Abstract
Study Objectives:
Angelman syndrome (AS) is a rare neurodevelopmental disorder that is characterized by developmental delay, intellectual disability, seizures, a characteristic happy personality, gait ataxia, tremulousness of the limbs, microcephaly, and anxiety. Severe sleep disturbances with the diminished need for sleep and abnormal sleep-wake cycles are seen in up to 90% of patients with AS. AS is caused by absent maternal expression of the gene UBE3A located in the 15q11.2-q13 locus. We hypothesized that selective antagonism of 5-HT2 and 5-HT3 serotonin receptors with mirtazapine would benefit sleep disturbances in patients with AS.
Methods:
Institutional Review Board approval was obtained at Vanderbilt University Medical Center. Medical records of individuals seen in the Comprehensive Angelman Syndrome clinic were retrospectively reviewed to determine the use of mirtazapine for disordered sleep. Parents were asked to respond to a survey to assess the phenotypic features of sleep and behavioral disturbances in AS. They were asked about the use of medications for sleep, focusing on the benefits and risks of mirtazapine.
Results:
A cohort of 8 individuals with AS, ranging in age from 3 to 16 years old with histories of sleep challenges, were treated with 3.75 to 30 mg of mirtazapine at bedtime for 0 to 36 weeks. Nocturnal awakenings were the most common sleep challenge reported. Seven of eight patients reported benefits from mirtazapine, including increased total sleep time, decreased nocturnal awakenings, and decreased time to fall asleep. The most significant side effects of mirtazapine were hyperphagia and weight gain.
Conclusions:
Individuals with AS have abnormal sleep-wake cycles and a high unmet medical need. Mirtazapine helped with sleep onset and nighttime awakenings in 7 of 8 patients, with 2 patients reporting a positive benefit with respect to behavior. These data suggest that mirtazapine may be considered for the treatment of sleep difficulties in patients with AS who remain refractory to more conventional therapies. Weight gain was a common side-effect and led to discontinuation of treatment in 1 patient.
Citation:
Hanzlik E, Klinger SA, Carson R, Duis J. Mirtazapine for sleep disturbances in Angelman syndrome: a retrospective chart review of 8 pediatric cases. J Clin Sleep Med. 2020;16(4):591–595.
Keywords: abnormal sleep-wake cycles, Angelman syndrome, imprinting, mirtazapine, neurodevelopmental disorder
BRIEF SUMMARY
Current Knowledge/Study Rationale: Disrupted sleep in patients with Angelman syndrome is a common complaint that adversely affects both patients and caregivers and does not respond well to typical sleep medications.
Study Impact: We report on a small case series that suggests a beneficial response to mirtazapine with respect to sleep latency, nocturnal awakenings, and sleep duration in patients with Angelman syndrome. These findings support a role for mirtazapine in treating sleep disorders in Angelman syndrome.
INTRODUCTION
Angelman syndrome (AS) is a neurodevelopmental syndrome with core features characterized by developmental delay, intellectual disability, lack of speech development, ataxic gait, tremulousness, epilepsy, a diminished need for sleep, abnormal sleep-wake cycles1 and typical features including an uncharacteristically happy demeanor. The etiology of AS is mutations in or loss of expression from the maternal allele of ubiquitin E3 ligase (UBE3A) in neurons.
Sleep disorders and disruption in sleep-wake cycles are common clinical features of AS, occurring in 20–80% of cases as defined by the most recent Clinical Guidelines Consensus,2 with an incidence of up to 90% in specific cohorts.3 A recent meta-analysis revealed an association with arousal during sleep, somnolence, and short sleep duration.1 Studies using sleep questionnaires have identified nocturnal awakenings, increased sleep latency, and decreased overall sleep time without increased daytime fatigue as the most common sleep-related concerns in children with AS.1,4–7 In addition to the effect on children, sleep disorders have been shown to have significant effects on caregivers. Survey data from Didden et al8 reported that 100% of patents of children with AS endorsed fatigue attributed to their child’s sleep difficulties, with 75% endorsing feelings of irritation toward their child, and over 60% of parents reported feeling powerless to cope with the problem. More recently, Trickett et al7 reported 94% of caregivers had increased daytime fatigue, stress, and a negative impact on work performance. Goldman et al4 directly evaluated sleep via actigraphy in parents of children with AS and found children’s sleep latency directly correlated to parental daytime fatigue and a higher number of nocturnal awakenings correlated to higher sleep fragmentation in parents. Lastly, given that approximately 80% of patients with AS have epilepsy, the association between sleep deprivation and a lowered seizure threshold is of additional concern.7,9
Nonpharmacological treatments for sleep-related disorders have been studied in AS, including ensuring a quality sleep environment, adjustment of sleep schedules, and managing parental/child interactions. These studies showed significantly improved independent sleep onset, improved sleep on actigraphy, and improved sleep by parental report.10 However, despite nonpharmacological interventions, children with AS, like many other children with neurodevelopmental disorders require medications for sleep. A survey of child psychiatrists reported that 30% of psychiatrists used medications for sleep in over 50% of their patients with neurodevelopmental disorders.11 In a multicenter study of patients with AS, 90% of patients had sleep disorders as children, with nearly 77% of those treated with medications for sleep.12
A randomized placebo-controlled clinical trial of melatonin in 8 patients with AS did report improvements in sleep latency, total sleep time, and nocturnal awakenings.13 Melatonin is commonly used in AS for this reason, but many patients are refractory to its benefits. Observational reports of clonidine, gabapentin, benzodiazepines, and trazodone suggest anecdotal, but variable benefits on sleep in patients with neurodevelopmental disorders. The majority of children with AS have tried at least 1 of the above medications with little efficacy.14,15 There remains no Food and Drug Administration-approved medication for sleep in children with AS. Evidence at this time relies on observational studies and clinical anecdotes.14
Although human data support that deletion of the genes coding for γ-Aminobutyric acid type A receptor (GABAA) subunits contributes to sleep dysregulation in AS and supports the use of GABAA subunit agonists for the treatment of sleep dysregulation,16 studies in animal models also support an association between mutations in the UBE3A gene and neurobiological changes in the sleep-wake cycle biology. Mouse models of AS have been shown to demonstrate no consistent rest periods and disrupted architecture of non-rapid eye movement sleep.17 A direct role for UBE3A in the regulation of circadian rhythms is suggested by an increased expression of the circadian clock protein Bmal1 in the AS model mouse.18 In animal models, the neurotransmitter serotonin has been shown to modulate both sleep onset and maintenance of sleep, also due in part to regulation of Bmal1.19
The convergence of findings that both UBE3A loss and serotonin modulate Bmal1, combined with clinical data that antagonism of 5-HT2A/2C serotonin receptors can increase slow-wave sleep in humans,20 led to the hypothesis that selective antagonism of 5-HT2/3 receptors may improve sleep in AS. Thus, we sought to determine whether the antidepressant medication mirtazapine was beneficial as adjunctive therapy for sleep in AS. We systematically collected retrospective data to further look into benefits versus risks in the population with AS. Herein, we present findings on the open-label benefits of mirtazapine for sleep in patients with AS.
METHODS
Medical records from patients seen at the Multidisciplinary Angelman Syndrome Clinic at Vanderbilt were identified based on the presence of a documented prescription for mirtazapine in the electronic medical record. This study was approved upon review by the Vanderbilt University Medical Center Institutional Review Board. Demographic information, including medical history and AS diagnosis, were obtained via chart review. After identification, a survey was presented to the patient’s guardian via conventional mail, electronic mail, and via telephone, all in the form of a RedCap survey. The survey included an explanation of the study and instructions for completing the survey. The survey was developed using questions adapted from standardized sleep surveys, addressing sleep issues and comorbidities seen in AS, as well as the timing of initiation, dose, results reported by a guardian, and side effects of mirtazapine. Comorbid conditions, including mood and anxiety disorders, epilepsy, and gastroesophageal reflux, were also addressed in the survey given their effect on sleep and prevalence in children with AS. Survey responses were collected electronically and via email responses.
RESULTS
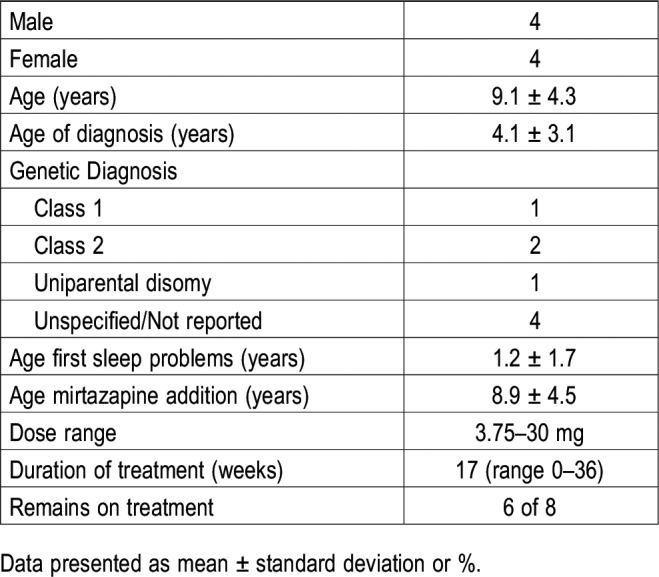
A cohort of patients seen at the Angelman Syndrome Foundation clinic at Vanderbilt was identified who were treated with mirtazapine for either mood or sleep. Data were collected from a variety of sources, including chart review, interviews, and surveys. Out of a total of 54 patients with AS, a total of 10 patients was identified who were treated with mirtazapine. One of the ten patients never started the medication. Of the remainder, 8 responded to survey requests (Table 1).
Table 1.
Demographics.
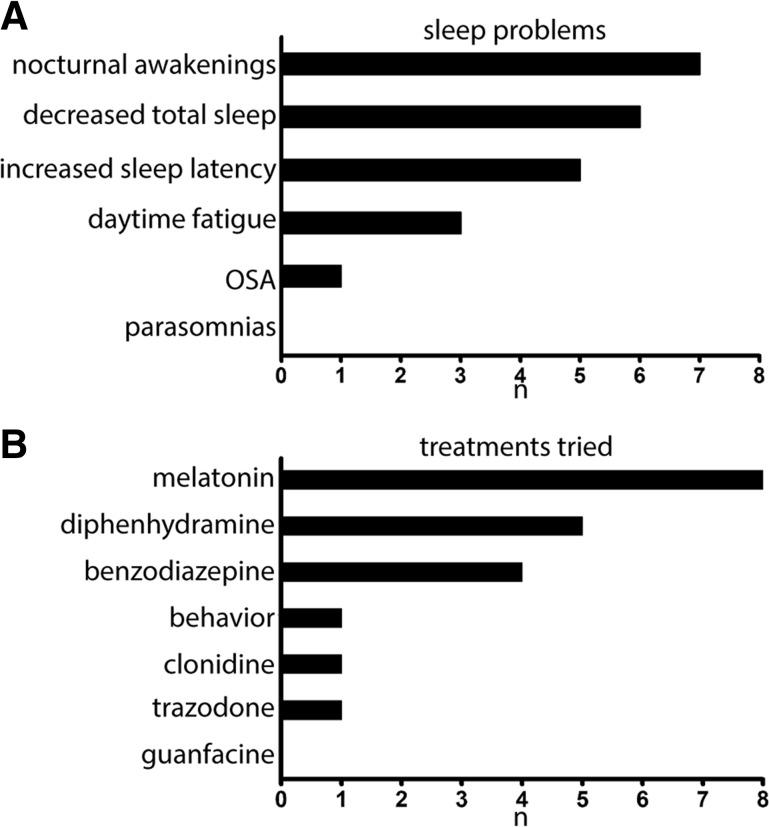
In a cohort of patients with AS who were treated with mirtazapine for persistent difficulties with sleep, nocturnal awakenings were the most common complaint, reported in 7 of 8 patients, followed by decreased total sleep time and increased time to fall asleep (sleep latency) as the top 3 most common complaints (Figure 1). All patients had previously been treated with medications to aid sleep, with melatonin and diphenhydramine used most commonly.
Figure 1. Sleep symptoms and medications tried.
(A) Primary sleep problems in patients with Angelman syndrome. (B) Medications used to improve sleep in our Angelman cohort. n is the number of patients who c/o of that symptoms in A and who had tried those various medications in B. OSA = obstructive sleep apnea.
At the time of mirtazapine addition, 5 of 8 patients were on medications for sleep, with benzodiazepines and trazodone noted most frequently, followed by an even distribution of medications, including melatonin, clonidine, guanfacine, and artisanal cannabidiol oil (Figure 2). No patients were currently using diphenhydramine. Of the 8 respondents, 6 patients remained on mirtazapine at the time of the survey. Seven of eight patients reported benefits from mirtazapine, including increased total sleep time, decreased nocturnal awakenings, and decreased time to fall asleep. The most frequent side effect of mirtazapine was weight gain, followed by hyperphagia. Of the patients who stopped mirtazapine, one stopped due to rapid weight gain and the other due to a combination of lack of efficacy, insomnia, and daytime sleepiness.
Figure 2. Patient medications and response to mirtazapine.
(A) Medications used during observational study. (B) Sleep improvements reported following the addition of mirtazapine. (C) Side effects reported in association with mirtazapine. n is number of patients. CBD = cannabidiol.
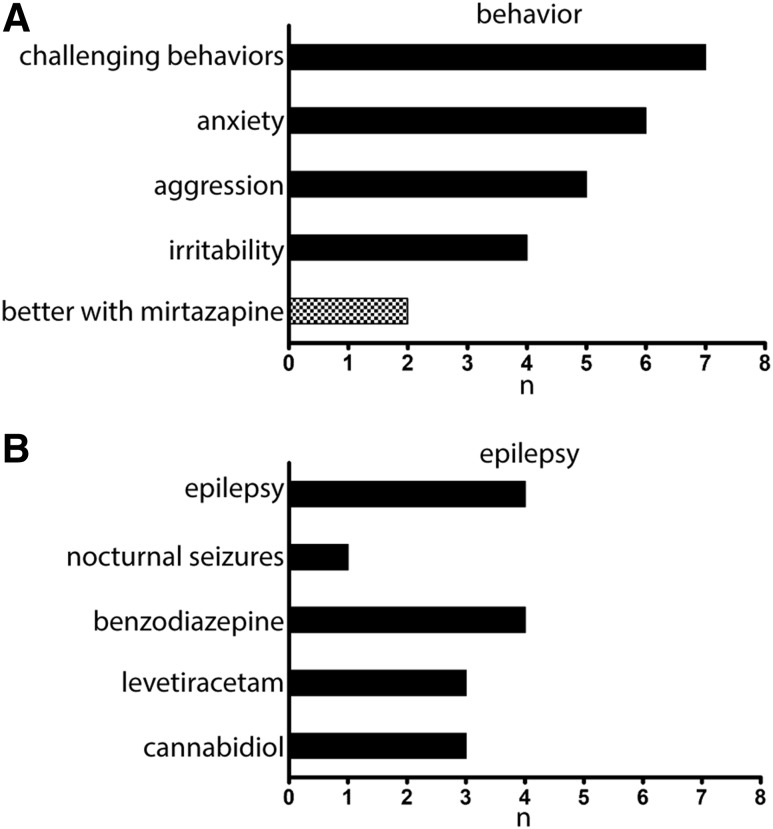
Consistent with the AS diagnosis, behavioral challenges were common in this cohort (Figure 3). Anxiety was reported in 6 of 8 patients, manifesting clinically by gagging, loss of focus, aggressive behaviors, and acting upset. Triggers included being out of routine, overstimulation, lack of sleep, new places, loud noises, and large crowds. Two of eight patients reported an improvement in behavior following the addition of mirtazapine. One patient had improved interactions with other children at school. The second patient had global improvements in behavior and was able to “resume activities that we had stopped due to anxiety and aggression.”
Figure 3. Incidence of behavior challenges and epilepsy in our cohort with Angelman syndrome (AS).
(A) Behavior challenges were common in our cohort with AS, with some benefit following mirtazapine addition. (B) Epilepsy is a common comorbidity that may contribute to sleep disruption in AS.
A history of epilepsy was present in 4 of 8 patients, 1 of which had a history of nocturnal seizures. Benzodiazepines were the most commonly used medication in patients with epilepsy. Clinically, no patients had a new onset of seizures or increase of seizure frequency following the addition of mirtazapine.
DISCUSSION
Based on the consensus criteria for AS, sleep difficulties are associated with AS, reported in 20–80% of patients.2 Given the disruptive effects of poor sleep on both patients with AS and their families and lack of medications to successfully treat sleep problems in AS, sleep problems rank highly among the most challenging aspects of AS, along with epilepsy and impaired expressive language. Thus, the identification of efficacious treatment options remains an unmet need for our patients and families. Improved sleep dramatically impacts the quality of life of the individual, decreases caregiver burden, and likely positively impacts other features, including seizures. We report a beneficial response to mirtazapine in a cohort of patients with AS for whom sleep problems have been a life-long issue and who have not responded to more conventional therapies.
In a small cohort of patients with AS-associated sleep difficulties, 7 of 8 patients had a positive response to mirtazapine, with improved sleep duration, fewer nighttime arousals, and decreased sleep latency. All patients had been previously treated with a variety of other sleep medications in addition to behavioral modifications targeted to improving sleep. Of the 2 patients who were no longer taking mirtazapine, only 1 stopped due to a lack of efficacy. Mirtazapine also seemed to benefit anxiety and behavioral concerns in some patients, with one parent noting reengagement in therapy and improved functioning in structured environments. Our findings in AS are consistent with acute treatment studies with mirtazapine in normal controls, which demonstrated increased sleep efficiency, increased total sleep time, and a 35–40% decrease in the number of awakenings, with shorter duration of awakenings.21,22 Similar findings concerning decreased sleep latency and increased sleep quality were seen in patients with major depressive disorder.23
Weight gain is a well described side effect of mirtazapine that was also seen in our cohort with AS, leading to discontinuation of the medication in 1 patient. Dry mouth is a reported side effect of mirtazapine; however, due to the drooling often seen in individuals with AS, this could be beneficial to our patient population with AS. It is notable that our AS patients, in general, seem to exhibit signs of hyperphagia, particularly cravings of carbohydrates (unpublished observation). Mirtazapine may be unmasking or worsening a feature of AS that requires more study.
The underlying cause of sleep disorders in AS and other neurodevelopmental disorders is being further investigated. Overall, up to 80% of children with neurodevelopmental disorders have an increased number of sleep-related issues, in contrast to 1–6% of the general population.14 Multiple mechanisms likely contribute to sleep issues in AS, including neurobiological changes, medical comorbidities, and behavioral issues. Gastroesophageal reflux disease has also been associated with an increased incidence of shortness of breath at night and parasomnias in AS.7 Endogenous melatonin levels in children with AS showed similar peak levels but later offset times compared with typically developing children or those with epilepsy.24
As noted above, there is a link between UBE3A expression and serotonin with modulation of circadian rhythms and sleep, which is further supported by UBE3A expression in the suprachiasmatic nucleus.17 Mirtazapine is an α2-adrenergic antagonist in the central nervous system as well as an antagonist of 5-HT2 and 5-HT3 serotonin receptors. The actions on presynaptic nerve terminals may lead to an overall enhancement of noradrenergic signaling as well as a selective enhancement of 5-HT1A neurotransmission. The finding that 1 of 3 patients with AS endorsed improved sleep with the 5-HT1A partial agonist buspirone may suggest some benefit due to enhancement of 5-HT1A signaling, with additional benefit from 5-HT2 and 5-HT3 receptor antagonism.25 The observation that no patients in our cohort report a sustained response to diphenhydramine, a H1-receptor antagonist, suggests histamine antagonism is not likely the primary mechanism of action for the beneficial sleep effects of mirtazapine.
Although our focus for this study was sleep, mirtazapine is predominantly used to treat behavior in patients with neurodevelopmental disorders, including autism spectrum disorders. Posey et al reported a cohort of 26 patients with pervasive developmental disorders in which nearly 35% of patients were reported to be “much improved” or “very much improved” on the Clinical Global Impressions scale following mirtazapine treatment.26 Although the overall benefit was considered modest and communication did not improve with treatment, the medication was well tolerated. Our findings are consistent with that reported by Posey et al, with 2 of 8 patients endorsing benefits concerning behavior and with 2 of 8 patients discontinuing the medication due to side effects. Mirtazapine is extensively liver metabolized by a variety of cytochromes, thus alterations in metabolism by other hepatically metabolized drugs are not profound. Of importance to patients with AS, the reported incidence of seizures associated with mirtazapine is <0.05%.27 Doses used in our cohort ranged from 3.75 to 30 mg at bedtime, with a “start low and go slow” approach. Notably, mirtazapine is available in a dissolvable tablet that can be crushed or halved to accommodate dosing in younger children and individuals with dysphagia who are at risk for aspiration.
Continued work is required to better define the underlying neurobiology of sleep disorders in AS for the development of rational and targeted therapies. In the interim, identification of safe and well-tolerated treatments for AS-related sleep difficulties is necessary. Our data suggest that serotonergic modulation by mirtazapine may serve as a therapeutic option that not only improves sleep in patients with AS but may improve behavior, anxiety, and potentially attenuate drooling. For some patients, the hyperphagia and the associated weight gain may prove intolerable. We recommend starting at a low dose and slowly increasing as tolerated, keeping in mind the long 20–40 hour half-life.27 Mirtazapine must be weaned when discontinued.
Our study is limited in that it is an open-label retrospective study; thus we can neither exclude potential bias given the lack of randomization and blinding nor exclude a placebo effect. The small cohort may also limit the power of our study. The high response rate within those who did respond to our survey requests suggests that mirtazapine may benefit patients with AS who have failed more conventional treatments for sleep. Given the huge unmet medical need in the AS community for a safe and effective sleep medication, we suggest mirtazapine as a candidate drug for future multicenter placebo-controlled trials to verify our results in a timely manner. A future study combining polysomnography, actigraphy, remote sleep monitoring, and sleep diaries with a validated sleep scale such as the Children’s Sleep Habits Questionnaire, will be required to establish fully mirtazapine's position in the AS armamentarium.
ABBREVIATIONS
- AS
Angelman syndrome
- 5-HT
serotonin
- UBE3A
ubiquitin E3 ligase
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Vanderbilt University Children’s Hospital. The authors report no conflict of interest. The authors report the off-label/investigational use of mirtazapine for the treatment of disordered sleep. At the time of the study Dr. Duis was affiliated with Vanderbilt University Medical Center, Nashville, TN. She is currently affiliated with Children’s Hospital Colorado, University of Colorado-Anschutz, Aurora, CO.
REFERENCES
- 1.Spruyt K, Braam W, Curfs LM. Sleep in Angelman syndrome: A review of evidence. Sleep Med Rev. 2018;37:69–84. doi: 10.1016/j.smrv.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Williams CA, Beaudet AL, Clayton-Smith J, et al. Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A. 2006;140A(5):413–418. doi: 10.1002/ajmg.a.31074. [DOI] [PubMed] [Google Scholar]
- 3.Clayton-Smith J. Clinical research on Angelman syndrome in the United Kingdom: observations on 82 affected individuals. Am J Med Genet. 1993;46(1):12–15. doi: 10.1002/ajmg.1320460105. [DOI] [PubMed] [Google Scholar]
- 4.Goldman SE, Bichell TJ, Surdyka K, Malow BA. Sleep in children and adolescents with Angelman syndrome: association with parent sleep and stress. J Intellect Disabil Res. 2012;56(6):600–608. doi: 10.1111/j.1365-2788.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelc K, Cheron G, Boyd SG, Dan B. Are there distinctive sleep problems in Angelman syndrome? Sleep Med. 2008;9(4):434–441. doi: 10.1016/j.sleep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler AC, Sacco P, Cabo R. Unmet clinical needs and burden in Angelman syndrome: a review of the literature. Orphanet J Rare Dis. 2017;12(1):164–209. doi: 10.1186/s13023-017-0716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trickett J, Heald M, Oliver C. Sleep in children with Angelman syndrome: parental concerns and priorities. Res Dev Disabil. 2017;69:105–115. doi: 10.1016/j.ridd.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Didden R, Korzilius H, Smits MG, Curfs LM. Sleep problems in individuals with Angelman syndrome. AM J Ment Retard. 2004;109(4):275–284. doi: 10.1352/0895-8017(2004)109<275:SPIIWS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Malow BA. Sleep deprivation and epilepsy. Epilepsy Curr. 2004;4(5):193–195. doi: 10.1111/j.1535-7597.2004.04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen KD, Kuhn BR, DeHaai KA, Wallace DP. Evaluation of a behavioral treatment package to reduce sleep problems in children with Angelman Syndrome. Res Dev Disabil. 2013;34(1):676–686. doi: 10.1016/j.ridd.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Owens JA, Rosen CL, Mindell JA, Kirchner HL. Use of pharmacotherapy for insomnia in child psychiatry practice: A national survey. Sleep Med. 2010;11(7):692–700. doi: 10.1016/j.sleep.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Sueri C, Ferlazzo E, Elia M, et al. Epilepsy and sleep disorders improve in adolescents and adults with Angelman syndrome: A multicenter study on 46 patients. Epilepsy Behav. 2017;75:225–229. doi: 10.1016/j.yebeh.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Braam W, Didden R, Smits MG, Curfs LM. Melatonin for chronic insomnia in Angelman syndrome: a randomized placebo-controlled trial. J Child Neurol. 2008;23(6):649–654. doi: 10.1177/0883073808314153. [DOI] [PubMed] [Google Scholar]
- 14.Blackmer AB, Feinstein JA. Management of sleep disorders in children with neurodevelopmental disorders: a review. Pharmacotherapy. 2016;36(1):84–98. doi: 10.1002/phar.1686. [DOI] [PubMed] [Google Scholar]
- 15.Veatch OJ, Maxwell-Horn AC, Malow BA. Sleep in Autism Spectrum Disorders. Curr Sleep Med Rep. 2015;1(2):131–140. doi: 10.1007/s40675-015-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDougle CJ, Keary CJ. Sleep and EEG biomarkers as avenues toward new treatment approaches in Angelman syndrome. Neuropsychopharmacology. 2020;45(1):238–239. doi: 10.1038/s41386-019-0517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlen JC, Jones KA, Pinckney L, et al. Maternal Ube3a loss disrupts sleep homeostasis but leaves circadian rhythmicity largely intact. J Neurosci. 2015;35(40):13587–13598. doi: 10.1523/JNEUROSCI.2194-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi SQ, Johnson CH. Circadian biology and sleep in monogenic neurological disorders and its potential application in drug discovery. Curr Opin Behav Sci. 2019;25:23–30. doi: 10.1016/j.cobeha.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morioka N, Sugimoto T, Sato K, et al. The induction of Per1 expression by the combined treatment with glutamate, 5-hydroxytriptamine and dopamine initiates a ripple effect on Bmal1 and Cry1 mRNA expression via the ERK signaling pathway in cultured rat spinal astrocytes. Neurochem Int. 2015;90:9–19. doi: 10.1016/j.neuint.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Thase ME. Depression, sleep, and antidepressants. J Clin Psychiatry. 1998;59(Suppl 4):55–65. [PubMed] [Google Scholar]
- 21.Aslan S, Isik E, Cosar B. The effects of mirtazapine on sleep: a placebo controlled, double-blind study in young healthy volunteers. Sleep. 2002;25(6):677–679. [PubMed] [Google Scholar]
- 22.Karsten J, Hagenauw LA, Kamphuis J, Lancel M. Low doses of mirtazapine or quetiapine for transient insomnia: A randomised, double-blind, cross-over, placebo-controlled trial. J Psychopharmacol. 2017;31(3):327–337. doi: 10.1177/0269881116681399. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Li Z, Li L, Hao W. Real-world, open-label study to evaluate the effectiveness of mirtazapine on sleep quality in outpatients with major depressive disorder. Asia-Pac Psychiatry. 2014;6(2):152–160. doi: 10.1111/appy.12060. [DOI] [PubMed] [Google Scholar]
- 24.Paprocka J, Kijonka M, Wojcieszek P, Pecka M, Emich-Widera E, Sokol M. Melatonin and Angelman syndrome: implications and mathematical model of diurnal secretion. Int J Endocrinol. 2017;2017:5853167. doi: 10.1155/2017/5853167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balaj K, Nowinski L, Walsh B, et al. Buspirone for the treatment of anxiety-related symptoms in Angelman syndrome: a case series. Psychiatr Genet. 2019;29(2):51–56. doi: 10.1097/YPG.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 26.Posey DJ, Guenin KD, Kohn AE, Swiezy NB, McDougle CJ. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11(3):267–277. doi: 10.1089/10445460152595586. [DOI] [PubMed] [Google Scholar]
- 27.Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]