Abstract
No specific and effective anti-viral treatment has been approved for COVID-19 so far. Systemic corticosteroid has been sometimes administered to severe infectious diseases combined with the specific treatment. However, as lack of the specific anti-SARS-CoV-2 drug, systemic steroid treatment has not been recommended for COVID-19. We report here three cases of the COVID-19 pneumonia successfully treated with ciclesonide inhalation. Rationale of the treatment is to mitigate the local inflammation with inhaled steroid that stays in the lung and to inhibit proliferation of the virus by antiviral activity. Larger and further studies are warranted to confirm the result of these cases.
Keywords: Ciclesonide, Inhalation, Coronavirus disease 2019
1. Introduction
Our hospital has been accepting the confirmed cases of coronavirus disease 2019 (COVID-19) since February 5, 2020 as a designated Type 2 Infectious Disease Medical Institution (Infectious disease wards that provide inpatient care have an independent negative pressure ventilation environment that is separate from general practice wards). The 8 patients were hospitalized by February 28, and 6 patients had physical symptoms and radiological findings of pneumonia. The possible efficacy of ciclesonide (trade name: Alvesco) was discussed at the “Emergency Expansion Meeting on New Coronavirus Infections” held on February 19. Then, ciclesonide inhalation was started on February 20 for 3 patients with poor oxygenation and CT findings in this hospital. In all three cases, clinical courses revealed favorable. Regarding this report, the consent of all patients was obtained.
2. Case reports
2.1. Case 1: Female, 73 years old
She embarked on the Diamond Princess on January 20, 2020 and landed in Hong Kong on January 25, 2020. Sore throat, malaise and loss of appetite were noted on February 4, and a fever of 38 °C appeared on February 7. A specimen was submitted on February 8, and the result of this throat swab PCR test on February 10 was positive for SARS-CoV-2. On February 11, she disembarked and was transported to our hospital.
-
-
Status at the first consultation
GCS E4V4M6, blood pressure 105/65 mmHg, pulse rate 103/min, respiratory rate 11/min, body temperature 36.7 °C, SpO2 94%/RA.
Physical findings: No pharyngeal redness, no lymphadenopathy, breathing sounds. A fine crackle was heard at the end of inspiration in the lower posterior lung fields.
Past history: Collagen disease was diagnosed, but no treatment was given and follow-up is ongoing. Due to anti-nuclear antibody (discrete type) 1280x at blood collection on admission, and poor color tone of the fingers, she was suspected of having scleroderma.
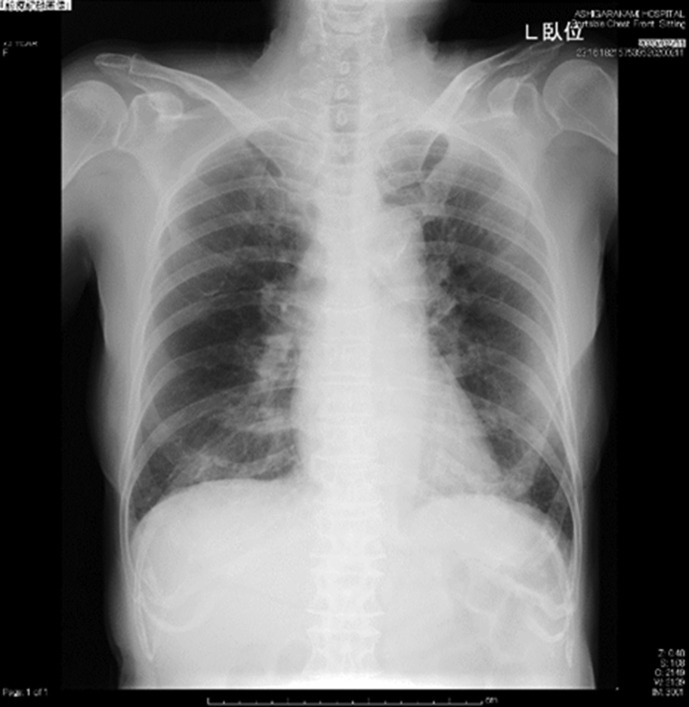
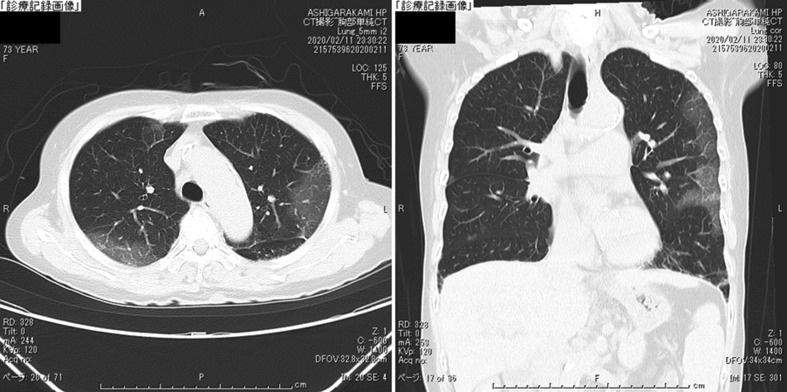
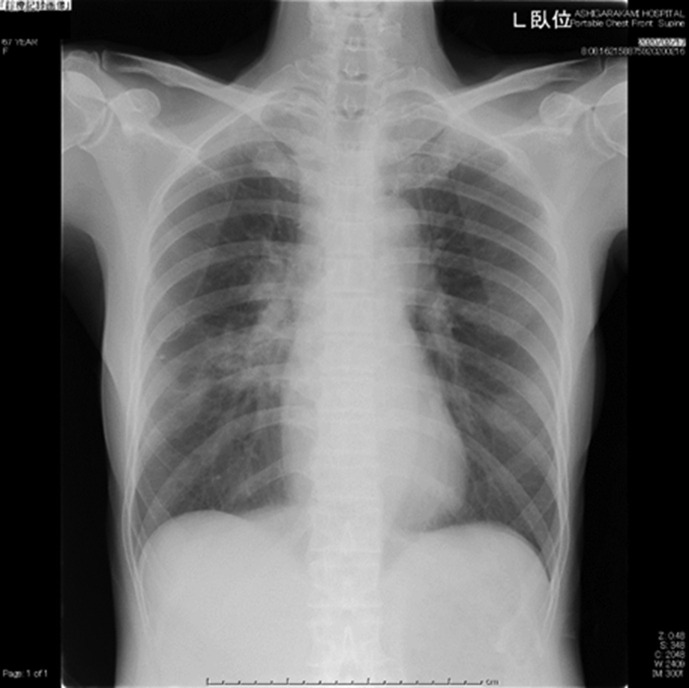
Laboratory findings were listed in Table 1 . Chest X-ray on admission (Feb 11) revealed infiltration shadow in lower right lung field (Fig. 1 ) and chest CT on the same day demonstrated GGO along the pleura over both middle and lower lung fields (Fig. 2 ).
-
-
Post-admission progress
Table 1.
Results of laboratory tests conducted on the day of admission (Case 1).
| Hematology | Biochemistry | ||
|---|---|---|---|
| WBC | 4200/μL | Alb | 4.0 g/dL |
| Neut | 74% | BUN | 15.6 mg/dL |
| Lym | 19% | Cr | 0.55 mg/dL |
| Mo | 8% | T-bill | 1.6 mg/dL |
| Eo | 0% | AST | 41 U/L |
| Baso | 0% | ALT | 23 U/L |
| LDH | 257 U/L | ||
| RBC | 426 × 106/μL | ALP | 450 U/L |
| Hb | 13.8 g/dL | ||
| Ht | 39.9% | Na | 132 mg/dL |
| Plt | 109 × 103/μL | K | 4.1 mg/dL |
| Cl | 93 mg/dL | ||
| Coagulation | CRP | 5.0 mg/dL | |
| PT | 12.4s | ||
| Fib | 5.3mg/dL | ||
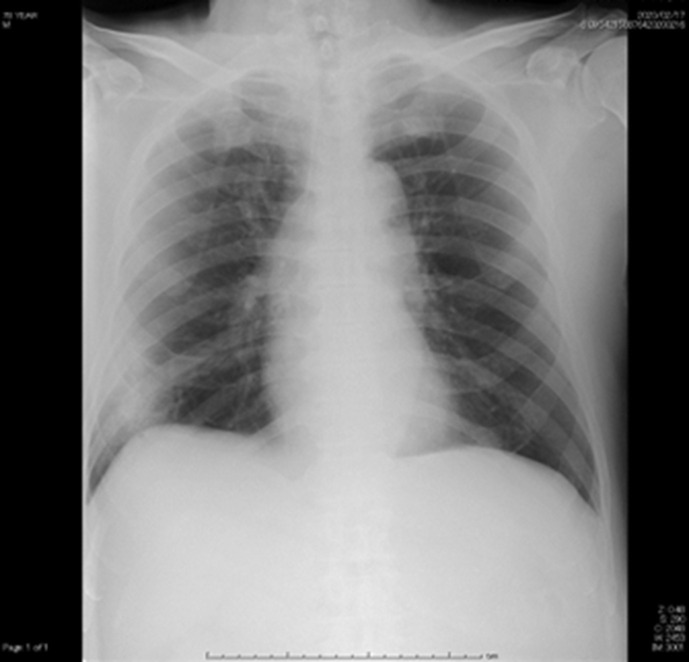
Fig. 1.
The chest X-ray on admission (Feb 11). Infiltrative shadows were shown in lower right lung field. L = left.
Fig. 2.
The chest CT on admission on admission (Feb 11). Axial (left) and coronal (right) sections of a chest X-ray demonstrating features of GGO along the pleura over both middle and lower lung fields.
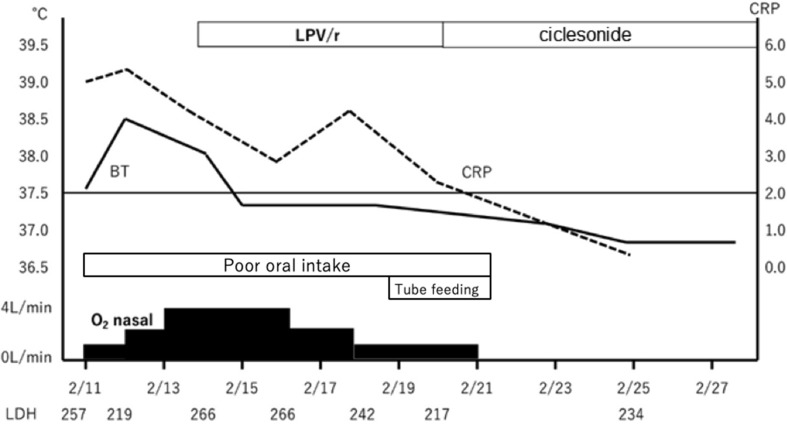
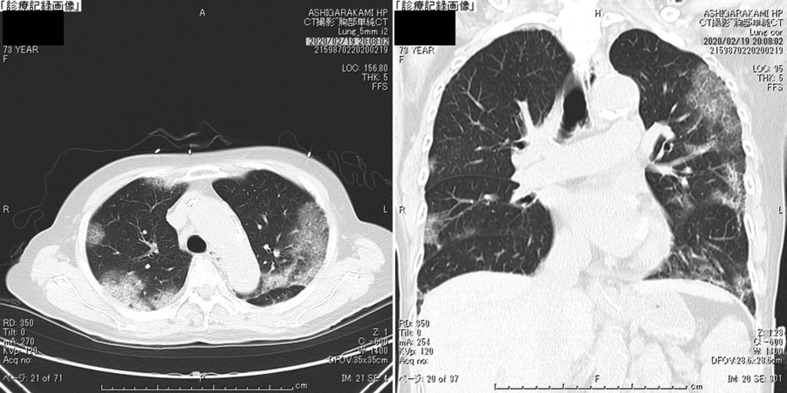
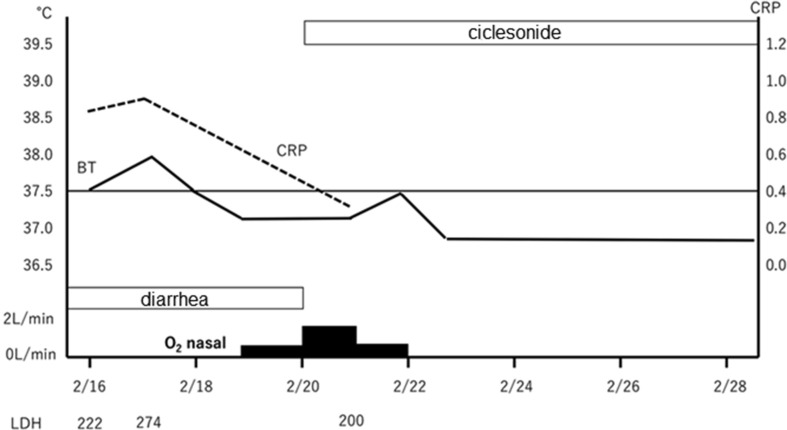
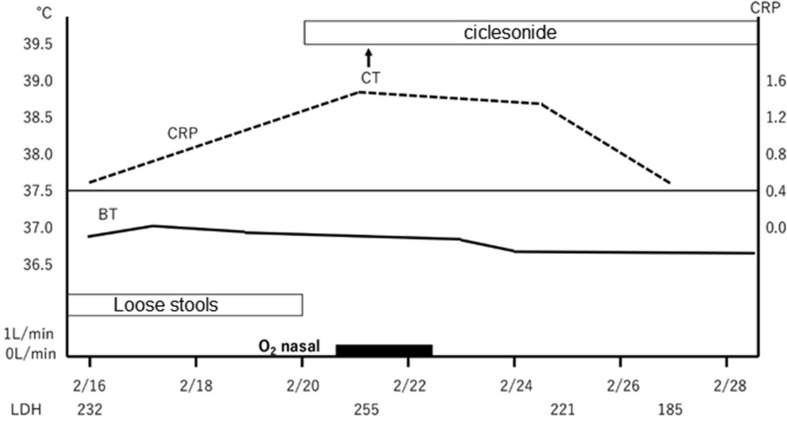
The clinical course is illustrated in Fig. 5 . On admission, she was started with O2 1L/min nasal to maintain SpO2 at 95% or more. She felt fatigue, lying down almost all the time, and could hardly take any food. There was poor communication and disorientation, and when interviewed later, she had no memory of the time before and after hospitalization. Although maintenance infusion, and CTRX, AZM were started, oxygenation gradually worsened, and fever of 38 °C or higher and poor food intake continued. On February 14, O2 4L/min was required to maintain SpO2 at 95% or more, and 4 tablets of Lopinavir/Ritonavir (LPV/r) (800/200 mg) were started. After the start of LPV/r, the fever tended to decrease and oxygenation also improved; but O2 1–2L nasal was required to maintain SpO2 at 95% or more, and it easily dropped to around SpO2 80% with body movement. Her appetite did not improve, and she had considerable fatigue. At CT on 2/19, the shadow of GGO became stronger and the area was enlarged (Fig. 3). In addition, diarrhea and elevation of liver enzymes appeared. Diarrhea, liver damage and loss of appetite were considered side effects of LPV/r; hence, LPV/r was discontinued on February 19. As poor food intake had continued for one week, nasal tube feeding was started. Ciclesonide inhalation (200 μg, twice a day) was started on February 20.
Fig. 5.
A clinical course of case 1.
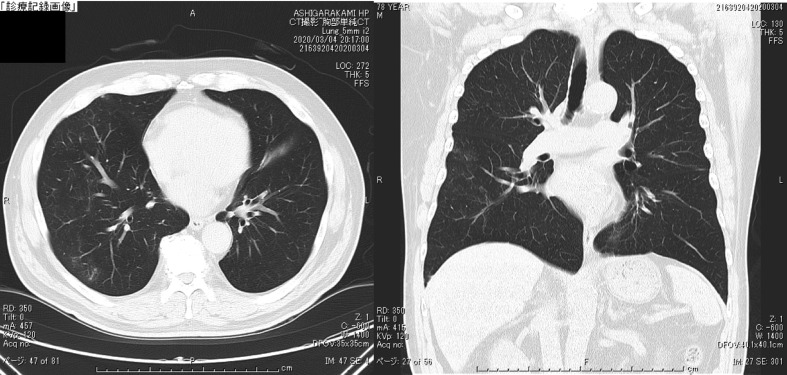
Fig. 3.
The chest CT on Day 9 of hospitalization (Feb 19). The shadow of GGO became stronger and the area was enlarged.
Within 2 days after starting ciclesonide, there was no fever above 37.5 °C, oxygenation had improved, SpO2 could be maintained at 95% or more with room air, and hypoxemia during bodily movement also improved. Her appetite remarkably recovered, and tube feeding was discontinued on February 22 following voluntary food intake of about 1200 kcal. Her general fatigue improved, and she became able to walk indoors without any aid. The patient was judged to have improvement from COVID-19. Nasal swab PCR tests were performed twice on February 25 and 26 were found both negative for SARS-CoV-2. She was discharged on February 28. On CT (February 27), the pneumonia markedly improved (Fig. 4). Currently, inhalation of 400 μg twice daily is being continued until the end of the kit, and outpatient follow-up is planned.
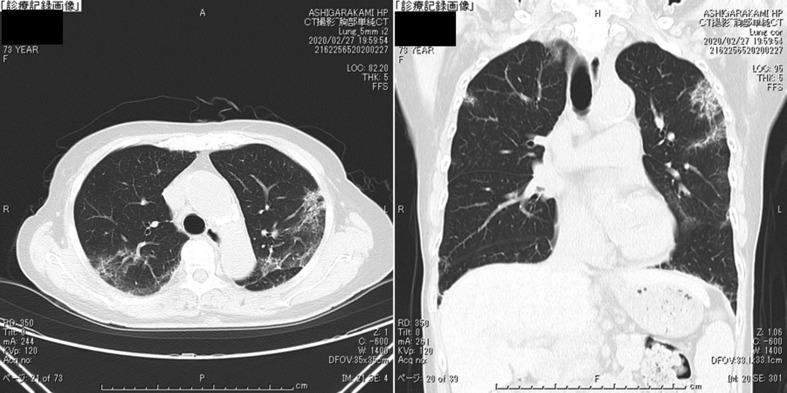
Fig. 4.
The chest CT on Day 18 of hospitalization (Feb 28). The observations of pneumonia findings markedly improved.
2.2. Case 2: Male, 78 years old
He embarked on the Diamond Princess on January 20, 2020. On February 6, dry cough, fatigue, loss of appetite and diarrhea appeared, and he was not able to eat almost any solid food. A fever of 37.4 °C was also noted. A throat swab PCR on February 16 was positive, and he was admitted to our hospital at 21:00 on February 16.
-
-
Status at the first consultation
Alert and conscious, but marked fatigue, blood pressure 146/83 mmHg, pulse 75/min, respiratory rate 16/min, body temperature 37.5 °C, SpO2 99%/RA.
Physical findings: No pharyngeal redness, no lymphadenopathy, no abnormal pulmonary sounds.
Past history: None in particular.
Smoking history: Previously smoked 80 cigarettes/day, but not currently smoking.
Laboratory findings were listed in Table 2 . Chest X-ray on 2/17 found Infiltrative shadow in lower right lung field (Fig. 6 ). Chest CT on 2/18 documented GGO observed in both middle and lower lung fields (Right > Left) (Fig. 7 ).
-
-
Post-admission progress
Table 2.
Results of laboratory tests conducted on the day of admission (Case 2).
| Hematology | Biochemistry | ||
|---|---|---|---|
| WBC | 8400/μL | Alb | 4.2 g/dL |
| Neut | 57% | BUN | 19.1 mg/dL |
| Lym | 24% | Cr | 1.03 mg/dL |
| Mo | 16% | T-bill | 0.4 mg/dL |
| Eo | 1% | AST | 30 U/L |
| Baso | 0% | ALT | 32 U/L |
| Atypical | 2% | LDH | 222 U/L |
| ALP | 202 U/L | ||
| RBC | 531 × 106/μL | ||
| Hb | 17.1 g/dL | Na | 137 mg/dL |
| Ht | 49.9% | K | 4.4 mg/dL |
| Plt | 216 × 103/μL | Cl | 99 mg/dL |
| CRP | 0.81 mg/dL | ||
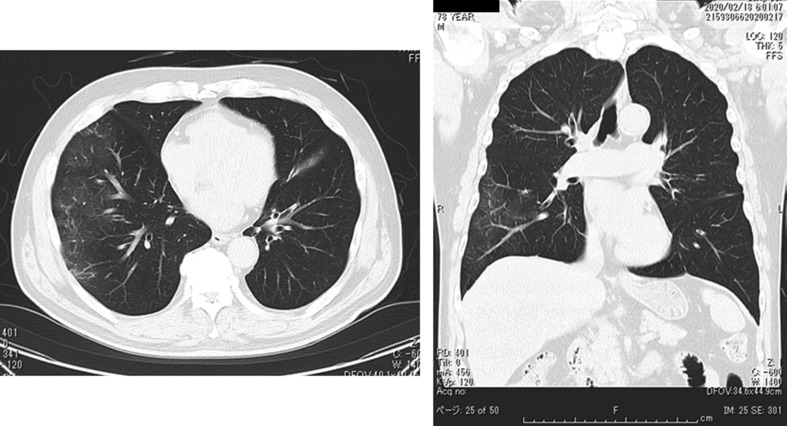
Fig. 6.
The chest X-ray on Day 2 (Feb 27) of hospitalization. Infiltrative shadows were shown in lower right lung field.
Fig. 7.
The chest CT on Day 3 (Feb 28) of hospitalization. GGO observed in both middle and lower lung fields (Right > Left).
The clinical course is illustrated in Fig. 9 . His body temperature on admission was 37.2 °C and increased to 38 °C next day. Oxygenation was also good at the time of admission but decreased to SpO2 90-88% from February 19. Nasal O2 1L/min was started to maintain SpO2 at 95% or more, but on February 20, O2 up to 2L was required. Watery stools had persisted, and he was almost unable to eat any food since admission. Ciclesonide (200 μg, twice a day) was started from February 20. From February 21, his appetite gradually improved, his diarrhea recovered, and his stools became normal. On February 22, oxygen was stopped, and fatigue was improved. He ate almost all of his meals and recovered to the point where he could do daily squats indoors. A pharyngeal swab PCR was performed on February 25 and February 27, and the result was positive. On Mar 3, a result of nasal swab PCR was still positive, but radiological finding in CT scan was improved (Fig. 8). Ciclesonide was increased to 1200 μg/day (400 μg, 3 times a day). On Mar 10 and 11, His nasal swab PCR results were both negative. He was discharged on Mar 13.
Fig. 9.
A clinical course of case 2.
Fig. 8.
The chest CT on Day 17 (Mar 3) of hospitalization. The observations of pneumonia findings improved.
2.3. Case 3: Female, 67 years old
She embarked on the Diamond Princess on January 20, 2020. She had a dry cough on February 6, fatigue and joint pain on February 8, and a fever of 38.9 °C on February 9, followed by loss of appetite and diarrhea, after which she could hardly eat any food. A throat swab for SARS-CoV-2 PCR was found positive on February 16, then she was transported to our hospital and admitted on February 16.
-
-
Status at the first consultation
Alert and conscious, blood pressure 125/86 mmHg, pulse 88/min, respiratory rate 18/min, body temperature 36.4 °C, SpO2 99%/RA.
Physical findings: No pharyngeal redness, no lymphadenopathy, no abnormal pulmonary sounds.
Past medical history: An abnormal X-ray shadow was found by a previous medical checkup, and is being followed up by CT.
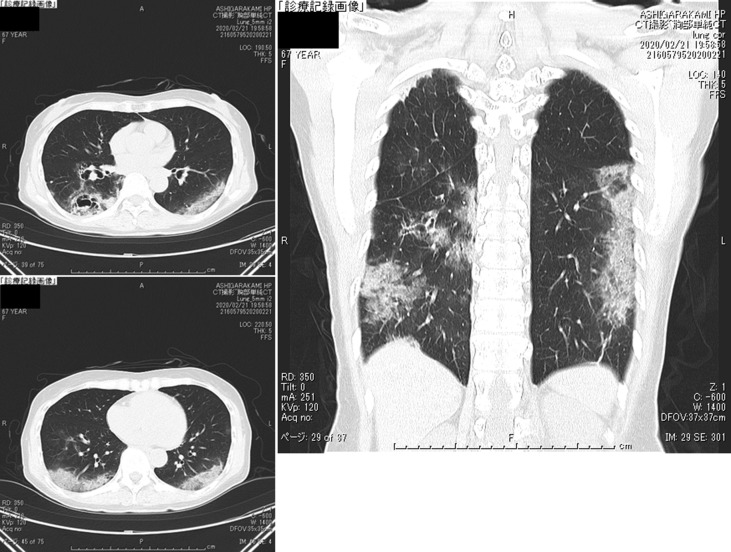
Laboratory findings were listed in Table 3 . Chest X-ray on Feb 17 revealed an infiltrative shadow in the hilar portion of the right middle lung field (Fig. 10 ). In chest CT on Feb 21, GGO was found in the posterior mid-lower lung field, on both sides. Bronchiectasis and cavitation with calcification were found in the right lung (Fig. 11 ). The cavernous lesion had been carefully examined in another hospital, and the course of the disease has been followed up without any sign of deterioration. At admission, expectoration of sputum was difficult, so a mycobacteria test was not performed.
-
-
Post-admission progress
Table 3.
Results of laboratory tests conducted on the day of admission (Case 3).
| Hematology | Biochemistry | ||
|---|---|---|---|
| WBC | 4400/μL | Alb | 4.0 g/dL |
| Neut | 60% | BUN | 16.0 mg/dL |
| Lym | 27% | Cr | 0.68 mg/dL |
| Mo | 12% | T-bill | 0.4 mg/dL |
| Eo | 1% | AST | 30 U/L |
| Baso | 0% | ALT | 17 U/L |
| LDH | 232 U/L | ||
| RBC | 418 × 106/μL | ALP | 175 U/L |
| Hb | 12.7 g/dL | ||
| Ht | 37.5% | Na | 136 mg/dL |
| Plt | 16.6 × 103/μL | K | 4.2 mg/dL |
| Cl | 98 mg/dL | ||
| CRP | 0.46 mg/dL | ||
Fig. 10.
The chest X-ray on Day 2 (Feb 17). An infiltrative shadow was found in the hilar portion of the right middle lung field.
Fig. 11.
The chest CT on Day 6 of hospitalization (Feb 21). GGO was found in the posterior mid-lower lung field, on both sides. Bronchiectasis and cavitation with calcification were found in the right lung.
The clinical course is illustrated in Fig. 13 . At the time of admission, her body temperature was 36.4 °C and oxygenation was good. She admitted fatigue and was often lying in bed. She ate about a half portion of her meals. To prevent exacerbation, ciclesonide was started on February 20. A fine crackle was heard from the back by chest auscultation from February 21, and CT showed GGO in the posterior lower lung field, on both sides. On the same day, SpO2 decreased to less than 90% during bodily movement, so O2 1L/min nasal was started. Ciclesonide (200 μg, twice a day) was administered alone, and on the follow-up on February 22, oxygenation improved, and administration of oxygen was discontinued. Her appetite began to improve from February 20 p.m. On February 22, she was able to eat complete meals, and her general fatigue improved rapidly. A pharyngeal swab PCR was performed on February 25 and 27, and the result was positive. A nasal swab PCR was performed on Mar 3 and was negative, but on Mar 4, it was positive. radiological finding on CT scan on Mar 4 was improved (Fig. 12). ciclesonide was increased to 1200 μg/day, in 3 doses. On Mar 10 and 11, She had negative PCR results on nasal swab. She was discharged on Mar 13.
Fig. 13.
A clinical Course of case 3.
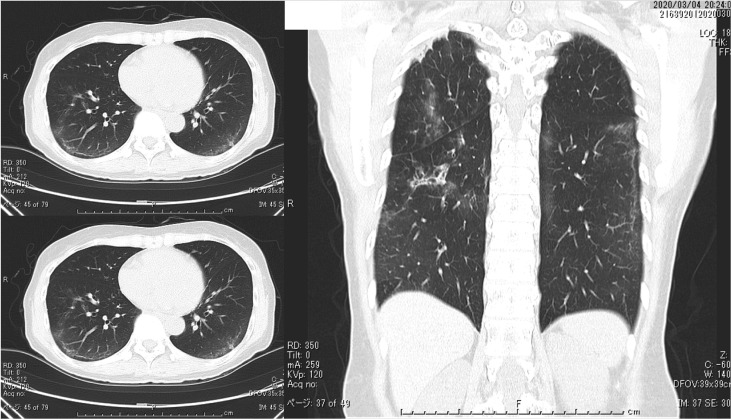
Fig. 12.
The chest CT on Day 18 of hospitalization (Mar 4). The observations of pneumonia findings improved.
3. Discussion
We treated 3 cases of mild to mid-stage COVID-19 with ciclesonide, inhaled steroid, and obtained favorable results. Although it was still very preliminary and unpublished data, the coronavirus laboratory at the National Institute of Infectious Diseases, Murayama Branch, had experimental data that among existing inhalation drugs for treatment of bronchial asthma, ciclesonide (trade name: Alvesco) has strong antiviral activity against SARS-CoV-2 [1]. In addition, according to another screening studies including FDA-approved drugs, ciclesonide also has the antiviral activity against MERS virus [2]. Ciclesonide is a safe drug that is widely used as a steroid for inhalation from premature babies and newborns to the elderly and is said to be effective in controlling chronic inflammation of the respiratory tract. Although the pathology of COVID-19 lung injury has not yet been elucidated, it has been estimated from MERS and SARS that the virus replicates in alveolar epithelial cells, causing lung damage and simultaneously infecting alveolar macrophages. It is thought to cause local inflammation, and it is expected that the antiviral and anti-inflammatory effects of ciclesonide will be effective in treating lung injury caused by coronavirus, which is becoming more severe. No antiviral effect against COVID-19 has currently been found for inhaled steroids other than ciclesonide. It has been reported that steroid treatment for COVID-19 is not recommended due to the possibility of prolonging viremia and complications such as diabetes [3], although systemic administration of hydrocortisone, methylprednisolone, dexamethasone and prednisolone are being considered. Ciclesonide is an inhaled prodrug that stays on the lung surface with only minor increases in blood levels. It is preferably given in pneumonia before the disease becomes severe, and is expected to have the effect of rapidly improving the patient symptoms and preventing progression to severe pneumonia.
The standard dose in adult is 400 μg/day (400 μg, once a day), and the maximum dose is 800 μg/day (400 μg, twice a day). Based on the viral replication time of 6–8hr, we considered frequent and high-dose administration is necessary to reach enough drug in alveoli. Furthermore, we thought it is desirable to continue for about 14 days or longer after starting to avoid reactivation of residual virus, and appearance of resistant virus. Since the virus replicates in alveolar epithelial cells, it is considered that inhaling as deeply as possible will enhance the effect. Based on the above considerations and the dosage form, it is proposed to use the following doses as standard at this time.
Indication: Pneumonia in confirmed COVID-19 positives (for long-term positive asymptomatic patients, further studies may be needed)
Dosage:
-
A.
Ciclesonide (Alvesco) 200 μg Inhaler (56 puffs/kit), 2 times a day, 2 inhalations each time
-
B.
Ciclesonide (Alvesco) 200 μg Inhaler (56 puffs/kit), 3 times a day, 2 inhalations each time
Take A as a basis, consider changing to B. for severe cases and cases where the effect is not sufficient. Considering that the drug must reach the infected area, “deep inhalation” is the most reasonable method of administration at present.
We accepted 8 patients with coronavirus positive from the cruise ship, all of whom were over 65 years old. Two patients were asymptomatic at the time of admission, became negative without any symptoms, and were discharged from the hospital. Six patients had poor oxygenation and pneumonia showing ground-glass shadow on chest CT. Four patients had pneumonia at the time of admission, and two had developed it post-admission. Three of the 6 patients who had not taken ciclesonide worsened and were transferred to a high-level medical institution, of whom 2 (including one who developed symptoms after admission) are on ventilator management.
Immediately after admission, symptoms of pneumonia became rather serious, and as noted in many reports, they seemed to worsen rapidly 7–10 days after onset, even if the initial symptoms were mild. At the time, our hospital was unable to provide a respirator in an infectious disease ward, and we felt a great danger in having pneumonia patients with worsening symptoms. We started administration of ciclesonide immediately after we received the information on February 19. In particular, case 1 developed pneumonia from the time of admission, started LPV/r and escaped the acute phase of inflammation, but poor oxygenation continued, and loss of appetite was prolonged. She became physically weak, was in danger of acute exacerbation, and CT showed a worsening trend. It was considered to transfer her to another hospital, but due to other seriously ill patients, we were forced to put her on standby. While we were trying to look for a transfer destination, we received the information of the possible favorable anti-SARS-CoV-2 effect of ciclesonide and started the administration, resulting rapid improvement described here. This was an impressive case.
Among former 3 pneumonia patients, 2 of them had required respiratory management beforehand; but after its introduction, these 3 cases were relieved of symptoms. However, the disease background was different in each patient, and efficacy cannot be discussed with only this limited number of observational cases.
In the case of COVID-19, it is mainly the elderly who show more severe symptoms. For elderly patients such as Case 1 who was weak due to pneumonia, its safety has been established. If this effect can be confirmed, early administration of ciclesonide is simple and inexpensive (∖2169/kit, LPV/r ∖1289.6/day), and is considered to have great merit. It is greatly anticipated that more cases of ciclesonide use will be collected and studied in future.
Authorship statement
Keisuke Iwabuchi treated all the patients, and wrote the initial draft of the manuscript. Tsuneo Morishima contributed to provide the basic research information about ciclesonide. All other authors have contributed to build a system of infectious disease ward in the hospital, backed up the usual practice, and critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors meet the ICMJE authorship criteria.
Declaration of Competing Interest
Authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Preprint server. https://www.biorxiv.org/content/10.1101/2020.03.11.987016v1
- 2.Preprint server. https://www.biorxiv.org/content/10.1101/2020.02.25.965582v1
- 3.Russell Clark D., Millar Jonathan E., Baillie J Kenneth. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30317-2/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]