Abstract:
Pharmacologic management of atrial fibrillation (AF) is a pressing problem. This arrhythmia afflicts >5 million individuals in the United States and prevalence is estimated to rise to 12 million by 2050. Although the pill-in-the-pocket regimen for self-administered AF cardioversion introduced over a decade ago has proven useful, significant drawbacks exist. Among these are the relatively long latency of effects in the range of hours along with potential for hypotension and other adverse effects. This experience prompted development of a new strategy for increasing plasma concentrations of antiarrhythmic drugs rapidly and for a limited time, namely, pulmonary delivery. In preclinical studies in Yorkshire pigs, intratracheal administration of flecainide was shown to cause a rapid, reproducible increase in plasma drug levels. Moreover, pulmonary delivery of flecainide converted AF to normal sinus rhythm by prolonging atrial depolarization, which slows intra-atrial conduction and seems to be directly correlated with efficacy in converting AF. The rapid rise in plasma flecainide levels optimizes its anti-AF effects while minimizing adverse influences on ventricular depolarization and contractility. A more concentrated and soluble formulation of flecainide using a novel cyclodextrin complex excipient reduced net drug delivery for AF conversion when compared to the acetate formulation. Inhalation of the beta-adrenergic blocking agent metoprolol slows ventricular rate and can also terminate AF. In human subjects, oral inhalation of flecainide acetate with a hand-held, breath-actuated nebulizer results in signature prolongation of the QRS complex without serious adverse events. Thus, pulmonary delivery is a promising advance in pharmacologic approach to management of AF.
Key Words: pulmonary delivery, atrial fibrillation, flecainide, cardioversion, dominant frequency, cyclodextrin
INTRODUCTION
Atrial fibrillation (AF) is a supraventricular tachyarrhythmia with fast uncoordinated atrial excitation and is associated with increased ventricular rates. It is the most frequently encountered sustained heart rhythm abnormality in clinical practice and afflicts more than 5 million individuals. Prevalence is projected to rise with the aging of the population to 12 million cases in the United States by 2050.1 Paroxysmal AF is among the most common types and is frequently associated with disabling symptoms that have a negative impact on quality of life and increase the risk of major cardiovascular events including stroke.
Flecainide is among the most effective agents for acute cardioversion of AF to normal sinus rhythm when delivered orally or intravenously (IV).2 Both routes of delivery have important drawbacks, including requirement of high doses (150–300 mg), 2- to 4-hour wait time for cardioversion with oral dosing, and hospitalization with specialized staff for IV administration. Over the past decade, individuals experiencing paroxysmal AF with recurring episodes of 1/mo to 1/yr have been increasingly managed with the so-called “pill-in-the-pocket” approach for “self-administered cardioversion.”3–6 The use of the pill-in-the-pocket antiarrhythmic drug regimen spans more than a decade and experience has generally been favorable with efficacy for class IC drugs reported in 84%3 and 70%6 of patients. An important drawback of the pill-in-the-pocket approach is the relatively long latency of effect in the range of hours.7–9
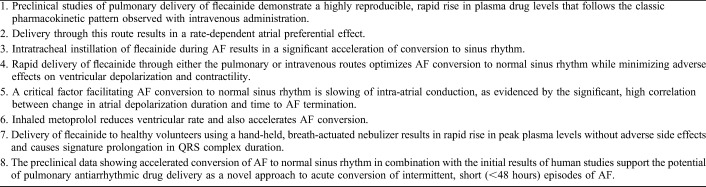
This limitation has prompted evaluation of a new approach, pulmonary delivery, for rapidly increasing plasma antiarrhythmic drug concentrations at the site of action. The basic rationale is that lung administration of antiarrhythmic agents benefits from the large surface area of the lung, ie, 100 m2, which avidly absorbs fluid, particularly in the aerosolized state. The net benefit is rapid transfer of drug into the pulmonary venous circulation. Blood containing the antiarrhythmic agent returning from the lungs traverses the luminal side of pulmonary veins and provides direct exposure of these structures to the active agent. However, the main transfer of drug occurs further downstream through the coronary arteries and capillaries, which perfuse the atrial myocardium and pulmonary vein sleeves, structures that are critical in the triggering and maintenance of AF. The anatomical structures involved in cardiopulmonary transfer of antiarrhythmic agents delivered through the lung are shown in Figure 1.
FIGURE 1.
Anatomical structures involved in pulmonary delivery of antiarrhythmic drugs. This mode of administration takes advantage of the large surface area of alveoli, ie, 100 m2, which permits avid absorption of fluid, particularly in the aerosolized state. The net benefit is rapid transfer of drug into the pulmonary venous circulation. Blood containing the antiarrhythmic agent returning from the lungs traverses the luminal side of pulmonary veins and results in direct exposure of these structures to the drug. However, the main transfer of drug occurs further downstream through the coronary arteries and capillaries, which perfuse the atrial myocardium and pulmonary vein sleeves, structures that are critical in the triggering and maintenance of AF.
The main objectives of this review are 3-fold: (1) to describe the preclinical studies that have been conducted to evaluate the efficacy of pulmonary compared to IV delivery of antiarrhythmic agents to convert AF; (2) to discuss new insights into electropharmacological mechanisms underlying the antiarrhythmic efficacy of pulmonary drug delivery; and (3) to summarize the available clinical data in normal subjects using a hand-held breath-actuated nebulizer.
PRECLINICAL STUDIES
Experimental Model
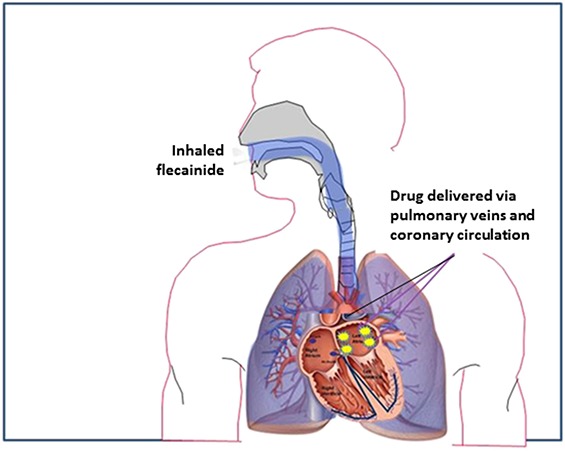
The experimental model in all protocols adhered to the Declaration of Helsinki and was approved by institutional animal care and use committee of Beth Israel Deaconess Medical Center (Boston, MA). Studies were performed in adolescent male Yorkshire pigs preanesthetized with intramuscular telazol and anesthetized with alpha-chloralose. The experimental setup for positioning the cardiac catheters is illustrated in Figure 2,10 which also shows positioning of the intratracheal catheter for pulmonary instillation of the drug. Specifically, the 5Fr angiography catheter was introduced into the trachea through a 7Fr endotracheal tube and extended ∼1 cm past the tube. The tip of the catheter was positioned under fluoroscopy at the level of the tracheal carina.
FIGURE 2.
Fluoroscopic image showing the complete set of intratracheal and cardiac catheters. On the left side, red arrows and lettering point to the intratracheal (IT) delivery system, which includes a 5 Fr modified angiography catheter positioned inside the endotracheal tube proximal to the bronchial bifurcation. LV, left ventricle; RA, right atrium. The catheter extends ∼1 cm past the end of the endotracheal tube to allow optimum distribution of the drug bolus. On the right side, yellow arrows and lettering show the RA bipolar catheter and the LV unipolar catheter used for recording electrograms and the LV pigtail catheter to record LV ventricular pressure and compute LV dP/dt as an index of contractility. The RA catheter is also used for pacing. An intrapericardial catheter is used for administering acetylcholine. Published with permission from J Cardiovasc Pharmacol from Marum et al,10 2020.
The parameters measured under this protocol included standard electrocardiographic variables (PR and QT intervals, QRS complex duration), heart rate, arterial blood pressure, left ventricular dP/dt as an index of contractility, atrial depolarization duration, and atrial PT interval. The latter has been shown, using monophasic action potential catheters, to be highly correlated with atrial action potential duration.11
Protocol for Induction of AF
AF was induced using a standard protocol used in a number of studies conducted over the past decade.12–15 Briefly, the procedure for inducing AF consisted of delivering a 1–3 mL bolus of a 102.5-mM solution of acetylcholine through the pericardial catheter (Fig. 2)10 followed by saline flush (2 mL). Burst pacing at a cycle length of 180 ms was performed at 1 minute after intrapericardial administration of acetylcholine. After AF initiation, lavage was performed with 20 mL of saline. The time to conversion of AF was the main metric used to assess the efficacy of the drug tested for terminating the arrhythmia.
Pharmacokinetic and Electrocardiographic Effects of Intratracheal and Intravenous Administration of Flecainide in Anesthetized Pigs
Stocco et al15 conducted the first preclinical study to evaluate the effects of intratracheal instillation compared to IV infusion of the class IC agent flecainide on standard electrocardiographic and hemodynamic parameters and plasma drug levels in anesthetized pigs. Two doses of flecainide were used for intratracheal delivery (0.75 and 1.5 mg/kg rapid bolus) compared to an IV infusion of 2 mg/kg over 2 or 10 minutes. In the clinic, flecainide at a dose of 2 mg/kg (to a maximum 150 mg) is typically administered IV over 10 minutes.
Intratracheal instillation of flecainide generates a pharmacokinetic (PK) profile similar to that of IV administration of flecainide. Specifically, a peak in plasma levels occurs at 2 minutes after delivery of the drug, consistent with rapid absorption of the solution through the lungs, airways, and alveoli. The distribution phase occurs between 2 and 5 minutes and is followed by the elimination phase throughout the remaining time. This PK profile is comparable to IV infusion of flecainide over 2 minutes, underscoring the efficiency of lung delivery using intratracheal instillation. Intratracheal instillation of flecainide also generates a concentration–response relationship between QRS complex and peak plasma drug levels that is similar to that of rapid IV infusion. Although none of the flecainide doses or delivery routes caused significant changes in the JTc or QTc intervals or in heart rate or mean arterial pressure, there were significant increases in the QRS complex duration after intratracheal instillation, ranging from 10% to 19% at 2 minutes and from 12% to 21% at 5 minutes with the 0.75 and 1.5 mg/kg doses, respectively.15 These findings are consistent with a clinical report by Hellestrand et al 16 of a greater effect of flecainide on His-Purkinje fibers and ventricular conduction than on atrioventricular nodal conduction.
These findings demonstrated that pulmonary delivery of flecainide produces the signature effects of this IC agent in prolonging QRS complex duration and PR interval. It is notable that the effects observed with the intratracheal doses of flecainide used are comparable to those observed after its IV administration, which is used clinically for acute cardioversion of AF to normal sinus rhythm.
Preclinical Evidence of Rapid Conversion of AF by Pulmonary Delivery of Flecainide and Potential Underlying Mechanisms
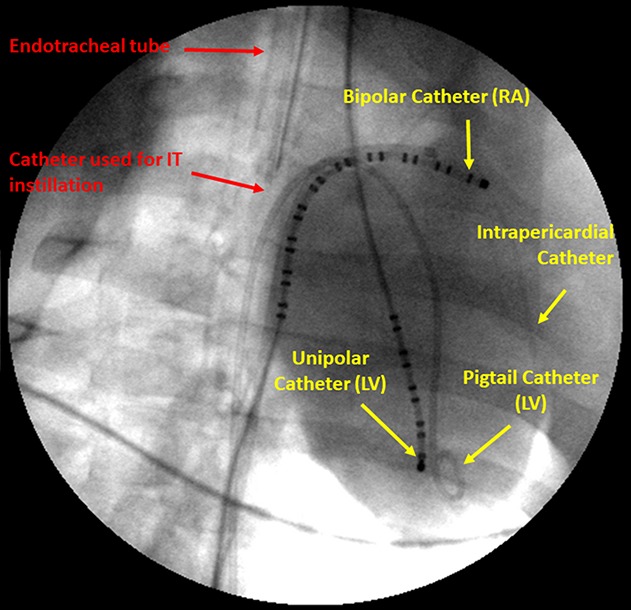
The next logical step in considering lung delivery as the route for antiarrhythmic drug therapy was to test the efficacy of intratracheal instillation of flecainide to convert AF. This goal was accomplished using the model of intrapericardial acetylcholine followed by burst pacing described above. Two intratracheal doses of flecainide were tested: 0.4 and 0.75 mg/kg.17 Both doses resulted in a relatively rapid (∼3.5 to ∼6.5 minutes) conversion of AF to normal sinus rhythm (P < 0.05 for both doses). The magnitude of the effect seemed to be dose-dependent, with shortening of AF duration by 35% and 54% for the 0.4- and 0.75-mg/kg doses, respectively, compared to no-drug spontaneous conversion. Relevant to the mechanisms whereby flecainide terminates AF, concurrent with the reduction in AF duration, intratracheal flecainide administration decreased the dominant frequency of the arrhythmia, an indicator of the level of organization of AF and propensity for spontaneous defibrillation.18 The transition from AF to normal sinus rhythm is illustrated in Figure 3.17 As shown in this figure, AF conversion to normal sinus rhythm is associated with a relatively rapid restoration of heart rate and mean arterial blood pressure.
FIGURE 3.
Atrial and ventricular electrograms from a representative experiment showing an episode of AF without drug (top), which converted to normal sinus rhythm at 4 minutes after intratracheal instillation of 0.4-mg/kg flecainide. Published with permission from Heart Rhythm Society from Verrier et al, 2018.17
The exact mechanisms whereby intratracheal flecainide leads to conversion of AF to normal sinus rhythm remain to be established. Flecainide has well-known effects on both triggered activity and reentrant rhythms.8,19 A fundamental insight into the electrophysiological mechanisms derives from the finding that the anti-AF effect is inversely correlated with the drug's prolongation of atrial depolarization duration (Fig. 4),17 which results from its slowing of intra-atrial conduction. Evidence implicates inhibition of the sodium current (INa) in suppression of early and delayed afterdepolarizations in atrial and pulmonary vein sites.20,21 The probable underlying mechanisms for flecainide's anti-AF action reside in decreasing the slope of action potential duration restitution and dispersion of repolarization. Both actions promote suppression of wavebreak.22
FIGURE 4.
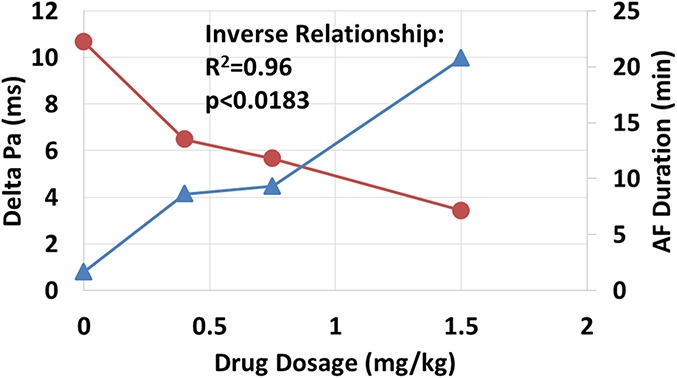
Strong inverse relationship between delta atrial depolarization (Pa) duration (blue filled triangles) and AF duration (red filled circles) in response to 3 doses of intratracheal flecainide. For the 0.4-, 0.75-, and 1.5-mg/kg doses, the number of pigs studied was 5, 6, and 5, respectively. Published with permission from Heart Rhythm Society from Verrier et al, 2018.17
Based on the plasma drug concentrations attained during conversion of AF to normal sinus rhythm, flecainide likely reduces not only peak INa but also late INa. Inhibition of the latter current may also contribute to the anti-AF action of flecainide because IV delivery of the prototypical agent ranolazine12,13 and the selective late INa inhibitor eleclazine14 is effective in converting AF to normal sinus rhythm in the same model used in the studies of intratracheal flecainide.
Optimizing Plasma Flecainide Concentration Profile for AF Cardioversion While Minimizing Adverse Mechanical and Electrophysiologic Effects on the Ventricle
A basic principle advanced by Deneer et al23 is that the probability of acute cardioversion of AF after an oral loading dose of flecainide is closely dependent on the absorption rate constant Ka. Marum et al24 tested the hypothesis that rapid delivery of low doses of flecainide would be effective not only in converting AF to normal sinus rhythm but would also minimize the adverse effects of reduced left ventricular inotropy and QRS complex prolongation. Rapid flecainide delivery was shown to prolong depolarization preferentially in atria compared to ventricles25 with attendant slowing of atrial conduction velocity, which has been found to be highly correlated (r2 = 0.87, P = 0.03) with the extent of reduction in AF duration.17
The precise factors responsible for the atrial predominant use‐dependence of flecainide delivered through intratracheal instillation are not completely understood. However, clues on other peak INa inhibitors are available from the literature.26–28 The main possibilities include the observations that atrial myocytes have a less negative resting membrane potential with a greater voltage dependence of steady‐state inactivation and a higher sodium channel density than ventricular myocytes. The use‐dependent relationships have been attributed to the fact that flecainide is an open‐state sodium channel blocker.
This rate-dependent preferential action is advantageous inasmuch as it contributes to increased effectiveness during rapid rhythms, particularly AF, and possibly decreases adverse effects of prolonged QRS complex duration, which has been linked to proarrhythmia and the negative inotropic effect of class I antiarrhythmic drugs. Furthermore, dyssynchrony of electrical activity can contribute to mechanical dyssynchrony and in turn to reduced contractility consistent with the principle of excitation-contraction coupling.
Intratracheal and IV delivery of flecainide elicits comparable reductions in AF duration with nearly similar peak plasma drug levels (Fig. 5).24 The area under the curve (AUC) of plasma drug levels is greater for higher-dose, slow IV flecainide infusion than for either intratracheal instillation or lower-dose, rapid IV infusion. The timecourse of the AUCs for intratracheal and IV delivery is shown in Figure 6.24 Collectively, these observations lead to the conclusion that lower-dose flecainide, rapidly delivered either intratracheally or IV, optimizes the plasma drug concentration profile for effective AF conversion while minimizing adverse effects on QRS complex duration and left ventricular contractility. As shown by Marum et al24 in their Figure 3, rapid delivery of flecainide through intratracheal instillation or IV administration markedly reduced the total time and the extent of depression of left ventricular contractility measured as the AUC (1006%, 323%, and 263%, for 2.0 mg/kg IV over 10 minutes, 1.0 mg/kg IV over 2 minutes, and 1.5 mg/kg intratracheal bolus). Thus, we introduced the term “negative inotropic burden” to refer to the net impact of an anti-AF agent on cardiac contractility.
FIGURE 5.
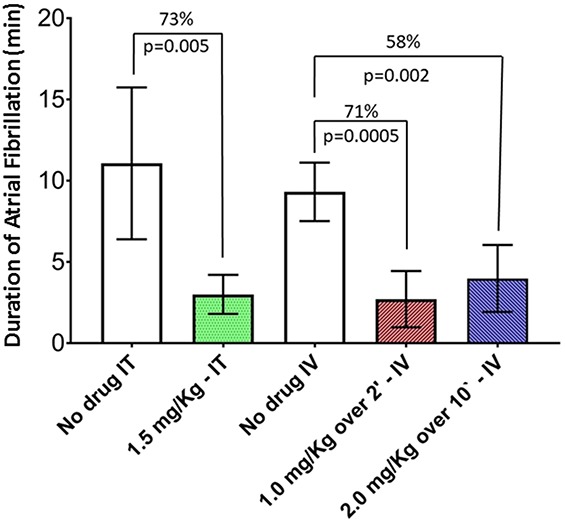
Duration of AF in the absence of drug and after 1.5-mg/kg bolus intratracheal instillation, 1.0-mg/kg IV infusion over 2 minutes, and 2.0-mg/kg IV infusion over 10 minutes of flecainide (n = 11). The reductions in AF duration were 73%, 71%, and 58%, respectively, compared to placebo. The lower dose, delivered using a rapid IV infusion, reduced AF duration to a greater extent than the higher dose, delivered at a slow IV infusion (P = 0.005). Values are represented as mean ± SEM. Published with permission from Elsevier from Marum et al 2018.24
FIGURE 6.
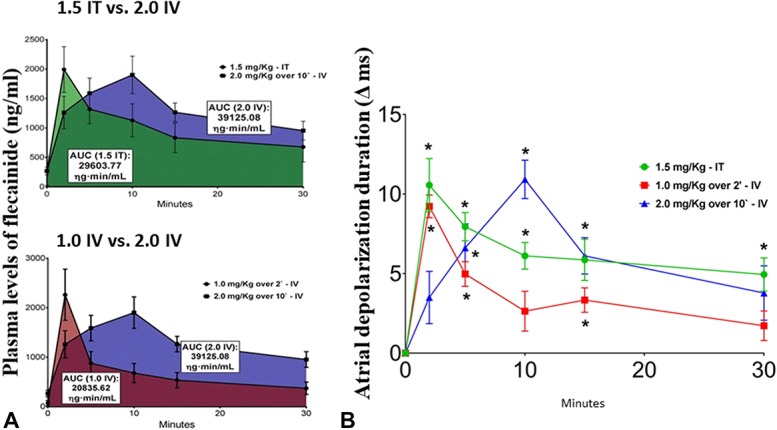
A, AUC for plasma flecainide levels after intratracheal (IT) instillation of 1.5-mg/kg bolus (N = 6) (green area) versus 2.0 mg/kg IV infusion over 10 minutes (blue area) (upper panel) as well as 1.0 mg/kg IV over 2 minutes (red area) versus 2.0 mg/kg IV infusion over 10 minutes (lower panel) (N = 6). The AUC is highest after the higher-dose, slow infusion. Each data point was calculated as mean ± SEM. B, Timecourse of changes in atrial depolarization (ΔPa) duration after administration of flecainide through intratracheal (IT) instillation of 1.5-mg/kg bolus (N = 6) (green filled circles), 1.0 mg/kg IV over 2 minutes (red filled squares), and 2.0 mg/kg IV infusion over 10 minutes (blue filled triangles) (N = 6) compared to no-drug baseline. Note the similar magnitudes of peak atrial depolarization prolongation after either intratracheal instillation or IV infusion of flecainide. Each data point represents mean ± SEM of the measurement. Significant differences from baseline using post hoc Dunnett's test are represented by the asterisk (P < 0.05). Published with permission from Elsevier from Marum et al 2018.24
Tessarolo Silva et al29 explored an additional approach to optimizing the effectiveness of pulmonary delivery of flecainide. The excipient hydroxypropyl-ß-cyclodextrin (HPßCD) was used to improve solubility of flecainide acetate to reduce the net delivered drug volume and to decrease the bitter taste of flecainide acetate. ß-cyclodextrin is a cyclic oligosaccharide that has been shown to have a wide range of applications because of its hydrophilic outer surface and lipophilic central cavity. The resulting benefit is to enhance solubility of drugs.30 Cyclodextrins have been shown to provide a means to form “host-guest” complexes with small molecules such as flecainide acetate. This novel formulation allowed drug concentrations in the inhalation solution to be increased from 35 to 45 mg/mL of flecainide acetate to 75 mg/mL of flecainide acetate in the flecainide HPßCD solution.
The flecainide cyclodextrin formulation was tested in the porcine model after induction of AF with intrapericardial acetylcholine and burst pacing. Plasma-concentration time-area analyses of flecainide HPßCD with intratracheal doses of 0.5 or 1.0 mg/kg were compared to the acetate formulation.29 Intratracheal instillation of flecainide HPßCD accelerated conversion of AF to normal sinus rhythm in a dose-proportional manner, shortening AF duration by 47% (P = 0.014) and 79% (P = 0.002) at the lower and higher doses, respectively, compared to intratracheal sterile water placebo. AF dominant frequency was also reduced as a function of dose. After intratracheal instillation of flecainide HPßCD, atrial depolarization duration was increased and PR interval was prolonged, indicating slowing of atrioventricular node conduction. There was a corresponding dose-dependent decrease in ventricular rate during AF.
Flecainide HPßCD solution seems to result in more efficient conversion of AF than the flecainide acetate solution, as indicated by a markedly reduced AUC. At the time of conversion, the AUC after intratracheal instillation of flecainide HPβCD (1.0 mg/kg) was 1759 ± 382 ng/mL/min or less than half of the AUC at the time of conversion after intratracheal instillation of flecainide acetate (0.5 mg/kg) at 4197 ± 756 ng/mL/min (*P = 0.03).29 Thus, although the dose of flecainide in the acetate formulation was 25% lower than the dose of flecainide in the HPβCD formulation, the time required to terminate AF (225%) and the AUC (239%) for flecainide acetate were more than double that of flecainide HPßCD.
On aggregate, these findings indicate that intratracheal instillation of the new flecainide HPßCD formulation can effectively terminate AF through efficient multimodal actions including slowing atrial conduction velocity and decreasing AF dominant frequency, allowing a greater net drug delivery and shorter inhalation time. Further implications of these findings are that the novel HPßCD formulation has the potential to deliver a higher dose of drug in a given time or even a shorter inhalation time, and thereby to facilitate administration of inhaled doses of flecainide.
Pulmonary Delivery of Metoprolol for AF Cardioversion
Beta-blockade is used primarily for heart rate control by slowing atrioventricular nodal conduction, which is often increased by enhanced adrenergic activity. Of note, increased adrenergic drive has been recurrently implicated in triggering and maintaining postoperative AF.31–34 The highest rate of postoperative AF occurs after pneumonectomy and lung transplantation, which can involve extensive dissection around the pulmonary veins,31,35 critical structures involved in the initiation of AF.36 Pulmonary vein sleeves are extensively innervated by sympathetic fibers37 and are exquisitely sensitive to adrenergic stimulation, thereby predisposing to AF.38,39 Beta-blockade, by suppressing these triggers, could potentially facilitate termination of AF. In our porcine model of AF, which induces markedly elevated ventricular rate and corresponding hypotension, it is reasonable to assume that sympathetic tone is elevated and thereby establishes the preconditions for enhanced efficacy of antiadrenergic agents.
In our studies, intratracheal instillation of metoprolol resulted in a 32-beat/min reduction in ventricular rate during AF (from 272 ± 13.7 to 240 ± 12.6 beats/min, P = 0.008) and a 2.3-minute decrease in AF duration (from 10.3 ± 2.0 to 8.0 ± 1.4 minutes, P = 0.018) compared to sterile water control.10 Conversion of AF to sinus rhythm was associated with rapid renormalization of heart rate and arterial blood pressure. Intratracheal metoprolol also significantly reduced AF dominant frequency.
Because of the well-known negative inotropic effects of beta-blockers, we monitored left ventricular contractile function to ascertain whether the dose and route of administration used might exhibit cardiodepressant actions. The study showed that the effect of the drug on left ventricular dP/dt was relatively minor, and accordingly, the beneficial effects of metoprolol to decrease ventricular rate during AF and to facilitate AF conversion were not associated with negative inotropy.
In summary, in this preclinical model, intratracheal delivery of metoprolol effectively reduced ventricular rate during AF and accelerated conversion to normal sinus rhythm. These salutary actions occurred without adverse effects on the contractile function of the heart.
CLINICAL EXPERIENCE WITH INHALED FLECAINIDE
The oral and IV routes for delivery of flecainide for acute cardioversion of AF to normal sinus rhythm have significant drawbacks, namely, relatively high doses and long latency of conversion, in the range of 2 to 4 hours with oral dosing and hospitalization for IV administration. These limitations have prompted the search for new methods for drug delivery, particularly oral inhalation of antiarrhythmic agents. Hence, the initial clinical studies investigated whether oral inhalation of flecainide could deliver sufficient drug to the systemic circulation to elicit its signature electrophysiologic effect, specifically, QRS complex prolongation.40,41
In these clinical studies, oral inhalation was performed using the AeroEclipse II breath-actuated nebulizer (BAN), which consists of a hand-held nebulizer that is driven by compressed medical air to convert liquid medication into an inhalable aerosol (Trudell Medical International, London, ON, Canada). The AeroEclipse-BAN set at the breath-actuate mode delivers a high respirable dose and an optimal particle size to reach the deeper lung regions, enabling faster drug absorption. Inhalable aerosolized medication or placebo is generated only when the subject inhales through the mouthpiece of the device.
This double-blind randomized, placebo-controlled study consisted of sequential single ascending estimated total lung doses (eTLDs) of flecainide solution or matched placebo administered to 34 normal healthy subjects.40,41 They were assigned to receive eTLDs of 20, 40, and 60 mg. The monitored variables included pulmonary and cardiac function using lung spirometry, continuous electrocardiogram, arterial blood pressure, and heart rate. The main findings were that inhaled flecainide caused dose-concentration-dependent increases in plasma drug levels and QRS complex prolongation. Specifically, the maximum increases in QRS complex duration were 3.5 ± 1.0, 10.1 ± 1.5, and 15.9 ± 1.4 ms. Prolongation of the QT interval was primarily due to widening of the QRS complex and hence there was minimum or no change in QT interval. These findings are similar to those reported for IV or oral flecainide.42 The half-lives for the distribution (3–4 minutes) and elimination (9–12 hours) phases of flecainide were dose-independent, similar to that reported for IV flecainide. Heart rate and blood pressure did not change. Subjects who received placebo exhibited minimum or no changes in QRS complex, QT interval duration, heart rate, and blood pressure. No serious adverse events were reported; the most common mild adverse events were oropharyngeal discomfort (40%), lightheadedness (12%), and cough (12%). These observations indicate that flecainide delivered through oral inhalation yields plasma drug levels that are sufficient to prolong the QRS complex in a dose-dependent manner, consistent with the established pharmacological and therapeutic effects of the drug when administered IV or orally.40,41
A second clinical study involved comparison of cardiovascular effects of inhaled versus IV flecainide.40,41 The design was open-label 2-period crossover study examining the effects on QRS complex and PR interval duration. Six healthy volunteers were randomized to receive flecainide either by IV infusion of 2 mg/kg over 10 minutes or oral inhalation over 4.2 ± 1.0 minutes for an eTLD of 30 mg. Subjects were monitored for pulmonary and cardiac function, and heart rate, blood pressure, and plasma flecainide levels were measured. The pharmacokinetic parameters for IV and inhaled flecainide had similar concentration-time profiles. The maximum increases in QRS complex duration were 36.2 ± 1.3 ms for IV compared to 14.5 ± 2.9 ms for inhaled flecainide. There were no serious adverse events. These observations indicate that low-dose inhaled flecainide is safe, well tolerated, and delivers flecainide into the systemic circulation within one minute with a PK profile similar to that of IV flecainide.15,42
Although these clinical studies did not examine the drug's effect of converting AF, the information derived from preclinical studies yielded promising inferences. In particular, although the primary measurement made was QRS complex duration, the experimental studies revealed that pulmonary delivery of flecainide has an even greater effect on atrial depolarization duration than on QRS complex duration. Specifically, in the intact porcine model, pulmonary delivery of flecainide has been shown to cause a rate-dependent predominant effect on atrial compared to ventricular depolarization duration.25 Thus, prolongation of QRS complex duration may have been associated with an even greater concomitant prolongation of atrial depolarization duration. This finding carries important implications because it has been shown that prolongation of atrial depolarization duration and the corresponding slowing of intra-atrial conduction are directly related to the efficacy of pulmonary delivery of flecainide in converting AF to normal sinus rhythm.17
GENERAL CONCLUSIONS AND FUTURE DIRECTIONS
Current preclinical data provide encouraging results regarding the effect of inhaled antiarrhythmic agents to convert AF rapidly to normal sinus rhythm. Multiple features inherent in this route of delivery are propitious and are summarized in Table 1. In particular, inhaled flecainide results in a rapid rise in plasma drug levels and presumably in atrial tissue drug levels. This action in turn confers the potential to flecainide or other antiarrhythmic drugs to alter atrial electrical properties, specifically, to reduce conduction velocity, suppress reentry, and facilitate termination of AF. The rapid rise and decline in plasma drug levels seems to reduce the adverse effects on ventricular electrical properties, specifically, QRS complex duration, and to minimize depression of left ventricular contractility, that is, the “negative inotropic burden,” as a result of reduced exposure time of the ventricular myocardium to the drug. Administering flecainide in solution containing the cyclodextrin incipient seems to allow an increase in the speed of drug delivery to atrial tissue with the potential to reduce inhalation time. Pulmonary delivery of metoprolol also seems to confer anti-AF effects in addition to the expected negative dromotropic effect, likely through antagonism of profibrillatory effects of adrenergic activity, which is prominent in the genesis of postoperative AF.
TABLE 1.
Summary of Key Findings
The next frontier is translation of these preclinical observations to the challenges of managing intermittent episodes of AF. The delivery of flecainide through oral inhalation yields plasma drug levels that are sufficient to prolong the QRS complex in a dose-concentration-dependent manner, consistent with the established pharmacological and therapeutic effects of the drug. The groundwork has thus been laid for studies in patients to determine the feasibility and potential efficacy of flecainide delivered through oral inhalation to convert acute AF episodes to normal sinus rhythm. Critical to this approach will be the tolerability and effectiveness of flecainide delivery through oral inhalation to patients while in AF. The goals of the phase II clinical trial “Inhalation of Flecainide to Convert Recent Onset SympTomatic AF to siNus rhyThm” (INSTANT) (NCT03539302) are the following: (1) to assess the feasibility of administration of various doses of flecainide (from 30 to 120 mg) delivered through oral inhalation; (2) to confirm safety and tolerability; and (3) to provide evidence of the potential efficacy to restore normal sinus rhythm in patients with recent onset (≤48 hours) of paroxysmal AF.
Footnotes
R. L. Verrier is principal investigator of grants from InCarda Therapeutics to Beth Israel Deaconess Medical Center. L. Belardinelli is an employee of InCarda Therapeutics.
REFERENCES
- 1.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. [DOI] [PubMed] [Google Scholar]
- 3.Alboni P, Botto GL, Baldi N, et al. Outpatient treatment of recent onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. [DOI] [PubMed] [Google Scholar]
- 4.Alboni P, Botto GL, Boriani G, et al. Intravenous administration of flecainide or propafenone in patients with recent-onset atrial fibrillation does not predict adverse effects during “pill-in-the-pocket” treatment. Heart. 2010;96:546–549. [DOI] [PubMed] [Google Scholar]
- 5.Murdock DK, Reiffel JA, Kaliebe J, et al. The conversion of paroxysmal or initial onset atrial fibrillation with oral ranolazine: implications for a new “pill-in-pocket” approach in structural heart disease. J Atr Fibrillation. 2010;2:705–710. [Google Scholar]
- 6.Andrade JG, MacGillivray J, Macle L, et al. Clinical effectiveness of a systematic pill-in-the-pocket approach for the management of paroxysmal atrial fibrillation. Heart Rhythm. 2018;15:9–16. [DOI] [PubMed] [Google Scholar]
- 7.Tjandra-Maga TB, Verbesselt R, Van Hecken A, et al. Flecainide: single and multiple oral dose kinetics, absolute bioavailability and effect of food and antacid in man. Br J Clin Pharmacol. 1986;22:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125:381–389. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhof P, Benussi S, Kotecha D, et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 10.Marum AA, Araujo Silva B, Bortolotto AL, et al. Pulmonary delivery of metoprolol reduces ventricular rate during atrial fibrillation and accelerates conversion to sinus rhythm. J Cardiovasc Pharmacol. 2020;75:135–140. [DOI] [PubMed] [Google Scholar]
- 11.Verrier RL, Fuller H, Justo FA, et al. Unmasking atrial repolarization to assess alternans, spatio-temporal heterogeneity, and susceptibility to atrial fibrillation. Heart Rhythm. 2016;13:953–961. [DOI] [PubMed] [Google Scholar]
- 12.Kumar K, Nearing BD, Carvas M, et al. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. [DOI] [PubMed] [Google Scholar]
- 13.Carvas M, Nascimento BCG, Acar M, et al. Intrapericardial ranolazine prolongs atrial refractory period and markedly reduces atrial fibrillation inducibility in the intact porcine heart. J Cardiovasc Pharmacol. 2010;55:286–291. [DOI] [PubMed] [Google Scholar]
- 14.Fuller H, Justo F, Nearing BD, et al. Eleclazine, a new selective cardiac late sodium current inhibitor, confers concurrent protection against autonomically induced atrial premature beats, repolarization alternans and heterogeneity, and atrial fibrillation in an intact porcine model. Heart Rhythm. 2016;13:1679–1686. [DOI] [PubMed] [Google Scholar]
- 15.Stocco FG, Evaristo E, Silva AC, et al. Comparative pharmacokinetic and electrocardiographic effects of intratracheal and intravenous administration of flecainide in anesthetized pigs. J Cardiovasc Pharmacol. 2018;72:129–135. [DOI] [PubMed] [Google Scholar]
- 16.Hellestrand KJ, Bexton RS, Nathan AW, et al. Acute electrophysiologic effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982;48:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verrier RL, Bortolotto AL, Silva BA, et al. Accelerated conversion of atrial fibrillation to normal sinus rhythm by pulmonary delivery of flecainide acetate in a porcine model. Heart Rhythm. 2018;15:1882–1888. [DOI] [PubMed] [Google Scholar]
- 18.Everett TH, Kok LC, Vaughn RH, et al. Frequency domain algorithm for quantifying atrial fibrillation organization to increase defibrillation efficacy. IEEE Trans Biomed Eng. 2001;48:969–978. [DOI] [PubMed] [Google Scholar]
- 19.Sardar MR, Saeed W, Kowey PR. Antiarrhythmic drug therapy for atrial fibrillation. Heart Fail Clin. 2016;12:205–221. [DOI] [PubMed] [Google Scholar]
- 20.Belardinelli L, Liu G, Smith-Maxwell C, et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. [DOI] [PubMed] [Google Scholar]
- 21.Sicouri S, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of the highly selective late sodium channel current blocker GS-458967. Heart Rhythm. 2013;10:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan SC, Antzelevitch C. Flecainide-induced arrhythmia in canine ventricular epicardium: phase 2 reentry? Circulation. 1993;87:562–572. [DOI] [PubMed] [Google Scholar]
- 23.Deneer VHM, Lie-A-Huen L, Kingma JH, et al. Absorption kinetics and pharmacodynamics of two oral dosage forms of flecainide in patients with an episode of paroxysmal atrial fibrillation. Eur J Clin Pharmacol. 2004;60:693–701. [DOI] [PubMed] [Google Scholar]
- 24.Marum AA, Araujo Silva B, Bortolotto AL, et al. Optimizing flecainide plasma concentration profile for atrial fibrillation conversion while minimizing adverse ventricular effects by rapid, low-dose intratracheal or intravenous administration. Int J Cardiol. 2018;274:170–174. [DOI] [PubMed] [Google Scholar]
- 25.De Antonio VZ, Silva AC, Stocco FG, et al. Pulmonary delivery of flecainide causes a rate-dependent predominant effect on atrial compared to ventricular depolarization duration revealed by intracardiac recordings in an intact porcine model. J Cardiovasc Electrophysiol. 2018;29:1563–1569. [DOI] [PubMed] [Google Scholar]
- 26.Burashnikov A, Di Diego JM, Zygmunt AC, et al. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin Emerg Drugs 2009;14:233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burashnikov A, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block strategy to suppress atrial fibrillation: ranolazine versus propafenone. J Pharmacol Exp Ther. 2012;340:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessarolo Silva F, Pedreira GC, Medeiros SA, et al. Multimodal mechanisms for rapid conversion of atrial fibrillation by pulmonary delivery of a novel flecainide formulation. J Cardiovasc Electrophysiol. 2020;31:205–213. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari G, Tiwari R, Rai AK. Cyclodextrins in delivery systems: Applications. J Pharm Bioallied Sci. 2010;2:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt RE, Shrager JB. Prophylaxis and management of atrial fibrillation after general thoracic surgery. Thorac Surg Clin. 2012;22:13–23. [DOI] [PubMed] [Google Scholar]
- 32.Omae T, Kanmura Y. Management of postoperative atrial fibrillation. J Anesth. 2012;26:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadava M, Hughey AB, Crawford TC. Postoperative atrial fibrillation: incidence, mechanisms, and clinical correlates. Cardiol Clin. 2014;32:627–636. [DOI] [PubMed] [Google Scholar]
- 34.Bessissow A, Khan J, Devereaux PJ, et al. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost. 2015;13(suppl 1):S304–S312. [DOI] [PubMed] [Google Scholar]
- 35.Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127:779–786. [DOI] [PubMed] [Google Scholar]
- 36.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 37.Tan AY, Li H, Wachsmann-Hogiu S, et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. [DOI] [PubMed] [Google Scholar]
- 38.Sicouri S, Glass A, Belardinelli L, et al. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen PS, Chen LS, Fishbein MC, et al. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belardinelli L, Rangachari NE, Verrier RL, et al. A novel approach to deliver flecainide to the heart: a study in healthy volunteers to compare the cardiovascular effects of inhaled vs intravenous flecainide (abstract). Circulation. 2017;136:A16888. [Google Scholar]
- 41.Belardinelli L, Rangachari NE, Schuler CA, et al. Hand-held breath-actuated nebulizer for delivery of flecainide to the heart: dose-concentration dependent pharmacokinetics of inhaled flecainide and QRS interval prolongation in healthy volunteers (abstract). Circulation. 2017;136:A16836. [Google Scholar]
- 42.Aliot E, Capucci A, Crijns HJ, et al. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]