Abstract
Monoacylglycerol lipase (MAGL) is a serine hydrolase that plays a crucial role catalysing the hydrolysis of monoglycerides into glycerol and fatty acids. It links the endocannabinoid and eicosanoid systems together by degradation of the abundant endocannabinoid 2-arachidaoylglycerol into arachidonic acid, the precursor of prostaglandins and other inflammatory mediators. MAGL inhibitors have been considered as important agents in many therapeutic fields, including anti-nociceptive, anxiolytic, anti-inflammatory, and even anti-cancer. Currently, ABX-1431, a first-in-class inhibitor of MAGL, is entering clinical phase 2 studies for neurological disorders and other diseases. This review summarizes the diverse (patho)physiological roles of MAGL and will provide an overview on the development of MAGL inhibitors. Although a large number of MAGL inhibitors have been reported, novel inhibitors are still required, particularly reversible ones.
KEY WORDS: Monoacylglycerol lipases, 2-Arachidaoylglycerol, Arachidonic acid, Drug discovery, Cancer, Neuroinflammation, Metabolic syndrome
Abbreviations: 2-AG, 2-arachidonoyl glycerol; 2-OG, 2-oleoylglycerol; 4-NPA, 4-nitrophenylacetate; 7-HCA, 7-hydroxycoumarinyl arachidonate; AA, arachidonic acid; ABHD6 and ABHD12, α/β-hydrolase 6 and 12; ABP, activity-based probes; ABPP, activity-based protein profiling; AD, Alzheimer's disease; AEA, anandamide; BCRP, breast cancer resistant protein; CB1R and CB2R, cannabinoid receptors; CC-ABPP, click chemistry activity-based protein profiling; CFA, complete Freund's adjuvant; CNS, central nervous system; COX, cyclooxygenases; cPLA2, cytosolic phospholipase A2; CYP, cytochrome P450 proteins; DAG, diacylglycerol; DAGLs, diacylglycerol lipases; DTT, dithiothreitol; EAE, encephalomyelitis; EI, enzyme–inhibitor complex; FAAH, amide hydrolase; FFAs, free fatty acids; FQ, fit quality; FP, fluorophosphonate; FP-Rh, fluorophosphonate-rhodamine; HFD, high-fat diet; HFIP, hexafluoroisopropyl; LFD, low-fat diet; LC–MS, liquid chromatographic mass spectrometry; MAGL, monoacylglycerol lipase; MAGs, monoglycerides; MS, multiple sclerosis; NAM, N-arachidonoyl maleimide; NHS, N-hydroxysuccinimidyl; OCT2, organic cation transporter 2; PA, phosphatidic acid; PD, Parkinson's disease; PET, positron emission tomography; P-gp, P-glycoprotein; PGs, prostaglandins; PGE2, prostaglandin; PLA2G7, phospholipase A2 group VII; PK, pharmacokinetic; SAR, structure–activity relationship; SBDD, structure-based drug design; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; THL, tetrahydrolipstatin
Graphical abstract
Monoacylglycerol lipase (MAGL) plays a crucial role catalysing the hydrolysis of monoglycerides. MAGL inhibitors have been considered as important agents in many therapeutic fields, including anti-nociceptive, anti-inflammatory and anti-cancer. The diverse (patho)physiological roles of MAGL and a comprehensive overview of reported MAGL inhibitors were summarized in this review.
1. Introduction
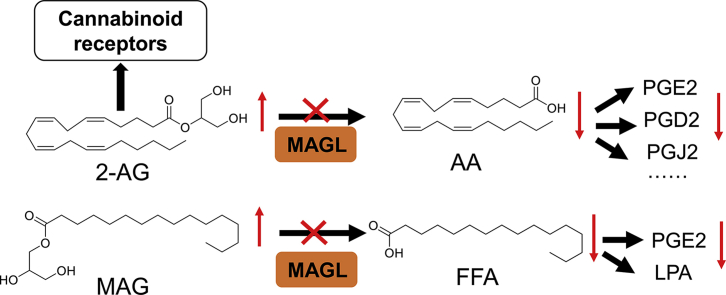
Monoacylglycerol lipase (MAGL) activity was initially discovered to hydrolyse monoglycerides (MAGs) into glycerol in the intestine and adipose tissue of rats1, 2. Subsequently, the purification, cloning and enzymatic characterization of MAGL have involved many research groups3, 4. MAGL, ∼33 kDa serine hydrolase, contains an α/β hydrolase fold and a catalytic triad with the nucleophilic serine in the highly conserved pentapeptide sequence Gly-X-Ser-X-Gly (GXSXG) found in lipases, where X represents any amino acid4. After around 40 years, MAGL was discovered to hydrolyse 2-arachidonoyl glycerol (2-AG) into arachidonic acid (AA) by Dinh et al5. 2-AG is an important endogenous signalling lipid that activates the cannabinoid receptors (CB1R and CB2R) and also serves as an important lipid precursor for the eicosanoid signalling pathway (Fig. 1a). Anandamide (AEA) is another main endogenous ligand for CB1R and CB2R. Both 2-AG and AEA, derivatives of AA, share some features of classical neurotransmitters but differ in others, for example, being produced on-demand rather than stored in vesicles6. 2-AG and AEA are produced and degraded by specific enzymatic pathways. For instance, the hydrolysis of 2-AG is mainly by MAGL, whereas AEA is hydrolysed mainly by fatty acid amide hydrolase (FAAH)6, 7. AA, the metabolite of 2-AG and AEA, is the major precursor for pro-inflammatory prostaglandin synthesis. Since physiological 2-AG levels are much higher than those of AEA7, MAGL generated more interest and was brought back to the spotlight of research again. Thereby, several selective inhibitors and genetic mouse models of MAGL were developed to study the (patho)physiological roles of MAGL. Using these powerful tools, Nomura et al.8 demonstrated that MAGL was the major enzyme that provides AA for eicosanoid biosynthesis in certain tissues. Furthermore, many studies, both genetic and pharmacologic, have demonstrated the important roles of MAGL in the regulation of endocannabinoid and eicosanoid signalling pathways9, 10, 11. Thus, MAGL is considered as a promising therapeutic target for the treatment of various disorders, including neurodegenerative12, inflammation13, 14, 15, metabolic diseases16, 17 and even cancer8, 18.
Figure 1.
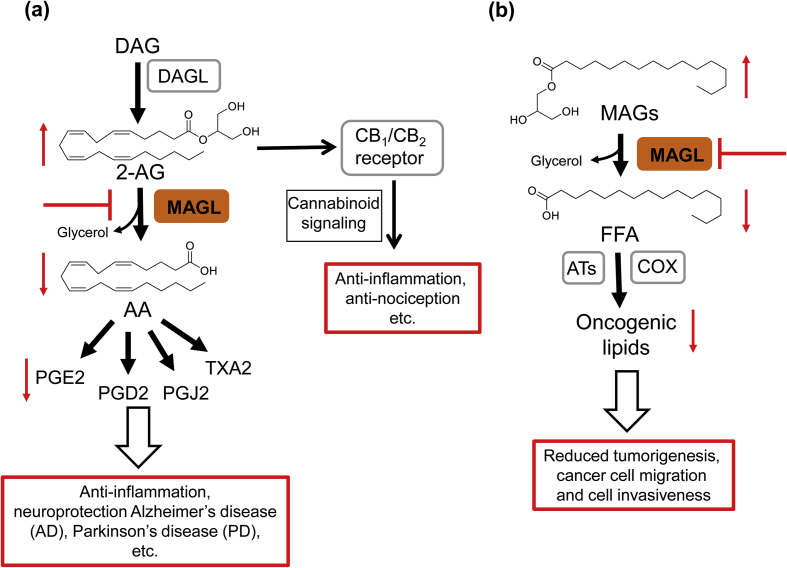
Signalling pathways regulated by monoacylglycerol lipase (MAGL) and their potential therapeutic roles. (a) MAGL inhibition induces an accumulation of the endocannabinoid 2-AG, which further enhances cannabinoid signalling by activation of CB1R and CB2R. MAGL modulates the production of the primary AA precursor pool for pro-inflammatory prostaglandins in tissues including brain, liver and lung. Thus, inactivation of MAGL has a variety of beneficial effects either by reducing eicosanoid production or enhancing endocannabinoid signalling; (b) MAGL also plays an important role in cancer cells by controlling FFA levels, which serves as sources for pro-tumorigenic signalling lipids (e.g., PGE2, lysophosphatidic acid) synthesis. MAGL inhibition reduced FFA production and attenuated cancer cell pathogenicity in aggressive cancer cells.
MAGL participates in the final lipolytic step of triacylglycerol catabolism and can broadly hydrolyse MAGs with different fatty acid chain length and saturation (e.g., 2-arachidonoyl glycerol, 2-palmitoylglycerol, 2-stearoylglycerol and 2-oleylglycerol)19. Among them, the degradation of 2-AG by MAGL has been studied extensively7. Since 2-AG is one of the most abundant endocannabinoids, it plays an essential role in the regulation of many physiological processes, including inflammation20, pain sensation21, neuroprotection22, food intake23, and addiction24. Although 2-AG can be metabolized by multiple enzymes, MAGL is still the predominant one via hydrolysing the ester bond into AA and glycerol (Fig. 1a). AA acts as a substrate for several enzymes such as cyclooxygenases (COX-1 and COX-2), and can be further converted into inflammatory prostaglandins (PGs) and thromboxanes (Fig. 1a)25, 26. For 2-AG biosynthesis, there are several proposed pathways. However, diacylglycerol lipases (DAGLs, containing two isoforms DAGLα and DAGLβ) are considered as the important enzymes for 2-AG production through the hydrolysis of diacylglycerol (DAG, Fig. 1a)7. In addition, studies have shown that Magl-deficient mice have dramatically increased 2-AG levels in brain and peripheral tissues11. However, these increased 2-AG levels did not induce the cannabimimetic effects that can be observed in the mice with acute inhibitor treatment. This discrepancy may be due to the desensitization of CB1R in the brain, which is caused by the chronic elevation of 2-AG in Magl-deficient mice11.
In 2010, Nomura et al.18 found that MAGL was highly expressed in aggressive human cancer cells and primary tumours, where it regulates an oncogenic signalling network of lipids that promotes cancer cell migration, invasion, survival and tumour growth (Fig. 1b). Both pharmacological and genetical blockade of MAGL have induced significant elevations in MAGs and reductions in free fatty acids (FFAs) in aggressive cancer cells (Fig. 1b)18. These alterations of FFAs mediated by MGAL are only observed in aggressive human cancer cells, but not normal tissue, where MAGL mainly controls the levels of MAGs, but not of FFAs18, 27. These results indicated that the MAGL–FFA pathway is essential in aggressive human cancer cell lines and primary tumours18. Additionally, the secondary lipid metabolites in the MAGL–FFA network can also be altered by suppression of MAGL. For example, the known oncogenic lipids including lysophospholipids (e.g., LPA, LPE and LPC), phosphatidic acid (PA), and prostaglandin (PGE2)28 have been reduced significantly by blockade of MAGL (Fig. 1b). These alterations contribute partially to the role of MAGL in cancer pathogenicity in aggressive human cancer cells. Of note, the exact pathways that FFAs are converted to oncogenic lipids are still unclear, although there are some proposed ones18. More studies about the role of MAGL in cancer cells will be discussed in detail in later section.
As summarized in Fig. 1, MAGL plays a critical role in lipid signalling: i) it is the major enzyme that controls the levels of 2-AG, an important lipid with various neuroprotective effects; ii) inactivation of MAGL induces an elevation in brain levels of 2-AG and a reduction of AA, a key precursor of pro-inflammatory prostaglandins, resulting in the reduction of neuroinflammation; iii) MAGL regulates the levels of FFAs in aggressive cancer cells, and this MAGL-promoted fatty acid network drives a number of pro-tumorigenic signalling pathways. Based on these, MAGL is emerging as a promising drug target for various diseases.
The determination of the crystal structure of MAGL provides the evidence how the enzyme interacts with substrate and inhibitors and gives insights to future MAGL inhibitor development29, 30. Substantial efforts by research groups and pharmaceutical companies have led to the development of MAGL inhibitors. In general, two types of inhibitors have been reported based on the reaction mechanisms: i) inhibitors that covalently and irreversibly bind on MAGL; ii) inhibitors that bind reversibly to MAGL31, 32. Among them, irreversible inhibitors are the majority for MAGL, and only a few reversible inhibitors have been reported recently. Here, we focus on an overview of MAGL, including its structural features and biochemical properties, tissue distribution and (patho)physiological roles, the current state of MAGL inhibitor development and their therapeutic potential.
2. Structure, distribution, biochemistry and physiology of MAGL
2.1. Structural features and biochemical properties
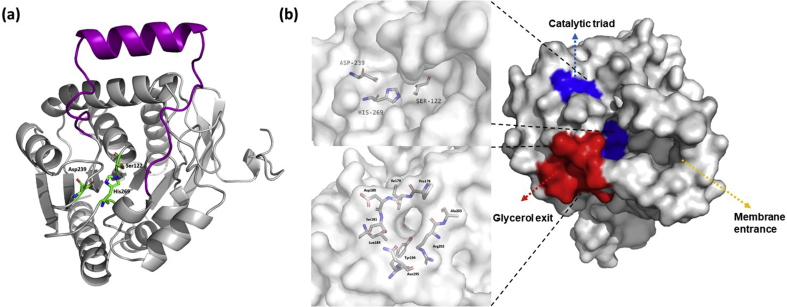
MAGL is a membrane-associated soluble enzyme, which belongs to the serine hydrolase superfamily. MAGL was first purified from rat adipose tissue in 197633 and cloned from mouse adipocytes in 19974. The X-ray crystal structure of MAGL was reported in 200929. MAGL crystalizes as a dimer and belongs to the α/β hydrolase superfamily. MAGL contains the cap domain in the structure, which is substantially different from that of other proteins in the superfamily (Fig. 2). Crystallographic data have revealed that the cap is flexible, indicating the existence of other conformations. Ser122-Asp239-His269 residues were identified as the catalytic triad for MAGL, with Ser122 identified as the nucleophile interacting with the carbonyl group of the substrate (Fig. 2). There is a wide hydrophobic entry to the catalytic site of MAGL, with the entry edge nearby hydrophobic helixes, which suggests the amphichroic character of MAGL and likely allows MAGL to interact with membranes and recruit lipophilic substrates. Additionally, three cysteines (Cys201, Cys208, and Cys242) located near the catalytic triad were proposed to stabilize the active conformation of MAGL34. These cysteine residues also provide opportunities to develop selective MAGL inhibitors over other serine hydrolases.
Figure 2.
(a) Overall structure of human MAGL, referred by X-ray crystal structure of MAGL, PDB code 3HJU29. The catalytic triad represented by sticks (Ser122-Asp239-His269), and cap domain is shown in magenta; (b) binding pockets of MAGL. The catalytic triad and glycerol exit channel are coloured by blue and red. The membrane entrance is indicated by arrow.
MAGL preferentially hydrolyses monoacylglycerols to glycerol and fatty acids with no positional preference for sn-1 (3) or 2-monoacylglycerols (MAGs)19, 35. MAGs are always short-lived lipids, which could be from both intra- and extracellular. One of the important MAGs is endocannabinoid 2-AG, which can be degraded into arachidonic acid and glycerol36. In most tissues including brain, more than 80% of 2-AG hydrolytic activity is prevented by inhibition of MAGL, this suggests the dominant role of MAGL for 2-AG degradation37, 38. FAAH, the key enzyme for degradation of AEA, has also been reported to contribute to the degradation of 2-AG to some extent in vitro39. Other studies have indicated that prostaglandin glycerol esters, the poorly characterized inflammatory mediators, could also be hydrolysed by MAGL40. More recently, MAGL has been identified to hydrolyse fatty acid ethyl esters that are generated in response to alcohol consumption41.
2.2. Tissue distribution and physiological roles of MAGL
MAGL is highly expressed in brain, liver, adipose tissue, intestine, and others, and that have been demonstrated by both genetic and pharmacological inhibition of MAGL in mice. In brain, MAGL is expressed in neurons, astrocytes, and oligodendrocytes, and in microglia (to a lower extent)5, 42. Western blot studies revealed heterogenetic of MAGL protein of multiple molecular weights4, 5. In mice, a single MAGL band is observed at ∼33 kDa in adipose tissue, liver, lung, heart, kidney, spleen, and adrenal glands, whereas in brain, testis, and skeletal muscle, MAGL migrates with another molecular weight37. In brain, MAGL bands were observed at ∼33 and 35 kDa. In testis, MAGL migrates as a single band of ∼30 kDa. In muscle, MAGL migrated at a molecular weight of ∼40 kDa. However, the source of this variation is unclear and might result either from alternative splicing or from posttranslational modifications4. Although phosphorylation or other modification of MAGL has not been reported, these process may regulate MAGL activity and localization4, 43. MAGL variation in subcellular localization can also be explained by alternative splicing, which would meet specific needs of a cell in the particular tissue of physiological process.
The understanding of the metabolic and physiological roles of MAGL has recently been accelerated by the development of selective MAGL inhibitors that are active in vivo such as JZL1849, and of Magl knock out mice (Magl−/−)17, 44. In brain, genetic or pharmacological inhibition of MAGL reduces 2-AG hydrolytic activity by at least 80%. The remaining ∼20% of 2-AG hydrolytic activity was reported to be responsible by other serine hydrolases, such as α/β-hydrolases 6 and 12 (ABHD6 and ABHD12)37. ABHD6 and ABHD12 contribute to approximately 20% of 2-AG hydrolysis, however, the exact roles in 2-AG metabolism and signalling are still unclear. MAGL is the primary enzyme responsible for 2-AG degradation, which is confirmed by MAGL inhibitors and Magl−/− mice model8, 9, 11. Recent reports suggest that ABHD6 acts as the dominant enzyme for 2-AG hydrolysis in cells where MAGL is not expressed26, 45, 46. In neurons, both ABHD6 and MAGL are expressed but with different subcellular distribution. ABHD6 is localized at post-synaptic membranes and MAGL is predominantly observed presynaptically. ABHD6 is suggested to contribute 2-AG formation at the post-synaptic site, while MAGL is mainly for pre-synaptic site46. In peripheral tissues, inhibition of MAGL by JZL184, a known potent and selective MAGL inhibitor, led to the accumulation of 2-AG to varying extents27. In testis and adipose tissue, there was 40%–50% reduction of 2-AG hydrolysing activity after JZL184 treatment, which suggests the presence of another hydrolase in these tissues, or the potential impact of alternative species on MAGL activity27. Of note, the complete inhibition of MAGL activity by JZL184 in these tissues is considered.
In addition to the reduction of monoacylglycerol levels such as 2-AG, MAGL inhibition was surprisingly found to decrease arachidonic acid (AA), prostaglandin and thromboxane production in mice brain8. Phospholipases have historically been considered as the major enzyme for AA-dependent prostaglandin production. Recent data suggests that cytosolic phospholipase A2 (cPLA2) is the major AA-releasing enzyme in gut, spleen and macrophages, whereas MAGL plays the dominant role to produce AA in brain, liver and lung8, 47. Magl-deficient mice or chronic pharmacological inhibition of MAGL leads to partial desensitization of the CB1R in the brain and loss of cannabinoid-mediated effects and produces cross-tolerance to exogenous CB1 agonists due to functional antagonism. Additionally, Magl−/− mice also have impaired CB1-dependent synaptic plasticity and physical dependence11. Therefore, it worth of interest to find an inhibition window for MAGL that maintains endocannabinoid signalling under chronic inhibition.
3. MAGL and diseases
3.1. MAGL in inflammation and neurological disorders
Cannabinoids have been used as analgesics for a quite long time, and only recently endocannabinoid system has been linked to inflammation48, 49. Inflammatory processes are always associated with multiple neurodegenerative disorders. Moreover, pain and inflammatory processes are considered to be a hallmark of neurological diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS) and stroke50. CB1R and CB2R agonists and cyclooxygenase (COX) inhibitors have been shown to have beneficial effects on various inflammatory diseases49, 51. However, use of COX1 and COX2 inhibitors have been limited because they can cause gastrointestinal and cardiovascular injury52, 53. MAGL has been discovered to reduce AA and prostaglandins levels in specific tissues, suggesting its potential as a therapeutic target for inflammation. In LPS-treated mice, administration of a MAGL inhibitor reduced pro-inflammatory prostaglandin and cytokine formation8, 54. MAGL inhibition has produced neuroprotective effects in animal models of Parkinson disease and multiple sclerosis. MAGL inhibition leads to accumulation of 2-AG and activation of cannabinoid receptors; however, these neuroprotective responses seem not to be driven via cannabinoid receptor dependent pathway, but lowering pro-inflammatory eicosanoids. The attenuated neuroinflammatory responses in animal models were not reversed upon cannabinoid receptor antagonists, indicating that the observed protective effects were mainly due to the reduction of prostaglandin and cytokine levels in brain. However, chronic MAGL inhibition that induces the functional desensitization of the cannabinoid system might also contribute to the neuroprotective response.
3.2. MAGL in metabolic disorders
Metabolic disorders are a major public health care concern that has been associated with the endocannabinoid signalling. CB1R activation induced the increase of lipogenesis and lipid deposition, hyperphagy and hypomotility55. In contrast, Cb1−/− mice showed hypophgia, leanness, hepatic steatosis and insulin resistance56, 57. Blockade of CB1R by the selective inverse agonist rimonabant reduced hepatic steatosis and dyslipidemia in animal models. Rimonabant (Acomplia®), a promising drug for obesity treatment, could reduce weight loss and improve cardiovascular risk factors, but it had to be removed from the market because of severe central psychiatric side effects58. Magl−/− mice were recently reported to have reduced body weight upon both low-fat diet (LFD) and high-fat diet (HFD). Besides, Magl−/− mice were found significantly leaner than WT mice, the serum lipid levels in Magl−/− mice were decreased as well17. Additionally, in pancreatic β-cells, MAGL was discovered to regulate insulin release and recent data also showed that glucose-stimulated and depolarization-induced insulin secretion were prevented by MAGL inhibitors59. Taken together, these data suggest that selective inhibition of MAGL might represent a new alternative therapeutic avenue to treat metabolic disorders.
3.3. MAGL in cancer
Beyond inflammation and metabolic disorders, MAGL was also implicated to play a pathophysiological role in cancer. Nomura et al.18 demonstrated that MAGL activity was highly elevated in multiple types of aggressive human cancer cells, including ovarian, breast and melanoma cancer cells. MAGL was discovered to be involved in several cellular processes in these cells, including cellular growth, survival, migration and invasion. These studies suggest that MAGL promotes cancer aggressiveness by providing a pool of FFAs for oncogenic signalling of lipid synthesis. Inhibition of MAGL induced the reduction of FFAs, lysophosphatidic acid and prostaglandins, leading to the decrease of cancer cell aggressiveness, reportedly independent on endocannabinoid signalling18. Nomura et al.60 further reported the high activity of MAGL in prostate cancer cells, and inhibition of MAGL activity impaired prostate cancer aggressiveness through FFAs reduction and CB1R activation. Other studies have demonstrated the high expression of MAGL in colorectal cancerous tissues, and tumorigenesis in colorectal cancer cell lines was impaired by MAGL inactivation61. Recently, a high expression of MAGL was also detected in nasopharyngeal and hepatocellular carcinoma, and knockdown of MAGL in these cells reduced cellular migration62, 63. Additionally, MAGL inhibition has been observed to have effects on cancer-associated symptoms, including alleviating pain and quelling nausea64. These studies suggest that MAGL plays a distinct role in driving cancer malignancy and is a potential therapeutic target for cancer treatment. However, more research is still required to explore the role of MAGL in malignant human cancer cells, for example, to determine whether the mechanism is cannabinoid signalling dependent or independent. In addition, inhibitors of MAGL have shown promise as anti-cancer agents, while alleviating cancer-associated symptoms, and may contribute to the understanding of the physiological role of MAGL in cancer aggressiveness.
4. Assays to measure MAGL activity
4.1. Surrogate substrate assay
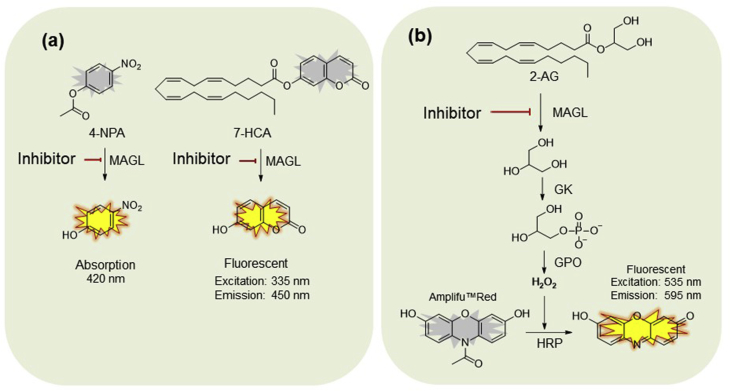
Several types of MAGL activity assays are currently available. The first type of assay employs surrogate substrates, for example, 4-nitrophenylacetate (4-NPA)65 and 7-hydroxycoumarinyl arachidonate (7-HCA)66, which mimics the reaction between MAGL and its natural substrate 2-AG (Fig. 3a). A surrogate substrate assay is generally used for inhibitor identification due to its cost-effectiveness and product easy detection. Surrogate substrate assays have multiple advantages. For example, enzymatic reaction progress can be monitored real-time by measuring absorption or fluorescence (Table 1). According to the product detection methods, radiometric assays have also been used to detect MAGL activity in vitro, utilizing radiolabelled substrate, such as [3H]-2-oleoylglycerol ([3H]-2-OG)5, 67. This method is more sensitive than previous methods using absorption or fluorescence detection, however, the wide-spread use of radiometric assays is limited by complex experimental procedures, including lipid extraction, fractionation on thin layer chromatography, and radiolabelled substrate ([3H]-2-OG) quantification. With the development of the 4-NPA-based surrogate substrate assay, Sanofi–Aventis68 identified a highly potent and selective MAGL inhibitor SAR127303. However, to some extent, surrogate substrate assays are limited in their ability to evaluate inhibitor activities, and also may affect the determination of inhibitor potency (e.g., IC50 values) with artificial substrates (Table 1). For example, binding affinities with MAGL usually have been attenuated compared with MAGL's natural substrate 2-AG. For this reason, additional assays are required to confirm the potency of inhibitors, for example, natural substrate assays are always preferred for inhibitor potency confirmation.
Figure 3.
The principles for substrate assays. (a) Surrogate substrate assays normally employ artificial substrates [e.g., 4-nitrophenylacetate (4-NPA), 7-hydroxycoumarinyl arachidonate (7-HCA)], which mimic the reaction between MAGL and its natural substrate 2-arachidonoyl glycerol (2-AG). The enzymatic reaction can be monitored real-time by absorption or fluorescence; (b) a natural substrate-based fluorometric glycerol assay for MAGL. Natural substrate 2-AG is degraded to AA and glycerol by MAGL. Subsequently, after an enzymatic cascade reaction, glycerol is converted to H2O2, which converts Amplifu™ Red to the fluorescent product resorufin in the presence of HRP.
Table 1.
A summarization of the pros and cons for surrogate substrate assays, natural substrate assays and ABPP assays.
| Assay | Pros | Cons |
|---|---|---|
| Surrogate substrate assays | Cost-effectiveness; easy detection of the product; enzymatic reaction progress can be monitored in real-time. | Binding affinities of enzymes can be attenuated due to artificial substrate; inhibitor potency (e.g., IC50 values) might be affected by use of different surrogate substrates. |
| LC–MS-based assays (natural substrate assays) | Highly sensitive and accurate. | Costly; less high throughput; cannot monitor enzymatic reaction progress in real-time; complex experimental procedures (e.g., lipid extraction); limited samples can be acquired and measured. |
| Fluorometric glycerol assay (natural substrate assays) | Using natural substrate (2-AG); enzyme inhibition can be tested in a more physiological condition; enzymatic reaction progress can be monitored in real-time; application in high throughput screening. | False-positive reduction: compounds interacting with glycerol should be excluded; experimental procedure is less straightforward. |
| ABPP | Without the need of substrate; activity and selectivity can be measured in one single experiment; both in vitro and in vivo activity/selectivity can be measured; a selectivity profile across entire proteome can be measured. | An effective activity-based probe is required; gel-based ABPP assay is less high throughput. |
4.2. Natural substrate assay
Several classes of natural substrate assays exist to measure MAGL activity. Liquid chromatographic methods coupled with mass spectrometry (LC–MS) have been used to directly measure AA formation69, 70. For example, Takeda70 has identified piperazinyl pyrrolidin-2-ones as a novel series of reversible MAGL inhibitors based on this type of assay. LC–MS-based assays are highly sensitive and accurate, but require lipid extraction and phase separation. LC–MS-based assays are more costly and less high throughput compared with surrogate substrate assays (Table 1). Therefore, LC–MS-based assays are not ideal for inhibitor screening. Furthermore, LC–MS-based assays cannot monitor an enzymatic reaction progress in real-time, because of the discontinuous setup. Besides, a coupled enzyme glycerol assay for MAGL has been developed based on an enzymatic cascade reaction that couples the conversion of the natural substrate 2-AG to the formation of a fluorescent signal (Fig. 3b)71. This assay allows 2-AG hydrolysis to be studied in real-time in 96-well (or even 384-well) plates using recombinant MAGL and avoiding the lipid extraction step. Natural substrate assays are generally used to further confirm inhibitor potency, and other more reliable results may be obtained from them compared with surrogate substrate assays.
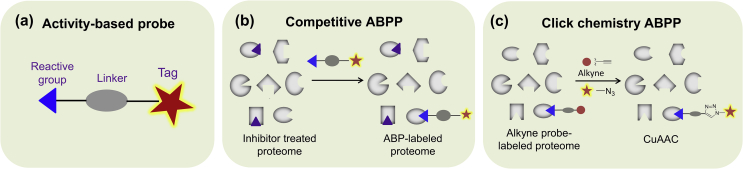
4.3. Activity-based protein profiling (ABPP)
Recently, competitive ABPP was employed as another class of assay to identify and optimize inhibitors for multiple enzymes (Fig. 4). ABPP is a powerful and robust chemical biology technique to study target engagement of inhibitors in a native system. Competitive ABPP makes use of activity-based probes (ABPs) that label the active sites of target enzymes from lysates, intact cells or even animal tissues to assess the activity and selectivity of inhibitors in a single experiment without the need of having substrate (Table 1)72, 73. In competitive ABPP, inhibitors are pre-incubated with a biological sample, and enzyme activities are subsequently detected and monitored by ABP (Fig. 4). Broad-spectrum ABPs that target a whole (or to a large extent) family of proteins, are generally used for inhibitor identification and optimization72. ABP generally consists of a warhead, a linker and a reporter tag (e.g., fluorophore, biotin, Fig. 4a). Fluorescent tags are usually used for gel-based ABPP assays, and biotin tags are used to identify and characterize the interacting proteins by mass spectrometry72. Competitive ABPP assays are able to assess activity and selectivity of inhibitors both in vitro and in vivo, and are highly valuable and complementary method to traditional substrate assays73. Currently, two structurally different ABPs have been developed for detection of MAGL activity in proteomes: fluorophosphonate (FP)-based probes72, 74 and JW91275. For example, the FP-based probe FP-rhodamine (FP-Rh), a broad-spectrum serine hydrolase probe, labels multiple enzymes, including MAGL. JW912, a tailor-made fluorescent probe, was developed based on a scaffold of MAGL inhibitors and is highly specific to MAGL and ABHD6. By using ABPP as the key method, Cisar et al.76 identified and developed the clinical MAGL inhibitor ABX-1431. In case the ABPs do not work well due to poor stability or lack enzyme specificity, click chemistry ABPP (CC-ABPP) may be utilized. The essence of this approach is to convert the inhibitor of interest into a clickable probe via introduction of a small ligation handle, such as an alkyne or an azide (Fig. 4c)73. Of note, click chemistry ABPP provides a more complete assessment of selectivity across the entire proteome (not limited by protein class or function), comparing with competitive ABPP.
Figure 4.
Overview of activity-based protein profiling (ABPP). (a) Representative cartoon structure of activity-based probe (ABP): reactive group (blue), linker (grey), and reporter tag (red); (b) in competitive ABPP, proteomes (tissue or cell lysates) are pre-incubated with inhibitors, and ABPs (broad-spectrum direct probes) are added subsequently; (c) click chemistry ABPP (CC-ABPP) provides a direct measurement of probe labelling events, and a more global map of covalent interaction.
5. MAGL inhibitors
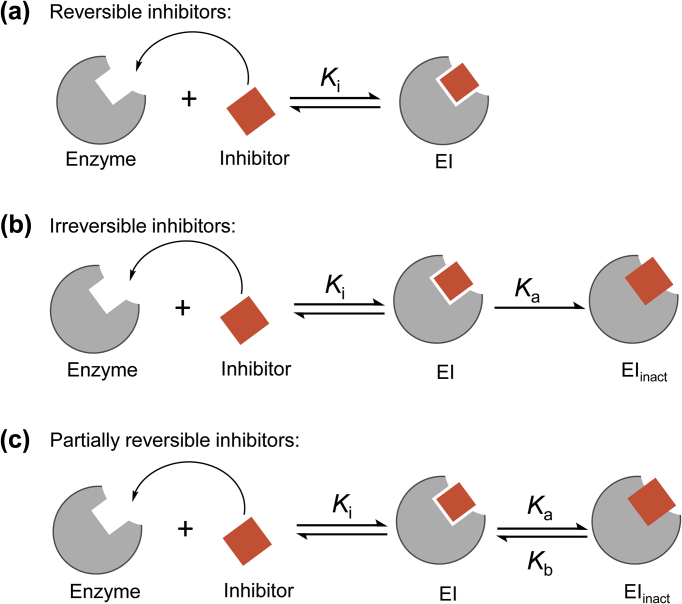
According to the interactive mechanism between inhibitors and enzyme, the inhibitors can generally be divided into two classes: reversible and irreversible (Fig. 5)77. Initially, the inhibitor binds the enzyme to form an enzyme–inhibitor (EI) complex. Subsequently, the EI complex can either i) reverse to release the free enzyme (reversible inhibitor), or ii) undergo a tightening interaction. For reversible inhibitors, enzyme activity is recovered due to the non-covalent association and the reversible equilibrium with the enzyme (Fig. 5a)77. For irreversible inhibitors, the complex shifts from EI to form EIinact, preventing the dissociation of EI (Fig. 5b)77. However, some inhibitors undergo a conversion from EIinact to the transition state EI, and are defined as partially/slowly reversible inhibitors69, 78 (Fig. 5c)77. To reverse the partially reversible inhibitor–enzyme complex EIinact, special treatments such as dialysis are required69, 77.
Figure 5.
Binding mechanism of reversible, irreversible and partially reversible inhibitors77. (a) Reversible inhibitors interact with the enzyme to form a transition state complex (EI) in a reversible way; (b) irreversible inhibitors initially bind to the enzyme to form the EI complex and subsequently irreversibly inactive the enzyme, generating a permanent inactive form EIinact, which prevents the dissociation of the EI complex (Ka > 0). (c) Partially reversible inhibitors undergo a conversion from EIinact to the transition state EI (Ka > 0, Kb > 0).
To date, a couple of chemotypes have been reported as MAGL inhibitors, which can be classified into (a) irreversible inhibitors: maleimides, disulfides, carbamates, ureas and arylthicarmide, and (b) reversible inhibitors: tetrahydrolipstatin-based derivatives (β-lactones), isothiazolines, natural terpenoids and amide-based derivatives, etc. Among them, the most reported MAGL inhibitors are irreversible ones, whereas only recently have reversible inhibitors been reported.
5.1. Irreversible inhibitors
5.1.1. Maleimides
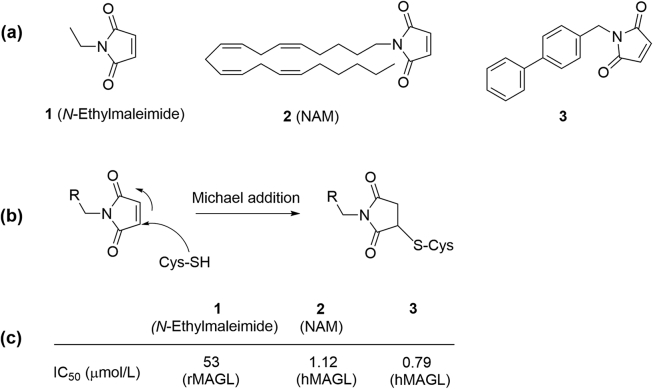
Unlike other serine hydrolase, MAGL has been demonstrated to interact with sulfhydryl-sensitive inhibitors, mostly due to the active cysteine residues near its active site (Cys201, Cys208 and Cys242)79. Maleimide derivatives such as N-ethylmaleimide (1) (Fig. 6a), comprising mercapto-specific functions, were identified as the starting points for MAGL inhibitor development. Structure–activity relationship (SAR) modification by increasing lipophilicity resulted in the identification of N-arachidonoyl maleimide (NAM, 2) with an IC50 of 1.12 μmol/L against human MAGL80 (Fig. 6a and c, Table 29, 34, 68, 70, 75, 76, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93). Further modification of NAM led to the discovery of compound 3 (Fig. 6a), which showed higher activity (IC50 = 0.79 μmol/L) and better selectivity against human MAGL over FAAH in comparison with NAM80. Maleimide derivatives are proposed to interact covalently and irreversibly with the sulfhydryl group of cysteine residues through a Michael addition to form a S-alkylated MAGL adduct (Fig. 6b)80. Although NAM and 3 are active and selective against MAGL, they have been rarely used for functional studies because the maleimide group, which is considered as a thiol-reactive electrophile, might react with other cysteine-containing proteins or enzymes.
Figure 6.
Maleimide-based MAGL inhibitors. (a) Chemical structures of maleimide-based MAGL inhibitors 1–3; (b) proposed cysteine-dependent interactive mechanism of MAGL and maleimides; (c) reported IC50 values of maleimide-based inhibitors 1–3 against MAGL.
Table 2.
Overview of known MAGL inhibitors classified as chemotype and assay type.
| Chemotype | Inhibitor | Reversibility | MAGL inhibition (IC50 nmol/L) |
||
|---|---|---|---|---|---|
| Surrogate substrate assay | Natural substrate assay | ABPP assay | |||
| Maleimides | NAM (2) | Irreversible | 112080 (hMAGL) | ||
| Disulfides | Disulfiram (4) | Irreversible | 36081 (hMAGL) | ||
| Compound 6 | Irreversible | 16681 (hMAGL) | |||
| Carbamates | URB602 (7) | Partially reversible | 1000080 (hMAGL) 2800082 (rMAGL) |
||
| JZL184 (8) | Irreversible | 48068 (hMAGL) | 89 (mMGAL) 9583 (mMAGL) 270083 (rMAGL) 69 (hMAGL) |
1084 (mMGAL) 26284 (rMAGL) 3.984 (hMAGL) |
|
| KML29 (9) | Irreversible | 2.584 (mMAGL) 0.8583 (mMAGL) 2.583 (rMAGL) 3.683 (hMAGL) |
1584 (mMAGL) 4384 (rMAGL) 5.984 (hMAGL) |
||
| JW651 (10) | Irreversible | 4.575 (mMAGL) | 3875 (mMAGL) | ||
| MJN110 (11) | Irreversible | 2.185 (mMAGL) | 9.575 (mMAGL) | ||
| SAR127303 (14) | Irreversible | 4868 (hMAGL) | |||
| PF06795071(20) | Irreversible | 386 (hMAGL) | |||
| ABX-1431 (21) | Irreversible | 876 (hMAGL) 2176 (hMAGL, brain cortex) 1476 (hMAGL, PC3 cells) 2776 (mMAGL) 2676 (rMAGL) |
|||
| Ureas | SAR629 (22) | Irreversible | 0.2283 (mMAGL) 1.183 (rMAGL) |
||
| ML30 (23) | Irreversible | 0.5487 (hMAGL) | 1.983 (mMAGL) 4.483 (rMAGL) 1.583 (hMAGL) |
||
| JJKK-048 (25) | Irreversible | 0.2883 (mMAGL) 0.2483 (rMAGL) 0.3683 (hMAGL) |
|||
| Arylthioamides | CK37 (30) | Irreversible | 15488 (hMAGL) | ||
| THL-based | OMDM169 (31) | Partial reversible (covalently) | 7.389 (mMAGL) 0.3489 (rMAGL) 89089 (hMAGL) |
||
| Natural terpenoids | Pristimerin (35) | Reversible | 9390 (rMAGL) | ||
| Euphol (36) | Reversible | 31590 (rMAGL) | |||
| Isothiazolines | Octhilinone (32) | Partial reversible | 8834 (rMAGL) | ||
| Others | Compound 41 | Reversible | 18091 (mMAGL) 24091 (rMAGL) |
||
| Compound 43 | Reversible | 84092 (hMAGL) | |||
| Compound 44 | Reversible | 8093 (hMAGL) | |||
| Compound (R)-49 | Reversible | 3.670 (hMAGL) | |||
Note: mMAGL, mouse brain MAGL; rMAGL, rat brain MAGL; hMAGL, human recombinant MAGL.
5.1.2. Disulfides
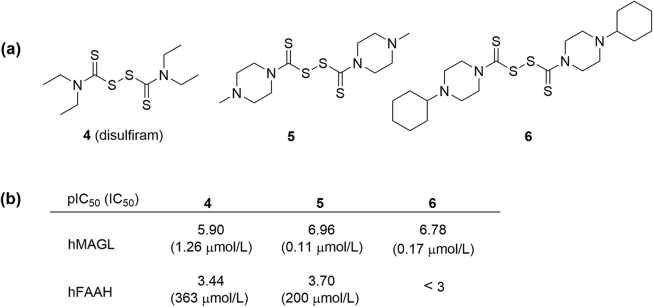
Disulfiram (4), an aldehyde dehydrogenase (ALDH) inhibitor used to treat alcoholism, was reported to show inhibitory activity against human MAGL with a pIC50 of 5.90 (IC50 = 0.36 μmol/L)81 (Fig. 7 and Table 2). Based on the scaffold of 4, a series of analogues with different N-substitutions have been synthesized to improve the activity and selectivity for human MAGL. To the end, compounds 5 and 6 were identified to exhibit optimal activity against human MAGL with pIC50 of 6.96 and 6.78 μmol/L, respectively (Fig. 7)81. Both of these compounds showed more than 1000-fold selectivity over human FAAH, particularly, compound 6 rarely inhibited human FAAH even at 1 mmol/L concentration (Fig. 7b)81. According to the exploration of the reversibility, disulfide derivatives were presumed to irreversibly interact with MAGL by formation of disulfide bonds with MAGL cysteine residues (Cys208 and Cys242).
Figure 7.
Disulfide-based MAGL inhibitors. (a) Chemical structures of disulfide-based MAGL inhibitors 4–6; (b) activities of disulfide-based inhibitors 4–6 against human MAGL and FAAH.
5.1.3. Carbamates
URB60282 (Fig. 8), the first-generation of MAGL O-aryl carbamate inhibitor, modestly inactivated rat brain MAGL with an IC50 of 28 μmol/L (Table 2). URB602 showed low potency in vivo, and increased (∼2-fold) 2-AG concentrations, but had no effect on AEA. However, the relative low potency against MAGL and cross-reactivity with FAAH94 make URB602 unsuitable to study the physiological role of MAGL. To determine the reversibility of URB602, dialysis experiments were performed, suggesting that URB602 is not a full irreversible agent, but partially reversibly binds to MAGL69. It was also proposed that an O-substituent of URB602 was the leaving group after hydrolysis.
Figure 8.
Chemical structures of known carbamate-based MAGL inhibitors (7–11, 14–16 and 18–21) and probes. Among them, compounds 12, 13 and 17 are clickable probes to determine proteome-wide selectivity profiles for MAGL inhibitors 10, 11 and 15.
In 2009, Long et al.9 identified the first selective and in vivo active MAGL inhibitor JZL184 (8) (Fig. 8) by using the robust technique ABPP (Fig. 4). Discovery of JZL184 is considered as one of the important breakthroughs for MAGL inhibitor development, and it accelerated our understanding of MAGL's physiological roles. JZL184, a piperidine carbamate, is regarded to inhibit enzyme activity covalently and irreversibly by carbamylating a serine residue in the active-site of MAGL. JZL1849 shows high inhibitory potency against MAGL at nanomolar range (IC50 = 8 nmol/L, mMAGL) (Table 2). Competitive ABPP indicated that JZL184 was around 100-fold more selective for MAGL over FAAH and other serine hydrolases in mouse brain. In peripheral tissues, however, JZL184 exhibited inhibitory effects on several other enzymes, including esterase 1, esterase 1-like, and triacylglycerol hydrolase27. In vivo experiments showed that 2-AG hydrolysis was inhibited ∼85% in mouse brain by inhibition of MAGL with JZL184, resulting in a dramatical increase of 2-AG levels in brain94. JZL184 exerted a long duration of action, and MAGL inhibition was up to 24 h. The maximal elevation of 2-AG induced by JZL184 (i.p. 16 mg/kg, single dose) lasted for 8 h9. Beneficial effects were observed by the administration of JZL184 in multiple animal models, including pain alleviation, inflammation, emesis, anxiety, neurodegeneration, and cancer pathogenicity11, 95, 96. JZL184 serves as an important chemical tool and drug candidate to pharmacologically explore the (patho)physiological roles of MAGL. However, subsequent studies have disclosed that chronic and complete inhibition of MAGL by JZL184 induced desensitization of CB1R in mouse brain. CB1R desensitization is a loss of cannabinoid-mediated effects and physical dependency, that is also observed in Magl knockout mice11. This might be due to the complete inhibition of MAGL, since further studies revealed that chronic and partial inhibition of MAGL maintained the CB1-mediated signalling, and avoided the functional antagonism of the cannabinoid system11. Of note, although JZL184 is a highly potent MAGL inhibitor, it also cross reacts with several other off-targets such as ABHD69. Thus, further structural modification of JZL184 was continued, resulting in the generation of several new carbamate derivatives, including the O-hexafluoroisopropyl (HFIP) and N-hydroxysuccinimidyl (NHS) analogues84 (compounds 9–11; Fig. 8 and Table 2). Among them, KML29 (9) (Fig. 8), a derivative of JZL184 with HFIP as a leaving group, displayed a complete selectivity for MAGL over FAAH in ABPP assays both in vitro and in vivo97, 98. Notably, KML29 was selective over ABHD6 to some extent (only observed ABHD6 inhibition at 10 μmol/L), comparing with JZL184 and other derivatives. Moreover, unlike JZL184, KML29 did not show any inhibition of carboxylesterases (e.g., esterase 1 and esterase 1-like) even at high doses (40 mg/kg) in mouse liver, and only exhibited minimal inhibition against carboxylesterase 1 in mouse lung97. Of note, JZL184 shows little inhibition of rat MAGL both in vitro and in vivo, however, KML29 maintains high potency and selectivity against rat MAGL and increases 2-AG levels in rat brain. As a carbamate-based inhibitor, KML29 was demonstrated to covalently and irreversibly react with MAGL by the formation of the carbamylated enzyme–inhibitor adduct84.
A subsequent modification of KML29 on the staying group led to the discovery of JW651 (10)75, which maintains similar activity but with improved selectivity for MAGL, compared with JZL184 and KML29. JW651 inhibited human MAGL at 100 nmol/L in vitro and complete inhibited MAGL in mouse brain at doses as low as 5 mg/kg75. JW651 was highly selective against MAGL even at high concentration (100 μmol/L) in vitro and high dose (40 mg/kg) in vivo, with ABHD6 the only identified off-target inhibition in mouse brain. Later on, Niphakis et al.85 reported MJN110 (11), a carbamate-based inhibitor with the replacement of the HFIP group in JW651 by a NHS group. MJN110 exhibited high potency and selectivity against MAGL. MJN110 was able to completely inhibit MAGL at 1 μmol/L and in vivo inhibition was observed as low as 1 mg/kg (oral or intraperitoneal injection)85. To comprehensively study the selectivity profiles of JW651 and MJN110 on a proteome-wide level, click chemistry ABPP was applied by using alkyne-bearing analogues of JW651 and MJN110 [clickable probes 12 (JW651yne) and 13 (MJN110yne), Fig. 8]. The results of click chemistry ABPP have confirmed that both of the inhibitors were selective across the entire proteome75, 85. Taken together, the discovery of highly potent, selective and irreversible carbamate-based MAGL inhibitors such as JW651 and MJN110 provides important tools for target validation, and these compounds also serve as useful templates for drug discovery.
In 2015, Sanofi–Aventis68 reported a new class of carbamate-base inhibitor SAR127303 (14) with little similarities to known MAGL inhibitors (Fig. 8). SAR127303 showed high potency towards recombinant human MAGL with an IC50 of 48 nmol/L in a biochemical assay with 4-nitrophenylacetate (4-NPA) as a substrate (Fig. 3a and Table 2). Meanwhile, SAR127303 exhibited high selectivity against MAGL over other serine enzymes (but interacted with ABHD6) and a variety of other potential targets, including 170 kinase, ion channels, neurotransmitter transporters, and receptors (e.g., CB1R and CB2R)68. Furthermore, SAR127303 was able to dramatically increase 2-AG levels in mice and produce analgesic effects in inflammation and pain animal models. However, CB1 antagonist can reverse the analgesic effects caused by SAR127303, but not CB2 antagonist, indicating the analgesia effect produced by SAR127303 is mainly due to a CB1-dependent mechanism. Importantly, in vivo experiments have demonstrated that SAR127303 did not produce hypothermia, catalepsy or hypomotility effects. Of note, this compound altered the learning and memory performances of animals, which might limit its clinical application.
In 2017, Pfizer66 reported a series of highly efficient MAGL inhibitors based on the carbamate scaffold. They identified the lead compounds through a parallel medicinal chemistry approach that highlighted the improved efficiency of azetidine and piperidine derived carbamates66. LipE (calculated as the pIC50–logP) and FQ (fit quality) were used to normalize the potency between lipophilicity and molecular weight. LipE normalizes potency enhancements of an inhibitor to lipophilicity alterations. FQ calculated as the ligand efficiency, correcting the potency values of a compound for its molecular weight by adjusting the number of heavy atoms. In their study, a series of 3-substituted azetidine inhibitors, including five- and six-membered heterocycles, were optimized by monitoring the improvements in FQ and LipE. The introduction of a pyrazole into 3-substituted azetidines resulted in a compound (15) with high efficiency (IC50 = 0.18 nmol/L, hMAGL), suggesting pyrazole could serve as an efficient linker of the azetidines. Continued optimization of the pyrazole substituent did not significantly improve the potency, but maximized LipE values of the inhibitors by substituting with pyrazine (compound 16). According to the in vitro data, compound 15 was selected as a well-optimized MAGL inhibitor, and the selectivity profile of 15 was evaluated by ABPP against a panel of 42 serine hydrolases. The results demonstrated that 15 significantly inhibited (>70%) ABHD6, CES1, CES2, MAGL and PLA2G7 at 1 μmol/L, and FAAH at 10 μmol/L66. Additionally, the activity and proteome-wide selectivity in human brain vascular pericytes (cellular context) were determined by click chemistry ABPP with alkyne-containing probe (17). Clickable probe 17 was selective against MAGL at low concentration (<IC50), whereas an additional protein band at ∼40 kDa molecular weight was observed at high concentrations from sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) results66. In vivo experimental studies of 15 have shown to elevate central 2-AG levels at a dose of 10 mg/kg, implicating the in vivo inhibitory activity of 1566. However, the increase of 2-AG levels in mouse brain by 15 was less robust comparing with the corresponding piperidine 18, suggesting the azetidine-derived analogues might exhibit reduced pharmacodynamics effects. The authors speculated that the azetidine adducts formed at the active site of MAGL might be rapidly hydrolysed from the enzyme, whereas compounds containing larger ring cores such as piperidine (18) might have improved MAGL adduct stability and produced prolonged pharmacodynamics effects. Thus, further effects were focused on the optimization of the core system of azetidine-derived inhibitors. To this end, compound 19, based on a [3.1.0] bicyclic core system, was discovered and retained high MAGL potency (IC50 = 1.4 nmol/L) and excellent selectivity86. Although compound 19 has a promising pharmacology profile, the high lipophilicity and poor kinetic solubility (<1 μmol/L) make it a less than ideal inhibitor. To address this, the leaving groups were systematically explored to improve drug-like properties, resulting in the identification of inhibitor PF-06795071 (20, IC50 = 3 nmol/L), which features a novel stereodefined trifluoromethyl glycol leaving group86. PF-06795071 has an excellent MAGL pharmacology profile, along with significantly improved physicochemical properties and LipE value. PF-06795071 also showed high selectivity over other serine hydrolases and a clean in vitro safety profile86. Moreover, PF06795071 showed good central nervous system (CNS) distribution in mouse brain and a suitable pharmacokinetic (PK) profile, and could be used as an ideal tool to study in vivo efficacy86. Administration of PF06795071 robustly increased mouse brain 2-AG accumulation, demonstrating the high in vivo efficacy of this compound. Notably, PF06795071 also reduced brain inflammatory markers in response to LPS challenge. The discovery of this glycol leaving group series provides an important scaffold for developing MAGL inhibitors, which can be used to treat neuro inflammatory conditions with injection of vein delivery.
Most recently, Abide Therapeutics76 reported a carbamate-based irreversible inhibitor ABX-1431, which is a first-in-class experimental drug for MAGL. To the discovery, optimization and profiling of ABX-1431, ABPP is the key technology that has been used. Particularly, competitive ABPP (Fig. 4b) was applied to study target engagement for covalent irreversible inhibitors in living systems99. Initially, HFIP carbamate-based MAGL inhibitors such as KML29 and JW651 were chosen as staring points for the rational design of novel MAGL inhibitors. To optimize both MAGL potency and serine hydrolase selectivity simultaneously, Cisar et al.76 used competitive gel-based ABPP with two distinct activity-based probes (FP-Rh and JW912) for the initial screening analysis. ABPP probe JW912 provides initial potency and selectivity information for MAGL inhibitors in multiple human proteomes. Of note, the selectivity assessment by JW912 is only limited to off-targets ABHD6 and phospholipase A2 group VII (PLA2G7). Therefore, they used an additional ABPP probe FP-Rh, which enabled detection of many serine hydrolases, to evaluate the interaction of their MAGL inhibitors on the serine hydrolase family. The first round of optimization starts with a symmetrical biaryl analogue JW651 (10); however, the replacement of the phenyl group by various heterocyclic rings did not significantly improve the potency or selectivity. To reduce the high lipophilicity of JW651 analogues, the authors evaluated the impacts of aryl groups by removing one of them, which resulted in the improved selectivity profile, while maintaining high MAGL potency. Further systematic SAR studies on the phenyl substitutions led to the discovery of pyrrolidine analogue ABX-1431 (21, Fig. 8), which displayed high potency and selectivity for MAGL in human PC3 lysates and rodent MAGL brain homogenates. ABX-1431 (IC50 = 8 nmol/L, recombinant human MAGL) is described as a highly selective, potent and CNS-penetrant MAGL inhibitor. Moreover, this compound has excellent drug-like property and is suitable for once-per-day oral administration. Mass spectrometry-based ABPP has demonstrated that ABX-1431 retains high activity and selectivity in human cellular assays and human prefrontal cortex proteomes76. Although ABX-1431 is a lipophilic molecule and has a basic amine, it showed only weak hHEG channel activity (IC20 ∼7 μmol/L). Additionally, ABX-1431 did not display any significant activity against a panel of 95 common off-targets including enzymes, receptors, transporters, and ion channels at 10 μmol/L, and had low propensity to inhibit human recombinant CYP (cytochrome P450 proteins) enzymes (IC50 > 50 μmol/L for CYP1A2, CYP2C9, CYP2C19, CYP3A4/5; IC50 = 6.5 μmol/L for CYP2D6). Furthermore, analysis in various transporter assays have shown that ABX-1431 was not an inhibitor or a substrate for P-gp (P-glycoprotein), BCRP (breast cancer resistant protein), and OCT2 (organic cation transporter 2) at 10 μmol/L76. In vivo experimental data revealed that ABX-1431 inhibited MAGL with an ED50 of 0.5–1.4 mg/kg (p.o.) and dose-dependently increased 2-AG levels in mouse brain76. Pharmacokinetic studies in rats and dogs have indicated that ABX-1431 has a low to moderate systemic clearance, moderate volume of distribution, and high oral bioavailability (64% in rat, 57% in dog, Table 3)76. Additionally, pharmacodynamic effects of ABX-1431 were assessed by using a rat inflammatory pain model, demonstrating that ABX-1431 exhibited potent antinociceptive effects in a formalin paw test at a single oral dose of 3 mg/kg (a dose produced near complete MAGL inhibition and maximal elevation of 2-AG)76. Other pharmacological effects have not been reported yet in the literature. Positron emission tomography (PET) studies have shown that ABX-1431 inhibited MAGL in the brain in a dose-dependent manner76. To the best of our knowledge, at least five different clinical trials have been tested for ABX-1431 (Table 4, www.clinicaltrials.gov). In phase 1 clinical studies, ABX-1431 was well-tolerated and safe, and the observed most common adverse effects were headache, somnolence, and fatigue. In a double-blind, randomized, placebo-controlled, cross-over phase 1b study, ABX-1431 showed positive effects on the symptoms of adult patients with Tourette's syndrome76, 99. Currently, ABX-1431 is entering clinical phase 2 studies for the treatment of several neurological disorders such as neuromyeltis optica and multiple sclerosis (Table 4). The results have shown that ABX-1431 had positive effects for patients suffering from neurological diseases. Table 4 summarized current clinical studies of ABX-1431. Hopefully, ABX-1431 may have positive clinical results, to speed up the development of MAGL inhibitors.
Table 3.
Pharmacokinetic parameters of MAGL inhibitor 21 (ABX-1431) in rat and dog.
| Species | Oral Cmax (μmol/L) | Tmax (h) | CL (mL/min/kg) | Vdss (L/kg) | i.v. t1/2 (h) | F (%) |
|---|---|---|---|---|---|---|
| Rat | 0.688 | 8.0 | 14.7 | 3.2 | 3.6 | 64 |
| Dog | 0.777 | 1.0 | 6.3 | 1.2 | 6.1 | 57 |
Table 4.
Overview of current clinical studies of MAGL inhibitor 21 (ABX-1431).
| Study phase | Status | Study title | Condition or disease | Intervention/treatment |
|---|---|---|---|---|
| Phase 1 | Active, not recruiting | A randomized, placebo-controlled, optimized titration study of ABX-1431 in adult patients with peripheral neuropathic pain. | Post herpetic neuralgia Diabetic peripheral neuropathy Small fibre neuropathy Post-traumatic neuralgia |
Drug: ABX-1431 Drug: placebo oral capsule |
| Phase 1 | Completed | A double-blind, placebo-controlled, crossover study to evaluate the safety and efficacy of ABX-1431 in patients with central pain. | Neuromyelitis optical spectrum disorder Transverse myelitis Multiple sclerosis Longitudinally extensive transverse myelitis |
Drug: ABX-1431 HCl Drug: placebo |
| Phase 1 | Completed | An fMRI study in healthy volunteers to investigate the effects of ABX-1431 on experimental hyperalgesia and its neural correlates. | Pain | Drug: ABX-1431 Drug: placebo |
| Phase 1 | Terminated (recruitment challenges) | A single-dose study to evaluate the effects of ABX-1431 on gastric accommodation and nutrient volume tolerance in patients with functional dyspepsia. | Dyspepsia | Drug: ABX-1431 Drug: placebo |
| Phase 1 | Completed | A randomized, placebo-controlled, single-dose crossover study of ABX-1431 HCl in adult patients with tourette syndrome (TS) and chronic motor tic disorder. | Tourette syndrome Chronic motor tic disorder |
Drug: ABX-1431 Drug: placebo comparator |
| Phase 2 | Recruiting | A randomized, placebo-controlled study of ABX-1431 in adult patients with tourette syndrome or chronic motor tic disorder. | Tourette syndrome Motor tic disorder |
Drug: ABX-1431 Drug: placebo |
5.1.4. Ureas
Sanofi–Aventis100 reported a triazole urea compound SAR629 (22, Fig. 9), which acts as a potent covalent MAGL inhibitor. The X-ray data has demonstrated that the urea moiety of SAR629 interacted with the serine residue within the catalytic site of MAGL, followed by the formation of a carbamylated enzyme adduct and subsequent release of the triazole moiety (Fig. 10)100. SAR629 is proposed to irreversibly react with MAGL, however, the irreversibility still need to be examined. Subsequently, a series of urea-based MAGL inhibitors have been identified and optimized, such as compound ML30 (23, Fig. 9), showing high potency against hMAGL with an IC50 of 0.54 nmol/L determined by the [3H]-2-oleoyl glycerol hydrolysis assay87. Further structural modifications of urea derivatives SAR629 and ML30 have been performed to improve the selectivity against MAGL, resulting in the identification of compounds JJKK-046 (24) and JJKK-048 (25, Fig. 9). Both 24 and 25 showed high inhibition activities against hMAGL with IC50 of 0.56 and 0.36 nmol/L, respectively, which were determined by a natural substrate assay83. Furthermore, ABPP assays confirmed that these compounds were selective over other serine hydrolases, including FAAH and ABHD683. Of note, 25 was able to selectively increase 2-AG levels in rat brain without affecting AEA levels. Additionally, urea-based compounds 26 and 27 were reported to selectively inhibit MAGL at submicromolar concentrations (Fig. 9). As 26 and 27 were optimized from the scaffold of loratadine (28, a histamine H1 receptor antagonist, Fig. 9), they still maintained antagonistic activities against the histamine H1 receptor101.
Figure 9.
Chemical structures of representative urea-based MAGL inhibitors 22–27 and loratadine 28 (histamine H1 receptor antagonist).
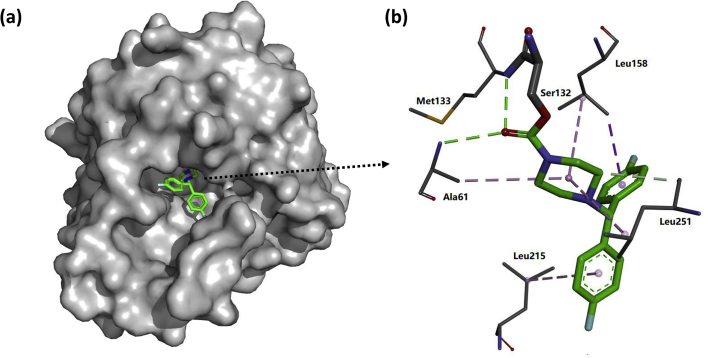
Figure 10.
(a) X-ray cocrystal structure of human MAGL (grey) with SAR629 (green), referred by PDB code 3JWE100. (b) Key interactions of MAGL–SAR629, catalytic Ser132 covalently bound to SAR629. Hydrogen bonds are depicted as green dashed lines, whereas π–σ and σ–σ interactions are depicted as purple dashed lines.
5.1.5. Arylthioamides
Arylthoamide derivatives such as CK16 (29) and CK37 (30) (Fig. 11) showed inhibition activities against MAGL with IC50 values of 355 and 154 nmol/L, respectively88. Although the inhibition activities of these inhibitors are moderate, the low logP values make them favourable for the further development of MAGL inhibitors. In vivo experiments revealed that 29 slightly increased 2-AG levels, however, 30 dramatically elevated 2-AG levels. The discrepancy between those in vivo and in vitro experiments might be due to the stability of compounds 29 and 30. Rapid dilution studies have suggested that 30 might inhibit MAGL irreversibly via a covalent binding88. To further investigate the inhibition mechanism, the authors conducted other studies such as the addition of dithiothreitol (DTT) studies and mutated hMAGL constructs studies, and found the potential formation of a DTT-sensitive covalent bond between compound 30 and Cys208 or Cys242 that are noncatalytic residues in MAGL88. According to their results, the formation of an adduct between inhibitor and the catalytic serine (Ser122) cannot be excluded.
Figure 11.
Chemical structures of representative arylthioamides-based MAGL inhibitors 29 and 30.
5.2. Reversible inhibitors
5.2.1. Tetrahydrolipstatin (THL)-based inhibitors
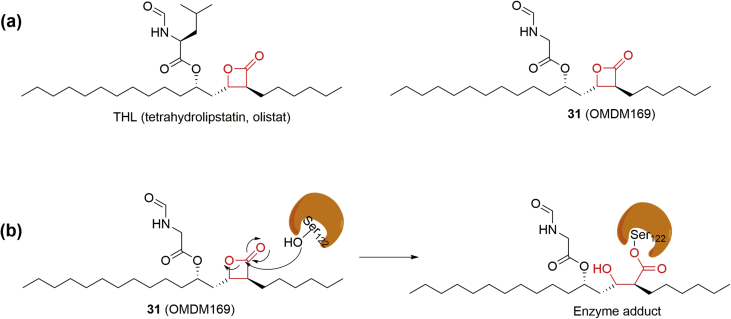
THL (Fig. 12a) is an approved drug for the treatment of obesity by the inhibition of lipases. In 2003, THL was found to show high potency against DAGLs, enzymes that responsible for 2-AG biosynthesis (Fig. 1a)102. Subsequently, a series of THL-based analogues were synthesized and screened leading to the identification of a MAGL inhibitor OMDM169 (31) (Fig. 12a)89, 103. OMDM169 inhibited MAGL with an IC50 of 0.89 μmol/L, and was more selective compared to its activity on DAGLα (∼7-fold) and FAAH (>7-fold)89. Of note, the inhibition activity of OMDM169 against MAGL varies among different enzyme species. For example, OMDM16989 exhibited better activity against rat MAGL than mouse MAGL in the brain (Table 2). In addition, OMDM169 was shown to modestly increase 2-AG levels without affecting AEA levels in neuroblastoma cells and in paws of formalin-treated mice. Initially, OMDM169 was reported as an irreversible inhibitor against β-lactone, however, subsequent studies have implicated that OMDM169 might covalently and reversibly interact with MAGL due to the hydrolysis of enzyme-inhibitor adduct (Fig. 12b)104, 105.
Figure 12.
(a) Chemical structures of tetrahydrolipstatin (THL, olistat) and MAGL inhibitor 31 (OMDM169); (b) plausible action mechanism of OMDM169 on MAGL by the formation of an acyl enzyme intermediate.
5.2.2. Isothiazolines
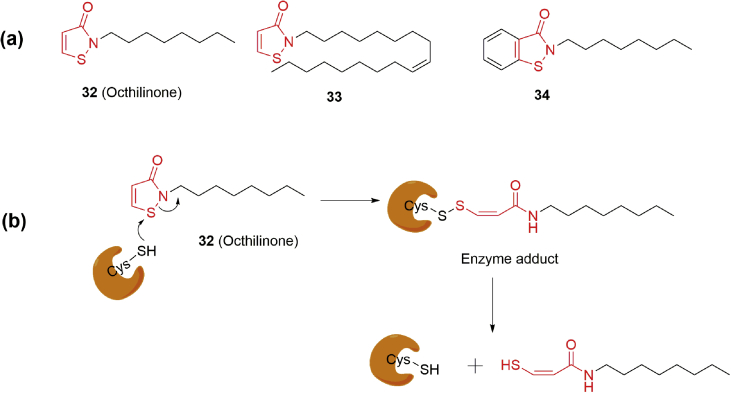
Isothiazoline derivatives are considered as promising scaffolds for MAGL inhibitors. Octhilinone (32)34 (Fig. 13a) was described to inhibit rat recombinant MAGL with an IC50 of 88 nmol/L through a partially reversible mechanism where enzyme recovery was observed after dilution. Subsequent structure modifications generated 33 and 34 by introduction of a N-substituted long hydrophobic alkyl group (33) or replacement of isothiazolineone moiety with benziosthiazolinone (34, Fig. 13a)34. The inhibition potencies of 33 (IC50 = 43 nmol/L) and 34 (IC50 = 20 nmol/L) against MAGL were slightly improved in comparison with 3234. The interaction mechanism of 32 was further investigated using a reducing agent DTT. The enzyme inhibition induced by 32 was blocked by the addition of DTT, but not other MAGL inhibitors34. The results indicated that 32 might form a reducible bond with amino acids in the active site of MAGL, which is different from the formation of a Michael addition product34. Accordingly, 32 was proposed to form a disulphide bond with MAGL (Fig. 13b).
Figure 13.
(a) Chemical structures of isothiazolinone-based MAGL inhibitors 32–34; (b) proposed inhibition mechanism by 32 at MAGL: formation of a disulphide adduct.
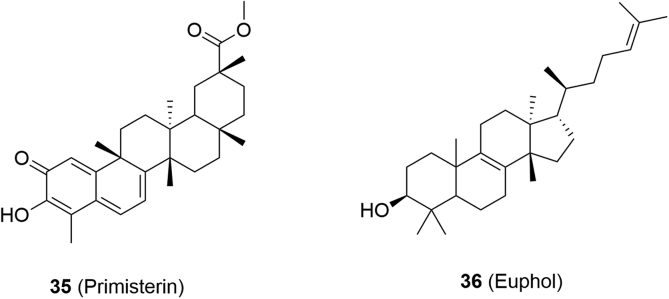
5.2.3. Natural terpenoids
The naturally occurring terpenoids pristimerin (35) and euphol (36) are described as reversible MAGL inhibitors (Fig. 14)90. Compound 35 was reported to inhibit purified recombinant rat MAGL with an IC50 of 93 nmol/L and increase 2-AG but not AEA levels in the brain90. On the other hand, compound 36 (IC50 = 315 nmol/L, purified recombinant rat MAGL), less potent than 35, whilst it did not alter 2-AG concentrations in the brain90. According to the chemical structure, the quinone methide group (35) is capable of reacting with the cysteine to form a covalent intermediate106. To verity the inhibition mechanism of 35, rapid dilution assays were performed. The catalytic activity of MAGL was recovered after rapid dilution of the MAGL–35 mixture, implicating a reversible inhibition mechanism. Moreover, time-course experiments and kinetic studies demonstrated that 35 inhibited MAGL in a rapid, reversible and non-competitive manner106. Terpenoids 35 and 36 were reported to occupy a common hydrophobic pocket located within the lid domain of MAGL and interact with the adjacent cysteines reversibly106. Notably, the discovery of terpenoids was important for the development of novel, potent and reversible MAGL inhibitors.
Figure 14.
(a) Chemical structures of MAGL inhibitors: natural terpenoids primisterin (35) and euphol (36).
5.2.4. Amide-based derivatives
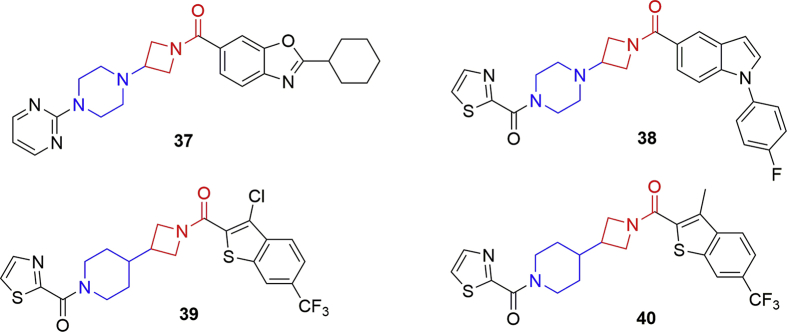
In 2010, Janssen Pharmaceutica107, 108 patented a series of potent and reversible amide-based MAGL inhibitors. These inhibitors all possess piperazine and azetidine cycles with carbonyl groups in their structures (Fig. 15). The binding mode of these compounds was resolved by the X-ray crystallography using human MAGL and a representative compound 3730. Structure optimization of 37 led to the generation of a high potent inhibitor 38 (IC50 < 5 nmol/L), which was able to increase 2-AG levels in the rat brain homogenate109. In 2013, compounds with a piperidine rather than piperazine ring were reported as MAGL inhibitors (e.g., compound 39 and 40; Fig. 15) in an US patent110. A surrogate assay using 4-methylumbelliferyl butyrate as substrate was applied to evaluate the activity of these inhibitors on MAGL31. The results revealed that these amide-based derivatives maintained high potency against MAGL with IC50 lower than 5 nmol/L. To evaluate the in vivo efficacy, compound 39 (30 mg/kg, p.o.) was administrated to the complete Freund's adjuvant (CFA)-induced cutaneous inflammation animal model, and ∼20% reversal of hypersensitivity was observed after 5 h110. Several other neuropathic pain animal models were also discussed in the patent, but no relative results were reported.
Figure 15.
Chemical structures of MAGL inhibitors: amide-based derivatives 37–40.
5.2.5. Other reversible inhibitors
In 2014, Hernande-Torres et al.91 found that compound c21 (41) was a potent, selective and reversible MAGL inhibitor with an IC50 of 180 nmol/L against mouse MAGL (Fig. 16a). To evaluate in vivo efficacy of 41, the experimental allergic encephalomyelitis (EAE) mouse model was applied. EAE model is broadly studied as an animal model of human CNS demyelinating diseases, including multiple sclerosis and acute disseminated encephalomyelitis. Compound 41 alleviated the clinical progression of a multiple sclerosis mouse model without inducing undesirable CB1-mediated side effects in disparate in vivo mouse models91. More importantly, as a reversible inhibitor, 41 did not induce catalepsy or other motor impairments that had been observed by irreversible inhibitors. In 2014, Tuccinardi et al.92, 111 identified benzoylpiperidine derivatives as a new type of reversible MAGL inhibitors by a virtual screening study. As a starting point, 42 (CL6a, Fig. 16a) proved to be a promising inhibitor with an IC50 of 11.7 μmol/L against recombinant human MAGL111. Probable binding poses from molecular modelling were used to guide the modification of 42, leading to the generation of compound 43 with improved potency on MAGL (IC50 = 840 nmol/L, hMAGL; Fig. 16a)92. Meanwhile, compound 43 displayed a high MAGL selectivity over FAAH as well as antiproliferative activity in a few cancer cells. Further optimization of 43 by replacing the p-chlorophenyl ring with a biphenyl ring led to ∼2-fold increase in its inhibition activity. This indicated that modification of the p-chlorophenyl region of these compounds is a promising strategy to improve the activity of inhibitors. Inspired by this, further structural optimization of 43 has been conducted by the same group, and identified compound 44 (Fig. 16a) as a new reversible MAGL inhibitor with high potency (IC50 = 80 nmol/L) and selectivity (IC50 values of MAGL against CB1R, CB2R, FAAH, ABHD6 and ABHD12 are all>10 μmol/L)93. Furthermore, the antiproliferative activities of 44 against aggressive cancer cells (e.g., human breast MDA-MB-231, colorectal HCT116, and ovarian CAOV3, OVCAR3 and SKOV3) have been evaluated, and 44 showed micromolar activities in these cells93. In addition, administration of 44 to mice (50 mg/kg, i.p.) significantly increased 2-AG levels in mouse brain and plasma, which confirmed the in vivo inhibitory activity of 4493. The preliminary results have indicated 44 was one of the most active and selective reversible MAGL inhibitors that has been reported in literature so far, however, the in vivo efficacy and ADME properties of this compound are still required to be explored and optimized. Recently, Aghazadeh Tabrizi et al.112 reported a diphenylpyrazole derivative 45, which has been characterized as a reversible mechanism-based MAGL inhibitor with good potency (Fig. 16a). 45 inhibited the activity of MAGL with an IC50 of 510 nmol/L, and exhibited a promising cell growth inhibitory activity in an antiproliferative assay in cancer cells that overexpress MAGL (e.g., OVCAR-3 and CAOV3). Moreover, the in vivo experiments have confirmed that 45 was effective for the treatment of neuropathic pain. In 2018, Takeda Pharmaceutical70 reported a series of novel piperazinyl pyrrolidine-2-one derivatives as reversible MAGL inhibitors using a structure-based drug design (SBDD) approach. Before the start of optimization, they identified a pyrrolidinone derivative 46 (Fig. 16b) through high-throughput screening campaign of their own compound library. However, compound 46 only showed moderate MAGL inhibitory activity (∼10% inhibition at 10 μmol/L). To improve the inhibitory activity, the cocrystal structure of amide-based inhibitor 37 and MAGL was applied to guide the structure optimization, and thus, the pyrimidinyl piperazine form 37 was introduced to the 4 position at the pyrrolidinone ring of 46, leading to the generation of compound 47 (Fig. 16b). Compound 47 displayed significantly increased MAGL inhibitory activity with an IC50 of 140 nmol/L. Subsequently, a systematic SAR study on 47 was performed, and compound 48 (Fig. 16b) was identified as the most potent MAGL inhibitor with a subnanomolar inhibition activity (IC50 = 0.64 nmol/L)70. However, the crystallography study has shown that a cocrystal structure was observed only for (R)-48, whereas the cocrystal experiments were performed using racemic 48. This indicates that MAGL might recognize the chirality at position 4 of the pyrrolidinone ring. Although 48 exhibited high potency against MAGL, the metabolic stability in human liver microsomes was poor (181 μL/min/mg). To develop an orally available MAGL inhibitor, further optimization yielded (R)-49 (Fig. 16b), which had a good balance between inhibition activity (IC50 = 5.0 nmol/L) and metabolic stability (65 μL/min/mg)70. For selectivity assessment, (R)-49 is selective against MAGL over FAAH (IC50 > 10 μmol/L), however, the selectivity profiles over other enzymes like ABHD6 and ABHD12, or even on a proteome-wide selectivity profile are still required for (R)-49. PK studies have revealed that high exposure level of (R)-49 was observed in both plasma and the brain after 1 h of oral administration to mice. To evaluate the pharmacodynamics properties of (R)-49, 2-AG and AA concentrations were measured from mice brains after 1 h of oral administration. It has shown that (R)-49 dramatically reduced AA (25%) and increased 2-AG (340%) in mice brain in vivo70. Taken together, these compounds would provide new perspectives for the development of reversible inhibitors against MAGL.
Figure 16.
(a) Chemical structures of representative reversible MAGL inhibitors 41–45; (b) development of new reversible MAGL inhibitors using structure-based drug design (SBDD).
6. Conclusion and perspectives
MAGL activity has central functions in various biological systems, particularly endocannabinoid signalling and AA metabolism. A number of studies in disease models, including intestinal inflammation, hepatic injury, insulin resistance, depression and stress, have indicated that inhibition of MAGL might serve as a powerful anti-inflammatory pharmacological strategy. Generally, two effects have been observed by MAGL inhibition: i) inducing anti-inflammatory and analgesic effects via activation of CBs receptors by increasing 2-AG levels, and ii) reducing the production of prostaglandin levels by the lowered pool of AA. Based on these effects, development of MAGL inhibitors may have significant effects for inflammation and pain treatment.
Over the past decades, a number of MAGL inhibitors, particularly irreversible inhibitors, have been reported by both academia and pharmaceutical companies. Various strategies, including high-throughput screening, de novo design as well as optimization of existing compounds, have been applied to search for novel potent and selective MAGL inhibitors. The reported MAGL inhibitors were found to have a large number of therapeutic applications, including pain and inflammation, metabolic disorders, neurodegenerative pathologies, anxiety, epilepsy, and cancer. During the development of MAGL inhibitors, another important concern is the selectivity profile. Several other serine hydrolases such as FAAH, ABHD6 and ABHD12 have similar binding site properties with MAGL, whereas these enzymes exert different functions and have different endogenous substrates in human. Thus, to exclude the effects caused by inhibition of other enzymes and to highlight the key role of MAGL inhibition in the in vivo results, it is necessary to determine the selectivity profiles of reported MAGL inhibitors. Assisted by the powerful technique ABPP, several high potent and selective inhibitors (e.g., JZL184, KLM29 and MJN110) were developed and considered as useful tools to explore a wide range of positive effects that induced by MAGL inhibition in animal models. ABPP is an efficient chemical biology strategy that can be used to discover and optimize inhibitors for multiple enzymes both in vitro and in vivo. Importantly, ABPP provides a global map of interactions for covalent irreversible inhibitors, which is not limited to protein class or functions. Most of the reported MAGL inhibitors contain similar structural motifs. For example, piperidine or piperazine rings and urea or carbamate groups are often connected together (e.g., JZL184, MJN110, and SAR629). This common scaffold from MAGL inhibitors may interact with residues that involved in the catalytic process of MAGL. These residues might be the same portion that interact with the glycerol part of 2-AG, and are located in a very polar area of MAGL. In general, the known inhibitors can be classified into irreversible and reversible. Repeat administration of irreversible MAGL inhibitors to mice was reported to induce cross-tolerance with CB1 agonists. Accordingly, the utility of reversible inhibitors that temporarily block the enzyme would provide an important strategy for MAGL inhibitor development. However, pharmacological studies are still required to confirm that reversible inhibition of MAGL has better therapeutic effects and fewer side effects than irreversible inhibition. Of note, covalent binding modes do not necessarily result in irreversible inhibition (e.g., OMDM169), and hence, it is important to determine the exact kinetic properties of the inhibitors. Finally, Abide Therapeutics76 reported a first-in-class MAGL irreversible inhibitor ABX-1431, which is discovered and optimized by applying ABPP. To the best of our knowledge, this molecule has completed a placebo-controlled phase 1 study successfully, and is being evaluated in phase 2 clinical trials, which also showed promising preliminary results in patients with a neurological disease. It will be interesting to see whether chronic dose of ABX-1431 could induce functional antagonism of CB1R, or whether this molecule could mimic some psychoactive effects of CB1 agonists. Hopefully, the positive clinical results of ABX-1431 would ultimately provide a new alternative for patients with neurological disease such as Tourette's syndrome, and thus, speed up the research of MAGL inhibitor development. To conclude, the therapeutic potential of MAGL inhibition is promising, but further research still requires.
Although MAGL inhibition was disclosed to have the potential for cancer treatment, how MAGL affects the metabolism of cancer cells is not well understood, and studies about the role of MAGL in cancer are also contradictory. Therefore, further comprehensive and systematic investigation on the role of MAGL in cancer is still required. Besides, according to the preclinical studies, MAGL inhibitors may have some side effects such as inducing CB1R desensitization in a chronic use. To avoid these potential adverse effects, lowering the dose of currently available irreversible inhibitors to ensure that MAGL is not completely inhibited will be required. Also, developing potent and selective MAGL reversible inhibitors would be another rational strategy. FAAH inhibitor BIA 10-2474 represents an encouraging lesson for the development of reversible MAGL inhibitors. BIA 10-2474, a covalent and irreversible inhibitor, has shown high potency and selectivity against FAAH in both in vitro and in vivo preclinical studies, however, it eventually led to failure in clinical trials. It has been postulated that off-target activities of BIA 10-2474 might have played a role. By application of the ABPP technique, Esbroeck et al.113 found that BIA 10-2474 displayed greater cross-reactivities with human serine hydrolases than other clinically tested FAAH inhibitor (e.g., PF04457845), suggesting the potential possibility to cause metabolic dysregulation in the nervous system. Therefore, the selectivity profile of MAGL inhibitors should also be seriously taken into account in the research field of MAGL inhibitor development in the future. A comprehensive selectivity profile is highly suggested for a MAGL inhibitor that is subject to clinical trials. During the development of MAGL inhibitors, their selectivity over FAAH is of particular importance, because inhibition of the off-target FAAH will induce the negative effects of dual activation of 2-AG and AEA metabolic pathways in vivo. In addition, the selectivity over ABHD6 and ABHD12 and other serine hydrolases linked in 2-AG degradation is also essential in the search of novel MAGL inhibitors. Although both ABHD6 and ABHD12 account only a small percentage of 2-AG hydrolysis, these enzymes are not well characterized yet, therefore, complete inhibition consequences are still difficult to be predicted. Currently, although several different reversible MAGL inhibitors were reported, their chemical structures are not versatile. Most of them contain similar motifs, particularly, piperidine or piperazine rings are often linked to amide groups, whereas aromatic or heteroaromatic groups of various sized are presented on the other sides of molecules. Therefore, to develop potent and selective reversible inhibitors, rational design from this general scaffold will be a good starting point. Additionally, several cocrystal structures of MAGL and its inhibitors have been reported, which would be beneficial for researchers to rationally develop novel reversible inhibitors with better activity and selectivity. On the other hand, a high-throughput screening of a large variety of compound library will be another attractive approach to identify novel starting points for MAGL inhibitor development, as this approach has the potential to discover new scaffolds. Hopefully, a new class of reversible MAGL inhibitors with high potency and selectivity will be generated and could be developed as effective therapeutic agents in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21807076), the Sichuan Natural Science Foundation, China (No. 2019YJ0112).
Hui Deng and Weimin Li designed and wrote the paper.
The authors have no conflicts of interest to declare.
Contributor Information
Hui Deng, Email: huideng0923@hotmail.com.
Weimin Li, Email: weimin003@163.com.
References
- 1.Senior J.R., Isselbacher K.J. Demonstration of an intestinal monoglyceride lipase: an enzyme with a possible role in the intracellular completion of fat digestion. J Clin Invest. 1963;42:187–195. doi: 10.1172/JCI104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan M., Berger J.E., Steinberg D. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J Biol Chem. 1964;239:401–409. [PubMed] [Google Scholar]
- 3.Kupiecki F.P. Partial purification of monoglyceride lipase from adipose tissue. J Lipid Res. 1966;7:230–235. [PubMed] [Google Scholar]
- 4.Karlsson M., Contreras J.A., Hellman U., Tornqvist H., Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 5.Dinh T.P., Carpenter D., Leslie F.M., Freund T.F., Katona I., Sensi S.L. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccarrone M., Bab I., Biró T., Cabral G.A., Dey S.K., Di Marzo V. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baggelaar M.P., Maccarrone M., van der Stelt M. 2-Arachidonoylglycerol: a signaling lipid with manifold actions in the brain. Prog Lipid Res. 2018;71:1–17. doi: 10.1016/j.plipres.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Nomura D.K., Morrison B.E., Blankman J.L., Long J.Z., Kinsey S.G., Marcondes M.C. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long J.Z., Li W., Booker L., Burston J.J., Kinsey S.G., Schlosburg J.E. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanda P.K., Gao Y., Mark L., Btesh J., Strassle B.W., Lu P. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- 11.Schlosburg J.E., Blankman J.L., Long J.Z., Nomura D.K., Pan B., Kinsey S.G. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass M., Abood M.E., Scotter E.L. The endocannabinoid system as a target for the treatment of neurodegenerative disease. Br J Pharmacol. 2010;160:480–498. doi: 10.1111/j.1476-5381.2010.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges D., Ahmad K., Rice A.S. The synthetic cannabinoid WIN55, 212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim M.M., Deng H., Zvonok A., Cockayne D.A., Kwan J., Mata H.P. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinsey S.G., Nomura D.K., O'Neal S.T., Long J.Z., Mahadevan A., Cravatt B.F. Inhibition of monoacylglycerol lipase attenuates nonsteroidal anti-inflammatory drug-induced gastric hemorrhages in mice. J Pharmacol Exp Ther. 2011;338:795–802. doi: 10.1124/jpet.110.175778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodhams S.G., Wong A., Barrett D.A., Bennett A.J., Chapman V., Alexander S.P. Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Br J Pharmacol. 2012;167:1609–1619. doi: 10.1111/j.1476-5381.2012.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglass J.D., Zhou Y.X., Wu A., Zadrogra J.A., Gajda A.M., Lackey A.I. Global deletion of MGL in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. J Lipid Res. 2015;56:1153–1171. doi: 10.1194/jlr.M058586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura D.K., Long J.Z., Niessen S., Hoover H.S., Ng S.W., Cravatt B.F. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandevoorde S., Saha B., Mahadevan A., Razdan R.K., Pertwee R.G., Martin B.R. Influence of the degree of unsaturation of the acyl side chain upon the interaction of analogues of 1-arachidonoylglycerol with monoacylglycerol lipase and fatty acid amide hydrolase. Biochem Biophys Res Commun. 2005;337:104–109. doi: 10.1016/j.bbrc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Rajesh M., Pan H., Mukhopadhyay P., Batkai S., Osei-Hyiaman D., Haskó G. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol. 2007;82:1382–1389. doi: 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calignano A., La Rana G., Giuffrida A., Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez A.J., García-Merino A. Neuroprotective agents: cannabinoids. Clin Immunol. 2012;142:57–67. doi: 10.1016/j.clim.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado R., Valverde O., Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Rouzer C.A., Marnett L.J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhouayek M., Masquelier J., Cani P.D., Lambert D.M., Muccioli G.G. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc Natl Acad Sci U S A. 2013;110:17558–17563. doi: 10.1073/pnas.1314017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long J.Z., Nomura D.K., Cravatt B.F. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills G.B., Moolenaar W.H. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 29.Labar G., Bauvois C., Borel F., Ferrer J.L., Wouters J., Lambert D.M. Crystal structure of the human monoacylglycerol lipase, a key actor in endocannabinoid signaling. ChemBioChem. 2010;11:218–227. doi: 10.1002/cbic.200900621. [DOI] [PubMed] [Google Scholar]
- 30.Schalk-Hihi C., Schubert C., Alexander R., Bayoumy S., Clemente J.C., Deckman I. Crystal structure of a soluble form of human monoglyceride lipase in complex with an inhibitor at 1.35 Å resolution. Protein Sci. 2011;20:670–683. doi: 10.1002/pro.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granchi C., Caligiuri I., Minutolo F., Rizzolio F., Tuccinardi T. A patent review of monoacylglycerol lipase (MAGL) inhibitors (2013–2017) Expert Opin Ther Pat. 2017;27:1341–1351. doi: 10.1080/13543776.2018.1389899. [DOI] [PubMed] [Google Scholar]
- 32.Viso A., Cisneros J.A., Ortega-Gutiérrez S. The medicinal chemistry of agents targeting monoacylglycerol lipase. Curr Top Med Chem. 2008;8:231–246. doi: 10.2174/156802608783498032. [DOI] [PubMed] [Google Scholar]
- 33.Tornqvist H., Belfrage P. Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem. 1976;251:813–819. [PubMed] [Google Scholar]
- 34.King A.R., Lodola A., Carmi C., Fu J., Mor M., Piomelli D. A critical cysteine residue in monoacylglycerol lipase is targeted by a new class of isothiazolinone-based enzyme inhibitors. Br J Pharmacol. 2009;157:974–983. doi: 10.1111/j.1476-5381.2009.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghafouri N., Tiger G., Razdan R.K., Mahadevan A., Pertwee R.G., Martin B.R. Inhibition of monoacylglycerol lipase and fatty acid amide hydrolase by analogues of 2-arachidonoylglycerol. Br J Pharmacol. 2004;143:774–784. doi: 10.1038/sj.bjp.0705948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisogno T., Melck D., De Petrocellis L., Di Marzo V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem. 1999;72:2113–2119. doi: 10.1046/j.1471-4159.1999.0722113.x. [DOI] [PubMed] [Google Scholar]
- 37.Blankman J.L., Simon G.M., Cravatt B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinh T.P., Kathuria S., Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- 39.Goparaju S.K., Ueda N., Yamaguchi H., Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- 40.Savinainen J.R., Kansanen E., Pantsar T., Navia-Paldanius D., Parkkari T., Lehtonen M. Robust hydrolysis of prostaglandin glycerol esters by human monoacylglycerol lipase (MAGL) Mol Pharmacol. 2014;86:522–535. doi: 10.1124/mol.114.094284. [DOI] [PubMed] [Google Scholar]
- 41.Heier C., Taschler U., Radulovic M., Aschauer P., Eichmann T.O., Grond S. Monoacylglycerol lipases act as evolutionarily conserved regulators of non-oxidative ethanol metabolism. J Biol Chem. 2016;291:11865–11875. doi: 10.1074/jbc.M115.705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulyas A.I., Cravatt B.F., Bracey M.H., Dinh T.P., Piomelli D., Boscia F. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 43.Labar G., Wouters J., Lambert D.M. A review on the monoacylglycerol lipase: at the interface between fat and endocannabinoid signalling. Curr Med Chem. 2010;17:2588–2607. doi: 10.2174/092986710791859414. [DOI] [PubMed] [Google Scholar]
- 44.Zhong P., Pan B., Gao X.P., Blankman J.L., Cravatt B.F., Liu Q.S. Genetic deletion of monoacylglycerol lipase alters endocannabinoid-mediated retrograde synaptic depression in the cerebellum. J Physiol. 2011;589:4847–4855. doi: 10.1113/jphysiol.2011.215509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabner G.F., Eichmann T.O., Wagner B., Gao Y., Farzi A., Taschler U. Deletion of monoglyceride lipase in astrocytes attenuates lipopolysaccharide-induced neuroinflammation. J Biol Chem. 2016;291:913–923. doi: 10.1074/jbc.M115.683615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrs W.R., Blankman J.L., Horne E.A., Thomazeau A., Lin Y.H., Coy J. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonventre J.V., Huang Z., Taheri M.R., O'Leary E., Li E., Moskowitz M.A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 48.Mechoulam R., Parker L.A. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 49.Turcotte C., Chouinard F., Lefebvre J.S., Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol. 2015;97:1049–1070. doi: 10.1189/jlb.3RU0115-021R. [DOI] [PubMed] [Google Scholar]
- 50.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 52.Ng S.C., Chan F.K. NSAID-induced gastrointestinal and cardiovascular injury. Curr Opin Gastroenterol. 2010;26:611–617. doi: 10.1097/MOG.0b013e32833e91eb. [DOI] [PubMed] [Google Scholar]
- 53.Cannon C.P., Cannon P.J. Physiology: COX-2 inhibitors and cardiovascular risk. Science. 2012;336:1386–1387. doi: 10.1126/science.1224398. [DOI] [PubMed] [Google Scholar]
- 54.Piro J.R., Benjamin D.I., Duerr J.M., Pi Y., Gonzales C., Wood K.M. A dysregulated endocannabinoid–eicosanoid network supports pathogenesis in a mouse model of Alzheimer's disease. Cell Rep. 2012;1:617–623. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quarta C., Mazza R., Obici S., Pasquali R., Pagotto U. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med. 2011;17:518–526. doi: 10.1016/j.molmed.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quarta C., Bellocchio L., Mancini G., Mazza R., Cervino C., Braulke L.J. CB1 signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 59.Berdan C.A., Erion K.A., Burritt N.E., Corkey B.E., Deeney J.T. Inhibition of monoacylglycerol lipase activity decreases glucose-stimulated insulin secretion in INS-1 (832/13) cells and rat islets. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nomura D.K., Lombardi D.P., Chang J.W., Niessen S., Ward A.M., Long J.Z. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye L., Zhang B., Seviour E.G., Tao K.X., Liu X.H., Ling Y. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011;307:6–17. doi: 10.1016/j.canlet.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Liu Z., Lian Z., Liao R., Chen Y., Qin Y. Monoacylglycerol lipase: a novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Sci Rep. 2016;6:35784. doi: 10.1038/srep35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu W., Zhao Y., Zhou J., Wang X., Pan Q., Zhang N. Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. J Hematol Oncol. 2016;9:127. doi: 10.1186/s13045-016-0361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sticht M.A., Long J.Z., Rock E.M., Limebeer C.L., Mechoulam R., Cravatt B.F. Inhibition of monoacylglycerol lipase attenuates vomiting in Suncus murinus and 2-arachidonoyl glycerol attenuates nausea in rats. Br J Pharmacol. 2012;165:2425–2435. doi: 10.1111/j.1476-5381.2011.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Granchi C., Rizzolio F., Bordoni V., Caligiuri I., Manera C., Macchia M. 4-Aryliden-2-methyloxazol-5(4H)-one as a new scaffold for selective reversible MAGL inhibitors. J Enzyme Inhib Med Chem. 2016;31:137–146. doi: 10.3109/14756366.2015.1010530. [DOI] [PubMed] [Google Scholar]
- 66.Butler C.R., Beck E.M., Harris A., Huang Z., McAllister L.A., Am Ende C.W. Azetidine and piperidine carbamates as efficient, covalent inhibitors of monoacylglycerol lipase. J Med Chem. 2017;60:9860–9873. doi: 10.1021/acs.jmedchem.7b01531. [DOI] [PubMed] [Google Scholar]
- 67.Labar G., Bauvois C., Muccioli G.G., Wouters J., Lambert D.M. Disulfiram is an inhibitor of human purified monoacylglycerol lipase, the enzyme regulating 2-arachidonoylglycerol signaling. ChemBioChem. 2007;8:1293–1297. doi: 10.1002/cbic.200700139. [DOI] [PubMed] [Google Scholar]
- 68.Griebel G., Pichat P., Beeské S., Leroy T., Redon N., Jacquet A. Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci Rep. 2015;5:7642. doi: 10.1038/srep07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.King A.R., Duranti A., Tontini A., Rivara S., Rosengarth A., Clapper J.R. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aida J., Fushimi M., Kusumoto T., Sugiyama H., Arimura N., Ikeda S. Design, synthesis, and evaluation of piperazinyl pyrrolidin-2-ones as a novel series of reversible monoacylglycerol lipase inhibitors. J Med Chem. 2018;61:9205–9217. doi: 10.1021/acs.jmedchem.8b00824. [DOI] [PubMed] [Google Scholar]
- 71.Navia-Paldanius D., Savinainen J.R., Laitinen J.T. Biochemical and pharmacological characterization of human α/β-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12) J Lipid Res. 2012;53:2413–2424. doi: 10.1194/jlr.M030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cravatt B.F., Wright A.T., Kozarich J.W. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 73.Niphakis M.J., Cravatt B.F. Enzyme inhibitor discovery by activity-based protein profiling. Annu Rev Biochem. 2014;83:341–377. doi: 10.1146/annurev-biochem-060713-035708. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Patricelli M.P., Cravatt B.F. Activity-based protein profiling: the serine hydrolases. Proc Natl Acad Sci U S A. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang J.W., Cognetta A.B., III, Niphakis M.J., Cravatt B.F. Proteome-wide reactivity profiling identifies diverse carbamate chemotypes tuned for serine hydrolase inhibition. ACS Chem Biol. 2013;8:1590–1599. doi: 10.1021/cb400261h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cisar J.S., Weber O.D., Clapper J.R., Blankman J.L., Henry C.L., Simon G.M. Identification of ABX-1431, a selective inhibitor of monoacylglycerol lipase and clinical candidate for treatment of neurological disorders. J Med Chem. 2018;61:9062–9084. doi: 10.1021/acs.jmedchem.8b00951. [DOI] [PubMed] [Google Scholar]
- 77.Sharma R. InTech; Rijeka, Croatia: 2012. Enzyme inhibition and bioapplications. [Google Scholar]
- 78.Ahn K., Johnson D.S., Fitzgerald L.R., Liimatta M., Arendse A., Stevenson T. Novel mechanistic class of fatty acid amide hydrolase inhibitors with remarkable selectivity. Biochemistry. 2007;46:13019–13030. doi: 10.1021/bi701378g. [DOI] [PubMed] [Google Scholar]
- 79.Saario S.M., Salo O.M., Nevalainen T., Poso A., Laitinen J.T., Järvinen T. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Matuszak N., Muccioli G.G., Labar G., Lambert D.M. Synthesis and in vitro evaluation of N-substituted maleimide derivatives as selective monoglyceride lipase inhibitors. J Med Chem. 2009;52:7410–7420. doi: 10.1021/jm900461w. [DOI] [PubMed] [Google Scholar]
- 81.Kapanda C.N., Muccioli G.G., Labar G., Poupaert J.H., Lambert D.M. Bis(dialkylaminethiocarbonyl)disulfides as potent and selective monoglyceride lipase inhibitors. J Med Chem. 2009;52:7310–7314. doi: 10.1021/jm901323s. [DOI] [PubMed] [Google Scholar]
- 82.Hohmann A.G., Suplita R.L., Bolton N.M., Neely M.H., Fegley D., Mangieri R. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 83.Aaltonen N., Savinainen J.R., Ribas C.R., Rönkkö J., Kuusisto A., Korhonen J. Piperazine and piperidine triazole ureas as ultrapotent and highly selective inhibitors of monoacylglycerol lipase. Chem Biol. 2013;20:379–390. doi: 10.1016/j.chembiol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 84.Chang J.W., Niphakis M.J., Lum K.M., Cognetta A.B., III, Wang C., Matthews M.L. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niphakis M.J., Cognetta A.B., III, Chang J.W., Buczynski M.W., Parsons L.H., Byrne F. Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci. 2013;4:1322–1332. doi: 10.1021/cn400116z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McAllister L.A., Butler C.R., Mente S., O'Neil S.V., Fonseca K.R., Piro J.R. Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation. J Med Chem. 2018;61:3008–3026. doi: 10.1021/acs.jmedchem.8b00070. [DOI] [PubMed] [Google Scholar]
- 87.Morera L., Labar G., Ortar G., Lambert D.M. Development and characterization of endocannabinoid hydrolases FAAH and MAGL inhibitors bearing a benzotriazol-1-yl carboxamide scaffold. Bioorg Med Chem. 2012;20:6260–6275. doi: 10.1016/j.bmc.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Kapanda C.N., Masquelier J., Labar G., Muccioli G.G., Poupaert J.H., Lambert D.M. Synthesis and pharmacological evaluation of 2,4-dinitroaryldithiocarbamate derivatives as novel monoacylglycerol lipase inhibitors. J Med Chem. 2012;55:5774–5783. doi: 10.1021/jm3006004. [DOI] [PubMed] [Google Scholar]
- 89.Bisogno T., Ortar G., Petrosino S., Morera E., Palazzo E., Nalli M. Development of a potent inhibitor of 2-arachidonoylglycerol hydrolysis with antinociceptive activity in vivo. Biochim Biophys Acta. 2009;1791:53–60. doi: 10.1016/j.bbalip.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 90.King A.R., Dotsey E.Y., Lodola A., Jung K.M., Ghomian A., Qiu Y. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem Biol. 2009;16:1045–1052. doi: 10.1016/j.chembiol.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hernández-Torres G., Cipriano M., Hedén E., Björklund E., Canales A., Zian D. A reversible and selective inhibitor of monoacylglycerol lipase ameliorates multiple sclerosis. Angew Chem Int Ed Engl. 2014;53:13765–13770. doi: 10.1002/anie.201407807. [DOI] [PubMed] [Google Scholar]
- 92.Granchi C., Rizzolio F., Palazzolo S., Carmignani S., Macchia M., Saccomanni G. Structural optimization of 4-chlorobenzoylpiperidine derivatives for the development of potent, reversible, and selective monoacylglycerol lipase (MAGL) inhibitors. J Med Chem. 2016;59:10299–10314. doi: 10.1021/acs.jmedchem.6b01459. [DOI] [PubMed] [Google Scholar]
- 93.Granchi C., Lapillo M., Glasmacher S., Bononi G., Licari C., Poli G. Optimization of a benzoylpiperidine class identifies a highly potent and selective reversible monoacylglycerol lipase (MAGL) inhibitor. J Med Chem. 2019;62:1932–1958. doi: 10.1021/acs.jmedchem.8b01483. [DOI] [PubMed] [Google Scholar]
- 94.Vandevoorde S., Jonsson K.O., Labar G., Persson E., Lambert D.M., Fowler C.J. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–191. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kinsey S.G., Wise L.E., Ramesh D., Abdullah R., Selley D.E., Cravatt B.F. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Z., Wang W., Zhong P., Liu S.J., Long J.Z., Zhao L. Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus. 2015;25:16–26. doi: 10.1002/hipo.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasquarelli N., Porazik C., Hanselmann J., Weydt P., Ferger B., Witting A. Comparative biochemical characterization of the monoacylglycerol lipase inhibitor KML29 in brain, spinal cord, liver, spleen, fat and muscle tissue. Neuropharmacology. 2015;91:148–156. doi: 10.1016/j.neuropharm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Ignatowska-Jankowska B.M., Ghosh S., Crowe M.S., Kinsey S.G., Niphakis M.J., Abdullah R.A. In vivo characterization of the highly selective monoacylglycerol lipase inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang M., van der Stelt M. Activity-based protein profiling delivers selective drug candidate ABX-1431, a monoacylglycerol lipase inhibitor, to control lipid metabolism in neurological disorders. J Med Chem. 2018;61:9059–9061. doi: 10.1021/acs.jmedchem.8b01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bertrand T., Augé F., Houtmann J., Rak A., Vallee F., Mikol V. Structural basis for human monoglyceride lipase inhibition. J Mol Biol. 2010;396:663–673. doi: 10.1016/j.jmb.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 101.Patel J.Z., Ahenkorah S., Vaara M., Staszewski M., Adams Y., Laitinen T. Loratadine analogues as MAGL inhibitors. Bioorg Med Chem Lett. 2015;25:1436–1442. doi: 10.1016/j.bmcl.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 102.Bisogno T., Howell F., Williams G., Minassi A., Cascio M.G., Ligresti A. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ortar G., Bisogno T., Ligresti A., Morera E., Nalli M., Di Marzo V. Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J Med Chem. 2008;51:6970–6979. doi: 10.1021/jm800978m. [DOI] [PubMed] [Google Scholar]
- 104.Borgstrom B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim Biophys Acta. 1988;962:308–316. doi: 10.1016/0005-2760(88)90260-3. [DOI] [PubMed] [Google Scholar]
- 105.Hadváry P., Sidler W., Meister W., Vetter W., Wolfer H. The lipase inhibitor tetrahydrolipstatin binds covalently to the putative active site serine of pancreatic lipase. J Biol Chem. 1991;266:2021–2027. [PubMed] [Google Scholar]
- 106.Bolton J.L., Turnipseed S.B., Thompson J.A. Influence of quinone methide reactivity on the alkylation of thiol and amino groups in proteins: studies utilizing amino acid and peptide models. Chem Biol Interact. 1997;107:185–200. doi: 10.1016/s0009-2797(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 107.Janssen Pharmaceutica N.V. 2010 Oct 28. Heteroaromatic and aromatic piperazinyl azetidinyl amides as monoacyglycerol lipase inhibitors. WO2010124122. [Google Scholar]
- 108.Janssen Pharmaceutica N.V. 2010 Oct 28. Heteroaromatic and aromatic piperazinyl azetidinyl amides as monoacyglycerol lipase inhibitors. WO2010124121. [Google Scholar]
- 109.Janssen Pharmaceutica N.V. 2013 Apr 04. Monoacylglycerol lipase inhibitors for the treatment of metabolic diseases and related disorders. WO2013049293. [Google Scholar]
- 110.Connolly P.J., Bian H.Y., Li X. 2012 Apr 26. Piperidin-4-yl-azetidine diamides as monoacylglycerol lipase inhibitors. WO2012054716A1. [Google Scholar]
- 111.Tuccinardi T., Granchi C., Rizzolio F., Caligiuri I., Battistello V., Toffoli G. Identification and characterization of a new reversible MAGL inhibitor. Bioorg Med Chem. 2014;22:3285–3291. doi: 10.1016/j.bmc.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 112.Aghazadeh Tabrizi M., Baraldi P.G., Baraldi S., Ruggiero E., De Stefano L., Rizzolio F. Discovery of 1,5-diphenylpyrazole-3-carboxamide derivatives as potent, reversible, and selective monoacylglycerol lipase (MAGL) inhibitors. J Med Chem. 2018;61:1340–1354. doi: 10.1021/acs.jmedchem.7b01845. [DOI] [PubMed] [Google Scholar]
- 113.van Esbroeck A.C., Janssen A.P., Cognetta A.B., III, Ogasawara D., Shpak G., van der Kroeg M. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science. 2017;356:1084–1087. doi: 10.1126/science.aaf7497. [DOI] [PMC free article] [PubMed] [Google Scholar]