluation of a multidrug chemotherapy protocol (ACOPA II) in dogs with lymphoma. J Vet Intern Med. 1997;11:333–339. doi: 10.1111/j.1939-1676.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 241.Rassnick K.M., Bailey D.B., Malone E.K. Comparison between L-CHOP and an L-CHOP protocol with interposed treatments of CCNU and MOPP (L-CHOP-CCNU-MOPP) for lymphoma in dogs. Vet Comp Oncol. 2010;8:243–253. doi: 10.1111/j.1476-5829.2010.00224.x. [DOI] [PubMed] [Google Scholar]
- 242.Siedlecki C.T., Kass P.H., Jakubiak M.J. Evaluation of an actinomycin-D-containing combination chemotherapy protocol with extended maintenance therapy for canine lymphoma. Can Vet J. 2006;47:52–59. [PMC free article] [PubMed] [Google Scholar]
- 243.Simon D., Moreno S.N., Hirschberger J. Efficacy of a continuous, multiagent chemotherapeutic protocol versus a short-term single-agent protocol in dogs with lymphoma. J Am Vet Med Assoc. 2008;232:879–885. doi: 10.2460/javma.232.6.879. [DOI] [PubMed] [Google Scholar]
- 244.Simon D., Nolte I., Eberle N. Treatment of dogs with lymphoma using a 12-week, maintenance-free combination chemotherapy protocol. J Vet Intern Med. 2006;20:948–954. doi: 10.1892/0891-6640(2006)20[948:todwlu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 245.Sorenmo K., Overley B., Krick E. Outcome and toxicity associated with a dose-intensified, maintenance-free CHOP-based chemotherapy protocol in canine lymphoma: 130 cases. Vet Comp Oncol. 2010;8:196–208. doi: 10.1111/j.1476-5829.2010.00222.x. [DOI] [PubMed] [Google Scholar]
- 246.Valerius K.D., Ogilvie G.K., Mallinckrodt C.H. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987-1995) J Am Vet Med Assoc. 1997;210:512–516. [PubMed] [Google Scholar]
- 247.Zemann B.I., Moore A.S., Rand W.M. A combination chemotherapy protocol (VELCAP-L) for dogs with lymphoma. J Vet Intern Med. 1998;12:465–470. doi: 10.1111/j.1939-1676.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 248.Cotter S.M.G.M. Treatment of lymphoma and leukemia with cyclophosphamide, vincristine and prednisone: I. Treatment of dog. J Am Anim Hosp Assoc. 1983;19:159–165. [Google Scholar]
- 249.Boyce K.L.K.B. Treatment of canine lymphoma with COPLA/LVP. J Am Anim Hosp Assoc. 2000;36:395–403. doi: 10.5326/15473317-36-5-395. [DOI] [PubMed] [Google Scholar]
- 250.Postorino N.C., Susaneck S.J., Withrow S.J. Single agent therapy with adriamycin for canine lymphosarcoma. J Am Anim Hosp Assoc. 1989;25:221–225. [Google Scholar]
- 251.Stone M.S.G.M., Cotter S.M. Comparison of two protocols for induction of remission in dogs with lymphoma. J Am Anim Hosp Assoc. 1991;27:315–321. [Google Scholar]
- 252.Fisher R.I., Gaynor E.R., Dahlberg S. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 253.Vail D.M., Michels G.M., Khanna C. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)—a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2010;8:28–37. doi: 10.1111/j.1476-5829.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 254.Tomiyasu H., Takahashi M., Fujino Y. Gastrointestinal and hematologic adverse events after administration of vincristine, cyclophosphamide, and doxorubicin in dogs with lymphoma that underwent a combination multidrug chemotherapy protocol. J Vet Med Sci. 2010;72:1391–1397. doi: 10.1292/jvms.10-0176. [DOI] [PubMed] [Google Scholar]
- 255.Vail D.M. Cytotoxic chemotherapy agents. Clin Brief. 2010;8:18–22. [Google Scholar]
- 256.Mellanby R.J., Herrtage M.E., Dobson J.M. Owners’ assessments of their dog’s quality of life during palliative chemotherapy for lymphoma. J Small Anim Pract. 2003;44:100–103. doi: 10.1111/j.1748-5827.2003.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 257.Bronden L.B., Rutteman G.R., Flagstad A. Study of dog and cat owners’ perceptions of medical treatment for cancer. Vet Rec. 2003;152:77–80. doi: 10.1136/vr.152.3.77. [DOI] [PubMed] [Google Scholar]
- 258.Lautscham E.M., Kessler M., Ernst T. Comparison of a CHOP-LAsp-based protocol with and without maintenance for canine multicentric lymphoma. Vet Rec. 2017;180:303. doi: 10.1136/vr.104077. [DOI] [PubMed] [Google Scholar]
- 259.Moore A.S., Cotter S.M., Rand W.M. Evaluation of a discontinuous treatment protocol (VELCAP-S) for canine lymphoma. J Vet Intern Med. 2001;15:348–354. [PubMed] [Google Scholar]
- 260.Piek C.J., Rutteman G.R., Teske E. Evaluation of the results of a L-asparaginase-based continuous chemotherapy protocol versus a short doxorubicin-based induction chemotherapy protocol in dogs with malignant lymphoma. Vet Q. 1999;21:44–49. doi: 10.1080/01652176.1999.9694990. [DOI] [PubMed] [Google Scholar]
- 261.Rassnick K.M., McEntee M.C., Erb H.N. Comparison of 3 protocols for treatment after induction of remission in dogs with lymphoma. J Vet Intern Med. 2007;21:1364–1373. doi: 10.1892/07-057.1. [DOI] [PubMed] [Google Scholar]
- 262.Zenker I., Meichner K., Steinle K. Thirteen-week dose-intensifying simultaneous combination chemotherapy protocol for malignant lymphoma in dogs. Vet Rec. 2010;167:744–748. doi: 10.1136/vr.c5081. [DOI] [PubMed] [Google Scholar]
- 263.Ruslander D., Moore A.S., Gliatto J.M. Cytosine arabinoside as a single agent for the induction of remission in canine lymphoma. J Vet Intern Med. 1994;8:299–301. doi: 10.1111/j.1939-1676.1994.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 264.Rassnick K.M., Frimberger A.E., Wood C.A. Evaluation of ifosfamide for treatment of various canine neoplasms. J Vet Intern Med. 2000;14:271–276. doi: 10.1892/0891-6640(2000)014<0271:eoifto>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 265.Smith A.A., Lejeune A., Kow K. Clinical response and adverse event profile of bleomycin chemotherapy for canine multicentric lymphoma. J Am Anim Hosp Assoc. 2017;53:128–134. doi: 10.5326/JAAHA-MS-6598. [DOI] [PubMed] [Google Scholar]
- 266.Turner A.I., Hahn K.A., Rusk A. Single agent gemcitabine chemotherapy in dogs with spontaneously occurring lymphoma. J Vet Intern Med. 2006;20:1384–1388. doi: 10.1892/0891-6640(2006)20[1384:sagcid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 267.Reiser H., Wang J., Chong L. GS-9219—a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin’s lymphoma. Clin Cancer Res. 2008;14:2824–2832. doi: 10.1158/1078-0432.CCR-07-2061. [DOI] [PubMed] [Google Scholar]
- 268.Thamm D.H., Vail D.M., Kurzman I.D. GS-9219/VDC-1101—a prodrug of the acyclic nucleotide PMEG has antitumor activity in spontaneous canine multiple myeloma. BMC Vet Res. 2014;10(30) doi: 10.1186/1746-6148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Morges M.A., Burton J.H., Saba C.F. Phase II evaluation of VDC-1101 in canine cutaneous T-cell lymphoma. J Vet Intern Med. 2014;28:1569–1574. doi: 10.1111/jvim.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Saba C.F., Vickery K.R., Clifford C.A. Rabacfosadine for relapsed canine B-cell lymphoma: Efficacy and adverse event profiles of 2 different doses. Vet Comp Oncol. 2018;16:E76–E82. doi: 10.1111/vco.12337. [DOI] [PubMed] [Google Scholar]
- 271.Jeffreys A.B., Knapp D.W., Carlton W.W. Influence of asparaginase on a combination chemotherapy protocol for canine multicentric lymphoma. J Am Anim Hosp Assoc. 2005;41:221–226. doi: 10.5326/0410221. [DOI] [PubMed] [Google Scholar]
- 272.MacDonald V.S., Thamm D.H., Kurzman I.D. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? J Vet Intern Med. 2005;19:732–736. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 273.Carter R.F., Harris C.K., Withrow S.J. Chemotherapy of canine lymphoma with histopathologial correlation: doxorubicin alone compared to COP as first treatment regimen. J Am Anim Hosp Assoc. 1987;23:587–596. [Google Scholar]
- 274.Lori J.C., Stein T.J., Thamm D.H. Doxorubicin and cyclophosphamide for the treatment of canine lymphoma: a randomized, placebo-controlled study. Vet Comp Oncol. 2010;8:188–195. doi: 10.1111/j.1476-5829.2010.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sauerbrey M.L., Mullins M.N., Bannink E.O. Lomustine and prednisone as a first-line treatment for dogs with multicentric lymphoma: 17 cases (2004-2005) J Am Vet Med Assoc. 2007;230:1866–1869. doi: 10.2460/javma.230.12.1866. [DOI] [PubMed] [Google Scholar]
- 276.Khanna C., Lund E.M., Redic K.A. Randomized controlled trial of doxorubicin versus dactinomycin in a multiagent protocol for treatment of dogs with malignant lymphoma. J Am Vet Med Assoc. 1998;213:985–990. [PubMed] [Google Scholar]
- 277.Marconato L., Stefanello D., Valenti P. Predictors of long-term survival in dogs with high-grade multicentric lymphoma. J Am Vet Med Assoc. 2011;238:480–485. doi: 10.2460/javma.238.4.480. [DOI] [PubMed] [Google Scholar]
- 278.Price G.S., Page R.L., Fischer B.M. Efficacy and toxicity of doxorubicin/cyclophosphamide maintenance therapy in dogs with multicentric lymphosarcoma. J Vet Intern Med. 1991;5:259–262. doi: 10.1111/j.1939-1676.1991.tb03131.x. [DOI] [PubMed] [Google Scholar]
- 279.Fournier Q., Serra J.C., Handel I. Impact of pretreatment neutrophil count on chemotherapy administration and toxicity in dogs with lymphoma treated with CHOP chemotherapy. J Vet Intern Med. 2018;32:384–393. doi: 10.1111/jvim.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mealey K.L., Fidel J., Gay J.M. ABCB1-1Delta polymorphism can predict hematologic toxicity in dogs treated with vincristine. J Vet Intern Med. 2008;22:996–1000. doi: 10.1111/j.1939-1676.2008.0122.x. [DOI] [PubMed] [Google Scholar]
- 281.Beaver L.M., Strottner G., Klein M.K. Response rate after administration of a single dose of doxorubicin in dogs with B-cell or T-cell lymphoma: 41 cases (2006-2008) J Am Vet Med Assoc. 2010;237:1052–1055. doi: 10.2460/javma.237.9.1052. [DOI] [PubMed] [Google Scholar]
- 282.Dobson J.M., Blackwood L.B., McInnes E.F. Prognostic variables in canine multicentric lymphosarcoma. J Small Anim Pract. 2001;42:377–384. doi: 10.1111/j.1748-5827.2001.tb02485.x. [DOI] [PubMed] [Google Scholar]
- 283.Rebhun R.B., Kent M.S., Borrofka S.A. CHOP chemotherapy for the treatment of canine multicentric T-cell lymphoma. Vet Comp Oncol. 2011;9:38–44. doi: 10.1111/j.1476-5829.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 284.Brown P.M., Tzannes S., Nguyen S. LOPP chemotherapy as a first-line treatment for dogs with T-cell lymphoma. Vet Comp Oncol. 2018;16:108–113. doi: 10.1111/vco.12318. [DOI] [PubMed] [Google Scholar]
- 285.Goodman I.H., Moore A.S., Frimberger A.E. Treatment of canine non-indolent T cell lymphoma using the VELCAP-TSC protocol: a retrospective evaluation of 70 dogs (2003-2013) Vet J. 2016;211:39–44. doi: 10.1016/j.tvjl.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 286.Brodsky E.M., Maudlin G.N., Lachowicz J.L. Asparaginase and MOPP treatment of dogs with lymphoma. J Vet Intern Med. 2009;23:578–584. doi: 10.1111/j.1939-1676.2009.0289.x. [DOI] [PubMed] [Google Scholar]
- 287.Gentilini F., Turba M.E., Forni M. Retrospective monitoring of minimal residual disease using hairpin-shaped clone specific primers in B-cell lymphoma affected dogs. Vet Immunol Immunopathol. 2013;153:279–288. doi: 10.1016/j.vetimm.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 288.Sato M., Yamazaki J., Goto-Koshino Y. Minimal residual disease in canine lymphoma: an objective marker to assess tumour cell burden in remission. Vet J. 2016;215:38–42. doi: 10.1016/j.tvjl.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 289.Sato M., Yamzaki J., Goto-Koshino Y. The prognostic significance of minimal residual disease in the early phases of chemotherapy in dogs with high-grade B-cell lymphoma. Vet J. 2013;195:319–324. doi: 10.1016/j.tvjl.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 290.Thilakaratne D.N., Mayer M.N., MacDonald V.S. Clonality and phenotyping of canine lymphomas before chemotherapy and during remission using polymerase chain reaction (PCR) on lymph node cytologic smears and peripheral blood. Can Vet J. 2010;51:79–84. [PMC free article] [PubMed] [Google Scholar]
- 291.Valerius K.D., Ogilvie G.K., Fettman M.J. Comparison of the effects of asparaginase administered subcutaneously versus intramuscularly for treatment of multicentric lymphoma in dogs receiving doxorubicin. J Am Vet Med Assoc. 1999;214:353–356. [PubMed] [Google Scholar]
- 292.Valli V.E., Jacobs R.M., Norris A. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest. 2000;12:295–306. doi: 10.1177/104063870001200401. [DOI] [PubMed] [Google Scholar]
- 293.Yamazaki J., Baba K., Goto-Koshino Y. Quantitative assessment of minimal residual disease (MRD) in canine lymphoma by using real-time polymerase chain reaction. Vet Immunol Immunopathol. 2008;126:321–331. doi: 10.1016/j.vetimm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 294.Nielsen L., Toft N., Eckersall P.D. Serum C-reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J Vet Intern Med. 2007;21:1231–1236. doi: 10.1892/07-058.1. [DOI] [PubMed] [Google Scholar]
- 295.Ito D., Endicott M.M., Jubala C.M. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. J Vet Intern Med. 2011;25:890–896. doi: 10.1111/j.1939-1676.2011.0756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tomiyasu H., Tsujimoto H. Comparative aspects of molecular mechanisms of drug resistance through ABC transporters and other related molecules in canine lymphoma. Vet Sci. 2015;2:185–205. doi: 10.3390/vetsci2030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zandvliet M., Teske E. Mechanisms of drug resistance in veterinary oncology- a review with an emphasis on canine lymphoma. Vet Sci. 2015;2:150–184. doi: 10.3390/vetsci2030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zandvliet M., Teske E., Schrickx J.A. A longitudinal study of ABC transporter expression in canine multicentric lymphoma. Vet J. 2015;205:263–271. doi: 10.1016/j.tvjl.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 299.Lee J.J., Hughes C.S., Fine R.L. P-glycoprotein expression in canine lymphoma: a relevant, intermediate model of multidrug resistance. Cancer. 1996;77:1892–1898. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1892::AID-CNCR20>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 300.Flory A.B., Rassnick K.M., Erb H.N. Evaluation of factors associated with second remission in dogs with lymphoma undergoing retreatment with a cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy protocol: 95 cases (2000-2007) J Am Vet Med Assoc. 2011;238:501–506. doi: 10.2460/javma.238.4.501. [DOI] [PubMed] [Google Scholar]
- 301.Back A.R., Schleis S.E., Smrkovski O.A. Mechlorethamine, vincristine, melphalan and prednisone (MOMP) for the treatment of relapsed lymphoma in dogs. Vet Comp Oncol. 2015;13:398–408. doi: 10.1111/vco.12055. [DOI] [PubMed] [Google Scholar]
- 302.Fahey C.E., Milner R.J., Barabas K. Evaluation of the University of Florida lomustine, vincristine, procarbazine, and prednisone chemotherapy protocol for the treatment of relapsed lymphoma in dogs: 33 cases (2003-2009) J Am Vet Med Assoc. 2011;239:209–215. doi: 10.2460/javma.239.2.209. [DOI] [PubMed] [Google Scholar]
- 303.Gillem J., Giuffrida M., Krick E. Efficacy and toxicity of carboplatin and cytarabine chemotherapy for dogs with relapsed or refractory lymphoma (2000-2013) Vet Comp Oncol. 2017;15:400–410. doi: 10.1111/vco.12176. [DOI] [PubMed] [Google Scholar]
- 304.Lenz J.A., Robat C.S., Stein T.J. Vinblastine as a second rescue for the treatment of canine multicentric lymphoma in 39 cases (2005 to 2014) J Small Anim Pract. 2016;57:429–434. doi: 10.1111/jsap.12500. [DOI] [PubMed] [Google Scholar]
- 305.Mastromauro M.L., Suter S.E., Hauck M.L. Oral melphalan for the treatment of relapsed canine lymphoma. Vet Comp Oncol. 2018;16:E123–E129. doi: 10.1111/vco.12356. [DOI] [PubMed] [Google Scholar]
- 306.Parsons-Doherty M., Poirier V.J., Monteith G. The efficacy and adverse event profile of dexamethasone, melphalan, actinomycin D, and cytosine arabinoside (DMAC) chemotherapy in relapsed canine lymphoma. Can Vet J. 2014;55:175–180. [PMC free article] [PubMed] [Google Scholar]
- 307.Tanis J.B., Mason S.L., Maddox T.W. Evaluation of a multi-agent chemotherapy protocol combining lomustine, procarbazine and prednisolone (LPP) for the treatment of relapsed canine non-Hodgkin high-grade lymphomas. Vet Comp Oncol. 2018 doi: 10.1111/vco.12387. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 308.Treggiari E., Elliott J.W., Baines S.J. Temozolomide alone or in combination with doxorubicin as a rescue agent in 37 cases of canine multicentric lymphoma. Vet Comp Oncol. 2018;16:194–201. doi: 10.1111/vco.12335. [DOI] [PubMed] [Google Scholar]
- 309.Alvarez F.J., Kisseberth W.C., Gallant S.L. Dexamethasone, melphalan, actinomycin D, cytosine arabinoside (DMAC) protocol for dogs with relapsed lymphoma. J Vet Intern Med. 2006;20:1178–1183. doi: 10.1892/0891-6640(2006)20[1178:dmadca]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 310.Bannink E.O., Sauerbrey M.L., Mullins M.N. Actinomycin D as rescue therapy in dogs with relapsed or resistant lymphoma: 49 cases (1999–2006) J Am Vet Med Assoc. 2008;233:446–451. doi: 10.2460/javma.233.3.446. [DOI] [PubMed] [Google Scholar]
- 311.Dervisis N.G., Dominguez P.A., Sarbu L. Efficacy of temozolomide or dacarbazine in combination with an anthracycline for rescue chemotherapy in dogs with lymphoma. J Am Vet Med Assoc. 2007;231:563–569. doi: 10.2460/javma.231.4.563. [DOI] [PubMed] [Google Scholar]
- 312.Flory A.B., Rassnick K.M., Al-Sarraf R. Combination of CCNU and DTIC chemotherapy for treatment of resistant lymphoma in dogs. J Vet Intern Med. 2008;22:164–171. doi: 10.1111/j.1939-1676.2007.0005.x. [DOI] [PubMed] [Google Scholar]
- 313.Griessmayr P.C., Payne S.E., Winter J.E. Dacarbazine as single-agent therapy for relapsed lymphoma in dogs. J Vet Intern Med. 2009;23:1227–1231. doi: 10.1111/j.1939-1676.2009.0376.x. [DOI] [PubMed] [Google Scholar]
- 314.Moore A.S., London C.A., Wood C.A. Lomustine (CCNU) for the treatment of resistant lymphoma in dogs. J Vet Intern Med. 1999;13:395–398. doi: 10.1892/0891-6640(1999)013<0395:lfttor>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 315.Moore A.S., Ogilvie G.K., Ruslander D. Evaluation of mitoxantrone for the treatment of lymphoma in dogs. J Am Vet Med Assoc. 1994;204:1903–1905. [PubMed] [Google Scholar]
- 316.Moore A.S., Ogilvie G.K., Vail D.M. Actinomycin D for reinduction of remission in dogs with resistant lymphoma. J Vet Intern Med. 1994;8:343–344. doi: 10.1111/j.1939-1676.1994.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 317.Northrup N.C., Gieger T.L., Kosarek C.E. Mechlorethamine, procarbazine and prednisone for the treatment of resistant lymphoma in dogs. Vet Comp Oncol. 2009;7:38–44. doi: 10.1111/j.1476-5829.2008.00170.x. [DOI] [PubMed] [Google Scholar]
- 318.Rassnick K.M., Mauldin G.E., Al-Sarraf R. MOPP chemotherapy for treatment of resistant lymphoma in dogs: a retrospective study of 117 cases (1989-2000) J Vet Intern Med. 2002;16:576–580. doi: 10.1892/0891-6640(2002)016<0576:mcftor>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 319.Saba C.F., Hafeman S.D., Vail D.M. Combination chemotherapy with continuous L-asparaginase, lomustine, and prednisone for relapsed canine lymphoma. J Vet Intern Med. 2009;23:1058–1063. doi: 10.1111/j.1939-1676.2009.0357.x. [DOI] [PubMed] [Google Scholar]
- 320.Saba C.F., Thamm D.H., Vail D.M. Combination chemotherapy with L-asparaginase, lomustine, and prednisone for relapsed or refractory canine lymphoma. J Vet Intern Med. 2007;21:127–132. doi: 10.1892/0891-6640(2007)21[127:ccwlla]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 321.Impellizeri J.A., Howell K., McKeever K.P. The role of rituximab in the treatment of canine lymphoma: an ex vivo evaluation. Vet J. 2006;171:556–558. doi: 10.1016/j.tvjl.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 322.Jubala C.M., Wojcieszyn J.W., Valli V.E. CD20 expression in normal canine B cells and in canine non-Hodgkin lymphoma. Vet Pathol. 2005;42:468–476. doi: 10.1354/vp.42-4-468. [DOI] [PubMed] [Google Scholar]
- 323.Rue S.M., Eckelman B.P., Efe J.A. Identification of a candidate therapeutic antibody for treatment of canine B-cell lymphoma. Vet Immunol Immunopathol. 2015;164:148–159. doi: 10.1016/j.vetimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 324.Ito D., Brewer S., Modiano J.F. Development of a novel anti-canine CD20 monoclonal antibody with diagnostic and therapeutic potential. Leuk Lymphoma. 2015;56:219–225. doi: 10.3109/10428194.2014.914193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Jain S., Aresu L., Comazzi S. The development of a recombinant scFv monoclonal antibody targeting canine CD20 for use in comparative medicine. PLoS One. 2016;11:e0148366. doi: 10.1371/journal.pone.0148366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Weiskopf K., Anderson K.L., Ito D. Eradication of canine diffuse large B-Cell lymphoma in a murine xenograft model with CD47 blockade and anti-CD20. Cancer Immunol Res. 2016;4:1072–1087. doi: 10.1158/2326-6066.CIR-16-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Crow S.E., Theilen G.H., Benjaminini E. Chemoimmunotherapy for canine lymphosarcoma. Cancer. 1977;40:2102–2108. doi: 10.1002/1097-0142(197711)40:5<2102::aid-cncr2820400519>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 328.Weller R.E., Theilen G.H., Madewell B.R. Chemoimmunotherapy for canine lymphosarcoma: a prospective evaluation of specific and nonspecific immunomodulation. Am J Vet Res. 1980;1980(41):516–521. [PubMed] [Google Scholar]
- 329.Jeglum K.A., Young K.M., Barnsley K. Chemotherapy versus chemotherapy with intralymphatic tumor cell vaccine in canine lymphoma. Cancer. 1988;61:2042–2050. doi: 10.1002/1097-0142(19880515)61:10<2042::aid-cncr2820611019>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 330.Turek M.M., Thamm D.H., Mitzey A. Human granulocyte-macrophage colony-stimulating factor DNA cationic-lipid complexed autologous tumour cell vaccination in the treatment of canine B-cell multicentric lymphoma. Vet Comp Oncol. 2007;5:219–231. doi: 10.1111/j.1476-5829.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 331.Gavazza A., Lubas G., Fridman A. Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Hum Gene Ther. 2013;24:728–738. doi: 10.1089/hum.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Peruzzi D., Gavazza A., Mesiti G. A vaccine targeting telomerase enhances survival of dogs affected by B-cell lymphoma. Mol Ther. 2010;18:1559–1567. doi: 10.1038/mt.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Marconato L., Frayssinet P., Rouquet N. Randomized, placebo-controlled, double-blinded chemoimmunotherapy clinical trial in a pet dog model of diffuse large B-cell lymphoma. Clin Cancer Res. 2014;20:668–677. doi: 10.1158/1078-0432.CCR-13-2283. [DOI] [PubMed] [Google Scholar]
- 334.Marconato L., Stefanello D., Sabattini S. Enhanced therapeutic effect of APAVAC immunotherapy in combination with dose-intense chemotherapy in dogs with advanced indolent B-cell lymphoma. Vaccine. 2015;33:5080–5086. doi: 10.1016/j.vaccine.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 335.Sorenmo K.U., Krick E., Coughlin C.M. CD40-activated B cell cancer vaccine improves second clinical remission and survival in privately owned dogs with non-Hodgkin’s lymphoma. PLoS One. 2011;6:e24167. doi: 10.1371/journal.pone.0024167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mason N.J., Coughlin C.M., Overley B. RNA-loaded CD40-activated B cells stimulate antigen-specific T-cell responses in dogs with spontaneous lymphoma. Gene Ther. 2008;15:955–965. doi: 10.1038/gt.2008.22. [DOI] [PubMed] [Google Scholar]
- 337.O’Connor C.M., Sheppard S., Hartline C.A. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Sci Rep. 2012;2(249) doi: 10.1038/srep00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Panjwani M.K., Smith J.B., Schutsky K. Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor t cells in dogs with spontaneous B cell lymphoma. Mol Ther. 2016;24:1602–1614. doi: 10.1038/mt.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.van Stee L.L., Boston S.E., Singh A. Outcome and prognostic factors for canine splenic lymphoma treated by splenectomy (1995-2011) Vet Surg. 2015;44:976–982. doi: 10.1111/vsu.12405. [DOI] [PubMed] [Google Scholar]
- 340.Brooks M.B., Matus R.E., Leifer C.E. Use of splenectomy in the management of lymphoma in dogs: 16 cases (1976-1985) J Am Vet Med Assoc. 1987;191:1008–1010. [PubMed] [Google Scholar]
- 341.Brown E.M., Ruslander D.M., Azuma C. A feasibility study of low-dose total body irradiation for relapsed canine lymphoma. Vet Comp Oncol. 2006;4:75–83. doi: 10.1111/j.1476-5810.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- 342.Deeg H.J., Appelbaum F.R., Weiden P.L. Autologous marrow transplantation as consolidation therapy for canine lymphoma: efficacy and toxicity of various regimens of total body irradiation. Am J Vet Res. 1985;46:2016–2018. [PubMed] [Google Scholar]
- 343.Gustafson N.R., Lana S.E., Mayer M.N. A preliminary assessment of whole-body radiotherapy interposed within a chemotherapy protocol for canine lymphoma. Vet Comp Oncol. 2004;2:125–131. doi: 10.1111/j.1476-5810.2004.00046.x. [DOI] [PubMed] [Google Scholar]
- 344.Lurie D.M., Gordon I.K., Theon A.P. Sequential low-dose rate half-body irradiation and chemotherapy for the treatment of canine multicentric lymphoma. J Vet Intern Med. 2009;23:1064–1070. doi: 10.1111/j.1939-1676.2009.0353.x. [DOI] [PubMed] [Google Scholar]
- 345.Lurie D.M., Kent M.S., Fry M.M. A toxicity study of low-dose rate half-body irradiation and chemotherapy in dogs with lymphoma. Vet Comp Oncol. 2008;6:257–267. doi: 10.1111/j.1476-5829.2008.00164.x. [DOI] [PubMed] [Google Scholar]
- 346.Mayer M.N., Larue S.M. Radiation therapy in the treatment of canine lymphoma. Can Vet J. 2005;46:842–844. [PMC free article] [PubMed] [Google Scholar]
- 347.Meleo K.A. The role of radiotherapy in the treatment of lymphoma and thymoma. Vet Clin North Am Small Anim Pract. 1997;27:115–129. doi: 10.1016/s0195-5616(97)50010-6. [DOI] [PubMed] [Google Scholar]
- 348.Santoro D., Kubicek L., Lu B. Total skin electron therapy as treatment for epitheliotropic lymphoma in a dog. Vet Dermatol. 2017;28:246–e265. doi: 10.1111/vde.12415. [DOI] [PubMed] [Google Scholar]
- 349.Williams L.E., Johnson J.L., Hauck M.L. Chemotherapy followed by half-body radiation therapy for canine lymphoma. J Vet Intern Med. 2004;18:703–709. doi: 10.1892/0891-6640(2004)18<703:cfbhrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 350.Williams L.E., Pruitt A.F., Thrall D.E. Chemotherapy followed by abdominal cavity irradiation for feline lymphoblastic lymphoma. Vet Radiol Ultrasound. 2010;51:681–687. doi: 10.1111/j.1740-8261.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 351.George R., Smith A., Schleis S. Outcome of dogs with intranasal lymphoma treated with various radiation and chemotherapy protocols: 24 cases. Vet Radiol Ultrasound. 2016;57:306–312. doi: 10.1111/vru.12318. [DOI] [PubMed] [Google Scholar]
- 352.Axiak S.M., Carreras J.K., Hahn K.A. Hematologic changes associated with half-body irradiation in dogs with lymphoma. J Vet Intern Med. 2006;20:1398–1401. doi: 10.1892/0891-6640(2006)20[1398:hcawhi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 353.Dank G., Rassnick K.M., Kristal O. Clinical characteristics, treatment, and outcome of dogs with presumed primary hepatic lymphoma: 18 cases (1992-2008) J Am Vet Med Assoc. 2011;239:966–971. doi: 10.2460/javma.239.7.966. [DOI] [PubMed] [Google Scholar]
- 354.Gustafson N.R., Lana S.E., Mayer M.N. A preliminary assessment of whole-body radiotherapy interposed within a chemotherapy protocol for canine lymphoma. Vet Comp Oncol. 2004;2:125–131. doi: 10.1111/j.1476-5810.2004.00046.x. [DOI] [PubMed] [Google Scholar]
- 355.Appelbaum F.R., Deeg H.J., Storb R. Marrow transplant studies in dogs with malignant lymphoma. Transplantation. 1985;39:499–504. doi: 10.1097/00007890-198505000-00008. [DOI] [PubMed] [Google Scholar]
- 356.Warry E.E., Willcox J.L., Suter S.E. Autologous peripheral blood hematopoietic cell transplantation in dogs with T-cell lymphoma. J Vet Intern Med. 2014;28:529–537. doi: 10.1111/jvim.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Willcox J.L., Pruitt A., Suter S.E. Autologous peripheral blood hematopoietic cell transplantation in dogs with B-cell lymphoma. J Vet Intern Med. 2012;26:1155–1163. doi: 10.1111/j.1939-1676.2012.00980.x. [DOI] [PubMed] [Google Scholar]
- 358.Escobar C., Grindem C., Neel J.A. Hematologic changes after total body irradiation and autologous transplantation of hematopoietic peripheral blood progenitor cells in dogs with lymphoma. Vet Pathol. 2012;49:341–343. doi: 10.1177/0300985811410721. [DOI] [PubMed] [Google Scholar]
- 359.Frimberger A.E., Moore A.S., Rassnick K.M. A combination chemotherapy protocol with dose intensification and autologous bone marrow transplant (VELCAP-HDC) for canine lymphoma. J Vet Intern Med. 2006;20:355–364. doi: 10.1892/0891-6640(2006)20[355:accpwd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 360.Rassnick K.M., Moore A.S., Collister K.E. Efficacy of combination chemotherapy for treatment of gastrointestinal lymphoma in dogs. J Vet Intern Med. 2009;23:317–322. doi: 10.1111/j.1939-1676.2008.0270.x. [DOI] [PubMed] [Google Scholar]
- 361.Frank J.D.R.S., Kass P.H. Clinical outcomes of 30 cases (1997-2004) of canine gastrointestinal lymphoma. J Am Anim Hosp Assoc. 2007;43:313–321. doi: 10.5326/0430313. [DOI] [PubMed] [Google Scholar]
- 362.Desmas I., Burton J.H., Post G. Clinical presentation, treatment and outcome in 31 dogs with presumed primary colorectal lymphoma (2001-2013) Vet Comp Oncol. 2017;15:504–517. doi: 10.1111/vco.12194. [DOI] [PubMed] [Google Scholar]
- 363.Van den Steen N., Berlato D., Polton G. Rectal lymphoma in 11 dogs: a retrospective study. J Small Anim Pract. 2012;53:586–591. doi: 10.1111/j.1748-5827.2012.01258.x. [DOI] [PubMed] [Google Scholar]
- 364.Long S.N., Johnston P.E., Anderson T.J. Primary T-cell lymphoma of the central nervous system in a dog. J Am Vet Med Assoc. 2001;218:719–722. doi: 10.2460/javma.2001.218.719. [DOI] [PubMed] [Google Scholar]
- 365.Deravi N., Berke O., Woods J.P. Specific immunotypes of canine T cell lymphoma are associated with different outcomes. Vet Immunol Immunopathol. 2017;2017(191):5–13. doi: 10.1016/j.vetimm.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 366.Risbon R.E., de Lorimier L.P., Skorupski K. Response of canine cutaneous epitheliotropic lymphoma to lomustine (CCNU): a retrospective study of 46 cases (1999-2004) J Vet Intern Med. 2006;20:1389–1397. doi: 10.1892/0891-6640(2006)20[1389:roccel]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 367.Berlato D., Schrempp D., Van Den Steen N. Radiotherapy in the management of localized mucocutaneous oral lymphoma in dogs: 14 cases. Vet Comp Oncol. 2012;10:16–23. doi: 10.1111/j.1476-5829.2011.00270.x. [DOI] [PubMed] [Google Scholar]
- 368.Holtermann N., Kiupel M., Kessler M. Masitinib monotherapy in canine epitheliotropic lymphoma. Vet Comp Oncol. 2016;14(suppl 1):127–135. doi: 10.1111/vco.12157. [DOI] [PubMed] [Google Scholar]
- 369.Laprais A., Olivry T. Is CCNU (lomustine) valuable for treatment of cutaneous epitheliotropic lymphoma in dogs? A critically appraised topic. BMC Vet Res. 2017;13:61. doi: 10.1186/s12917-017-0978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Williams L.E., Rassnick K.M., Power H.T. CCNU in the treatment of canine epitheliotropic lymphoma. J Vet Intern Med. 2006;20:136–143. doi: 10.1892/0891-6640(2006)20[136:cittoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 371.White S.D., Rosychuk R.A., Scott K.V. Use of isotretinoin and etretinate for the treatment of benign cutaneous neoplasia and cutaneous lymphoma in dogs. J Am Vet Med Assoc. 1993;202:387–391. [PubMed] [Google Scholar]
- 372.Moriello K.A.M.E., Schultz K.T. PEG-asparaginase in the treatment of canine epitheliotrophic lymphoma and histiocytic proleferative dermatitis. In: Ihrke P.J.M.I., White S.D., editors. Advances in Veterinary Dermatology. Perggamon Press; New York: 1993. [Google Scholar]
- 373.Tzannes S., Ibarrola P., Batchelor D.J. Use of recombinant human interferon alpha-2a in the management of a dog with epitheliotropic lymphoma. J Am Anim Hosp Assoc. 2008;44:276–282. doi: 10.5326/0440276. [DOI] [PubMed] [Google Scholar]
- 374.Rechner K.N., Weeks K.J., Pruitt A.F. Total skin electron therapy technique for the canine patient. Vet Radiol Ultrasound. 2011;52:345–352. doi: 10.1111/j.1740-8261.2011.01799.x. [DOI] [PubMed] [Google Scholar]
- 375.Childress M.O., Ramos-Vara J.A., Ruple A. Retrospective analysis of factors affecting clinical outcome following CHOP-based chemotherapy in dogs with primary nodal diffuse large B-cell lymphoma. Vet Comp Oncol. 2018;16:E159–E168. doi: 10.1111/vco.12364. [DOI] [PubMed] [Google Scholar]
- 376.Davies O., Szladovits B., Polton G. Prognostic significance of clinical presentation, induction and rescue treatment in 42 cases of canine centroblastic diffuse large B-cell multicentric lymphoma in the United Kingdom. Vet Comp Oncol. 2018;16:276–287. doi: 10.1111/vco.12378. [DOI] [PubMed] [Google Scholar]
- 377.Fontaine S.J., McCulloch E., Eckersall P.D. Evaluation of the modified Glasgow Prognostic Score to predict outcome in dogs with newly diagnosed lymphoma. Vet Comp Oncol. 2017;15:1513–1526. doi: 10.1111/vco.12296. [DOI] [PubMed] [Google Scholar]
- 378.Koshino A., Goto-Koshino Y., Setoguchi A. Mutation of p53 gene and its correlation with the clinical outcome in dogs with lymphoma. J Vet Intern Med. 2016;30:223–229. doi: 10.1111/jvim.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Romano F.R., Heinze C.R., Barber L.G. Association between body condition score and cancer prognosis in dogs with lymphoma and osteosarcoma. J Vet Intern Med. 2016;30:1179–1186. doi: 10.1111/jvim.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Sierra Matiz O.R., Santilli J., Anai L.A. Prognostic significance of Ki67 and its correlation with mitotic index in dogs with diffuse large B-cell lymphoma treated with 19-week CHOP-based protocol. J Vet Diagn Invest. 2017 doi: 10.1177/1040638717743280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wang S.L., Lee J.J., Liao A.T. Chemotherapy-induced neutropenia is associated with prolonged remission duration and survival time in canine lymphoma. Vet J. 2015;205:69–73. doi: 10.1016/j.tvjl.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 382.Marconato L., Martini V., Stefanello D. Peripheral blood lymphocyte/monocyte ratio as a useful prognostic factor in dogs with diffuse large B-cell lymphoma receiving chemoimmunotherapy. Vet J. 2015;206:226–230. doi: 10.1016/j.tvjl.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 383.Wilson-Robles H., Budke C.M., Miller T. Geographical differences in survival of dogs with non-Hodgkin lymphoma treated with a CHOP based chemotherapy protocol. Vet Comp Oncol. 2017;15:1564–1571. doi: 10.1111/vco.12302. [DOI] [PubMed] [Google Scholar]
- 384.Zamani-Ahmadmahmudi M., Aghasharif S., Ilbeigi K. Prognostic efficacy of the human B-cell lymphoma prognostic genes in predicting disease-free survival (DFS) in the canine counterpart. BMC Vet Res. 2017;13(17) doi: 10.1186/s12917-016-0919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zamani-Ahmadmahmudi M., Najafi A., Nassiri S.M. Detection of critical genes associated with overall survival (OS) and progression-free survival (PFS) in reconstructed canine B-cell lymphoma gene regulatory network (GRN) Cancer Invest. 2016;34:70–79. doi: 10.3109/07357907.2015.1114120. [DOI] [PubMed] [Google Scholar]
- 386.Abbo A.H., Lucroy M.D. Assessment of anemia as an independent predictor of response to chemotherapy and survival in dogs with lymphoma: 96 cases (1993-2006) J Am Vet Med Assoc. 2007;231:1836–1842. doi: 10.2460/javma.231.12.1836. [DOI] [PubMed] [Google Scholar]
- 387.Jagielski D., Lechowski R., Hoffmann-Jagielska M. A retrospective study of the incidence and prognostic factors of multicentric lymphoma in dogs (1998-2000) J Vet Med A Physiol Pathol Clin Med. 2002;49:419–424. doi: 10.1046/j.1439-0442.2002.00458.x. [DOI] [PubMed] [Google Scholar]
- 388.Kiupel M., Bostock D., Bergmann V. The prognostic significance of AgNOR counts and PCNA-positive cell counts in canine malignant lymphomas. J Comp Pathol. 1998;119:407–418. doi: 10.1016/s0021-9975(98)80035-2. [DOI] [PubMed] [Google Scholar]
- 389.Larue S.M., Fox M.H., Ogilvie G.K. Tumour cell kinetics as predictors of response in canine lymphoma treated with chemotherapy alone or combined with whole body hyperthermia. Int J Hyperthermia. 1999;15:475–486. doi: 10.1080/026567399285477. [DOI] [PubMed] [Google Scholar]
- 390.Miller A.G., Morley P.S., Rao S. Anemia is associated with decreased survival time in dogs with lymphoma. J Vet Intern Med. 2009;23:116–122. doi: 10.1111/j.1939-1676.2008.00210.x. [DOI] [PubMed] [Google Scholar]
- 391.Rao S., Lana S., Eickhoff J. Class II major histocompatibility complex expression and cell size independently predict survival in canine B-cell lymphoma. J Vet Intern Med. 2011;25:1097–1105. doi: 10.1111/j.1939-1676.2011.0767.x. [DOI] [PubMed] [Google Scholar]
- 392.Vaughan A., Johnson J.L., Williams L.E. Impact of chemotherapeutic dose intensity and hematologic toxicity on first remission duration in dogs with lymphoma treated with a chemoradiotherapy protocol. J Vet Intern Med. 2007;21:1332–1339. doi: 10.1892/06-197.1. [DOI] [PubMed] [Google Scholar]
- 393.Yamazaki J., Takahashi M., Setoguchi A. Monitoring of minimal residual disease (MRD) after multidrug chemotherapy and its correlation to outcome in dogs with lymphoma: a proof-of-concept pilot study. J Vet Intern Med. 2010;24:897–903. doi: 10.1111/j.1939-1676.2010.0536.x. [DOI] [PubMed] [Google Scholar]
- 394.Williams M.J., Avery A.C., Lana S.E. Canine lymphoproliferative disease characterized by lymphocytosis: immunophenotypic markers of prognosis. J Vet Intern Med. 2008;22:596–601. doi: 10.1111/j.1939-1676.2008.0041.x. [DOI] [PubMed] [Google Scholar]
- 395.Cook A.K., Wright Z.M., Suchodolski J.S. Prevalence and prognostic impact of hypocobalaminemia in dogs with lymphoma. J Am Vet Med Assoc. 2009;235:1437–1441. doi: 10.2460/javma.235.12.1437. [DOI] [PubMed] [Google Scholar]
- 396.von Euler H., Einarsson R., Olsson U. Serum thymidine kinase activity in dogs with malignant lymphoma: a potent marker for prognosis and monitoring the disease. J Vet Intern Med. 2004;18:696–702. doi: 10.1892/0891-6640(2004)18<696:stkaid>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 397.Von Euler H.P., Rivera P., Aronsson A.C. Monitoring therapy in canine malignant lymphoma and leukemia with serum thymidine kinase 1 activity—evaluation of a new, fully automated non-radiometric assay. Int J Oncol. 2009;34:505–510. [PubMed] [Google Scholar]
- 398.Baskin C.R., Couto C.G., Wittum T.E. Factors influencing first remission and survival in 145 dogs with lymphoma: a retrospective study. J Am Anim Hosp Assoc. 2000;36:404–409. doi: 10.5326/15473317-36-5-404. [DOI] [PubMed] [Google Scholar]
- 399.Adam F., Villiers E., Watson S. Clinical pathological and epidemiological assessment of morphologically and immunologically confirmed canine leukaemia. Vet Comp Oncol. 2009;7:181–195. doi: 10.1111/j.1476-5829.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 400.Comazzi S., Gelain M.E., Martini V. Immunophenotype predicts survival time in dogs with chronic lymphocytic leukemia. J Vet Intern Med. 2011;25:100–106. doi: 10.1111/j.1939-1676.2010.0640.x. [DOI] [PubMed] [Google Scholar]
- 401.Roode S.C., Rotroff D., Avery A.C. Genome-wide assessment of recurrent genomic imbalances in canine leukemia identifies evolutionarily conserved regions for subtype differentiation. Chromosome Res. 2015;23:681–708. doi: 10.1007/s10577-015-9475-7. [DOI] [PubMed] [Google Scholar]
- 402.Gioia G., Mortarino M., Gelain M.E. Immunophenotype-related microRNA expression in canine chronic lymphocytic leukemia. Vet Immunol Immunopathol. 2011;142:228–235. doi: 10.1016/j.vetimm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 403.Stokol T., Schaefer D.M., Shuman M. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet Clin Pathol. 2015;44:79–93. doi: 10.1111/vcp.12227. [DOI] [PubMed] [Google Scholar]
- 404.Bennett A.L., Williams L.E., Ferguson M.W. Canine acute leukaemia: 50 cases (1989-2014) Vet Comp Oncol. 2017;15:1101–1114. doi: 10.1111/vco.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Leifer C.E., Matus R.E. Chronic lymphocytic leukemia in the dog: 22 cases (1974-1984) J Am Vet Med Assoc. 1986;189:214–217. [PubMed] [Google Scholar]
- 406.Kleiter M., Hirt R., Kirtz G. Hypercalcaemia associated with chronic lymphocytic leukaemia in a Giant Schnauzer. Aust Vet J. 2001;79:335–338. doi: 10.1111/j.1751-0813.2001.tb12007.x. [DOI] [PubMed] [Google Scholar]
- 407.Workman H.C., Vernau W. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. Vet Clin North Am Small Anim Pract. 2003;33:1379–1399. doi: 10.1016/s0195-5616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 408.Matus R.E., Leifer C.E., MacEwen E.G. Acute lymphoblastic leukemia in the dog: a review of 30 cases. J Am Vet Med Assoc. 1983;183:859–862. [PubMed] [Google Scholar]
- 409.Adams J., Mellanby R.J., Villiers E. Acute B cell lymphoblastic leukaemia in a 12-week-old greyhound. J Small Anim Pract. 2004;45:553–557. doi: 10.1111/j.1748-5827.2004.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 410.Hodgkins E.M., Zinkl J.G., Madewell B.R. Chronic lymphocytic leukemia in the dog. J Am Vet Med Assoc. 1980;177:704–707. [PubMed] [Google Scholar]
- 411.Comazzi S., Aresu L., Marconato L. Transformation of canine lymphoma/leukemia to more aggressive diseases: anecdotes or reality? Front Vet Sci. 2015;2(42) doi: 10.3389/fvets.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Comazzi S., Martini V., Riondato F. Chronic lymphocytic leukemia transformation into high-grade lymphoma: a description of Richter’s syndrome in eight dogs. Vet Comp Oncol. 2017;15:366–373. doi: 10.1111/vco.12172. [DOI] [PubMed] [Google Scholar]
- 413.Januszewicz E., Cooper I.A., Pilkington G. Blastic transformation of chronic lymphocytic leukemia. Am J Hematol. 1983;15:399–402. doi: 10.1002/ajh.2830150412. [DOI] [PubMed] [Google Scholar]
- 414.Harvey J.W., Terrell T.G., Hyde D.M. Well-differentiated lymphocytic leukemia in a dog: long-term survival without therapy. Vet Pathol. 1981;18:37–47. doi: 10.1177/030098588101800105. [DOI] [PubMed] [Google Scholar]
- 415.Boyce K.L., Kitchell B.E. Treatment of canine lymphoma with COPLA/LVP. J Am Anim Hosp Assoc. 2000;36:395–403. doi: 10.5326/15473317-36-5-395. [DOI] [PubMed] [Google Scholar]
- 416.Sato M., Yamazaki J., Goto-Koshino Y. Increase in minimal residual disease in peripheral blood before clinical relapse in dogs with lymphoma that achieved complete remission after chemotherapy. J Vet Intern Med. 2011;25:292–296. doi: 10.1111/j.1939-1676.2010.0675.x. [DOI] [PubMed] [Google Scholar]
- 417.Gentilini F., Calzolari C., Turba M.E. Prognostic value of serum vascular endothelial growth factor (VEGF) and plasma activity of matrix metalloproteinase (MMP) 2 and 9 in lymphoma-affected dogs. Leuk Res. 2005;29:1263–1269. doi: 10.1016/j.leukres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 418.Hahn K.A., Barnhill M.A., Freeman K.P. Detection and clinical significance of plasma glutathione-S-transferases in dogs with lymphoma, In. Vivo. 1999;13:173–175. [PubMed] [Google Scholar]
- 419.Heath H., 3rd, Weller R.E., Mundy G.R. Canine lymphosarcoma: a model for study of the hypercalcemia of cancer. Calcif Tissue Int. 1980;30:127–133. doi: 10.1007/BF02408617. [DOI] [PubMed] [Google Scholar]
- 420.weller R.H.C., Theilen G.H. Canine lymphosarcoma and hypercalcemia: clinical laboratory and pathologic evaluation of twenty-four cases. J Small Anim Pract. 1982;23:649–658. [Google Scholar]
- 421.Couto C.G. Oncology. In: R.G.S., editor. The cat: diseases and clinical management. Churchill Livingstone; New York: 1989. [Google Scholar]
- 422.Hardy W.J. Hematopoietic tumors of cats. J Am Anim Hosp Assoc. 1981;17:921–940. [Google Scholar]
- 423.Essex M.F.D. The risk to humans from malignant diseases of their pets: an unsettled issue. J Am Anim Hosp Assoc. 1976;12:386–390. [Google Scholar]
- 424.Theillen G., Madewell B.R. Feline hematopoietic neoplasms. In: Theilen G.H.M.B., editor. Veterinary cancer medicine. ed 2. Lea & Febiger; Philadelphia: 1987. [Google Scholar]
- 425.Beatty J. Viral causes of feline lymphoma: retroviruses and beyond. Vet J. 2014;201:174–180. doi: 10.1016/j.tvjl.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 426.Meichner K., Kruse D.B., Hirschberger J. Changes in prevalence of progressive feline leukaemia virus infection in cats with lymphoma in Germany. Vet Rec. 2012;171:348. doi: 10.1136/vr.100813. [DOI] [PubMed] [Google Scholar]
- 427.Louwerens M., London C.A., Pedersen N.C. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19:329–335. doi: 10.1892/0891-6640(2005)19[329:flitpl]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 428.Vail D.M., Moore A.S., Ogilvie G.K. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med. 1998;12:349–354. doi: 10.1111/j.1939-1676.1998.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 429.S.M.C. Feline viral neoplasia. In: Greene C., editor. Infectious diseases of the dog and cat. WB Saunders; Philadelphia: 1998. [Google Scholar]
- 430.Meichner K., Fogle J.E., English L. Expression of apoptosis-regulating proteins Bcl-2 and Bax in lymph node aspirates from dogs with lymphoma. J Vet Intern Med. 2016;30:819–826. doi: 10.1111/jvim.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Meichner K., von Bomhard W. Patient characteristics, histopathological findings and outcome in 97 cats with extranodal subcutaneous lymphoma (2007-2011) Vet Comp Oncol. 2016;14(Suppl 1):8–20. doi: 10.1111/vco.12081. [DOI] [PubMed] [Google Scholar]
- 432.Rissetto K.V.J., Selting K.A. Recent trends in feline intestinal neoplasia: an epidemiologic study of 1,129 cases in the veterinary medicanl database from 1964 to 2004. J Am Anim Hosp Assoc. 2011;47:28–36. doi: 10.5326/JAAHA-MS-5554. [DOI] [PubMed] [Google Scholar]
- 433.Schmidt J.M., North S.M., Freeman K.P. Feline paediatric oncology: retrospective assessment of 233 tumours from cats up to one year (1993 to 2008) J Small Anim Pract. 2010;51:306–311. doi: 10.1111/j.1748-5827.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 434.Gabor L.J., Malik R., Canfield P.J. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J. 1998;76:725–732. doi: 10.1111/j.1751-0813.1998.tb12300.x. [DOI] [PubMed] [Google Scholar]
- 435.Gabor L.J., Canfield P.J., Malik R. Immunophenotypic and histological characterisation of 109 cases of feline lymphosarcoma. Aust Vet J. 1999;77:436–441. doi: 10.1111/j.1751-0813.1999.tb12085.x. [DOI] [PubMed] [Google Scholar]
- 436.Barrs V.R., Beatty J.A. Feline alimentary lymphoma: 1. Classification, risk factors, clinical signs and non-invasive diagnostics. J Feline Med Surg. 2012;14:182–190. doi: 10.1177/1098612X12439265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Crouse Z., Phillips B., Flory A. Post-chemotherapy perforation in cats with discrete intermediate- or large-cell gastrointestinal lymphoma. J Feline Med Surg. 2017 doi: 10.1177/1098612X17723773. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Finotello R., Vasconi M.E., Sabattini S. Feline large granular lymphocyte lymphoma: an Italian Society of Veterinary Oncology (SIONCOV) retrospective study. Vet Comp Oncol. 2018;16:159–166. doi: 10.1111/vco.12325. [DOI] [PubMed] [Google Scholar]
- 439.Gustafson T.L., Villamil A., Taylor B.E. A retrospective study of feline gastric lymphoma in 16 chemotherapy-treated cats. J Am Anim Hosp Assoc. 2014;50:46–52. doi: 10.5326/JAAHA-MS-5989. [DOI] [PubMed] [Google Scholar]
- 440.Rau S.E., Burgess K.E. A retrospective evaluation of lomustine (CeeNU) in 32 treatment naive cats with intermediate to large cell gastrointestinal lymphoma (2006-2013) Vet Comp Oncol. 2017;15:1019–1028. doi: 10.1111/vco.12243. [DOI] [PubMed] [Google Scholar]
- 441.Sabattini S., Bottero E., Turba M.E. Differentiating feline inflammatory bowel disease from alimentary lymphoma in duodenal endoscopic biopsies. J Small Anim Pract. 2016;57:396–401. doi: 10.1111/jsap.12494. [DOI] [PubMed] [Google Scholar]
- 442.Ahmad S., Levy L.S. The frequency of occurrence and nature of recombinant feline leukemia viruses in the induction of multicentric lymphoma by infection of the domestic cat with FeLV-945. Virology. 2010;403:103–110. doi: 10.1016/j.virol.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Fuhino Y.S.H., Ohno K. Molecular cytogenetic analysis of feline leukemia virus insertions in cat lymphoid tumor cells. J Virol Methods. 2010;163:344–352. doi: 10.1016/j.jviromet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 444.Stutzer B., Simon K., Lutz H. Incidence of persistent viraemia and latent feline leukaemia virus infection in cats with lymphoma. J Feline Med Surg. 2011;13:81–87. doi: 10.1016/j.jfms.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Fujino Y., Liao C.P., Zhao Y.S. Identification of a novel common proviral integration site, flit-1, in feline leukemia virus induced thymic lymphoma. Virology. 2009;386:16–22. doi: 10.1016/j.virol.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 446.Fujino Y., Ohno K., Tsujimoto H. Molecular pathogenesis of feline leukemia virus-induced malignancies: insertional mutagenesis. Vet Immunol Immunopathol. 2008;123:138–143. doi: 10.1016/j.vetimm.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 447.Weiss A.T., Klopfleisch R., Gruber A.D. Prevalence of feline leukaemia provirus DNA in feline lymphomas. J Feline Med Surg. 2010;12:929–935. doi: 10.1016/j.jfms.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Beatty J.A., Lawrence C.E., Callanan J.J. Feline immunodeficiency virus (FIV)-associated lymphoma: a potential role for immune dysfunction in tumourigenesis. Vet Immunol Immunopathol. 1998;65:309–322. doi: 10.1016/s0165-2427(98)00164-0. [DOI] [PubMed] [Google Scholar]
- 449.Callanan J.J., McCandlish I.A., O’Neil B. Lymphosarcoma in experimentally induced feline immunodeficiency virus infection. Vet Rec. 1992;130:293–295. doi: 10.1136/vr.130.14.293. [DOI] [PubMed] [Google Scholar]
- 450.Endo Y., Cho K.W., Nishigaki K. Molecular characteristics of malignant lymphomas in cats naturally infected with feline immunodeficiency virus. Vet Immunol Immunopathol. 1997;57:153–167. doi: 10.1016/s0165-2427(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 451.Gabor L.J., Love D.N., Malik R. Feline immunodeficiency virus status of Australian cats with lymphosarcoma. Aust Vet J. 2001;79:540–545. doi: 10.1111/j.1751-0813.2001.tb10742.x. [DOI] [PubMed] [Google Scholar]
- 452.Hutson C.A., Rideout B.A., Pedersen N.C. Neoplasia associated with feline immunodeficiency virus infection in cats of southern California. J Am Vet Med Assoc. 1991;199:1357–1362. [PubMed] [Google Scholar]
- 453.Poli A., Abramo F., Baldinotti F. Malignant lymphoma associated with experimentally induced feline immunodeficiency virus infection. J Comp Pathol. 1994;110:319–328. doi: 10.1016/s0021-9975(08)80309-x. [DOI] [PubMed] [Google Scholar]
- 454.Shelton G.H., Grant C.K., Cotter S.M. Feline immunodeficiency virus and feline leukemia virus infections and their relationships to lymphoid malignancies in cats: a retrospective study (1968-1988) J Acquir Immune Defic Syndr. 1990;3:623–630. [PubMed] [Google Scholar]
- 455.Terry A., Callanan J.J., Fulton R. Molecular analysis of tumours from feline immunodeficiency virus (FIV)-infected cats: an indirect role for FIV? Int J Cancer. 1995;61:227–232. doi: 10.1002/ijc.2910610215. [DOI] [PubMed] [Google Scholar]
- 456.Wang J., Kyaw-Tanner M., Lee C. Characterisation of lymphosarcomas in Australian cats using polymerase chain reaction and immunohistochemical examination. Aust Vet J. 2001;79:41–46. doi: 10.1111/j.1751-0813.2001.tb10639.x. [DOI] [PubMed] [Google Scholar]
- 457.Rosenberg M.P.H.A., Matus R.E. Monoclonal gammopathy and lymphoma in a cat infected with feline immunodeficiency virus. J Am Anim Hosp Assoc. 1991;27:335–337. [Google Scholar]
- 458.Rassnick K.M., Mauldin G.N., Moroff S.D. Prognostic value of argyrophilic nucleolar organizer region (AgNOR) staining in feline intestinal lymphoma. J Vet Intern Med. 1999;13:187–190. doi: 10.1892/0891-6640(1999)013<0187:pvoano>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 459.Slawienski M.J., Mauldin G.E., Mauldin G.N. Malignant colonic neoplasia in cats: 46 cases (1990-1996) J Am Vet Med Assoc. 1997;211:878–881. [PubMed] [Google Scholar]
- 460.Zwahlen C.H., Lucroy M.D., Kraegel S.A. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993-1997) J Am Vet Med Assoc. 1998;213:1144–1149. [PubMed] [Google Scholar]
- 461.Mahony O.M., Moore A.S., Cotter S.M. Alimentary lymphoma in cats: 28 cases (1988-1993) J Am Vet Med Assoc. 1995;207:1593–1598. [PubMed] [Google Scholar]
- 462.McLuckie A.J., Barrs V.R., Lindsay S. Molecular diagnosis of Felis catus gammaherpesvirus 1 (FcaGHV1) infection in cats of known retrovirus status with and without lymphoma. Viruses. 2018;10 doi: 10.3390/v10030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Mayr B., Winkler G., Schaffner G. N-ras mutation in a feline lymphoma. Low frequency of N-ras mutations in a series of feline, canine and bovine lymphomas. Vet J. 2002;163:326–328. doi: 10.1053/tvjl.2001.0691. [DOI] [PubMed] [Google Scholar]
- 464.Cadile C.D., Kitchell B.E., Biller B.J. Telomerase activity as a marker for malignancy in feline tissues. Am J Vet Res. 2001;62:1578–1581. doi: 10.2460/ajvr.2001.62.1578. [DOI] [PubMed] [Google Scholar]
- 465.Yazawa M., Okuda M., Uyama R. Molecular cloning of the feline telomerase reverse transcriptase (TERT) gene and its expression in cell lines and normal tissues. J Vet Med Sci. 2003;65:573–577. doi: 10.1292/jvms.65.573. [DOI] [PubMed] [Google Scholar]
- 466.Kano R., Sato E., Okamura T. Expression of Bcl-2 in feline lymphoma cell lines. Vet Clin Pathol. 2008;37:57–60. doi: 10.1111/j.1939-165X.2008.00013.x. [DOI] [PubMed] [Google Scholar]
- 467.Madewell B., Griffey S., Walls J. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 in feline lymphoma. Vet Pathol. 2001;38:698–702. doi: 10.1354/vp.38-6-698. [DOI] [PubMed] [Google Scholar]
- 468.Dank G., Lucroy M.D., Griffey S.M. bcl-2 and MIB-1 labeling indexes in cats with lymphoma. J Vet Intern Med. 2002;16:720–725. [PubMed] [Google Scholar]
- 469.Bertone E.R., Snyder L.A., Moore A.S. Environmental tobacco smoke and risk of malignant lymphoma in pet cats. Am J Epidemiol. 2002;156:268–273. doi: 10.1093/aje/kwf044. [DOI] [PubMed] [Google Scholar]
- 470.Marconato L., Leo C., Girelli R. Association between waste management and cancer in companion animals. J Vet Intern Med. 2009;23:564–569. doi: 10.1111/j.1939-1676.2009.0278.x. [DOI] [PubMed] [Google Scholar]
- 471.Beatty J., Terry A., MacDonald J. Feline immunodeficiency virus integration in B-cell lymphoma identifies a candidate tumor suppressor gene on human chromosome 15q15. Cancer Res. 2002;62:7175–7180. [PubMed] [Google Scholar]
- 472.Durham A.C., Mariano A.D., Holmes E.S. Characterization of post transplantation lymphoma in feline renal transplant recipients. J Comp Pathol. 2014;150:162–168. doi: 10.1016/j.jcpa.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 473.Schmiedt C.W., Grimes J.A., Holzman G. Incidence and risk factors for development of malignant neoplasia after feline renal transplantation and cyclosporine-based immunosuppression. Vet Comp Oncol. 2009;7:45–53. doi: 10.1111/j.1476-5829.2008.00172.x. [DOI] [PubMed] [Google Scholar]
- 474.Wooldridge J.D., Gregory C.R., Mathews K.G. The prevalence of malignant neoplasia in feline renal-transplant recipients. Vet Surg. 2002;31:94–97. doi: 10.1053/jvet.2002.30540. [DOI] [PubMed] [Google Scholar]
- 475.Carreras J.K., Goldschmidt M., Lamb M. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997-2000) J Vet Intern Med. 2003;17:326–331. doi: 10.1111/j.1939-1676.2003.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 476.Hart J.F.S.E., Patnaik A.K. Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988-1990) J Am Anim Hosp Assoc. 1994;30:505–514. [Google Scholar]
- 477.Hoehne S.N., McDonough S.P., Rishniw M. Identification of mucosa-invading and intravascular bacteria in feline small intestinal lymphoma. Vet Pathol. 2017;54:23–241. doi: 10.1177/0300985816664792. [DOI] [PubMed] [Google Scholar]
- 478.Bridgeford E.C., Marini R.P., Feng Y. Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol. 2008;123:106–113. doi: 10.1016/j.vetimm.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Martini V., Bernardi S., Marelli P. Flow cytometry for feline lymphoma: a retrospective study regarding pre-analytical factors possibly affecting the quality of samples. J Feline Med Surg. 2017 doi: 10.1177/1098612X17717175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hammer S.E., Groiss S., Fuchs-Baumgartinger A. Characterization of a PCR-based lymphocyte clonality assay as a complementary tool for the diagnosis of feline lymphoma. Vet Comp Oncol. 2017;15:1354–1369. doi: 10.1111/vco.12277. [DOI] [PubMed] [Google Scholar]
- 481.Valli V.E., Jacobs R.M., Norris A. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest. 2000;12:295–306. doi: 10.1177/104063870001200401. [DOI] [PubMed] [Google Scholar]
- 482.Wolfesberger B., Skor O., Hammer S.E. Does categorisation of lymphoma subtypes according to the World Health Organization classification predict clinical outcome in cats? J Feline Med Surg. 2017;19:897–906. doi: 10.1177/1098612X16666119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Valli V.E.J.R., Parodi A.L. Armed Forces Institute of Pathology and The World health Organization; Washington, DC: 2002. Histological classification of hematopoietic tumors of domestic animals. [Google Scholar]
- 484.Sato H., Fujino Y., Chino J. Prognostic analyses on anatomical and morphological classification of feline lymphoma. J Vet Med Sci. 2014;76:807–811. doi: 10.1292/jvms.13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Chino J., Fujino Y., Kobayashi T. Cytomorphological and immunological classification of feline lymphomas: clinicopathological features of 76 cases. J Vet Med Sci. 2013;75:701–707. doi: 10.1292/jvms.12-0246. [DOI] [PubMed] [Google Scholar]
- 486.Lingard A.E., Briscoe K., Beatty J.A. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg. 2009;11:692–700. doi: 10.1016/j.jfms.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Moore P.F., Rodriguez-Bertos A., Kass P.H. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol. 2012;49:658–668. doi: 10.1177/0300985811404712. [DOI] [PubMed] [Google Scholar]
- 488.Barrs V.R., Beatty J.A. Feline alimentary lymphoma: 2. Further diagnostics, therapy and prognosis. J Feline Med Surg. 2012;14:191–201. doi: 10.1177/1098612X12439266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Lalor S., Schwartz A.M., Titmarsh H. Cats with inflammatory bowel disease and intestinal small cell lymphoma have low serum concentrations of 25-hydroxyvitamin D. J Vet Intern Med. 2014;28:351–355. doi: 10.1111/jvim.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Russell K.J., Beatty J.A., Dhand N. Feline low-grade alimentary lymphoma: how common is it? J Feline Med Surg. 2012;14:910–912. doi: 10.1177/1098612X12454861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kiupel M., Smedley R.C., Pfent C. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol. 2011;48:212–222. doi: 10.1177/0300985810389479. [DOI] [PubMed] [Google Scholar]
- 492.Vezzali E., Parodi A.L., Marcato P.S. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet Comp Oncol. 2010;8:38–49. doi: 10.1111/j.1476-5829.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 493.Swerdlow S.H.C.E., Harris N.L. International Agency for Research on Cancer; France: 2008. WHO classification of tumor of the haematopoietic and lymphoid tissues. [Google Scholar]
- 494.Briscoe K.A., Krockenberger M., Beatty J.A. Histopathological and immunohistochemical evaluation of 53 cases of feline lymphoplasmacytic enteritis and low-grade alimentary lymphoma. J Comp Pathol. 2011;145:187–198. doi: 10.1016/j.jcpa.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 495.Warren A., Center S., McDonough S. Histopathologic features, immunophenotyping, clonality, and eubacterial fluorescence in situ hybridization in cats with lymphocytic cholangitis/cholangiohepatitis. Vet Pathol. 2011;48:627–641. doi: 10.1177/0300985810384409. [DOI] [PubMed] [Google Scholar]
- 496.Gouldin E.D., Mullin C., Morges M. Feline discrete high-grade gastrointestinal lymphoma treated with surgical resection and adjuvant CHOP-based chemotherapy: retrospective study of 20 cases. Vet Comp Oncol. 2017;15:328–335. doi: 10.1111/vco.12166. [DOI] [PubMed] [Google Scholar]
- 497.Pohlman L.M., Higginbotham M.L., Welles E.G. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Pathol. 2009;46:259–268. doi: 10.1354/vp.46-2-259. [DOI] [PubMed] [Google Scholar]
- 498.Sapierzynski R., Jankowska U., Jagielski D. Large granular lymphoma in six cats. Pol J Vet Sci. 2015;18:163–169. doi: 10.1515/pjvs-2015-0021. [DOI] [PubMed] [Google Scholar]
- 499.Ezura K., Ezura K., Nomura I. Natural killer-like T cell lymphoma in a cat. Vet Rec. 2004;154:268–270. doi: 10.1136/vr.154.9.268. [DOI] [PubMed] [Google Scholar]
- 500.Krick E.L., Little L., Patel R. Description of clinical and pathological findings, treatment and outcome of feline large granular lymphocyte lymphoma (1996-2004) Vet Comp Oncol. 2008;6:102–110. doi: 10.1111/j.1476-5829.2007.00146.x. [DOI] [PubMed] [Google Scholar]
- 501.Roccabianca P., Vernau W., Caniatti M. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8(alpha) phenotype. Vet Pathol. 2006;43:15–28. doi: 10.1354/vp.43-1-15. [DOI] [PubMed] [Google Scholar]
- 502.Congress ‘99: what’s new in the treatment of lymphoma? J Small Anim Pract. 1999;40:51. doi: 10.1111/j.1748-5827.1999.tb03259.x. [DOI] [PubMed] [Google Scholar]
- 503.Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract. 2011;41:419–432. doi: 10.1016/j.cvsm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 504.Kiselow M.A., Rassnick K.M., McDonough S.P. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995-2005) J Am Vet Med Assoc. 2008;232:405–410. doi: 10.2460/javma.232.3.405. [DOI] [PubMed] [Google Scholar]
- 505.Fondacaro J.V.R.K., Carpenter J.L. Feline gastrointestinal lymphoma: 67 cases. Eur J Comp Gastroenterol. 1999;4:199. [Google Scholar]
- 506.Gabor L.J., Canfield P.J., Malik R. Haematological and biochemical findings in cats in Australia with lymphosarcoma. Aust Vet J. 2000;78:456–461. doi: 10.1111/j.1751-0813.2000.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 507.Gerou-Ferriani M., McBrearty A.R., Burchmore R.J. Agarose gel serum protein electrophoresis in cats with and without lymphoma and preliminary results of tandem mass fingerprinting analysis. Vet Clin Pathol. 2011;40:159–173. doi: 10.1111/j.1939-165X.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- 508.Daniaux L.A., Laurenson M.P., Marks S.L. Ultrasonographic thickening of the muscularis propria in feline small intestinal small cell T-cell lymphoma and inflammatory bowel disease. J Feline Med Surg. 2014;16:89–98. doi: 10.1177/1098612X13498596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Evans S.E., Bonczynski J.J., Broussard J.D. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447–1450. doi: 10.2460/javma.229.9.1447. [DOI] [PubMed] [Google Scholar]
- 510.Zwingenberger A.L., Marks S.L., Baker T.W. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med. 2010;24:289–292. doi: 10.1111/j.1939-1676.2009.0457.x. [DOI] [PubMed] [Google Scholar]
- 511.Jergens A.E., Willard M.D., Allenspach K. Maximizing the diagnostic utility of endoscopic biopsy in dogs and cats with gastrointestinal disease. Vet J. 2016;214:50–60. doi: 10.1016/j.tvjl.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 512.Kleinschmidt S., Harder J., Nolte I. Chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract in cats: diagnostic advantages of full-thickness intestinal and extraintestinal biopsies. J Feline Med Surg. 2010;12:97–103. doi: 10.1016/j.jfms.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Awaysheh A., Wilcke J., Elvinger F. Evaluation of supervised machine-learning algorithms to distinguish between inflammatory bowel disease and alimentary lymphoma in cats. J Vet Diagn Invest. 2016;28:679–687. doi: 10.1177/1040638716657377. [DOI] [PubMed] [Google Scholar]
- 514.Sawa M., Yabuki A., Setoguchi A. Development and application of multiple immunofluorescence staining for diagnostic cytology of canine and feline lymphoma. Vet Clin Pathol. 2015;44:580–585. doi: 10.1111/vcp.12300. [DOI] [PubMed] [Google Scholar]
- 515.Malik R., Gabor L.J., Foster S.F. Therapy for Australian cats with lymphosarcoma. Aust Vet J. 2001;79:808–817. doi: 10.1111/j.1751-0813.2001.tb10923.x. [DOI] [PubMed] [Google Scholar]
- 516.Tzannes S., Hammond M.F., Murphy S. Owners ‘perception of their cats’ quality of life during COP chemotherapy for lymphoma. J Feline Med Surg. 2008;10:73–81. doi: 10.1016/j.jfms.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Pope K.V., Tun A.E., McNeill C.J. Outcome and toxicity assessment of feline small cell lymphoma: 56 cases (2000-2010) Vet Med Sci. 2015;1:51–62. doi: 10.1002/vms3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Stein T.J., Pellin M., Steinberg H. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc. 2010;46:413–417. doi: 10.5326/0460413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Dutelle A.L., Bulman-Fleming J.C., Lewis C.A. Evaluation of lomustine as a rescue agent for cats with resistant lymphoma. J Feline Med Surg. 2012;14:694–700. doi: 10.1177/1098612X12448017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Collette S.A., Allstadt S.D., Chon E.M. Treatment of feline intermediate- to high-grade lymphoma with a modified university of Wisconsin-Madison protocol: 119 cases (2004-2012) Vet Comp Oncol. 2016;14(Suppl 1):136–146. doi: 10.1111/vco.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Limmer S., Eberle N., Nerschbach V. Treatment of feline lymphoma using a 12-week, maintenance-free combination chemotherapy protocol in 26 cats. Vet Comp Oncol. 2016;14(Suppl 1):21–31. doi: 10.1111/vco.12082. [DOI] [PubMed] [Google Scholar]
- 522.Waite A.H., Jackson K., Gregor T.P. Lymphoma in cats treated with a weekly cyclophosphamide-, vincristine-, and prednisone-based protocol: 114 cases (1998-2008) J Am Vet Med Assoc. 2013;242:1104–1109. doi: 10.2460/javma.242.8.1104. [DOI] [PubMed] [Google Scholar]
- 523.Hadden A.G., Cotter S.M., Rand W. Efficacy and toxicosis of VELCAP-C treatment of lymphoma in cats. J Vet Intern Med. 2008;22:153–157. doi: 10.1111/j.1939-1676.2007.0031.x. [DOI] [PubMed] [Google Scholar]
- 524.Jeglum K.A., Whereat A., Young K. Chemotherapy of lymphoma in 75 cats. J Am Vet Med Assoc. 1987;190:174–178. [PubMed] [Google Scholar]
- 525.Milner R.J., Peyton J., Cooke K. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin-Madison chemotherapy protocol: 38 cases (1996-2003) J Am Vet Med Assoc. 2005;227:1118–1122. doi: 10.2460/javma.2005.227.1118. [DOI] [PubMed] [Google Scholar]
- 526.Mooney S.C., Hayes A.A., MacEwen E.G. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977-1981) J Am Vet Med Assoc. 1989;194:696–702. [PubMed] [Google Scholar]
- 527.Moore A.S., Cotter S.M., Frimberger A.E. A comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma. J Vet Intern Med. 1996;10:372–375. doi: 10.1111/j.1939-1676.1996.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 528.Simon D., Eberle N., Laacke-Singer L. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med. 2008;22:394–400. doi: 10.1111/j.1939-1676.2008.0057.x. [DOI] [PubMed] [Google Scholar]
- 529.Krick E.L., Cohen R.B., Gregor T.P. Prospective clinical trial to compare vincristine and vinblastine in a COP-based protocol for lymphoma in cats. J Vet Intern Med. 2013;27:134–140. doi: 10.1111/jvim.12006. [DOI] [PubMed] [Google Scholar]
- 530.Kristal O., Lana S.E., Ogilvie G.K. Single agent chemotherapy with doxorubicin for feline lymphoma: a retrospective study of 19 cases (1994-1997) J Vet Intern Med. 2001;15:125–130. doi: 10.1892/0891-6640(2001)015<0125:sacwdf>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 531.Peaston A.E., Maddison J.E. Efficacy of doxorubicin as an induction agent for cats with lymphosarcoma. Aust Vet J. 1999;77:442–444. doi: 10.1111/j.1751-0813.1999.tb12087.x. [DOI] [PubMed] [Google Scholar]
- 532.Poirier V.J., Thamm D.H., Kurzman I.D. Liposome-encapsulated doxorubicin (Doxil) and doxorubicin in the treatment of vaccine-associated sarcoma in cats. J Vet Intern Med. 2002;16:726–731. [PubMed] [Google Scholar]
- 533.Teske E., van Straten G., van Noort R. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med. 2002;16:179–186. doi: 10.1892/0891-6640(2002)016<0179:cwcvap>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 534.Vajdovich P., Psader R., Toth Z.A. Use of the argyrophilic nucleolar region method for cytologic and histologic examination of the lymph nodes in dogs. Vet Pathol. 2004;41:338–345. doi: 10.1354/vp.41-4-338. [DOI] [PubMed] [Google Scholar]
- 535.Teske E., van Lankveld A.J., Rutteman G.R. Intraperitoneal antineoplastic drug delivery: experience with a cyclophosphamide, vincristine and prednisolone protocol in cats with malignant lymphoma. Vet Comp Oncol. 2014;12:37–46. doi: 10.1111/j.1476-5829.2012.00329.x. [DOI] [PubMed] [Google Scholar]
- 536.Elliott J., Finotello R. A dexamethasone, melphalan, actinomycin-D and cytarabine chemotherapy protocol as a rescue treatment for feline lymphoma. Vet Comp Oncol. 2018;16:E144–E151. doi: 10.1111/vco.12360. [DOI] [PubMed] [Google Scholar]
- 537.Martin O.A., Price J. Mechlorethamine, vincristine, melphalan and prednisolone rescue chemotherapy protocol for resistant feline lymphoma. J Feline Med Surg. 2017 doi: 10.1177/1098612X17735989. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Parshley D.L., Larue S.M., Kitchell B. Abdominal irradiation as a rescue therapy for feline gastrointestinal lymphoma: a retrospective study of 11 cats (2001-2008) J Feline Med Surg. 2011;13:63–68. doi: 10.1016/j.jfms.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Williams L.E., Pruitt A.F., Thrall D.E. Chemotherapy followed by abdominal cavity irradiation for feline lymphoblastic lymphoma. Vet Radiol Ultrasound. 2010;51:681–687. doi: 10.1111/j.1740-8261.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 540.Williams L.E., Johnson J.L., Hauck M.L. Chemotherapy followed by half-body radiation therapy for canine lymphoma. J Vet Intern Med. 2004;18:703–709. doi: 10.1892/0891-6640(2004)18<703:cfbhrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 541.Day M.J., Kyaw-Tanner M., Silkstone M.A. T-cell-rich B-cell lymphoma in the cat. J Comp Pathol. 1999;120:155–167. doi: 10.1053/jcpa.1998.0267. [DOI] [PubMed] [Google Scholar]
- 542.Walton R.M., Hendrick M.J. Feline Hodgkin’s-like lymphoma: 20 cases (1992-1999) Vet Pathol. 2001;38:504–511. doi: 10.1354/vp.38-5-504. [DOI] [PubMed] [Google Scholar]
- 543.Elliott J. Temporary spontaneous regression of feline non-Hodgkin’s lymphoma. Aust Vet J. 2018;96:83–85. doi: 10.1111/avj.12672. [DOI] [PubMed] [Google Scholar]
- 544.Newton J.A., de Vicente F., Haugland S.P. Extra-nodal subcutaneous Hodgkin’s-like lymphoma and subsequent regression in a cat. J Feline Med Surg. 2015;17:543–547. doi: 10.1177/1098612X14541262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Holt E., Goldschmidt M.H., Skorupski K. Extranodal conjunctival Hodgkin’s-like lymphoma in a cat. Vet Ophthalmol. 2006;9:141–144. doi: 10.1111/j.1463-5224.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 546.Davies C., Forrester S.D. Pleural effusion in cats: 82 cases (1987 to 1995) J Small Anim Pract. 1996;37:217–224. doi: 10.1111/j.1748-5827.1996.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 547.Day M.J. Review of thymic pathology in 30 cats and 36 dogs. J Small Anim Pract. 1997;38:393–403. doi: 10.1111/j.1748-5827.1997.tb03492.x. [DOI] [PubMed] [Google Scholar]
- 548.Fabrizio F., Calam A.E., Dobson J.M. Feline mediastinal lymphoma: a retrospective study of signalment, retroviral status, response to chemotherapy and prognostic indicators. J Feline Med Surg. 2014;16:637–644. doi: 10.1177/1098612X13516621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Court E.A., Watson A.D., Peaston A.E. Retrospective study of 60 cases of feline lymphosarcoma. Aust Vet J. 1997;75:424–427. doi: 10.1111/j.1751-0813.1997.tb14347.x. [DOI] [PubMed] [Google Scholar]
- 550.Forrester S.D., Fossum T.W., Rogers K.S. Diagnosis and treatment of chylothorax associated with lymphoblastic lymphosarcoma in four cats. J Am Vet Med Assoc. 1991;198:291–294. [PubMed] [Google Scholar]
- 551.Fossum T.W., Jacobs R.M., Birchard S.J. Evaluation of cholesterol and triglyceride concentrations in differentiating chylous and nonchylous pleural effusions in dogs and cats. J Am Vet Med Assoc. 1986;188:49–51. [PubMed] [Google Scholar]
- 552.Savary K.C., Price G.S., Vaden S.L. Hypercalcemia in cats: a retrospective study of 71 cases (1991-1997) J Vet Intern Med. 2000;14:184–189. doi: 10.1892/0891-6640(2000)014<0184:hicars>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 553.Avery P.R., Avery A.C. Molecular methods to distinguish reactive and neoplastic lymphocyte expansions and their importance in transitional neoplastic states. Vet Clin Pathol. 2004;33:196–207. doi: 10.1111/j.1939-165x.2004.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 554.Henrich M., Hecht W., Weiss A.T. A new subgroup of immunoglobulin heavy chain variable region genes for the assessment of clonality in feline B-cell lymphomas. Vet Immunol Immunopathol. 2009;130:59–69. doi: 10.1016/j.vetimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 555.Moore P.F., Woo J.C., Vernau W. Characterization of feline T cell receptor gamma (TCRG) variable region genes for the molecular diagnosis of feline intestinal T cell lymphoma. Vet Immunol Immunopathol. 2005;106:167–178. doi: 10.1016/j.vetimm.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 556.Weiss A.T., Klopfleisch R., Gruber A.D. T-cell receptor gamma chain variable and joining region genes of subgroup 1 are clonally rearranged in feline B- and T-cell lymphoma. J Comp Pathol. 2011;144:123–134. doi: 10.1016/j.jcpa.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 557.Werner J.A., Woo J.C., Vernau W. Characterization of feline immunoglobulin heavy chain variable region genes for the molecular diagnosis of B-cell neoplasia. Vet Pathol. 2005;42:596–607. doi: 10.1354/vp.42-5-596. [DOI] [PubMed] [Google Scholar]
- 558.Mooney S.C., Hayes A.A. Lymphoma in the cat: an approach to diagnosis and management. Semin Vet Med Surg (Small Anim) 1986;1:51–57. [PubMed] [Google Scholar]
- 559.Taylor S.S., Goodfellow M.R., Browne W.J. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract. 2009;50:584–592. doi: 10.1111/j.1748-5827.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 560.Little L., Patel R., Goldschmidt M. Nasal and nasopharyngeal lymphoma in cats: 50 cases (1989-2005) Vet Pathol. 2007;44:885–892. doi: 10.1354/vp.44-6-885. [DOI] [PubMed] [Google Scholar]
- 561.Santagostino S.F., Mortellaro C.M., Boracchi P. Feline upper respiratory tract lymphoma: site, cyto-histology, phenotype, FeLV expression, and prognosis. Vet Pathol. 2015;52:250–259. doi: 10.1177/0300985814537529. [DOI] [PubMed] [Google Scholar]
- 562.Demko J.L., Cohn L.A. Chronic nasal discharge in cats: 75 cases (1993-2004) J Am Vet Med Assoc. 2007;230:1032–1037. doi: 10.2460/javma.230.7.1032. [DOI] [PubMed] [Google Scholar]
- 563.Henderson S.M., Bradley K., Day M.J. Investigation of nasal disease in the cat—a retrospective study of 77 cases. J Feline Med Surg. 2004;6:245–257. doi: 10.1016/j.jfms.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Mukaratirwa S., van der Linde-Sipman J.S., Gruys E. Feline nasal and paranasal sinus tumours: clinicopathological study, histomorphological description and diagnostic immunohistochemistry of 123 cases. J Feline Med Surg. 2001;3:235–245. doi: 10.1053/jfms.2001.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Haney S.M., Beaver L., Turrel J. Survival analysis of 97 cats with nasal lymphoma: a multi-institutional retrospective study (1986-2006) J Vet Intern Med. 2009;23:287–294. doi: 10.1111/j.1939-1676.2008.0243.x. [DOI] [PubMed] [Google Scholar]
- 566.Nemanic S., Hollars K., Nelson N.C. Combination of computed tomographic imaging characteristics of medial retropharyngeal lymph nodes and nasal passages aids discrimination between rhinitis and neoplasia in cats. Vet Radiol Ultrasound. 2015;56:617–627. doi: 10.1111/vru.12279. [DOI] [PubMed] [Google Scholar]
- 567.Detweiler D.A., Johnson L.R., Kass P.H. Computed tomographic evidence of bulla effusion in cats with sinonasal disease: 2001-2004. J Vet Intern Med. 2006;20:1080–1084. doi: 10.1892/0891-6640(2006)20[1080:cteobe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 568.Tromblee T.C., Jones J.C., Etue A.E. Association between clinical characteristics, computed tomography characteristics, and histologic diagnosis for cats with sinonasal disease. Vet Radiol Ultrasound. 2006;47:241–248. doi: 10.1111/j.1740-8261.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- 569.Nagata K., Lamb M., Goldschmidt M.H. The usefulness of immunohistochemistry to differentiate between nasal carcinoma and lymphoma in cats: 140 cases (1986-2000) Vet Comp Oncol. 2014;12:52–57. doi: 10.1111/j.1476-5829.2012.00330.x. [DOI] [PubMed] [Google Scholar]
- 570.Fu D.R., Kato D., Endo Y. Apoptosis and Ki-67 as predictive factors for response to radiation therapy in feline nasal lymphomas. J Vet Med Sci. 2016;78:1161–1166. doi: 10.1292/jvms.15-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Fujiwara-Igarashi A., Fujimori T., Oka M. Evaluation of outcomes and radiation complications in 65 cats with nasal tumours treated with palliative hypofractionated radiotherapy. Vet J. 2014;202:455–461. doi: 10.1016/j.tvjl.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 572.Moore A. Extranodal lymphoma in the cat: prognostic factors and treatment options. J Feline Med Surg. 2013;15:379–390. doi: 10.1177/1098612X13483236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Elmslie RE Ogilvie G.K., Gillette E.L. Radiotherapy with and without chemotherapy for localized lymphoma in 10 cats. Vet Radiol Ultrasound. 1991;32:277–280. [Google Scholar]
- 574.Sfiligoi G., Theon A.P., Kent M.S. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet Radiol Ultrasound. 2007;48:388–393. doi: 10.1111/j.1740-8261.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- 575.Mooney S.C., Hayes A.A., Matus R.E. Renal lymphoma in cats: 28 cases (1977-1984) J Am Vet Med Assoc. 1987;191:1473–1477. [PubMed] [Google Scholar]
- 576.Valdes-Martinez A., Cianciolo R., Mai W. Association between renal hypoechoic subcapsular thickening and lymphosarcoma in cats. Vet Radiol Ultrasound. 2007;48:357–360. doi: 10.1111/j.1740-8261.2007.00256.x. [DOI] [PubMed] [Google Scholar]
- 577.Troxel M.T., Vite C.H., Van Winkle T.J. Feline intracranial neoplasia: retrospective review of 160 cases (1985-2001) J Vet Intern Med. 2003;17:850–859. doi: 10.1111/j.1939-1676.2003.tb02525.x. [DOI] [PubMed] [Google Scholar]
- 578.Marioni-Henry K., Van Winkle T.J., Smith S.H. Tumors affecting the spinal cord of cats: 85 cases (1980-2005) J Am Vet Med Assoc. 2008;232:237–243. doi: 10.2460/javma.232.2.237. [DOI] [PubMed] [Google Scholar]
- 579.Mandara M.T., Motta L., Calo P. Distribution of feline lymphoma in the central and peripheral nervous systems. Vet J. 2016;216:109–116. doi: 10.1016/j.tvjl.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 580.Tomek A., Cizinauskas S., Doherr M. Intracranial neoplasia in 61 cats: localisation, tumour types and seizure patterns. J Feline Med Surg. 2006;8:243–253. doi: 10.1016/j.jfms.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Marioni-Henry K., Vite C.H., Newton A.L. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med. 2004;18:851–858. doi: 10.1892/0891-6640(2004)18<851:podots>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 582.Lane S.B., Kornegay J.N., Duncan J.R. Feline spinal lymphosarcoma: a retrospective evaluation of 23 cats. J Vet Intern Med. 1994;8:99–104. doi: 10.1111/j.1939-1676.1994.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 583.Spodnick G.J., Berg J., Moore F.M. Spinal lymphoma in cats: 21 cases (1976-1989) J Am Vet Med Assoc. 1992;200:373–376. [PubMed] [Google Scholar]
- 584.Palus V., Volk H.A., Lamb C.R. MRI features of CNS lymphoma in dogs and cats. Vet Radiol Ultrasound. 2012;53:44–49. doi: 10.1111/j.1740-8261.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- 585.Fontaine J., Heimann M., Day M.J. Cutaneous epitheliotropic T-cell lymphoma in the cat: a review of the literature and five new cases. Vet Dermatol. 2011;22:454–461. doi: 10.1111/j.1365-3164.2011.00972.x. [DOI] [PubMed] [Google Scholar]
- 586.Caciolo P.L.N.G., Patnaik A.K. Cutaneous lymphosarcoma in the cat: a report of nine cases. J Am Anim Hosp Assoc. 1984;20:491–496. [Google Scholar]
- 587.Gilbert S., Affolter V.K., Gross T.L. Clinical, morphological and immunohistochemical characterization of cutaneous lymphocytosis in 23 cats. Vet Dermatol. 2004;15:3–12. doi: 10.1111/j.1365-3164.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 588.Day M.J. Immunophenotypic characterization of cutaneous lymphoid neoplasia in the dog and cat. J Comp Pathol. 1995;112:79–96. doi: 10.1016/s0021-9975(05)80091-x. [DOI] [PubMed] [Google Scholar]
- 589.Schick R.O., Murphy G.F., Goldschmidt M.H. Cutaneous lymphosarcoma and leukemia in a cat. J Am Vet Med Assoc. 1993;203:1155–1158. [PubMed] [Google Scholar]
- 590.Wood C., Almes K., Bagladi-Swanson M. Sezary syndrome in a cat. J Am Anim Hosp Assoc. 2008;44:144–148. doi: 10.5326/0440144. [DOI] [PubMed] [Google Scholar]
- 591.Komori S., Nakamura S., Takahashi K. Use of lomustine to treat cutaneous nonepitheliotropic lymphoma in a cat. J Am Vet Med Assoc. 2005;226:237–239. doi: 10.2460/javma.2005.226.237. [DOI] [PubMed] [Google Scholar]
- 592.Burr H.D., Keating J.H., Clifford C.A. Cutaneous lymphoma of the tarsus in cats: 23 cases (2000-2012) J Am Vet Med Assoc. 2014;244:1429–1434. doi: 10.2460/javma.244.12.1429. [DOI] [PubMed] [Google Scholar]
- 593.Roccabianca P., Avallone G., Rodriguez A. Cutaneous lymphoma at injection sites: pathological, immunophenotypical, and molecular characterization in 17 cats. Vet Pathol. 2016;53:823–832. doi: 10.1177/0300985815623620. [DOI] [PubMed] [Google Scholar]
- 594.Taylor S.S., Harvey A.M., Barr F.J. Laryngeal disease in cats: a retrospective study of 35 cases. J Feline Med Surg. 2009;11:954–962. doi: 10.1016/j.jfms.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Nerschbach V., Eule J.C., Eberle N. Ocular manifestation of lymphoma in newly diagnosed cats. Vet Comp Oncol. 2016;2016(14):58–66. doi: 10.1111/vco.12061. [DOI] [PubMed] [Google Scholar]
- 596.Peiffer R.L., Jr. Wilcock BP: Histopathologic study of uveitis in cats: 139 cases (1978-1988) J Am Vet Med Assoc. 1991;198:135–138. [PubMed] [Google Scholar]
- 597.Malmberg J.L., Garcia T., Dubielzig R.R. Canine and feline retinal lymphoma: a retrospective review of 12 cases. Vet Ophthalmol. 2017;20:73–78. doi: 10.1111/vop.12356. [DOI] [PubMed] [Google Scholar]
- 598.McCowan C., Malcolm J., Hurn S. Conjunctival lymphoma: immunophenotype and outcome in five dogs and three cats. Vet Ophthalmol. 2014;17:351–357. doi: 10.1111/vop.12083. [DOI] [PubMed] [Google Scholar]
- 599.Ota-Kuroki J., Ragsdale J.M., Bawa B. Intraocular and periocular lymphoma in dogs and cats: a retrospective review of 21 cases (2001-2012) Vet Ophthalmol. 2014;17:389–396. doi: 10.1111/vop.12106. [DOI] [PubMed] [Google Scholar]
- 600.Wiggans K.T., Skorupski K.A., Reilly C.M. Presumed solitary intraocular or conjunctival lymphoma in dogs and cats: 9 cases (1985-2013) J Am Vet Med Assoc. 2014;244:460–470. doi: 10.2460/javma.244.4.460. [DOI] [PubMed] [Google Scholar]
- 601.Grindem C.B. Ultrastructural morphology of leukemic cells in the cat. Vet Pathol. 1985;22:147–155. doi: 10.1177/030098588502200209. [DOI] [PubMed] [Google Scholar]
- 602.Gorman N.T., Evans R.J. Myeloproliferative disease in the dog and cat: clinical presentations, diagnosis and treatment. Vet Rec. 1987;121:490–496. doi: 10.1136/vr.121.21.490. [DOI] [PubMed] [Google Scholar]
- 603.Blue J.T., French T.W., Kranz J.S. Non-lymphoid hematopoietic neoplasia in cats: a retrospective study of 60 cases. Cornell Vet. 1988;78:21–42. [PubMed] [Google Scholar]
- 604.Facklam N.R., Kociba G.J. Cytochemical characterization of feline leukemic cells. Vet Pathol. 1986;23:155–161. doi: 10.1177/030098588602300208. [DOI] [PubMed] [Google Scholar]
- 605.Grindem C. Morphological and clinical an pathological characteristics of spontaneous leukemia in 10 cats. J Am Anim Hosp Assoc. 1985;21:227. [Google Scholar]
- 606.Campbell M.W., Hess P.R., Williams L.E. Chronic lymphocytic leukaemia in the cat: 18 cases (2000-2010) Vet Comp Oncol. 2013;11:256–264. doi: 10.1111/j.1476-5829.2011.00315.x. [DOI] [PubMed] [Google Scholar]
- 607.Weiss D.J. Differentiating benign and malignant causes of lymphocytosis in feline bone marrow. J Vet Intern Med. 2005;19:855–859. doi: 10.1892/0891-6640(2005)19[855:dbamco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 608.Hisasue M., Okayama H., Okayama T. Hematologic abnormalities and outcome of 16 cats with myelodysplastic syndromes. J Vet Intern Med. 2001;15:471–477. doi: 10.1892/0891-6640(2001)015<0471:haaooc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 609.Essex M.E. Feline leukemia: a naturally occurring cancer of infectious origin. Epidemiol Rev. 1982;4:189–203. doi: 10.1093/oxfordjournals.epirev.a036246. [DOI] [PubMed] [Google Scholar]
- 610.Tebb A.J., Cave T., Barron R. Diagnosis and management of B cell chronic lymphocytic leukaemia in a cat. Vet Rec. 2004;154:430–433. doi: 10.1136/vr.154.14.430. [DOI] [PubMed] [Google Scholar]
- 611.Workman H.C., Vernau W. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. Vet Clin North Am Small Anim Pract. 2003;33:1379–1399. doi: 10.1016/s0195-5616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 612.Cotter S.M. Treatment of lymphoma and leukemia with cyclophosphamide, vincristine and prednisone: I. Treatment of dogs. J Am Anim Hosp Assoc. 1983;19:159–165. [Google Scholar]
- 613.Lichtman M.A. Classification and clinical manifestations of the clonal myeloid disorders. In: Lichtman MA Kaushansky K., Seligsohn U., editors. Williams hematology. ed 8. McGraw-Hill; New York: 2011. [Google Scholar]
- 614.Breen M., Modiano J.F. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans—man and his best friend share more than companionship. Chromosome Res. 2008;16:145–154. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 615.Jain N.C., Blue JT., Grindem C.B. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet Clin Pathol. 1991;20:63–82. doi: 10.1111/j.1939-165x.1991.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 616.Juopperi T.A., Bienzle D., Bernreuter D.C. Prognostic markers for myeloid neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol. 2011;48:182–197. doi: 10.1177/0300985810389317. [DOI] [PubMed] [Google Scholar]
- 617.Nielson S.W. myeloproliferative disorders in animals. In: Clarke WJ Hacett E.B., Hackett P.L., editors. Myeloproliferative disorders in animals and man. USAEC Division of Technical Information Extension; Oak Ridge, Tenn: 1970. [Google Scholar]
- 618.Bennett A.L., Williams L.E., Ferguson M.W. Canine acute leukaemia: 50 cases (1989-2014) Vet Comp Oncol. 2017;15:1101–1114. doi: 10.1111/vco.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Davis L.L., Hume K.R., Stokol T. A retrospective review of acute myeloid leukaemia in 35 dogs diagnosed by a combination of morphologic findings, flow cytometric immunophenotyping and cytochemical staining results (2007-2015) Vet Comp Oncol. 2018;16:268–275. doi: 10.1111/vco.12377. [DOI] [PubMed] [Google Scholar]
- 620.Novacco M., Comazzi S., Marconato L. Prognostic factors in canine acute leukaemias: a retrospective study. Vet Comp Oncol. 2016;14:409–416. doi: 10.1111/vco.12136. [DOI] [PubMed] [Google Scholar]
- 621.Stokol T., Nickerson G.A., Shuman M. Dogs with acute myeloid leukemia have clonal rearrangements in T and B Cell receptors. Front Vet Sci. 2017;4:76. doi: 10.3389/fvets.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Grindem C.B.S.J., Perman V. Morphologic classification and clinical and pathological charactersitics of spontaneous leukemia in 17 dogs. J Am Anim Hosp Assoc. 1985;21:219–226. [Google Scholar]
- 623.Jandl J. Blackwell Scientific; Boston: 1991. Hematopoietic malignancies. blood: pathophysiology. [Google Scholar]
- 624.Grindem C.B., Buoen L.C. Cytogenetic analysis of leukaemic cells in the dog. A report of 10 cases and a review of the literature. J Comp Pathol. 1986;96:623–635. doi: 10.1016/0021-9975(86)90059-9. [DOI] [PubMed] [Google Scholar]
- 625.Reimann N., Bartnitzke S., Bullerdiek J. Trisomy 1 in a canine acute leukemia indicating the pathogenetic importance of polysomy 1 in leukemias of the dog. Cancer Genet Cytogenet. 1998;101:49–52. doi: 10.1016/s0165-4608(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 626.Roode S.C., Rotroff D., Avery A.C. Genome-wide assessment of recurrent genomic imbalances in canine leukemia identifies evolutionarily conserved regions for subtype differentiation. Chromosome Res. 2015;23:681–708. doi: 10.1007/s10577-015-9475-7. [DOI] [PubMed] [Google Scholar]
- 627.Culver S., Ito D., Borst L. Molecular characterization of canine BCR-ABL-positive chronic myelomonocytic leukemia before and after chemotherapy. Vet Clin Pathol. 2013;42:314–322. doi: 10.1111/vcp.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Breen M. Canine cytogenetics—from band to basepair. Cytogenet Genome Res. 2008;120:50–60. doi: 10.1159/000118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Breen M. Update on genomics in veterinary oncology. Top Companion Anim Med. 2009;24:113–121. doi: 10.1053/j.tcam.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Reimann N., Bartnitzke S., Nolte I. Working with canine chromosomes: current recommendations for karyotype description. J Hered. 1999;90:31–34. doi: 10.1093/jhered/90.1.31. [DOI] [PubMed] [Google Scholar]
- 631.Andersen A.C., Johnson R.M. Erythroblastic malignancy in a beagle. J Am Vet Med Assoc. 1962;141:944–946. [PubMed] [Google Scholar]
- 632.Seed T.M., Tolle D.V., Fritz T.E. Irradiation-induced erythroleukemia and myelogenous leukemia in the beagle dog: hematology and ultrastructure. Blood. 1977;50:1061–1079. [PubMed] [Google Scholar]
- 633.Tolle D.V., Seed T.M., Fritz T.E. Acute monocytic leukemia in an irradiated Beagle. Vet Pathol. 1979;16:243–254. doi: 10.1177/030098587901600210. [DOI] [PubMed] [Google Scholar]
- 634.Sykes G.P., King J.M., Cooper B.C. Retrovirus-like particles associated with myeloproliferative disease in the dog. J Comp Pathol. 1985;95:559–564. doi: 10.1016/0021-9975(85)90025-8. [DOI] [PubMed] [Google Scholar]
- 635.Quesenberry P. Hemopoietic stem cells, progenitro cells and cytokines. In: Beutler E.L.M., Coller B.S., editors. Williams hematology. ed 5. McGraw-Hill; New York: 1995. [Google Scholar]
- 636.Metcalf D. Elsevier; New York: 1984. The hemopoietic colony stimulating factors. [Google Scholar]
- 637.Lok S., Kaushansky K., Holly R.D. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 638.Boone L.I., Knauer K.W., Rapp S.W. Use of human recombinant erythropoietin and prednisone for treatment of myelodysplastic syndrome with erythroid predominance in a dog. J Am Vet Med Assoc. 1998;213:999–1001. [PubMed] [Google Scholar]
- 639.Couto C.G. Clinicopathologic aspects of acute leukemias in the dog. J Am Vet Med Assoc. 1985;186:681–685. [PubMed] [Google Scholar]
- 640.Couto C.G., Kallet A.J. Preleukemic syndrome in a dog. J Am Vet Med Assoc. 1984;184:1389–1392. [PubMed] [Google Scholar]
- 641.Weiss D.J., Raskin R., Zerbe C. Myelodysplastic syndrome in two dogs. J Am Vet Med Assoc. 1985;187:1038–1040. [PubMed] [Google Scholar]
- 642.Degen M.A., Feldman B.F., Turrel J.M. Thrombocytosis associated with a myeloproliferative disorder in a dog. J Am Vet Med Assoc. 1989;194:1457–1459. [PubMed] [Google Scholar]
- 643.Evans R.J., Gorman N.T. Myeloproliferative disease in the dog and cat: definition, aetiology and classification. Vet Rec. 1987;121:437–443. doi: 10.1136/vr.121.19.437. [DOI] [PubMed] [Google Scholar]
- 644.Jain N.C., Madewell B.R., Weller R.E. Clinical-pathological findings and cytochemical characterization of myelomonocytic leukaemia in 5 dogs. J Comp Pathol. 1981;91:17–31. doi: 10.1016/0021-9975(81)90041-4. [DOI] [PubMed] [Google Scholar]
- 645.Clark P., Swenson C.L., Drenen C.M. A 6-year-old rottweiler with weight loss. Aust Vet J. 1997;75(709) doi: 10.1111/j.1751-0813.1997.tb12249.x. [DOI] [PubMed] [Google Scholar]
- 646.Graves T.K., Swenson C.L., Scott M.A. A potentially misleading presentation and course of acute myelomonocytic leukemia in a dog. J Am Anim Hosp Assoc. 1997;33:37–41. doi: 10.5326/15473317-33-1-37. [DOI] [PubMed] [Google Scholar]
- 647.Keller P., Sager P., Freudiger U. Acute myeloblastic leukaemia in a dog. J Comp Pathol. 1985;95:619–632. doi: 10.1016/0021-9975(85)90031-3. [DOI] [PubMed] [Google Scholar]
- 648.Barthel C.H. Acute myelomonocytic leukemia in a dog. Vet Pathol. 1974;11:79–86. doi: 10.1177/030098587401100109. [DOI] [PubMed] [Google Scholar]
- 649.Bolon B., Buergelt C.D., Harvey J.W. Megakaryblastic leukemia in a dog. Vet Clin Pathol. 1989;18:69–74. doi: 10.1111/j.1939-165x.1989.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 650.Green R.A.B.C. Acute myelomonocytic leukemia in a dog. J Am Anim Hosp Assoc. 1977;13:708–712. [Google Scholar]
- 651.Hayashi A., Tanaka H., Kitamura M. Acute myelomonocytic leukemia (AML-M4) in a dog with the extradural lesion. J Vet Med Sci. 2011;73:419–422. doi: 10.1292/jvms.10-0324. [DOI] [PubMed] [Google Scholar]
- 652.Hisasue M., Nishimura T., Neo S. A dog with acute myelomonocytic leukemia. J Vet Med Sci. 2008;70:619–621. doi: 10.1292/jvms.70.619. [DOI] [PubMed] [Google Scholar]
- 653.Linnabary R.D.H.M., Glick A.D. Acute myelomonocytic leukemia in a dog. J Am Anim Hosp Assoc. 1978;14:71–75. [Google Scholar]
- 654.Madewell B. Unusual cytochemical reactivity in canine acute myeloblastic leukemia. Comp Haematol Int. 1991;1:117–120. [Google Scholar]
- 655.Moulton J.E.D.D. Tumors of the lymphoid and hemopoietic tissues. In: JE M., editor. Tumors in domestic animals. ed 2. University of California Press; Berkeley: 1978. [Google Scholar]
- 656.Ragan H.A., Hackett P.L., Dagle G.E. Acute myelomonocytic leukemia manifested as myelophthisic anemia in a dog. J Am Vet Med Assoc. 1976;169:421–425. [PubMed] [Google Scholar]
- 657.Rohrig K.E. Acute myelomonocytic leukemia in a dog. J Am Vet Med Assoc. 1983;182:137–141. [PubMed] [Google Scholar]
- 658.Cain G.R., Feldman B.F., Kawakami T.G. Platelet dysplasia associated with megakaryoblastic leukemia in a dog. J Am Vet Med Assoc. 1986;188:529–530. [PubMed] [Google Scholar]
- 659.Cain G.R., Kawakami T.G., Jain N.C. Radiation-induced megakaryoblastic leukemia in a dog. Vet Pathol. 1985;22:641–643. doi: 10.1177/030098588502200622. [DOI] [PubMed] [Google Scholar]
- 660.Canfield P.J.C.D., Russ I.G. Myeloproliferative disorder involving the megakaryocytic line. J Small Anim Pract. 1993;34:296–301. [Google Scholar]
- 661.Colbatzky F., Hermanns W. Acute megakaryoblastic leukemia in one cat and two dogs. Vet Pathol. 1993;30:186–194. doi: 10.1177/030098589303000212. [DOI] [PubMed] [Google Scholar]
- 662.Comazzi S.G.M., Belfanti U. Acute megakaryoblastic leukemia in a dog: a report of 3 cases and review of the literature. J Am Anim Hosp Assoc. 2010;46:327–335. doi: 10.5326/0460327. [DOI] [PubMed] [Google Scholar]
- 663.Ferreira H.M.T., Smith S.H., Schwartz A.M. Myeloperoxidase-positive acute megakaryoblastic leukemia in a dog. Vet Clin Pathol. 2011;40:530–537. doi: 10.1111/j.1939-165X.2011.00363.x. [DOI] [PubMed] [Google Scholar]
- 664.Holscher M.A., Collins R.D., Glick A.D. Megakaryocytic leukemia in a dog. Vet Pathol. 1978;15:562–565. doi: 10.1177/030098587801500416. [DOI] [PubMed] [Google Scholar]
- 665.Messick J., Carothers M., Wellman M. Identification and characterization of megakaryoblasts in acute megakaryoblastic leukemia in a dog. Vet Pathol. 1990;27:212–214. doi: 10.1177/030098589002700314. [DOI] [PubMed] [Google Scholar]
- 666.Park H.M., Doster A.R., Tashbaeva R.E. Clinical, histopathological and immunohistochemical findings in a case of megakaryoblastic leukemia in a dog. J Vet Diagn Invest. 2006;18:287–291. doi: 10.1177/104063870601800311. [DOI] [PubMed] [Google Scholar]
- 667.Shull R.M., DeNovo R.C., McCracken M.D. Megakaryoblastic leukemia in a dog. Vet Pathol. 1986;23:533–536. doi: 10.1177/030098588602300431. [DOI] [PubMed] [Google Scholar]
- 668.Suter S.E., Vernau W., Fry M.M. CD34+, CD41+ acute megakaryoblastic leukemia in a dog. Vet Clin Pathol. 2007;36:288–292. doi: 10.1111/j.1939-165x.2007.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 669.Willan M.M.L., Schwendenwein I. Chemotherapy in canine acute megakaryoblastic leukemia: a case report and review of the literature. Vivo. 2009;23:911–918. [PubMed] [Google Scholar]
- 670.Mylonakis M.E., Kritsepi-Konstantinou M., Vernau W. Presumptive pure erythroid leukemia in a dog. J Vet Diagn Invest. 2012;24:1004–1007. doi: 10.1177/1040638712452731. [DOI] [PubMed] [Google Scholar]
- 671.Valentini F., Tasca S., Gavazza A. Use of CD9 and CD61 for the characterization of AML-M7 by flow cytometry in a dog. Vet Comp Oncol. 2012;10:312–318. doi: 10.1111/j.1476-5829.2011.00290.x. [DOI] [PubMed] [Google Scholar]
- 672.Latimer K.S., Dykstra M.J. Acute monocytic leukemia in a dog. J Am Vet Med Assoc. 1984;184:852–854. [PubMed] [Google Scholar]
- 673.Mackey L.J. Monocytic leukaemia in the dog. Vet Rec. 1975;96:27–30. doi: 10.1136/vr.96.2.27. [DOI] [PubMed] [Google Scholar]
- 674.Campbell M.W., Hess P.R., Williams L.E. Chronic lymphocytic leukaemia in the cat: 18 cases (2000-2010) Vet Comp Oncol. 2013;11:256–264. doi: 10.1111/j.1476-5829.2011.00315.x. [DOI] [PubMed] [Google Scholar]
- 675.Tomiyasu H., Fujino Y., Takahashi M. Spontaneous acute erythroblastic leukaemia (AML-M6Er) in a dog. J Small Anim Pract. 2011;52:445–447. doi: 10.1111/j.1748-5827.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- 676.Hejlasz Z. Three cases of erythroleukemia in a dog. Medycyna Weterynaryjna. 1986;42:346–349. [Google Scholar]
- 677.Grindem C.B. Blood cell markers. Vet Clin North Am Small Anim Pract. 1996;26:1043–1064. doi: 10.1016/s0195-5616(96)50055-0. [DOI] [PubMed] [Google Scholar]
- 678.Usher S.G., Radford A.D., Villiers E.J. RAS, FLT3, and C-KIT mutations in immunophenotyped canine leukemias. Exp Hematol. 2009;37:65–77. doi: 10.1016/j.exphem.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 679.Figueiredo J.F., Culver S., Behling-Kelly E. Acute myeloblastic leukemia with associated BCR-ABL translocation in a dog. Vet Clin Pathol. 2012;41:362–368. doi: 10.1111/j.1939-165X.2012.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Passamonti F., Maffioli M. The role of JAK2 inhibitors in MPNs 7 years after approval. Blood. 2018;131:2426–2435. doi: 10.1182/blood-2018-01-791491. [DOI] [PubMed] [Google Scholar]
- 681.Beurlet S., Krief P., Sansonetti A. Identification of JAK2 mutations in canine primary polycythemia. Exp Hematol. 2011;39:542–545. doi: 10.1016/j.exphem.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 682.Prchal J. Primary polycythemias In JW A, editor. Curr Opin Hematol. 1995;2:146–152. doi: 10.1097/00062752-199502020-00007. [DOI] [PubMed] [Google Scholar]
- 683.Bush B.M., Fankhauser R. Polychthaemia vera in a bitch. J Small Anim Pract. 1972;13:75–89. doi: 10.1111/j.1748-5827.1972.tb06833.x. [DOI] [PubMed] [Google Scholar]
- 684.Carb A.V. Polycythemia vera in a dog. J Am Vet Med Assoc. 1969;154:289–297. [PubMed] [Google Scholar]
- 685.Diogo C.C., Fabretti A.K., Camassa J.A. Diagnosis and treatment of primary erythrocytosis in a dog: a case report. Top Companion Anim Med. 2015;30:65–67. doi: 10.1053/j.tcam.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 686.Holden A.R. Polycythaemia vera in a dog. Vet Rec. 1987;120:473–475. doi: 10.1136/vr.120.20.473. [DOI] [PubMed] [Google Scholar]
- 687.McGrath C.J. Polycythemia vera in dogs. J Am Vet Med Assoc. 1974;164:1117–1122. [PubMed] [Google Scholar]
- 688.Meyer H.P., Slappendel R.J., Greydanus-van der Putten S.W. Polycythaemia vera in a dog treated by repeated phlebotomies. Vet Q. 1993;15:108–111. doi: 10.1080/01652176.1993.9694385. [DOI] [PubMed] [Google Scholar]
- 689.Miller R.M. Polycythemia vera in a dog. Vet Med Small Anim Clin. 1968;63:222–223. [PubMed] [Google Scholar]
- 690.Peterson M.E., Randolph J.F. Diagnosis of canine primary polycythemia and management with hydroxyurea. J Am Vet Med Assoc. 1982;180:415–418. [PubMed] [Google Scholar]
- 691.Quesnel A.D., Kruth S.A. Polycythemia vera and glomerulonephritis in a dog. Can Vet J. 1992;33:671–672. [PMC free article] [PubMed] [Google Scholar]
- 692.Wysoke J.M., Van Heerden J. Polycythaemia vera in a dog. J S Afr Vet Assoc. 1990;61:182–183. [PubMed] [Google Scholar]
- 693.Dunn J.K.J.A., Evans R.J. Chronic granulocytic leukaemia in a dog with assoiciated bacterial endocarditis, thrombocytopenia and preretinal and retinal hemorrhages. J Small Anim Pract. 1987;28:1079–1086. [Google Scholar]
- 694.Fine D.M., Tvedten H.W. Chronic granulocytic leukemia in a dog. J Am Vet Med Assoc. 1999;214:1809–1812. [PubMed] [Google Scholar]
- 695.Grindem C.B., Stevens J.B., Brost D.R. Chronic myelogenous leukaemia with meningeal infiltration in a dog. Comp Haematol Int. 1992;2:170–174. [Google Scholar]
- 696.Leifer C.E., Matus R.E., Patnaik A.K. Chronic myelogenous leukemia in the dog. J Am Vet Med Assoc. 1983;183:686–689. [PubMed] [Google Scholar]
- 697.Jain N.C. ed 4. Lea & Febiger; Philadelphia: 1986. The leukemia complex. Schalm’s veterinary hematology. [Google Scholar]
- 698.Pollet L., Van Hove W., Mattheeuws D. Blastic crisis in chronic myelogenous leukaemia in a dog. J Small Anim Pract. 1978;19:469–475. doi: 10.1111/j.1748-5827.1978.tb05524.x. [DOI] [PubMed] [Google Scholar]
- 699.Liesveld J.L.L.M. Chronic myelogenous leukemia and related disorders. In: Lichtman M.A., Kaushansky K., Seligsohn U., editors. Williams hemtology. ed 8. McGraw Hill; New York: 2010. [Google Scholar]
- 700.Marino C.L., Tran J., Stokol T. Atypical chronic myeloid leukemia in a German Shepherd Dog. J Vet Diagn Invest. 2017;29:338–345. doi: 10.1177/1040638716689581. [DOI] [PubMed] [Google Scholar]
- 701.Perez M.L., Culver S., Owen J.L. Partial cytogenetic response with toceranib and prednisone treatment in a young dog with chronic monocytic leukemia. Anticancer Drugs. 2013;24:1098–1103. doi: 10.1097/CAD.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 702.Cruz Cardona J.A., Milner R., Alleman A.R. BCR-ABL translocation in a dog with chronic monocytic leukemia. Vet Clin Pathol. 2011;40:40–47. doi: 10.1111/j.1939-165X.2010.00277.x. [DOI] [PubMed] [Google Scholar]
- 703.Hiraoka H., Hisasue M., Nagashima N. A dog with myelodysplastic syndrome: chronic myelomonocytic leukemia. J Vet Med Sci. 2007;69:665–668. doi: 10.1292/jvms.69.665. [DOI] [PubMed] [Google Scholar]
- 704.Rossi G., Gelain M.E., Foroni S. Extreme monocytosis in a dog with chronic monocytic leukaemia. Vet Rec. 2009;165:54–56. doi: 10.1136/vetrec.165.2.54. [DOI] [PubMed] [Google Scholar]
- 705.MacEwen E.G., Drazner F.H., McClelland A.J. Treatment of basophilic leukemia in a dog. J Am Vet Med Assoc. 1975;166:376–380. [PubMed] [Google Scholar]
- 706.Mahaffey E.A., Brown T.P., Duncan J.R. Basophilic leukaemia in a dog. J Comp Pathol. 1987;97:393–399. doi: 10.1016/0021-9975(87)90017-x. [DOI] [PubMed] [Google Scholar]
- 707.Mears E.A., Raskin R.E., Legendre A.M. Basophilic leukemia in a dog. J Vet Intern Med. 1997;11:92–94. doi: 10.1111/j.1939-1676.1997.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 708.Jensen A.L.N.O. Eosinophilic leukemoid reaction in a dog. J Small Anim Pract. 1992;33:337–340. [Google Scholar]
- 709.Ndikuwera J.S.D., Obwolo M.J. Chronic granulocytic leukaemia/eosinophilic leukaemia in a dog. J Small Anim Pract. 1992;33:553–557. [Google Scholar]
- 710.Beer P.A.G.A. Essential thrombocythemia. In: Lichtman MA Kaushansky K., Seligsohn U., editors. Williams hematology. ed 8. McGraw-Hill; New York: 2010. [Google Scholar]
- 711.Bass M.C.S.A. Essential thrombocythemia in a dog: case report and literature review. J Am Anim Hosp Assoc. 1998;34:197–203. doi: 10.5326/15473317-34-3-197. [DOI] [PubMed] [Google Scholar]
- 712.Dunn J.K., Heath M.F., Jefferies A.R. Diagnostic and hematologic features of probable essential thrombocythemia in two dogs. Vet Clin Pathol. 1999;28:131–138. doi: 10.1111/j.1939-165x.1999.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 713.Hopper P.E., Mandell C.P., Turrel J.M. Probable essential thrombocythemia in a dog. J Vet Intern Med. 1989;3:79–85. doi: 10.1111/j.1939-1676.1989.tb03083.x. [DOI] [PubMed] [Google Scholar]
- 714.Simpson J.W.E.R., Honeyman P. Successful treatment of suspected essential thrombocythemia in the dog. J Small Anim Pract. 1990;31:345–348. [Google Scholar]
- 715.Reagan W.J. A review of myelofibrosis in dogs. Toxicol Pathol. 1993;21:164–169. doi: 10.1177/019262339302100208. [DOI] [PubMed] [Google Scholar]
- 716.Weiss D.J. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004) J Vet Intern Med. 2006;20:955–961. doi: 10.1892/0891-6640(2006)20[955:arsoti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 717.Castro-Malaspina, Berk P.D. Pathogenesis of myelofibrosis: role of ineffective megakaryopoiesis and megakaryocyte components. In: Castro-Malaspina H., Wasserman L.R., editors. Myelofibrosis and the biology of connective tissue. Alan R. Liss; New York: 1984. [PubMed] [Google Scholar]
- 718.Dungworth D.L., Goldman M., Switzer J. Development of a myeloproliferative disorder in beagles continuously exposed to 90Sr. Blood. 1969;34:610–632. [PubMed] [Google Scholar]
- 719.Prasse K.W., Crouser D., Beutler E. Pyruvate kinase deficiency anemia with terminal myelofibrosis and osteosclerosis in a beagle. J Am Vet Med Assoc. 1975;166:1170–1175. [PubMed] [Google Scholar]
- 720.Rudolph R.H.C. Megakaryozytenleukose beim hund. Kleintier-Praxis. 1972;17:9–13. [Google Scholar]
- 721.Thompson J.C.J.A. Myelofibrosis in the dog: three case reports. J Small Anim Pract. 1983;24:589–601. [Google Scholar]
- 722.McManus P.M. Classification of myeloid neoplasms: a comparative review. Vet Clin Pathol. 2005;34:189–212. doi: 10.1111/j.1939-165x.2005.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 723.Thomsen M.K., Jensen A.L., Skak-Nielsen T. Enhanced granulocyte function in a case of chronic granulocytic leukemia in a dog. Vet Immunol Immunopathol. 1991;28:143–156. doi: 10.1016/0165-2427(91)90136-z. [DOI] [PubMed] [Google Scholar]
- 724.Facklam N.R., Kociba G.J. Cytochemical characterization of leukemic cells from 20 dogs. Vet Pathol. 1985;22:363–369. doi: 10.1177/030098588502200411. [DOI] [PubMed] [Google Scholar]
- 725.Grindem C.B., Stevens J.B., Perman V. Cytochemical reactions in cells from leukemic dogs. Vet Pathol. 1986;23:103–109. doi: 10.1177/030098588602300201. [DOI] [PubMed] [Google Scholar]
- 726.Mochizuki H., Goto-Koshino Y., Takahashi M. Demonstration of the cell clonality in canine hematopoietic tumors by X-chromosome inactivation pattern analysis. Vet Pathol. 2015;52:61–69. doi: 10.1177/0300985814528217. [DOI] [PubMed] [Google Scholar]
- 727.Goldman E.E.G.J. Clinical diagnosis and management of acute nonlymphoid leukemias and chronic myeloproliferative disorders. In: Feldman BF Zinkel J., Jain N.C., editors. Schalm’s veterinary hematology. ed 5. Lippincott Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- 728.Stokol T., Schaefer D.M., Shuman M. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet Clin Pathol. 2015;44:79–93. doi: 10.1111/vcp.12227. [DOI] [PubMed] [Google Scholar]
- 729.Cobbold S., Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW) Tissue Antigens. 1994;43:137–154. doi: 10.1111/j.1399-0039.1994.tb02315.x. [DOI] [PubMed] [Google Scholar]
- 730.Comazzi S., Gelain M.E., Spagnolo V. Flow cytometric patterns in blood from dogs with non-neoplastic and neoplastic hematologic diseases using double labeling for CD18 and CD45. Vet Clin Pathol. 2006;35:47–54. doi: 10.1111/j.1939-165x.2006.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 731.Tasca S., Carli E., Caldin M. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002-2006) Vet Clin Pathol. 2009;38:2–12. doi: 10.1111/j.1939-165X.2008.00099.x. [DOI] [PubMed] [Google Scholar]
- 732.Villiers E., Baines S., Law A.M. Identification of acute myeloid leukemia in dogs using flow cytometry with myeloperoxidase, MAC387, and a canine neutrophil-specific antibody. Vet Clin Pathol. 2006;35:55–71. doi: 10.1111/j.1939-165x.2006.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 733.Peterson M.E., Zanjani E.D. Inappropriate erythropoietin production from a renal carcinoma in a dog with polycythemia. J Am Vet Med Assoc. 1981;179:995–996. [PubMed] [Google Scholar]
- 734.Scott R.C.P.A. Renal carcinoma associated with secondary polycythemia in a dog. J Am Anim Hosp Assoc. 1972;8:275–283. [Google Scholar]
- 735.Waters D.J.P.J. Secondary polycythemia associated with renal disease in the dog: two case reports and review of literature. J Am Anim Hosp Assoc. 1988;24:109–114. [Google Scholar]
- 736.Cook S.M., Lothrop C.D., Jr. Serum erythropoietin concentrations measured by radioimmunoassay in normal, polycythemic, and anemic dogs and cats. J Vet Intern Med. 1994;8:18–25. doi: 10.1111/j.1939-1676.1994.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 737.Giger U. Serum erythropoietin concentrations in polycythemic and anemic dogs. Proc 9th Annual Vet Med Forum (ACVIM) 1991:143–145. [Google Scholar]
- 738.Reimann K.A., Knowlen G.G., Tvedten H.W. Factitious hyperkalemia in dogs with thrombocytosis. The effect of platelets on serum potassium concentration. J Vet Intern Med. 1989;3:47–52. doi: 10.1111/j.1939-1676.1989.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 739.Weiss D.J., Aird B. Cytologic evaluation of primary and secondary myelodysplastic syndromes in the dog. Vet Clin Pathol. 2001;30:67–75. doi: 10.1111/j.1939-165x.2001.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 740.Friedrichs K.R.Y.K. How to collect diagnostic bone marrow samples. Vet Med. 2005;8:578–588. [Google Scholar]
- 741.Feeney D.A., Sharkey L.C., Steward S.M. Applicability of 3T body MRI in assessment of nonfocal bone marrow involvement of hematopoietic neoplasia in dogs. J Vet Intern Med. 2013;27:1165–1171. doi: 10.1111/jvim.12151. [DOI] [PubMed] [Google Scholar]
- 742.Feeney D.A., Sharkey L.C., Steward S.M. Parenchymal signal intensity in 3-T body MRI of dogs with hematopoietic neoplasia. Comp Med. 2013;63:174–182. [PMC free article] [PubMed] [Google Scholar]
- 743.Schmidt J.M., North S.M., Freeman K.P. Feline paediatric oncology: retrospective assessment of 233 tumours from cats up to one year (1993 to 2008) J Small Anim Pract. 2010;51:306–311. doi: 10.1111/j.1748-5827.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 744.Gorman N.T., Evans R.J. Myeloproliferative disease in the dog and cat: clinical presentations, diagnosis and treatment. Vet Rec. 1987;121:490–496. doi: 10.1136/vr.121.21.490. [DOI] [PubMed] [Google Scholar]
- 745.Hamlin R.H., Duncan R.C. Acute nonlymphocytic leukemia in a dog. J Am Vet Med Assoc. 1990;196:110–112. [PubMed] [Google Scholar]
- 746.Theilen GH MB., Gardner MB. Hematopoietic neoplasms, sarcomas and related conditions. In: Theilen G.H., Madewell, editors. Veterinary cancer medicine. ed 2. Lea & Febiger; Philadelphia: 1987. [Google Scholar]
- 747.Lawless S., McMullin M.F., Cuthbert R. (32)P in the treatment of myeloproliferative disorders. Ulster Med J. 2016;85:83–85. [PMC free article] [PubMed] [Google Scholar]
- 748.Smith M., Turrel J.M. Radiophosphorus (32P) treatment of bone marrow disorders in dogs: 11 cases (1970-1987) J Am Vet Med Assoc. 1989;194:98–102. [PubMed] [Google Scholar]
- 749.Lyss A.P. Enzymes and random synthetics. In: Perry M.C., editor. The chemotherapy source book. Williams & Wilkins; Baltimore: 1992. pp. 398–412. [Google Scholar]
- 750.Bolin R.W., Robinson W.A., Sutherland J. Busulfan versus hydroxyurea in long-term therapy of chronic myelogenous leukemia. Cancer. 1982;50:1683–1686. doi: 10.1002/1097-0142(19821101)50:9<1683::aid-cncr2820500904>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 751.Rosenthal S., Canellos G.P., Whang-Peng J. Blast crisis of chronic granulocytic leukemia. Morphologic variants and therapeutic implications. Am J Med. 1977;63:542–547. doi: 10.1016/0002-9343(77)90199-1. [DOI] [PubMed] [Google Scholar]
- 752.Ganser A., Hoelzer D. Treatment of myelodysplastic syndromes with hematopoietic growth factors. Hematol Oncol Clin North Am. 1992;6:633–653. [PubMed] [Google Scholar]
- 753.Liesveld JL., Lichtman MA. Myelodysplastic syndromes (clonal cytopenias and oligoblastic myelogenous leukemia) In: Lichtman MA., Kaushansky K., Seligsohn U., editors. Williams hematology. ed 8. McGraw-Hill; New York: 2010. [Google Scholar]
- 754.Jacobs A. Treatment for the myelodysplastic syndromes. Haematologica. 1987;72:477–480. [PubMed] [Google Scholar]
- 755.Kelsey S.M., Newland A.C., Cunningham J. Sustained haematological response to high-dose oral alfacalcidol in patients with myelodysplastic syndromes. Lancet. 1992;340:316–317. doi: 10.1016/0140-6736(92)92414-b. [DOI] [PubMed] [Google Scholar]
- 756.Ohno R., Naoe T., Hirano M. Treatment of myelodysplastic syndromes with all-trans retinoic acid. Leukemia Study Group of the Ministry of Health and Welfare. Blood. 1993;81:1152–1154. [PubMed] [Google Scholar]
- 757.Matus R.E., Leifer C.E., MacEwen E.G. Prognostic factors for multiple myeloma in the dog. J Am Vet Med Assoc. 1986;188:1288–1292. [PubMed] [Google Scholar]
- 758.Liu S., Dorfman H.D., Hurvitz A.I. Primary and secondary bone tumors in the dog. J Small Anim Pract. 1977;18:313–326. doi: 10.1111/j.1748-5827.1977.tb05890.x. [DOI] [PubMed] [Google Scholar]
- 759.Weiss D.J. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996-2004) J Vet Intern Med. 2006;20:955–961. doi: 10.1892/0891-6640(2006)20[955:arsoti]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 760.Tappin S.W., Taylor S.S., Tasker S. Serum protein electrophoresis in 147 dogs. Vet Rec. 2011;168:456. doi: 10.1136/vr.d88. [DOI] [PubMed] [Google Scholar]
- 761.Fernandez R., Chon E. Comparison of two melphalan protocols and evaluation of outcome and prognostic factors in multiple myeloma in dogs. J Vet Intern Med. 2018;32:1060–1069. doi: 10.1111/jvim.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Thamm D.H., Vail D.M., Kurzman I.D. GS–9219/VDC-1101-a prodrug of the acyclic nucleotide PMEG has antitumor activity in spontaneous canine multiple myeloma. BMC Vet Res. 2014;10(30) doi: 10.1186/1746-6148-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Osborne C.A., Perman V., Sautter J.H. Multiple myeloma in the dog. J Am Vet Med Assoc. 1968;153:1300–1319. [PubMed] [Google Scholar]
- 764.MacEwen E.G., Hurvitz A.I. Diagnosis and management of monoclonal gammopathies. Vet Clin North Am. 1977;7:119–132. doi: 10.1016/s0091-0279(77)50010-x. [DOI] [PubMed] [Google Scholar]
- 765.Carpenter J., Andrews L.K., Holzworth J. Tumors and tumor like lesions. In: Holzworth J., editor. Diseases of the cat: medicine and surgery. WB Saunders; Philadelphia: 1987. [Google Scholar]
- 766.Engle G., Brodey R.S. A retrospective study of 395 feline neoplasms. J Am Anim Hosp Assoc. 1969;5:21–31. [Google Scholar]
- 767.Patel R.T., Caceres A., French A.F. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol. 2005;34:341–352. doi: 10.1111/j.1939-165X.2005.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Taylor S.S., Tappin S.W., Dodkin S.J. Serum protein electrophoresis in 155 cats. J Feline Med Surg. 2010;12:643–653. doi: 10.1016/j.jfms.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Cannon C.M., Knudson C., Borgatti A. Clinical signs, treatment, and outcome in cats with myeloma–related disorder receiving systemic therapy. J Am Anim Hosp Assoc. 2015;51:239–248. doi: 10.5326/JAAHA-MS-6216. [DOI] [PubMed] [Google Scholar]
- 770.Hanna F. Multiple myelomas in cats. J Feline Med Surg. 2005;7:275–287. doi: 10.1016/j.jfms.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Mellor P.J., Haugland S., Murphy S. Myeloma-related disorders in cats commonly present as extramedullary neoplasms in contrast to myeloma in human patients: 24 cases with clinical follow-up. J Vet Intern Med. 2006;20:1376–1383. doi: 10.1111/j.1939-1676.2006.tb00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Qurollo B.A., Balakrishnan N., Cannon C.Z. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J Feline Med Surg. 2014;16:713–720. doi: 10.1177/1098612X13519632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Geigy C., Riond B., Bley C.R. Multiple myeloma in a dog with multiple concurrent infectious diseases and persistent polyclonal gammopathy. Vet Clin Pathol. 2013;42:47–54. doi: 10.1111/vcp.12018. [DOI] [PubMed] [Google Scholar]
- 774.Cangul I.T., Wijnen M., Van Garderen E. Clinico–pathological aspects of canine cutaneous and mucocutaneous plasmacytomas. J Vet Med A Physiol Pathol Clin Med. 2002;49:307–312. doi: 10.1046/j.1439-0442.2002.00456.x. [DOI] [PubMed] [Google Scholar]
- 775.Imahori S., Moore G.E. Multiple myeloma and prolonged stimulation of reticuloendothelial system. N Y State J Med. 1972;72:1625–1628. [PubMed] [Google Scholar]
- 776.London C.A., Hannah A.L., Zadovoskaya R. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768. [PubMed] [Google Scholar]
- 777.Porter D.D., Dixon F.J., Larsen A.E. The development of a myeloma-like condition in mink with Aleutian disease. Blood. 1965;25:736–742. [PubMed] [Google Scholar]
- 778.Potter M. A resume of the current status of the development of plasma-cell tumors in mice. Cancer Res. 1968;28:1891–1896. [PubMed] [Google Scholar]
- 779.Potter M., Morrison S., Miller F. Induction of plasmacytomas in genetically susceptible mice with silicone gels. Curr Top Microbiol Immunol. 1995;194:83–91. doi: 10.1007/978-3-642-79275-5_11. [DOI] [PubMed] [Google Scholar]
- 780.Bourguet C.C., Grufferman S., Delzell E. Multiple myeloma and family history of cancer. A case–control study. Cancer. 1985;56:2133–2139. doi: 10.1002/1097-0142(19851015)56:8<2133::aid-cncr2820560842>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 781.Cuzick J., De Stavola B. Multiple myeloma-a case-control study. Br J Cancer. 1988;57:516–520. doi: 10.1038/bjc.1988.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Linet M.S., Harlow S.D., McLaughlin J.K. A case-control study of multiple myeloma in whites: chronic antigenic stimulation, occupation, and drug use. Cancer Res. 1987;47:2978–2981. [PubMed] [Google Scholar]
- 783.Ehrensing G., Craig L.E. Intravascular neoplastic cells in canine cutaneous plasmacytomas. J Vet Diagn Invest. 2018;30:329–332. doi: 10.1177/1040638717752781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Burnett R.C., Blake M.K., Thompson L.J. Evolution of a B-cell lymphoma to multiple myeloma after chemotherapy. J Vet Intern Med. 2004;18:768–771. doi: 10.1892/0891-6640(2004)18<768:eoablt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 785.Radhakrishnan A., Risbon R.E., Patel R.T. Progression of a solitary, malignant cutaneous plasma-cell tumour to multiple myeloma in a cat. Vet Comp Oncol. 2004;2:36–42. doi: 10.1111/j.1476-5810.2004.00032.x. [DOI] [PubMed] [Google Scholar]
- 786.Mellor P.J., Haugland S., Smith K.C. Histopathologic, immunohistochemical, and cytologic analysis of feline myeloma–related disorders: further evidence for primary extramedullary development in the cat. Vet Pathol. 2008;45:159–173. doi: 10.1354/vp.45-2-159. [DOI] [PubMed] [Google Scholar]
- 787.Valli V.E.J.R., Parodi A.L. Armed Forces Institute of Pathology and The World health Organization; Washington, DC: 2002. Histological classification of hematopoietic tumors of domestic animals. [Google Scholar]
- 788.Drazner F. Multiple myeloma in the cat. Comp Cont Ed Pract Vet. 1982;4:206–216. [Google Scholar]
- 789.Igase M., Shimokawa Miyama T., Kambayashi S. Bimodal immunoglobulin A gammopathy in a cat with feline myeloma–related disorders. J Vet Med Sci. 2016;78:691–695. doi: 10.1292/jvms.15-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Bienzle D., Silverstein D.C., Chaffin K. Multiple myeloma in cats: variable presentation with different immunoglobulin isotypes in two cats. Vet Pathol. 2000;37:364–369. doi: 10.1354/vp.37-4-364. [DOI] [PubMed] [Google Scholar]
- 791.Facchini R.V., Bertazzolo W., Zuliani D. Detection of biclonal gammopathy by capillary zone electrophoresis in a cat and a dog with plasma cell neoplasia. Vet Clin Pathol. 2010;39:440–446. doi: 10.1111/j.1939-165X.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- 792.Giraudel J.P.J., Guelfi J.F. Monoclonal gammopathie in the dog: a retrospective study of 18 cases (1986–1999) and literature review. J Am Anim Hosp Assoc. 2002;38:135–147. doi: 10.5326/0380135. [DOI] [PubMed] [Google Scholar]
- 793.Jacobs R.M., Couto C.G., Wellman M.L. Biclonal gammopathy in a dog with myeloma and cutaneous lymphoma. Vet Pathol. 1986;23:211–213. doi: 10.1177/030098588602300220. [DOI] [PubMed] [Google Scholar]
- 794.Peterson E.N., Meininger A.C. Immunoglobulin A and immunoglobulin G biclonal gammopathy in a dog with multiple myeloma. J Am Anim Hosp Assoc. 1997;33:45–47. doi: 10.5326/15473317-33-1-45. [DOI] [PubMed] [Google Scholar]
- 795.Ramaiah S.K., Seguin M.A., Carwile H.F. Biclonal gammopathy associated with immunoglobulin A in a dog with multiple myeloma. Vet Clin Pathol. 2002;31:83–89. doi: 10.1111/j.1939-165x.2002.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 796.Souchon F., Koch A., Sohns A. Multiple myeloma with significant multifocal osteolysis in a dog without a detectible gammopathy, Tierarztl Prax Ausg K. Kleintiere Heimtiere. 2013;41:413–420. [PubMed] [Google Scholar]
- 797.MacEwen E.G., Patnaik A.K., Hurvitz A.I. Nonsecretory multiple myeloma in two dogs. J Am Vet Med Assoc. 1984;184:1283–1286. [PubMed] [Google Scholar]
- 798.Seelig D.M., Perry J.A., Avery A.C. Monoclonal gammopathy without hyperglobulinemia in 2 dogs with IgA secretory neoplasms. Vet Clin Pathol. 2010;39:447–453. doi: 10.1111/j.1939-165X.2010.00262.x. [DOI] [PubMed] [Google Scholar]
- 799.Braund K.G., Everett R.M., Bartels J.E. Neurologic complications of IgA multiple myeloma associated with cryoglobulinemia in a dog. J Am Vet Med Assoc. 1979;174:1321–1325. [PubMed] [Google Scholar]
- 800.Hickford F.H., Stokol T., vanGessel Y.A. Monoclonal immunoglobulin G cryoglobulinemia and multiple myeloma in a domestic shorthair cat. J Am Vet Med Assoc. 2000;217:1029–1033. doi: 10.2460/javma.2000.217.1029. [DOI] [PubMed] [Google Scholar]
- 801.Hurvitz A.I., MacEwen E.G., Middaugh C.R. Monoclonal cryoglobulinemia with macroglobulinemia in a dog. J Am Vet Med Assoc. 1977;170:511–513. [PubMed] [Google Scholar]
- 802.Cowgill E.S., Neel J.A., Ruslander D. Light-chain myeloma in a dog. J Vet Intern Med. 2004;18:119–121. doi: 10.1892/0891-6640(2004)18<119:lmiad>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 803.Yamada O., Tamura K., Yagihara H. Light-chain multiple myeloma in a cat. J Vet Diagn Invest. 2007;19:443–447. doi: 10.1177/104063870701900421. [DOI] [PubMed] [Google Scholar]
- 804.Gentilini F., Calzolari C., Buonacucina A. Different biological behaviour of Waldenstrom macroglobulinemia in two dogs. Vet Comp Oncol. 2005;3:87–97. doi: 10.1111/j.1476-5810.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- 805.Hurvitz A.I., Haskins S.C., Fischer C.A. Macroglobulinemia with hyperviscosity syndrome in a dog. J Am Vet Med Assoc. 1970;157:455–460. [PubMed] [Google Scholar]
- 806.Hill R.R., Clatworthy R.H. Macroglobulinaemia in the dog, the canine analogue of gamma M monoclonal gammopathy. J S Afr Vet Med Assoc. 1971;42:309–313. [PubMed] [Google Scholar]
- 807.Anderson K. Plasma cell tumors. In: Holland J., Frei E., Bast R.C., editors. Cancer medicine. ed 3. Lea & Febiger; Philadelphia: 1993. [Google Scholar]
- 808.Salon W., Cassady J.F. Plasma cell neoplasms. In: DeVita V., Hellman S., Rosenberg S.A., editors. Cancer: principles and practice of oncology. ed 5. Lippincott; Philadelphia: 1997. [Google Scholar]
- 809.Shull R.M. Serum hyperviscosity syndrome associated with IgA multiple myeloma in two dogs. J Am Anim Hosp Assoc. 1978;14:58–70. [Google Scholar]
- 810.Center S.A., Smith J.F. Ocular lesions in a dog with serum hyperviscosity secondary to an IgA myeloma. J Am Vet Med Assoc. 1982;181:811–813. [PubMed] [Google Scholar]
- 811.Hendrix D.V., Gelatt K.N., Smith P.J. Ophthalmic disease as the presenting complaint in five dogs with multiple myeloma. J Am Anim Hosp Assoc. 1998;34:121–128. doi: 10.5326/15473317-34-2-121. [DOI] [PubMed] [Google Scholar]
- 812.Kirschner S.E., Niyo Y., Hill B.L. Blindness in a dog with IgA-forming myeloma. J Am Vet Med Assoc. 1988;193:349–350. [PubMed] [Google Scholar]
- 813.Violette N.P., Ledbetter E.C. Punctate retinal hemorrhage and its relation to ocular and systemic disease in dogs: 83 cases. Vet Ophthalmol. 2018;21:233–239. doi: 10.1111/vop.12496. [DOI] [PubMed] [Google Scholar]
- 814.Boyle T., Holowaychuk M.K., Adams A.K. Treatment of three cats with hyperviscosity syndrome and congestive heart failure using plasmapheresis. J Am Anim Hosp Assoc. 2011;47:50–55. doi: 10.5326/JAAHA-MS-5635. [DOI] [PubMed] [Google Scholar]
- 815.Forrester S.D., Greco D.S., Relford R.L. Serum hyperviscosity syndrome associated with multiple myeloma in two cats. J Am Vet Med Assoc. 1992;200:79–82. [PubMed] [Google Scholar]
- 816.Hawkins E.C., Feldman B.F., Blanchard P.C. Immunoglobulin A myeloma in a cat with pleural effusion and serum hyperviscosity. J Am Vet Med Assoc. 1986;188:876–878. [PubMed] [Google Scholar]
- 817.Hribernik T.N., Barta O., Gaunt S.D. Serum hyperviscosity syndrome associated with IgG myeloma in a cat. J Am Vet Med Assoc. 1982;181:169–170. [PubMed] [Google Scholar]
- 818.Williams D., Goldschmidt M.H. Hyperviscosity syndrome with IgM monoclonal gammopathy and hepatic plasmcytoid lymphosarcoma in a cat. J Small Anim Pract. 1982;23:311–323. [Google Scholar]
- 819.Mundy G.R., Bertolini D.R. Bone destruction and hypercalcemia in plasma cell myeloma. Semin Oncol. 1986;13:291–299. [PubMed] [Google Scholar]
- 820.Rosol T.J., Nagode L.A., Couto C.G. Parathyroid hormone (PTH)-related protein, PTH, and 1,25-dihydroxyvitamin D in dogs with cancer–associated hypercalcemia. Endocrinology. 1992;131:1157–1164. doi: 10.1210/endo.131.3.1505457. [DOI] [PubMed] [Google Scholar]
- 821.Sheafor S.E., Gamblin R.M., Couto C.G. Hypercalcemia in two cats with multiple myeloma. J Am Anim Hosp Assoc. 1996;32:503–508. doi: 10.5326/15473317-32-6-503. [DOI] [PubMed] [Google Scholar]
- 822.Twomey J.J. Infections complicating multiple myeloma and chronic lymphocytic leukemia. Arch Intern Med. 1973;132:562–565. [PubMed] [Google Scholar]
- 823.Dunbar M.D., Lyles S. Hemophagocytic syndrome in a cat with multiple myeloma. Vet Clin Pathol. 2013;42:55–60. doi: 10.1111/vcp.12015. [DOI] [PubMed] [Google Scholar]
- 824.Webb J., Chary P., Northrup N. Erythrophagocytic multiple myeloma in a cat. Vet Clin Pathol. 2008;37:302–307. doi: 10.1111/j.1939-165X.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 825.Yearley J.H., Stanton C., Olivry T. Phagocytic plasmacytoma in a dog. Vet Clin Pathol. 2007;36:293–296. doi: 10.1111/j.1939-165x.2007.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 826.Dewhirst M.W., Stamp G.L., Hurvitz A.I. Idiopathic monoclonal (IgA) gammopathy in a dog. J Am Vet Med Assoc. 1977;170:1313–1316. [PubMed] [Google Scholar]
- 827.Hoenig M., O’Brien J.A. A benign hypergammaglobulinemia mimicking plasma cell myeloma. J Am Anim Hosp Assoc. 1988;24:688–690. [Google Scholar]
- 828.Rusbridge C., Wheeler S.J., Lamb C.R. Vertebral plasma cell tumors in 8 dogs. J Vet Intern Med. 1999;13:126–133. doi: 10.1892/0891-6640(1999)013<0126:vpctid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 829.Van Bree H., Pollet L., Cousemont W. Cervical cord compresion as a neurologic complication in an IgG multiple myeloma in a dog. J Am Anim Hosp Assoc. 1983;19:317–323. [Google Scholar]
- 830.DiBartola S.P., Reynolds H.A. Hypoglycemia and polyclonal gammopathy in a dog with plasma cell dyscrasia. J Am Vet Med Assoc. 1982;180:1345–1349. [PubMed] [Google Scholar]
- 831.Villiers E., Dobson J. Multiple myeloma with associated polyneuropathy in a German shepherd dog. J Small Anim Pract. 1998;39:249–251. doi: 10.1111/j.1748-5827.1998.tb03644.x. [DOI] [PubMed] [Google Scholar]
- 832.Mitcham S.A., McGillivray S.R., Haines D.M. Plasma cell sarcoma in a cat. Can Vet J. 1985;26:98–100. [PMC free article] [PubMed] [Google Scholar]
- 833.O’Keefe D.A., Couto C.G. Fine–needle aspiration of the spleen as an aid in the diagnosis of splenomegaly. J Vet Intern Med. 1987;1:102–109. doi: 10.1111/j.1939-1676.1987.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 834.Werner J.A., Woo J.C., Vernau W. Characterization of feline immunoglobulin heavy chain variable region genes for the molecular diagnosis of B–cell neoplasia. Vet Pathol. 2005;42:596–607. doi: 10.1354/vp.42-5-596. [DOI] [PubMed] [Google Scholar]
- 835.Munshi N., Anderon K.C. Plasma cell neoplasms. In: DeVita V., Hellman S., Rosenberg S.A., editors. Cancer: principles and practice of oncology. ed 7. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 836.Breitschwerdt E.B., Woody B.J., Zerbe C.A. Monoclonal gammopathy associated with naturally occurring canine ehrlichiosis. J Vet Intern Med. 1987;1:2–9. doi: 10.1111/j.1939-1676.1987.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 837.Font A., Closa J.M., Mascort J. Monoclonal gammopathy in a dog with visceral leishmaniasis. J Vet Intern Med. 1994;8:233–235. doi: 10.1111/j.1939-1676.1994.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 838.MacEwen E.G., Hurvitz A.I., Hayes A. Hyperviscosity syndrome associated with lymphocytic leukemia in three dogs. J Am Vet Med Assoc. 1977;170:1309–1312. [PubMed] [Google Scholar]
- 839.Matus R.E., Leifer C.E., Hurvitz A.I. Use of plasmapheresis and chemotherapy for treatment of monoclonal gammopathy associated with Ehrlichia canis infection in a dog. J Am Vet Med Assoc. 1987;190:1302–1304. [PubMed] [Google Scholar]
- 840.Fan T.M., Kitchell B.E., Dhaliwal R.S. Hematological toxicity and therapeutic efficacy of lomustine in 20 tumor–bearing cats: critical assessment of a practical dosing regimen. J Am Anim Hosp Assoc. 2002;38:357–363. doi: 10.5326/0380357. [DOI] [PubMed] [Google Scholar]
- 841.Farhangi M., Osserman E.F. The treatment of multiple myeloma. Semin Hematol. 1973;10:149–161. [PubMed] [Google Scholar]
- 842.Lippi I., Perondi F., Ross S.J. Double filtration plasmapheresis in a dog with multiple myeloma and hyperviscosity syndrome. Open Vet J. 2015;5:108–112. [PMC free article] [PubMed] [Google Scholar]
- 843.Bartges J.W. Therapeutic plasmapheresis. Semin Vet Med Surg (Small Anim) 1997;12:170–177. doi: 10.1016/s1096-2867(97)80030-8. [DOI] [PubMed] [Google Scholar]
- 844.Farrow B.R., Penny R. Multiple myeloma in a cat. J Am Vet Med Assoc. 1971;158:606–611. [PubMed] [Google Scholar]
- 845.Anderson K., Ismaila N., Flynn P.J. Role of bone-modifying agents in multiple myeloma: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2018;36:812–818. doi: 10.1200/JCO.2017.76.6402. [DOI] [PubMed] [Google Scholar]
- 846.Mhaskar R., Kumar A., Miladinovic B. Bisphosphonates in multiple myeloma: an updated network meta–analysis. Cochrane Database Syst Rev. 2017;12:CD003188. doi: 10.1002/14651858.CD003188.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Kisseberth W.C., MacEwen E.G., Helfand S.C. Response to liposome–encapsulated doxorubicin (TLC D-99) in a dog with myeloma. J Vet Intern Med. 1995;9:425–428. doi: 10.1111/j.1939-1676.1995.tb03304.x. [DOI] [PubMed] [Google Scholar]
- 848.Ito K., Kobayashi M., Kuroki S. The proteasome inhibitor bortezomib inhibits the growth of canine malignant melanoma cells in vitro and in vivo. Vet J. 2013;198:577–582. doi: 10.1016/j.tvjl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 849.Araujo K.P., Bonuccelli G., Duarte C.N. Bortezomib (PS-341) treatment decreases inflammation and partially rescues the expression of the dystrophin–glycoprotein complex in GRMD dogs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Boostrom B.O., Moore A.S., DeRegis C.J. Canine cutaneous plasmacytosis: 21 cases (2005-2015) J Vet Intern Med. 2017;31:1074–1080. doi: 10.1111/jvim.14729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.McHale B., Blas–Machado U., Oliveira FN. A divergent pseudoglandular configuration of cutaneous plasmacytoma in dogs. J Vet Diagn Invest. 2018;30:260–262. doi: 10.1177/1040638717735868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Mikiewicz M., Otrocka–Domagala I., Pazdzior–Czapula K. Morphology and immunoreactivity of canine and feline extramedullary plasmacytomas. Pol J Vet Sci. 2016;19:345–352. doi: 10.1515/pjvs-2016-0042. [DOI] [PubMed] [Google Scholar]
- 853.Stilwell J.M., Rissi D.R. Immunohistochemical labeling of multiple myeloma oncogene 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine cutaneous histiocytoma. Vet Pathol. 2018;55:517–520. doi: 10.1177/0300985818759770. [DOI] [PubMed] [Google Scholar]
- 854.Baer K.E., Patnaik A.K., Gilbertson S.R. Cutaneous plasmacytomas in dogs: a morphologic and immunohistochemical study. Vet Pathol. 1989;26:216–221. doi: 10.1177/030098588902600305. [DOI] [PubMed] [Google Scholar]
- 855.Clark G., Berg J., Engler S.J. Extramedullary plasmacytomas in dogs: results of surgical excision in 131 cases. J Am Anim Hosp Assoc. 1992;28:105–111. [Google Scholar]
- 856.Kyriazidou A., Brown P.J., Lucke V.M. An immunohistochemical study of canine extramedullary plasma cell tumours. J Comp Pathol. 1989;100:259–266. doi: 10.1016/0021-9975(89)90103-5. [DOI] [PubMed] [Google Scholar]
- 857.Lucke V. Primary cutaneous plasmacytomas in the dog and cat. J Small Anim Pract. 1987;28:49–55. [Google Scholar]
- 858.Platz S.J., Breuer W., Pfleghaar S. Prognostic value of histopathological grading in canine extramedullary plasmacytomas. Vet Pathol. 1999;36:23–27. doi: 10.1354/vp.36-1-23. [DOI] [PubMed] [Google Scholar]
- 859.Rakich P.M., Latimer K.S., Weiss R. Mucocutaneous plasmacytomas in dogs: 75 cases (1980–1987) J Am Vet Med Assoc. 1989;194:803–810. [PubMed] [Google Scholar]
- 860.Rowland P.H., Valentine B.A., Stebbins K.E. Cutaneous plasmacytomas with amyloid in six dogs. Vet Pathol. 1991;28:125–130. doi: 10.1177/030098589102800204. [DOI] [PubMed] [Google Scholar]
- 861.Culp W.T., Ehrhart N., Withrow S.J. Results of surgical excision and evaluation of factors associated with survival time in dogs with lingual neoplasia: 97 cases (1995–2008) J Am Vet Med Assoc. 2013;242:1392–1397. doi: 10.2460/javma.242.10.1392. [DOI] [PubMed] [Google Scholar]
- 862.Sykes S.E., Byfield V., Sullivan L. Feline respiratory extramedullary plasmacytoma with lymph node metastasis and intrahistiocytic amyloid. J Comp Pathol. 2017;156:173–177. doi: 10.1016/j.jcpa.2016.11.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Aoki M., Kim T., Shimada T. A primary hepatic plasma cell tumor in a dog. J Vet Med Sci. 2004;66:445–447. doi: 10.1292/jvms.66.445. [DOI] [PubMed] [Google Scholar]
- 864.Chaffin K., Cross A.R., Allen S.W. Extramedullary plasmacytoma in the trachea of a dog. J Am Vet Med Assoc. 1998;212:1579–1581. [PubMed] [Google Scholar]
- 865.Choi Y.K., Lee J.Y., Kim D.Y. Uterine extramedullary plasmacytoma in a dog. Vet Rec. 2004;154:699–700. doi: 10.1136/vr.154.22.699. [DOI] [PubMed] [Google Scholar]
- 866.Hayes A., Gregory S.P., Murphy S. Solitary extramedullary plasmacytoma of the canine larynx. J Small Anim Pract. 2007;48:288–291. doi: 10.1111/j.1748-5827.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 867.Perlmann E., Dagli M.L., Martins M.C. Extramedullary plasmacytoma of the third eyelid gland in a dog. Vet Ophthalmol. 2009;12:102–105. doi: 10.1111/j.1463-5224.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- 868.Schoniger S., Bridger N., Allenspach K. Sinonasal plasmacytoma in a cat. J Vet Diagn Invest. 2007;19:573–577. doi: 10.1177/104063870701900521. [DOI] [PubMed] [Google Scholar]
- 869.Van Wettere A.J., Linder K.E., Suter S.E. Solitary intracerebral plasmacytoma in a dog: microscopic, immunohistochemical, and molecular features. Vet Pathol. 2009;46:949–951. doi: 10.1354/vp.08-VP-0012-V-BC. [DOI] [PubMed] [Google Scholar]
- 870.Witham A.I., French A.F., Hill K.E. Extramedullary laryngeal plasmacytoma in a dog. N Z Vet J. 2012;60:61–64. doi: 10.1080/00480169.2011.621876. [DOI] [PubMed] [Google Scholar]
- 871.Smithson C.W., Smith M.M., Tappe J. Multicentric oral plasmacytoma in 3 dogs. J Vet Dent. 2012;29:96–110. doi: 10.1177/089875641202900205. [DOI] [PubMed] [Google Scholar]
- 872.Fukumoto S., Hanazono K., Kawasaki N. Anaplastic atypical myeloma with extensive cutaneous involvement in a dog. J Vet Med Sci. 2012;74:111–115. doi: 10.1292/jvms.11-0243. [DOI] [PubMed] [Google Scholar]
- 873.Mayer M.N., Kerr M.E., Grier C.K. Immunoglobulin A multiple myeloma with cutaneous involvement in a dog. Can Vet J. 2008;49:694–702. [PMC free article] [PubMed] [Google Scholar]
- 874.Atherton M.J., Vazquez–Sanmartin S., Sharpe S. A metastatic secretory gastric plasmacytoma with aberrant CD3 expression in a dog. Vet Clin Pathol. 2017;46:520–525. doi: 10.1111/vcp.12503. [DOI] [PubMed] [Google Scholar]
- 875.Hamilton T.A., Carpenter J.L. Esophageal plasmacytoma in a dog. J Am Vet Med Assoc. 1994;204:1210–1211. [PubMed] [Google Scholar]
- 876.MacEwen E.G., Patnaik A.K., Johnson G.F. Extramedullary plasmacytoma of the gastrointestinal tract in two dogs. J Am Vet Med Assoc. 1984;184:1396–1398. [PubMed] [Google Scholar]
- 877.Jackson M.W., Helfand S.C., Smedes S.L. Primary IgG secreting plasma cell tumor in the gastrointestinal tract of a dog. J Am Vet Med Assoc. 1994;204:404–406. [PubMed] [Google Scholar]
- 878.Bellezza E., Bianchini E., Pettinelli S. Intestinal plasmacytoma causing colocolic double intussusception in an adult dog. J Small Anim Pract. 2016;57:718. doi: 10.1111/jsap.12599. [DOI] [PubMed] [Google Scholar]
- 879.kupanoff P., Popovitch C.A., Goldschmidt M.H. Colorectal plasmacytomas: a retrospective study of nine dogs. J Am Anim Hosp Assoc. 2006;42:37–43. doi: 10.5326/0420037. [DOI] [PubMed] [Google Scholar]
- 880.Trevor P.B., Saunders G.K., Waldron D.R. Metastatic extramedullary plasmacytoma of the colon and rectum in a dog. J Am Vet Med Assoc. 1993;203:406–409. [PubMed] [Google Scholar]
- 881.Meis J.M., Butler J.J., Osborne B.M. Solitary plasmacytomas of bone and extramedullary plasmacytomas. A clinicopathologic and immunohistochemical study. Cancer. 1987;59:1475–1485. doi: 10.1002/1097-0142(19870415)59:8<1475::aid-cncr2820590814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 882.Breuer W., Colbatzky F., Platz S. Immunoglobulin-producing tumours in dogs and cats. J Comp Pathol. 1993;109:203–216. doi: 10.1016/s0021-9975(08)80246-0. [DOI] [PubMed] [Google Scholar]
- 883.Kyriazidou A., Brown P.J., Lucke V.M. Immunohistochemical staining of neoplastic and inflammatory plasma cell lesions in feline tissues. J Comp Pathol. 1989;100:337–341. doi: 10.1016/0021-9975(89)90113-8. [DOI] [PubMed] [Google Scholar]
- 884.Majzoub M., Breuer W., Platz S.J. Histopathologic and immunophenotypic characterization of extramedullary plasmacytomas in nine cats. Vet Pathol. 2003;40:249–253. doi: 10.1354/vp.40-3-249. [DOI] [PubMed] [Google Scholar]
- 885.Michau T.M., Proulx D.R., Rushton S.D. Intraocular extramedullary plasmacytoma in a cat. Vet Ophthalmol. 2003;6:177–181. doi: 10.1046/j.1463-5224.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- 886.Carothers M.A., Johnson G.C., DiBartola S.P. Extramedullary plasmacytoma and immunoglobulin-associated amyloidosis in a cat. J Am Vet Med Assoc. 1989;195:1593–1597. [PubMed] [Google Scholar]
- 887.Brunnert S.R., Altman N.H. Identification of immunoglobulin light chains in canine extramedullary plasmacytomas by thioflavine T and immunohistochemistry. J Vet Diagn Invest. 1991;3:245–251. doi: 10.1177/104063879100300312. [DOI] [PubMed] [Google Scholar]
- 888.Fernandez N.J., West K.H., Jackson M.L. Immunohistochemical and histochemical stains for differentiating canine cutaneous round cell tumors. Vet Pathol. 2005;42:437–445. doi: 10.1354/vp.42-4-437. [DOI] [PubMed] [Google Scholar]
- 889.Ramos–Vara J.A., Miller M.A., Valli V.E. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: comparison with CD79a and CD20. Vet Pathol. 2007;44:875–884. doi: 10.1354/vp.44-6-875. [DOI] [PubMed] [Google Scholar]
- 890.Marcos R., Canadas A., Leite-Martins L. What is your diagnosis? Cutaneous nodules and atypical blood cells in a dog. Vet Clin Pathol. 2018 doi: 10.1111/vcp.12586. [DOI] [PubMed] [Google Scholar]
- 891.Rout E.D., Shank A.M., Waite A.H. Progression of cutaneous plasmacytoma to plasma cell leukemia in a dog. Vet Clin Pathol. 2017;46:77–84. doi: 10.1111/vcp.12463. [DOI] [PubMed] [Google Scholar]
- 892.Lee A.R., Lee M.S., Jung I.S. Imaging diagnosis-FDG-PET/CT of a canine splenic plasma cell tumor. Vet Radiol Ultrasound. 2010;51:145–147. doi: 10.1111/j.1740-8261.2009.01639.x. [DOI] [PubMed] [Google Scholar]
- 893.Wright Z.M., Rogers K.S., Mansell J. Survival data for canine oral extramedullary plasmacytoma: a retrospectve analysis (1996–2006) J Am Anim Hosp Assoc. 2008;44:75–81. doi: 10.5326/0440075. [DOI] [PubMed] [Google Scholar]
- 894.Ware K., Gieger T. Use of strontium-90 plesiotherapy for the treatment of a lingual plasmacytoma in a dog. J Small Anim Pract. 2011;52:220–223. doi: 10.1111/j.1748-5827.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 895.Mellor P.J., Polton G.A., Brearley M. Solitary plasmacytoma of bone in two successfully treated cats. J Feline Med Surg. 2007;9:72–77. doi: 10.1016/j.jfms.2006.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Zikes C.D., Spielman B., Shapiro W. Gastric extramedullary plasmacytoma in a cat. J Vet Intern Med. 1998;12:381–383. doi: 10.1111/j.1939-1676.1998.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 897.Frazier K.S., Hines M.E., 2nd, Hurvitz A.I. Analysis of DNA aneuploidy and c-myc oncoprotein content of canine plasma cell tumors using flow cytometry. Vet Pathol. 1993;30:505–511. doi: 10.1177/030098589303000603. [DOI] [PubMed] [Google Scholar]


