Abstract
Background
Ipomoea cairica (L.) Sweet is a destructive invasive weed in South China but rarely infected with pathogens in nature. Its pathogen resistance mechanism is largely unknown at present. Some non-pathogenic isolates of Fusarium oxysporum and Fusarium fujikuroi are prevalent on many plant species and function as pathogen resistance inducers of host plants. The objective of the present research is to investigate whether the symbiosis between the both fungi and I. cairica is present, and thereby induces pathogen resistance of I. cairica.
Methods
Through field investigation, we explored the occurrence rates of F. oxysporum and F. fujikuroi on leaf surfaces of I. cairica plants in natural habitats and compared their abundance between healthy leaves and leaves infected with Colletotrichum gloeosporioides, a natural pathogen. With artificial inoculation, we assessed their pathogenicity to I. cairica and studied their contribution of pathogen resistance to I. cairica against C. gloeosporioides.
Results
We found that F. oxysporum and F. fujikuroi were widely epiphytic on healthy leaf surfaces of I. cairica in sunny non-saline, shady non-saline and sunny saline habitats. Their occurrence rates reached up to 100%. Moreover, we found that the abundance of F. oxysporum and F. fujikuroi on leaves infected with C. gloeosporioides were significantly lower than that of healthy leaves. With artificial inoculation, we empirically confirmed that F. oxysporum and F. fujikuroi were non-pathogenic to I. cairica. It was interesting that colonization by F. fujikuroi, F. oxysporum alone and a mixture of both fungi resulted in a reduction of C. gloeosporioides infection to I. cairica accompanied by lower lesion area to leaf surface area ratio, increased hydrogen peroxide (H2O2) concentration and salicylic acid (SA) level relative to the control. However, NPR1 expression, chitinase and β-1,3-glucanase activities as well as stem length and biomass of I. cairica plant only could be significantly improved by F. oxysporum and a mixture of both fungi but not by F. fujikuroi. In addition, as compared to colonization by F. oxysporum and a mixture of both fungi, F. fujikuroi induced significantly higher jasmonic acid (JA) level but significantly lower β-1,3-glucanase activity in leaves of I. cairica plants. Thus, our findings indicated the symbiosis of epiphytic fungiF. fujikuroi and F. oxysporum induced systemic resistance of I. cairica against C. gloeosporioides. F. oxysporum played a dominant role in inducing pathogen resistance of I. cairica. Its presence alleviated the antagonism of the JA signaling on SA-dependent β-1,3-glucanase activity and enabled I. cairica plants to maintain relatively higher level of resistance against C. gloeosporioides.
Keywords: Symbiosis; Non-pathogen; NPR1 gene; Hormone; β-1,3-glucanase; Signaling molecules; Hydrogen peroxide
Introduction
Fungal epiphytes are a group of microbes which colonize the surface of the plants and establish various relationships with their hosts. These associations range from epiphytic commensals, mutualistic symbionts to pathogens (Kowalski et al., 2015). Fusarium oxysporum and Fusarium fujikuroi are polytypic species complex with anamorphs in Fusarium, which are prevalent on the leaf, stem, root, seed and inflorescence surfaces of many economically-important plants such as Ananas comosus (Dianese et al., 1981), Ipomoea batatas (Clark, Hoy & Nelson, 1995) and Oryza sativa (Choi et al., 2018). Some isolates within F. fujikuroi or F. oxysporum species can trigger gibberellin-induced bakanae disease of O. sativa (Hwang et al., 2013), pitch canker of Pinus spp. (Herron et al., 2015), stalk rot of Zea mays and Sorghum bicolor (Leslie, 1995), stem wilt and root rot of Schlumbergera truncata (Lops et al., 2013) and crown disease of oil palm (Hafizi, Salleh & Latiffah, 2013). However, it was reported that the isolates of F. fujikuroi and F. oxysporum were entirely nonpathogenic and avirulent to their hosts, such as A. comosus (Dianese et al., 1981), O. sativa (Choi et al., 2018; Amatulli et al., 2010) and Glycine max (Lanubile et al., 2015). It is known that many pathogenic and nonpathogenic F. oxysporum or F. fujikuroi isolates elicited the systemic acquired resistance (SAR) or induced systemic resistance (ISR) of their plant hosts to confer resistance against a broad spectrum of pathogens (Paparu et al., 2007; Patil et al., 2011; Veloso & Díaz, 2012; Matić et al., 2016; Miyaji et al., 2017). These Fusarium spp. studied earlier are plant endophytes or soil-borne fungi. Epiphytic F. oxysporum or F. fujikuroi involving ISR and SAR of plants to date is little known. Pathogen resistance is induced through the accumulation of salicylic acid (SA) or jasmonic acid (JA) (Mandal, Mallick & Mitra, 2009; Chen et al., 2018; Jin et al., 2018) and the expression of non-expressor of pathogenesis-related genes-1 (NPR1) as well as pathogenesis-related (PR) proteins (Stein et al., 2008; Nic-Matos et al., 2017; Ali et al., 2017). Cytosolic hydrolytic enzymes such as β-1,3-glucanases and chitinases are members of PR1 proteins (Fagoaga et al., 2001; Park et al., 2004), and exert inhibitory effects on the fungal growth through degrading chitin and glucan in the cell wall of pathogenic fungi (Balasubramanian et al., 2012; Vieira et al., 2010). In addition, the accumulation of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) is frequently involved in the defense responses, which may kill pathogens directly (Lin & Ishii, 2009).
Ipomoea cairica (L.) Sweet is native to tropical Africa and is causing a serious invasive ecological problem in South China (Huang et al., 2009). This weed usually occurs in non-arable lands, wastelands, forests edges and farmlands where it invades the original diverse community which is always reduced to a monoculture (Li et al., 2012). Owing to its strong salt tolerance, I. cairica has been found to have successfully invaded into mangrove wetland in the coastal areas (Liu et al., 2012; Liu et al., 2016) and seriously threatened local eco-systematic functions. In addition, I. cairica has strong pathogen resistance and is scarcely infected with pathogens in nature. Only a disease symptom caused by Colletotrichum gloeosporioides was observed sporadically on I. cairica plants in previous field investigations (Lin & Liu, 2010). Although several herbicides have shown significant efficacies in controlling I. cairica, their application may lead to environmental pollution (Sun et al., 2015). Thus, the use of biological control is an attractive option. To help with the biological control of I. cairica, it is important to understand its mechanisms of pathogen resistance which to date remain relatively unknown.
Considering the omnipresence of F. oxysporum and F. fujikuroi on the surface of plants in natural surroundings and their roles in inducing plant pathogen resistance, we hypothesized that the occurrence of F. oxysporum or F. fujikuroi on leaves might be involved in the antagonistic character of I. cairica against plant pathogen. The objectives of the present study were therefore to elucidate the following questions: (i) whether F. oxysporum or F. fujikuroi widely occur on the leaf surface of I. cairica in the field; (ii) whether there is different abundance of F. oxysporum or F. fujikuroi between leaves infected with C. gloeosporioides and healthy leaves; (iii) if the former two questions are positive then whether F. oxysporum or F. fujikuroi can induce the pathogen resistance of I. cairica against C. gloeosporioides.
Materials & Methods
The occurrence rate and abundance of F. oxysporum and F. fujikuroi on leaves of I. cairica in the field
To investigate the occurrence of epiphytic F. oxysporum and F. fujikuroi, sunny saline, sunny non-saline and shady non-saline habitats with I. cairica were selected for leaf sampling. The saline habitat was located in intertidal zones of Yakou village, Zhongshan city (22°28′1.03″N, 113°32′42.56″E). The non-saline habitat was located in Huitong village, Zhuhai city (22°21′26.60″N, 113°30′46.18″E). The linear distance between the saline and non-saline habitats is about 20 km. In each habitat, five I. cairica populations covering more than 50 m2 were selected as sample plots. Distances between sample plots are more than 500 m. In each sample plot, three sample sites covering about 4 m2 were selected randomly. In each sample site, three healthy leaves of I. cairica were excised and pooled together as a sample, then stored in a sterile plastic Ziploc® (Racine, WI) bag and returned to the laboratory. A total of 45 samples (3 habitats ×5 sample plots ×3 sample sites) were used for analyzing the occurrence rate of F. oxysporum and F. fujikuroi.
Fungal epiphytes were isolated from the leaves of I. cairica following the method of Salazar-Cerezo et al. (2018). Briefly, each sample was dipped into 75 ml of sterile distilled water contained in a 250 ml sterile conical flask. The conical flask was set on a shaker (IKA, Staufen, Germany) at 170 rpm for 60 min. The resulting suspension (200 µl) was plated onto sterile potato dextrose agar (PDA) contained in a sterile Petri dish. The plating was performed in triplicate. Control treatments only contained sterile water and PDA. All plates were incubated in dark at 26 °C for 15 days. Plates were checked daily and each emerging fungal colony was transferred onto a fresh PDA until axenic cultures were obtained. These cultures were classified into diverse morphotypes according to color, texture and colony morphology, and were used for species identification. The value 1 and 0 indicated the presence or absence of F. oxysporum and F. fujikuroi, respectively on each sample. These values were used to assess the occurrence of F. oxysporum and F. fujikuroi.
In addition, the abundance of F. oxysporum and F. fujikuroi on I. cairica in the field was compared between healthy leaves and leaves infected with C. gloeosporioides. In Huitong village, Zhuhai city, three sample plots covering more than 100 m2 were selected for leaf sampling. Six healthy leaves and equal number of infected leaves of I. cairica were collected from each sample plot and separately stored in a sterile plastic Ziploc® bag as a healthy and infected sample, then returned to the laboratory for further analyses. After weighing of each sample, fungal isolation, purification and morphotype classification were performed using the above methods. The colony-forming units (CFU) of F. oxysporum and F. fujikuroi were recorded. The abundance of F. oxysporum and F. fujikuroi was expressed as CFU per gram fresh weight (FW).
Fungal identification
The internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA (rDNA) have been successfully used in resolving phylogenetic relationships of the fungi in the genera Fusarium (Lin et al., 2014) and Colletotrichum (Weir, Johnston & Damm, 2012). Combining with morphological characteristics, these fungi can be identified well to species level (Kvas et al., 2009; Lin et al., 2014; Rabha et al., 2016; Moya-Elizondo et al., 2019). In this work, the identification of F. fujikuroi and F. oxysporum along with a natural pathogen of I. cairica, C. gloeosporioides was performed with a combined method of molecular and morphological analyses. C. gloeosporioides was isolated directly from leaves of I. cairica infected with C. gloeosporioides in the field. Briefly, a leaf spot was excised from infected leaf and transferred on sterile PDA contained in a sterile Petri dish, and then incubated in dark at 26 °C. Subsequent process of fungal purification was performed using the above method. According to the differentiation of colony morphology, the cultures of these three fungi were divided into three morphotypes.
Three representative isolates (JY1, JY2 and JY3) were selected from their respective related morphotypes, and were used for analyzing the ITS sequences. Genomic DNA was extracted from 0.1 g of mycelia using CTAB method (Séne et al., 2015). Following the methods of Lin et al. (2014) and Rabha et al. (2016), PCR amplification of the ITS region was performed using fungus-specific primers ITS1-F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4-R (5′-TCCTCCGCTTATTGATATGC-3′). PCR amplification reactions were conducted with 10 ng of template DNA, 0.5 µM of each primer, 17.5 µl of the Premix Taq (TaKaRa, Dalian, China) and double distilled water (ddH2O) in a final volume of 35 µl. The program used for PCR was as follows: 95 °C for 5 min (1 cycle); 95 °C for 30 s, 51.6 °C for 45 s, 72 °C for 90 s (30 cycles); 72 °C for 7 min (1 cycle). Amplification products were sequenced using the services provided by Sangon Biotech Co., Ltd. (Shanghai, China). These sequences were submitted to GenBank under accession numbers MN704851.1, MN704852.1 and MN704853.1, and were compared against those sequences published in GenBank using the BLAST search program (http://www.ncbi.nlm.nih.gov/BLAST). The sequences of related fungi were obtained from GenBank as follows: Colletotrichum siamense (MN296060.1, MN296066.1 and KP635210.1), C. gloeosporioides (JQ936115.1, MF314168.1 and MH930419.1), F. oxysporum (FJ867936.1, KY305290.1 and MK156682.1) and F. fujikuroi (KT192276.1, KP998524.1 and LS422781.1). All of sequences were aligned with using CLUSTAL W (Thompson, Higgins & Gibson, 1994) present in MEGA 5 software (Tamura et al., 2011). Aligned sequences were used to construct phylogenetic tree using the neighbor-joining (NJ) and Kimura 2-parameter methods (Saitou & Nei, 1987; Kimura, 1980). Bootstrap resampling (1,000 replications) was used as a statistical support for nodes in the phylogenetic tree. Penicillium oxalicum (MH634489.1) was used as an outgroup.
Fourteen days old fungal cultures were used for morphological identification of F. fujikuroi and F. oxysporum according to the Fusarium Laboratory Manual (Leslie & Summerell, 2006). Morphological analysis of C. gloeosporioides was performed following the method of Rabha et al. (2016). Morphological characteristics including conidial length, width and septation were measured in a photonic microscope (Nikon, Tokyo, Japan).
Pathogenicity identification of F. oxysporum and F. fujikuroi
In order to identify the pathogenicity of F. oxysporum and F. fujikuroi to I. cairica, with artificial inoculation, the lesion areas caused by the two fungi were compared with a positive control and a negative control. The positive control was inoculated with C. gloeosporioides. The negative control was sprayed with sterile potato dextrose broth (Huankai Co., Ltd., Guangzhou, China).
Plant materials
Two hundred cuttings (10 cm length, three mm diameter) each with two healthy leaves were clipped from an I. cairica population in the field in Huitong village, Zhuhai city. These cuttings were cultivated with sterile Hoagland nutrient solution for a week.
Fungi materials
Fungal isolates identified as F. oxysporum, F. fujikuroi and C. gloeosporioides in the previous experiments were subcultivated on fresh PDA and used as experiment materials. Twenty days old cultures of F. oxysporum, F. fujikuroi and C. gloeosporioides were used for preparation of their respective conidial suspensions. Briefly, the mycelia were transferred to 200 ml of sterile potato dextrose broth contained in a 250 ml sterile conical flask, which was then sealed with parafilm, shaken repeatedly and incubated in dark at 26 °C. After 24 h, the fungal suspension was filtered with three layers of sterile gauze to obtain the conidial suspension. Conidial concentration of each fungal species was determined using hemocytometer (Shanghai Medical Optical Instrument Plant, Shanghai, China) and photonic microscope, then adjusted to 1 × 107 ml−1 with sterile potato dextrose liquid broth according to the methods of Matić et al. (2016) and Aimé et al. (2008) with some modification.
Pathogenicity assessment
Thirty two cuttings of I. cairica were selected for assessing pathogenicities of F. oxysporum, F. fujikuroi and C. gloeosporioides. The leaf surface was sterilized by cleaning twice with the degreased cotton immersed by 75% (v/v) ethanol. These cuttings were divided evenly into four groups including three treatments and one control. Thus, each treatment was repeated eight times. One hour later, the three treatments were inoculated with conidial suspension of F. oxysporum, F. fujikuroi and C. gloeosporioides, respectively. Inoculation volume of conidial suspension on each leaf was 2.5 ml. Leaves in the control group were sprayed with an equal volume of sterile potato dextrose broth. All inoculated cuttings were then cultivated with sterile Hoagland nutrient solution in sterile illuminating incubator at 28 ± 1 °C with a 14/10 h photoperiod (cool- white neon tube (200 µmol m−2 s−1). The relative humidity in the illuminating incubator was maintained at 75%–80%. After 7 days, all cuttings were harvested, and then the inoculated leaves of each cutting were photographed with a digital camera. Total number of pixel of lesions and pixels of the whole leaf were measured with Adobe Photoshop CS6 software (Adobe System Inc., San Jose, CA, USA). The lesion area ratio was calculated as the % of the whole leaf area.
Plant pathogen resistance induced by F. oxysporum, F. fujikuroi alone and in a mixture
One hundred and twenty cuttings of I. cairica were divided evenly into four groups including three treatments and one control. The leaf surface was sterilized as above. One hour later, three treatments were pre-inoculated with the conidial suspension of F. oxysporum, F. fujikuroi alone or a mixture of both fungi, respectively. The conidial suspension of the fungal mixture of the two species was prepared with an equal volume of conidial suspension of F. oxysporum and F. fujikuroi. Pre-inoculation volume of conidial suspension on each leaf was 2.5 ml. The leaves in the control group were sprayed with an equal volume of sterile potato dextrose broth. All cuttings were then cultivated with sterile Hoagland nutrient solution in sterile illuminating incubator at 28 ± 1 °C with a 14/10 h photoperiod (cool- white neon tube (200 µmol m−2 s−1). The relative humidity in the illuminating incubator was maintained at 75%–80%. After 3 days, these cuttings were removed and inoculated with 2.5 ml of conidial suspension of C. gloeosporioides per leaf, then returned to illuminating incubator. After 15 days, these cuttings were harvested.
Growth parameter determination: Stem length, biomass and lesion area ratio
The stem length of five random replicates was measured from the harvested cuttings of each treatment and the control group. Each cutting was then clipped into small pieces and dried at 75 °C in a drying oven for 10 h to weigh the biomass. Five random replicates of harvested cuttings from each treatment and the control were sampled to determine the lesion area ratio according to the method described above.
Physiological characteristics measurement
Four random replicates of the harvested cuttings in each treatment and the control group were sampled to determine H2O2 concentration, β-1,3-glucanase and chitinase activity. The leaf was excised from each cutting of I. cairica, deveined and stored at −80 °C.
H2O2 was extracted according to the method of Ferguson, Watkins & Harman (1983). The deveined leaf (0.2 g FW) was homogenized in 5 ml cold acetone in a mortal. The extract and washings were centrifuged (4,000 rpm) at 4 °C for 10 min. The supernatant was used to measure H2O2 concentration by modification of the method of Brennan & Frenkel (1977). One milliliter of the supernatant was added to 250 µl of 50 mg ml−1 Ti(SO4)2 in concentrated H2SO4. The solution was shaken, followed by the addition of 2 ml concentrated NH4OH and thoroughly mixed. After centrifugation (20 min at 4,000 rpm), the supernatant was discarded and the precipitate washed repeatedly with 4 ml acetone until the supernatant was colorless. The precipitate was solubilized in 4 ml 2 NH2SO4. The absorbance of the obtained solutions was recorded at 415 nm against a water blank. The concentration of H2O2 in the extracts was determined by comparing the absorbance against a standard curve representing 0–80 µmol ml−1H2O2.
The extraction of β-1,3-glucanase and chitinase was based on the method of Magnin Robert et al. (2007). Deveined leaf (0.2 g FW) was homogenized in 5 ml cold sodium acetate buffer, PH 5.0 containing one mmol dithiothreitol and 10 mg phenylmethysulfonyl fluoride in a cold mortal. The crude extracts were centrifuged at 4,000 rpm for 50 min at 4 °C and supernatants were used in enzymatic activity assays.
β-1,3-glucanase activity was measured according to the method of De la Cruz et al. (1995). The reaction was started by mixing 200 µl of the supernatant and 200 µl of laminarin (1 mg ml−1). The mixture was incubated at 37 °C for 30 min, followed by the addition of 2 ml of dinitrosalicylic acid (DNS) reagent (Sangon Biotech Co., Ltd., Shanghai, China), then boiled for 5 min. Enzyme and substrate blank were also included. The absorbance of the obtained solution was recorded at 600 nm. A standard curve was established with 0 to 80 mg ml−1 glucose. A unit of β-1,3-glucanase activity was defined as the amount of enzyme catalyzing the release of 1 µmol of glucose equivalent per minute.
Chitinase activity was measured according to the method of Chen & Lee (1994). The mixture containing 400 µl of the supernatant and 400 µl of colloidal chitin (10 mg ml−1) was incubated at 37 °C for 1 h, followed by the addition of 1.5 ml of DNS reagent, then boiled for 5 min. Enzyme and substrate blank were also included. The absorbance of the obtained solution was recorded at 530 nm. A standard curve was established with 0–1 mg ml−1 N-acetylglucosamine (NAG). A unit of chitinase activity was defined as the amount of enzyme catalyzing the release of 0.5 µmol of NAG equivalent per hour.
Hormone measurement
Nine harvested cuttings in each treatment and the control group were sampled to determine SA and JA. Leaves of three cuttings in each treatment or the control group were cut into pieces and pooled together as a sample, stored at −80 °C. Thus, each treatment was repeated 3 times.
SA and JA were extracted following the method of Engelberth et al. (2003), with some modification. Frozen leaves (1.0 g) in each sample were weighed and ground in liquid nitrogen to a fine powder. Extraction was done by adding 10 ml of methanol and transferring the mixture to a 50 ml centrifuge tube, then set on a shaker at 300 rpm for 2 h. After centrifugation at 4,000 rpm for 5 min, the supernatant was transferred to another centrifuge tube and concentrated under a flow of nitrogen gas. The residue was reconstituted with 1 ml of methanol, then was filtered through a 0.2- µm-Teflon filter into an autosampler vials.
According to the method of Ratzinger et al. (2009), with some modification, an AB Sciex Qtrap® 5500 LC/MS/MS system (AB Sciex, Foster City, CA, USA) with multiple reaction monitoring mode was used to quantify SA and JA. The sample was injected onto a reverse-phase column PAK C18-ARC (150 × 2.0 mm, 3 µm, Shiseido, Tokyo, Japan) kept at 25 °C and eluted isocratically with the mobile phase consisting of 5 mM ammonium acetate (mobile phase A) and acetonitrile (mobile phase B) at a flow rate of 0.3 ml min−1. The injection volume was 0.2 µl. The eluate was subjected to positive electrospray ionization, and the ions were detected using the following mass transitions: SA m/z 137.0 → m/z 93; JA m/z 209.0 → m/z 59.0.
The external standard working fluids for calibration curves were established with 2–100 ng ml−1 of SA and JA in methanol. The standards of SA and JA were purchased from ZZBIO Co., Ltd (Shanghai, China).
Real-time RT-PCR analysis of NPR1 expression
Three replicates of the harvested cuttings in each treatment and the control group were sampled to analyze NPR1 expression. Total RNA was extracted from leaves using Total RNA Purification Reagent Kit (Sangon Biotech Co., Ltd., Shanghai, China) according to manufacturer’s instructions. First strand cDNA was synthesized from 1 µg of total RNA using Reverse Transcription System (DaAn Gene Co., Ltd., Guangzhou, China) according to the manufacturer’s instructions. Following the method of Aimé et al. (2008), the actin gene was used as a reference gene. Based on NPR1 (accession numbers: EF190039.1, XM_019312052.1, XM_019317156.1 and XM_019317920.1) and actin gene (accession numbers: AY905538.1, GU395493.1, XM_019297139.1 and D78205.1) mRNA sequences of homogenous species Ipomoea nil and Ipomoea batatas deposited in GenBank, NPR1 primers (5′-CTTCAGGAGCGTATTTAGTGG-3′and 5′- AAAACAGTCACTACGGCATCA-3′) and actin gene primers (5′- GCGGATAGAATGAGCAAGG-3′and 5′- GAGCCTCCAATCCAGACAC-3′) of I. cairica were designed respectively by Primer3 software (http://fokker.wi.mit.edu/primer3/input.htm).
Real-time PCR reactions were conducted with 10 ng of cDNA, 200 nM of each primer, 10 µl of the SYBR green master mix (TaKaRa, Dalian, China) and double distilled water (ddH2O) in a final volume of 20 µl. In the negative control, cDNA was replaced by ddH2O. Reactions were performed on an ABI PRISM 7500HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The program used for real-time PCR was as follow: 10 s at 95 °C, 45 cycles of 5 s at 95 °C, 30 s at 53 °C and 34 s at 72 °C. Two replicates of real-time PCR reactions were performed for each sample.
The melting curve analysis was performed to verify the sensitivity and specificity of primers. After the real-time PCR finished, Ct number was extracted for both actin gene and NPR1 gene with auto baseline and manual threshold. The relative expression of NPR1 gene and actin gene were calculated according to the 2−ΔCt method provided by Schmittgen & Livak (2008). Δ Ct = CtNPR1 − Ctactin.
Data analyses
Statistical analyses were performed on SPSS 16.0 software (IBM, Chicago, IL, USA) using one-way analysis of variance (ANOVA) followed by LSD’s post-hoc test. The values were expressed as the means ± standard errors and P values < 0.05 were considered statistically significant.
Results
The identification of representative isolates JY1, JY2 and JY3
The total size of ITS regions of JY1, JY2 and JY3 were 561, 557 and 588 bp, respectively. The phylogenetic analysis showed that the ITS sequences of JY1 and F. fujikuroi were clustered into the same group with 98% of bootstraps (Fig. 1). The ITS sequences of JY2 and JY3 were respectively clustered together with that of F. oxysporum and C. gloeosporioides into the same groups with 100% of bootstraps (Fig. 1). Morphologically, JY1 produced abundant orange sporodochia, the colony color was white to orange on PDA (Fig. 2A). The macroconidia were sparse, hyaline, sickle-shaped, with two to three septations, and measured 22.7 to 40.8 × 3.1 to 4.1 µm (n = 20). Microconidia were formed in chains or scattered, hyaline, aseptate or one septate. They were generally slender, clavate with a flattened base, and measured 5.2 to 15.0 × 1.5 to 3.8 (n = 20). Colony color of JY2 was white to purple on PDA (Fig. 2B). The macroconidia were sparse, hyaline, slightly sickle-shaped, with two septations, and measured 30.2 to 42.5 × 3.4 to 4.5 µm (n = 20). Microconidia were abundant, hyaline, aseptate, and formed abundantly in false heads. They were generally oval-ellipsoid to kidney-shaped and measured 5.2 to 15.0 × 1.5 to 3.8 (n = 20). Based on the morphological criteria presented in the Fusarium Laboratory Manual (Leslie & Summerell, 2006) and phylogenetic analysis (Fig. 1), JY1 and JY2 were identified as F. fujikuroi and F. oxysporum, respectively. Colony color of JY3 was white to pale grey with orange conidial masses near the inoculums point (Fig. 2C). The conidia were abundant and cylinder-shaped, and measured 9.0 to 14.7 × 3.1 to 4.1 µm (n = 20). These morphological characteristics were highly in agreement with a previous description of C. gloeosporioides (Rabha et al., 2016). Combining the morphological with phylogenetic analyses (Fig. 1), JY3 was identified as C. gloeosporioides.
Figure 1. Phylogenetic tree of JY1, JY2 and JY3 as well as reference isolates.
JY1, JY2 and JY3 were representative isolates used for identifying F. fujikuroi, F. oxysporum and C. gloeosporioides. The analysis involved 16 internal transcribed spacer sequences of nuclear ribosomal DNA. Penicillium oxalicum (MH634489.1) was used as an outgroup. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The optimal tree with the sum of branch length = 0.57566860 is shown.
Figure 2. Fungal colonies of JY1, JY2 and JY3 on PDA.
JY1 (A), JY2 (B) and JY3 (C) were representative isolates used for identifying F. fujikuroi, F. oxysporum and C. gloeosporioides. PDA, Potato dextrose agar.
Occurrence rate of F. fujikuroi and F. oxysporum in the field
In the field, the occurrence rates of F. fujikuroi and F. oxysporum on the surfaces of healthy leaves did not vary amongst habitats occupied by I. cairica. F. fujikuroi and F. oxysporum always coexisted, and their occurrence rates were 100 ±0.00% on I. cairica.
Comparison of F. oxysporum and F. fujikuroi abundance between infected and healthy leaves of I. cairica in the field
In the field, the abundance of F. oxysporum (n = 3, P = 0.000) and F. fujikuroi (n = 3, P = 0.000) on the surfaces of healthy leaves of I. cairica was significantly higher than that of C. gloeosporioides infected leaves, respectively (Fig. 3).
Figure 3. Comparison of F. oxysporum and F. fujikuroi abundance between infected and healthy leaves of I. cairica.
The leaf infected naturally by C. gloeosporioides in the field was defined as infected leaf. Healthy leaf had no any disease symptom. Each value is the mean ± standard error of three replicates. Error bars indicate standard errors. Different letters above error bars indicate significant difference (P < 0.05) as determined by LSD test. CFU, colony-forming units; FW, fresh weight.
The pathogenicity of F. oxysporum and F. fujikuroi to I. cairica
Inoculation with F. oxysporum and F. fujikuroi did not cause lesions on the leaves of I. cairica, whereas inoculation with C. gloeosporioides led to obvious infection symptom. The lesion area ratio caused by C. gloeosporioides was 2.11 ± 0.48% and significantly higher than that of inoculation with F. oxysporum (n = 8, P = 0.000) and F. fujikuroi (n = 8, P = 0.000) in addition to the negative control (n = 8, P = 0.000). The results showed that F. oxysporum and F. fujikuroi were non-pathogens of I. cairica.
Effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on growth parameters of I. cairica infected with C. gloeosporioides
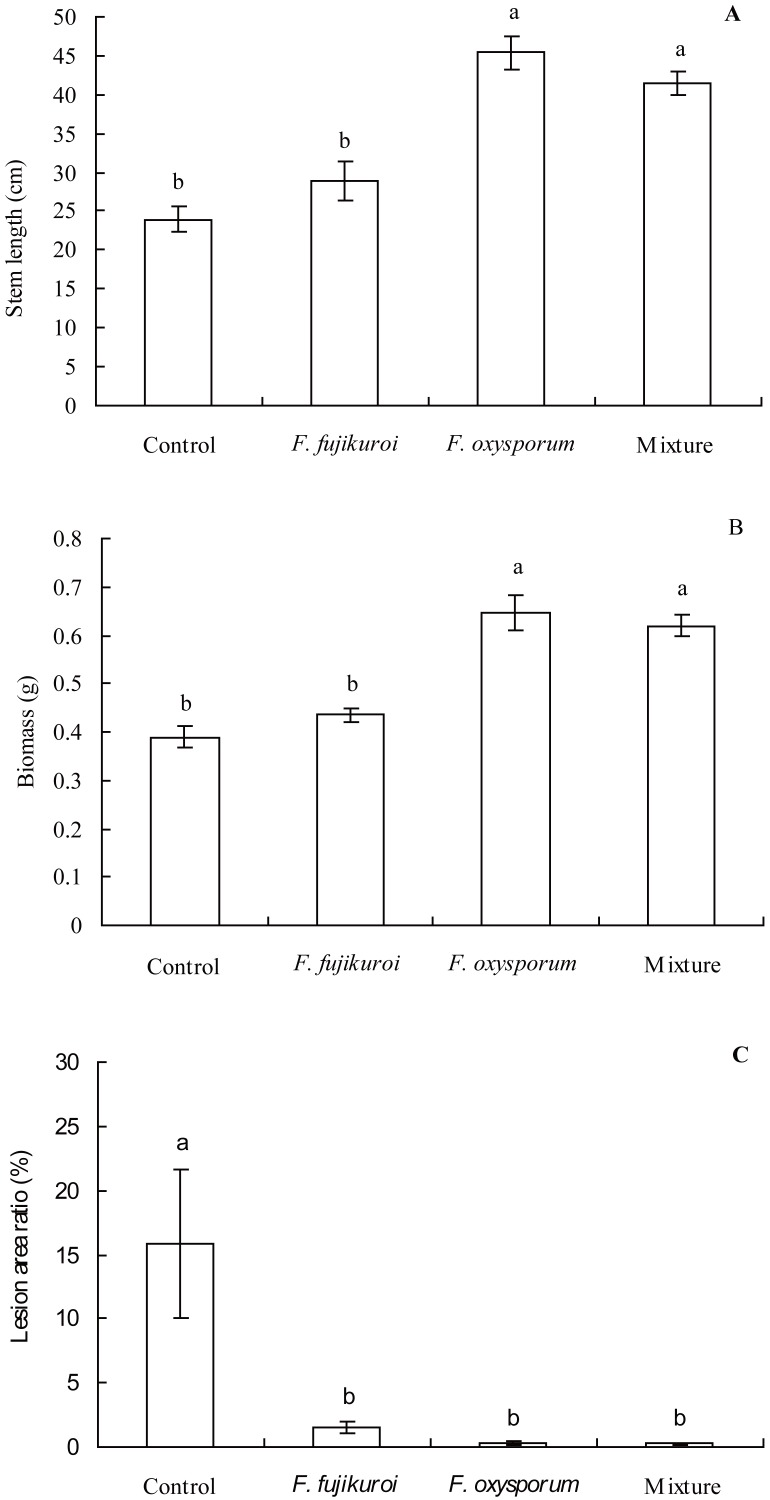
The growth of I. cairica within treatment and control groups responded differentially to the infection of C. gloeosporioides (Fig. 4). There were significant differences in the leaf lesion area ratio (df = 3, 16, F = 6.863, P = 0.003; Fig. 5C), stem length (df = 3, 16, F = 25.633, P = 0.000; Fig. 5A) and biomass (df = 3, 16, F = 25.129, P = 0.000; Fig. 5B) of I. cairica within treatment and control groups. Compared to the control, pre-inoculation with F. oxysporum (n = 5, P = 0.02), F. fujikuroi (n = 5, P = 0.03) alone and in a mixture (n = 5, P = 0.02) significantly reduced leaf lesion area ratio of I. cairica plants caused by C. gloeosporioides (Fig. 5C). Moreover, pre-inoculation with F. oxysporum and mixture of both fungi significantly increased the stem length (F. oxysporum: n = 5, P = 0.000; mixture of both fungi: n = 5, P = 0.000; Fig. 5A) and biomass (F. oxysporum: n = 5, P = 0.000; mixture of both fungi: n = 5, P = 0.000; Fig. 5B) of I. cairica plants. However, the two growth parameters were not promoted by pre-inoculation with F. fujikuroi (biomass: n = 5, P = 0.220, Fig. 5B; stem length: n = 5, P = 0.103, Fig. 5A), and significantly lower than that of I. cairica plants inoculated with F. oxysporum (biomass: n = 5, P = 0.000, Fig. 5B; stem length: n = 5, P = 0.000, Fig. 5A) and mixture of both fungi (biomass: n = 5, P = 0.000, Fig. 5B; stem length: n = 5, P = 0.000, Fig. 5A).
Figure 4. Growth responses of I. cairica inoculated with F. oxysporum, F. fujikuroi alone and a mixture of both to the infectionof C. gloeosporioides.

The control group was sprayed with sterile potato dextrose broth.
Figure 5. Effects of pre-inoculation with F. oxysporum,F. fujikuroi alone and a mixture of both on growth parameters of I. cairica infected with C. gloeosporioides.
(A) Stem length. (B) Biomass. (C) Lesion area ratio. Each value is the mean ± standard error of five replicates per treatment. Error bars indicate standard errors. Different letters above error bars indicate significant difference (P < 0.05) as determined by LSD test.
Effects of pre-inoculation with F. oxysporum,F. fujikuroialone and a mixture of both on physiological characteristics ofI. cairicainfected withC. gloeosporioides
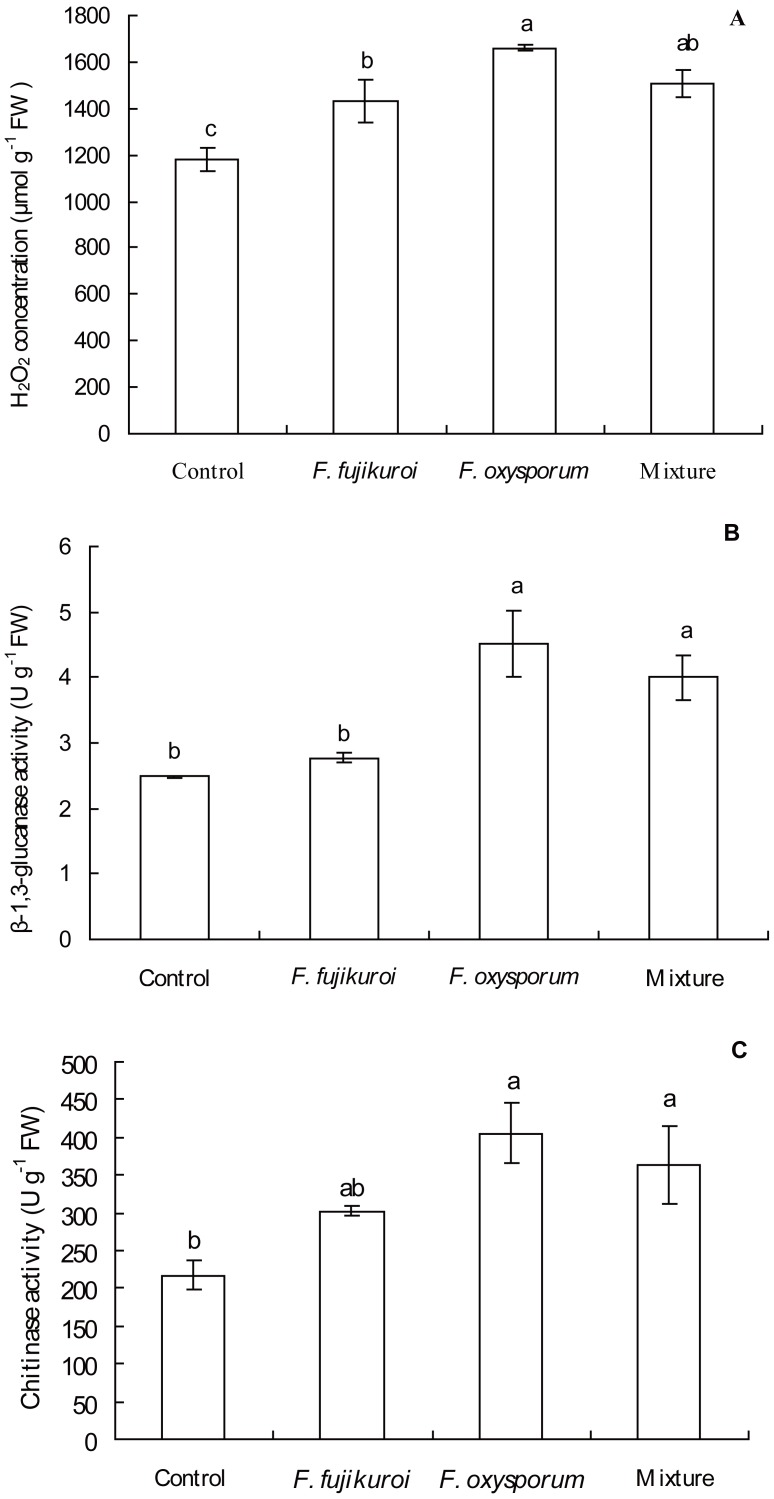
There were significant differences in H2O2 concentration (df = 3, 12, F = 11.025, P = 0.001; Fig. 6A), β-1,3-glucanase (df = 3, 12, F = 10.318, P = 0.001; Fig. 6B) and chitinase activities (df = 3, 12, F = 5.650, P = 0.012; Fig. 6C) in leaves of I. cairica within treatment and control groups. F. oxysporum (n = 4, P = 0.000), F. fujikuroi (n = 4, P = 0.011) alone and as a mixture (n = 4, P = 0.002) significantly increased H2O2 concentration in leaves of I. cairica plants infected with C. gloeosporioides, compared with the control (Fig. 6A). Furthermore, pre-inoculation with F. oxysporum and mixture of both fungi significantly improved β-1,3-glucanase (F. oxysporum: n = 4, P = 0.000; mixture of both fungi: n = 4, P = 0.004; Fig. 6B) and chitinase activities (F. oxysporum: n = 4, P = 0.002; mixture of both fungi: n = 4, P = 0.011; Fig. 6C) in leaves, whereas pre-inoculation with F. fujikuroi did not enhance the two enzymes activities (β-1,3-glucanase activity: n = 4, P = 0.511, Fig. 6B; chitinase activity: n = 4, P = 0.106, Fig. 6C). β-1,3-glucanase activity (Fig. 6B) in leaves pre-inoculated with F. fujikuroi was significantly lower than that in leaves pre-inoculated with F. oxysporum (n = 4, P = 0.002) and mixture of both fungi (n = 4, P = 0.014).
Figure 6. Effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on physiological characteristics of I. cairica infected with C. gloeosporioides.
(A) Hydrogen peroxide (H2O2) concentration. (B) β-1,3-glucanase activity. (C) Chitinase activity. Each value is the mean ± standard error of four replicates per treatment. Error bars indicate standard errors. Different letters above error bars indicate significant difference (P < 0.05) as determined by LSD test. FW: Fresh weight.
Effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on hormone content and NPR1 expression in leaves of I. cairica infected with C. gloeosporioides
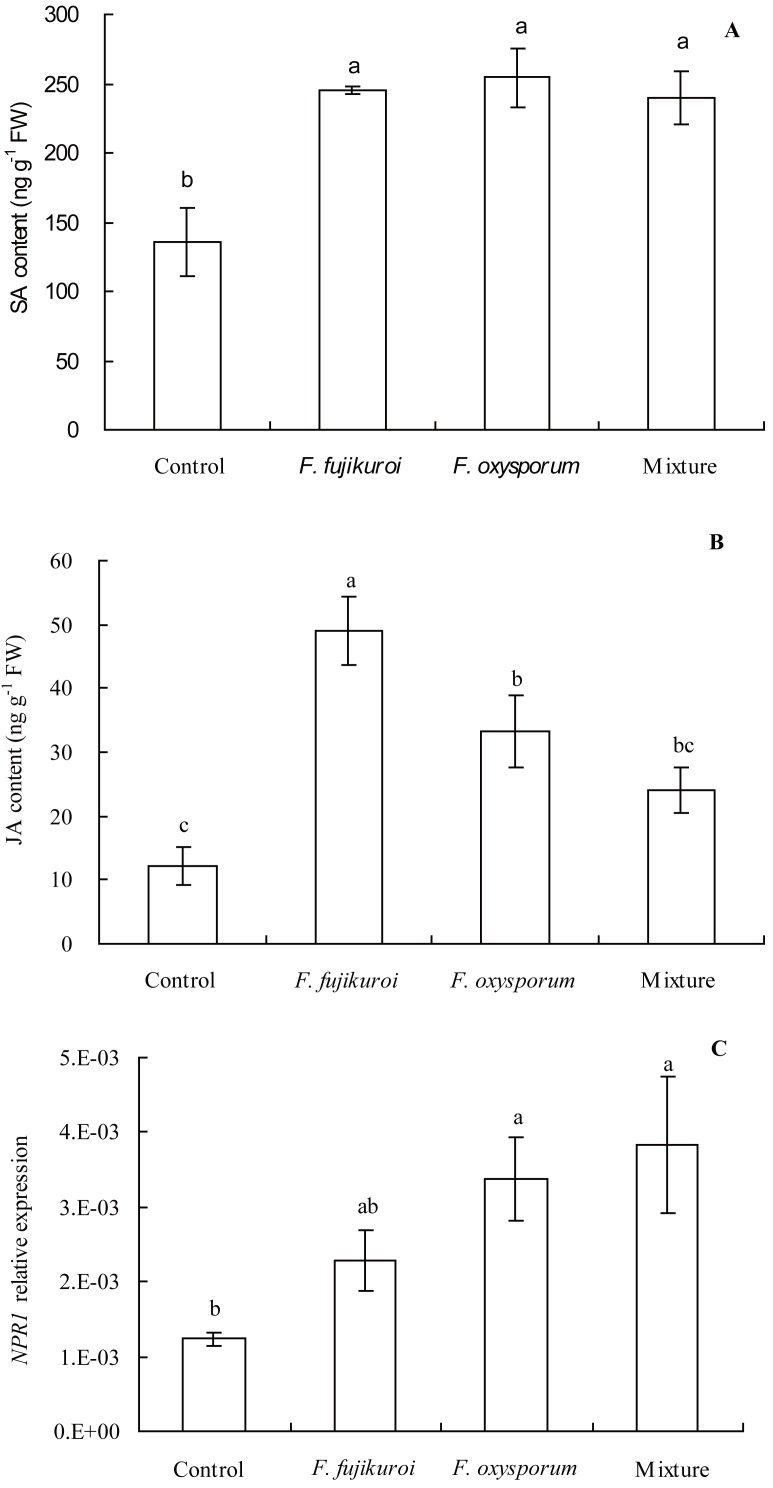
There were significant differences in NPR1 expression (df = 3, 8, F = 4.112, P = 0.049; Fig. 7C), SA (df = 3, 8, F = 8.637, P = 0.007; Fig. 7A) and JA content (df = 3, 8, F = 11.751, P = 0.003; Fig. 7B) in leaves of I. cairica within treatment and control groups. In contrast with the control, pre-inoculation with F. oxysporum (n = 3, P = 0.002), F. fujikuroi (n = 3, P = 0.004) alone and a mixture of both (n = 3, P = 0.005) significantly elevated SA content in leaves of I. cairica plants infected with C. gloeosporioides (Fig. 7A). Moreover, except for pre-inoculation with mixture, pre-inoculation with F. oxysporum (n = 3, P = 0.011) and F. fujikuroi (n = 3, P = 0.000) significantly enhanced JA content in leaves (Fig. 7B). JA content in leaves pre-inoculated with F. fujikuroi was significantly higher than that in leaves pre-inoculated with F. oxysporum (n = 3, P = 0.038) and mixture of both fungi (n = 3, P = 0.005). Furthermore, in contrast with the control, pre-inoculation with F. oxysporum (n = 3, P = 0.030) or a mixture of both fungi (n = 3, P = 0.012) significantly increased NPR1 expression of leaves infected with C. gloeosporioides (Fig. 7C). However, there was no significant upgrade following pre-inoculation with F. fujikuroi in NPR1 expression (n = 3, P = 0.232, Fig. 7C).
Figure 7. Effects of pre-inoculation with F. oxysporum F. fujikuroi alone and a mixture of both on hormone content and NPR1 expression in leaves of I. cairica infected with C. gloeosporioides.
(A) Salicylic acid (SA) content. (B) Jasmonic acid (JA) content. (C) Non-expressor of pathogenesis-related genes-1 (NPR1) expression. Each value is the mean ± standard error of three replicates per treatment. Error bars indicate standard errors. Different letters above error bars indicate significant difference (P < 0.05) as determined by LSD test. FW, fresh weight.
Discussion
The occurrence, abundance and pathogen resistance attributes of F. oxysporumandF. fujikuroi
Symbiosis between plants and microbes is a very common ecological relationship. Host plants obtain diverse benefits from the symbiosis involving the improvement of nutrition availability (Ngwene et al., 2016;Bertolazi et al., 2019), yields (Xia et al., 2016) and tolerance against abiotic as well as biotic stresses (Daneshkhah, Grundler & Wieczorek, 2018; Song et al., 2015). In this study, the occurrence rates of F. oxysporum and F. fujikuroi on leaf surface of I. cairica reached up to 100% regardless of habitat. The results indicated that the symbiosis between F. oxysporum, F. fujikuroi and I. cairica was established naturally in habitats and was considerably stable. Moreover, with artificial inoculation, we found that F. oxysporum and F. fujikuroi were not pathogenic to I. cairica, conversely, they enhanced pathogen resistance of I. cairica against C. gloeosporioides and significantly reduced lesion area ratio of leaves (Fig. 5C). Previous studies have shown that pathogen resistance of plants can be induced by F. oxysporum and F. fujikuroi (Patil et al., 2011; Veloso & Díaz, 2012). Interestingly, under natural conditions, the abundance of F. oxysporum and F. fujikuroi on healthy leaves was significantly higher than that of C. gloeosporioides infected leaves (Fig. 3). The results further suggested that the health of I. cairica plants was relevant to high abundance of symbiotic F. oxysporum and F. fujikuroi. Since I. cairica first invaded Hong Kong as an exotic species in 1912 (Yuan et al., 2019), it has experienced an invasive history spanning 100 years in China. Saikkonen et al. (2016) reported that symbiosis was the outcome of long-term co-evolution between microbes and host plants. To our knowledge, the symbiosis between microbes and I. cairica was first reported in the present study. Thus, it is not clear whether the symbiosis between F. oxysporum, F. fujikuroi and I. cairica is inherent in its native location or established afterwards via co-evolution in the invasive regions. We believe that, by investigating the presence or absence of F. oxysporum and F. fujikuroi on I. cairica in its native locations, the origin of their symbiosis can be better understood. If I. cairica in its native locations harbors F. oxysporum and F. fujikuroi, we can conclude that their symbiosis is inherent. If not, then it is likely that their symbiosis is established in invasive regions of I. cairica. Nevertheless, our findings suggested that the symbiosis had important ecological significance in alleviating the pathogen pressure of C. gloeosporioides imposed on I. cairica in nature.
Physiological mechanism of pathogen resistance induced by F. oxysporumandF. fujikuroi
H2O2 was used to measure ROS. When plants are attacked by pathogens, hypersensitive responses will be elicited and H2O2 will be accumulated in plants (Lin & Ishii, 2009). As H2O2 may directly kill pathogens at infection sites (Lin & Ishii, 2009), we inferred that significant increase of H2O2 concentration in leaves of I. cairica induced by F. oxysporum, F. fujikuroi alone and a mixture of both fungi (Fig. 6A) might have strengthened inhibitory effects on C. gloeosporioides at infection sites and prevented further expansion of leaf lesion, resulting in significantly less lesion area ratio relative to the control (Fig. 5C). In addition, H2O2 also can be employed as a signal molecule to mediate the levels of downstream signal of SA and JA (Ren & Dai, 2012) and induces pathogen resistance of plants (Keshavarz-Tohid et al., 2016; Deng et al., 2016). In our study, significantly increased SA and JA content (Figs. 7A and 7B) in leaves of I. cairica pre-inoculated with F. oxysporum, F. fujikuroi alone and mixture should be relevant to H2O2 accumulation in leaves (Fig. 6A).
In plants, SA- or JA-dependent defense responses are generally activated by non-pathogens and pathogens with different lifestyles, such as biotrophy and necrotrophy (Paparu et al., 2007; Chen et al., 2018). SA and JA are important signaling molecules in plant defense responses. Through signaling transduction, SA and JA signaling mediates NPR1 expression (Stein et al., 2008; Nic-Matos et al., 2017; Ali et al., 2017), further eliciting distinct sets of resistance gene expression. SA signaling involves PR genes encoding PR proteins including β-1,3-glucanase and chitinase (Stein et al., 2008). JA signaling involves some genes encoding defense-related proteins, such as defensin (Tiwari et al., 2017; Sarkar, Jana & Sikdar, 2017; Brown et al., 2003). Previous studies have shown that non-pathogenic F. oxysporum and F. fujikuroi induce up-regulation of PR1 genes expression (Veloso & Díaz, 2012) and activities of chitinase and β-1,3-glucanase (Fuchs, Moënne-Loccoz & Défago, 1997; Patil et al., 2011) and improve pathogen resistance of host plants. However, our results showed that, in contrast to the control, although F. oxysporum and F. fujikuroi alone and in mixture induced significantly higher SA content in leaves of I. cairica (Fig. 7A), the transmission efficiency of SA signaling between the three treatments was largely different. Colonization by F. fujikuroi failed to transmit SA signaling and did not up-regulate NPR1 expression (Fig. 7C), chitinase and β-1,3-glucanase activities (Figs. 6B and 6C), whereas colonization by F. oxysporum and the mixture of both fungi successfully transmitted SA signaling, significantly up-regulated NPR1 expression (Fig. 7C), chitinase and β-1,3-glucanase activities (Figs. 6B and 6C). Interestingly, compared with F. oxysporum and the mixture of both fungi, F. fujikuroi induced significantly higher JA content but significantly lower β-1,3-glucanase activity in leaves of I. cairica plants (Figs. 6B and 7B). The results showed that excessive JA content in I. cairica plants induced with F. fujikuroi antagonized SA signaling defense pathway and suppressed SA-dependent β-1,3-glucanase activity. Previous studies have suggested JA signaling cross-talk with SA signaling defense pathways via NPR1 (Spoel et al., 2003; Withers & Dong, 2016) antagonizes SA signaling and suppresses SA-dependent genes expression (Kachroo et al., 2001). Therefore, our findings showed that F. oxysporum played a dominant role in inducing pathogen resistance of I. cairica against C. gloeosporioides because its presence alone or coexistence with F. fujikuroi alleviated the antagonism of JA signaling on SA-dependent β-1,3-glucanase activity.
It is well known that β-1,3-glucanase inhibit fungal growth through degrading glucan in the cell wall of pathogenic fungi (Balasubramanian et al., 2012; Vieira et al., 2010; Li et al., 2015). In our study, compared to the control and F. fujikuroi treatment, with higher β-1,3-glucanase activities (Fig. 6B), I. cairica plants induced with F. oxysporum and a mixture of both fungi strengthened pathogen resistance against C. gloeosporioides and achieved greater stem length and biomass (Figs. 5A and 5B).
Conclusions
In natural habitats, healthy leaves of I. cairica plants established stable symbiosis with non-pathogenic F. fujikuroi and F. oxysporum and had a higher abundance of the both fungi relative to C. gloeosporioides infected leaves. Although F. fujikuroi and F. oxysporum could induce pathogen resistance of I. cairica against C. gloeosporioides, F. oxysporum played a dominant role in inducing pathogen resistance. Its presence alleviated the antagonism of JA on the SA signaling defense pathway and enabled I. cairica plants to maintain relatively higher level of resistance against C. gloeosporioides.
The interactions between plants and symbiotic microbes have been well studied in plant invasion ecology (Soares et al., 2016; Shearin et al., 2018). Some microbial symbionts have been identified as drivers in successful plant invasions owing to their plant growth promoting effects (Dai et al., 2016). The results obtained in the present study provide new evidence that epiphytic F. fujikuroi and F. oxysporum act as pathogen resistance inducers of the invasive plant I. cairica. However, in this study, we only targeted the selected epiphytic F. fujikuroi and F. oxysporum to explore their contributions of pathogen resistance to I. cairica, which might have overlooked other microbial symbionts associated with pathogen resistance against C. gloeosporioides. Therefore, future works should systematically investigate the overall symbiotic microbial community (endophytes and epiphytes) of I. cairica, screen microbial species functioning as plant pathogen resistance inducers, and thereby extend the study of ecological and physiological mechanisms inducing pathogen resistance. In addition, in relation to the management and control of I. cairica, the disruption of the symbiosis between I. cairica and mutualistic microbes might provide a potentially effective strategy.
Supplemental Information
The occurrence rate of F. oxysporum and F. fujikuroi on I. cairica in different habitats. The comparison of the abundance of F. oxysporum and F. fujikuroi between infected and healthy leaves of I. cairica in the field. The pathogenicity of F. oxysporum and F. fujikuroi to I. cairica. The effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on growth parameters of I. cairica infected with C. gloeosporioides. The effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on physiological characteristics of I. cairica infected with C. gloeosporioides. The effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on hormone content and NPR1 expression in leaves of I. cairica. The morphological characteristic of representative isolates JY1, JY2 and JY3.
Acknowledgments
We are grateful to Professor Paul Giller (University College Cork, Ireland) for the English editing of the manuscript.
Funding Statement
This research was financially supported by the Natural Science Foundation of China (NSFC, 31670266), Guangdong Natural Science Foundation (Project No. 2017A030313115) and Guangdong Pearl River Scholar Funded Science (2012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Hua Xu and Minjie Zhu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Shaoshan Li conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Weibin Ruan conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Can Xie performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The ITS sequences of representative isolates JY1, JY2 and JY3 are available at GenBank: MN704851.1, MN704852.1 and MN704853.1.
Data Availability
The following information was supplied regarding data availability:
The raw data measurements are available in the Supplemental Files.
References
- Aimé et al. (2008).Aimé S, Cordier C, Alabouvette C, Olivain C. Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. oxysporum Fo47. Physiological and Molecular Plant Pathology. 2008;73:9–15. doi: 10.1016/j.pmpp.2008.10.001. [DOI] [Google Scholar]
- Ali et al. (2017).Ali S, Mir ZA, Tyagi A, Mehari H, Meena RP, Bhat JA, Yadav P, Papalou P, Rawat S, Grover A. Overexpression of NPR1 in Brassica juncea confers broad spectrum resistance to fungal pathogens. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.01693. Article 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatulli et al. (2010).Amatulli MT, Spadaro D, Gullino ML, Gaaribaldi A. Molecular identification of Fusarium spp, associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathology. 2010;59:839–844. doi: 10.1111/j.1365-3059.2010.02319.x. [DOI] [Google Scholar]
- Balasubramanian et al. (2012).Balasubramanian V, Vashisht D, Cletus J, Sakthivel N. Plant β-1, 3-glucanases: their biological functions and transgenic expression against phytopathogenic fungi. Biotechnology Letters. 2012;34:1983–1990. doi: 10.1007/s10529-012. [DOI] [PubMed] [Google Scholar]
- Bertolazi et al. (2019).Bertolazi AA, De Souza SB, Ruas KF, Campostrini E, De Rezende CE, Cruz C, Melo J, Colodete CM, Varma A, Ramos AC. Inoculation with piriformospora indica is more efficient in wild-type rice than in transgenic rice over-expressing the vacuolar H+-PPase. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.01087. Article 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan & Frenkel (1977).Brennan T, Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiology. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown et al. (2003).Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM. A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiology. 2003;32:1020–1032. doi: 10.1104/pp.102.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen & Lee (1994).Chen JP, Lee MS. Simultaneous production and partition of chitinase during growth of Serratia marcescens in an aqueous two-phase system. Biotechnology Techniques. 1994;8:783–788. doi: 10.1007/BF00152884. [DOI] [Google Scholar]
- Chen et al. (2018).Chen Z, Wang J, Li Y, Zhong Y, Liao J, Lu S, Zhong Y, Liao J, Lu S, Wang L, Wang X, Chen S. Dry mycelium of Penicillium chrysogenum activates defense via gene regulation of salicylic acid and jasmonic acid signaling in Arabidopsis. Physiological and Molecular Plant Pathology. 2018;103:54–61. doi: 10.1016/j.pmpp.2018.04.006. [DOI] [Google Scholar]
- Choi et al. (2018).Choi HW, Hong SK, Lee YK, Kim WG, Chun S. Taxonomy of Fusarium fujikuroi species complex associated with bakanae on rice in Korea. Australasian Plant Pathology. 2018;47:23–24. doi: 10.1007/s13313-017-0536-6. [DOI] [Google Scholar]
- Clark, Hoy & Nelson (1995).Clark CA, Hoy MW, Nelson PE. Variation among isolated of Fusarium lateritium from sweetpotato for pathogenicity and vegetative compatibility. Phytopathology. 1995;85:624–629. doi: 10.1094/Phyto-85-624. [DOI] [Google Scholar]
- Dai et al. (2016).Dai ZC, Fu W, Wan LY, Cai HH, Wang N, Qi SS, Du DL. Different growth promoting effects of endophytic bacteria on invasive and native clonal plants. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00706. Article 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz et al. (1995).De la Cruz J, Pintor TJA, Benitez T, Romero LC. A novel endo-beta-1, 3-glucanase, BGN13. 1, involved in the mycoparasitism of Trichoderma harzianum. Journal of Bacteriology. 1995;177:6937–6945. doi: 10.1128/jb.177.23.6937-6945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshkhah, Grundler & Wieczorek (2018).Daneshkhah R, Grundler FMW, Wieczorek K. The role of MPK6 as mediator of ethylene/jasmonic acid signaling in Serendipita indica-colonized Arabidopsis roots. Plant Molecular Biology Reporter. 2018;36:284–294. doi: 10.1007/s11105-018-1077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al. (2016).Deng XG, Zhu T, Zou LJ, Han XY, Zhou X, Xi DH, Zhang DW, Lin HH. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant Journal. 2016;85:478–493. doi: 10.1111/tpj.13120. [DOI] [PubMed] [Google Scholar]
- Dianese et al. (1981).Dianese JC, Bolkan HA, Da Silva CB, Couto FAA. Pathogenicity of epiphytic Fusarium moniliformevar.subglutinansto pineapple. Phytopathology. 1981;71:1145–1149. doi: 10.1094/Phyto-71-1145. [DOI] [Google Scholar]
- Engelberth et al. (2003).Engelberth J, Schmelz EA, Alborn HT, Cardoza YJ, Huang J, Tumlinson JH. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Analytical Biochemistry. 2003;312:242–250. doi: 10.1016/S0003-2697(02)00466-9. [DOI] [PubMed] [Google Scholar]
- Fagoaga et al. (2001).Fagoaga C, Rodrigo I, Conejero V, Hinarejos C, Tuset JJ, Arnau J, Pina JA, Navarro L, Peña L. Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Molecular Breeding. 2001;7:175–185. doi: 10.1023/A:1011358005054. [DOI] [Google Scholar]
- Ferguson, Watkins & Harman (1983).Ferguson IB, Watkins CB, Harman JE. Inhibition by calcium of senescence of detached cucumber cotyledons: effect on ethylene and hydroperoxide production. Plant Physiology. 1983;71:182–186. doi: 10.1104/pp.71.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, Moënne-Loccoz & Défago (1997).Fuchs JG, Moënne-Loccoz Y, Défago G. Nonpathogenic Fusarium oxysporum strain Fo47 induces resistance to Fusarium wilt in tomato. Plant Disease. 1997;81:492–496. doi: 10.1094/PDIS.1997.81.5.492. [DOI] [PubMed] [Google Scholar]
- Hafizi, Salleh & Latiffah (2013).Hafizi R, Salleh B, Latiffah Z. Morphological and molecular characterization of Fusarium solani and F. oxysporum associated with crown disease of oil palm. Brazilian Journal of Microbiology. 2013;44:959–968. doi: 10.1590/S1517-83822013000300047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron et al. (2015).Herron DA, Wingfield MJ, Wingfield BD, Rodas CA, Marincowitz S, Steenkamp ET. Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Studies in Mycology. 2015;80:131–150. doi: 10.1016/j.simyco.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2009).Huang QQ, Wu JM, Bai YY, Zhou L, Wang GX. Identifying the most noxious invasive plants in China: role of geographical origin, life form and means of introduction. Biodiversity and Conservation. 2009;18:305–316. doi: 10.1007/s10531-008-9485-2. [DOI] [Google Scholar]
- Hwang et al. (2013).Hwang IS, Kang WR, Hwang DJ, Bae SC, Yun SH, Ahn IP. Evaluation of bakanae disease progression caused by Fusarium fujikuroi in Oryza sativa L. Journal of Microbiology. 2013;51:858–865. doi: 10.1007/s12275-013-3472-3. [DOI] [PubMed] [Google Scholar]
- Jin et al. (2018).Jin H, Choi SM, Kang MJ, Yun SH, Kwon DJ, Noh YS, Noh B. Salicylic acid-induced transcriptional reprogramming by the HAC–NPR1–TGA histone acetyltransferase complex in Arabidopsis. Nucleic Acids Research. 2018;46:11712–11725. doi: 10.1093/nar/gky847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo et al. (2001).Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz-Tohid et al. (2016).Keshavarz-Tohid V, Taheri P, Taghavi SM, Tarighi S. The role of nitric oxide in basal and induced resistance in relation with hydrogen peroxide and antioxidant enzymes. Journal of Plant Physiology. 2016;119:29–38. doi: 10.1016/j.jplph.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Kimura (1980).Kimura M. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kowalski et al. (2015).Kowalski KP, Bacon C, Bickford W, Braun H, Clay K, Leduc-Lapierre M, Lillard E, McCormick MK, Nelson E, Torres M, White J, Wilcox DA. Advancing the science of microbial symbiosis to support invasive species management: a case study on Phragmites in the Great Lakes. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.00095. Article 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvas et al. (2009).Kvas M, Marasas WFO, Wingfield BD, Wingfield MJ, Steenkamp ET. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Diversity. 2009;34:1–21. [Google Scholar]
- Lanubile et al. (2015).Lanubile A, Muppirala UK, Severin AJ, Marocco A, Munkvold GP. Transcriptome profiling of soybean (Glycine max) roots challenged with pathogenic and non-pathogenic isolates of Fusarium oxysporum. BMC Genomics. 2015;16:1089. doi: 10.1186/s12864-015-2318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie (1995).Leslie JF. Gibberella fujikuroi: available populations and variable traits. Canadian Journal of Botany. 1995;73:282–291. doi: 10.1139/b95-258. [DOI] [Google Scholar]
- Leslie & Summerell (2006).Leslie JF, Summerell BA. The Fusarium laboratory manual. Blackwell Publishing; Iowa: 2006. [DOI] [Google Scholar]
- Li et al. (2015).Li J, Liu W, Luo L, Dong D, Liu T, Zhang T, Lu C, Liu D, Zhang D, Wu H. Expression of Paenibacillus polymyxaβ-1, 3-1, 4-glucanase in Streptomyces lydicus A01 improves its biocontrol effect against Botrytis cinerea. Biological Control. 2015;90:141–147. doi: 10.1016/j.biocontrol.2015.06.008. [DOI] [Google Scholar]
- Li et al. (2012).Li WH, Luo JN, Tian XS, Peng CL, Zhou XY. Patterns of defoliation and their effect on the plant growth and photosynthetic characteristics of Ipomoea cairica. Weed Biology and Management. 2012;12:40–46. doi: 10.1111/j.1445-6664.2012.00432.x. [DOI] [Google Scholar]
- Lin & Liu (2010).Lin C, Liu GK. Main disease and insect pests of I. cairica in Fuzhou region. Subtropical Agriculture Research. 2010;6:98–101. doi: 10.13321/j.cnki.subtrop.agric.res.2010.02.002. [DOI] [Google Scholar]
- Lin & Ishii (2009).Lin TC, Ishii H. Accumulation of H2O2 in xylem fluids of cucumber stems during ASM-induced systemic acquired resistance (SAR) involves increased LOX activity and transient accumulation of shikimic acid. European Journal of Plant Pathology. 2009;125:119–130. doi: 10.1007/s10658-009-9464-9. [DOI] [Google Scholar]
- Lin et al. (2014).Lin Z, Xu S, Que Y, Wang J, Comstock JC, Wei J, McCord PH, Chen B, Chen R, Zhang M. Species-specific detection and identification of Fusarium species complex, the causal agent of sugarcane pokkah boeng in China. PLOS ONE. 2014;9:e104195. doi: 10.1371/journal.pone.0104195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2016).Liu G, Gao Y, Huang FF, Yuan MY, Peng SL. The invasion of coastal areas in south china by Ipomoea cairica may be accelerated by the ecotype being more locally adapted to salt stress. PLOS ONE. 2016;11:e0149262. doi: 10.1371/journal.pone.0149262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu G, Huang QQ, Lin ZG, Huang FF, Liao HX, Peng SL. High tolerance to salinity and herbivory stresses may explain the expansion of Ipomoea cairica to salt marshes. PLOS ONE. 2012;7:e48829. doi: 10.1371/journal.pone.0048829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lops et al. (2013).Lops F, Cibelli F, Raimondo ML, Carlucci A. First report of stem wilt and root rot of Schlumbergera truncata caused by Fusarium oxysporumf. sp. opuntiarumin Southern Italy. Plant Disease. 2013;97:846. doi: 10.1094/PDIS-11-12-1092-PDN. [DOI] [PubMed] [Google Scholar]
- Magnin Robert et al. (2007).Magnin-Robert M, Trotel-Aziz P, Quantinet D, Biagianti S, Aziz A. Biological control of Botrytis cinerea by selected grapevine-associated bacteria and stimulation of chitinase and β-1,3 glucanase activities under field conditions. European Journal of Plant Pathology. 2007;118:43–57. doi: 10.1007/s10658-007-9111-2. [DOI] [Google Scholar]
- Mandal, Mallick & Mitra (2009).Mandal S, Mallick N, Mitra A. Salicylic acid-induced resistance to Fusarium oxysporumf, sp. lycopersici in tomato. Plant Physiology and Biochemistry. 2009;47:642–649. doi: 10.1016/j.plaphy.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Matić et al. (2016).Matić S, Bagnaresi P, Biselli C, Orru L, Carneiro GA, Siciliano I, Valé G, Gullino LM, Spadaro D. Comparative transcriptome profiling of resistant and susceptible rice genotypes in response to the seedborne pathogen Fusarium fujikuroi. BMC Genomics. 2016;17:608. doi: 10.1186/s12864-016-2925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji et al. (2017).Miyaji N, Shimizu M, Miyazaki J, Osabe K, Sato M, Ebe Y, Takada S, Kaji M, Dennis ES, Fujimoto R, Okazaki K. Comparison of transcriptome profiles by Fusarium oxysporum inoculation between Fusarium yellows resistant and susceptible lines in Brassica rapa L. Plant Cell Reports. 2017;36:1841–1854. doi: 10.1007/s00299-017-2198-9. [DOI] [PubMed] [Google Scholar]
- Moya-Elizondo et al. (2019).Moya-Elizondo EA, Doussoulin H, San Martin J, Ruiz B, Del Valle P. First report of Fusarium oxysporum causing Fusarium wilt on blueberry (Vaccinium corymbosum) in Chile. Plant Disease. 2019;103:2669. doi: 10.1094/PDIS-02-19-0275-PDN. [DOI] [Google Scholar]
- Ngwene et al. (2016).Ngwene B, Boukail S, Söllner L, Franken P, Andrade-Linares DR. Phosphate utilization by the fungal root endophyte Piriformospora indica. Plant and Soil. 2016;405:231–241. doi: 10.1007/s11104-015-2779-8. [DOI] [Google Scholar]
- Nic-Matos et al. (2017).Nic-Matos G, Narváez M, Peraza-Echeverría S, Sáenz L, Oropeza C. Molecular cloning of two novel NPR1 homologue genes in coconut palm and analysis of their expression in response to the plant defense hormone salicylic acid. Genes and Genomics. 2017;39:1007–1019. doi: 10.1007/s13258-017-0566-z. [DOI] [Google Scholar]
- Paparu et al. (2007).Paparu P, Dubois T, Coyne D, Viljoen A. Defense-related gene expression in susceptible and tolerant bananas (Musa spp.) following inoculation with non-pathogenic Fusarium oxysporum endophytes and challenge with Radopholus similis. Physiological and Molecular Plant Pathology. 2007;71:149–157. doi: 10.1016/j.pmpp.2007.12.001. [DOI] [Google Scholar]
- Park et al. (2004).Park CJ, Kim KJ, Shin R, Park JM, Shin YC, Paek KH. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant Journal. 2004;37:186–198. doi: 10.1046/j.1365-313X.2003.01951.x. [DOI] [PubMed] [Google Scholar]
- Patil et al. (2011).Patil S, Sriram S, Savitha MJ, Arulmani N. Induced systemic resistance in tomato by non-pathogenic Fusarium species for the management of Fusarium wilt. Archives of Phytopathology and Plant Protection. 2011;44:1621–1634. doi: 10.1080/03235408.2010.526774. [DOI] [Google Scholar]
- Rabha et al. (2016).Rabha AJ, Naglot A, Sharma GD, Gogoi HK, Gupta VK, Shreemali DD, Veer V. Morphological and molecular diversity of endophytic Colletotrichum gloeosporioides from tea plant, Camellia sinensis (L.) O. Kuntze of Assam, India. Journal of Genetic Engineering and Biotechnology. 2016;14:181–187. doi: 10.1016/j.jgeb.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger et al. (2009).Ratzinger A, Riediger N, Von Tidemann A, Karlovsky P. Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum. Journal of Plant Research. 2009;122:571–579. doi: 10.1007/s10265-009-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren & Dai (2012).Ren CG, Dai CC. Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biology. 2012;12:128. doi: 10.1186/1471-2229-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikkonen et al. (2016).Saikkonen K, Young CA, Helander M, Schardl CL. Endophytic Epichloë species and their grass hosts: from evolution to applications. Plant Molecular Biology. 2016;90:665–675. doi: 10.1007/s11103-015-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou & Nei (1987).Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salazar-Cerezo et al. (2018).Salazar-Cerezo S, Martinez-Montiel N, Cruz-Lopez MC, Martinez-Contreras RD. Fungal diversity and community composition of culturable fungi in Stanhopea trigrinacast gibberellin producers. Frontiers in Microbiology. 2018;9 doi: 10.3389/fmicb.2018.00612. Article 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, Jana & Sikdar (2017).Sarkar P, Jana K, Sikdar SR. Overexpression of biologically safe Rorippa indica defensin enhances aphid tolerance in Brassica juncea. Planta. 2017;246:1029–1044. doi: 10.1007/s00425-017-2750-4. [DOI] [PubMed] [Google Scholar]
- Schmittgen & Livak (2008).Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Séne et al. (2015).Séne S, Avril R, Chaintreuil C, Geoffroy A, Ndiaye C, Diédhiou AG, Sadio O, Courtecuisse R, Sylla SN, Selosse MA, Bâ A. Ectomycorrhizal fungal communities of Coccoloba uvifera (L.) L. mature trees and seedlings in the neotropical coastal forests of Guadeloupe (Lesser Antilles) Mycorrhiza. 2015;25:547–559. doi: 10.1007/s00572-015-0633-8. [DOI] [PubMed] [Google Scholar]
- Shearin et al. (2018).Shearin ZRC, Filipek M, Desai R, Bickford WA, Bickford WA, Kowalski KP, Clay K. Fungal endophytes from seeds of invasive, non-native Phragmites australis and their potential role in germination and seedling growth. Plant and Soil. 2018;422:183–194. doi: 10.1007/s11104-017-3241-x. [DOI] [Google Scholar]
- Soares et al. (2016).Soares MA, Li HY, Kowalski KP, Bergen M, Torres MS, White JF. Evaluation of the functional roles of fungal endophytes of Phragmites australis from high saline and low saline habitats. Biological Invasions. 2016;18:2689–2702. doi: 10.1007/s10530-016-1160-z. [DOI] [Google Scholar]
- Song et al. (2015).Song M, Li X, Saikkonen K, Li C, Nan Z. An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecology. 2015;13:44–52. doi: 10.1016/j.funeco.2014.07.004. [DOI] [Google Scholar]
- Spoel et al. (2003).Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CMJ. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell. 2003;15:760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein et al. (2008).Stein E, Molitor A, Kogel KH, Waller F. Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant and Cell Physiology. 2008;49:1747–1751. doi: 10.1093/pcp/pcn147. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2015).Sun ZY, Zhang TJ, Su JQ, Chow WS, Liu JQ, Chen LL, Li WH, Peng SL, Peng CL. A novel role of ethephon in controlling the noxious weed Ipomoea cairica (Linn.) Sweet. Scientific Reports. 2015;5:11372. doi: 10.1038/srep11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura et al. (2011).Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, Higgins & Gibson (1994).Thompson JD, Higgins DG, Gibson TJ. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari et al. (2017).Tiwari S, Pandey D, Gaur M, Kumar A. Effect of methyl jasmonate on disease severity and expression of plant defensin gene during Alternaria brassicae infection in Arabidopsis. International Journal of Current Microbiology and Applied Sciences. 2017;6:857–865. doi: 10.20546/ijcmas.2017.607.105. [DOI] [Google Scholar]
- Veloso & Díaz (2012).Veloso J, Díaz J. Fusarium oxysporum Fo47 confers protection to pepper plants against Verticillium dahliae and Phytophthora capsici, and induces the expression of defence genes. Plant Pathology. 2012;61:281–288. doi: 10.1111/j.1365-3059.2011.02516.x. [DOI] [Google Scholar]
- Vieira et al. (2010).Vieira FA, Carvalho AO, Vitória ÂP, Retamal CA, Gomes VM. Differential expression of defence-related proteins in Vigna unguiculata (L. Walp.) seedlings after infection with Fusarium oxysporum. Crop Protection. 2010;29:440–447. doi: 10.1016/j.cropro.2009.10.010. [DOI] [Google Scholar]
- Weir, Johnston & Damm (2012).Weir BS, Johnston PR, Damm U. The Colletotrichum gloeosporioides species complex. Studies in Mycology. 2012;73:115–180. doi: 10.3114/sim0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers & Dong (2016).Withers J, Dong X. Posttranslational modifications of NPR1: a single protein playing multiple roles in plant immunity and physiology. PLOS Pathogens. 2016;12:e1005707. doi: 10.1371/journal.ppat.1005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2016).Xia C, Li N, Zhang X, Feng Y, Christensen MJ, Nan Z. An epichloë endophyte improves photosynthetic ability and dry matter production of its host Achnatherum inebrians infected by Blumeria graminis under various soil water conditions. Fungal Ecology. 2016;22:26–34. doi: 10.1016/j.funeco.2016.04.002. [DOI] [Google Scholar]
- Yuan et al. (2019).Yuan BQ, Li SS, Xiong TT, Zhang T. Cytogenetic and genotoxic effects of Ipomoea cairica (L.) Sweet leaf aqueous extract on root growth of Allium cepa var. agrogarum (L.) Allelopathy Journal. 2019;46:205–214. doi: 10.26651/allelo.j/2019-46-2-1209. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The occurrence rate of F. oxysporum and F. fujikuroi on I. cairica in different habitats. The comparison of the abundance of F. oxysporum and F. fujikuroi between infected and healthy leaves of I. cairica in the field. The pathogenicity of F. oxysporum and F. fujikuroi to I. cairica. The effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on growth parameters of I. cairica infected with C. gloeosporioides. The effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on physiological characteristics of I. cairica infected with C. gloeosporioides. The effects of pre-inoculation with F. oxysporum, F. fujikuroi alone and a mixture of both on hormone content and NPR1 expression in leaves of I. cairica. The morphological characteristic of representative isolates JY1, JY2 and JY3.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data measurements are available in the Supplemental Files.