Abstract
Background/Purpose
Current studies on pediatric coronavirus disease 2019 (COVID-19) are rare. The clinical characteristics and spectrum are still unknown. Facing this unknown and emerging pathogen, we aimed to collect current evidence about COVID-19 in children.
Methods
We performed a systematic review in PubMed and Embase to find relevant case series. Because some reports were published in Chinese journals, the journals and publications of the Chinese Medical Association related to COVID-19 were completely reviewed. A random effects model was used to pool clinical data in the meta-analysis.
Results
Nine case series were included. In the pooled data, most of patients (75%) had a household contact history. The disease severity was mainly mild to moderate (98%). Only 2 children (2%) received intensive care. Fever occurred in 59% of the patients, while cough in 46%. Gastrointestinal symptoms (12%) were uncommon. There are 26% children are asymptomatic. The most common radiographic finding was ground glass opacities (48%). Currently, there is no evidence of vertical transmission to neonates born to mothers with COVID-19. Compared with the most relevant virus, SARS-CoV, SARS-CoV-2 causes less severe disease.
Conclusion
COVID-19 has distinct features in children. The disease severity is mild. Current diagnosis is based mainly on typical ground glass opacities on chest CT, epidemiological suspicion and contact tracing.
Keywords: Children, COVID-19, SARS-CoV-2
Introduction
In December 2019, an outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) occurred in Wuhan, Hubei Province, China.1 COVID-19 has spread rapidly to other provinces of China and now internationally. On January 30, 2020, the World Health Organization (WHO) declared COVID-19 a public health emergency of international concern (PHEIC). As of April 7, 1,279,722 cases were confirmed, and 72,616 (5.7%) patients died in 211 countries. In the beginning of the outbreak, COVID-19 was considered a zoonotic disease with limited human-to-human transmission. Initially, pneumonia cases were linked to the Huanan Seafood Wholesale Market. Soon after, this emerging coronavirus was found to be highly infectious. The basic reproductive number ranged from 2.2 to 3.58, with a mean serial interval of 7.5 days.1, 2, 3
Children are more susceptible than adults to some infectious diseases, which may cause fatal outcomes. For instance, young children, especially infants aged less than 6 months, have higher mortality rates due to seasonal influenza infection than individuals in other age groups.4 During enterovirus outbreaks, enterovirus 71 and echoviruses lead to death in different age groups of children.5 , 6 Streptococcus pneumoniae and Haemophilus influenzae type b were associated with high childhood mortality in the era without conjugate vaccines.7
The clinical spectrum of COVID-19 ranges from asymptomatic to critically ill cases. Fever and cough are the most common manifestations in symptomatic adults.8 , 9 It seems that SARS-CoV-2 is less invasive in children than in adults. However, reports about the clinical characteristics of pediatric COVID-19 are limited. Facing this unknown and emerging pathogen, we aimed to collect current evidence about COVID-19 in children.
Method
We performed a systematic literature search in PubMed and Embase using the following terms: “coronavirus or COVID-19 or 2019-nCoV or SARS-CoV-2” and “pediatric or neonate or newborn or infant or children or adolescence” to find reports of pediatric COVID-19. Since the novel coronavirus was identified in January 2020, the time period was restricted to not include literature published prior to this time point. Available full texts and the reference lists of the relevant studies were reviewed. There was no language restriction in our search. Since some reports were published in Chinese journals, the journals and publications of the Chinese Medical Association related to COVID-19 were completely reviewed. Google Scholar was searched manually for possible missing articles. The last update of the study was on March 15, 2020.
Two independent reviewers (THC, JLW) screened all titles and abstracts for eligibility. Studies were eligible for inclusion if they met the following criteria: 1) patients were younger than 18 years old, and 2) COVID-19 was diagnosed by PCR. Repeated calculations in other publications or clinical diagnoses of COVID-19 cases without laboratory confirmation were excluded. Correspondences or letters fulfilling the above criteria were also included.
Data extraction and quality assessment
After full-text screening for eligibility and review, two authors (THC, LYC) independently extracted data. Disagreements were resolved by consensus. The following items were extracted from each study, if available: author, journal, date of publication, study design, study country, time period, contact or travel history, clinical symptoms, laboratory results, complications, and chest computed tomography (CT) findings. The publication date, author, hospital and case report content were carefully examined to avoid repeated calculation during data pooling. The quality of studies included in meta-analysis was assessed using the Newcastle–Ottawa Scale (NOS). Articles with poor quality (score = 0–3) were excluded.
Data synthesis and analysis
We presented the data with descriptive statistics and pooled the available data for overall demonstration. Disease severity was categorized into four types according to officially published guideline in China: 1) Mild type: Asymptomatic or some upper respiratory tract infection signs; 2) Moderate type: The above manifestations with pneumonia in imaging study; 3) Severe type: Disease progression with danger signs; 4) Critical type: Shock or organ failure needing intensive care.10 The pooled data were further compared with those for laboratory-confirmed severe acute respiratory syndrome (SARS) children reported in previous studies. Categorical data were compared with the chi-square test. A p-value less than 0.05 by a two-tailed test was considered statistically significant. When at least 3 studies with available data reported the same characteristic, we used Comprehensive Meta-Analysis version 3 (Biostat, Englewood, NJ) to conduct meta-analysis. We used random effects models to calculate the pooled frequency among each clinical manifestation or outcome. The results are expressed as incidences and 95% CIs. I2 was calculated to examine statistical heterogeneity across the included studies. I2 > 50% and a p < 0.05 were considered substantial heterogeneity. Egger precision weighted linear regression tests and funnel plots were used to test potential publication bias. If publication bias was present, the trim and fill method would be performed and Rosenthal's Fail-safe N would be calculated to evaluate the effect.
Results
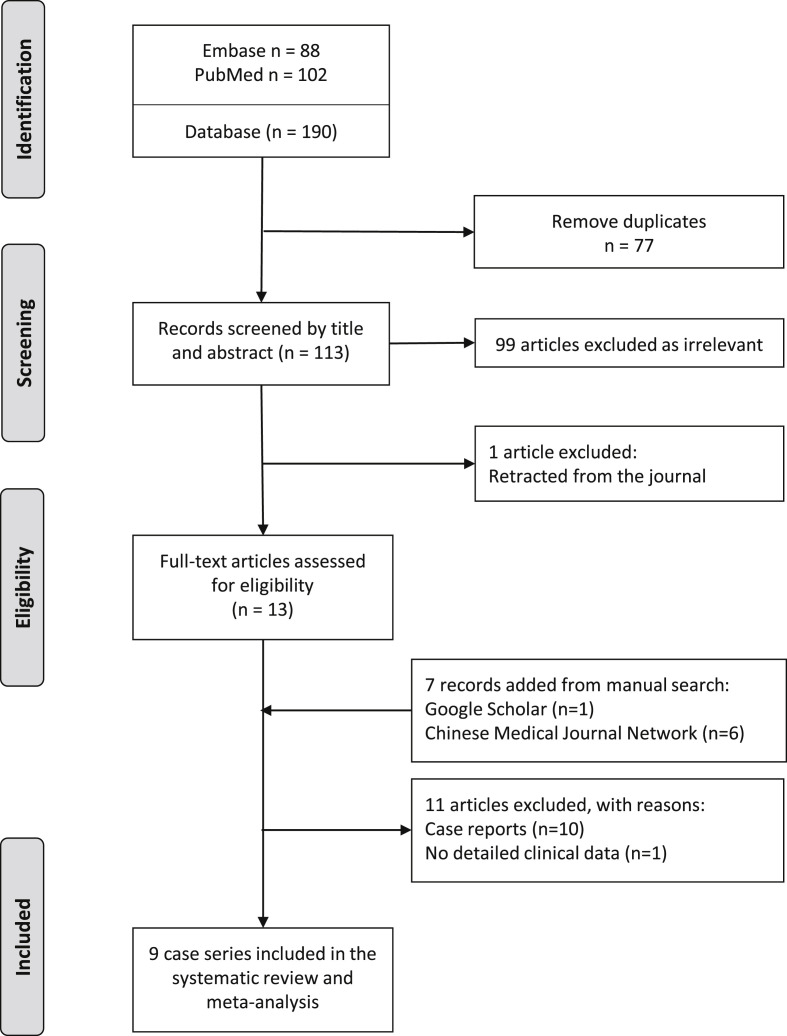
Fig. 1 shows the flow diagram of the study selection process. A total of 190 articles were identified in the initial search. After removing duplicates, 113 were screened by titles and abstracts. Obviously irrelevant articles were excluded. The remaining 13 were retrieved for full text assessment. Seven records were identified through manual searches of Google Scholar (n = 1) and the Chinese Medical Journal Network (n = 6). After qualitative synthesis, we excluded 11 articles. Ten articles were case reports. One study investigating infant cases was excluded because the patient profile was extracted from a national database. Considering repeated calculations and missing data, we did not include this study in the pooled data. In our search, we found 9 case series related to pediatric COVID-19.
Figure 1.
Flow diagram of the study selection process.
Epidemiological and clinical features
In Table 1 and Table 2 , we summarize the epidemiological and clinical features of pediatric COVID-19. The study period ranged from January 2020 to February 2020. The reported population included all age groups, from neonates to adolescents. Most of them (75%) had a clear household contact history. No gender difference among these studies. The majority of patients were categorized as having mild to moderate disease severity (98%). More than half (59%, 95% CI 0.41–0.72; I2 = 44%, p = 0.10) of the patients presented with fever. About 46% (95% CI 0.27–0.66; I2 = 57%, p = 0.03) of the patients had a cough. Only a few (12%, 95% CI 0.06–0.32; I2 = 45%, p = 0.09) patients had gastrointestinal manifestations. Approximately 26% (95% CI 0.13–0.52; I2 = 63%, p = 0.01) of patients showed no specific symptoms initially. However, the result revealed moderate heterogeneity. Regarding laboratory results, lymphopenia (lymphocytes < 1500 × 109/L) was found in 32% (95% CI 0.05–0.83; I2 = 72%, p = 0.03) of infected children. Some children (9%, 95% CI 0.04–0.46; I2 = 20%, p = 0.28) even developed severe lymphopenia (lymphocytes < 1000 × 109/L).
Table 1.
Characteristics and summary data of included studies.
| Liu et al.11 | Feng et al.12 | Cai et al.13 | Xia et al.14 | Wang et al.15 | Jiang et al.16 | Li et al.17 | |
|---|---|---|---|---|---|---|---|
| Case number | 6 | 15 | 10 | 20 | 31 | 6 | 5 |
| Regions | Hubei | Shenzhen | Multiple | Wuhan | Multiple | Chongqing | Zhuhai |
| Median age, y | 3 y | 7 y | 6.5 y | 2 y | 7 y | 7 y | 3 y |
| Age range | 1 y–7 y | 4 y–14 y | 3 m–10 y | 1 d–14 y | 6 m–17 y | 7 m–14 y | 10 m–6 y |
| Male No. (%) | 2 (33) | 5 (33) | 4 (40) | 13 (65) | 15 (48) | 5 (83) | 4 (80) |
| Household contact |
0 (0) |
12 (80) |
7 (70) |
13 (65) |
28 (90) |
6 (100) |
4 (80) |
| Disease severity |
No. (%) |
||||||
| Mild | 2 (33) | 3 (20) | 6 (60) | 4 (20) | 17 (55) | 3 (50) | 2 (40) |
| Moderate | 3 (50) | 12 (80) | 4 (40) | 15 (75) | 14 (45) | 3 (50) | 3 (60) |
| Severe | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Critical | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Clinical features | |||||||
| Asymptomatic | 0 (0) | 8 (53) | 0 (0) | 2 (10) | 7 (23) | 3 (50) | 4 (80) |
| Fever | 6 (100) | 5 (33) | 8 (80) | 12 (60) | 20 (65) | 3 (50) | 1 (20) |
| Cough | 6 (100) | 1 (7) | 6 (60) | 13 (65) | 14 (45) | 2 (33) | 1 (20) |
| GI symptoms | 4 (67) | 0 (0) | 0 (0) | 3 (15) | 3 (10) | 1 (17) | 0 (0) |
| Lymphocyte <1500, x109/L | 5 (83) | – | 1 (10) | – | – | 1 (17) | – |
| Lymphocyte <1000, x109/L | 2 (33) | – | 0 (0) | – | – | 0 (0) | – |
| Chest CT findings | |||||||
| Patchy consolidations | 3/5a (60) | 2 (13) | – | 20 | – | 1 (17) | 0 (0) |
| Ground glass opacities | 1/5a (20) | 7 (47) | – | 10 (50) | – | 2 (33) | 3 (60) |
| No lesion | 1/5a (20) | 6 (40) | – | 12 (60) | – | 1 (17) | 2 (40) |
| Outcomes | |||||||
| Intensive care | 1 (17) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Complication | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
Data are shown as numbers (%).
Number with positive results/number with available data.
Table 2.
Pooled epidemiological and clinical features of COVID-19 in pediatric case series.
| Pooled value | ||||
|---|---|---|---|---|
| Epidemiological data | ||||
| Case numbers | 93 (100) | |||
| Male | 48 (52) | |||
| Household contact | 70 (75) | |||
| Disease severityMild to moderate |
91 (98) |
|||
| Severe | 1 (1) | |||
| Critical | 1 (1) | |||
| Clinical features | Pooled value | 95% CI | I2 | p value |
| Asymptomatic | 24 (26) | 0.13 – 0.52 | 63 % | 0.01 |
| Fever | 55 (59) | 0.41 – 0.72 | 44 % | 0.10 |
| Cough | 43 (46) | 0.27 – 0.66 | 57 % | 0.03 |
| GI symptoms | 11 (12) | 0.06 – 0.32 | 45 % | 0.09 |
| Lymphocyte <1500, x109/L | 7/22a (32) | 0.05 – 0.83 | 72 % | 0.03 |
| Lymphocyte <1000, x109/L | 2/22a (9) | 0.04 – 0.46 | 20 % | 0.28 |
| Outcome | ||||
| Intensive care | 2 (2) | 0.02 – 0.14 | 0 % | 0.88 |
| Complication | 1 (1) | 0.02 – 0.12 | 0 % | 0.98 |
Data are shown as numbers (%).
Number with positive results/number with available data.
Radiographic findings
Table 3 shows the pooled frequency of initial chest CT findings in 3 pediatric COVID-19 case series. Patchy consolidation and ground glass opacities were the most common radiographic features, which occurred in 31% (95% CI 0.13–0.55; I2 = 51%, p = 0.09) and 48% (95% CI 0.36–0.64; I2 = 5%, p = 0.52) of these patients. In 27% (95% CI 0.18–0.43; I2 = 0%, p = 0.64) of the patients, there was no definite lung lesion.
Table 3.
Pooled frequency of initial chest CT findings in pediatric COVID-19.
| Pooled value | 95% CI | I2 | p value | |
|---|---|---|---|---|
| Patchy consolidations | 16 (31) | 0.13–0.55 | 51% | 0.09 |
| Ground glass opacities | 25 (48) | 0.36–0.64 | 0% | 0.52 |
| No lesion | 14 (27) | 0.18–0.43 | 0% | 0.64 |
Data are shown as numbers (%).
Neonates born to mothers with COVID-19
In Table 4 , we provide the current data about neonates born to mothers with COVID-19. Most of the pregnant women (78–89%) had fever at some point during hospitalization and received a Cesarean section (78–100%). Approximately half (44–60%) of the babies were preterm. Hence, gestational age affected birth weight. There was no difference between the two cohorts in initial Apgar score. No neonatal death was mentioned. In addition, all neonates tested negative for SARS-CoV-2.
Table 4.
Demographics and outcomes of neonates born to mothers with COVID-19.
| Chen et al.18 | Zhu et al.19 | |
|---|---|---|
| Number |
9 |
10 (1 twin) |
| Maternal history |
No. (%) |
|
| Cesarean-section | 9 (100) | 7 (78) |
| Fever |
7 (78) |
8 (89) |
| Neonate outcomes |
No. (%) |
|
| Gestational age (weeks) | 37 (36–39) | 34 (31–39) |
| Preterm birth | 4 (44) | 6 (60) |
| Low birth weight (<2500 g) | 2 (22) | 7 (70) |
| 1 min Apgar score | 9 | 9 |
| 5 min Apgar score | 10 | 10 |
| Positive for SARS-CoV-2 | 0 (0) | 0 (0) |
| Neonatal death | 0 (0) | NA |
Data are shown as numbers (%).
Comparison of the clinical features between SARS and COVID-19 in children
In Table 5 , we compare laboratory-confirmed SARS children in previous studies20, 21, 22, 23 and COVID-19 children in our review. Household or direct contact represents the major transmission route for both coronavirus diseases. However, SARS in children is more severe than COVID-19. Children infected with SARS-CoV tended to have more fever (98% vs. 59%, p < 0.001) than those with COVID-19. Symptomatic infection is also more common with SARS than with COVID-19 (100% vs. 74%, p < 0.001). Overall, COVID-19 seems less invasive than SARS in pediatric patients. However, the proportion of patients requiring intensive care shows no statistical difference (10% vs. 2%, p = 0.06).
Table 5.
Comparing the clinical features between SARS and COVID-19 in children.
| SARS (N=80) | COVID-19 (N=93) | ||
|---|---|---|---|
| Years of outbreak | 2002–2003 | 2020 | |
| Household or direct contact | 66–100% | 75% | |
| Clinical features | p value | ||
| Asymptomatic | 0 (0) | 24 (26) | <0.001 |
| Fever | 78 (98) | 55 (59) | <0.001 |
| Cough | 48 (60) | 43 (46) | 0.02 |
| Lymphopenia (<1500, x109/L) | 37 (46) | 7/22∗ (32) | 0.33 |
| Intensive care | 6/59a (10) | 2 (2) | 0.06 |
| Mortality rate | 0 (0) | Very low | – |
Data are shown as numbers (%).
Number with positive results/number with available data.
Discussion
This review summarized currently available observational studies about pediatric COVID-19, from neonates to adolescents. The number of COVID-19 cases has increased worldwide. According to the largest epidemiologic survey, the age distribution of these patients is mainly 30–79 years (87%).24 The elderly and people with chronic diseases are most susceptible to critical forms of COVID-19. There are relatively few cases among children. Patients under 19 years old accounted for 2.2% of 44,672 confirmed cases.24 Only one death was recorded. The crude mortality rate is extremely low (0.1%) compared with that in current published data for adults (2.3%–14.6%).8 , 9 , 24 , 25
The clinical data in pediatric COVID-19 have to be reviewed with caution. Most of the authors in our review used the “Diagnosis and Treatment Protocol for 2019-nCoV. 5th ed” to categorize disease severity.10 We unified the pooled data according to this classification. Based on this rule, the disease severity in children seems to be mild or moderate. Mild disease makes the clinical features subtle and transient. Hence, the proportion of asymptomatic patients varied among studies. Also, one retrospective study was conducted before mid-January.11 The result might be representative of the early stage of COVID-19 outbreak. Therefore, more symptomatic or severe cases were reported, the same as adults,8 , 9 which make moderate heterogeneity of the pooled data in clinical features. Despite the benign presentation of pediatric COVID-19, a 1-year-old boy, who was classified as critical in one report,26 presented with vomiting and diarrhea for 6 days. There was no obvious cough or respiratory tract symptom initially. His condition deteriorated quickly after admission. Shock developed and soon progressed to acute respiratory distress syndrome (ARDS), so he received mechanical ventilation. Acute kidney injury with hemodialysis also occurred during admission. Prompt diagnosis is more challenging in pediatric cases with unusual presentations.
Compared with other pandemic or epidemic viral illnesses, the small numbers of cases and low mortality rate in pediatric COVID-19 are of great interest. Compared with the most relevant virus, SARS-CoV, SARS-CoV-2 causes less severe disease. Human angiotensin-converting enzyme 2 (ACE2) is proposed to be the key factor. ACE2 is the primary entry receptor for both coronaviruses. After replacing four out of five important interface amino acid residues from SARS-CoV, the affinity of SARS-CoV-2 for ACE2 is weaker.27 Nevertheless, SARS-CoV-2 still exhibits significant binding affinity to human ACE2. The binding strength of SARS-CoV-2 to ACE2 partially explains why it causes less severe disease than SARS-CoV but remains highly infectious. However, this result did not answer the question of why children are spared from severe COVID-19. In previous studies, SARS-CoV induced higher ACE2 shedding than human coronavirus NL63. The differential downregulation of ACE2 is related to lung injury.28 In one study about the pathophysiology of acute respiratory distress syndrome (ARDS), increasing age led to an imbalance of the pulmonary renin-angiotensin system, which correlates with aggravated inflammation and enhanced lung injury in a rat model.29 Nevertheless, no age-dependent differences in ACE and ACE2 were seen in a prospective observational cohort study.30 There is one hypothesis that smoking increases ACE2 expression and thus enhances coronavirus entry into pulmonary epithelial cells.31 Undoubtedly, smoking is more prevalent among adults than among children. Nonetheless, some controversies exist.32 Another important fact is that children rarely have underlying diseases, such as diabetes mellitus, chronic obstructive pulmonary disease or cardiovascular diseases, as adults do. These underlying diseases may predispose patients to critical conditions during COVID-19. However, the definite pathogenesis is unknown. Therefore, the severity difference between adults and children with COVID-19 needs more investigation.
The diagnosis of pediatric COVID-19 is a challenge. In China, clinicians should have a suspicion of COVID-19 when patients meet one epidemiological history with two clinical manifestations. According to our literature review, the main symptoms in pediatric COVID-19 are fever and cough, resembling common viral illness frequently encountered in school or kindergarten.33 Epidemiological features are the main diagnostic clue. Children with familial clustering, contact with infected cases, travel or living in areas with persistent local transmission within 14 days prior to disease onset are at risk of being infected with SARS-CoV-2. Since COVID-19 has spread to many other countries, the epidemiological history should be modified outside mainland China. In addition, asymptomatic cases are a potential source of infection. Although only 1% of the patients had been reported as asymptomatic,24 26% of infected children were asymptomatic in the pooled data. Namely, children may act as silent carriers or spreaders.34 Notably, an asymptomatic infection does not always mean a carrier. Chan et al. presented a familial cluster with one family member, a 10-year-old boy, who tested positive for SARS-CoV-2. Without symptoms, he had ground glass lung opacities identified incidentally by CT scan.35 A similar scenario occurred in another report: chest CT performed in one asymptomatic child revealed patchy density.16 This finding raised another issue that needs to be further considered. According to our review, the radiographic findings in pediatric COVID-19 are similar to those in adults.36 In China, chest CT has been used as a screening tool for COVID-19. The disease severity and clinical suspicion are associated with radiographic findings. However, this diagnostic policy might not be applicable in all countries.
Household contact and epidemic area travel history are two major transmission routes for SARS. SARS-CoV-2 shows the same characteristics. Familial clustering is a strong epidemiological link with COVID-19, especially in children. Some reports have already demonstrated that asymptomatic infections in children occurred in family clusters.35 , 37 , 38 Because of the high asymptomatic ratio, pediatric COVID-19 cases are usually detected by contact tracing. Unfortunately, presymptomatic or asymptomatic transmission of SARS-CoV-2 has been reported.38 , 39 The transmission of SARS-CoV-2 via asymptomatic children is reasonable. To date, no clustering or asymptomatic transmission among children has been documented. There are some possible explanations. First, the outbreak of COVID-19 occurred during winter vacation for students. Moreover, the Chinese government imposed lockdowns and travel restrictions in Hubei Province beginning on January 24, 2020. Furthermore, the government announced that the Lunar New Year holiday would be extended to February 2, 2020 across the country. The new semester in school will also be postponed indefinitely. These events limited the community spread of SARS-CoV-2 within school-age children. However, contact tracing and case isolation alone may be unlikely to control the SARS-CoV-2 outbreak in pediatric groups when a new semester starts.
There are some important issues not addressed in our review. First, the outbreak of COVID-19 occurred 2 months ago, so detailed case descriptions and clinical courses are limited. In addition, some patients are still hospitalized and quarantined. The long-term outcome and sequelae may need further follow-up. Second, critical or fatal pediatric COVID-19 cases are rare, indicating that the risk factors and protective factors are still unknown. Third, there are often multiple viral infections that mimic COVID-19 circulating in the same class or the same school. In areas outside China, how to recognize COVID-19 early in children and implement infection control is an urgent problem. Fourth, the treatment strategy in children may need additional debate and caution. In some pediatric cases in China, lopinavir, ritonavir and inhaled recombinant human interferon alpha have been applied. However, the efficacy and safety have not yet been reported.
In conclusion, compared with SARS, COVID-19 has distinct features in children. There are more asymptomatic and mild cases, which make diagnosis and infection source control more challenging. Current diagnosis is based mainly on typical ground glass opacities on chest CT, epidemiological suspicion and contact tracing.
Declaration of Competing Interest
The authors have no conflicts of interest relevant to this article.
Acknowledgment
This research was supported in part by Ministry of Science and Technology with grant no. MOST 108-2321-B-002-016 and MOST 109-2634-F-002-029 to LY Chang. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W., et al. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J Evid Base Med. 2020;13(1):3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W., et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang M., Blanton L., Brammer L., Olsen S.J., Fry A.M. Influenza-associated pediatric deaths in the United States, 2010–2016. Pediatrics. 2018;141(4) doi: 10.1542/peds.2017-2918. [DOI] [PubMed] [Google Scholar]
- 5.Lin T.Y., Twu S.J., Ho M.S., Chang L.Y., Lee C.Y. Enterovirus 71 outbreaks, Taiwan: occurrence and recognition. Emerg Infect Dis. 2003;9(3):291–293. doi: 10.3201/eid0903.020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin T.Y., Kao H.T., Hsieh S.H., Huang Y.C., Chiu C.H., Chou Y.H., et al. Neonatal enterovirus infections: emphasis on risk factors of severe and fatal infections. Pediatr Infect Dis J. 2003;22(10):889–894. doi: 10.1097/01.inf.0000091294.63706.f3. [DOI] [PubMed] [Google Scholar]
- 7.Wahl B., O'Brien K.L., Greenbaum A., Majumder A., Liu L., Chu Y., et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.General Office of the National Health Commission of China . 5th ed. National Health Commission of China; Beijing, China: 2020. Diagnosis and treatment Protocol for 2019-nCoV. [Google Scholar]
- 11.Liu W., Zhang Q., Chen J., Xiang R., Song H., Shu S., et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng K., Yun Y.X., Wang X.F., Yang G.D., Zheng Y.J., Lin C.M., et al. Analysis of CT features of 15 Children with 2019 novel coronavirus infection. Zhonghua er ke za zhi. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 13.Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z., et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020 Feb 28 doi: 10.1093/cid/ciaa198. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia W., Shao J., Guo Y., Peng X., Li Z., Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Ju X.L., Xie F., Lu Y., Li F.Y., Huang H.H., et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua er ke za zhi. 2020;58(4):E011. doi: 10.3760/cma.j.cn112140-20200225-00138. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 16.Jiang J.Y., Duan L., Xiong D.X., Feng Y., Liu X.J., Yu J., et al. Epidemiological and clinical characteristics of novel coronavirus infection in children: Thoughts on the diagnostic criteria of suspected cases outside Hubei Province. Chin Pediatr Emerg Med. 2020;27 [Article in Chinese] [Google Scholar]
- 17.Li W., Cui H., Li K., Fang Y., Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020 Mar 11 doi: 10.1007/s00247-020-04656-7. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang L.Y., Huang F.Y., Wu Y.C., Su I.J., Chiu N.C., Chen K.T., et al. Childhood severe acute respiratory syndrome in Taiwan and how to differentiate it from childhood influenza infection. Arch Pediatr Adolesc Med. 2004;158(11):1037–1042. doi: 10.1001/archpedi.158.11.1037. [DOI] [PubMed] [Google Scholar]
- 21.Leung C.W., Kwan Y.W., Ko P.W., Chiu S.S., Loung P.Y., Fong N.C., et al. Severe acute respiratory syndrome among children. Pediatrics. 2004;113(6):e535–e543. doi: 10.1542/peds.113.6.e535. [DOI] [PubMed] [Google Scholar]
- 22.Chiu W.K., Cheung P.C., Ng K.L., Ip P.L., Sugunan V.K., Luk D.C., et al. Severe acute respiratory syndrome in children: experience in a regional hospital in Hong Kong. Pediatr Crit Care Med. 2003;4(3):279–283. doi: 10.1097/01.PCC.0000077079.42302.81. [DOI] [PubMed] [Google Scholar]
- 23.Stockman L.J., Massoudi M.S., Helfand R., Erdman D., Siwek A.M., Anderson L.J., et al. Severe acute respiratory syndrome in children. Pediatr Infect Dis J. 2007;26(1):68–74. doi: 10.1097/01.inf.0000247136.28950.41. [DOI] [PubMed] [Google Scholar]
- 24.Wu Z., McGoogan J.M. Characteristics of and important Lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [published online ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen F., Liu Z.S., Zhang F.R., Xiong R.H., Chen Y., Cheng X.F., et al. Frist case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58(3):179–182. doi: 10.3760/cma.j.issn.0578-1310.2020.03.003. [Article in Chinese] [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020:1–4. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schouten L.R., Helmerhorst H.J., Wagenaar G.T., Haltenhof T., Lutter R., Roelofs J.J., et al. Age-dependent Changes in the pulmonary renin-angiotensin system Are associated with severity of lung injury in a model of acute lung injury in rats. Crit Care Med. 2016;44(12):e1226–e1235. doi: 10.1097/CCM.0000000000002008. [DOI] [PubMed] [Google Scholar]
- 30.Schouten L.R., van Kaam A.H., Kohse F., Veltkamp F., Bos L.D., de Beer F.M., et al. Age-dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann Intensive Care. 2019;9(1):55. doi: 10.1186/s13613-019-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung Y.H., Hsieh W.Y., Hsieh J.S., Liu F.C., Tsai C.H., Lu L.C., et al. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by Cigarette smoke Exposure in ACE2 Knockout Mice. Int J Biol Sci. 2016;12(4):454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu C.Y., Huang L.M., Fan T.Y., Cheng A.L., Chang L.Y. Incidence of respiratory viral infections and associated factors among children attending a public kindergarten in Taipei City. J Formos Med Assoc. 2018;117(2):132–140. doi: 10.1016/j.jfma.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 34.Cao Q., Chen Y.C., Chen C.L., Chiu C.H. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong Z.D., Tang A., Li K.F., Li P., Wang H.L., Yi J.P., et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang province, China, 2020. Emerg Infect Dis. 2020;26:1052–1054. doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21 doi: 10.1001/jama.2020.2565. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]