Abstract
Background and Purpose
β‐Caryophyllene (BCP) is a plant‐derived terpenoid used as a food additive for many decades. Recent studies indicate that BCP is a cannabinoid CB2 receptor agonist with medical benefits for a number of human diseases. However, little is known about its therapeutic potential for drug abuse and addiction.
Experiment Approach
We used pharmacological, transgenic, and optogenetic approaches to systematically evaluate the effects of BCP on nicotine‐taking and nicotine‐seeking behaviour in animal models of drug self‐administration, electrical, and optical brain‐stimulation reward.
Key Results
Systemic administration of BCP dose‐dependently inhibited nicotine self‐administration and motivation for nicotine seeking in rats and mice. The reduction in nicotine self‐administration was blocked by AM630, a selective CB2 receptor antagonist, but not by AM251, a selective CB1 receptor antagonist, suggesting involvement of a CB2 receptor mechanism. Genetic deletion of CB2 receptors in mice blocked the reduction in nicotine self‐administration produced only by low doses, but not by high doses, of BCP, suggesting involvement of both CB2 and non‐CB2 receptor mechanisms. Furthermore, in the intracranial self‐stimulation paradigm, BCP attenuated electrical brain‐stimulation reward and nicotine‐enhanced brain‐stimulation reward in rats. Lastly, BCP also attenuated brain‐stimulation reward maintained by optogenetic stimulation of dopaminergic neurons in the ventral tegmental area in DAT‐cre mice, suggesting the involvement of a dopamine‐dependent mechanism in BCP's action.
Conclusions and Implications
The present findings suggest that BCP has significant anti‐nicotine effects via both CB2 and non‐CB2 receptor mechanisms and, therefore, deserves further study as a potential new pharmacotherapy for cigarette smoking cessation.
Abbreviations
- BCP
β‐caryophyllene
- BSR
brain‐stimulation reward
- CB2‐KO
CB2 receptor‐knockout
- CPP
conditioned place preference
- eCB
endocannabinoid
- FAAH
fatty acid amide hydrolase
- FR
fixed ratio
- ICSS
intracranial self‐stimulation
- NIDA
National Institute on Drug Abuse
- VTA
ventral tegmental area
What is already known
Although several medications for smoking cessation are available, the rate of relapse remains extremely high.
There is an urgent need to develop novel and more effective pharmacotherapies for smoking cessation.
What this study adds
BCP, an FDA‐approved dietary terpenoid, has therapeutic potential in reducing nicotine‐taking and nicotine‐seeking behaviours.
Both CB2 and non‐CB2 receptor mechanisms are involved in BCP's action on nicotine self‐administration.
What is the clinical significance
BCP may have highly translational potential as a repurposed pharmacotherapy for cigarette smoking cessation.
1. INTRODUCTION
Tobacco use is a serious health threat globally. Every year, approximately 7 million people die worldwide from tobacco‐related health problems (World Health Organization, 2019). In the United States alone, 16 million individuals are living with a disease caused by smoking (Centers for Disease Control and Prevention, 2019). Cigarette smoking is the second most costly health problem in the United States with an estimated cost of 300 billion U.S. dollars per year (Centers for Disease Control and Prevention, 2019). The addictive properties of tobacco are attributed to the https://www.sciencedirect.com/topics/neuroscience/nicotine of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2585 in the mesolimbic dopamine system, where nicotine is thought to increase dopaminergic neuron activity in the ventral tegmental area (VTA) by activating nicotinic ACh receptors. To date, the U.S. Food and Drug Administration (FDA) has approved several medications for smoking cessation, including nicotine replacement therapies (nicotine patches and gums), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7135, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5459 (Jordan & Xi, 2018). Although these treatments display significant efficacies in reducing cigarette use and in mitigating withdrawal symptoms, a rate of relapse after termination of treatment remains high. Over 90% of individuals in treatment who achieve abstinence relapse to smoking within a year post‐treatment (Jordan & Xi, 2018). Therefore, there continues to be a significant need for the development of novel and more effective pharmacotherapies for nicotine dependence.
Growing evidence indicates that the endocannabinoid (eCB) system is involved in motivation for rewards, including the rewarding effects of nicotine (Parsons & Hurd, 2015). The eCB system consists of two major cannabinoid receptor subtypes (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57), endogenous ligands, and the enzymes responsible for their biosynthesis and degradation (Di Marzo, 2009). Early studies focused on the CB1 receptor (Le Foll & Goldberg, 2004; Merritt, Martin, Walters, Lichtman, & Damaj, 2008). However, clinical trials with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=743, a CB1 receptor antagonist/inverse agonist, were terminated worldwide due to serious adverse effects such as depression and suicidality (Le Foll, Gorelick, & Goldberg, 2009). Since then, cannabinoid‐based medication development has shifted to other targets in the eCB system such as the CB2 receptor (Jordan & Xi, 2019), fatty acid amide hydrolase (FAAH), or phytocannabinoids without psychotomimetic effects (Galaj, Bi, Yang, & Xi, 2019).
β‐Caryophyllene (BCP) is a natural bicyclic sesquiterpene with a sweet, woody, spicy, clove‐like smell. It is a unique terpene due to its large size and structure (Gertsch et al., 2008). BCP is a constituent of many essential oils derived from a large number of spice and food plants including black pepper, thyme, and cloves (Sharma et al., 2016) and is also present in high proportions in some strains of cannabis (Mediavilla & Steinemann, 1997). Due to its distinctive flavour, BCP has been used for many decades as a food or cosmetic additive. BCP falls under the FDA's “generally recognized as safe” classification, so large doses can be safely consumed. BCP was first synthesized in 1964 (Corey, Mitra, & Uda, 1964) and later identified as a selective agonist of CB2 receptors (K i = 155 nM; Gertsch et al., 2008). When administered orally, BCP can reduce inflammation in wild‐type (WT) mice, but not in mice lacking CB2 receptors (CB2‐KO mice), implying action at CB2 receptors mediated the effects of BCP in vivo (Cho et al., 2007; Gertsch et al., 2008; Klauke et al., 2014).
In recent years, the CB2 receptors have become a new target in medication development for the treatment of substance use disorders as this receptor has been identified on midbrain dopaminergic neurons and implicated in drug reward and addiction (Jordan & Xi, 2019; Liu et al., 2017; Manzanares et al., 2018). In support of this premise are the findings that, in CB2‐KO mice, there was attenuation of nicotine‐induced conditioned place preference (CPP; Canseco‐Alba et al., 2018; Ignatowska‐Jankowska, Muldoon, Lichtman, & Damaj, 2013; Navarrete et al., 2013), nicotine self‐administration (Navarrete et al., 2013), and nicotine withdrawal symptoms (Navarrete et al., 2013, but see Ignatowska‐Jankowska et al., 2013), suggesting the involvement of CB2 receptors in nicotine reward and dependence. This is further supported by the finding that O‐1966 (a selective CB2 receptor agonist) enhanced nicotine‐induced CPP, when given in combination with a subthreshold dose of nicotine (Ignatowska‐Jankowska et al., 2013). Paradoxically, it was also reported that systemic administration of CB2 receptor agonists (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=747 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3316) or antagonists/inverse agonists (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=750 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=751) blocked cocaine‐ or nicotine‐induced CPP in mice (Canseco‐Alba et al., 2018; Ignatowska‐Jankowska et al., 2013; Navarrete et al., 2013), although the mechanisms underlying such conflicting findings are unclear.
Given that BCP has been shown to be safe in humans and has been identified as a selective CB2 receptor agonist both in vitro and in vivo, we explored the potential utility of BCP in the treatment of nicotine use disorders. In this study, we used pharmacological, transgenic, and optogenetic approaches to systematically evaluate the effects of BCP on nicotine reward and motivation for nicotine seeking in models of nicotine addiction in rats and mice. We also explored potential receptor mechanisms underlying BCP action. Our findings support the potential utility of BCP as a pharmacotherapy for smoking cessation.
2. METHODS
2.1. Animals
All animal care and experimental procedures were consistent with the Guide for the Care and Use of Laboratory Animals and were approved by the National Institute on Drug Abuse (NIDA) Animal Care and Use Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
Male WT, CB2‐KO, and https://www.jax.org/strain/020080 (RRID:IMSR_JAX:020080) mice with https://www.jax.org/strain/000664 (IMSR_IAX:000664) genetic backgrounds were bred at the National Institute on Drug Abuse Intramural Research Program. CB2+/− breeders were originally created by Buckley et al. (2000) and donated by Dr Kunos at the National Institute on Alcoholism and Alcohol Abuse and DAT‐cre breeders were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Male https://rgd.mcw.edu/rgdweb/report/strain/main.html?id=2308852 (RRID:RGD_2308850) rats (purchased from Charles River Laboratories, Frederick, MD, USA) were also used. Animals were housed in climate‐controlled animal colony rooms on a reversed light–dark cycle (lights on at 7:00 p.m., lights off at 7:00 a.m.) with free access to food and water, except for WT and CB2‐KO mice that received daily rations of food in order to maintain their weights at 85% of free feeding values 1 week prior to and during the food self‐administration experiments.
2.2. Surgery
The procedures for jugular catheter surgery and nicotine self‐administration were as previously reported (Wang et al., 2015). Briefly, animals (rats or mice) were anaesthetized by an i.p. injection of ketamine/xylazine, and catheters, constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA), were inserted into the right jugular vein. After being sutured into place, the catheter was passed s.c. to the top of the skull and exited and attached to a connector (a modified 24‐g cannula; Plastics One, Roanoke, VA, USA). The connector was then mounted onto the skull using jeweller's stainless‐steel screws and dental acrylic.
For the electrical intracranial self‐stimulation (ICSS) experiment, a unilateral monopolar stainless‐steel stimulation electrode was implanted into the rat medial forebrain bundle at the level of the lateral hypothalamus (AP −2.56, ML ±1.9, and DV −8.6) under ketamine/xylazine anaesthesia, as described previously (Wang et al., 2015). For the optical ICSS experiment, DAT‐cre mice were injected with adeno‐associated virus carrying ChR2‐EGFP (AAV5‐EF1α‐DIO‐ChR2‐EGFP) and implanted with bilateral custom‐made optical fibres (200‐μm inner diameter, N.A. 0.22; Doric Lenses, Quebec, Canada) targeted at the VTA (AP − 3.1, ML ± 0.8, DV − 4.25; Han et al., 2017; Jordan, Humburg, et al., 2019; Newman et al., 2019).
2.3. Drug treatments
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2585 hydrochloride (provided by NIDA) was dissolved in 0.9% saline to achieve a final dose of 0.25 mg·kg−1 for s.c. injections or 0.03 mg·kg−1 for i.v. deliveries. BCP was dissolved in 5% Cremophor to achieve doses of 3, 10, 25, 50, and 100 mg·kg−1 and was administered 30 min prior to the test sessions (except locomotor activity and rotarod experiments that involved immediate administration). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3317 (a CB1 receptor antagonist) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=750 (a selective CB2 receptor antagonist) were purchased from Tocris Bioscience (Minneapolis, MN, USA), dissolved in 5% Cremophor to achieve a final dose of 3 mg·kg−1 and administered 30 min prior to the BCP administration.
2.4. Apparatus
Rat or mouse self‐administration was conducted in standard operant chambers (Med‐Associates, Fairfax, VT, US), each housed in a sound‐attenuating box. Each chamber was equipped with two retractable levers, a white light above the active lever, drug tubing connected to a syringe pump and a food dispenser (Wang et al., 2015).
Rat electrical ICSS experiments were conducted in standard operant chambers (Med‐Associates, Fairfax, VT, USA), each of which contained a wall‐mounted retractable lever and a cue light above the lever. The operant chambers were enclosed in ventilated, sound‐attenuating cabinets. Depression of the operant lever activated a brain stimulator (Spiller et al., 2019).
Mouse optical ICSS experiments were conducted in standard operant conditioning chambers (Med‐Associates, Fairfax, VT, USA). Each chamber was equipped with two wall‐mounted levers, two cue lights, a house light, and an audio stimulus generator. Mice were gently connected to a cable attached to an optical swivel, which was in turn connected to a 473‐nm laser tuned for channelrhodopsin (ChR2) stimulation. Computer software controlled a pulse generator that controlled the lasers (Han et al., 2017; Jordan, Humburg, et al., 2019; Newman et al., 2019).
2.5. Experimental procedures
2.5.1. The effects of BCP on nicotine self‐administration in rats
The experimental designs, analysis, and reporting were according to new guidance of BJP. In each instance, all ratings of behavioural tests were performed by observers blinded to the experimental treatments. In this experiment, two groups of rats were used to evaluate the effects of BCP on nicotine self‐administration under fixed‐ratio 1 (FR1; n = 9) and FR5 (n = 9) schedules of reinforcement. A third group of rats (n = 9) was used to study the receptor mechanisms underlying BCP action under an FR5 schedule of reinforcement. Two different schedules (FR1 and FR5) were chosen since the FR5 schedule of reinforcement yielded higher basal active lever responding than the FR1 schedule, providing higher confidence in evaluating drug‐induced effects on nicotine reward. In this experiment, all rats were trained to lever press for nicotine (0.03 mg·kg−1 per infusion) initially under an FR1 schedule of reinforcement during daily 3‐hr sessions (Wang et al., 2015). Responding on the active lever resulted in the i.v. delivery of 0.08‐ml of nicotine solution and the illumination of the light above the active lever. There was a 20‐s post‐injection timeout during which no additional drug infusions could be earned. Responses on the inactive lever were counted but had no consequences. Once animals demonstrated a stable pattern of responding, defined as <20% variability in daily nicotine intake across three consecutive sessions, and an active/inactive lever press ratio exceeding 2:1, they were tested with either of two doses of BCP (25 and 50 mg·kg−1, i.p.). The effects of BCP on FR1 nicotine self‐administration were assessed. The second and third groups of rats were trained first under an FR1 schedule of reinforcement for 1 week and then switched to an FR5 schedule of reinforcement for the remainder of the experiment. After stable self‐administration was observed, the effects of a wide dose range of BCP (0, 3, 10, 25, and 50 mg·kg−1) on nicotine self‐administration under the FR5 schedule were assessed in the second group of rats. To determine whether a CB2 receptor mechanism was involved in the effects of BCP, the third group (n = 9) of rats self‐administering nicotine was treated with either vehicle (5% cremophor), BCP alone (25 mg·kg−1), 3 mg·kg−1 of AM630 + 25 mg·kg−1 of BCP, or AM630 alone (3 mg·kg−1). The sequence of these tests was random with intervals of 3–5 days between tests.
2.5.2. The effects of BCP on nicotine self‐administration in WT and CB2‐KO mice
To further elucidate the above findings in rats, we used CB2‐KO mice (n = 9) and their WT littermates (n = 9) in a self‐administration paradigm. Briefly, animals were trained to self‐administer nicotine (0.03 mg·kg−1 per infusion, i.v.) under an FR1 schedule of reinforcement during daily 3‐hr sessions for approximately 1 week, and then their reinforcement schedule was changed to FR5 in order to increase workload (active lever responses) for nicotine reward. Responding on the active lever activated the syringe pump producing an i.v. infusion of nicotine (0.015 ml) and presentation of the light/tone cue above the active lever. Responses on an inactive lever were counted but had no consequences. During the 4.2‐s infusion period, additional responses on the active lever were recorded but did not lead to additional infusions. Animals were tested with BCP (0, 25, and 50 mg·kg−1, i.p., 30 min prior to the test session) once stable nicotine self‐administration was achieved, defined as (a) at least 20 nicotine infusions during the 3‐hr session, (b) less than 20% variability in daily nicotine infusions across two consecutive days, and (c) an active/inactive lever press ratio exceeding 2:1.
We then followed up with a pharmacological approach to investigate the potential involvement of CB1 and CB2 receptors in the actions of BCP action in WT mice. In this experiment, each animal received pretreatment with vehicle (5% cremophor), 3 mg·kg−1 of AM251, or 3 mg·kg−1 of AM630 thirty minutes prior to BCP administration (25 mg·kg−1) after stable nicotine self‐administration was demonstrated. After each test, animals continued daily nicotine self‐administration. The sequence of drug testing was counterbalanced with time intervals of 3–5 days between tests.
2.5.3. The effects of BCP on nicotine seeking during extinction
An additional group of mice (n = 12) was used in this experiment. The procedures for nicotine self‐administration (FR5) were the same as described above. After stable self‐administration was achieved, the extinction phase began during which active and inactive lever pressing had no consequence (no drug, no cues). After 2 weeks of extinction training, the mice displayed significant extinction resistance—failure to meet the extinction criteria that we previously used in rats (≤10 lever presses for three consecutive days; Wang et al., 2015). Therefore, we evaluated whether BCP can reduce nicotine seeking during the extinction training. After each test, daily extinction training continued. The active BCP doses (0, 25, and 50 mg·kg−1, i.p.) were administered in a random order with 2–5 days between tests (Figure 3a).
Figure 3.
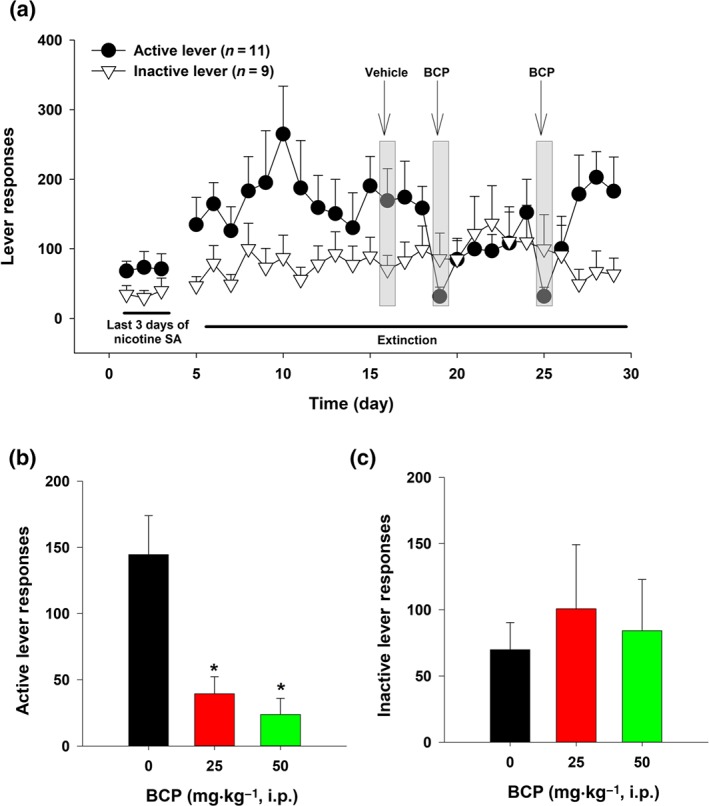
The effects of BCP on nicotine seeking during extinction in mice (n = 12). (a) Time course of active and inactive lever responding during the last three sessions of nicotine self‐administration followed by 4 weeks of extinction. Each animal received a vehicle and two BCP injections, with BCP doses (25 and 50 mg·kg−1) counterbalanced. (b) Mean (±SEM) numbers of active lever responding. (c) Mean (±SEM) numbers of inactive lever responding, illustrating that BCP pretreatment dose‐dependently reduced cue‐induced nicotine‐seeking during extinction from previous nicotine self‐administration. *P < .05, significantly different from the vehicle control group; one‐way ANOVA followed by post hoc Student–Newman–Keuls tests for multiple group comparisons
2.5.4. The effect of BCP and nicotine on electrical ICSS in rats
In this experiment, we further assessed the effects of BCP on brain‐stimulation reward (BSR) itself and on nicotine‐enhanced BSR in rats (n = 12). Each lever press resulted in a 500‐ms train of 0.1‐ms rectangular cathodal pulses through the electrode, followed by retraction of the lever and a 500‐ms “timeout” in which further lever presses did not produce brain stimulation. After acquiring brain stimulation‐reinforced lever pressing, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in a descending order. At each pulse frequency, animals could lever press for two 30‐s trials, after which the pulse frequency was decreased by 0.05 log units. The response rate for each frequency was defined as the average number of lever presses during the two 30‐s trials at that frequency. The BSR threshold (θ 0) was defined as the minimum frequency at which an animal responded for rewarding stimulation, calculated using the Gompertz sigmoidal model (Coulombe & Miliaressis, 1987). In addition, M 50 was measured as stimulation frequency for half maximal reward efficacy, and Y max was defined as the maximal rate of responding (number of lever presses for BSR per unit of time). The testing phase began once stable BSR responding was achieved (<10% variation in θ 0 over five consecutive days; Spiller et al., 2019). Thirty minutes before the test session, animals were injected systemically with one of the BCP doses (0, 25, 50, or 100 mg·kg−1). During the test session, animals could lever press for BSR in the presence or absence of nicotine (0.25 mg·kg−1, s.c.). After each test, animals received additional self‐stimulation sessions until a new θ 0 baseline was established and then re‐tested with a different dose of BCP.
2.5.5. The effects of BCP on food self‐administration in WT and CB2‐KO mice
We also assessed the effects of BCP pretreatment on non‐drug (i.e., food pellet) self‐administration in WT (n = 8) and CB2‐KO (n = 8) mice to determine whether BCP might inhibit non‐drug reward, presumably via a CB2 receptor mechanism. Procedures for food self‐administration were similar to i.v. nicotine self‐administration described above, with the exception that there is no i.v. catheter surgery and lever presses led to the delivery of a 20‐mg food pellet (TestDiet, St. Lois, MO, USA) into a food tray installed in the self‐administration chamber. To prevent food satiation, we set the maximum number of food pellet deliveries to 30 for each 3‐hr session. Animals were tested with different doses of BCP (0, 10, 25, and 50 mg·kg−1) once stable food self‐administration was achieved. After each test, animals continued with daily food self‐administration sessions. The order of drug testing was random with tests occurring to 3–5 days apart. The effects of BCP on food self‐administration were assessed in WT and CB2‐KO mice.
2.5.6. The effects of BCP on open‐field locomotion and rotarod performance
To determine whether any reduction in nicotine or food self‐administration after BCP administration was due to locomotor impairment, we examined the effects of BCP on open‐field locomotion and rotarod locomotor performance in rats and mice.
In the first assay, rats (n = 9) or mice (n = 8) were placed in open‐field locomotor chambers (Accuscan, Columbus, OH, USA) and habituated for 1 hr. After 2–3 days of habituation (3 hr·day−1), mice were randomly treated with one of the BCP doses (0, 25, or 50 mg·kg−1, i.p.) and rats with 0, 3, 10, 25, 50, or 100 mg·kg−1 with 1–3 days of intervals between tests. Following each injection, locomotor activity was recorded for an additional 2 hr in 10‐min bins, and the travelled distance was used to evaluate the effects of BCP on locomotor activity.
Given that the baseline level of open‐field locomotion before BCP injection was low, we then used rotarod test to further evaluate the effects of BCP on fast‐running rotarod locomotor performance. In this assay, WT mice (n = 5) were trained on a rotarod device. Mobility was measured by the latency (s) to fall from the rotarod rotating at increasing speed from 4 to 20 rpm over 5 min. After stable baselines were established (defined as less than 20% variability in latency to fall across at least three consecutive sessions), mice were treated with BCP (50 mg·kg−1, i.p.) or vehicle 30 min prior to rotarod testing. Data were collected at 30, 60, 120, and 180 min following BCP administration. The latency to fall off the rotarod was recorded.
2.5.7. The effects of BCP on optical ICSS in DAT‐cre mice
Lastly, to determine whether BCP alters nicotine or BSR through a dopamine‐dependent mechanism, we observed the effects of BCP on BSR maintained by optical stimulation of VTA DA neurons in DAT‐cre mice. The procedures for optical intracranial self‐stimulation (oICSS) were the same as reported previously (Han et al., 2017). DAT‐cre mice (n = 6), with ChR2 expression targeting the VTA dopaminergic cells, were connected to a 473‐nm wavelength laser (OEM Laser Systems, UT) by two sheathed optic fibres (200‐μm core diameter, Precision Fiber Products, Chula Vista, CA, USA) and FC/FC fibre rotary joint (Doric Lenses Inc., Quebec, Canada). The total output of the laser was adjusted to ∼10‐mW transmittance into the brain. During each 1‐hr session, each press on the active lever activated the light above the lever and delivered a 1‐s pulse train of blue light (473 nm, 10 mW, 5‐ms pulse duration, and 50 Hz) depolarizing VTA dopaminergic cells. Pressing the inactive lever yielded no stimulation. Once animals' responding stabilized (<20% variability in responding for at least three consecutive sessions), a multiple stimulation frequency schedule was introduced. Every 10 min, stimulation frequency was decreased from 100 to 50, 25, 10, 5, and finally to 1 Hz, and lever presses at each frequency were counted. The testing phase began once stable oICSS responding was achieved with <20% variation across three consecutive sessions. Mice received a s.c. injection of BCP (0, 50, or 100 mg·kg−1) 30 min prior to the test session and allowed to lever press for oICSS. After each test, mice received additional oICSS sessions until a new baseline was established and later were re‐tested with a different dose of BCP. After completion of the above behavioural experiment, immunohistochemistry assay was used to verify AAV‐ChR2‐EGFP expression in VTA dopaminergic neurons in DAT‐cre mice using the methods we reported previously (Han et al., 2017) and followed the BJP Guidelines (Alexander et al., 2018).
2.6. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Animal group sizes were chosen on the basis of extensive previous experience with the animal models used. No data points were excluded from the analysis in any experiment. Where variation in group size occurred, this was due to animals being dropped from the experiment due to obstruction or clogging of i.v. catheters.
To validate the use of parametric statistics, we ensured that (a) the residuals were normally distributed (Shapiro Wilk Test for normality; P values >.05) and (b) variances of the differences across all groups were equal (Levene's test for homogeneity for between‐subject ANOVA, P values >.05 and Mauchly test for sphericity for repeated measures/mixed design ANOVA; P values >.05). The Greenhouse Geisser correction was applied to the degrees of freedom, when necessary. The group size (n > 5) is the number of independent values (individual animals), and statistical analysis was done using these independent values. All data were expressed as means ± SEM and were analysed using one or two‐way ANOVA, as appropriate. Significant main effects and interactions were followed by post hoc Student–Newman–Keuls tests for multiple group comparisons. Statistical analyses were performed using https://www.ebay.com/itm/303395151379 (Systat Software, Inc., San Jose, CA, USA) with a threshold for statistical significance of P < .05.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Cidlowski et al., 2019; Alexander, Fabbro et al., 2019).
3. RESULTS
3.1. BCP reduces nicotine self‐administration in rats
As shown in Figure 1a, systemic administration of BCP produced a dose‐dependent reduction in nicotine self‐administration under an FR1 schedule of reinforcement. Figure 1b shows the time course of nicotine self‐administration within a daily 3‐hr sessions, illustrating that BCP significantly inhibited nicotine self‐administration throughout the session. Figure 1c shows that BCP, over a wider dose range, lowered nicotine self‐administration under an FR5 schedule of reinforcement. Post hoc tests for multiple group comparisons revealed significant reductions in nicotine self‐administration after 10, 25, and 50 mg·kg−1 BCP administration as compared to the vehicle group.
Figure 1.
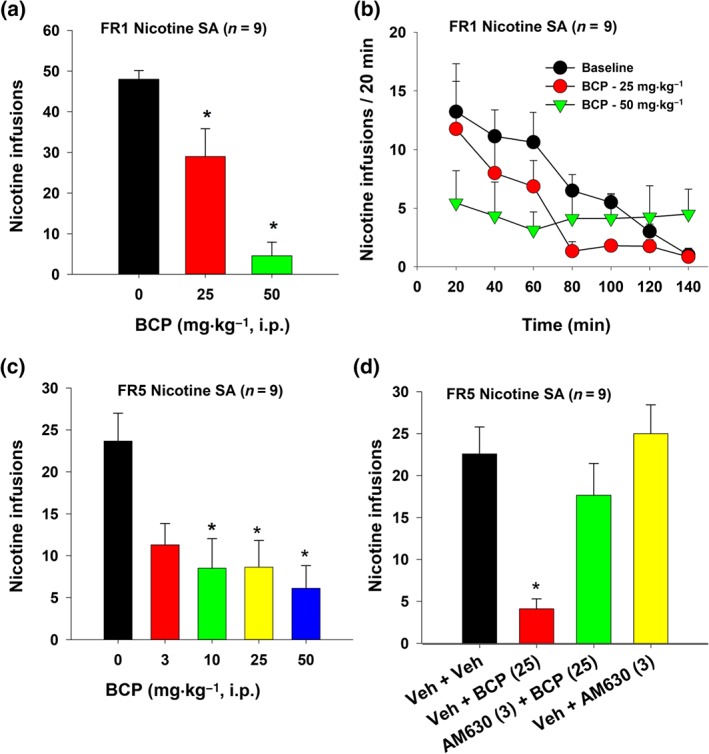
The effects of BCP on nicotine self‐administration in rats. (a) The effects of BCP on mean (±SEM) number of nicotine infusions under an FR1 schedule of reinforcement (n = 9). (b) Time courses of FR1 nicotine self‐administration within a daily 3‐hr session in the presence of vehicle or BCP pretreatment. (c) The effects of BCP on mean (±SEM) numbers of nicotine infusions under FR5 schedule of reinforcement (n = 9). (d) The effects of BCP and/or AM630 on nicotine self‐administration under FR5 schedule of reinforcement (n = 9), illustrating that pretreatment with AM630 blocked BCP‐induced reduction in nicotine self‐administration. AM630 alone had no effect on nicotine self‐administration. *P < .05, significantly different from the vehicle control group; one‐way ANOVA followed by post hoc Student–Newman–Keuls tests for multiple group comparisons
In vitro cell‐line receptor binding assays suggest that BCP is a selective CB2 receptor agonist (Gertsch et al., 2008). To determine whether the observed reduction in nicotine self‐administration in rats was mediated by stimulation of CB2 receptors in vivo, we evaluated the effects of AM630, a selective CB2 receptor antagonist, on BCP's action on nicotine self‐administration. Figure 1d shows that pretreatment with AM630 (3 mg·kg−1, i.p.) blocked the attenuating effects of BCP (25 mg·kg−1) on nicotine self‐administration, implying that the effects of BCP were mediated by stimulation of CB2 receptors. AM630 alone had no effect on nicotine self‐administration (Figure 1d).
3.2. CB2 receptor and non‐CB2 receptor mechanisms underlying BCP action in mice
To further explore the above findings, we evaluated the effects of BCP on nicotine self‐administration in WT and CB2‐KO mice. There are two important findings in this experiment. First, vehicle‐treated CB2‐KO mice showed significantly lower (~50%) nicotine self‐administration than their WT littermates (Figure 2a,b), a finding that is consistent with a previous report (Ignatowska‐Jankowska et al., 2013). Second, both CB2 and non‐CB2 receptor mechanisms underlie BCP‐induced reduction in nicotine self‐administration. In WT mice, treatment with BCP (25 or 50 mg·kg−1) significantly reduced nicotine intake (Figure 2a, P < .05) and responding on the active lever (Figure 2b) but had no effect on inactive lever responding (Figure 2c). In CB2‐KO mice, only a high dose of BCP (50 mg·kg−1 dose) caused significant reductions in nicotine intake (Figure 2a). This was accompanied by a significant reduction in responding on the active lever (Figure 2b) and no effect on the inactive lever responding (Figure 2c). Figure 2d shows representative nicotine self‐administration records in a WT mouse and a CB2‐KO mouse, illustrating that both mouse genotypes displayed evenly distributed self‐administration rates during a 3‐hr session with lower numbers of infusions in CB2‐KO mice than in their WT littermates and that BCP (25 mg·kg−1) inhibited nicotine self‐administration only in WT mice. These findings suggest that the receptor mechanisms through which BCP inhibits nicotine self‐administration depend on a BCP dose. The effects produced by low doses of BCP (25 mg·kg−1 or lower) are mediated mainly by stimulation of CB2 receptors, while the effects produced by high doses (50 mg·kg−1 or above) of BCP may be mediated by both CB2 and non‐CB2 receptor mechanisms.
Figure 2.
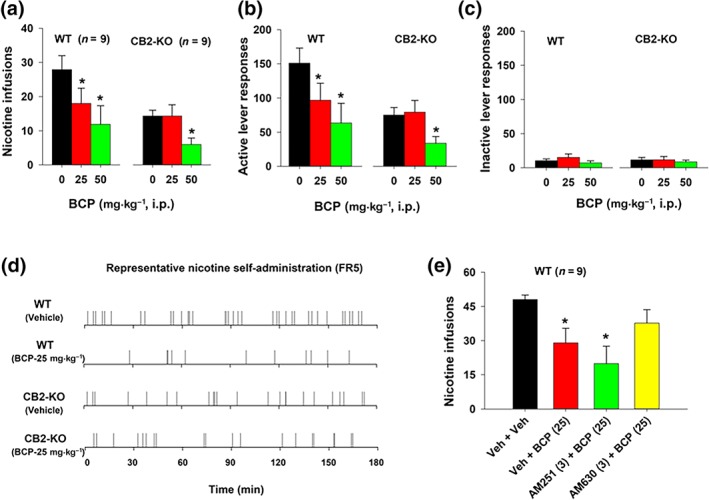
The effects of BCP on nicotine self‐administration in WT (n = 9) and CB2‐KO (n = 9) mice under FR5 schedule of reinforcement. (a–c) Mean (±SEM) numbers of nicotine infusions (a), active (b), and inactive (c) lever responding during a 3‐hr session, illustrating that CB2‐KO mice self‐administered less nicotine than WT littermates, and BCP inhibited nicotine self‐administration in WT and CB2‐KO mice. (d) Representative event records from a WT mouse and a CB2‐KO mouse, illustrating the patterns of nicotine self‐administration and the effects of BCP on nicotine self‐administration in WT and CB2‐KO mice. (e) The effects of BCP, AM251, and AM630 on nicotine self‐administration, illustrating that pretreatment with AM630, but not AM251, blocked BCP‐induced reduction in nicotine self‐administration. *P < .05, significantly different from the vehicle control group; one‐way ANOVA followed by post hoc Student–Newman–Keuls tests for multiple group comparisons
We then used pharmacological approaches to further explore the above findings. Figure 2e shows that pretreatment with AM251 (a selective CB1 receptor antagonist) failed to alter, while AM630 (a selective CB2 receptor antagonist) blocked the attenuating effects of BCP on nicotine self‐administration produced in WT mice, suggesting that the effects produced by a low dose of BCP are mediated by stimulation of CB2 receptors.
3.3. BCP inhibits nicotine seeking during extinction in mice
We also observed the effects of BCP on nicotine seeking in WT mice during extinction (voluntary abstinence from previous nicotine self‐administration). Unexpectedly, mice did not show extinction of responding, which typically occurs within 2 weeks of extinction training in rats (Wang et al., 2015). In contrast, mice displayed persistently high levels of active and inactive lever responding during the 4‐week extinction training. This observation is in line with previous reports that mice with a history of cocaine self‐administration show the persistence of responding during the extinction training (Thomsen & Caine, 2011), suggesting either a lack of extinction of lever pressing in the absence of a drug reinforcer, or conditioned reinforcing effects of the stimulus lights and tones previously associated with a drug in mice. Figure 3a shows the time course of extinction responding at both active and inactive levers. Systemic administration of BCP (25 and 50 mg·kg−1) significantly decreased active lever responding in a dose‐dependent manner (Figure 3). Separate one‐way ANOVAs with repeated measures for BCP dose revealed a significant BCP treatment effect on active lever responses (Figure 3b), but not on inactive lever responses (Figure 3c). Post hoc tests for multiple group comparisons revealed a significant reduction in active lever responding after 25 or 50 mg·kg−1 of BCP, compared with the vehicle control group.
3.4. BCP reduces food self‐administration
To determine whether BCP is similarly effective in attenuating non‐drug reinforcement, we evaluated the effects of BCP on food self‐administration. Figure 4a shows that BCP, only at high doses (50 mg·kg−1), caused significant reductions in food self‐administration in both WT and CB2‐KO mice, suggesting non‐CB2 receptor mechanisms.
Figure 4.
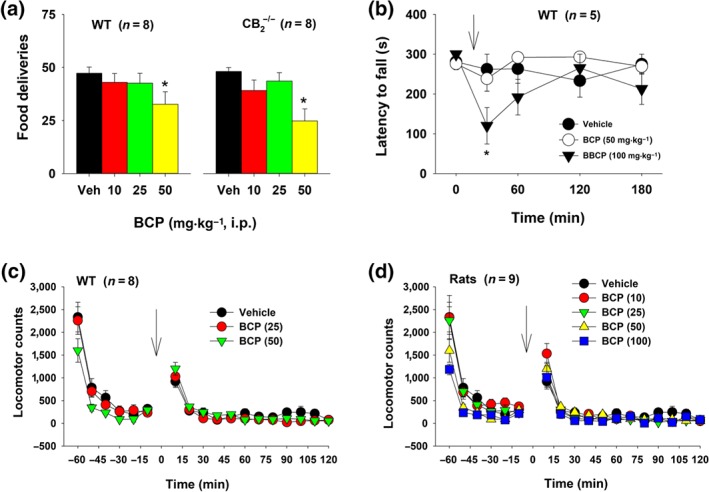
The effect of BCP treatment on food self‐administration and locomotion. (a) Mean (±SEM) self‐administration of food pellets in WT (n = 8) and CB2‐KO (n = 8) mice. At the highest dose, BCP inhibited food self‐administration in both genotypes of mice. (b) Mean (±SEM) latency to fall from the rotarod in WT mice (n = 5), illustrating that BCP, at the highest dose (100 mg·kg−1), produced a transient reduction in rotarod locomotor performance. (c) Mean (±SEM) locomotor counts during the open‐field test in mice. (d) Mean (±SEM) locomotor counts during the open‐field locomotor test in rats (n = 9), illustrating that BCP had no significant effect on basal locomotor activity. *P < .05, significantly different from the vehicle control group; one‐way ANOVA (a) and two‐way ANOVA for repeated measures over time (b), followed by post hoc Student–Newman–Keuls tests for multiple group comparisons. Arrows indicate drug injections
3.5. The effects of BCP on locomotor behaviour
To determine whether the observed reductions in nicotine or food self‐administration were due to sedation or locomotor impairment, we measured the effects of BCP on rotarod locomotor performance and open‐field locomotor activity. Figure 4b shows that BCP produced a transient (~30 min) dose‐dependent reduction in rotarod performance, as assessed by a reduction in the latencies to fall. A two‐way ANOVA with repeated measures for time and BCP dose factors did not reveal a main effect of the BCP treatment or Treatment × Time interaction, although it did reveal a significant main effect of time.
Further, systemic administration of a wide dose range (10–100 mg·kg−1, i.p.) of BCP failed to alter open‐field locomotor activity in WT mice (Figure 4c) or rats (Figure 4d). Separate two‐way ANOVAs with BCP dose and time as repeated‐measures factors revealed that WT mice treated with various doses of BCP showed similar activity levels during habituation (from −60 to −10 min) and after the BCP treatment. Likewise, rats assigned to different BCP dose groups showed similar locomotor activity prior to and after BCP administration. These findings suggest that the BCP treatment does not produce sedation or alter motor coordination.
3.6. BCP reduces brain‐stimulation reward (BSR) and nicotine‐enhanced BSR
We then evaluated the effects of BCP on electrical BSR and nicotine‐enhanced BSR in rats. Figure 5a shows the experimental method with the location of a stimulation electrode in the medial forebrain bundle at the anterior–posterior level of the hypothalamus. Figure 5b shows that systemic administration of nicotine (0.25 mg·kg−1, s.c.) shifted the stimulation frequency response curve leftward and decreased the BSR stimulation threshold (θ 0) and M 50, while BCP produced an opposite effect—a rightward shift of the frequency–response curve and an increase in θ 0 and M 50 values. Figure 5c–e shows that BCP pretreatment dose‐dependently decreased nicotine‐enhanced BSR as assessed by a reduction in the enhanced θ 0 value (Figure 5c) and M 50 (Figure 5d) but had no effect on Y max (Figure 5e). Figure 5f–h shows that BCP, at high doses (50 and 100 mg·kg−1), dose‐dependently attenuated electrical BSR as assessed by increased θ 0 value (Figure 4f) or M 50 frequency (Figure 4g). A reduction in Y max was also observed (Figure 5h). Student–Newman–Keuls tests revealed that the 50 and 100 mg·kg−1 dose groups were significantly different from the vehicle‐treated group.
Figure 5.
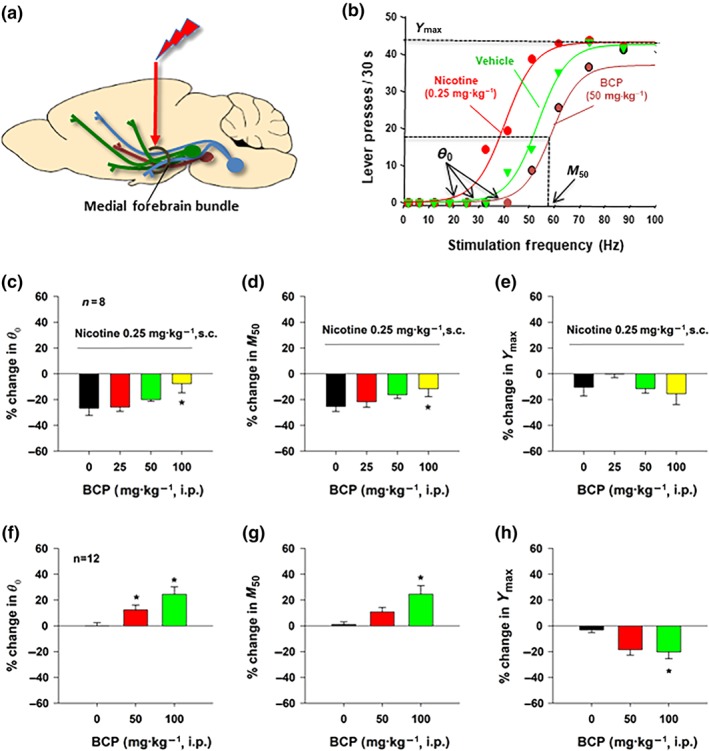
The effects of nicotine and BCP on electrical brain‐stimulation reward (BSR) in rats (n = 12). (a) A diagram illustrates the location of a stimulation electrode in the medial forebrain bundle at the anterior–posterior level of the lateral hypothalamus. (b) Representative stimulation–response curves, indicating that nicotine shifted the stimulation–response curve to the left and decreased the BSR stimulation threshold (θ 0) and M 50, while BCP shifted the stimulation–response curve to the right and increased the stimulation threshold (θ 0) and M 50. (c–e) The effects of nicotine and BCP on the mean (±SEM) values of BSR stimulation threshold (θ 0) (c) and M 50 (d). BCP, at a high dose, attenuated nicotine‐enhanced θ 0 (c) and M 50 (d) values. Co‐administration of nicotine and BCP failed to alter Y max (e). (f–h) BCP alone, at 50–100 mg·kg−1, dose‐dependently attenuated BSR, as assessed by increased θ 0 (f) and M50 (g). BCP, at high dose (100 mg·kg−1), also significantly decreased Y max (h). *P < .05, significantly different from the vehicle control group; one‐way ANOVA followed by post hoc Student–Newman–Keuls tests for multiple group comparisons
3.7. BCP reduces the rewarding effects of stimulating dopaminergic neurons
Lastly, we examined whether a dopamine‐dependent mechanism underlies the capacities of BCP to attenuate nicotine and BSR. Figure 6a shows the experimental methods, illustrating that AAV‐ChR2 was microinjected into the VTA (unilaterally) followed by the surgical implantation of an optrode (fibre) into the midbrain (1 mm above the VTA) in DAT‐cre mice. Figure 6b shows fluorescent ChR2 expression in VTA dopaminergic neurons. Figure 6c depicts representative active lever responding maintained by different frequencies (from 100 to 1 Hz) of laser stimulation. Systemic administration of BCP (50 and 100 mg·kg−1) dose‐dependently shifted the frequency‐rate response curve downward (Figure 5d). Post hoc tests for multiple group comparisons revealed that BCP caused significant dose‐dependent reductions in active lever pressing for stimulation at 10, 25, 50, and 100 Hz.
Figure 6.
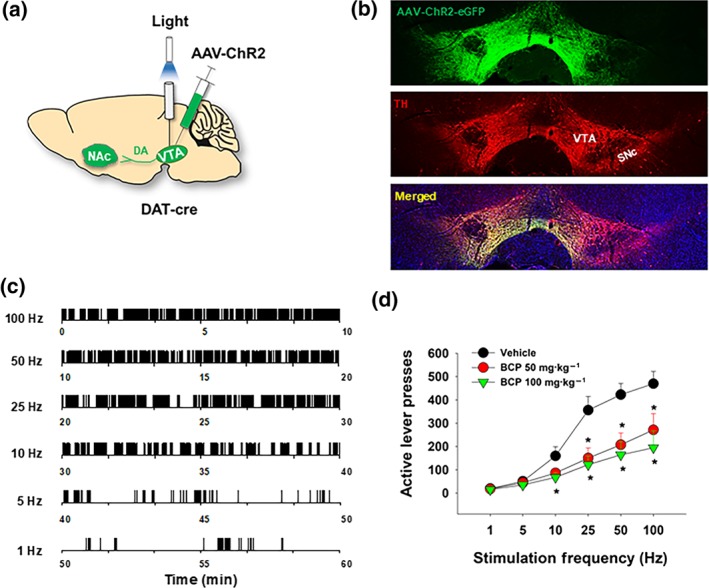
The effects of BCP on brain‐stimulation reward maintained by optical stimulation of VTA dopaminergic neurons in mice. (a) A diagram showing the experimental methods for optogenetic intracranial self‐stimulation (oICSS). AAV‐ChR2‐eGFP virus was microinjected into the VTA of DAT‐cre mice, and then a fibre optrode was implanted into the VTA to optically excite VTA dopaminergic neurons contingently upon lever response. (b) Representative images of TH‐immunostaining (red) and fluorescent ChR2‐EGFP expression (green) in the VTA, illustrating ChR2‐EGFP expression in VTA dopaminergic neurons. (c) Representative oICSS records in a single session from a single mouse under descending stimulation frequency conditions (10 min per frequency), indicating that photoactivation of VTA dopaminergic neurons induced robust oICSS behaviour (lever presses) in a stimulation frequency‐dependent manner. (d) Systemic administration of BCP dose‐dependently shifted the stimulation–response curve downward, indicating a reduction in brain‐stimulation reward. *P < .05, significantly different from the vehicle control group; two‐way ANOVAfor repeated measures over stimulation frequency, followed by post hoc Student–Newman–Keuls tests for multiple group comparisons
4. DISCUSSION
The major findings of the present study include the following: (a) BCP significantly and dose‐dependently inhibited nicotine self‐administration in rats and mice as well as nicotine seeking during extinction in mice; (b) pharmacological blockade (by AM630) or genetic deletion of CB2 receptors (in CB2‐KO mice) blocked BCP (25 mg·kg−1)‐induced reduction in nicotine self‐administration, suggesting CB2 receptor‐mediated effects. However, at a high dose (50 mg·kg−1), BCP inhibited nicotine or food self‐administration in both WT and CB2‐KO mice, suggesting the involvement of non‐CB2 receptor mechanisms at higher doses; (c) BCP dose‐dependently reduced the rewarding effects of electrical BSR and nicotine‐enhanced BSR. A dopamine‐dependent mechanism appears to be involved in this action since BCP dose‐dependently attenuated the rewarding effects of brain‐stimulation caused by optogenetic stimulation of VTA dopaminergic neurons in DAT‐cre mice. Together, these findings suggest that BCP, a dietary terpene, has promise as a novel pharmacotherapy for cigarette smoking cessation and should be further explored for such possible therapeutic utility—especially as it is already a FDA‐approved compound.
4.1. BCP inhibits nicotine reward and nicotine seeking
The most important finding in this study is that systemic administration of BCP attenuated the rewarding effects of nicotine as assessed by nicotine self‐administration and BSR experiments in both rats and mice. In addition, BCP was effective in attenuating cue‐induced nicotine seeking during extinction in mice and also effective in reducing food self‐administration at high doses. The reduction in nicotine‐ or food‐taking behaviour is unlikely to be due to sedation or motor impairment as BCP, at a wide dose range, failed to alter open‐field locomotion. Although BCP, at a very high dose (100 mg·kg−1), produced a transient reduction (<30 min) in rotarod coordination performance, we do not believe that this contributed to the observed reduction in nicotine or food self‐administration as BCP was given 30 min prior to the self‐administration sessions. In addition, slight increases in responding on the inactive lever during the extinction test argues against BCP‐induced sedative effects and suggest that the animals under treatment with BCP were actively engaged in looking for alternative means to obtain the drug. These findings support the therapeutic utility of BCP for nicotine use disorder and overeating or obesity. Given that nicotine or cocaine self‐administration mice displayed a lack of extinction of lever pressing in the absence of a reinforcer as shown in the present study and others (Thomsen & Caine, 2011), more studies are required to determine whether BCP is also effective in reducing drug‐, cue‐, or stress‐induced reinstatement of extinguished nicotine seeking in rats. This work needs to be done in alcohol‐preferring rats (P‐rats) as this strain of rats shows more robust reinstatement of responding than Long‐Evans rats (Wang et al., 2015).
During the past decade, BCP has gained scientific attention after it was identified as a selective CB2 receptor agonist (Gertsch et al., 2008). Since then, BCP has been shown to have significant anti‐inflammatory, anti‐carcinogenic, antioxidative, anti‐depressive, anxiolytic, neuroprotective, and analgesic effects (Corey et al., 1964; Guo, Mou, Huang, Xiong, & Li, 2014; Katsuyama et al., 2013; Klauke et al., 2014). In addition, BCP decreases alcohol consumption and alcohol‐induced CPP in rodents (Al Mansouri et al., 2014). In a clinical study, nicotine smokers inhaling vapour from an essential oil of black pepper (Piper nigrum), which contains high concentrations of BCP, reported reduced nicotine cravings (Rose & Behm, 1994). Although this effect was attributed to irritation of the bronchial tree and no mechanisms of action were proposed, it is possible that the effects were pharmacologically mediated by BCP action. Given that BCP shows good oral bioavailability (Varga et al., 2018), favourable pharmacokinetics (Liu et al., 2013), and low toxicity at doses up to 100 mg·kg−1 for 14 days in mice or 700 mg·kg−1 for 90 days in rats (Oliveira et al., 2018; Schmitt, Levy, & Carroll, 2016), these findings support the potential utility of BCP for nicotine use disorder. Notably, BCP is devoid of the psychotomimetic effects associated with CB1 receptor activation (Galaj & Xi, 2019). Further, unlike CB1 receptor antagonists, BCP has low propensity to induce nausea and anxiogenesis (Schmitt et al., 2016). These unique pharmacological profiles make BCP a desirable therapeutic candidate for the treatment of substance use disorder.
4.2. CB2 receptor involvement in nicotine reward
The second important finding in this study is the involvement of CB2 receptors in nicotine self‐administration in mice. An exciting body of literature provides compelling evidence that the eCB system interacts with brain reward circuits and is implicated in drug reward and addiction (Jordan & Xi, 2019; Manzanares et al., 2018). As CB1 receptors are highly expressed in the brain, early studies focused on the involvement of these receptors in drug reward, including nicotine reward and relapse. For example, stimulation of CB1 receptors increases nicotine self‐administration and precipitates relapse in rats (Gamaleddin, Zvonok, Makriyannis, Goldberg, & Le Foll, 2012), while blockade of CB1 receptors by rimonabant reduces nicotine self‐administration and reinstatement of nicotine seeking (Cohen, Perrault, Griebel, & Soubrié, 2005; Diergaarde, de Vries, Raasø, Schoffelmeer, & De Vries, 2008). Furthermore, CB1 receptor antagonists block nicotine‐induced CPP (Forget, Hamon, & Thiébot, 2005; Hashemizadeh, Sardari, & Rezayof, 2014; Le Foll & Goldberg, 2004), disrupt the reconsolidation of nicotine memory (Fang et al., 2011), and ameliorate nicotine withdrawal symptoms (Merritt et al., 2008), suggesting an important role for CB1 receptors in a number of nicotine‐related behaviours. Paradoxically, mice lacking CB1 receptors acquire nicotine self‐administration (Cossu et al., 2001) but fail to develop nicotine CPP (Castañé et al., 2002). The mechanisms underlying these conflicting findings are unknown.
Recent studies indicate that, in addition to CB1 receptors, the CB2 receptors are also involved in drug reward, including nicotine reward and dependence (Jordan & Xi, 2019; Manzanares et al., 2018). In the present study, we found that CB2‐KO mice displayed significantly lower nicotine self‐administration than their WT littermates (Figure 2). This is consistent with previous reports that constitutive CB2‐KO mice or mice with conditional CB2‐KO in dopaminergic neurons failed to show nicotine‐induced CPP (Canseco‐Alba et al., 2018; Ignatowska‐Jankowska et al., 2013), self‐administered less nicotine (Ignatowska‐Jankowska et al., 2013), and showed no somatic signs of nicotine withdrawal as compared to WT mice (Navarrete et al., 2013, but see Ignatowska‐Jankowska et al., 2013), suggesting that the CB2 receptor is necessary for nicotine reward and possibly play a role in the emergence of somatic withdrawal symptoms. The present finding that mice lacking CB2 receptors self‐administered less nicotine provides additional evidence supporting the involvement of CB2 receptors in nicotine action.
In contrast to these findings, it was also reported that the CB2 receptor agonist (JWH133) or antagonist (AM630 and SR144528) blocked nicotine‐induced CPP (Canseco‐Alba et al., 2018; Ignatowska‐Jankowska et al., 2013; Navarrete et al., 2013) and reversed the anti‐depressive effects of nicotine in mice subjected to chronic mild stress (Pekala, Michalak, Kruk‐Slomka, Budzynska, & Biala, 2018). It was also reported that systemic administration of the CB2 receptor agonist (AM1241) or CB2 receptor antagonist (AM630) was ineffective at reducing nicotine self‐administration and reinstatement of nicotine seeking in rats (Gamaleddin et al., 2012). The reasons underlying such conflicting findings are unclear. It may be related to differences in drug doses, affinities of different ligands for CB2 receptors, their specificities for CB2 receptors, ligand pharmacological profiles in vivo versus in vitro, or species differences in CB2 receptor gene structures or in CB2 receptor responses to different ligands (Bingham et al., 2007; Zhang et al., 2015).
4.3. CB2 receptor mechanisms in BCP's action
A third important finding in this study is the involvement of CB2 receptors in BCP's action on nicotine reward. Pharmacological blockade or genetic deletion of CB2 receptors blocked BCP‐induced reduction in nicotine self‐administration produced by a low dose (25 mg·kg−1) of BCP. However, a high dose of BCP (50 mg·kg−1 or higher) also inhibited nicotine or food self‐administration in both WT and CB2‐KO mice, suggesting the involvement of non‐CB2 receptor mechanisms. These findings suggest that BCP's selectivity as a CB2 receptor agonist depends on the BCP dose and that both CB2 and non‐CB2 receptor mechanisms might be responsible for BCP's action in various behavioural assays. The finding that low doses of BCP inhibited nicotine self‐administration by stimulation of CB2 receptors is consistent with our previous reports that CB2 receptor stimulation by JWH133 or GW405833 inhibited cocaine self‐administration, cocaine‐induced hyperactivity, and cocaine‐enhanced nucleus accumbens DA release (Xi et al., 2011; Zhang et al., 2014). It is also congruent with recent reports that a CB2 receptor mechanism mediates the analgesic, anxiolytic and anti‐depressant, metabolic, neurobehavioural, and anti‐inflammatory effects of BCP (Bahi et al., 2014; Klauke et al., 2014; Youssef, El‐Fayoumi, & Mahmoud, 2019). The present finding that BCP inhibited both nicotine taking and seeking, is consistent with a recent report that BCP, administered at 50–100 mg·kg−1, s.c., for 7 days, inhibited electroshock‐ or kainic acid‐induced seizures in mice without affecting their rotarod performance (Tchekalarova et al., 2018).
4.4. Non‐CB2R mechanisms in BCP's action
The last significant finding in the present study is that additional non‐CB2 receptor mechanisms appear to be involved in BCP's action at high doses. This is based on the finding that BCP, at a high dose (50 mg·kg−1), significantly inhibited nicotine and food self‐administration in both WT and CB2‐KO mice. This finding is of particular significance given that high doses (50 mg·kg−1 or higher) of BCP are commonly used in other studies. Thus, it might be incorrect to attribute pharmacological effects produced by high doses of BCP exclusively to a CB2 receptor mechanism simply because BCP has a moderate CB2 receptor agonist profile (Gertsch et al., 2008).
To date, it is unknown which non‐CB2 receptor mechanisms are responsible for BCP‐induced reductions in nicotine or food self‐administration. Among potential non‐CB2 receptor targets are fatty acid amide hydrolase (FAAH; Chicca et al., 2014), the major eCB degrading enzyme (Chicca et al., 2014), https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319(Katsuyama et al., 2013; Paula‐Freire, Andersen, Gama, Molska, & Carlini, 2014), and peroxisome proliferator‐activated receptors ( https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=593&familyId=86&familyType=NHR; Justinova et al., 2015; Youssef et al., 2019), all of which have been implemented in nicotine‐related behaviours (Justinova et al., 2015; Krause et al., 2018; Merritt et al., 2008) and food consumption (Fu et al., 2003; King et al., 1979). Thus, our findings of attenuating effects of BCP on nicotine and food self‐administration in CB2‐KO mice might be partly explained by BCP‐induced inhibition of FAAH or stimulation of PPAR/μ opioid receptors. It is noteworthy that although AM251 did not reverse the action of BCP on nicotine self‐administration, we cannot completely exclude the possible involvement of CB1 receptors as CB1 receptor antagonists are known for their attenuating effects on nicotine self‐administration (Shoaib, 2008). Additional studies are clearly needed to investigate the potential involvement of these mechanisms in BCP's action.
In conclusion, in the present study, we provide convincing evidence from gene to behaviour indicating that BCP, a dietary cannabinoid or terpene, has potent pharmacological efficacy in reducing nicotine reward and nicotine seeking without producing significant sedative effects. Both CB2 and non‐CB2 receptor mechanisms may be involved in these effects. We note that only male rats and mice were used in this study. Thus, further studies should determine whether the present findings observed in males can be expanded to females. Given that BCP is an FDA‐approved food additive with excellent safety and pharmacokinetic profiles, BCP appears to have high translational potential as a repurposed pharmacotherapy for the treatment of substance use disorders, including cigarette smoking cessation and obesity.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
E.L.G. and Z.X.X. designed the experiments. Y.H., E.G., G.H.B., and X.F.W. conducted the experiments. Y.H., E.G., G.H.B., and Z.X.X. performed data analyses. E.G. and Z.X.X. wrote the manuscript. E.L.G. revised the manuscript. All authors have approved the final version of this article.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
ACKNOWLEDGEMENT
This research was supported by NIDA‐IRP (Z1A DA000620‐02). All rights are reserved by NIH.
He Y, Galaj E, Bi G‐H, Wang X‐F, Gardner E, Xi Z‐X. β‐Caryophyllene, a dietary terpenoid, inhibits nicotine taking and nicotine seeking in rodents. Br J Pharmacol. 2020;177:2058–2072. 10.1111/bph.14969
This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Present Address Yi He, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA, 15260.
REFERENCES
- Al Mansouri, S. , Ojha, S. , Al Maamari, E. , Al Ameri, M. , Nurulain, S. M. , & Bahi, A. (2014). The cannabinoid receptor 2 agonist, β‐caryophyllene, reduced voluntary alcohol intake and attenuated ethanol‐induced place preference and sensitivity in mice. Pharmacology, Biochemistry, and Behavior, 124, 260–268. 10.1016/j.pbb.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi, A. , Al Mansouri, S. , Al Memari, E. , Al Ameri, M. , Nurulain, S. M. , & Ojha, S. (2014). β‐Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiology & Behavior, 135, 119–124. 10.1016/j.physbeh.2014.06.003 [DOI] [PubMed] [Google Scholar]
- Bingham, B. , Jones, P. G. , Uveges, A. J. , Kotnis, S. , Lu, P. , Smith, V. A. , … Kennedy, J. D. (2007). Species‐specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. British Journal of Pharmacology, 151, 1061–1070. 10.1038/sj.bjp.0707303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, N. E. , McCoy, K. L. , Mezey, E. , Bonner, T. , Zimmer, A. , Felder, C. C. , … Zimmer, A. (2000). Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. European Journal of Pharmacology, 396, 141–149. 10.1016/s0014-2999(00)00211-9 [DOI] [PubMed] [Google Scholar]
- Canseco‐Alba, A. , Schanz, N. , Sanabria, B. , Zhao, J. , Lin, Z. , Liu, Q.‐R. , & Onaivi, E. S. (2018). Behavioral effects of psychostimulants in mutant mice with cell‐type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behavioural Brain Research, 360, 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañé, A. , Valjent, E. , Ledent, C. , Parmentier, M. , Maldonado, R. , & Valverde, O. (2002). Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology, 43, 857–867. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019). Fast Facts and Fact Sheets|Smoking & Tobacco Use|CDC.
- Chicca, A. , Caprioglio, D. , Minassi, A. , Petrucci, V. , Appendino, G. , Taglialatela‐Scafati, O. , & Gertsch, J. (2014). Functionalization of β‐caryophyllene generates novel polypharmacology in the endocannabinoid system. ACS Chemical Biology, 9, 1499–1507. 10.1021/cb500177c [DOI] [PubMed] [Google Scholar]
- Cho, J. Y. , Chang, H.‐J. , Lee, S.‐K. , Kim, H.‐J. , Hwang, J.‐K. , & Chun, H. S. (2007). Amelioration of dextran sulfate sodium‐induced colitis in mice by oral administration of β‐caryophyllene, a sesquiterpene. Life Sciences, 80, 932–939. 10.1016/j.lfs.2006.11.038 [DOI] [PubMed] [Google Scholar]
- Cohen, C. , Perrault, G. , Griebel, G. , & Soubrié, P. (2005). Nicotine‐associated cues maintain nicotine‐seeking behavior in rats several weeks after nicotine withdrawal: Reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology, 30, 145–155. [DOI] [PubMed] [Google Scholar]
- Corey, E. J. , Mitra, R. B. , & Uda, H. (1964). Total Synthesis of d,l‐Caryophyllene and d,l‐Isocaryophyllene. Journal of the American Chemical Society, 86, 485–492. [Google Scholar]
- Cossu, G. , Ledent, C. , Fattore, L. , Imperato, A. , Böhme, G. A. , Parmentier, M. , & Fratta, W. (2001). Cannabinoid CB1 receptor knockout mice fail to self‐administer morphine but not other drugs of abuse. Behavioural Brain Research, 118, 61–65. 10.1016/s0166-4328(00)00311-9 [DOI] [PubMed] [Google Scholar]
- Coulombe, D. , & Miliaressis, E. (1987). Fitting intracranial self‐stimulation data with growth models. Behavioral Neuroscience, 2, 209–214. [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br J Pharmacology, 175, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo, V. (2009). The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological Research, 60, 77–84. 10.1016/j.phrs.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Diergaarde, L. , de Vries, W. , Raasø, H. , Schoffelmeer, A. N. M. , & De Vries, T. J. (2008). Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid‐1 receptor antagonist Rimonabant (SR141716A). Neuropharmacology, 55, 712–716. 10.1016/j.neuropharm.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Fang, Q. , Li, F.‐Q. , Li, Y.‐Q. , Xue, Y.‐X. , He, Y.‐Y. , Liu, J.‐F. , … Wang, J. S. (2011). Cannabinoid CB1 receptor antagonist rimonabant disrupts nicotine reward‐associated memory in rats. Pharmacology, Biochemistry, and Behavior, 99, 738–742. [DOI] [PubMed] [Google Scholar]
- Forget, B. , Hamon, M. , & Thiébot, M.‐H. (2005). Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology, 181, 722–734. 10.1007/s00213-005-0015-6 [DOI] [PubMed] [Google Scholar]
- Fu, J. , Gaetani, S. , Oveisi, F. , Lo Verme, J. , Serrano, A. , Rodríguez De Fonseca, F. , … Piomelli, D. (2003). Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR‐alpha. Nature, 425, 90–93. 10.1038/nature01921 [DOI] [PubMed] [Google Scholar]
- Galaj, E. , Bi, G.‐H. , Yang, H.‐J. , & Xi, Z.‐X. (2019). Cannabidiol attenuates the rewarding effects of cocaine by CB2, 5‐HT1A and TRPV1 receptor mechanisms. Neuropharmacology, 107740 10.1016/j.neuropharm.2019.107740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj, E. , & Xi, Z. X. (2019). Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders. CNS Drugs, 33, 1001–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin, I. , Zvonok, A. , Makriyannis, A. , Goldberg, S. R. , & Le Foll, B. (2012). Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS ONE, 7, e29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch, J. , Leonti, M. , Raduner, S. , Racz, I. , Chen, J.‐Z. , Xie, X.‐Q. , … Zimmer, A. (2008). β‐caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences of the United States of America, 105, 9099–9104. 10.1073/pnas.0803601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, K. , Mou, X. , Huang, J. , Xiong, N. , & Li, H. (2014). Trans‐caryophyllene suppresses hypoxia‐induced neuroinflammatory responses by inhibiting NF‐κB activation in microglia. Journal of Molecular Neuroscience, 54, 41–48. 10.1007/s12031-014-0243-5 [DOI] [PubMed] [Google Scholar]
- Han, X. , He, Y. , Bi, G.‐H. , Zhang, H.‐Y. , Song, R. , Liu, Q.‐R. , … Xi, Z.‐X. (2017). CB1 receptor activation on VgluT2‐expressing glutamatergic neurons underlies Δ9‐tetrahydrocannabinol (Δ9‐THC)‐induced aversive effects in mice. Scientific Reports, 7, 12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemizadeh, S. , Sardari, M. , & Rezayof, A. (2014). Basolateral amygdala CB1 cannabinoid receptors mediate nicotine‐induced place preference. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 51, 65–71. 10.1016/j.pnpbp.2014.01.010 [DOI] [PubMed] [Google Scholar]
- Ignatowska‐Jankowska, B. M. , Muldoon, P. P. , Lichtman, A. H. , & Damaj, M. I. (2013). The cannabinoid CB2 receptor is necessary for nicotine‐conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology, 229, 591–601. 10.1007/s00213-013-3117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, C. J. , Humburg, B. , Rice, M. , Bi, G.‐H. , You, Z.‐B. , Shaik, A. B. , … Xi, Z. X. (2019). The highly selective dopamine D3R antagonist, R‐VK4‐40 attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology, 107597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, C. J. , & Xi, Z.‐X. (2018). Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov, 13, 671–683. 10.1080/17460441.2018.1458090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, C. J. , & Xi, Z.‐X. (2019). Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neuroscience and Biobehavioral Reviews, 98, 208–220. 10.1016/j.neubiorev.2018.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova, Z. , Panlilio, L. V. , Moreno‐Sanz, G. , Redhi, G. H. , Auber, A. , Secci, M. E. , … Goldberg, S. R. (2015). Effects of fatty acid amide hydrolase (FAAH) inhibitors in non‐human primate models of nicotine reward and relapse. Neuropsychopharmacology, 40, 2185–2197. 10.1038/npp.2015.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama, S. , Mizoguchi, H. , Kuwahata, H. , Komatsu, T. , Nagaoka, K. , Nakamura, H. , … Sakurada, S. (2013). Involvement of peripheral cannabinoid and opioid receptors in β‐caryophyllene‐induced antinociception. European Journal of Pain, 17, 664–675. 10.1002/j.1532-2149.2012.00242.x [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, B. M. , Castellanos, F. X. , Kastin, A. J. , Berzas, M. C. , Mauk, M. D. , Olson, G. A. , & Olson, R. D. (1979). Naloxone‐induced suppression of food intake in normal and hypothalamic obese rats. Pharmacology, Biochemistry, and Behavior, 11, 729–732. [DOI] [PubMed] [Google Scholar]
- Klauke, A.‐L. , Racz, I. , Pradier, B. , Markert, A. , Zimmer, A. M. , Gertsch, J. , & Zimmer, A. (2014). The cannabinoid CB₂ receptor‐selective phytocannabinoid β‐caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. European Neuropsychopharmacology, 24, 608–620. 10.1016/j.euroneuro.2013.10.008 [DOI] [PubMed] [Google Scholar]
- Krause, D. , Warnecke, M. , Schuetz, C. G. , Soyka, M. , Manz, K. M. , Proebstl, L. , … Koller, G. (2018). The impact of the opioid antagonist naloxone on experimentally induced craving in nicotine‐dependent individuals. European Addiction Research, 24, 255–265. [DOI] [PubMed] [Google Scholar]
- Le Foll, B. , & Goldberg, S. R. (2004). Rimonabant, a CB1 antagonist, blocks nicotine‐conditioned place preferences. Neuroreport, 15, 2139–2143. 10.1097/00001756-200409150-00028 [DOI] [PubMed] [Google Scholar]
- Le Foll, B. , Gorelick, D. A. , & Goldberg, S. R. (2009). The future of endocannabinoid‐oriented clinical research after CB1 antagonists. Psychopharmacology, 205, 171–174. 10.1007/s00213-009-1506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Yang, G. , Tang, Y. , Cao, D. , Qi, T. , Qi, Y. , & Fan, G. (2013). Physicochemical characterization and pharmacokinetics evaluation of β‐caryophyllene/β‐cyclodextrin inclusion complex. International Journal of Pharmaceutics, 450, 304–310. 10.1016/j.ijpharm.2013.04.013 [DOI] [PubMed] [Google Scholar]
- Liu, Q.‐R. , Canseco‐Alba, A. , Zhang, H.‐Y. , Tagliaferro, P. , Chung, M. , Dennis, E. , … Onaivi, E. S. (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Scientific Reports, 7, 17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares, J. , Cabañero, D. , Puente, N. , García‐Gutiérrez, M. S. , Grandes, P. , & Maldonado, R. (2018). Role of the endocannabinoid system in drug addiction. Biochemical Pharmacology, 157, 108–121. 10.1016/j.bcp.2018.09.013 [DOI] [PubMed] [Google Scholar]
- Mediavilla, V. , & Steinemann, S. (1997). Essential oil of Cannabis sativa L. strains. J Intl Hemp Assoc., 82–84. [Google Scholar]
- Merritt, L. L. , Martin, B. R. , Walters, C. , Lichtman, A. H. , & Damaj, M. I. (2008). The endogenous cannabinoid system modulates nicotine reward and dependence. The Journal of Pharmacology and Experimental Therapeutics, 326, 483–492. 10.1124/jpet.108.138321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete, F. , Rodríguez‐Arias, M. , Martín‐García, E. , Navarro, D. , García‐Gutiérrez, M. S. , Aguilar, M. A. , … Manzanares, J. (2013). Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology, 38, 2515–2524. 10.1038/npp.2013.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A. H. , Cao, J. , Keighron, J. D. , Jordan, C. J. , Bi, G.‐H. , Liang, Y. , … Xi, Z. X. (2019). Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology, 44, 1435–1444. 10.1038/s41386-019-0366-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, G. L. D. S. , Machado, K. C. , Machado, K. C. , da Silva, A. P. D. S. C. L. , Feitosa, C. M. , & de Castro Almeida, F. R. (2018). Non‐clinical toxicity of β‐caryophyllene, a dietary cannabinoid: Absence of adverse effects in female Swiss mice. Regulatory Toxicology and Pharmacology, 92, 338–346. 10.1016/j.yrtph.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Parsons, L. H. , & Hurd, Y. L. (2015). Endocannabinoid signalling in reward and addiction. Nature Reviews. Neuroscience, 16, 579–594. 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula‐Freire, L. I. G. , Andersen, M. L. , Gama, V. S. , Molska, G. R. , & Carlini, E. L. A. (2014). The oral administration of trans‐caryophyllene attenuates acute and chronic pain in mice. Phytomedicine, 21, 356–362. [DOI] [PubMed] [Google Scholar]
- Pekala, K. , Michalak, A. , Kruk‐Slomka, M. , Budzynska, B. , & Biala, G. (2018). Impacts of cannabinoid receptor ligands on nicotine‐ and chronic mild stress‐induced cognitive and depression‐like effects in mice. Behavioural Brain Research, 347, 167–174. 10.1016/j.bbr.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Rose, J. E. , & Behm, F. M. (1994). Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug and Alcohol Dependence, 34, 225–229. 10.1016/0376-8716(94)90160-0 [DOI] [PubMed] [Google Scholar]
- Schmitt, D. , Levy, R. , & Carroll, B. (2016). Toxicological evaluation of β‐caryophyllene oil: Subchronic toxicity in rats. International Journal of Toxicology, 35, 558–567. [DOI] [PubMed] [Google Scholar]
- Sharma, C. , Al Kaabi, J. M. , Nurulain, S. M. , Goyal, S. N. , Kamal, M. A. , & Ojha, S. (2016). Polypharmacological properties and therapeutic potential of β‐caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Current Pharmaceutical Design, 22, 3237–3264. [DOI] [PubMed] [Google Scholar]
- Shoaib, M. (2008). The cannabinoid antagonist AM251 attenuates nicotine self‐administration and nicotine‐seeking behavior in rats. Neuropharmacology, 54, 438–444. 10.1016/j.neuropharm.2007.10.011 [DOI] [PubMed] [Google Scholar]
- Spiller, K. J. , Bi, G.‐H. , He, Y. , Galaj, E. , Gardner, E. L. , & Xi, Z.‐X. (2019). Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. British Journal of Pharmacology, 176, 1268–1281. 10.1111/bph.14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchekalarova, J. , da Conceição Machado, K. , Gomes Júnior, A. L. , de Carvalho Melo Cavalcante, A. A. , Momchilova, A. , & Tzoneva, R. (2018). Pharmacological characterization of the cannabinoid receptor 2 agonist, β‐caryophyllene on seizure models in mice. Seizure, 57, 22–26. 10.1016/j.seizure.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Thomsen, M. , & Caine, S. B. (2011). False positive in the intravenous drug self‐administration test in C57BL/BJ mice. Behavioural Pharmacology, 22, 239–247. 10.1097/FBP.0b013e328345f8f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga, Z. V. , Matyas, C. , Erdelyi, K. , Cinar, R. , Nieri, D. , Chicca, A. , … Pacher, P. (2018). β‐Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. British Journal of Pharmacology, 175, 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.‐F. , Bi, G.‐H. , He, Y. , Yang, H.‐J. , Gao, J.‐T. , Okunola‐Bakare, O. M. , … Newman, A. H. (2015). R‐modafinil attenuates nicotine‐taking and nicotine‐seeking behavior in alcohol‐preferring rats. Neuropsychopharmacology, 40, 1762–1771. 10.1038/npp.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2019). Tobacco.
- Xi, Z.‐X. , Peng, X.‐Q. , Li, X. , Song, R. , Zhang, H.‐Y. , Liu, Q.‐R. , … Gardner, E. L. (2011). Brain cannabinoid CB₂ receptors modulate cocaine's actions in mice. Nature Neuroscience, 14, 1160–1166. 10.1038/nn.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, D. A. , El‐Fayoumi, H. M. , & Mahmoud, M. F. (2019). β‐caryophyllene alleviates diet‐induced neurobehavioral changes in rats: The role of CB2 and PPAR‐γ receptors. Biomedicine & Pharmacotherapy, 110, 145–154. 10.1016/j.biopha.2018.11.039 [DOI] [PubMed] [Google Scholar]
- Zhang, H.‐Y. , Bi, G.‐H. , Li, X. , Li, J. , Qu, H. , Zhang, S.‐J. , … Liu, Q. R. (2015). Species differences in cannabinoid receptor 2 and receptor responses to cocaine self‐administration in mice and rats. Neuropsychopharmacology, 40, 1037–1051. 10.1038/npp.2014.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.‐Y. , Gao, M. , Liu, Q.‐R. , Bi, G.‐H. , Li, X. , Yang, H.‐J. , … Xi, Z. X. (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine‐related behavior in mice. Proceedings of the National Academy of Sciences of the United States of America, 111, E5007–E5015. 10.1073/pnas.1413210111 [DOI] [PMC free article] [PubMed] [Google Scholar]


