Abstract
Autophagy, defined as a scavenging process of protein aggregates and damaged organelles mediated by lysosomes, plays a significant role in the quality control of macromolecules and organelles. Since protein kinases are integral to the autophagy process, it is critically important to understand the role of kinases in autophagic regulation. At present, intervention of autophagic processes by small-molecule modulators targeting specific kinases has becoming a reasonable and prevalent strategy for treating several varieties of human disease, especially cancer. In this review, we describe the role of some autophagy-related kinase targets and kinase-mediated phosphorylation mechanisms in autophagy regulation. We also summarize the small-molecule kinase inhibitors/activators of these targets, highlighting the opportunities of these new therapeutic agents.
KEY WORDS: Autophagy, Protein kinases, Autophagy-related kinase, Phosphorylation, Small-molecule kinase inhibitors/activators, Human disease therapy
Abbreviations: 4E-BP1, eukaryotic translation initiation factor 4E-binding protein; AKT1, AKT serine/threonine kinase 1; AMBRA1, autophagy/beclin-1 regulator 1; AMPK, AMP-activated protein kinase; ARF, auxin response factor gene; ATG, autophagy-related protein; CaMKK2, calcium/calmodulin-dependent protein kinase kinase 2; DAPK, death associated protein kinase; FIP200, FAK family kinase-interacting protein of 200 kDa; GAP, GTPase-activating protein; GO, gene ontology; GSK3α, glycogen synthase kinase 3 alpha; HMGB1, high mobility group protein B1; JNK1, C-Jun N-terminal kinase; LC3, microtubule-associated protein 1 light chain 3; LKB1, serine/threonine-protein kinase stk11; LPS, lipopolysaccharide; LRRK2, leucine rich repeat kinase 2; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PD, Parkinson's disease; PI, phosphatidylinositol; PI3 kinase, phosphoinositide 3-kinase; PI3P, phosphatidylinositol triphosphate; PIM2, proviral insertion in murine lymphomas 2; PINK1, PTEN-induced putative kinase 1; PIP2, phosphatidylinositol-4,5-bisphosphate; PKACα, a protein kinase cAMP-activated catalytic subunit alpha; PKCα, protein kinase C alpha type; PKD1, polycystin-1; PPIs, protein–protein interactions; PROTAC, proteolysis targeting chimeras; PTMs, post-translational modifications; Rheb, the RAS homolog enriched in brain; TAK1, transforming growth factor activated kinase-1; TFEB, transcription factor EB; TNBC, triple-negative breast cancer; TSC1/2, tuberous sclerosis complex proteins 1/2; ULK complex, ULK1–mATG13–FIP200–ATG101 complex; ULK1, unc-51-like kinase 1; UVRAG, ultraviolet resistance-associated gene
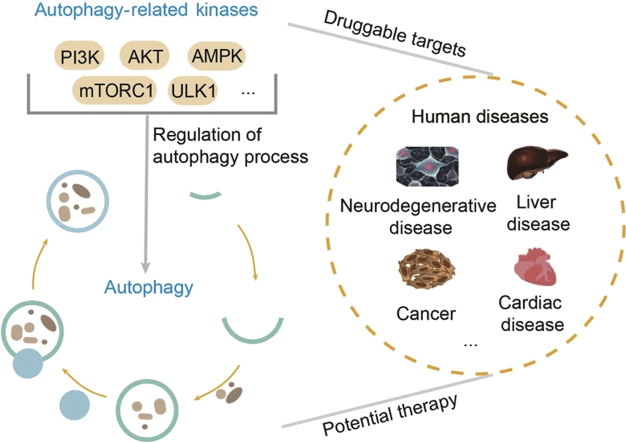
Graphical abstract
This review elaborates in detail the role of some autophagy-related kinase targets and phosphorylation in autophagy regulation, and further summarizes the application of small-molecule kinase inhibitors/activators for autophagy inhibition and induction. Understanding how these autophagy-related kinases regulate autophagy machinery is critical for the potential therapeutic application of autophagy.
1. Introduction
Autophagy, first proposed in 1963 at the International Conference of Lysosomes by Belgian scientist Christian de Duve, refers to a highly conserved cellular self-digestion process by which cellular components are targeted for degradation via lysosomes. Autophagy in mammalian cells can be categorized into three main ways: macroautophagy, microautophagy, and chaperone-mediated autophagy1, 2, 3. Of these, macroautophagy (henceforth, autophagy) is the most intensively studied. In general, autophagy plays a Janus role and is implicated in certain human diseases4, 5. For one thing, moderate autophagy is regarded as a cytoprotective mechanism. It governs the degradation of denatured proteins and nucleic acids in damaged, denatured, aging cells, organelles and biomacromolecules, which provide raw materials for cell regeneration and repair6, 7. Also, autophagy can resist the invasion of pathogens and protect cells from detrimental cellular components. For another, excessive autophagy can contribute to metabolic stress, cell death, etc.
Accumulating research has indicated that protein kinases are integral to autophagy. Both autophagy initiation and autophagy signaling pathways utilize kinase mechanisms. An example of the latter is mammalian target of rapamycin (mTOR). Furthermore, the activity of these initiation complexes and signaling pathways is also highly dependent on post-translational modifications (PTMs)8, 9, 10 including phosphorylation, ubiquitination, acetylation, glycosylation and lipidation. The PTMs can occur at multiple stages of autophagosome formation, leading to the induction, regulation and fine-tuning of autophagic responses. In particular, kinase-catalyzed phosphorylation reactions are by far the most thoroughly investigated components of autophagic PTMs11. Phosphorylation plays a role in regulating catalytic activity and protein–protein interactions (PPIs), and almost every signal transduction process (autophagy and beyond) is linked with a phosphate transport cascade. Thus, a specific physiological response can be induced by changing the activity of kinases, demonstrating their essential nature for human physiology. Typically, unc-51-like kinase 1 (ULK1, mammalian homologue of the yeast Atg1 kinase) has been identified as a significant autophagic initiator. ULK1 is the sole serine/threonine protein kinase in all known 38 autophagy-related proteins (ATGs). As an indispensable constituent of autophagy vesicles, ULK1 constitutes ULK1 complex with ATG13, FAK family kinase-interacting protein of 200 kDa (FIP200) and ATG101 to induce autophagy12, 13. In the presence of amino acids, mammalian target of rapamycin complex 1 (mTORC1) is activated to inhibit autophagy by phosphorylating ULK1 and ATG13. However, during nutrient deficiency, mTORC1 on the lysosomal surface is inhibited thereby allowing ULK1 and ATG13 to be rapidly dephosphorylated, thus leading to the activation of ULK1 kinase and induction of autophagy14.
Another case in point is phosphoinositide 3-kinase (PI3 kinase, the ortholog of yeast Vps34). Phosphorylation of phosphatidylinositol (PI) by PI3K produces phosphatidylinositol triphosphate (PI3P), a key membrane marker for both intracellular trafficking and autophagosome formation15. PI3K is activated by binding to serine/threonine-protein kinase Vps15 and further binding to beclin-1 to form the PI3K–Vps15–beclin1 complex. Within this complex, beclin-1 is phosphorylated by ULK1, which then acts as a scaffold of PI3K complex, promoting localization of autophagy protein to autophagy vesicles16. As such, PI3K kinase interacts with various regulatory proteins to form multiple complexes which will selectively participate in different stages of autophagy. For example, a complex of PI3K kinase and ATG14 is involved in the formation of autophagy vesicles17. When combined with ultraviolet resistance-associated gene protein (UVRAG), PI3K participated in the maturation and transportation of autophagic vesicles18. These findings indicate that decrypting the regulatory role of kinases in autophagy can facilitate a deeper understanding of these important mechanisms.
In this review, 49 autophagy-related kinases were mined by gene ontology (GO) analysis. These kinases are involved in autophagy regulation, mainly in autophagy initiation and the formation of autolysosome. Furthermore, we have interpreted in detail the role of some kinases in autophagy, and summarized related small-molecule kinase inhibitors/activators for autophagy induction and inhibition.
2. Identification of autophagy-related kinases
To identify kinases that are associated with autophagy, the keyword “autophagy” was used to perform a search for related GO terms on the Gene Ontology Consortium19 website (http://www.geneontology.org). With the designated species as Homo sapiens, 499 resultant protein targets among 57 autophagy-related GO terms were obtained and then normalized, followed by a comparison between the normalized proteins and all 518 kinase proteins20. These results identified a total of 49 proteins as autophagy-related kinases (Table 1). Some of these kinases (e.g., mTOR) are well studied, but little is known about many of the others. Their potential for autophagy regulation remains to be evaluated.
Table 1.
Autophagy-related kinases in different kinase families.
| Family | Autophagy-related kinase |
|---|---|
| AGC | AKT1, ROCK1, PKACα, PKCα, PKCβ |
| CAMK | AMPKα1, AMPKα2, LKB1, MARK2, DAPK1, DAPK2, DAPK3, PIM2, PKD1 |
| CK1 | VRK1 |
| CMGC | CDC2, CDK5, CK2α2, GSK3A, ERK1, ERK7, JNK1 |
| TK | ABL1, ABL2, FAK, MET, SRC, KDR |
| TKL | LRRK2, TAK1, RIPK2 |
| Other | CaMKK2, TBK1, IRE1, NEK6, NEK9, PINK1, NRBP2, GCN2, PLK1, PLK2, PLK3, TLK2, ULK1, ULK2, ULK3, PIK3R4, FRAP, ATM |
3. Autophagy-related protein kinases in autophagy regulation
Autophagic processes (Fig. 1), can be divided into five stages: autophagy initiation, membrane nucleation and phagophore formation, phagophore expansion, fusion with the lysosome to form autolysosome, and degradation of contents of the package21. These processes correspondingly are regulated by multiple ATGs and kinases. However, kinase participation in autophagy mainly occurs during autophagy initiation and in the first stage of autolysosome formation. Therefore, we describe below the role of autophagy-related kinases in autophagic mechanisms from upstream to downstream of autophagy signaling transduction (from PI3K/AKT to ULK1). In addition, we separate the kinases that regulate beclin-1 and these aforementioned targets. The regulatory phosphorylation mechanisms in autophagy are underlined in Table 215, 16, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 19.
Figure 1.
The relationship between autophagy processes and autophagy-related kinases. Autophagic processes can be divided into five stages: autophagy initiation, membrane nucleation and phagophore formation, phagophore expansion, fusion with the lysosome to form autolysosome, and degradation of contents of the package. Regulation of kinases in autophagy mainly occurs in autophagy initiation. During this step, several kinases are implicated in autophagy regulation, such as PI3K, AKT, AMPK, mTOR, and ULK1.
Table 2.
Phosphorylation regulation of autophagy-related kinases.
| Kinase | Substrate | Site | Function | Ref. |
|---|---|---|---|---|
| PI3K | PIP2 | – | Forms second messenger PIP3 | 15 |
| PDK1 | AKT | Thr308 | Activates AKT signaling | 22 |
| AKT | TSC2 | Ser939/Thr1462 | Dissociates TSC1/2 from lysosome and activates Rheb | 23 |
| AMPK | TSC2 | Thr1227/Ser1345 | Regulates cell size and cell survival under energy starvation condition | 24 |
| Raptor | Ser722/Ser792 | Mediates a metabolic checkpoint | 25 | |
| ULK1 | Ser317/Ser555/Ser777 | Induces autophagy under glucose starvation | 26, 27 | |
| PRAS40 | Thr246 | Regulates the activity of mTORC1 | 28 | |
| FOXO3a | Ser413/Ser588 | Transcriptionally represses SKP2 | 29 | |
| LKB1 | AMPKα | Thr172 | Mediates the prolonged and adaptive activation of AMPK following energy stress | 30 |
| CaMKKβ | AMPKα | Thr172 | Activates AMPK in response to increase in cellular Ca2+ | 31 |
| TAK1 | AMPKα1/2 | Thr183/Thr172 | Activates AMPKα1/2 | 32 |
| mTORC1 | ULK1 | Ser757 | Disrupts the ULK1–AMPK interaction | 26 |
| DAP1 | Ser3/Ser51 | Inhibits autophagy indirectly | 33 | |
| AMBRA1 | Ser52 | Inhibits autophagy indirectly | 33, 34 | |
| TFEB | Ser142 | Inhibits autophagy indirectly | 33, 34 | |
| ULK1 | Beclin-1 | Ser14 | Induces autophagy | 16 |
| ATG9 | Ser14 | Promotes ATG9 trafficking in response to starvation | 35 | |
| ATG4B | Ser316 | Inhibits ATG4B activity and LC3 processing | 36 | |
| AMBRA1 | Ser465/Ser635 | Regulates dissociation of AmpRa1–Vps34–beclin-1 from the dynein complex | 37 | |
| Raptor | Ser855/Ser859/Ser792 | Inhibits mTORC1 during starvation | 38 | |
| ATG13 | Ser318 | Promotes its release to damaged mitochondria | 39 | |
| PKCα | ULK1 | Ser423 | Prevents autolysosome formation | 40 |
| DAPK1 | Beclin-1 | Thr119 | Liberates beclin-1 from BCL-2 and BCL-XL | 41 |
| DAPK3 | Beclin-1 | Ser90 | Regulates autophagy in skeletal muscle | 42 |
| DAPK2 | Raptor | Ser721 | Modulates mTORC1 activity and autophagy level | 43 |
| JNK1 | BCL-2 | Thr69/Ser70/Ser87 | Dissociates BCL-2 from beclin1 | 44 |
| PIM2 | TSC2 | Ser1798 | Relieves the suppression of TSC2 on mTORC1 | 45 |
| BAD | Ser112 | Prevents dissociation of BCL-2 from beclin-1 | 46 | |
| GSK-3 | Raptor | Ser859 | Regulates mTORC1 | 47 |
| LRRK2 | EndoA | Ser75 | Modulates the membrane curvature | 48 |
| PINK1 | Parkin | Ser65 | Leads to the aggregation of parkin from cytoplasm to damaged mitochondria | 49 |
– Not applicable.
3.1. PI3K/AKT
PI3K kinases, the most upstream molecules, act as triggers of autophagy signaling cascades, which are activated by tyrosine kinase receptors (RTKs), G protein-coupled receptors (GPCR) or Ras-like protein (RAS)50. Importantly, activation of PI3K leads to the generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3), an anchor for phosphoinositide-dependent kinases 1 (PDK1), converted from phosphatidylinositol-4,5-bisphosphate (PIP2). Subsequently, PDKs phosphorylate AKT at Thr30822. The activated AKT signaling pathway is thus directly phosphorylated and thereby inhibits tuberous sclerosis complex proteins 1/2 (TSC1/2), a GTPase-activating protein (GAP) for the Ras homolog enriched in brain (Rheb) GTPase. The AKT-dependent phosphorylation causes the dissociation of TSC1/2 from lysosome and resultant activation of Rheb23. Since GTP-bound Rheb is a potent mTORC1 activator, suppression of TSC1/2 by AKT-dependent phosphorylation results in mTORC1 activation.
3.2. AMPK/mTOR/ULK1
Another major mTOR-related signaling pathway is the AMPK–mTOR pathway. AMPKs are a type of evolutionarily conserved serine/threonine protein kinases which are composed of 13 members51. The AMPKs serve as master sensors of cellular energy status that is of great significance in energy homeostasis. AMPK is activated by a low energy state and its role in autophagy initiation has been clearly shown. On the one hand, the activation of AMPK can phosphorylate TSC2 and subunit Raptor at Ser792 of mTORC124, 25. On the other hand, AMPK initiates autophagy by direct phosphorylation of ULK1 at Ser317 and Ser777 under glucose starvation26. Additionally, a new signal axis of AMPK–SKP2–CARM1 has been discovered which can regulate autophagy induced by nutrient starvation29. The regulation mechanism of AMPK is extremely complex. The αβγ trimeric AMPK complexes are allosterically regulated mainly by the ratio of AMP/ATP52. AMPK is also subject to the regulation by upstream kinases like serine/threonine-protein kinase stk11 (LKB1) and calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ). Another kinase, transforming growth factor activated kinase-1 (TAK1) has also been identified, which is able to activate Snf1 kinase in yeast and phosphorylates AMPKα1/2 at Thr183/Thr172 in vitro53, 54, 55.
mTOR, known as the mammalian target of rapamycin (also known as FRAP), serves as a core component of two structurally and functionally distinct protein complexes, mTORC1 and mTORC2. The former senses and responds to fluctuations in the levels of intra- and extracellular nutrients, primarily amino acids as well as growth factor signaling, cellular energy and oxygen levels. It has been validated as a primary node in coordinating the respective anabolic and catabolic processes in response to various stresses. In mammals, mTORC1 regulates autophagy under nutrient-rich conditions through directly phosphorylating and suppressing ULK1 complex, which is required for autophagy initiation23, 56, 57. Additionally, under nutrient-deprived conditions, mTORC1 also can negatively regulate autophagy via phosphorylating autophagy/beclin-1 regulator 1 (AMBRA1) at Ser52 and phosphorylating DAP1 at Ser3 and Ser5133, 58. Other work has shown a new link between mTORC1 and autophagy regulation: mTORC1 directly phosphorylates the transcription factor EB (TFEB) at Ser142, which is required for lysosome biogenesis59, 60. mTORC2 was reported to indirectly suppress autophagy through AKT/mTORC1 signaling axis activation61, 62.
As another pivotal node in autophagy14, 63, ULK1 is a component of the ULK1–ATG13–FIP200–ATG101 complex (ULK complex) required for autophagy induction. Specifically, under starvation conditions, as mentioned above, ULK1 is directly and indirectly activated by AMPK and subsequently phosphorylates beclin-1 on Ser14 and activates Vps34 lipid kinase16. The latter is essential for full autophagic induction. Besides, ULK1 can phosphorylate ATG9 at Ser14 synergistically with the proto-oncogene tyrosine-protein kinase SRC, thus promoting translocation of ATG9-positive vesicles to the autophagy initiation sites35. Also, ULK1 phosphorylates ATG4B at Ser316, and ATG4B phosphorylation is responsible for the conversion of pro-LC3 to LC3-I and LC3-II back into LC3-I36. Additionally, ULK1 also can modulate autophagy via phosphorylation of other substrates like AMBRA1 and Raptor37, 38. Apart from phosphorylation of ULK1 by mTOR and AMPK, ULK1 activity is also fine-tuned by additional mechanisms and other kinases (e.g., PKCα and P38). PKCα phosphorylates ULK1 at Ser423 and prevents autolysosome formation without a direct change of ULK1 activity40. P38α-dependent phosphorylation of ULK1 triggered by lipopolysaccharide (LPS) leads to the inhibition of ULK1, preventing it from binding to the downstream effector ATG13. This pathway was shown to eventually reduce autophagy in microglia64.
3.3. Kinases regulate autophagy via beclin-1 and its interactomes
Beclin-1 (the mammalian orthologue of yeast Atg6) is another key node of autophagy regulation in mammalian cell which interacts with several interactomes (such as ATG14L, UVRAG, BIF-1, Ambra1, HMGB1 and PINK) to regulate Vps34 and promote formation of PI3KIII core complexes, thereby inducing autophagy65, 66. Although not a kinase itself, beclin-1 and its interactomes are regulated by several kinase-mediated phosphorylation reactions in autophagy. The section below describes how other kinases regulate autophagy though beclin-1 and its interactomes, such as DAPKs and JNK1.
Death-associated protein kinase (also known as DAPK), a Ca2+-calmodulin regulated kinase, was reported to stimulate autophagy and membrane blebbing by binding to LC3. Specifically, DAPK1 phosphorylates beclin-1 on Thr119 at the BH3 domain, thus liberating beclin-1 from BCL-2 and BCL-XL, and, in turn, promoting autophagy41, 67. Also, DAPK1 stimulates autophagy via ARF-dependent accumulation of P5368. DAPK3 is also reported to control autophagy by directly phosphorylating beclin-1 at Ser90 in skeletal muscle tissues, providing an enhanced understanding for the mechanism through which metabolism and autophagy are linked42. Compared to DAPK1 and DAPK3, DAPK2 was shown to phosphorylate raptor at Ser721 to modulate mTORC1 activity and autophagy levels under stress and steady-state conditions43.
JNK1 controls autophagy via phosphorylating the antiapoptotic protein BCL-2 at residues Thr69, Ser70 and Ser87 of the non-structured loop which causes dissociation of BCL-2 from beclin-144. Moreover, this process has also been implicated in the induction of apoptosis. A model has been proposed to explain how cells balance the interaction between autophagy and apoptosis via JNK1-mediated BCL-2 phosphorylation69.
3.4. Other kinases in autophagy regulation
Previous work has shown that serine/threonine-protein kinase PIM-2 (one of three PIM kinases) is involved in autophagy regulation by activation of the mTOR pathway. Aberrant PIM-2 expression has been observed in a variety of malignancies. Evidence showed that PIM2 can directly phosphorylate TSC2 on Ser1798 and relieve the suppression of TSC2 on mTORC145. Furthermore, PIM2 promotes autophagy and PIM2-suppression decreases the autophagic response and prevents dissociation of BCL-2 from beclin-1 and enhancing lysosomal acidification46. Other work demonstrated that phosphorylation of hexokinase-II by PIM2 was required for autophagy during glucose starvation70.
GSK-3, an ubiquitously expressed serine/threonine kinase, was initially discovered as a regulator of glycogen synthesis, has also been found to be involved in autophagy modulation. In MCF-7 cells, GSK-3α/β overexpression activates mTORC1 and inhibits autophagy via phosphorylating Ser859 on raptor, resulting in reduced p70S6K1 and ULK1 phosphorylation along with increased autophagic flux47. In a prostate cancer cell model, GSK-3β was found to control autophagy by modulating the LKB1–AMPK pathway71. Thus, GSK-3β inhibition caused a rapid cellular ATP decline, and subsequently LKB1-depedent AMPK activation and mTOR pathway inactivation were associated with autophagy induction.
ERK1 (also known as p44MAPK or MAPK3), one isoform of extracellular signal-regulated kinase (ERK) that belongs to mitogen-activated protein kinases (MAPKs), plays a role in autophagic regulation in various tumor cells72, 73. ERK1 was phosphorylated and activated to regulate autophagy via the RAS–RAF–MEK axis74. Another lab reported that non-classic activation of MEK/ERK can also modulate beclin-1 expression to stimulate autophagy75. Acute activation of MEK/ERK causes cytoprotective autophagy by inhibition of either mTORC1 or mTORC2 with a moderate increase of beclin-1 expression. However, prolonged activation of MEK/ERK leads to dual inhibition of mTORC1 and mTORC2, with a definitive increase in beclin-1 expression and cytodestructive autophagy.
Leucine-rich repeat kinase 2 (LRRK2), a member of the leucine-rich repeat kinase family, has also been implicated in autophagy. LRRK2 regulates autophagy by phosphorylating EndoA at Ser75, which in turn modulates the membrane curvature, thus controlling the recruitment of the autophagy machinery to the nascent autophagosome48. Besides, prolonged LRRK2 kinase inhibition increases phosphorylation on Ser758 ULK1 via an unknown regulatory feedback loop76. Another lab found that membrane-associated LRRK2 inactivated beclin-1 and consequently inhibited autophagy, supporting LRRK2 as a primary inhibitor of autophagy77.
PTEN-induced putative kinase 1 (PINK1) and parkin RBR E3 ubiquitin protein ligase (Parkin/PARK2) mediate mitophagy, which can clear dysfunctional mitochondria. Mounting evidence has shown that PINK1 acts as a gatekeeper of mitochondrial quality control78. PINK1 directly phosphorylates Parkin at a highly conserved Ser6579, leading to the aggregation of parkin from cytoplasm to damaged mitochondria, and finally clears the organelles depending on mitophagy49.
4. Kinase inhibitors/activators for autophagy inhibition and induction
Modulation of the autophagy-related kinases discussed above has the potential to modify autophagy processes. Of note, autophagy inhibition and induction are achievable by small-molecule kinase inhibitors/activators. Therefore, in this following section, we review some kinase modulators applied to autophagy induction (Table 3 31, 76, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116) and inhibition (Table 4117, 118, 119, 120, 121, 122, 123, 124).
Table 3.
Kinase inhibitors/activators for autophagy induction.
| Name | Mechanism | Cell line | Disease | Clinical status | Ref. |
|---|---|---|---|---|---|
| GDC-0941 | PI3K inhibition | MCF-7, T47D and ZR75-1 cells | ER+ breast cancer | Phase 1 | 80 |
| Taselisib | PI3K inhibition | Human KPL-4 breast cancer cell | Advanced solid tumors | Phase 3 | 81 |
| PX-866 | PI3K inhibition | LNZ308 and LN229 cells | Glioblastoma | Phase 1 | 82 |
| PKI-587 | PI3K/mTOR inhibition | A431-CR and FaDu-CR cells | Breast cancer, non-small cell lung cancer, ovarian cancer, etc. | Phase 1/2 | 83 |
| BEZ235 | PI3K/mTOR inhibition | 786-0 and Caki-1 cells | Metastatic renal cell carcinoma | Phase 1 | 84, 85 |
| PF-04691502 | PI3K/mTOR inhibition | A549 cells | Non-small cell lung cancer | Phase 1 | 86 |
| Perifosine | AKT inhibition | T98G and U373MG cells | Glioblastoma, anaplastic astrocytoma, mixed glioma, etc. | Phase 2 | 87, 88 |
| MK2206 | AKT inhibition | UL cells | Colon mucinous adenocarcinoma, colon signet ring cell adenocarcinoma, rectal mucinous adenocarcinoma, etc. | Phase 2 | 89 |
| BI-69A11 | AKT inhibition | HT-29 cell | – | – | 90 |
| Metformin | AMPK activation | EC109 cells | Diabetes mellitus type 2 | Phase 4 | 91 |
| Salicylate | AMPK activation | HEK-293 cells | – | – | 31 |
| Hernandezine | AMPK activation | HeLa cells | – | – | 92 |
| Simvastatin | AMPK activation | Mouse coronary arterial myocytes | Coronary arterial myocytes | Phase 1/2 | 93 |
| A-769662 | AMPK activation | U373 cells | – | – | 94 |
| Rapamycin | mTOC1 inhibition | SK-N-SH and SH-SY5Y cells | Primitive neurotodermal tumor and mast cell leukemia | – | 95, 96 |
| AZD8055 | mTOC1 inhibition | H838 and A549 cells | Glioblastoma multiforme, advanced hepatocellular carcinoma, advanced solid malignancies, etc. | Phase 1 | 97 |
| PP242 | mTOC1 inhibition | HeLa cells | – | – | 98 |
| RapaLink-1 | mTOC1 inhibition | MCF-7, RR1 and RR2 cells | – | – | 99 |
| LYN-1604 | ULK1 activation | MDA-MB-231 cells | Triple negative breast cancer | – | 100 |
| BL-918 | ULK1 activation | SH-SY5Y cells | Parkinson's disease | 101 | |
| JNK-IN-8 | JNK1 inhibition | Primary hepatocytes and primary epithelial cells | – | – | 102 |
| HJ-PI01 | PIM2 inhibition | MDA-MB-231 cells | – | – | 103 |
| SMI-4a | PIM2 inhibition | A375 and G361 cells | – | – | 104 |
| SGI-1776 | PIM2 inhibition | MM.1S cells | Relapsed/refractory leukemias | Phase 1 | 105 |
| AZD1208 | PIM2 inhibition | Primary chronic lymphocytic leukemia cells | Primary chronic lymphocytic leukemia | Phase 1 | 106 |
| SB216763 | GSK3β inhibition | Human pancreatic cancer cells | – | – | 107 |
| CHIR99021 | GSK3 inhibition | Human pancreatic cancer cells | – | – | 108 |
| TDZD8 | GSK3β inhibition | PC-3 cells | – | – | 109 |
| 9-ING-41 | GSK3 inhibition | PC-3 cells | Lymphoma pancreatic cancer | Phase 1/2 | 110 |
| SCH7 72984 | ERK1 inhibition | HPAC and PANC-1 cells | – | – | 111 |
| BVD-523 | ERK1 inhibition | HPAC and PANC-1 cells | Advanced solid tumors | Phase 1/2 | 111, 112 |
| GDC-0994 | ERK1 inhibition | Epithelial Caco-2 and HT-29 cells | – | – | 113 |
| LRRK2-IN-1 | LRRK2 inhibition | H4 astroglioma cells | – | – | 76 |
| GSK2578215A | LRRK2 inhibition | SH-SY5Y cells | – | – | 114 |
| SB202190 | MAPK inhibition | HT29 cells | – | – | 115 |
| SB203580 | MAPK inhibition | HCC cells | – | – | 116 |
– Not applicable.
Table 4.
Kinase inhibitors for autophagy inhibition.
| Name | Mechanism | Cell line | Disease | Clinical status | Ref. |
|---|---|---|---|---|---|
| LY294002 | PI3K inhibition | Mel Z and Mel IL cells | Neuroblastoma | Phase 1 | 117 |
| Wortmannin | PI3K inhibition | CHO cells | – | – | 118 |
| 3-MA | PI3K inhibition | Mouse embryonic fibroblast | – | – | 119 |
| Dimeric quinacrines | mTORC1/autophagy dual inhibition | PANC1, A375P and C8161 cells | – | – | 120 |
| SBI0206965 | ULK1 inhibition | PDAC cell lines | – | – | 121 |
| MRT67307 | ULK1 inhibition | HeLa cells | – | – | 122 |
| MRT6892 | ULK1 inhibition | HeLa cells | – | – | 122 |
| ULK-101 | ULK1 inhibition | U2OS cells | – | – | 123 |
| SP600125 | JNK inhibition | HT29 cells | – | – | 124 |
4.1. Kinase inhibitors/activators for autophagy induction
As discussed above, PI3K inhibition can lead to autophagy induction. In a case of ER+ breast cancer treatment, autophagy was induced by the PI3K inhibitor GDC-094180. Taselisib and PX-866 were also reported to induce autophagy respectively in ovarian cancer cells and in glioblastoma cells81, 82, 125. Additionally, since the catalytic subunit structure of mTOR resembles that of PI3K, many PI3K inhibitors can also potently inhibit mTOR. After the combination treatment of the dual PI3K/mTOR inhibitor PKI-587 with cetuximab, A431-CR and FaDu-CR cell lines displayed an increased Beclin-1 expression and a decrease in p62 levels126. Consistently, the dual PI3K/mTOR competitive inhibitor BEZ235 induced autophagy in human glioma and hepatocellular carcinoma cells84, 127. Another study showed that dual PI3K/mTOR inhibitor PF-04691502 induced autophagy in non-small-cell lung cancer cell lines in vitro, demonstrated by upregulated LC3-II and beclin-1 expression86. These dual inhibitors were used to prevent drug resistance effectively in curing various cancers like breast cancer, T-cell acute lymphoblastic leukemia, gastric cancer and lymphomas83, 85, 128.
As an AKT inhibitor, the phosphatidylinositol analog perifosine87 induces protective autophagy and upregulation of ATG5 in human chronic myelogenous leukemia cells88. The allosteric inhibitor MK2206 was reported to induce autophagy89, which showed potent activity against multiple cancers in clinical trials129, 130. In addition, a novel AKT inhibitor BI-69A11 was reported to induce autophagy at earlier time point through the inhibition of AKT/mTOR/p70S6 kinase pathway90.
Autophagy can also be induced by AMPK activation. To date, more than 100 different natural plant products and derivatives originating from traditional medicines can activate AMPK by several different mechanisms. Metformin and salicylate are the most successful and widely used AMPK activators31, 91, both of which commonly appear in the autophagy-related research for AMPK activation131, 132, 133. Also, hernandezine, a novel AMPK activator, was reported to induce autophagic cell death in drug-resistant cancers such as HeLa cells92. Significant progress has been made in the development of direct small molecule AMPK activators over the last few years. For instance, an AMPK activator, simvastatin increases autophagy in coronary arterial myocytes via inhibiting the RAC1-mTOR pathway93. Another study has found that activation of AMPK by A-769662 led to an increased expression of the autophagosomal markers LC3 and P62/SQSTM, suggesting an efficient induction of autophagy94. Conversely, AMPK inhibitor dorsomorphin was reported to induce autophagy in T24 cells via AMPK-independent inhibition134.
It is well established that mTORC1 inhibition can induce autophagy. For example, rapamycin and its derivatives are well known as autophagy inducers by inhibiting mTORC1 and are widely used in autophagy related research95. Furthermore, many other mTOR inhibitors have been developed. In a variety of cancer cells like H838 and A549 cells, AZD8055 can induce the formation of acidic vesicles associated with LC3, consistent with the induction of autophagosome formation, suggesting an activation of autophagic flux97. The ATP competitive mTOR inhibitor pp242, a stronger autophagy inducer than rapamycin, is able to induce lysosomal activation via blockade of mTORC1 activity98. RapaLink-1, a third-generation mTORC1 inhibitor, overcomes resistance to existing first- and second-generation inhibitors through exploiting the unique juxtaposition of two drug-binding pockets to create a bivalent interaction that allows inhibition of resistant mutants99. A recent study also found that RapaLink-1 can activate autophagy, but the specific mechanism is unknown135. Additionally, LYN-1604, the first ULK1 agonist, can induce cell death associated with autophagy in MDA-MB-231 cells by interfering with the formation of the ULK complex. This drug showed potential for good therapeutic effects on TNBC100. BL-918, a potent activator of ULK1, induced autophagy via the ULK complex, and displayed a cytoprotective effect on MPP+-treated SH-SY5Y cells, which may be a candidate drug for Parkinson's disease (PD) treatment101.
Treatment with the JNK1 inhibitor JNK-IN-8 increased the accumulation of LC3B-II following lysosomal inhibition and reduced the accumulation of SQSTM1, demonstrating increased autophagic flux102. Additionally, among the few PIM inhibitors identified by far, HJ-PI01 was able to induce autophagic death in MDA-MB-231 cells103. The pan-PIM inhibitor SMI-4a also induced autophagy through inhibition of the PI3K/AKT/mTOR axis in melanoma cells104. Induction of autophagy was also interrupted by the PIM inhibitor SGI-1776 in MM cell lines and bone marrow CD138+ cells105. The PIM kinase inhibitor AZD1208, inhibits protein translation by decreasing phosphorylation levels of eukaryotic translation initiation factor 4E-binding protein (4E-BP1) and induces autophagy in primary CLL cells106.
Modulation of other kinase targets can also alter autophagy. Inhibition of GSK3β with SB216763 promotes autophagy induced by starvation in vitro107. Also, GSK3 inhibition with SB216763 or CHIR99021 induces an autophagic response in human pancreatic cancer cells108. GSK-3β suppression by TDZD8, a non-ATP competitive small molecule, promotes autophagy during serum starvation109. In renal cancer cells, the GSK-3 inhibitor 9-ING-41 leads to AMPK activation and autophagy induction110. In a recent study, the ERK1-selective inhibitor SCH7 72984 (an analog of the clinical candidate MK-8353) and the clinical candidate BVD-523 were reported to elevate autophagic flux. These results suggest that concurrent blockade of both ERK and autophagic processes that are upregulated in response to ERK inhibition, may be an effective approach for treating pancreatic ductal adenocarcinoma111. GDC-0994 is also an ERK inhibitor, which suppressed ERK phosphorylation, and thus inhibiting p-mTOR and activating autophagy113. The LRRK2 inhibitor LRRK2-IN-1 was reported to stimulate autophagy in a non-canonical fashion. This mechanism was independent of mTOR and ULK1, but dependent upon the activation of class III PI3-kinase136. GSK2578215A also induces protective autophagy in SH-SY5Y cells114. Other p38a inhibitors like SB202190 and SB203580 can induce autophagy via different mechanisms; the former acts via p38a blockade115, whereas the latter activates AMPK and DAPK116.
4.2. Kinase inhibitors/activators for autophagy inhibition
In recent years, some small-molecule kinase inhibitors have been used to inhibit autophagy. For instance, Wortmannin, LY294002 and 3-MA, the first classical generation non-selective pan-PI3K inhibitors117, 118, 119, are known as autophagy suppressors. Dimeric quinacrines (DQ) were reported to concurrently inhibit both mTORC1 and autophagy as a unified approach to targeting lysosomes degradation and growth signaling roles120. These differ from normal mTOR inhibitors which induce autophagy. ULK1 inhibitors can also inhibit autophagy. For example, compound SBI0206965 exhibits good inhibitory potency of ULK1 and can be used as an autophagy inhibitor121. MRT67307 and MRT6892 share the same scaffold and the ability to inhibit ULK1 and ULK2 in vitro and subsequently block autophagy in cells122. Another inhibitor, ULK-101, exhibits a similar degree of ULK1 inhibition as MRT6892, and suppresses autophagy induction and autophagic flux in response to different stimuli such as nutrients, including amino acids and growth factors123. In addition, JNK inhibitors have also been used for autophagy inhibition. For instance, the first reported effective JNK inhibitor SP600125 inhibited autophagy via reduction of beclin-1124.
5. Conclusions and prospects
Both cell survival and cell death are regulated by autophagy. Precise positioning of autophagy in the specific disease context confers a rational basis for deciding the proper direction for modulation of autophagy. For example, numerous previous studies have indicated autophagy determines the fate of cancer cells depending on the type, stage and genetic context137, 138, 139, 140, 141. Autophagy inducers like rapamycin contribute to lower oncogenic risk caused by deficiencies in autophagy function required for the initiation of cancer142. However, when cancer is established, autophagy inhibition is needed to cope with the pro-survival effects of autophagy. For instance, a combination of autophagy inhibitors can sensitize tumor cells to metabolic stress induced by chemotherapy (e.g., angiogenesis inhibitors). Furthermore, as in the case of neurodegenerative disorders, activation of moderate protective autophagy for degradation of accumulated misfolded proteins serves as a reasonable therapeutic approach143, 144. Thus the use of autophagy inducers is likely to have benefit in this situation.
Nearly all signal transduction processes are linked to phosphate transport cascades, suggesting that a true physiological response (autophagy and beyond) can be induced by changing the activity of kinases. Indeed, autophagy-related kinases are commonly multi-functional. For instance, mTOR, the gatekeeper of autophagy, is also implicated with cell growth, cell metabolism and protein synthesis145. Additionally, ULK1, as the autophagy initiator, not only regulates multiple steps in the autophagy pathways, but also modulates cellular processes such as ER-to-Golgi trafficking and axonal growth, as well as PARP1 activation related to programmed cell death146. Nevertheless, there is no doubt of the essential regulatory roles of these kinases in autophagy. In particular, mTOR and ULK1 play pivotal roles in autophagy induction and their kinase activities are closely associated with autophagy initiation. Although other autophagy-related kinases, such as AKT, PI3K, and AMPK, mainly act as important regulators of cell proliferation and metabolism, inhibition/activation of these kinases are also significant to autophagy signal transduction147, 148. Whether other kinases (not emphasized in this review) have the potential to regulate autophagy and become drug targets need to be validated by future studies. Furthermore, as for the unknown side effects caused by kinase inhibition/activation, it has not been determined whether these autophagy-related kinases can be used as a breakthrough point of autophagy intervention.
Despite the potential therapeutic benefits of autophagy-related kinase inhibitors/activators in animal models of disease, their clinical development into useful drugs will be challenging. First of all, due to the lack of organ-specificity, utilization of autophagy-related kinase inhibitors/activators may lead to unwanted and uncontrolled side effects, unless the side effects are tolerable for the duration of intended use. Considering the protective effects of autophagy on neurons, it is sensible to improve the brain-specificity of autophagy-related treatments for neurodegenerative diseases. To achieve the goal of organ-specificity, different delivery strategies and photodynamic chemotherapy are proposed. Additionally, these small-molecule autophagy-related kinase inhibitors/activators share the problems of resistance and target selectivity149, as known for the common kinase drugs. The occurrence of drug resistance of autophagy-related kinase inhibitors/activators is a formidable obstacle to surmount because of factors such as gene mutation, kinase up-regulation, and compensatory mechanisms and bypass effects. New therapies are needed to approach these limitations and treat the evolving diseases, especially cancer. For instance, RapaLink-1, a third-generation mTOR inhibitor, exploits the unique juxtaposition of two drug-binding pockets to create a bivalent interaction that reverses the resistance to existing first- and second-generation inhibitors. So far, several strategies have been proposed to solve the problem of target selectivity. First, compared to target ATP sites shared in kinase family, targeting allosteric sites of kinases takes distinct advantages like enhanced specificity, reduced side effects, and lower toxicity. Another approach is covalent targeting of kinases, used to design reversible and irreversible covalent kinase inhibitors/activators with a better pharmacokinetic characteristics150.
At present, mounting innovations contribute to a better use of small-molecule kinase inhibitors/activators. The popular proteolysis targeting chimeras (PROTAC) approach provides a new profile for the application of autophagy-related kinase inhibitors/activators. And new computational methods such as available 3D-e-Chem-VM help to predict selectivity profiles. Meanwhile, artificial intelligence holds great promise in discovery, transformation and application of kinase agents. Based on the current trends discussed above, we believe that autophagy-based kinase targeting therapy presents a fascinating direction of autophagy research and is a promising beneficial therapeutic approach.
Acknowledgments
This work was supported by grants from National Key R&D Program of China (Grants 2017YFC0909301 and 2017YFC0909302), National Natural Science Foundation of China (Grants 81874290, 81673290, 81673455, 81602953, and 81903502) and the Opening Foundation of State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, China (Grant KF-GN-201904).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Lan Zhang, Bo Liu, and Liang Ouyang were responsible for the conception and design of the review. Honggang Xiang, Jifa Zhang and Congcong analyzed the literatures, summarized the results and drafted the manuscript. Lan Zhang, Bo Liu, and Liang Ouyang revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
The authors claim that the researchers in this study have no conflict of interest.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.10.003.
Contributor Information
Lan Zhang, Email: zhanglanx_9@126.com.
Bo Liu, Email: liubo2400@163.com.
Liang Ouyang, Email: ouyangliang@scu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Klionsky D.J. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7–18. doi: 10.1242/jcs.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massey A., Kiffin R., Cuervo A.M. Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36:2420–2434. doi: 10.1016/j.biocel.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Parzych K.R., Klionsky D.J. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tekirdag K.A., Korkmaz G., Ozturk D.G., Agami R., Gozuacik D. MIR181A regulates starvation- and rapamycin-induced autophagy through targeting of ATG5. Autophagy. 2013;9:374–385. doi: 10.4161/auto.23117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmeisser H., Bekisz J., Zoon K.C. New function of type I IFN: induction of autophagy. J Interferon Cytokine Res. 2014;34:71–78. doi: 10.1089/jir.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Wang S., Chen Y., Liu G., Yang X. miR-22 targets the 3′ UTR of HMGB1 and inhibits the HMGB1-associated autophagy in osteosarcoma cells during chemotherapy. Tumor Biol. 2014;35:6021–6028. doi: 10.1007/s13277-014-1797-0. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Vos K.E., Eliasson P., Proikas-Cezanne T., Vervoort S.J., Van Boxtel R., Putker M. Modulation of glutamine metabolism by the PI(3)K–PKB–FOXO network regulates autophagy. Nat Cell Biol. 2012;14:829. doi: 10.1038/ncb2536. [DOI] [PubMed] [Google Scholar]
- 8.McEwan D.G., Dikic I. The three musketeers of autophagy: phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011;21:195–201. doi: 10.1016/j.tcb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boya P., Reggiori F., Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y., Kang R., Sun X., Zhong M., Huang J., Klionsky D.J. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy. 2014;11:28–45. doi: 10.4161/15548627.2014.984267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wani W.Y., Boyer-Guittaut M., Dodson M., Chatham J., Darley-Usmar V., Zhang J. Regulation of autophagy by protein post-translational modification. Lab Invest. 2015;95:14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., He J., Tian M., Zhang S.Y., Guo M.R., Kasimu R. UNC51-like kinase 1, autophagic regulator and cancer therapeutic target. Cell Prolif. 2014;47:494–505. doi: 10.1111/cpr.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley J.H., Young L.N. Mechanisms of autophagy initiation. Annu Rev Biochem. 2017;86:225–244. doi: 10.1146/annurev-biochem-061516-044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zachari M., Ganley Ian G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017;61:585–596. doi: 10.1042/EBC20170021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backer Jonathan m. The intricate regulation and complex functions of the class III phosphoinositide 3-kinase Vps34. Biochem J. 2016;473:2251. doi: 10.1042/BCJ20160170. [DOI] [PubMed] [Google Scholar]
- 16.Russell R.C., Tian Y., Yuan H., Park H.W., Chang Y.-Y., Kim J. ULK1 induces autophagy by phosphorylating beclin-1 and activating Vps34 lipid kinase. Nat Cell Biol. 2013;15:741. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nascimbeni A.C., Codogno P., Morel E. Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 2017;284:1267–1278. doi: 10.1111/febs.13987. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.-M., Jung Chang H., Seo M., Kim Eun K., Park J.-M., Bae Sun S. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol Cell. 2015;57:207–218. doi: 10.1016/j.molcel.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denny P., Feuermann M., Hill D.P., Lovering R.C., Plun-Favreau H., Roncaglia P. Exploring autophagy with gene ontology. Autophagy. 2018;14:419–436. doi: 10.1080/15548627.2017.1415189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 21.Hansen M., Rubinsztein D.C., Walker D.W. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19:579–593. doi: 10.1038/s41580-018-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cen L., Hsieh F.C., Lin H.J., Chen C.S., Qualman S.J., Lin J. PDK-1/AKT pathway as a novel therapeutic target in rhabdomyosarcoma cells using OSU-03012 compound. Br J Cancer. 2007;97:785–791. doi: 10.1038/sj.bjc.6603952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J., Manning Brendan D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoki K., Zhu T., Guan K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 25.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J., Kundu M., Viollet B., Guan K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan X.-Y., Tian C., Wang H., Xu Y., Ren K., Zhang B.-Y. Activation of the AMPK–ULK1 pathway plays an important role in autophagy during prion infection. Sci Rep. 2015;5:14728. doi: 10.1038/srep14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sancak Y., Thoreen C.C., Peterson T.R., Lindquist R.A., Kang S.A., Spooner E. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Shin H.-J.R., Kim H., Oh S., Lee J.-G., Kee M., Ko H.-J. AMPK–SKP2–CARM1 signaling cascade in transcriptional regulation of autophagy. Nature. 2016;534:553–557. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shackelford D.B., Shaw R.J. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J., Yang G., Kim Y., Kim J., Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48:e224. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q., Liu S., Zhai A., Zhang B., Tian G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull. 2018;41:985–993. doi: 10.1248/bpb.b17-00724. [DOI] [PubMed] [Google Scholar]
- 33.Nazio F., Strappazzon F., Antonioli M., Bielli P., Cianfanelli V., Bordi M. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 34.Paquette M., El-Houjeiri L., Pause A. mTOR Pathways in cancer and autophagy. Cancers. 2018;10:18. doi: 10.3390/cancers10010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou C., Ma K., Gao R., Mu C., Chen L., Liu Q. Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res. 2016;27:184. doi: 10.1038/cr.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pengo N., Agrotis A., Prak K., Jones J., Ketteler R. A reversible phospho-switch mediated by ULK1 regulates the activity of autophagy protease ATG4B. Nat Commun. 2017;8:294. doi: 10.1038/s41467-017-00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egan D.F., Chun M.G.H., Vamos M., Zou H., Rong J., Miller C.J. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunlop E.A., Hunt D.K., Acosta-Jaquez H.A., Fingar D.C., Tee A.R. ULK1 inhibits mTORC1 signaling, promotes multisite raptor phosphorylation and hinders substrate binding. Autophagy. 2011;7:737–747. doi: 10.4161/auto.7.7.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joo J.H., Dorsey F.C., Joshi A., Hennessy-Walters K.M., Rose K.L., Mccastlain K. Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell. 2011;43:572–585. doi: 10.1016/j.molcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C., Wang H., Zhang D., Luo W., Liu R., Xu D. Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat Commun. 2018;9:3492. doi: 10.1038/s41467-018-05449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalckvar E., Berissi H., Eisenstein M., Kimchi A. Phosphorylation of beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy. 2009;5:720–722. doi: 10.4161/auto.5.5.8625. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara N., Usui T., Ohama T., Sato K. Regulation of beclin 1 protein phosphorylation and autophagy by protein phosphatase 2A (PP2A) and death-associated protein kinase 3 (DAPK3) J Biol Chem. 2016;291:10858–10866. doi: 10.1074/jbc.M115.704908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ber Y., Shiloh R., Gilad Y., Degani N., Bialik S., Kimchi A. DAPK2 is a novel regulator of mTORC1 activity and autophagy. Cell Death Differ. 2014;22:465. doi: 10.1038/cdd.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y.-Y., Li Y., Jiang W.-Q., Zhou L.-F. MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep. 2015;35 doi: 10.1042/BSR20140141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohensky J., Shapiro I.M., Leshinsky S., Watanabe H., Srinivas V. PIM-2 is an independent regulator of chondrocyte survival and autophagy in the epiphyseal growth plate. J Cell Physiol. 2007;213:246–251. doi: 10.1002/jcp.21117. [DOI] [PubMed] [Google Scholar]
- 46.Bin Y. The PIM-2 kinase phosphorylates BAD on serine 112 and reverses BAD-induced cell death. J Biol Chem. 2003;46:45358–45367. doi: 10.1074/jbc.M307933200. [DOI] [PubMed] [Google Scholar]
- 47.Mancinelli R., Carpino G., Petrungaro S., Mammola C.L., Tomaipitinca L., Filippini A. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev. 2017;2017:14. doi: 10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soukup S.-F., Kuenen S., Vanhauwaert R., Manetsberger J., Hernández-Díaz S., Swerts J. A LRRK2-dependent endophilin A phosphoswitch is critical for macroautophagy at presynaptic terminals. Neuron. 2016;92:829–844. doi: 10.1016/j.neuron.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C.A. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ersahin T., Tuncbag N., Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11:1946–1954. doi: 10.1039/c5mb00101c. [DOI] [PubMed] [Google Scholar]
- 51.Inoki K., Kim J., Guan K.-L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 52.Schimmack G., Defronzo R.A., Musi N. AMP-activated protein kinase: role in metabolism and therapeutic implications. Diabetes Obes Metab. 2006;8:591–602. doi: 10.1111/j.1463-1326.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 53.Shaw R.J., Kosmatka M., Bardeesy N., Hurley R.L., Witters L.A., Depinho R.A. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawley S.A., Pan D.A., Mustard K.J., Ross L., Bain J., Edelman A.M. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Momcilovic M., Hong S.-P., Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh P., Wu M., Zhang H., Sun H. mTORC1 signaling requires proteasomal function and the involvement of CUL4-DDB1 ubiquitin E3 ligase. Cell Cycle. 2008;7:373–381. doi: 10.4161/cc.7.3.5267. [DOI] [PubMed] [Google Scholar]
- 57.Gulati P., Thomas G. Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem Soc Trans. 2007;35:236–238. doi: 10.1042/BST0350236. [DOI] [PubMed] [Google Scholar]
- 58.Koren I., Reem E., Kimchi A. DAP1, a novel substrate of mTOR, negatively regulates autophagy. Curr Biol. 2010;20:1093–1098. doi: 10.1016/j.cub.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 59.Martin P., Poggi M.C., Chambard J.C., Boulukos K.E., Pognonec P. Low dose cadmium poisoning results in sustained ERK phosphorylation and caspase activation. Biochem Biophys Res Commun. 2006;350:803–807. doi: 10.1016/j.bbrc.2006.09.126. [DOI] [PubMed] [Google Scholar]
- 60.El-Ashry D., Miller D.L., Kharbanda S., Lippman M.E., Kern F.G. Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene. 1997;15:423–435. doi: 10.1038/sj.onc.1201198. [DOI] [PubMed] [Google Scholar]
- 61.Polson H.E.J., De Lartigue J., Rigden D.J., Reedijk M., Urbé S., Clague M.J. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–522. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- 62.Krick R., Tolstrup J., Appelles A., Henke S., Thumm M. The relevance of the phosphatidylinositolphosphat-binding motif FRRGT of Atg18 and Atg21 for the Cvt pathway and autophagy. FEBS Lett. 2006;580:4632–4638. doi: 10.1016/j.febslet.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 63.Pasquier B. Autophagy inhibitors. Cell Mol Life Sci. 2016;73:985–1001. doi: 10.1007/s00018-015-2104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Y., She H., Zhang T., Xu H., Cheng L., Yepes M. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 2018;217:315–328. doi: 10.1083/jcb.201701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang R., Zeh H.J., Lotze M.T., Tang D. The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menon M.B., Dhamija S. Beclin 1 phosphorylation—at the center of autophagy regulation. Front Cell Dev Biol. 2018;6:137. doi: 10.3389/fcell.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gozuacik D., Kimchi A. DAPk protein family and cancer. Autophagy. 2006;2:74–79. doi: 10.4161/auto.2.2.2459. [DOI] [PubMed] [Google Scholar]
- 68.Farag A.K., Roh E.J. Death-associated protein kinase (DAPK) family modulators: current and future therapeutic outcomes. Med Res Rev. 2019;39:349–385. doi: 10.1002/med.21518. [DOI] [PubMed] [Google Scholar]
- 69.Wei Y., Sinha S.C., Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4:949–951. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang T., Ren C., Qiao P., Han X., Wang L., Lv S. PIM2-mediated phosphorylation of hexokinase 2 is critical for tumor growth and paclitaxel resistance in breast cancer. Oncogene. 2018;37:5997–6009. doi: 10.1038/s41388-018-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki T., Bridges D., Nakada D., Skiniotis G., Morrison S.J., Lin J.D. Inhibition of AMPK catabolic action by GSK3. Mol Cell. 2013;50:407–419. doi: 10.1016/j.molcel.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sivaprasad U., Basu A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-α-induced cell death in MCF-7 cells. J Cell Mol Med. 2008;12:1265–1271. doi: 10.1111/j.1582-4934.2008.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ellington A.A., Berhow M.A., Singletary K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis. 2005;27:298–306. doi: 10.1093/carcin/bgi214. [DOI] [PubMed] [Google Scholar]
- 74.Mckay M.M., Morrison D.K. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 75.Wang J., Whiteman M.W., Lian H., Wang G., Singh A., Huang D. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem. 2009;284:21412–21424. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manzoni C., Mamais A., Dihanich S., Soutar M.P.M., Plun-Favreau H., Bandopadhyay R. mTOR independent alteration in ULK1 Ser758 phosphorylation following chronic LRRK2 kinase inhibition. Biosci Rep. 2018;38 doi: 10.1042/BSR20171669. BSR20171669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takagawa T., Kitani A., Fuss I., Levine B., Brant S.R., Peter I. An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leites E.P., Morais V.A. Mitochondrial quality control pathways: PINK1 acts as a gatekeeper. Biochem Biophys Res Commun. 2018;500:45–50. doi: 10.1016/j.bbrc.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 79.Mcwilliams Thomas G., Barini E., Pohjolan-Pirhonen R., Brooks Simon P., Singh F., Burel S. Phosphorylation of Parkin at serine 65 is essential for its activation in vivo. Open Biol. 2018;8 doi: 10.1098/rsob.180108. 180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang W., Hosford S.R., Traphagen N.A., Shee K., Demidenko E., Liu S. Autophagy promotes escape from phosphatidylinositol 3-kinase inhibition in estrogen receptor-positive breast cancer. FASEB J. 2018;32:1222–1235. doi: 10.1096/fj.201700477R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Juric D., Krop I., Ramanathan R.K., Wilson T.R., Ware J.A., Sanabria Bohorquez S.M. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017;7:704–715. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gwak H.-S., Shingu T., Chumbalkar V., Hwang Y.-H., Dejournett R., Latha K. Combined action of the dinuclear platinum compound BBR3610 with the PI3-K inhibitor PX-866 in glioblastoma. Int J Cancer. 2011;128:787–796. doi: 10.1002/ijc.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gazi M., Moharram S.A., Marhäll A., JU Kazi. The dual specificity PI3K/mTOR inhibitor PKI-587 displays efficacy against T-cell acute lymphoblastic leukemia (T-ALL) Cancer Lett. 2017;392:9–16. doi: 10.1016/j.canlet.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 84.Liu T.-J., Koul D., Lafortune T., Tiao N., Shen R.J., Maira S.-M. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen D., Lin X., Zhang C., Liu Z., Chen Z., Li Z. Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis. 2018;9:123. doi: 10.1038/s41419-017-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fei H.-R., Tian H., Zhou X.-L., Yang M.-F., Sun B.-L., Yang X.-Y. Inhibition of autophagy enhances effects of PF-04691502 on apoptosis and DNA damage of lung cancer cells. Int J Biochem Cell Biol. 2016;78:52–62. doi: 10.1016/j.biocel.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 87.Momota H., Nerio E., Holland E.C. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 88.Tong Y., Liu Y.-Y., You L.-S., Qian W.-B. Perifosine induces protective autophagy and upregulation of ATG5 in human chronic myelogenous leukemia cells in vitro. Acta Pharmacol Sin. 2012;33:542–550. doi: 10.1038/aps.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sefton E.C., Qiang W., Serna V., Kurita T., Wei J.-J., Chakravarti D. MK-2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth. Endocrinology. 2013;154:4046–4057. doi: 10.1210/en.2013-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pal I., Parida S., Prashanth Kumar B.N., Banik P., Kumar Dey K., Chakraborty S. Blockade of autophagy enhances proapoptotic potential of BI-69A11, a novel Akt inhibitor, in colon carcinoma. Eur J Pharmacol. 2015;765:217–227. doi: 10.1016/j.ejphar.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 91.Feng Y., Ke C., Tang Q., Dong H., Zheng X., Lin W. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating STAT3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Law B.Y.K., Mok S.W.F., Chan W.K., Xu S.W., Wu A.G., Yao X.J. Hernandezine, a novel AMPK activator induces autophagic cell death in drug-resistant cancers. Oncotarget. 2016;7:8090–8104. doi: 10.18632/oncotarget.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei Y.-M., Li X., Xu M., Abais J.M., Chen Y., Riebling C.R. Enhancement of autophagy by simvastatin through inhibition of Rac1-mTOR signaling pathway in coronary arterial myocytes. Cell Physiol Biochem. 2013;31:925–937. doi: 10.1159/000350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vlachaki Walker J.M., Robb J.L., Cruz A.M., Malhi A., Weightman Potter P.G., Ashford M.L.J. AMP-activated protein kinase (AMPK) activator A-769662 increases intracellular calcium and ATP release from astrocytes in an AMPK-independent manner. Diabetes Obes Metab. 2017;19:997–1005. doi: 10.1111/dom.12912. [DOI] [PubMed] [Google Scholar]
- 95.Sarkar S., Ravikumar B., Floto R.A., Rubinsztein D.C. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2008;16:46. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 96.Lin X., Han L., Weng J., Wang K., Chen T. Rapamycin inhibits proliferation and induces autophagy in human neuroblastoma cells. Biosci Rep. 2018;38 doi: 10.1042/BSR20181822. BSR20181822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chresta C.M., Davies B.R., Hickson I., Harding T., Cosulich S., Critchlow S.E. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 98.Zhou J., Tan S.-H., Nicolas V., Bauvy C., Yang N.-D., Zhang J. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome–lysosome fusion. Cell Res. 2013;23:508. doi: 10.1038/cr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodrik-Outmezguine V.S., Okaniwa M., Yao Z., Novotny C.J., Mcwhirter C., Banaji A. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272. doi: 10.1038/nature17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L., Fu L., Zhang S., Zhang J., Zhao Y., Zheng Y. Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chem Sci. 2017;8:2687–2701. doi: 10.1039/c6sc05368h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ouyang L., Zhang L., Zhang S., Yao D., Zhao Y., Wang G. Small-molecule activator of UNC-51-like kinase 1 (ULK1) that induces cytoprotective autophagy for Parkinson's disease treatment. J Med Chem. 2018;61:2776–2792. doi: 10.1021/acs.jmedchem.7b01575. [DOI] [PubMed] [Google Scholar]
- 102.Barutcu S.A., Girnius N., Vernia S., avis RJ D. Role of the MAPK/cJun NH2-terminal kinase signaling pathway in starvation-induced autophagy. Autophagy. 2018;14:1586–1595. doi: 10.1080/15548627.2018.1466013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao Y.-Q., Yin Y.-Q., Liu J., Wang G.-H., Huang J., Zhu L.-J. Characterization of HJ-PI01 as a novel Pim-2 inhibitor that induces apoptosis and autophagic cell death in triple-negative human breast cancer. Acta Pharmacol Sin. 2016;37:1237–1250. doi: 10.1038/aps.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L., Lv D.L., Liu W.B., Ding W., Zhang W., Wang H.L. Inhibition of autophagy enhances SMI-4a-induced growth inhibition and apoptosis of melanoma cells. Trop J Pharm Res. 2018;17:401–407. [Google Scholar]
- 105.Cervantes-Gomez F., Chen L.S., Orlowski R.Z., Gandhi V. Biological effects of the Pim kinase inhibitor, SGI-1776, in multiple myeloma. Clin Lymph Myelom Leuk. 2013;13:S317–S329. doi: 10.1016/j.clml.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cervantes-Gomez F., Stellrecht C.M., Ayres M.L., Keating M.J., Wierda W.G., Gandhi V. PIM kinase inhibitor, AZD1208, inhibits protein translation and induces autophagy in primary chronic lymphocytic leukemia cells. Oncotarget. 2019;10:2793–2809. doi: 10.18632/oncotarget.26876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ren F., Zhang L., Zhang X., Shi H., Wen T., Bai L. Inhibition of glycogen synthase kinase 3β promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor α. Cell Death Dis. 2016;7:e2151. doi: 10.1038/cddis.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marchand B., Arsenault D., Raymond-Fleury A., Boisvert F.-M., Boucher M.-J. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem. 2015;290:5592–5605. doi: 10.1074/jbc.M114.616714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang J., Takahashi Y., Cheng E., Liu J., Terranova P.F., Zhao B. GSK-3β promotes cell survival by modulating Bif-1-dependent autophagy and cell death. J Cell Sci. 2010;123:861. doi: 10.1242/jcs.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun A., Li C., Chen R., Huang Y., Chen Q., Cui X. GSK-3β controls autophagy by modulating LKB1–AMPK pathway in prostate cancer cells. The Prostate. 2016;76:172–183. doi: 10.1002/pros.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bryant K.L., Stalnecker C.A., Zeitouni D., Klomp J.E., Peng S., Tikunov A.P. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25:628–640. doi: 10.1038/s41591-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sullivan R.J., Infante J.R., Janku F., Wong D.J.L., Sosman J.A., Keedy V. First-in-Class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 2018;8:184. doi: 10.1158/2159-8290.CD-17-1119. [DOI] [PubMed] [Google Scholar]
- 113.Zhou M., Xu W., Wang J., Yan J., Shi Y., Zhang C. Boosting mTOR-dependent autophagy via upstream TLR4–MyD88–MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–360. doi: 10.1016/j.ebiom.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saez-Atienzar S., Bonet-Ponce L., Blesa J.R., Romero F.J., Murphy M.P., Jordan J. The LRRK2 inhibitor GSK2578215A induces protective autophagy in SH-SY5Y cells: involvement of Drp-1-mediated mitochondrial fission and mitochondrial-derived ROS signaling. Cell Death Dis. 2014;5:e1368. doi: 10.1038/cddis.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Comes F., Matrone A., Lastella P., Nico B., Susca F.C., Bagnulo R. A novel cell type-specific role of p38α in the control of autophagy and cell death in colorectal cancer cells. Cell Death Differ. 2006;14:693. doi: 10.1038/sj.cdd.4402076. [DOI] [PubMed] [Google Scholar]
- 116.Zhang H., Chen G G., Zhang C.Z., Chun S., Leung B., Lai P. Induction of autophagy in hepatocellular carcinoma cells by SB203580 requires activation of AMPK and DAPK but not p38 MAPK. Apoptosis. 2011;17:325–334. doi: 10.1007/s10495-011-0685-y. [DOI] [PubMed] [Google Scholar]
- 117.Ryabaya O.O., Inshakov A.N., Egorova A.V., Emelyanova M.A., Nasedkina T.V., Zasedatelev A.S. Autophagy inhibitors chloroquine and LY294002 enhance temozolomide cytotoxicity on cutaneous melanoma cell lines in vitro. Anti Cancer Drugs. 2017;28:307–315. doi: 10.1097/CAD.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 118.Munafó D.B., Colombo M.I. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci. 2001;114:3619. doi: 10.1242/jcs.114.20.3619. [DOI] [PubMed] [Google Scholar]
- 119.Wu Y.-T., Tan H.-L., Shui G., Bauvy C., Huang Q., Wenk M.R. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rebecca V.W., Nicastri M.C., Mclaughlin N., Fennelly C., Mcafee Q., Ronghe A. A unified approach to targeting the lysosome and growth signaling roles. Cancer Discov. 2017;7:1266. doi: 10.1158/2159-8290.CD-17-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang F., Hu P., Yang Z., Xue C., Gong J., Sun S. SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. J Thorac Oncol. 2017;37:3449–3458. doi: 10.3892/or.2017.5635. [DOI] [PubMed] [Google Scholar]
- 122.Lazarus M.B., Shokat K.M. Discovery and structure of a new inhibitor scaffold of the autophagy initiating kinase ULK1. Bioorg Chem. 2015;23:5483–5488. doi: 10.1016/j.bmc.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin K.R., Celano S.L., Solitro A.R., Gunaydin H., Scott M., O'hagan R.C. A potent and selective ULK1 inhibitor suppresses autophagy and sensitizes cancer cells to nutrient stress. iScience. 2018;8:74–84. doi: 10.1016/j.isci.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vasilevskaya I.A., Selvakumaran M., Roberts D., O'dwyer P.J. JNK1 inhibition attenuates hypoxia-induced autophagy and sensitizes to chemotherapy. Mol Cancer Res. 2016;14:753–763. doi: 10.1158/1541-7786.MCR-16-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zorea J., Prasad M., Cohen L., Li N., Schefzik R., Ghosh S. IGF1R upregulation confers resistance to isoform-specific inhibitors of PI3K in PIK3CA-driven ovarian cancer. Cell Death Dis. 2018;9:944. doi: 10.1038/s41419-018-1025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D'amato V., Rosa R., D'amato C., Formisano L., Marciano R., Nappi L. The dual PI3K/mTOR inhibitor PKI-587 enhances sensitivity to cetuximab in EGFR-resistant human head and neck cancer models. Br J Cancer. 2014;110:2887–2895. doi: 10.1038/bjc.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thomas H.E., Mercer C.A., Carnevalli L.S., Park J., Andersen J.B., Conner E.A. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blunt M.D., Carter M.J., Larrayoz M., Smith L.D., Aguilar-Hernandez M., Cox K.L. The PI3K/mTOR inhibitor PF-04691502 induces apoptosis and inhibits microenvironmental signaling in CLL and the Eμ-TCL1 mouse model. Blood. 2015;125:4032–4041. doi: 10.1182/blood-2014-11-610329. [DOI] [PubMed] [Google Scholar]
- 129.Ma C.X., Sanchez C., Gao F., Crowder R., Naughton M., Pluard T. A phase I study of the AKT inhibitor MK-2206 in combination with hormonal therapy in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22:2650–2658. doi: 10.1158/1078-0432.CCR-15-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma C.X., Suman V., Goetz M.P., Northfelt D., Burkard M.E., Ademuyiwa F. A phase II trial of neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA-mutant ER-positive and HER2-negative breast cancer. Clin Cancer Res. 2017;23:6823. doi: 10.1158/1078-0432.CCR-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dite T.A., Ling N.X.Y., Scott J.W., Hoque A., Galic S., Parker B.L. The autophagy initiator ULK1 sensitizes AMPK to allosteric drugs. Nat Commun. 2017;8:571. doi: 10.1038/s41467-017-00628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niu C., Chen Z., Kim K.T., Sun J., Xue M., Chen G. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 2019;15:843–870. doi: 10.1080/15548627.2019.1569913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pietrocola F., Castoldi F., Maiuri M.C., Kroemer G. Aspirin—another caloric-restriction mimetic. Autophagy. 2018;14:1162–1163. doi: 10.1080/15548627.2018.1454810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song Y., Zhang P., Sun Y., Li X., Chen L., Xiao Y. AMPK activation-dependent autophagy compromises oleanolic acid-induced cytotoxicity in human bladder cancer cells. Oncotarget. 2017;8:67942–67954. doi: 10.18632/oncotarget.18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang S., Zhang D., Ye X., He Y., Cai Y., Xia Z. Activation of autophagy protects against myocardial ischemic reperfusion injury by inhibition of NLRP3 inflammasome-mediated pyroptosis and inflammatory responses in diabetic rats. FASEB J. 2019;33:lb398. [Google Scholar]
- 136.Manzoni C., Mamais A., Roosen D.A., Dihanich S., Soutar M.P.M., Plun-Favreau H. mTOR independent regulation of macroautophagy by leucine rich repeat kinase 2 via beclin-1. Sci Rep. 2016;6:35106. doi: 10.1038/srep35106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mathew R., Karantza-Wadsworth V., White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singh S.S., Vats S., Chia A.Y.-Q., Tan T.Z., Deng S., Ong M.S. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142–1158. doi: 10.1038/s41388-017-0046-6. [DOI] [PubMed] [Google Scholar]
- 141.Hippert M.M., Toole P.S., Thorburn A. Autophagy in cancer: good, bad, or both?. Cancer Res. 2006;66:9349. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 142.Nassour J., Radford R., Correia A., Fusté J.M., Schoell B., Jauch A. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–663. doi: 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nah J., Yuan J., Jung Y.-K. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells. 2015;38:381–389. doi: 10.14348/molcells.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo F., Liu X., Cai H., Le W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol. 2018;28:3–13. doi: 10.1111/bpa.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang B., Kundu M. Canonical and noncanonical functions of ULK/Atg1. Curr Opin Cell Biol. 2017;45:47–54. doi: 10.1016/j.ceb.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lien E.C., Dibble C.C., Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–37. doi: 10.1016/j.ceb.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 149.Ferguson F.M., Gray N.S. Kinase inhibitors: the road ahead. Nat Rev Drug Discov. 2018;17:353. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 150.Fischer P.M. Approved and experimental small-molecule oncology kinase inhibitor drugs: a mid-2016 overview. Med Res Rev. 2017;37:314–367. doi: 10.1002/med.21409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.