Abstract
Study Objectives:
The objective of this study was to assess the narratives from the recalled accounts of cataplexy attacks of patients with narcolepsy type 1 (NT1).
Methods:
Twenty-two drug-naive adult patients meeting the International Classification of Sleep Disorders criteria for the diagnosis of NT1 referring to the Narcolepsy Center of the University of Bologna in the year 2017 underwent a semistructured interview focusing on their personal experiences during the cataplectic attacks. Verbatim transcripts were analyzed by thematic analysis of elementary contexts using T-LAB software.
Results:
The thematic analysis performed on the entire body text showed 3 clusters that explained 36.2%, 34.1%, and 29.7% of the variance, respectively: a cluster related to situations that trigger attacks of cataplexy, a cluster to bodily sensations, and another control strategies during episodes. The thematic content highlighted a tendency to disregard emotional experiences, thus affecting the ability to name, recognize, and regulate critical emotions.
Conclusions:
The study showed that patients with NT1 spoke of their cataplectic attacks in terms of self-reported bodily experiences, trigger situations, and their management. Therefore, patients may have developed strategies of symptom management focused on emotion avoidance and/or inhibition.
Citation:
Franceschini C, Fante C, Folli MC, et al. Giving a voice to cataplectic experience: recollections from patients with narcolepsy type 1. J Clin Sleep Med. 2020;16(4):597–603.
Keywords: cataplexy attack, chronic illness, narrative medicine, self-reported experience
BRIEF SUMMARY
Current Knowledge/Study Rationale: The narrative approach to illness proved to be effective to help patients with chronic diseases to become aware of their status in terms of acceptance, control, and general well-being. Understanding what facing the disease truly means for the patients with narcolepsy type 1 could reveal a key to engage them in long-term management treatments.
Study Impact: This is the first original article that applied a narrative approach to enlighten this neglected aspect in the daily life of patients. The patients seem to talk about their cataplectic attacks in terms of self-reported bodily experiences, trigger situations, and their management. Therefore, the results suggest a tendency to disregard emotional experiences that affect the ability to name, recognize, and regulate critical emotions.
INTRODUCTION
Cataplexy is the clinical hallmark of narcolepsy type 1 (NT1), a rare disabling central disorder of hypersomnolence affecting 0.02–0.06% of adults in United States and Europe.1,2 Besides cataplexy, NT1 is clinically characterized by severe, irresistible daytime sleepiness, hypnagogic hallucinations, and disrupted nocturnal sleep.3 The age of NT1 onset varies from early childhood to the 6th decade of life, with a bimodal incidence peak around 15 and around 36 years of age.4–6 The disease is chronic and likely caused by the autoimmune destruction of hypothalamic neurons producing hypocretin.7 Cataplexy is defined as sudden and transient episodes of loss of muscle tone, generally bilateral, triggered while facing emotions (usually laughing and joking). During these episodes, which can last from a few seconds up to 10 minutes, the patient remains fully conscious. Loss of muscle tone may range from partial loss to generalized atonia, resulting in a fall.8 Because of its unusual symptoms and the spontaneous modification of cataplexy through the disease course in children,9 the disease is often diagnosed with a huge delay from its onset4,10 or misdiagnosed and also because of the scarce awareness of NT1 among health care professionals.11 Once diagnosed, patients with NT1 can benefit from behavioral (ie, scheduled naps for daytime sleepiness) and pharmacological treatments, such as stimulant drugs to suppress daytime sleepiness, off-label antidepressants (imipramine, clomipramine, fluoxetine), or selective norepinephrine reuptake inhibitor (namely venlafaxine) and for cataplexy, sodium oxybate and pitolisant that act on both symptoms.12
Despite these treatments, patients with NT1 present several challenges in everyday life caused by the disease, such as in education, home, work, social life,13,14 car accidents (55% of drivers with narcolepsy),15 relations with friends, and leisure activities (especially in adolescents),16 difficulties in coping with babies during the puerperium in mothers with narcolepsy,17 and parental fitness during divorce and custody litigation.18 Kapella et al (2015)19 noticed higher levels of health-related stigma in young adults with narcolepsy. This stigma is linked to difficulties in social relationships, economic disparities, and negative effects on therapeutic adherence and accessibility to high-quality health care.20
Moreover, a disabling psychiatric comorbidity (depression, anxiety, eating disorders, and psychotic disturbances) appears commonly to affect patients with narcolepsy and negatively influence their quality of life (QoL) and the burden of disease.21–24 However, little data about the personal experiences of patients with NT1 during cataplexy exist and can be found only in internet blogs (http://www.narcolessia.org; https://www.anc-narcolepsie.com/forums/).
To cast some light on this neglected aspect, here we applied a narrative approach using a widely adopted technique25–27 that allows gathering insight into the effects of the irruption of disease in the everyday life of patients (and into how the medical team manages it). Oral or written narratives, indeed, have proven to be capable of helping patients with chronic diseases to become aware fully of their status and then rebuild their current identity.28,29
In this explorative study, we attempted to enlighten the perception of cataplexy attacks by means of textual analysis of reports provided by the patients with NT1 themselves.
METHODS
Participants
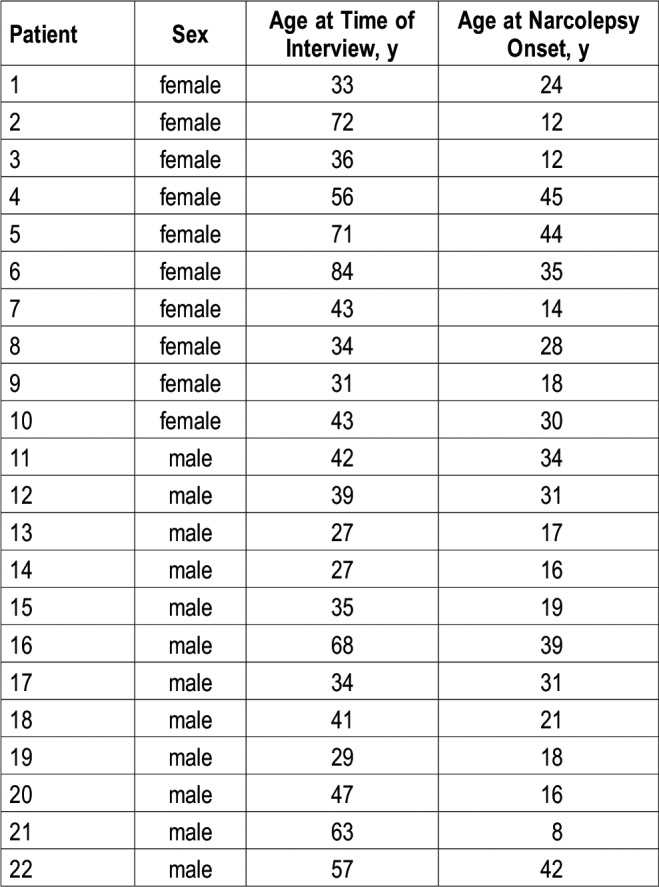
The studied population included a continuous series of 22 drug-naive adult patients with NT1 (12 men and 10 women), diagnosed according to the International Classification of Sleep Disorders criteria.3 Patients were recruited from January to December 2017 at the Outpatient Clinic for Narcolepsy of the University of Bologna. The average age of the patients was 47 years (range from 27 to 84 years), and the average age at the disease onset was 25.2 years (range from 8 to 45 years) (Table 1).
Table 1.
Demographic characteristics of the patients.
Method
The study was qualitative and designed according to the narrative inquiry approach.30 The narrative inquiry approach has the intent to elucidate how people frame, remember, and report their personal experiences. For this reason, the information collected may contribute to reducing false common beliefs by providing a glance of the complexity of human lives and the daily fights of people with a chronic disease.31
Each patient underwent a semistructured face-to-face interview conducted by a trained psychologist (C.F.) to gather self-described descriptions of individual experiences on cataplectic attacks. The interview included open questions on the first recalled cataplectic attack and on the current set of symptoms. The interviews were recorded, and their entire transcribed text has been analyzed. Qualitative textual data were submitted to the thematic analysis of elementary contexts with the support of T-LAB software (T-LAB di Lancia Franco, Roccasecca, Italy).32 This kind of analysis not only provides the researcher a hypothesis on what the participant’s lexical choices may hint at but also highlights possible keyword patterns that repeatedly appear across the narratives. The resulting themes can be considered latent shared themes, which can be influenced by the topics solicited by the interviewer but do not correspond to them; a particular association of concepts/words may in fact appear in response to different topics, thus what emerges as a main theme will be the issue expressed via such an association.33
Participation was voluntary and written informed consent was obtained from all participants. All procedures were in accordance with the ethical standards of the institutional and/or national research committee of each country and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data analysis
The textual analysis was conducted by T-LAB di Lancia Franco software tool that allows the exploration and mapping of the contents in texts of various kinds. The analyses were conducted on the transcripts in the original language; only later, the outputs (the results) were translated into English by a native English speaker for this paper.32 T-LAB di Lancia Franco uses a text-driven automatic analysis approach, based on statistical algorithms, which cluster significant patterns of words and themes (for an example application of this software-based analysis see Bellardita and colleagues, 2012).34
Since the software has a sensitivity to the occurrence of words, we decided to discard the interviewer’s questions and collect only the interview in a single text.
For the analyses, the function “Thematic Analysis of the Elementary Contexts” was used to reconstruct a thread of discourse within the overall structure of the text. Specifically, the function allows the corpus to be represented in a few, significant thematic clusters that possess the following features: each cluster consists of a set of Elementary Context Units (ECUs), ie, sentences or short texts characterized by the same patterns of keywords, and is described through lemmatized keywords (or, in some case, by modalities of variables) that co-occur in the same portions of text (ECU) ranked according to the decreasing value of χ2.32 Each cluster has a different weight according to the percentage of text (sentences) that it includes with respect to the whole amount of ECUs and is, therefore, a theme more or less representative for the participants. All the authors analyzed the statistical outputs obtained while redefining the interpretations and naming each cluster.
RESULTS
The entire corpus was made up of 7,152 keywords and 138 ECUs.
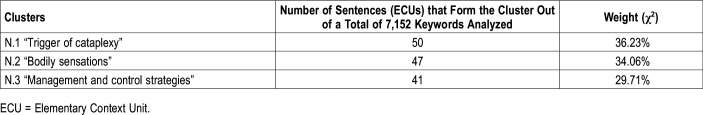
The topic analysis of ECUs carried out on the entire set of data showed 3 clusters that explain 36.2%, 34.1%, and 29.7% of the “variance”, respectively (the percentage of context units indicating their relevance in the overall textual corpus; Table 2). A cluster was related to situations that trigger attacks of cataplexy, 1 to bodily sensations and 1 to control strategies. Table 2, Table 3, Table 4 and Table 5 report the cluster weight, the typical lemmatized keywords for each theme, according to their χ2 values, and some illustrative examples in which they co-occur (ECUs).
Table 2.
Number of sentences forming each cluster and weight percentage compared with the total.
Table 3.
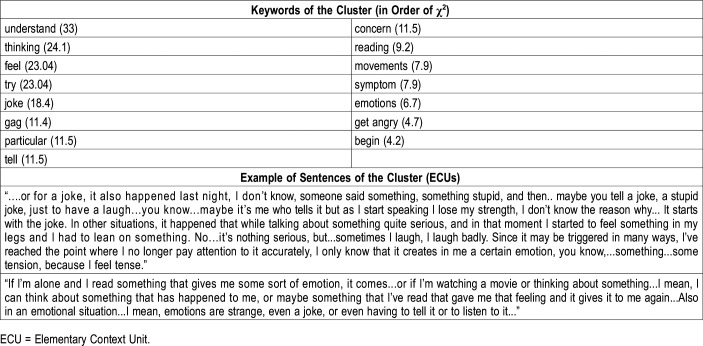
Keywords in order of χ2 and examples of sentences of cluster no.1: trigger of cataplexy.
Table 4.
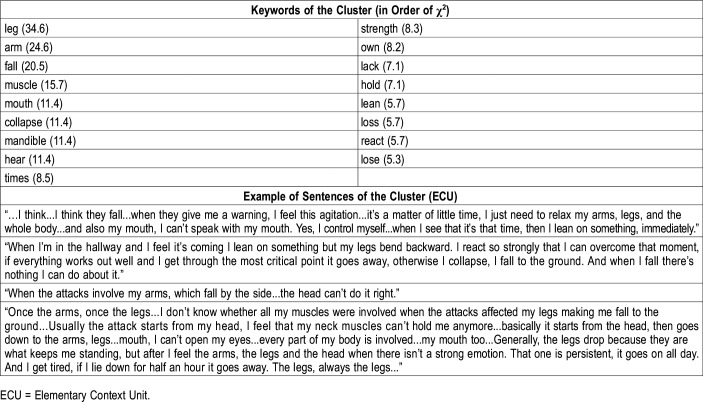
Keywords in order of χ2 and examples of sentences of cluster no.2: bodily sensations.
Table 5.
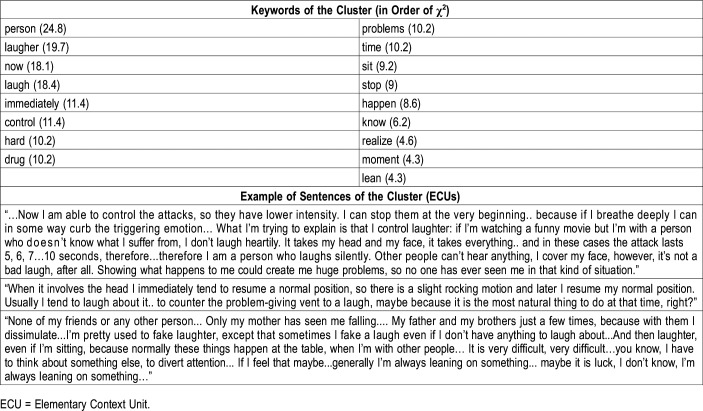
Keywords in order of χ2 and examples of sentences of the cluster no.3: management and control strategies.
Each cluster is briefly described in terms of “keywords” (lexical units together with the corresponding value of χ2. Threshold value of χ2 for each lemma was 3.84; P ≤ 0.05) (Table 2).
Cluster 1: trigger of cataplexy
The first theme, the most representative, concerned self-reported situations that trigger cataplexy attacks, mostly referring to situations related to laughter (joke, gag, telling, reading) (Table 3).
Cluster 2: bodily sensations
The second theme was related to the description of cataplexy attacks at the body level. The attacks are perceived as sudden lack of strength (feel, strength, muscle) in specific areas of the body easy to localize (arms, legs, mouth, jaw). The first keywords in this cluster, according to the χ2 values, were “leg” and “arm” (Table 4).
Cluster 3: management and control strategies
The most relevant issue in the third theme was the management of the attacks. The thematic core was mostly characterized by words referring to relational situations involving laughter (person, laughter, laugh, problems, down, stop) and the attempt to self-control (block, control). Several keywords with high χ2 values were related to “person,” “laughter,” “hard,” “problems,” “sitting” (Table 5).
DISCUSSION
The patients’ own viewpoint about cataplexy experience has never been investigated, despite a potential impact on the global well-being and on the relationships of patients and on professional health care communication strategies by adopting a psychosocial and cross-cultural perspective. With this study, we aimed at unraveling the patient's point of view on his/her emotional and experiential experience of cataplexy.
The results showed 3 dominant themes through which patients with narcolepsy spoke of their cataplectic attacks: triggering situations (36.2% of total ECUs), bodily sensations (34.1% of total ECUs), and control strategies during episodes (29.7% of total ECUs).
When describing the situations that most likely trigger cataplexy attacks, the patients refer more to generic emotional situations (entry “emotion”) and laughter (laugh, laughter, joke), than to anger. From the reports of some patients, it appears difficult to identify and to name emotions, suggesting a difficulty in recognizing emotions at cognitive level (“Also in an emotional situation... I mean, emotions are strange”, “In other situations it happened that while talking... I felt an emotion, in a moment I could feel an emotion while I was talking about something quite serious”). It is possible that some patients could have developed a strategy to minimize their emotional experiences by inhibiting the ability to process emotionally connoted accounts. Accordingly, de Zambotti and colleagues35 disclosed that patients with NT1 had a cerebral hemodynamic response (transcranial doppler) to emotional stimulation (picture viewing) similar to control patients, but inhibited emotion-related behaviors and evaluated the stimuli as less arousing and pleasant than control patients. Conversely, when the emotional stimulation is more intense, cataplexy occurs and is associated with a discrete pattern of cerebral activation (in the amygdala, the nucleus accumbens, the pregenual anterior cingulate cortex, and the ventromedial prefrontal cortex) detected by means of functional magnetic resonance imaging (fMRI).36,37
This result confirmed previous questionnaire-based studies: a questionnaire focusing on cataplexy showed that the symptom was most commonly triggered by “positive” emotions, such as joking and laughing, and that the most commonly reported situations inducing cataplexy were laughing and anger38,39 reported that 40% of patients recognized the presence of “bad days,” certain days in which the frequency of cataplexy was higher, a finding mirrored by our patients reporting days with subcontinuous cataplexy occurrence. Plazzi et al (2011)5 pointed out that the laughter or joke was always the “gold standard” that induced hypotonia or muscular weakness, while anger was a less strong trigger evoking a cataplectic attack.5,8,40 Although this phenomena possibly depends on the fact that the frequency of laughing in daily life is higher than that of getting angry, another hypothesis is that cataplexy is mainly generated by an activity dysregulation of the cerebral territories controlling laughter behavior. In fact, the aforementioned fMRI studies on patients with NT1 showed that some of the regions activated during cataplexy, in particular the nucleus accumbens and the pregenual anterior cingulate cortex, are the same in which the electrical stimulations elicit laughter.41 These two cerebral regions are connected to the hypothalamus, a structure damaged in patients with NT1, but also involved in epileptic manifestations characterized by incontrollable laughter (gelastic seizure).42 Since recent fMRI studies on young patients with NT1 showed that during cataplexy other cerebral structures are also involved,37 future investigations should better clarify the exact cerebral circuitry whose dysregulation is at the origin of narcolepsy.
As concerns the description of what patients feel in their body, the cataplectic attack was mostly described as a “loss of strength” felt at the level of arms, legs, head, and jaw. Anic-Labat et al (1999)38 reported that episodes such as jaw dropping, facial flickering, or head dropping were the most characteristic attacks; in another study, the review of the narrative description of typical attacks revealed that the jaw and the face were the most frequently affected, even more than the knee or the legs.39
The issue of symptom management suggests that some patients have developed a sort of ability to control the attacks by focusing on the inhibition/avoidance of emotional experiences connected to specific situations that, mostly, imply laughter: “I say stopping them, because if you start breathing you can stop the emotion, good or bad... What I want to explain is that I control laughter, because even if there is a movie that makes me laugh but there is a person who doesn't know what I suffer from, I don't laugh heartily...Showing what happens to me could create me huge problems, so no one has ever seen me…I'm pretty used to fake laughter, then a few times...except that sometimes I have this fake laugh even if I don't have anything to laugh about…It’s very difficult, very difficult... I have to think about something else, to divert attention...”
Other patients, however, claim to have developed management strategies mostly aimed at a better body control, as they seek support or try to sit down: “And then laughter, even if I'm sitting, because normally these things happen at the table, when I'm with other people. If I feel that maybe...generally I'm always leaning on something...or it is luck, I don't know, I'm always leaning on something.”
About the management of attacks, Overeem et al (2011)39 reported that 57% of the sample of patients examined could perceive the arrival of a cataplexy attack, making it possible to take countermeasures, such as sitting down, and that almost 60% of patients developed some “trick” to prevent cataplexy occurrence, such as “trying to think about something else” and “pressing against a support.”
Finally, the patients’ reports also provided new insights about how cataplexy may affect their QoL. Patients tend to hide or even simulate their emotions for the fear of having an attack, and feelings of shame for their condition are evident, especially in the presence of strangers. This may in part account for the difficulties in interpersonal relationships, in meeting a partner,13,43,44 and the ability to search for emotional support during critical moments45 reported by people with narcolepsy, who were found to marry less often than the general population.22,46 About this, a study of Vignatelli and collaborators (2011)47 revealed that QoL was directly associated with disease duration: the longer the duration of the disease, the better QoL. This finding suggests that patients learn over time to manage cataplexy attacks using better coping strategies instead of those of avoidance/inhibition of emotion.47 Indeed, emotional inhibition and avoidance strategies are contributing factors to depressed mood, symptoms that are often found in people with narcolepsy.21 This hypothesis can be confirmed by further studies investigating coping strategies used by patients to deal with emotional and potentially stressful situations along disease course, using quantitative and standardized data.
CONCLUSIONS
Over the past decade, the theoretical premises of narrative medicine have been effectively translated into specific programs for different medical subspecialties. Megan Alcauskas and Rita Charon, in 2008,48 claimed that neurology could benefit from these strategies, because it heavily relies on the patient's history and helps people “to discover things they would not otherwise have noticed.”
To date, this is the first original study to adopt a narrative approach to NT1 patients aimed at exploring how NT1 patients describe cataplectic attacks in terms of self-reported bodily experiences, trigger situations, and their management.
The data seem to indicate that some patients may have developed a tendency to disregard emotional experiences, thus affecting the ability to name, recognize, and regulate critical emotions. Writing about their experience can help patients to rebuild their identity after the disruption caused by the disease. Although very few data are available on the role of narrative medicine,49,50 this represents a novel approach to patient care and management focusing on disease burden.
A narrative approach to illness may be useful to plan efficacious treatments that cover multidisciplinary global care. This way it would be easier to reduce the negative impact that the disease has on the QoL of patients with NT1 and their families. A free and sincere expression of the daily feelings and difficulties related to the disease is a valuable instrument for the patient in terms of acceptance, control, and general well-being. Not less important, understanding what facing their disease truly means for the patients could reveal a key to engage them in the long-term management treatments and help clinicians to build a more participatory approach to health care, especially in dealing with NT1 and other chronic diseases.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at the Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy and IRCCS Istituto delle Scienze Neurologiche di Bologna, Italy. Giuseppe Plazzi participated in advisory board for UCB pharma, Jazz pharmaceuticals, and Bioproject. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
We are indebted to all the participants of the study, most notably the Italian association of narcoleptic patients (Associazione Italiana Narcolettici e Ipersonni, AIN). Without their contributions, this study would not have been possible. The authors thank Cecilia Baroncini for editing the English text.
ABBREVIATIONS
- ECUs
Elementary Context Units
- fMRI
functional magnetic resonance imaging
- NT1
narcolepsy type 1
- QoL
quality of life
REFERENCES
- 1.Mignot E, Kimura A, Lattermann A, et al. Extensive HLA class II studies in 58 non-DRB1*15 (DR2) narcoleptic patients with cataplexy. Tissue Antigens. 1997;49(4):329–341. doi: 10.1111/j.1399-0039.1997.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 2.Kornum B, Knudsen S, Ollila H, Pizza F, Jennum P, Dauvilliers Y, Overeem S. Narcolepsy. Nat Rev Dis Primers. 2017;3(1):1–19. doi: 10.1038/nrdp.2016.100. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 4.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep. 2014;15(5):502–507. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134(12):3480–3492. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the innsbruck narcolepsy cohort. J Clin Sleep Med. 2013;9(8):805–812. doi: 10.5664/jcsm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyagawa T, Tokunaga K. Genetics of narcolepsy. Hum Genome Var. 2019;6(4) doi: 10.1038/s41439-018-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillen S, Pizza F, Dhondt K, Scammell TE, Overeem S. Cataplexy and its mimics: clinical recognition and management. Curr Treat Options Neurol. 2017;19(6):23. doi: 10.1007/s11940-017-0459-0. [DOI] [PubMed] [Google Scholar]
- 9.Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136(12):3787–3795. doi: 10.1093/brain/awt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taddei RN, Werth E, Poryazova R, Baumann CR, Valko PO. Diagnostic delay in narcolepsy type 1: combining the patients’ and the doctors’ perspectives. J Sleep Res. 2016;25(6):709–715. doi: 10.1111/jsr.12420. [DOI] [PubMed] [Google Scholar]
- 11.Vignatelli L, Antelmi E, Ceretelli I, et al. Red flags for early referral of people with symptoms suggestive of narcolepsy: a report from a national multidisciplinary panel. Neurol Sci. 2019;40(3):447–456. doi: 10.1007/s10072-018-3666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini C, Pizza F, Antelmi E, Folli MC, Plazzi G. Narcolepsy treatment: pharmacological and behavioral strategies in adults and children. Sleep Breath. 2019;Jul 10 doi: 10.1007/s11325-019-01894-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10(1):75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 14.Rovere H, Rossini S, Reimão R. Quality of life in patients with narcolepsy: a WHOQOL-brief study. Arq Neuropsiquiatr. 2008;66(2a):163–167. doi: 10.1590/s0004-282x2008000200004. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki A, Inoue Y, Nakajima T, et al. Health-related quality of life among drug-naïve patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time. J Clin Sleep Med. 2008;4(6):572–578. [PMC free article] [PubMed] [Google Scholar]
- 16.Inocente CO, Gustin M-P, Lavault S, et al. Quality of life in children with narcolepsy. CNS Neurosci Ther. 2014;20(8):763–771. doi: 10.1111/cns.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurovich-Horvat E, Kemlink D, Högl B, et al. Narcolepsy and pregnancy: a retrospective European evaluation of 249 pregnancies. J Sleep Res. 2013;22(5):496–512. doi: 10.1111/jsr.12047. [DOI] [PubMed] [Google Scholar]
- 18.Barbero L, Govi A, Pizza F, Plazzi G, Ingravallo F. Parental Fitness questioned on the grounds of narcolepsy: presentation of two cases. J Clin Sleep Med. 2017;13(08):1017–1018. doi: 10.5664/jcsm.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapella MC, Berger BE, Vern BA, Vispute S, Prasad B, Carley DW. Health-related stigma as a determinant of functioning in young adults with narcolepsy. PLoS One. 2015;10(4):e0122478. doi: 10.1371/journal.pone.0122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. Am J Public Health. 2013;103(5):813–821. doi: 10.2105/AJPH.2012.301069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Droogleever Fortuyn HA, Fronczek R, Smitshoek M, et al. Severe fatigue in narcolepsy with cataplexy. J Sleep Res. 2012;21(2):163–169. doi: 10.1111/j.1365-2869.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 22.Ingravallo F, Gnucci V, Pizza F, et al. The burden of narcolepsy with cataplexy: how disease history and clinical features influence socio-economic outcomes. Sleep Med. 2012;13(10):1293–1300. doi: 10.1016/j.sleep.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. doi: 10.1016/j.sleep.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med. 2018;43:14–18. doi: 10.1016/j.sleep.2017.11.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charon R. Literature and medicine: origins and destinies. Acad Med. 2000;75(1):23–27. doi: 10.1097/00001888-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Charon R. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897–1902. doi: 10.1001/jama.286.15.1897. [DOI] [PubMed] [Google Scholar]
- 27.Charon R. What to do with stories: the sciences of narrative medicine. Can Fam Physician. 2007;53:1265–1267. [PMC free article] [PubMed] [Google Scholar]
- 28.Graffigna G, Cecchini I, Breccia M, et al. Recovering from chronic myeloid leukemia: the patients’ perspective seen through the lens of narrative medicine. Qual Life Res. 2017;26(10):2739–2754. doi: 10.1007/s11136-017-1611-8. [DOI] [PubMed] [Google Scholar]
- 29.Potì S, Palareti L, Cassis FR, Brondi S. Health care professionals dealing with hemophilia: insights from the international qualitative study of the HERO initiative. J Multidiscip Healthc. 2019;12:361–375. doi: 10.2147/JMDH.S201759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank AW. The Wounded Storyteller. Chicago, IL: University of Chicago Press;1995. [Google Scholar]
- 31.Riley T, Hawe P. Researching practice: the methodological case for narrative inquiry. Health Educ Res. 2005;20(2):226–236. doi: 10.1093/her/cyg122. [DOI] [PubMed] [Google Scholar]
- 32.Lancia F. Strumenti per l’analisi dei testi. Introduzione all’uso di T-LAB. Milano, Italy: Franco Angeli; 2004. [Google Scholar]
- 33.Potì S, Emiliani F, Palareti L. Subjective experience of illness among adolescents and young adults with diabetes: a qualitative research study. J Patient Exp. 2018;5(2):140–146. doi: 10.1177/2374373517738234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellardita L, Graffigna G, Donegani S, et al. Patient’s choice of observational strategy for early-stage prostate cancer. Neuropsychological Trends. 2012;12:107–116. [Google Scholar]
- 35.de Zambotti M, Pizza F, Covassin N, et al. Facing emotions in narcolepsy with cataplexy: haemodynamic and behavioural responses during emotional stimulation. J Sleep Res. 2014;23(4):432–440. doi: 10.1111/jsr.12133. [DOI] [PubMed] [Google Scholar]
- 36.Meletti S, Vaudano AE, Pizza F, et al. The brain correlates of laugh and cataplexy in childhood narcolepsy. J Neurosci. 2015;35(33):11583–11594. doi: 10.1523/JNEUROSCI.0840-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaudano AE, Pizza F, Talami F, Plazzi G, Meletti S. The neuronal network of laughing in young patients with untreated narcolepsy. Neurology. 2019;92(5):e504–e515. doi: 10.1212/WNL.0000000000006853. [DOI] [PubMed] [Google Scholar]
- 38.Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 39.Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12(1):12–18. doi: 10.1016/j.sleep.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Mattarozzi K, Bellucci C, Campi C, et al. Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy. Sleep Med. 2008;9(4):425–433. doi: 10.1016/j.sleep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Caruana F, Gerbella M, Avanzini P, et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain. 2018;141(10):3035–3051. doi: 10.1093/brain/awy219. [DOI] [PubMed] [Google Scholar]
- 42.Gerbella M, Caruana F, Rizzolatti G. Pathways for smiling, disgust and fear recognition in blindsight patients. Neuropsychologia. 2019;128:6–13. doi: 10.1016/j.neuropsychologia.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 43.Teixeira VG, Faccenda JF, Douglas NJ. Functional status in patients with narcolepsy. Sleep Med. 2004;5(5):477–483. doi: 10.1016/j.sleep.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Kim LJ, Coelho FM, Hirotsu C, et al. Frequencies and associations of narcolepsy-related symptoms: a cross-sectional study. J Clin Sleep Med. 2015;11(12):1377–1384. doi: 10.5664/jcsm.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiappa C, Scarpelli S, D’Atri A, Gorgoni M, De Gennaro L. Narcolepsy and emotional experience: a review of the literature. Behav Brain Funct. 2018;14(1):19. doi: 10.1186/s12993-018-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dodel R, Peter H, Spottke A, et al. Health-related quality of life in patients with narcolepsy. Sleep Med. 2007;8(7-8):733–741. doi: 10.1016/j.sleep.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Vignatelli L, Plazzi G, Peschechera F, Delaj L, D’Alessandro R. A 5-year prospective cohort study on health-related quality of life in patients with narcolepsy. Sleep Med. 2011;12(1):19–23. doi: 10.1016/j.sleep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Alcauskas M, Charon R. Right brain: reading, writing, and reflecting: making a case for narrative medicine in neurology. Neurology. 2008;70(11):891–894. doi: 10.1212/01.wnl.0000304945.48551.13. [DOI] [PubMed] [Google Scholar]
- 49.Vaccarella M. Narrative epileptology. Lancet. 2011;377(9764):460–461. doi: 10.1016/S0140-6736(11)60150-5. [DOI] [PubMed] [Google Scholar]
- 50.Vaccarella M. Disembodiment and identity in literary depictions of epilepsy surgery. Lit Med. 2015;33(1):1–22. doi: 10.1353/lm.2015.0004. [DOI] [PubMed] [Google Scholar]