Abstract
INTRODUCTION:
Stroke causes physiologic functional changes such as vascular resistance and arterial remodeling. This study aimed to explore the effects of 3-month regular home-based exercise rehabilitation on brachial flow-mediated dilation (FMD), daily physical activity, and upper and lower extremity sensorimotor of the acute ischemic stroke patients.
MATERIALS AND METHODS:
This randomized control trial was done from August 2017 to September 2018. Patients with unilateral ischemic stroke were recruited from inpatient wards at an educational hospital. Patients were randomly assigned to the home-based rehabilitation program (intervention group) or usual care (control group). Fugl-Meyer upper and lower extremity sensorimotor score and Barthel score were evaluated in both the groups before and 3 months after baseline assessment. Furthermore, brachial artery vasomotor reactivity (FMD) hemiparetic arm assessed vascular health. The intervention group received home-based rehabilitation exercise program for 3 months. The control group did not receive home-based rehabilitation program and incentive telephone call. All data were collected and analyzed by SPSS software (version 20) and appropriate statistical tests.
RESULTS:
Forty ischemic stroke patients (twenty in the intervention group and twenty in the control group) were examined. Results showed that Barthel score and Fugl-Meyer upper and lower extremity score and FMD in the intervention group were significantly higher than the control group after 3-month home-based exercise rehabilitation (P < 0/001).
CONCLUSION:
Twelve-week regular home-based exercise training was well tolerated by the intervention group. After this period, improvements were reported in brachial FMD as well as daily physical activity and upper and lower extremity functional capacity.
Keywords: Flow-mediated dilation, health promotion, stroke
Introduction
Stroke is mainly a disease of the arterial blood flow of the brain. Changes in the vascular system in the stroke-affected limb may impact physical performance. Impairment in endothelium-dependent vasodilatation could be a risk factor for stroke or transient ischemic attack.[1,2] The endothelium is an essential organ in regulation of vasomotor tone and vascular homeostasis. Endothelial dysfunction was evaluated by flow-mediated dilation (FMD). Brachial artery FMD reflects endothelium-dependent vasodilation function. FMD is an important stimulus-regulating vascular tone and homeostasis of the peripheral circulation by synthesizing nitric oxide (NO). NO has been shown to play a key role in the maintenance of vasodilator tone of the blood vessels. Endothelial dysfunction results in reduced NO availability, causing reduced vasodilatation. In acute ischemic stroke, a reduction FMD is an independent predictor for new-onset vascular events.[3,4] Brachial FMD is a physiological, noninvasive, and validated assessment for detecting endothelial dysfunction.[5]
FMD is expressed as the peak percentage change in arterial diameter (%FMD) in response to a hyperemic shear stress after temporary flow occlusion. Shear stress is directly related to the velocity and the viscosity of the blood but inversely related to the vessel diameter.[5,6]
Stroke causes physiologic functional changes such as vascular resistance and arterial remodeling. Moreover, peripheral vascular adaptations such as reduced blood flow, decreased arterial diameter, and endothelial dysfunction in the hemiparetic side have been observed as a result of stroke.[7]
It is confirmed that regular exercise training has a protective effect on endothelial function through the activation of endothelial NO synthase.[8]
An article reported that 8-week moderate-high exercise training using a recumbent stepper has significant improvements in brachial artery vasomotor reactivity (FMD) as well as physical performance (6-min walk test) in the subacute stroke patients.[9]
Another study revealed that exercise increases cerebral perfusion and reduces infarction size by expression of endothelial NO after experimental ischemia in the mouse model.[10]
Another article demonstrated that home-based rehabilitation produced greater gains and higher rates of functional independence, ability, and quality of life than did usual care.[11,12]
This study aimed to explore the effects of 3-month regular home-based exercise rehabilitation on brachial FMD, daily physical activity, and upper and lower extremity sensorimotor of the acute ischemic stroke patients.
Materials and Methods
Study design and patients
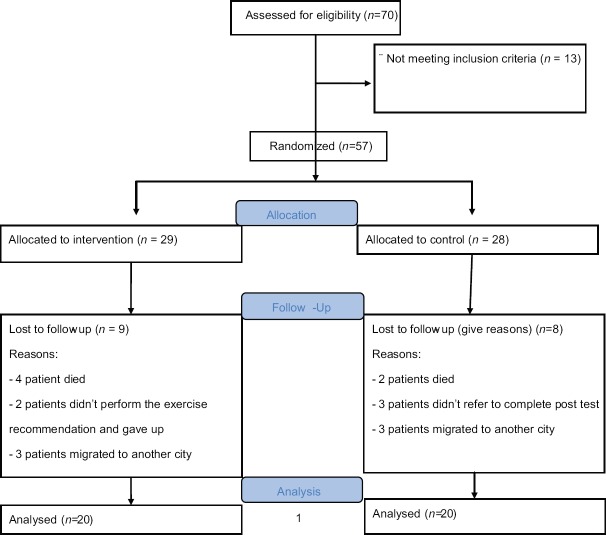
This prospective, randomized, parallel, controlled trial was done from August 2017 to September 2018 in Alzahra Educational Hospital, Isfahan, Iran. Forty patients with unilateral ischemic stroke were recruited using convenience sampling [Figure 1]. A computer random number generator was used to generate the random sequence for group allocation (twenty patients in each group). An investigator who was blinded to patient selection kept the random sequence and allocated the participants to intervention and control at a 1:1 ratio. The main inclusion criteria for the trial were the following: patients and their caregivers' agreement to participate, first ischemic stroke within 24–72 h, persistent hemiparesis in the upper and lower extremities, and 7 ≤National Institutes of Health Stroke Scale (NIHSS) ≤20.[13] Patients with uncontrolled hypertension, heart diseases, severe dysphasia and cognitive impairment, pulmonary embolus, and dependency before stroke were excluded from the study.
Figure 1.
CONSORT Flow Diagram
Measures
Baseline demographic data including age, gender, medical history, physical examination, and phone number were asked by the assessor. Then, NIHSS, Fugl-Meyer upper and lower extremity sensorimotor score, and FMD were evaluated in both the groups before and 3 months after baseline assessment.[14,15]
Flow-mediated dilation measurement
All measurements of brachial artery diameter and FMD were performed in the morning in the quiet room. Patients were asked to be overnight fasting for 12 h (water was permitted). They were resting supine for 20 min before the FMD procedure. The hemiparetic arm was immobilized in the extending position, and a cuff was placed just distal to the olecranon process and allowing for ultrasound scanning of the brachial artery 2–3 cm above the antecubital fossa. 30.31 The cuff was inflated to suprasystolic pressure (220 mmHg) and maintained for 5 min. Twenty seconds before cuff deflation, recording of diameter and blood flow velocity was resumed. At 5 min, the cuff was deflated while ultrasound images continued to be recorded for another 3 min. All images were collected on a computer and analyzed offline using specialized software (Brachial Analyzer, Medical Imaging Applications, Coralville, Iowa). The brachial artery was scanned longitudinally using an HDI 5000 Ultrasound Instrument (Philips Medical Systems, Bothell, WA, USA) with a 5–12 MHz linear array transducer. Once a satisfactory image of the brachial artery was obtained, the transducer was stabilized using a custom-designed holder. If needed, minor adjustments were made to the transducer placement for optimal imaging. We also captured Doppler velocity measurements at an insonation angle of 60° using the ultrasound system. Baseline diameter and blood flow velocity were recorded continuously for 10 s.
This edge-detection software allows the operator to identify a region of interest at a specific, designated area of the vessel. The automated software identified the near and far wall properties of the vessel and track diameter changes.
Absolute FMD (mm) = (peak postocclusion diameter) − (preocclusion diameter)
Relative FMD (%) = (absolute FMD ÷ [preocclusion diameter]) ×100[16]
Intervention
The intervention group received home-based rehabilitation exercise program which was recorded on DVD and delivered to the patients at discharge time. Rehabilitation program was designed by the expert exercise physiologist and physical therapist for 3 months. The home-based rehabilitation program was progressive exercise training which includes passive exercise, active exercise, resistance exercise, and endurance exercise to improve flexibility, strength, endurance, and balance and to encourage more use of the affected extremity. The 1st-month exercises, which included stretching and flexibility, were done with the help of caregivers. In the 2nd month, endurance and little resistance exercises were designed in the sitting position that according to the development of the patient's functional capacity, most of the movements were performed independently by the patient himself. In the 3rd month, exercises were balance and slow walking. Each stage of exercise program lasted approximately 1 h, and patients performed it twice a day. Patients and their caregivers were instructed for home-based rehabilitation program before discharge from the hospital. Caregivers were given instruction for how to assist patients in ways that permitted patients to use their functional skills as much as possible. The intervention group was either received physical therapy in the duration of hospital stay, and they might receive outpatient rehabilitation according to the neurologist description. The patients received the exercise physiologist's telephone number for consultation about the home exercise rehabilitation program. The assessor appraised each patient once a month and called them for motivation.
Patients in the control group had physical therapy once a day when they were hospitalized. After discharge, they might receive usual care such as outpatient rehabilitation and other treatment services as prescribed by their physicians. They did not receive home-based rehabilitation program and incentive telephone call.
Ethical consideration
The present study was confirmed by the Ethics Committee of Isfahan University of Medical Sciences (Confirmation code: IR.MUI.1395.3.046) and was also registered on the website of the Iranian Registry of Clinical Trials (IRCT) with a code of IRCT20180827040885N1. After explaining the objectives of the study, participants completed the written consent forms and were ensured for the confidentiality of information.
Statistical analysis
All data were collected and analyzed by SPSS (version 20) to evaluate the effects of home-based exercise rehabilitation. Numerical values were presented as mean and standard deviation. Chi-square test was used to compare the distribution of sex frequency and hemiparesis in the intervention and control groups. Independent t-test and paired t-test were used to compare the measures between and within the groups in different time points, respectively.
Results
Forty ischemic stroke patients (twenty in the intervention group and twenty in the control group) were examined in this research. The age range of the patients was 33–80 years with the mean of 62. ±12.4 in the intervention group and 40–80 years with the mean of 66 ± 10.3 years in the control group. Independent t-test did not show any significant difference in the mean of the age between the two groups (P = 0.48).
Chi-square test showed that there was no significant difference in the distribution of gender (P = 0.10) and hemiparesis side (P = 0.40) between the two groups [Table 1].
Table 1.
Characteristics of the patients
| Variable | Intervention group, n (%) | Control group, n (%) | P |
|---|---|---|---|
| Gender | |||
| Female | 11 (55) | 12 (60) | 0.10 |
| Male | 9 (45) | 8 (40) | |
| Hemiparesis side | |||
| Right | 12 (60) | 10 (50) | 0.40 |
| Left | 8 (40) | 10 (50) |
Independent t-test revealed that there were no significant differences in Barthel score (P = 0.53), Fugl-Meyer upper extremity sensorimotor score (P = 0.71) and Fugl-Meyer lower extremity sensorimotor score (P = 0.98) and FMD score (P = 0.67) between the two groups before the intervention. The covariance analysis test showed that the mean of the Barthel score, the mean of the Fugl-Meyer upper extremity sensorimotor score, the mean of the Fugl-Meyer lower extremity sensorimotor score, and the mean of FMD score in the intervention group were significantly higher than the control group after 3-month home-based exercise rehabilitation (P < 0.001) [Table 2].
Table 2.
Comparison the mean scores of Barthel*, Fugl-Meyer,** and flow-mediated dilatation score before and 3 months after exercise rehabilitation between the two groups
| Time | Variable | Mean±SD | P | |
|---|---|---|---|---|
| Intervention group | Control group | |||
| Baseline | Barthel score | 12.6±4.1 | 9.7±1.9 | 0.53 |
| Fugl-Meyer upper extremity sensorimotor score | 16.9±3.9 | 14.8±4 | 0.71 | |
| Fugl-Meyer lower extremity sensorimotor score | 26.3±5.1 | 26.5±3.9 | 0.98 | |
| FMD% | 0.005±0.02 | 0.01±0.08 | 0.67 | |
| Follow-up | Barthel score | 83.1±2.7 | 14.1±2.8 | <0.001 |
| Fugl-Meyer upper extremity sensorimotor score | 88.2±4.5 | 19.6±3.8 | <0.001 | |
| Fugl-Meyer lower extremity sensorimotor score | 92.6±3.8 | 27.6±4.1 | <0.001 | |
| FMD% | 0.17±0.12 | 0.02±0.12 | <0.001 | |
*An index for activities of daily living, **An assessment of sensory motor recovery after stroke. SD=Standard deviation, FMD=Flow-mediated dilatations
Paired t-test showed that the mean of the Barthel score (P = 0.21), the mean of the Fugl-Meyer upper extremity sensorimotor score (P = 0.19), the mean of the Fugl-Meyer lower extremity sensorimotor score (P = 0.76), and the mean of FMD score (P = 0.84) did not have significant differences before and after 3 months in the control group. However, in the intervention group, all of the mentioned assessments were significantly higher after 3-month home-based exercise rehabilitation (P < 0.001) [Table 3].
Table 3.
Comparison the mean scores of Barthel*, Fugl-Meyer,**and flow-mediated dilatation before and 3 months after exercise rehabilitation within groups
| Group | Variable | Mean±SD | P | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Intervention group | Barthel score | 12.6±4.1 | 83.1±2.7 | <0.001 |
| Fugl-Meyer upper extremity sensorimotor score | 16.9±3.9 | 88.2±4.5 | <0.001 | |
| Fugl-Meyer lower extremity sensorimotor score | 26.3±5.1 | 92.6±3.8 | <0.001 | |
| FMD% | 0.005±0.02 | 0.17±0.12 | <0.001 | |
| Control group | Barthel score | 9.7±2.7 | 14.1±2.8 | 0.21 |
| Fugl-Meyer upper extremity sensorimotor score | 14.8±4.5 | 19.6±3.8 | 0.19 | |
| Fugl-Meyer lower extremity sensorimotor score | 26.5±4.1 | 27.6±4.1 | 0.76 | |
| FMD% | 0.01±0.08 | 0.02±0.12 | 0.84 | |
*An index for activities of daily living, **An assessment of sensory motor recovery after stroke. SD=Standard deviation, FMD=Flow-mediated dilatation
Discussion
The main purpose of this study was the effect of 3-month home-based exercise rehabilitation on brachial artery vasomotor reactivity evaluated by FMD during the subacute stage of stroke recovery. The other targets examined whether 3-month home-based exercise could improve daily physical activities (Barthel index [BI]) and upper and lower extremity functional capacity.
The primary objective of the intervention was to encourage patients to perform exercise recommendations according to DVD education program and increase their functional capacity. Since many stroke patients are not familiar with regular exercise training, therefore, the intervention must be simple, motivating, not time-consuming, and easy to perform.
Previous studies have been shown the beneficial effects of exercise training in improving brachial artery vasomotor reactivity in patients with acute myocardial infarction and other cardiovascular and cerebrovascular diseases[7,8] but rarely have been considered the effectiveness of home-based exercise training on endothelial function and FMD in the subacute stroke stage. Our finding demonstrated that this early initiation of a 3-month structured, physiologically based, progressive home-based exercise rehabilitation program has provided the remarkable improvements in brachial FMD due to increasing diameter in the hemiparetic forearm on ischemic stroke survivors in the acute phase of recovery.
According to the other studies after stroke, if the hemiparetic side has a reduced metabolic demand due to the lack of physical activity, vascular dysfunction may occur.[17] Endothelial dysfunction plays a key role in the progression of atherosclerosis; accordingly, ischemic stroke is also associated with endothelial dysfunction. Impaired FMD adversely related to stroke severity and associated with functional disability in the acute ischemic patients.[18]
Improvement on endothelial function as a result of regular exercise training contributes to cardiovascular and cerebrovascular health and physical capacity enhancement.[3]
Billinger et al. have been shown a 4-week single-limb exercise training program that increases muscular activity in the hemiparetic limb and improves femoral artery blood flow.[9]
A randomized controlled study has been explored that 3-month intensive rehabilitation twice a week has a significant effect on arterial function (cardio-ankle vascular index and ankle-brachial pressure index) of chronic stroke patients, despite no changes on physical function.[19]
According to other findings of the current study, this intervention produced greater improvements on functional capacity of upper and lower extremities, activities of daily livings, and functional independence than did usual care. This superiority of home-based exercise rehabilitation may result from some factors such as the patients and their family education before discharge from hospital, the family support, the initiation earlier exercise rehabilitation, the home environment of exercise, and close follow-up. Furthermore, no adverse event was seen for patients during the intervention period. A randomized controlled trial consistent with our study revealed that a home-based physical activity program was feasible, safe, and capable of inducing improvements in functional capacity.[20]
Some studies have shown patients and their caregivers' satisfaction with the home-based rehabilitation to reduce the impact of the disease, to avoid exacerbation of the diseases, and to be closer to their families, although personal factors of stroke patients, such as age, education, socioeconomic status, medical history, and family relationships have an effect on successful rehabilitation[21,22]
A pervious article like our finding revealed early home rehabilitation service during the first 3-month period in patients with ischemic stroke provides a better outcome for improving function in BI and reducing disability compared to the usual care group.[12]
Another study proposed early rehabilitation which can effectively improve stroke patients' daily living activities, especially in eating, bed-chair transfer, and short-distance walking activities, and decrease the rate of complications' incidence within 1 month after stroke.[23]
Chaparro et al.'s study has shown the valuable effects of a home-based physical activity incentive and education program on functional capacity of the lower limb and 6 min walking test in subacute stroke patients.[24]
A systematic review evaluated 11 trials which compared the functional benefits of home based versus center for community-dwelling people with stroke and exposed a significant effect of home-based rehabilitation at 6 weeks (P = 0.03) and 3–6 months (P = 0.01). Furthermore, cost benefits and caregivers' satisfaction in favor of home-based rehabilitation have been mentioned.[25] An article implied that home-based rehabilitation is at least as good as the outpatient rehabilitation programs in a hospital setting, in terms of functionality achievement in poststroke patients.[26] This study is one of the few home-based interventions in the field of rehabilitation of patients with stroke in Iran that can be considered as an innovation and strength point of the current study. Taking all patients from one hospital can be considered as a main limitation of the study.
Conclusion
Twelve-week regular home-based exercise training was well tolerated by the intervention group.
After this period, improvements were reported in brachial FMD as well as daily physical activity and upper and lower extremity functional capacity. In fact, regular exercise training enhances these repairing processes. Hence, home-based rehabilitation could be performed consistently across the Iran geography, considering both social and health needs by providing supervised and progressive exercises for acute stroke patients with functional limitations, and where the transportation to care services is difficult.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to express our special thanks to the patients and their families for their kind participation in the current study.
References
- 1.Stenborg A, Terent A, Lind L. Endothelium-dependent vasodilatation in forearm is impaired in stroke patients. J Intern Med. 2006;259:569–75. doi: 10.1111/j.1365-2796.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- 2.Billinger SA, Kluding PM. Use of Doppler ultrasound to assess femoral artery adaptations in the hemiparetic limb in people with stroke. Cerebrovasc Dis. 2009;27:552–8. doi: 10.1159/000214218. [DOI] [PubMed] [Google Scholar]
- 3.Phillips SA, Andaku DK, Mendes RG, Caruso FR, Cabiddu R, Jaenisch RB, et al. Exploring vascular function biomarkers: Implications for rehabilitation. Braz J Cardiovasc Surg. 2017;32:125–35. doi: 10.21470/1678-9741-2016-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos-García D, Blanco M, Serena J, Rodríguez-Yáñez M, Leira R, Castillo J. Impaired brachial flow-mediated dilation is a predictor of a new-onset vascular event after stroke. Cerebrovasc Dis. 2011;32:155–62. doi: 10.1159/000328651. [DOI] [PubMed] [Google Scholar]
- 5.Barone-Rochette G, Vanzetto G, Detante O, Quesada JL, Hommel M, Mallion JM, et al. Imaging of functional and structural alterations of large arteries after acute ischaemic atherothrombotic stroke or acute coronary syndromes. Arch Cardiovasc Dis. 2014;107:443–51. doi: 10.1016/j.acvd.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Iwata T, Mori T, Tanno Y, Kasakura S, Yoshioka K. Impaired brachial flow-mediated dilatation may predict symptomatic intracranial arterial dissections. J Stroke Cerebrovasc Dis. 2018;27:2691–5. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 7.Kolmos M, Krawcyk RS, Kruuse C. Effect of high-intensity training on endothelial function in patients with cardiovascular and cerebrovascular disease: A systematic review. SAGE Open Med. 2016;4:2050312116682253. doi: 10.1177/2050312116682253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Li M, Dong F, Zhang J, Zhang F. Physical exercise-induced protection on ischemic cardiovascular and cerebrovascular diseases. Int J Clin Exp Med. 2015;8:19859–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G, Rippee MA, et al. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther. 2012;36:159–65. doi: 10.1097/NPT.0b013e318274d082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt W, Endres M, Dimeo F, Jungehulsing GJ. Train the vessel, gain the brain: Physical activity and vessel function and the impact on stroke prevention and outcome in cerebrovascular disease. Cerebrovasc Dis. 2013;35:303–12. doi: 10.1159/000347061. [DOI] [PubMed] [Google Scholar]
- 11.Pallesen H, Bjerk M, Pedersen AR, Nielsen JF, Evald L. The effects of high-intensity aerobic exercise on cognitive performance after stroke: A pilot randomised controlled trial. J Cent Nerv Syst Dis. 2019;11:1179573519843493. doi: 10.1177/1179573519843493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaiyawat P, Kulkantrakorn K, Sritipsukho P. Effectiveness of home rehabilitation for ischemic stroke. Neurol Int. 2009;1:e10. doi: 10.4081/ni.2009.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyden P. Using the national institutes of health stroke scale: A cautionary tale. Stroke. 2017;48:513–9. doi: 10.1161/STROKEAHA.116.015434. [DOI] [PubMed] [Google Scholar]
- 14.Oveisgharan S, Shirani S, Ghorbani A, Soltanzade A, Baghaei A, Hosseini S, et al. Barthel index in a middle-East country: Translation, validity and reliability. Cerebrovasc Dis. 2006;22:350–4. doi: 10.1159/000094850. [DOI] [PubMed] [Google Scholar]
- 15.Santisteban L, Térémetz M, Bleton JP, Baron JC, Maier MA, Lindberg PG. Upper limb outcome measures used in stroke rehabilitation studies: A systematic literature review. PLoS One. 2016;11:e0154792. doi: 10.1371/journal.pone.0154792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie KD, McKelvie RS, Macdonald MJ. Brachial artery endothelial responses during early recovery from an exercise bout in patients with coronary artery disease. Biomed Res Int. 2014;2014:591918. doi: 10.1155/2014/591918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billinger SA, Gajewski BJ, Guo LX, Kluding PM. Single limb exercise induces femoral artery remodeling and improves blood flow in the hemiparetic leg poststroke. Stroke. 2009;40:3086–90. doi: 10.1161/STROKEAHA.109.550889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omisore AD, Ayoola OO, Ibitoye BO, Fawale MB, Adetiloye VA. Sonographic evaluation of endothelial function in brachial arteries of adult stroke patients. J Ultrasound Med. 2017;36:345–51. doi: 10.7863/ultra.16.03100. [DOI] [PubMed] [Google Scholar]
- 19.Takatori K, Matsumoto D, Okada Y, Nakamura J, Shomoto K. Effect of intensive rehabilitation on physical function and arterial function in community-dwelling chronic stroke survivors. Top Stroke Rehabil. 2012;19:377–83. doi: 10.1310/tsr1905-377. [DOI] [PubMed] [Google Scholar]
- 20.Malagoni AM, Cavazza S, Ferraresi G, Grassi G, Felisatti M, Lamberti N, et al. Effects of a “test in-train out” walking program versus supervised standard rehabilitation in chronic stroke patients: A feasibility and pilot randomized study. Eur J Phys Rehabil Med. 2016;52:279–87. [PubMed] [Google Scholar]
- 21.Jhaveri MM, Benjamin-Garner R, Rianon N, Sherer M, Francisco G, Vahidy F, et al. Telemedicine-guided education on secondary stroke and fall prevention following inpatient rehabilitation for texas patients with stroke and their caregivers: A feasibility pilot study. BMJ Open. 2017;7:e017340. doi: 10.1136/bmjopen-2017-017340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studenski S, Duncan PW, Perera S, Reker D, Lai SM, Richards L, et al. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke. 2005;36:1764–70. doi: 10.1161/01.STR.0000174192.87887.70. [DOI] [PubMed] [Google Scholar]
- 23.Zhang NX, Liu GZ, Yao QH, Li WJ, Huang Y, Wang AM, et al. Effects of warming-reinforcing acupuncture combined with rehabilitation training on the early motor function of hemiparalysis patients caused by ischemic brain stroke: A randomized and controlled study. Zhongguo Zhen Jiu. 2010;30:441–5. [PubMed] [Google Scholar]
- 24.Chaparro D, Daviet JC, Borel B, Kammoun B, Salle JY, Tchalla A, et al. Home-based physical activity incentive and education program in subacute phase of stroke recovery (Ticaa'dom): Study protocol for a randomized controlled trial. Trials. 2018;19:68. doi: 10.1186/s13063-017-2410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillier S, Inglis-Jassiem G. Rehabilitation for community-dwelling people with stroke: Home or centre based? A systematic review. Int J Stroke. 2010;5:178–86. doi: 10.1111/j.1747-4949.2010.00427.x. [DOI] [PubMed] [Google Scholar]
- 26.López-Liria R, Vega-Ramírez FA, Rocamora-Pérez P, Aguilar-Parra JM, Padilla-Góngora D. Comparison of two post-stroke rehabilitation programs: A follow-up study among primary versus specialized health care. PLoS One. 2016;11:e0166242. doi: 10.1371/journal.pone.0166242. [DOI] [PMC free article] [PubMed] [Google Scholar]