Abstract
Background and Purpose
Age‐related macular degeneration (AMD) is a complex neurodegenerative disease treated by anti‐VEGF intravitreal injections. As inflammation is potentially involved in retinal degeneration, the pro‐inflammatory kallikrein–kinin system is a possible alternative pharmacological target. Here, we investigated the effects of anti‐VEGF and anti‐B1 receptor treatments on the inflammatory mechanisms in a rat model of choroidal neovascularization (CNV).
Experimental Approach
Immediately after laser‐induced CNV, Long–Evans rats were treated by eye‐drop application of a B1 receptor antagonist (R‐954) or by intravitreal injection of B1 receptor siRNA or anti‐VEGF antibodies. Effects of treatments on gene expression of inflammatory mediators, CNV lesion regression and integrity of the blood‐retinal barrier was measured 10 days later in the retina. B1 receptor and VEGF‐R2 cellular localization was assessed.
Key Results
The three treatments significantly inhibited the CNV‐induced retinal changes. Anti‐VEGF and R‐954 decreased CNV‐induced up‐regulation of B1 and B2 receptors, TNF‐α, and ICAM‐1. Anti‐VEGF additionally reversed up‐regulation of VEGF‐A, VEGF‐R2, HIF‐1α, CCL2 and VCAM‐1, whereas R‐954 inhibited gene expression of IL‐1β and COX‐2. Enhanced retinal vascular permeability was abolished by anti‐VEGF and reduced by R‐954 and B1 receptor siRNA treatments. Leukocyte adhesion was impaired by anti‐VEGF and B1 receptor inhibition. B1 receptors were found on astrocytes and endothelial cells.
Conclusion and Implications
B1 receptor and VEGF pathways were both involved in retinal inflammation and damage in laser‐induced CNV. The non‐invasive, self‐administration of B1 receptor antagonists on the surface of the cornea by eye drops might be an important asset for the treatment of AMD.
Abbreviations
- AMD
age‐related macular degeneration
- BK
bradykinin
- CNV
choroidal neovascularization
- FITC
fluorescein isothiocyanate
- GCL
ganglion cell layer
- GFAP
glial fibrillary acid protein
- HIF‐1α
hypoxia‐induced factor‐1α
- Iba1
ionized calcium binding adapter molecule‐1
- ICAM‐1
intercellular adhesion molecule‐1
- INL
inner nuclear layer
- IVT
intravitreal injection
- KKS
kallikrein–kinin system
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- PFA
paraformaldehyde
- PlGF
placental growth factor
- qRT‐PCR
quantitative real‐time PCR
- R‐954
AcOrn[Oic2,(αMe)Phe5,DβNal7,Ile8]desArg9‐BK
- RNV
retinal neovascularization
- RPE
retinal pigment epithelium
- STZ
streptozotocin
- VCAM‐1
vascular cell adhesion molecule‐1
What is already known
Intravitreal injection of anti‐VEGF antibodies is the common treatment of neovascular age‐related macular degeneration (AMD).
Choroidal neovascularization (CNV) is associated with chronic inflammation in the retina.
What this study adds
The kallikrein–kinin system, particularly the B1 receptors, contribute to the development of laser‐induced CNV.
B1 receptor blockade decreases CNV and retinal inflammatory responses, notably leukocyte adhesion and vascular hyperpermeability.
What is the clinical significance
B1 receptor antagonists and anti‐VEGF antibodies reduce retinal inflammation through distinct and complementary mechanisms.
Topical application of B1 receptor antagonists is a promising approach to treat neovascular AMD.
1. INTRODUCTION
Ocular pathologies involving angiogenesis are particularly devastating in terms of visual acuity. Among these, age‐related macular degeneration (AMD) is one of the leading causes of severe vision loss in the elderly of industrialized countries (Bashshur et al., 2006; Nguyen et al., 2006). The predominant pathological feature of the wet form of AMD is choroidal neovascularization (CNV), which corresponds to the formation of new branches from pre‐existing choroidal vessels that penetrate the Bruch's membrane and develop under the retinal pigment epithelium (RPE) and/or in the subretinal space (Lu & Adamis, 2006). This results in discrete loss of RPE areas and decreased retinal thickness in the RPE/Bruch's membrane complex (Rutar & Provis, 2016).
CNV is associated with chronic inflammation in the retina, which provokes further decrease in oxygenation and cell death. The inflammatory process includes the breakdown of the blood‐retinal barrier, leukocyte adhesion on the blood vessels wall, macrophage and microglia activation, and cytokines and chemokine production (Dorrell, Uusitalo‐Jarvinen, Aguilar, & Friedlander, 2007). These chemokines, by participating in the recruitment of macrophages, spread the degeneration to the retina (Rutar & Provis, 2016). The overexpression of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074‐α, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5085, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 along with increased density of macrophages has been shown in excised choroidal neovascular membranes of AMD patients, as well as in laser‐induced CNV (Grossniklaus et al., 2002; Sheridan et al., 2009; Xie et al., 2012; Zou, Xu, & Chiou, 2006). These molecules may promote angiogenesis (Lavalette et al., 2011; Yang, Wang, Qian, Zhang, & Huang, 2011) and vascular hyperpermeability (Clermont et al., 2016). Leukocyte adhesion and the breakdown of the blood‐retinal barrier involve adhesion molecules such as vascular cell adhesion molecule‐1 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6758), E‐selectin, and intercellular adhesion molecule‐1 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6757), which are overexpressed in the presence of VEGF in endothelial cells (Kim et al., 2001). The chemokine https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771 and ICAM‐1 are both heavily involved in the recruitment and extravasation of leukocytes and are induced in cultured vascular endothelial cells by VEGF via NF‐κB activation (Kim et al., 2001; Marumo, Schini‐Kerth, & Busse, 1999). Both VEGF and CCL2 are overexpressed in RPE‐choroid in laser‐induced CNV animal models (Xie et al., 2011) and neovascular AMD patients (Jonas, Tao, Neumaier, & Findeisen, 2010). Hence, several studies have shown the deleterious effect of leukocyte adhesion in neovascular AMD (Penfold, Provis, & Billson, 1987; Takeda et al., 2009) and VEGF‐induced ocular angiogenesis (Nakao et al., 2005). The presence of inflammatory cells and cytokines can thus be considered an integral part of an active inflammatory phase that keeps CNV active.
VEGF is considered the main mediator of choroidal (Lu & Adamis, 2006) and retinal neovascularization (Aiello et al., 1995; Campochiaro, 2013). The main isoform https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5085 can bind to either of its receptors, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1812 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1813&familyId=324&familyType=CATALYTICRECEPTOR. VEGF‐R1 is a regulator of monocyte and macrophage migration and a positive and negative regulator of VEGF‐R2 (Witmer, Vrensen, Van Noorden, & Schlingemann, 2003). However, most in vitro studies have shown a weak or undetectable response of VEGF‐R1 in angiogenesis of endothelial cells. VEGF activity on endothelial cells proliferation and migration is mediated by VEGF‐R2 (Witmer et al., 2003).
VEGF has become the target of choice for the treatment of pathological ocular angiogenesis using anti‐VEGF antibodies (Fontaine et al., 2011; Mitchell, 2011; Sarwar et al., 2016). The most common therapy approved by the U.S. Food and Drug Administration for the treatment of patients with wet AMD is https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6786, administered monthly by intravitreal injection (Hernandez et al., 2018). Aflibercept is a recombinant soluble VEGF receptor protein that shows a high affinity for all VEGF isoforms and for the placental growth factor (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5314), a VEGF‐R1 ligand. Several studies suggest that PlGF and VEGF can act synergistically to promote angiogenesis (Balser, Wolf, Herb, & Langmann, 2019; Cunningham et al., 2019). However, it is not clear if this synergy is clinically relevant. Indeed, targeting PlGF does not seem to confer superiority to aflibercept, relative to https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6779, a strictly anti‐VEGF agent (Cunningham et al., 2019).
Despite the efficacy of these molecules in the improvement of visual acuity, repeated intravitreal injections of anti‐VEGF may cause long‐term ocular complications given the important role of VEGF in maintaining vascular integrity and survival of neuronal cells (Fontaine et al., 2011; Park, Kim, & Park, 2014; Saint‐Geniez et al., 2008; Wilkinson‐Berka & Miller, 2008). VEGF appears to be neuroprotective, and a loss of this factor could result in retinal ganglion cell loss potentiated by decreased retinal blood flow (Le, 2017). The anti‐VEGF treatment can potentially increase the risk of endophthalmitis (Reibaldi et al., 2018) and development of geographical atrophy (Gemenetzi, Lotery, & Patel, 2017). Furthermore, the latter treatment is successful in only 40% of AMD patients and many patients suffering from neovascular AMD also develop resistance to anti‐VEGF therapy (Yang, Zhao, & Sun, 2016).
The https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2379&familyId=751&familyType=ENZYME–kinin system (KKS) is highly activated during inflammatory processes. Bradykinin (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=649), kallidin (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=650), and their C‐terminal metabolites (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=646 and Lys‐des‐Arg9‐BK) induce increased vascular permeability, vasodilation, and the expression of pro‐inflammatory cytokines. These effects are mediated by two GPCRs, the bradykinin https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=41, and the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=42. The B2 receptor is constitutive while the B1 receptor is virtually absent in healthy tissue, but it is induced by tissue damage and pro‐inflammatory cytokines via activation of NF‐κB (Couture, Blaes, & Girolami, 2014). The kinin receptors are expressed in the retina and ocular tissues of several species, including humans (Bhat, Pouliot, Couture, & Vaucher, 2014; Ma et al., 1996). A role for the KKS was previously described in diabetic retinopathy (Feener, 2010; Pouliot et al., 2012). For example, activation of B1 receptors enhances vascular permeability, leukocyte adhesion, and the expression of pro‐inflammatory molecules such as VEGF‐A, VEGF‐R2, ICAM‐1, VCAM‐1, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376, and IL‐1β in the diabetic retina (Hachana et al., 2018; Pouliot et al., 2012). In addition to its effects on inflammation, BK promotes angiogenesis by up‐regulating basic FGF (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4924) via B1 receptors and by stimulating VEGF formation via B2 receptors in a non‐ocular model of neovascularization (Colman, 2006). The B1 receptors promote reparative angiogenesis in murine models of occlusive vascular disease and activation of these receptors also induces the proliferation and survival of endothelial cells, while B1 receptor antagonists induce apoptosis (Emanueli et al., 2001; Emanueli et al., 2002; Hillmeister et al., 2011). Thus far, only three studies have explored the KKS in AMD aetiology (Fukuhara et al., 2013; Nagai et al., 2007; Nakamura et al., 2011). However, the specific role of kinin receptors and their interactions with VEGF in retinal neovascular AMD are still poorly understood.
The present study has examined the activation of kinin B1 receptors and VEGF in relation to retinal inflammation in a rat model of laser‐induced CNV. Molecular (qRT‐PCR) and cellular (immunohistochemistry) expressions of these two mediators were studied in the retina after CNV. The effects of anti‐VEGF therapy was compared with that of a selective B1 receptor antagonist (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=3906) or B1 receptor siRNA on the expression of inflammatory mediators, the size of CNV lesions, leukocyte adhesion, and vascular permeability. Our results indicate that B1 receptors exert substantial effects on CNV‐induced inflammation within the retina.
2. METHODS
2.1. Animal model and procedures
All experimental methods and animal care procedures were approved by the animal care committee of Université de Montréal (Protocols 15‐063, 16‐059, 17‐057, and 18‐061), in compliance with the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-visual-research/. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath & Lilley, 2015) and with the recommendations made by the British Journal of Pharmacology. Male Long–Evans rats (200–250 g, 6–8 weeks old, RRID:RGD_60991) were purchased from Charles River Laboratories (Charles River Laboratories, RRID:SCR_003792, St‐Constant, QC, Canada) and housed two per cage in a room under standard conditions of temperature (23°C) and lighting (12‐hr light/dark cycle), with food and water provided ad libitum. Rats were subjected to a CNV in the left eye while the right eye (without CNV) was used as the control. Every day, the rats were visually inspected for any redness, porphyrin secretion, or corneal opacity. The Long–Evans rats were randomly divided in groups of equal size (n = 6) for each pharmacological treatment, except for immunostaining (n = 4 per group). The experimenter was blinded to assess immunohistological parameters (B1 receptors, glial, endothelial, microglia, and VEGF‐R2 distribution) and to measure retinal vascular permeability, leukocyte recruitment, expression of inflammatory mediators, and estimation of CNV lesions.
2.2. Laser‐induced CNV in rats
Rats were anaesthetized with an intramuscular injection of a mixture of ketamine (80 mg·kg−1) and xylazine (20 mg·kg−1). The pupils were dilated with tropicamide 1%, and the cornea was kept moisturized with an ointment. Five regions of 100 μm were targeted using an argon laser, with 0.1‐s duration and energy of 110 mW (Coherent Novus 2000; Carl Zeiss Meditec, Oberkochen, Germany). The CNV was successful if a typical bubble at the choroid/RPE interface was seen, corresponding to the breakdown of Bruch's membrane. The development of laser‐induced CNV is manifested by an early phase occurring during the first week and mature membranes develop between 10 and 14 days post‐CNV in most studies (Edelman & Castro, 2000; Pennesi, Neuringer, & Courtney, 2012; Tobe et al., 1998). Based on these studies, the experimental protocols were designed to be carried out at 10 days post‐CNV.
2.3. Pharmacological treatments
Immediately after laser‐induced CNV, rats received intravitreal (IVT) injection while they were still under anaesthesia with an intramuscular injection of a mixture of ketamine (80 mg·kg−1) and xylazine (20 mg·kg−1). A dose of 125‐μg polyclonal goat IgG anti‐VEGF (AF564, R&D systems, Oakville, Canada) was injected as a single IVT injection (5 μl) to neutralize vitreal and retinal VEGF. The same dosage of a normal goat IgG (AB‐108C, R&D systems) was injected as control. Induction and up‐regulation of B1 receptors was blocked with 10 nmol of an IVT injection of B1 receptor siRNA (Ambion Invitrogen Life Technologies, Canada; Hachana et al., 2018). The same dosage of scrambled siRNA (Invitrogen Life Technologies)—same nucleotides as siRNA but in a random sequence—was injected IVT as control. This treatment with IVT B1 receptor siRNA (10 nmol) resulted in the absence of B1 receptor protein, by immunohistochemistry, in the streptozotocin (STZ)‐diabetic rat retina and confirmed the specificity of the polyclonal rabbit antiserum for rat B1 receptors (Hachana et al., 2018). The latter study also showed the efficacy of the B1 receptor siRNA in reducing the mRNA levels of kinin receptors (B1 receptors and B1 receptors), VEGF‐A, and VEGFR‐2 in the retinas of STZ‐diabetic rats by quantitative RT‐PCR.
Pharmacological blockade of the B1 receptors was achieved with R‐954, a highly selective and metabolically stable peptide antagonist obtained from Dr. Fernand Gobeil Jr (Pharmacology, Université de Sherbrooke, Canada; Gobeil, Sirois, & Regoli, 2014). It was topically administered (≈100 μg/10 μl−1) twice a day to the surface of the eye in non‐anaesthetized rats for 10 days, starting immediately after the CNV. Using radiolabelled [3H]‐R‐954, R‐954 was found to diffuse to the retina within 1 hr and to remain in the tissue for up to 12 hr after its administration to the ocular surface (Hachana et al., 2018). As R‐954 is not metabolized in rat tissue homogenates at room temperature (Gobeil et al., 2014), only its intact form can be found in retina. Eye drop application of sterile saline solution (vehicle) served as control. At the end of each experiment, animals were killed with an overdose of sodium pentobarbital.
2.4. Observation of laser‐induced CNV with Heidelberg retinal angiograph
Rats were anaesthetized with an intramuscular injection of ketamine (80 mg·kg−1) and xylazine (10 mg·kg−1). Fundus fluorescein angiography was performed using a commercial camera and imaging system (KOWA GENESIS‐D system; KOWA Company, Tokyo, Japan) at 10 days after laser photocoagulation. The photographs were captured with a 20‐D lens in contact with the fundus camera lens, after an i.p. injection of 0.1 ml of 1% fluorescein sodium (Akorn, Decatur, IL). CNV was monitored in untreated and anti‐VEGF‐treated rat eyes with a laser ophthalmoscope (Heidelberg, Dossenheim, Germany).
2.5. Measurement of retinal inflammatory mediators by quantitative RT‐PCR
After anaesthesia with sodium pentobarbital (60 mg·kg−1, i.p.), enucleated eyes were bisected equatorially, and after removal of the cornea and peeling the sclera and choroid under the dissecting microscope, the entire retina was carefully removed. Dissected retina including the RPE was visually inspected to exclude cross‐contamination with the vitreous and choroid and was immediately submerged in RNAlater stabilization reagent (QIAGEN, Valencia, CA, USA). SYBR green‐based real‐time quantitative PCR using the Mx3000p device for signal detection was performed as described previously (Hachana et al., 2018; Pouliot et al., 2012). Duplicates were used to ensure the reliability of single values. The primer pairs designed by Vector NTI software (Vector NTI, RRID:SCR_014265) are shown in Table 1. PCR conditions were as follows: 95°C for 15 min, followed by 46 cycles at 94°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The cycle threshold value represents the cycle number at which a fluorescent signal rises statistically above background. For standardization and quantification, rat 18S was amplified simultaneously. The relative quantification of gene expression was analysed by the 2−ΔΔCt method and normalized with respect to the control values (fold = 1).
Table 1.
List of primers used in this study
| Sequence | Position | GGenBanq | ||
|---|---|---|---|---|
| B1 receptor | Forward | 5′GCAGCGCTTAACCATAGCGGAAAT3′ | 367–390 | NM_030851 |
| Reverse | 5′CCAGTTGAAACGGTTCCCGATGTT3′ | 454–431 | ||
| B2 receptor | Forward | 5′AGGTGCTGAGGAACAACGAGATGA3′ | 882–905 | NM_173100 |
| Reverse | 5′TCCAGGAAGGTGCTGATCTGGAAA3′ | 990–967 | ||
| VEGF‐A | Forward | 5′TCACCAAAGCCAGCACATAGGAGA3′ | 1219–1242 | BC168708 |
| Reverse | 5′TTACACGTCTGCGGATCTTGGACA3′ | 1371–1348 | ||
| VEGF‐R2 | Forward | 5′AGTGGCTAAGGGCATGGAGTTCTT3′ | 3269–3292 | U93306 |
| Reverse | 5′GGGCCAAGCCAAAGTCACAGATTT3′ | 3387–3364 | ||
| HIF‐1α | Forward | 5′TAGACTTGGAAATGCTGGCTCCCT3′ | 1693–1716 | NM_024359 |
| Reverse | 5′TGGCAGTGACAGTGATGGTAGGTT3′ | 1863–1840 | ||
| IL‐8 | Forward | 5′GAAGATAGATTGCACCGA3′ | 351‐368 | NM_030845 |
| Reverse | 5′CATAGCCTCTCACACATTTC3′ | 715‐696 | ||
| IL‐1β | Forward | 5′TGTCACTCATTGTGGCTGTGGAGA3′ | 247–270 | NM_031512 |
| Reverse | 5′TGGGAACATCACACACTAGCAGGT3′ | 411‐388 | ||
| TNF‐α | Forward | 5′ACGGAAAGCATGATCCGAGATGTG3′ | 151–174 | NM_012675 |
| Reverse | 5′TTG GGA ACT TCT CCT CCT TGT TGG3′ | 340–317 | ||
| COX‐2 | Forward | 5′GCATTCTTTGCCCAGCACTTCACT3′ | 677–700 | U03389 |
| Reverse | 5′TTTAAGTCCACTCCATGGCCCAGT3′ | 744–751 | ||
| ICAM‐1 | Forward | 5′TGCAGGTGAACTGCTCTTCCTCTT3′ | 161–184 | NM_012967 |
| Reverse | 5′AGCTTCCAGTTGTGTCCACTCGAT3′ | 263–240 | ||
| CCL2 | Forward | 5′ATGCAGGTCTCTGTCACG3′ | 76‐93 | NM_031530 |
| Reverse | 5′CTAGTTCTCTGTCATACT3′ | 522‐505 | ||
| VCAM‐1 | Forward | 5′GAACCCAAACAAAGGCAGAG3′ | 1540–1559 | NM_012889 |
| Reverse | 5′GAAAACCATCACTTGAGCAGG3′ | 1675‐1655 | ||
| 18S | Forward | 5′TCAACTTTCGATGGTAGTCGCCGT3′ | 363–385 | X01117 |
| Reverse | 5′TCCTTGGATGTGGTAGCCGTTTCT3′ | 470–447 |
2.6. Measurement of retinal adherent leukocytes and vascular permeability
Retinal adherent leukocytes were determined in sodium pentobarbital anaesthetized rats (60 mg·kg−1, i.p.), as described in previous studies (Hachana et al., 2018; Pouliot et al., 2012). Briefly, rats were perfused using a 16G cannula inserted into the left heart ventricle, with phosphate buffer saline (PBS; 35 ml·min−1) to eliminate non‐adherent leukocytes. FITC‐conjugated concanavalin A (FITC‐ConA 20 μg·ml−1 in PBS, 5 mg·kg−1; Vector Laboratories, Burlingame, CA, USA) was used to label the adherent leukocytes and vascular endothelium. Subsequently, rats were perfused with 4% paraformaldehyde (PFA) followed by 1% albumin in PBS (35 ml·min−1). Then, FITC‐Concanavalin A‐stained leukocytes were counted in each flat‐mounted retina with a fluorescence microscope at 40× and 60× magnification (Leica microsystems, Concord, ON, Canada). The total number of leukocytes in each retina was determined.
Retinal vascular permeability was assessed using the Evans blue dye extravasation technique (Hachana et al., 2018; Pouliot et al., 2012). Rats were anaesthetized with sodium pentobarbital (60 mg·kg−1, i.p.). Evans blue dye (45 mg·ml−1 in saline; Sigma‐Aldrich, Oakville, ON, Canada) was injected using a catheter (Micro‐Renathane, I.D. 0.0400, O.D. 0.0200, Braintree Scientific, Braintree, MA, USA) into the right femoral vein. The dye was allowed to circulate for 1 hr 30 min, and then 25 ml of saline was infused through the left ventricle. After enucleation, retinas were collected, weighed, and incubated in 1‐ml formamide for 18 hr at 70–75°C. The fluorescence of extracted Evans blue was measured with a spectrofluorometer (Spex 1681, Horiba JobinYvon Inc., Edison, NJ, USA; 620 nm for excitation and 680 nm for emission).
2.7. Evaluation of the retinal vascular leakage to assess neovascular area
Rats were anaesthetized with an overdose of ketamine (100 mg·kg−1) and xylazine (40 mg·kg−1, i.m.). A solution of FITC‐dextran (2 × 106 Da; Sigma‐Aldrich Canada) was prepared at a concentration of 10 mg·ml−1 in PBS. After an injection of 0.5 ml of FITC‐dextran solution into the left heart ventricle, the eyes were removed and fixed for 1 hr in PFA (4%). The retina was then dissected out and visually inspected to exclude cross‐contamination with the vitreous, limbus, and choroid and then flat‐mounted on a gelatinized microscope slide with cover slip. Neovascularization lesions were visualized using a fluorescence microscope (Leica microsystems Co., Concord, ON, Canada). A digital camera was used to take micrographs (40×) for each of the five laser burn sites seen on the retina. Blinded data analysis for retinal neovascular area was outlined in red and performed using Java‐based image processing software (ImageJ; National Institutes of Health, Bethesda, USA).
2.8. Immunofluorescence staining
The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). Rats were perfused with PFA 2%, and the eyes were embedded in paraffin and cut into 5‐μm‐thick sections as described previously (Hachana et al., 2018; Pouliot et al., 2012). The sections were pretreated with citrate buffer solution (10‐mM citric acid, 0.05% Tween 20, pH 6.0) at 80°C for 20 min to break down the protein cross‐links, unmasking the antigens and epitopes. Sections from all groups were incubated with either the selective rabbit polyclonal antiserum anti‐B1 receptor 1:500 (Hachana et al., 2018), the mouse monoclonal anti‐endothelial cells antibody RECA‐1 (ab 9774, 1:500, Abcam, USA, RRID:AB_296613), the mouse monoclonal anti‐glial fibrillary acid protein GFAP antibody (ABIN284434, 1:500, Millipore Sigma, USA, RRID:AB_10800083), the mouse polyclonal anti‐ionized calcium binding adapter molecule‐1 antibody Iba‐1 (NCNP24; 1:500, Wako, USA, RRID:AB_2811160), or the chicken monoclonal anti‐VEGF‐R2 antibody (GW21181, 1:250, Sigma‐Aldrich, USA, RRID:AB_741295). The slides were incubated with Alexa Fluor 488 donkey anti‐rabbit IgG (A21206, Invitrogen) to visualize B1 receptors, with Alexa Fluor 555 donkey anti‐mouse IgG (A31570, Invitrogen) to visualize endothelial and glial cells and microglia or with Alexa Fluor 647 goat anti‐chicken IgY (ab150175, Abcam) to visualize VEGF‐R2. Slides were then washed and mounted using ProLong® Gold Antifade Reagent (Invitrogen) or Fluoroshield with DAPI (Sigma). Images were obtained with a confocal microscope Zeiss‐LSM800 equipped with an argon laser.
The total number of ameboid and ramified‐shaped microglial cells marked with Iba1 was counted within a circular region of 200‐μm diameter adjacent to the CNV in retinal sections (Luckoff, Scholz, Sennlaub, Xu, & Langmann, 2017). Cellular morphology was analysed using a grid system to determine the number of grid cross per cell (Chen et al., 2012).
2.9. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Data were expressed as mean ± SEM. Statistical analysis was undertaken only for studies where each group size was at least n = 5. The declared group size is the number of independent values (n = 6 rats) on which statistical analysis was done. The group size selection for each protocol was determined based on our previous study (Hachana et al., 2018) and the power analysis . For immunohistochemistry, no statistical analysis was performed as n = 4 rats (16 retinal sections per rat). However, quantitative values were presented to show the trend of changes as exploratory (Figure 4). Multiple comparisons between groups were performed using the non‐parametric ANOVA Kruskal–Wallis test and post hoc Dunn's test for quantification of leukostasis and area of CNV lesion. One‐way ANOVA followed by the Bonferroni test was used for vascular permeability and mRNA levels. For parametric variables, post hoc test was conducted only if F in ANOVA achieved P < .05, and there was no significant variance in homogeneity. No outliers were excluded in data analysis and presentation. Statistical analysis was performed using Prism™ version 5.0 (GraphPad Prism, RRID:SCR_002798, GraphPad Software Inc., La Jolla, CA, USA). For determining whether groups differ, the level of probability (P) was set at P < .05 to constitute the threshold for statistical significance.
Figure 4.
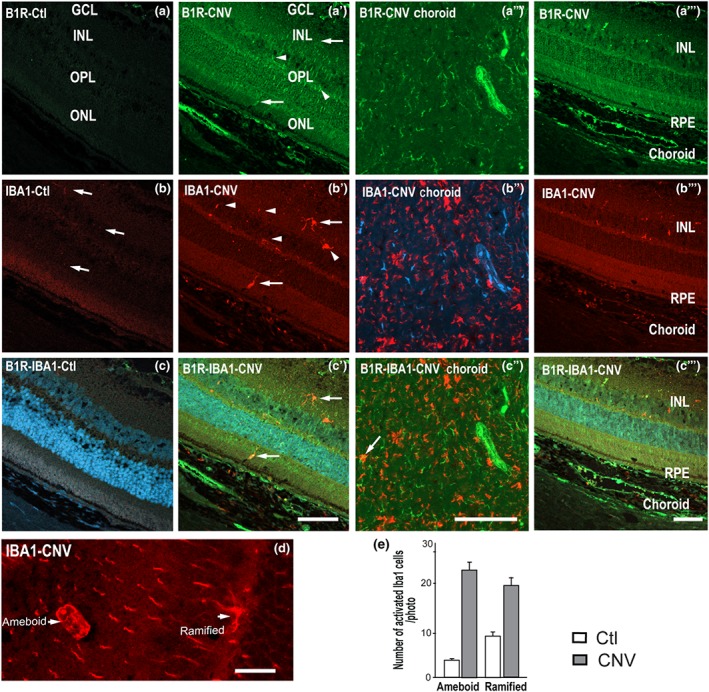
Micrographs of cellular distribution of B1 receptors on microglia. Representative micrographs of immunolabeling for B1 receptors (green) and Iba‐1 (red) in control (Ctl) and CNV retinas. When appropriate, sections are counterstained for DAPI (blue) which labels cell nuclei. Iba‐1(+) cells were sparsely distributed in the control inner retina (panel b, arrows) but more intensively in CNV inner retina (panels b′, b‴, arrows) RPE and choroid (panels b″, b‴). No visible co‐localization was seen in most sections for B1 receptors and Iba‐1 in control (panel c). Note that B1 receptors partly co‐localized (yellow, arrows) with Iba‐1 in inner retina, subretinal space, RPE, and of CNV (panels c′, c″, c‴). CNV rat retina shows a network of ramified and ameboid Iba‐1 microglia in the ONL (panel d). Counting of ramified and ameboid‐shaped microglia in the ONL, subretinal space, and RPE (panel e). Data are from four rats. Scale bar: 75 μm (panels a–c‴) and 150 μm (panel d). B1R, B1 receptors
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org (Guide to Pharmacology, RRID:SCR_013077), the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY IUPHAR/BPS (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019a, 2019b).
3. RESULTS
3.1. Effect of anti‐VEGF on the expression of retinal inflammatory mediators, retinal vascular permeability, and retinal leukocyte adhesion
To assess the inflammatory component of laser‐induced CNV, we first measured mRNA expression of selected mediators related to the kinin and VEGF systems (B1 receptors, B2 receptors, VEGF‐A, VEGF‐R2, and HIF‐1α), the inflammation pathway (IL‐8, IL‐1β, COX‐2, CCL2, and TNF‐α), and adhesion molecules (ICAM‐1 and VCAM‐1; Figure 1a–l). All these inflammatory mediators (B1 and B2 receptors, VEGF‐A, VEGF‐R2, HIF‐1α, IL‐8, IL‐1β, TNF‐α, COX‐2, ICAM‐1, CCL2 and VCAM‐1) were significantly increased in the CNV retina (fourfold to 10‐fold) compared to the control retina. The up‐regulation of kinin receptors (B1 and B2 receptors) in the CNV retina was significantly prevented by IVT anti‐VEGF therapy (Figure 1a,b). Similarly, the CNV‐induced overexpression of VEGF‐A and VEGF‐R2 was blocked by the anti‐VEGF therapy (Figure 1c,d). The up‐regulation of the hypoxia‐induced factor HIF‐1α involved in the expression of VEGF in CNV was also blocked by the anti‐VEGF therapy (Figure 1e). The pro‐inflammatory cytokine TNF‐α was down‐regulated by the anti‐VEGF therapy (Figure 1h), which had, however, no effects on expression of IL‐8, IL‐1β, and COX‐2 (Figure 1f,g,i). The up‐regulation of leukocyte recruitment markers ICAM‐1, CCL2 and VCAM‐1 was blocked by anti‐VEGF (Figure 1j–l).
Figure 1.
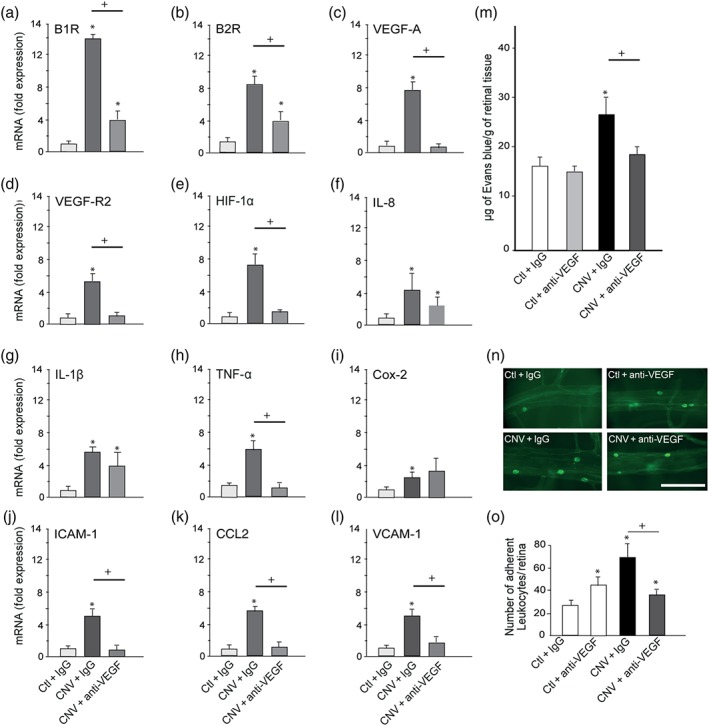
Effect of anti‐VEGF on the expression of retinal inflammatory mediators, retinal vascular permeability, and retinal leukocyte adhesion. In the control and CNV retina treated with IgG or anti‐VEGF are shown (a–l) mRNA levels of B1 receptors, B2 receptors, VEGF‐A, VEGF‐R2, HIF‐1α, IL‐8, IL‐1β, TNF‐α, COX‐2, ICAM‐1, CCL2 and VCAM‐1, (m) Evans blue dye extravasation, (n) representative images of adherent leukocytes in retinal vessels. Scale bar = 100 μm. (o) Number of adherent leukocytes. Data are means ± SEM of values obtained from six rats per group. Ctl, control without lesion; CNV, laser‐induced choroidal neovascularization; IgG, immunoglobulin; *P ≤ .05, significantly different from control + IgG. + P ≤ .05, significantly different from CNV + IgG. B1R, B2R; B1 receptors, B2 receptors
CNV significantly increased retinal extravasation of Evans blue dye compared to the control retina (CNV + IgG compared to Ctl + IgG; Figure 1m). These changes as well as effects of treatments were significant. IVT injection of anti‐VEGF in control eyes did not significantly change the baseline values of retinal vascular permeability. In contrast, IVT injection of anti‐VEGF in the CNV group restored vascular permeability to baseline values, compared to the CNV group receiving IgG IVT injection.
Leukocyte adhesion to the retinal vasculature among the four groups of animals was counted under fluorescence microscopy (Figure 1n,o). There was a significant difference between groups (Figure 1o). The total number of labelled adherent leukocytes was significantly increased in laser‐induced CNV compared to the control retina injected with IgG. One single IVT injection of anti‐VEGF concomitant to the CNV induction significantly reduced the number of retinal adherent leukocytes compared to the CNV + IgG group and to the control retina. The residual retinal adherent leukocytes after anti‐VEGF in CNV can be related to the direct stimulatory effect of anti‐VEGF as measured in control retina (Figure 1o).
3.2. Effect of R‐954 or B1 receptor siRNA on the expression of retinal inflammatory mediators, retinal vascular permeability, and retinal leukocyte adhesion
Likewise, laser‐induced CNV enhanced mRNA levels of the inflammatory mediators (B1 and B2 receptors, VEGF‐A, VEGF‐R2, HIF‐1α, IL‐8, IL‐1β, TNF‐α, COX‐2, ICAM‐1, CCL2 and VCAM‐1) in the retina (fourfold to 10‐fold) compared to the control retina in this set of experiments (Figure 2a–l). The up‐regulation of kinin receptors (B1 and B2 receptors) in the CNV retina was significantly prevented by topical administration of the B1 receptor antagonist R‐954 (Figure 2a,b). In contrast, the CNV‐induced overexpression of VEGF‐A and VEGF‐R2 and HIF‐1α remained unaffected by the B1 receptor antagonist (Figure 2c–e). Pro‐inflammatory cytokine markers (IL‐1β and TNF‐α) were significantly reduced by the R‐954 antagonist, but not IL‐8 (Figure 2f–h). Also, COX‐2 mRNA levels were blocked by R‐954 (Figure 2i). The leukocyte recruitment markers CCL2 and VCAM‐1 were not affected by R‐954 (Figure 2k,l), whereas ICAM‐1 was down‐regulated by R‐954 (Figure 2j).
Figure 2.
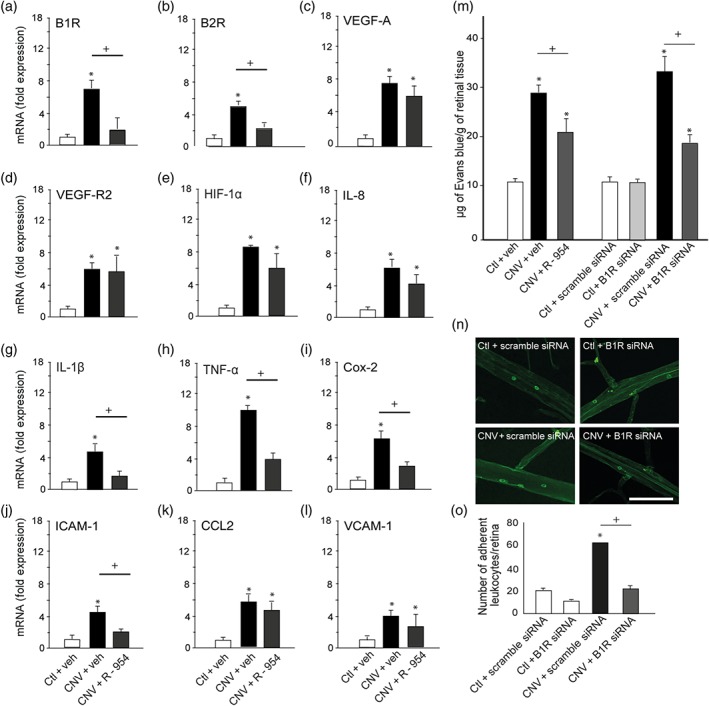
Effect of R‐954 or B1 receptor siRNA on the expression of retinal inflammatory mediators, retinal vascular permeability, and retinal leukocyte adhesion. In the control and CNV retina treated with vehicle or R‐954, scrambled siRNA or B1 receptor siRNA are shown (a–l) mRNA levels of B1 receptors, B2 receptors, VEGF‐A, VEGF‐R2, HIF‐1α, IL‐8, IL‐1β, TNF‐α, COX‐2, ICAM‐1, CCL2, and VCAM‐1, (m) Evans blue dye extravasation, (n) representative images of adherent leukocytes in retinal vessels. Scale bar = 100 μm. (o) Number of adherent leukocytes. Data are means ± SEM of values obtained from six rats per group. Ctl, control without lesion; CNV, choroidal neovascularization; veh, vehicle; * P ≤ .05, significantly different from control + veh or control + scrambled siRNA. + P ≤ .05, significantly different from CNV + veh or CNV + scrambled siRNA. B1R, B2R; B1 receptors, B2 receptors
CNV significantly increased by twofold to threefold the extravasation of Evans blue dye compared to the control retina (Figure 2m). These changes as well as effects of treatments were significant. A 10‐day topical treatment with R‐954 significantly decreased vascular permeability in CNV retina compared to the CNV group treated with the vehicle. The permeability remained, however, higher than control retinal values. IVT injection of B1 receptor siRNA in control eyes did not significantly change the baseline values of retinal vascular permeability. However, IVT treatment with the B1 receptor siRNA significantly decreased, although not totally, vascular permeability in CNV retina, compared to that after treatment with scrambled B1 receptor siRNA.
Leukocyte adhesion to the retinal vasculature was counted in four groups of animals (Figure 2n,o). The total number of labelled adherent leukocytes was significantly increased in laser‐induced CNV compared to the control retina. One single IVT injection with B1 receptor siRNA significantly abolished leukocyte adherence in laser‐induced CNV, compared with that in CNV treated with scrambled siRNA.
3.3. Cellular distribution of B1 receptors on glial and vascular endothelial cells in the retina
In contrast to the weak immunostaining for B1 receptors in control retina, (Figure 3a), there was a heavy staining for B1 receptors in the neovascularization area, observed 10 days after laser‐induced CNV, particularly in the ganglion cell layer (Figure 3a′, white arrows). The GFAP labelling in the CNV retina was highly ramified, and hypertrophic glial extensions were seen (Figure 3b′), which was not the case in the control retina (Figure 3b). In CNV and control retinas, B1 receptors were co‐localized with astrocytes labelled with the GFAP antibody (Figure 3c,c′).
Figure 3.
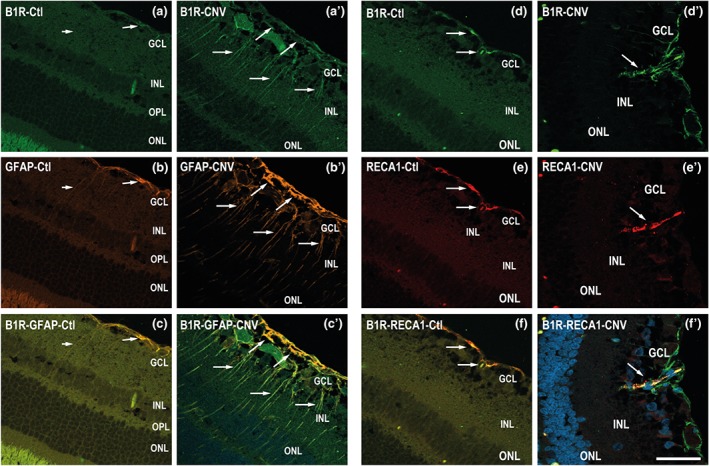
Micrographs of cellular distribution of B1 receptors on glial and endothelial cells. Representative micrographs of immunolabeling for B1 receptors (green), GFAP (orange), and RECA1 (red) in control (Ctl) and CNV retinas. Sections are counterstained for DAPI (blue) which labels cell nuclei. Note that B1 receptors co‐localized (yellow, arrows) with GFAP (panel c′) and RECA‐1 (panel f′) in CNV retina and to some extent in control retina (c, f). Data are from four rats. Scale bar: 75 μm. B1R, B1 receptors
Confocal microscopy revealed dense retinal vessels in CNV retina (Figure 3e′ indicated by an arrow). B1 receptor immunostaining was markedly increased in retinal vessels (Figure 3d′, arrows) and was co‐localized with the endothelial marker (RECA‐1) in control and CNV retinas (Figure 3f,f′).
3.4. Cellular distribution of B1 receptors on microglia in the retina
B1 receptor labelling after CNV was more intense in GCL, INL retinal layers, and RPE (Figure 4a′,a″) in comparison to control retina (Figure 4a). In order to investigate the effects of activated microglia in retina, we used the specific Iba‐1 marker. Activated microglia, characterised by a hypertrophic cell body and fewer processes, were more abundant in CNV retina (Figure 4b′,b‴) compared to control retina (Figure 4b). After CNV, ramified and ameboid microglia accumulated in the subretinal space, in all the retinal layers (Figure 4b′,b‴) and strongly in the choroid (Figure 4b″). Counting of ramified and ameboid‐shaped microglia in the ONL (Figure 4d), subretinal space and choroid showed increase of both types of microglia in the CNV compared to control (Figure 4e). Most microglia cells were not labelled for B1 receptors in the retina, yet there was a partial colocalization in the subretinal space/choroid close to the experimental laser spots (Figure 4c′,c″).
3.5. Cellular distribution of B1 receptors and VEGF‐R2 in the retina
In comparison with the low immunostaining for VEGF‐R2 in the control retina (Figure 5b), VEGF‐R2 immunostaining was more striking in blood vessels of the CNV retina (Figure 5b′). The immunostaining of B1 receptors—and VEGF‐R2—merged together, especially in the GCL layer in CNV retina (Figure 5c′). Such co‐localization of B1 receptors with VEGF‐R2 was not seen in the morphologically identified extensions of Müller cells.
Figure 5.
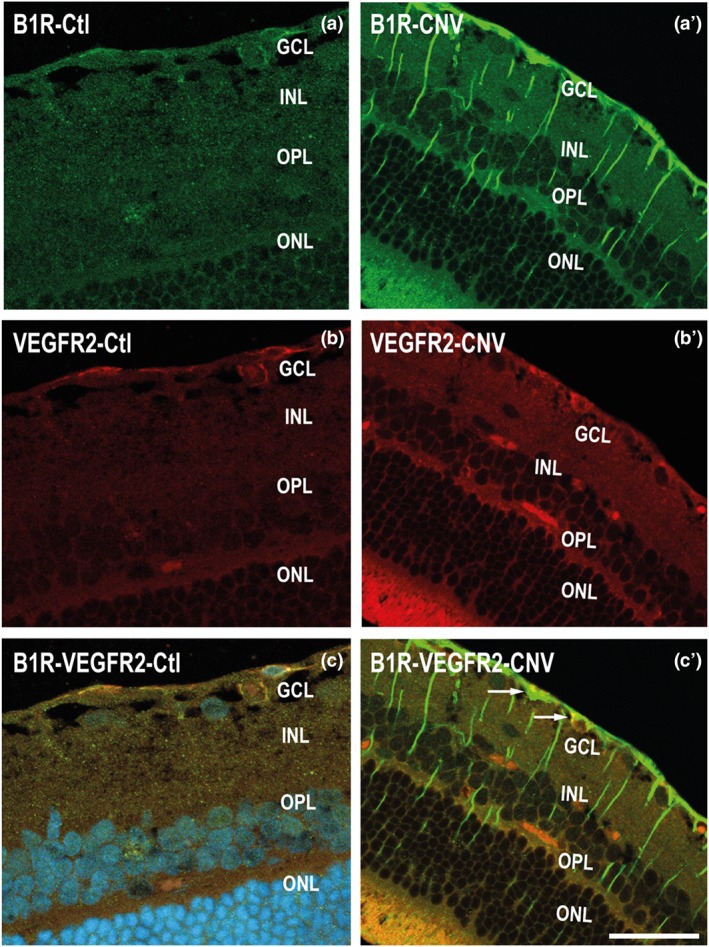
Micrographs of cellular distribution of B1 receptors on VEGF‐R2. Representative micrographs of immunolabelling for B1 receptors (green) and VEGF‐R2 (red) in control (Ctl) and CNV retinas. B1 receptors and VEGF‐R2 are co‐expressed partly (yellow) in the GCL layer of CNV retina (panel c′). Data are from four rats. Scale bar: 75 μm. B1R, B1 receptors
3.6. Effect of anti‐VEGF, R‐954, and B1 receptor siRNA on the CNV lesion
CNV was first monitored with a fundus camera and angiography. Laser‐induced spots of CNV were marked by visible hyperfluorescence in the late phase of angiography (Figure 6a). The lesion area was more visible in the untreated eye (left pictures, indicated by red circles and arrows) than in the treated eye with IVT injection of anti‐VEGF (right pictures). To evaluate the retinal vascular damage, leakage of the FITC‐Dextran dye was observed ex vivo in CNV retina (Figure 6b). We determined also whether the pharmacological treatments could attenuate the retinal damage. All laser spots demonstrated evident fluorescein leakage 10 days post‐CNV compared to the control eye. The anti‐VEGF treatment significantly decreased the surface of retinal damage by 72% (2.6 ± 1.1 mm2), while R‐954 and B1 receptor siRNA treatments reduced it by 45% (5.2 ± 0.8 mm2) and 78% (2.0 ± 0.6 mm2), respectively, compared with vehicle‐treated lesions (9.6 ± 1.1 mm2, significant for each comparison, Figure 6c).
Figure 6.
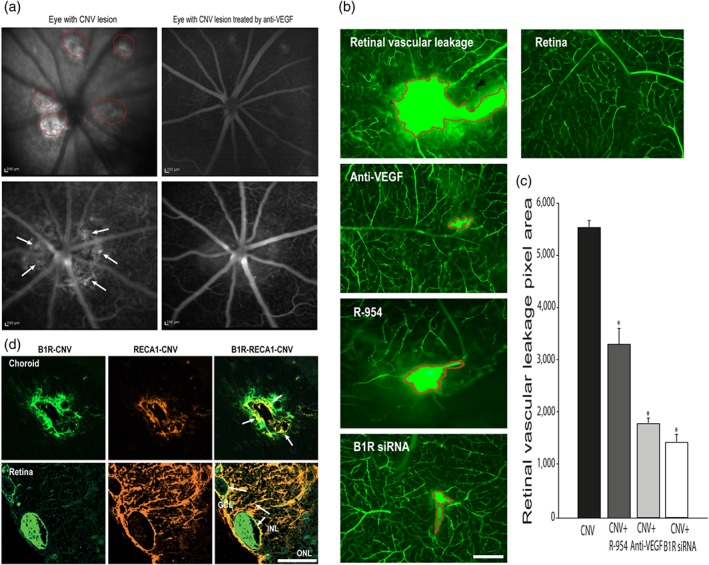
Effect of anti‐VEGF, R‐954, and B1 receptor siRNA on CNV lesion. CNV is shown as visible hyperfluorescence in the late phase of rat eyes with occult CNV on fluorescein angiography (panel a). The lesion area was more visible in the untreated eyes (left pictures, indicated by red circles and arrows) than in the treated eyes with IVT injection of anti‐VEGF (right pictures). Effects of IVT anti‐VEGF or B1 receptor siRNA and ocular administration of R‐954 on retinal vascular leakage are shown in representative micrographs of the FITC‐dextran (panel b) and quantified by the number of green pixels (panel c). The area of retinal lesions is identified in red in flat‐mounted CNV‐retinas. Data are means ± SEM of values from six rats (five laser spots for each group). * P ≤ .05, significantly different from CNV without treatment. Microphotographs of immunolocalization of B1 receptors (panel d, green) are shown on endothelial cells (panel d, red) in choroid (upper panels) and retina (bottom panels). Scale bar: 200 μm (a), 50 μm (b), 75 μm (d). B1R, B1 receptors
Immunostaining of RECA‐1 (red) supports laser‐induced neovascularization. RECA‐1 and B1 receptor (green) staining merged together (yellow) showing their colocalization in CNV choroid and retina (Figure 6d). The immunodetection of B1 receptors by fluorescence revealed an increased protein expression of these receptors in both the CNV choroid and retina (Figure 6d). Topical application of R‐954, IVT B1 receptor siRNA treatment, and anti‐VEGF treatment significantly decreased the severity of laser‐induced CNV (Figure 6b,c).
4. DISCUSSION
This study provides the first demonstration that, in addition to VEGF, the KKS contributes to the inflammatory process following laser‐induced CNV. Blockade of B1 receptors with IVT B1 receptor siRNA or topical application of the B1 receptor antagonist R‐954 significantly decreased CNV in the retina and inflammatory responses such as leukocyte adhesion and enhanced retinal vascular permeability. Moreover, the topical administration of the B1 receptor antagonist R‐954 markedly reduced the expression of inflammation mediators (IL‐1β, TNF‐α, and COX‐2). On the other hand, treatment with anti‐VEGF also reduced CNV‐induced up‐regulation of B1 and B2 receptors, VEGF‐A, and VEGF‐R2, as well as vascular adhesion molecules resulting in leukocyte adhesion. These findings suggest that both the VEGF and B1 receptor pathways are involved in complementary mechanisms in the development of CNV‐induced retinal changes.
4.1. Contribution of B1 receptors along with VEGF in retinal damage induced by CNV
Our study clearly shows up‐regulation of B1 and B2 receptors in retina after laser‐induced CNV. Moreover, B1 receptor blockade prevented most of the pathological events linked to CNV, to an extent similar to that after anti‐VEGF treatment. These results suggest a possible contribution of the B1 receptor in the retinal damage induced by CNV, either directly by its action on the vascular bed or through its interaction with VEGF. Moreover, the pathological consequences of raised VEGF might possibly be mediated through the KKS system.
The size of retinal damage as shown by FITC Dextran leakage through pathological vessels was substantially reduced by B1 receptor blockade. As shown by immunostaining, the B1 receptors were co‐localized with endothelial cells and partly with the microglia. The overexpression of B1 receptors in endothelial cells is in accordance with the enhanced vascular permeability observed in this study. Additionally, B1 receptors were detected on astrocytes. These glial cells are in close relationship with ganglion cells and capillaries contributing to the blood‐retinal barrier (Hachana et al., 2018; Wang, Xu, Elliott, Zhu, & Le, 2010). Moreover, astrocytes are strongly implicated in retinal angiogenesis, providing the necessary template for vessel proliferation (O'Sullivan et al., 2017). A 10‐day treatment by eye‐drop application of R‐954 decreased vascular permeability and up‐regulation of B1 and B2 receptors without affecting VEGF and VEGF‐R2 up‐regulation. One can infer that the B1 receptors could activate the inflammatory process in the retina, independently of the VEGF pathway. Accordingly, both effects of the B1 receptor antagonist on vascular permeability and size of the CNV are slightly less than those after anti‐VEGF, and a previous study showed that plasma kallikrein is involved, as a VEGF‐independent mediator, in macular oedema (Kita et al., 2015). This is congruent with the role of the KKS in vasodilation, inflammation, and retinal vascular hyperpermeability in retinal diseases (Abdulaal, Haddad, Sun, & Silva, 2016; Clermont et al., 2011; Pouliot et al., 2012).
The cellular distribution of B1 receptors also overlapped VEGF‐R2 in the retina in the CNV model. Whereas B1 receptors and VEGF may interact in the retina (Clermont et al., 2016; Kita et al., 2015), only three studies have explored the role of the KKS in AMD aetiology. The first showed that the KKS was a positive regulator of CNV through the additive effects of a B2 receptor antagonist and ACE inhibition on the reduction of CNV (Nagai et al., 2007). The second showed that tissue kallikrein attenuates CNV formation via the cleavage of VEGF and VEGF164 isoform in RPE‐choroid complexes (Fukuhara et al., 2013). In the third one, tissue kallikrein inhibited retinal neovascularization in laser‐induced CNV via the cleavage of VEGF‐165 (Nakamura et al., 2011). However, this effect was modest and did not involve plasma kallikrein, which is strongly induced in retinal vascular disease (Bhat et al., 2014; Pouliot et al., 2012). The co‐localization of VEGF‐R2 and B1 receptors on retinal blood vessels is congruent with the possibility that VEGF acts partly through the KKS system. Both VEGF and B1 receptors caused breakdown of the blood‐retinal barrier in numerous ocular diseases, including neovascular AMD (Clermont et al., 2016; Gragoudas et al., 2004; Qaum et al., 2001; Saishin et al., 2003). A single anti‐VEGF IVT injection reduced the formation of vascular damage in retina and prevented the increase in retinal vascular permeability in laser‐induced CNV, concomitant with a down‐regulation of B1 and B2 receptors. Accordingly, a recent study has shown that the full effect of VEGF on retinal vascular hyperpermeability is dependent on plasma kallikrein (Clermont et al., 2016).
4.2. Contribution of inflammatory mediators in retinal damage induced by CNV
The profile of inflammatory mediators remains less investigated in AMD. An association has been shown between cytokine levels in the aqueous humour and the pathogenesis of AMD patients (Liu et al., 2016). Although BK can up‐regulate IL‐8 expression in human fibroblasts (Bastian et al., 1998; Brunius, Domeij, Gustavsson, & Yucel‐Lindberg, 2005), the expression of this cytokine is not altered by R‐954 or anti‐VEGF in the CNV retina. Therefore, our data would not support a primary role for IL‐8, a potent chemoattractant, involved in CNV (Qu, Zhou, & Xu, 2009), in the therapeutic effects of anti‐VEGF and B1 receptor antagonism in laser‐induced CNV. The local production of IL‐1β and TNF‐α is one of the most prominent processes at the beginning of inflammation. Both are involved in the induction and up‐regulation of B1 receptors (Leeb‐Lundberg, Marceau, Muller‐Esterl, Pettibone, & Zuraw, 2005). In vivo and in vitro studies have shown that angiogenesis is induced by IL‐1β via the COX‐2 pathway (Kuwano et al., 2004).
TNF‐α is known as a potent mediator of increased retinal endothelial cell permeability (Aveleira, Lin, Abcouwer, Ambrosio, & Antonetti, 2010) that is partly mediated by the KKS system (Clermont et al., 2016). Macrophages recruited during a CNV express TNF‐α (Oh et al., 1999), and TNF‐α produced by macrophages stimulates RPE and overexpression of VEGF (Beatty, Koh, Phil, Henson, & Boulton, 2000). Interestingly, anti‐VEGF injection and R‐954 topical application both reduced TNF‐α expression in CNV retina. In contrast, COX‐2 and IL‐1β up‐regulation in CNV retina was normalized with R‐954, but not with anti‐VEGF treatment. These findings suggest that B1 receptor antagonism and anti‐VEGF reduced inflammation through distinctive and complementary mechanisms in the development of CNV‐induced retinal damage.
4.3. Regulation and interaction between kinin B1 receptors and VEGF in the inflammatory cascade associated with neovascularization
Kinin B1 receptors and VEGF appear to promote retinal inflammation through complementary but distinctive mechanisms that may work in concert as a loop of auto‐amplification (Figure 7). In this possible scheme, VEGF‐A, which binds to both VEGF‐R1/R2, operates upstream through the up‐regulation of the B1 receptor by releasing TNF‐α. While VEGF‐R1 was shown to be involved in inflammation, VEGF‐R2 mediates angiogenesis (Witmer et al., 2003); thus, the major and direct angiogenic signals are generated from VEGF‐R2. Studies have shown that B1 receptors can be up‐regulated by cytokines, such as TNF‐α, IL‐1β, and by its own agonists (via cytokines), yet it is down‐regulated by B1 receptor antagonists in several paradigms (Couture et al., 2014). Such a regulatory mechanism is also proposed here to explain the suppressive effect of R‐954 on expression of B1 receptors (most likely mediated by a down‐regulation of cytokines) in the CNV retina. Because anti‐VEGF treatment results in inhibition of cytokine production, particularly TNF‐α, it is conceivable that a mechanism similar to that for the B1 receptors, is also effective for VEGF. Hence, a decrease of inflammatory mediators by anti‐VEGF is likely to block the positive feedback loop of autoregulation on B1 receptors, but also on VEGF‐A and its receptors.
Figure 7.
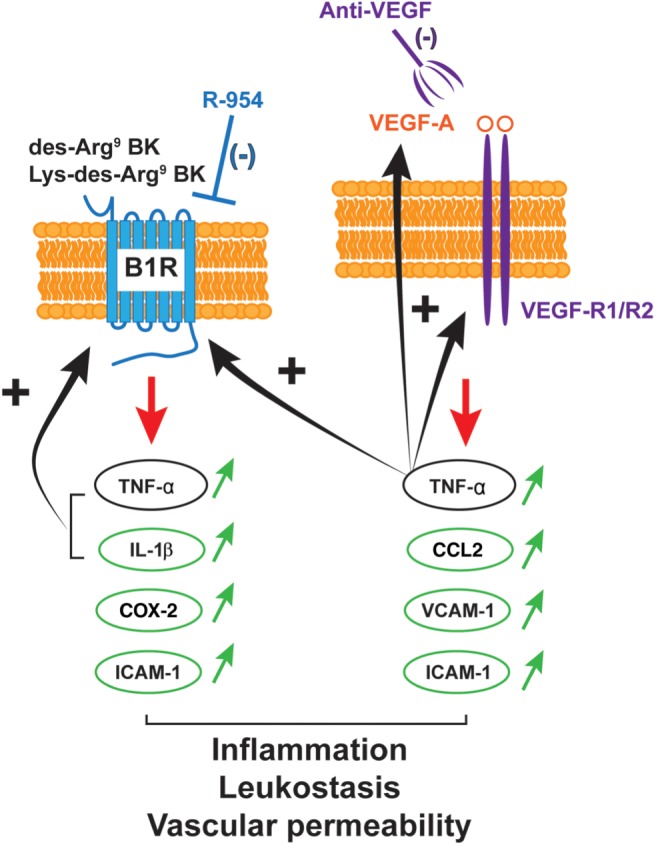
Possible interaction between kinin‐B1 receptor (B1R) and VEGF signalling in retinal inflammation. Data suggest that the pro‐inflammatory mediators (IL‐1β, CCL2, TNF‐α, ICAM‐1, VCAM‐1, and COX‐2) are differently up‐regulated (↑) by the B1 receptors and VEGF‐A, which can bind to both VEGF‐R1 and VEGF‐R2. These two main systems could favour pathological effects in a complementary but distinct manner in the vascular and inflammatory processes. B1 receptors and VEGF may work in concert as a loop of auto‐amplification mediated by TNF‐α known to up‐regulate B1 receptors and VEGF. In that scenario, the B1 receptor is instrumental for the inflammatory effects of VEGF. Anti‐VEGF IVT injection and topical application of R‐954 were both able to reduce TNF‐α expression in CNV retina. Thus, anti‐VEGF therapy prevents the up‐regulation of B1 receptors. Conversely, the anti‐B1 receptor antagonist does not affect the expression of the VEGF system and may exert its effects independently of VEGF
4.4. Contribution of leukocytes to the retinal damage induced by CNV
The inflammatory process in CNV and its spread to retina include leukocyte infiltration and leukocytes have been described in excised neovascular membranes (Gehrs, Heriot, & de Juan, 1992; Lopez et al., 1991; Seregard, Algvere, & Berglin, 1994). Under physiological conditions, the quiescent vascular endothelium prevents the adhesion of leukocytes and platelets to its apical surface. Under inflammatory conditions, leukocyte adhesion is promoted by pro‐adhesive molecules such as ICAM‐1, VCAM‐1, and CCL2. Human choroidal neovascular membranes express these leukocyte adhesion molecules (Yeh, Bula, Miller, Gragoudas, & Arroyo, 2004). As expected, we observed that the increased number of adherent leukocytes in the CNV retina is accompanied by an increase in mRNA levels of CCL2, ICAM‐1, and VCAM‐1. Targeted disruption of ICAM‐1 and CCL2 in mice leads to a significant decrease in CNV development (Sakurai et al., 2003; Schmack et al., 2009), suggesting that adherent leukocytes have a prominent role in the angiogenic process. In the present study, anti‐VEGF reduced the expression of these adhesion molecules and leukocyte adhesion, which is consistent with the reduction of the retinal CNV. Blockade of B1 receptors also reduced leukocyte adhesion and the size of the CNV by reducing the overexpression of ICAM‐1. This occurs without affecting the overexpression of CCL2 and VCAM‐1 in the CNV retina, again supporting that B1 receptors and VEGF operate through distinct and complementary mechanism on leukocyte adhesion and the development of CNV complications.
4.5. Contribution of microglia in retinal damage induced by CNV
Microglia act as resident macrophage‐related cells and are classified in two major populations, namely, ramified resting and activated ameboid (Hickey & Kimura, 1988). Ramified microglial cells are derived from monocytes, but pass through an intermediate stage of ameboid microglia as active macrophages of the perinatal period (Karperien, Ahammer, & Jelinek, 2013). In the retina, microglia are resident immune cells that can release a range of cytokines including IL‐1α, IL‐1β, IL‐6, and TNF‐α (McGeer, Klegeris, & McGeer, 2005; McGeer & McGeer, 2004). Activated microglia have been found in the subretinal space of patients with AMD or in mouse models (Checchin, Sennlaub, Levavasseur, Leduc, & Chemtob, 2006; Gupta, Brown, & Milam, 2003; Ma et al., 1996; Ma, Zhao, Fontainhas, Fariss, & Wong, 2009). Under normal conditions, retinal microglia are totally absent from the subretinal space, largely excluded from the external retina and present in small amounts in the inner retina (Lee, Liang, Fariss, & Wong, 2008). Here, we have shown that microglia cells accumulated in the GCL, INL, OPL, the subretinal space close to the RPE layer, and choroid. The accumulation and morphological changes of activated microglia can probably fuel inflammation, neovascularization, and possible apoptosis of retinal neuronal cells. A study revealed that eyes with subretinally transplanted microglia develop large and prominent CNV in the subretinal space (Ma et al., 2009). Local retinal microglia participate in the vascularization of the retina by interacting with signals coming from retinal vessels in response to local tissue hypoxia (Checchin et al., 2006). When the blood‐retinal barrier is compromised, microglia infiltrate the subretinal space with their protrusions. These cells become ameboid phagocytes that can produce a variety of inflammatory mediators, including ROS and cytokines (Luckoff et al., 2017; Madeira, Boia, Santos, Ambrosio, & Santiago, 2015).VEGF receptor blockade reduced retinal microglia/macrophage infiltration in laser‐induced CNV (Huang, Parlier, Shen, Lutty, & Vinores, 2013). In our study, B1 receptors were party colocalized with microglia, close to the experimental laser spots, particularly in the choroid. Whether B1 receptor blockade can also interfere with retinal microglia infiltration and reactivity in CNV remains to be determined.
In conclusion, this study has highlighted the beneficial effect of topical ocular application of a B1 receptor antagonist to avert CNV development and inflammation in the retina immediately subsequent to a laser burn in the choroid. From a clinical point of view, the non‐invasive and self‐administration of B1 receptor antagonists on the surface of the cornea by eye drops might be an important asset for the treatment of AMD or a complementary approach to patients currently resistant to anti‐VEGF therapy. This possibility warrants further investigation.
AUTHOR CONTRIBUTIONS
S.H. and O.F. performed the experiments, S.H., O.F., R.C., and E.V. designed the experiments, analysed the data, and interpreted the results. S.H. wrote the first version of the paper and contributed significantly to the last version edited by E.V. and R.C. All authors approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
The authors greatly appreciate the technical assistance of Jacques Sénécal for the immunohistochemistry experiments. This work was supported by a grant from the Canadian Institutes of Health Research (MOP‐125962) to E.V. and R.C. and from the FRQS Vision Health Research Network (AMD program/Fondation Antoine Turmel) to E.V., R.C., and M.S. S.H. was recipient of a Scholarship from the GRUM‐Université de Montréal. O.F. was a recipient of a Scholarship from the FROUM.
Hachana S, Fontaine O, Sapieha P, Lesk M, Couture R, Vaucher E. The effects of anti‐VEGF and kinin B1 receptor blockade on retinal inflammation in laser‐induced choroidal neovascularization. Br J Pharmacol. 2020;177:1949–1966. 10.1111/bph.14962
REFERENCES
- Abdulaal, M. , Haddad, N. M. , Sun, J. K. , & Silva, P. S. (2016). The role of plasma kallikrein–kinin pathway in the development of diabetic retinopathy: Pathophysiology and therapeutic approaches. Seminars in Ophthalmology, 31, 19–24. 10.3109/08820538.2015.1114829 [DOI] [PubMed] [Google Scholar]
- Aiello, L. P. , Pierce, E. A. , Foley, E. D. , Takagi, H. , Chen, H. , Riddle, L. , … Smith, L. E. (1995). Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF‐receptor chimeric proteins. Proceedings of the National Academy of Sciences of the United States of America, 92, 10457–10461. 10.1073/pnas.92.23.10457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveleira, C. A. , Lin, C. M. , Abcouwer, S. F. , Ambrosio, A. F. , & Antonetti, D. A. (2010). TNF‐α signals through PKCζ/NF‐κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes, 59, 2872–2882. 10.2337/db09-1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser, C. , Wolf, A. , Herb, M. , & Langmann, T. (2019). Co‐inhibition of PGF and VEGF blocks their expression in mononuclear phagocytes and limits neovascularization and leakage in the murine retina. Journal of Neuroinflammation, 16(1), 26 10.1186/s12974-019-1419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashshur, Z. F. , Bazarbachi, A. , Schakal, A. , Haddad, Z. A. , El Haibi, C. P. , & Noureddin, B. N. (2006). Intravitreal bevacizumab for the management of choroidal neovascularization in age‐related macular degeneration. American Journal of Ophthalmology, 142, 1–9. 10.1016/j.ajo.2006.02.037 [DOI] [PubMed] [Google Scholar]
- Bastian, S. , Paquet, J. L. , Robert, C. , Cremers, B. , Loillier, B. , Larrivee, J. F. , … Pruneau, D. (1998). Interleukin 8 (IL‐8) induces the expression of kinin B1 receptor in human lung fibroblasts. Biochemical and Biophysical Research Communications, 253, 750–755. 10.1006/bbrc.1998.9848 [DOI] [PubMed] [Google Scholar]
- Beatty, S. , Koh, H. , Phil, M. , Henson, D. , & Boulton, M. (2000). The role of oxidative stress in the pathogenesis of age‐related macular degeneration. Survey of Ophthalmology, 45, 115–134. 10.1016/s0039-6257(00)00140-5 [DOI] [PubMed] [Google Scholar]
- Bhat, M. , Pouliot, M. , Couture, R. , & Vaucher, E. (2014). The kallikrein–kinin system in diabetic retinopathy. Progress in Drug Research, 69, 111–143. 10.1007/978-3-319-06683-7_5 [DOI] [PubMed] [Google Scholar]
- Brunius, G. , Domeij, H. , Gustavsson, A. , & Yucel‐Lindberg, T. (2005). Bradykinin upregulates IL‐8 production in human gingival fibroblasts stimulated by interleukin‐1β and tumor necrosis factor α. Regulatory Peptides, 126, 183–188. 10.1016/j.regpep.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Campochiaro, P. A. (2013). Ocular neovascularization. Journal of Molecular Medicine (Berlin, Germany), 91, 311–321. 10.1007/s00109-013-0993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checchin, D. , Sennlaub, F. , Levavasseur, E. , Leduc, M. , & Chemtob, S. (2006). Potential role of microglia in retinal blood vessel formation. Investigative Ophthalmology & Visual Science, 47, 3595–3602. 10.1167/iovs.05-1522 [DOI] [PubMed] [Google Scholar]
- Chen, M. , Zhao, J. , Luo, C. , Pandi, S. P. , Penalva, R. G. , Fitzgerald, D. C. , & Xu, H. (2012). Para‐inflammation‐mediated retinal recruitment of bone marrow‐derived myeloid cells following whole‐body irradiation is CCL2 dependent. Glia, 60, 833–842. 10.1002/glia.22315 [DOI] [PubMed] [Google Scholar]
- Clermont, A. , Chilcote, T. J. , Kita, T. , Liu, J. , Riva, P. , Sinha, S. , & Feener, E. P. (2011). Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes, 60, 1590–1598. 10.2337/db10-1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont, A. , Murugesan, N. , Zhou, Q. , Kita, T. , Robson, P. A. , Rushbrooke, L. J. , … Feener, E. P. (2016). Plasma kallikrein mediates vascular endothelial growth factor‐induced retinal dysfunction and thickening. Investigative Ophthalmology & Visual Science, 57, 2390–2399. 10.1167/iovs.15-18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman, R. W. (2006). Regulation of angiogenesis by the kallikrein–kinin system. Current Pharmaceutical Design, 12, 2599–2607. 10.2174/138161206777698710 [DOI] [PubMed] [Google Scholar]
- Couture, R. , Blaes, N. , & Girolami, J. P. (2014). Kinin receptors in vascular biology and pathology. Current Vascular Pharmacology, 12, 223–248. 10.2174/1570161112666140226121627 [DOI] [PubMed] [Google Scholar]
- Cunningham, F. , Van Bergen, T. , Canning, P. , Lengyel, I. , Feyen, J. H. M. , & Stitt, A. W. (2019). The placental growth factor pathway and its potential role in macular degenerative disease. Current Eye Research, 44, 813–822. 10.1080/02713683.2019.1614197 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell, M. , Uusitalo‐Jarvinen, H. , Aguilar, E. , & Friedlander, M. (2007). Ocular neovascularization: Basic mechanisms and therapeutic advances. Survey of Ophthalmology, 52(Suppl 1), S3–S19. 10.1016/j.survophthal.2006.10.017 [DOI] [PubMed] [Google Scholar]
- Edelman, J. L. , & Castro, M. R. (2000). Quantitative image analysis of laser‐induced choroidal neovascularization in rat. Experimental Eye Research, 71, 523–533. 10.1006/exer.2000.0907 [DOI] [PubMed] [Google Scholar]
- Emanueli, C. , Bonaria Salis, M. , Stacca, T. , Pintus, G. , Kirchmair, R. , Isner, J. M. , … Madeddu, P. (2002). Targeting kinin B1 receptor for therapeutic neovascularization. Circulation, 105, 360–366. 10.1161/hc0302.102142 [DOI] [PubMed] [Google Scholar]
- Emanueli, C. , Minasi, A. , Zacheo, A. , Chao, J. , Chao, L. , Salis, M. B. , … Madeddu, P. (2001). Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation, 103, 125–132. 10.1161/01.cir.103.1.125 [DOI] [PubMed] [Google Scholar]
- Feener, E. P. (2010). Plasma kallikrein and diabetic macular edema. Current Diabetes Reports, 10, 270–275. 10.1007/s11892-010-0127-1 [DOI] [PubMed] [Google Scholar]
- Fontaine, O. , Olivier, S. , Descovich, D. , Cordahi, G. , Vaucher, E. , & Lesk, M. R. (2011). The effect of intravitreal injection of bevacizumab on retinal circulation in patients with neovascular macular degeneration. Investigative Ophthalmology & Visual Science, 52, 7400–7405. 10.1167/iovs.10-6646 [DOI] [PubMed] [Google Scholar]
- Fukuhara, J. , Noda, K. , Murata, M. , Namba, S. , Kinoshita, S. , Dong, Z. , … Ishida, S. (2013). Tissue kallikrein attenuates choroidal neovascularization via cleavage of vascular endothelial growth factor. Investigative Ophthalmology & Visual Science, 54, 274–279. 10.1167/iovs.12-10512 [DOI] [PubMed] [Google Scholar]
- Gehrs, K. M. , Heriot, W. J. , & de Juan, E. Jr. (1992). Transmission electron microscopic study of a subretinal choroidal neovascular membrane due to age‐related macular degeneration. Archives of Ophthalmology, 110, 833–837. 10.1001/archopht.1992.01080180105036 [DOI] [PubMed] [Google Scholar]
- Gemenetzi, M. , Lotery, A. J. , & Patel, P. J. (2017). Risk of geographic atrophy in age‐related macular degeneration patients treated with intravitreal anti‐VEGF agents. Eye (London, England), 31, 1–9. 10.1038/eye.2016.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil, F. Jr. , Sirois, P. , & Regoli, D. (2014). Preclinical pharmacology, metabolic stability, pharmacokinetics and toxicology of the peptidic kinin B1 receptor antagonist R‐954. Peptides, 52, 82–89. 10.1016/j.peptides.2013.12.009 [DOI] [PubMed] [Google Scholar]
- Gragoudas, E. S. , Adamis, A. P. , Cunningham, E. T. Jr. , Feinsod, M. , Guyer, D. R. , & Group VISiONCT (2004). Pegaptanib for neovascular age‐related macular degeneration. The New England Journal of Medicine, 351, 2805–2816. 10.1056/NEJMoa042760 [DOI] [PubMed] [Google Scholar]
- Grossniklaus, H. E. , Ling, J. X. , Wallace, T. M. , Dithmar, S. , Lawson, D. H. , Cohen, C. , … Sternberg P Jr (2002). Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Molecular Vision, 8, 119–126. [PubMed] [Google Scholar]
- Gupta, N. , Brown, K. E. , & Milam, A. H. (2003). Activated microglia in human retinitis pigmentosa, late‐onset retinal degeneration, and age‐related macular degeneration. Experimental Eye Research, 76, 463–471. 10.1016/S0014-4835(02)00332-9 [DOI] [PubMed] [Google Scholar]
- Hachana, S. , Bhat, M. , Senecal, J. , Huppe‐Gourgues, F. , Couture, R. , & Vaucher, E. (2018). Expression, distribution and function of kinin B1 receptor in the rat diabetic retina. British Journal of Pharmacology, 175, 968–983. 10.1111/bph.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, L. , Lanitis, T. , Cele, C. , Toro‐Diaz, H. , Gibson, A. , & Kuznik, A. (2018). Intravitreal aflibercept versus ranibizumab for wet age‐related macular degeneration: A cost‐effectiveness analysis. Journal of Managed Care & Specialty Pharmacy, 24, 608–616. 10.18553/jmcp.2018.24.7.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey, W. F. , & Kimura, H. (1988). Perivascular microglial cells of the CNS are bone marrow‐derived and present antigen in vivo. Science, 239, 290–292. 10.1126/science.3276004 [DOI] [PubMed] [Google Scholar]
- Hillmeister, P. , Gatzke, N. , Dulsner, A. , Bader, M. , Schadock, I. , Hoefer, I. , … Urban, D. (2011). Arteriogenesis is modulated by bradykinin receptor signaling. Circulation Research, 109, 524–533. 10.1161/CIRCRESAHA.111.240986 [DOI] [PubMed] [Google Scholar]
- Huang, H. , Parlier, R. , Shen, J. K. , Lutty, G. A. , & Vinores, S. A. (2013). VEGF receptor blockade markedly reduces retinal microglia/macrophage infiltration into laser‐induced CNV. PLoS ONE, 8, e71808 10.1371/journal.pone.0071808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas, J. B. , Tao, Y. , Neumaier, M. , & Findeisen, P. (2010). Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age‐related macular degeneration. Archives of Ophthalmology, 128, 1281–1286. 10.1001/archophthalmol.2010.227 [DOI] [PubMed] [Google Scholar]
- Karperien, A. , Ahammer, H. , & Jelinek, H. F. (2013). Quantitating the subtleties of microglial morphology with fractal analysis. Frontiers in Cellular Neuroscience, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , Altman, D. G. , & Group NCRRGW (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, I. , Moon, S. O. , Kim, S. H. , Kim, H. J. , Koh, Y. S. , & Koh, G. Y. (2001). Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), and E‐selectin through nuclear factor‐κB activation in endothelial cells. The Journal of Biological Chemistry, 276, 7614–7620. 10.1074/jbc.M009705200 [DOI] [PubMed] [Google Scholar]
- Kita, T. , Clermont, A. C. , Murugesan, N. , Zhou, Q. , Fujisawa, K. , Ishibashi, T. , … Feener, E. P. (2015). Plasma kallikrein–kinin system as a VEGF‐independent mediator of diabetic macular edema. Diabetes, 64, 3588–3599. 10.2337/db15-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano, T. , Nakao, S. , Yamamoto, H. , Tsuneyoshi, M. , Yamamoto, T. , Kuwano, M. , & Ono, M. (2004). Cyclooxygenase 2 is a key enzyme for inflammatory cytokine‐induced angiogenesis. The FASEB Journal, 18, 300–310. 10.1096/fj.03-0473com [DOI] [PubMed] [Google Scholar]
- Lavalette, S. , Raoul, W. , Houssier, M. , Camelo, S. , Levy, O. , Calippe, B. , … Sennlaub, F. (2011). Interleukin‐1β inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. The American Journal of Pathology, 178, 2416–2423. 10.1016/j.ajpath.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, Y. Z. (2017). VEGF production and signaling in Muller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vision Research, 139, 108–114. 10.1016/j.visres.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. E. , Liang, K. J. , Fariss, R. N. , & Wong, W. T. (2008). Ex vivo dynamic imaging of retinal microglia using time‐lapse confocal microscopy. Investigative Ophthalmology & Visual Science, 49, 4169–4176. 10.1167/iovs.08-2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb‐Lundberg, L. M. , Marceau, F. , Muller‐Esterl, W. , Pettibone, D. J. , & Zuraw, B. L. (2005). International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacological Reviews, 57, 27–77. 10.1124/pr.57.1.2 [DOI] [PubMed] [Google Scholar]
- Liu, F. , Ding, X. , Yang, Y. , Li, J. , Tang, M. , Yuan, M. , … Lu, L. (2016). Aqueous humor cytokine profiling in patients with wet AMD. Molecular Vision, 22, 352–361. [PMC free article] [PubMed] [Google Scholar]
- Lopez, P. F. , Grossniklaus, H. E. , Lambert, H. M. , Aaberg, T. M. , Capone, A. Jr. , Sternberg, P. Jr. , & L'Hernault, N. (1991). Pathologic features of surgically excised subretinal neovascular membranes in age‐related macular degeneration. American Journal of Ophthalmology, 112, 647–656. 10.1016/s0002-9394(14)77270-8 [DOI] [PubMed] [Google Scholar]
- Lu, M. , & Adamis, A. P. (2006). Molecular biology of choroidal neovascularization. Ophthalmology Clinics of North America, 19, 323–334. 10.1016/j.ohc.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Luckoff, A. , Scholz, R. , Sennlaub, F. , Xu, H. , & Langmann, T. (2017). Comprehensive analysis of mouse retinal mononuclear phagocytes. Nature Protocols, 12, 1136–1150. 10.1038/nprot.2017.032 [DOI] [PubMed] [Google Scholar]
- Ma, J. X. , Song, Q. , Hatcher, H. C. , Crouch, R. K. , Chao, L. , & Chao, J. (1996). Expression and cellular localization of the kallikrein–kinin system in human ocular tissues. Experimental Eye Research, 63, 19–26. 10.1006/exer.1996.0087 [DOI] [PubMed] [Google Scholar]
- Ma, W. , Zhao, L. , Fontainhas, A. M. , Fariss, R. N. , & Wong, W. T. (2009). Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: A potential cellular interaction relevant to AMD. PLoS ONE, 4, e7945 10.1371/journal.pone.0007945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, M. H. , Boia, R. , Santos, P. F. , Ambrosio, A. F. , & Santiago, A. R. (2015). Contribution of microglia‐mediated neuroinflammation to retinal degenerative diseases. Mediators of Inflammation, 2015, 673090 10.1155/2015/673090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo, T. , Schini‐Kerth, V. B. , & Busse, R. (1999). Vascular endothelial growth factor activates nuclear factor‐κB and induces monocyte chemoattractant protein‐1 in bovine retinal endothelial cells. Diabetes, 48, 1131–1137. 10.2337/diabetes.48.5.1131 [DOI] [PubMed] [Google Scholar]
- McGeer, E. G. , Klegeris, A. , & McGeer, P. L. (2005). Inflammation, the complement system and the diseases of aging. Neurobiology of Aging, 26(Suppl 1), 94–97. 10.1016/j.neurobiolaging.2005.08.008 [DOI] [PubMed] [Google Scholar]
- McGeer, P. L. , & McGeer, E. G. (2004). Inflammation and the degenerative diseases of aging. Annals of the New York Academy of Sciences, 1035, 104–116. 10.1196/annals.1332.007 [DOI] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP . British Journal of Pharmacology, 172, 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, P. (2011). A systematic review of the efficacy and safety outcomes of anti‐VEGF agents used for treating neovascular age‐related macular degeneration: Comparison of ranibizumab and bevacizumab. Current Medical Research and Opinion, 27, 1465–1475. 10.1185/03007995.2011.585394 [DOI] [PubMed] [Google Scholar]
- Nagai, N. , Oike, Y. , Izumi‐Nagai, K. , Koto, T. , Satofuka, S. , Shinoda, H. , … Ishida, S. (2007). Suppression of choroidal neovascularization by inhibiting angiotensin‐converting enzyme: Minimal role of bradykinin. Investigative Ophthalmology & Visual Science, 48, 2321–2326. 10.1167/iovs.06-1296 [DOI] [PubMed] [Google Scholar]
- Nakamura, S. , Morimoto, N. , Tsuruma, K. , Izuta, H. , Yasuda, Y. , Kato, N. , … Hara, H. (2011). Tissue kallikrein inhibits retinal neovascularization via the cleavage of vascular endothelial growth factor‐165. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 1041–1048. 10.1161/ATVBAHA.111.223594 [DOI] [PubMed] [Google Scholar]
- Nakao, S. , Kuwano, T. , Tsutsumi‐Miyahara, C. , Ueda, S. , Kimura, Y. N. , Hamano, S. , … Ono, M. (2005). Infiltration of COX‐2‐expressing macrophages is a prerequisite for IL‐1β‐induced neovascularization and tumor growth. The Journal of Clinical Investigation, 115, 2979–2991. 10.1172/JCI23298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, Q. D. , Shah, S. M. , Hafiz, G. , Quinlan, E. , Sung, J. , Chu, K. , … CLEAR‐AMD 1 Study Group (2006). A phase I trial of an IV‐administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age‐related macular degeneration. Ophthalmology, 113, 1522 e1521‐1522 e1514. [DOI] [PubMed] [Google Scholar]
- Oh, H. , Takagi, H. , Takagi, C. , Suzuma, K. , Otani, A. , Ishida, K. , … Honda, Y. (1999). The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investigative Ophthalmology & Visual Science, 40, 1891–1898. [PubMed] [Google Scholar]
- O'Sullivan, M. L. , Punal, V. M. , Kerstein, P. C. , Brzezinski, J. A. , Glaser, T. , Wright, K. M. , & Kay, J. N. (2017). Astrocytes follow ganglion cell axons to establish an angiogenic template during retinal development. Glia, 65, 1697–1716. 10.1002/glia.23189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. Y. , Kim, J. H. , & Park, C. K. (2014). Neuronal cell death in the inner retina and the influence of vascular endothelial growth factor inhibition in a diabetic rat model. The American Journal of Pathology, 184, 1752–1762. 10.1016/j.ajpath.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Penfold, P. L. , Provis, J. M. , & Billson, F. A. (1987). Age‐related macular degeneration: ultrastructural studies of the relationship of leucocytes to angiogenesis. Graefe's Archive for Clinical and Experimental Ophthalmology, 225, 70–76. 10.1007/bf02155808 [DOI] [PubMed] [Google Scholar]
- Pennesi, M. E. , Neuringer, M. , & Courtney, R. J. (2012). Animal models of age related macular degeneration. Molecular Aspects of Medicine, 33, 487–509. 10.1016/j.mam.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot, M. , Talbot, S. , Senecal, J. , Dotigny, F. , Vaucher, E. , & Couture, R. (2012). Ocular application of the kinin B1 receptor antagonist LF22‐0542 inhibits retinal inflammation and oxidative stress in streptozotocin‐diabetic rats. PLoS ONE, 7, e33864 10.1371/journal.pone.0033864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaum, T. , Xu, Q. , Joussen, A. M. , Clemens, M. W. , Qin, W. , Miyamoto, K. , … Adamis, A. P. (2001). VEGF‐initiated blood‐retinal barrier breakdown in early diabetes. Investigative Ophthalmology & Visual Science, 42, 2408–2413. [PubMed] [Google Scholar]
- Qu, Y. , Zhou, F. , & Xu, X. Y. (2009). Selective non‐peptide CXCR2 antagonist SB225002 inhibits choroidal neovascularization in rat model. Zhonghua Yan Ke Za Zhi, 45, 742–745. [PubMed] [Google Scholar]
- Reibaldi, M. , Pulvirenti, A. , Avitabile, T. , Bonfiglio, V. , Russo, A. , Mariotti, C. , … Longo, A. (2018). Pooled estimates of incidence of endophthalmitis after intravitreal injection of anti‐vascular endothelial growth factor agents with and without topical antibiotic prophylaxis. Retina, 38, 1–11. 10.1097/IAE.0000000000001583 [DOI] [PubMed] [Google Scholar]
- Rutar, M. , & Provis, J. M. (2016). Role of chemokines in shaping macrophage activity in AMD. Advances in Experimental Medicine and Biology, 854, 11–16. 10.1007/978-3-319-17121-0_2 [DOI] [PubMed] [Google Scholar]
- Saint‐Geniez, M. , Maharaj, A. S. , Walshe, T. E. , Tucker, B. A. , Sekiyama, E. , Kurihara, T. , … D'Amore, P. A. (2008). Endogenous VEGF is required for visual function: Evidence for a survival role on muller cells and photoreceptors. PLoS ONE, 3, e3554 10.1371/journal.pone.0003554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saishin, Y. , Saishin, Y. , Takahashi, K. , Lima e Silva, R. , Hylton, D. , Rudge, J. S. , … Campochiaro, P. A. (2003). VEGF‐TRAP(R1R2) suppresses choroidal neovascularization and VEGF‐induced breakdown of the blood‐retinal barrier. Journal of Cellular Physiology, 195, 241–248. 10.1002/jcp.10246 [DOI] [PubMed] [Google Scholar]
- Sakurai, E. , Taguchi, H. , Anand, A. , Ambati, B. K. , Gragoudas, E. S. , Miller, J. W. , … Ambati, J. (2003). Targeted disruption of the CD18 or ICAM‐1 gene inhibits choroidal neovascularization. Investigative Ophthalmology & Visual Science, 44, 2743–2749. 10.1167/iovs.02-1246 [DOI] [PubMed] [Google Scholar]
- Sarwar, S. , Clearfield, E. , Soliman, M. K. , Sadiq, M. A. , Baldwin, A. J. , Hanout, M. , … Nguyen, Q. D. (2016). Aflibercept for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews, 2, CD011346 10.1002/14651858.CD011346.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack, I. , Berglin, L. , Nie, X. , Wen, J. , Kang, S. J. , Marcus, A. I. , … Grossniklaus, H. E. (2009). Modulation of choroidal neovascularization by subretinal injection of retinal pigment epithelium and polystyrene microbeads. Molecular Vision, 15, 146–161. [PMC free article] [PubMed] [Google Scholar]
- Seregard, S. , Algvere, P. V. , & Berglin, L. (1994). Immunohistochemical characterization of surgically removed subfoveal fibrovascular membranes. Graefe's Archive for Clinical and Experimental Ophthalmology, 232, 325–329. 10.1007/bf00175983 [DOI] [PubMed] [Google Scholar]
- Sheridan, C. M. , Pate, S. , Hiscott, P. , Wong, D. , Pattwell, D. M. , & Kent, D. (2009). Expression of hypoxia‐inducible factor‐1α and ‐2α in human choroidal neovascular membranes. Graefe's Archive for Clinical and Experimental Ophthalmology, 247, 1361–1367. 10.1007/s00417-009-1133-3 [DOI] [PubMed] [Google Scholar]
- Takeda, A. , Baffi, J. Z. , Kleinman, M. E. , Cho, W. G. , Nozaki, M. , Yamada, K. , … Ambati, J. (2009). CCR3 is a target for age‐related macular degeneration diagnosis and therapy. Nature, 460, 225–230. 10.1038/nature08151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe, T. , Ortega, S. , Luna, J. D. , Ozaki, H. , Okamoto, N. , Derevjanik, N. L. , … Campochiaro, P. A. (1998). Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. The American Journal of Pathology, 153, 1641–1646. 10.1016/S0002-9440(10)65753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Xu, X. , Elliott, M. H. , Zhu, M. , & Le, Y. Z. (2010). Muller cell‐derived VEGF is essential for diabetes‐induced retinal inflammation and vascular leakage. Diabetes, 59, 2297–2305. 10.2337/db09-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson‐Berka, J. L. , & Miller, A. G. (2008). Update on the treatment of diabetic retinopathy. ScientificWorldJournal, 8, 98–120. 10.1100/tsw.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer, A. N. , Vrensen, G. F. , Van Noorden, C. J. , & Schlingemann, R. O. (2003). Vascular endothelial growth factors and angiogenesis in eye disease. Progress in Retinal and Eye Research, 22, 1–29. 10.1016/s1350-9462(02)00043-5 [DOI] [PubMed] [Google Scholar]
- Xie, P. , Kamei, M. , Suzuki, M. , Matsumura, N. , Nishida, K. , Sakimoto, S. , … Nishida, K. (2011). Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS ONE, 6, e28933 10.1371/journal.pone.0028933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, P. , Zhang, W. , Yuan, S. , Chen, Z. , Yang, Q. , Yuan, D. , … Liu, Q. H. (2012). Suppression of experimental choroidal neovascularization by curcumin in mice. PLoS ONE, 7, e53329 10.1371/journal.pone.0053329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H. , Wang, Y. , Qian, H. , Zhang, P. , & Huang, C. (2011). Pim protein kinase‐3 is regulated by TNF‐α and promotes endothelial cell sprouting. Molecules and Cells, 32, 235–241. 10.1007/s10059-011-1026-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. , Zhao, J. , & Sun, X. (2016). Resistance to anti‐VEGF therapy in neovascular age‐related macular degeneration: A comprehensive review. Drug Design, Development and Therapy, 10, 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, D. C. , Bula, D. V. , Miller, J. W. , Gragoudas, E. S. , & Arroyo, J. G. (2004). Expression of leukocyte adhesion molecules in human subfoveal choroidal neovascular membranes treated with and without photodynamic therapy. Investigative Ophthalmology & Visual Science, 45, 2368–2373. 10.1167/iovs.03-0981 [DOI] [PubMed] [Google Scholar]
- Zou, Y. , Xu, X. , & Chiou, G. C. (2006). Effect of interleukin‐1 blockers, CK112, and CK116 on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. Journal of Ocular Pharmacology and Therapeutics, 22, 19–25. 10.1089/jop.2006.22.19 [DOI] [PubMed] [Google Scholar]


