Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is thought to be associated with dyslipidemia. However, differences concerning dyslipidemia during rapid eye movement (REM) and non-REM (NREM) sleep have yet to be determined. This study was designed to explore the association between lipid profiles and OSA during REM or NREM sleep.
Methods:
This is a clinical cohort. A total of 2,619 participants with at least 30 minutes of REM sleep were included. Sleep variables and fasting lipid profiles [total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein (apo)A-I, apoB, apoE, and lipoprotein(a) (Lp(a))] were obtained from each participant. Apnea-hypopnea indices in REM and NREM sleep (AHIREM and AHINREM, respectively) were recorded. Linear regression analysis was used to assess the associations of AHIREM and AHINREM with lipid profiles.
Results:
When stratified by the AHIREM severity of OSA, all demographics, clinical variables, and sleep parameters differed between the groups except for apoA-I. In fully-adjusted multivariate linear regression models, AHIREM was independently associated with increasing levels of TG, HDL-C, and apoE (P = .04, P = .01 and P = .01, respectively). AHINREM was independently associated with increasing levels of TC, TG, LDL, and apoB, and lower level of HDL-C (all P < .05). In sensitivity analyses by only exploring associations in patients who had an AHINREM or AHIREM < 5 events/h in separate regression models, AHIREM was not associated with all-lipid profile in almost all adjusted models (all P > .05), whereas AHINREM was associated with elevated TC, LDL-C, and apoB (P = .03, P = .01 and P = .01, respectively).
Conclusions:
AHINREM was independently associated with the greatest alterations in serum lipids, including TC, LDL-C, and apoB.
Citation:
Xu H, Xia Y, Li X, et al. Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. J Clin Sleep Med. 2020;16(4):475–482.
Keywords: dyslipidemia, lipid profile, obstructive sleep apnea, rapid eye movement
INTRODUCTION
Rapid eye movement (REM) sleep accounts for almost 25% of the total sleep time in humans and is associated with distinct physiologic alterations (ie, sympathetic activity, lower vagal tone, and cardiovascular instability).1 During REM sleep, pharyngeal dilator muscles and genioglossus activity are suppressed, which can lead to increased upper airway collapse.2 Such physiologic features during REM sleep can increase the likelihood of obstructive sleep apnea (OSA). In a previous study, patients with OSA experienced a longer duration of decreased oxygen desaturation, with more frequent and worse episodes, during REM sleep compared to non-REM (NREM) sleep.3
In line with the pathophysiologic differences that exist between REM and NREM sleep in terms of OSA, the associations of OSA with early cardiovascular disease (CVD) and CVD also appears to differ according to sleep stage (REM versus NREM). In women, severe OSA during REM sleep was independently associated with the early signs of atherosclerosis, as evidenced by intima thickness.4 The apnea-hypopnea index (AHI) during REM sleep (AHIREM) was independently associated with peripheral arterial stiffness in patients with OSA.5 In addition, the occurrence of severe OSA during REM sleep is associated with a higher incidence of common CVD.6,7
Because metabolic syndrome is an independent predictor of CVD and all-cause mortality,8 an exploration of the individual components of metabolic syndrome (ie, hypertension, diabetes, and dyslipidemia) during REM and NREM sleep in patients with OSA could help to clarify the possible mechanisms regarding OSA and OSA-related CVD risk. Previously, cross-sectional and longitudinal data from the Wisconsin Sleep Cohort Study determined that OSA during REM sleep was independently associated with hypertension.9 In another study, REM OSA was associated with a nondipping pattern of nocturnal blood pressure.10 In a sample of men without a prior diagnosis of OSA, undiagnosed OSA during REM sleep, but not during NREM sleep, was independently associated with hypertension.11 OSA during REM sleep could influence long-term glycemic control in patients with type 2 diabetes.12 In the Sleep Heart Health Study, AHIREM was associated with insulin resistance, but not with fasting glycemia or glucose intolerance.13 In the HypnoLaus cohort, REM OSA is independently associated with metabolic syndrome and diabetes.14 However, it remains unclear whether OSA during REM sleep could promote dyslipidemia.
Therefore, compared with non-REM OSA, REM-related OSA appears to have more detrimental clinical outcomes (ie, hypertension, diabetes, and CVD). Many rodent studies showed that altered serum lipids play a pivotal role in the development of atherosclerosis under conditions of intermittent hypoxia. Our previous clinical studies also showed that OSA was independently associated with impaired dyslipidemia.15,16 Of the various lipids studied, only low-density lipoprotein cholesterol (LDL-C) was independently associated with OSA.16 Thus, whether REM-related OSA leads to more severe dyslipidemia compared to NREM-related OSA, similar to hypertension and glucose metabolism, remains to be determined. In this study, we aimed to assess the relationship between OSA and lipid profiles during REM or NREM sleep in a clinical setting with a large sample size.
METHODS
Study population
From 2007 to 2014, participants who experienced primary snoring and/or daytime sleepiness were consecutively enrolled from the Sleep Center of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Prior to the study, each participant completed a questionnaire capturing basic information, such as health status and medical history. Patients were excluded if they met the following criteria: (1) age younger than 18 years (n = 26); (2) previous anti-OSA therapy such as continuous positive airway pressure (CPAP) treatment, upper airway surgery, or application of an oral appliance (n = 122); (3) history of dyslipidemia and/or on lipid-lowering drugs (n = 198); (4) unstable systemic diseases such as hepatic, pulmonary, or cardiac failure (n = 38); (5) other common sleep disorders (eg, central sleep apnea, restless leg syndrome, upper airway resistance syndrome, or narcolepsy) (n = 26); (6) alcoholism or drug addiction (n = 8); and (7) REM sleep time < 30 minutes (n = 466).
The study was conducted according to the World Medical Association Declaration of Helsinki in 1975 (trial registration number: ChiCTR1900025714), as revised in 1983, and was approved by the Ethical Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. All participants provided their informed written consent.
Anthropometric and biochemical measurements
Well-trained physicians measured common anthropometric indices, such as height, weight, neck circumference (NC), waist circumference (WC), and hip circumference (HC) in each participant. Height was measured by a portable stadiometer having an accuracy of 0.1 cm, with the patient in a standing position. Weight was measured by a digital scale with an accuracy of 0.1 kg, with the patient wearing only undergarments. NC was measured at the level of the cricothyroid membrane. WC was measured at the midpoint between the lowest rib and the iliac crest, with an accuracy of 1 mm. HC was measured at the widest girth at the greater trochanter, with an accuracy of 1 mm. All measurements were recorded twice and the mean value was noted.
Fasting venous blood was collected from each participant. Serum lipid profiles [high-density lipoprotein cholesterol (HDL-C), LDL-C, total cholesterol (TC) and triglycerides (TG), apolipoprotein (apo)A-I, apoB, apoE, and lipoprotein(a) (Lp(a))] were measured using routine procedures in a hospital laboratory.
Sleep evaluation
The Chinese version of the Epworth Sleepiness Scale was used to evaluate the daytime sleepiness of each participant. The total Epworth Sleepiness Scale scores ranged from 0 to 24.17 Hospital-based polysomnography (PSG) (Alice 4 or 5; Respironics, Pittsburgh, Pennsylvania, USA) was used to evaluate the objective sleep status. Standard PSG equipment includes electroencephalography channels (C3-M2 and C4-M1) and allows for electrooculography, submental electromyography, electrocardiography, oronasal airflow monitoring (using both a nasal pressure transducer and oronasal thermistor), monitoring of thoracoabdominal movements (using piezoelectric belts) and snoring sounds, and pulse oximetry. The PSG equipment used herein could automatically record sleep parameters such as apnea, hypopnea, pulse oxygen desaturation, and microarousals. A skilled technician, following the 2007 alternative American Academy of Sleep Medicine (AASM) criteria definition,18 also manually checked the scores.
Calculation and definition
Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). The waist/hip ratio (WHR) was calculated as WC divided by HC. Hypertension was defined as a systolic blood pressure of > 140 mmHg or a diastolic blood pressure of > 90 mmHg. A history of hypertension and current antihypertensive drug treatment were considered to be additional indicators of hypertension. Diabetes was defined as a fasting plasma glucose concentration of ≥ 7.0 mmol/L or the known use of antidiabetic medication before the measurement.
Apnea was defined as reduction in oronasal airflow by at least 90% for at least 10 seconds. Hypopnea was defined as an at least 50% decrease in oronasal airflow for 10 seconds or longer, associated with at least a 3% reduction in oxygen saturation or arousal according to the AASM 2007 alternative criteria.18 The AHI was defined as the sum of apnea and hypopnea events/h of sleep.19 The oxygen desaturation index (ODI) was defined as the total number of oxyhemoglobin desaturation ≥ 3% events/h of sleep. Arousal was defined as abrupt shifts in electroencephalographic frequency lasting at least 3 seconds, and the microarousal index was defined as the mean number of arousal events/h of sleep according to the AASM 2007 alternative criteria. OSA was classified into 4 classes: no OSA (AHI < 10 events/h), mild OSA (AHI 10 < 20 events/h), moderate OSA (AHI 20 < 30 events/h), and severe OSA (AHI ≥ 30 events/h).18 AHIREM and AHINREM were calculated as the number of apnea and hypopnea events/h of REM and NREM sleep, respectively.
Statistical analysis
All statistical analyses in this study were performed using SPSS software (version 22.0; SPSS Inc., Chicago, Illinois, USA). Data are presented as medians (interquartile range), means, and standard deviation (SD), or percentages (for skewed, normally distributed, and categorical data, respectively). Differences in basic characteristics among the 4 OSA groups were examined using the Kruskal-Wallis H-test, 1-way analysis of variance, or the chi-square test, as appropriate. If distributions of variables were skewed, the variables [AHIREM, AHINREM, TG, apoE and Lp(a)] were natural log transformed. The associations of AHIREM [we treated AHIREM as a continuous variable: log (AHIREM + 1)], AHINREM [we treated AHINREM as a continuous variable: log (AHINREM + 1)] with lipid profile were evaluated using multiple linear regression. AHI in either REM or NREM sleep was always included as a covariate in all models. In models for evaluating the association of AHIREM with lipid metabolism, AHINREM was adjusted. Similarly, in models for evaluating the association of AHINREM with lipid metabolism, AHIREM was adjusted. The associations were first explored in a model adjusted for age, sex, and BMI, and then in a multivariable-adjusted model additionally adjusted for WHR, presence of diabetes and presence of hypertension. We also performed a sensitivity analysis by only exploring associations in patients who had an AHINREM or AHIREM < 5 events/h, respectively. Values of P for linear trends across the four groups (non, mild, moderate, and severe OSA) were calculated using the polynomial linear trend test for continuous variables. A value of P < .05 was considered to indicate statistical significance.
RESULTS
Baseline characteristics
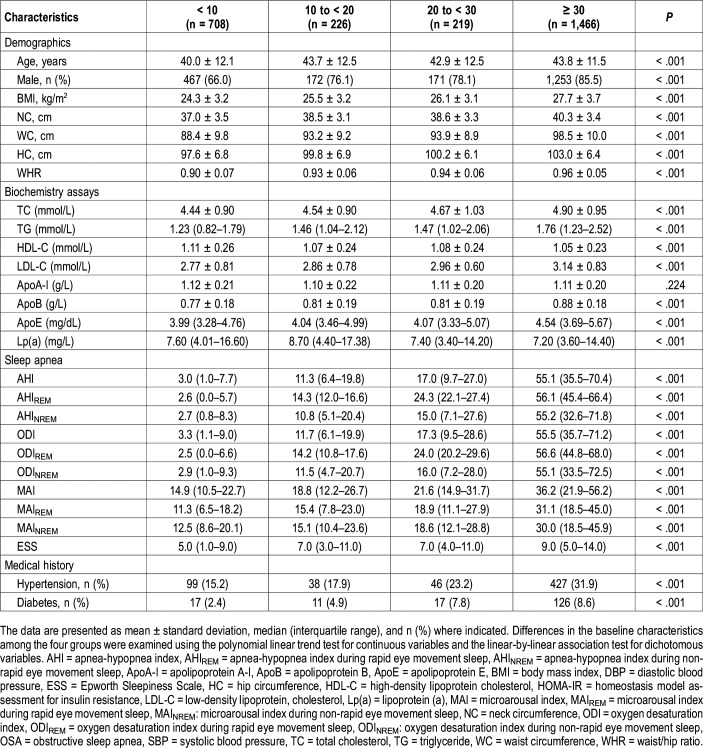
A total of 2,619 participants were enrolled in this study. The demographic and clinical characteristics of the study participants are presented in Table 1. Patients suspected of having OSA were stratified by OSA severity according to AHIREM quartiles. Table 1 shows that participants with more severe REM-related OSA were older and more likely to be male and had a higher BMI and other obesity indices such as NC, WC, HC, and WHR. Moreover, all indices of arousal or apnea frequency, and nearly all biochemical indices, were increased with OSA severity, but not with the presence of apoA-I. Fasting serum levels of TC, TG, LDL-C, apoA-I, apoB, and apoE were generally elevated with OSA severity. However, other lipids, including HDL-C and Lp(a), were decreased with OSA severity.
Table 1.
Basic characteristics of the overall population by the AHIREM severity of OSA.
Relationship between AHIREM and the lipid profile
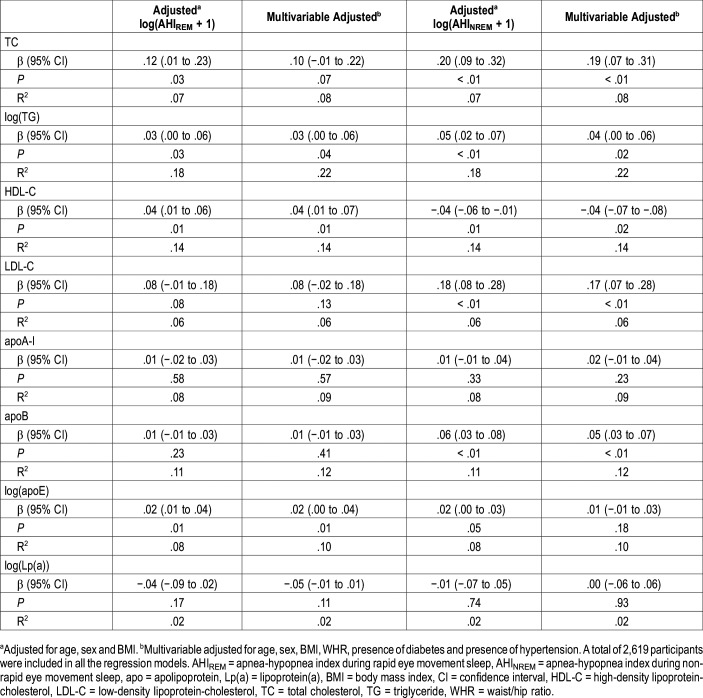
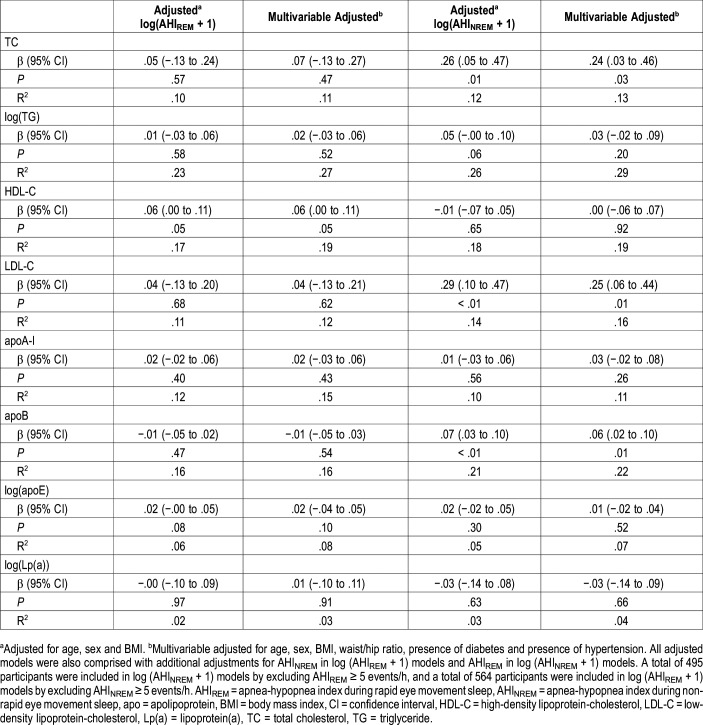
AHIREM was independently associated with increasing levels of TG (β = .029; P = .042), HDL-C (β = .040; P = .005), and apoE (β = .021; P = .014) after fully adjusting for confounders including age, sex, BMI, WHR, presence of diabetes, presence of hypertension and AHINREM (Table 2). However, AHIREM was not associated with TC, LDL-C, apoA-I, apoB and Lp(a) (all P > .05, after adjustment including AHINREM) (Table 2). In sensitivity analyses, we excluded patients who had an AHINREM > 5 events/h and only explored associations in AHINREM < 5 events/h. AHIREM was not associated with all lipid profile in almost all adjusted models (Table 3).
Table 2.
Associations between AHIREM and AHINREM with serum lipid levels (AHIREM and AHINREM analyzed in same regression models).
Table 3.
Associations between AHIREM and AHINREM with serum lipid levels (AHIREM and AHINREM analyzed in separate regression models) by excluding AHINREM ≥ 5 events/h in log (AHIREM + 1) models and AHIREM ≥ 5 events/h in log (AHINREM + 1) models.
Relationship between AHINREM and the lipid profile
AHINREM was independently associated with increasing levels of TC, TG, LDL and apoB, and inversely correlated with levels of HDL-C after adjustment for confounders including AHIREM (all P < .05) (Table 2). We also performed sensitivity analyses by only exploring associations between AHINREM and lipid profile in patients who had an AHIREM < 5 events/h. AHINREM was associated with elevated TC (β = .244; P = .029), LDL-C (β = .246; P = .012), and apoB (β = .055; P = .008) levels (Table 3). There was no relationship between AHINREM and other lipids such as TG, HDL-C, apoA-I, apoE/Lp(a) (all P > .05, after adjustment including AHIREM) (Table 3).
DISCUSSION
This study is the first to reveal a positive association between OSA during REM and NREM sleep and the lipid profile. In this large-scale observational study, we found that AHIREM was independently associated with increasing levels of TG, HDL-C, and apoE. AHINREM was independently associated with increasing levels of TC, TG, LDL, and apoB, and a lower level of HDL-C. However, only AHINREM was associated with elevated levels of TC, LDL-C, and apoB in further sensitivity analyses.
OSA has been associated with an altered lipid profile in both clinical and community-based studies.16,20 However, few current studies have focused on the relationship between the lipid profile and REM or NREM sleep. Previous studies showed that REM OSA, as opposed to NREM OSA, was independently associated with hemoglobin A1c, insulin resistance, and hypertension.11–13 Similarly, OSA during REM sleep might also contribute to dyslipidemia, which may also be attributable to elevated sympathetic activity and the accompanying intermittent hypoxia and recurrent arousal/sleep fragmentation. However, association did not persist in REM OSA and lipids when sensitivity analyses were performed. This seems that lipids were more prone to be related to AHINREM. The results were largely inconsistent with our original hypothesis that AHINREM might be a better predictor for dyslipidemia. Because sympathetic tone is already elevated during REM sleep, there may be a ceiling effect, such that further bursts in sympathetic tone from apneas/hypopneas in REM have limited effect on further freeing circulating lipids during this stage. Contrary to our expectations, we observed a positive association between AHIREM and HDL (which one might interpret as being cardioprotective), though the association did not persist after sensitivity analysis in which participants with AHINREM > 5 events/h were excluded. This might be partly explained by other potential residual confounders not adjusted for in our study.
During sleep, patients with OSA exhibited a marked increase in free fatty acids compared to control participants, and this increase persisted throughout the entire sleep period (mostly in NREM sleep).21 Increased levels of free fatty acids might decrease the production of growth hormone, which is mainly secreted during deep sleep.22 Reduced growth hormone appears to contribute independently to dyslipidemia.23 Thyrotropin also increased in NREM sleep and is closely associated with dyslipidemia.24 These altered hormone levels might partly explain why lipids (including TC, LDL-C, and apoB) are independently associated with AHINREM. Interestingly, we found that other lipids such as TG and apoE were not associated with AHINREM. This lack of a relationship between TG, apoE, and AHINREM was seen after adjusting for the obesity indices. Thus, obesity had a much greater effect on TG/apoE compared to AHINREM. A previous study also showed that OSA had no independent association with lipid profile abnormalities, with obesity being the major determinant of metabolic abnormalities.25 HDL-C, apoA-I, and Lp(a) were also uncorrelated with AHINREM. Recently, the European Sleep Apnea Database cohort with the largest sample size revealed that OSA severity was independently associated with TC, not HDL-C.26 This was consistent with our findings. Because apoA-I is the main component of HDL-C, the change of apoA-I is in agreement with that of LDL-C. Similar to our previous reports that Lp(a) was not correlative with AHI, level of Lp(a) was also unaltered in this study.15
From meta-analysis of observational studies, patients with OSA appeared to have an increased risk of dyslipidemia, evidenced by high TC, LDL-C, and TG levels, and a low HDL-C level.27 However, in interventional studies, the effects of CPAP on lipid levels were equivocal. Our previous meta-analysis showed that CPAP could decrease the TC level, especially in patients with OSA who use CPAP over a relatively long period.28 Recent clinical studies drew different conclusions. In one such study, 3 months of CPAP treatment did not alter any metabolic variable, including the lipid profile, in women.29 Recently, a randomized controlled trial compared the effects of 2 months of CPAP usage with sham-CPAP on Lp(a), and a negative result was found [Lp(a) was not changed]. However, Lp(a) improved in those adherent to CPAP.30 Such conflicting results might be partly explained by the duration of CPAP usage. In our study, NREM OSA was mainly associated with elevated lipid levels. CPAP adherence is greater in the earlier part of the night where NREM sleep predominates. Therefore, longer CPAP usage might lead to lower lipid levels. Thus, recommendations regarding CPAP usage need to encompass a sufficiently long period to cover all NREM sleep time, to achieve a greater lipid-lowing effect.
The strengths of this study include its large sample size, rigorous quality control measures, recording of standard PSG data using strict protocols, and adequate adjustment for confounding factors. However, some limitations in the study also need to be mentioned. First, the cross-sectional design meant that we were unable to identify causal relationships. Second, the study used a hospital-based design, which has limited generalizability to the large number of people in whom OSA remains undiagnosed. Third, although obesity is among the most important confounding factors, we only adjusted for variables such as BMI and WHR; subcutaneous adipose tissue and visceral adipose tissue were not measured. Fourth, specific medications for either diabetes (such as metformin) or hypertension could be affecting blood lipid levels. Fifth, other residual confounders such as lifestyle, exercise, and diet also affect the serum lipid profile; however, it was difficult to consider all of these factors thoroughly.
In conclusion, we found that AHINREM is more strongly associated with lipid metabolism than AHIREM.
DISCLOSURE STATEMENT
Work for this study was performed at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China. This study was supported by grants-in-aid from National Key R&D Program of China (2017YFC0112500); The Joint Project of New Frontier Technology of Shanghai Shen-kang Hospital Development Center (SHDC 12014240); Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-02-E00047); National Natural Science Foundation of China (81700896, 81770987, 81701306, 81770988); Shanghai Sailing Program (17YF1414300); multi-center clinical research project from School of Medicine, Shanghai Jiao Tong University (DLY201502), and Shanghai Shen-Kang Hospital Management Center Project (SHDC12015101). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the help of all staff members who work at Department of Otolaryngology Head and Neck Surgery & Center of Sleep Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. We are grateful for their assistance with sample collection. Author contributions: The authors take responsibility and vouch for the accuracy and completeness of the data and analyses. Prof. SY, JG and HY had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Study design: HX, JG and SY; Data collection: HX, XL, YQ, FF, HW, JG, HY, YX and JZ; Statistical analysis: HX, YX, JG, HY, YS; Manuscript draft: HX, JG, HY and SY. All authors have seen and approved the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AHIREM
apnea-hypopnea index during rapid eye movement sleep
- AHINREM
apnea-hypopnea index during non-rapid eye movement sleep
- ApoA-I
apolipoprotein A-I
- ApoB
apolipoprotein B
- ApoE
apolipoprotein E
- BMI
body mass index
- CVD
cardiovascular diseases
- DBP
diastolic blood pressure
- HC
hip circumference
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment for insulin resistance
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
lipoprotein (a)
- MAI
microarousal index
- MAIREM
micro-arousal index during rapid eye movement sleep
- MAINREM
micro-arousal index during non-rapid eye movement sleep
- NC
neck circumference
- ODI
oxygen desaturation index
- ODIREM
oxygen desaturation index during rapid eye movement sleep
- ODINREM
oxygen desaturation index during non-rapid eye movement sleep
- OSA
obstructive sleep apnea
- SBP
systolic blood pressure
- TC
total cholesterol
- TG
triglyceride
- WC
waist circumference
- WHR
waist/hip ratio.
REFERENCES
- 1.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McSharry DG, Saboisky JP, Deyoung P, et al. Physiological mechanisms of upper airway hypotonia during REM sleep. Sleep. 2014;37(3):561–569. doi: 10.5665/sleep.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180(8):788–793. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljunggren M, Lindberg E, Franklin KA, et al. Obstructive sleep apnea during rapid eye movement sleep is associated with early signs of atherosclerosis in women. Sleep. 2018;41(7) doi: 10.1093/sleep/zsy099. [DOI] [PubMed] [Google Scholar]
- 5.Lin CY, Ho CS, Tsai WC, Chen JY. Different effects of apnea during rapid eye movement period on peripheral arterial stiffness in obstructive sleep apnea. Atherosclerosis. 2018;269:166–171. doi: 10.1016/j.atherosclerosis.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Aurora RN, Crainiceanu C, Gottlieb DJ, Kim JS, Punjabi NM. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med. 2018;197(5):653–660. doi: 10.1164/rccm.201706-1112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokhlesi B, Varga AW. Obstructive sleep apnea and cardiovascular disease. REM sleep matters! Am J Respir Crit Care Med. 2018;197(5):554–556. doi: 10.1164/rccm.201710-2147ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax. 2015;70(11):1062–1069. doi: 10.1136/thoraxjnl-2015-207231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appleton SL, Vakulin A, Martin SA, et al. Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest. 2016;150(3):495–505. doi: 10.1016/j.chest.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Grimaldi D, Beccuti G, Touma C, Van Cauter E, Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–363. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chami HA, Gottlieb DJ, Redline S, Punjabi NM. Association between glucose metabolism and sleep-disordered breathing during REM sleep. Am J Respir Crit Care Med. 2015;192(9):1118–1126. doi: 10.1164/rccm.201501-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Castro P, Hirotsu C, Marti-Soler H, et al. REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J. 2018;52(2) doi: 10.1183/13993003.02484-2017. [DOI] [PubMed] [Google Scholar]
- 15.Guan J, Yi H, Zou J, et al. Distinct severity stages of obstructive sleep apnoea are correlated with unique dyslipidaemia: large-scale observational study. Thorax. 2016;71(4):347–355. doi: 10.1136/thoraxjnl-2015-207403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Guan J, Yi H, et al. Elevated low-density lipoprotein cholesterol is independently associated with obstructive sleep apnea: evidence from a large-scale cross-sectional study. Sleep Breath. 2016;20(2):627–634. doi: 10.1007/s11325-015-1262-3. [DOI] [PubMed] [Google Scholar]
- 17.Chung KF. Use of the Epworth Sleepiness Scale in Chinese patients with obstructive sleep apnea and normal hospital employees. J Psychosom Res. 2000;49(5):367–372. doi: 10.1016/s0022-3999(00)00186-0. [DOI] [PubMed] [Google Scholar]
- 18.Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep. 2015;38(12):1887–1892. doi: 10.5665/sleep.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyama Y, Chin K, Chihara Y, et al. Association between sleep apnea, sleep duration, and serum lipid profile in an urban, male, working population in Japan. Chest. 2013;143(3):720–728. doi: 10.1378/chest.12-0338. [DOI] [PubMed] [Google Scholar]
- 21.Jun JC, Drager LF, Najjar SS, et al. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34(9):1207–1213. doi: 10.5665/SLEEP.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanfranco F, Motta G, Minetto MA, Ghigo E, Maccario M. Growth hormone/insulin-like growth factor-I axis in obstructive sleep apnea syndrome: an update. J Endocrinol Invest. 2010;33(3):192–196. doi: 10.1007/BF03346580. [DOI] [PubMed] [Google Scholar]
- 23.Stanley TL, Grinspoon SK. Effects of growth hormone-releasing hormone on visceral fat, metabolic, and cardiovascular indices in human studies. Growth Horm IGF Res. 2015;25(2):59–65. doi: 10.1016/j.ghir.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delitala AP, Fanciulli G, Maioli M, Delitala G. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. Eur J Intern Med. 2017;38:17–24. doi: 10.1016/j.ejim.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SK, Kumpawat S, Goel A, et al. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8(1):12–17. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Gündüz C, Basoglu OK, Hedner J, et al. Obstructive sleep apnoea independently predicts lipid levels: data from the European Sleep Apnea Database. Respirology. 2018;23(12):1180–1189. doi: 10.1111/resp.13372. [DOI] [PubMed] [Google Scholar]
- 27.Nadeem R, Singh M, Nida M, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10(5):475–489. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234(2):446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Campos-Rodriguez F, Gonzalez-Martinez M, Sanchez-Armengol A, et al. Effect of continuous positive airway pressure on blood pressure and metabolic profile in women with sleep apnoea. Eur Respir J. 2017;50(2) doi: 10.1183/13993003.00257-2017. [DOI] [PubMed] [Google Scholar]
- 30.Paz y Mar HL, Hazen SL, Tracy RP, et al. Effect of continuous positive airway pressure on cardiovascular biomarkers: the sleep apnea stress randomized controlled trial. Chest. 2016;150(1):80–90. doi: 10.1016/j.chest.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]