ABSTRACT
Background:
Ivabradine selectively inhibits the If current, reducing the heart rate and protecting against myocardial ischemia/reperfusion injury. We investigated the effects of ivabradine on post-resuscitation myocardial function in a porcine model of cardiopulmonary resuscitation.
Methods and Results:
Ventricular fibrillation was induced and untreated for 8 min while defibrillation was attempted after 6 min of cardiopulmonary resuscitation in anesthetized domestic swine. Then the animals were randomized into ivabradine and placebo groups (n = 5 each). Ivabradine and saline were administered at the same volume 5 min after Return of Spontaneous Circulation, followed by continuous intravenous infusion at 0.5 mg/kg for 480 min. Hemodynamic parameters were continuously recorded. Myocardial function was assessed by echocardiography at baseline and at 60, 120, 240, 480 min and 24 h after resuscitation. The serum levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiac troponin I (cTnI) were measured by commercial enzyme-linked immunosorbent assay kits. Animals were killed 24 h after resuscitation, and all myocardial tissue was removed for histopathological analysis. The heart rate was significantly reduced from 1 h after resuscitation in the ivabradine group (all P < 0.05). The post-resuscitation mitral E/A and E/e′ velocity ratios and left ventricular ejection fraction were significantly better in the ivabradine than placebo group (P < 0.05). The serum levels of myocardial injury biomarkers (NT-proBNP, cTnI) and the myocardial biopsy scores were significantly lower in the ivabradine than placebo group (P < 0.05). Neurological deficit scores were lower in the IVA group at PR 24 h (P < 0.05).
Conclusions:
Ivabradine improved post-resuscitation myocardial dysfunction, myocardial injury, and post-resuscitation cerebral function, and also slowed the heart rate in this porcine model.
Keywords: Cardiac arrest, cardioprotection, ivabradine, myocardial dysfunction, porcine
INTRODUCTION
Cardiac arrest (CA) remains a major public health concern worldwide. Even though many efforts to improve the outcomes of CA, survival rate still low. Global ischemia and reperfusion injury induced by cardiopulmonary resuscitation cardiopulmonary resuscitation (CPR) causes so-called post-resuscitation syndrome (1). Furthermore, post-resuscitation myocardial dysfunction (PRMD) is considered the leading cause of death within 72 h after successful CPR in the animal studies (2, 3). Therefore, studies of new medications that aim to improve PRMD are of great urgency and importance.
The β1-adrenergic actions of epinephrine increase the heart rate (HR), increase myocardial oxygen consumption, and decrease myocardial perfusion in the rat model (4). Our previous animal experiments showed that epinephrine increased the severity of post-resuscitation (PR) myocardial tissue injury [cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP)] and dysfunction. Additionally, β-blocker and β-plus α1-blocker pretreatment significantly reduced the severity of PR myocardial tissue injury and myocardial dysfunction with better neurologic function and a prolonged duration of survival (5, 6). However, β-adrenergic blockers have been associated with higher survival rates only in animal studies and observational clinical studies. Administration of β-blockers can induce hemodynamic instability and bradyarrhythmia and exacerbate heart failure. Therefore, the 2015 American Heart Association guideline suggested that inadequate evidence exists to support the routine use of β-blockers after cardiac arrest. Ivabradine is FDA-approved for use in adults for the following indication to provide clinical relevance: “Ivabradine was indicated to reduce the risk of hospitalization for worsening heart failure in adult patients with stable, symptomatic chronic heart failure with left ventricular ejection fraction ≤ 35%, who are in sinus rhythm with resting heart rate ≥ 70 beats per minute and either are on maximally tolerated doses of beta-blockers or have a contraindication to beta-blocker use.” Ivabradine (IVA) selectively inhibits the If current by delaying the diastolic depolarization slope of sinoatrial node cells to reduce the HR and protect against myocardial ischemia/reperfusion injury. IVA has been demonstrated to decrease the morbidity and mortality associated with cardiovascular diseases (7) with significantly decreased myocardial oxygen consumption, improved left ventricular systolic function, and decreased left ventricular hypertrophy, fibrosis, and inflammation markers (8–10). And therefore, in the present study, we investigated the protective effects and potential mechanisms of IVA on PRMD in a porcine model of CA and CPR.
METHODS
All animals were treated with humane care in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People's Republic of China. Our protocol was approved by the Institutional Animal Care and Use Committee of Anhui Medical University and Sun Yat-sen University.
Animal preparation
Ten healthy male domestic pigs weighing 35 kg to 40 kg (3–4 mo) were used in this study. The strain of those pigs is mixed breed. Those animals were supplied by a single source breeder (Experimental Animal Center of Sun Yat-sen University, Guangzhou, China). Anesthesia was induced by intramuscular administration of midazolam (0.5 mg/kg), the calculated dose of pentobarbital (30 mg/kg) was then injected by ear vein, and an additional dose of sodium pentobarbital (8 mg/kg) was administered hourly. Endotracheal tubes (TaperGuard Evac; Covidien, Dublin, Ireland) were advanced into the trachea. All animals were mechanically ventilated by a volume-controlled ventilator (Viasys T-Bird VELA; Vyaire Medical, Mettawa, Ill). End-tidal carbon dioxide (ETCO2) was monitored by an infrared capnometer (BeneView T5; Mindray Inc, Shenzhen, China) and maintained at 35 mm Hg to 40 mm Hg before CA. The preparation of animals was performed according to the same procedure in our previous study (11). Three adhesive electrodes were adhered to the shaved skin for continuous recording of the electrocardiogram. A 6-Fr transducer-tipped Millar catheter (Vista Brite Tip Guiding Catheter; Cordis, Fremont, Calif) was advanced from the right femoral artery into the thoracic aorta for the measurement of the aortic pressure and blood gases. A 7-Fr catheter (774HF75 Swan-Ganz TD Catheter; Edwards Lifesciences, Irvine, Calif) was advanced to the right atrium for the measurement of the right atrial pressure and blood temperature. Each of the catheters was intermittently flushed with saline containing 5 IU/mL of bovine heparin. A 5-Fr pacing catheter (EP Technologies, Akron, Ohio) was advanced from the right external jugular vein into the right ventricle to induce ventricular fibrillation. The compression position was located at the midline at the level of the fifth interspace with a black marking pen. The body temperature of the animals was maintained at 38.0°C ± 0.5°C during the experiment by a cooling/warming blanket (Model HGT-200 II; Hejia Medical Equipment Co, Ltd, Zhuhai, China).
Experimental procedures
Twenty minutes before inducing ventricular fibrillation, 10 animals were randomized into two groups (IVA and placebo groups, n = 5 each) by the sealed envelope method. In the IVA group, a 5-mg loading dose of IVA was administered by right femoral vein injection 5 min after return of spontaneous circulation (ROSC), and IVA was then infused at 0.5 mg/kg for 480 min (12, 13). In the placebo group, saline at the same volume as in the IVA group was administered as a bolus injection into the right femoral vein at the same time. Thirty minutes before inducing ventricular fibrillation, baseline measurements and arterial blood gas values were obtained. Fifteen minutes before inducing ventricular fibrillation, mechanical ventilation was established at a tidal volume of 10 mL/kg, peak flow of 40 L/min, and FiO2 of 0.21. Ventricular fibrillation was then induced by a 1-mA alternating current through a 5-Fr pacing catheter delivered to the ventricular endocardium, and untreated ventricular fibrillation was maintained for 8 min. Mechanical ventilation was discontinued after the onset of ventricular fibrillation. The pacing catheter was evacuated from the right ventricle during precordial compression to avoid heart injury. CPR was started after 8 min of untreated ventricular fibrillation. A mechanical chest compressor (Weil SCC; Sunlife Science Shanghai, China) was used to provide 100 compressions/min at the fifth intercostal space. The compression depth was adjusted to decrease the anteroposterior diameter of the chest by 25%. With the beginning of precordial compression, all animals were mechanically ventilated at a tidal volume of 10 mL/kg, FiO2 of 1.0, and rate of 10 breaths/min. After 2 min of precordial compression, 20 μg/kg of epinephrine was administered via the femoral vein. After 6 min of precordial compression, defibrillation was attempted with a single 120-J biphasic electrical shock delivered between the right infraclavicular electrode and the precordial electrode with a defibrillator (ZOLL M Series; ZOLL Medical Corporation, Chelmsford, Mass) to terminate VF. ROSC was defined as restoration of an organized cardiac rhythm with a mean arterial pressure (MAP) of > 50 mm Hg and persistence for at least 5 min. If ROSC was not achieved, chest compression and ventilation were immediately resumed for 2 min again prior to the second single shock. The procedure was repeated for a maximum of five cycles. Additional doses of epinephrine were given at 3-min intervals after the first administration. If signs of circulation were still absent, the resuscitation maneuvers were terminated. After ROSC, the animals were monitored for an additional 480 min. Mechanical ventilation was continued with 100% inspired oxygen for the first 30 min, 50% for the second 30 min, and 21% until the end of the observation. After the 480 min period, all catheters were removed. The wounds were surgically repaired and the animals were extubated and returned to their cages. After PR 24 h, the animals were killed with an intravenous injection of 150 mg/kg of pentobarbital, followed by a lethal dose of potassium chloride, and the hearts were excised. Left ventricular myocardial tissues were removed for hematoxylin and eosin (HE) biopsy score analysis.
Measurements
The HR, MAP, and ETCO2 were monitored and recorded on a personal computer-based data acquisition system containing WinDaq hardware/software (DATAQ Instruments Inc, Akron, Ohio). Blood samples were taken at baseline and at PR 60, 120, 240, and 480 min. Arterial blood gases were measured by a Stat Profile pHOx Plus analyzer (Model RADIOMETER ABL80 FLEX; Radiometer Medical APS, Copenhagen, Denmark) at baseline and at PR 60, 120, 240, and 480 min with a 0.5-mL arterial blood sample. The coronary perfusion pressure was digitally calculated based on the difference between the time-coincident diastolic aortic and right atrial pressures.
Myocardial systolic and diastolic function parameters including the LV ejection fraction (LVEF), mitral E/A ratio, and E/e′ velocity ratio were measured by echocardiography (ACUSON sc2000; Siemens, Munich, Germany) at baseline and at PR 60, 120, 240, 480 min and 24 h (14). The blood samples were withdrawn at baseline and at PR 60, 240 min and 24 h and centrifuged at 3,000 r/min for 15 min, and the serum was stored for further analysis. The serum levels of cTnI and NT-proBNP were measured by commercial enzyme-linked immunosorbent assay kits (Wuhan CUSABIO Biotech Industry Co, Ltd, Wuhan, China).
Neurological function was evaluated according to the neurological deficit score, which ranged from 0 (no observed neurological deficit) to 400 (death or brain death) (15). The neurological deficit scores were confirmed by two investigators blinded to the treatment at PR 24 h.
Myocardial tissues were fixed in formalin and embedded in paraffin. These blocks were sectioned at 5-μm thickness and subjected to HE staining. Myocardial morphological injury was observed under a light microscope (ZEISS Axioskop 2 plus; ZEISS, Oberkochen, Germany) by a pathologist who was blinded to our study. We calculated the myocardial histological injury according to previously described morphological criteria (16). Eight random fields of the LV endomyocardium for each sample were evaluated.
Statistical analysis
Normally distributed data are presented as mean ± standard deviation, and non-normally distributed data are presented as median (25th, 75th percentiles). Time-based measurements within each group were compared by the paired t test. All data were analyzed using one-way analysis of variance. A P value of < 0.05 was considered statistically significant.
RESULTS
Ten animals were used in our research. There were no significant differences in the baseline characteristics between the IVA and placebo groups (Table 1).
Table 1.
Baseline measurements and primary resuscitation outcomes
| Measurements | CPR+IVA | CPR+placebo |
| Body wt. (kg) | 38.00 ± 1.00 | 38.06 ± 1.40 |
| HR (beats/min) | 144.00 ± 15.03 | 153.80 ± 6.42 |
| MAP (mm Hg) | 112.40 ± 7.92 | 109.20 ± 9.76 |
| ETCO2 (mm Hg) | 37.60 ± 1.14 | 40.60 ± 2.70 |
| ROSC | 5/5 | 5/5 |
| Total shocks | 2.20 ± 0.84 | 2.40 ± 0.89 |
| LVEF% | 62.41 ± 2.76 | 62.05 ± 2.96 |
| E/A | 0.93 ± 0.29 | 0.96 ± 0.18 |
| E/e′ | 6.19 ± 1.11 | 6.75 ± 1.14 |
Values are presented as mean ± standard deviation.
Body wt. indicates body weight; E/A and E/e′, mitral E/A ratio and E/e′ velocity ratio; ETCO2, end-tidal carbon dioxide; HR, heart rate; LVEF, left ventricular ejection fraction; MAP, mean arterial pressure; ROSC, return of spontaneous circulation; Total shocks, the number of shocks during CPR.
The HR was remarkably higher after ROSC than at baseline in both groups. In the IVA group, the HR significantly decreased from PR 60 to 480 min with a reduction of 30 to 50 beats/min (P < 0.05). There were no significant differences in the MAP, ETCO2, or and pH between the two groups at PR 60, 120, 240, and 480 min (P < 0.05) (Fig. 1).
Fig. 1.
IVA reduced the heart rate without an impact on hemodynamic parameter outcomes after return of spontaneous circulation.
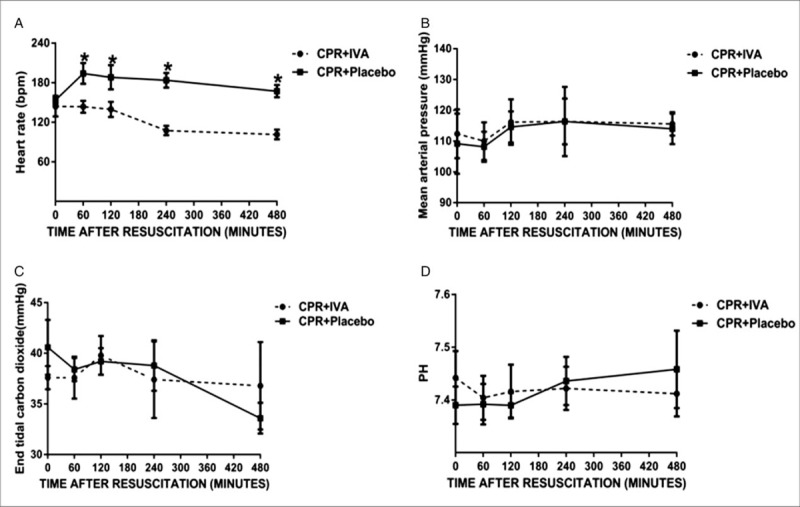
(A) Heart rate, (B) mean arterial pressure, (C) end-tidal carbon dioxide, (D) pH. ∗P < 0.05 versus CPR + Placebo group with administration of saline (5 mg) at 5 min after return of spontaneous circulation, followed by continuous infusion (0.5 mg/kg) for 480 min. IVA indicates ivabradine; CPR, cardiopulmonary resuscitation.
At PR 24 h, the HE biopsy score was significantly increased in the placebo group (P < 0.05) (Fig. 2A). The IVA group showed significantly better morphological characteristics than the placebo group as indicated by alleviation of myocardial cell swelling, interstitial edema, necrosis, contraction band, and infiltration of inflammatory cells (Fig. 2B).
Fig. 2.
IVA improved post-resuscitation myocardial morphological injury.
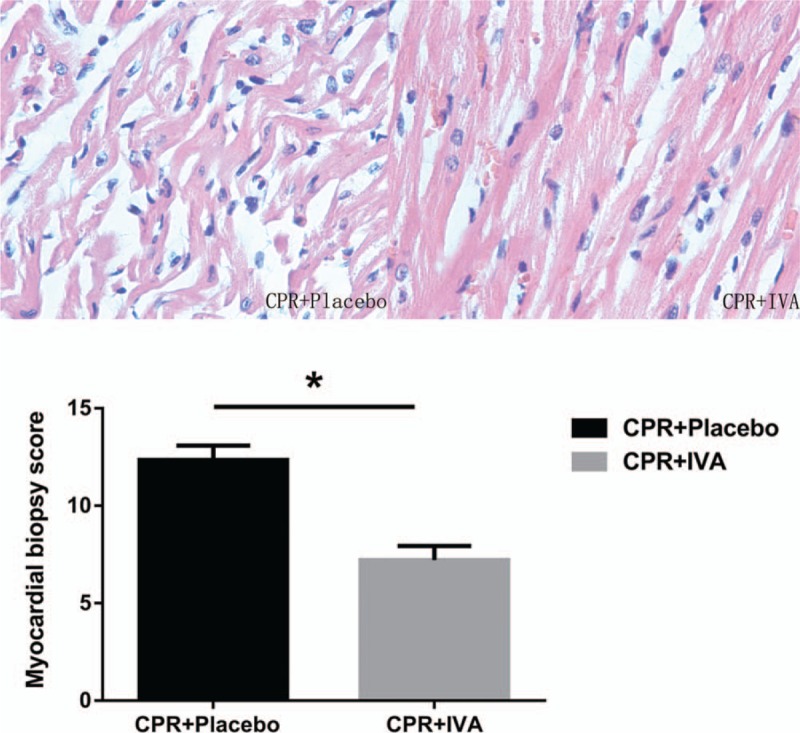
Images represent hematoxylin–eosin staining of left ventricular myocardial tissues (×400). ∗P < 0.05 versus CPR + Placebo group at post-resuscitation 24 h. IVA indicates ivabradine; CPR, cardiopulmonary resuscitation.
The parameters of cardiac function (LVEF, E/A, and E/e′) after ROSC were significantly worse than those at baseline in all animals. LVEF, E/A, and E/e′ were significantly better in the IVA group at PR 60, 120, 240, 480 min and 24 h (P < 0.05) (Fig. 3). E/A, E/e′, and LVEF were well correlated with HR at PR 240 min (r = −0.832, P < 0.01; r = 0.905, P < 0.01; and r = −0.70, P < 0.05, respectively).
Fig. 3.
IVA improved post-resuscitation myocardial dysfunction.
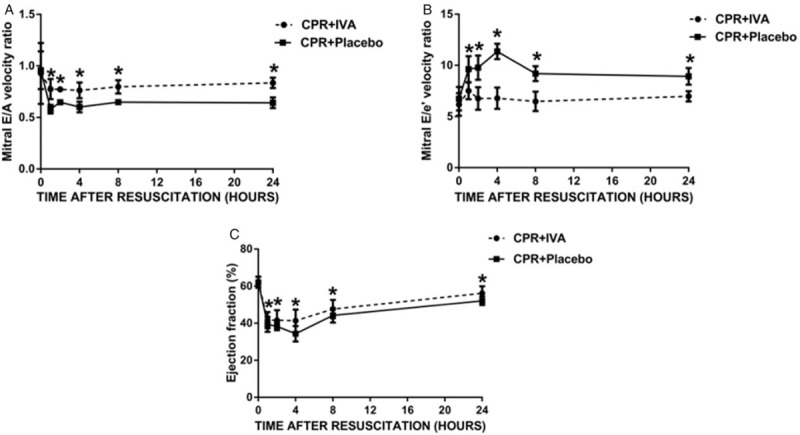
A, Mitral E/A velocity ratio. B, Mitral E/e′ velocity ratio. C, Ejection fraction. ∗P < 0.05 versus CPR + Placebo group at post-resuscitation 1, 2, 4, and 8 h. IVA indicates ivabradine; CPR, cardiopulmonary resuscitation.
The serum levels of cTnI and NT-proBNP were dramatically elevated from PR 60 to 240 min and then decreased at PR 24 h in both groups (Fig. 4). The levels of cTnI and NT-proBNP were significantly lower in the IVA than placebo group at the same time points (P < 0.05).
Fig. 4.
IVA improved post-resuscitation myocardial injury biomarkers.
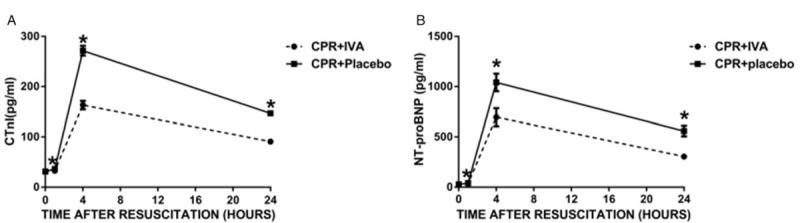
(A) cTnI, (B) NT-proBNP. ∗P < 0.05 versus CPR + Placebo group at post-resuscitation 1, 4, and 24 h. cTnI indicates cardiac troponin I; NT-proBNP, N-terminal pro-brain natriuretic peptide; IVA, ivabradine; CPR, cardiopulmonary resuscitation.
There was no significant difference in the duration of survival between the two groups (P > 0.05). Lower neurological deficit scores were observed in the IVA group at PR 24 h (Fig. 5).
Fig. 5.
IVA improved post-resuscitation cerebral function.
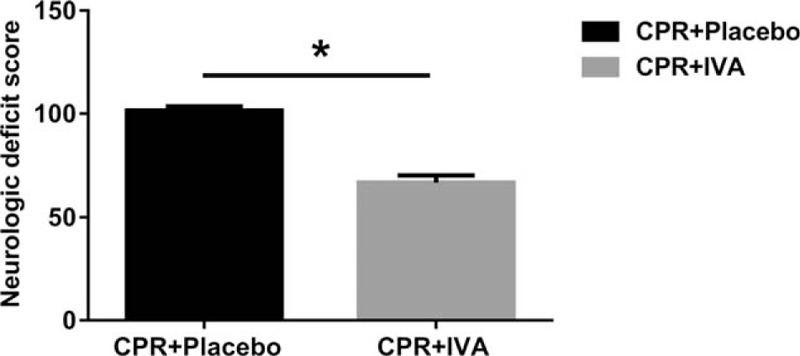
∗P < 0.05 versus CPR + Placebo group at post-resuscitation 24 h. IVA indicates ivabradine; CPR, cardiopulmonary resuscitation.
DISCUSSION
The present study demonstrated that administration of IVA significantly reduced the PR HR, improved the PR LV systolic and diastolic function, and attenuated myocardial-neurological injury in a porcine model of CA and resuscitation. The serum levels of cTnI and NT-proBNP were significantly decreased in the IVA group. Myocardial function was significantly correlated with the alterations in the PR HR in the IVA group.
The severity of PRMD is the major cause of early death, and no effective treatment method has been established. Actively protective treatment to myocardial function is urgent to improve postresuscitation outcomes. Both animal and clinical studies have consistently demonstrated that the severity of PRMD is closely associated with the duration of ischemia, number of electrical defibrillations, and dose of epinephrine (5, 17). Our study demonstrated dramatic impairments in the LVEF, E/A, and E/e′ at 60 min after resuscitation in the placebo group. Our study focused on the deterioration of diastolic dysfunction. Tissue Doppler (TD) imaging is a practical clinical method to assess myocardial relaxation function by measuring the mitral annulus velocity during diastole (18). The mitral flow velocity reflects LV diastolic function and is acquired from the apical four-chamber view at the mitral annulus level. LV diastolic filling abnormalities show a restrictive-type filling pattern, including high E, low A, and short deceleration time, and these findings are helpful in the diagnosis of dilated cardiomyopathy (19). An elevated E/e′ is recognized as a clinical marker of elevated LV diastolic pressure, and an abnormal E/e′ is evidence of diastolic heart failure (20). In previous studies, because the E and e’ increased proportionally, the E/e’ ratio of the middle-aged healthy group did not change after exercise, while the E/e′ ratio increased significantly in patients with impaired myocardial relaxation because the increase in e′ with exercise is much less than the increase in mitral E velocity (18, 21). Our study showed that mitral E/A was decreased and E/e′ was increased after CA and resuscitation. We also demonstrated that all animals had different degrees of myocardial injury after CA and resuscitation. Our study showed that the impairment of myocardial dysfunction and injury could be reversed by IVA.
In the present study, we further investigated the potential mechanisms underlying the protective effect of IVA against PRMD. The myocardial oxygen consumption of every heart cycle is quite constant and increases proportionally with the HR. The duration of the cardiac diastolic phase, during which coronary blood flow occurs, shortens over proportionately with an increase in the HR. PR syndrome has been suggested to be a sepsis-like syndrome that induces sympatho-adrenal hyperactivation. Excessive endogenous and exogenous epinephrine during CA and CPR accelerates the HR, and this is probably the cause of the tachycardia in PR syndrome. A retrospective study showed that tachycardia after ROSC was significantly associated with a poor prognosis (22). Myocardial oxygen extraction increasing and following metabolic coronary vasodilation are difficult to match an increased oxygen supply to the increased oxygen consumption with increased HR. The β-adrenergic effect of epinephrine dramatically increased the HR, increased myocardial oxygen consumption, reduced the isovolumetric relaxation time and diastolic filling, impaired coronary perfusion, and finally resulted in LV dysfunction (23) and heart failure (24, 25). The animal studies also demonstrated that the accelerated HR exacerbated vascular oxidative stress, endothelial dysfunction, atherogenesis, and vascular stiffness (8, 26), which are harmful to the recovery of heart function. To some extent, HR reduction is a preferred treatment trend.
The multivariate regression analysis indicated that a reduced HR was independently associated with a better outcome (27). Administration of β-blockers is associated with good outcomes in patients with myocardial infarction (28). A cohort study showed that PR patients with bradycardia had a better LVEF and less heart failure than PR patients without bradycardia (29). A lower HR during PR hypothermia therapy protected the heart probably by prolonging the diastolic time and reducing oxygen consumption (30, 31).
IVA reduces the HR by selectively inhibiting the If current, which prolongs myocardial diastole and simultaneously enhances coronary blood flow and myocardial perfusion. This in turn reduces the myocardial oxygen demand and improves the oxygen supply (32–34). IVA decreases the HR without a negative inotropic or lusitropic effect. The study demonstrated that IVA significantly decreased the HR with a reduced LV contraction diameter, increased the stroke volume, and improved LV function. Our results confirmed that IVA significantly decreased the PR HR and severity of PRMD without an impact on MAP. We also demonstrated that LVEF, E/A, and E/e′ were significantly related to PR HR. Thus, we hypothesized that IVA improved PR myocardial systolic and diastolic function by decreasing the HR.
Our previous study demonstrated that the serum levels of cTnI and NT-proBNP were significantly increased after ROSC in a rat CPR model (6). Our study also demonstrated that cTnI and NT-proBNP were significantly increased after ROSC in porcine CPR model. The concentrations of cTnI and NT-proBNP were significantly lower in the IVA group at each time point after ROSC. These results confirm that administration of IVA reduces myocardial ischemia/reperfusion injury. Our experiment not only provided new ideas and targeting point for the treatment of PRMD, but also indicated important significance and clinical application for improving the prognosis of CA and CPR patients.
An additional hypothesis to explain the cardioprotective effects of IVA after CA and CPR is a reduction in the formation of reactive oxygen species in the mitochondria, which in turn reduces myocardial oxidative damage and vascular endothelial dysfunction (35, 36).
This study had several limitations. First, our porcine model of CA and resuscitation does not fully represent clinical conditions in humans, although it has some cardiovascular and pathophysiologic features similar to those in humans. Second, the survival rate of animals needs to be investigated, and larger randomized studies should be performed. Third, the long-term treatment outcomes and prognosis of IVA therapy after ROSC require further studies. Finally, the optimal dose and target HR management of IVA in the clinical setting are worthy of further studies.
CONCLUSIONS
IVA improved the severity of PRMD and myocardial injury in a porcine CPR model, especially diastolic function (mitral E/A and E/e′ velocity ratio), as measured by a noninvasive tissue Doppler. IVA has the potential to improve PRMD in patients with clinical conditions induced by CPR.
Footnotes
This study was supported by the National Natural Science Foundation of China (No. 81601661), Natural Science Foundation of Anhui Province of China (No. 1608085MH195), and Science Foundation for Post-doctoral Researchers in Anhui Province of China (No. 2016B140).
The authors report no conflicts of interest.
REFERENCES
- 1.Hassager C, Nagao K, Hildick-Smith D. Out-of-hospital cardiac arrest: in-hospital intervention strategies. Lancet 391 (10124):989–998, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Xu T, Tang W, Ristagno G, Wang H, Sun S, Weil MH. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med 36 (1):188–192, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Liang LN, Zhong X, Zhou Y, Hou ZQ, Hu HR, Zhu FF, Chen JB, Ji XF, Shang DY. Cardioprotective effect of nicorandil against myocardial injury following cardiac arrest in swine. Am J Emerg Med 35 (8):1082–1089, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Hua T, Li H, Wu X, Bradley J, Peberdy MA, Ornato JP, Tang W. Decreased cAMP level and decreased downregulation of β1-adrenoceptor expression in therapeutic hypothermia-resuscitated myocardium are associated with improved post-resuscitation myocardial function. J Am Heart Assoc 7 (6):e006573, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang M, Hu X, Lu X, Wu X, Xu J, Yang Z, Qian J, Sun S, Cahoon J, Tang W. The effects of α- and β-adrenergic blocking agents on postresuscitation myocardial dysfunction and myocardial tissue injury in a rat model of cardiac arrest. Transl Res 165 (5):589–598, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation 92 (10):3089–3093, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Riccioni G. Ivabradine: recent and potential applications in clinical practice. Expert Opin Pharmacother 12 (3):443–450, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Custodis F, Reil JC, Laufs U, Böhm M. Heart rate: a global target for cardiovascular disease and therapy along the cardiovascular disease continuum. J Cardiol 62 (3):183–187, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Roubille F, Tardif JC. New therapeutic targets in cardiology: heart failure and arrhythmia: HCN channels. Circulation 127 (19):1986–1996, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Becher PM, Lindner D, Miteva K, Savvatis K, Zietsch C, Schmack B, Van Linthout S, Westermann D, Schultheiss HP, Tschöpe C. Role of heart rate reduction in the prevention of experimental heart failure: comparison between If-channel blockade and β-receptor blockade. Hypertension 59 (5):949–957, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Tang D, Wu X, Hu J, Qian J, Yang M, Tang W. A tourniquet assisted cardiopulmonary resuscitation augments myocardial perfusion in a porcine model of cardiac arrest. Resuscitation 86:49–53, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Vaillant F, Timour Q, Descotes J, Manati W, Belhani D, Bui-Xuan B, Tabib A, Bricca G, Chevalier P. Ivabradine induces an increase in ventricular fibrillation threshold during acute myocardial ischemia: an experimental study. J Cardiovasc Pharmacol 52 (6):548–554, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Thollon C, Bidouard JP, Cambarrat C, Lesage L, Reure H, Delescluse I, Vian J, Peglion JL, Vilaine JP. Stereospecific in vitro and in vivo effects of the new sinus node inhibitor (+)-S 16257. Eur J Pharmacol 339 (1):43–51, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29 (4):277–314, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Berg RA, Otto CW, Kern KB, Sanders AB, Hilwig RW, Hansen KK, Ewy GA. High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit Care Med 22 (2):282–290, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Suh GJ, Kwon WY, Kim KS, Rhee JE, Kim MA, Park MH. Protective effects of therapeutic hypothermia in post-resuscitation myocardium. Resuscitation 83 (5):633–639, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Palmer BS, Hadziahmetovic M, Veci T, Angelos MG. Global ischemic duration and reperfusion function in the isolated perfused rat heart. Resuscitation 62 (1):97–106, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 18 (1):63–68, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation 90 (6):2772–2779, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28 (20):2539–2550, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ha JW, Lulic F, Bailey KR, Pellikka PA, Seward JB, Tajik AJ, Oh JK. Effects of treadmill exercise on mitral inflow and annular velocities in healthy adults. Am J Cardiol 91 (1):114–115, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Torgersen C, Meichtry J, Schmittinger CA, Bloechlinger S, Jakob SM, Takala J, Dünser MW. Haemodynamic variables and functional outcome in hypothermic patients following out-of-hospital cardiac arrest. Resuscitation 84 (6):798–804, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H. Influence of the force-frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J 15 (2):164–170, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Böhm M. Pathophysiology of heart failure today. Herz 27 (2):75–91, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Umana E, Solares CA, Alpert MA. Tachycardia-induced cardiomyopathy. Am J Med 114 (1):51–55, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Custodis F, Schirmer SH, Baumhäkel M, Heusch G, Böhm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 56 (24):1973–1983, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Oksanen T, Tiainen M, Vaahersalo J, Bendel S, Varpula T, Skrifvars M, Pettilä V, Wilkman E. FINNRESUSCI Study Group. Lower heart rate is associated with good one-year outcome in post-resuscitation patients. Resuscitation 128:112–118, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Sterling LH, Filion KB, Atallah R, Reynier P, Eisenberg MJ. Intravenous beta-blockers in ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Int J Cardiol 228:295–302, 2017. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen JH, Nielsen N, Hassager C, Wanscher M, Pehrson S, Køber L, Bro-Jeppesen J, Søholm H, Winther-Jensen M, Pellis T, et al. Bradycardia during targeted temperature management: an early marker of lower mortality and favorable neurologic outcome in comatose out-of-hospital cardiac arrest patients. Crit Care Med 44 (2):308–318, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Post H, Schmitto JD, Steendijk P, Christoph J, Holland R, Wachter R, Schöndube FW, Pieske B. Cardiac function during mild hypothermia in pigs: increased inotropy at the expense of diastolic dysfunction. Acta Physiol (Oxf) 199 (1):43–52, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Jacobshagen C, Pelster T, Pax A, Horn W, Schmidt-Schweda S, Unsöld BW, Seidler T, Wagner S, Hasenfuss G, Maier LS. Effects of mild hypothermia on hemodynamics in cardiac arrest survivors and isolated failing human myocardium. Clin Res Cardiol 99 (5):267–276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riccioni G. Ivabradine: from molecular basis to clinical effectiveness. Adv Ther 27 (3):160–167, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Mengesha HG, Tafesse TB, Bule MH. If channel as an emerging therapeutic target for cardiovascular diseases: a review of current evidence and controversies. Front Pharmacol 8:874, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucchi A, Barbuti A, Baruscotti M, DiFrancesco D. Heart rate reduction via selective ’funny’ channel blockers. Curr Opin Pharmacol 7 (2):208–213, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Kleinbongard P, Gedik N, Witting P, Freedman B, Klöcker N, Heusch G. Pleiotropic, heart rate-independent cardioprotection by ivabradine. Br J Pharmacol 172 (17):4380–4390, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Custodis F, Baumhäkel M, Schlimmer N, List F, Gensch C, Böhm M, Laufs U. Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 117 (18):2377–2387, 2008. [DOI] [PubMed] [Google Scholar]


