Abstract
Lappaconitine (LA), a natural compound with a novel C18-diterpenoid alkaloid skeleton, displayed extensive biological profile. Recent research on LA is focused mainly on its anti-tumor and analgesic effects, and therefore we aimed to investigate its anti-inflammatory potential. A series of novel LA derivatives with various substituents on the 20-N position was designed and synthesized. In the initial screening of LA derivatives against NO production, all the target compounds, except compound E2, exhibited excellent inhibitory ability relative to that of LA. Particularly, compound A4 exhibited the most potent inhibition with IC50 of 12.91 μmol/L. The elementary structure–activity relationships (SARs) of NO inhibitory activity indicated that replacement of the benzene ring with an electron donating group could improve the anti-inflammatory efficacy. Furthermore, compound A4 shows an anti-inflammatory mechanism by inhibiting NO, PGE2, and TNF-α generation via the suppression of NF-κB and MAPK signaling pathways. Notably, compound A4 could exert a significant therapeutic effect on LPS-induced acute lung injury (ALI) in vivo. Based on the above research, we further investigated the preliminary pharmacokinetic property of A4 in rats. Therefore, compound A4 could be a promising candidate for the development of anti-inflammatory agents in the future.
Key words: Lappaconitine, Anti-inflammatory activity, NF-κB, MAPK, Acute lung injury, Pharmacokinetic study
Graphical abstract
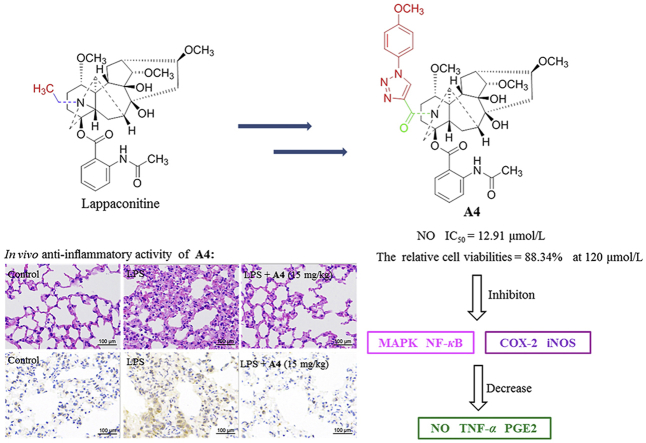
Lappaconitine derivative A4 significantly reduced the levels of pro-inflammatory cytokines NO. The anti-inflammatory mechanism of A4 might be associated with the inhibition of NO, TNF-α and PGE2 generation as a result of suppressing NF-κB and MAPK signaling pathway. Notably, A4 showed outstanding anti-inflammatory activity both in vivo and in vitro.
1. Introduction
Inflammation is one of the most important defense responses of the body to tissue damage or microbial invasion. Under normally physiological conditions, an immune response is beneficial to the host. However, persistent inflammation can lead to severe cell injury and release of inflammatory mediators inducing tissue and organ dysfunction1. Inflammation is closely related to many human diseases, including rheumatoid arthritis, inflammatory bowel disease, atherosclerosis and neurodegenerative disorders2, 3. Currently, nonsteroidal anti-inflammatory drugs (NSAIDs) and anti-cytokine biologics are widely used to treat inflammation; however, their side effects or excessive costs limit their clinical application4, 5. In the past decade, safer and more effective anti-inflammatory drugs have been developed to reduce the severity of inflammation6. However, the human immune system is a complex process involving many factors and prone to errors. Moreover, progress in the development of anti-inflammatory drugs is very slow in the pharmaceutical industry. Development of novel anti-inflammatory drugs, with improved pharmaceutical profiles and reduced toxicity, is still of great significance.
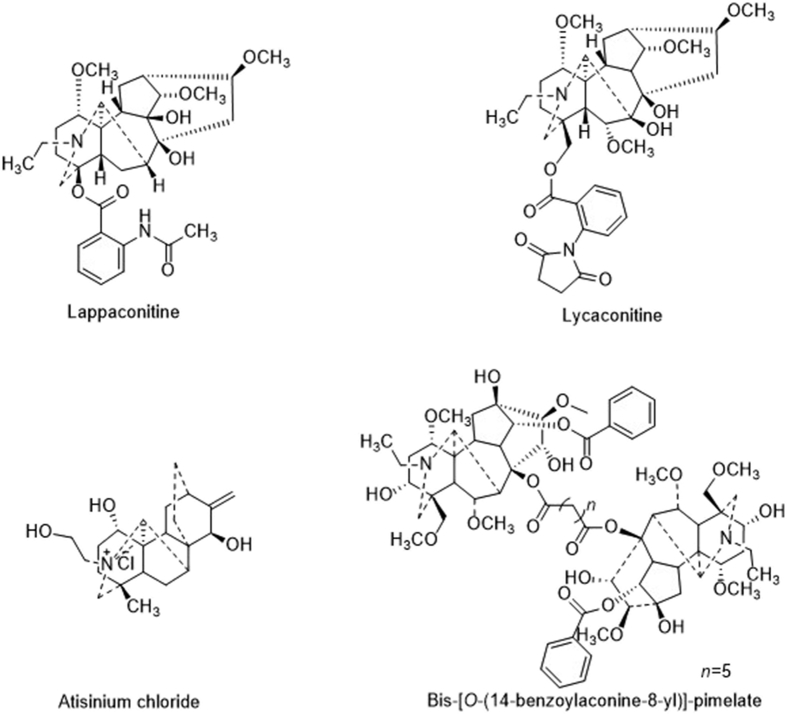
In recent years, natural products continue to play a highly significant role in drug discovery and development7. The drugs obtained from natural products or derivatives represent 62% of all small molecule drugs approved by the U. S. Food and Drug Administration (FDA) from 1981 to 2014, and 2% of these natural molecules are anti-inflammatory drugs8. Anti-inflammatory drugs derived from naturally occurring products are receiving greater attention. Diterpenoid alkaloids are a class of natural products with complex structures and extensive biological activity9, 10. They primarily exist in the genera Aconitum, Delphinium, and Consolida of the Ranunculaceae family and the genera Spiraea of the Rosaceae family. According to their structural characteristics, diterpenoid alkaloids are classified as C18-diterpenoid alkaloids (representative compound: lappaconitine), C19-diterpenoid alkaloids (representative compound: lycaconitine), C20-diterpenoid alkaloids (representative compound: atisinium chloride), and bis-diterpenoid alkaloids (representative compound: bis-[O-(14-benzoylaconine-8-yl)]-pimelate)11, 12 (Fig. 1). Lappaconitine (LA), extracted from the roots of Aconitum sinomontanum Nakai, has a wide range of pharmacological activities13, 14, 15, 16. However, research on LA is very limited. Most of the pharmacological activity studies have focused on its anti-tumor and analgesic effects, and some of its derivatives have been designed and synthesized17. There are relatively few reports on lappaconitine against LPS-induced NO production16. Hence, we selected LA as a lead compound and synthesized some its derivatives with anti-inflammatory activity.
Figure 1.
Structural types of diterpenoid alkaloids.
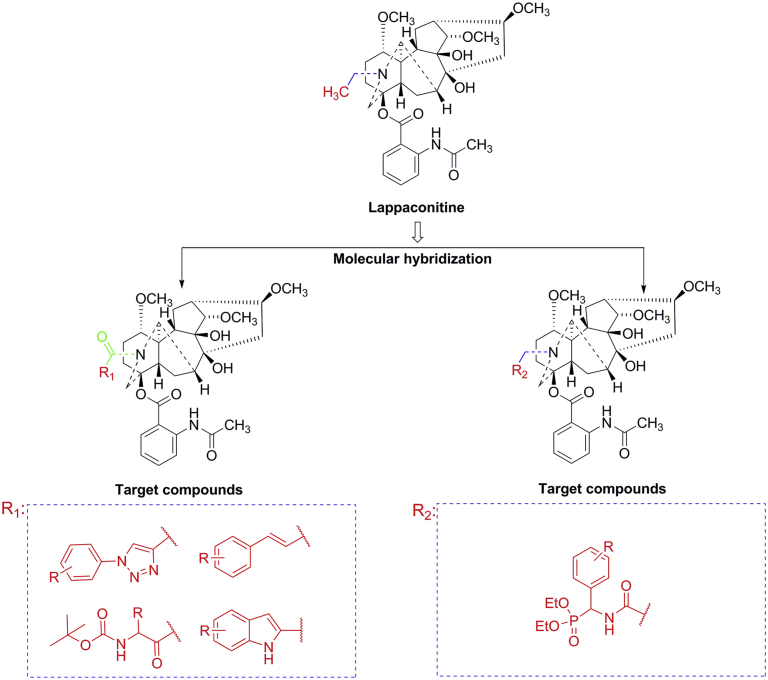
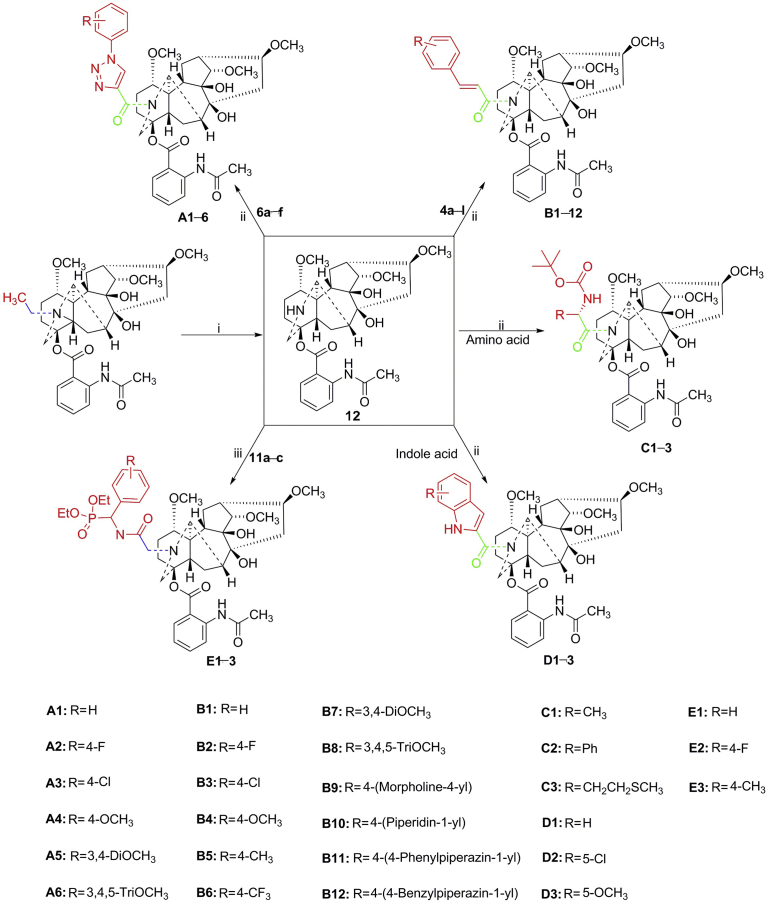
In the past few decades, 1,2,3-triazoles and their derivatives have received considerable attention, owing to their chemotherapeutic value18, 19. They have been considered as indispensable structural fragments in terms of biological activity and are found in many natural drug products20. Various 1,2,3-triazoles with potent anti-bacterial21, 22, anti-inflammatory23, 24, anti-cancer25, anti-malarial26, and antiviral27 effects have been reported. Thus, 1,2,3-triazoles have emerged as powerful pharmacophores. In addition, these functional structure fragments, including cinnamic acid28, 29, indole30, 31, and amino acid32, 33, are widely distributed in many biologically active molecules and show better anti-inflammatory activity. Thus far, it has been demonstrated that biologically active small fragments integrated into natural products improve bioactivity, inherent toxicity, and drug-forming properties34, 35, 36. Based on the above findings, a series of novel LA derivatives bearing amide of 1,2,3-triazoles, cinnamic acid moiety, indole moiety, and amino acid moiety were designed and synthesized (Fig. 2). Further, to change the structure type of the target compound, adjust physicochemical properties of the whole molecule and to find more potent anti-inflammatory drugs with low toxicity, we introduced phospholipid skeletons showing anti-inflammatory activity to the 20-N position of LA37. Another series of novel LA derivatives was designed and synthesized. Subsequently, we investigated the cytotoxicity of 27 LA derivatives and further evaluated their anti-inflammatory activity. Their elementary structure–activity relationships (SARs) of nitric oxide (NO) inhibitory activity were also assessed. The effects of the most promising compound A4 on NF-κB and MAPK signaling pathways were examined using Western blot assay. In addition, the in vivo anti-inflammatory efficacy and the preliminary pharmacokinetic property of compound A4 were investigated.
Figure 2.
Illustration of the design strategy for target compounds.
2. Results and discussions
2.1. Chemistry
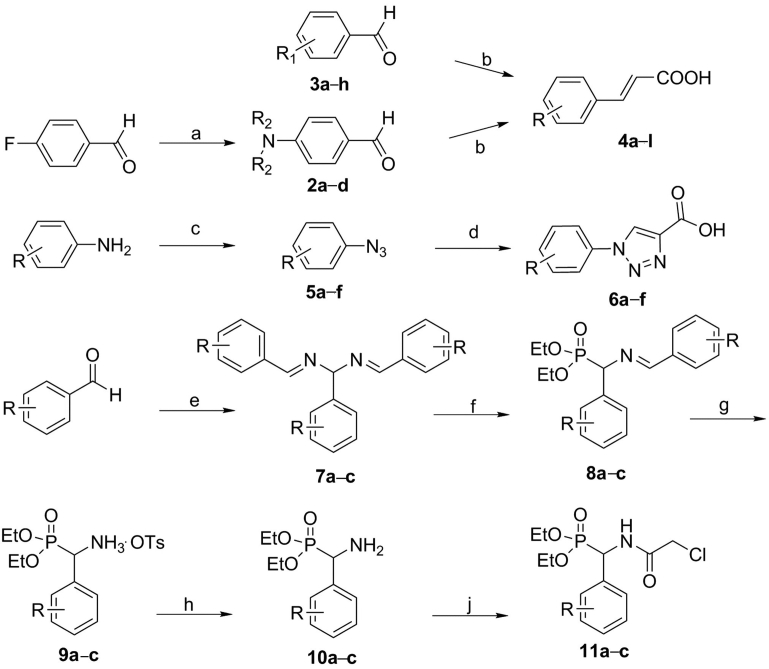
The synthetic route of the intermediates is shown in Scheme 1. Synthesis of the cinnamic acid derivatives 4a−l is considered the most classical application of the Knoevenagel condensation reaction38. The specific method is the reaction of aromatic aldehydes with malonic acid, and piperidine as a catalyst in a pyridine solution. The triazole acid derivatives 6a−f were constructed via 1,3-dipolar cycloaddition of propiolic acid with various substituted azide compounds 5a−f in the presence of l-ascorbic acid sodium salt and CuSO4·5H2O in a mixed solution of n-butanol and water39, 40. The chlorophospholipid derivatives 11a−c were prepared by an acylation reaction of phospholipid amine compounds 10a−c, which were obtained by a simple and efficient method41. Aromatic aldehydes were treated with ammonium hydroxide, and the resulting imine 7a−c was then reacted with dialkyl phosphite to generate compounds 8a−c, which could be easily hydrolyzed to phospholipid amine compounds 10a−c in the presence of 4-methylbenzenesulfonic acid (p-TSOH).
Scheme 1.
Synthetic route of the intermediates. Reagents and conditions: (a) secondary amine, K2CO3, DMF, 90 °C, 10–15 h; (b) malonic acid, piperidine in pyridine, reflux, 3 h; (c) (i) HCl, NaNO2, H2O, 0–5 °C, 30 min; (ii) NaN3, H2O, 0–5 °C, 2–4 h; (d) propiolic acid, l-ascorbic acid sodium salt, CuSO4·5H2O, n-BuOH/H2O, r.t., 24 h; (e) NH3·H2O, reflux, 5 h; (f) diethyl phosphite, 70–75 °C, 2–5 h; (g) p-TsOH, THF, 0 °C, 2 h; (h) NH3·H2O, r.t., 30 min; (j) chloroacetyl chloride, TEA, r.t., 3 h.
The N(20)-deethyllappaconitine 12 was prepared by reacting LA with N-bromosuccinimide (NBS) in glacial acetic acid solution at room temperature for 3 h42. To improve the anti-inflammatory activity of target compounds, the compound 12 obtained above was reacted with various natural products, such as cinnamic acid derivatives 4a−l, triazole acid derivatives 6a−f, amino acid derivatives 12a−c, and indole acid derivatives 13a−c (Scheme 2), showing anti-inflammatory activity. In this reaction, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydrocarbonation (HOBt) were used as condensing agents to obtain target compounds through a condensation reaction. On the other hand, the N-hydrocarbonation reaction of compound 12 with chlorophospholipid derivatives 11a−c was used to yield different types of target compounds.
Scheme 2.
Synthetic route of target compounds. Reagents and conditions: (i) NBS, CH3COOH, r.t., 3 h; (ii) carboxylic acid derivative, EDC, HOBt, DCM, r.t., 5 h; (iii) K2CO3, CH3CN, 70 °C, 6 h.
2.2. Biological evaluation
2.2.1. Cell viability assay
The cytotoxicity of novel LA derivatives was evaluated in mouse RAW264.7 macrophages by the MTT assay to investigate the possible correlation between inflammatory inhibitory activity and cell viability. As shown in Supporting Information Fig. S1, 27 target products were screened at the 30 μmol/L concentration. Among them, 17 target products showed no significant cytotoxicity in RAW264.7 cells, and the relative cell viabilities of the dosing test cells exceeded 85%. Compounds A3, A5, A6, B1, B3, B5, D1, D3, C1, and E3 were toxic for macrophages at 30 μmol/L. Based on the result, the non-toxic concentrations were further used to evaluate anti-inflammatory activity of the 17 target products.
2.2.2. Initial screening of LA derivatives against LPS-induced NO production
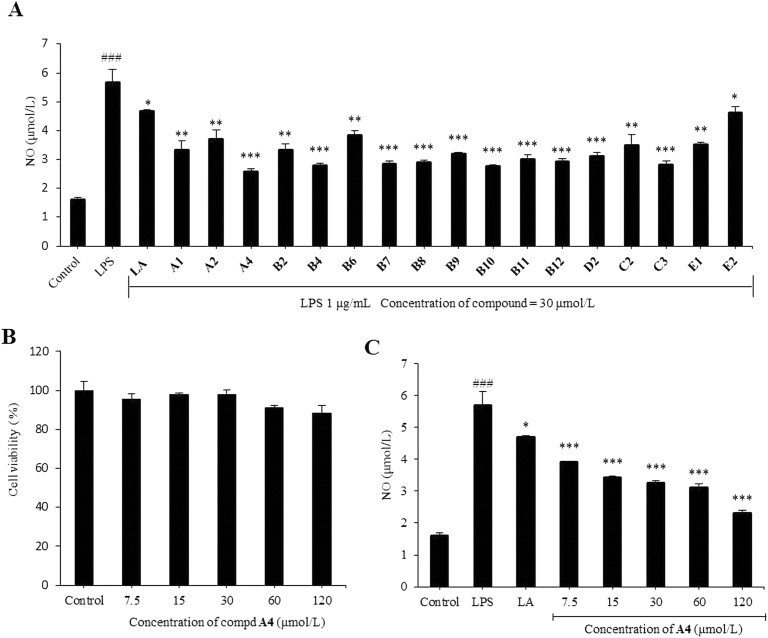
High levels of inflammatory mediators NO have been shown to be closely related to the occurrence and development of inflammation43. Hence, in preliminary screening studies on anti-inflammatory activity, the newly synthetic compounds were tested for their inhibitory activity on LPS-induced NO production in RAW264.7 macrophages. The anti-inflammatory activity of all tested compounds to reduce NO production is described in Fig. 3A. All target compounds exhibited excellent inhibitory ability relative to LA, except compound E2. Compounds A4, B4, B10, and C3 exhibited better inhibition against NO production than the other target compounds. Particularly, compound A4 showed the most remarkable inhibitory activity at the concentration of 30 μmol/L. The elementary SARs of NO inhibitory activity indicated that replacement of the benzene ring with an electron donating group (4-(morpholine-4-yl)<4-(4-phenylpiperazin-1-yl)<4-(4-benzylpiperazin-1-yl)<3,4,5-triOCH3<3,4-diOCH3<4-OCH3<4-(piperidin-1-yl)) could improve anti-inflammatory efficacy, and replacement of the benzene ring with an electron withdrawing group (4-CF3<4-F) could reduce anti-inflammatory efficacy. To investigate its toxicity, compound A4 was tested for its effect on RAW264.7 cell viability (Fig. 3B). The result indicated that compound A4 showed no significant cytotoxicity in RAW264.7 cells at concentrations ranging from 7.5 to 120 μmol/L. Subsequently, we further studied the IC50 value of compound A4 against NO production, which was observed as 12.91 μmol/L at concentrations ranging from 15 to 120 μmol/L (Fig. 3C).
Figure 3.
Initial screening of lappaconitine derivatives for anti-inflammatory activity in activated RAW264.7 macrophage. Nitric oxide (NO) production was determined by Griess assay (A) lappaconitine (LA) derivatives inhibits LPS-induced NO production at the concentration of 30 μmol/L; (B) compound A4 was tested for its effect on RAW264.7 cell viability at 7.5–120 μmol/L; (C) the production NO in the medium of RAW264.7 cells after treatment with different concentrations of compound A4 and LA (30 μmol/L) for 30min and LPS (1 μg/mL) for 24 h, The results are shown as means ± SD (n = 3). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with LPS-induced group.
2.2.3. Compound A4 inhibits TNF-α and PGE2 production in RAW264.7 cells
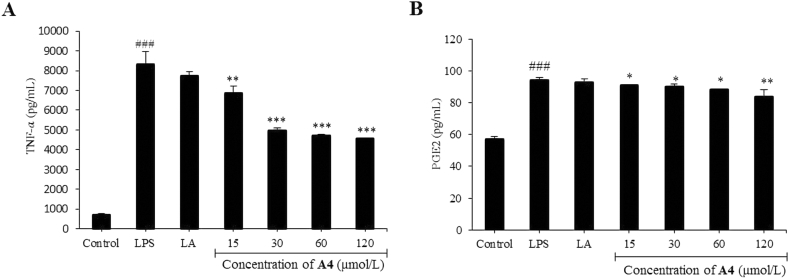
A variety of inflammatory mediators, including tumor necrosis factor (TNF-α), prostaglandins (PGs), and NO, play important roles in host defensive responses and maintain normal cellular conditions44. Based on the initial screening results of anti-inflammatory activity, compound A4 was evaluated for its inhibitory ability against TNF-α and PGE2 production in RAW264.7 cells. As shown in Fig. 4, LPS stimulation markedly increased levels of inflammatory mediators TNF-α and PGE2 compared to normal macrophages. In the dose group, LPS-induced TNF-α and PGE2 production were decrease by compound A4 treatment in a concentration-dependent manner (Fig. 4A and B). The IC50 values of compound A4 against TNF-α and PGE2 production were 89.66 and > 100 μmol/L, respectively. These results indicated that compound A4 certainly attenuated LPS-induced inflammatory reactions in RAW264.7 cells.
Figure 4.
Compound A4 and LA (30 μmol/L) inhibits TNF-α (A) and PGE2 (B) production in RAW264.7 cells. RAW264.7 cells were incubated for 24 h and treated with different concentrations of compound A4 for 30 min, and LPS (1 μg/mL) for 24 h, The results are shown as means ± SD (n = 3). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with LPS-induced group.
2.2.4. Compound A4 inhibits LPS-induced expression of COX-2 and iNOS in RAW264.7 cells
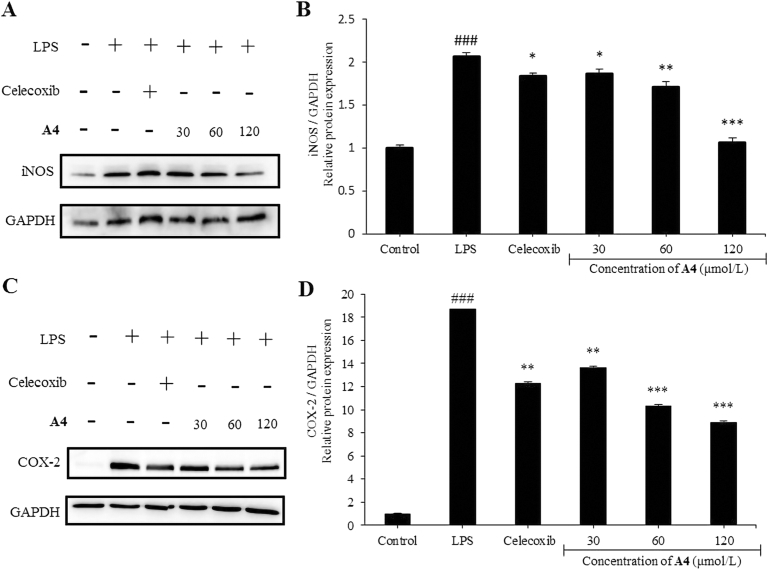
During inflammation, increased production/activity of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) promotes release of various inflammatory mediators, including NO and PGE245. Therefore, we further investigated the impact of compound A4 treatment on protein expression in LPS-induced RAW264.7 cells. As expected, LPS induction increased COX-2 and iNOS protein expression, which was significantly decreased by compound A4 (Fig. 5). These results indicated that compound A4 may participate in signaling pathways activated by LPS in macrophages.
Figure 5.
Compound A4 inhibits LPS-induced expression of COX-2 and iNOS in RAW264.7 cells. (A) Western blot for iNOS; (B) relative ratio of iNOS; (C) Western blot for COX-2; (D) relative ratio of COX-2. Celecoxib (5 μmol/L) was used as positive control. The results are shown as means ± SD (n = 3). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with LPS-induced group.
2.2.5. Compound A4 inhibits LPS-induced NF-κB activation in RAW264.7 cells
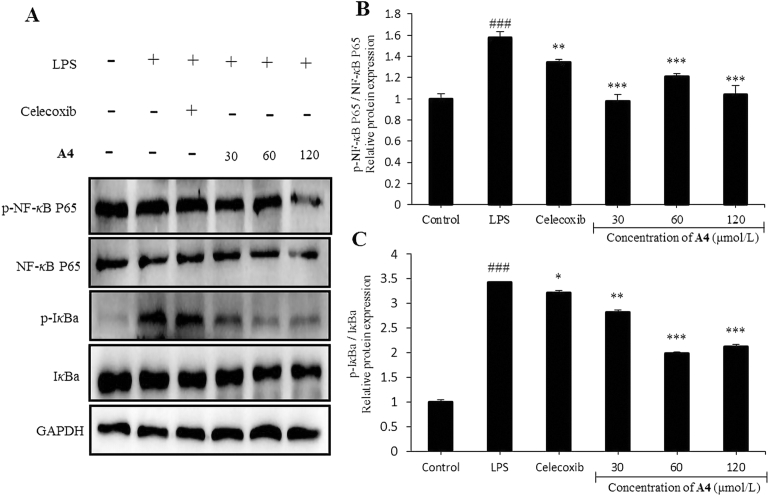
NF-κB is recognized as a crucial signaling pathway in many immune responses, including NF-κB-mediated macrophage secretion of pro-inflammatory cytokines46. The phosphorylation and degradation of IκBa exert an important effect on NF-κB activation, and IκBa is a cognate regulatory subunit of NF-κB47. Based on the above, compound A4 was tested for effects on LPS-induced transcriptional activity of NF-κB in RAW264.7 cells. As shown in Fig. 6, the relative protein expression of p-NF-κB, P65/NF-κB P65, and p-IκBa/IκBa was significantly down-regulated by compound A4 treatment. Compared to the LPS-induced group, phosphorylated IκBa and NF-κB P65 protein expression levels were also decreased. Further, the Western blot results demonstrated that compound A4 exerted its anti-inflammatory activity through inhibition of NF-κB signaling pathways.
Figure 6.
Compound A4 inhibits LPS-induced NF-κB activation in RAW264.7 cells. (A) The levels of NF-κB P65 and IκBa proteins, and their phosphorylated forms were analyzed using Western blotting. (B) and (C) The relative protein expression of p-NF-κB P65/NF-κB P65 and p-IκBa/IκBa. Celecoxib (5 μmol/L) was used as positive control. The results are shown as means ± SD (n = 3). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with LPS-induced group.
2.2.6. Compound A4 inhibits LPS-induced MAPK activation in RAW264.7 cells
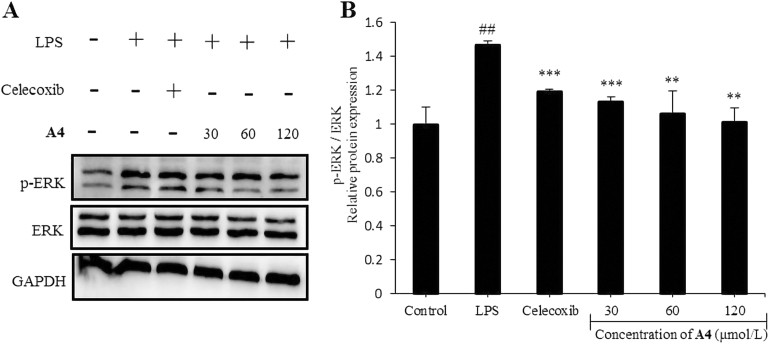
Mitogen-activated protein kinases (MAPKs) are a family of signal transduction proteins including extracellular signal regulated kinase (ERK), the P38 isoform (P38) and c-Jun N-terminal kinases (JNK), which play crucial roles in the regulation of inflammation, cell survival and apoptosis48. Therefore, we evaluated whether compound A4 had an impact on LPS-induced MAPK activation by Western blot in RAW264.7 cells (Fig. 7). The results confirmed that compound A4 inhibited relative protein expression of p-ERK/ERK compared to the LPS-induced group. Thus, the anti-inflammatory mechanism of compound A4 might be associated with its negative effects on p-ERK/ERK activation.
Figure 7.
Compound A4 inhibits LPS-induced MAPK activation in RAW264.7 cells. (A) The levels of ERK proteins, and their phosphorylated forms were analyzed using Western blotting. (B) The relative protein expression of p-ERK/ERK. Celecoxib (5 μmol/L) was used as positive control. The results are shown as means ± SD (n = 3). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with LPS-induced group.
2.2.7. Compound A4 attenuates LPS-induced ALI in vivo
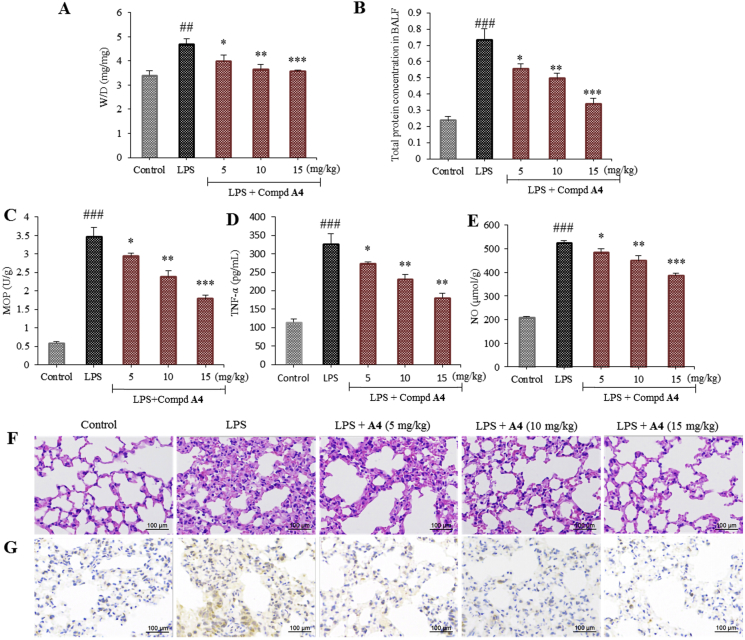
Acute lung injury (ALI) is an illness of critical pulmonary inflammation, which is mainly characterized by noncardiogenic edema, decreased neutrophil apoptosis in the lung, severe systemic hypoxemia, and multiple organ failure49. Thus, compound A4 with the highest inhibitory potency was selected to test its anti-inflammatory activity in vivo in LPS-induced ALI mouse model. As shown in Fig. 8A and B, the lung wet/dry ratio (W/D ratio) and total protein concentration in the bronchoalveolar lavage fluid (BALF) were significantly increased by LPS challenge compared to the control group. In contrast, pretreatment with compound A4 at 5, 10 and 15 mg/kg effectively prevented this increase. The results demonstrated that pulmonary edema was attenuated. To assess histological changes in ALI mice following compound A4 treatment, hematoxylin and eosin (H&E) staining were performed (Fig. 8F). LPS induced histopathological changes, including inflammatory cell infiltration, lung edema, alveolar hemorrhage, interalveolar septal thickening, and alveolar structure damage. These changes improved with compound A4 treatment. In addition, compound A4 inhibited the LPS-induced increase in myeloperoxidase (MPO) activity compared to the LPS group, indicating reduced neutrophil extravasation (Fig. 8C).
Figure 8.
Compound A4 attenuates LPS-induced ALI in mice. ICR mice (n = 8 per group) were treated by orally administered with A4 (5, 10, 15 mg/kg). After 1 h, the mice were anesthetized with ether and given LPS (0.5 mg/kg) by dripping nose. (A) Lung wet/dry weight ratio; (B) total protein in BALF; (C) myeloperoxidase (MPO) activity of lung tissue; (D) concentrations of TNF-α determined by ELISA assay; (E) NO production determined by the Griess assay; (F) hematoxylin and eosin (H&E) staining; and (G) lung CD68 immunohistochemistry. The results are shown as means ± SD (n = 8). #P < 0.05, ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with LPS-induced group.
In the early phase of ALI, pro-inflammatory cytokines are critical factors in promoting lung injury50. Therefore, we measured the levels of pro-inflammatory cytokines TNF-α and NO in the BALF of mice (Fig. 8D and E). Cytokine release was found to be elevated by LPS challenge compared to the control group. However, administration of compound A4 remarkably down-regulated LPS-induced TNF-α and NO levels, indicating that the protective effects of compound A4 may be associated with inhibition of inflammatory cytokines. To further evaluate A4 against macrophage infiltration in the lung tissues, immunohistochemical analysis of CD68 was performed (Fig. 8G). The number of CD68-immunostained positive macrophages in the lung tissues was significantly increased by LPS challenge, whereas this accumulation was decreased by compound A4 pretreatment in mice. Collectively, these results showed that compound A4 could exert a significant therapeutic effect on pulmonary inflammation and potentially other inflammatory injuries in vivo.
2.2.8. Preliminary pharmacokinetic properties of compound A4
In view of above anti-inflammatory activity both in vivo and in vitro, compound A4 was selected for in vivo pharmacokinetic properties in SD rats. Blood plasma samples were collected prior to drug delivery (0 h) and 0.083, 0.167, 0.25, 0.5, 1, 2, 4, 8 and 10 h after tail vein injection (i.v.) of compound A4 at 2 mg/kg. The peak area of compound A4 in plasmas was analyzed by using chromatography–mass spectrometry (LC–MS/MS). The pharmacokinetic parameters were calculated using a non-compartmental model in Phoenix WinNonlin 8.0 software (Pharsight Corporation, Cary, USA). The results show that t1/2 of compound A4 is 0.385±0.224 h, AUC0–t is 37.8 ± 18.4 ng h/mL, CL is 969 ± 610 mL/kg·min (Table 1). The plasma concentrations of compound A4 vs. time profiles are shown in Supporting Information Fig. S2. The pharmacokinetic experiment was used as a guide for subsequent studies for compound A4.
Table 1.
Pharmacokinetic (PK) parameters (mean±SD) of A4 in plasma following tail-vein administration to rats (n = 3).
| PK parameter | Mean ± SD |
|---|---|
| C0 (ng/mL) | 140±75.6 |
| Kel (/h) | 2.30±1.36 |
| t1/2 (h) | 0.385±0.224 |
| MRT0−inf (h) | 0.484±0.303 |
| MRT0–t (h) | 0.320±0.220 |
| AUC0−t (ng·h/mL) | 37.8±18.4 |
| AUC0−inf (ng·h/mL) | 42.9±20.4 |
| CL (mL/kg·min) | 969±610 |
| Vdss (L/kg) | 22.8±9.23 |
C0, initial concentration; Kel, elimination rate constant; t1/2, apparent terminal elimination half-life; MRT0−inf, mean residence time from time 0 extrapolated to infinity; MRT0−t, mean residence time from time 0 to the time of the last quantifiable concentration; AUC0−t, area under the concentration–time curve from time 0 to the time of the last quantifiable concentration; AUC0−inf, area under the concentration–time curve from time 0 extrapolated to infinity; CL, clearance rate; Vdss, steady-state apparent volume of distribution.
3. Conclusions
In this study, a series of novel LA derivatives bearing various substituents on the 20-N position were designed and synthesized. The cytotoxicity of 27 novel LA derivatives was investigated in mouse RAW264.7 macrophages at the concentration of 30 μmol/L. Among them, 17 target products showed no significant cytotoxicity in RAW264.7 cells, and the relative cell viabilities of the dosing test cells exceeded 85%. Subsequently, we further evaluated anti-inflammatory activity against NO production. All target compounds exhibited excellent inhibitory ability compared to LA, except compound E2. Particularly, compound A4 exhibited the most potent inhibition with an IC50 of 12.91 μmol/L. Furthermore, compound A4 significantly reduced the levels of pro-inflammatory cytokines TNF-α and PGE2, and their IC50 values were 89.66 and > 100 μmol/L, respectively. Western blotting showed that compound A4 decreased iNOS and COX-2 expression and down-regulated the relative protein expression of p-NF-κB, P65/NF-κB P65, p-IκBa/IκBa, and p-ERK/ERK. Taken together, these results indicated that the anti-inflammation mechanism of compound A4 might be associated with inhibition of NO, TNF-α, and PGE2 generation through suppression of NF-κB and MAPK signaling pathways. In the ALI mouse model, compound A4 effectively improved LPS-induced inflammatory cell infiltration, lung edema, alveolar hemorrhage, interalveolar septal thickening, and alveolar structure damage. Therefore, compound A4 could exert a significant therapeutic effect on pulmonary inflammation. In conclusion, compound A4 showed outstanding anti-inflammatory activity both in vivo and in vitro. Therefore, the compound A4 provides a promising scaffold for the development of new anti-inflammatory agents including the treatment of acute lung injury.
4. Experimental
4.1. Chemistry
All commercially purchased raw materials and solvents were chemical pure and used without further purification. The progress of reactions was checked by TLC analysis carried out on silica gel plates GF254 (Yantai Huanghai chemical, China). 1H NMR and 13C NMR spectra recorded on AV-300 and AV-500 (Bruker BioSpin, Switzerland) in CDCl3 or DMSO-d6, and using TMS as internal standard. Chemical shifts are reported in ppm (δ) and stated relative to TMS. Coupling constants are reported in Hz. High resolution mass spectra (Bremen, Germany) of all target compounds were recorded on a Thermo Scientific LTQ Orbitrap XL spectrometer by electrospray ionization (ESI). The purity of all target compounds was determined by reversed phase HPLC at 254 nm. The HPLC system applied a C18 phase (Nucleosil, 5 μm, 4.6 mm × 150 mm, Shim-pack, SHIMADZU, Japan) eluting the compounds with a gradient of 10%–90% MeCN/H2O over 30 min. All compounds were judged as >95% pure by HPLC.
4.1.1. General procedure for preparation of compounds 4a−l
A mixture of p-fluorobenzaldehyde (1.24 g, 10 mmol), appropriate amine (11 mmol), anhydrous potassium carbonate (1.66 g, 12 mmol) in 40 mL of DMF is stirred at 90 °C for 10–15 h until the starting material disappeared (monitored by TLC). The mixture was extracted with ethyl acetate (3 × 10 mL). After washing with brine (2 × 10 mL), the solvent was evaporated in vacuum. The residue was crystallized with ethyl acetate/petroleum ether (1/10, v/v) to yield compounds 2a−d. To a solution of respective aldehyde 2a−d or 3a−h (10 mmol) and malonic acid (1.05 g, 10 mmol) in pyridine (40 mL) were added catalytic amount of piperidine. The reaction mixture was stirred for 3 h under reflux. After the starting material disappeared, the reaction mixture was cooled and poured into cold water. To the mixture was slowly dropped hydrochloric acid at stirring and acidified to pH 2. The formed solid was filtered, washed with ice water to yield compounds 4a−l.
4.1.2. General procedure for preparation of compounds 6a−f
To a solution of aromatic amine (4 mmol) in dilute hydrochloric acid solution (10 mL, 1.2 mmol/mL) was dropped sodium nitrite solution (2 mL, 2.4 mmol/mL) at 0 °C. The reaction mixture was stirred over a period of 30 min, followed by slowly added sodium azide (312 mg, 4.8 mmol) with stirring in room temperature for 2–4 h. The mixture was extracted with ethyl acetate (3 × 10 mL). After washing with brine (2 × 10 mL), the solvent was evaporated in vacuum to afford the crude compounds 5a−f. An n-butanol/water solution (10 mL, 2 mL/1 mL) containing the crude compounds 5a−f (2 mmol), propiolic acid (154 mg, 2.2 mmol), l-ascorbic acid sodium salt (19.81 mg, 0.1 mmol), CuSO4·5H2O (99.87 mg, 0.4 mmol) was stirred at room temperature for 24 h until the starting material disappeared (monitored by TLC). The mixture was extracted with n-butanol (3 × 10 mL). After washing with brine (2 × 10 mL), the solvent was evaporated in vacuum. The residue was crystallized with ethyl acetate to yield compounds 6a−f.
4.1.3. General procedure for preparation of compounds 11a−c
A mixture of aldehyde (6 mmol), ammonium hydroxide (30%, 6 mL) is stirred for 5 h at reflux. During this time, a white precipitate was formed and filtered, washed with water to obtain compounds 7a−c. Diethyl phosphite (3 mmol) was added to compounds 7a−c and the reaction mixture was stirred at 70 °C for 2–5 h. After the starting material disappeared, the mixture was cooled and poured into a solution of p-toluenesulfonic acid (3 mmol) in 25 mL THF. Then the resulting solution stirred at 0 °C for 2 h. The formed solid was filtered, washed with THF to obtain compounds 9a−c. Aqueous ammonium hydroxide (15 mL, 10%) was added compounds 9a−c with stirring for 30 min at room temperature. The mixture was extracted with ether (3 × 20 mL). After washing with brine (2 × 20 mL), the solvent was evaporated in vacuum. The crude was purified by chromatography on silica gel with EtOAc/petroleum ether (9:1) to afford compounds 10a−c. To a solution of compounds 10a−c (1 mmol) in dichloromethane (10.0 mL) were dropped chloroacetyl chloride (1.2 mmol) at 0 °C. The reaction mixture was stirred for 3 h at room temperature. Then the mixture was extracted with dichloromethane (3 × 20 mL). After washing with brine (2 × 20 mL), the solvent was evaporated in vacuum. The residue was crystallized with methanol/water (1/10, v/v) to yield compounds 11a−c.
4.1.3.1. Diethyl ((2-chloroacetamido) (phenyl)methyl)phosphonate (11a)
1H NMR (300 MHz, DMSO-d6) δ 9.30 (d, J = 9.6 Hz, 1H), 7.51–7.27 (m, 5H), 5.39 (dd, J = 21.1, 9.7 Hz, 1H), 4.19 (q, J = 12.6 Hz, 2H), 4.09–3.95 (m, 2H), 3.95–3.83 (m, 1H), 3.77 (ddd, J = 10.2, 8.6, 7.1 Hz, 1H), 1.20 (t, J = 7.0 Hz, 3H), 1.07 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.60, 134.43, 128.72, 128.70, 128.36, 128.10, 128.02, 63.39, 51.59, 49.54, 42.44, 16.38, 16.11.
4.1.3.2. Diethyl ((2-chloroacetamido) (4-fluorophenyl)methyl)phosphonate (11b)
1H NMR (300 MHz, DMSO-d6) δ 9.31 (d, J = 9.4 Hz, 1H), 7.55–7.42 (m, 2H), 7.22 (t, J = 8.7 Hz, 2H), 5.41 (dd, J = 21.0, 9.6 Hz, 1H), 4.18 (q, J = 12.6 Hz, 2H), 4.09–3.97 (m, 2H), 3.96–3.86 (m, 1H), 3.85–3.72 (m, 1H), 1.20 (t, J = 7.0 Hz, 3H), 1.07 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.78, 130.41, 129.93, 129.84, 129.74, 115.85, 115.56, 63.45, 63.33, 50.88, 48.82, 42.38, 16.38, 16.15.
4.1.3.3. Diethyl ((2-chloroacetamido) (p-tolyl)methyl)phosphonate (11c)
1H NMR (300 MHz, DMSO-d6) δ 9.25 (d, J = 9.5 Hz, 1H), 7.32 (d, J = 6.7 Hz, 2H), 7.17 (d, J = 7.8 Hz, 2H), 5.34 (dd, J = 20.9, 9.7 Hz, 1H), 4.17 (q, J = 12.6 Hz, 2H), 4.08–3.95 (m, 2H), 3.94–3.84 (m, 1H), 3.83–3.71 (m, 1H), 2.29 (s, 3H), 1.20 (t, J = 7.0 Hz, 3H), 1.08 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.50, 138.23, 131.37, 129.42, 129.40, 128.00, 127.92, 63.32, 51.29, 49.24, 42.46, 21.15, 16.39, 16.14.
4.1.4. Preparation of N(20)-deethyllappaconitine 12
To a solution of LA (58.46 mg, 0.1 mmol) in glacial acetic acid (5 mL) was added NBS (53.39 mg, 0.3 mmol). The resulting mixture was stirred for 3 h at room temperature, followed by added aqueous ammonium hydroxide (15 mL, 10%) to pH 9–10. The mixture was extracted with dichloromethane (3 × 10 mL). After washing with brine (2 × 10 mL), the solvent was evaporated in vacuum. The crude was purified by chromatography on silica gel with methanol/dichloromethane (20:1) to afford N(20)-deethyllappaconitine (28 mg, 51%). m.p. 144–146 °C, 1H NMR (300 MHz, DMSO-d6) δ 10.51 (s, 1H), 8.19 (d, J = 8.1 Hz, 1H), 7.84 (dd, J = 7.9, 1.4 Hz, 1H), 7.56 (dd, J = 11.4, 4.3 Hz, 1H), 7.16 (t, J = 7.1 Hz, 1H), 4.48 (s, 1H), 4.29 (s, 1H), 3.29–3.16 (m, 11H), 3.02 (d, J = 13.7 Hz, 1H), 2.85 (s, 1H), 2.65 (dd, J = 14.4, 7.6 Hz, 1H), 2.41 (d, J = 6.9 Hz, 1H), 2.33–2.25 (m, 1H), 2.24–2.14 (m, 2H), 2.10 (s, 3H), 2.03 (s, 2H), 1.97 (dd, J = 16.3, 7.7 Hz, 3H), 1.81 (d, J = 7.7 Hz, 1H), 1.67–1.57 (m, 2H), 1.40 (s, 1H), 1.24 (d, J = 3.4 Hz, 1H), 0.90–0.79 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 169.05, 167.38, 141.79, 134.61, 130.98, 122.36, 120.29, 115.45, 90.11, 82.47, 82.34, 77.35, 76.06, 57.99, 57.47, 56.26, 56.01, 52.56, 52.10, 50.78, 49.05, 44.40, 44.33, 37.01, 30.08, 26.93, 26.42, 25.58, 24.47, 23.70.
4.1.5. General procedure for the reaction of N(20)-deethyll-appaconitine 12 with different intermediates containing-carboxy
A mixture of N(20)-deethyllappaconitine (55.67 mg, 0.1 mmol), carboxylic acid derivative (0.11 mmol), EDC (38.34 mg, 0.2 mmol), HOBt (27.02 mg, 0.2 mmol) in 10 mL of anhydrous dichloromethane is stirred for 5 h at room temperature. The mixture was extracted with dichloromethane (3 × 10 mL). After washing with brine (2 × 10 mL), the solvent was evaporated in vacuum. The crude was purified by chromatography on silica gel with methanol/dichloromethane (60:1) to afford target compounds.
4.1.5.1. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-(1-phenyl-1H-1,2,3-triazole-4-carbonyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (A1)
m.p. 140–142 °C, white powder; Yield 71%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 9.25 (s, 1H), 8.18 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 7.9 Hz, 2H), 7.89 (d, J = 7.8 Hz, 1H), 7.69–7.45 (m, 4H), 7.20 (t, J = 7.6 Hz, 1H), 5.07 (d, J = 14.4 Hz, 1H), 4.56 (d, J = 9.5 Hz, 2H), 4.33 (s, 1H), 3.31–3.16 (m, 6H), 3.09 (s, 5H), 2.81 (dt, J = 29.7, 9.2 Hz, 2H), 2.39 (dd, J = 15.5, 7.7 Hz, 2H), 2.26–1.98 (m, 8H), 1.83 (d, J = 16.0 Hz, 4H), 1.58–1.42 (m, 1H), 1.23 (s, 1H), 0.84 (d, J = 8.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.17, 167.24, 160.55, 145.81, 141.94, 136.60, 134.71, 131.00, 129.96, 129.22, 125.83, 122.36, 120.37, 115.24, 89.96, 82.84, 81.53, 81.34, 78.08, 75.84, 59.14, 58.00, 56.37, 55.83, 53.81, 51.49, 49.88, 47.82, 47.06, 43.83, 37.78, 31.32, 26.05, 25.61, 25.48, 24.72. HR-MS (ESI) m/z Calcd. for C39H46N5O9+ [M+H]+ 728.3290, Found 728.3292. HPLC: tR = 20.087 min; purity 97.90%.
4.1.5.2. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-1-(1-(4-Fluorophenyl)-1H-1,2,3-triazole-4-carbonyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (A2)
m.p. 145–146 °C, white powder; Yield 74%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 9.24 (s, 1H), 8.31–7.77 (m, 4H), 7.54 (dt, J = 16.6, 7.8 Hz, 3H), 7.27–7.10 (m, 1H), 5.07 (d, J = 13.8 Hz, 1H), 4.58 (d, J = 18.5 Hz, 2H), 4.33 (s, 1H), 3.23 (dd, J = 22.7, 11.8 Hz, 6H), 3.09 (d, J = 6.5 Hz, 5H), 2.82 (ddd, J = 24.5, 16.6, 9.5 Hz, 2H), 2.44–2.32 (m, 2H), 2.30–1.95 (m, 8H), 1.86 (s, 4H), 1.57–1.40 (m, 1H), 1.23 (s, 1H), 0.84 (d, J = 7.2 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.15, 167.24, 160.35, 145.97, 141.93, 134.71, 132.86, 130.99, 126.11, 122.46, 122.35, 120.34, 117.12, 116.81, 115.21, 89.94, 82.81, 81.53, 81.34, 78.06, 75.80, 59.08, 58.00, 56.34, 55.84, 53.83, 51.47, 49.87, 47.86, 47.04, 43.82, 37.74, 31.31, 26.04, 25.60, 25.47, 24.72. HR-MS (ESI) m/z Calcd. for C39H45FN5O9+ [M+H]+ 746.3196, Found 746.31901. HPLC: tR = 20.453 min; purity 97.55%.
4.1.5.3. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-(1-(4-Chlorophenyl)-1H-1,2,3-triazole-4-carbonyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (A3)
m.p. 130–132 °C, white powder; Yield 62%. 1H NMR (300 MHz, CDCl3) δ 11.08 (s, 1H), 8.70 (d, J = 8.5 Hz, 1H), 8.52 (s, 1H), 7.97 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 8.8 Hz, 2H), 7.54 (d, J = 8.6 Hz, 3H), 7.06 (t, J = 7.6 Hz, 1H), 5.51 (s, 1H), 5.20 (d, J = 14.7 Hz, 1H), 3.64 (s, 1H), 3.54 (d, J = 7.6 Hz, 1H), 3.45 (d, J = 9.8 Hz, 5H), 3.37 (s, 1H), 3.24 (t, J = 7.7 Hz, 1H), 3.12 (s, 2H), 3.00 (dd, J = 14.3, 8.6 Hz, 1H), 2.87 (dd, J = 15.6, 7.4 Hz, 1H), 2.66 (d, J = 7.5 Hz, 2H), 2.38–2.18 (m, 8H), 2.08–1.86 (m, 4H), 1.71–1.57 (m, 2H), 0.86 (dd, J = 13.2, 7.1 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.18, 167.23, 160.29, 146.02, 141.90, 135.09, 135.03, 134.71, 130.99, 130.15, 125.90, 122.37, 121.55, 120.33, 115.20, 89.91, 82.79, 81.54, 81.30, 78.06, 75.77, 59.10, 57.99, 56.33, 55.84, 53.83, 51.46, 49.87, 47.85, 47.02, 43.75, 37.70, 31.30, 26.03, 25.60, 25.45, 24.71. HR-MS (ESI) m/z Calcd. for C39H45ClN5O9+ [M+H]+ 762.2900, Found 762.2901. HPLC: tR = 21.993 min; purity 98.47%.
4.1.5.4. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-(1-(4-methoxyphenyl)-1H-1,2,3-triazole-4-carbonyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (A4)
m.p. 125–126 °C, white powder; Yield 53%. 1H NMR (300 MHz, CDCl3) δ 11.09 (s, 1H), 8.70 (d, J = 8.6 Hz, 1H), 8.44 (s, 1H), 7.98 (d, J = 8.0 Hz, 1H), 7.67 (d, J = 9.0 Hz, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.05 (d, J = 8.9 Hz, 3H), 5.52 (s, 1H), 5.20 (d, J = 14.6 Hz, 1H), 3.88 (s, 3H), 3.65 (s, 1H), 3.57–3.50 (m, 1H), 3.42 (d, J = 7.3 Hz, 6H), 3.37 (s, 1H), 3.25 (s, 1H), 3.13 (s, 2H), 3.01 (dd, J = 14.9, 8.9 Hz, 1H), 2.86 (dd, J = 15.0, 7.0 Hz, 1H), 2.66 (d, J = 7.7 Hz, 2H), 2.33 (dd, J = 13.1, 6.0 Hz, 2H), 2.28–2.16 (m, 5H), 2.09–1.86 (m, 4H), 1.73–1.67 (m, 1H), 1.61 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 169.17, 167.23, 160.65, 160.14, 145.58, 141.91, 134.69, 131.00, 129.99, 125.89, 122.36, 122.00, 120.32, 115.24, 114.96, 89.95, 82.86, 81.52, 81.36, 78.07, 75.82, 59.08, 57.99, 56.36, 55.84, 55.68, 53.83, 51.46, 49.87, 47.83, 47.05, 43.80, 37.76, 31.33, 26.04, 25.60, 25.49, 24.71. HR-MS (ESI) m/z Calcd. for C40H48N5O10+ [M+H]+ 758.3396, Found 758.3394. HPLC: tR = 20.207 min; purity 95.45%.
4.1.5.5. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-(1-(3,4-Dimethoxyphenyl)-1H-1,2,3-triazole-4-carbonyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (A5)
m.p. 140–142 °C, white powder; Yield 53%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 9.21 (s, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.89 (d, J = 7.3 Hz, 1H), 7.65–7.49 (m, 3H), 7.19 (dd, J = 16.1, 8.1 Hz, 2H), 5.08 (d, J = 14.2 Hz, 1H), 4.63 (s, 1H), 4.54 (s, 1H), 4.32 (s, 1H), 3.85 (d, J = 10.6 Hz, 6H), 3.23 (d, J = 12.2 Hz, 6H), 3.11 (d, J = 14.7 Hz, 5H), 2.94–2.75 (m, 2H), 2.37 (d, J = 7.3 Hz, 2H), 2.23–1.98 (m, 8H), 1.85 (d, J = 13.3 Hz, 4H), 1.49 (d, J = 10.2 Hz, 1H), 1.19 (dd, J = 16.1, 8.9 Hz, 1H), 0.91–0.83 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.18, 167.23, 160.64, 149.87, 149.74, 145.55, 141.89, 134.69, 130.99, 130.08, 125.92, 122.37, 120.32, 115.23, 112.47, 111.35, 104.66, 89.92, 82.84, 81.57, 81.36, 78.06, 75.78, 59.12, 58.00, 56.26, 56.24, 55.86, 53.84, 51.45, 49.87, 47.85, 47.05, 43.75, 37.65, 31.33, 26.04, 25.59, 25.47, 24.70. HR-MS (ESI) m/z Calcd. for C41H50N5O11+ [M+H]+ 788.3501, Found 788.3505. HPLC: tR = 19.260 min; purity 98.50%.
4.1.5.6. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-(1-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazole-4-carbonyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (A6)
m.p. 116–118 °C, white powder; Yield 73%. 1H NMR (300 MHz, CDCl3) δ 11.08 (s, 1H), 8.71 (d, J = 8.5 Hz, 1H), 8.49 (s, 1H), 8.02–7.94 (m, 1H), 7.54 (t, J = 7.9 Hz, 1H), 7.10–7.02 (m, 1H), 7.00 (d, J = 8.8 Hz, 2H), 5.50 (s, 1H), 5.20 (d, J = 14.5 Hz, 1H), 4.00–3.85 (m, 9H), 3.65 (s, 1H), 3.56–3.49 (m, 1H), 3.44 (t, J = 8.2 Hz, 5H), 3.39–3.33 (m, 2H), 3.25 (s, 2H), 3.14 (s, 2H), 3.00 (dd, J = 14.8, 8.5 Hz, 1H), 2.87 (dd, J = 15.1, 6.9 Hz, 1H), 2.67 (d, J = 7.4 Hz, 2H), 2.38–2.30 (m, 2H), 2.27–2.14 (m, 5H), 2.03–1.84 (m, 4H), 1.72 (dd, J = 18.3, 9.0 Hz, 1H), 1.63 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 169.16, 167.23, 160.52, 154.04, 153.68, 145.67, 141.91, 134.71, 132.34, 130.99, 126.05, 122.37, 120.34, 115.22, 101.22, 98.28, 89.92, 82.81, 81.60, 81.38, 78.05, 75.77, 61.09, 59.14, 58.01, 56.45, 56.22, 55.88, 53.84, 51.44, 49.87, 47.87, 47.06, 43.76, 37.62, 31.33, 26.04, 25.60, 25.46, 24.71. HR-MS (ESI) m/z Calcd. for C42H52N5O12+ [M+H]+ 818.3607, Found 818.3598. HPLC: tR = 20.107 min; purity 96.05%.
4.1.5.7. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-Cinnamoyl-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B1)
m.p. 115–116 °C, white powder; yield 73%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.59 (t, J = 7.8 Hz, 3H), 7.45–7.35 (m, 3H), 7.21 (dd, J = 21.6, 12.1 Hz, 2H), 6.95 (d, J = 15.9 Hz, 1H), 4.77 (d, J = 14.7 Hz, 1H), 4.61 (s, 1H), 4.30 (s, 1H), 4.10 (s, 1H), 3.25 (dt, J = 24.0, 8.0 Hz, 10H), 3.10 (t, J = 8.3 Hz, 1H), 2.92 (s, 3H), 2.78 (dd, J = 14.5, 7.8 Hz, 1H), 2.54 (s, 3H), 2.36 (d, J = 7.5 Hz, 1H), 2.25 (d, J = 7.9 Hz, 3H), 2.12 (d, J = 6.7 Hz, 5H), 2.02–1.93 (m, 1H), 1.86 (d, J = 7.5 Hz, 1H), 1.80–1.70 (m, 1H), 1.43 (dd, J = 17.3, 8.0 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 169.20, 167.19, 166.94, 141.94, 140.22, 135.52, 134.71, 130.95, 129.44, 128.87, 128.01, 127.24, 122.34, 120.36, 120.22, 115.21, 89.89, 82.84, 82.78, 81.13, 77.96, 75.45, 58.94, 58.00, 56.07, 55.86, 53.80, 51.03, 49.80, 47.86, 47.27, 44.79, 37.26, 31.31, 26.30, 25.60, 25.54, 24.42. HR-MS (ESI) m/z Calcd. for C39H47N2O9+ [M+H]+ 687.3276, Found 687.3277. HPLC: tR = 20.873 min; purity 98.20%.
4.1.5.8. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-((E)-3-(4-Fluorophenyl)acryloyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B2)
m.p. 112–114 °C, white powder; Yield 84%. 1H NMR (300 MHz, CDCl3) δ 11.06 (s, 1H), 8.70 (d, J = 8.2 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.60–7.36 (m, 4H), 7.13–6.98 (m, 3H), 6.85 (d, J = 15.6 Hz, 1H), 4.92 (d, J = 14.8 Hz, 1H), 4.24 (s, 1H), 3.52–3.26 (m, 10H), 3.08 (s, 2H), 2.83 (dd, J = 14.9, 6.3 Hz, 2H), 2.63 (d, J = 8.1 Hz, 2H), 2.47–2.37 (m, 3H), 2.25 (s, 5H), 1.97 (dd, J = 43.1, 7.8 Hz, 4H), 1.67–1.54 (m, 2H), 1.26 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 169.18, 167.17, 166.72, 141.92, 138.99, 134.71, 131.71, 130.93, 129.77, 128.99, 128.88, 122.33, 120.35, 119.99, 116.09, 115.80, 115.16, 89.85, 82.95, 82.70, 81.11, 77.93, 75.39, 58.92, 57.99, 56.09, 55.87, 53.78, 50.99, 49.78, 47.86, 47.26, 44.76, 37.17, 31.27, 26.29, 25.59, 25.54, 24.39. HR-MS (ESI) m/z Calcd. for C39H46FN2O9+ [M+H]+ 705.3182, Found 705.3186. HPLC: tR = 21.133 min; purity 98.278%.
4.1.5.9. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-1-((E)-3-(4-Chlorophenyl)acryloyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B3)
m.p. 120–122 °C, white powder; Yield 61%. 1H NMR (300 MHz, DMSO-d6) δ 10.49 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.95–7.68 (m, 2H), 7.63–7.52 (m, 2H), 7.47 (d, J = 8.3 Hz, 2H), 7.21 (dd, J = 15.3, 7.8 Hz, 2H), 6.96 (d, J = 16.1 Hz, 1H), 4.77 (d, J = 14.8 Hz, 1H), 4.61 (s, 1H), 4.28 (s, 1H), 4.06 (s, 1H), 3.24 (dt, J = 21.4, 8.7 Hz, 10H), 3.10–3.02 (m, 1H), 2.92 (s, 2H), 2.78 (dd, J = 14.7, 7.3 Hz, 1H), 2.36 (d, J = 7.2 Hz, 1H), 2.23 (d, J = 11.0 Hz, 4H), 2.17–2.05 (m, 6H), 1.97 (dd, J = 13.1, 7.4 Hz, 1H), 1.86 (d, J = 7.5 Hz, 1H), 1.78–1.69 (m, 1H), 1.40 (s, 1H), 0.85 (d, J = 6.7 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.17, 167.17, 166.58, 141.93, 138.83, 135.23, 134.72, 134.01, 130.93, 129.11, 128.37, 122.33, 120.84, 120.35, 115.14, 89.85, 82.95, 82.67, 81.08, 77.92, 75.40, 58.96, 58.00, 56.11, 55.86, 53.76, 51.00, 49.77, 47.85, 47.27, 44.79, 37.14, 31.26, 26.30, 25.60, 25.52, 24.41. HR-MS (ESI) m/z Calcd. for C39H46ClN2O9+ [M+H]+ 721.2886, Found 721.2882. HPLC: tR = 22.700 min; purity 95.61%.
4.1.5.10. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(4-methoxyphenyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B4)
m.p. 118–120 °C, white powder; Yield 74%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 8.2 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.69–7.50 (m, 3H), 7.21 (dd, J = 20.6, 11.9 Hz, 2H), 6.97 (d, J = 8.6 Hz, 2H), 6.81 (d, J = 15.8 Hz, 1H), 4.75 (d, J = 14.2 Hz, 1H), 4.60 (s, 1H), 4.30 (s, 1H), 4.11 (s, 1H), 3.79 (d, J = 5.2 Hz, 3H), 3.32–3.10 (m, 11H), 3.00 (s, 2H), 2.78 (dd, J = 14.5, 7.8 Hz, 1H), 2.36 (d, J = 7.4 Hz, 1H), 2.27 (d, J = 4.9 Hz, 3H), 2.18–2.05 (m, 6H), 1.99 (dd, J = 13.9, 7.5 Hz, 1H), 1.84 (d, J = 7.5 Hz, 1H), 1.78–1.69 (m, 1H), 1.40 (s, 1H), 1.24 (s, 1H), 0.85 (d, J = 6.9 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.18, 167.16, 167.10, 160.71, 141.93, 140.25, 134.68, 130.95, 129.62, 128.77, 128.23, 122.32, 120.33, 117.50, 115.21, 114.26, 89.90, 82.92, 82.82, 81.16, 77.96, 75.45, 58.77, 58.00, 56.35, 55.85, 55.39, 53.82, 51.00, 49.80, 47.91, 47.26, 44.79, 37.27, 31.33, 26.28, 25.61, 25.57, 24.40. HR-MS (ESI) m/z Calcd. for C40H49N2O10+ [M+H]+ 717.3382, Found 717.3387. HPLC: tR = 20.693 min; purity 95.26%.
4.1.5.11. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(p-tolyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B5)
m.p. 120–122 °C, white powder; Yield 63%. 1H NMR (300 MHz, DMSO-d6) δ 10.49 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.53 (dt, J = 17.4, 8.3 Hz, 3H), 7.29–7.12 (m, 4H), 6.90 (d, J = 15.9 Hz, 1H), 4.85–4.71 (m, 1H), 4.59 (s, 1H), 4.30 (s, 1H), 4.10 (s, 1H), 3.28–3.10 (m, 9H), 2.99 (s, 3H), 2.78 (dd, J = 14.8, 7.7 Hz, 1H), 2.37–2.19 (m, 8H), 2.10 (dd, J = 16.4, 4.0 Hz, 6H), 1.98 (dd, J = 13.0, 8.7 Hz, 2H), 1.85 (d, J = 6.6 Hz, 1H), 1.79–1.70 (m, 1H), 1.40 (dd, J = 8.0, 5.4 Hz, 1H), 0.89–0.76 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 169.19, 167.18, 167.03, 141.95, 140.47, 139.68, 134.69, 132.76, 130.95, 129.55, 128.01, 127.26, 122.33, 120.35, 118.91, 115.22, 89.92, 82.86, 82.81, 81.15, 77.96, 75.47, 58.83, 58.00, 56.11, 55.83, 53.80, 51.02, 49.80, 47.88, 47.27, 44.81, 37.27, 31.32, 26.29, 25.61, 25.55, 24.43, 21.37. HR-MS (ESI) m/z Calcd. for C40H49N2O9+ [M+H]+ 701.3433, Found 701.3436. HPLC: tR = 22.220 min; purity 97.50%.
4.1.5.12. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(4-(trifluoromethyl)phenyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B6)
m.p. 136–140 °C, white powder; Yield 59%. 1H NMR (300 MHz, DMSO-d6) δ 10.49 (s, 1H), 8.18 (d, J = 8.1 Hz, 1H), 7.84 (ddd, J = 23.2, 18.4, 8.1 Hz, 5H), 7.63–7.52 (m, 1H), 7.17 (dt, J = 34.8, 16.3 Hz, 3H), 4.80 (d, J = 14.1 Hz, 1H), 4.61 (s, 1H), 4.27 (s, 1H), 4.05 (s, 1H), 3.32–3.15 (m, 10H), 2.98 (t, J = 8.2 Hz, 1H), 2.80 (s, 3H), 2.36 (d, J = 7.2 Hz, 1H), 2.29–2.19 (m, 3H), 2.16–2.03 (m, 6H), 2.00–1.94 (m, 1H), 1.89 (d, J = 7.7 Hz, 1H), 1.80–1.73 (m, 1H), 1.67–1.54 (m, 1H), 1.48–1.35 (m, 1H), 0.89–0.76 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.16, 167.18, 166.32, 141.95, 139.00, 138.98, 138.06, 134.75, 130.92, 128.13, 127.29, 125.90, 125.85, 123.22, 122.33, 120.36, 115.11, 89.82, 82.94, 82.58, 81.05, 77.92, 75.39, 59.11, 57.98, 55.96, 55.87, 53.71, 51.02, 49.75, 47.78, 47.29, 44.80, 37.04, 31.23, 26.32, 25.59, 25.49, 24.41. HR-MS (ESI) m/z Calcd. for C40H46F3N2O9+ [M+H]+ 755.3150, Found 755.3142. HPLC: tR = 23.333 min; purity 96.27%.
4.1.5.13. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-1-((E)-3-(3,4-Dimethoxyphenyl)acryloyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B7)
m.p. 118–120 °C, white powder; Yield 71%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 8.2 Hz, 1H), 7.58 (t, J = 7.8 Hz, 1H), 7.31–7.10 (m, 4H), 6.98 (d, J = 8.3 Hz, 1H), 6.82 (d, J = 16.0 Hz, 1H), 4.76 (d, J = 14.2 Hz, 1H), 4.60 (s, 1H), 4.28 (s, 1H), 4.12 (s, 1H), 3.79 (d, J = 4.8 Hz, 6H), 3.29–3.08 (m, 9H), 2.94 (s, 2H), 2.78 (dd, J = 13.7, 7.2 Hz, 1H), 2.36 (d, J = 6.9 Hz, 1H), 2.32–2.22 (m, 3H), 2.20–2.05 (m, 6H), 1.98 (dd, J = 13.4, 8.1 Hz, 1H), 1.87 (d, J = 7.9 Hz, 1H), 1.73 (dd, J = 13.9, 8.1 Hz, 1H), 1.66–1.53 (m, 1H), 1.51–1.33 (m, 2H), 1.23 (s, 1H), 0.96–0.78 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 169.18, 167.18, 167.14, 150.41, 149.26, 141.95, 140.17, 134.70, 130.95, 128.60, 122.33, 120.63, 120.35, 118.16, 115.20, 111.24, 110.11, 89.92, 82.91, 82.80, 81.24, 78.00, 75.38, 58.91, 58.03, 56.35, 56.02, 56.00, 55.86, 53.84, 51.00, 49.80, 47.96, 47.32, 44.84, 36.80, 31.35, 26.28, 25.61, 25.54, 24.44. HR-MS (ESI) m/z Calcd. for C41H51N2O11+ [M+H]+ 747.3487, Found 747.3482. HPLC: tR = 19.227 min; purity 97.07%.
4.1.5.14. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(2,3,4-trimethoxyphenyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B8)
m.p. 110–112 °C, white powder; Yield 76%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.59 (t, J = 7.1 Hz, 1H), 7.17 (dd, J = 18.7, 11.7 Hz, 2H), 7.04 (d, J = 13.2 Hz, 1H), 6.97–6.85 (m, 2H), 4.78 (d, J = 14.3 Hz, 1H), 4.61 (s, 1H), 4.27 (s, 1H), 4.10 (s, 1H), 3.82 (d, J = 6.4 Hz, 6H), 3.68 (d, J = 8.0 Hz, 3H), 3.26 (dd, J = 18.0, 7.2 Hz, 8H), 3.09–3.01 (m, 1H), 2.85 (s, 2H), 2.78 (dd, J = 15.1, 7.8 Hz, 1H), 2.36 (d, J = 7.1 Hz, 1H), 2.25 (d, J = 5.4 Hz, 4H), 2.16–2.06 (m, 5H), 2.02–1.83 (m, 3H), 1.82–1.67 (m, 2H), 1.61 (dd, J = 15.0, 10.1 Hz, 1H), 1.41 (dd, J = 8.2, 3.1 Hz, 1H), 0.91–0.76 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.19, 167.18, 167.02, 153.52, 141.92, 139.70, 139.49, 134.72, 131.14, 130.93, 122.33, 120.34, 119.99, 117.02, 115.16, 105.37, 104.56, 89.86, 82.84, 82.71, 81.21, 77.90, 75.35, 60.95, 59.05, 58.03, 56.24, 55.87, 53.81, 53.64, 50.98, 49.77, 47.89, 47.31, 44.83, 36.30, 31.31, 26.25, 25.59, 25.44, 24.44. HR-MS (ESI) m/z Calcd. for C42H53N2O12+ [M+H]+ 777.3593, Found 777.3597. HPLC: tR = 19.493 min; purity 96.09%.
4.1.5.15. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(4-morpholinophenyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B9)
m.p. 130–132 °C, light yellow powder; Yield 73%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 7.6 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.66–7.38 (m, 3H), 7.21 (dd, J = 19.4, 11.4 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 6.76 (d, J = 15.7 Hz, 1H), 4.75 (d, J = 14.4 Hz, 1H), 4.58 (s, 1H), 4.30 (s, 1H), 4.12 (s, 1H), 3.73 (s, 4H), 3.29–3.12 (m, 14H), 3.06 (s, 3H), 2.78 (dd, J = 14.5, 7.0 Hz, 1H), 2.37–2.24 (m, 4H), 2.13 (s, 5H), 2.00 (dd, J = 11.2, 6.0 Hz, 2H), 1.79 (dd, J = 26.1, 11.7 Hz, 3H), 1.68–1.54 (m, 1H), 1.39 (dd, J = 9.5, 5.0 Hz, 2H), 0.85–0.76 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.20, 167.23, 167.14, 152.03, 141.91, 140.61, 134.65, 130.95, 129.44, 128.67, 128.61, 126.77, 122.32, 120.32, 116.42, 115.23, 115.02, 89.89, 82.86, 81.72, 81.18, 77.96, 75.43, 66.69, 58.69, 57.99, 56.33, 56.19, 55.83, 53.85, 50.99, 49.83, 48.48, 47.93, 47.24, 44.72, 37.28, 31.34, 26.26, 25.61, 25.56, 24.40. HR-MS (ESI) m/z Calcd. for C43H54N3O10+ [M+H]+ 772.3804, Found 772.3809. HPLC: tR = 19.120 min; purity 96.37%.
4.1.5.16. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(4-(piperidin-1-yl)phenyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B10)
m.p. 146–148 °C, light yellow powder; Yield 69%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 8.1 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.64–7.38 (m, 3H), 7.20 (dd, J = 16.0, 11.4 Hz, 2H), 6.89 (dd, J = 18.9, 8.5 Hz, 2H), 6.72 (d, J = 15.6 Hz, 1H), 4.74 (d, J = 14.6 Hz, 1H), 4.59 (s, 1H), 4.31 (s, 1H), 4.12 (s, 1H), 3.33 (s, 4H), 3.25–3.15 (m, 9H), 3.07 (s, 2H), 2.81–2.74 (m, 1H), 2.33 (dd, J = 20.0, 9.1 Hz, 4H), 2.11 (d, J = 16.3 Hz, 6H), 2.04–1.91 (m, 2H), 1.82 (d, J = 8.0 Hz, 2H), 1.76–1.70 (m, 1H), 1.57 (s, 7H), 1.40 (s, 1H), 0.83 (dd, J = 9.9, 5.2 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.21, 167.38, 167.14, 152.64, 143.88, 141.90, 141.06, 134.65, 130.95, 129.46, 128.64, 125.46, 122.31, 120.31, 115.34, 115.25, 115.09, 89.91, 82.92, 81.73, 81.21, 77.98, 75.45, 58.62, 57.99, 56.33, 56.24, 55.83, 53.87, 50.98, 49.83, 49.58, 47.94, 47.23, 44.73, 37.31, 31.35, 26.26, 25.61, 25.49, 24.39, 24.30. HR-MS (ESI) m/z Calcd. for C44H56N3O9+ [M+H]+ 770.4011, Found 770.4008. HPLC: tR = 18.373 min; purity 96.16%.
4.1.5.17. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-((E)-3-(4-(4-phenylpiperazin-1-yl)phenyl)acryloyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B11)
m.p. 144–146 °C, light yellow powder; Yield 63%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 8.2 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.58 (t, J = 7.9 Hz, 1H), 7.47 (d, J = 8.4 Hz, 2H), 7.22 (dt, J = 16.0, 7.8 Hz, 4H), 7.05–6.95 (m, 4H), 6.84–6.73 (m, 2H), 4.75 (d, J = 14.5 Hz, 1H), 4.58 (s, 1H), 4.47 (s, 1H), 4.12 (s, 1H), 3.41–3.32 (m, 8H), 3.18 (d, J = 7.7 Hz, 5H), 3.07 (s, 3H), 2.81–2.73 (m, 1H), 2.40–2.25 (m, 5H), 2.13 (s, 5H), 2.00 (dd, J = 12.7, 5.8 Hz, 3H), 1.83 (d, J = 8.3 Hz, 2H), 1.78–1.68 (m, 2H), 1.64–1.51 (m, 1H), 1.40 (s, 2H), 0.88–0.78 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.20, 167.26, 167.15, 151.06, 141.91, 140.66, 134.67, 130.96, 129.48, 129.24, 129.20, 128.64, 126.65, 122.32, 120.32, 120.28, 116.40, 116.36, 116.33, 115.50, 115.23, 89.90, 82.92, 82.87, 81.18, 77.97, 75.45, 58.69, 58.01, 56.23, 55.84, 53.85, 50.99, 49.82, 49.77, 49.25, 48.49, 48.29, 47.93, 47.26, 44.75, 37.28, 31.34, 26.27, 25.62, 25.57, 24.40. HR-MS (ESI) m/z Calcd. for C49H59N4O9+ [M+H]+ 847.4277, Found 847.4270. HPLC: tR = 25.187 min; purity 95.30%.
4.1.5.18. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-((E)-3-(4-(4-benzylpiperazin-1-yl)phenyl)acryloyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (B12)
m.p. 134–136 °C, light yellow powder; Yield 77%. 1H NMR (300 MHz, DMSO-d6) δ 10.50 (s, 1H), 8.19 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 7.9 Hz, 1H), 7.56 (dd, J = 18.3, 10.9 Hz, 2H), 7.43 (d, J = 8.5 Hz, 2H), 7.36–7.19 (m, 6H), 6.99–6.86 (m, 2H), 6.74 (d, J = 16.2 Hz, 1H), 4.74 (d, J = 14.3 Hz, 1H), 4.59 (s, 1H), 4.31 (s, 1H), 4.11 (s, 1H), 3.52 (s, 2H), 3.30–3.13 (m, 14H), 3.05 (s, 3H), 2.77 (dd, J = 14.9, 7.8 Hz, 2H), 2.41–2.21 (m, 5H), 2.19–2.05 (m, 6H), 2.03–1.89 (m, 2H), 1.82 (d, J = 7.6 Hz, 2H), 1.72 (dd, J = 14.8, 9.2 Hz, 1H), 1.60 (dd, J = 10.8, 4.9 Hz, 1H), 1.47–1.33 (m, 1H), 0.88–0.78 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.19, 167.29, 167.15, 152.09, 141.91, 140.79, 137.77, 134.65, 130.95, 129.41, 129.21, 128.59, 128.32, 127.24, 126.19, 122.32, 120.32, 115.98, 115.18, 89.90, 82.89, 81.20, 81.07, 77.97, 75.45, 63.02, 58.65, 57.99, 56.33, 56.21, 55.83, 53.85, 52.82, 50.99, 49.81, 48.23, 47.93, 47.24, 44.74, 37.29, 31.35, 26.26, 25.60, 25.54, 24.40. HR-MS (ESI) m/z Calcd. for C50H61N4O9+ [M+H]+ 861.4433, Found 861.4438. HPLC: tR=12.007 min; purity 97.01%.
4.1.5.19. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-((tert-butoxycarbonyl)-L-alanyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (C1)
m.p. 136–138 °C, white powder; Yield 54%. 1H NMR (300 MHz, DMSO-d6) δ 10.46 (s, 1H), 8.16 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 7.4 Hz, 1H), 7.58 (d, J = 6.9 Hz, 1H), 7.18 (s, 1H), 7.03 (d, J = 7.8 Hz, 1H), 4.59–4.46 (m, 2H), 4.37 (s, 2H), 3.89 (s, 1H), 3.29–3.17 (m, 11H), 2.73 (dd, J = 14.4, 6.2 Hz, 1H), 2.37–2.21 (m, 5H), 2.11 (s, 4H), 1.96 (dd, J = 25.5, 9.8 Hz, 3H), 1.84–1.74 (m, 1H), 1.66 (dd, J = 14.8, 9.1 Hz, 2H), 1.36 (s, 9H), 1.26 (d, J = 6.4 Hz, 3H), 1.13 (d, J = 5.9 Hz, 1H), 0.84 (d, J = 5.4 Hz, 2H).13C NMR (126 MHz, CDCl3) δ 171.81, 169.13, 167.20, 155.17, 141.93, 134.73, 130.94, 122.33, 120.33, 115.11, 89.95, 82.74, 82.29, 81.48, 79.47, 77.93, 75.43, 58.04, 57.96, 56.28, 55.71, 54.22, 53.33, 50.62, 49.94, 48.69, 47.11, 44.49, 36.59, 31.42, 28.39, 26.35, 25.67, 25.57, 24.54, 18.48. HR-MS (ESI) m/z Calcd. for C38H54N3O11+ [M+H]+ 728.3753, Found 728.3751. HPLC: tR=20.667 min; purity 99.40%.
4.1.5.20. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-((S)-2-((tert-butoxycarbonyl)amino)-2-phenylacetyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (C2)
m.p. 138–140 °C, white powder; Yield 58%. 1H NMR (300 MHz, DMSO-d6) δ 10.45 (s, 1H), 8.12 (s, 1H), 7.83 (s, 1H), 7.57 (s, 1H), 7.48–7.01 (m, 7H), 4.75–4.41 (m, 2H), 4.28 (d, J = 26.2 Hz, 1H), 3.94 (d, J = 52.7 Hz, 1H), 3.22 (d, J = 20.8 Hz, 10H), 2.91 (s, 2H), 2.73 (s, 1H), 2.30 (dd, J = 10.5, 4.1 Hz, 3H), 2.11 (s, 5H), 2.03–1.79 (m, 4H), 1.73–1.58 (m, 2H), 1.31 (d, J = 41.1 Hz, 10H), 0.85 (s, 2H), 0.57 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 171.36, 169.15, 168.81, 154.97, 141.95, 134.83, 130.92, 129.27, 128.97, 128.65, 128.29, 127.87, 127.53, 122.34, 120.36, 115.12, 89.82, 82.91, 82.38, 81.39, 79.60, 77.77, 75.24, 58.06, 58.00, 57.83, 56.33, 56.11, 52.91, 51.31, 49.88, 49.55, 49.35, 47.46, 36.50, 31.52, 28.38, 25.82, 25.53, 24.42, 24.12. HR-MS (ESI) m/z Calcd. for C43H56N3O11+ [M+H]+ 790.3909, Found 790.3905. HPLC: tR=23.280 min; purity 96.74%.
4.1.5.21. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-((tert-butoxycarbonyl)-L-methionyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (C3)
m.p. 118–120 °C, white powder; Yield 61%. 1H NMR (300 MHz, DMSO-d6) δ 10.46 (s, 1H), 8.17 (d, J = 8.2 Hz, 1H), 7.85 (d, J = 7.9 Hz, 1H), 7.57 (t, J = 7.8 Hz, 1H), 7.26–6.99 (m, 2H), 4.62–4.41 (m, 3H), 4.31 (s, 1H), 3.94 (s, 1H), 3.28 (s, 6H), 3.22 (d, J = 5.1 Hz, 6H), 2.78–2.70 (m, 1H), 2.43 (dd, J = 7.7, 5.3 Hz, 2H), 2.35 (d, J = 7.2 Hz, 3H), 2.23 (d, J = 19.8 Hz, 3H), 2.09 (d, J = 13.0 Hz, 8H), 2.01 (s, 1H), 1.94 (d, J = 7.7 Hz, 2H), 1.80 (dd, J = 13.1, 9.5 Hz, 2H), 1.65 (dd, J = 14.3, 7.7 Hz, 2H), 1.38 (d, J = 6.3 Hz, 9H), 1.29–1.21 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 171.60, 169.10, 167.23, 155.36, 141.92, 134.74, 130.94, 122.33, 120.33, 115.09, 89.77, 82.78, 82.29, 81.66, 79.60, 77.90, 75.37, 60.38, 58.03, 56.33, 56.25, 55.92, 54.50, 50.46, 49.94, 49.14, 47.03, 44.51, 37.06, 32.51, 31.48, 30.50, 28.33, 26.42, 25.54, 24.65, 21.04, 15.76. HR-MS (ESI) m/z Calcd. for C40H58N3O11S+ [M+H]+ 788.3787, Found 788.3785. HPLC: tR=22.567 min; purity 95.25%.
4.1.5.22. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-1-(1H-indole-2-carbonyl)-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (D1)
m.p. 180–182 °C, white powder; yield 61%. 1H NMR (300 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.50 (s, 1H), 8.17 (d, J = 8.1 Hz, 1H), 7.88 (d, J = 7.7 Hz, 1H), 7.60 (t, J = 6.5 Hz, 2H), 7.41 (d, J = 8.2 Hz, 1H), 7.27–7.09 (m, 2H), 7.03 (t, J = 7.5 Hz, 1H), 6.79 (s, 1H), 4.88 (d, J = 14.2 Hz, 1H), 4.50 (d, J = 29.1 Hz, 2H), 4.26 (s, 1H), 3.46–3.31 (m, 4H), 3.23–3.14 (m, 4H), 2.78 (dd, J = 14.9, 7.1 Hz, 1H), 2.53 (s, 2H), 2.43–2.36 (m, 2H), 2.28 (s, 2H), 2.14 (s, 3H), 2.07–1.95 (m, 4H), 1.84 (dd, J = 22.3, 13.7 Hz, 4H), 1.59 (dd, J = 9.5, 3.3 Hz, 2H), 1.40 (s, 1H), 0.84 (dd, J = 5.8, 2.4 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 169.16, 167.24, 164.26, 141.95, 135.73, 134.83, 130.96, 127.50, 124.09, 122.38, 121.95, 120.48, 120.39, 115.06, 111.19, 105.62, 105.55, 89.68, 83.12, 82.49, 81.80, 78.01, 75.27, 57.89, 56.30, 55.65, 54.42, 50.86, 49.75, 48.63, 48.61, 48.09, 44.38, 37.26, 31.38, 26.75, 25.83, 25.60, 24.20. HR-MS (ESI) m/z Calcd. for C39H46N3O9+ [M+H]+ 700.3229, Found 700.3223. HPLC: tR=20.547 min; purity 97.19%.
4.1.5.23. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-(5-Chloro-1H-indole-2-carbonyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (D2)
m.p. 258–260 °C, white powder; Yield 56%. 1H NMR (300 MHz, DMSO-d6) δ 11.70 (s, 1H), 10.49 (s, 1H), 8.16 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 7.2 Hz, 1H), 7.67 (s, 1H), 7.59 (t, J = 7.1 Hz, 1H), 7.42 (d, J = 8.8 Hz, 1H), 7.26–7.12 (m, 2H), 6.76 (s, 1H), 4.87 (d, J = 14.9 Hz, 1H), 4.56 (s, 1H), 4.32 (s, 1H), 4.23 (s, 1H), 3.42 (d, J = 14.6 Hz, 1H), 3.26 (s, 3H), 3.23–3.17 (m, 4H), 2.77 (dd, J = 14.1, 7.0 Hz, 1H), 2.54 (s, 3H), 2.39–2.24 (m, 4H), 2.14 (s, 3H), 2.06–1.98 (m, 3H), 1.94 (t, J = 8.5 Hz, 3H), 1.78 (dd, J = 14.1, 8.4 Hz, 3H), 1.57 (dd, J = 9.2, 3.6 Hz, 1H), 1.19 (dd, J = 15.9, 8.7 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 169.15, 163.67, 157.25, 142.00, 134.89, 133.98, 132.65, 130.95, 128.52, 126.20, 124.46, 122.39, 121.22, 120.44, 115.00, 112.23, 105.01, 89.65, 83.24, 82.32, 81.98, 77.98, 75.26, 60.02, 57.94, 56.37, 55.66, 54.46, 50.86, 49.70, 48.60, 48.16, 44.47, 37.20, 31.37, 26.82, 25.87, 25.61, 24.20. HR-MS (ESI) m/z Calcd. for C39H45ClN3O9+ [M+H]+ 734.2839, Found 734.2835. HPLC: tR=22.300 min; purity 98.39%.
4.1.5.24. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-7a,11a-Dihydroxy-6,8,10-trimethoxy-1-(5-methoxy-1H-indole-2-carbonyl)dodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (D3)
m.p. 184–186 °C, white powder; Yield 59%. 1H NMR (300 MHz, DMSO-d6) δ 11.31 (s, 1H), 10.50 (s, 1H), 8.18 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 7.1 Hz, 1H), 7.59 (t, J = 7.9 Hz, 1H), 7.30 (d, J = 8.8 Hz, 1H), 7.21 (t, J = 7.6 Hz, 1H), 7.07 (d, J = 2.1 Hz, 1H), 6.88–6.68 (m, 2H), 4.85 (d, J = 14.5 Hz, 1H), 4.47 (s, 2H), 4.28 (s, 1H), 3.73 (s, 3H), 3.41 (d, J = 14.9 Hz, 4H), 3.19 (s, 4H), 2.80–2.73 (m, 1H), 2.62 (s, 3H), 2.38 (d, J = 7.4 Hz, 1H), 2.27 (s, 1H), 2.14 (s, 3H), 2.09–2.00 (m, 3H), 1.92 (s, 2H), 1.88–1.72 (m, 4H), 1.58 (dd, J = 8.3, 5.4 Hz, 2H), 1.40 (s, 1H), 0.88–0.76 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 169.16, 167.23, 164.22, 154.63, 141.94, 134.82, 131.01, 130.96, 127.91, 127.89, 122.38, 120.40, 115.07, 112.02, 112.01, 105.36, 102.64, 89.70, 83.10, 82.49, 81.92, 78.01, 75.28, 57.91, 56.29, 55.81, 55.73, 54.41, 50.87, 49.75, 48.61, 48.58, 48.09, 44.38, 37.12, 31.39, 26.70, 25.84, 25.59, 24.20. HR-MS (ESI) m/z Calcd. for C40H48N3O10+ [M+H]+ 730.3334, Found 730.3332. HPLC: tR=19.800 min; purity 97.38%.
4.1.6. General procedure for the reaction of N(20)-deethyllapp-aconitine 12 with different intermediates containing-chlorine
A mixture of N(20)-deethyllappaconitine (55.67 mg, 0.1 mmol), chlorinated hydrocarbon (0.11 mmol), potassium carbonate (27.64 mg, 0.2 mmol) in 10 mL of acetonitrile is stirred for 6 h at 70 °C. The mixture was extracted with ethyl acetate (3 × 10 mL). After washing with brine (2 × 10 mL), the solvent was evaporated in vacuum. The crude was purified by chromatography on silica gel with methanol/dichloromethane (60:1) to afford target compounds.
4.1.6.1. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-(2-(((Diethoxyphosphoryl) (phenyl)methyl)amino)-2-oxoethyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (E1)
m.p. 106–108 °C, white powder; Yield 71%. 1H NMR (300 MHz, CDCl3) δ 10.98 (s, 1H), 8.67 (d, J = 8.3 Hz, 1H), 8.41 (d, J = 9.6 Hz, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.52 (dd, J = 20.0, 7.4 Hz, 3H), 7.40–7.30 (m, 3H), 7.02 (t, J = 7.7 Hz, 1H), 5.79 (dd, J = 20.6, 10.0 Hz, 1H), 4.32–3.90 (m, 4H), 3.57 (s, 1H), 3.48 (d, J = 11.7 Hz, 3H), 3.41 (s, 3H), 3.36 (s, 3H), 3.29–3.24 (m, 1H), 3.12 (d, J = 17.5 Hz, 1H), 2.98 (s, 1H), 2.82–2.72 (m, 2H), 2.47 (d, J = 8.0 Hz, 3H), 2.38–2.28 (m, 3H), 2.21 (s, 3H), 2.13 (s, 2H), 2.05 (s, 2H), 1.84–1.75 (m, 1H), 1.62 (s, 4H), 1.31 (t, J = 7.0 Hz, 3H), 1.18 (t, J = 7.0 Hz, 3H), 0.85 (dd, J = 9.3, 5.0 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 170.35, 169.02, 167.38, 157.72, 141.80, 134.77, 134.66, 130.98, 128.51, 128.50, 128.20, 128.15, 128.00, 122.35, 120.29, 115.30, 90.04, 83.33, 82.96, 81.80, 78.35, 75.54, 63.14, 63.10, 62.39, 58.09, 57.97, 57.15, 57.03, 55.72, 51.01, 49.91, 49.59, 48.09, 46.83, 45.12, 35.53, 31.06, 27.48, 25.54, 24.75, 24.39, 16.38, 16.28. HR-MS (ESI) m/z Calcd. for C43H59N3O12P+ [M+H]+ 840.3831, Found 840.3825. HPLC: tR=13.133 min; purity 95.81%.
4.1.6.2. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12S,12aR,14S)-1-(2-(((Diethoxyphosphoryl) (4-fluorophenyl)methyl)amino)-2-oxoethyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (E2)
m.p. 108–110 °C, white powder; yield 83%. 1H NMR (300 MHz, CDCl3) δ 10.96 (s, 1H), 8.67 (d, J = 8.3 Hz, 1H), 8.37 (d, J = 9.9 Hz, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.51 (d, J = 17.6 Hz, 3H), 7.10–6.99 (m, 3H), 5.76 (dd, J = 20.5, 9.9 Hz, 1H), 4.20–3.95 (m, 4H), 3.56 (s, 1H), 3.49 (d, J = 10.2 Hz, 3H), 3.41 (s, 3H), 3.35 (s, 3H), 3.27 (s, 1H), 3.12 (d, J = 17.4 Hz, 1H), 2.96 (s, 1H), 2.78 (d, J = 10.9 Hz, 2H), 2.51–2.42 (m, 3H), 2.38–2.28 (m, 3H), 2.21 (s, 3H), 2.11 (d, J = 8.9 Hz, 2H), 2.04 (d, J = 10.0 Hz, 2H), 1.81 (dd, J = 16.0, 4.6 Hz, 1H), 1.61 (s, 4H), 1.32 (t, J = 7.0 Hz, 3H), 1.19 (t, J = 7.0 Hz, 3H), 0.86 (d, J = 6.8 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 170.37, 169.03, 167.38, 141.78, 134.67, 130.97, 130.73, 130.02, 129.91, 129.82, 122.34, 120.29, 115.59, 115.31, 115.25, 89.98, 83.21, 82.97, 81.83, 78.32, 75.48, 63.20, 63.12, 62.45, 58.08, 57.95, 57.13, 56.98, 55.71, 50.98, 49.88, 48.13, 47.25, 46.86, 45.06, 35.55, 31.03, 27.53, 25.51, 24.76, 24.37, 16.43, 16.35. HR-MS (ESI) m/z Calcd. for C43H58FN3O12P+ [M+H]+ 858.3737, Found 858.3738. HPLC: tR=13.480 min; purity 95.01%.
4.1.6.3. (3S,6S,6aS,7S,7aS,8S,9R,10S,11aS,12R,12aR,14S)-1-(2-(((Diethoxyphosphoryl) (p-tolyl)methyl)amino)-2-oxoethyl)-7a,11a-dihydroxy-6,8,10-trimethoxydodecahydro-2H-3,6a,12-(epiethane[1,1,2]triyl)-7,9-methanonaphtho[2,3-b]azocin-3(4H)-yl 2-acetamidobenzoate (E3)
m.p. 116–118 °C, white powder; Yield 81%. 1H NMR (300 MHz, CDCl3) δ 11.00 (s, 1H), 8.68 (d, J = 8.5 Hz, 1H), 8.39 (d, J = 10.0 Hz, 1H), 7.91 (d, J = 8.1 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.45 (d, J = 6.7 Hz, 2H), 7.19 (d, J = 7.9 Hz, 2H), 7.04 (t, J = 7.6 Hz, 1H), 5.77 (dd, J = 20.3, 10.0 Hz, 1H), 4.24–3.91 (m, 4H), 3.59 (s, 1H), 3.52–3.47 (m, 2H), 3.43 (s, 3H), 3.37 (d, J = 8.9 Hz, 4H), 3.32–3.24 (m, 1H), 3.13 (d, J = 17.5 Hz, 1H), 2.99 (s, 1H), 2.78 (dd, J = 13.9, 5.6 Hz, 2H), 2.47 (dd, J = 11.2, 6.2 Hz, 3H), 2.37 (dd, J = 19.6, 9.8 Hz, 6H), 2.21 (d, J = 9.1 Hz, 3H), 2.13 (d, J = 8.0 Hz, 2H), 2.04 (dd, J = 15.3, 7.2 Hz, 2H), 1.83 (dd, J = 17.4, 9.2 Hz, 1H), 1.65 (d, J = 14.3 Hz, 4H), 1.33 (t, J = 7.0 Hz, 3H), 1.20 (t, J = 7.0 Hz, 3H), 0.88 (d, J = 7.5 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 170.27, 169.01, 167.36, 141.78, 137.75, 137.72, 134.64, 131.64, 130.98, 129.17, 128.09, 128.01, 122.35, 120.28, 115.31, 90.02, 83.36, 82.97, 81.78, 78.35, 75.52, 63.02, 62.33, 58.06, 57.94, 57.18, 57.03, 55.70, 50.99, 49.91, 49.71, 48.07, 47.66, 46.80, 45.09, 35.49, 31.06, 27.47, 25.53, 24.74, 24.35, 21.13, 16.30, 16.22. HR-MS (ESI) m/z Calcd. for C44H61N3O12P+ [M+H]+ 854.3987, Found 854.3983. HPLC: tR=14.087 min; purity 95.81%.
4.2. Biology assays
4.2.1. Animals
Male ICR mice weighing 18–22 g were purchased from the Changchun Yisi experimental animal technology Co., Ltd. (Changchun, China). Animals were housed in standard conditions with a 12:12 h light–dark cycle, fed with a standard rodent diet and water, and adapted to the laboratory environment for at least 7 days before initiating experiments. Protocols involving animal care and experimental procedures were approved by Yanbian University Animal Policy and Welfare Committee (Yanji, China).
Male SD rat weighing 275–317 g were purchased from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. (Zhejiang, China). Animal quality certificate number: 1906050052. Animals were fasted overnight before intravenous administration. On the day of the experiment, the animals resumed feeding after 4 h of administration. Animals are free to drink water during the experiment. Protocols involving animal care and experimental procedures were approved by 3D BioOptima Co., Ltd. Laboratory Animal Use Management Committee (Suzhou, China).
4.2.2. Cell culture and reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and LPS (Escherichia coli serotype 055:B5) were purchased from Sigma–Aldrich, Saint Louis, MO, USA. Mouse TNF-α ELISA Kit, Mouse NO ELISA Kit and Mouse PGE2 ELISA Kit were obtained from Biolegend, San Diego, CA, USA. Protein COX-2, iNOS, p-ERK, ERK, p-NF-κB P65, NF-κB P65, p-IκBa and IκBa were purchased from CST, Danvers, MA, USA. Cell line RAW264.7 was provided by Cell Resource Center (IBMS, CAMS/PUMC, Beijing, China). BCA protein quantification kit was purchased from Beyotime company (Tianjin, China). PVDF membrane was provided by Roche company (Basel, Switzerland). Mouse RAW264.7 cells were cultured in Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum, 100 U/mL penicillin and 100 pg/mL streptomycin. The cells were incubated at 37 °C under a 5% CO2 and 90% relative humidity (RH) atmosphere.
4.2.3. Cell viability assay
Cell viability studies induced by synthesized compounds were evaluated by 3 MTT assay. RAW264.7 macrophages were seeded in 96-well plates at a density of 5 × 104 cells/mL in complete medium and incubated for 24 h (100 μL/well). Then the cells were treated with different concentrations of synthesized compounds (7.5–120 μmol/L, 50 μL/well) for 30 min, followed by stimulation with LPS (1 mg/L) for 24 h. 150 μL MTT (5 g/L in PBS) was added to each well and the cells were further incubated for 4 h. The supernatant was removed and the cells were lysed with 150 μL/well DMSO. The optical density was measured at 570 nm on a microplate reader (Thermo Scientific, Waltham, MA, USA).
4.2.4. Assay for NO production
NO production was determined by the level of nitrite (NO2) in the culture medium using the Griess assay. Briefly, RAW264.7 cells were treated with the tested compounds for 30 min, and then stimulated with or without LPS (1 μg/mL) for 24 h. The isolated supernatant was then added to an equal volume of Griess reagent and incubated for 15 min at room temperature. Nitrite production was determined by measuring the absorbance at 540 nm by a microplate reader.
4.2.5. Measurement of PEG2 and TNF-α
The RAW264.7 macrophages were seeded at 5 × 104 cells/mL in 48-well plates. Cells were incubated for 24 h and treated with 15, 30, 60 or 120 μmol/L of LA derivatives 30 min and then stimulated with 1 μg/mL of LPS for 24 h. Cell culture supernatants were centrifuged at 5000×g for 10 min at 4 °C to remove insoluble material and the supernatants were collected and stored at −20 °C until assayed for cytokines. Secreted TNF-α, PEG2 were measured in cell culture supernatants using commercially-available ELISA kits (BioLegend) following the instructions provided by the manufacturers. The absorbance (450 nm) for each sample was analyzed using microplate reader and was interpolated with a standard curve. Results of three independent experiments were used for statistical analysis.
4.2.6. Western blot analysis
RAW264.7 cells (5 × 104 cells/well) plated onto 6-well plates were incubated for 24 h. The cells treated with 30, 60 or 120 μmol/L of LA derivatives and 5 μmol/L of celecoxib 30 min and then stimulated with 1 μg/mL of LPS for 24 h. The cells were collected and washed three times with ice-cold PBS. The cells were treated with a cell lysis buffer [PIPA:PMSF = 9:1 (Sigma)] and kept on ice for 30 min. The cell lysates were centrifuged for 5 min at 4 °C to obtain a cytosolic fraction. The protein concentration was determined by BCA protein assay kit (Beyotime, Haimen, China). Aliquots of the lysates were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and then electro blotted onto a polyvinylidene difluoride (PVDF) membrane. The blots were blocked with 5% (w/v) non-fat dry milk for 2 h at 37 °C, followed by incubation with specific primary antibody at 4 °C overnight. Blots were washed with Tween 20/Tris-buffered saline [TTBS, 20 mmol/L Tris–HCl buffer, pH 7.6, containing 137 mmol/L NaCl and 0.05% (v/v) Tween 20] and incubated with a peroxidase-conjugated secondary antibody for 1 h. Blots were again washed with TTBS and the immune active proteins were detected using ECL plus (Thermo Scientific). All Western blot analyses were carried out at least three times. Results are expressed as the relative ratio of the specific band compared with the internal reference.
4.2.7. LPS-induced ALI
40 male mice weighing 18–22 g were randomly divided into five groups: control group, LPS group, LPS + A4 low-dose group (A4: 5 mg/kg), LPS + A4 medial-dose group (A4: 10 mg/kg), LPS + A4 high-dose group (A4: 15 mg/kg). The drug group was given different doses of the compound A4 by administered orally. The control group and the LPS group were orally administered with the corresponding volume of PBS. After 1 h, the mice were anesthetized with ether and given LPS (0.5 mg/kg) by dripping nose. The control group was administered with the corresponding volume of PBS. Broncho alveolar lavage fluid (BALF), blood and lung tissues were collected for further analysis after 6 h.
4.2.8. BALF analysis
BALF was collected by irrigating the left lung with saline, and centrifuged at 1000 rpm (Medical centrifuge HC-20C, Jiangsu, China) for 10 min, and the supernatant was used for protein detection and cytokine determinations. Total protein was determined by BCA protein assay (Beyotime Biotechnology, Shanghai, China). Cytokine TNF-α was tested by ELISA kits (BioLegend). Assay for NO production use the Griess assay.
4.2.9. Myeloperoxidase activity
Tissuemyeloperoxidase activity (MPO) was determined by commercial MPO detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Briefly, Lung tissue was homogenized in 1 mL of 50 mmol/L potassium PBS (pH 6.0) and centrifuged for 20 min (Medical centrifuge HC-20C). The supernatants were incubated with 0.01% H2O2 in the company of O-dianisidine dihydrochloride (0.167 mg/mL). MPO activity was determined by measuring absorbance values at 460 nm.
4.2.10. Wet/dry weight ratio
The middle lobe of the right lung was collected, blotted dry. The weight was recorded as the wet weight. Then the lungs were heated in a thermostatic oven at 65 °C for 72 h until getting constant weight recorded as dry weight. The wet/dry weigh ratio use to assess lung edema.
4.2.11. Histopathologic analysis
The collected right lung was and fixed in 4% paraformaldehyde, then embedded in paraffin, and cut into 5 mm. The sections were stained with hematoxylin and eosin (H&E). The stained cells were examined under light microscopy (Nikon, Tokyo, Japan).
4.2.12. Immunohistochemistry
Paraffin tissue sections at 5 mm were deparaffinized in xylene, and hydrated in ethanol gradient. Pressure cooker was used for heat-induced antigen retrieval. The lung sections were treated with 30% H2O2, then blocked in 5% bovine serum albumin (BSA) and incubated with primary anti-CD68 antibody overnight at 4 °C. The sections were next incubated with HRP-labeled secondary antibody for 1 h at 37 °C, stained with 3,3-diaminobenzidine tetrahydrochloride (DAB), the slides were evaluated under a microscope.
4.2.13. Preliminary pharmacokinetic study of compound A4 in rats
Intravenous injection of compound A4: to a glass bottle containing 2.03 mg of compound A4 was added 0.508 mL of DMA, and the solid substance was completely dissolved by vortex mixing; then 1.015 mL of 30% solutol HS-15 was added. After vortex mixing, 3.553 mL of saline was added. In solution, DMA:30% solutol HS-15:saline = 10:20:70 (v/v/v). The solution was mixed evenly by vortex oscillation, and filtered using a filter membrane (PALL, Nylon, 0.45 μm). A colorless clear solution was obtained. Pipette 100 μL × 2 filter and store in 1.5 mL EP tube, store at 2–8 °C.
Compound A4 was administered to SD rats via tail vein injection at a dose of 2 mg/kg (n = 3). Blood (0.15 mL) were taken from the jugular vein prior to drug delivery (0 h) and 0.083, 0.167, 0.25, 0.5, 1, 2, 4, 8 and 10 h after administration of drugs. Blood samples were placed in an anticoagulation tube containing EDTA-K2. All blood samples were centrifuged at 1500×g for 10 min to allow the collection of plasma which was stored at −40 to −20 °C.
Preparation of plasma samples: an aliquot of 30 μL of sample was added with 150 μL ACN which contains of verapamil (5 ng/mL) and glibenclamide (50 ng/mL) for protein precipitation. The mixture was vortexed for 5 min, then centrifuged at 3700 rpm (Medical centrifuge HC-20C) for 10 min. Then 70 μL of supernatant was added with 70 μL water, then vortexed for 5 min. An aliquot of 20 μL of the mixture was injected into the LC–MS/MS system. All data collected in pharmacokinetic experiment was shown in Supporting Information Tables S1−8 and Fig. S2.
4.2.14. Statistical analysis
All values are presented as mean ± SEM. Data were analyzed using IBM SPSS Statistics 20.0 (Chicago, CA, USA) and comparison between groups was made with one-way ANOVA (Dunnett's t-test) and Student's t-test. The P values of 0.05 or less were considered statistically significant. Phoenix WinNonlin 8.0 software was used to calculate the pharmacokinetic parameters of tested compounds using a non-compartmental model.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.21662036). Thanks to 3D BioOptima Co., Ltd. (Suzhou, China) for the help of pharmacokinetic experiments.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.09.002.
Contributor Information
Guo-Hua Gong, Email: gongguohua0211@163.com.
Zhe-Shan Quan, Email: zsquan@ybu.edu.cn.
Author contributions
Lei Pang synthesized the target compounds, analyzed experimental data and results, wrote the manuscript; Chun-Yan Liu performed the biological experiment; Zhe-Shan Quan designed the research; Guo-Hua Gong amended the manuscript; Zhe-Shan Quan and Guo-Hua Gong provided the financial support for this project.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamoorthy S., Honn K.V. Inflammation and disease progression. Cancer Metastasis Rev. 2006;25:481–491. doi: 10.1007/s10555-006-9016-0. [DOI] [PubMed] [Google Scholar]
- 3.Khoshneviszadeh M., Ghahremani M.H., Foroumadi A., Miri R., Firuzi O., Madadkar-Sobhani A. Design, synthesis and biological evaluation of novel anti-cytokine 1,2,4-triazine derivatives. Bioorg Med Chem. 2013;21:6708–6717. doi: 10.1016/j.bmc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Ratsimandresy R.A., Rappaport J., Zagury J.F. Anti-cytokine therapeutics: history and update. Curr Pharmaceut Des. 2009;15:1998–2025. doi: 10.2174/138161209788453130. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R.S., Antonisamy P., Almansour A.I., Arumugam N., Periyasami G., Altaf M. Functionalized spirooxindole-indolizine hybrids: stereoselective green synthesis and evaluation of anti-inflammatory effect involving TNF-α and nitrite inhibition. Eur J Med Chem. 2018;152:417–423. doi: 10.1016/j.ejmech.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Singh P., kaur J., Singh G., Bhatti R. Triblock conjugates: identification of a highly potent antiinflammatory agent. J Med Chem. 2015;58:5989–6001. doi: 10.1021/acs.jmedchem.5b00952. [DOI] [PubMed] [Google Scholar]
- 7.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 9.Khan H., Nabavi S.M., Sureda A., Mehterov N., Gulei D., Berindan-Neagoe I. Therapeutic potential of songorine, a diterpenoid alkaloid of the genus Aconitum. Eur J Med Chem. 2018;153:29–33. doi: 10.1016/j.ejmech.2017.10.065. [DOI] [PubMed] [Google Scholar]
- 10.Ren M.Y., Yu Q.T., Shi C.Y., Luo J.B. Anticancer activities of C18-, C19-, C20-, and bis-diterpenoid alkaloids derived from genus Aconitum. Molecules. 2017;22:267. doi: 10.3390/molecules22020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F.P., Chen Q.H., Liu X.Y. Diterpenoid alkaloids. Nat Prod Rep. 2010;27:529–570. doi: 10.1039/b916679c. [DOI] [PubMed] [Google Scholar]
- 12.Wang F.P., Chen Q.H. The C19-diterpenoid alkaloids. Alkaloids Chem Biol. 2010;69:1–577. doi: 10.1016/s1099-4831(10)69001-3. [DOI] [PubMed] [Google Scholar]
- 13.Sun M.L., Ao J.P., Wang Y.R., Huang Q., Li T.F., Li X.Y. Lappaconitine, a C18-diterpenoid alkaloid, exhibits antihypersensitivity in chronic pain through stimulation of spinal dynorphin a expression. Psychopharmacology (Berlin) 2018;235:2559–2571. doi: 10.1007/s00213-018-4948-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.Z., Xiao Y.Q., Zhang C., Sun X.M. Study of analgesic and anti-inflammatory effects of lappaconitine gelata. J Tradit Chin Med. 2009;29:141–145. doi: 10.1016/s0254-6272(09)60051-0. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad M., Ahmad W., Ahmad M., Zeeshan M., Obaidullah Shaheen F. Norditerpenoid alkaloids from the roots of Aconitum heterophyllum Wall with antibacterial activity. J Enzym Inhib Med Chem. 2008;23:1018–1022. doi: 10.1080/14756360701810140. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Li N., Sui Z.Y., Bi K.S., Li Z.J. An investigation on the quantitative structure–activity relationships of the anti-inflammatory activity of diterpenoid alkaloids. Molecules. 2017;22:363. doi: 10.3390/molecules22030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada K., Ohkoshi E., Morris-Natschke S.L., Bastow K.F., Lee K.H. Cytotoxic esterified diterpenoid alkaloid derivatives with increased selectivity against a drug-resistant cancer cell line. Bioorg Med Chem Lett. 2012;22:249–252. doi: 10.1016/j.bmcl.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Journet M., Cai D.W., Kowal J.J., Larsen R.D. Highly efficient and mild synthesis of variously 5-substituted-4-carbaldehyde-1,2,3-triazole derivatives. Tetrahedron Lett. 2001;42:9117–9118. [Google Scholar]
- 19.Sanghvi Y.S., Bhattacharya B.K., Kini G.D., Matsumoto S.S., Larson S.B., Jolley W.B. Growth inhibition and induction of cellular differentiation of human myeloid leukemia cells in culture by carbamoyl congeners of ribavirin. J Med Chem. 1990;33:336–344. doi: 10.1021/jm00163a054. [DOI] [PubMed] [Google Scholar]
- 20.Genin M.J., Allwine D.A., Anderson D.J., Barbachyn M.R., Emmert D.E., Garmon S.A. Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms Haemophilus influenzae and Moraxella catarrhalis. J Med Chem. 2000;43:953–970. doi: 10.1021/jm990373e. [DOI] [PubMed] [Google Scholar]
- 21.Hou W., Zhang G.J., Luo Z., Li D., Ruan H.Q., Ruan B.H. Identification of a diverse synthetic abietane diterpenoid library and insight into the structure–activity relationships for antibacterial activity. Bioorg Med Chem Lett. 2017;27:5382–5386. doi: 10.1016/j.bmcl.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Lal K., Yadav P., Kumar A. Synthesis, characterization and antimicrobial activity of 4-((1-benzyl/phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzaldehyde analogues. Med Chem Res. 2016;25:644–652. [Google Scholar]
- 23.De Simone R., Chini M.G., Bruno I., Riccio R., Mueller D., Werz O. Structure-based discovery of inhibitors of microsomal prostaglandin E2 synthase-1,5-lipoxygenase and 5-lipoxygenase-activating protein: promising hits for the development of new anti-inflammatory agents. J Med Chem. 2011;54:1565–1575. doi: 10.1021/jm101238d. [DOI] [PubMed] [Google Scholar]
- 24.Naaz F., Preeti Pallavi M.C., Shafi S., Mulakayala N., Shahar Yar M., Sampath Kumar H.M. 1,2,3-Triazole tethered indole-3-glyoxamide derivatives as multiple inhibitors of 5-LOX, COX-2 & tubulin: their anti-proliferative & anti-inflammatory activity. Bioorg Chem. 2018;81:1–20. doi: 10.1016/j.bioorg.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Dheer D., Singh V., Shankar R. Medicinal attributes of 1,2,3-triazoles: current developments. Bioorg Chem. 2017;71:30–54. doi: 10.1016/j.bioorg.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Brandão G.C., Rocha Missias F.C., Arantes L.M., Soares L.F., Roy K.K., Doerksen R.J. Antimalarial naphthoquinones. Synthesis via click chemistry, in vitro activity, docking to PfDHODH and SAR of lapachol-based compounds. Eur J Med Chem. 2018;145:191–205. doi: 10.1016/j.ejmech.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 27.Kaoukabi H., Kabri Y., Curti C., Taourirte M., Rodriguez-Ubis J.C., Snoeck R. Dihydropyrimidinone/1,2,3-triazole hybrid molecules: synthesis and anti-varicella-zoster virus (VZV) evaluation. Eur J Med Chem. 2018;155:772–781. doi: 10.1016/j.ejmech.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 28.Takeda Y., Tanigawa N., Sunghwa F., Ninomiya M., Hagiwara M., Matsushita K. Morroniside cinnamic acid conjugate as an anti-inflammatory agent. Bioorg Med Chem Lett. 2010;20:4855–4857. doi: 10.1016/j.bmcl.2010.06.095. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Chávez M.M., Pineda-Urbina K., Pérez D.J., Obledo-Benicio F., Flores-Parra A., Gómez-Sandoval Z. Organotin(IV) compounds derived from ibuprofen and cinnamic acids, an alternative into design of anti-inflammatory by the cyclooxygenases (COX-1 and COX-2) pathway. J Organomet Chem. 2018;862:58–70. [Google Scholar]
- 30.Fatahala S.S., Khedr M.A., Mohamed M.S. Synthesis and structure activity relationship of some indole derivatives as potential anti-inflammatory agents. Acta Chim Slov. 2017;64:865–876. doi: 10.17344/acsi.2017.3481. [DOI] [PubMed] [Google Scholar]
- 31.Swathi K., Manasa C., Sarangapani M. Development of novel indole molecules for the screening of anti-inflammatory activity. Adv Exp Med Biol. 2017;988:139–147. doi: 10.1007/978-3-319-56246-9_11. [DOI] [PubMed] [Google Scholar]
- 32.Chen X., Leng J., Rakesh K.P., Darshini N., Shubhavathi T., Vivek H.K. Synthesis and molecular docking studies of xanthone attached amino acids as potential antimicrobial and anti-inflammatory agents. MedChemComm. 2017;8:1706–1719. doi: 10.1039/c7md00209b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leite A.C.L., Barbosa F.F., De Oliveira Cardoso M.V., Moreira D.R.M., Coêlho L.C.D., Da Silva E.B. Phthaloyl amino acids as anti-inflammatory and immunomodulatory prototypes. Med Chem Res. 2014;23:1701–1708. [Google Scholar]
- 34.Reddyrajula R., Dalimba U., Madan Kumar S. Molecular hybridization approach for phenothiazine incorporated 1,2,3-triazole hybrids as promising antimicrobial agents: design, synthesis, molecular docking and in silico ADME studies. Eur J Med Chem. 2019;168:263–282. doi: 10.1016/j.ejmech.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Bérubé G. An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov. 2016;11:281–305. doi: 10.1517/17460441.2016.1135125. [DOI] [PubMed] [Google Scholar]
- 36.Harrison J.R., Brand S., Smith V., Robinson D.A., Thompson S., Smith A. A molecular hybridization approach for the design of potent, highly selective, and brain-penetrant N-myristoyltransferase inhibitors. J Med Chem. 2018;61:8374–8389. doi: 10.1021/acs.jmedchem.8b00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y., Dial E.J., Doyen R., Lichtenberger L.M. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G722–G731. doi: 10.1152/ajpgi.00387.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdel-Atty M.M., Farag N.A., Kassab S.E., Serya R.A.T., Abouzid K.A.M. Design, synthesis, 3D pharmacophore, QSAR, and docking studies of carboxylic acid derivatives as histone deacetylase inhibitors and cytotoxic agents. Bioorg Chem. 2014;57:65–82. doi: 10.1016/j.bioorg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Meldal M., Tornøe C.W. Cu-catalyzed azide-alkyne cycloaddition. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 40.Himo F., Lovell T., Hilgraf R., Rostovtsev V.V., Noodleman L., Sharpless K.B. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 41.Kaboudin B., Moradi K. A simple and convenient procedure for the synthesis of 1-aminophosphonates from aromatic aldehydes. Tetrahedron Lett. 2005;46:2989–2991. [Google Scholar]
- 42.Wang J.L., Shen X.L., Chen Q.H., Qi G., Wang W., Wang F.P. Structure–analgesic activity relationship studies on the C18- and C19-diterpenoid alkaloids. Chem Pharm Bull (Tokyo) 2009;57:801–807. doi: 10.1248/cpb.57.801. [DOI] [PubMed] [Google Scholar]
- 43.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 44.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B., Cai S., Yang Y.A., Chen S.C., Chen R., Shi J.B. Novel unsaturated glycyrrhetic acids derivatives: design, synthesis and anti-inflammatory activity. Eur J Med Chem. 2017;139:337–348. doi: 10.1016/j.ejmech.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.B., Kang M.J., Kang C.W., Kim N.H., Choi H.W., Jung H.A. Anti-inflammatory effects of 6-formyl umbelliferone via the NF-κB and ERK/MAPK pathway on LPS-stimulated RAW264.7 cells. Int J Mol Med. 2019;43:1859–1865. doi: 10.3892/ijmm.2019.4078. [DOI] [PubMed] [Google Scholar]
- 49.Amaral A.C., Amado V.M. Fluid-management strategies in acute lung injury. N Engl J Med. 2006;355:1175–1176. [PubMed] [Google Scholar]
- 50.Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Zoheir K.M.A., Iqbal M. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol Res. 2015;102:1–11. doi: 10.1016/j.phrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.