Abstract
Background and Purpose
Melatonin is a neurohormone involved in bone homeostasis. Melatonin directs bone remodelling and the role of bone marrow mesenchymal stem cells (BMMSCs) in the regulating melatonin‐mediated bone formation–resorption balance remains undefined.
Experimental Approach
Osteoporosis models were established and bone tissue and serum were collected to test the effects of melatonin on bone homeostasis. Melatonin receptors were knocked down, the NF‐κB signalling pathway and receptor activator of NF‐κB ligand (RANKL) expression were investigated. Communication between bone marrow mesenchymal stem cells and osteoclasts was detected with direct‐contact or indirect‐contact system.
Key Results
Bone loss and microstructure disorder in mice were reversed after melatonin treatment, as a result of anabolic and anti‐resorptive effects. In vitro, a physiological (low) concentration of melatonin promoted the bone marrow mesenchymal stem cells, osteogenic lineage commitment and extracellular mineralization but had no impact on extracellular matrix synthesis. After MT knockdown, especially MT2, the positive effects of melatonin on osteogenesis were attenuated. The canonical NF‐κB signalling pathway was the first discovered downstream signalling pathway after MT receptor activation and was found to be down‐regulated by melatonin during osteogenesis. Melatonin suppressed BMMSC‐mediated osteoclastogenesis by inhibiting RANKL production in BMMSCs and this effect only occurred when BMMSCs and osteoclast precursors were co‐cultured in an indirect‐contact manner.
Conclusion and Implications
Our work suggests that melatonin plays a crucial role in bone balance, significantly accelerates the osteogenic differentiation of bone marrow mesenchymal stem cells by suppressing the MT2‐dependent NF‐κB signalling pathway, and down‐regulates osteoclastogenesis via RANKL paracrine secretion.
Abbreviations
- ALP
alkaline phosphatase
- BMD
bone mineral density
- BMMs
bone marrow monocytes
- BMMSCs
bone marrow mesenchymal stem cells
- BV/TV
trabecular bone volume per total volume
- Col‐I
collagen I
- CTX‐I
C‐terminal telopeptide α1 chain of type I collagen
- H&E
haematoxylin and eosin
- Mel
melatonin
- OCN
osteocalcin
- OPG
osteoprotegerin
- OVX
ovariectomy
- PINP
N‐terminal propeptide of type I procollagen
- RANK
receptor activator of NF‐κB
- RANKL
receptor activator of NF‐κB ligand
- TRAP
tartrate‐resistant acid phosphatase
- Tb. N
trabecular bone number
- Tb. Sp
trabecular bone separation
- Tb. Th
trabecular bone thickness
- μCT
micro‐CT
What is already known
Melatonin participates in regulating bone homeostasis
Bone marrow mesenchymal stem cells can differentiate into osteoblasts and regulate osteoclasts by secreting cytokines
What this study adds
Melatonin up‐regulates the osteogenesis of bone marrow mesenchymal stem cells by inactivating MT2‐mediated NF‐κB signalling
Melatonin suppressed bone marrow mesenchymal stem cell‐mediated osteoclastogenesis by indirectly inhibiting the production of RANKL.
What is the clinical significance
At the physiological level, melatonin could enhance bone mass by biasing bone formation over resorption.
Melatonin and bone marrow mesenchymal stem cells may become potential therapeutic targets for osteoporosis.
1. INTRODUCTION
Osteoporosis is a metabolic bone disease characterized by bone mass loss and microarchitectural deterioration as the result of an imbalance between resorption and formation. In addition, postmenopausal women are most likely to suffer from osteoporosis (Rachner, Khosla et al., 2011). In the bone micro‐environment, the changing framework of cytokines, receptors and transcription factors is closely associated with bone loss; these factors include https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5066) and pro‐inflammatory factors, such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 (Barbour, Lui et al., 2014; Tilg, Moschen, Kaser, Pines, & Dotan, 2008). The present clinical therapies for osteoporosis are limited to anti‐resorptive therapies or the promotion of anabolic pathways to maintain bone homeostasis, but these therapies are not that effective and have many side effects (Rachner, Khosla, & Hofbauer, 2011). Therefore, it is urgent to find a multimodal, more efficient and safer therapy.
As an endogenous neurohormone mainly produced by the pineal gland, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224 is capable of exerting immune regulation, anti‐inflammation, cell protection, antioxidant function and bone homeostasis modulation (Hu et al., 2017; Luchetti et al., 2014; Yang et al., 2015; Yu et al., 2014; Yu et al., 2017; Zhao et al., 2015). In line with these findings, light/dark cycle‐mediated melatonin secretion is tensely associated with human bone physiology and melatonin supplementation could profoundly improve bone mass in perimenopause period (Witt‐Enderby et al., 2012). Accumulating evidence has also confirmed that melatonin (1–50 μM) could successfully promote anabolic effects on skeleton (Han, Kim, Kim, & Lee, 2017; Xu et al., 2018) and that much higher concentration (100 μM) of melatonin can reduce the negative effects of pro‐inflammatory cytokines on bone (Lian et al., 2016). However, the concentrations that are used are much higher than physiological levels and do not represent physiological situation, which limits the exploration of real phenomenon and its clinical use.
Most melatonin‐mediated functions are activated by coupling melatonin with https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39 and MT receptor deficiency will destroy bone homeostasis by affecting osteogenesis, osteoclastogenesis and the RANKL/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1881/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1882) axis (Maria et al., 2018; Sharan, Lewis, Furukawa, & Yadav, 2017). In bone homeostasis, the RANKL/RANK/OPG axis contributes to a stable counterbalance in bone formation and resorption (Cao, 2011). The canonical NF‐κB signalling pathway could be activated by the osteoclastogenic cytokine RANKL and, although previous studies have paid much attention to its functions in osteoclasts, recent studies have revealed that NF‐κB signalling is also essential in regulating osteogenesis (Chang et al., 2009; Novack, 2011). These results imply that crosstalk exists between osteogenesis and osteoclastogenesis after melatonin treatment and NF‐κB signalling is a critical pathway in bridging these two processes. Therefore, although the preventive effect of melatonin on the osteoporotic process and the underlying mechanisms has long been investigated in different models, whether NF‐κB signalling directly or indirectly participates in melatonin‐mediated bone metabolism and the role of MT receptors have not been fully explored.
In the present work, we aimed to demonstrate whether osteoporosis amelioration by melatonin depended on both anabolic and anti‐resorptive effects on bone. Furthermore, we also wondered how the cytokine framework changed after melatonin treatment, whether the MT receptors were selectively activated and mediated signal transduction, whether NF‐κB inactivation was mainly responsible for the favourable effects of melatonin on bone mass recovery in oestrogen‐deficient osteoporosis and whether there was an interaction between osteogenic and osteoclastic activities.
2. METHODS
2.1. Reagents and antibodies
Melatonin, β‐glycerol phosphate, ascorbic acid, and tartrate‐resistant acid phosphatase (TRAP) Kit were purchased from Sigma‐Aldrich (St. Louis, USA). The NF‐κB inhibitor JSH‐23 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2779 were purchased from MedChem Express (Monmouth Junction, USA). Alizarin Red S staining kit was purchased from ScienCell (Carlsbad, USA). Murine macrophage colony‐stimulating factor (M‐CSF), murine RANKL and murine TNF‐α were purchased from Peprotech (Rock Hill, USA). Primary antibodies included rabbit monoclonal anti‐Runx2 (Cell Signaling Technology, Cat# 12556S, RRID:AB_2732805), rabbit polyclonal anti‐IKKα (Cell Signaling Technology Cat# 2682, RRID:AB_331626), rabbit monoclonal anti‐IKKβ (Cell Signaling Technology Cat# 8943, RRID:AB_11024092), rabbit monoclonal anti‐phospho–IKKα (Ser176)/IKKβ (Ser177) (Cell Signaling Technology, Cat# 2078, RRID:AB_2079379), mouse monoclonal anti‐IkBα (Cell Signaling Technology Cat# 4814, RRID:AB_390781), rabbit monoclonal anti‐phospho–IκBα (Ser32) (Cell Signaling Technology Cat# 2859, RRID:AB_561111), rabbit monoclonal anti‐p65 (Cell Signaling Technology Cat# 8242, RRID:AB_10859369) and rabbit monoclonal anti‐phospho‐NF‐κB p65 (Ser536) (Cell Signaling Technology, Cat# 3033, RRID:AB_331284), rabbit polyclonal anti‐collagen I (Col‐I) (Proteintech Cat# 14695‐1‐AP, RRID:AB_2082037), primary antibodies mouse monoclonal anti‐RANKL (Abcam, Cat# ab45039, RRID:AB_2205935), rabbit monoclonal anti‐Osterix (Abcam, Cat# ab22552, RRID:AB_2194492), rabbit polyclonal anti‐cathepsin K (Abcam, Cat# ab19027, RRID:AB_2261274), rabbit polyclonal anti‐https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=288 (Abcam, Cat# ab203346, RRID:AB_2783824), rabbit polyclonal anti‐c‐Fos (Abcam, Cat# ab190289, RRID:AB_2737414), rabbit polyclonal anti‐osteocalcin (OCN) (Abcam, Cat# ab93876, RRID:AB_10675660) and rabbit monoclonal anti‐glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) (Abcam, Cat# ab181602, RRID:AB_2630358) and mouse monoclonal anti‐https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287 (Santa Cruz Biotechnology, Cat# sc‐390328, RRID:AB_2810849).
2.2. Osteoporosis mouse model establishment and melatonin treatment
Eight‐week‐old C57BL/6J female mice (20–25 g) were purchased from the Animal Resource Center of Zhejiang University. All animal experiments were approved by the Institutional Animal Care and Use Committee of Zhejiang University (Approval Number: ZJU20170806) and were performed in compliance with the Guide for the Care and Use of Laboratory Animals–Eighth Edition (National Institutes of Health publication, 2011). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
Twenty‐four mice were housed under controlled light conditions (12‐hr day/night cycle) in a clean vivarium (specific pathogen free) and fed standard mouse chow. Mice were randomly divided into four groups (n = 6 in each group); 18 mice underwent bilateral ovariectomy (OVX) surgery and six mice were subjected to a sham operation. Six weeks after the surgery, a 6‐week drug treatment was started. The sham group and OVX groups were treated with vehicle reagents, and the OVX + Mel(L) and OVX + Mel(H) groups were treated with melatonin (10 or 100 mg·kg−1 body weight per day) oral gavage as previously reported (Sharan et al., 2017). Drug administration and follow‐up measurements were performed blindly. At the end of the experiments, mice were killed by cervical dislocation after blood was collected from the lateral canthus vein and the fresh long bones were frozen with liquid nitrogen or fixed with 4% paraformaldehyde.
2.3. Bone μCT analysis
Excised right femurs were analysed by the SkyScan‐1176 micro‐CT (μCT) system (Bruker micro CT, Kontich, Belgium), using an 8.96‐μm pixel size, 45‐kV voltage and 500‐μA current. The region of interest was the 1‐mm‐thick area 0.5 mm proximal away from the growth plate. The bone mineral density (BMD), trabecular bone volume per total volume (BV/TV), trabecular bone thickness (Tb. Th), trabecular bone number (Tb. N) and trabecular bone separation (Tb. Sp) were calculated with the CT software (1.13 version, Bruker micro CT, Kontich, Belgium). The 2D and 3D images of bone structure were also re‐established by this software.
2.4. Bone histology and immunohistochemistry
The decalcified tibias were embedded with paraffin and sliced in sagittal plane for haematoxylin and eosin (H&E) and immunohistochemistry staining according to the standard procedures. H&E staining was used to analyse the bone trabecula structure. Immunohistochemistry staining was used to evaluate and locate the expression of osteocalcin and Osterix in bone. The sections of interest were photographed using a microscope. Image‐Pro Plus 6.0 software (Rockville, USA) was used to assay the immunohistochemistry OD.
For immunohistochemistry staining, after the paraffin sections were deparaffinized, antigen retrieval was followed by submersing slides in EDTA buffer solution. Then the sections were incubated in 3% hydrogen peroxide for 10 min and blocked with 5% BSA for 1 hr at room temperature. Thereafter, the primary antibody against osteocalcin (1:200) or Osterix (1:500) was added to the sections at 4°C overnight. After that, sections were incubated with the anti‐rabbit HRP‐conjugated antibody for 30 min at room temperature. Sections were stained with the EnVision Detection Systems Peroxidase/DAB kit (DAKO, Glostrup, Denmark) and counterstained with haematoxylin. Finally, sections were mounted with coverslips and observed under a microscope. The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
2.5. Cell culture and differentiation
Bone marrow mesenchymal stem cells (BMMSCs) and bone marrow monocytes (BMMs) were isolated and harvested from 6‐week‐old to 8‐week‐old C57BL/6J mice as previously described (Soleimani & Nadri, 2009). Briefly, the bone marrow mesenchymal stem cells from the tibias and femurs were flushed out with the syringe. Cells were seeded in α‐minimum essential medium (α‐MEM) with 15% FBS (Bovogen Biologicals, East Keilor, Australia), 100 U·ml−1 penicillin and 100 μg·ml−1 streptomycin and were cultured at 37°C with 5% CO2. Non‐adherent cells were removed by frequent medium change during the first 72 hr and then culture medium was replaced every other day. Bone marrow mesenchymal stem cells were passaged using 0.25% trypsin when the cells reached 70–90% confluence. Cells from passage one were used for our experiments. To determine their osteogenic differentiation, bone marrow mesenchymal stem cells were cultured in osteogenic differentiation medium (α‐MEM with 10−2 M β‐glycerol phosphate, 50 mg·ml−1 ascorbic acid, and 15% FBS).
Bone marrow monocytes were also collected from tibias and femurs and obtained by density gradient centrifugation using Ficoll‐Paque (Dakewe Biotech, Beijing, China). Bone marrow monocytes were cultured for 7 days in α‐MEM medium containing 10% FBS with 25 ng·ml−1 M‐CSF and 100 ng·ml−1 RANKL for osteoclast differentiation.
2.6. Co‐culture experiments
In the direct‐contact co‐culture system, bone marrow mesenchymal stem cells (3 × 105 cells) were seeded onto the bottom of a six‐well plate and were cultured in osteogenic differentiation medium with or without melatonin for 7 days. On the last day, bone marrow monocytes (3 × 106 cells) were seeded directly on the top of bone marrow mesenchymal stem cells in the co‐culture medium for another 7 days.
In the indirect‐contact co‐culture system, bone marrow mesenchymal stem cells (3 × 105 cells) were seeded in the upper chamber of a Transwell plate with osteogenic differentiation medium. After 7 days, bone marrow monocytes (3 × 106 cells) were seeded in the bottom chamber with the co‐culture medium. The co‐culture medium was α‐MEM containing 10% FBS, 10−8 M 1,25‐dihydroxyvitamin D3 and 25 ng·ml−1 M‐CSF (Liu et al., 2017), with or without 10−8 M melatonin.
To confirm the role of the NF‐κB signalling pathway, the inhibitor JSH‐23 (10 nM) was used to pretreat bone marrow mesenchymal stem cells for 1 hour in the first 7 days and before every culture medium change, which occurred every 3 days.
2.7. Cell proliferation and apoptosis assays
The cell counting kit‐8 (CCK‐8, Dojindo Laboratories, Kumamoto, Japan) was used to assess cell proliferation. Bone marrow mesenchymal stem cells (1 × 104cells per well) were seeded into 96‐well plates with melatonin treatment for 3, 5, or 7 days. After removing the supernatants, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=864 solution was added into each well for 2 hr at 37°C, and the absorbance at 450 nm was measured using a Multiskan Spectrum Microplate Spectrophotometer (Thermo Scientific, Waltham, USA).
For apoptosis detection, bone marrow mesenchymal stem cells were treated with different doses of melatonin for 48 hr. After harvesting and staining with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3568 V‐FITC/PI apoptosis detection kit (Multi Sciences, Hangzhou, China) according to the manufacturer's instructions, the cells were analysed using a CytoFLEX S Flow Cytometer (Beckman Coulter, Indianapolis, USA).
2.8. Lentiviral shRNA transfection
To knock down the MT1/MT2 gene, lentiviral shRNAs (GenePharma, Shanghai, China) were used to transfect bone marrow mesenchymal stem cells. Cells were infected with lentiviruses for 48 hr and non‐infected cells were excluded with 0.25 μg·ml−1 puromycin according to the manufacturer's protocol. The knockdown efficiency was confirmed by quantitative real‐time RT‐PCR at the gene level and western blotting at the protein level. The sequences of shMT1, shMT2 and shNC are listed in Table S1.
2.9. Quantitative real‐time RT‐PCR
The gene expression levels were detected by quantitative real‐time RT‐PCR as described previously (Wang, Zhou, Guan, Yu, & Wang, 2018). Briefly, the total RNA of milled bone tissues or cells was extracted using the RNAiso Plus (TaKaRa Biotechnology, Kusatsu, Japan) and reverse transcribed using the PrimeScript RT Reagent Kit (TaKaRa Biotechnology, Kusatsu, Japan). Quantitative real‐time RT‐PCR assays were performed using a ViiATM7 Real‐Time PCR System (Applied Biosystems, Carlsbad, USA) with SYBR® Premix Ex Taq™ Kit (TaKaRa Biotechnology, Kusatsu, Japan) according to the manufacturer's instructions. The detailed sequences of the primers for each gene are listed in Table S2. The housekeeping gene gapdh was used as the internal reference and the ΔCT method was used to analyse the gene expression levels.
2.10. Western blotting
Protein expression levels of cells were detected by western blotting as described previously (Wang et al., 2018). Cells were lysed in RIPA mixed with protease and phosphatase inhibitors and incubated for half an hour at 4°C. Then cellular lysates were centrifuged at 16,000 rpm for 30 min at 4°C. The supernatants were collected as total cellular proteins and were quantified using BCA protein kit (Beyotime, Shanghai, China). Samples were boiled at 100°C for 5 min after mixing with the 4× sodium dodecyl sulfate (SDS) loading buffer (Invitrogen, Waltham, USA). Equal amounts of proteins (20–40 μg) were separated on 10% SDS‐PAGE gels and transferred onto polyvinylidene difluoride membranes (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% skim milk for 1 to 2 hr at room temperature. Then the blots were incubated with primary antibodies against Runx2 (1:1,000), Osterix (1:1,000), Col‐I (1:1,000), RANKL (1:1,000), phospho–IKKα (Ser176)/IKKβ (Ser177) (1:1,000), IKKα (1:1,000), IKKβ (1:1,000), phospho–IκBα (Ser32) (1:1,000), IkBα (1:1,000), phospho‐NF‐κB p65 (Ser536) (1:1,000), p65 (1:1,000), cathepsin K (1:500), c‐Fos (1:2,000), MT1 (1:500), MT2 (1:500), and GAPDH (1:10,000) overnight at 4°C. After washed with Tris‐buffered saline with Tween (TBST) buffer three times for 10 min, the blots were incubated with the anti‐mouse or anti‐rabbit HRP‐conjugated antibody (1:10,000) for 1 hr, followed by three times TBST washing (10 min once). Finally, the protein bands were finally visualized by an ECL kit (Thermo Scientific, Waltham, USA) with the ChemDoc MP Imaging System (Bio‐Rad, Hercules, USA). The images were analysed with Image Lab 5.2.1 software. GAPDH was defined as an internal reference. The western blot was conducted, and the experimental details provided conform to the British Journal of Pharmacology guidelines (Alexander, Roberts et al., 2018).
2.11. ELISA
ELISA was used to measure the concentrations of the C‐terminal telopeptide α1 chain of type I collagen (RatLaps™ [CTX‐I] EIA, IDS, Boldon, UK), N‐terminal propeptide of type I procollagen (Rat/Mouse PINP EIA, IDS, Boldon, UK), TRAP (Mouse TRAP ELISA kit, IDS, Boldon, UK), RANKL (Mouse TRANCE ELISA kit, RayBio, Norcross, GA, USA) and TNF‐α (Mouse TNF‐α ELISA kit, Dakewe Biotech, Beijing, China) in mouse serum according to the manufacturer's instructions. The level of osteocalcin produced by mouse Bone marrow mesenchymal stem cells was measured by the Mouse Osteocalcin ELISA Kit (Elabscience Biotechnology, Wuhan, China).
2.12. Alkaline phosphatase analysis
Bone marrow mesenchymal stem cells were seeded into osteogenic differentiation medium for 7 days and fixed with 4% paraformaldehyde for 15 min, and a BCIP/NBT Alkaline Phosphatase Colour Development Kit (Beyotime, Shanghai, China) was used for alkaline phosphatase (ALP) staining. The alkaline phosphatase activity was measured by an Alkaline Phosphatase Assay Kit (Beyotime, Shanghai, China) according to the manufacturer's instructions and the amount of activity was read at 405 nm by a microplate spectrophotometer.
2.13. Mineralization assay
At the end of the 21‐day incubation within osteogenic differentiation medium, bone marrow mesenchymal stem cells were fixed with 4% paraformaldehyde for 15 min and stained with alizarin red S for 30 min. The mineralization nodes were visualized. For quantitative evaluation, we destained the cells with 10% cetylpyridinium chloride for 15 min, and the supernatants were read at 540 nm.
2.14. TRAP staining
Cells were fixed with 4% paraformaldehyde for 15 min and treated with a TRAP Kit according to the manufacturer's protocol. After 15–20 min of staining, the TRAP‐positive cells (containing more than three nuclei) were counted as osteoclasts. When assessing the number of osteoclasts, all the osteoclasts were counted in the 96‐well plate. If cells were seeded onto 24‐well or 6‐well plates, we randomly selected five visual fields and calculated the average values after counting. The osteoclast area was quantified using ImageJ software (National Institutes of Health, Washington, DC, USA).
2.15. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
Experimental data were expressed as the mean ± SD and assessed by Student's t test, one‐way ANOVA, or two‐way ANOVA procedure. Tukey's post hoc tests were performed only if the F value in ANOVA achieved the necessary level of statistical significance (P < .05), and there was no significant variance inhomogeneity. GraphPad Prism 6.01 statistical software (GraphPad, San Diego, USA) was used for the statistical analysis, and the threshold for statistical significance was set at the level of P being .05. Therefore, in all cases, P < .05 was considered statistically significant.
Equalization, randomization, and blinding were used for each group in both in vivo and in vitro experiments, including mouse allocation, experimental performance, data extraction, and data analysis. As the periods of in vivo animal experiments were long, six mice were allocated to each group, so as to ensure the suitable group size for statistical analysis; for the in vitro study, the group size was set as at least five to ensure the implementation of statistical analysis. All group sizes represent the numbers of experimental independent biological repeats, and statistical analysis was performed using these independent values.
The statistical analysis was performed only for studies where each group size was at least five and no data points were excluded from the statistical analysis in any test. To reduce variation, data normalization was done. For most results of quantitative real‐time RT‐PCR, alkaline phosphatase activity, quantitative evaluation of alizarin red S staining, ELISA, and osteoclasts TRAP staining, each value was divided by the mean of the control values and expressed in the form of “fold change.” For these results, the Y axis in the figures was labelled with “fold mean of the controls.” Specifically, for quantitative real‐time RT‐PCR, the data were normalized to fold mean of the controls by ΔCT method; for the alkaline phosphatase activity, the data were normalized to fold mean of the controls by the formula “p‐nitrophenol amount per total protein amount per minute”; when analysing the protein levels in cells by ELISA, we first divided the detected concentration by the total protein and then normalized the parameters to fold mean of the controls.
2.16. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019a; Alexander, Fabbro et al., 2019b; Alexander, Kelly et al., 2019).
3. RESULTS
3.1. Melatonin transformed the cytokine framework and improved bone mass in osteoporotic mice
We successfully mimicked the pathological bone condition of postmenopausal women in mice by bilateral ovariectomy, which was confirmed by μCT scanning (Figure 1a) and H&E staining (Figure 1b). Melatonin (10 or 100 mg·kg−1 body weight per day) improved bone mass effectively but not in a concentration‐dependent manner. Although they share similar tendency, compared with high concentration, melatonin in low dose resulted in more obvious bone mineral density increase (Figure 1c), in accordance with the results of efficiently elevated BV/TV (Figure 1d), higher trabecular bone number (Tb. N) (Figure 1e) and decreased trabecular bone separation (Tb. Sp) (Figure 1f). However, no influence was observed on trabecular bone thickness (Tb.Th) (Figure 1g). In addition, the bone anabolic marker PINP level in serum was consistent with the μCT result (Figure 1h). All of the above results suggested that melatonin could rescue the osteoporosis process, especially at a lower dose.
Figure 1.
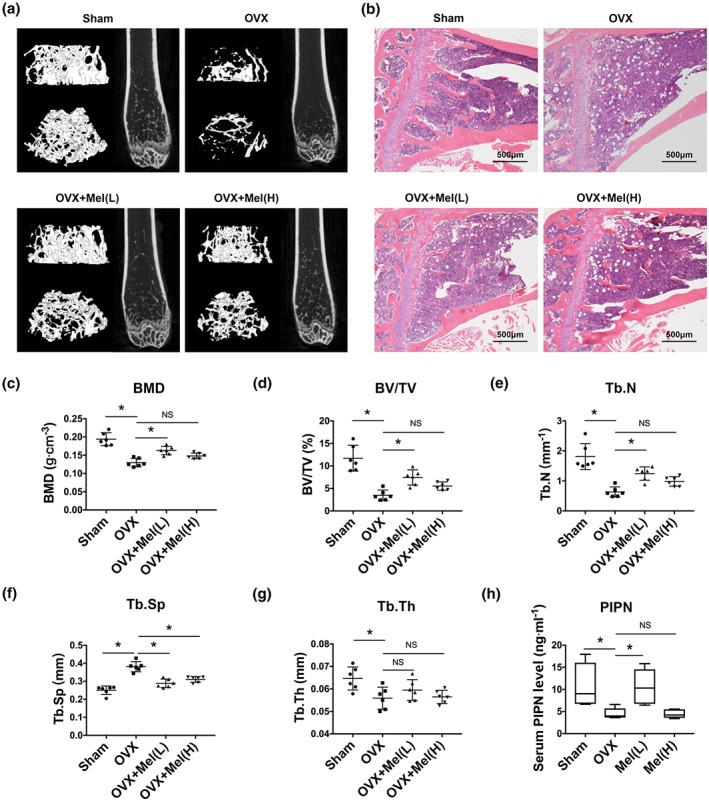
Melatonin stunted bone loss and increased bone formation in osteoporotic mice. (a–b) μCT imaging of distal femurs (a) and H&E staining (40×) of proximal tibias (b) of ovariectomy (OVX) mice followed by melatonin treatment for 6 weeks, L = low dose (10 mg·kg−1 body weight per day); H = high dose (100 mg·kg−1 body weight per day). (c–g) Bone microstructure parameters such as bone mineral density (BMD, c), trabecular bone volume per total volume (BV/TV, d), trabecular bone number (Tb. N, e), trabecular bone separation (Tb. Sp, f), and trabecular bone thickness (Tb. Th, g) were measured by μCT scanning. (h) The bone formation marker N‐terminal propeptide of type I procollagen (PINP) level in serum was detected by ELISA. Data are expressed as the mean ± SD and n = 6 in each group; *P < .05, significant differences between each indicated group. NS, not significant
To determine the underlying mechanisms of melatonin's positive effects, we next isolated the bone tissue and detected the profile of osteogenic markers. As expected, the levels of the osteogenic genes runx2, osterix, alp, col‐I and ocn were all up‐regulated after drug administration, especially runx2 and alp in the low‐dose melatonin group (Figure 2a). The opg/rankl gene ratio, a major negative correlation parameter for osteoclast activity, was raised with melatonin treatment (Figure 2a). In this regard, immunohistopathology also confirmed the same results of the matricellular molecular osteocalcin and osteogenic marker Osterix in the bone tissue (Figure 2b).
Figure 2.
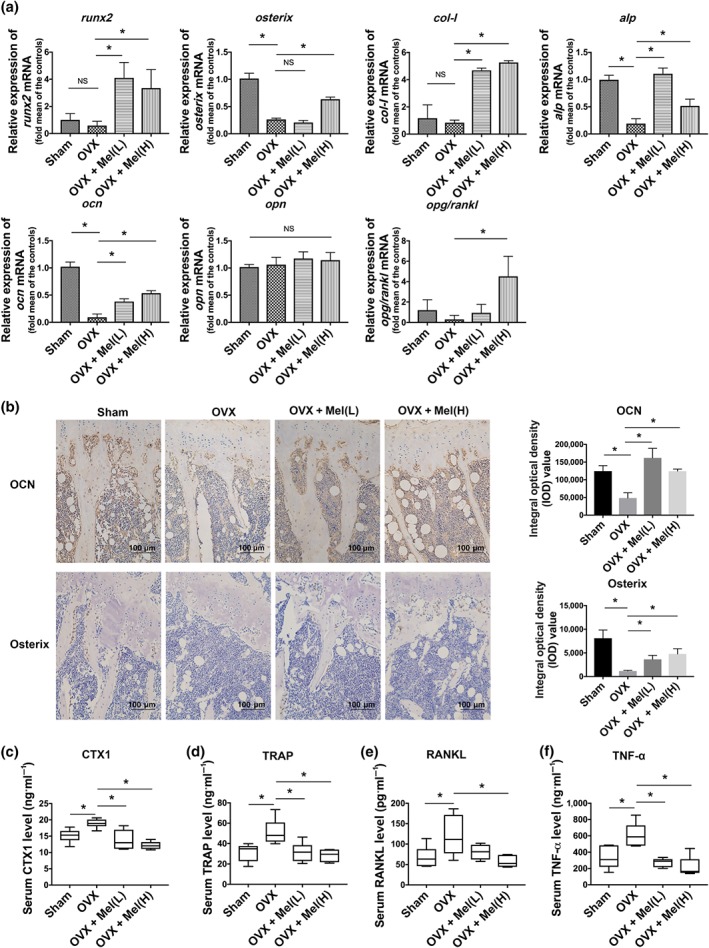
Melatonin transformed the cytokine framework in osteoporosis mice. (a) Gene presentations of osteogenic markers in bone tissue were detected. (b) Immunohistopathology (200×) showed the protein presentations of matricellular molecular osteocalcin (OCN) and osteogenic marker Osterix in proximal tibias bone tissue. (c–f) The concentrations of bone resorption markers CTX1 (c) and TRAP (d), osteoclast maturation‐related cytokine RANKL (e), and inflammation cytokine TNF‐α (f) in serum were determined by ELISA. Data are expressed as the mean ± SD, and n = 6 in each group; *P < .05, significant differences between each indicated group. NS, not significant
Osteoporosis is generally regarded as an osteoimmune disease with overexpressed inflammatory cytokines. To explore the extensive reciprocal interactions between the skeletal system and the immune system, we studied the cytokine framework by ELISA. We found that the bone resorption markers CTX‐I and TRAP were suppressed by melatonin in a concentration‐dependent manner (Figure 2c,d) and OVX‐induced osteoclast maturation‐related cytokine RANKL up‐regulation was corrected to the normal range after being treated with a high dose of melatonin (Figure 2e). To our surprise, the low concentration of melatonin was sufficient to normalize the serum TNF‐α level (Figure 2f).
By changing the cytokine framework, melatonin exerted both anabolic and anti‐resorptive effects on bone, implying the double effects of melatonin on bone‐forming cells such as bone marrow mesenchymal stem cells and osteoclastic cells. Furthermore, it was also supposed that melatonin at the low concentration was in favour of bone anabolic effects and that the high melatonin concentration was more likely to have an inhibitory impact on bone absorption.
3.2. Melatonin promoted osteogenesis of bone marrow mesenchymal stem cells at low concentrations (10–100 nM) and inhibited RANKL expression involved in osteoclastogenesis
To determine the minimum concentration that is required for melatonin to augment the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro, alkaline phosphatase staining was used to observe the treatment effects. Interestingly, low doses of melatonin (10 nM or 100 nM) elevated alkaline phosphatase levels, whereas excessive doses (1–100 μM) almost had no effect on osteogenesis and even exerted a negative role (Figure S1A). These effects may partly result from the inhibitory effect of excessive dose melatonin on cell proliferation (Figure S1B). Therefore, we chose concentrations of 10 nM and 100 nM in subsequent experiments. Both doses did not affect cell proliferation after a short or long period of exposure (Figure S2A) or apoptosis (Figure S2B,C). Despite both alkaline phosphatase assays (Figure 3a–c) and alizarin red staining (Figure 3d) revealed the positive effects of melatonin on extracellular mineralization, there was no visible difference in the deposited calcium after 10‐ to 100‐nM melatonin treatment. Consistent with the in vivo data, a low concentration of melatonin was more beneficial for promoting osteogenesis.
Figure 3.
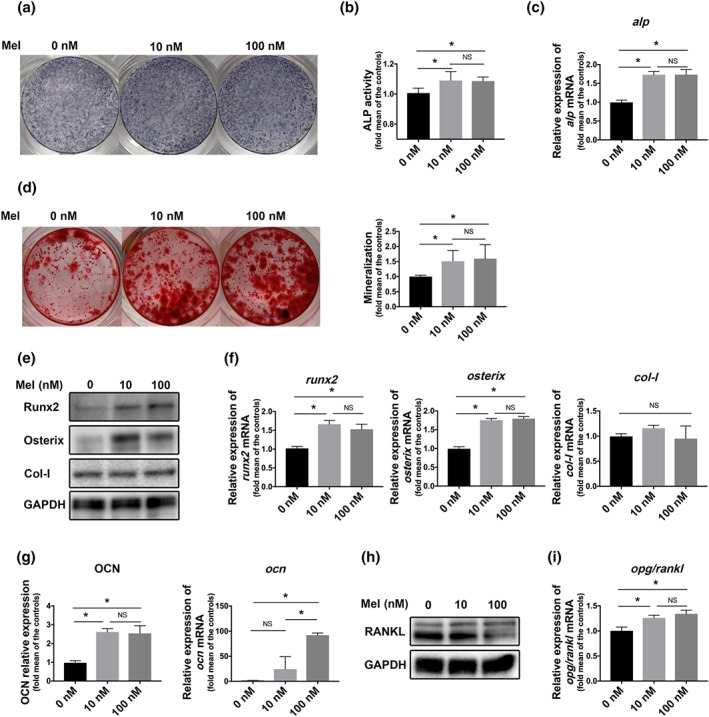
Melatonin promoted osteogenesis of bone marrow mesenchymal stem cells (BMMSCs) and inhibited RANKL expression which is involved in osteoclastogenesis. (a–c) alkaline phosphatase (ALP) levels were detected after osteogenic induction with melatonin for 7 days. (d) Mineralization nodes were observed by alizarin red staining after 21 days of osteogenic induction. (e, f) Protein (e) and gene (f) levels of Runx2, Osterix, and collagen I (Col‐I) were detected on Day 3 and Day 7 after melatonin treatment. (g) osteocalcin (OCN) expression was detected on Day 14 after melatonin treatment. (h, i) RANKL protein level (h) and the ratio of opg/rankl gene (i) were detected in bone marrow mesenchymal stem cells after melatonin treatment. Data are expressed as the mean ± SD and n = 5 in each group; *P < .05 significant differences between each indicated group. NS, not significant
At the molecular level, melatonin increased the levels of early‐expressed osteogenic markers, including Runx2 and Osterix, at both the protein and gene levels, especially at 10 nM (Figure 3e,f). Expression of osteocalcin, a matricellular molecule that usually shows high expression in mature osteoblasts, was also enhanced as expected (Figure 3g). However, melatonin failed to impact the expression of another bone matrix marker, collagen I (Figure 3e,f). Furthermore, RANKL, which was secreted by stromal cells, such as bone marrow mesenchymal stem cells, and could activate osteoclastogenesis, was inhibited by melatonin (Figure 3h). In line with these findings, the opg/rankl ratio was elevated by melatonin in bone marrow mesenchymal stem cells (Figure 3i).
Both 10 nM and 100 nM were close to the physiological concentration of melatonin, so this study is more likely to reflect the real in vivo micro‐environment than other studies (Han, Kim, Kim, Bae, & Kim, 2017; Maria et al., 2018; Nakade, Koyama, Ariji, Yajima, & Kaku, 1999; Xu et al., 2018), in which much higher doses were used to demonstrate the functions of melatonin on bone. Our data indicated that melatonin at its physiological concentration was sufficient to promote the osteogenic differentiation of bone marrow mesenchymal stem cells and affect bone marrow mesenchymal stem cell‐mediated osteoclastogenesis, especially at 10 nM, which was used the concentration used in these experiments. In addition, TNF‐α‐induced osteogenesis suppression was attenuated in the presence of 10 nM melatonin (Figure S3).
3.3. MT2 receptor played the main role in melatonin‐regulated osteogenesis
To gain further insight into the mechanisms of how MT receptors modulated osteogenic differentiation, we knocked down MT1 or MT2 and validated the silencing efficiency (Figure S4).
After 7 days of osteogenic differentiation with melatonin, the alkaline phosphatase level increased significantly, but the improvement was absent after MT knockdown and more obviously in MT2‐deficient cells (Figure 4a–c). In line with these findings, the numbers of mineralization nodes were reduced in the MT‐deficient groups, especially in the shMT2 group, which was even fewer than the control group without melatonin (Figure 4d). The classical osteogenic markers, including Runx2, Osterix and osteocalcin,were enhanced with melatonin, but melatonin lost its positive influence on MT2‐deficient cells (Figure 4e–g). However, Osterix protein level also dropped obviously after MT1 knockdown, in addition to MT2 knockdown (Figure 4e), which suggested that there might be crosstalk between MT1 and MT2, which possibly led to this phenomenon.
Figure 4.
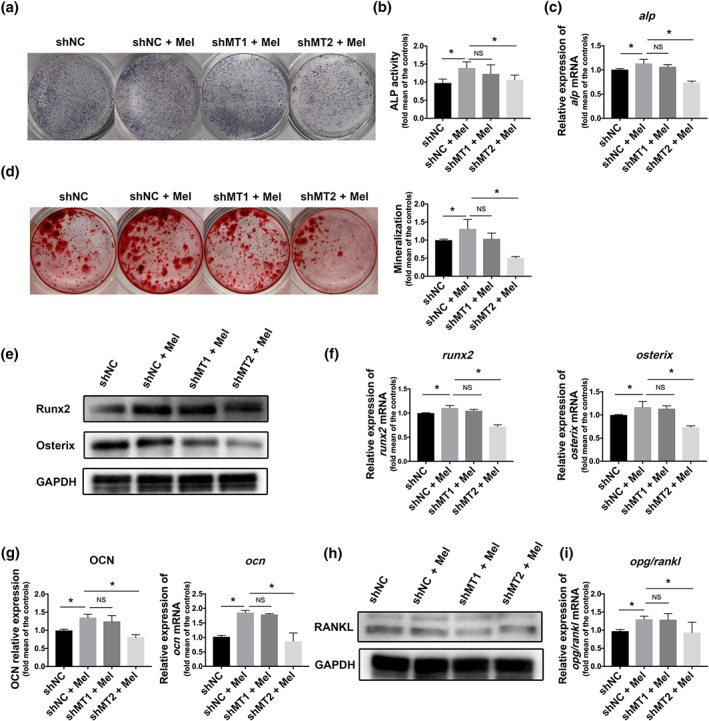
MT2 receptor played the main role in melatonin‐regulated osteogenesis. After MT1 or MT2 silencing with shRNA, bone marrow mesenchymal stem cells (BMMSCs) were treated with melatonin for different days as indicated before parameter determination. (a–c) alkaline phosphatase (ALP) staining (a), ALP activity (b), and alp mRNA (c) were determined in Bone marrow mesenchymal stem cells after melatonin treatment for 7 days. (d) Mineralization nodes were measured by alizarin red staining on the Day 21. (e, f) The expressions of Runx2 and Osterix were detected on Day 3 at the protein (e) and gene level (f). (g) osteocalcin (OCN) was detected on Day 14 at the protein and gene level. (h) RANKL production was measured by western blot. (i) The opg/rankl ratio was measured by qRT‐PCR, respectively. Data are expressed as the mean ± SD and n = 5 in each group; *P < .05, significant differences between each indicated group. NS, not significant. NC, negative control
For the RANKL, MT2 knockdown was more able to counteractthe function of melatonin than in the MT1 knockdown (Figure 4h), while the elevation in the opg/rankl ratio induced by melatonin was cancelled in the shMT2 knockdown cells (Figure 4i).
These data implied that for melatonin‐regulated osteogenesis and bone marrow mesenchymal stem cell‐mediated osteoclastogenesis, MT2 receptor possibly had a more predominant role than MT1 receptor.
3.4. MT2‐mediated NF‐κB inactivation was responsible for melatonin‐regulated osteogenesis
To confirm our supposition that NF‐κB signalling was involved in melatonin‐induced osteogenesis, we treated bone marrow mesenchymal stem cells with melatonin and found that NF‐κB signalling was obviously suppressed, as phosphorylated IκBα, p65, and IKKα/β were decreased (Figure 5a). Wondering the complete signalling network, we tested the status of NF‐κB signalling with MT1 or MT2 knockdown and surprisingly found that the suppressive effects on NF‐κB activation by melatonin were weakened predominantly in shMT2 group (Figure 5b).
Figure 5.
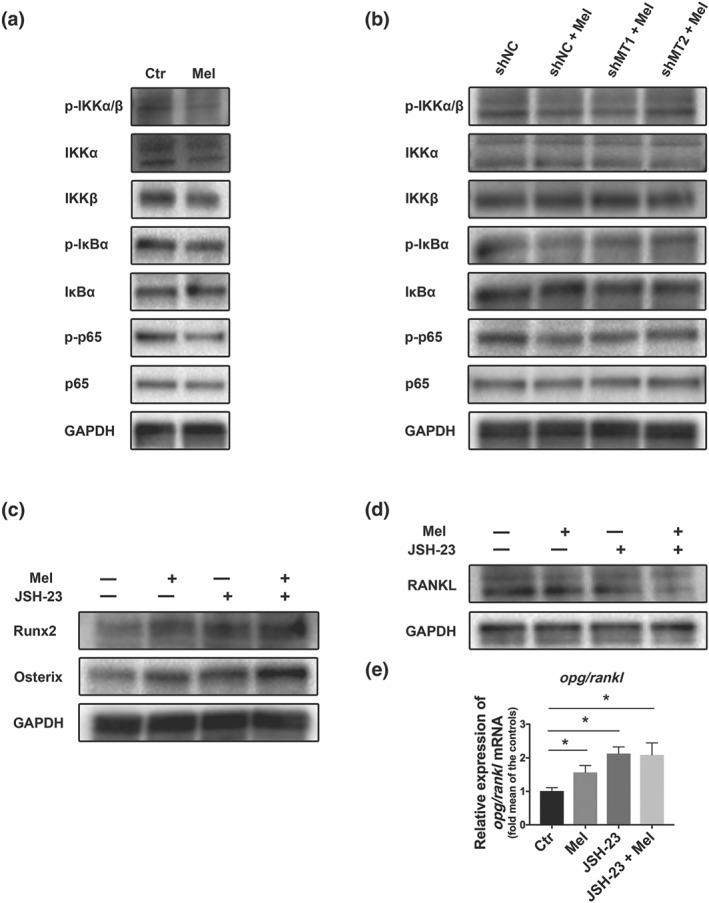
Melatonin promoted osteogenesis by inhibiting the MT2‐mediated NF‐κB pathway. (a–b) Expression levels of NF‐keppa B pathway proteins including p‐p65, p65, p‐IκBα, IκBα, p‐IKKα/β, IKKα, and IKKβ were detected in bone marrow mesenchymal stem cells (BMMSCs) treated without or with melatonin (a) or in MT1/MT2 pre‐silenced Bone marrow mesenchymal stem cells (b). (c–e) bone marrow mesenchymal stem cells were pretreated with or without the NF‐κB inhibitor JSH‐23 for 1 hr and then treated with or without melatonin before parameter detection. Runx2, Osterix (c) and RANKL (d) were examined by western blot, and the opg/rankl ratio (e) was examined by qRT‐PCR. Data are expressed as the mean ± SD and n = 5 in each group; *P < .05, significant differences between each indicated group
To further investigate the role of NF‐κB signals in bone marrow mesenchymal stem cells, we firstly inhibited the signalling using JSH‐23 (10 nM), and then treated the cells with melatonin, and it is meaningful to find much more NF‐κB inactivation and an increased osteogenic ability, as testified by elevated Runx2 and Osterix (Figure 5c). At the same time, with the NF‐κB signalling pathway inhibited by JSH‐23, RANKL production further declined (Figure 5d) and the opg/rankl ratio climbed up concomitantly (Figure 5e).
These analyses revealed that MT2‐mediated NF‐κB inactivation was an important axis during melatonin‐regulated osteogenesis and bone marrow mesenchymal stem cell‐mediated osteoclastogenesis.
3.5. Melatonin negatively regulated osteoclastogenesis by indirectly affecting the secreted cytokines of bone marrow mesenchymal stem cells through the MT2‐mediated NF‐κB pathway
The number of signals orchestrated by the complex interactions between osteoblasts and osteoclasts and the RANKL‐RANK axis, one of most classical signalling pathways, is unclear in the presence context. Although our above experiments have already demonstrated that melatonin reduced RANKL production through MT2‐mediated NF‐κB inactivation in bone marrow mesenchymal stem cells, how the RANKL‐RANK axis bridges bone marrow mesenchymal stem cells and osteoclasts still needs further investigation. Therefore, both direct‐contact and indirect‐contact co‐culture systems were established to gain a better understanding of the relationship between the above two kinds of cells.
The TRAP staining results showed that melatonin significantly decreased the osteoclast number and area in the indirect‐contact co‐culture system, did not in the direct‐contact co‐culture system (Figure 6b). In line with these findings, the expressions of several osteoclast‐related transcription factors, including cathepsin K and c‐Fos, declined with melatonin treatment in the indirect‐contact co‐culture system, but did not change in the direct‐contact co‐culture system (Figure 6c). However, melatonin at low concentrations did not change the differentiation and maturation of osteoclast precursors alone (Figure 6a. Therefore, we believed that melatonin suppressed osteoclastogenesis mainly by modulating the profile of cytokines secreted by bone marrow mesenchymal stem cells, which supports the view that melatonin is acting in a paracrine manner, not in a cell‐to‐cell direct‐contact manner.
Figure 6.
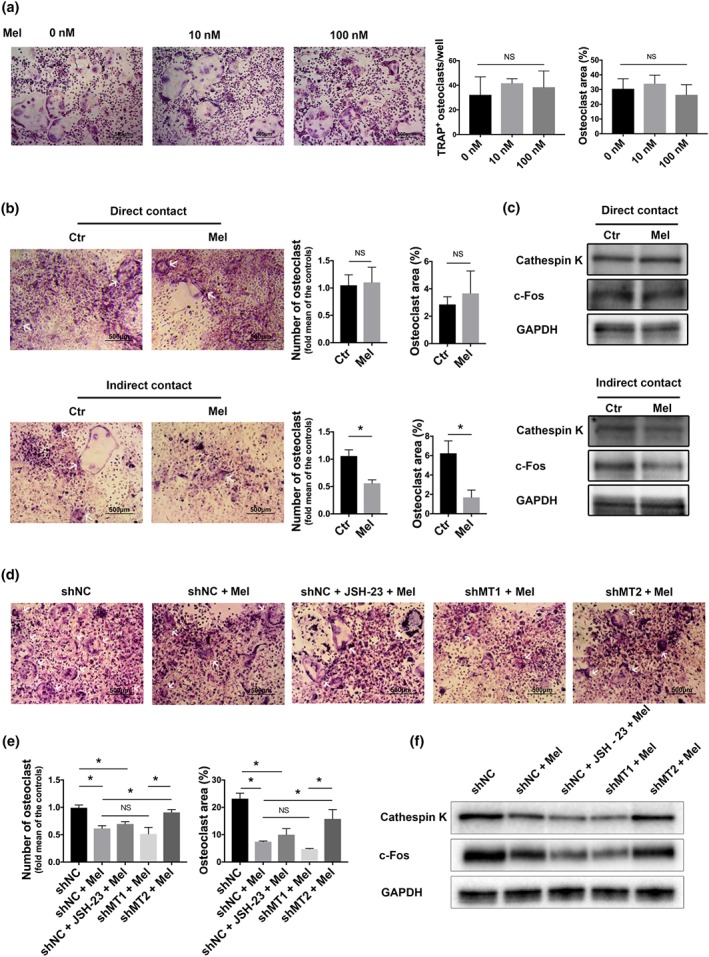
Osteoclastogenesis negatively regulated by melatonin was BMMSC dependent and was mediated by the MT2/NF‐κB pathway. (a) Osteoclastogenesis of primary bone marrow monocytes treated with different concentrations of melatonin was visualized by TRAP staining, and the number of osteoclasts and their area were determined. (b–c) In direct‐contact and indirect‐contact co‐culture systems, osteoclastogenesis of primary BMMs was visualized by TRAP staining, the number of osteoclasts and their area were determined (b), and the expression of cathepsin K and c‐Fos (osteoclastogenic markers) was measured by western blot (c). (d) In the indirect‐contact co‐culture system, bone marrow mesenchymal stem cells (BONE MARROW MONOCYTESCs)were pretreated with lentiviral shRNA transfer or JSH‐23, and the osteoclastogenesis of Bone marrow monocytes was determined by TRAP staining (d, e) and western blot (f). In b–f, the concentration of melatonin was 10 nM. Data are expressed as the mean ± SD, and n = 5 in each group; *P < .05, significant differences between each indicated group. NS, not significant. NC, negative control
To further identify whether the NF‐κB signalling pathway and melatonin receptors controlled osteoclastogenesis in a paracrine manner, we pretreated bone marrow mesenchymal stem cells with JSH‐23 or lentiviral shMT1/2. Keeping the same production pattern of RANKL in bone marrow mesenchymal stem cells (Figures 4h and 5d), mature osteoclasts became few after MT1‐knockdown or JSH‐23 treatment but not in the MT2‐knockdown group (Figure 6d–e). Collectively, these data demonstrated that melatonin affected bone marrow mesenchymal stem cell‐mediated osteoclastogenesis via the MT2‐mediated NF‐κB signalling pathway and possibly required the participation of the RANKL‐RANK axis.
4. DISCUSSION
Mounting evidence indicates that melatonin promotes osteogenic differentiation of bone marrow mesenchymal stem cells and osteoblasts at excessive concentrations (>1 μM) (Han, Kim et al., 2017; Xu, Zhang et al., 2018). However, it should be noted that these concentrations are much higher than the concentration of melatonin circulating in human blood (fluctuates around 10−9 M) (Man, Wang et al., 2010). Thus, in our work, we chose a relatively lower concentration of melatonin (10 nM), which was close to the physiological concentration, to imitate the in vivo conditions. Regarding varying concentrations of melatonin on osteogenesis, previous reports have revealed that melatonin dose‐dependently enhanced cell proliferation and the alkaline phosphatase activity of human osteoblasts and bone marrow mesenchymal stem cells without any cellular toxicity, even at the excessive concentration of 200 μM (Nakade et al., 1999; Satomura et al., 2007). Intriguingly and inconsistent with these studies, we found that only low doses (10–100 nM) stimulated osteogenesis, while excessive doses (1–100 μM) made no changes to osteogenesis and even inhibited this ability. We suggest the following reasons for this conflicting observation. First, the origins of cells were different, the bone marrow mesenchymal stem cells we used were isolated from C57BL/6J mice, and the high sensitivity of these primary cells enabled them to detect any small changes. Therefore, as Knani et al. reported, low‐dose melatonin was sufficient to affect the osteogenic/adipogenic differentiation balance in primary human adipose mesenchymal stem cells (Knani, Bartolini, et al., 2019). Second, ethanol was needed to dissolve the melatonin powder, but a higher concentrations of melatonin required more ethanol, which would actually inhibit cell proliferation (Figure S1B). However, the outcome of cell proliferation (Figure S1B) indicated that in addition to the negative effect of ethanol, other reasons must exist. As many pathways, such as MAPK, Wnt and PPAR‐γ, regulate the functions of melatonin in various biological processes (Luchetti et al., 2010; Luchetti et al., 2014; Murdolo et al., 2017), they may also contribute to these different responses between physiological and excessive melatonin levels. Moreover, our in vivo experiments also indicated that the low dose of melatonin administered (10 mg·kg−1 body weight per day), which approximated to physiological concentration, appeared to have better therapeutic effects than treatment with high drug dose (100 mg·kg−1 body weight per day).
It is well known that in certain conditions, bone marrow mesenchymal stem cells are able to differentiate into osteoblasts and trigger bone formation. Generally, the process of osteogenic differentiation is orderly divided into three stages: osteogenic lineage commitment, matrix synthesis and matrix mineralization (Coutu, Kokkaliaris, Kunz, & Schroeder, 2017; Franceschi, 1999). Notably, the expression of collagen I, a typical collagenous extracellular‐regulated molecule, increases in the initial stage and reaches the peak in the middle stage (Coutu et al., 2017; Franceschi, 1999). However, in our study, expression of collagen I remained unchanged by low doses (10 nM and 100 nM) of melatonin stimulation of osteogenic induction (Figure 3e,f), although several research articles have shown that melatonin could elevate the collagen I level (Satomura et al., 2007; Xu et al., 2018). The different specific microenvironments, varying drug concentrations (low or high) and diverse origins of cells, might be the reasons for the contradictory observations. We thus inferred that melatonin may facilitate osteogenesis mainly by affecting cell commitment and mineralization abilities at physiological concentrations, with no influence on extracellular matrix maturation.
In view of osteoimmunology, osteoporosis is considered as an immunological disorder with chronic inflammation. Except for anabolic effects on bone, melatonin is able to suppress bone loss by regulating immune disorder and reversing the abnormal cytokine framework back to normal status. Many studies have suggested that an excessive dose (1–100 μM) of melatonin could alleviate foreigner antigen‐induced negative nonspecific responses and inflammatory cytokines, including TNF‐α and IL‐1β, which helped to inhibit the acceleration of bone loss (Lian et al., 2016; Liu et al., 2013; Ping et al., 2017). However, Zhang et al. found that a lower concentration of melatonin played a more beneficial role (50 mg·kg−1 was better than 100 mg·kg−1) in improving bone microstructure in type 2 diabetic osteoporosis rats by regulating bone immunity (Zhang et al., 2016). Our work confirmed that the anti‐inflammatory effect of melatonin in osteoporotic mice functioned in a concentration‐dependent manner by suppressing serum TNF‐α (Figure 2f). In contrast to low concentration (10 mg·kg−1 body weight per day) of melatonin having more significant bone anabolic effects, melatonin at high concentration (100 mg·kg−1 body weight per day) was more helpful in exerting anti‐inflammatory properties and anti‐resorption effects to regain bone loss (Figures 1 and 2).
Melatonin receptors are GPCRs with seven transmembrane structures. Melatonin stimulation will induce various G proteins, including Gαi/o, Gαq and Gαs, to localize to MT receptors; this process is controlled by the regulators of G protein signalling (RGS) (Alkozi, Sanchez Montero, Doadrio, & Pintor, 2018; Druey, 2017). Based on the intimate relationship between Gαi and adenylate cyclase, it is not surprising to find that the cAMP/PKA pathway takes part in the downstream events of melatonin (Alkozi et al., 2018). Moreover, the PKC/ERK, PI3K/Akt and PLC/IP3 pathways also help to orchestrate melatonin‐mediated signalling cascades (Alkozi et al., 2018; Oishi, Cecon, & Jockers, 2018). For the NF‐κB pathway, although there is no direct evidence supporting that it can regulate the melatonin response, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2818, whose target is Gαi/o, negatively regulates the NF‐κB pathway (Lee, Chung, McAlpine, & Tansey, 2011), and Gαq is also relevant to NF‐κB signalling in controlling cancer cell functions (Peeters et al., 2015). Thus, NF‐κB signalling might be a crucial downstream pathway once MT receptors are activated. As a first step, we showed that NF‐ κB signalling, a classic and essential axis in osteoclastogenesis, was not only involved in melatonin‐induced bone anti‐absorption as reported (Kim et al., 2017) but also participated in bone anabolic effects (Figure 5). Therefore, NF‐κB signalling coupled the process of osteogenesis and osteoclastogenesis after melatonin treatment.
There are three defined subtypes of MT receptors, among which MT1 and MT2 share a high degree (60%) of sequence homology and are found in humans and other mammals (Luo et al., 2019). However, non‐mammalian MT3 has a high affinity to https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5451 (the precursor of melatonin) but not for melatonin (Jang et al., 2010). As reported, MT2 is a main receptor subtype in regulating bone metabolism by melatonin treatment and MT2 gene polymorphism (Man et al., 2010) or MT2 knockout (Sharan, Lewis et al., 2017) is majorially associated with abnormal skeletal growth, low bone mineral density and increased bone loss by affecting bone‐forming cell proliferation and differentiation (Man et al., 2010; Sethi et al., 2010). Although MT1 receptor is also expressed in bone tissue (Luo et al., 2019), it seems to have little impact on bone metabolism (Sharan et al., 2017). Consistent with the integral involvement of MT2 receptor in bone metabolism, we have also confirmed the major role of MT2 receptor. Furthermore, the dominant capacity of MT2 receptor in the bone system is actually predictable, as the different distributions of MT receptors indicate there different functions and MT1 receptor is always regarded as a vital target in CNS diseases, while MT2 receptor is widely distributed in various organs including the bone, brain, liver, and retina (Luo et al., 2019). In addition, in the immune system, MT1 receptor is the primary target in acquired immune responses, while MT2 receptor is mainly for innate immune responses (Jockers et al., 2016). Therefore, we believe that in osteoporosis, a chronic inflammatory disease, the role of MT2 receptor is more striking.
In the bone micro‐environment, a complicated signalling network, such as the OPG/RANKL/RANK axis, bridges osteoblast lineage cells and osteoclast lineage cells. By examining serum from osteoporosis mice, we found that the RANKL level increased in osteoporotic conditions and that melatonin treatment could effectively reduce this level to the normal state (Figure 2E). Regrettably, we failed to detect the serum OPG level by ELISA, but bone tissue PCR data showed a potential tendency of melatonin to able to rescue the opg/rankl gene expression ratio (Figure 2a). Therefore, we speculated that melatonin might affect the interaction between osteoblast lineage cells and osteoclast lineage cells via OPG/RANKL/RANK axis. As expected, melatonin in vitro also dampened the production and secretion of RANKL via the MT2‐mediated NF‐κB signalling pathway (Figures 3h, 4h, and 5b,d). Since cells can communicate with each other via direct cell‐to‐cell contact or by the secretion of paracrine factor, we imitated these two communication modes and demonstrated that the paracrine secretion model of cytokines (such as RANKL) was responsible for osteoblastic and osteoclastic signal exchange (Figure 6b). In a direct‐contact model, the existence of cell‐to‐cell contact, such as EphrinB2‐EphB2/B4 and gap junctions between osteoclasts and osteoblasts, may impair RANKL/RANK signalling and lead to the indifference in osteoclastogenesis (Zhu et al., 2018). Inconsistent with our results, Maria et al. believed that melatonin (50 nM) inhibited osteoclastogenesis only through direct cell contact in osteogenic induction condition (Maria et al., 2018). Perhaps, the different substances are involved in osteogenic induction along with different concentrations of melatonin and there may be different ways in using Transwell plates which result in different outcomes.
Although the positive effect of melatonin on the osteogenic differentiation of human mesenchymal stem cells has been investigated and affirmed in several studies (Knani, Bartolini et al., 2019; Lian, Wu et al., 2016), interspecies interpretation and application from mice to humans should be done with great care, and our work further consolidated the perspective of the bone protection effects of melatonin on osteoporosis and we have determine more completely mechanisms involved. In conclusion, our study shows that melatonin exerts dual anti‐inflammatory and bone‐protective effects in osteoporotic mice. Mechanistically, to our knowledge, this is the first study demonstrating that the MT2‐mediated NF‐κB signalling pathway in bone marrow mesenchymal stem cells is involved in the osteogenic and anti‐osteoclastogenic effects of melatonin and it is the paracrine cytokine secretion which regulates the RANKL‐RANK system, by which melatonin indirectly affects osteoclastogenesis by influencing RANKL production (Figure 7). Taken together, we demonstrated that melatonin could successfully rescue the osteoporotic process by accelerating bone marrow mesenchymal stem cells osteogenic differentiation via suppressing MT2 receptor‐dependent NF‐κB signalling pathway and down‐regulating osteoclastogenesis via RANKL paracrine secretion in the physiological environment. More validations and conclusive supporting evidence from clinical trials are urgently needed before melatonin can be selected as a more effective alternative in the therapy of postmenopausal osteoporosis, in addition to the previous anti‐resorptive and anabolic drugs, such as bisphosphonates and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6886.
Figure 7.
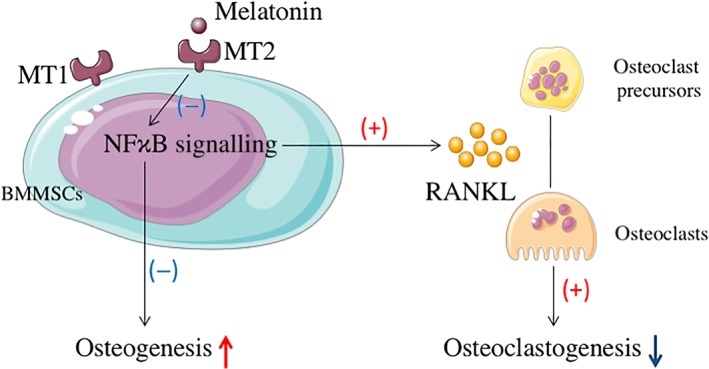
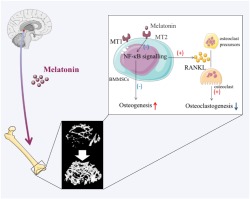
A schematic model depicting MT2‐mediated NF‐κΒ signalling regulates the functions of bone marrow mesenchymal stem cells (BMMSCs) and the relationship between bone marrow mesenchymal stem cells and osteoclasts in the bone micro‐environment. Melatonin augments the osteogenic differentiation of Bone marrow mesenchymal stem cells and declines the production of RANKL in Bone marrow mesenchymal stem cells by inhibiting MT2‐mediated NF‐κΒ signalling. Then bone marrow mesenchymal stem cells affect the maturation and functions of osteoclasts via RANKL paracrine secretion
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Y.Z. C.W., and H.W. conceived and designed the experiments. Y.Z. and H.W. supervised the study. C.W. and J.S. performed the experiments and analysed the data. D.Z. and B.W. helped in vitro experiments. D.D. and J.Z. helped in vivo experiments. C.W. wrote the manuscript. Y.Z. and H.W. made manuscript revisions.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1. The sequences of shMT1, shMT2 and shNC
Table S2. Quantitative real‐time RT–PCR primers
Figure S1. Melatonin regulated osteogenic differentiation and the cell proliferation of Bone marrow mesenchymal stem cells. (A) Bone marrow mesenchymal stem cells (BMMSCs) were treated with different concentrations of melatonin in osteogenic induction medium for 7 days, and then alkaline phosphatase (ALP) staining was analysed. (B) BMMSC proliferation was detected by CCK8 after treatment with PBS (Ctr group), ethanol (equal dose to that in Mel 100 μM group) or 100 μM melatonin (Mel 100 μM group) for 3, 5 or 7 days. Data are expressed as the mean ± SD, and n = 5 in each group; * P < 0.05, significant differences between each indicated group. NS, not significant.
Figure S2. Bone marrow mesenchymal stem cells(BMMScs) proliferation and apoptosis were investigated with various low‐dose melatonin treatments. (A) BMMSC proliferations was determined by CCK8 after different doses of melatonin treatment for 3, 5 or 7 days. (B, C) Apoptosis was detected by flow cytometry after melatonin treatment for 48 hours. Data are expressed as the mean ± SD, and n = 5 in each group; * P < 0.05, significant differences between each indicated group. NS, not significant.
Figure S3. Melatonin alleviated TNF‐α‐induced osteogenic downregulation. Bone marrow mesenchymal stem cells were treated with TNF‐α in osteogenic induction medium with or without melatonin supplementation. (A) Alkaline phosphatase (ALP) activity was determined on day 7. (B) Runx2 and Osterix protein expressions were detected on day 3. Data are expressed as the mean ± SD, and n = 6 in each group; * P < 0.05, significant differences between each indicated group.
Figure S4. Efficiency of lentiviral shRNA transfection. Three sequences were designed for each melatonin receptor (MT). (A‐B) The protein and gene levels of MT1 (A) and MT2 (B) were detected. MT1–367 and MT2–306 were finally used in the following experiments. Data are expressed as the mean ± SD, and n = 5 in each group; * P < 0.05, significant differences between each indicated group. NS, not significant. NC, negative control.
Data S1
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grants 31671763, 81801640, and 81600909) and the National Natural Science Foundation of Zhejiang Province of China (Grant Y19H140018). We thank Qingxiao Chen, Jing Chen, Ruyi Xu, and Xi Huang for their technical helps.
Zhou Y, Wang C, Si J, et al. Melatonin up‐regulates bone marrow mesenchymal stem cells osteogenic action but suppresses their mediated osteoclastogenesis via MT2‐inactivated NF‐κB pathway. Br J Pharmacol. 2020;177:2106–2122. 10.1111/bph.14972
Yi Zhou and Chaowei Wang should be considered joint first author.
Contributor Information
Yi Zhou, Email: zyuthscsa@zju.edu.cn.
Huiming Wang, Email: whmwhm@zju.edu.cn.
REFERENCES
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Pawson, A. J. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Sharman, J. L. (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176(Suppl 1), S247–S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Sharman, J. L. (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176(Suppl 1), S297–S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … Southan, C. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkozi, H. A. , Sanchez Montero, J. M. , Doadrio, A. L. , & Pintor, J. (2018). Docking studies for melatonin receptors. Expert Opin Drug Discov, 13(3), 241–248. 10.1080/17460441.2018.1419184 [DOI] [PubMed] [Google Scholar]
- Barbour, K. E. , Lui, L. Y. , Ensrud, K. E. , Hillier, T. A. , LeBlanc, E. S. , Ing, S. W. , … Study of Osteoporotic Fractures (SOF) Research Group (2014). Inflammatory markers and risk of hip fracture in older white women: The study of osteoporotic fractures. Journal of Bone and Mineral Research, 29(9), 2057–2064. 10.1002/jbmr.2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X. (2011). Targeting osteoclast‐osteoblast communication. Nature Medicine, 17(11), 1344–1346. 10.1038/nm.2499 [DOI] [PubMed] [Google Scholar]
- Chang, J. , Wang, Z. , Tang, E. , Fan, Z. , McCauley, L. , Franceschi, R. , … Wang, C. Y. (2009). Inhibition of osteoblastic bone formation by nuclear factor‐κB. Nature Medicine, 15(6), 682–689. 10.1038/nm.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu, D. L. , Kokkaliaris, K. D. , Kunz, L. , & Schroeder, T. (2017). Three‐dimensional map of nonhematopoietic bone and bone‐marrow cells and molecules. Nature Biotechnology, 35(12), 1202–1210. 10.1038/nbt.4006 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druey, K. M. (2017). Emerging roles of regulators of G protein signaling (RGS) proteins in the immune system. Advances in Immunology, 136, 315–351. [DOI] [PubMed] [Google Scholar]
- Franceschi, R. T. (1999). The developmental control of osteoblast‐specific gene expression: Role of specific transcription factors and the extracellular matrix environment. Critical Reviews in Oral Biology and Medicine, 10(1), 40–57. 10.1177/10454411990100010201 [DOI] [PubMed] [Google Scholar]
- Han, Y. , Kim, Y. M. , Kim, H. S. , & Lee, K. Y. (2017). Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Scientific Reports, 7(1), 5716‐5726. 10.1038/s41598-017-06304-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Bryant, C. (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. , Zhang, L. , Yang, Y. , Guo, Y. , Fan, Y. , Zhang, M. , … Wang, H. (2017). Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. Journal of Pineal Research, 62(1), 12368‐12380. [DOI] [PubMed] [Google Scholar]
- Jang, S. W. , Liu, X. , Pradoldej, S. , Tosini, G. , Chang, Q. , Iuvone, P. M. , & Ye, K. (2010). N‐acetylserotonin activates TrkB receptor in a circadian rhythm. Proceedings of the National Academy of Sciences of the United States of America, 107(8), 3876–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers, R. , Delagrange, P. , Dubocovich, M. L. , Markus, R. P. , Renault, N. , Tosini, G. , … Zlotos, D. P. (2016). Update on melatonin receptors: IUPHAR review 20. British Journal of Pharmacology, 173(18), 2702–2725. 10.1111/bph.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160(7), 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Kim, H. J. , Bae, M. K. , & Kim, Y. D. (2017). Suppression of osteoclastogenesis by melatonin: A melatonin receptor‐independent action. International Journal of Molecular Sciences, 18(6), 1142‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knani, L. , Bartolini, D. , Kechiche, S. , Tortoioli, C. , Murdolo, G. , Moretti, M. , … Galli, F. (2019). Melatonin prevents cadmium‐induced bone damage: First evidence on an improved osteogenic/adipogenic differentiation balance of mesenchymal stem cells as underlying mechanism. Journal of Pineal Research, 67(3), 12597‐12610. 10.1111/jpi.12597 [DOI] [PubMed] [Google Scholar]
- Lee, J. K. , Chung, J. , McAlpine, F. E. , & Tansey, M. G. (2011). Regulator of G‐protein signaling‐10 negatively regulates NF‐κB in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats. The Journal of Neuroscience, 31(33), 11879–11888. 10.1523/JNEUROSCI.1002-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian, C. , Wu, Z. , Gao, B. , Peng, Y. , Liang, A. , Xu, C. , … Huang, D. (2016). Melatonin reversed tumor necrosis factor‐α‐inhibited osteogenesis of human mesenchymal stem cells by stabilizing SMAD1 protein. Journal of Pineal Research, 61(3), 317–327. 10.1111/jpi.12349 [DOI] [PubMed] [Google Scholar]
- Liu, P. , Lee, S. , Knoll, J. , Rauch, A. , Ostermay, S. , Luther, J. , … Tuckermann, J. P. (2017). Loss of menin in osteoblast lineage affects osteocyte‐osteoclast crosstalk causing osteoporosis. Cell Death and Differentiation, 24(4), 672–682. 10.1038/cdd.2016.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Gong, Y. , Xiong, K. , Ye, Y. , Xiong, Y. , Zhuang, Z. , … He, F. (2013). Melatonin mediates protective effects on inflammatory response induced by interleukin‐1β in human mesenchymal stem cells. Journal of Pineal Research, 55(1), 14–25. 10.1111/jpi.12045 [DOI] [PubMed] [Google Scholar]
- Luchetti, F. , Canonico, B. , Bartolini, D. , Arcangeletti, M. , Ciffolilli, S. , Murdolo, G. , … Galli, F. (2014). Melatonin regulates mesenchymal stem cell differentiation: A review. Journal of Pineal Research, 56(4), 382–397. [DOI] [PubMed] [Google Scholar]
- Luchetti, F. , Canonico, B. , Betti, M. , Arcangeletti, M. , Pilolli, F. , Piroddi, M. , … Galli, F. (2010). Melatonin signaling and cell protection function. The FASEB Journal, 24(10), 3603–3624. 10.1096/fj.10-154450 [DOI] [PubMed] [Google Scholar]
- Luo, C. , Yang, Q. , Liu, Y. , Zhou, S. , Jiang, J. , Reiter, R. J. , … Wang, X. (2019). The multiple protective roles and molecular mechanisms of melatonin and its precursor N‐acetylserotonin in targeting brain injury and liver damage and in maintaining bone health. Free Radical Biology & Medicine, 130, 215–233. 10.1016/j.freeradbiomed.2018.10.402 [DOI] [PubMed] [Google Scholar]
- Man, G. C. W. , Wang, W. W. J. , Yeung, B. H. Y. , Lee, S. K. M. , Ng, B. K. A. , Hung, W. Y. , … Cheng, J. C. (2010). Abnormal proliferation and differentiation of osteoblasts from girls with adolescent idiopathic scoliosis to melatonin. Journal of Pineal Research, 49(1), 69–77. [DOI] [PubMed] [Google Scholar]
- Maria, S. , Samsonraj, R. M. , Munmun, F. , Glas, J. , Silvestros, M. , Kotlarczyk, M. P. , … Lassila, H. (2018). Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin‐mediated osteoblastogenesis. Journal of Pineal Research, 64(3), 12465‐12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdolo, G. , Bartolini, D. , Tortoioli, C. , Piroddi, M. , Torquato, P. , & Galli, F. (2017). Selenium and cancer stem cells. Advances in Cancer Research, 136, 235–257. [DOI] [PubMed] [Google Scholar]
- Nakade, O. , Koyama, H. , Ariji, H. , Yajima, A. , & Kaku, T. (1999). Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. Journal of Pineal Research, 27(2), 106–110. [DOI] [PubMed] [Google Scholar]
- Novack, D. V. (2011). Role of NF‐κB in the skeleton. Cell Research, 21(1), 169–182. 10.1038/cr.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi, A. , Cecon, E. , & Jockers, R. (2018). Melatonin receptor signaling: Impact of receptor oligomerization on receptor function. International Review of Cell and Molecular Biology, 338, 59–77. [DOI] [PubMed] [Google Scholar]
- Peeters, M. C. , Fokkelman, M. , Boogaard, B. , Egerod, K. L. , van de Water, B. , IJzerman, A. P. , & Schwartz, T. W. (2015). The adhesion G protein‐coupled receptor G2 (ADGRG2/GPR64) constitutively activates SRE and NFκB and is involved in cell adhesion and migration. Cellular Signalling, 27(12), 2579–2588. [DOI] [PubMed] [Google Scholar]
- Ping, Z. , Hu, X. , Wang, L. , Shi, J. , Tao, Y. , Wu, X. , … Geng, D. (2017). Melatonin attenuates titanium particle‐induced osteolysis via activation of Wnt/β‐catenin signaling pathway. Acta Biomaterialia, 51, 513–525. 10.1016/j.actbio.2017.01.034 [DOI] [PubMed] [Google Scholar]
- Rachner, T. D. , Khosla, S. , & Hofbauer, L. C. (2011). Osteoporosis: Now and the future. Lancet, 377(9773), 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomura, K. , Tobiume, S. , Tokuyama, R. , Yamasaki, Y. , Kudoh, K. , Maeda, E. , & Nagayama, M. (2007). Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. Journal of Pineal Research, 42(3), 231–239. 10.1111/j.1600-079X.2006.00410.x [DOI] [PubMed] [Google Scholar]
- Sethi, S. , Radio, N. M. , Kotlarczyk, M. P. , Chen, C. T. , Wei, Y. H. , Jockers, R. , & Witt‐Enderby, P. A. (2010). Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. Journal of Pineal Research, 49(3), 222–238. [DOI] [PubMed] [Google Scholar]
- Sharan, K. , Lewis, K. , Furukawa, T. , & Yadav, V. K. (2017). Regulation of bone mass through pineal‐derived melatonin‐MT2 receptor pathway. Journal of Pineal Research, 63(2), 12423‐12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani, M. , & Nadri, S. (2009). A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nature Protocols, 4(1), 102–106. 10.1038/nprot.2008.221 [DOI] [PubMed] [Google Scholar]
- Tilg, H. , Moschen, A. R. , Kaser, A. , Pines, A. , & Dotan, I. (2008). Gut, inflammation and osteoporosis: Basic and clinical concepts. Gut, 57(5), 684–694. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Zhou, Y. , Guan, X. , Yu, M. , & Wang, H. (2018). β‐Estradiol antagonizes the inhibitory effects of caffeine in Bone marrow mesenchymal stem cells via the ERβ‐mediated cAMP‐dependent PKA pathway. Toxicology, 394, 1–10. 10.1016/j.tox.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Witt‐Enderby, P. A. , Slater, J. P. , Johnson, N. A. , Bondi, C. D. , Dodda, B. R. , Kotlarczyk, M. P. , … Davis, V. L. (2012). Effects on bone by the light/dark cycle and chronic treatment with melatonin and/or hormone replacement therapy in intact female mice. Journal of Pineal Research, 53(4), 374–384. 10.1111/j.1600-079X.2012.01007.x [DOI] [PubMed] [Google Scholar]
- Xu, L. , Zhang, L. , Wang, Z. , Li, C. , Li, S. , Li, L. , … Zheng, L. (2018). Melatonin suppresses estrogen deficiency‐induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome. Calcified Tissue International, 103(4), 400–410. 10.1007/s00223-018-0428-y [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Jiang, S. , Dong, Y. , Fan, C. , Zhao, L. , Yang, X. , … Qu, Y. (2015). Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1‐dependent mechanism during ischemic‐stroke in mice. Journal of Pineal Research, 58(1), 61–70. 10.1111/jpi.12193 [DOI] [PubMed] [Google Scholar]
- Yu, L. , Gong, B. , Duan, W. , Fan, C. , Zhang, J. , Li, Z. , … Zhang, M. (2017). Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: Role of AMPK‐PGC‐1α‐SIRT3 signaling. Scientific Reports, 7, 41337‐41349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L. , Sun, Y. , Cheng, L. , Jin, Z. , Yang, Y. , Zhai, M. , … Zhang, Y. (2014). Melatonin receptor‐mediated protection against myocardial ischemia/reperfusion injury: Role of SIRT1. Journal of Pineal Research, 57(2), 228–238. [DOI] [PubMed] [Google Scholar]
- Zhang, W. L. , Meng, H. Z. , Yang, R. F. , Yang, M. W. , Sun, G. H. , Liu, J. H. , … Yang, B. (2016). Melatonin suppresses autophagy in type 2 diabetic osteoporosis. Oncotarget, 7(32), 52179–52194. 10.18632/oncotarget.10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , An, R. , Yang, Y. , Yang, X. , Liu, H. , Yue, L. , … Qu, Y. (2015). Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: The role of SIRT1 signaling. Journal of Pineal Research, 59(2), 230–239. [DOI] [PubMed] [Google Scholar]
- Zhu, S. , Ehnert, S. , Rouss, M. , Haussling, V. , Aspera‐Werz, R. H. , Chen, T. , & Nussler, A. K. (2018). From the clinical problem to the basic research—Co‐culture models of osteoblasts and osteoclasts. International Journal of Molecular Sciences, 19(8), 2284‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The sequences of shMT1, shMT2 and shNC
Table S2. Quantitative real‐time RT–PCR primers
Figure S1. Melatonin regulated osteogenic differentiation and the cell proliferation of Bone marrow mesenchymal stem cells. (A) Bone marrow mesenchymal stem cells (BMMSCs) were treated with different concentrations of melatonin in osteogenic induction medium for 7 days, and then alkaline phosphatase (ALP) staining was analysed. (B) BMMSC proliferation was detected by CCK8 after treatment with PBS (Ctr group), ethanol (equal dose to that in Mel 100 μM group) or 100 μM melatonin (Mel 100 μM group) for 3, 5 or 7 days. Data are expressed as the mean ± SD, and n = 5 in each group; * P < 0.05, significant differences between each indicated group. NS, not significant.
Figure S2. Bone marrow mesenchymal stem cells(BMMScs) proliferation and apoptosis were investigated with various low‐dose melatonin treatments. (A) BMMSC proliferations was determined by CCK8 after different doses of melatonin treatment for 3, 5 or 7 days. (B, C) Apoptosis was detected by flow cytometry after melatonin treatment for 48 hours. Data are expressed as the mean ± SD, and n = 5 in each group; * P < 0.05, significant differences between each indicated group. NS, not significant.
Figure S3. Melatonin alleviated TNF‐α‐induced osteogenic downregulation. Bone marrow mesenchymal stem cells were treated with TNF‐α in osteogenic induction medium with or without melatonin supplementation. (A) Alkaline phosphatase (ALP) activity was determined on day 7. (B) Runx2 and Osterix protein expressions were detected on day 3. Data are expressed as the mean ± SD, and n = 6 in each group; * P < 0.05, significant differences between each indicated group.
Figure S4. Efficiency of lentiviral shRNA transfection. Three sequences were designed for each melatonin receptor (MT). (A‐B) The protein and gene levels of MT1 (A) and MT2 (B) were detected. MT1–367 and MT2–306 were finally used in the following experiments. Data are expressed as the mean ± SD, and n = 5 in each group; * P < 0.05, significant differences between each indicated group. NS, not significant. NC, negative control.
Data S1


