Abstract
Study Objectives:
To determine whether an intensive weight-loss program (IWLP) is effective for reducing weight, the severity of obstructive sleep apnea (OSA), and metabolic variables in patients with obesity and severe OSA undergoing continuous positive airway pressure treatment.
Methods:
Forty-two patients were randomized to the control (CG, n = 20) or the intervention group (IG, n = 22), who followed a 12-month IWLP. The primary outcome was a reduction in the apnea-hypopnea index (AHI) as measured at 3 and 12 months by full polysomnography. Metabolic variables, blood pressure, body fat composition by bioimpedance, carotid intima media thickness, and visceral fat by computed tomography were also assessed.
Results:
Mean age was 49 (6.7) years, body mass index 35 (2.7) kg/m2, and AHI 69 (20) events/h. Weight reduction was higher for the IG than the CG at 3 and 12 months, −10.5 versus −2.3 kg (P < .001), and −8.2 versus −0.1 kg (P < .001), respectively, as was loss of visceral fat at 12 months. AHI decreased more in the IG at 3 months (−23.72 versus −9 events/h) but the difference was not significant at 12 months, though 28% of patients from the IG had an AHI < 30 events/h compared to none in the CG (P = .046). At 12 months, the IG showed a reduction in C-reactive protein (P = .013), glycated hemoglobin (P = .031) and an increase in high density lipoprotein cholesterol (P = .027).
Conclusions:
An IWLP in patients with obesity and severe OSA is effective for reducing weight and OSA severity. It also results in an improvement in lipid profiles, glycemic control, and inflammatory markers.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Effectiveness of an Intensive Weight Loss Program for Obstructive Sleep Apnea Syndrome (OSAS) Treatment; Identifier: NCT02832414; URL: https://clinicaltrials.gov/ct2/show/record/NCT02832414
Citation:
López-Padrós C, Salord N, Alves C, et al. Effectiveness of an intensive weight-loss program for severe OSA in patients undergoing CPAP treatment: a randomized controlled trial. J Clin Sleep Med. 2020;16(4):503–514.
Keywords: apnea-hypopnea index, intensive weight-loss program, obesity, obstructive sleep apnea syndrome, weight loss
BRIEF SUMMARY
Current Knowledge/Study Rationale: Few studies have demonstrated a significant improvement in obstructive sleep apnea (OSA) severity through weight-loss programs, and there is still a lack of evidence regarding the effect of these programs on populations of patients with severe OSA.
Study Impact: An intensive weight-loss program reduces the severity of OSA in patients with severe OSA undergoing continuous positive airway pressure treatment, results in a reduction in apnea-hypopnea index to below 30 events/h in a significant percentage of patients, and achieves small but significant benefits in patients' metabolic profile. Weight-loss programs should therefore be considered for patients with severe OSA, even if they respond well to continuous positive airway pressure, in order to optimize their global health.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder affecting 14% of men and 5% of women when defined by an apnea-hypopnea index (AHI) ≥ 5 events/h and symptoms of daytime sleepiness.1,2 Obesity, particularly central adiposity, is one of the main risk factors for sleep apnea.3 Obesity predisposes patients to OSA through multiple mechanisms.4 The prevalence of OSA has increased as a result of a progressive increase in obesity.1,2 The gold standard treatment for OSA is continuous positive airway pressure (CPAP) therapy.5 This therapy is a highly efficacious treatment that prevents main airway collapse, corrects oxyhemoglobin saturation, and reduces the cortical arousals associated with apneic/hypopneic events. When there is good adherence, CPAP improves OSA-related symptoms and quality of life and reduces traffic accidents, high blood pressure, and cardiovascular risk in individuals with severe OSA.6 However, because it is a long-term treatment, low adherence to CPAP can limit its overall effectiveness.7
Weight loss is considered to be an adjuvant treatment for OSA.8 However, although most patients with OSA undergoing CPAP treatment are obese, only a few manage to lose weight. In fact, a meta-analysis of randomized controlled studies demonstrated that patients undergoing CPAP treatment experienced a small but significant increase in body mass index (BMI) and weight.9 In many health care settings, such as ours, this failure to lose weight is due to a lack of access to adequate protocols addressing weight reduction.10 A small number of studies have shown that intensive diet programs, accompanied by exercise and especially behavioral counseling, have an effect on OSA severity in different OSA populations.11–19 These studies focused mainly on patients with mild to moderate OSA who were naive to any OSA treatment, and a few also included severe cases. These studies revealed a dose-response association between weight loss and AHI11–13 and greater improvements in patients with severe disease.12,13 Whether or not an intensive weight-loss program (IWLP) provides effective long-term improvements in cases of severe OSA has yet to be demonstrated.
The interrelationships between obesity and OSA are complex and bidirectional. Obesity causes metabolic syndrome and multiple epidemiologic and clinical studies suggest an independent association of OSA with the different components of metabolic syndrome, particularly insulin resistance, hypertension, and abnormal lipid metabolism.20 OSA may increase visceral fat dysfunction, which could play a fundamental role in the relationship between obesity and metabolic dysfunction.20 Whether treatment with CPAP alone is able to control metabolic alterations is still under study and a few studies have demonstrated an improvement in oral glucose tolerance and insulin sensitivity.21–23 A number of positive effects on lipid metabolism have also been described.24 A recent study has shown that CPAP, associated with a weight-loss program, increases insulin sensitivity and reduces serum triglyceride levels, but no improvement was observed with CPAP treatment alone,25 which raises the important issue of the usefulness of adding weight-loss programs to CPAP treatment in order to improve the cardiovascular risk factor profile of obese patients with OSA.
We hypothesized that patients with obesity and OSA who are already undergoing CPAP treatment are able to achieve weight loss and subsequently a general improvement in OSA, as well as obtaining beneficial effects on metabolic syndrome and subclinical cardiovascular disease. This study therefore aimed to analyze the effect on weight loss, OSA severity, and metabolic variables of a 12-month IWLP with patients with severe OSA undergoing CPAP treatment.
METHODS
Trial design
A randomized controlled parallel-group prospective study was designed. Patients were randomly assigned to a group either undergoing an IWLP or receiving standard lifestyle recommendations over a period of 12 months. Randomization was performed using a computer-generated automated program including block randomization with a block size of 5.
At baseline, all variables were measured and the patients in the intervention group began the IWLP, while the patients in the control group continued with their regular medical visits. Sleep studies were repeated at 3 months and at 1 year and all variables were measured again.
Participants
Patients were recruited from the Sleep Unit at the Bellvitge University Hospital outpatient clinic. Recruitment started in November 2014 and ended in April 2016. The inclusion criteria were as follows: age 25–60 years, class I and II obesity (BMI 30–40 kg/m2), severe OSA (AHI > 30 events/h), and treatment with CPAP for a minimum of 6 months previous to inclusion. Exclusion criteria were: contraindications for physical activity or diet, cognitive impairment, or psychiatric disorders that impeded patients’ understanding of the program; severe diseases; major cardiovascular disease; clinical instability within the previous month; prior bariatric surgery; refusal to participate in the study; and participation in another clinical trial. The study protocol was approved by the local ethical committee (PR209/14). All participants provided written informed consent. The clinical trial registration number was NCT02832414.
Procedures and measurements
Sleep study
All patients underwent polysomnography (PSG) at baseline, at 3 months, and at 12 months. Patients were instructed to cease CPAP treatment for 3 nights prior to PSG. Apnea was defined as an absence or a 90% decrease in airflow for at least 10 seconds.26 Hypopnea was defined as a reduction in airflow with a minimum duration of 10 seconds, in association with 3% oxygen desaturation or an arousal.26 The AHI was defined as the total number of apnea or hypopnea events/h of sleep. Supine time was defined as the percentage of time spent in supine position in relation to recording time.
Other assessments
Sociodemographic data were assessed at baseline. Measurement of visceral fat by abdominal computed tomography was performed at baseline and at 12 months.27 The following assessments were carried out at baseline and at 3 and 12 months: anthropometric variables; body fat composition; general medical history and OSA-related symptoms; self-reported sleepiness assessed by the Epworth Sleepiness Scale28; health-related quality of life measured by the Spanish language version of the Functional Outcomes of Sleep Questionnaire29 and the Spanish language version of the Quebec Sleep Questionnaire30; assessment of well-being by EuroQol31 and the visual analogical well-being scale; measurement of pain by the visual analog scale; subclinical cardiovascular disease measured as carotid intima-media thickness (IMT) by ultrasonography of the supra-aortic vessels; blood pressure; CPAP adherence by the mean hours of usage per night recorded by the time counters; CPAP related side-effects by means of a brief questionnaire; routine laboratory tests including complete blood count, blood coagulation tests, and basic biochemical tests.
Intensive weight-loss program
Patients randomized to the intervention group followed an IWLP under the supervision of an expert nutritionist who conducted behavioral counseling during all the visits. The program consisted of a very low calorie diet (600–800 kcal) with low-calorie liquid meal replacements during 15 days and a 1,200 kcal diet during the rest of the initial intensive diet phase lasting 12 weeks, followed by a hypocaloric (1,200–1,800 kcal) Mediterranean diet32 for the remaining 36 weeks. Unsupervised physical activity was introduced after 15 days. The full protocol is described in the supplemental material.
Control group
The participants in the control group were given general oral and written information about diet and exercise at baseline. To estimate nutrient intake, patients completed a 24-hour food record at baseline, and again at 3 and 12 months.
Statistical analysis
The primary endpoint was a reduction in AHI at 12 months compared to baseline. The null hypothesis was that there would be no differences between the means of the intervention group and the control group. Secondary end points included an improvement in blood pressure levels, lipid profile and glycemic control, a reduction in the risk of subclinical cardiovascular disease, and a reduction in visceral adiposity as well as in other measures of obesity.
The main variable was the reduction in participants’ final AHI with respect to baseline. Accepting an alpha risk of 0.05 and a power of 80% in a bilateral approach, a final sample of 42 patients was deemed sufficient to detect a difference of 15 points in the AHI between the control and intervention groups (a clinically relevant difference), considering a standard deviation of 15 and a loss of 25%. Balanced groups were estimated.
Categorical variables were presented as the number of cases and percentages, and continuous variables as mean and standard deviation or median and interquartile rank. Continuous variables were compared using the t test or the Mann-Whitney U test, as appropriate. Fisher exact test or Pearson χ2 test were applied to assess the relationship between categorical variables. To identify the factors associated with AHI, we estimated a linear mixed-effects model, using the lme4 package33 for R. This method accounts for clustered data within the same participant, using repeated AHI measurements over time. To show the magnitude of the association, we reported regression coefficients corresponding to the fixed effects and their corresponding 95% confidence intervals, and values of P. All model assumptions were assessed graphically and analytically. Full models were built by adding adjusting covariates one at a time, which were finally included in the model if the modified estimated effect > 10%. The adjusting variables considered were treatment groups; sex; variables related to weight and its distribution, such as basal weight, BMI, waist circumference and neck circumference, and weight difference at 3 and 12 months; and supine time at baseline, 3 and 12 months, and variables related to the PSG that could influence on the AHI variation such as percentage of supine time, positional OSA, and sleep efficiency.
Analyses were performed with R software 3.4.0.34 The level of statistical significance was set at 5%.
RESULTS
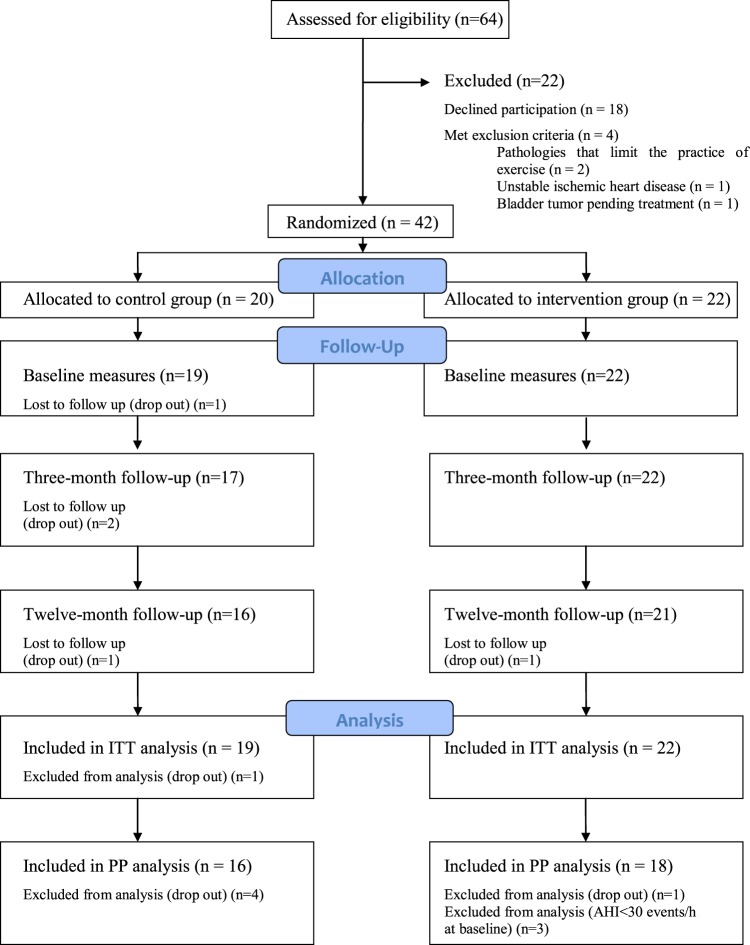
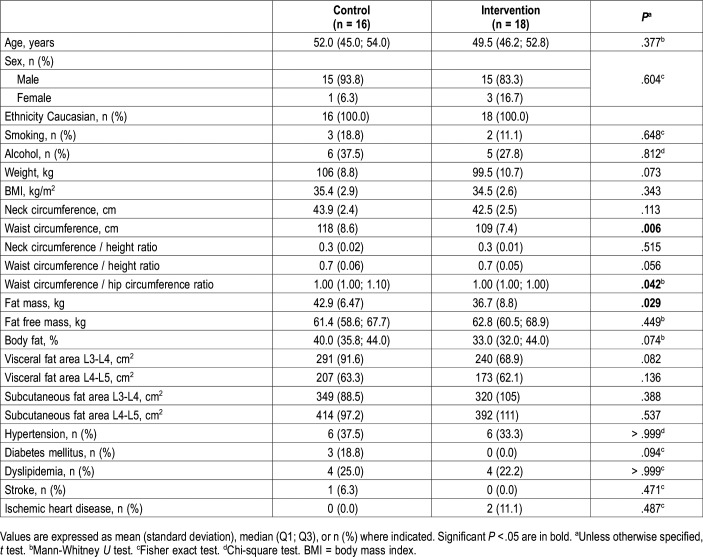
A total of 42 patients (38 male and 4 female) with a mean age of 49 (6.7) years, a mean baseline BMI of 35 (2.7) kg/m2, and a mean AHI of 69 (20) events/h were randomized to the intervention (n = 22) or the control (n = 20) group. Four patients from the control group left the study, whereas only 1 patient in the intervention group left (Figure 1). Three patients from the intervention group had an AHI below 30 events/h in the baseline PSG of the trial and were therefore not included in the per-protocol analysis. There were no differences in the main characteristics between per-protocol and intention-to-treat analyses; therefore, the data shown are from the per-protocol analysis, for which all data are available. The baseline characteristics of the participants are shown in Table 1. Even when patients were randomized, those in the control group, despite having a similar BMI, had a predominance of central adiposity (higher waist circumference, waist/hip ratio, and fat mass that reached statistical significance). No differences were found regarding comorbidities in the two groups. Hypertension was the most prevalent comorbidity, followed by dyslipidemia.
Figure 1. Study flowchart.
ITT = intention-to-treat, PP = per-protocol.
Table 1.
Characteristics of the study population at baseline (n = 34).
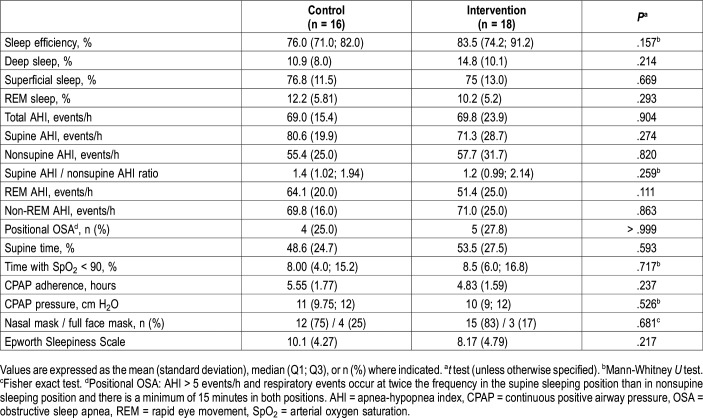
There were no differences between the two treatment groups in respiratory variables (Table 2). CPAP adherence was higher in control group without reaching statistical significance.
Table 2.
Respiratory variables at baseline (n = 34).
Anthropometric parameters
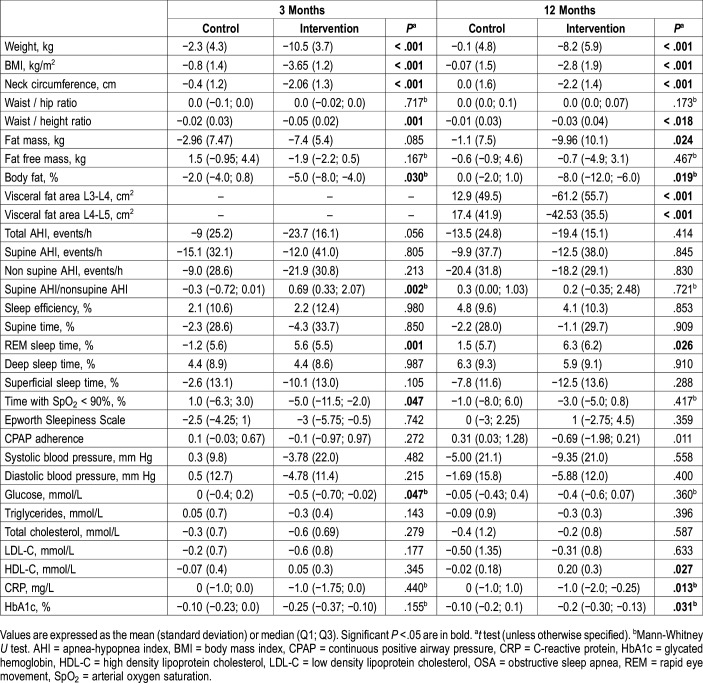
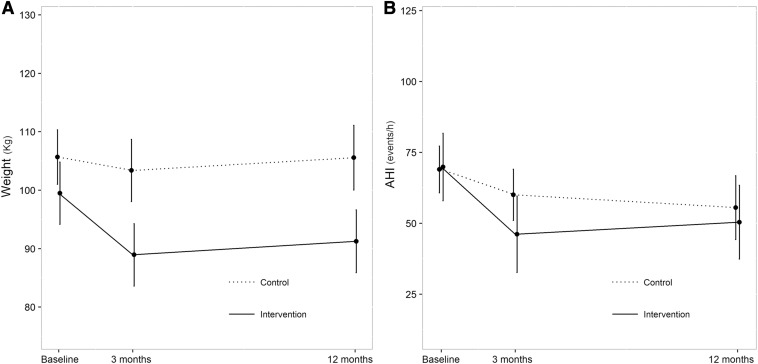
Changes in anthropometric parameters are shown in Table 3. Between baseline and 3 months, patients in the intervention group achieved a total loss of 10.6% with respect to their initial weight. This figure was 8.2% at 12 months. Changes were significant compared to the control group (Figure 2A). BMI, neck circumference, and waist/height ratio showed a greater reduction at 3 months, which was sustained at 12 months in the intervention group. In terms of body composition measured by bioimpedance, the percentage of body fat registered a significant reduction at 3 and 12 months in the intervention group. Visceral obesity measured by abdominal computed tomography was significantly lower in the intervention group than in the control group.
Table 3.
Changes in anthropometric, respiratory and metabolic variables between baseline and 3 months and baseline and 12 months (n = 34).
Figure 2. Change in weight and change in AHI.
(A) Change in weight over the follow-up period. The mean values are represented by dots. (B) Change in apnea-hypopnea index over the follow-up period. AHI = apnea-hypopnea index.
Adherence to diet and physical exercise program
During the first 2 weeks, ketone levels were positive in all patients. During the following 10 weeks, all patients maintained a restriction of 500–700 kcal according to their 24-hour food records. During the past 9 months, only 22% of patients maintained a restriction of 500–700 kcal.
At 3 months, 7 patients (43.8%) from the intervention group met the goal of > 150 minutes of physical exercise per week, decreasing to 3 patients (17.6%) at 12 months. However, 33.3% of patients from the control group practiced > 150 minutes of physical exercise per week at 3 months, decreasing to 4 patients (26.7%) at 12 months.
Respiratory and sleep parameters
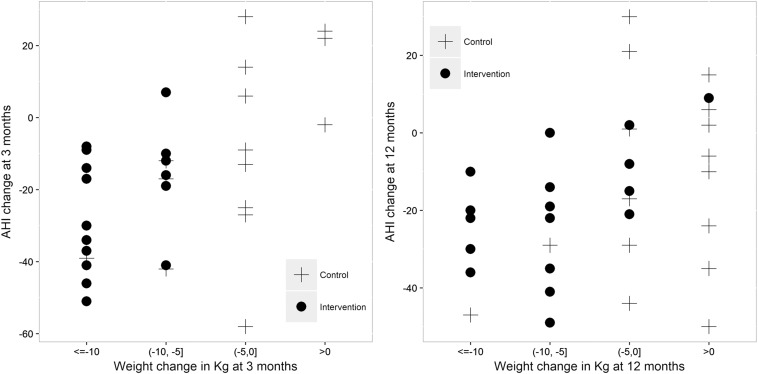
At 3 months, the mean change in AHI was significantly higher in the intervention group. At 12 months, AHI improvement was maintained in the intervention group but there was no significant difference in AHI reduction between groups due to an unexpected improvement in the control group (Table 3 and Figure 2B).
Regarding OSA severity, using AHI cutoffs for mild, moderate, and severe OSA, at 12 months, 1 patient in the intervention group had improved by 2 categories and 4 patients had improved by 1. No one in the control group had improved their OSA category at 12 months. As a result, 28% of patients in the treatment group changed from severe OSA to mild-moderate, compared to none in the control group (P = .046, Fisher exact test).
A significant improvement was observed in the percentage of rapid eye movement (REM) sleep time in the intervention group compared with the control group at 3 and 12 months. No other differences were found regarding sleep variables.
Over the follow-up, the patients in the control group improved CPAP adherence, whereas adherence decreased in the intervention group, reaching statistical significance at 12 months.
Association between AHI reduction and weight change categories
At 3 months of follow-up, all of the patients in the intervention group showed a weight reduction of at least 5 kg (Figure 3). Moreover, a positive association was observed between AHI reduction and weight loss at that time. Hence, patients with a higher weight decrease were those with a greater AHI reduction.
Figure 3. Association between AHI and weight reduction at 3 and 12 months.
AHI = apnea-hypopnea index.
In contrast, at 12 months of follow-up weight reduction was less pronounced in the intervention group and an improvement in AHI was observed in some patients in the control group, even in cases without weight loss.
The correlation between weight change and AHI change was .522 (95% confidence interval .223, .731) from baseline to 3 months and .360 (95% confidence interval .025, .622) from baseline to 12 months.
Mixed-effects models estimating change in AHI over time
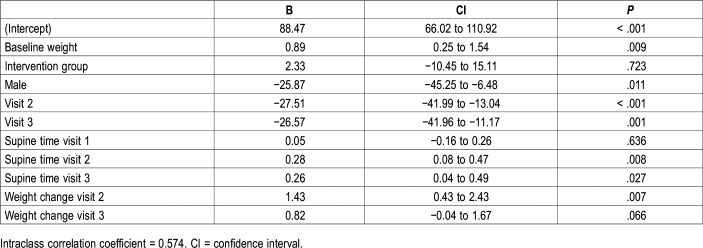
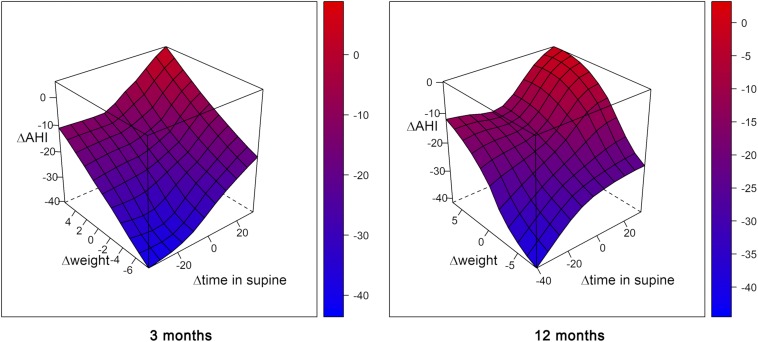
To compare the value of AHI at the baseline visit, at 3 months, and at 12 months, we fitted a mixed-effects model adjusted for potential confounders. The change in AHI (outcome variable) adjusted by sex and baseline weight was related to changes in weight and in supine time (predictor variables). Decreasing the time spent in the supine position decreased the AHI (for each decrease of 10% of supine time, AHI is expected to decrease by almost 3 points). Losing weight also decreases the AHI; for every kilogram of weight lost, the AHI is expected to decrease by 1.43 points (Table 4). Figure 4 shows a graphic representation of the change in AHI considering its interaction with the change in weight and the change in time spent in supine position.
Table 4.
Mixed-effects models estimating change in apnea-hypopnea index over time (n = 34).
Figure 4. 3D surface plot contrasting apnea-hypopnea index change, weight change, and time in supine change at 3 and 12 months.
It can be observed that both weight change and supine change have a certain effect on the change in AHI. On the one hand, with an equal weight loss, AHI improvement is higher the less time is spent in the supine position, while, on the other hand, despite no weight loss, AHI improvement occurs if there is a reduction in the time spent in the supine position. AHI = apnea-hypopnea index.
Comorbidities
Systolic and diastolic blood pressure improved more in the intervention group (Table 3), but this difference did not reach statistical significance.
In terms of metabolic improvement, there was a significant increase in high-density lipoprotein cholesterol and a notable decrease in glycated hemoglobin in the intervention group compared with the control group. With regard to the inflammatory profile, C-reactive protein achieved a significant reduction in the intervention group but not in the control group (Table 3).
Subclinical cardiovascular disease
There was no difference in the reduction in median carotid IMT in the 2 groups. The mean change in IMT during the first 3 months was −0.06 in the control group versus −0.01 in the intervention group (P = .220). At 12 months, IMT decreased −0.08 in the control group versus −0.04 in the intervention group (P = .338).
Quality of life
Between baseline and 12 months, the average Functional Outcomes of Sleep Questionnaire score decreased in the control group and increased in the intervention group. No significant differences were observed. The average Quebec Sleep Questionnaire score increased at 12 months in both the control and the intervention group, though without reaching statistically significant differences. The EuroQoL score and the Well-being EuroQoL scale did not change between baseline and 12 months in either of the two groups. These data are summarized in Table S1 in the supplemental material.
Adverse events
During the first 3 months, there were 2 cases of iron deficiency anemia that were reversed with oral iron treatment. Four patients experienced transient constipation that was solved by hygienic-dietetic measures. Regarding physical activity, 2 patients experienced knee pain as evaluated by rehabilitation doctors. No unwanted effects were recorded in the control group.
DISCUSSION
To the best of our knowledge, this is the first randomized study to evaluate a weight-loss program as a treatment for OSA in a population of patients with severe OSA who were already undergoing CPAP treatment. The program proved to be highly effective at 3 months, achieving greater weight loss and a reduction in the AHI in the intervention group compared to the control group. At 12 months, 28% of the patients in the intervention group managed to reduce their AHI to under 30 events/h and withdrawal of CPAP treatment could therefore be considered, compared to no participants in the control group. In addition, small but clinically relevant benefits at the metabolic level were also observed in the intervention group.
Our program included a low-calorie diet, physical activity, and behavioral therapy, as recommended by recent guidelines.35 As would be expected, weight loss was greater at 3 months, immediately following the most intensive phase, than at 12 months when the diet was less intensive and the visits with the dietician were less frequent. Weight loss was 10.6% of the initial weight at 3 months and 8.2% at 12 months, values similar to those achieved in other studies.11–13
In our study, it should be noted that the improvement in the degree of obesity was mainly due to an improvement in the degree of central obesity. We observed a significant reduction in visceral fat measured by abdominal computed tomography at 12 months, together with a reduction in fat mass and body fat percentage as measured by bioelectrical impedance analysis in the intervention group. We can therefore state that the weight loss achieved was linked directly to a reduction in central obesity. The close association between OSA and visceral obesity has been known for some time36 and a reduction in visceral fat will have beneficial effects on both OSA and the metabolic consequences of obesity.
The effect of weight loss on the AHI was clear at 3 months (Figure 3). However, at 12 months there was a greater variability (Figure 3). We observed that some patients in the control group had improved their AHI without losing weight. We analyzed the different factors that influence an improvement in AHI at 12 months using mixed-effects models. Variables related to weight and its distribution, such as basal weight, BMI, waist circumference, and neck circumference, along with variables related to PSG that could influence the AHI variation, such as percentage of supine time, positional OSA and sleep efficiency, were included in the model. In the model with the greatest explanatory capacity, basal weight, weight loss at 3 months, and the percentage of time in the supine position at 3 and 12 months showed a statistically significant association with changes in AHI. It is known that there is an increased severity of OSA in the supine position37 and an association between supine time and total AHI.38 This may explain, though only in part, the improvement in AHI observed in some patients in the control group who did not lose weight.
Other factors that could influence AHI change, in addition to the model, were also considered: first, the possible influence of physical activity,39 though this was ruled out due to the absence of any increase in physical activity among control group participants; second, the possible residual effect of CPAP,40 which was excluded because only one patient in the control group had discontinued CPAP treatment for fewer than 3 nights before PSG at 12 months; and third, the role of intraindividual variations in AHI when PSG was repeated, which cannot be excluded.41,42
Even though the reduction in AHI observed in the intervention group at 12 months is not significant with respect to that of the control group, it should be noted that 28% of the patients in the intervention group reduced their AHI to below 30 events/h at 12 months compared to no one in the control group. The fact that 28% of the patients in the intervention group transitioned from a severe to a moderate/mild form of the disease may have important implications in terms of morbidity and mortality43 in addition to the possibility of considering discontinuing CPAP treatment. This can be considered to be a successful result, especially when patients’ severe initial conditions are taken into account.
Regarding previous studies that have analyzed the effect of weight loss on OSA, this study includes patients with serious OSA. Most studies11–13,17–19 include patients with AHI > 15 events/h with a basal mean AHI much lower than in our study. The participants in a study by Tuomilehto et al11 had mild OSA (AHI 5–15 events/h), and other studies12,13,18–20 included patients with higher OSA having an average basal AHI between 23–43 events/h, which is considerably lower than the average basal AHI of participants assessed in the present study (AHI 69 events/h).
A meta-analysis that included four randomized controlled trials assessing the effect of intensive lifestyle interventions on weight change and AHI reduction found that a weight loss of 14 kg was associated with a reduction in AHI of 16 events/h.44 The reduction achieved in the AHI of patients in the intervention group in our study was more than 20 points at 3 months, with a difference of almost 15 points with respect to the control group. Therefore, the effect of weight loss was similar in terms of the reduction of the AHI despite initial severity.
Another difference compared to most previous studies is that all the patients we included were undergoing CPAP treatment. We included patients with severe disease based on the results of two previous studies that had demonstrated greater improvements after weight loss in patients with a severe form of the disease.12,13 Additionally, by including patients undergoing CPAP treatment we avoided having to exclude patients for ethical issues due to the severity of their conditions. The success of a diet-based approach is less well studied in patients with OSA that has already been corrected by CPAP and our study shows similar results to those including patients who have not been treated with CPAP. If we take into account that they are affected by severe OSA, it is possible that undergoing CPAP treatment, and theoretically having a better quality of sleep, allows them to follow a diet and achieve a weight loss that, without CPAP and with sleep fragmentation, would be very difficult to achieve. The results of our study are therefore applicable to patients who are already undergoing treatment with CPAP with the aim of being able to suspend this treatment, reduce pressure requirements, or simply improve the comorbidities associated with obesity.
Although not statistically significant, an important clinical reduction in blood pressure was observed in the intervention group at 12 months. In a recent study,25 a reduction in blood pressure was observed in patients with moderate or severe OSA undergoing either CPAP treatment or on a weight-loss program, or both, which suggests that both OSA and obesity have different causal relationships with high blood pressure. Regarding metabolic profile, the patients who took part in the IWLP managed to increase their levels of high-density lipoprotein cholesterol and reduce their levels of glycated hemoglobin, though they did start with levels within the normal range. It should be pointed out that all patients were undergoing CPAP treatment, which means that the observed improvement was due to the effect of weight loss alone. Furthermore, an admittedly modest reduction in C-reactive protein was achieved by the intervention group in comparison with the control group. These results are in line with those of other published studies,45 which reported a reduction in chronic secondary inflammation following weight loss in obese patients. There was no significant reduction in the thickness of carotid IMT in the intervention group compared to the control group. It should be borne in mind that baseline values were less than 0.9 mm in most cases, a figure above which there is considered to exist an increase in cardiovascular risk.46 There is known to be an association between the thickness of carotid IMT and OSA47 and a recent meta-analysis48 concluded that the thickness of carotid IMT is modified by undergoing CPAP treatment only in patients with very severe OSA (AHI > 50 events/h) and with > 6 months of treatment with CPAP. It is therefore unrealistic to expect extra weight loss benefit in our patients.
The benefits we observed in patients’ metabolic profile, though admittedly modest, are important enough to be taken into account in the management of patients with OSA, because our goal is not only to control patients’ symptoms but also to improve their cardiovascular risk profile. This is congruent with an approach that emphasizes the holistic treatment of the patient, focusing on both controlling OSA and the metabolic consequences of the underlying factor, which is obesity. Treating obesity and reducing OSA severity is important not only to enable some patients to discontinue CPAP treatment but also so that the other patients can benefit from a reduction in disease severity and its consequences while they are not undergoing CPAP treatment. It is well known that many patients discontinue CPAP in the last hours of the night and therefore go untreated during the last hours of the sleep period when REM sleep prevails. REM sleep OSA has been associated with an increased risk of hypertension49 and cardiovascular disease50 and glucose metabolism impairment.51 Moreover, although it is well known that OSA in itself contributes to weight gain,52 a meta-analysis of randomized controlled studies demonstrated that CPAP treatment is not only not accompanied by weight loss but may also be associated with a slight but significant increase in weight.9 Therefore weight loss strategies are mandatory for treating obesity in patients with OSA in order to improve their general health status. In our study, these improvements can only be attributed to weight loss, because patients were previously undergoing CPAP treatment, and, regarding adherence with treatment, there were no significant changes at 3 months, whereas at 12 months adherence was better in the control group.
The strengths of this study include its randomized controlled design, which entails a low probability of selection bias and residual confounding. Furthermore, the study had a low dropout rate in the intervention group, which highlights the fact that the program is realistic and able to foster good adherence rates—the dropout rate in most weight-loss programs is higher. Unlike most studies, we used the gold standard PSG for OSA diagnosis. Additionally, the patients included were cases with severe OSA who, clinically speaking, can obtain the greatest benefits from a reduction in AHI. In this work, we carefully assessed anthropometrics, body composition, visceral fat, metabolic parameters and intima media thickness in order to assess the cardiometabolic effect of the program. Additionally, the low- cost, low-energy diet treatment program we used is simple and realistic and could potentially be implemented in primary care.
Limitations
It should be noted that the sample size of this work, though in line with the figure calculated initially, is not particularly large, which may have an influence on the variability of the results and the unexpected improvement in AHI in the control group. When compared with other studies assessing the effect of diet on OSA, the current study included patients with a greater disease severity, and this could also explain in part the variability of the results. Also, most patients were male, so results need to be replicated in female predominant samples. Regarding physical activity, it must be borne in mind that the physical activity program was carried out without supervision, which probably helps to explain the low adherence with the rules set out. Additionally, physical activity adherence was assessed by a simple questionnaire that may not have been sensitive enough to detect intergroup differences. However, we do know that most of the studies that have demonstrated a beneficial effect of physical activity on OSA did so by applying a program with monitored training sessions.
The visits in our program were not as tightly scheduled as in other studies with a view to its reproducibility in our health system. Given the weight increase that occurred in the second part of the program, and especially after the 9-month mark, when visits were further apart in time (data not shown), we would now consider implementing more intensive follow-up during the entire year in any future study.
CONCLUSIONS
An IWLP in patients with obesity and severe OSA was effective at reducing weight, achieving a reduction of 10.6% at 3 months and of 8.2% at 12 months. This reduction had a direct effect on central obesity, with a marked decrease in visceral fat. A reduction in AHI was achieved and was significant at 3 months though not at 12 months, partly due to an improvement in the control group. It was possible to consider withdrawing CPAP treatment in 28% of the patients in the intervention group, given the change from severe to mild-moderate OSA. Weight reduction also resulted in an improvement in lipid profiles, glycemic control and inflammatory markers. These results highlight the need to incorporate weight-loss programs in the treatment of patients with severe OSA undergoing CPAP treatment with the aim of improving their general health status.
DISCLOSURE STATEMENT
All authors have read and approved the final version of this manuscript. Work for this study was performed at Bellvitge University Hospital. This research was partly supported by a grant by Sociedad Española de Neumología y Cirugía Torácica [Grant: SEPAR 72/2015]. Projects fees were partially supported by LINDE Healthcare España. The authors report no conflicts of interest. M Inmaculata Ramos is a LINDE Healthcare España employee.
ACKNOWLEDGMENTS
Biostatistical support was provided by Cristian Tebé and Natàlia Pallarès (statistical advisor service, Bellvitge Biomedical Research Institute-IDIBELL). David Bridgewater assisted with the English in versions of the manuscript. The authors thank all the staff of the Sleep Unit at Bellvitge University Hospital for their inestimable collaboration.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- IMT
intima-media thickness
- IWLP
intensive weight loss program
- OSA
obstructive sleep apnea
- PP
per-protocol
- PSG
polysomnography
- REM
rapid eye movement
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7(5):920–929. doi: 10.3978/j.issn.2072-1439.2015.04.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99(4):1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDaid C, Duree KH, Griffin SC, et al. A systematic review of continuous positive airway pressure for obstructive sleep apnoea-hypopnoea syndrome. Sleep Med Rev. 2009;13(6):427–436. doi: 10.1016/j.smrv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Sanders MH, Montserrat JM, Farre R, Givelber RJ. Positive pressure therapy: a perspective on evidence-based outcomes and methods of application. Proc Am Thorac Soc. 2008;5(2):161–172. doi: 10.1513/pats.200709-150MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veasey SC, Guilleminault C, Strohl KP, Sanders MH, Ballard RD, Magalang UJ. Medical therapy for obstructive sleep apnea: a review by the Medical Therapy for Obstructive Sleep Apnea Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 2006;29(8):1036–1044. doi: 10.1093/sleep/29.8.1036. [DOI] [PubMed] [Google Scholar]
- 9.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70(3):258–264. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]
- 10.Capehorn MS, Haslam DW, Welbourn R. Obesity treatment in the UK health system. Curr Obes Rep. 2016;5(3):320–326. doi: 10.1007/s13679-016-0221-z. [DOI] [PubMed] [Google Scholar]
- 11.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179(4):320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 12.Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169(17):1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomilehto H, Gylling H, Peltonen M, et al. Sustained improvement in mild obstructive sleep apnea after a diet- and physical activity-based lifestyle intervention: postinterventional follow-up. Am J Clin Nutr. 2010;92(4):688–696. doi: 10.3945/ajcn.2010.29485. [DOI] [PubMed] [Google Scholar]
- 15.Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ. 2011;342:d3017. doi: 10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuna ST, Reboussin DM, Borradaile KE, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36(5):641–649. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148(5):1193–1203. doi: 10.1378/chest.14-3016. [DOI] [PubMed] [Google Scholar]
- 18.Igeström H, Asenlöf P, Emtner M, Lindberg E. Improvement in obstructive sleep apnea after a tailored behavioural sleep medicine intervention targeting healthy eating and physical activity: a randomised controlled trial. Sleep Breath. 2018;22(3):653–661. doi: 10.1007/s11325-017-1597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cayanan EA, Marshall NS, Hoyos CM, et al. Maintenance diets following rapid weight loss in obstructive sleep apnea: a pilot 1-year clinical trial. J Sleep Res. 2018;27(2):244–251. doi: 10.1111/jsr.12572. [DOI] [PubMed] [Google Scholar]
- 20.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyos CM, Killick R, Yee BJ, Phillips CL, Grunstein RR, Liu PY. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67(12):1081–1089. doi: 10.1136/thoraxjnl-2011-201420. [DOI] [PubMed] [Google Scholar]
- 22.Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung. 2009;187(1):17–22. doi: 10.1007/s00408-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 23.Salord N, Fortuna AM, Monasterio C, et al. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. Sleep. 2016;39(1):35–41. doi: 10.5665/sleep.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234(2):446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370(24):2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211(1):283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer M, Vilagut G, Monasterio C, et al. [Measurement of the perceived impact of sleep problems: the Spanish version of the functional outcomes sleep questionnaire and the Epworth Sleepiness Scale] Med Clin (Barc) 1999;113(7):250–255. [PubMed] [Google Scholar]
- 30.Lacasse Y, Bureau MP, Series F. A new standardised and self-administered quality of life questionnaire specific to obstructive sleep apnoea. Thorax. 2004;59(6):494–499. doi: 10.1136/thx.2003.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EuroQol Group EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 32.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 33.Bates D, Maechler M, Ben Bolker SW. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 34.R Development Core Team The R Project for Statistical Computing. https://www.r-project.org/. Accessed July 3, 2019.
- 35.Hudgel DW, Patel SR, Ahasic AM, et al. The role of weight management in the treatment of adult obstructive sleep apnea an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;198(6):e70–e87. doi: 10.1164/rccm.201807-1326ST. [DOI] [PubMed] [Google Scholar]
- 36.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 37.Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18(1):7–17. doi: 10.1016/j.smrv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Yalciner G, Babademez MA, Gul F. Association of sleep time in supine position with apnea-hypopnea index as evidenced by successive polysomnography. Sleep Breath. 2017;21(2):289–294. doi: 10.1007/s11325-016-1401-5. [DOI] [PubMed] [Google Scholar]
- 39.Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184(10):1192–1199. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 41.White LH, Lyons OD, Yadollahi A, Ryan CM, Bradley TD. Night-to-night variability in obstructive sleep apnea severity: relationship to overnight rostral fluid shift. J Clin Sleep Med. 2015;11(2):149–156. doi: 10.5664/jcsm.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Bon O, Hoffmann G, Tecco J, et al. Mild to moderate sleep respiratory events: one negative night may not be enough. Chest. 2000;118(2):353–359. doi: 10.1378/chest.118.2.353. [DOI] [PubMed] [Google Scholar]
- 43.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell LJ, Davidson ZE, Bonham M, O’Driscoll DM, Hamilton GS, Truby H. Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis. Sleep Med. 2014;15(10):1173–1183. doi: 10.1016/j.sleep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 46.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28(12):1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 47.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 48.Chen LD, Lin L, Lin XJ, et al. Effect of continuous positive airway pressure on carotid intima-media thickness in patients with obstructive sleep apnea: a meta-analysis. PLoS One. 2017;12(9):e0184293. doi: 10.1371/journal.pone.0184293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aurora RN, Crainiceanu C, Gottlieb DJ, Kim JS, Punjabi NM. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med. 2018;197(5):653–660. doi: 10.1164/rccm.201706-1112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chami HA, Gottlieb DJ, Redline S, Punjabi NM. Association between glucose metabolism and sleep-disordered breathing during REM sleep. Am J Respir Crit Care Med. 2015;192(9):1118–1126. doi: 10.1164/rccm.201501-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ong CW, O’Driscoll DM, Truby H, Naughton MT, Hamilton GS. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2013;17(2):123–131. doi: 10.1016/j.smrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.