Abstract
The novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a pandemic threatening global public health. In the current paper, we describe our successful treatment of three COVID-19 pneumonia patients cases including severe cases and cases with mortality risk factors. One 32-year-old male COVID-19 patient was diagnosed with severe COVID-19 pneumonia and moderate ARDS. The second COVID-19 pneumonia patient had a history of diabetes and chronic bronchitis. The third case of COVID-19 pneumonia was an 82-year old female patient. All three cases had severe COVID pneumonia and therefore were aggressively managed with a multidisciplinary and personalized therapeutic approach that included nutritional support, antiviral pharmacotherapy, active control of comorbidities, prevention of complication development and psychological intervention. Our experience highlights the importance of the use of a multidisciplinary therapeutic approach that tailors to the specific condition of the patient in achieving a favorable clinical outcome.
Keywords: COVID-19, SARS-CoV-2, Multidisciplinary therapeutic approach
Introduction
Since the outbreak of infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), formerly known as 2019 novel coronavirus (2019-nCoV), in late December, 2019 in Wuhan, Hubei Province, China, the number of infected persons has exceeded 136 895 with 5077 deaths globally.1 The novel coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has already surpassed that of the previous severe acute respiratory syndrome (SARS) epidemic and has so far spread to 123 countries and territories.2 , 3 On March 11, 2020, the World health Organization has characterized COVID-19 as a pandemic. The mortality rate is 10% for SARS-CoV4 and 37% for MERS-CoV5 while the overall case-fatality rate of COVID-19 was 2.3% (1023 deaths among 44 672 confirmed cases.6 Wang et al. reported a mortality of 4.3% in 138 hospitalized COVID-19 patients.7 Zhou et al. analyzed 191 adult COVID-19 patients and showed that older age (odds ratio 1·10, 95% CI 1·03–1·17, per year increase; p = 0·0043), higher Sequential Organ Failure Assessment (SOFA) score (5·65, 2·61–12·23; p < 0·0001), and D-dimer greater than 1 μg/mL (18·42, 2·64–128·55; p = 0·0033) on admission were independent risk factors of mortality in COVID-19 patients.8
It is well known that the Center for Disease Prevention and Control (CDC) and hospital infection control play an important role in preventing and controlling an epidemic.9 In the current paper, we describe three cases of severe COVID-19 pneumonia who were managed by a multidisciplinary and personalized approach that included nutritional support, antiviral pharmacotherapy, active control of comorbidities, prevention of complication development and psychological intervention. Our successful treatment of the three patients highlights the importance of a multidisciplinary approach in managing the disease.
Case reports
Case No. 1
A 32-year-old male was admitted to our hospital on February 4, 2020 due to diarrhea and fatigue for 8 days and cough and breathing difficulty for 3 days and worsening for 1 day. The patient resided within one kilometer of Huanan Seafood Wholesale Market in Wuhan city, China, the epicenter of the COVID-19 outbreak. On January 27, 2020, the patient developed fatigue without an apparent cause and had 5-6 watery stools/day. He had no fever, nausea, vomiting, or abdominal pain. Cough with scant foamy sputum and difficulty in breathing during activity developed on February 1, 2020. The patient took over the counter (OTC) cold medicine for 2 days to no avail. On February 3, his breathing difficulty was worsened, chest x ray at a local hospital revealed lung infection and was referred to our hospital.
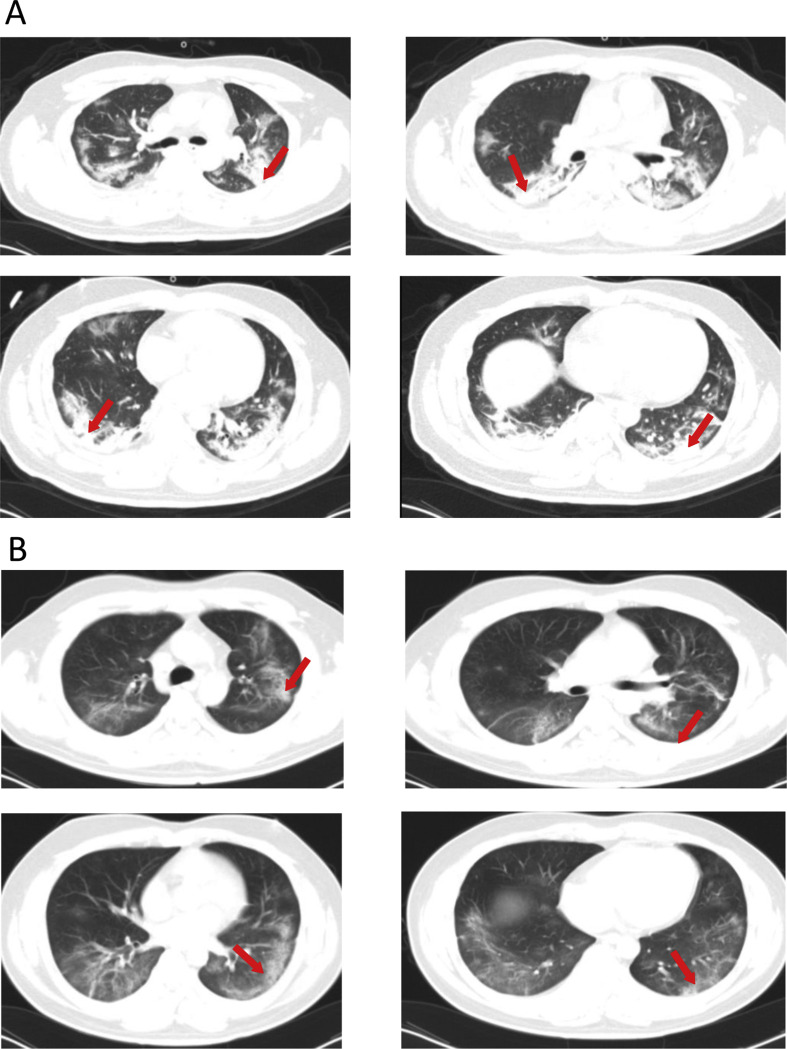
Admission examination showed a temperature of 36 °C, a pulse of 85/min, a respiratory rate of 35/min, a blood pressure of 117/84 mmHg. Physical examination showed no abnormalities. Laboratory studies showed increased white blood cell (WBC) count (17.64 × 109/L; normal references: 4–10 × 109), neutrophil count (15.45 × 109/L; normal references: 2.0–7.0 × 109), and lymphocyte count (0.6 × 109/L; normal references: 0.8–4.0 × 109). Blood chemistries revealed elevations in C-reactive protein (CRP) (348.56 mg/L; normal references: 0.00–8.20 mg/L), and erythrocyte sedimentation rate (ESR,140 mm/h; normal references: 0–15 mm/h), fasting plasma glucose (FPG, 7.13 mmol/L; normal references: 3.9–6.10 mmol/L), alanine aminotransferase (ALT, 70 U/L; normal references: 5–40 U/L), aspartate aminotransferase (AST, 63 U/L; normal references: 8–40 U/L), blood urea nitrogen (BUN, 11.21 mmol/L; normal references: 2.9–8.2 mmol/L), and procalcitonin (PCT, 0.162 ng/mL; normal references: <0.046 ng/mL). Chest computed tomography (CT) scan revealed diffuse multiple patchy exudates, which were more marked in both lower lungs, suggesting infectious lesions (Fig. 1 A). Echocardiography showed no apparent abnormalities. Blood gas analysis showed pH 7.287, PCO2 48.6 mmHg (normal references: 35–45 mmHg), PO2 69.8 mmHg (normal references: 80–100 mmHg), BE: −4.3 mmol/L (normal reference: −3.0 to 3.0 mmol/L), and HCO3 - 20.8 mmol/L. His influenza B test was negative, and the nucleic acid test of nasopharyngeal swabs was positive on two occasions. A diagnosis of severe COVID-19 pneumonia and moderate acute respiratory distress syndrome (ARDS) was made and the patient was placed under isolation.
Figure 1.
(A) Chest CT scan of a 32-year-old male with cough and breathing difficulty on February 6 reveals diffuse multiple patchy exudates, which are more marked in both lower lungs, suggesting infectious lesions. (B) Chest CT scan on February 20, 2020 shows diffuse bilateral ground glass opacities and partial exudation, which are markedly improved compared with admission CT scan findings.
The patient received assisted continuous positive airway pressure (CPAP) ventilation on February 4, 2020. Apart from supplemental oxygen (Fi: 0.4–0.6) for 5 days, he was treated with ceftazidime (2.0 g twice daily) for 13 days, and levofloxacin (0.5 g/day) for 3 days, recombinant interferon α aerosol (500 × 106 U, twice daily) for 17 days, lopinavir-ritonavir (0.2 g twice daily) for 5 days, and oral umifenovir (0.2 g, three times daily), intravenous esomeprazole (40 mg twice daily), injectable Xuebijing for 13 days, budesonide aerosol twice daily and methylprednisolone (40 mg/day q12h) for 5 days. Meanwhile, the patient received enteral nutritional support and psychological intervention was also provided to lessen anxiety and depression. Traditional Chinese medicine therapy was further provided. The patient showed improvement (Fig. 1B and Table 1 ) and mechanical ventilation was discontinued on February 9, 2020. The nucleic acid test for COVID-19 was negative on two separate occasions and the patient was discharged from the hospital on February 27, 2020.
Table 1.
Demographic and baseline variables of the study patients with COVID-19 pneumonia.
| CASE NO. 1 | CASE NO. 2 | CASE 3 | |
|---|---|---|---|
| Age, years | 32 | 48 | 84 |
| Sex | Male | Male | Female |
| Exposure history | Residing in epidemic area | No | Yes |
| Current smoker | NO | Yes | NO |
| Comorbidities | |||
| Hypertension | No | No | No |
| Diabetes | No | Yes | No |
| COPD | No | Yes | No |
| Cancer | No | No | No |
| CKD | No | No | No |
| Others | No | No | Osteoporosis |
| Clinical manifestations | |||
| Respiratory rate, breaths per min | 35 | 20 | 22 |
| Pulse rate, beats per min | 85 | 88 | 80 |
| Fever ≥37.3 °C | No | Yes | No |
| Cough | Yes | Yes | No |
| Sputum | Foamy | Foamy | No |
| Myalgia | No | No | No |
| Fatigue | Yes | No | No |
| Diarrhea | Watery | No | Yes |
| Nausea or vomiting | No | No | Yes |
| Disease severity status (general/severe/critical) | Severe | Severe | Severe |
| Time from illness onset to admission, days | 8 | 9 | 4 |
Case No. 2
A 48-year old male patient was admitted on February 1, 2020 because of fever and cough for 9 days. The patient developed fever (highest temperature 39 °C) with shivering and cough with scant whitish foamy sputum without apparent causes 9 days ago. Neither fatigue nor generalized muscle pain was present. The patient had no exposure history to SARS-CoV-2 infected persons. He was prescribed some unknown oral medications, but intermittent fever was present and there was no apparent improvement in his symptoms. He was admitted to a local hospital on January 26, 2020. Blood test revealed WBCs at 8.28 × 109/L, neutrophils 72.8% and lymphocytes 17.9%. Chest CT scan showed multi-lobar multiple interstitial changes in bilateral lungs. The nucleic acid test conducted on January 28, 2020 at the local CDC laboratory for SARS-CoV-2 ORF1ab and gene for nucleocapsid N protein was positive. The patient was diagnosed with COVID-19 pneumonia and placed under isolation and was given lopinavir antiviral therapy. The patient was referred to our hospital for further treatment.
The patient was a current smoker and had a history of type 2 diabetes for 2 years and chronic bronchitis for 8 years.
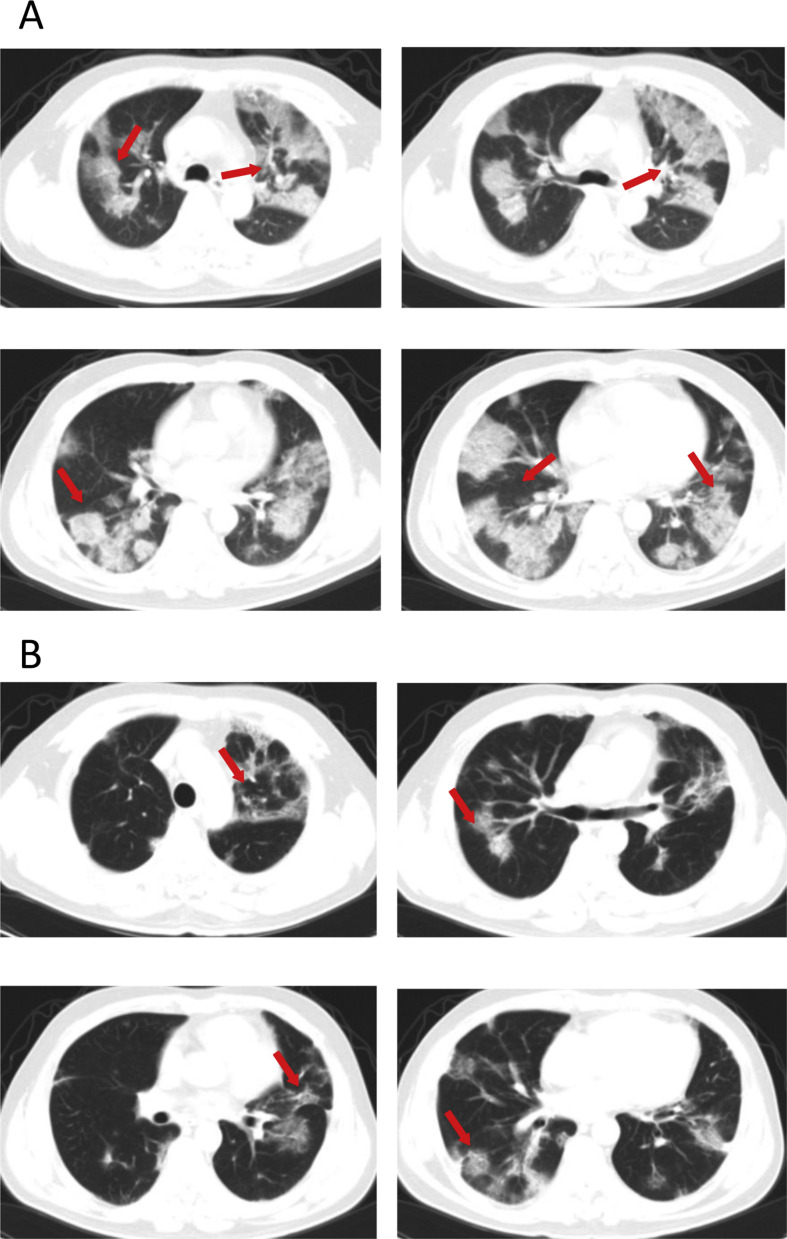
Admission examination showed a temperature of 36.6 °C, a pulse of 88/min, a respiratory rate of 20/min, and a blood pressure of 138/85 mmHg (Table 1). No obvious abnormality was found on physical examination. Blood test revealed elevations in WBCs (10.11 × 109/L) and neutrophils (9.19 × 109/L) and reductions in lymphocytes (0.6 × 109/L) (Table 2 ). Laboratory studies showed increases in FPG (20.76 mmol/L) pro-BNP (170 pg/mL; normal references: 0–125 pg/mL), fibrinogen C (5.22; normal references: 2.03–4.72 g/L), fibrin degradation product (FDP, 2.06 mg/L; normal references: 0.00–2.01 mg/L), total bilirubin (20.5, mmol/L; normal references: 3.4–17.1 mmol/L) and lactate dehydrogenase (LDH, 374 U/L; normal references: 105–245 U/L) and decreased IgG levels (8.0 g/L; normal references: 11.5–14.2 g/L). His PO2 was reduced (76.6 mmHg) and PCO2 was elevated (47.8 mmHg), and the oxygen saturation was unnormal (94.6%). Chest CT scan on February 6, 2020 showed diffuse multiple patchy exudates with partial interlobar septation, suggesting viral pneumonia (Fig. 2 A). Apart from nutritional support and oxygen supplementation (5 mL/min), the patient was given recombinant interferon α aerosol (500 × 106 U twice daily), lopinavir-ritonavir, metformin (0.5 g twice daily), intravenous ceftazidime (2 g twice daily), and oral glimepiride (2 mg once daily). The patient's condition worsened despite treatment. On February 8, the patient received mechanical ventilation. Blood coagulation test on February 9 showed marked elevations in fibrinogen C (6.75 g/L), FDP (20.55 mg/L) and D-dimer (2744 ng/mL; normal references: 0–231 ng/mL) and subcutaneous low molecular weight heparin was started on February 10. Intravenous insulin (20IU/daily) and intravenous moxifloxacin (0.4 g once daily) were given on February 16. The patient gradually improved. CT scan on February 23, 2020 showed multiple bilateral patchy shadows, partial exudates and fibrotic changes, which markedly improved versus changes at admission (Fig. 2B) and laboratory studies on February 23, 2020 revealed normal WBC count, lymphocyte and neutrophil count and coagulation parameters were also normal. The patient was discharged from the hospital on March 8, 2020.
Table 2.
Laboratory findings of the study patients with COVID-19 pneumonia.
| CASE NO. 1 | CASE NO. 2 | CASE NO. 3 | NORMAL REERENCES | |
|---|---|---|---|---|
| White blood cell count × 109/L | 17.64 | 10.11 | 1.77 | 4.0–10.0 |
| Lymphocyte count × 109/L | 0.6 | 0.6 | 0.34 | 0.8–4.0 |
| Neutrophil count × 109/L | 15.45 | 9.19 | 1.18 | 2.0–7.0 |
| Hemoglobin, g/L | 116 | 150 | 122 | 120–160 |
| Platelet × 109/L | 399 | 203 | 69 | 100–300 |
| Albumin | 31 | 41.14 | 33.64 | 34–48 |
| Lactate | 5.48 | 4.14 | 3.10 | 0.60–2.20 |
| Alanine aminotransferase, U/L | 70 | 41 | 14 | 5–40 |
| Aspartate aminotransferase, U/L | 63 | 29 | 30 | 8–40 |
| Total bilirubin, mmol/L | 19.9 | 20.5 | 6.60 | 3.4–17.1 |
| LDH, U/L | – | 374 | – | 105–245 |
| Blood urea nitrogen, mmol/L | 11.21 | 7.17 | 3.78 | 2.9–8.2 |
| Creatinine, μmol/L | 80.8 | 68.7 | 61.1 | 59–104 |
| High sensitivity cardiac troponin | Normal | Normal | Normal | 0.000~0.014 |
| Prothrombin time, s | Normal | Normal | Normal | 8.8~14.0 |
| Activated partial thromboplastin time, s | Normal | 27.8 | 28.1 | 26.5–39.3 |
| D-dimer, μg/L | Normal | 205 | 163 | 0.00–231 |
| Fibrinogen C, g/L | Normal | 5.22 | 3.70 | 2.03–4.72 |
| Fibrin degradation product, μg/L | Normal | 2.06 | 2.41 | 0.00–2.01 |
| Serum ferritin | Normal | Normal | Normal | |
| C-reactive protein, g/L | 348.56 | 21.5 | 8.6 | 0.00–8.20 |
| Procalcitonin, ng/mL | 0.162 | 0.039 | – | <0.046 |
| ESR, mm/h | 140 | – | – | 0–15 |
| Immunoglobulin G, g/L | Normal | 8.0 | 9.8 | 11.5–14.2 |
| Imaging features | ||||
| Bilateral distribution of patchy shadows or ground glass opacity | Yes | Yes | Yes | |
Abnormal values are shown in bold.
Figure 2.
(A) Chest CT scan of a 48-year old male patient with fever and cough for 9 days who had confirmed COVID-19 pneumonia on February 6 diffuse multiple patchy exudates with partial interlobar septation, suggesting viral pneumonia. (B) CT scan on February 23, 2020 reveals multiple bilateral patchy shadows, partial exudation and fibrotic changes, which are markedly improved versus changes at admission.
Case No. 3
An 82-year old female patient was admitted to our hospital on February 16, 2020 upon referral with a confirmed diagnosis of COVID-19 for 5 days. She had a contact history with a COVID-19 patient for 5 days (from January 23) before she was placed under isolation on February 4, 2020. The nucleic acid test on February 12, 2020 for COVID-19 by Dazhou City CDC laboratory was positive. The patient had no fever, cough or difficulty in breathing. She was given oral lopinavir-ritonavir, which was discontinued after severe diarrhea occurred. She received no further antiviral treatment. On February 16, 2020, the patient had poor appetite and developed severe upper abdominal distension and her oxygen saturation was 80% and was transferred to our hospital by ambulance.
She received surgery due to osteoporosis in 2019 and underwent gallbladder resection in 2000 and surgery for lumbar fracture due to a fall in 2015.
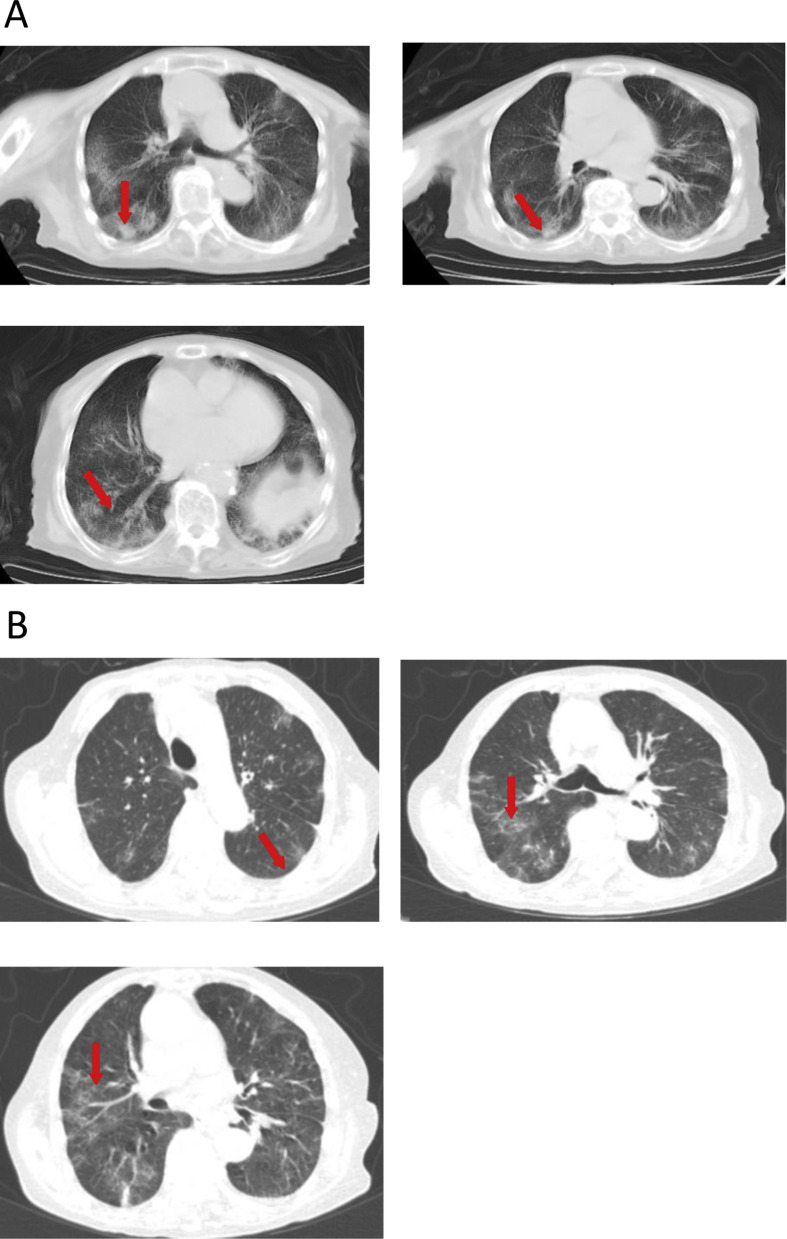
Admission examined showed a temperature of 36.9 °C, a pulse of 80/min, a respiratory rate of 22 min, and a blood pressure of 126/66 mmHg (Table 1). Bilateral lung sounds were coarse upon auscultation. No other remarkable abnormalities were observed. Routine blood test showed a reduction in WBCs (1.77 × 109/L), lymphocytes (0.34 × 109/L) and neutrophils (1.18 × 109/L) and a decrease in IgG levels (9.8 g/L). Blood gas analysis showed elevated pH (7.6), reduced PaO2 (58 mmHg) and decreased HCO3 - (20.6 mmol/L) (Table 3 ). Admission chest CT scan revealed multiple bilateral ground glass opacities and mainly peripheral exudative changes (Fig. 3 A).
Table 3.
Results of blood gases in the study patients with COVID-19 pneumonia.
| CASE NO. 1 | CASE NO. 2 | CASE 3 | NORMAL REFERENCES | |
|---|---|---|---|---|
| pH | 7.287 | 7.345 | 7.6 | 7.35–7.45 |
| PaO2, mmHg | 69.8 | 76.6 | 58 | 80–100 |
| O2 saturation | 93 | 94.6 | 91 | 80–100 |
| PaCO2, mmHg | 48.6 | 47.8 | 35.5 | 35–45 |
| HCO3-, mmol/L | 20.8 | 25.5 | 20.6 | 21.4–27.3 |
| Base excess, mmol/L | −4.3 | −0.7 | −3.0 | −3.0 to 3.0 |
Abnormal values are shown in bold.
Figure 3.
(A) Chest CT scan on February 16, 2020 of an 82-year old female patient referred with a confirmed diagnosis of COVID-19 for 5 days shows multiple bilateral ground glass opacities and mainly peripheral exudative changes. (B) Chest CT scan on February 23, 2020 reveals multiple bilateral ground glass opacities and scant cord-like shadows, which are mainly peripheral.
The patient received recombinant interferon α aerosol (500 × 106 U twice daily), intravenous methylprednisolone (40 mg twice daily), intravenous esomeprazole (40 mg twice daily), and oral umifenovir (0.2 g, three times daily). Laboratory study on February 19, 2020 revealed lymphocytes at 0.34 × 109/L, increased proBNP (1219 pg/mL), decreased aPPT (24.2 s, normal references 26.5–39.3 s), increased D-dimers (280 ng/mL) and FDP (3.13 μg/L). The patient was given oxygen supplementation (4 L/min) to correct hypoxemia on February 19, 2020 and human immunoglobulin (10 g/day) for 6 days. Plasma glucose control was managed by consultation with endocrinologists at the hospital and chronic bronchitis was treated by pulmonologists. Nutritional support and traditional Chinese medicine were also provided. The patient showed improvement and chest CT scan on February 23, 2020 showed multiple bilateral ground glass opacities and scant cord-like shadows, which were mainly peripheral (Fig. 3B). The patient was discharged from the hospital on March 12, 2020.
Discussion
In this paper, we report three patients with confirmed COVID-19 pneumonia. Two of the patients had a clear history of exposure to SARS-CoV-2. One patient with severe COVID-19 pneumonia developed ARDS and received invasive mechanical ventilation therapy. All three patients showed bilateral distribution of patchy shadows or lung consolidations on chest CT scans. All of them had gradually recovered and were discharged from the hospital after multidisciplinary treatment including enteral nutritional support, mechanical ventilation, antiviral therapy and active management of comorbidities.
Variable hematologic findings have been described in adult patients with COVID-19 and indicated conditions, such as lymphopenia, prolonged prothrombin time, and elevated LDH.10 One prominent feature of COVID is the presence of ARDS in 17%–29% of documented COVID-19 cases.11 Moderate ARDS was also documented in our case No. 1. The production of proinflammatory cytokines/chemokines12 is elevated in SARS-CoV and MERS-CoV infections13 , 14 which contribute to acute lung injury and development of ARDs.12 We did not determine cytokine/chemokine levels in this patient, but we observed a marked increase in WBC count and neutrophil count and a noticeable rise in CRP and PCT in the patient. The patient was aggressively managed with CPAP, antiviral therapy, antibiotics for possible bacterial infections, glucocorticoid therapy and enteral nutrition.
COVID-19 affects disproportionally the elderly and those with preexisting conditions such as diabetes, which increase the risk of death in these patients.8 Case No. 2 in our report had diabetes and chronic bronchitis. Apart from enteral nutrition glucocorticoid therapy and antiviral therapy, the patient also received antidiabetic treatment to bring abnormally high FPG under control with glimepiride and subsequently intravenous insulin. Despite our aggressive therapy, the patient's condition worsened, with aberrant fibrinolysis as fibrinogen, FDP and D-dimer saw dramatic increase. Due to elevated fibrinogens and comorbidity with diabetes and an advanced age, the risk of deep vein thrombosis became accentuated, we consulted deep vein thrombosis experts in the hospital was made with regards to optimal therapy and initiated anticoagulation therapy with low molecular weight heparin. Zhou et al. found that D-dimer greater than 1 μg/mL was an independent risk factor of mortality in COVID-19 patients. Both case 2 and 3 in our study had increased D-dimer levels over the course of illness. In addition, case No. 3 was at an advanced age, which is another risk factor of morality for COVID-19 patients.15
Currently, no specific treatment is effective for treating COVID-19 despite the treatment guideline16 by the National Health Commission of the People's Republic of China. Our experience in managing COVID-19 patients suggests that for mild COVID-19 cases, early screening, early recognition, early diagnosis, early treatment and early prevention of development of complications are critical to a favorable clinical outcome. For severe COVID-19 cases, we implement a personalized multidisciplinary approach tailoring to the condition of each patient. Apart from antiviral therapy, active infection control, glucocorticoid therapy, we carry out enteral nutritional support by consulting clinical nutritionists, immune support, psychological counseling, and herbal therapy. More importantly, appropriate oxygen therapy such as gradient oxygen therapy, high flow humidified oxygen therapy, mechanical ventilation, ventilation in the prone position and extracorporeal membrane oxygenation (ECMO), and physical therapy. We have introduced the ventilation in the prone position method in which the patient is placed in the prone position, holds breath and then takes a deep breath to expand the lungs in order to reduce lung consolidation and improve oxygenation.
In conclusion, our successful management of COVID19 cases including severe cases and cases with mortality risk factors shows that early screening and prompt diagnosis and a multidisciplinary therapeutic approach that includes nutritional support, pharmacotherapy, and psychotherapy and tailors to the specific condition of the patient are critical to achieving a favorable clinical outcome.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was funded by Sichuan City Science and Technology Bureau Key Research and Development Projects (No. 20ZDYF0001).
References
- 1.WHO situation reports 2020. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd [March 13, 2020]. Available from:
- 2.Joob B., Wiwanitkit V. COVID-19 pneumonia in Taiwan. J Formos Med Assoc. 2020;119:998. doi: 10.1016/j.jfma.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S. 2020. The 2019 novel coronavirus disease (COVID-19) pandemic: a zoonotic prospective. [Google Scholar]
- 4.Singh S.K. Middle East respiratory syndrome virus pathogenesis. Semin Respir Crit Care Med. 2016;37(4):572–577. doi: 10.1055/s-0036-1584796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh V.C.-R. Putting resiliency of a health system to the test: COVID-19 in Taiwan. J Formos Med Assoc. 2020;119:884–885. doi: 10.1016/j.jfma.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019 doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Channappanavar R., Perlman S., editors. Seminars in immunopathology. Springer; 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171(8):850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 14.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.[!!! INVALID CITATION !!!].
- 16.Lin L., Li T. Interpretation of guidelines for the diagnosis and treatment of novel coronavirus (2019-ncov) infection by the national health commission (trial version 5) Zhonghua Yixue Zazhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [DOI] [PubMed] [Google Scholar]