Abstract
Mechanical and microstructural evaluations of cortical bone using ultrashort echo time magnetic resonance imaging (UTE-MRI) have been performed increasingly in recent years. UTE-MRI acquires considerable signal from cortical bone and enables quantitative bone evaluations. Fitting bone apparent transverse magnetization (T2*) decay using a bicomponent model has been regularly performed to estimate bound water (BW) and pore water (PW) in the quantification of bone matrix and porosity, respectively. Human cortical bone possesses a considerable amount of fat, which appears as MRI T2* signal oscillation and can subsequently lead to BW overestimation when using a bicomponent model. Tricomponent T2* fitting model has been developed to improve BW and PW estimations by accounting for fat contribution in the MRI signal. This study aimed to investigate the correlations of microstructural and mechanical properties of human cortical bone with water pool fractions obtained from a tricomponent T2* model. 135 cortical bone strips (~4×2×40 mm3) from tibial and femoral midshafts of 37 donors (61±24 years old) were scanned using ten sets of dual-echo 3D-UTE-Cones sequences (TE=0.032–24.0 ms) on a 3T MRI scanner for T2* fitting analyses. Average bone porosity and pore size were measured using microcomputed tomography (μCT) at 9 μm voxel size. Bone mechanical properties were measured using 4-point bending tests. Using a tricomponent model, bound water fraction (FracBW) showed significant strong (R=0.70, P<0.01) and moderate (R=0.58–0.62, P<0.01) correlations with porosity and mechanical properties, respectively. Correlations of bone microstructural and mechanical properties with water pool fractions were higher for tricomponent model results compared with the bicomponent model. The tricomponent T2* fitting model is suggested as a useful technique for cortical bone evaluation where the MRI contribution of bone fat is accounted for.
Keywords: cortical bone, MRI, ultrashort echo time, fat, bone mechanics, T2* fitting model
Graphical Abstract

Tricomponent UTE-MRI T2* fitting model has been investigated for its correlations with microstructural and mechanical properties of human cortical bone specimens. Using tricomponent model, bound water fraction (FracBW) showed significant strong (R=0.70, P<0.01) and moderate (R=0.58–0.62, P<0.01) correlations with porosity and mechanical properties, respectively.
Introduction
Cortical bone assessment in patients has been based historically on evaluating the bone mineral density (BMD) by x-ray based techniques such as dual-energy X-ray absorptiometry (DEXA), quantitative computed tomography (QCT), and high-resolution peripheral quantitative computed tomography (HR-pQCT)1,2.
Recently, magnetic resonance imaging (MRI)-based assessment of cortical bone has received a great deal of attention because it enables investigation of the bone water content as a surrogate for bone microstructural properties and avoids potential harms associated with x-ray-based techniques 1,3–5. Employing MRI techniques to evaluate bone may also provide excellent assessment of the surrounding soft tissue, a property which is not available in x-ray-based techniques. However, clinical MRI is not able to detect considerable signal of cortical bone because of bone’s very short apparent transverse relaxation time (T2*). From recently advanced MRI techniques, ultrashort echo time (UTE) MRI can image cortical bone, thus allowing for quantitative assessment of cortical bone 3,4,6–11. Using UTE-MRI techniques, signal can be acquired quickly (tens of microseconds) after radiofrequency (RF) excitation and before a major decay in transverse magnetization.
Most water in cortical bone exists as bound water (BW), either loosely bound to the organic matrix or tightly bound to mineral; however, a smaller fraction of water exists as pore water (PW), which resides in the microscopic pores of cortical bone 3,4,12. The BW pool provides information on bone matrix density. Higher BW content and potentially lower T2* likely indicate a more dense bone with higher mechanical properties. On the other hand, the PW pool provides information on intracortical bone porosity. Higher PW content and potentially higher T2* indicate higher bone porosity and lower mechanical properties. It is of critical importance to distinguish BW and PW components using quantitative UTE-MRI analysis in order to provide complementary information about bone microstructure and mechanics 12,13.
T2* of BW is about ten times shorter than that of PW; thus, BW and PW can be separated using UTE acquisitions combined with bicomponent T2* analysis 3,6,12–14. Bicomponent exponential T2* fitting has been investigated extensively as a method to separate BW and PW in order to quantify matrix density and porosity, respectively 3,6,12–15. Bae et al. 13 and Seifert et al. 15 reported significant correlations of BW and PW fractions from bicomponent T2* analysis with human cortical bone porosity measured with micro-computed tomography (μCT). Bae et al. also reported moderately significant correlations between bicomponent T2* results and mechanical properties of human cortical bone strips 13. Recently, the upper limit of UTE-MRI bicomponent T2* analysis and its efficacy were investigated through comparisons with histomorphometric measures of bone porosity. Bicomponent T2* was found capable of detecting bone porosities comprised of pores below the range detectable by μCT 6.
Human cortical bone may contain a considerable amount of fat 16–18, particularly in the regions near the endosteum where larger pores can be found. Fat in the pores of cortical bone likely has similar properties and roles as the fat of bone marrow located in the endosteum. It is believed that adipocytes secrete cytokines and adipokines which either stimulate or inhibit osteoblastic activities in nearby bone sites 17,18. This could simply suggest that the greater the formation of fat cells, the lower the bone remodeling rate. It should be noted that bone fat is rare in neonates and accumulates steadily with age 17. Average signal oscillation of the multi-echo MRI in T2 fitting analyses has been observed by several studies 8,15,19,20, and has been explained as a phenomenon most likely due to the chemical shift caused by the fat in human cortical bone 20. Lu et al. 8 recently proposed a tricomponent fitting model that incorporates a modeled fat NMR spectrum 21,22, enabling improved estimation of BW and PW fractions in cortical bone. This tricomponent T2* fitting model has been examined by scanning entire cross-sections of eight tibial cortical bone using a clinical knee coil on a clinical 3T scanner. Estimated water fractions by the tricomponent T2* fitting demonstrated improved correlations with μCT-based porosity compared with bicomponent analyses 8. It is hypothesized that providing higher correlations with bone porosity by tricomponent T2* analysis can also lead to an improved correlation with bone mechanical properties. However, the relationship between tricomponent T2* analyses and mechanical properties of cortical bone has not been investigated.
The goal of this study was to investigate the correlations between water fractions obtained from tricomponent T2* analysis in human cortical bone with microstructural and mechanical properties. Bone specimens were scanned using the UTE-MRI technique, and the results were analyzed using bicomponent and tricomponent models to be compared with microstructural and mechanical properties. This study highlights the potential applications of tricomponent T2* model for detecting the mechanical properties of cortical bone in the human skeleton.
2. Materials and methods
2.1. Sample preparation
A total of 135 cortical bone specimens was harvested from the tibial and femoral midshafts of 37 donors (61±24 years old, 52 specimens from 19 females, 83 specimens from 18 males) provided by a non-profit whole-body donation company (United Tissue Network, Phoenix, AZ). The middle part of the tibial and femoral shafts was cut into 40 mm segments using a commercial band saw. 1–3 rectangular bone strips were excised from each specimen using a low-speed diamond saw (Isomet 1000, Buehler, IL). The specimens were harvested mainly from anterior and posterior regions of the tibial and femoral bone cross-sections where the bone was thicker, enabling more accurate MRI measurements. The final dimensions of the specimens were approximately 4mm×2mm×40mm. Specimens were immersed in phosphate-buffered saline (PBS) overnight at room temperature before the MRI scans in order to compensate for potential dehydration occurring throughout specimen preparation. Next, specimens were randomly distributed (to avoid any bias due to arrangements of samples) into eight groups and placed in 30-mL syringes (15–20 specimens per syringe) filled with perfluoropolyether (Fomblin, Ausimont, Thorofare, NJ) to minimize dehydration and susceptibility artifacts. It should be noted that each specimen number was labeled with paper tape at one end of the specimen to enable accurate identification of the bone specimens.
2.2. UTE-MR protocol
The UTE-MRI scans were performed on a 3T clinical scanner (GE Healthcare, Waukesha, WI) using a homemade 1-inch diameter transmit/receive birdcage coil. The UTE-MRI scans involved ten sets of dual-echo 3D-UTE-Cones sequences (repetition time (TR) = 28 ms; flip angle (FA) = 10˚; echo times (TEs) = [0.032, 4.2], [0.2, 4.8], [0.4, 5.6], [0.6, 6.4], [0.8, 7.2], [1.2, 8.0], [1.8, 10.0], [2.2, 12.0] [2.4, 18.0], and [3.2, 24] ms; rectangular RF excitation pulse with a duration of 26 μs; cones stretching factor = 1) with a total scan time of 35 minutes for bicomponent and tricomponent T2* decay analyses. Other imaging parameters included: field of view (FOV) = 40 mm, matrix size = 160×160, in-plane pixel size = 0.25 mm, slice thickness = 2 mm, receiver bandwidth = ±62.5 kHz. Features of the 3D-UTE-Cones sequence have been described in previous studies 23–25.
2.3. Bicomponent and tricomponent T2* signal model
In the bicomponent fitting model, a short T2* component and a long T2* component were assumed to represent BW protons and PW protons, respectively. UTE-MRI T2* can be given by Eq.1 using bicomponent fitting model
| Eq.1 |
where is the normalized UTE-MR signal, Frac BW and Frac PW are the fractions of the BW and PW protons, respectively, and noise is white Gaussian noise. To normalize the UTE-MRI signal, all images were sorted in an ascending TE order and then all signal values of each voxel at different TEs were divided by the signal value of the same voxel at TE = 0.032 ms.
The recently proposed tricomponent fitting model 8 considers a fat component in addition to the water components. Specifically, multi-spectral lipid peaks in bone were considered in the tricomponent model, as shown in Eq.2
| Eq.2 |
where is the relative amplitude of the nth spectral peak of fat, is the corresponding multi-spectral peak frequency shift, and Frac Fat is fat fraction. Based on Ren’s study, no significant difference was assumed between the fat in bone marrow and the fat available in other adipose tissues 26. Therefore, a 9-peak fat model was adopted in the presented tricomponent fitting model 21,22.
The bi- and tricomponent fitting analyses were performed in MATLAB (The Mathworks Inc., Natick, MA, USA) based on a least square curve fitting algorithm and executed offline on MRI magnitude images in DICOM format. The initial fraction values were set to 0.33, while initial T2*Fat, T2*BW, and T2*PW were set to 5, 0.3, and 10 ms, respectively. After selecting one of the slices in the middle of each bone specimen, a region of interest (ROI) was selected to cover the whole bone cross-section (4mm×2mm). Average MRI signal intensity within each ROI was used for subsequent curve fitting. It should be noted that the mechanical failure via 4-point bending tests on the scanned specimens would be expected to occur near the middle of the specimens, where the applied mechanical stresses are maximum.
2.4. Micro-computed tomography (μCT)
Bone specimens were scanned using a Skyscan 1076 (Kontich, Belgium) μCT scanner at 9 μm isotropic voxel size. Other scanning parameters were as follows: a 0.05 mm aluminum filter in addition to a 0.038 mm copper filter, 100 kV, 100 mA, 0.3˚ rotation step, and 5 frame-averaging. The total μCT scan time was twelve hours for all specimens.
The μCT image segmentation was performed though gray level thresholding. The gray level threshold was selected for each set of μCT data using the gray level histograms and visual investigation of the bone-pore interface in raw μCT images. Microstructural properties of each bone strip was calculated in 200 μCT slices at the middle of the bone strips. The stack of μCT images corresponded to the 2mm slices of MRI images. Bone porosity was estimated as the ratio of number of voxels in pores to the total number of voxels. Pore size at each voxel in pores was defined as the diameter of the largest covering sphere, which is a standard definition for semispherical or ellipsoidal pores in the literature 27–29.
2.5. Mechanical properties measurement
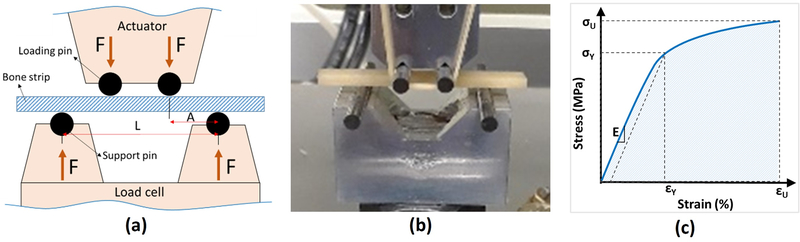
Tensile mechanical properties of the bone strips were measured using a 4-point bending set up 30. It is assumed that the tensile side of the specimens fail in brittle specimens under bending because of lower tensile properties compared with compressive properties in such materials. The schematics of and actual assembled 4-point bending setup are shown in Figures 1a and b, respectively. The 4-point bending setup was comprised of four tungsten carbide pins (3-mm diameter) mounted on two aluminum holders. The upper holder was connected to the hydraulic actuator of a mechanical testing machine (model 8511.20, Instron, Norwood, MA, USA), while the lower aluminum holder was connected to a 4500 N load cell (Sensotec 1000 LBS). Bone strips were positioned on the lower pins, and contact between loading pins and bone specimens was achieved by manually lowering the actuator. A mechanical test was performed in displacement-controlled mode for 30 to 60 seconds at 0.1 mm/s until specimen failure. Simultaneously, total force was continuously recorded.
Figure 1:
(a) Schematics of the four-point bending jigs at the longitudinal cross-section (pin diameter=3 mm, L=24 mm, A=8 mm, bone strip thickness=approx. 2 mm). The experiments were displacement-controlled at 0.1 mm/s rate when the force was recorded. (b) Prepared bone cortical bone strips (approx. 40 mm length) mounted on the fabricated four-point bending jigs (aluminum seats and tungsten carbide pins) mounted on an Instron 8511.20 machine. (c) Schematics stress–strain curve for calculating the mechanical properties such as Young’s modulus (E), yield stress (σY), yield strain (εY), ultimate stress (σU), ultimate strain (εU), and failure energy (Wf).
Stress-strain (σ-ε) relation on the beam’s surface between loading pins (middle of the beam length) was determined from the measured force and displacement data, as well as from the accurate μCT-based specimen dimensions. It should be noted that the beam surface experiences the highest mechanical stress in bending. Using the estimated stresses at the surface of the beam based on standard approaches ( where σ, M, and I, are stress, the force moment at the middle of the beam, and second moment of area of the beam’s cross-section, respectively) 31 was not appropriate for porous cortical bone specimens 32,33.
Routine 4-point bending stress calculations leads to dramatic overestimations of mechanical properties for bone specimens with relatively large pores (pore/thickness >15) 34, as described in ASTM C1674 for ceramic beams with engineered porosity 33. ASTM C1674 was used to modify the estimated stresses after measuring the effective second moment of area (i.e., I) of the beam’s cross-section using μCT data. To consider size dependency of mechanical properties in cortical bone specimens (brittle material), a Weibull modulus 35,36 of four was considered. It should be noted that the harvested bone specimens for old donors were thinner than specimens for younger donors. The selected Weibull modulus was in the range of reported values for cortical bone in the literature 37–39.
Modified stresses were used in determining the stress-strain curves (Figure 1c). Young’s modulus of elasticity (E) was determined from a straight line fit to initial part of the stress–strain curve. A yield point was defined at a point on the curve where the curve deviated by a strain of 0.002 from the linear part of the curve described by the Young’s modulus 30. The yield point was used to determine both yield stress (σY) and yield strain (εY). The maximum stress and its corresponding strain were assigned to the ultimate stress (σU) and ultimate strain (εU), respectively. The failure energy or work to failure (WF) was defined as the area below the stress-strain curve.
2.6. Statistical analyses
Student t-test was used to compare the water proton pool fractions and their T2* values between bicomponent and tricomponent fitting models. Pearson’s correlation coefficients were calculated between UTE-MRI T2* fitting results (bicomponent and tricomponent models) and μCT-based measures (porosity and pore size), as well as the mechanical properties (young modulus, yield stress, ultimate stress and failure energy). Correlations with P-values below 0.05 were considered significant. All measurements and models were performed using MATLAB (version 2017, The Mathworks Inc., Natick, MA, USA) codes developed in-house.
3. Results
Figure 2a shows the UTE-MRI image (TE=0.032 ms) of a set of bone specimens in the 30-ml syringe scanned in axial plane, showing the 4mm x 2mm cross section of samples. Samples I and II indicated with yellow boxes were harvested from a 47-year-old male and a 57-year-old female, respectively. Figures 2b and 2c show the μCT images of samples I and II with 15% and 33% average porosities, respectively. Bicomponent fitting analysis of T2* signal decay along differing TEs from 0 to 24ms are demonstrated in Figures 2d and 2e for samples I and II, respectively. Tricomponent fitting analysis are shown in Figures 2f and 2g for samples I and II, respectively. The bone specimen from the female donor (sample II) showed a large oscillating signal initiating around TE=2ms. The signal oscillating phenomenon was negligible for the bone specimen from the male donor with lower porosity (sample I). The tricomponent fitting model fitted the oscillating signal decay better and presented a higher R2 value. The tricomponent fitting model resulted in higher fat fraction in the specimen from the female donor with higher porosity (Figure 2g).
Figure 2:
UTE-MRI image and μCT images of two representative cortical bone strips harvested from different donors possessing different levels of porosities, in addition to bicomponent and tricomponent T2* fitting results. (a) UTE-MRI (TE=0.032ms) image of a set of cortical bone strips with 4×2 mm2 cross-sections on average soaked in fomblin, which has no signal in MRI. (b, c) μCT images of representative cortical bone strips from a 47-year-old male and 57-year-old female, respectively. (d, e) Bicomponent T2* fittings for the bone strips shown in (b) and (c), respectively. (f, g) Tricomponent T2* fitting for bone strips shown in (a) and (b), respectively. The oscillating signal decay in cortical bone specimens was fitted better by including the signal contribution of fat using tricomponent model.
To determine the level of system variations during the scanning process, one human bone specimen was scanned seven times using one dual echo UTE sequence (TE=0 and 4.4ms), with the same setup as described above in the Methods section. The average signal variation and frequency shifts in the bone region are presented in Supplemental Table 1. The maximum frequency shift during scans in the bone region was below 2Hz. The phase and the frequency shift maps are shown in Supplemental Figures 1 and 2, respectively.
Average, standard deviation, and range of all calculated proton pool fractions and T2* values in addition to goodness of fittings obtained from bicomponent and tricomponent T2* models are presented in Table 1. R2 fitting was significantly higher in the tricomponent fitting model. FracBW was significantly lower when the tricomponent fitting model was used. The tricomponent model resulted in significantly lower pore water T2* values. Fitting models did not show significantly different results when measuring T2*BW and FracPW values.
Table 1:
Average, standard deviation (SD), and range of the measured parameters from bicomponent and tricomponent T2* fitting in the studied cortical bone specimens. The significance level of the differences were calculated t student test.
| Bicomponent model | |||||||
|---|---|---|---|---|---|---|---|
| Frac BW | T2* BW | Frac PW | T2* PW | R2-fitting | |||
| Ave±SD | 73.9±7.2 | 0.26±0.04 | 26.1±7.2 | 4.93±1.45 | 0.9798±0.0238 | ||
| [range] | [55.0, 88.8] | [0.18, 0.42] | [11.2, 45.0] | [1.75, 10.38] | [0.9112,0.9990] | ||
| Tricomponent model | |||||||
| Frac BW | T2* BW | Frac PW | T2* PW | Frac Fat | T2* Fat | R2-fitting | |
| Ave±SD | 59.6±11.3 | 0.21±0.06 | 28.3±7.2 | 4.04±1.43 | 12.1±6.2 | 5.46±5.43 | 0.9966±0.0045 |
| [range] | [32.8, 84.7] | [0.10, 0.43] | [12.1, 45.8] | [1.77, 12.29] | [0.8, 24.6] | [1.27, 29.98] | [0.9811,0.9998] |
|
p-value t-test |
<0.01 | 0.17 | 0.11 | <0.01 | <0.01 | ||
Pearson’s correlation coefficients and statistical significance of bicomponent and tricomponent T2* fitting results with μCT measures and with mechanical properties are presented in Table 2. From bicomponent model results, FracBW and FracPW showed significantly moderate correlations with bone porosity and pore size (R=0.61–0.65, P<0.01). These fractions also demonstrated moderate correlations with young modulus, yield stress, and ultimate stress from mechanical properties (R=0.52–0.54, P<0.01). T2* value correlations with μCT-based microstructural and mechanical measures were low, but significant (R<0.5, P<0.01). Correlations between T2* values and mechanical properties were lower than correlations between T2* values and microstructural properties. From the tricomponent model results, FracBW showed significantly strong correlation with bone porosity (R=0.70, P<0.01). Pore size correlation with tricomponent FracBW was moderate, similar to bicomponent FracBW results. FracBW from the tricomponent model also demonstrated moderate correlation with young modulus, yield stress, and ultimate stress from mechanical properties in a level higher than the correlations presented by bicomponent analysis (R=0.58–0.62, P<0.01). The correlation levels of FracPW and FracFat with microstructural and mechanical properties were at levels similar to those demonstrated by fractions from the bicomponent model. T2*BW from the tricomponent model showed significant moderate correlations with bone porosity; however, its correlations with pore size and mechanical properties were low, albeit significant. T2*Fat did not show significant correlations with bone microstructure; however, its correlation with mechanical properties was low, but significant.
Table 2:
Pearson’s correlation coefficients of UTE-MRI bicomponent and tricomponent T2* results with microstructural and mechanical properties of cortical bone strips
| μCT | 4-point bending test | ||||||
|---|---|---|---|---|---|---|---|
| Porosity | Pore size | Young modulus | Yield stress | Ultimate stress | Failure energy | ||
| Bicomponent model | Frac BW | −0.65 P<0.01 |
−0.61 P<0.01 |
0.52 P<0.01 |
0.52 P<0.01 |
0.54 P<0.01 |
0.44 P<0.01 |
| T2* BW | −0.47 P<0.01 |
−0.34 P<0.01 |
0.14 P=0.11 |
0.15 P=0.08 |
0.18 P=0.04 |
0.20 P=0.02 |
|
| Frac PW | 0.65 P<0.01 |
0.61 P<0.01 |
−0.52 P<0.01 |
−0.52 P<0.01 |
−0.54 P<0.01 |
−0.44 P<0.01 |
|
| T2* PW | 0.42 P<0.01 |
0.35 P<0.01 |
−0.13 P=0.12 |
−0.19 P=0.03 |
−0.20 P=0.02 |
−0.12 P=0.48 |
|
| Tricomponent model | Frac Fat | 0.55 P<0.01 |
0.41 P<0.01 |
−0.41 P<0.01 |
−0.47 P<0.01 |
−0.46 P<0.01 |
−0.33 P<0.01 |
| T2* Fat | −0.14 P=0.11 |
−0.10 P=0.26 |
0.25 P<0.01 |
0.28 P<0.01 |
0.27 P<0.01 |
0.19 P<0.01 |
|
| Frac BW | −0.70 P<0.01 |
−0.61 P<0.01 |
0.58 P<0.01 |
0.61 P<0.01 |
0.62 P<0.01 |
0.48 P<0.01 |
|
| T2* BW | −0.58 P<0.01 |
−0.40 P<0.01 |
0.32 P<0.01 |
0.33 P<0.01 |
0.35 P<0.01 |
0.31 P<0.01 |
|
| Frac PW | 0.62 P<0.01 |
0.60 P<0.01 |
−0.55 P<0.01 |
−0.55 P<0.01 |
−0.57 P<0.01 |
−0.47 P<0.01 |
|
| T2* PW | 0.35 P<0.01 |
0.29 P<0.01 |
−0.04 P=65 |
−0.05 P=0.56 |
−0.05 P=0.53 |
0.00 P=0.27 |
|
Figures 3a and 3b demonstrate the scatter plots and linear regressions of μCT-based bone porosity on FracBW from bicomponent and tricomponent models, respectively. Figures 3c, e, g, and i, illustrate the scatter plots and linear regressions of Young modulus, yield stress, ultimate stress, and failure energy on FracBW from the bicomponent model. Scatter plots and linear regressions of the same mechanical properties on FracBW from the tricomponent model are depicted in Figures 3d, f, h, and j, respectively. As mentioned in Table 2, higher correlations coefficients were obtained using the tricomponent model. Obviously, FracBW in tricomponent analysis was lower compared with bicomponent analysis.
Figure 3:
Scatter plots and linear regression analyses with significant correlations (p<0.01) of microstructural and mechanical properties on bound water fraction (FracBW) estimated from bicomponent and tricomponent T2* fitting models. (a) μCT-porosity, (c) Young modulus, (e) yield stress, (g) ultimate stress, and (i) failure energy versus FracBW from bicomponent fitting model. (b) μCT-porosity, (d) Young modulus, (f) yield stress, (h) ultimate stress, and (j) failure energy versus FracBW from tricomponent fitting model. Estimated correlation coefficients from tricomponent model were higher compared with bicomponent model.
4. Discussion
This study examined the correlations of a recently developed tricomponent T2* fitting model with microstructural and mechanical properties of human cortical bone strips, where the results were compared with the widely used bicomponent T2* fitting model. UTE-MRI-based techniques for bone assessment 7,40–44 are considered non-invasive, x-ray-free, and, importantly, in vivo-translatable techniques. Moreover, such MRI-based bone evaluation may provide excellent assessment of the surrounding soft tissue, which can improve diagnosis.
The implemented tricomponent model for T2* model took the chemical shifts from fat to water protons into consideration. Chemical shifts result in the oscillation of the UTE-MRI signal with differing TEs 8,15,19,20. Such signal oscillations were well-fitted using the proposed tricomponent model, whereas the bicomponent model lacked accuracy (Table 1). It was hypothesized that considering the fat chemical shift in UTE-MRI signal would improve the estimations of water fractions, which in turn could predict the microstructural and mechanical properties of human cortical bone with higher accuracy. Bone microstructural and mechanical properties of the studied bone specimens presented higher correlations with the tricomponent model results compared with the bicomponent model (Table 2, Figure 3). Specifically, using the tricomponent model, the summation of FracPW and FracFat (100-FracBW) showed a strong correlation with bone porosity, versus moderate correlations with pore size and bone biomechanics. No strong correlations were found between bicomponent T2* fitting results and bone microstructural or mechanical properties. Pore size correlations with MRI properties were similar using both bi- and tricomponent fitting models. The presented higher correlations of the tricomponent model suggest that the tricomponent model provides a more accurate estimation of the bone water proton pool fractions compared with the bicomponent model. BW fraction in cortical bone is assumed to represent the bone matrix (FracBW=100 - FracPW - FracFat) and is expected to decrease in regions of cortical bone with higher porosity and lower mechanical properties. It should be noted that the studied bone specimens were not harvested from the same human long bone site that enables investigating the performance of UTE-MRI technique at different bone sites (e.g., anterior and posterior tibial and femoral bone).
BW fraction in tricomponent analysis was lower than the bicomponent model results. As explained in an earlier study using MRI signal simulations, the MRI T2* signal in cortical bone drops significantly at ultrashort echo times (0–1 ms), which becomes sharper for bone with oscillating T2* signal caused by fat chemical shift. In the bicomponent fitting model, this sharp signal drop is interpreted as the contribution of the BW protons, resulting in an overestimated BW fraction 8. FracBW overestimation in bicomponent fitting is inevitable, even with optimized TE selection. Tricomponent fitting can likely model fat and PW oscillation accurately and, thusly, provide correct fat and PW proton fractions, respectively. In the bicomponent fitting model, fat and PW are considered as one single component (i.e., the long T2* component), with BW being considered the other component (i.e., the short T2* component). Fat and PW oscillations lead to signal cancellation, thereby producing a reduced long T2* fraction with contribution from both fat and PW, as well as an increased short T2* fraction from BW.
The MRI-based estimations of water pools have received a great deal of attention for their potential correlations with mechanical properties. The majority of such studies were performed at high magnetic field strengths. Fernandez et al. 45 found negative correlations between bone mechanical properties and total water content in whole cross-sections of long bones measured by NMR spectroscopy at 9.4T magnetic field (n=11, R=0.72–0.77). Horch et al. and Nyman et al. 46,47 demonstrated positive significant correlations between estimated BW pool from NMR spectroscopy at 4.7T magnetic field and cortical bone mechanical properties in bone strips (n=18, R=0.60, and n=40, R=0.82). They also showed significant negative correlations between PW pool and mechanical properties (n=18, R=0.45 and n=40, R=0.78). Later, Horch et al. 48 used UTE-MRI at 4.7T magnetic field for direct imaging of BW and PW, and reported significant correlations with mechanical properties of bone strips (n=14, R=0.68–0.83). Granke et al. 49 investigated the correlations between bone NMR spectroscopy results of PW and BW peaks at 4.7T magnetic field with human bone fracture toughness. They found significant correlations between NMR-derived BW fraction and fracture toughness properties of cortical bone strips (n=62, R=0.63). In general, the reported correlation coefficients of water pools with bone mechanical properties were slightly higher in this group of studies compared with our study, likely because of the higher obtainable SNR at higher magnetic field strengths. For the first time, Bae et al. 13 presented significant correlations between bicomponent T2* fitting results performed at 3T magnetic field and the mechanical properties of human cortical bone strips (n=44, R=0.54). Their reported correlation coefficients were similar to our correlations using bicomponent T2* modeling. Later, Manhard et al. 50 studied the correlations between direct imaging of PW and BW contents at 3T with bone fracture toughness in whole cross-sections of long cortical bone. They found that BW content significantly correlated with toughness defined as the energy dissipated during fracture (n=20, R=0.51). Chang et al. 51 found significant correlations between bone mechanical properties and magnetization transfer (MT) ratio between collagen and water pools at 3T (n=122, R=0.55). Recently, we employed a UTE-based two-pool MT modeling to measure collagen proton fraction at 3T and observed significant correlations with bone strips’ mechanical properties (n=156, R=0.60–0.61) 52. In spite of an increasing number of MRI-based bone evaluation techniques, the effect of intracortical bone fat on different MRI-based bone evaluation techniques is not well understood. As highlighted in this study, the intracortical bone fat content needs to be considered when analyzing the bone water fractions, which in turn may lead to better prediction of mechanical properties using any of reported UTE-MRI based techniques.
This study had a number of limitations. First, tricomponent UTE-T2* fitting used twenty MRI mages with different TEs that required 35 minutes of scan time. Employing a lower number of images with optimized TEs or accelerated imaging techniques such as readout stretching 53 would be required for future clinical studies. Second, the selected short TR (i.e., 28 ms) and low flip angle (i.e., 10°) may have introduced some T1 weighting and, therefore, errors in fraction calculations. However, the T1 weighting is relatively small, with errors less than 10% in estimating bound and pore water fractions relative to fat. Furthermore, such errors influence bicomponent and tricomponent models similarly, and are therefore unlikely to affect the presented comparisons in this paper. Nevertheless, for future investigations of the presented tricomponent fitting model, longer TRs or correction criteria using reported T1 values for fat54,55, BW15,56, and PW 56,57, should be employed to ensure accuracy in fraction measurements within an acceptable total scan time. The correction criteria can be made more accurate and precise if the T1 values are measured simultaneously55. Third, this study was performed ex vivo on bone specimens cut from pure cortical bone layers, where there was low bone marrow and no surrounding muscle. A higher fat presence in cortical bone in vivo may further emphasize the improved performance of tricomponent T2* fitting compared with bicomponent T2* fitting. However, the presence of other soft tissues, lower spatial in vivo resolutions, temperature difference 11, and subject motion during scan will likely reduce the performance of all MRI-based imaging techniques in vivo compared with ex vivo studies. Fourth, the estimated fat fraction through tricomponent fitting model does not determine the accurate volumetric fractions of fat in cortical bone as a result of the different volumetric proton densities in water and fat. A well-designed phantom study is required to derive the actual relationship between the volumetric fat fraction with the estimated fat fractions using tricomponent T2* analysis. Fifth, despite the efforts in keeping bone specimens wet, some degree of bone dehydration was to be expected during sample preparation steps. Although PBS may be different from the actual intraosseous liquid in bone, soaking the specimens in PBS would presumably compensate for the potential hydration with negligible impacts on the MRI properties. Soaking bone in PBS for rehydration is a common procedure in the study of bone, as can be seen from extensive publications in the literature6,44,58.
5. Conclusion
A recently developed tricomponent T2* fitting model was used to assess the water proton pools in human cortical bone specimens by considering the oscillating signal contributions from fat. The correlations of tricomponent model results with microstructural and mechanical properties of the bone specimens were compared with correlation of the bicomponent model. The tricomponent model results showed significant strong correlations with microstructural properties and showed significant moderate correlations with mechanical properties. Bone microstructural and mechanical properties presented higher correlations with water pool fractions obtained from the tricomponent model rather than from the bicomponent model. This study highlighted the importance of the intracortical bone fat contribution in UTE-MRI bone evaluations. Moreover, tricomponent T2* fitting is suggested as a useful MRI technique to assess cortical bone mechanical properties and intracortical bone microstructure.
Supplementary Material
7. Acknowledgements
The authors acknowledge grant support from NIH (1R21AR073496, 1R01NS092650, R01AR062581-06) and VA Clinical Science and Rehabilitation R&D (I01CX001388 and I01RX002604).
Abbreviations:
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- 3D
three-dimensional
- 3D-UTE
three-dimensional ultrashort echo time imaging
- RF
radio frequency
- FOV
field of view
- ROI
region of interest
- TE
echo time
- TR
repetition time
- CT
computed tomography
- μCT
micro-computed tomography
- FA
flip angle
- BMD
bone mineral density
- PBS
phosphate-buffered saline
- DEXA
dual-energy X-ray absorptiometry
- HR-pQCT
peripheral quantitative computed tomography
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
8. References
- 1.Manhard MK, Nyman JS, Does MD. Advances in imaging approaches to fracture risk evaluation. Transl Res. 2017;181:1–14. doi: 10.1016/j.trsl.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moser E, Rejnmark L, Sikjaer T, Mosekilde L, Amstrup AK, Jakobsen NFB. Association between bone indices assessed by DXA, HR-pQCT and QCT scans in post-menopausal women. J Bone Miner Metab. 2015;34(6):638–645. doi: 10.1007/s00774-015-0708-9 [DOI] [PubMed] [Google Scholar]
- 3.Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2013;26(5):489–506. doi: 10.1002/nbm.2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging. 2015;41(4):870–883. doi: 10.1002/jmri.24713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehrli FW. Magnetic resonance of calcified tissues. J Magn Reson. 2013;229:35–48. doi: 10.1016/j.jmr.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerban S, Ma Y, Wong JH, et al. Ultrashort echo time magnetic resonance imaging (UTE-MRI) of cortical bone correlates well with histomorphometric assessment of bone microstructure. Bone. 2019;123(December 2018):8–17. doi: 10.1016/j.bone.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X, Song HK, Seifert AC, Li C, Wehrli FW. Feasibility of assessing bone matrix and mineral properties in vivo by combined solidstate 1H and 31P MRI. PLoS One. 2017;12(3):1–16. doi: 10.1371/journal.pone.0173995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Jerban S, Wan L, et al. Three Dimensional Ultrashort Echo Time Imaging with Tri-component Analysis for Human Cortical Bone. Magn Reson Med. 2019;82(1):348–355. doi: 10.1002/mrm.27718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jerban S, Lu X, Jang H, et al. Significant correlations between human cortical bone mineral density and quantitative susceptibility mapping (QSM) obtained with 3D Cones ultrashort echo time magnetic resonance imaging (UTE-MRI). Magn Reson Imaging. 2019;62(October):104–110. doi: 10.1016/j.mri.2019.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerban S, Ma Y, Li L, et al. Volumetric Mapping of Bound and Pore Water as well as Collagen Protons in Cortical Bone Using 3D Ultrashort Echo Time Cones MR Imaging Techniques. Bone. 2019;127(October):120–128. doi: 10.1016/j.bone.2019.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerban S, Szeverenyi N, Ma Y, et al. Ultrashort echo time MRI (UTE-MRI) quantifications of cortical bone varied significantly at body temperature compared with room temperature. Investig Magn Reson Imaging. 2019;23(3):00. doi: 10.13104/imri.2019.23.3.000 [DOI] [Google Scholar]
- 12.Du J, Diaz E, Carl M, Bae WC, Chung CB, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med. 2012;67(3):645–649. doi: 10.1002/mrm.23047 [DOI] [PubMed] [Google Scholar]
- 13.Bae WC, Chen PC, Chung CB, Masuda K, D’Lima D, Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: Correlation with porosity and biomechanical properties. J Bone Miner Res. 2012;27(4):848–857. doi: 10.1002/jbmr.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Hermida JC, Diaz E, et al. Assessment of cortical bone with clinical and ultrashort echo time sequences. Magn Reson Med. 2013;70(3):697–704. doi: 10.1002/mrm.24497 [DOI] [PubMed] [Google Scholar]
- 15.Seifert AC, Wehrli SL, Wehrli FW. Bi-component T2* analysis of bound and pore bone water fractions fails at high field strengths. NMR Biomed. 2015;(January):861–872. doi: 10.1002/nbm.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundh D, Rudäng R, Zoulakis M, Nilsson AG, Darelid A, Lorentzon M. A High Amount of Local Adipose Tissue Is Associated with High Cortical Porosity and Low Bone Material Strength in Older Women. J Bone Miner Res. 2016;31(4):749–757. doi: 10.1002/jbmr.2747 [DOI] [PubMed] [Google Scholar]
- 17.Devlin MJ, Rosen CJ. The bone-fat interface: Basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141–147. doi: 10.1016/S2213-8587(14)70007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kremer R, Gilsanz V. Fat and Bone: An Odd Couple. Front Endocrinol (Lausanne). 2016;6(March). doi: 10.3389/fendo.2015.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz E, Chung CB, Bae WC, et al. Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR Biomed. 2012;25(1):161–168. doi: 10.1002/nbm.1728 [DOI] [PubMed] [Google Scholar]
- 20.Li S, Ma L, Chang EY, et al. Effects of inversion time on inversion recovery prepared ultrashort echo time (IR-UTE) imaging of bound and pore water in cortical bone. NMR Biomed. 2015;28(1):70–78. doi: 10.1002/nbm.3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton G, Smith DL, Bydder M, Nayak KS, Hu HH. MR properties of brown and white adipose tissues. J Magn Reson Imaging. 2011;34(2):468–473. doi: 10.1002/jmri.22623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton G, Yokoo T, Bydder M, et al. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011;24(7):784–790. doi: 10.1002/nbm.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55(3):575–582. doi: 10.1002/mrm.20796 [DOI] [PubMed] [Google Scholar]
- 24.Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med. 2015;76(2):577–582. doi: 10.1002/mrm.25823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Zhu Y, Lu X, Carl M, Chang EY, Du J. Short T 2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn Reson Med. 2017;00:1–9. doi: 10.1002/mrm.26908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1 H NMR at 7 Tesla. J Lipid Res. 2008;49(9):2055–2062. doi: 10.1194/jlr.d800010-jlr200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildebrand T, Rüegsegger P. A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc. 1997;185(1):67–75. doi: 10.1046/j.1365-2818.1997.1340694.x [DOI] [Google Scholar]
- 28.Darabi A, Chandelier F, Baroud G. Morphometric analysis of trabecular bone thickness using different algorithms. Can J Electr Comput Eng. 2007;32(3):157–163. doi: 10.1109/CJECE.2007.4413127 [DOI] [Google Scholar]
- 29.Bashoor-Zadeh M, Baroud G, Bohner M. Geometric analysis of porous bone substitutes using micro-computed tomography and fuzzy distance transform. Acta Biomater. 2010;6(3):864–875. doi: 10.1016/j.actbio.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 30.ASTM. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials 1. Annu B ASTM Stand. 2011;(C):1–11. doi: 10.1520/D0790-10. [DOI] [Google Scholar]
- 31.Hammant B The use of 4-point loading tests to determine mechanical properties. Composites. 1971;2(4):246–249. doi: 10.1016/0010-4361(71)90154-6 [DOI] [Google Scholar]
- 32.Baratta FI. Requirements for Flexure Testing of Brittle Materials. Army Mater Mech Res Cent. 1982:46. doi: 10.1520/STP35245S [DOI] [Google Scholar]
- 33.ASTM. ASTM C1674 – 11 Standard Test Method for Flexural Strength of Advanced Ceramics with Engineered Porosity (Honeycomb Cellular Channels) at Ambient Temperatures. Annu B ASTM Stand. 2015;i:10. doi: 10.1520/C1674-11.2 [DOI] [Google Scholar]
- 34.Koudelka P, Jiroušek O, Valach J. Determination of mechanical properties of materials with complex inner structure using microstructural models. Mach Technol Mater. 2011;1(3):39–42. http://mech-ing.com/journal/3-2011.html. [Google Scholar]
- 35.Weibull W A Statistical Theory of the Strength of Materials; 1939. [Google Scholar]
- 36.Quinn JB, Quinn GD. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent Mater. 2010;26(2):135–147. doi: 10.1016/j.dental.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khandaker M, Ekwaro-Osire S. Weibull analysis of fracture test data on bovine cortical bone: Influence of orientation. Int J Biomater. 2013;2013. doi: 10.1155/2013/639841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigley RF, Gibeling JC, Stover SM, Hazelwood SJ, Fyhrie DP, Martin RB. Volume effects on yield strength of equine cortical bone. J Mech Behav Biomed Mater. 2008;1(4):295–302. doi: 10.1016/j.jmbbm.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 39.Bigley RF, Gibeling JC, Stover SM, Hazelwood SJ, Fyhrie DP, Martin RB. Volume effects on fatigue life of equine cortical bone. J Mech Behav Biomed Mater. 2008;1(4):295–302. doi: 10.1016/j.jmbbm.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 40.Jerban S, Ma Y, Wan L, et al. Collagen proton fraction from ultrashort echo time magnetization transfer (UTE‐MT) MRI modelling correlates significantly with cortical bone porosity measured with micro‐computed tomography (μCT). NMR Biomed. 2019;32(e4045.):1–10. doi: 10.1002/nbm.4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jerban S, Ma Y, Nazaran A, et al. Detecting stress injury (fatigue fracture) in fibular cortical bone using quantitative ultrashort echo time-magnetization transfer (UTE-MT): An ex vivo study. NMR Biomed. 2018;31(11):e3994. doi: 10.1002/nbm.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Jang H, Ma Y, Jerban S, Chang EY, Du J. Ultrashort Echo Time Quantitative Susceptibility Mapping (UTE-QSM) of Highly Concentrated Magnetic Nanoparticles: A Comparison Study about Different Sampling Strategies. Molecules. 2019;24(6):1143. doi: 10.3390/molecules24061143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manhard MK, Horch RA, Gochberg DF, Nyman JS, Does MD. In Vivo Quantitative MR Imaging of Bound and Pore Water in cortical bone. Radiology. 2015;277(1):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajapakse CS, Bashoor-Zadeh M, Li C, Sun W, Wright AC, Wehrli FW. Volumetric Cortical Bone Porosity Assessment with MR Imaging: Validation and Clinical Feasibility. Radiology. 2015;276(2):526–535. doi: 10.1148/radiol.15141850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Seara MA, Wehrli SL, Takahashi M, Wehrli FW. Water Content Predicts Bone Mineral Density and Mechanical Properties. J Bone Jt Surg Am Vol. 2004;19:289–295. doi: 10.1359/JBMR.0301227 [DOI] [PubMed] [Google Scholar]
- 46.Horch RA, Gochberg DF, Nyman JS, Does MD. Non-invasive predictors of human cortical bone mechanical properties: T2-Discriminated 1H NMR compared with high resolution X-ray. PLoS One. 2011;6(1):1–5. doi: 10.1371/journal.pone.0016359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nyman JS, Ni Q, Nicolella DP, Wang X. Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone. 2008;42(1):193–199. doi: 10.1016/j.bone.2007.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horch RA, Gochberg DF, Nyman JS, Does MD. Clinically compatible MRI strategies for discriminating bound and pore water in cortical bone. Magn Reson Med. 2012;68(6):1774–1784. doi: 10.1002/mrm.24186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS. Identifying Novel Clinical Surrogates to Assess Human Bone Fracture Toughness. J Bone Miner Res. 2015;30(7):1290–1300. doi: 10.1002/jbmr.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manhard MK, Uppuganti S, Granke M, Gochberg DF, Nyman JS, Does MD. MRI-derived bound and pore water concentrations as predictors of fracture resistance. Bone. 2016;87:1–10. doi: 10.1016/j.bone.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang EY, Bae WC, Shao H, et al. Ultrashort echo time magnetization transfer ( UTE-MT ) imaging of cortical bone. NMR Biomed. 2015;28(April):873–880. doi: 10.1002/nbm.3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jerban S, Ma Y, Dorthe EW, et al. Assessing cortical bone mechanical properties using collagen proton fraction from ultrashort echo time magnetization transfer (UTE-MT) MRI modeling. Bone Reports. 2019;8(2). doi: 10.1016/j.bonr.2019.100220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan L, Zhao W, Ma Y, et al. Fast quantitative three-dimensional ultrashort echo time (UTE) magnetic resonance imaging of cortical bone using extended cones sampling. Magn Reson Med. 2019. doi: 10.1002/mrm.27715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T and 7.0 T: A comparison of relaxation times and image contrast. Am Roentgen Ray Soc. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343 [DOI] [PubMed] [Google Scholar]
- 55.Le Ster C, Gambarota G, Lasbleiz J, Guillin R, Decaux O, Saint-Jalmes H. Breath-hold MR measurements of fat fraction, T1, and T2* of water and fat in vertebral bone marrow. J Magn Reson Imaging. 2016;44(3):549–555. doi: 10.1002/jmri.25205 [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Chang EY, Carl M, et al. Measurement of bound and pore water T1 relaxation times in cortical bone using three-dimensional ultrashort echo time cones sequences. Magn Reson Med. 2016;77(6):2136–2145. doi: 10.1002/mrm.26292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manhard MK, Horch RA, Harkins KD, Gochberg DF, Nyman JS, Does MD. Validation of quantitative bound- and pore-water imaging in cortical bone. Magn Reson Med. 2014;71(6):2166–2171. doi: 10.1002/mrm.24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, Seifert AC, Rad HS, et al. Cortical Bone Water Concentration: Dependence of MR Imaging Measures on Age and Pore Volume Fraction. Radiology. 2014;272(3):796–806. doi: 10.1148/radiol.14132585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.