Abstract
The protozoan genus Leishmania, causative agent of human leishmaniasis, results in significant death, disability, and disfigurement around the world. The parasite is transmitted to a mammalian host by a sand fly vector where it develops as an intracellular parasite within macrophages. This process requires the acquisition of nutritional iron and heme from the host as Leishmania lack the capacity for de novo heme synthesis and do not contain cytosolic iron storage proteins. Proteins involved in Leishmania iron and heme transport and metabolism have been identified and shown to be crucial for parasite growth and replication within the host. Consequently, a detailed understanding of how these parasites harness host pathways for survival may lay the foundation for promising new therapeutic intervention against leishmaniasis.
Keywords: nutritional immunity, macrophage, parasites, porphyrins, anemia
Leishmania parasites have dual requirements for nutritional iron and heme
Leishmania spp. are the protozoan parasites from the Trypanosomatidae family that cause leishmaniasis (reviewed in [1]), a disease spectrum that can vary from cutaneous lesions to visceralizing disease. Visceral leishmaniasis is fatal if untreated in over 95% of cases. However, the cutaneous form of the disease is often self-healing in humans, but in some cases can progress to severe disseminated cutaneous leishmaniasis. Leishmania is endemic in 98 countries throughout the world and has been spreading from rural to urban areas in recent years. Currently, an estimated 12 million people are infected with Leishmania and close to one billion people may be at risk worldwide. There is no established vaccine and the treatments available can be highly toxic, expensive and/or of limited effectiveness.
Leishmania alternates between insect and mammalian hosts, going through dramatic changes as they transition from the midgut and proboscis of the sand fly to the phagolysosomal vacuoles of the mammalian macrophages with shifts in temperature, pH, and availability of nutrients such as iron and heme (Box1) [2–4]. Iron and heme are essential in many conserved metabolic pathways including electron transport, gas synthesis and sensing, and signal transduction [5, 6]. Leishmania must acquire these nutrients from their hosts since they do not have cytosolic iron storage proteins [7] and lack the capacity for heme synthesis [8, 9]. Furthermore, free iron and heme are cytotoxic due to their ability to generate reactive radicals [10]. Consequently, Leishmania must not only rely on host iron and heme for survival but they must also regulate intracellular iron and heme levels to prevent toxicity as they lack any identifiable means to store these nutrients. In the current review we will discuss recently discovered players of iron and heme metabolism pathways. We will specifically address the following main topics:
Box 1. Leishmania life cycle.
Leishmania parasites can be morphologically distinguished into two distinct life-forms during their life cycle, the amastigote and the promastigote, associated with two hosts (Figure I). In the mammalian host, the obligate intracellular amastigote form is typically found in the PV, a compartment analogous to phagolysosomes, of macrophages. Leishmania is transmitted by hematophagous insects from the Psychodidae family during a blood meal by ingesting peripheral macrophages harboring Leishmania amastigotes along with blood or lymph. Once in the insect’s digestive tract, these forms are released and differentiate into procyclic promastigotes. These forms multiply intensely by binary division and colonize the digestive tract of the insect vector. When they reach a large number, they differentiate into infectious, fast-moving metacyclic promastigote forms, and invade the anterior portions of the esophagus and proventriculum of the sand fly. In the next blood meal, regurgitation of the aspirated material ensures the inoculation of infective forms into a new vertebrate host. In the tegument of this new host, the parasites are phagocytosed by macrophages and differentiate into amastigotes, completing the life cycle of the parasites and their propagation [13, 91]. In addition to macrophages, neutrophils, monocyte-derived dendritic cells and fibroblasts have also been reported to be involved in the uptake of Leishmania [92–94].
Iron and heme availability for the intracellular parasite
Iron and heme acquisition, intracellular regulation and metabolism by Leishmania
Leishmania iron and heme pathways as drug targets
Host iron and heme availability during the Leishmania life cycle
Leishmania are heme auxotrophs (see Glossary) as they contain only the last three steps of heme synthesis and therefore must rely on host heme, or heme precursors, for sustenance [8]. In the insect midgut, heme is abundant following blood meals as a result of the breakdown of hemoglobin inside the peritrophic matrix, which contains the ingested erythrocytes alongside macrophages harboring parasites. The gradual decrease of the hemoglobin levels entails the secretion of Leishmania chitinases that are able to degrade the peritrophic membrane, allowing parasite migration to the anterior midgut [11]. Interestingly, hemoglobin digestion and depletion of heme and iron [12] provides exogenous signals that trigger parasite differentiation from dividing noninfective forms into nondividing infective metacyclic parasites. The insect regurgitates metacyclic promastigotes during blood-feeding along with other salivary compounds on to the skin of a mammalian host. These forms are phagocytosed by macrophages where they differentiate into amastigotes in the parasitophorous vacuole (PV) of macrophages (Box 1). Therefore, intracellular replication within macrophages is critical for leishmaniasis pathogenesis, and requires hijacking host iron and heme [13].
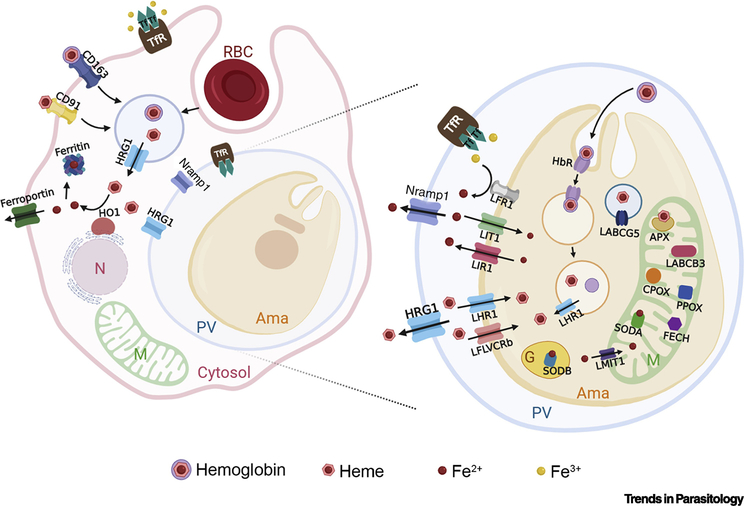
Macrophages play a critical role in iron storage and recycling of senescent red blood cells (RBCs) [14]. About 90% of the body iron is obtained by recycling of RBCs (erythrophagocytosis), at a rate of about five million senescent RBC per second. Since each RBC contains about 1.2 billion molecules of heme, macrophages recycle an astonishing 5×1015 heme each second [15]. During erythrophagocytosis, hemoglobin digestion in the phagolysosome leads to heme release and translocation to the cytosol through the Heme-Responsive Gene 1 (HRG1/SLC48A1) [16] (Figure 1, Key Figure). Cytosolic heme is then broken down by heme oxygenase 1 (HMOX1) (Figure 1) [17, 18]. Macrophages can also scavenge hemoglobin and heme released during intravascular hemolysis [19]. Hemoglobin binds to haptoglobin and is cleared by the macrophage CD163 receptor, while heme binds to hemopexin and is internalized by the macrophage CD91 receptor (Figure 1) [20, 21]. Interestingly, mice infected with L. donovani, the causative agent of visceral leishmaniasis, are anemic and show increased erythrophagocytosis [22]. Leishmania infection also induces HMOX1 and attenuates production of reactive oxygen species (ROS) aiding parasite survival and replication [23, 24], while upregulating CD163 expression [25].
Figure 1, Key Figure. Leishmania replicates within host macrophages.
When hemoglobin is degraded through erythrophagocytosis, heme is translocated to the cytosol by HRG1 and broken down by HO1 (HMOX1). The released iron is stored in ferritin or exported by ferroportin. Hemoglobin and heme can also be internalized via CD163 and CD91 endocytosis, respectively. Leishmania PVs resemble phagolysosomes that contain HRG1, which is then likely to compete with the parasites for heme-iron. NRAMP1 is postulated to transport iron from the phagolysosome although the source of this iron is unknown. Transferrin (Tf) bound iron is internalized by the transferrin receptor (TfR) via endocytosis which fuses with the phagosome. Fe+3 is released by the low pH into the phagosomal lumen. Leishmania utilizes a ferric reductase (LFR1) to reduce Fe+3 to Fe+2, and a ferrous iron transporter (LIT1) transport Fe+3 into the cytosol. Cellular iron levels in the amastigotes are maintained by the iron exporter LIR1. Leishmania must also acquire heme, via LHR1 and LFLVCRb, or hemoglobin, via endocytosis of the hemoglobin receptor (HbR). In the parasite, iron is translocated into the mitochondria by Leishmania mitoferrin 1 (LMIT1). In the mitochondria, heme and iron are required by Leishmania ascorbate peroxidase (APX) and superoxide dismutase A (SODA), respectively. The parasite mitochondria also contains coproporphyrinogen oxidase (CPOX), protoporphyrinogen oxidase (PPOX), ferrochelatase (FECH) and LABCB3, all required for heme biosynthesis from a precursor. Abbreviations: RBC, red blood cell; N, nucleus; M, mitochondria; PV, parasitophorous vacuole; Ama, amastigote; G, glycosome; Tf, transferrin; TfR, transferrin receptor; HRG1, heme-responsive gene 1; HO1; heme oxygenase 1; LFR1, Leishmania ferric reductase 1; LIT1, Leishmania iron transporter 1; LIR1, Leishmania iron regulator 1; LHR1, Leishmania heme response 1; LFLVCRb, Leishmania FLVCRb; HbR, hemoglobin receptor; LMIT1, Leishmania mitoferrin 1; CPOX, coproporphyrinogen oxidase; PPOX, protoporphyrinogen oxidase; FECH, ferrochelatase; SODA, superoxide dismutase A; SODB, superoxide dismutase B, APX, Leishmania ascorbate peroxidase, LABCB3, Leishmania ABCB3; LABCG5, Leishmania ABCG5.
Intracellular Leishmania are at the center of this critical hub of heme and iron metabolism residing within macrophages and replicating within PVs with access to different iron and heme sources. Although the phagosome containing engulfed RBC is not supposed to directly fuse with the PV [26], they may share components. For example, lysosomes containing hemoglobin/haptoglobin and/or heme/hemopexin complexes could also bind the PV as “exogenous” heme is important for the replication of the intracellular amastigotes [27]. Furthermore, macrophages synthetize their own heme and so, “endogenous” heme or its precursors may be redirected to the PV through a yet-to-be identified transporter. This pathway is indeed important for the multiplication of the parasite, at least in vitro [28]. Finally, heme could be also obtained from the proteolysis of macrophage heme-containing proteins in the PV [29].
The vertebrate host restricts iron and heme availability to the pathogen – a process termed nutritional immunity [14]. Thus, successful colonization requires the pathogen to circumvent host iron and heme-related defense mechanisms. Leishmania PVs resemble phagolysosomes that contain HRG1 (Figure 1) [16]. Therefore, macrophage HRG1 is likely to compete with the parasite heme acquisition pathway. Moreover, Nramp1 is proposed to deplete the iron from the PV denying the parasites access to iron for survival. Nramp1 is a divalent cation transporter that mediates the pH-dependent efflux of iron from the phagolysosome (Figure 1), so the transferrin-bound iron internalized by the transferrin receptor (TFR) (Figure 1) is transported out of the phagolysosome by Nramp-1 [30]. It was shown that mutations in Nramp-1 confers susceptibility to intracellular pathogens such as Leishmania, Mycobacteria and Salmonella [31–33]. These findings support the importance of optimal iron levels for parasite virulence.
Leishmania iron and heme acquisition pathways
In the past decade, several parasite pathways involved in iron and heme acquisition were identified and characterized. In the PV, iron is in the oxidized ferric (Fe3+) form complexed with transferrin. Despite the capability of using Fe3 + from transferrin, lactoferrin and other chelators, Leishmania prefers reduced ferrous (Fe2+) iron. Studies with L. chagasi promastigotes, provided the first evidence that these parasites are capable of converting intracellular Fe3 + into soluble iron (Fe2+) in a NADPH-dependent way for direct Fe2+ uptake by the parasite [34].
Analysis of the L. major genome, led to the identification of the Leishmania Iron Transporter 1 (LIT1), characterized as a ZIP-family iron transporter expressed in the plasma membrane of intracellular amastigotes [35] (Figure 1). LIT1 knockout parasites revealed the importance of this transporter for Leishmania virulence, since LIT1 absence led to the inability of the parasite to replicate in macrophages, and to generate lesions in in vivo infections [36]. Later, Leishmania Ferric Reductase 1 (LFR1) (Figure 1) was identified as the parasite membrane protein that reduces the Fe3+ to Fe2+, the biologically active soluble form translocated across membranes [37, 38]. LFR1 is essential for iron transport by LIT1, and both are induced by iron deficiency [7, 39]. Notably, LIT1 and LFR1 share significant homology with plant iron-regulated transporter 1 (IRT1) [35] and ferric oxidoreductase 1 (FRO1), respectively.
Beyond iron, Leishmania lacks a complete heme synthesis pathway and are therefore dependent on host heme [40]. The first parasite heme importer was discovered in L. amazonensis due to its homology with the Caenorhabditis elegans Heme-Responsive Gene 4 (HRG4) [41]. This transmembrane permease, termed Leishmania Heme Response 1 (LHR1) (Figure 1) localizes to the parasite plasma membrane and endocytic compartments, and promotes heme import into the cytosol [42]. A LHR1 null mutant (double knockout) is not viable, but mice infected with parasites in which one LHR1 allele was knocked-out (single knockout) developed smaller lesions with lower parasite load, confirming the importance of LHR1 in parasite virulence [43]. Recently, a new transporter involved in heme uptake has been identified in L. major [44]. This major facilitator superfamily (MFS) member, named LFLVCRb (Figure 1), has significant homology with the mammalian heme transporter FLVCR2 [45]. LFLVCRb, is located at the plasma membrane of the parasite and promotes the uptake of free heme, a function confirmed by its heterologous expression in Xenopus laevis oocytes. LFLVCRb is essential for the parasites as CRISPR/Cas9-mediated knockout cells were only obtained when LFLVCRb was expressed from an episome. Furthermore, single knockout of LFLVCRb showed replication defects and impaired virulence in vivo. The presence of two heme importers is perplexing. A possible explanation for this could be that each heme transporter contributes heme to distinct subcellular pools within the parasite.
Leishmania promastigotes can also obtain heme derived from hemoglobin, which is internalized through a receptor-mediated [46], clathrin-dependent endocytic process [47]. This hemoglobin receptor (HbR) is a 46 kDa protein located in the flagellar pocket of the parasite [46] (Figure 1) that is also functional in the amastigote stage [48]. Its sequence and biochemical characterization suggest that HbR is a hexokinase involved in hemoglobin binding and rapid endocytosis [49]. This is surprising as trypanosomatid hexokinases are glycolytic enzymes expected to be located in the glycosome of the parasite. It remains to be established if HbR ATP binding and/or hexokinase activity are required for hemoglobin endocytosis. Hemoglobin binding to the extracellular N-terminal part of HbR, results in rapid internalization through the endocytic pathway. Rab5 mediates the early endosomal fusion of hemoglobin-containing vesicles [50] whereas their fusion with late endosomes and the lysosome requires Rab7 [51] mediated in part by the HbR cytoplasmic C-terminus [50]. Once in the lysosome, hemoglobin is digested [51] and the released heme moiety could be imported into the cytosol through LHR1 [52]. Since heme and hemoglobin can also be a source of iron for the intracellular amastigote [48], LHR1 is upregulated after iron deprivation [42]. The co-existence of iron and heme acquisition pathways could facilitate parasite survival under conditions of iron limitation [29].
Intracellular iron and heme homeostasis in Leishmania
Heme and iron intracellular pool
Heme is a hydrophobic cytotoxic macrocycle and is unlikely to exist as a “free” molecule in the cell. Most of the heme is inaccessible as it is bound to hemoproteins that are essential for the survival of the cell (P450, globin, cytochrome). The rest of the heme resides in a “labile pool”, i.e. available for trafficking by as yet unspecified mechanisms, and can be used for regulation [53, 54]. Currently, it is unknown in what form the heme exists in this labile pool, or how it is mobilized in Leishmania. Probably, heme distribution involves the presence of heme carriers or chaperones to avoid its toxicity, but the nature of these parasite proteins has not been elucidated. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been shown to play a heme-chaperone role in mammal cells, driving labile heme to its protein targets [55]. Although GADPH is a glycosomal enzyme (gGADPH) in Leishmania, a cytosolic form (cGADPH) exits in many species such as L donovani, L. mexicana and L. infantum, whereas cGADPH has evolved into a pseudogene in L. major and is absent in L. braziliensis [56]. All Leishmania GADPHs (gGADPH, cGADPH and pseudo cGADPH) conserve the histidine known to interact with heme in mammalian GADPH [55], so they could function as a heme chaperone. Recently, it has been shown that G-quadruplexes DNA sequences sequester heme in vivo, protecting cells from the pathophysiological consequences of free heme [57]. G-quadruplexes are DNA secondary structures that take part in the regulation of gene expression [57]. Interestingly, G-quadruplexes have been recently described in Leishmania and other heme auxotrophic parasites [58], and proposed as novel drug targets [58, 59]. Therefore, Leishmania G-quadruplexes could also sequester heme regulating gene expression in the parasite [57]. In addition, Leishmania could store heme reserves to be used during heme, and maybe iron, starvation [28]. Although the nature of these heme reserves remains to be elucidated, they could be equivalent to the heme storage in the reservosomes of the related protozoan parasite Trypanosoma cruzi [60]. Indeed, it is known that Leishmania can accumulate porphyrins in discrete vesicles called porphyrinosomes [61].
Intracellular trafficking of iron and heme
Once in the parasite cytosol, iron must be translocated into the mitochondria, where it is required as a cofactor of several enzymes and for the biosynthesis of iron/sulfur clusters. This is carried out by Leishmania Mitoferrin 1 (LMIT1) (Figure 1), a homolog of the mitochondrial iron importer genes mrs3 (yeast) and mitoferrin-1 (human) that is highly conserved among trypanosomatids [62]. Iron uptake into the mitochondria is essential for this organelle’s proper function, and a single knockout of LMIT1 impairs parasite growth, differentiation, virulence and enhances promastigote sensitivity to ROS [62]. Iron is also required in the glycosomes, which contain iron superoxide dismutase (Fe-SOD) (Figure 1)[63]. Glycosomes are trypanosomatid-exclusive organelles compartmentalizing several essential pathways [64, 65]. How iron is imported into the glycosomes is currently unknown.
Unlike intracellular iron metabolism pathways in eukaryotes, heme trafficking pathways are not well understood. In mammals, ABCB6 has been proposed to transport heme intermediates into the mitochondria. Leishmania LABCB3 (Figure 1), an essential protein required for the virulence of the parasite, has significant homology with human ABCB6, localizes to mitochondrial membranes, and supports heme synthesis when supplemented with exogenous protoporphyrin IX [66]. However, complementation assays in ATM1 yeast mutants suggested that LABCB3 may not be responsible for mitochondria heme import but in the biogenesis of cytosolic iron/sulfur clusters, as implied for yeast ATM1 and human ABCB7 [66].
Iron and heme utilization
Several Leishmania enzymes use iron as a cofactor, including the distinct isoforms of superoxide dismutase (SOD). In the mitochondria, SODA protects this organelle from oxidative stress [67] and deletion of one allele impairs parasite differentiation and virulence [68]. The glycosomal isoforms SODB1 and SODB2 are differentially expressed along the parasite life cycle and were also shown to be important for Leishmania virulence protecting this parasite organelle from superoxide toxicity [63]. Unlike host SODs, trypanosomatid SOD isoforms rely exclusively on iron as an essential cofactor [69].
Leishmania heme enzymes are involved in redox reactions (detailed in [9]). Some of them are required for essential metabolic pathways such as the respiratory complexes in the mitochondrion and the synthesis of sterols and polyunsaturated fatty acids in the endoplasmic reticulum (reviewed in [70]). In the mitochondria, several cytochromes form part of the respiratory chain being involved in electron transfer during oxidative phosphorylation. These include the c-type cytochromes, Cytc and Cytc1, in which heme is covalently attached to the apoprotein. In Leishmania (and the rest of trypanosomatids), this link is via a single-thioether bond within a F/AXXCH heme binding motif in contrast to the two thioether bonds with a CXXCH heme binding motif that occurs in most other cases, including mammals [70]. Cytochrome c oxidase (CcO, Complex IV) contain heme a, which is synthesized from heme. In contrast to the parasite-specific cytochrome c biogenesis, heme a synthesis is similar between trypanosomatids and mammals, as demonstrated for T. cruzi [71]. In the endoplasmic reticulum, heme also forms part of essential cytochromes. Cytochrome b5 (Cytb5) is the electron donor required for the biosynthesis of polyunsaturated fatty acids (PUFAs), an essential process in the parasite. Lanosterol-14-α-demethylase (CYP51), member of the cytochrome P450 (cytP450) superfamily, is involved in the synthesis of ergosterol in the endoplasmic reticulum (ER), an essential component of parasite membranes since these parasites do not produce cholesterol. Some antifungal azoles that inhibit ergosterol biosynthesis by binding to CYP51 have antileishmanial activity. In addition, heme is also the prosthetic group of the Leishmania ascorbate peroxidase (APX), which is located in the inner membrane of the mitochondrion [72]. In the absence of catalase and glutathione (GSH) peroxidases in Leishmania, APX plays a central role in redox homeostasis by detoxifying mitochondrial hydrogen peroxide (H2O2) (reviewed in [73]). APX protects the parasite from the highly oxidizing environment of the PV [73]. In addition, APX-regulated mitochondrial H2O2 has a role in promoting differentiation of virulent forms in L. major [74] and L. amazonensis [72]. However, APX-mediated control of ROS levels is essential for survival during all life-cycle stages in L. amazonensis but not in L. major, indicating potential differences in the mechanisms for pathogenesis development between these two Leishmania species. Interestingly, APX null L. major parasites are viable and even have increased virulence in macrophage and animal models [74], but APX KO promastigotes of L. amazonensis cannot be generated and the deletion of a single APX allele inhibits their replication inside the macrophage and their ability to induce cutaneous lesions in mice [72].
Iron and heme intracellular balance
The Leishmania genome appears to encode a frataxin homolog, which could function in mitochondrial iron-sulfur biogenesis, but not a ferritin homolog, a gene encoding for cytosolic iron storage protein [7, 75]. The recently identified Leishmania Iron Regulator 1 (LIR1) (Figure 1) showed how these parasites can prevent accumulation of free iron in the cytosol to avoid toxicity. LIR1 is a major facilitator superfamily plasma membrane protein that was identified due to its similarity to plant nodulin-like proteins [75–77]. Nodulin-like proteins are implicated in the transmembrane transport of iron and share sequence similarity with vacuolar iron transporters (VITs) of plants that mediate sequestration of cytosolic iron into the vacuoles [78]. Also, VITs are homologous to the yeast CCC1 membrane transporter characterized by its role in translocating manganese and iron into vacuoles – the yeast equivalent of lysosomes [79, 80]. Heterologous expression of LIR1 in Arabidopsis thaliana causes decrease in the iron in the leaves. LIR1 knockout parasites show a significant impairment in iron efflux and virulence, and increased sensitivity to iron toxicity demonstrating a critical role of LIR1 in maintaining iron homeostasis in Leishmania [27, 75].
At present, heme efflux has not been described in the parasite, although its existence cannot be ruled out as Leishmania genome contains several genes encoding putative homologs of mammalian heme exporters from the FLVCR and ABC families. Heme can also be used as an iron source by Leishmania amastigotes [48] suggesting that the parasite could degrade heme. However, the mechanism by which Leishmania cleaves the heme moiety to release iron remains elusive. Leishmania genome does not contain any obvious homologs for heme oxygenases, enzymes responsible for heme breakdown, despite a report suggesting the existence of heme oxygenase enzyme activity in the parasite [81]. It is possible that the Leishmania enzyme may be a non-canonical heme degrading enzyme. It has also been suggested that since the PV compartment is acidic, contains proteases and/or ROS, breakdown of the heme moiety from hemoglobin may not require an enzyme [48, 82, 83].
Heme metabolism
The heme auxotrophy of trypanosomatids is due to the complete loss in the early kinetoplastids evolution of the broadly conserved eight-step heme biosynthetic pathway present in most aerobic organisms. Unlike Trypanosoma, in Leishmania the heme pathway was partially rescued by lateral gene transfer (LGT) from γ-proteobacteria of genes coding the last three enzymes of the pathway: coproporphyrinogen oxidase (CPOX), protoporphyrinogen oxidase (PPOX) and ferrochelatase (FECH) (Figure 2) [9, 84, 85]. The function of L. major PPOX [86] and FECH [28] have been confirmed in complementation assays performed in Escherichia coli. Although the presence of this partial pathway may seem enigmatic, as Leishmania cannot produce an earlier precursor of the route, it allows intracellular amastigotes to use heme precursors scavenged from the macrophage to fulfill their heme requirements [28] (Figure 2). Indeed, L. major lmfech knockout intracellular amastigotes were unable to replicate in vitro [28]. However, these knockout parasites were still able to form normal lesions in a murine model of cutaneous leishmaniasis, suggesting that exogenous heme sources could be more relevant in this experimental model. In addition, lmfech knockout parasites developed normally inside the insect vector. Together, these studies demonstrate that even though L. major can synthesize heme de novo from host heme precursors, this activity is dispensable and therefore not a suitable target for leishmaniasis treatment [28].
Figure 2. Leishmania heme auxotrophy.
Schematic illustration of mammalian and Leishmania heme biosynthesis pathway. Enzymatic substrates and products are depicted in black/white boxes. Heme synthesis in the mammalian mitochondria starts in the mitochondrial matrix with the synthesis of 5-aminolevelunic acid (ALA) by 5-aminolevulinate synthase (ALAS). ALA is exported to the cytosol where it undergoes enzymatic modifications for generation of coproporphyrinogen III (COPRO III). COPRO III is transported back to mitochondrial matrix where the last three enzymatic reactions occur. Leishmania mitochondria contains only the last three enzymes of this pathway, reacquired by LGT by from γ-proteobacteria: coproporphyrinogen oxidase (CPOX), protoporphyrinogen oxidase (PPOX) and ferrochelatase (FECH). Abbreviations: PV, parasitophorous vacuole; Ama, amastigote; ALA, 5-aminolevelunic acid; ALAS, 5-aminolevulinate synthase; PGB, porphobilinogen; PGBD, porphobilinogen synthase; HMB, hydroxymethylbilane; UROS, uroporphyrinogen III synthase; URO, uroporphyrinogen III; UROD, uroporphyrinogen decarboxylase; COPRO III, coproporphyrinogen III; CPOX, coproporphyrinogen oxidase; PPGIX, protoporphyrinogen IX; PPOX, protoporphyrinogen oxidase; PPIX, protoporphyrin IX; FECH, ferrochelatase.
Concluding Remarks
Given that most identified iron and heme metabolism-related proteins were shown to be critical for Leishmania homeostasis and virulence, these pathways could be promising drug targets for leishmaniasis therapeutic intervention. LIT1, LFR1, LIR1, LHR1, LFLVCRb and LABCB3 have low sequence conservation with their corresponding mammalian orthologs [35, 37, 42, 44, 66, 75] establishing them as strong candidates for the design of molecules that specifically target these Leishmania membrane proteins without impacting host metabolism. Proteins involved in the uptake of heme could be also interesting vaccine candidates [87]. Anti-HbR antibodies were detected in kala-azar patients’ sera. Immunization of hamsters and mice with bifunctional HbR-encoding DNA induced sterile protection against visceral leishmaniasis [88]. This complete protection was due to the protective Th1 response and the suppression of disease-promoting cytokines induced by HbR. Moreover, anti-HbR antibodies generated were able to kill extracellular parasites, and could have a role in blocking the transmission of the parasite, although it remains to be established if this effect is due to the blocking of hemoglobin endocytosis or by complement-mediated lysis [88].
Iron deficiency is the most common nutritional disorder in the world and a top ten risk factor for losing healthy years of life in developed and developing countries [89]. A current discussion regarding the treatment of iron deficiency anemia with iron supplementation points that this treatment may increase the risk of infections [90]. Although estimates of the prevalence of anemia vary widely and accurate data are often lacking, it can be assumed that in resource-poor areas, like many areas affected by leishmaniasis, significant proportions of the population are anemic. Therefore, uncovering host-pathogen factors in this “tug-of-war” (see Outstanding Questions) is crucial for defining novel therapeutic strategies for overcoming iron deficiency anemia while thwarting parasitic infections, such as leishmaniasis.
OUTSTANDING QUESTIONS.
What is the function of the last three enzymes of the heme synthesis pathway in Leishmania?
How is iron released from heme in Leishmania?
What form of heme do intracellular amastigotes hijack from their host? Are these pathways similar between visceral and cutaneous leishmaniasis?
How may heme regulate parasite differentiation?
Does Leishmania have mechanisms for heme storage, for example, heme reservosomes?
Can Leishmania G-quadruplex sequester heme or can heme alter gene expression in the parasite through its binding to G-quadruplex sequences?
What is the impact of host iron and heme status on leishmaniasis progression?
Will iron supplementation increase the risk of leishmaniasis?
How is the PV generated and what host and parasite proteins are directed to the PV?
Is iron essential for Leishmania glycosome function? How is iron and/or heme translocated into this organelle?
Will elucidating iron and heme homeostasis pathways in Leishmania help illuminate analogous pathways in other Neglected Tropical Disease parasites?
Figure I (in Box 1). Leishmania life cycle stages.
During blood feeding, the sand fly acquires Leishmania infected macrophages. The Leishmania amastigotes then convert into procyclic promastigotes in the sand fly midgut. The procyclic promastigotes differentiate into infective, non-dividing metacyclic promastigotes, which invade the anterior portions of the esophagus and proventriculum of the sand fly. In the next blood feeding, the sand fly regurgitates metacyclic promastigotes in the mammal host tegument, together with various salivary components. The metacyclic promastigotes are then acquired by macrophages, or one of several possible cell types at the bite site, such as neutrophils, fibroblasts and monocyte-derived dendritic cells. In neutrophils, the engulfed metacyclics may be later released and acquired by other cell types. In the macrophage phagolysome, the parasites differentiate into amastigotes and replicate allowing reinfection of local cells.
HIGHLIGHTS.
Leishmania are heme auxotrophs and do not have any obvious iron storage systems, making scavenging for host heme and iron an essential adaptation for survival and virulence.
The infected macrophage restricts iron and heme availability to the parasite – a process termed nutritional immunity.
Uncovering crucial components that permit Leishmania to extract these nutrients from the host could define novel therapeutic strategies to combat leishmaniasis and other parasites that are heme auxotrophs.
Acknowledgements
We thank Ahyun Hong for assistance with the figures. This work was supported in part by funding from the National Institutes of Health DK85035, AI06797 and ES025661 (IH); the Spanish Ministerio de Economía y Competitividad SAF2016-80228-R and from Spanish Junta de Andalucía BIO1786 (JMPV); and the Brazilian Research Foundation Fundação de Amparo à Pesquisa do Estado de São Paulo FAPESP 2017/23933-3 (MFLS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
GLOSSARY
- Aerobic
an organism that requires oxygen
- Auxotroph
an organism that is unable to synthesize a particular compound and must obtain it form the environment
- Cofactor
a factor such as a coenzyme or a metallic ion required for an enzyme’s activity
- Endocytosis
the uptake by a cell of particles, fluids or specific macromolecules through plasma membrane invaginations
- Endogenous
originated within the cell
- Episome
an extrachromosomal genetic element
- Exogenous
originated outside the cell
- G-quadruplexes
secondary structures formed in nucleic acids by sequences that are rich in guanine. They can be located in the telomeres, vulnerable terminal ends of chromosomes
- Hematophagous
an organism that feeds on blood
- Heterologous
derived from a different organism
- Homologous
that share a common evolutionary origin
- Isoforms
families of functionally related proteins that differ slightly in their amino acid sequences, encoded by genes derived from a single ancestral gene
- Labile pool
transitory, active, available pool of ions
- Lateral gene transfer (LGT)
is the “horizontal” movement of genetic material between organisms, which is not through the “vertical” transmission of parents’ DNA to their offspring
- Parasitophorous Vacuole (PV)
a membrane-enclosed intracellular structure that harbors Leishmania (or amastigote) parasites in the host
- Phagolysosome
a membrane-enclosed intracellular structure formed during phagocytosis by the fusion of phagosome with lysosome
- Senescent
that is deteriorating
Footnotes
Author Information.
IH is the President and Founder of Rakta Therapeutics Inc. (College Park, MD), a company involved in the development of heme transporter-related diagnostics. He declares no other competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burza S et al. (2018) Leishmaniasis. Lancet 392 (10151), 951–970. [DOI] [PubMed] [Google Scholar]
- 2.Schaible UE and Kaufmann SH (2004) Iron and microbial infection. Nat Rev Microbiol 2, 946–953. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Othman R et al. (2014) Leishmania-mediated inhibition of iron export promotes parasite replication in macrophages. PLoS Pathog 10, e1003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki JI et al. (2019) The impact of arginase activity on virulence factors of Leishmania amazonensis. Curr Opin Microbiol 52, 110–115. [DOI] [PubMed] [Google Scholar]
- 5.Hamza I and Dailey HA (2012) One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta 1823 (9), 1617–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponka P (1999) Cell biology of heme. Am J Med Sci 318 (4), 241–56. [DOI] [PubMed] [Google Scholar]
- 7.Flannery AR et al. (2013) Pathways of iron acquisition and utilization in Leishmania. Curr Opin Microbiol 16 (6), 716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CS and Chang KP (1985) Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol Biochem Parasitol 16 (3), 267–76. [DOI] [PubMed] [Google Scholar]
- 9.Koreny L et al. (2013) Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog 9 (1), e1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon SJ and Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10, 9–17. [DOI] [PubMed] [Google Scholar]
- 11.Schlein Y and Jacobson RL (1994) Haemoglobin inhibits the development of infective promastigotes and chitinase secretion in Leishmania major cultures. Parasitology 109 ( Pt 1), 23–8. [DOI] [PubMed] [Google Scholar]
- 12.Mittra B et al. (2013) Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J Exp Med 210, 401–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossage SM et al. (2003) Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. Int J Parasitol 33 (10), 1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares MP and Hamza I (2016) Macrophages and Iron Metabolism. Immunity 44 (3), 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korolnek T and Hamza I (2015) Macrophages and iron trafficking at the birth and death of red cells. Blood 125 (19), 2893–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White C et al. (2013) HRG1 Is Essential for Heme Transport from the Phagolysosome of Macrophages during Erythrophagocytosis. Cell Metab 17 (2), 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaby C et al. (2005) A physiological model to study iron recycling in macrophages. Exp Cell Res 310 (1), 43–53. [DOI] [PubMed] [Google Scholar]
- 18.Delaby C et al. (2012) Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis. PloS one 7 (7), e42199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen MJ et al. (2010) Hemoglobin and heme scavenger receptors. Antioxid Redox Signal 12 (2), 261–73. [DOI] [PubMed] [Google Scholar]
- 20.Kristiansen M et al. (2001) Identification of the haemoglobin scavenger receptor. Nature 409 (6817), 198–201. [DOI] [PubMed] [Google Scholar]
- 21.Hvidberg V et al. (2005) Identification of the receptor scavenging hemopexin-heme complexes. Blood 106 (7), 2572–2579. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto A et al. (2016) Hemophagocytosis in Experimental Visceral Leishmaniasis by Leishmania donovani. PLoS Negl Trop Dis 10 (3), e0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham NK et al. (2005) Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infect Immun 73 (12), 8322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha S et al. (2019) Exploits Macrophage Heme Oxygenase-1 To Neutralize Oxidative Burst and TLR Signaling-Dependent Host Defense. J Immunol 202 (3), 827–840. [DOI] [PubMed] [Google Scholar]
- 25.Silva RL et al. (2017) sCD163 levels as a biomarker of disease severity in leprosy and visceral leishmaniasis. PLoS Negl Trop Dis 11 (3), e0005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veras PS et al. (1992) Transfer of zymosan (yeast cell walls) to the parasitophorous vacuoles of macrophages infected with Leishmania amazonensis. J Exp Med 176 (3), 639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar A et al. (2019) Intracellular iron availability modulates the requirement for Leishmania Iron Regulator 1 (LIR1) during macrophage infections. Int J Parasitol 49 (6), 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orrego LM et al. (2019) Heme synthesis through the life cycle of the heme auxotrophic parasite. FASEB J, fj201901274RR. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho S et al. (2009) Heme as a source of iron to Leishmania infantum amastigotes. Acta Trop 109 (2), 131–5. [DOI] [PubMed] [Google Scholar]
- 30.Cellier MF (2012) Nutritional immunity: homology modeling of Nramp metal import. Adv Exp Med Biol 946, 335–51. [DOI] [PubMed] [Google Scholar]
- 31.Canonne-Hergaux F et al. (1999) The Nramp1 protein and its role in resistance to infection and macrophage function. Proc Assoc Am Physicians 111 (4), 283–9. [DOI] [PubMed] [Google Scholar]
- 32.Fritsche G et al. (2007) Modulation of macrophage iron transport by Nramp1 (Slc11a1). Immunobiology 212 (9–10), 751–7. [DOI] [PubMed] [Google Scholar]
- 33.Nairz M et al. (2009) Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol 11 (9), 1365–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson ME et al. (2002) Leishmania chagasi: uptake of iron bound to lactoferrin or transferrin requires an iron reductase. Exp Parasitol 100 (3), 196–207. [DOI] [PubMed] [Google Scholar]
- 35.Huynh C et al. (2006) A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med 203, 2363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacques I et al. (2010) Functional characterization of LIT1, the Leishmania amazonensis ferrous iron transporter. Mol Biochem Parasitol 170 (1), 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flannery AR et al. (2011) LFR1 ferric iron reductase of Leishmania amazonensis is essential for the generation of infective parasite forms. J Biol Chem 286, 23266–23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rocco-Machado N et al. (2019) Leishmania amazonensis ferric iron reductase (LFR1) is a bifunctional enzyme: Unveiling a NADPH oxidase activity. Free Radic Biol Med 143, 341–353. [DOI] [PubMed] [Google Scholar]
- 39.Mittra B and Andrews NW (2013) IRONy OF FATE: role of iron-mediated ROS in Leishmania differentiation. Trends Parasitol 29 (10), 489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos-Salinas J et al. (2011) A new ATP-binding cassette protein is involved in intracellular haem trafficking in Leishmania. Mol Microbiol 79 (6), 1430–44. [DOI] [PubMed] [Google Scholar]
- 41.Rajagopal A et al. (2008) Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature 453 (7198), 1127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huynh C et al. (2012) Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog 8 (7), e1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miguel DC et al. (2013) Heme uptake mediated by LHR1 is essential for Leishmania amazonensis virulence. Infect Immun 81 (10), 3620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabello-Donayre M et al. (2019) Leishmania heme uptake involves LmFLVCRb, a novel porphyrin transporter essential for the parasite. Cell Mol Life Sci 10.1007/s00018-019-03258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy SP et al. (2010) The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol Cell Biol 30 (22), 5318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sengupta S et al. (1999) Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J Biol Chem 274 (5), 2758–65. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal S et al. (2013) Clathrin-mediated hemoglobin endocytosis is essential for survival of Leishmania. Biochim Biophys Acta 1833 (5), 1065–77. [DOI] [PubMed] [Google Scholar]
- 48.Carvalho S et al. (2009) Heme as a source of iron to Leishmania infantum amastigotes. Acta Tropica 109 (2), 131–135. [DOI] [PubMed] [Google Scholar]
- 49.Krishnamurthy G et al. (2005) Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J Biol Chem 280 (7), 5884–91. [DOI] [PubMed] [Google Scholar]
- 50.Singh SB et al. (2003) Rab5-mediated endosome-endosome fusion regulates hemoglobin endocytosis in Leishmania donovani. EMBO J 22 (21), 5712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel N et al. (2008) Leishmania requires Rab7-mediated degradation of endocytosed hemoglobin for their growth. Proc Natl Acad Sci U S A 105 (10), 3980–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cabello-Donayre M et al. (2016) Trypanosomatid parasites rescue heme from endocytosed hemoglobin through lysosomal HRG transporters. Mol Microbiol 101 (6), 895–908. [DOI] [PubMed] [Google Scholar]
- 53.Yuan X et al. (2016) Regulation of intracellular heme trafficking revealed by subcellular reporters. Proc Natl Acad Sci U S A 113 (35), E5144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanna DA et al. (2016) Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc Natl Acad Sci U S A 113 (27), 7539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sweeny EA et al. (2018) Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J Biol Chem 293 (37), 14557–14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang WW et al. (2013) Role of cytosolic glyceraldehyde-3-phosphate dehydrogenase in visceral organ infection by Leishmania donovani. Eukaryot Cell 12 (1), 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray LT et al. (2019) G-quadruplexes Sequester Free Heme in Living Cells. Cell Chem Biol 10.1007/s00018-019-03258-3. [DOI] [PubMed] [Google Scholar]
- 58.Belmonte-Reche E et al. (2018) G-Quadruplex Identification in the Genome of Protozoan Parasites Points to Naphthalene Diimide Ligands as New Antiparasitic Agents. J Med Chem 61 (3), 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuffo M et al. (2019) Carbohydrate-naphthalene diimide conjugates as potential antiparasitic drugs: Synthesis, evaluation and structure-activity studies. Eur J Med Chem 163, 54–66. [DOI] [PubMed] [Google Scholar]
- 60.Lara FA et al. (2007) Heme requirement and intracellular trafficking in Trypanosoma cruzi epimastigotes. Biochem Biophys Res Commun 355 (1), 16–22. [DOI] [PubMed] [Google Scholar]
- 61.Dutta S et al. (2008) Transgenic Leishmania model for delta-aminolevulinate-inducible monospecific uroporphyria: cytolytic phototoxicity initiated by singlet oxygen-mediated inactivation of proteins and its ablation by endosomal mobilization of cytosolic uroporphyrin. Eukaryot Cell 7 (7), 1146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittra B et al. (2016) A Trypanosomatid Iron Transporter that Regulates Mitochondrial Function Is Required for Leishmania amazonensis Virulence. PLoS Pathog 12, e1005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plewes KA et al. (2003) Iron superoxide dismutases targeted to the glycosomes of Leishmania chagasi are important for survival. Infect Immun 71 (10), 5910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.da Silva MF et al. (2012) Leishmania amazonensis arginase compartmentalization in the glycosome is important for parasite infectivity. PLoS One 7 (3), e34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith DF and Parsons M (1996) Molecular biology of parasitic protozoa, IRL Press at Oxford University Press. [Google Scholar]
- 66.Martínez-García M et al. (2016) LmABCB3, an atypical mitochondrial ABC transporter essential for Leishmania major virulence, acts in heme and cytosolic iron/sulfur clusters biogenesis. Parasit Vectors 9, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Getachew F and Gedamu L (2012) Leishmania donovani mitochondrial iron superoxide dismutase A is released into the cytosol during miltefosine induced programmed cell death. Mol Biochem Parasitol 183 (1), 42–51. [DOI] [PubMed] [Google Scholar]
- 68.Mittra B et al. (2017) The iron-dependent mitochondrial superoxide dismutase SODA promotes Leishmania virulence. J Biol Chem 292 (29), 12324–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor MC and Kelly JM (2010) Iron metabolism in trypanosomatids, and its crucial role in infection. Parasitology 137 (6), 899–917. [DOI] [PubMed] [Google Scholar]
- 70.Tripodi KE et al. (2011) Role of heme and heme-proteins in trypanosomatid essential metabolic pathways. Enzyme Res 2011, 873230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchensky C et al. (2010) The Trypanosoma cruzi proteins TcCox10 and TcCox15 catalyze the formation of heme A in the yeast Saccharomyces cerevisiae. FEMS Microbiol Lett 312 (2), 133–41. [DOI] [PubMed] [Google Scholar]
- 72.Xiang L et al. (2019) Ascorbate-Dependent Peroxidase (APX) from Leishmania amazonensis Is a Reactive Oxygen Species-Induced Essential Enzyme That Regulates Virulence. Infect Immun 87 (12), e00193–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adak S and Pal S (2013) Ascorbate peroxidase acts as a novel determiner of redox homeostasis in Leishmania. Antioxid Redox Signal 19 (7), 746–54. [DOI] [PubMed] [Google Scholar]
- 74.Pal S et al. (2010) Ascorbate peroxidase from Leishmania major controls the virulence of infective stage of promastigotes by regulating oxidative stress. PLoS One 5 (6), e11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laranjeira-Silva MF et al. (2018) A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog 14 (6), e1007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denancé N et al. (2014) Emerging functions of nodulin-like proteins in non-nodulating plant species. Plant Cell Physiol 55, 469–474. [DOI] [PubMed] [Google Scholar]
- 77.Gollhofer J et al. (2011) Members of a small family of nodulin-like genes are regulated under iron deficiency in roots of Arabidopsis thaliana. Plant Physiol Biochem 49, 557–564. [DOI] [PubMed] [Google Scholar]
- 78.Kim SA et al. (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314 (5803), 1295–8. [DOI] [PubMed] [Google Scholar]
- 79.Lapinskas PJ et al. (1996) The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol Microbiol 21 (3), 519–28. [DOI] [PubMed] [Google Scholar]
- 80.Li L et al. (2001) CCC1 Is a transporter that mediates vacuolar iron storage in yeast. J Biol Chem 276, 29515–29519. [DOI] [PubMed] [Google Scholar]
- 81.Srivastava P et al. (1997) Heme metabolism in promastigotes of Leishmania donovani. Mol Cell Biochem 171 (1–2), 65–8. [DOI] [PubMed] [Google Scholar]
- 82.Gabay T and Ginsburg H (1993) Hemoglobin denaturation and iron release in acidified red blood cell lysate--a possible source of iron for intraerythrocytic malaria parasites. Exp Parasitol 77 (3), 261–72. [DOI] [PubMed] [Google Scholar]
- 83.Nagababu E and Rifkind JM (1998) Formation of fluorescent heme degradation products during the oxidation of hemoglobin by hydrogen peroxide. Biochem Biophys Res Commun 247 (3), 592–6. [DOI] [PubMed] [Google Scholar]
- 84.Cenci U et al. (2016) Heme pathway evolution in kinetoplastid protists. BMC Evol Biol 16 (1), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koreny L et al. (2010) Evolution of the haem synthetic pathway in kinetoplastid flagellates: an essential pathway that is not essential after all? Int J Parasitol 40 (2), 149–56. [DOI] [PubMed] [Google Scholar]
- 86.Zwerschke D et al. (2014) Leishmania major possesses a unique HemG-type protoporphyrinogen IX oxidase. Biosci Rep 34 (4), e00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar R and Engwerda C (2014) Vaccines to prevent leishmaniasis. Clin Transl Immunology 3 (3), e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guha R et al. (2013) Vaccination with leishmania hemoglobin receptor-encoding DNA protects against visceral leishmaniasis. Sci Transl Med 5 (202), 202ra121. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization (2019) Micronutrient deficiencies - Iron deficiency anaemia: The chalenge.
- 90.Jonker FAM et al. (2017) Anaemia, iron deficiency and susceptibility to infection in children in sub-Saharan Africa, guideline dilemmas. Br J Haematol 177 (6), 878–883. [DOI] [PubMed] [Google Scholar]
- 91.Sacks D and Kamhawi S (2001) Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol 55, 453–83. [DOI] [PubMed] [Google Scholar]
- 92.Kaye P and Scott P (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9 (8), 604–15. [DOI] [PubMed] [Google Scholar]
- 93.Paduch K et al. (2019) Resolution of Cutaneous Leishmaniasis and Persistence of. J Immunol 202 (5), 1453–1464. [DOI] [PubMed] [Google Scholar]
- 94.Cavalcante-Costa VS et al. (2019) Leishmania amazonensis hijacks host cell lysosomes involved in plasma membrane repair to induce invasion in fibroblasts. J Cell Sci 132 (6), jcs226183. [DOI] [PubMed] [Google Scholar]