Supplemental Digital Content is available in the text.
Keywords: clinical decision-making, critical care, Delphi technique, intensive care units, patient care planning
Objectives:
To develop a consensus framework that can guide the process of decision-making on continuing or limiting life-sustaining treatments in ICU patients, using evidence-based items, supported by caregivers, patients, and surrogate decision makers from multiple countries.
Design:
A three-round web-based international Delphi consensus study with a priori consensus definition was conducted with experts from 13 countries. Participants reviewed items of the decision-making process on a seven-point Likert scale or with open-ended questions. Questions concerned terminology, content, and timing of decision-making steps. The summarized results (including mean scores) and expert suggestions were presented in the subsequent round for review.
Setting:
Web-based surveys of international participants representing ICU physicians, nurses, former ICU patients, and surrogate decision makers.
Patients:
Not applicable.
Interventions:
Not applicable.
Measurements and Main Results:
In three rounds, respectively, 28, 28, and 27 (of 33 invited) physicians together with 12, 10, and seven (of 19 invited) nurses participated. Patients and surrogates were involved in round one and 12 of 27 responded. Caregivers were mostly working in university affiliated hospitals in Northern Europe. During the Delphi process, most items were modified in order to reach consensus. Seven items lacked consensus after three rounds. The final consensus framework comprises the content and timing of four elements; three elements focused on caregiver-surrogate communication (admission meeting, follow-up meeting, goals-of-care meeting); and one element (weekly time-out meeting) focused on assessing preferences, prognosis, and proportionality of ICU treatment among professionals.
Conclusions:
Physicians, nurses, patients, and surrogates generated a consensus-based framework to guide the process of decision-making on continuing or limiting life-sustaining treatments in the ICU. Early, frequent, and scheduled family meetings combined with a repeated multidisciplinary time-out meeting may support decisions in relation to patient preferences, prognosis, and proportionality.
On almost 75,000 ICU beds across Europe, critically ill patients are cared for every day (1). On most of these days, a decision to continue treatment is taken, implicitly or explicitly. In at least 10% of all ICU patients, however, the decision is made to limit life-sustaining ICU treatments (2, 3). Although some decisions concerning life-prolonging therapies may seem straightforward, most decisions are the result of a complex process of decision-making.
In 2003 and 2019, the prospective multicenter ETHICUS studies on end-of-life decisions in the ICU gave valuable insights into the variability and complexity of life-sustaining treatment decisions across European ICUs (3, 4). The variability between and within countries on those decisions as well as heterogeneity in prevalence of withdrawal of life-sustaining treatments within one ICU is only decreasing slowly (2, 4–6). The reason for this variation is multifactorial. Patient-related factors (i.e., age, comorbidities), geographic factors (e.g., southern Europe vs central/northern Europe), and ICU characteristics all impact the tendency to make or avoid decisions on life-sustaining therapy (3, 4, 7–9). In addition, physician-related factors can cause discordances in prognostic estimates, and unknown preferences of the patient can cause hesitation in decision-making (10–12).
Although decisions are inevitably individual, they should always be the result of a careful process. The decision-making process minimally involves a stepwise practice of gathering and interpreting information, weighing different options, and ultimately taking a (shared-) evidence-based and personalized decision (13). Engaging in this process can both minimize subjectivity and biases and maximize ICU team, patient, and surrogate involvement (10, 14).
There is limited evidence on the required elements of the decision-making process on continuing or limiting ICU treatment and how each element contributes to a careful process and decision (15, 16). Strategies based on frequent caregiver-family meetings with predefined topics and integrated within standard ICU care may improve process measures like “time to decision” and reduce nonbeneficial treatment days (16). In addition, it has been shown that adequate communication, including the opportunity to challenge the appropriateness of care within ICU teams, is a prerequisite for the delivery of appropriate care (17).
Because several strategies contributing to careful decision-making have been described in the past, we hypothesized that it would be possible to integrate this knowledge into a framework that can support the process of decision-making on treatment intensity in the ICU. The use of a framework promotes transparency of the process for all stakeholders, minimizes the effect of coincidence, and encourages patients and surrogates to participate in decision-making (18).
The aim of this study was to develop a framework that can support the decision-making process to continue, withhold, or withdraw life-sustaining treatments in ICU patients.
MATERIALS AND METHODS
We conducted a three-round web-based Delphi consensus study, based on evidence on decision-making strategies, in accordance with guidelines and in analogy with recently performed ICU Delphi studies (16, 19–22). The Delphi technique is widely used in health research to obtain consensus in serial surveys, referred to as rounds (23). Key elements of the technique are: 1) expert participants, 2) anonymity and individuality, and 3) providing a summary of results of the former round at the start of the subsequent round.
Ethical approval was granted from the Institutional Review Board of the University Medical Centre Utrecht, The Netherlands (protocol number: 16/508), University Hospital Ghent, Belgium (BC/2368 LBG), and the University Hospital Oslo, Norway (17/16124). Consent was waived, except for the Norwegian and Belgian participants who provided a written consent.
Expert Panel Recruitment
Experts were defined as having theoretical knowledge or practical experience with the decision-making process on continuing, withholding, or withdrawing life-sustaining treatments in the ICU. To develop a framework that would comply with the needs of all stakeholders, the expert panel consisted of: 1) ICU physicians, 2) ICU nurses, and 3) former ICU patients and surrogate decision makers. Physicians who were either member of the section on Ethics of the European Society of Intensive Care Medicine or known for their interest in ethics (clinically or scientifically) were primarily invited to participate by email. Snowball sampling, participants recommending acquaintances, was used to recruit additional physicians and nurses. Intended participants received an email containing a summary of the relevant literature and the purpose, design, and time investment of the study.
The former ICU patients and surrogate decision makers were invited through five participating hospitals in four countries. Patients were eligible if they had been admitted to the ICU for more than 7 days in the past 2 years. Surrogate decision makers were eligible when their family member met the former criteria and was discharged alive. For practical reasons, Dutch or English proficiency was required. Patients and surrogate decision makers were treated as one expert group.
Delphi Design
The study was designed to consist of three rounds. Between rounds, data were summarized and analyzed anonymously by an independent researcher (J.S.) using Typeform and IBM SPSS Statistics for Windows, version 25.0 (IBM, New York, NY) (Fig. 1). Refinements made to the survey or framework were discussed within the core research group (J.S., M.C.K., J.v.D., D.v.D.). Only participants who completed the previous round were invited to subsequent rounds. Participation could be withdrawn at any time without reason. No financial compensation was offered. Consensus was a priori defined as more than 70% agreement or a mean Likert score more than or equal to 4 (on a scale of 1–7). No consensus encompassed less than 70% agreement or a mean Likert score of less than 4. Items with 30–70% consensus were refined based on recommendations and feedback from participants and presented in the subsequent round. Elements with less than 30% agreement were regarded as irrelevant and excluded.
Figure 1.
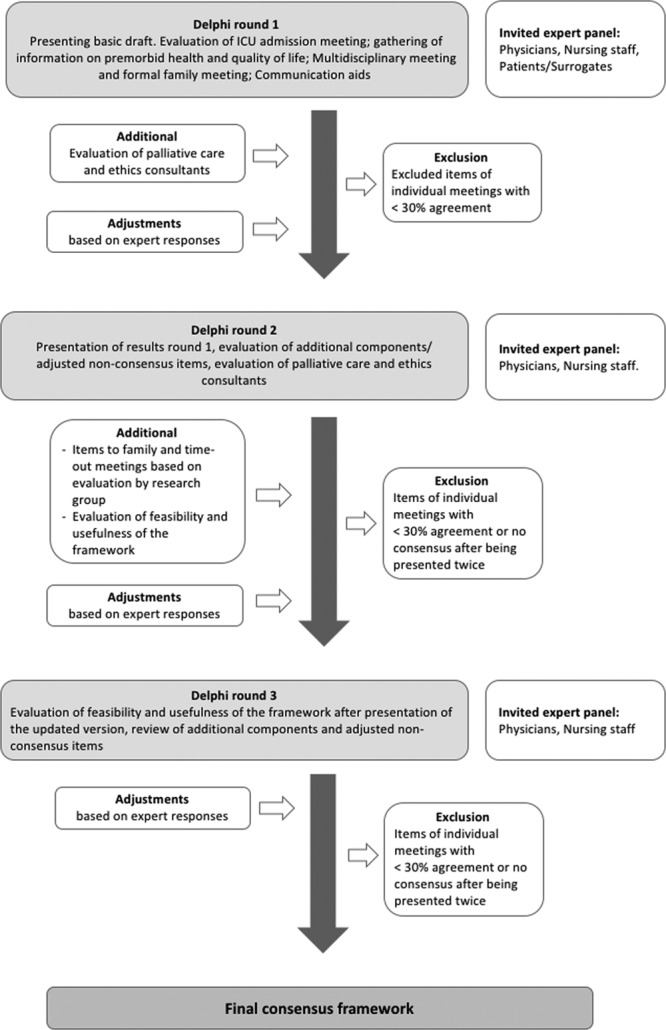
Flowchart Delphi process. Graphical representation of the methods of the Delphi consensus process.
Survey Design
A complete description of the survey design is described in the supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/F308).
An online survey was designed based on an outline of decision-making steps (elements) for which the participants were asked to specify the content through defining “items,” timing, and contributors (Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CCM/F308). The survey consisted of statements, questions using a seven-point Likert scale (seven is most positive), and open-ended questions.
The online survey was pilot-tested in eight experts, professionals, and surrogate decision makers, not participating in the Delphi study itself. The survey could be completed via a personal access-code ensuring single completion.
Patients and surrogate decision makers received a paper copy of the first round of the survey in laymen’s terms with a prepaid return-envelop. They were given the opportunity to reflect on the basic draft and encouraged to share their view on the decision-making process. It was prespecified to involve them only in round one to minimize the burden. To maximize their input, their survey was enriched with more open-ended questions.
“Round one” aimed at getting insight into expert’s views on the optimal ICU decision-making process, focused on communication, gathering of background information, and the use of communication aids and tools. Importance and feasibility of each element were assessed.
During “round two,” the ICU physicians and nurses were provided the results and feedback from the first round, revised items (without consensus), and additional items based on recommendations. Questions on the importance, feasibility, role, and triggers to involve palliative care teams and ethics consultants were added.
During “round three,” the refined version of the decision-making framework was presented for feedback regarding usefulness and feasibility. Round three also finalized evaluation of items without consensus as well as additional recommendations that had been made by participants.
Statistical Reporting
Descriptive statistics (SPSS, version 25.0; IBM Corp) were used to analyze and report data, including percentage agreement, mean Likert score, equimedian (defined as the median weighted by size of group), and interquartile ranges when appropriate.
RESULTS
Seventy-nine experts consented to participate after the invitational email, of which 28 physicians, 12 nurses, and 12 patients and surrogate decision makers completed round one (Table 1; details in Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/F308). The physicians and nurses were experienced caregivers, predominantly working in university affiliated hospitals. Ninety-two percent of all experts were from Europe. Former ICU patients and surrogate decision makers completing the survey were from the Netherlands, United Kingdom, and Belgium. Results are presented in Figure 2, A and B, Table 2, and in the following paragraphs. Detailed results on each element are presented in Figure S2 and Table S2 (Supplemental Digital Content 1, http://links.lww.com/CCM/F308).
TABLE 1.
Characteristics of Experts
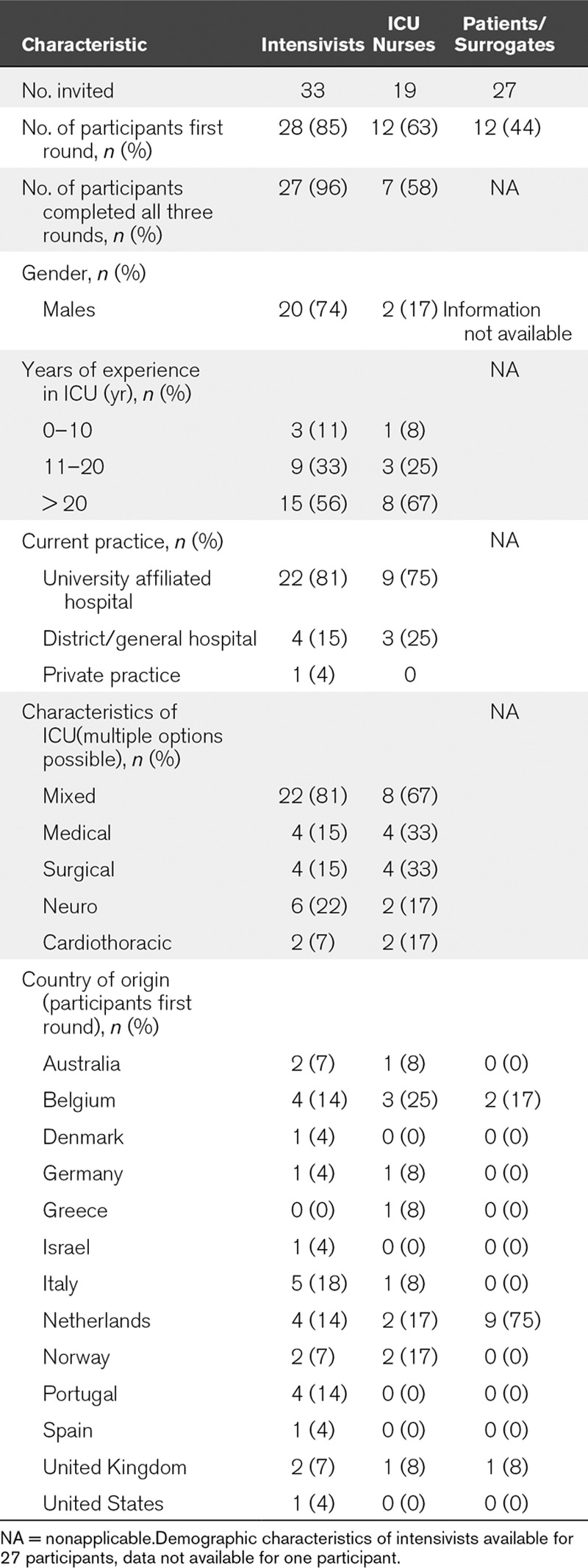
Figure 2.
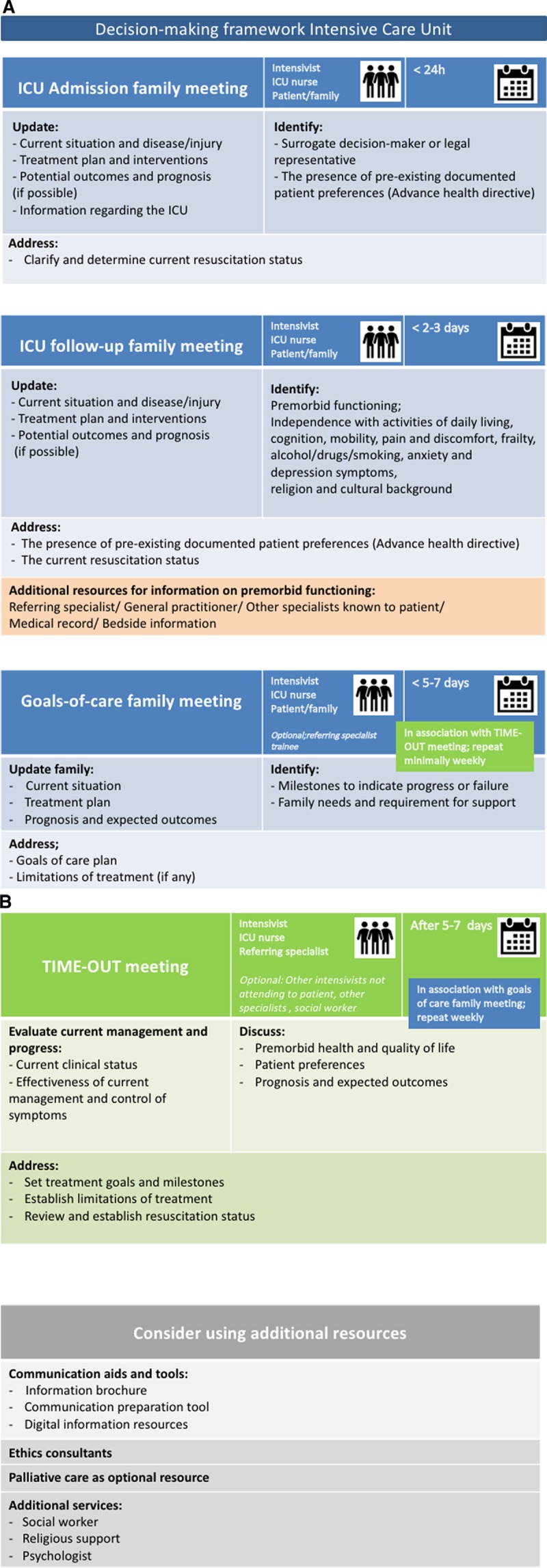
Suggested format of the decision-making framework to use in practice. A, The three family meetings (admission, ICU follow-up and goals-of-care). B, The TIME-OUT meeting and additional resources.
TABLE 2.
Description of the Results of the Consensus Process Per Round
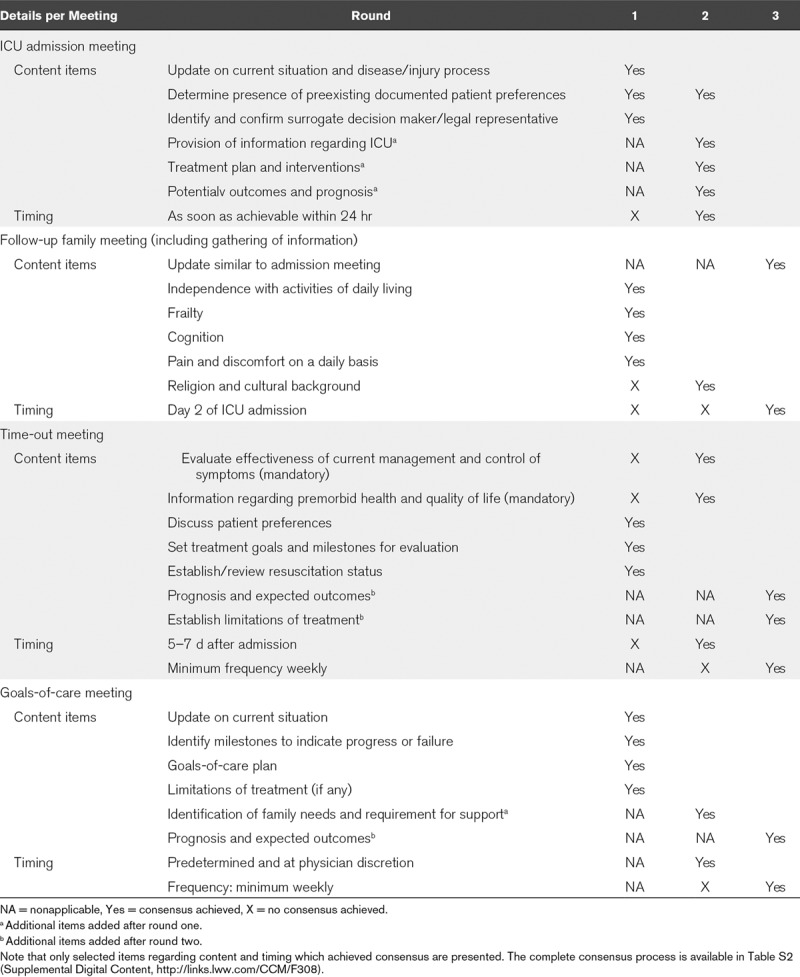
“In round one,” 52 of the 79 invited experts responded (66%). Consensus was reached on importance and feasibility of an admission meeting, gathering of information regarding premorbid health, and quality of life, a multidisciplinary meeting and a formal family meeting. No consensus was reached on their timing. Consulting the general practitioner and other specialists to gather background information was considered important. In addition, using family meetings was chosen over questionnaires as a source of information. There was consensus on the use of an informational brochure and communication preparation tool, as opposed to the use of video. Excluded items referred to mandatory participants of individual meetings.
“After round one,” the framework was modified to a structure of four elements; three family meetings and a multidisciplinary caregivers meeting. This meeting was retitled “time-out meeting” to elucidate the goal of the meeting, namely to address whether to continue, withhold, or withdraw life-sustaining treatments. The item on religion and cultural background was rephrased to assess inclusion in the framework. New propositions regarding the timing of the individual meetings were made based on responses. Four additional items of the admission meeting and family meetings, additional options to support the gathering of information, as well as a recommendation for additional services available to patients/surrogates were added based on recommendations from participants.
“In round two,” 38 of 40 experts responded (95%). Consensus was reached on the four additional items included in the admission and formal family meeting and on three of four additional options to support the gathering of information. Neither additional services to support the family nor the use of validated questionnaires to support gathering of information reached consensus. Six of the 13 previous nonconsensus items, which were adjusted to choose optional versus mandatory inclusion in the framework, remained without consensus.
Importance, but not feasibility, of palliative care was established together with futility as a trigger. All other suggestions regarding palliative care did not achieve consensus. The importance and feasibility of the availability of an ethics consultant was established in an advisory role regarding ethics, policies, and legal implications, as well as resolution of conflict.
Only the timing of the admission- and time-out meeting reached consensus.
“After round two,” the gathering of information was included as an item of the follow-up family meeting based on the suggestions of participants, where the timing was proposed to be day 2 or 3. The “formal family meeting” was retitled to “goals-of-care meeting” to emphasize the objective of this meeting. Three additional items related to prognosis and treatment limitations were added to the family and time-out meeting.
The described role of palliative care was adjusted to evaluate whether a protocolized role in the decision-making process would be appropriate. The previously presented triggers were combined into “poor prognosis” as a single trigger. The trigger for ethics involvement was proposed to be conflict where other means had been ineffective.
The use of video was rephrased to “include digital information resources,” in order to include the use of internet resources.
“In round three,” 34 of 38 experts responded (89%). The framework was found useful and feasible to support the decision-making process in the ICU, and it was established that the framework should be made available to patients and surrogate decision makers.
The three items on discussing prognosis and limitations of treatment during time-out- and goals-of-care meeting achieved consensus. Consensus was reached on recommending the availability of religious support, a social worker, and psychologist to support the family as well as the use of digital information resources. It was established that the palliative care team should not have a mandatory protocolled role in the ICU.
The minimum frequency of both the time-out and the goals-of-care meetings was agreed upon to be weekly.
Statistics
For each option provided in the various rounds, the mean and equimedian on the seven-point Likert scale and percentage agreement on dichotomous questions were presented in the subsequent rounds. Data are presented in the Table S2 (Supplemental Digital Content 1, http://links.lww.com/CCM/F308).
Practical Implications Concerning the Use of the Suggested Framework
The final result of the three rounds is presented in Figure 2, A and B and consists of (in summary): 1) an ICU admission meeting, aimed to inform the family and clarify treatment goals and resuscitation status; 2) a family follow-up meeting, inform and gather information on premorbid functioning; 3) a goals-of-care meeting, defining milestones and treatment goals in relation to patient preferences and values; and 4) a (recurrent) time-out meeting where caregivers address prognosis and proportionality together with goals and/or limitations of treatment. At admission, the framework would be offered to the patient/surrogate, informing them about the process of communication and decision-making steps including the agenda for each meeting. The local version of the framework can be enriched with available, recommended measures such as family support services.
DISCUSSION
We conducted a three-round International Delphi consensus study with physicians, nurses, former ICU patients, and surrogate decision makers from 13 countries, who cocreated a decision-making framework describing the content and timing of four evidence- and practice-based decision-making steps.
Interpretation of the Framework
The created framework should be considered as an indicative aid to enhance timely, personalized, and proportional decision-making, allowing the opportunity to modify the timing of the various elements according to patient trajectory. Although our expert sample was relatively small and skewed toward experienced European caregivers, the content and timing of the four decision-making steps largely align with international evidence and practice.
Framework in Relation to Scientific Evidence
The admission meeting, the follow-up meeting, and the weekly goals-of-care meeting all emphasize the importance of communication with patients or surrogate decision makers. This is supported by previous studies, as early patient and family participation improves satisfaction with decision-making, decreases decisional conflict (24–27), and is associated with a decreased length of stay for patients who ultimately die in the ICU (28). Furthermore, it may lead to a reduction in depressive symptoms in family members and ICU caregivers (29).
Another consistent item in the framework for each family meeting is eliciting and reassessing patient treatment preferences. Studies have shown that this is currently often omitted (15, 30, 31).
The time-out meeting, as the fourth element of the framework, can work as a cognitive aid. By discussing factors known to influence prognosis, physicians are stimulated to use analytical thinking before they use their intuition to estimate outcome for a specific patient (32, 33). In addition, weighing risks and benefits helps to structure the decision-making process, promotes more unbiased decisions, and improves prognostic accuracy (34).
The absence of palliative care team involvement in the framework is remarkable because previous studies have shown its effect on length of stay and quality of communication (35–38). A possible explanation is that evidence on palliative care interventions mainly arises from Northern America, whereas our experts were predominantly working in Europe. Delphi participants mentioned that intensivists should have these competences themselves.
Framework in Practice
The timing of the various meetings may appear late. After multiple Delphi rounds, only a small minority perceived the meetings as being too late. Therefore, the suggested timing could be regarded as the latest suitable timing, also granting the opportunity to advance meetings according to patient trajectory.
In addition, the formal role of family members in decision-making varies across countries and our expert sample may have not covered all strategies. Shared decision-making strategies, as opposed to paternalistic strategies, allows all stakeholders including the patient’s substitutes to share responsibility (38). This approach may promote earlier surrogate involvement than is suggested in the framework.
Since the experts were encouraged to draft a framework applicable to most ICU patients, some items were rejected for mandatory inclusion. However, addressing premorbid functioning at admission or evaluating burden and rehabilitation planning during the time-out meeting can be very important and might be already valuable practice in some ICUs.
Strengths and Weaknesses
This study has several strengths. First, we followed the reporting standard for Conducting and Reporting of Delphi Studies ensuring clear consensus criteria and descriptions of designing subsequent rounds (22). Second, the survey was first piloted, to test understanding of the participants. Third, the items included in the framework are evidence-based (16). Fourth, the Delphi experts originated from 13 countries reflecting different regions, various cultural backgrounds, and several types of ICUs. Fifth, former ICU patients and surrogate decision makers participated in the development of the framework ensuring compliance with patients’ needs and expectations. Sixth, the use of refining comments, for example, the use of additional resources to provide information on ICU admission was valued very important by patients and surrogate decision makers, therefore represented in the next round and subsequently included in the framework.
This study also has limitations. First, by design, the results are influenced by the participating experts. The snowball sampling method might have introduced a sampling bias resulting in the majority of representatives being overall very experienced, from North-European countries and working in university affiliated hospitals, which might impede generalization in other regions worldwide. The lack of consensus on some items could reflect cultural differences regarding end-of-life decisions. Second, we are not informed about the background of the patient and surrogates and how decision-making was perceived in their situation. In addition, their response rate was lower than expected and they were only involved in the first round. Including patients and surrogate decision makers in subsequent rounds could have influenced the framework, although most of their remarks are incorporated in the final version. Third, only 58% of nurses completed all three rounds.
Future research should especially consider the applicability of the framework in various regions throughout the world and the different perspectives of patients and surrogates (including surrogates of deceased patients).
CONCLUSIONS
International physicians, nurses, patients, and surrogates generated a consensus-based framework to guide the process of decision-making on continuing or limiting life-sustaining treatments in the ICU. Early, frequent, and scheduled family meetings combined with a repeated multidisciplinary time-out meeting may support decisions in relation to patient preferences, prognosis, and proportionality.
ACKNOWLEDGMENTS
We would like to acknowledge all participants: T. Aasmundstad, J. van den Akker, T. S. Andersen, A. Artigas, J. Benbenishty, D. Benoit, T. Boeter, V. Bosschem, E. Bull, J. Butler, A. Carneiro, G. Citerio, R. Curtis, C. Danbury, L. De Bus, P. Depuyt, B. Frommlett, A. Galazzi, L. Gale, R. Gerritsen, G Grasselli, C. Groeninx van Zoelen, E. Indseth, N. Latronico, A. Michalsen, J. Millo, L. Mavrommati, M. Mol, R. Moreno, S. Oeyen, F. Pais Silva, E. Picetti, D. Poole, P. Povoa, M. Reade, A. Robertsen, D. Tjan, M. Trout, F. Van Lierde, C. Verdonck, and all patients and surrogate decision makers.
Supplementary Material
Footnotes
Drs. Kerckhoffs and Senekal contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the LIFE-PRIORITY foundation of the European Society of Intensive Care Medicine (King Baudouin Foundation: 2017-j5920780-208088). There was no involvement in design, conduction, or reporting of the study.
Drs. Kerckhoffs and van Dijk’s institutions received funding from the LIFEPRIORITY foundation of the European Society of Intensive Care Medicine (ESICM) (King Baudouin Foundation 2017-j5920780-208088). Dr. Michalsen received funding from ESICM. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rhodes A, Ferdinande P, Flaatten H, et al. The variability of critical care bed numbers in Europe. Intensive Care Med 2012; 38:1647–1653 [DOI] [PubMed] [Google Scholar]
- 2.Mark NM, Rayner SG, Lee NJ, et al. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: A systematic review. Intensive Care Med 2015; 41:1572–1585 [DOI] [PubMed] [Google Scholar]
- 3.Sprung CL, Cohen SL, Sjokvist P, et al. ; Ethicus Study Group: End-of-life practices in European intensive care units: The Ethicus study. JAMA 2003; 290:790–797 [DOI] [PubMed] [Google Scholar]
- 4.Sprung CL, Ricou B, Hartog CS, et al. Changes in end-of-life practices in European intensive care units from 1999 to 2016. JAMA 2019; 322:1692–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnato AE, Tate JA, Rodriguez KL, et al. Norms of decision making in the ICU: A case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med 2012; 38:1886–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piers RD, Azoulay E, Ricou B, et al. ; APPROPRICUS Study Group of the Ethics Section of the ESICM: Perceptions of appropriateness of care among European and Israeli intensive care unit nurses and physicians. JAMA 2011; 306:2694–2703 [DOI] [PubMed] [Google Scholar]
- 7.Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med 1998; 158:1163–1167 [DOI] [PubMed] [Google Scholar]
- 8.Azoulay E, Metnitz B, Sprung CL, et al. ; SAPS 3 investigators: End-of-life practices in 282 intensive care units: Data from the SAPS 3 database. Intensive Care Med 2009; 35:623–630 [DOI] [PubMed] [Google Scholar]
- 9.Benoit DD, Jensen HI, Malmgren J, et al. ; DISPROPRICUS study group of the Ethics Section of the European Society of Intensive Care Medicine: Outcome in patients perceived as receiving excessive care across different ethical climates: A prospective study in 68 intensive care units in Europe and the USA. Intensive Care Med 2018; 44:1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson DJ, Truog RD. The luck of the draw: physician-related variability in end-of-life decision-making in intensive care. Intensive Care Med 2013; 39:1128–1132 [DOI] [PubMed] [Google Scholar]
- 11.Poulton B, Ridley S, Mackenzie-Ross R, et al. Variation in end-of-life decision making between critical care consultants. Anaesthesia 2005; 60:1101–1105 [DOI] [PubMed] [Google Scholar]
- 12.Wilson ME, Rhudy LM, Ballinger BA, et al. Factors that contribute to physician variability in decisions to limit life support in the ICU: A qualitative study. Intensive Care Med 2013; 39:1009–1018 [DOI] [PubMed] [Google Scholar]
- 13.Kon AA, Davidson JE, Morrison W, et al. Shared decision-making in intensive care units. Executive summary of the American College of Critical Care Medicine and American Thoracic Society Policy Statement. Am J Respir Crit Care Med 2016; 193:1334–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook D, Rocker G, Marshall J, et al. ; Level of Care Study Investigators and the Canadian Critical Care Trials Group: Withdrawal of mechanical ventilation in anticipation of death in the intensive care unit. N Engl J Med 2003; 349:1123–1132 [DOI] [PubMed] [Google Scholar]
- 15.Scheunemann LP, Cunningham TV, Arnold RM, et al. How clinicians discuss critically ill patients’ preferences and values with surrogates: An empirical analysis. Crit Care Med 2015; 43:757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerckhoffs MC, Kant M, van Delden JJM, et al. Selecting and evaluating decision-making strategies in the intensive care unit: A systematic review. J Crit Care 2019; 51:39–45 [DOI] [PubMed] [Google Scholar]
- 17.Piers RD, Azoulay E, Ricou B, et al. ; Appropricus Study Group of the Ethics Section of the European Society of Intensive Care Medicine: Inappropriate care in European ICUs: Confronting views from nurses and junior and senior physicians. Chest 2014; 146:267–275 [DOI] [PubMed] [Google Scholar]
- 18.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014; 1:CD001431. [DOI] [PubMed] [Google Scholar]
- 19.Turnbull AE, Sepulveda KA, Dinglas VD, et al. Core domains for clinical research in acute respiratory failure survivors: An international modified Delphi consensus study. Crit Care Med 2017; 45:1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Needham DM, Sepulveda KA, Dinglas VD, et al. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med 2017; 196:1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 2014; 67:401–409 [DOI] [PubMed] [Google Scholar]
- 22.Jünger S, Payne SA, Brine J, et al. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat Med 2017; 31:684–706 [DOI] [PubMed] [Google Scholar]
- 23.Linstone HA, Turoff M. The Delphi Method: Techniques and Applications. Digital copy of original version (1975). 2002Newark, NJ, Addison-Wesley. [Google Scholar]
- 24.Chiarchiaro J, Buddadhumaruk P, Arnold RM, et al. Prior advance care planning is associated with less decisional conflict among surrogates for critically ill patients. Ann Am Thorac Soc 2015; 12:1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med 2007; 356:469–478 [DOI] [PubMed] [Google Scholar]
- 26.Kodali S, Stametz RA, Bengier AC, et al. Family experience with intensive care unit care: Association of self-reported family conferences and family satisfaction. J Crit Care 2014; 29:641–644 [DOI] [PubMed] [Google Scholar]
- 27.Heyland DK, Rocker GM, Dodek PM, et al. Family satisfaction with care in the intensive care unit: Results of a multiple center study. Crit Care Med 2002; 30:1413–1418 [DOI] [PubMed] [Google Scholar]
- 28.Majesko A, Hong SY, Weissfeld L, et al. Identifying family members who may struggle in the role of surrogate decision maker. Crit Care Med 2012; 40:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quenot JP, Rigaud JP, Prin S, et al. Suffering among carers working in critical care can be reduced by an intensive communication strategy on end-of-life practices. Intensive Care Med 2012; 38:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiarchiaro J, Ernecoff NC, Scheunemann LP, et al. Physicians rarely elicit critically ill patients’ previously expressed treatment preferences in intensive care units. Am J Respir Crit Care Med 2017; 196:242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheunemann LP, Ernecoff NC, Buddadhumaruk P, et al. Clinician-family communication about patients’ values and preferences in intensive care units. JAMA Intern Med 2019; 179:676–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: A systematic review. BMC Med Inform Decis Mak 2016; 16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiegler MP, Ruskin KJ. Decision-making and safety in anesthesiology. Curr Opin Anaesthesiol 2012; 25:724–729 [DOI] [PubMed] [Google Scholar]
- 34.Kahneman D. Thinking Fast and Slow. 2011New York, NY, Farrar, Straus and Giroux. [Google Scholar]
- 35.Braus N, Campbell TC, Kwekkeboom KL, et al. Prospective study of a proactive palliative care rounding intervention in a medical ICU. Intensive Care Med 2016; 42:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslakson R, Cheng J, Vollenweider D, et al. Evidence-based palliative care in the intensive care unit: A systematic review of interventions. J Palliat Med 2014; 17:219–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercadante S, Gregoretti C, Cortegiani A. Palliative care in intensive care units: Why, where, what, who, when, how. BMC Anesthesiol 2018; 18:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis JR, Treece PD, Nielsen EL, et al. Randomized trial of communication facilitators to reduce family distress and intensity of end-of-life care. Am J Respir Crit Care Med 2016; 193:154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprung CL, Truog RD, Curtis JR, et al. Seeking worldwide professional consensus on the principles of end-of-life care for the critically ill. The Consensus for Worldwide End-of-Life Practice for Patients in Intensive Care Units (WELPICUS) study. Am J Respir Crit Care Med 2014; 190:855–866 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


